Abstract
Background
The angiotensin receptor-neprilysin inhibitor sacubitril/valsartan is known to improve outcomes of cardiac death and hospitalization due to heart failure in patients with heart failure and reduced ejection fraction (HFrEF). However, data on improvements in ejection fraction after using sacubitril/valsartan are still lacking in Taiwan.
Methods
We conducted this prospective, single armed, observation cohort study to evaluate changes in left ventricular ejection fraction (LVEF) in patients with heart failure and reduced LVEF treated with sacubitril/valsartan. This was an all-comer study. We prescribed sacubitril/valsartan as both first-line and second-line therapy to every eligible patient regardless of whether they were already on standard therapy or newly-diagnosed with HFrEF. The primary outcome was improvements in LVEF. We also collected data about changes in left ventricular chamber size, blood pressure, N-terminal pro-B-type natriuretic peptide (NT-proBNP), and renal function according to serum creatinine level.
Results
During March 2016 to April 2018, 93 patients were enrolled. The mean LVEF improved from 35 ± 6.1% to 50 ± 8.8% at 6 months use of sacubitril/valsartan (p < 0.001). The left ventricular end-diastolic diameter, left ventricular end-systolic diameter, and left atrial diameter all decreased. The average NT-proBNP level decreased from 6379 pg/mL to 1661 pg/dL.
Conclusions
Sacubitril/valsartan demonstrated a significant effect in improving LVEF, left ventricular reverse remodeling, and reduction of NT-proBNP in this Taiwanese cohort.
Keywords: Heart failure, Left ventricular remodeling, Reduced ejection fraction, Sacubitril/valsartan
INTRODUCTION
Heart failure is a complex clinical syndrome related to many diseases, including coronary artery disease, diabetes mellitus, hypertension, valvular heart disease, cardiomyopathy, and even aging and neurohormonal status. Patients with heart failure may experience dyspnea, dyspepsia, weakness, insomnia, edema, and depression.1 Among many independent prognostic factors associated with heart failure, ejection fraction is strongly correlated with mortality.2 The angiotensin receptor-neprilysin inhibitor sacubitril/valsartan blocks the renin-angiotensin-aldosterone system (RAAS) and enhances the natriuretic peptide (NP) system by inhibiting neprilysin. Both inhibitions facilitate vasodilatation, diuresis, natriuresis, and have synergistic effects on each other. This may improve heart function by reducing cardiac fibrosis, inflammation, and suppressing cardiac remodeling. Sacubitril/valsartan was shown to reduce death and hospitalization rates in patients with heart failure and reduced ejection fraction (HFrEF) in the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM-HF) trial,3 however data about improvements in ejection fraction after using sacubitril/valsartan are still lacking in Taiwan. Moreover, the long-term treatment results have yet to be established in an Asian population. Therefore, this study aimed to evaluate the efficacy of sacubitril/valsartan in an Asian population, especially with regards to left ventricular ejection fraction (LVEF).
METHODS
Study subjects
This was a single-facility, practice-based, all-comer, prospective cohort study. Sacubitril/valsartan was prescribed to every eligible patient with an ejection fraction less than 40% and heart failure symptoms at medical contact. Heart failure symptoms were defined based on the Framingham criteria.4 If the patient was already receiving standard heart failure therapy with angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs), they were replaced by sacubitril/valsartan. For the patients who were naïve to ACEis and ARBs, sacubitril/valsartan was prescribed at first medical contact. This strategy is different to the 2016 European Society of Cardiology (ESC) heart failure guidelines which considers sacubitril/valsartan to be treatment failure bailout.5 We believe that there is an irreversible point for heart failure, and once cardiac fibrosis goes into the end stage, no treatment may work. Therefore, we tried to treat the patients with sacubitril/valsartan as early as possible.
