Abstract Abstract
The genus Melanconis (Melanconidaceae, Diaporthales) in the strict sense is here re-evaluated regarding phylogenetic structure, taxonomy, distribution and ecology. Using a matrix of sequences from ITS, LSU, ms204, rpb2, tef1 and tub2, eight species are recognised and their phylogenetic positions are determined. Based on phylogenetic, morphological and geographical differentiation, Melanconis marginalis is subdivided into four subspecies. Melanconis italica is reduced to a subspecies of Melanconis marginalis. The two species Melanconis larissae from Betula sp. and M. pacifica from Alnus rubra are described as new. Melanconis alni and M. stilbostoma are lectotypified and M. alni, M. marginalis and M. stilbostoma are epitypified. All GenBank sequences deposited as Melanconis alni are shown to actually represent M. marginalis and those as M. marginalis belong to the newly described M. pacifica. Currently, Alnus and Betula are the sole host genera of Melanconis. All species and subspecies are (re-)described and illustrated. In addition, the neotypification of Melanconium pterocaryae is here validated.
Keywords: Juglanconis , Melanconiella , Melanconium , multigene phylogeny, pyrenomycetes, systematics, 1 new combination, 2 new species
Introduction
Melanconis, the type genus of the family Melanconidaceae (Diaporthales), was originally described by Tulasne (1856) with M. stilbostoma as its generic type, but without a generic diagnosis. His inclusion of species like M. spodiaea made the genus heterogeneous from the beginning. Since then, many species names have been erected in the genus. In his generic revision, Wehmeyer (1941) treated the genus in a very wide sense, organising the species in subgenera and sections, which themselves were heterogeneous, containing species of genera like Chapeckia, Coryneum (Pseudovalsa), Macrodiaporthe, Massariovalsa, Melanconiella or Pseudovalsella. Barr (1978) accepted Melanconis roughly in the sense of Wehmeyer´s subgenus Eumelanconis, which included Melanconiella. In this sense, the genus Melanconis was one of many genera of the large family Melanconidaceae and was defined by a distinct ectostromatic disc, a more or less well-developed entostroma, two-celled hyaline or brown ascospores with or without appendages, in combination with melanconium- or discosporium-like asexual morphs (Barr 1978). The first phylogenetic analyses of the Diaporthales (Castlebury et al. 2002; see also Jaklitsch et al. 2016, Senanayake et al. 2018), however, suggested that Melanconidaceae should be confined to its type genus Melanconis with a restricted number of species. This phylogenetic generic concept corresponds, apart from a few exceptions, with Wehmeyer’s (1941) section Stilbostomae of his subgenus Eumelanconis. Subsequently, many names have been combined in other genera in various families following morphological and/or phylogenetic analyses (Barr 1978; Jaklitsch and Voglmayr 2004; Voglmayr and Jaklitsch 2008; De Silva et al. 2009). Melanconiella was extensively studied by Voglmayr et al. (2012), who determined that species of Melanconis cause more conspicuous bumps in the host bark than those of Melanconiella and form light-coloured, white or yellowish ectostromatic discs. Wehmeyer (1941) had used this trait to distinguish his section Stilbostomae from his Chrysostromae, which are characterised by dark coloured discs. Although light coloured discs are not uncommon in Melanconiella, Wehmeyer’s (1941) section Chrysostromae of his subgenus Eumelanconis basically matches the phylogenetically conceived genus Melanconiella, except for a few species, which belong elsewhere. For some of these species, the new genus Juglanconis was established in the new family Juglanconidaceae (Voglmayr et al. 2017, 2019). Two other species were segregated from Melanconis to Alnecium and Phaeodiaporthe by Voglmayr and Jaklitsch (2014). Voglmayr et al. (2012) found an unexpectedly high species diversity in Melanconiella, particularly on Carpinus spp. and showed that its species either have a melanconium- or a discosporina-like asexual morph, but never both morph types. They gave also information of taxonomic placement of other Melanconis spp. Here we treat the residual species of Melanconis in the strict sense.
Materials and methods
Sample sources
All isolates included in this study originated from ascospores or conidia of freshly collected specimens derived from recently dead branches or twigs. Details of the strains including NCBI GenBank accession numbers of gene sequences used to compute the phylogenetic trees are listed in Table 1. Strain acronyms, other than those of official culture collections, are used here primarily as strain identifiers throughout the work. Representative isolates have been deposited at the Westerdijk Fungal Biodiversity Centre (CBS-KNAW), Utrecht, The Netherlands. Details of the specimens, used for morphological investigations, are listed in the Taxonomy section under the respective descriptions. Herbarium acronyms are according to Thiers (2019). Freshly collected specimens have been deposited in the Fungarium of the Department of Botany and Biodiversity Research, University of Vienna (WU) and in the Fungarium of the Natural History Museum of Vienna (W).
Table 1.
Isolates and accession numbers of sequences used in the phylogenetic analyses.
| Taxon | Strain1 | Origin | Host | GenBank accession no.2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| ITS | LSU | ms204 | rpb2 | tef1 | tub2 | ||||
| Juglanconis appendiculata | MC | Greece | Juglans regia | KY427141 | KY427141 | KY427159 | KY427191 | KY427210 | KY427227 |
| Juglanconis japonica | MAFF 410079 = ME20* | Japan | Pterocarya rhoifolia | KY427155 | KY427155 | KY427172 | KY427205 | KY427224 | KY427240 |
| Juglanconis juglandina | CBS 133343 = ME22 | Austria | Juglans regia | KY427149 | KY427149 | KY427166 | KY427199 | KY427218 | KY427234 |
| Juglanconis oblonga | CBS 133344 = ME14 | USA | Juglans cinerea | KY427151 | KY427151 | KY427168 | KY427201 | KY427220 | KY427236 |
| Juglanconis pterocaryae | CBS 144326 = D272* | Austria | Pterocarya fraxinifolia | MK229175 | MK229175 | MK238314 | MK238324 | MK238332 | MK238338 |
| Melanconis alni | CBS 131693 = MAMI | Austria | Alnus glutinosa | MN784962 | MN784962 | MN780721 | MN780745 | MN780774 | MN780803 |
| CBS 131695 = MAW* (from ascospores) | Austria | Alnus glutinosa | MN784963 | MN784963 | MN780722 | MN780746 | MN780775 | MN780804 | |
| MEW*(from conidia) | Austria | Alnus glutinosa | MN784964 | MN784964 | MN780723 | MN780747 | MN780776 | MN780805 | |
| MAIV | France | Alnus incana | MN784965 | MN784965 | MN780724 | MN780748 | MN780777 | MN780806 | |
| D156 | Poland | Alnus glutinosa | MN784966 | MN784966 | MN780725 | MN780749 | MN780778 | MN780807 | |
| Melanconis betulae | CFCC 50471* | China | Betula albosinensis | KT732952 | KT732971 | – | KT732984 | KT733001 | KT733022 |
| CFCC 50472 | China | Betula albosinensis | KT732953 | KT732972 | – | KT732985 | KT733002 | KT733023 | |
| CFCC 50473 | China | Betula albosinensis | KT732954 | KT732973 | – | KT732986 | KT733003 | KT733024 | |
| Melanconis groenlandica | CBS 116450 = UPSC 3407* | Denmark (Greenland) | Betula nana | KU878552 | KU878553 | – | – | KU878554 | KU878555 |
| MAFF 410219 = M4-2 = ME1 | Japan | Betula maximowicziana | MN784967 | MN784967 | MN780726 | MN780750 | MN780779 | MN780808 | |
| CBS 133341 = LCM191.01 = ME10 | USA | Betula papyrifera | MN784968 | MN784968 | MN780727 | MN780751 | MN780780 | MN780809 | |
| CBS 133339 = LCM 02.02 = ME13 | USA | Betula sp. | MN784969 | MN784969 | MN780728 | MN780752 | MN780781 | MN780810 | |
| CBS 133340 = LCM 185.01 | USA | Betula papyrifera | MN784970 | MN784970 | MN780729 | MN780753 | MN780782 | MN780811 | |
| Melanconis itoana | MAFF 410080 = LFP-M4-9 = ME8 | Japan | Betula ermanii | MN784971 | MN784971 | MN780730 | MN780754 | MN780783 | MN780812 |
| CFCC 50474 | China | Betula albosinensis | KT732955 | KT732974 | – | KT732987 | KT733004 | KT733025 | |
| CFCC 52876 | China | Betula albosinensis | MK096324 | MK096364 | – | MK096409 | MK096284 | – | |
| CFCC 52877 | China | Betula albosinensis | MK096326 | MK096366 | – | MK096411 | MK096286 | – | |
| CFCC 52878 | China | Betula albosinensis | MK096327 | MK096367 | – | MK096412 | MK096287 | – | |
| Melanconis larissae | CBS 123196 = AR 3886 = ME7* | USA | Betula sp. | MN784972 | MN784972 | MN780731 | MN780755 | MN780784 | MN780813 |
| Melanconis marginalis subsp. europaea | D157 | Austria | Alnus alnobetula | MN784973 | MN784973 | – | MN780756 | MN780785 | – |
| D158 | Austria | Alnus alnobetula | MN784974 | MN784974 | MN780732 | MN780757 | MN780786 | MN780814 | |
| D257 | Austria | Alnus incana | MN784975 | MN784975 | – | MN780758 | MN780787 | MN780815 | |
| CBS 131692 = MAI* | Austria | Alnus incana | MN784976 | MN784976 | MN780733 | MN780759 | MN780788 | MN780816 | |
| CBS 131694 = MAV | Austria | Alnus alnobetula | MN784977 | MN784977 | MN780734 | MN780760 | MN780789 | MN780817 | |
| MAV1 | Austria | Alnus alnobetula | MN784978 | MN784978 | MN780735 | MN780761 | MN780790 | MN780818 | |
| Melanconis marginalis subsp. italica | MFLUCC 16-1199* | Italy | Alnus cordata | MF190151 | MF190096 | – | – | – | – |
| MFLUCC 17-1659* | Italy | Alnus cordata | MF190152 | MF190097 | – | MF377602 | – | – | |
| Melanconis marginalis subsp. marginalis | D321 (from ascospores)* | Canada | Alnus alnobetula subsp. crispa | MN784979 | MN784979 | – | MN780762 | MN780791 | MN780819 |
| D321a (from α-conidia)* | Canada | Alnus alnobetula subsp. crispa | MN784980 | MN784980 | – | MN780763 | MN780792 | MN780820 | |
| D321b (from β-conidia)* | Canada | Alnus alnobetula subsp. crispa | MN784981 | MN784981 | – | MN780764 | MN780793 | MN780821 | |
| CBS 109496 = AR 3529 = ME2 | Russia | Alnus alnobetula subsp. maximowiczii | MN784982 | MN784982 | MN780736 | MN780765 | MN780794 | MN780822 | |
| AR 4864 = ME5 | USA | Alnus alnobetula | MN784983 | MN784983 | MN780737 | MN780766 | MN780795 | MN780823 | |
| CBS 133346 = AR 4865 = ME6 | USA | Alnus alnobetula | MN784984 | MN784984 | MN780738 | MN780767 | MN780796 | MN780824 | |
| MAFF 410218 = M4-6 = ME9 | Japan | Alnus alnobetula subsp. maximowiczii | MN784985 | MN784985 | MN780739 | MN780768 | MN780797 | MN780825 | |
| Melanconis marginalis subsp. tirolensis | CBS 122310 = AR 3748 = ME4* | Austria | Alnus alnobetula | MN784986 | MN784986 | MN780740 | MN780769 | MN780798 | MN780826 |
| D322a | Austria | Alnus alnobetula | MN959458 | MN959458 | – | MN989415 | MN989416 | MN989417 | |
| Melanconis pacifica | CBS 109744 = AR 3442 = AFTOL-ID 2128 | Canada | Alnus rubra | EU199197 | AF408373 | – | DQ862022 | DQ862038 | EU219103, DQ862038 |
| Melanconis stilbostoma | D143 | Poland | Betula pendula | KY427156 | KY427156 | KY427173 | KY427206 | KY427225 | KY427241 |
| D258 | Italy | Betula aetnensis | MN784987 | MN784987 | – | MN780770 | MN780799 | MN780827 | |
| CBS 109778 = AR 3501 = AFTOL-ID 936 = ME11* | Austria | Betula pendula | MN784988 | MN784988 | MN780741 | MN780771 | MN780800 | MN780828 | |
| MAFF 410225 = M3-9 = ME12 | Japan | Betula platyphylla var. japonica | MN784989 | MN784989 | MN780742 | MN780772 | MN780801 | MN780829 | |
| CBS 121894 = MS | Austria | Betula pendula | KY427156 | KY427156 | MN780743 | JQ926302 | JQ926368 | MN780830 | |
| CBS 133338 = DMW 514.3 | USA | Betula papyrifera | MN784990 | MN784990 | MN780744 | MN780773 | MN780802 | MN780831 | |
| CFCC 50475 | China | Betula platyphylla | KT732956 | KT732975 | – | KT732988 | KT733005 | KT733026 | |
| CFCC 50476 | China | Betula platyphylla | KT732957 | KT732976 | – | KT732989 | KT733006 | KT733027 | |
| CFCC 50477 | China | Betula platyphylla | KT732958 | KT732977 | – | KT732990 | KT733007 | KT733028 | |
| CFCC 50478 | China | Betula platyphylla | KT732959 | KT732978 | – | KT732991 | KT733008 | KT733029 | |
| CFCC 50479 | China | Betula platyphylla | KT732960 | KT732979 | – | KT732992 | KT733009 | KT733030 | |
