Abstract
Background
The durable polymers (DP) used in first-generation drug-eluting stents (DESs) were associated with long-term cardiovascular events, and thus biodegradable polymer DESs (BP-DESs) and second-generation DP-DESs were designed to overcome this problem. In this study, we compared angiographic follow-up and long-term clinical outcomes between patients who received BP-DESs or second-generation DP-DESs.
Methods
We enrolled 436 patients with single coronary lesions who received a second-generation DP-DES or BP-DES between June 2009 and October 2012. All patients received follow-up angiography when new clinical events developed or at 9 months after index stenting. All participants received follow-up for 5 years.
Results
There were no significant differences in patient and lesion characteristics between the two groups. The 9-month angiographic follow-up showed a lower net gain in the second-generation DP-DES group (2.19 mm vs. 2.41 mm, p = 0.040), but a similar binary restenosis rate between the two groups (5.4% vs. 8.7%, p = 0.276). During the 5-year follow-up period, no significant differences were observed between the two groups in major adverse cardiac events (MACEs), cardiovascular death, nonfatal myocardial infarction (MI), target vessel revascularization (TVR), all revascularization, stent thrombosis (ST), or MACE-free survival.
Conclusions
No significant differences were observed in cardiovascular death, nonfatal MI, TVR, all revascularization, ST, or MACE-free survival between the patients undergoing single coronary artery stenting with BP-DESs and second-generation DP-DESs.
Keywords: Coronary artery disease, Drug-eluting stent, Major adverse cardiac events, Polymer
INTRODUCTION
Drug-eluting stents (DESs) are widely used in the treatment of coronary artery disease and have demonstrated significantly better outcomes than traditional bare-metal stents.1 The use of DESs in patient requiring stenting has increased in recent decades,2 however, the follow-up results have varied according to the patients’ underlying conditions, lesion characteristics, and stent design. Differences among stents including strut thickness, stent design, eluted drugs, and materials used as the drug carrier can influence the lesion outcomes. Differences in the polymers used for DESs have been reported to be a factor affecting the long-term results of post-percutaneous transluminal coronary angioplasty (PTCA).3 In first-generation DESs, durable polymers (DPs) were used as a drug carrier. However, the risks of late stent thrombosis (ST) were increased. DPs have been associated with long-term inflammation of the peri-stent tissue leading to late ST or a higher restenosis rate,4-6 and consequently biodegradable polymer DESs (BP-DESs) were developed to overcome this problem. Second-generation DP-DESs with a modified design for the stent strut and the carrier polymer were developed later, however comparisons between BP-DESs and DP-DESs have yielded varying results.7-9
Although previous studies have compared the results of BP-DESs and DP-DESs, long-term studies comparing BP-DESs and second-generation DP-DESs are limited.10 To understand the long-term differences between the clinical and angiographic results of second-generation DP-DESs and BP-DESs, we investigated the 5-year outcomes of these stents in an East Asian population.
MATERIAL AND METHODS
Patient population
This study was a retrospective analysis conducted using data from the Cardiovascular Atherosclerosis and Percutaneous TrAnsluminal INterventions (CAPTAIN) registry, which includes consecutive patients who underwent elective or emergent percutaneous coronary interventions (PCIs) at a single center. Clinical and procedural data of these patients were prospectively entered into a database. In total, 436 patients with single coronary lesions who received second-generation DP-DESs with zotarolimus eluting stent (ZES, Resolute IntegrityTM, Medtronic, Inc.) or everolimus eluting stent (EES, Xience PrimeTM, Abbott, Inc.), or BP-DESs (NoboriTM, Terumo Inc. and BioMatrixTM, Biosensors Inc.) between June 2009 and October 2012 were enrolled. All patients received follow-up angiography when new clinical events developed or at 9 months after index stenting. This study was conducted in accordance with the provisions of the Declaration of Helsinki and local regulations. All patients provided informed consent for the index stenting procedure and subsequent data collection and analysis. This study was approved by the Institutional Review Board of the Chang Gung Medical Foundation.
Interventional procedure
All patients were pretreated with a loading dose of aspirin (300 mg) and clopidogrel (300-600 mg) before catheterization. Heparin was also administered as an initial bolus dose, and the dose was adjusted according to the activated clotting time, with a target of 250-350 s. The procedures were performed according to standard techniques.11 Dual antiplatelet therapy was maintained for at least 9 months after stenting.