The patients were enrolled when they were admitted for heart failure or when they visited our outpatient department. In the first month post-discharge, the patients returned to the clinic every 1 to 2 weeks to evaluate the tolerability to sacubitril/valsartan and assess the possibility of up-titration. All patients were carefully followed, and telephone interviews were used if the patients stopped attending the clinic.
The inclusion criteria included an age ≥ 18 years, and chronic symptomatic heart failure with New York Heart Association (NYHA) functional class II to IV.6 Reduced ejection fraction was defined as < 40% by echocardiography or left ventriculography. The exclusion criteria were a history of angioedema, estimated glomerular filtration rate < 30 mL/min/1.73 m2 at screening, and systolic pressure < 100 mmHg with symptomatic hypotension. Baseline characteristics, laboratory data, underlying diseases, blood pressure, and echocardiographic findings of the eligible patients were abstracted from medical records by a trained chart review assistant.
Endpoints
The primary outcome was changes in LVEF. We use M mode, biplane method on transthoracic echocardiography and left ventriculography to measure LVEF. Biplane LVEF was determined using two-dimensional echocardiographic imaging according to the report by Simpson.7 Apical four chamber and apical two chamber views were obtained to calculate average LVEF. For the patients with atrial fibrillation, we used the average ejection fraction over five measurements.
The reproducibility of echocardiographically determined LVEF may be not be good enough, as serial LVEF measurements are limited by high interindividual and intraindividual variability. 3D volumetry8 and optimized endocardial border detection in contrast echocardiography9 may diminish intraindividual variability, however we do not routinely use this method in our daily practice. To minimize interindividual and intraindividual bias, two different sonographers measured the LVEF and chamber size twice for each examination of the patients enrolled in this trial. At the end of the study, we randomly selected and deidentified the LVEF and left ventricular end-diastolic diameter (LVEDd) data of 15 patients. LVEF and LVEDd were measured again by our first author. The paired T-test was used to compare the measurements from the first author and the original data, and no statistical difference was noted between different observers. Overall, the differences were mostly within 5% for LVEF and 5 mm for LVEDd.
LVEDd was also recorded as one of the secondary endpoints to evaluate the effects of reverse remodeling of the left ventricle. LVEDd, left ventricular end-systolic diameter (LVEDs) and left atrial diameter (LAD) were obtained via the parasternal long axis on echocardiography.
We also recorded blood pressure and laboratory data such as serum N-terminal pro-B-type natriuretic peptide (NT-proBNP), creatinine, potassium level and HbA1c. All data were collected at baseline, and at the second and sixth months after initiating sacubitril/valsartan.
Statistical analysis
Baseline characteristics, laboratory data, underlying diseases, and echocardiographic findings were presented as mean ± standard deviation and frequencies. The χ2 test and paired t-test were used to evaluate statistical associations between categorical and continuous data, respectively. In addition to continuous changes in average LVEF, we defined non-responders and responders based on an improvement in LVEF < 10% or ≥ 10%, respectively. Clinical presentation and pathophysiology are used as variables to evaluate their effects on the response to sacubitril/valsartan. Subgroup analysis of the patients who had and had not used ACEis/ARBs was also performed. Among all tests, a p-value ≤ 0.05 was considered to be statistically significant. Statistical analyses were performed with SPSS 12.0 (SPSS Inc. Chicago, IL, USA).
RESULTS
Baseline characteristics
From March 2016 to April 2018, 93 patients were enrolled. The mean follow-up period was 231.7 ± 153.9 days. The demographic data of the patients are presented in Table 1. Ischemic cardiomyopathy (ICM) accounted for 54% of all patients, and 34% had diabetes. The mean ejection fraction was 34.4 ± 5.0% (range: 19-39%), and the mean serum NT-proBNP level was 6379 pg/mL (range: 38-27960 pg/mL).