| CFCC 50480 | China | Betula platyphylla | KT732961 | KT732980 | – | KT732993 | KT733010 | KT733031 | |
| CFCC 50481 | China | Betula platyphylla | KT732962 | KT732981 | – | KT732994 | KT733011 | KT733032 | |
| CFCC 50482 | China | Betula platyphylla | KT732963 | KT732982 | – | KT732995 | KT733012 | KT733033 | |
| CFCC 50483 | China | Betula platyphylla | KT732964 | KT732983 | – | KT732996 | KT733013 | KT733034 | |
| CFCC 52843 | China | Betula platyphylla | MK096338 | MK096378 | – | MK096423 | MK096298 | – | |
| CFCC 52844 | China | Betula platyphylla | MK096341 | MK096381 | – | MK096426 | MK096301 | – | |
| CFCC 52845 | China | Betula platyphylla | MK096343 | MK096383 | – | MK096428 | MK096303 | – | |
| Melanconis stilbostoma | CFCC 52846 | China | Betula platyphylla | MK096347 | MK096387 | – | MK096432 | MK096307 | – |
| CFCC 52847 | China | Betula platyphylla | MK096348 | MK096388 | – | MK096433 | MK096308 | – | |
| CFCC 52848 | China | Betula platyphylla | MK096349 | MK096389 | – | MK096434 | MK096309 | – | |
| CFCC 52849 | China | Betula platyphylla | MK096328 | MK096368 | – | MK096413 | MK096288 | – | |
| CFCC 52850 | China | Betula platyphylla | MK096329 | MK096369 | – | MK096414 | MK096289 | – | |
| CFCC 52851 | China | Betula platyphylla | MK096330 | MK096370 | – | MK096415 | MK096290 | – | |
| CFCC 52852 | China | Betula platyphylla | MK096331 | MK096371 | – | MK096416 | MK096291 | – | |
| CFCC 52853 | China | Betula platyphylla | MK096332 | MK096372 | – | MK096417 | MK096292 | – | |
| CFCC 52854 | China | Betula platyphylla | MK096333 | MK096373 | – | MK096418 | MK096293 | – | |
| CFCC 52855 | China | Betula platyphylla | MK096334 | MK096374 | – | MK096419 | MK096294 | – | |
| CFCC 52856 | China | Betula platyphylla | MK096335 | MK096375 | – | MK096420 | MK096295 | – | |
| CFCC 52857 | China | Betula platyphylla | MK096336 | MK096376 | – | MK096421 | MK096296 | – | |
| CFCC 52858 | China | Betula platyphylla | MK096337 | MK096377 | – | MK096422 | MK096297 | – | |
| CFCC 52859 | China | Betula platyphylla | MK096339 | MK096379 | – | MK096424 | MK096299 | – | |
| CFCC 52860 | China | Betula platyphylla | MK096340 | MK096380 | – | MK096425 | MK096300 | – | |
| CFCC 52861 | China | Betula platyphylla | MK096342 | MK096382 | – | MK096427 | MK096302 | – | |
| CFCC 52862 | China | Betula platyphylla | MK096344 | MK096384 | – | MK096429 | MK096304 | – | |
| CFCC 52863 | China | Betula platyphylla | MK096345 | MK096385 | – | MK096430 | MK096305 | – | |
| CFCC 52864 | China | Betula platyphylla | MK096346 | MK096386 | – | MK096431 | MK096306 | – | |
| CFCC 52865 | China | Betula platyphylla | MK096316 | MK096356 | – | MK096401 | MK096276 | – | |
| CFCC 52866 | China | Betula platyphylla | MK096317 | MK096357 | – | MK096402 | MK096277 | – | |
| CFCC 52867 | China | Betula platyphylla | MK096318 | MK096358 | – | MK096403 | MK096278 | – | |
| CFCC 52868 | China | Betula platyphylla | MK096319 | MK096359 | – | MK096404 | MK096279 | – | |
| CFCC 52869 | China | Betula platyphylla | MK096320 | MK096360 | – | MK096405 | MK096280 | – | |
| CFCC 52870 | China | Betula platyphylla | MK096321 | MK096361 | – | MK096406 | MK096281 | – | |
| CFCC 52871 | China | Betula platyphylla | MK096322 | MK096362 | – | MK096407 | MK096282 | – | |
| CFCC 52872 | China | Betula platyphylla | MK096323 | MK096363 | – | MK096408 | MK096283 | – | |
| CFCC 52873 | China | Betula platyphylla | MK096350 | MK096390 | – | MK096435 | MK096310 | – | |
| CFCC 52874 | China | Betula platyphylla | MK096351 | MK096391 | – | MK096436 | MK096311 | – | |
| CFCC 52875 | China | Betula platyphylla | MK096325 | MK096365 | – | MK096410 | MK096285 | – | |
1 Ex-type strains marked by an asterisk; 2 Sequences in bold were generated in the present study
Morphology
Microscopic observations were made in tap water, except where noted. Morphological analyses of microscopic characters were carried out as described by Jaklitsch (2009). Methods of microscopy included stereomicroscopy using a Nikon SMZ 1500 and Nomarski differential interference contrast (DIC), using the compound microscopes Nikon Eclipse E600 or Zeiss Axio Imager.A1 equipped with a Zeiss Axiocam 506 colour digital camera. Images and data were gathered using a Nikon Coolpix 4500 or a Nikon DS-U2 digital camera and measured by using the NIS-Elements D v. 3.0 or 3.22.15 or Zeiss ZEN Blue Edition software packages. For certain images of ascomata, the stacking software Zerene Stacker v. 1.04 (Zerene Systems LLC, Richland, WA, USA) was used. Measurements are reported as maxima and minima in parentheses and the range representing the mean plus and minus the standard deviation of the number of measurements given in parentheses.
Culture preparation, DNA extraction, PCR and sequencing
Ascospore isolates were prepared and grown on 2% corn meal dextrose agar (CMD; CMA: Sigma, St Louis, Missouri; supplemented with 2% (w/v) D(+)-glucosemonohydrate) or 2% malt extract agar (MEA; 2% w/v malt extract, 2% w/v agar-agar; Merck, Darmstadt, Germany). Growth of liquid cultures and extraction of genomic DNA was performed as reported previously (Voglmayr and Jaklitsch 2011; Jaklitsch et al. 2012) using the DNeasy Plant Mini Kit (QIAgen GmbH, Hilden, Germany). The following loci were amplified and sequenced: a ca. 1.6 kb fragment containing the terminal part of the small subunit nuclear ribosomal DNA (nSSU rDNA), the complete internal transcribed spacer region (ITS1-5.8S-ITS2) and a ca. 900 bp fragment of the large subunit nuclear ribosomal DNA (nLSU rDNA), amplified and sequenced as a single fragment with primers V9G (De Hoog and Gerrits van den Ende 1998) and LR5 (Vilgalys and Hester 1990); a ca. 1 kb fragment of the guanine nucleotide-binding protein subunit beta (ms204) gene with primers MS-E1F1 and MS-E5R1 (Walker et al. 2012); a ca. 1.2 kb fragment of the RNA polymerase II subunit 2 (rpb2) gene with primers fRPB2-5F and fRPB2-7cR (Liu et al. 1999) or dRPB2-5f and dRPB2-7r (Voglmayr et al. 2016); and a ca. 1.3–1.5 kb fragment of the translation elongation factor 1-alpha (tef1) gene with primers EF1-728F (Carbone and Kohn 1999) and TEF1LLErev (Jaklitsch et al. 2005). For the β-tubulin (tub2) gene, either a ca. 0.45 kb fragment was amplified with primers T1 (O’Donnell and Cigelnik 1997) and BtHV2r (Voglmayr et al. 2016) or a ca. 1.6 kb fragment with primer pairs T1 and T22 (O’Donnell and Cigelnik 1997) or T1D and T22D (Voglmayr et al. 2019).
PCR products were purified using an enzymatic PCR cleanup (Werle et al. 1994), as described in Voglmayr and Jaklitsch (2008). DNA was cycle-sequenced using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit v. 3.1 (Applied Biosystems, Warrington, UK) and the PCR primers; in addition, primers ITS4 (White et al. 1990), LR2R-A (Voglmayr et al. 2012) and LR3 (Vilgalys and Hester 1990) were used for the SSU-ITS-LSU region, TEF1_INTF (forward, Jaklitsch 2009) and TEFD_iR1 (reverse, Jaklitsch and Voglmayr 2019) or TEF1_INT2 (reverse, Voglmayr and Jaklitsch 2017) for tef1 and BtHVf (Voglmayr and Mehrabi 2018) and BtHV2r for the long fragment of tub2. Sequencing was performed on an automated DNA sequencer (3730xl Genetic Analyzer, Applied Biosystems).
Phylogenetic analyses
The newly generated sequences were aligned with the Melanconis sequences of Fan et al. (2016, 2018) and a few additional GenBank sequences. Species of Juglanconis were selected as outgroup (Voglmayr et al. 2017, 2019); the GenBank accession numbers of the sequences, used in the phylogenetic analyses, are given in Table 1. All alignments were produced with the server version of MAFFT (www.ebi.ac.uk/Tools/mafft), checked and refined using BioEdit v. 7.2.6 (Hall 1999). For phylogenetic analyses, all sequence alignments (ITS, LSU, ms204, rpb2, tef1 and tub2) were combined.
Maximum Likelihood (ML) analyses were performed with RAxML (Stamatakis 2006) as implemented in raxmlGUI 1.3 (Silvestro and Michalak 2012), using the ML + rapid bootstrap setting and the GTRGAMMA substitution model with 1000 bootstrap replicates. The matrix was partitioned for the different gene regions and substitution model parameters were calculated separately for them.
Maximum Parsimony (MP) analyses were performed with PAUP v. 4.0a166 (Swofford 2002). All molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data; the COLLAPSE command was set to MINBRLEN. MP analysis of the combined multilocus matrix was done, using a parsimony ratchet approach. For this, a nexus file was prepared using PRAP v. 2.0b3 (Müller 2004), implementing 10000 ratchet replicates with 25% of randomly chosen positions upweighted to 2, which were then run with PAUP. MP bootstrap analyses were performed with 1000 replicates, using 5 rounds of random sequence addition and subsequent TBR branch swapping (MULTREES option in effect, steepest descent option not in effect) during each bootstrap replicate, with each replicate limited to 100000 rearrangements.
In the Results and Discussion sections, bootstrap values (BS) below 70% are considered low, between 70–90% medium and above 90% high.
Results
Revision of Melanconis sequences deposited in GenBank
Comparison of our sequences with GenBank sequences revealed that all accessions of Melanconis alni and M. marginalis, deposited in GenBank, were misidentified. All GenBank accessions of M. alni were shown to actually represent M. marginalis, while the single isolate of M. marginalis turned out to be a new species, described as M. pacifica below. These misidentifications were also confirmed by morphological re-investigation of specimens from which these sequences were generated.
Phylogenetic analyses
Of the 6052 characters included in the combined multilocus analyses, 925 were parsimony informative (133 from ITS-LSU, 142 from ms204, 214 from rpb2, 245 from tef1 and 191 from tub2). The best ML tree (lnL = −18240.558) revealed by RAxML is shown as Fig. 1. The MP analysis revealed 3394 MP trees 1647 steps long, which were identical except for some differences within species and a polytomy at the M. groenlandica-M. larissae-M. stilbostoma node (not shown). Tree topology of the MP strict consensus tree was compatible with the ML tree, except for a sister group relationship of M. marginalis subsp. europaea and M. marginalis subsp. marginalis and some minor topological differences within species and subspecies (not shown).
Figure 1.
Phylogram of the best ML tree (lnL = −18240.558) revealed by RAxML from an analysis of the ITS-LSU-ms204-rpb2-tef1-tub2 matrix of Melanconis, with 5 species of Juglanconis (Juglanconidaceae) selected as outgroup. ML and MP bootstrap support above 50% are given at the first and second position, respectively, above or below the branches. Strain numbers are given following the taxon names; strains formatted in bold were sequenced in the current study. Melanconis taxa occurring on Alnus are marked blue, those on Betula in green. The broken branches to the outgroup were scaled to 10%.
All species of Melanconis received high (M. itoana, M. groenlandica) to maximum (M. alni, M. betulae, M. marginalis, M. stilbostoma) support in both analyses (Fig. 1). Sister group relationship of M. alni and M. pacifica and monophyly of the three betulicolous species M. groenlandica, M. larissae and M. stilbostoma received maximum support as well. Within Melanconis marginalis, two main subclades were evident with ML and MP BS above 85%, one containing accessions from eastern Canada, Alaska, Japan and the Russian Far East and another with accessions from Central Europe; in addition to these two main subclades, the Melanconis marginalis clade contained two deviating lineages, an Italian collection from ?Alnus cordata described as M. italica by Senanayake et al. (2017) and two accessions from eastern Tyrol from Alnus alnobetula. In light of this geographical differentiation, a substantial genetic variability within these clades (Fig. 1) and minor morphological differences, these four lineages are formally recognised on the subspecies level.