Angiographic analysis
Coronary angiograms were obtained in multiple views after intracoronary nitrate administration. Quantitative coronary angiographic analysis was performed by two experienced interventional cardiologists, and images were selected with an end-diastolic cine frame of the most severe stenosis and non-foreshortened projection. Reference vessel diameter, minimal luminal diameter (MLD), and percentage of diameter stenosis were measured before and after the intervention.
After index stenting, clinical follow-up was performed by telephone contact or clinic visit every 3 months for 5 years or until April 2016. All patients received follow-up angiography 9 months after the index stenting. Restenosis at 9 months was defined as stenosis of at least 50% of the luminal diameter. Myocardial infarction (MI) was defined as any typical increase above the upper range limit of biochemical markers of myocardial necrosis, with at least one of the following: ischemic symptoms, development of Q waves on electrocardiography (ECG), and ECG changes indicative of ischemia. The patients received repeat coronary revascularizations only when they had recurrent ischemic symptoms and evidence of myocardial ischemia in noninvasive stress tests. Clinically driven target vessel revascularization (TVR) was defined as any repeat revascularization at the index stenting vessel. All revascularization was defined as whether any revascularization (PCI or coronary artery bypass graft) was required at the index stenting vessel. Cardiovascular death was defined as death due to a cardiac cause (e.g., MI, heart failure, or fatal arrhythmia). ST was identified on the basis of the Academic Research Consortium definition of definite ST. We defined major adverse cardiac events (MACEs) as composite endpoints of clinically driven TVR, nonfatal MI, cardiovascular death, and ST during 5 years of clinical follow-up. Overall, 90.8% of our participants received complete follow-up for more than 2 years, and the mean follow-up period was 44.4 ± 22.4 months.
Statistical analysis
Data were prospectively collected and analyzed using the SPSS statistical software package (version 22.0, IBM, Armonk, New York, USA) for all statistical analyses. Continuous variables were expressed as mean ± standard deviation, and categorical variables were presented as numbers and relative percentages. For continuous data, the groups were compared using the t-test or Wilcoxon rank-sum test on the basis of the distribution. Categorical variables were compared using the chi-square test. The patients’ underlying condition and lesion characteristics including age, gender, hypertension, diabetes mellitus, smoking, dyslipidemia, family history of coronary artery disease, chronic kidney disease, previous stroke, previous myocardial infarction, left ventricular ejection fraction, acute coronary syndrome, multi-vessel disease, small vessel size (< 3.0 mm), lesion length (> 30 mm), polymer type, in-stent restenosis, chronic total occlusion, calcified lesion, bifurcation lesion, Ostia lesion, and complex lesion (B2/C type) were selected as risk predictors for subgroup analysis of 5-year MACEs after stenting. Multivariate Cox proportional hazard regression analysis with the forward stepwise selection process was used to determine independent predictors. MACE-free survival curves were depicted using the Kaplan-Meier method, and differences between the two groups were assessed using the log-rank test. All p values of < 0.05 were considered to be statistically significant.
RESULTS
Regarding the baseline clinical characteristics of the study population, there were no significant differences in age, sex, or underlying diseases between the two groups. The indications for coronary stenting and severity of coronary artery disease between the two groups were also similar (Table 1). The baseline angiographic characteristics and quantitative coronary analysis are listed in Table 2. No differences were observed in the treated vessel, lesion type, severity, restenotic lesions, calcification, bifurcation, ostium, and type B2/C lesions. No significant difference was observed in the stent length between the two groups (23.4 mm vs. 20.4 mm, p = 0.056). Although the stent diameter of DP-DESs was smaller than that of BP-DESs (3.12 mm vs. 3.25 mm, p = 0.001), no difference in post-PCI MLD was observed between the two groups (2.97 mm vs. 3.07 mm, p = 0.123). The prescription rate and coverage duration of dual-antiplatelet therapy (DAPT) after stenting were similar in both groups (99.5% vs. 100%, p = 1.00; 15.3 ± 12.6 months vs. 15.3 ± 13.8 months, p = 1.00). There was no significant difference in the statin prescription rate between the two groups (85.6% vs. 88.4%, p = 0.705).