Table 1. Basic characters.
| Sacubitril/valsartan, n = 93 | |
| Follow-up days | 231.7 ± 153.9 (2016/03/28-2018/01/11) |
| Duration of CHF (mean ± SD) | 1242.1 ± 1483.6 (2003/07/07-2018/01/04) |
| Male | 67 (72%) |
| Female | 26 (28%) |
| Age (mean ± SD) | 67.1 ± 12.4 (37-93) |
| SBP (mmHg) | 127 ± 19 |
| NYHA functional class – no. (%) | |
| NYHA I-II | 62 (67%) |
| NYHA III-IV | 31 (33%) |
| Clinical feature of heart failure | |
| Dilated cardiomyopathy | 43 (46%) |
| Ischemic cardiomyopathy | 50 (54%) |
| Range of LVEF (%) | 19-39 |
| LVEF (mean ± %) | 34.4 ± 5.0 |
| Lab data | |
| NT-proBNP (pg/ml) | 6379 (38-27960) |
| Creatinine (mg/dl) | 1.5 ± 0.3 |
| Potassium (mEq/l) | 4.29 ± 0.58 |
| eGFR (ml/min/1.73 m2) | 63 ± 21.3 |
| BUN (mg/dl) | 20.6 ± 5.1 |
| Medical history – no. (%) | |
| Atrial fibrillation | 21 (23%) |
| Hypertension | 28 (30%) |
| Diabetes mellitus | 32 (34%) |
BUN, blood urea nitrogen; CHF, congestive heart failure; eGFR, estimated glomerular filtration rate; LVEF, left ventricule ejection fraction; NT-proBNP, N-terminal pro–B-type natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation.
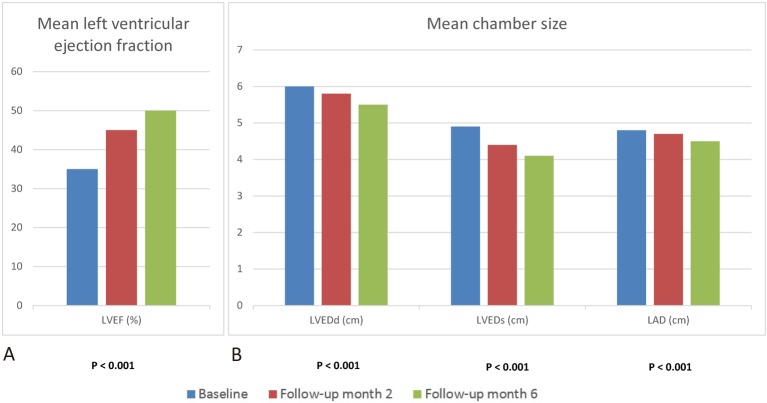
After a mean follow-up of 231.7 ± 153.9 days, echocardiography was arranged at baseline, and at the second and sixth months. The echocardiographic findings including primary and secondary endpoints are presented in Figure 1.
Figure 1.
Echocardiographic findings with primary and secondary end-points. (A) Mean ejection fraction improves from 35 ± 6.1% to 50 ± 8.8% at 6 months post treatment of sacubitril/valsartan. (B) Echocardiographic follow up showed consistent reduction of chamber size including LVEDd, LVEDs, and LAD. The p values were tested for comparison of baseline and final assessment. LAD, left atrial diameter; LVEDd, left ventricular end-diastolic diameter; LVEDs, left ventricular end-systolic diameter.
Primary endpoint
The mean ejection fraction improved from 35 ± 6.1% to 50 ± 8.8% at 6 months of sacubitril/valsartan treatment (p < 0.001) (Figure 1A). After 12 months of treatment, the mean LVEF was 54 ± 10.8%.
Secondary endpoints
The chamber size is presented in Figure 1B. The LVEDd decreased from 6.0 ± 0.7 cm to 5.5 ± 0.8 cm (p < 0.001), with similar findings in LVEDs and LAD (Figure 1B).