Culture characteristics
Culture images of seven studied Melanconis species, grown on MEA and CMD, are illustrated in Figure 2. Culture descriptions are given under the respective species.
Figure 2.
Melanconis cultures. a–cM. alni (a, b D156, c MAW) dM. groenlandica ME13 eM. itoana ME8 fM. larissae ME7 (after irregular rehydration) g–iM. marginalis subsp. europaea (g, h D257, i MAI) j–lM. marginalis subsp. marginalis (j, k D321, l ME5) mM. marginalis subsp. tirolensis ME4 nM. pacifica ME3 o, pM. stilbostoma (o D143, p ME11) a, b, g, h, j, o on CMDc–f, i, k, l–n, p on MEAa, b, g, h, j at 16 °C, j, k at 22 °C c–f, i, k, l–n, p at room temperature a, g, j, k after 3 weeks b, h after 3 c, i 5 d–f, l–n, p 3.7 o 2 months.
Taxonomy
Melanconis
Tul. & C. Tul., Select. fung. carpol. (Paris) 2: 115 (1863).
31474D83-9B28-5A4C-A9FE-277EB3A3BC31
?= Melanconium Link : Fr., Mag. Gesell. naturf. Freunde, Berlin 3(1–2): 9 (1809).
Type species.
Melanconis stilbostoma (Fr. : Fr.) Tul. & C. Tul., Select. fung. carpol. (Paris) 2: 115 (1863).
Notes.
Tulasne (1856) had already mentioned Melanconis, but did not give a generic diagnosis. Hence, the species he newly described were invalid, but became validated by reference in Tulasne and Tulasne (1863) (Paul Kirk, pers. comm.).
In contrast to Diaporthe, species of Melanconis always develop in bark, never in wood and lack stromatic zones. Pseudostromata are pulvinate to conical, circular to elliptic in outline and usually slightly project beyond the bark surface with perithecial contours remaining indistinct. Ectostromatic discs usually project distinctly from the surface of the pseudostromata and are bright, white to yellowish, to brown when old.
Nomenclaturally, the older genus Melanconium potentially competes with the younger genus Melanconis. However, as outlined in Rossman et al. (2015), the generic concept of Melanconium and the true identity of its generic type, M. atrum, are obscure and they therefore recommended to protect the well-defined Melanconis over Melanconium, which was formally adopted in the last ICN (Turland et al. 2018, Appendix III).
Melanconis alni
Tul. & C. Tul., Select. fung. carpol. (Paris) 2: 122 (1863).
3EC30B0B-3179-50CE-B3FD-C1E6BC24605E
Figure 3.
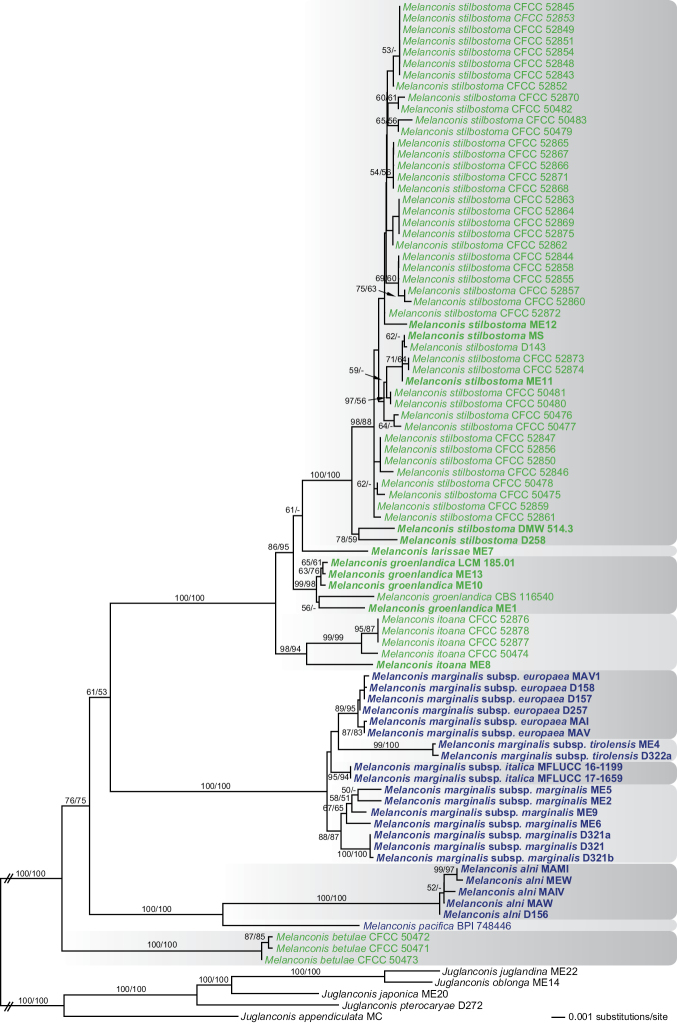
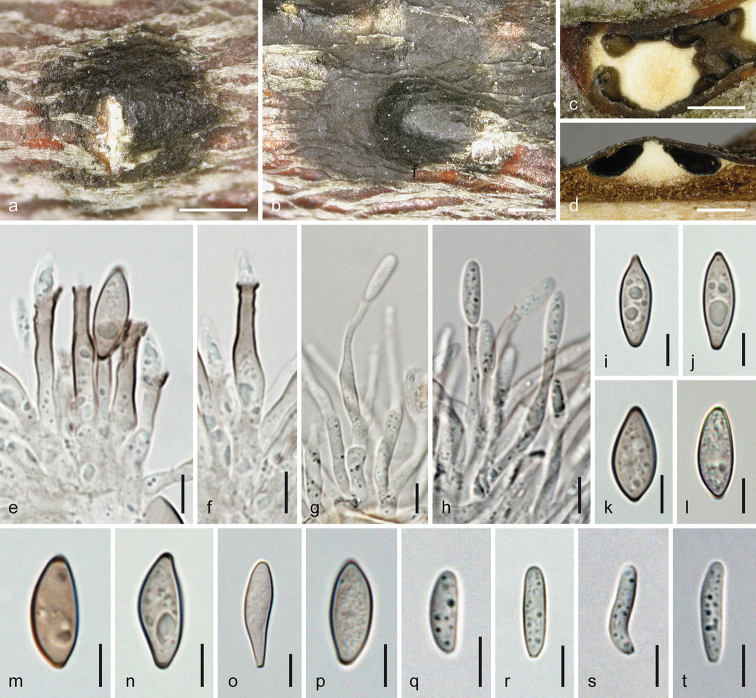
Melanconis alni. Sexual morph a, b ectostromatic discs c pseudostroma with ectostromatic disc in face view d cross section showing remnants of asexual morph at the sides of the sexual pseudostroma e cross section showing perithecia with lateral ostiolar necks and central column f vertical section showing perithecium with central ostiolar neck g–j asci k, l ascus apices showing apical ring m–x ascospores j, l, w in aqueous Congo Red a, b, iWU 31885 = W.J. 148 c–f, j, o–q epitype WU 31884 = MAIV g, h, k, l, xWU 37043 = J.F. 10104 m lectotype PC 0723592 nWU 37042 = D156 r, sWU 31882 = MAMI t, uWU 31883 = MAW vWU 31887 = W.J. 1194 wWU 31886 = W.J. 178. Scale bars: 400 µm (a, b, d–f), 500 µm (c), 10 µm (g–j, n, s–u), 7 µm (k–m, o–r, v–x).
Figure 4.
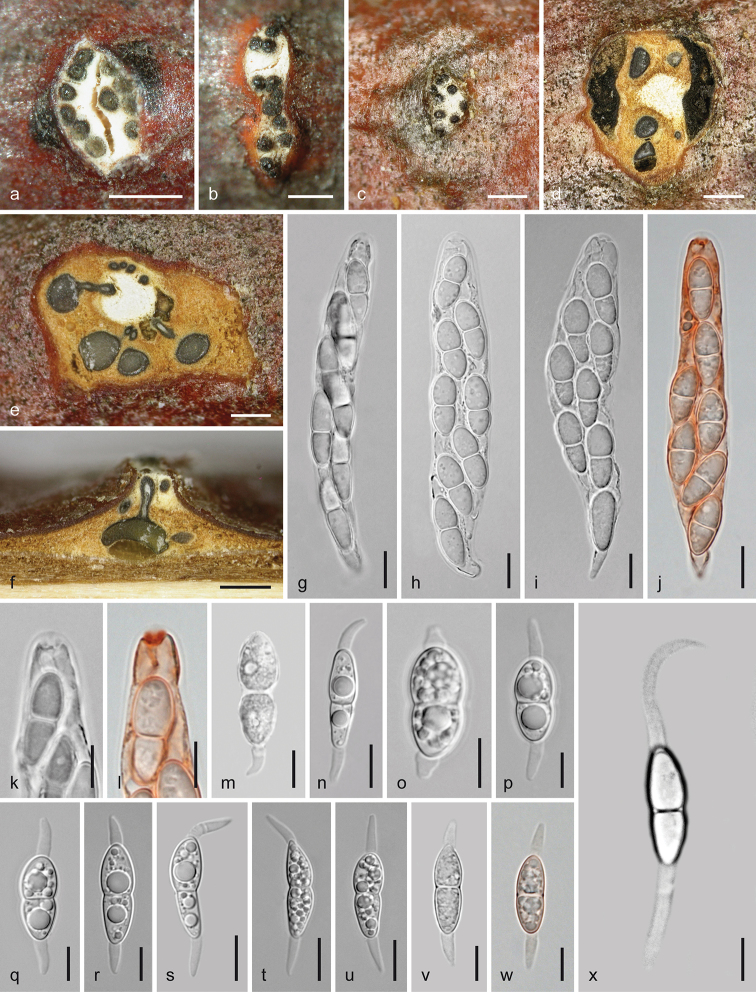
Melanconis alni. Asexual morph a, b conidiomata in face view c conidioma in cross section d conidioma in vertical section e–i conidiophores and conidiogenous cells j–p α-conidia q–t β-conidia a, fWU 31885 = W.J. 148 b–d, h, m, q, s epitype WU 31884 = MAIV e, i PC0723596 g, j, k lectotype PC0723592 l, rWU 37043 = J.F. 10104 n, t PC0723595 oWU 31886 = W.J. 178 pM. atrum isotype K(M) 171588 e–o, q–t in 3% KOH. Scale bars: 300 µm (a–d), 10 µm (e), 7 µm (f–i), 5 µm (j–t).
≡ Melanconis alni Tul., Annls Sci. Nat., Bot., sér. 4, 5: 109 (1856). (Nom. inval., Art. 35.1).
= Rehm, Ascom. exs. 148 (1872).
?= Melanconium apiocarpum Link, in Willdenow, Sp. pl., Edn 4 6(2): 90 (1825).
?= M. sphaeroideum Link, in Willdenow, Sp. pl., Edn 4 6(2): 92 (1825).
?= Stilbospora microsperma Pers., Observ. mycol. (Lipsiae) 1: 31 (1796).
Diagnosis.
Melanconis alni is recognised by ascospores having filiform, tapering appendages and dark brown α-conidia with a pale to subhyaline median area.
Type material.
Lectotype, here designated: France, Hauts-de-Seine, Chaville, on Alnus glutinosa, 1 Feb 1856, Tulasne (PC 0723592; MBT390380). Epitype, here designated: Austria, Oberösterreich, Raab, Wetzlbach, grid square 7648/1, on Alnus glutinosa, 4 Jun 2011, H. Voglmayr (WU 31883; ex-epitype cultures CBS 131695 = MAW (from ascospores), MEW (from α-conidia); MBT390381).
Description.