Table 1. Baseline patient characteristics.
| DP-DES (n = 367) | BP-DES (n= 69) | p value | |
| Age, years old | 61.5 ± 11.7 | 60.9 ± 10.7 | 0.695 |
| Male, n (%) | 307 (83.7) | 56 (81.2) | 0.600 |
| Hypertension, n (%) | 204 (55.6) | 42 (60.9) | 0.431 |
| Diabetes mellitus, n (%) | 107 (29.2) | 21 (30.4) | 0.886 |
| Dyslipidemia, n (%) | 164 (44.7) | 23 (33.8) | 0.110 |
| Smoking, n (%) | 144 (39.2) | 28 (40.6) | 0.893 |
| Family history of CAD, n (%) | 7 (1.9) | 0 (0.0) | 0.603 |
| Chronic kidney disease, n (%) | 22 (6.0) | 7 (10.1) | 0.195 |
| Previous stroke, n (%) | 21 (5.7) | 4 (5.8) | 1.000 |
| Previous MI, n (%) | 28 (7.6) | 8 (11.6) | 0.337 |
| LVEF, (%) | 59.9 ± 12.4 | 59.4 ± 11.6 | 0.769 |
| ACS for stenting, n (%) | 174 (47.4) | 32 (46.4) | 0.896 |
| Multi-vessel disease, n (%) | 210 (57.2) | 41 (59.4) | 0.791 |
ACS, acute coronary syndrome; BP-DES, biodegradable polymer drug-eluting stent; CAD, coronary artery disease; DP-DES, second-generation durable polymer drug-eluting stent; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
Table 2. Baseline angiographic characteristics and quantitative coronary analysis.
| DP-DES (n = 367) | BP-DES (n = 69) | p value | |
| Treated vessel, n (%) | |||
| LM | 21 (5.7) | 3 (4.3) | 1.000 |
| LAD | 134 (37.1) | 32 (46.4) | 0.177 |
| LCx | 148 (40.3) | 24 (34.8) | 0.423 |
| RCA | 62 (16.9) | 10 (14.5) | 0.725 |
| In-stent restenosis lesion, n (%) | 20 (5.4) | 4 (5.8) | 0.781 |
| Chronic total occlusion, n (%) | 24 (6.5) | 4 (5.8) | 1.000 |
| Calcified lesion, n (%) | 51 (13.9) | 7 (10.1) | 0.561 |
| Bifurcation, n (%) | 32 (8.7) | 5 (7.2) | 0.817 |
| Ostial lesion, n (%) | 37 (10.1) | 7 (10.1) | 1.000 |
| B2/C lesion, n (%) | 306 (83.4) | 52 (75.4) | 0.124 |
| Stent diameter, mm | 3.12 ± 0.50 | 3.25 ± 0.50 | 0.001* |
| Stent length, mm | 23.4 ± 7.3 | 20.4 ± 5.5 | 0.056 |
| Diameter stenosis, % | |||
| Pre-PCI | 87.3 ± 12.3 | 88.1 ± 11.2 | 0.610 |
| Post-PCI | 6.8 ± 5.1 | 6.3 ± 4.2 | 0.419 |
| MLD, mm | |||
| Pre-PCI | 0.41 ± 0.41 | 0.39 ± 0.38 | 0.796 |
| Post-PCI | 2.97 ± 0.46 | 3.07 ± 0.48 | 0.123 |
| Acute gain, mm | 2.56 ± 0.55 | 2.68 ± 0.53 | 0.126 |
| DAPT, n (%) | 365 (99.5) | 69 (100) | 1.000 |
| DAPT duration, months | 15.3 ± 12.6 | 15.3 ± 13.8 | 1 |
| Statin, n (%) | 314 (85.6) | 61 (88.4) | 0.705 |
BP-DES, biodegradable polymer drug-eluting stent; DAPT, dual antiplatelet therapy; DP-DES, second-generation durable polymer drug-eluting stent; LAD, left anterior descending artery; LCX, left circumflex artery; LM, left main artery; MLD, minimal lumen diameter; PCI, percutaneous coronary intervention; RCA, right coronary artery.
We performed follow-up angiography in all patients 9 months after the index stenting. No differences were observed in acute gain and late loss; however, net gain in the second-generation DP-DES group was lower than that in the BP-DES group (2.19 mm vs. 2.41 mm, p = 0.040). Moreover, the restenosis rate between the two groups was similar (5.4% vs. 8.7%, p = 0.276). The patients received mean clinical follow-up for 44.4 ± 22.4 months, and no significant differences were observed in cardiovascular death, nonfatal MI, TVR, all revascularization, or ST (Table 3). In Kaplan-Meier analysis, there was no significant difference in MACE-free survival rate between the two groups (log-rank test, p = 0.631) (Figure 1). Furthermore, we found that in-stent restenosis (ISR) lesions [hazard ratio (HR) = 2.72, 95% confidence interval (CI) = 1.34-5.55], chronic total occlusion (CTO) lesions (HR = 2.75, 95% CI = 1.29-5.82), patients with chronic kidney disease (CKD) (HR = 2.08, 95% CI = 1.01-4.27), and multivessel disease (HR = 4.05, 95% CI = 2.05-8.02) were predictors of 5-year MACEs (Table 4).