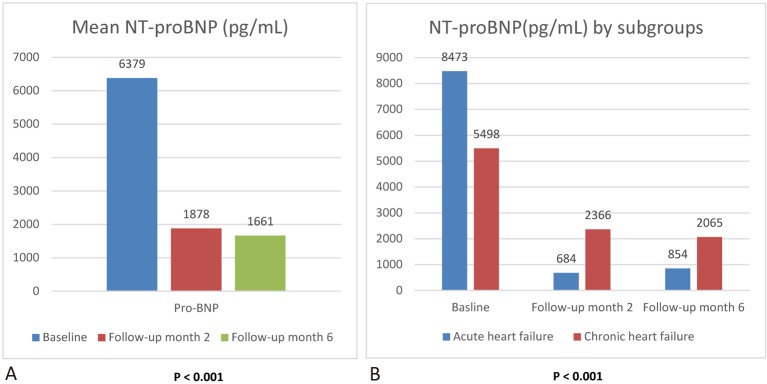
The biochemical study showed a significant reduction in mean NT-proBNP from 6379 pg/mL to 1878 pg/dL after 65 days follow-up. The average NT-proBNP level was 1661 pg/mL at 151 days of follow-up (Figure 2A).
Figure 2.
NT-proBNP during follow up. (A) Mean NT-proBNP at baseline, month 2, and month 6 post treatment. (B) Mean NT-proBNP at baseline, month 2, and month 6 post treatment by acute and chronic heart failure. The p values were tested for comparison of baseline and final assessment.
The baseline NT-proBNP level was higher in the patients with acute heart failure than in those with chronic heart failure. After 151 days follow-up, there was a greater reduction in NT-proBNP in the acute heart failure group (Figure 2B). The mean NT-proBNP level at 62 days follow-up was reduced by 92.1% in the acute heart failure group (from 8473 pg/mL to 684 pg/mL), and this reduction was greater than that in the PIONEER-HF trial.10 Compared to the PIORNEER-HF trial, more patients used mineralocorticoid receptor antagonist (MRA) and beta-blockers in the present study. The PIORNEER-HF trial did not mention concomitant revascularization or controlling for other factors leading to heart failure. Besides heart failure medication, we also aggressively controlled for all factors that can lead to heart failure.
Subgroup analysis
Prior use of ACEis/ARBs
We divided the patients two groups as those with (n = 68) or without (n = 25) ACEi and ARB pre-treatment. There were no significant differences in other background therapies between the two groups. Okumura et al. found that background therapy did not affect the effect of sacubitril/valsartan in the PARADIGM-HF trial.11 Baseline ejection fraction and chamber size were also not statistically different between the two groups. Both groups had similar improvements in ejection fraction and chamber size after using sacubitril/valsartan. The results are presented in Table 2.
Table 2. Background therapy and echocardiographic changes of subgroups with and without prior ACEi/ARB treatment.
| ACEI/ARB (n = 68) | Non-ACEI/ARB (n = 25) | p value | |
| Background therapy | |||
| Statin | 45 (66%) | 13 (52%) | 0.221 |
| Antiplatelet | 53 (78%) | 19 (75%) | 0.183 |
| Aspirin | 32 (47%) | 11 (43%) | 0.144 |
| Clopidogrel | 7 (10%) | 0 | 0.193 |
| Aspirin +Ticagrelor | 6 (9%) | 3 (13%) | 0.854 |
| Aspirin + Clopidogrel | 8 (12%) | 5 (20%) | 0.440 |
| Anticoagulant | |||
| Warfarin | 1 (1%) | 0 | 0.607 |
| NOAC | 6 (9%) | 5 (20%) | 0.754 |
| Spironolactone | 52 (77%) | 19 (75%) | 0.986 |
| Furosemide | 35 (52%) | 13 (54%) | 0.886 |
| Digitalis | 1 (1%) | 0 | 0.607 |
| β-blocker | |||
| Carvedilol | 23 (33%) | 6 (24%) | 0.781 |
| Bisoprolol | 45 (67%) | 19 (75%) | 0.761 |
| Echocardiography baseline | |||