Sexual morph: Pseudostromata developing in bark after the asexual morph and sometimes with acervuli of the asexual morph still present within their sides, 0.9–2.7 mm diam., scattered, pulvinate, more or less circular in outline, slightly projecting from the bark surface and then causing a greyish bark surface; consisting of an ectostromatic disc and perithecia embedded in an entostroma. Ectostromatic discs 0.3–1.4 mm diam., white to yellowish, brownish when old, flat to convex, circular, fusoid, angular or elongate in section, projecting up to 0.6 mm. Ostiolar necks cylindrical, laterally attached on perithecia and convergent in the disc, centrally only on centrally arranged perithecia, 1–15(–20) per disc, in the disc plane, convex to papillate and slightly projecting, with dark rounded tips; first pale brownish to greyish-brown, turning black, (70–)93–162(–210) µm (n = 33) diam. apically, mostly present at the margins but often also randomly within the disc. Entostroma bark-coloured, not or only slightly paler than the surrounding bark, consisting of bark cells and some light-coloured hyphae. Perithecia (390–)450–645(–765) µm (n = 24) diam., formed below overmature conidiomata in valsoid configuration, globose to subglobose, collapsing up- or laterally inwards upon drying. Hamathecium of wide multiguttulate paraphyses, collapsing, dissolving and usually absent amongst mature asci. Asci floating free at maturity, (68–)79–97(–110) × (10.5–)12.5–16.5(–21) µm (n = 114), narrowly clavate, fusoid, oblong to nearly ellipsoid, with an apical ring staining in Congo Red but invisible or indistinct in the strongly thickened apex in 3% potassium hydroxide (KOH), containing 8 biseriate ascospores. Ascospores (14.5–)16–21(–25.3) × (4.7–)6–7.8(–9) µm, l/w (1.9–)2.3–3.2(–4.8) (n = 198), hyaline, ellipsoid, clavate or inequilaterally fusoid, bicellular with upper cell usually slightly wider, slightly or strongly constricted at the median septum, thick-walled, multiguttulate or with one large and several small guttules when fresh, with a filiform, tapering and acute, less commonly short and stout rounded, triangular or truncate appendage (2.5–)4.7–10(–24.3) × (1.7–)2.3–3(– 4) µm, l/w (1–)1.8–3.8(–8.4) (n = 224) at one or both ends; in 3% KOH, appendages invisible and cells tending to be more equal.
Asexual morph acervular, often conspicuous due to thick black conidial masses, first subperidermal, after ejection forming deposits 0.5–3.6 mm diam., sometimes confluent from 2–3 conidiomata and then up to 5 mm long, projecting to 0.5 mm. Conidiomata scattered, gregarious, sometimes confluent, pulvinate to conical, (0.6–) 0.8–2.5 mm diam., consisting of a superficial, ca. 0.2–1.3 mm wide, flat, white to yellowish, slightly projecting disc becoming concealed by dark brown to black conidial deposits, a whitish to yellowish, when old orange-brown, compact, more or less pseudoparenchymatous base, in the centre arising as central column with circular to longish outline and sometimes wavy margin, surrounded by conidiophores and black conidial chambers. Conidiophores emerging radially from the pseudoparenchymatous base and column surface, filiform, to ca. 50 × 4 µm, branching 1–3 times from their bases producing whorls of conidiogenous cells. Conidiogenous cells (10–)12–43 × 2–4 µm, annellidic, more or less cylindrical, hyaline, turning brown with age, forming more or less simultaneously two types of conidia on top. Conidia dimorphic, α-conidia (9–)10.5–12.2(–14) × (4.8–) 6.8–8(–9) µm, l/w (1.2–)1.4–1.7(–2.4) (n = 301), dark brown, more or less cuboid or subglobose and often with pinched sides or oval, oblong to broadly ellipsoid, with a diffuse or more or less well-defined, paler to subhyaline median area or stripe; β-conidia produced in small numbers, (5.3–)7.3–10.3(–11.5) × (2–)2.5–3.2(–3.7) µm, l/w (2–)2.6–3.9(–4.7) (n = 38), oblong, mostly straight, hyaline to subhyaline, turning dilute brownish with age, containing few minute guttules, with a distinct basal abscission scar.
Culture: Colony on CMD at 16 °C first hyaline, turning yellowish-brown from the centre, becoming covered by flocks of white aerial hyphae and conidiomata forming around the centre or colony irregular, with limited growth, turning green to black due to conidiomata; on MEA first hyaline, circular, with short aerial hyphae, forming concentric zones, the outer white, the inner turning brown, black conidiomata forming between the zones, margin becoming diffuse and the entire colony turning brown. Odour indistinct.
Distribution and ecology.
Melanconis alni occurs in Europe on dead twigs and branches of Alnus glutinosa and A. incana, mainly at lower elevations.
Additional material examined.
Austria, Kärnten, Eisenkappel, Bad Vellach, Vellacher Kotschna, grid square 9653/1, on Alnus incana, 7 Sep 1998, W. Jaklitsch W.J. 1194 (WU 31887); St. Margareten im Rosental, village area, at the brook Tumpfi, grid square 9452/4, on Alnus glutinosa, 18 Jul 1994, W. Jaklitsch W.J. 148 (WU 31885); Trieblach, Drau-Auen, near Kucher, grid square 9452/2, on Alnus incana, 7 Aug 1994, W. Jaklitsch W.J. 178 (WU 31886); Niederösterreich, Michelbach, Mayerhöfen, on Alnus glutinosa, 18 Jun 2011, H. Voglmayr (WU 31882, culture CBS 131693 = MAMI). FRANCE, Alpes-de-Haute-Provence, Trigance SE Castellane, at the river Jabron ca. 500 m elev. before entering the Verdon river, on Alnus incana, 4 Aug 2011, H. Voglmayr (WU 31884; culture MAIV); Ariége-Rimont, Peyrau, on Alnus glutinosa, soc. Diplodia sp., 26 Jul 2010, J. Fournier J.F. 10104 (WU 37043); Hauts-de-Seine, Chaville, on Alnus glutinosa, 11 Oct 1852, Tulasne (PC 0723589, PC 0723596); Meudon, on Alnus glutinosa, 13 May 1856, Tulasne (PC 0723593); Oise, Pierrefonds, on Alnus glutinosa, 30 Jul 1857, Tulasne (PC 0723590, PC 0723591); Yvelines, Viroflay, on Alnus glutinosa, July 1860, Tulasne (PC 0723594, PC 0723595); no collection data, Tulasne (PC 0723588). POLAND, S Kuligi, Biebrzański Park Narodowy, on Alnus glutinosa, 28 Jul 2015, H. Voglmayr (WU 37042, culture D156).
Notes.
Melanconis alni was described by Tulasne from Alnus glutinosa in 1856 after a presentation of the topic in April 1856. Tulasne and Tulasne (1863) validated the name in Melanconis, illustrated ascospores with typical long acute appendages and mentioned material from Meudon and Chaville. In PC, nine specimens of Tulasne are extant in the Melanconis alni folder; three of them were collected after its description in 1856 and, for one, no collection data are available. PC 0723590, PC 0723591, PC 0723593, PC 0723594 and PC 0723595 were collected after the publication date. PC 0723588 (no data) and PC 0723589, PC 0723596 from 1852 only contain asexual morph, but in the protologue, the sexual morph is also described. Therefore, we select PC 0723592, which also contains few pseudostromata of the sexual morph, as the lectotype. In PC 0723592 and PC 0723595, both α- and β-conidia are present. Generally, β-conidia are inconspicuous and produced in small numbers, i.e. they are easily overlooked. Asci in old herbarium material are shrunk and difficult to rehydrate, therefore significantly smaller than those of fresh material. In KOH, the ascus apex becomes very thick and the ring disappears; also ascospore appendages disappear in KOH.
Tulasne and Tulasne (1863) and Wehmeyer (1941) listed the following asexual morph names, amongst others, as linked to M. alni: Stilbospora microsperma Pers. Material with this name is not accessible in L; Melanconium sphaeroideumLink (1825) is more generally given as the name of the asexual morph. Sieber et al. (1991) used another name described by Link (1825), Melanconium apiocarpum, for the asexual morph of Melanconis alni. As Link´s type material of these taxa is not extant in B, we are unable to draw a conclusion about their identity; in addition, the descriptions in Link (1825) are vague and he gave no hosts. Therefore, we continue to use the name M. alni, which is generally well-known. Type material of Melanconium atrum Link, the generic type of Melanconium, described from Germany (K(M) 171588, slide from Melanconium atrum type material from Persoon´s herbarium) has conidia of the same shape, size and lighter median band (Fig. 4p) and may thus be conspecific with M. alni, but it was described from Fagus sylvatica. According to Sutton (1964), Link had sent his material to Persoon, because in the herbarium of the latter 3 specimens labelled M. atrum were extant. The host of one of these materials was identified as Fagus, based on bark structure. This specimen was selected as lectotype. The slide K(M) 171588 (= IMI 102914) was prepared from the lectotype and is thus an isotype. Accordingly, Melanconium atrum is a different species, despite its morphological similarity with M. alni, because the latter only occurs on Alnus spp. We have not seen any Melanconium on Fagus, but Petrini and Fisher (1988), Sieber et al. (1991) and Kowalski and Kehr (1992) reported and isolated M. atrum as an endophyte of Fagus. For α-conidia of isolates from Fagus sylvatica and Quercus robur, Sieber et al. (1991) reported mean sizes of 11.7–12 × 8.5–8.9 µm, which were similar to those from Alnus glutinosa (on average, 10.1–12.3 × 5.9–7.4 µm). However, the protein profiles revealed by isozyme electrophoresis differed markedly between the isolates from Alnus glutinosa and those from Fagus/Quercus, confirming them to represent distinct species that may not even be congeneric. Another fact may support the presence of morphologically similar but rare taxa on Fagaceae, as, for example, Melanconium gourdaeforme with similar conidia was described by Kobayashi (1968) from Castanea. A narrow light band is also characteristic for conidia of Melanconiella ostryae (Voglmayr et al. 2012).
Ascospore appendages of Melanconis alni may sometimes be similar to those of M. marginalis, at least in fractions, although truncate appendages in M. alni are rather a consequence of microscopic mount preparation. On Alnus incana both species occur, therefore the asexual morph should be sought for to reliably identify the species.
Melanconis betulae
C.M. Tian & X.L. Fan, in Fan, Du, Liang & Tian, Mycol. Progr. 15(4/40): 4 (2016).
5AEFCE1D-17E7-5177-875D-5C05A90BEE9D
Note.
According to Fan et al. (2016), who described this species as an asexual morph from Gansu Province in China on Betula albosinensis, Melanconis betulae can be distinguished from M. stilbostoma by the smaller average length of its alpha conidia (10 vs. 12 μm). Phylogenetically, M. betulae is remote from the other betulicolous Melanconis species (Fig. 1).
Melanconis groenlandica
(M. Bohn) L. Lombard & Crous, in Lombard et al. Persoonia 36: 234 (2016).
B5143FA8-EACB-5704-8D5A-FBDFCB2A0DEE
≡ Myrothecium groenlandicum M. Bohn, Mycotaxon 46: 336 (1993) (Basionym).
Type material.
Holotype (not examined): Greenland, Qaqortoq, (isolated from) twigs of Betula nana, July 1991, M. Bohn (C; dried culture UPSC 3416; isotype in UPS; living cultures CBS 116450 = UPSC 3407, UPSC 3416).
Description
(after Bohn 1993): Colonies on PDA and MEA 30–33 mm after 10 d (52–62 mm after 20 d), appearing leathery, at first whitish to greyish, later becoming greyish-orange, particularly on MEA; margin superficial, entire on MEA but fimbriate to lobate on PDA; exudate and diffusible pigment absent; reverse greyish-orange, especially at the margin; brownish, thick-walled, chlamydospore-like swollen portions 6–18 µm diam. present. Conidiomata appearing after ca. 14 d as dark green pustules of various sizes, irregularly scattered over the colony surface, but sometimes arranged in concentric rings, particularly in old cultures, initially covered by mycelium but becoming almost black and shiny at later stages due to the mass of conidia; conidiomata sporodochial (acervular?), irregular, dark green, up to 2 mm diam., scattered, gregarious or coalescent, composed of a 50–70 µm high stroma of textura intricata and conidiophores. Marginal hyphae and setae absent. Conidiophores arising from the stroma, branched, septate, yellowish to brownish, ca. 40–75 µm × 2–4 µm. Conidiogenous cells cylindrical to subulate, 15–25 × 2–3 µm, arranged in verticils of 2–4 at the top of the conidiophore, sometimes also intercalary, provided with conspicuous, pigmented collarettes and producing conidia by percurrent growth. Conidia black and shiny in mass, olivaceous to brownish under the microscope, straight, cylindrical with rounded ends, sometimes slightly narrowing towards the base or apiculate, (9–)10–12(–15) × (5–)6(–7) µm, with smooth wall. Teleomorph not formed after 3 months incubation.
Culture (own observations): Colony on MEA circular, first hyaline, turning and long remaining whitish, with age forming narrow concentric zones with tooth-like margins and turning pale brownish. Odour indistinct to unpleasant.
Distribution and ecology.
Melanconis groenlandica is known from North America (Greenland, USA) and Japan from Betula maximowicziana, B. nana and B. papyrifera.
Additional collections sequenced.
Japan, Hokkaido, Sorachi, Furano, Hokkaido Experimental Forest of Univ. Tokyo, on B. maximowicziana, 25 Sep 1964, T. Kobayashi (TFM FPH2478, culture MAFF 410219 = M4-2, ME1). USA: New Hampshire, close to the top of Mount Washington, on Betula sp., 28 Jul 2006, L. Mejia (BPI 879597; culture CBS 133339 = LCM 02.02 = ME13); New York, Adirondack High Peaks Region, Marcy Dam, on Betula papyrifera, 2 Jun 2007, L. Mejia (BPI 881485; culture CBS 133341 = LCM191.01 = ME10); ibidem, same host, 9 Jun 2007, L. Mejia (BPI 881515; culture CBS 133340 = LCM 185.01).
Note.
This species was isolated as a putative endophyte from Betula nana and described from MEA and potato dextrose agar as a species of Myrothecium. In our phylogenetic analyses, three isolates from North America and one from Japan grouped with the ex-type isolate of M. groenlandica with high support.