Table 3. Outcomes of 9-month angiography and 5-year clinical follow-up.
| DP-DES (n = 367) | BP-DES (n = 69) | p value | |
| Diameter stenosis (9 m), % | 18.9 ± 20.0 | 14.8 ± 19.9 | 0.120 |
| MLD (9 m), mm | 2.61 ± 0.76 | 2.79 ± 0.77 | 0.058 |
| Late loss (9 m), mm | 0.38 ± 0.62 | 0.26 ± 0.59 | 0.147 |
| Net gain (9 m), mm | 2.19 ± 0.80 | 2.41 ± 0.84 | 0.040* |
| Restenosis (9 m), n (%) | 20 (5.4) | 6 (8.7) | 0.276 |
| Cardiovascular death (5 yrs), n (%) | 4 (1.1) | 1 (1.4) | 0.579 |
| Non-fatal MI (5 yrs), n (%) | 3 (0.8) | 1 (1.4) | 0.499 |
| TVR (5 yrs), n (%) | 13 (3.5) | 2 (2.9) | 1.000 |
| All revascularization (5 yrs), n (%) | 52 (14.2) | 7 (10.1) | 0.446 |
| Stent thrombosis (5 yrs), n (%) | 0 (0.0) | 0 (0.0) | - |
| MACEs (5 yrs), n (%) | 55 (15.0) | 8 (11.6) | 0.577 |
BP-DES, biodegradable polymer drug-eluting stent; DP-DES, second-generation durable polymer drug-eluting stent; MACEs, major adverse cardiac events; MI, myocardial infarction; MLD, minimal lumen diameter; TVR, target vessel revascularization.
Figure 1.
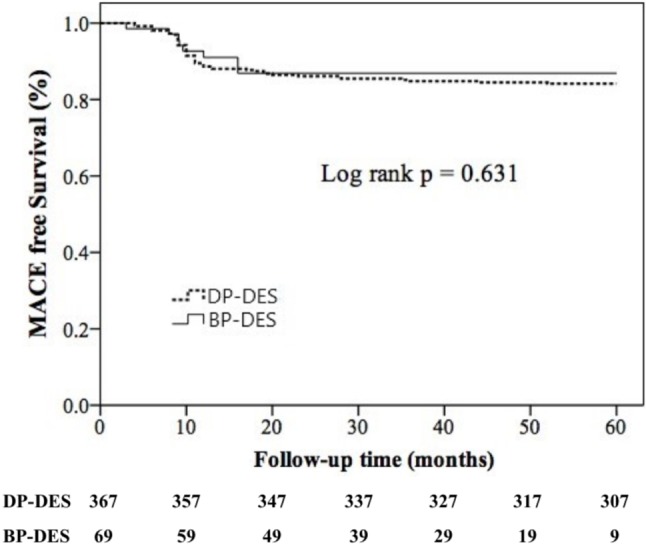
MACE-free survival between the two stent groups during 5-year follow-up. BP-DES, biodegradable polymer drug-eluting stent; DP-DES, second-generation durable polymer drug-eluting stent; MACE, major adverse cardiac events.
Table 4. Predictors of MACEs after 5-year follow-up.