| LVEF (%) | 36.2 ± 7.5 | 34.8 ± 5.4 | 0.192 |
| LVEDd (cm) | 5.9 ± 0.7 | 5.9 ± 0.6 | 0.441 |
| LVEDs (cm) | 4.8 ± 0.7 | 4.7 ± 0.9 | 0.879 |
| LAD (cm) | 4.8 ± 0.6 | 4.6 ± 0.5 | 0.394 |
| 2 months | |||
| LVEF (%) | 46.1 ± 9.3 | 43.1 ± 4.8 | 0.213 |
| LVEDd (cm) | 5.6 ± 0.6 | 5.7 ± 0.6 | 0.815 |
| LVEDs (cm) | 4.2 ± 0.7 | 4.4 ± 0.5 | 0.572 |
| LAD (cm) | 4.6 ± 0.6 | 4.5 ± 0.5 | 0.493 |
| 6 months | |||
| LVEF (%) | 51.6 ± 9.4 | 50.9 ± 6.4 | 0.270 |
| LVEDd (cm) | 5.4 ± 0.7 | 5.6 ± 0.7 | 0.358 |
| LVEDs (cm) | 3.9 ± 0.6 | 4.2 ± 0.6 | 0.433 |
| LAD (cm) | 4.5 ± 0.5 | 4.3 ± 0.4 | 0.615 |
ACEI, angiotensin-converting-enzyme inhibitors; ARB, angiotensin receptor blocker; LAD, left atrial diameter; LVEDd, left ventricular end-diastolic diameter; LVEDs, left ventricular end-systolic diameter; NOAC, novel oral anticoagulants; LVEF, left ventricular ejection fraction.
Clinical presentation and pathophysiology
Subgroup analyses stratified by clinical presentation (acute heart failure, chronic heart failure, and de novo heart failure) and pathophysiology (dilated cardiomyopathy and ischemic cardiomyopathy) are presented in Table 3. In our cohort, 7 patients had de novo heart failure, all of whom presented with acute heart failure. Seventeen patients had acute decompensation heart failure and received chronic heart failure treatment, and 69 patients had chronic stable heart failure with NYHA functional class II or III.
Table 3. Logistic regression for LVEF response according to clinical presentation, revascularization and pathophysiology.
| N = 93 | Odds ratio (95% CI) | p |
| Acute HF (26%)/chronic HF (74%) | 0.72 (0.16-3.16) | 0.667 |
| Complete revascularization (78%)/not complete revascularization (22%) | 3.50 (0.46-26.61) | 0.226 |
| DCM (46%)/ICM (54%) | 1.33 (0.41-4.26) | 0.625 |
DCM, dilated cardiomyopathy; HF, heart failure; ICM, ischemic cardiomyopathy.
Overall, 43 patients had dilated cardiomyopathy (DCM) and 50 had ICM. In the ICM group, some patients received sacubitril/valsartan before complete revascularization and some received sacubitril/valsartan after complete revascularization. The time span between using sacubitril/valsartan and complete revascularization was mostly within 2 months. Therefore, our results are more likely to represent a synergistic effect. Both the ICM and DCM group showed significant improvements in LVEF at 151 days follow-up compared to baseline LVEF (33 ± 7.5% to 52 ± 7.4%; 33 ± 4.3% to 47 ± 7.4% for the ICM and DCM groups, respectively).
Responders were defined as having an improvement ≥ 10% in LVEF, they were analyzed using logistic regression for different subgroups (Table 3). The p value is insignificant for other variables; larger patient number is needed to evaluate the effects of sacubitril/valsartan with more clinical variables.