Melanconis itoana
Tak. Kobay., Bull. Govt Forest Exp. Stn Meguro 226: 19 (1970).
C9A1C7C8-4C22-5F69-B964-A8C79CE731AE
Type material.
Holotype: Japan, Shizuoka, Fujinomiya, Mt. Fuji, on Betula ermanii, 6 Aug 1968, T. Kobayashi (TFM FPH3375; ex-type culture MAFF 410080 = LFP-M4-9 = ME8).
Description.
See Kobayashi (1970) and Fan et al. (2016).
Culture: Colony on MEA circular, first hyaline, forming a white outer and brown inner zone, with radial stripes; conidiomata forming mostly in the inner zone. Odour indistinct.
Note.
This species occurs on Betula ermanii in Japan and Betula albosinensis in China and is particularly well characterised by its long narrow fusoid conidia, which are more or less pointed at each end. It was originally described by Kobayashi (1970) in detail and the asexual morph was redescribed by Fan et al. (2016), who gave slightly shorter measurements of conidia (12–13.5(–14) × 3.5–4(–4.5) μm). Our measurements of conidia are (13–)14.7–17.5(–20) × (3–)3.5–4.3(–4.7) µm, l/w (3–)3.6–4.7(– 5.4) (n = 100), upon examination of the holotype, which corresponds with Kobayashi (1970). The Chinese accessions genetically differ significantly from the ex-type culture from Japan (Fig. 1) and may therefore merit separation.
Melanconis larissae
Jaklitsch & Voglmayr sp. nov.
F825EFBE-AD44-5C5F-8AD3-BA5C8F3A027C
834108
Figure 5.
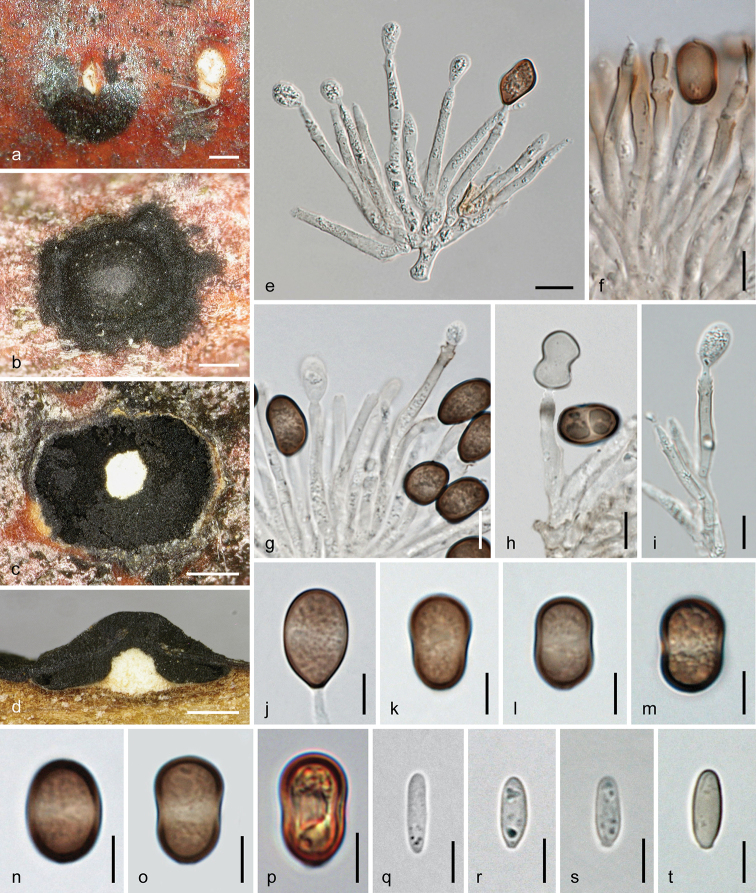
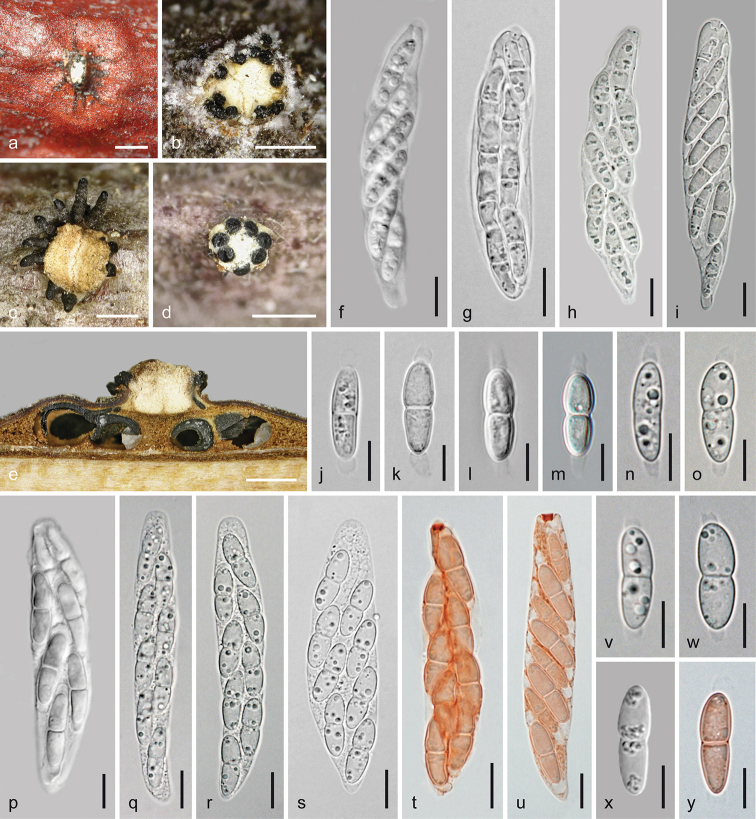
Melanconis larissae holotype (BPI 870998) a–o sexual morph a, b ectostromatic discs c, d cross sections showing white upper and yellow lower parts of central columns, ostiolar necks and perithecia e–g asci h–o ascospores f, g in aqueous Congo Red p–y asexual morph p conidial deposit q conidioma in cross section r, s conidiophores and conidiogenous cells (showing annellations in s) t–y α-conidia r–y in 3% KOH. Scale bars: 500 µm (a–d, p, q), 15 µm (e–g), 7 µm (h–o, r), 5 µm (s–y).
Diagnosis.
Melanconis larissae differs from M. stilbostoma by α-conidia having a broad diffuse light-coloured zone.
Type material.
Holotype. USA, New York, Adirondack Mts., Cranberry Lake, on Betula sp., 13 Jun 2002, L. Vasilyeva (BPI 870998; ex-type culture CBS 123196 = A.R. 3886, ME7).
Etymology.
Named after the collector Larissa Vasilyeva.
Description.
Sexual morph: Pseudostromata 1.8–2.7 mm diam., scattered to aggregated, not or only scarcely projecting from the bark surface, pulvinate, circular to elliptical in outline; consisting of an ectostromatic disc and perithecia embedded in an entostroma around a central column and sometimes conidial locules present on the ostiolar level. Ectostromatic discs 0.5–1.4 mm diam. or long, slightly projecting, fusoid to circular, flat or concave, white to yellow, often concealed by ostioles; central column beneath disc brightly white at upper levels, yellow amongst ostiolar necks at lower levels, consisting of hyaline hyphae and colourless crystals. Ostiolar necks cylindrical, laterally or centrally attached on perithecia, convergent and irregularly inserted in the disc; visible part (88–)130–204(–230) µm (n = 32) diam., 1–12 per disc, black, subglobose to subconical with flat or pointed tips, projecting to 200 µm. Entostroma consisting of hyaline hyphae and bark cells. Perithecia (420–)490–650(–690) µm (n = 14) diam., arranged in valsoid configuration around and below central column, globose to subglobose, collapsing up- or laterally inwards upon drying. Peridium pseudoparenchymatous, consisting of a dark brown small-celled outer and a hyaline to brownish, large-celled inner layer. Hamathecium absent at maturity. Asci floating free at maturity, (69–)84–106(–117) × (11–)13–17.5(–19.7) µm (n = 22), fusoid to oblong, with an apical ring distinct in water and staining in Congo Red, but invisible or indistinct in 3% KOH, containing (2–)4–8 ascospores in biseriate or obliquely uniseriate arrangement. Ascospores (14.8–)17–21.5(–25) × (5.8–)6.5–8.3(–9.7) µm, l/w (1.9–)2.3–3(–3.7) (n = 93), ellipsoid to subfusoid, symmetric or inequilateral, bicellular, hyaline, dilute brownish when old, slightly constricted at the central to slightly eccentric septum, thick-walled, becoming verruculose with age, devoid of appendages.
Asexual morph acervular, intermingled with pseudostromata of the sexual morph or developing separately, conspicuous. First white tissue (central column) forming within the bark, becoming surrounded by a yellow margin and narrow whitish to yellowish discs emerging through bark cracks, followed by the production of conidia in olivaceous to dark brown chambers. Conidiomata 1.3–2.7 mm diam., pulvinate, more or less circular in outline, scattered or crowded. Covering discs 0.25–1.2 mm long, narrowly fusoid or longish to circular, flat to convex, whitish to yellowish, becoming obscured by large, coppery to olivaceous brown conidial deposits 1–4 mm diam., projecting to 1.2 mm, also confluent from two or several conidiomata; discs and pulvinate or conical columns beneath consisting of textura intricata of hyaline hyphae and numerous colourless crystals, becoming brittle with age. Conidiophores emerging around the central column from a pseudoparenchymatous base, ca. 40–70 µm long, filiform, branched near the base and usually 1–3 fold asymmetrically at higher levels, first hyaline, turning brown from their tips; terminal conidiogenous cells (10.5–)14.5–28(–36.5) × (1.7–)2.5–3.5(–4.2) µm (n = 70), cylindrical and often widened towards base, with funnel-shaped collarette and up to 5 or 6 annellations, densely arranged, repetitive, producing α-conidia. Conidia (9.7–)11–13(–14.5) × (6.5–)7.7–9(–9.5) µm, l/w (1.1–)1.3–1.6(–2.2) (n = 66), oval, subglobose to drop-like, unicellular, dark brown, thick-walled, with a broad lighter coloured median zone and a small scar, smooth. No β-conidia detected.
Culture: Colony on MEA at room temperature circular, dense, first hyaline, turning rosy. Odour indistinct to musty.
Distribution and ecology.
Melanconis larissae is known from a single specimen collected in New York State from an unidentified species of Betula.
Notes.
The description of this taxon is based on a single specimen with over-mature sexual morph and well-developed asexual morph with thick masses of conidia. Melanconis larissae differs from M. stilbostoma by the broad light-coloured zone of its conidia. No β-conidia have been detected in this specimen, but oblong to ellipsoid, hyaline to dilute brownish conidia 5–9 × 1.7–5 µm, which we interpret as immature α-conidia.
Melanconis marginalis
(Peck) Wehm., Pap. Michigan Acad. I. 6: 382 (1926).
666B05B5-C5E8-5E3B-8C20-3C32A9B2E128
Notes.
This species is here subdivided into four subspecies below. See under subsp. marginalis for the original species.
Although Wehmeyer (1926a) combined Diaporthe marginalis in Melanconis, he later (Wehmeyer 1941) argued that the conidia only differ from those of M. alni in depth of pigmentation and, therefore, reduced M. marginalis to a subspecies of the latter. In Europe, where, owing to Wehmeyer (1941), Melanconis on Alnus was always identified as M. alni, Petrak (1941) reported Melanconium dimorphum for the first time, described both conidial types, but still found it probable that Melanconium dimorphum was an abnormal form of M. sphaeroideum, the putative name of the asexual morph of M. alni. Kobayashi (1970) and Jensen (1984), however, were convinced that Melanconis marginalis should be treated as a species separate from M. alni, which is here confirmed phylogenetically. In addition, ascospores of M. marginalis are narrower, usually more oblong and symmetric than those of M. alni and appendages shorter, stouter and rounded or truncate at the ends, which swell and become diffuse in mounts.
Melanconis marginalis subsp. europaea
Jaklitsch & Voglmayr subsp. nov.
3D3B8B2D-87A7-57C0-9C66-6025066F1EC7
834109
Figure 6.