| Variables | Univariate analysis | Multivariate analysis | ||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age, > 65 yrs | 0.90 | 0.54-1.50 | 0.688 | |||
| Male | 1.96 | 0.85-4.55 | 0.117 | |||
| Hypertension | 1.46 | 0.87-2.45 | 0.115 | |||
| Diabetes mellitus | 1.42 | 0.85-2.37 | 0.181 | |||
| Smoking | 0.75 | 0.45-1.27 | 0.285 | |||
| Dyslipidemia | 1.23 | 0.75-2.01 | 0.419 | |||
| Family history of CAD | 2.56 | 0.63-10.5 | 0.192 | |||
| Chronic kidney disease | 2.70 | 1.33-5.47 | 0.006 | 2.08 | 1.01-4.27 | 0.046 |
| Previous stroke | 0.58 | 0.14-2.39 | 0.453 | |||
| Previous MI | 0.72 | 0.26-1.98 | 0.525 | |||
| LVEF < 40 | 1.25 | 0.57-2.75 | 0.574 | |||
| ACS for stenting | 0.70 | 0.42-1.16 | 0.164 | |||
| Multi-vessel disease | 4.18 | 2.12-8.21 | < 0.001 | 4.05 | 2.05-8.02 | < 0.001 |
| Vessel size < 3.0 mm | 1.48 | 0.90-2.44 | 0.118 | |||
| Lesion length > 30 mm | 1.22 | 0.52-2.82 | 0.649 | |||
| BP-DES vs. DP-DES | 0.84 | 0.40-1.75 | 0.634 | |||
| In-stent restenosis lesion | 3.23 | 1.60-6.54 | 0.001 | 2.72 | 1.34-5.55 | 0.006 |
| CTO lesion | 2.49 | 1.18-5.23 | 0.016 | 2.75 | 1.29-5.82 | 0.009 |
| Calcified lesion | 1.29 | 0.66-2.54 | 0.460 | |||
| Bifurcation lesion | 0.73 | 0.27-2.01 | 0.541 | |||
| Ostial lesion | 1.32 | 0.63-2.76 | 0.467 | |||
| B2/C type lesion | 1.34 | 0.66-2.71 | 0.416 |
ACS, acute coronary syndrome; BP-DES, biodegradable polymer drug-eluting stent; CAD, coronary artery disease; CI, confidence interval; CTO, chorionic total occlusion; DP-DES, second-generation durable polymer drug-eluting stent; HR, hazard ratio; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
DISCUSSION
This study was designed to elucidate the angiographic and clinical differences between second-generation DP-DESs and BP-DESs in an East Asian population. During the 5 years of follow-up period, no significant differences were observed in cardiovascular death, nonfatal MI, TVR, all revascularization, ST, or MACE-free survival rates between the two groups.
In first-generation DESs, DPs were used as the drug carrier. The intrastent restenosis rate was significantly decreased with first-generation DESs compared with bare metal stents (BMSs), however evaluation of very late ST became a challenge. DP coatings have been reported to cause hypersensitivity vasculitis at the site of stent implantation, thereby altering the healing process.4 Furthermore, human autopsy studies have suggested that the DPs of first-generation DESs triggered chronic vessel inflammation, delayed hypersensitivity reactions, and caused chronic fibrin deposition, resulting in an increased risk of impaired endothelialization and very late ST.5,6 Concerns over the safety of DPs are therefore based on long-term in vivo results.
The absence of DPs from the DES platform seems to mitigate late restenosis and thrombosis, and a BP coating on DESs is regarded to be a promising step forward in polymer technology.12,13 A BP-DES is designed to provide antiproliferative benefits before functionally transforming into a BMS once drug delivery and polymer biodegradation are complete.14 Thus, BP-DESs can provide a non-thrombogenic coating for exposed stent surfaces and reduce the risk of adverse cardiac events related to ST after the first year of stenting, whereas DPs may be related to ST.15-17 In the Limus Eluted from A Durable versus ERodable Stent Coating (LEADERS) trial, biolimus-eluting stents with BPs successfully showed significantly lower 5-year definite very late ST rates than Cypher stents (relative risk = 0.31; 95% CI = 0.14-0.69; p = 0.002).18 In a pooled analysis of three randomized trials, Stefanini et al. reported a significant reduction in target lesion revascularization, MI, and very late ST with BP-DESs compared with sirolimus-eluting stents.19 A meta-analysis also demonstrated a lower rate of very late ST and an equivalent risk of MACEs with BP-DESs compared with sirolimus-based DP-DESs.20
Unlike the previous trials comparing the results of BP-DESs and first-generation DP-DESs, we enrolled patients with biolimus-eluting BP-DESs and second-generation DP-DESs in this study. Second-generation DESs were developed with a thinner strut and were coated with a thinner durable and biocompatible fluorocopolymer as a drug carrier.21,22 These second-generation fluorinated DP-based EESs and ZESs have been associated with a favorable safety profiles and lower rates of early, late, and very late ST compared with first-generation DESs and BMSs.23,24 Although BP-DESs are superior in many aspects to the first-generation DP-DESs, the results of studies comparing BP-DESs with newer DP-DESs have varied. Palmerini et al. demonstrated a higher rate of 1-year definite ST but similar rates of cardiac death, MI, and TVR with BP-DESs compared with EESs.9 Furthermore, Banagalore et al. reported a higher 1-year mortality rate with BP-DESs than with EESs.7 BPs have also been reported to require active bioresorption, which has been associated with a higher rate of inflammation during the absorption process.25,26 However, very late ST and neoatherosclerosis with associated adverse clinical outcomes have also been observed with second-generation DP-DESs.27 Optical coherence tomography (OCT) has recently been used to evaluate angiographic results. Although Tada et al. showed that the use of BP-DESs substantially decreased the risk of uncovered struts (6.8% vs. 17.5%) compared with DP-DESs,28 results of the ISAR-TEST 6 OCT study29 demonstrated similar stent coverage and apposition with BP-DESs and everolimus-based DP-DESs, as assessed through OCT at 6-8 months. During the follow-up period in the present study, the risk of ST or TVR was equally low between the two groups, which is comparable to the findings of other such studies.