Safety profiles
The adverse effects and major cardiovascular events noted in this study are presented in Table 4. The mean systolic blood pressure decreased from 122 mmHg to 117 mmHg at the first month, and then gradually increased to 129 mmHg at 6 months follow-up. This is consistent with our clinical experience about sacubitril/valsartan, in that patients may experience hypotension during the first month of treatment. Once cardiac function gradually improves, the blood pressure gradually elevates and hypotension diminishes. Hyperkalemia and worsening renal function (WRF) accounted for 1%, respectively. WRF was defined as an increase in serum creatinine concentration of 0.5 mg/dL or a decrease in estimated glomerular filtration rate of 25% or more. The 1-year all-cause mortality rate was about 3% in our cohort. Rates of cardiovascular death and rehospitalization for heart failure were also around 1%, respectively (Table 4). Compared to another Taiwanese HFrEF registry,12 three major adverse cardiovascular events including all-cause mortality, cardiovascular death and rehospitalization for heart failure were lower in our cohort.
Table 4. Adverse events and MACE after Sacubitril/Valsartan.
| Varsartan/sacubitril, n = 93 | |
| Adverse effect | |
| Symptomatic hypotension | 4 (4%) |
| Hyperkalemia | 1 (1%) |
| Worsening renal function | 1 (1%) |
| MACE | |
| All-cause mortality | 3 (3%) |
| CV death | 1 (1%) |
| Rehospitalization of HF | 1 (1%) |
CV death, cardiovascular death; HF, heart failure; MACE, major adverse cardiac events.
DISCUSSION
Medications for heart failure and concomitantly controlling the underlying condition are equally important. Complete revascularization also plays an important role in treating heart failure. In our study, most of our patients with ICM received complete revascularization. We encouraged the patients to have all their diseases underlying the heart failure under good control, including diabetes, hypertension, coronary artery disease, and arrhythmia. Complete revascularization has been shown to reduce the rates of death from cardiovascular causes and hospitalization for cardiovascular causes.13 In addition, complete revascularization after myocardial infarction has been shown to be effective to improve heart function. Reibis et al. demonstrated that complete revascularization beyond background therapy with beta-blockers and RAAS inhibition improved heart function.14
In our study, both reduction in heart size and increased LVEF were observed, and the LVEDd decreased from 6.0 ± 0.7 cm to 5.5 ± 0.8 cm (p < 0.001). Reverse remodeling15 could be one of the most important mechanisms by which sacubitril/valsartan improves mortality and morbidity in patients with HFrEF.
Some reported shrinkage of LVEDd may be associated with the use of diuretics.16 However the use of diuretics decreased over time in this study, and therefore the effects of diuretics on LVEDd and LVEF may have diminished during follow-up.
NT-proBNP is an important disease parameter and has been associated with major adverse cardiovascular events in past studies,17-19 and to be positively related to volume status of the patient. The decreased in NT-proBNP may have improved the clinical condition in our cohort. The mean serum NT-proBNP level was 6379 pg/mL (38-27960 pg/mL), which is higher than the average NT-proBNP level in the PARADIGM-HF trial (1631 pg/mL, 885-3154 pg/mL). Natriuretic peptide can reduce blood pressure by decreasing plasma volume20 and inducing vasodilatation through endothelial nitric oxide synthesis.21 Sacubitril/valsartan caused more hypotension relative to enalapril in the PARADIGM-HF trial.22 This side effect can be overcome by minimizing the initial dose and careful up-titration. The PIORNEER-HF trial10 also started sacubitril/valsartan at alow dose.
In our cohort, there are 16 non-responders (defined as LVEF improvement < 10%). Among them, two patients received cardiac resynchronizing therapy, one patient had coronary artery disease without complete revascularization, and six patients had old myocardial infarction without viable tissue. It could be too late for patients with end-stage heart disease [cardiac resynchronization therapy (CRT) and old myocardial infarction groups] to respond to sacubitril/valsartan or other treatment. For ICM, sacubitril/valsartan itself may not be effective enough without complete revascularization.