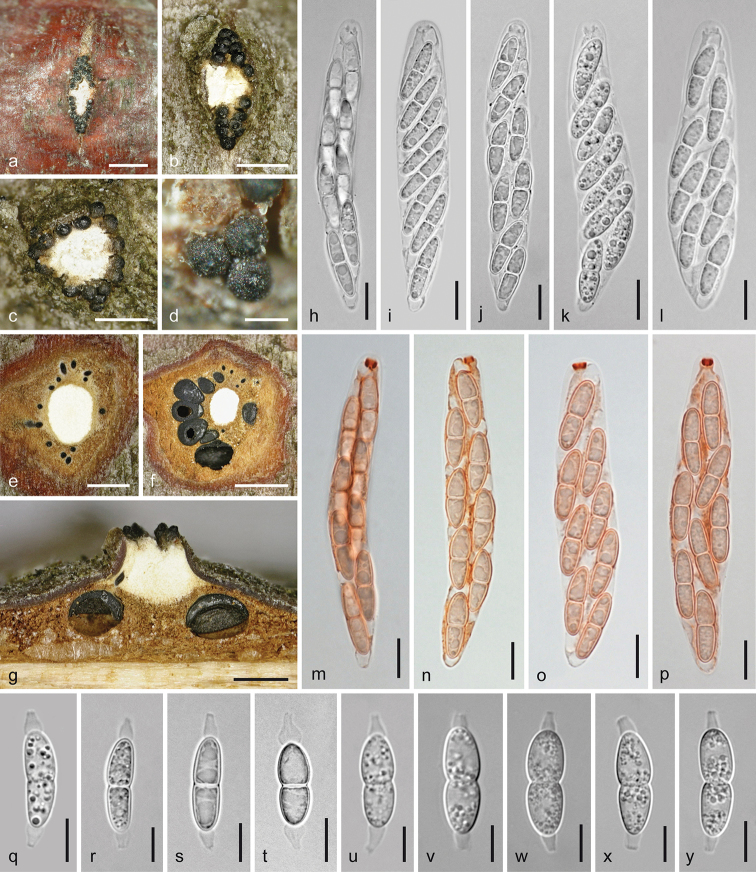
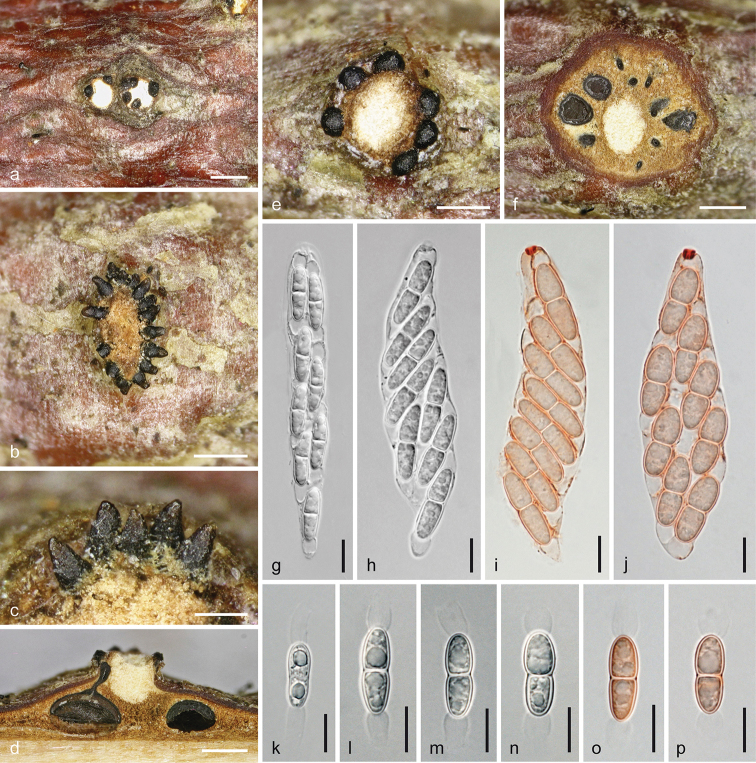
Melanconis marginalis subsp. europaea. Sexual morph a pseudostroma in face view b, c ectostromatic discs d subglobose visible part of ostiolar necks e, f cross sections (e showing central column and marginal ostioles f showing central column and perithecia) g vertical section showing central column and two perithecia h–p asci q–y ascospores m–p in aqueous Congo Red aWU 31890 = MAV1 b–g, j, n, q, s, t, w–y holotype WU 31888 = MAI h, i, mWU 37045 = D158 k, rWU 36699 l, pWU 31172 oWU 29888 uWU 31889 = MAV vWU 38243. Scale bars: 1 mm (a, f), 500 µm (b, c, e, g), 150 µm (d), 10 µm (h–q, t), 7 µm (r, s, u–y).
Figure 7.
Melanconis marginalis subsp. europaea. Asexual morph a, b conidiomata and conidial deposits in face view c conidioma with β-conidia in cross section d conidioma with α-conidia in vertical section e–h conidiophores and conidiogenous cells (producing α-conidia in e, f, β-conidia in g, h) i–p α-conidia q–t β-conidia e–t in 3% KOH a, b, d–f, i–k, q–sWU 37044 = D157 c, g, h, l, tWU 31893 mWU 31891 = W.J. 1542 nWU 31888 = MAI o, pWU 31889 = MAV. Scale bars: 500 µm (a–d), 5 µm (e–t).
Diagnosis.
This subspecies of Melanconis marginalis occurs in Europe and differs from the American subsp. marginalis phylogenetically and by slightly larger asci, ascospores and ascospore appendages.
Type material.
Holotype. Austria, Steiermark, Judenburg, Pusterwald, Hinterwinkel, grid square 8651/4, on Alnus incana, 11 Jun 2011, H. Voglmayr (WU 31888, culture CBS 131692 = MAI).
Etymology.
For its occurrence in Europe.
Description.
Sexual morph: Pseudostromata 1.5–3.6 mm diam., usually conspicuous and numerous, scattered to tightly aggregated, forming pustules, pulvinate, circular to elliptical in outline, typically elevated beyond bark surface; consisting of an ectostromatic disc and perithecia embedded in an entostroma around a central column. Ectostromatic discs 0.5–2.1 diam. or long, discrete, less commonly confluent, bright white to yellowish, turning brownish with age, variable, fusoid, elliptic or circular in outline, flat, convex, concave, entire or coarsely fissured and crumbly, projecting up to 1 mm including projecting part of the pseudostroma; central column beneath disc whitish to yellowish, consisting of hyaline hyphae and colourless crystals. Ostiolar necks cylindrical, laterally attached on perithecia, centrally attached only on centrally arranged perithecia, convergent in the disc margin or crowded at the ends of fusoid discs, 1–25(–35) per disc. Visible part of the ostiolar necks (53–)103–167(–212) µm (n = 90) diam., black or brown with black tips, usually circular in section, sometimes plane with the disc, but much more frequently papillate and projecting to 250 µm, often resembling minute perithecia with pointed tips or discoid with depressed centre to nearly ring-like, sometimes conical to bristle-like and projecting to 0.4 mm. Entostroma bark coloured, not or only slightly paler than the surrounding bark, consisting of bark cells and some light-coloured hyphae. Perithecia (450–)515–680(–810) µm (n = 58) diam., arranged in valsoid configuration around and below central column, globose to subglobose, collapsing up- or laterally inwards upon drying. Peridium pseudoparenchymatous, consisting of a dark brown small-celled outer and a hyaline to brownish, large-celled inner layer. Hamathecium of broad multiguttulate paraphyses, collapsing, dissolving and usually absent amongst mature asci. Asci floating free at maturity, (52–)68–85(–98) × (8.7–)10.5–15.5(–18.7) µm (n = 126), narrowly fusoid to oblong or narrowly ellipsoid, with an apical ring distinct in water and staining in Congo Red, but invisible or indistinct in 3% KOH, containing 8 biseriate or obliquely uniseriate ascospores. Ascospores (13.8–)17–20(–22.8) × (3.5–)4.7–6.5(–7.7) µm, l/w (2.5–)2.9–3.8(–5.5) (n = 242), hyaline, mostly oblong or narrowly ellipsoid, sometimes broadly ellipsoid upon release, symmetric or inequilateral, bicellular with nearly equal cells, slightly or strongly constricted at the median septum, multiguttulate or with few large and several small guttules when fresh, with a short and broad, rounded, sometimes tapering, angular or bell-shaped and typically terminally truncate appendage (1.8–)2.7–4.7(–8.4) × (2–)2.5–4(–5.5) µm, l/w (0.4–)0.9–1.5(–2.8) (n = 318), at one or both ends becoming invisible in 3% KOH and Congo Red after release.
Asexual morph acervular, intermingled with pseudostromata of the sexual morph or more frequently developing separately, usually inconspicuous, but sometimes becoming conspicuous due to greyish-brown to dark brown conidial deposits 0.2–0.6 mm diam., rarely confluent from 2 conidiomata and then up to more than 1 cm long. First, white to yellow tissue (central column) forming within the bark, becoming visible by pustulate bark and narrow whitish to yellowish or brownish slit-like discs emerging through bark cracks, usually first followed by the production of β-conidia in olivaceous chambers and later α-conidia on the same or similar conidiophores turning the contents brown and oozing out from ends of the discs or perithecia of the sexual morph formed below the acervulus. Conidiomata 1–2 mm diam., pulvinate, more or less circular in outline, scattered or aggregated in lines. Covering discs 0.3–0.9(–1.6) mm (n = 45) long, narrowly fusoid or longish to rounded, plane to convex, becoming covered and obscured by conidial deposits; discs and pulvinate or conical columns beneath, consisting of compact textura intricata of hyaline hyphae and numerous colourless crystals. Conidiophores emerging around the central column or directly on bark in dense palisades, up to ca. 50 µm long, filiform, branched near the base or sometimes 1–2 fold asymmetrically at higher levels, hyaline, turning brown from their tips; terminal conidiogenous cells (10–)14.5–23(–27) × (1.8–)2.3–3.5(–5) µm (n = 90), cylindrical and often widened in the middle or towards base and at the funnel-shaped tips beyond its width, with up to 3 annellations, producing β-conidia and/or α-conidia. Conidia dimorphic, α-conidia (9–)11–14(–16.3) × (3.2–)4.5–5.5(–6.2) µm, l/w (1.7–)2.2–2.9(–3.6) (n = 172), first hyaline, soon turning pale to medium brown or greyish-brown, unicellular, mostly fusoid, but also oblong, oval or ellipsoid, straight, less commonly slightly curved, upper end usually subacute and sometimes elongated, lower end narrowly truncate, containing several guttules, smooth; β-conidia (8–)9–11.5(–12.7) × (2–)2.5–3(–3.3) µm, l/w (2.8–)3.3–4.6(–5.8) (n = 39), hyaline to dilute brownish, unicellular, oblong to cylindrical, straight or slightly curved, thick-walled in water, with few guttules to eguttulate, smooth.
Culture: Colony on CMD at 16 °C first hyaline, partly or entirely turning brownish or ochre, either covered by a dense white mat of aerial hyphae or not, sometimes becoming indistinctly zonate, sometimes forming irregularly disposed conidiomata; on MEA at room temperature, first hyaline to whitish, soon forming a few broad zones with uneven margins forming teeth, the latter partly turning brown.
Distribution and ecology.
Common on Alnus alnobetula (syn. A. viridis) and A. incana in mountainous areas of Central and Eastern Europe (confirmed for Austria, the Czech Republic fide Podlahová 1973, Romania fide Szász 1966 and Switzerland fide Sieber et al. 1991).
Other material examined.
Austria, Burgenland, Forchtenstein, Kohlstatt, on Alnus incana, 24 Sep 2016, H. Voglmayr & W. Jaklitsch (WU 37046, culture D257); Kärnten, Hüttenberg, Knappenberg, grid square 9053/3, on Alnus alnobetula, 10 Jun 1992, W. Jaklitsch (WU 15093); Niederösterreich, Aspangberg-St. Peter, Mariensee, grid square 8461/4, on Alnus alnobetula, 23 Sep 2009, H. Voglmayr (WU 29888); Steiermark, Hartberg, Pinggau, Schaueregg, Alte Glashütte, on Alnus alnobetula, 28 Jul 2012, W. Jaklitsch & H. Voglmayr (WU 38243); Judenburg, Pusterwald, grid square 8652/3, on Alnus alnobetula, 11 Jun 2011, H. Voglmayr (WU 31890, culture MAV1); Liezen, Kleinsölk, walking path between Breitlahnhütte and Schwarzensee, grid square 8649/3, on Alnus alnobetula, 6 Aug 2003, W. Jaklitsch W.J. 2296 (BPI 843621; culture CBS 121480 = A.R. 4013); St. Nikolai im Sölktal, Sölker Paß, grid square 8750/1, on Alnus alnobetula, 14 Jun 2011, H. Voglmayr (WU 31889, culture CBS 131694 = MAV); Spital am Semmering, near Pfaffensattel, grid square 8460/2, on Alnus alnobetula, 15 Aug 2003, W. Jaklitsch W.J. 2331 (BPI 872072; culture A.R. 4032); ibidem, same host, 8 Jul 2010, I. Krisai-Greilhuber & H. Voglmayr (WU 31172); ibidem, same host, 7 Apr 2015, H. Voglmayr (WU 36699); Tirol, Kühtai, between Haggen and Kühtai, near Zirmbachalm, grid square 8732/3, on Alnus alnobetula, 3 Sep 2003, W Jaklitsch W.J. 2368 (W 2004-0000062); Prägraten, Bodenalm, on Alnus alnobetula, 18 Jun 2015, H. Voglmayr & W. Jaklitsch (WU 37044; culture D157); Umbalfälle, grid square 8939/4, on Alnus alnobetula, 28 Aug 2000, W. Jaklitsch W.J. 1542 (WU 31891, BPI 748444; culture CBS 109773 = A.R. 3500; AFTOL-ID 2127); same area and host, 17 Jun 2015, H. Voglmayr & W. Jaklitsch (WU 37045; culture D158); Vienna, Landstraße, Botanical garden, Alpinum, grid square 7864/1, on Alnus alnobetula, 21 Aug 1994, H. Voglmayr (WU 12976); same place and host, 6 Jan 2012, H. Voglmayr (WU 31893).