Although the 9-month net gain was significantly higher in the BP-DES group in this study, no significant differences were observed in angiographic 9-month restenosis rates or clinical events up to 5 years between the two groups. This result is comparable to the finding of a meta-analysis of seven randomized clinical trials, which demonstrated equivalent safety and efficacy at 1 year with BP-DESs (Nobori) and DP-DESs.30 Other meta-analyses focusing on BP-DESs and second-generation DP-DESs have also concluded that these DESs have similar safety and efficacy profiles.10,31 In addition, a recent report of 5-year MACEs with BP-DESs and DP-DESs in the COMPARE II Trial (Abluminal Biodegradable Polymer Biolimus-Eluting Stent Versus Durable Polymer Everolimus-Eluting Stent) described similar MACE rates and results to our study.32 Moreover, the subgroup analysis in the present study showed that patients with CKD (HR = 2.08, 95% CI = 1.01-4.27), multivessel disease (HR = 4.05, 95% CI = 2.05-8.02), and lesion characteristics such as ISR lesions (HR = 2.72, 95% CI = 1.34-5.55) and CTO lesions (HR = 2.75, 95% CI = 1.29-5.82) were better predictors of post-PTCA events than polymer type. In the era of new-generation stents, due to the newer design of the stent and polymer, the difference between polymers may be a minor factor in predicting events. Compared to other factors, stent type was a less significant predictor of MACEs (HR = 0.84, 95% CI = 0.40-1.75) in this study. These results support our conclusion of similar outcomes between current BP-DESs and second-generation DP-DESs.
Limitations
This single-center observational study has several limitations. First, we used a nonrandomized real-world registry. The BP-DESs in this study included Nobori and BioMatrix, which contain stainless steel struts; however the DP-DESs in our study used cobalt chromium struts. It may be difficult to control for all stent designs and materials in the real-world, and this may have led to bias in the results. Second, we measured angiographic diameter for the vessels and lesions size instead of using intravascular imaging such as intravascular ultrasound (IVUS) or OCT, which may provide better lesion and diameter evaluation. Third, the BP-DES group had fewer participants than the second-generation DP-DES group in this study, which may have resulted in a nonsignificant difference in the outcome analysis. Finally, comprehensive medical records including medications of the patients were not obtained. Periprocedural conditions such as systemic inflammation status could also have influenced the results.33,34 In addition, compliance and controlled status of underlying diseases were unclear, which may have affected the study outcomes.
CONCLUSIONS
In this single-center clinical-based long-term follow-up study, we demonstrated similar safety and efficacy profiles for BP-DESs and second-generation DP-DESs regarding cardiovascular death, nonfatal MI, TVR, all revascularization, ST, and MACE-free survival rates.
FUNDING
This work was supported by the grant from Chang Gung Memorial Hospital [grant number: CORPG3G0291, CORPG3C0162].