Drug dosage
Most of our patients did not receive the target dose of sacubitril/valsartan according to the PARAGIDM-HF trial. Vardeny et al. analyzed dose reduction effects with enalapril and sacubitril/valsartan,23 and found that dose reduction could identify the patients at higher risk. Dose reduction of sacubitril/valsartan still provided more benefits compared to a dose reduction of enalapril. If the patients can tolerate sacubitril/valsartan without hypotension and have the dose up-titrated, they usually have a good response to sacubitril/valsartan. Most of our patients initiated sacubitril/valsartan at a dose of 24/26 mg once daily or 24/26 mg twice daily depending on their tolerability. Instead of following a fixed protocol, we up-titrated the dosage of sacubitril/valsartan based on individual conditions. A target sacubitril/valsartan dose of 48/52 mg twice daily has shown effectiveness in improving both functional class and ejection fraction. In our cohort, 82 patients (88.1%) took 48/52 mg of sacubitril/valsartan twice per day at the end of follow-up. We did not up-titrate the dose if the effect was already satisfactory. The target dose in Taiwanese patients may be lower compared to those in the PARADIGM-HF trial. This titration strategy decreased the hypotensive side effects of sacubitril/valsartan and increased drug compliance of the patients. The same titration strategy was also reported in the TITRATION trial.24
One reason why we did not up-titrate sacubitril/valsartan to the target dose in the PARADIGM-HF trial is due to the high cost of sacubitril/valsartan. Most patients received 200 mg sacubitril/valsartan per day by dividing a 200 mg tablet into half to halve the cost. Health economics is a concern. If the patient’s symptoms and ejection fraction reached our goal, we think it was reasonable not to push up to the target dose. For those who could tolerate the target dose with residual symptoms or unsatisfactory ejection fraction, we up-titrated their dose to the target dose. It is unclear whether up-titrating the drugs to the target dose would provide clear benefits for patients with restored LVEF and NYHA Fc I.
Limitations
The sample size was small in this study, and there may have been selection bias. In addition, this study was not a randomized control trial, and the enrolled patients included those with both acute and chronic heart failure. This may explain why our patients had higher baseline NT-proBNP than those in the PARADIGM-HF trial. On the other hand, this also reveals the usefulness of sacubitril/valsartan in patients with acute, subacute and chronic heart failure. After reviewing the non-responders, improvements in ejection fraction may not have simply been due to sacubitril/valsartan. A multidisciplinary approach for heart failure and early treatment are essential.
Our titration strategy effectively improved tolerability to sacubitril/valsartan. In our subgroup analysis, sacubitril/valsartan was still beneficial for patients with heart failure symptoms on the basis of ACEi/ARB treatment. First- and second-line use of sacubitril/valsartan were both effective in our study. Despite a good response in our study, some patients did not have an improvement in ejection fraction with sacubitril/valsartan. Further studies are needed to identify the potential non-responders from responders.
CONCLUSIONS
In conclusion, our data demonstrate the effectiveness of sacubitril/valsartan in a Taiwanese population. The latest TSOC guidelines25 for heart failure recommend angiotensin receptor neprilysin inhibitors.