Notes.
This subspecies differs mainly in its occurrence in (Central) Europe and by forming a clade of its own in phylogenetic analyses (Fig. 1). While the differences of the European accessions in each marker included are few, they are consistent, resulting in a well-delimited clade in the multigene analyses. As the morphological differences from M. marginalis subsp. marginalis are only small, we prefer to classify the European taxon as a subspecies rather than a separate species.
Under the name Melanconis alni, Podlahová (1973) described both sexual and asexual morphs of a Czech collection from Alnus alnobetula which clearly represents M. marginalis, and Szász (1966) listed and described the species (as Melanconium dimorphum) from Romania, again from Alnus alnobetula. In his isozyme studies of Melanconium, Sieber et al. (1991) included a Swiss isolate from Alnus alnobetula (as Melanconium sp. 1). This isolate showed a distinct but similar isozyme pattern to North American collections of Melanconis marginalis and had a mean conidial size of 11.7 × 4.3 µm, indicating that this isolate also represents Melanconis marginalis subsp. europaea.
Melanconis marginalis subsp. italica
(Senan., Camporesi & K.D. Hyde) Jaklitsch & Voglmayr, comb. et stat. nov.
AAD697A5-5463-5546-829D-7C87147BFD66
834110
≡ Melanconis italica Senan., Camporesi & K.D. Hyde, in Senanayake et al., Stud. Mycol. 86: 273 (2017) (Basionym).
Type material.
Holotype. Italy, Province of Forlì-Cesena, Fiumicello di Premilcuore, on dead branch of Alnus cordata, 4 Dec 2013, E. Camporesi IT 1557 (MFLU 17–0879; ex-type cultures MFLUCC 16–1199, MFLUCC 17–1659; isotype BBH 42441).
Notes.
It is presently unclear, whether this poorly described and illustrated taxon that is only known from a single collection is simply Melanconis marginalis subsp. europaea or merits a subspecies name of its own. First, the host given by the authors, Alnus cordata, naturally occurs in southern Italy and Corsica and, thus, may be correct only if planted in the collection area, which is not given by the authors. Secondly, the ascospores are in the range of other subspecies and appendages are neither mentioned nor illustrated, although a few are visible in their ascus images. Apparently, ascospores were mounted in KOH, where appendages are invisible. Thirdly, they describe the asexual morph from culture and include only a poor image of conidia without giving any measurements. Last but not least, only LSU, ITS and rpb2 are available, which are insufficient to reliably resolve its true phylogenetic position. In addition, instead of comparing their taxon with M. marginalis, they compare it with M. alnicola (Jaap 1917), which is a synonym of Alnecium auctum.
Melanconis marginalis subsp. marginalis
(Peck) Wehm., Pap. Michigan Acad. I. 6: 382 (1926).
90BC257D-7D5B-5002-862C-B8F41D37BC32
Figure 8.
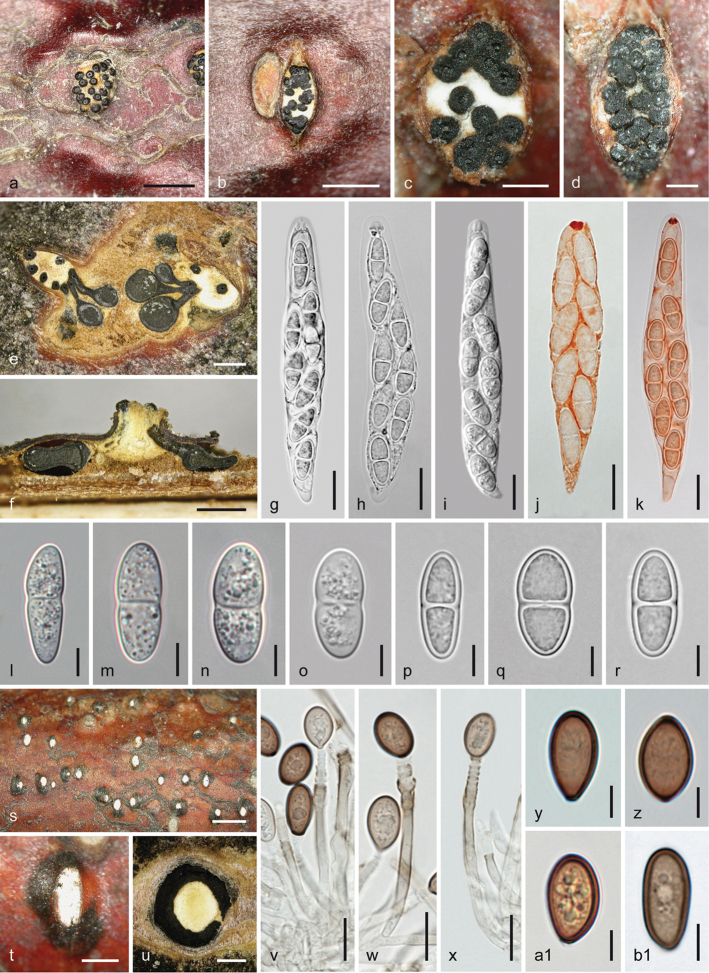
Melanconis marginalis subsp. marginalis. Sexual morph a pseudostroma in face view b–d ectostromatic discs (note conical to bristle-like ostiolar necks in c discoid in d; e vertical section showing central column and perithecia f–i, p–u asci j–o, v–y ascospores t, u, y in aqueous Congo Red x in 3% KOH a, f BPI 614844 b, g, h, t holotype NYSf 1859 c, k, j BPI 748233 d, e, n, o, q–s, v, w, y epitype WU 37850 i, u DAOM 227767 l, m DAOM 202917 p BPI 614977 x DAOM 86074. Scale bars: 500 µm (a–e), 10 µm (f–i, q–u), 7 µm (j–p, v–y).
Figure 9.
Melanconis marginalis subsp. marginalis. Asexual morph a early stage of covering disc b, c conidiomata and conidial deposits in face view d, e conidiomata in cross section (d with β-conidia, e with α-conidia) f conidioma with α-conidia in vertical section g–k conidiophores and conidiogenous cells (producing α-conidia in g, h β-conidia in i–k) l–w α-conidia x–e1 β-conidia g–e1 in 3% KOH a, b, d–g, i–k, n–s, x–b1 epitype WU 37850 = D321 c, t–w, c1–e1 DAOM 227767 h, l, m BPI 614844. Scale bars: 300 µm (a, e, f), 500 µm (b, d), 1 mm (c), 10 µm (g, h), 7 µm (i, t–v), 5 µm (j–l, n, s, w, y–e1), 3 µm (m, o–r, x).
≡ Diaporthe marginalis Peck, Rep. (Annual) Trustees State Mus. Nat. Hist., New York 39: 52 (1887) [1886] (Basionym).
≡ Melanconis alni var. marginalis (Peck) Wehm., Revision of Melanconis, Pseudovalsa, Prosthecium & Titania: 27 (1941).
= Diaporthe nivosa Ellis & Everh., Proc. Acad. nat. Sci. Philad. 42: 222 (1890).
= Melanconium dimorphum Peck, Ann. Rep. New York State Mus. Nat. Hist. 40: 62 (1887).
Type material.
Holotype of Diaporthe marginalis: USA, New York, Essex County, Elisabethtown, on Alnus alnobetula subsp. crispa (given as Alnus viridis), May 1885, C.H. Peck (NYSf 1859!; material separated into 2 envelopes NYSf 1859.1 and NYSf 1859.2). Epitype, here designated: Canada, New Brunswick, Charlotte Co., 1.5 km SW of Little Lepreau, on Alnus alnobetula subsp. crispa attached to the tree, soc. Tortilispora aurantiaca, 3 Sep 2019, D. Malloch (WU 37850; ex-epitype cultures CBS 146200 = D321 (from ascospores), D321a (from α-conidia), D321b (from β-conidia); MBT390382).
Description.
Sexual morph: Pseudostromata immersed in bark causing pustules, scattered or aggregated, sometimes fused in pairs, 1.2–3.2 mm diam., pulvinate, circular to elliptic in outline, often elevated beyond bark surface; consisting of an ectostromatic disc and perithecia embedded in an entostroma around a central column, sometimes also acervuli containing α-conidia on the ostiolar level. Ectostromatic discs 0.3–1.5(–2) mm diam. or long, bright white to yellowish or cream, flat, convex or concave, sometimes fissured or with dark stellate stripes around disc on the bark surface, sometimes concealed by ostioles, circular, elliptic or fusoid in outline, typically distinctly projecting up to 1 mm including projecting part of the pseudostroma; central column beneath disc white to yellowish, consisting of hyaline hyphae and colourless crystals. Ostiolar necks cylindrical, laterally attached on perithecia, centrally attached only on centrally arranged perithecia, convergent in the disc margin or crowded at the ends of fusoid discs, sometimes completely filling disc, 1–15(–22) per disc. Visible part of the ostiolar necks (55–)87–153(–230) µm (n = 128) diam., shiny black or brown with black tip, flat discoid to ring-like, papillate to subglobose with pointed tip or conical, sometimes bristle-like and projecting up to 0.6 mm. Entostroma bark coloured, not or only slightly paler than the surrounding bark, consisting of bark cells and some light-coloured hyphae. Perithecia (420–)480–650(–750) µm (n = 34) diam., arranged in valsoid configuration around and below central column, depressed subglobose, collapsing up- or laterally inwards upon drying. Peridium pseudoparenchymatous, consisting of a dark brown small-celled outer and a hyaline to brownish, large-celled inner layer. Hamathecium of broad multiguttulate paraphyses, collapsing, dissolving and usually absent amongst mature asci. Asci floating free at maturity, (46–)56–69(–82) × (10–)11–14.5(–18) µm (n = 116), mostly oblong to fusoid, but also clavate or narrowly ellipsoid, with an apical ring distinct in water and staining in Congo Red but invisible or indistinct in 3% KOH, containing 8 ascospores in bi- or obliquely uniseriate arrangement. Ascospores (13.8–)15.5–18(–20.7) × (3.7–)4.5–5.7(–7.7) µm, l/w (2.4–)2.9–3.7(–4.4) (n = 236), hyaline to yellowish, oblong to ellipsoid, bicellular with equal or slightly unequal cells, slightly to distinctly constricted at the more or less median septum, multiguttulate or with few large and several small guttules when fresh, with a roundish to triangular or broadly oblong to beak-like and truncate appendage (1.1–)1.8–3.5(–6.1) × (2.2–)2.5–3.5(–4.2) µm, l/w (0.4–)0.6–1.2(–2.1) (n = 140) at each end; in 3% KOH, ascospores wider and more ellipsoid; appendages mostly invisible.
Asexual morph acervular, intermingled with pseudostromata of the sexual morph or more frequently developing separately, usually inconspicuous but sometimes becoming conspicuous due to greyish-brown to dark brown conidial deposits to 2.7 mm diam., sometimes confluent from 2 conidiomata and then up to 7 mm long. First white to yellow tissue (central column) forming within the bark, becoming visible by pustulate bark and narrow whitish to yellowish or brownish slit-like discs emerging through bark cracks, usually first followed by the production of β-conidia in olivaceous chambers, followed by fusion of the chambers and production of α-conidia on the same or similar conidiophores, turning the cavity brown and oozing out from ends of the discs or perithecia of the sexual morph formed beneath. Conidiomata ca. 0.9–3 mm long or diam., pulvinate, more or less circular in outline, scattered or aggregated in lines. Covering discs 0.3–0.7 mm long or diam., narrowly fusoid or longish to circular, plane to convex, white-yellowish-brownish, becoming covered and obscured by conidial deposits; discs and pulvinate or conical columns beneath consisting of compact textura intricata of hyaline hyphae and numerous colourless crystals. Conidiophores emerging around the central column from a textura intricata, fasciculate, filiform, branched near the base or sometimes 1–2 fold asymmetrically at higher levels, hyaline, turning brown from their tips; terminal conidiogenous cells (10–)13.5–23(–31) × (1.7–)2–3(–3.5) µm (n = 68), cylindrical and often widened in the middle or towards the base and at the funnel-shaped tips beyond its width, annellidic, producing α- and/or β-conidia. Conidia dimorphic, α-conidia (9–)10.5–13.3(–16.8) × (3.8–)4.5–5.3(– 6) µm, l/w (1.7–)2–2.8(–3.9) (n = 171), first hyaline, soon turning light to medium brown, unicellular, mostly fusoid, but also oblong, oval, citriform or ellipsoid, straight or slightly curved to sigmoid, upper end often subacute, lower end narrowly truncate, containing several guttules or eguttulate, smooth; β-conidia (6–)8–10.5(–12.2) × (1.7–)2.2–2.8(– 3) µm, l/w (2.4–)3–4.6(–6.4) (n = 46), hyaline to dilute brownish, unicellular, oblong to cylindrical, sometimes reniform, straight or curved, thick-walled in water, with few guttules to eguttulate, smooth.