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Sung SH, Chen TC, Cheng HM, et al. Comparison of clinical outcomes in patients undergoing coronary intervention with drug-eluting stents or bare-metal stents: a nationwide population study. Acta Cardiol Sin. 2017;33:10–19. doi: 10.6515/ACS20160608A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li YH, Chiu YW, Cheng JJ, et al. Changing practice pattern of acute coronary syndromes in Taiwan from 2008 to 2015. Acta Cardiol Sin. 2019;35:1–10. doi: 10.6515/ACS.201901_35(1).20180716B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sammel AM, Chen D, Jepson N. New generation coronary stent technology--is the future biodegradable? Heart Lung Circ. 2013;22:495–506. doi: 10.1016/j.hlc.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Commandeur S, van Beusekom HM, van der Giessen WJ. Polymers, drug release, and drug-eluting stents. J Interv Cardiol. 2006;19:500–506. doi: 10.1111/j.1540-8183.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- 5.Virmani R, Guagliumi G, Farb A, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004;109:701–705. doi: 10.1161/01.CIR.0000116202.41966.D4. [DOI] [PubMed] [Google Scholar]
- 6.Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 7.Bangalore S, Toklu B, Amoroso N, et al. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. BMJ. 2013;347:f6625. doi: 10.1136/bmj.f6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang SH, Park KW, Kang DY, et al. Biodegradable-polymer drug-eluting stents vs. bare metal stents vs. durable-polymer drug-eluting stents: a systematic review and Bayesian approach network meta-analysis. Eur Heart J. 2014;35:1147–1158. doi: 10.1093/eurheartj/eht570. [DOI] [PubMed] [Google Scholar]
- 9.Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Clinical outcomes with bioabsorbable polymer- versus durable polymer-based drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2014;63:299–307. doi: 10.1016/j.jacc.2013.09.061. [DOI] [PubMed] [Google Scholar]
- 10.El-Hayek G, Bangalore S, Casso Dominguez A, et al. Meta-analysis of randomized clinical trials comparing biodegradable polymer drug-eluting stent to second-generation durable polymer drug-eluting stents. JACC Cardiovasc Interv. 2017;10:462–473. doi: 10.1016/j.jcin.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh IC, Chang HJ, Chern MS, et al. Late coronary artery stenting in patients with acute myocardial infarction. Am Heart J. 1998;136:606–612. doi: 10.1016/s0002-8703(98)70006-7. [DOI] [PubMed] [Google Scholar]
- 12.Byrne RA, Iijima R, Mehilli J, et al. Durability of antirestenotic efficacy in drug-eluting stents with and without permanent polymer. JACC Cardiovasc Interv. 2009;2:291–299. doi: 10.1016/j.jcin.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Byrne RA, Kastrati A. No country for old stents? Improving long-term patient outcomes with biodegradable polymer drug-eluting stents. Expert Rev Cardiovasc Ther. 2012;10:429–432. doi: 10.1586/erc.12.29. [DOI] [PubMed] [Google Scholar]
- 14.Vorpahl M, Finn AV, Nakano M, Virmani R. The bioabsorption process: tissue and cellular mechanisms and outcomes. EuroIntervention. 2009;5 Suppl F:F28–F35. doi: 10.4244/EIJV5IFA5. [DOI] [PubMed] [Google Scholar]
- 15.Hamilos MI, Ostojic M, Beleslin B, et al. Differential effects of drug-eluting stents on local endothelium-dependent coronary vasomotion. J Am Coll Cardiol. 2008;51:2123–2129. doi: 10.1016/j.jacc.2007.12.059. [DOI] [PubMed] [Google Scholar]
- 16.Stefanini GG, Kalesan B, Serruys PW, et al. Long-term clinical outcomes of biodegradable polymer biolimus-eluting stents versus durable polymer sirolimus-eluting stents in patients with coronary artery disease (LEADERS): 4 year follow-up of a randomised non-inferiority trial. Lancet. 2011;378:1940–1948. doi: 10.1016/S0140-6736(11)61672-3. [DOI] [PubMed] [Google Scholar]
- 17.Koppara T, Joner M, Bayer G, et al. Histopathological comparison of biodegradable polymer and permanent polymer based sirolimus eluting stents in a porcine model of coronary stent implantation. Thromb Haemost. 2012;107:1161–1171. doi: 10.1160/TH12-01-0043. [DOI] [PubMed] [Google Scholar]
- 18.Serruys PW, Farooq V, Kalesan B, et al. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. JACC Cardiovasc Interv. 2013;6:777–789. doi: 10.1016/j.jcin.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Stefanini GG, Byrne RA, Serruys PW, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. 2012;33:1214–1222. doi: 10.1093/eurheartj/ehs086. [DOI] [PubMed] [Google Scholar]