REFERENCES
- 1.Heo S, Lennie TA, Okoli C, Moser DK. Quality of life in patients with heart failure: ask the patients. Heart Lung. 2009;38:100–108. doi: 10.1016/j.hrtlng.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curtis JP, Sokol SI, Wang Y, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003;42:736–742. doi: 10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 4.Maestre A, Gil V, Gallego J, et al. Diagnostic accuracy of clinical criteria for identifying systolic and diastolic heart failure: cross-sectional study. J Eval Clin Pract. 2009;15:55–61. doi: 10.1111/j.1365-2753.2008.00954.x. [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Otterstad JE, Froeland G, St John Sutton M, Holme I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J. 1997;18:507–513. doi: 10.1093/oxfordjournals.eurheartj.a015273. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins C, Bricknell K, Chan J, et al. Comparison of two- and three-dimensional echocardiography with sequential magnetic resonance imaging for evaluating left ventricular volume and ejection fraction over time in patients with healed myocardial infarction. Am J Cardiol. 2007;99:300–306. doi: 10.1016/j.amjcard.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Olszewski R, Timperley J, Szmigielski C, et al. The clinical applications of contrast echocardiography. Eur J Echocardiogr. 2007;8:S13–S23. doi: 10.1016/j.euje.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 11.Okumura N, Jhund PS, Gong J, et al. Effects of sacubitril/valsartan in the PARADIGM-HF Trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) according to background therapy. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.116.003212. [DOI] [PubMed] [Google Scholar]
- 12.Chang HY, Wang CC, Wu YW, et al. One-year outcomes of acute decompensated systolic heart failure in Taiwan: lessons from TSOC-HFrEF Registry. Acta Cardiol Sin. 2017;33:127–138. doi: 10.6515/ACS20170202A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velazquez EJ, Lee KL, Deja MA, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reibis R, Salzwedel A, Bonaventura K, et al. Improvement of left ventricular ejection fraction in revascularized postmyocardial patients: indication for statistical fallacy. BMC Res Notes. 2017;10:244. doi: 10.1186/s13104-017-2562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim GH, Uriel N, Burkhoff D. Reverse remodelling and myocardial recovery in heart failure. Nat Rev Cardiol. 2018;15:83–96. doi: 10.1038/nrcardio.2017.139. [DOI] [PubMed] [Google Scholar]
- 16.Chen ZH, Jiang YR, Peng JQ, et al. Clinical effects of combined treatment by optimal dose of furosemide and spironolactone on diastolic heart failure in elderly patients. Exp Ther Med. 2016;11:890–894. doi: 10.3892/etm.2015.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishra RK, Beatty AL, Jaganath R, et al. B-type natriuretic peptides for the prediction of cardiovascular events in patients with stable coronary heart disease: the Heart and Soul Study. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doust JA, Pietrzak E, Dobson A, Glasziou P. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ. 2005;330:625. doi: 10.1136/bmj.330.7492.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodges M, Bailey JJ, Church TR. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2003;107:e13. doi: 10.1161/01.cir.0000046777.74753.f8. [DOI] [PubMed] [Google Scholar]
- 20.Wijeyaratne CN, Moult PJ. The effect of alpha human atrial natriuretic peptide on plasma volume and vascular permeability in normotensive subjects. J Clin Endocrinol Metab. 1993;76:343–346. doi: 10.1210/jcem.76.2.8432776. [DOI] [PubMed] [Google Scholar]
- 21.Elesgaray R, Caniffi C, Ierace DR, et al. Signaling cascade that mediates endothelial nitric oxide synthase activation induced by atrial natriuretic peptide. Regul Pept. 2008;151:130–134. doi: 10.1016/j.regpep.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Vardeny O, Claggett B, Kachadourian J, et al. Incidence, predictors, and outcomes associated with hypotensive episodes among heart failure patients receiving sacubitril/valsartan or enalapril: The PARADIGM-HF Trial (Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor With Angiotensin-Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure). Circ Heart Fail. 2018;11:e004745. doi: 10.1161/CIRCHEARTFAILURE.117.004745. [DOI] [PubMed] [Google Scholar]
- 23.Vardeny O, Claggett B, Packer M, et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016;18:1228–1234. doi: 10.1002/ejhf.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senni M, McMurray JJV, Wachter R, et al. Impact of systolic blood pressure on the safety and tolerability of initiating and up-titrating sacubitril/valsartan in patients with heart failure and reduced ejection fraction: insights from the TITRATION study. Eur J Heart Fail. 2018;20:491–500. doi: 10.1002/ejhf.1054. [DOI] [PubMed] [Google Scholar]
- 25.Wang CC, Wu CK, Tsai ML, et al. 2019 focused update of the guidelines of the Taiwan Society of Cardiology for the diagnosis and treatment of heart failure. Acta Cardiol Sin. 2019;35:244–283. doi: 10.6515/ACS.201905_35(3).20190422A. [DOI] [PMC free article] [PubMed] [Google Scholar]