- 20.Zhu L, Lv YN, Wang LY. Stent thrombosis with biodegradable polymer drug-eluting stents versus durable polymer sirolimus-eluting stents: an update meta-analysis. Cardiology. 2015;130:96–105. doi: 10.1159/000368073. [DOI] [PubMed] [Google Scholar]
- 21.Jukema JW, Ahmed TA, Verschuren JJ, et al. Restenosis after PCI. Part 2: prevention and therapy. Nat Rev Cardiol. 2011;9:79–90. doi: 10.1038/nrcardio.2011.148. [DOI] [PubMed] [Google Scholar]
- 22.Stefanini GG, Holmes DR., Jr. Drug-eluting coronary-artery stents. N Engl J Med. 2013;368:254–265. doi: 10.1056/NEJMra1210816. [DOI] [PubMed] [Google Scholar]
- 23.Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393–1402. doi: 10.1016/S0140-6736(12)60324-9. [DOI] [PubMed] [Google Scholar]
- 24.Navarese EP, Tandjung K, Claessen B, et al. Safety and efficacy outcomes of first and second generation durable polymer drug eluting stents and biodegradable polymer biolimus eluting stents in clinical practice: comprehensive network meta-analysis. BMJ. 2013;347:f6530. doi: 10.1136/bmj.f6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Scheerder IK, Wilczek KL, Verbeken EV, et al. Biocompatibility of biodegradable and nonbiodegradable polymer-coated stents implanted in porcine peripheral arteries. Cardiovasc Intervent Radiol. 1995;18:227–232. doi: 10.1007/BF00239417. [DOI] [PubMed] [Google Scholar]
- 26.van der Giessen WJ, Lincoff AM, Schwartz RS, et al. Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation. 1996;94:1690–1697. doi: 10.1161/01.cir.94.7.1690. [DOI] [PubMed] [Google Scholar]
- 27.Naidu SS, Krucoff MW, Rutledge DR, et al. Contemporary incidence and predictors of stent thrombosis and other major adverse cardiac events in the year after XIENCE V implantation: results from the 8,061-patient XIENCE V United States study. JACC Cardiovasc Interv. 2012;5:626–635. doi: 10.1016/j.jcin.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Tada T, Byrne RA, Schuster T, et al. Early vascular healing with rapid breakdown biodegradable polymer sirolimus-eluting versus durable polymer everolimus-eluting stents assessed by optical coherence tomography. Cardiovasc Revasc Med. 2013;14:84–89. doi: 10.1016/j.carrev.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Tada T, Kastrati A, Byrne RA, et al. Randomized comparison of biolimus-eluting stents with biodegradable polymer versus everolimus-eluting stents with permanent polymer coatings assessed by optical coherence tomography. Int J Cardiovasc Imaging. 2014;30:495–504. doi: 10.1007/s10554-014-0376-1. [DOI] [PubMed] [Google Scholar]
- 30.Danzi GB, Piccolo R, Galasso G, Piscione F. Nobori biolimus-eluting stent vs. permanent polymer drug-eluting stents in patients undergoing percutaneous coronary intervention. Circ J. 2014;78:1858–1866. doi: 10.1253/circj.cj-13-1558. [DOI] [PubMed] [Google Scholar]
- 31.Pandya B, Gaddam S, Raza M, et al. Biodegradable polymer stents vs second generation drug eluting stents: a meta-analysis and systematic review of randomized controlled trials. World J Cardiol. 2016;8:240–246. doi: 10.4330/wjc.v8.i2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vlachojannis GJ, Smits PC, Hofma SH, et al. Biodegradable polymer biolimus-eluting stents versus durable polymer everolimus-eluting stents in patients with coronary artery disease: final 5-year report from the COMPARE II Trial (Abluminal Biodegradable Polymer Biolimus-Eluting Stent Versus Durable Polymer Everolimus-Eluting Stent). JACC Cardiovasc Interv. 2017;10:1215–1221. doi: 10.1016/j.jcin.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 33.Huang WC, Chou RH, Chang CC, et al. Systemic inflammatory response syndrome is an independent predictor of one-year mortality in patients with acute myocardial infarction. Acta Cardiol Sin. 2017;33:477–485. doi: 10.6515/ACS20170603A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin HJ, Wang TD. Profiling the evolution of inflammatory response and exploring its prognostic significance in acute myocardial infarction: the first step to establishing anti-inflammatory strategy. Acta Cardiol Sin. 2017;33:486–488. doi: 10.6515/ACS20170731A. [DOI] [PMC free article] [PubMed] [Google Scholar]