Culture: Colony on CMD at 22 °C circular with slightly uneven margin, hyaline to whitish, forming a broad inner white zone with tooth-like margin and narrow hyaline outer zones; on MEA at room temperature circular, first hyaline to white, margin becoming diffuse, narrow or coarse concentric zones formed, turning brown from the margins, aerial hyphae short, dense, surface sometimes becoming imbricate, sometimes growth limited and ceasing after a few weeks.
Distribution and ecology.
Widespread in North America and also occurring in Japan and eastern Russia on various subspecies of Alnus alnobetula and A. incana; recorded also from A. rubra (Sieber et al. 1991; see also material cited below).
Additional material examined.
Canada, British Columbia, Kelowna, June Springs road, June Springs trail, on Alnus incana, 18 Jul 1999, J. Ginns 10834 (DAOM 227767; measurements separately given, see below under Notes); Nelson, on Alnus incana subsp. tenuifolia, soc. Cryptosporella sp., 26 Jun 1930, G.G. Hedgcock (BPI 614844, F.P. 50704); Victoria, Lake Cowichan, Mesachie Lake, 48.7942N 124.1573W, on Alnus rubra, 14 Sep 1988, C. Dorworth (DAVFP 24976, dried culture PFC-051 only); Victoria, Ucluelet, Kennedy Lake, 49.0416N 125.5315W, on Alnus rubra, 16 May 1987, C. Dorworth (DAVFP 24972, dried culture PFC-025 only); Manitoba, W Hawk Lake, on Alnus sp., 5 Jun 1932, G.R. Bisby 4593 (DAOM 202917); Nova Scotia, Kings Co., Glenmont, on Alnus alnobetula subsp. crispa (as Alnus crispa var. mollis), 25 Jul 1936, I.L. Conners (Ottawa 3798 (DAOM)); Kentville, on Alnus alnobetula subsp. crispa, 11 May 1953, D. Creelman (DAOM 54346); Ontario, District of Nipissing, Temagami Forest Reserve, Lake Temagami, Bear Island, on Alnus alnobetula subsp. crispa (as Alnus viridis var. mollis), 19 Jun 1933, R.F. Cain 2686 (DAOM 86075); trail at Matagama Point, on Alnus alnobetula subsp. crispa (as Alnus crispa var. mollis), 23 Jun 1933, R.F. Cain 2687 (DAOM 86074); Sharp Rock Inlet, on Alnus alnobetula subsp. crispa (as Alnus crispa var. mollis), 29 Jun 1933, R.F. Cain (BPI 614977, F.P. 69748). Japan, Hokkaido, Shirikinai, on Alnus alnobetula subsp. maximowiczii, 1 Sept 1967, T. Oguchi (TFM FPH3290; culture MAFF 410218 = M4-6, ME9). RUSSIA, Sakhalin Island, Lake Dvoynoe, on Alnus alnobetula subsp. maximowiczii, 3 Aug 2000, A. Bogachova, comm. L. Vasilyeva (BPI 748233; culture CBS 109496 = A.R. 3529, ME2). USA, Alaska, Fairbanks, Large Animal Research Station, on Alnus alnobetula, 5 Aug 2011, L. Mejia (BPI 884096; culture A.R. 4864, ME5); same area, on Alnus alnobetula (given as Betula neoalaskana), 5 Aug 2011, L. Mejia (BPI 884097; culture CBS 133346 = A.R. 4865, ME6); Juneau, on Alnus alnobetula subsp. sinuata, 6 Sep 1936, D.V. Baxter (BPI 615125).
Notes.
The asexual morph of Melanconis marginalis subsp. marginalis is inconspicuous with usually only thin greyish patches of α-conidia. The two types of conidia may be present at the same time or only one is present; acervuli containing α-conidia are sometimes present in pseudostromata of the sexual morph. The specimen DAOM 227767 from Alnus incana differs from all others by very large and conspicuous conidial deposits (Fig. 9c), slightly larger α-conidia, (13–)14.5–16.5(–17.5) × (5–)5.8–7(–8) µm, l/w (1.8–)2.1–2.8(–3.4) (n = 70) and longer and more slender β-conidia, (7.5–)12.5–16(–17.3) × (1.7–)2.2–3(–3.5) µm, l/w (4–)4.6–6.7(–9) (n = 35) and also by slightly larger asci, (68–)74–88(–95.5) × (10–)12–15.5(–18.2) µm (n = 26), which approach the European subspecies. Although Jensen (1984) gave a range of 9–17 × 3–7 µm for α-conidia and 10–18 × 2–3 µm for β-conidia of M. marginalis, it is unclear, whether all examined specimens, including DAOM 227767, phylogenetically belong to M. marginalis subsp. marginalis or a different subspecies or even species. Jensen (1984) reported exceptionally long ascospores (19–32 µm) for four of his collections from Idaho, which also differed in their colony characters; due to lack of DNA data, the taxonomic status of these collections is unclear. While all our North American and Eastern Asian accessions of M. marginalis subsp. marginalis sequences originated from various subspecies of Alnus alnobetula, the accessions investigated by Jensen (1984) originated from Alnus incana subsp. tenuifolia. Sieber et al. (1991), who investigated M. marginalis from British Columbia, recorded mean conidial sizes of 11.2–11.8 × 4.4–4.7 µm for two isolates from A. rubra, while those from three isolates of Alnus alnobetula were slightly larger (13.6–14 × 5.6–5.9 µm). These data demonstrate the need of additional detailed investigations of the M. marginalis complex in western North America. Kobayashi (1970) determined the following sizes for Japanese collections of M. marginalis: asci 70–93 × 10–15 µm, ascospores 15–23 × 4–6.5 µm, mostly 17–20 × 4.5–5.5 µm, α-conidia 11.5–15 × 4–6.5 µm, β-conidia 7.5–12.5 × 1.5–2.5 µm. He also mentioned that the Japanese collections usually lacked ascospore appendages, which, however, may be due to the use of a mounting medium instead of water in his microscope mounts. This is supported by the fact that he also reported a lack of appendages in his M. pterocaryae, which was disproved by re-investigation of the type (Voglmayr et al. 2017).
Sizes of asci depend on the age of the material. They shrink with time and in specimens, which are 20 or more years old, they are smaller and do not obtain the original size even in KOH; also, it is very difficult to release ascospores from asci. In fresher specimens, asci are easily separable and ascospores are readily released. Vital asci open readily in mounts. Nonetheless, fresh asci of the epitype of subsp. marginalis were distinctly smaller than fresh asci of subsp. europaea.
Poor representation of the asexual morph in fungarium specimens may be due to the fact that the sexual morph is usually abundant, with numerous white ectostromatic discs; thus, the asexual morph may have been neglected during collecting or even discarded. β-conidia are often absent or scant and old amongst α-conidia in dark conidial deposits, hence they are either not formed or produced before α-conidia.
Melanconis marginalis subsp. tirolensis
Jaklitsch & Voglmayr subsp. nov.
A68202E1-7A05-5A20-949B-1D27F9B9545F
834111
Figure 10.
Melanconis marginalis subsp. tirolensis. Sexual morph a, b pseudostromata with ectostromatic discs c conical ostioles d vertical section showing central column and two perithecia e ectostromatic disc with subglobose ostiolar tips f cross section showing central column, marginal ostioles and upper parts of perithecia g–j asci (compressed in j) k–p ascospores; i, j, o, p in aqueous Congo Red a, c, k–p holotype BPI 872035 b, d–j isotype WU 31892. Scale bars: 500 µm (a, b, d, f), 150 µm (c), 300 µm (e), 10 µm (g–p).
Figure 11.
Melanconis marginalis subsp. tirolensis (isotype WU 31892). Asexual morph a, b conidiomata showing covering discs in face view c, d conidiomata in cross section (c with β-conidia d with α-conidia) e conidioma with α-conidia in vertical section f–l conidiophores and conidiogenous cells (k, l producing β-conidia) m–r α-conidia s–y β-conidia f–y in 3% KOH. Scale bars: 500 µm (a–e), 15 µm (f), 10 µm (g–l), 5 µm (m–y).
Diagnosis.
This subspecies differs from Melanconis marginalis subsp. europaea and subsp. marginalis phylogenetically and by slightly larger α-conidia, asci, ascospores and ascospore appendages.
Type material.
Holotype: Austria, Tirol, Osttirol, Prägraten am Großvenediger, Umbalfälle, grid square 8939/4, on Alnus alnobetula, 10 Sep 2001, W. Jaklitsch W.J. 1796 (BPI 872035; ex-type culture CBS 122310 = A.R. 3748 = ME4; part preserved as isotype WU 31892, asexual morph only present in the latter).
Etymology.
Named after its occurrence in Tirol, Austria.
Description.
Sexual morph: Pseudostromata 1.3–5.5 mm diam., conspicuous and numerous, scattered to aggregated, pulvinate, circular to elliptical in outline, elevated beyond bark surface forming pustules; consisting of an ectostromatic disc and perithecia embedded in an entostroma around a central column. Ectostromatic discs 0.35–1.55 mm (n = 43) diam. or long, bright white to yellowish, turning brownish with age, mostly fusoid, also elliptic or circular in outline, mostly flat, crumbly, distinctly projecting up to 1.3 mm, including projecting part of the pseudostroma; central column beneath disc white to yellowish, consisting of hyaline hyphae and colourless crystals. Ostiolar necks cylindrical, laterally or centrally attached on perithecia, convergent in the disc margin or crowded at the ends of fusoid discs, 1–15 per disc. Visible part of the ostiolar necks (53–)85–180(–240) µm (n = 56) diam., black, often with olivaceous tips, frequently conical to bristle-like and projecting to 0.4 mm, but also papillate, resembling minute perithecia or discoid with depressed centre. Entostroma bark coloured, not or only slightly paler than the surrounding bark, consisting of bark cells and some light-coloured hyphae. Perithecia (510–)570–780(–900) µm (n = 36) diam., arranged in valsoid configuration around and below central column, globose to subglobose, collapsing up- or laterally inwards upon drying. Peridium pseudoparenchymatous, consisting of a dark brown small-celled outer and a hyaline to brownish, large-celled inner layer. Hamathecium absent at maturity. Asci floating free at maturity, (74–)86–102(–115) × (11.3–)13–20(–25) µm (n = 61), fusoid to oblong or clavate, short-stipitate prior to full maturation, with an apical ring distinct in water and staining in Congo Red, but invisible or indistinct in 3% KOH, containing 8 biseriate or obliquely uniseriate ascospores. Ascospores (15.8–)17.8–21.2(–24) × (4.5–)5.5–7(–8) µm, l/w (2.5–)2.8–3.5(–4) (n = 123), hyaline, turning pale brown with age, oblong to ellipsoid, symmetric to slightly inequilateral with nearly equal cells, slightly or strongly constricted at the median septum, multiguttulate or with 1–2 large and several small guttules when fresh, with a short and broad, rounded, parabolic or vesicular, sometimes tapering but typically terminally broadly truncate appendage (2–)3.8–6.2(–9.5) × (3–)4–5.7(–7.2) µm, l/w (0.4–)0.8–1.4(–2) (n = 104) at each end, after release becoming invisible in 3% KOH, but partly persistent in Congo Red.
Asexual morph acervular, intermingled with pseudostromata of the sexual morph or developing separately, inconspicuous. First white to yellowish tissue (central column) forming within the bark, becoming visible by slightly pustulate bark and narrow whitish to yellowish discs emerging through bark cracks, usually first followed by the production of β-conidia in olivaceous chambers and later α-conidia or both more or less simultaneously on the same or similar conidiophores, chambers fusing into a single locule, turning brown and dark conidial patches 0.5–1.5 mm diam. or perithecia of the sexual morph forming. Conidiomata 1.2–3.2 mm diam., pulvinate, more or less circular in outline, scattered or crowded. Covering discs 0.2–1.5 mm (n = 14) diam. or long, narrowly fusoid or longish to circular, flat to convex, whitish, yellowish to brownish; discs and pulvinate or conical columns beneath consisting of compact textura intricata of hyaline hyphae and numerous colourless crystals, becoming brittle with age. Conidiophores emerging around the central column in dense palisades, up to ca. 65 µm long, filiform, branched near the base and usually 1–3 fold asymmetrically at higher levels, first hyaline, turning brown from their tips; terminal conidiogenous cells (9–)15–25(–28) × (1.7–)2.3–3.2(–3.7) µm (n = 63), cylindrical and often widened towards base, even wider at the funnel-shaped tips, with up to 3 annellations, proliferating and producing α- or β-conidia. Conidia dimorphic, α-conidia (10–)11.5–16.3(–21.8) × (2.5–)4.5–6.3(–7.5) µm, l/w (1.8–)2.1–3.2(–5.3) (n = 70), first hyaline, soon turning light to medium brown, mostly fusoid, also oblong, oval or ellipsoid, straight or slightly curved, upper end usually subacute and sometimes elongated, lower end narrowly truncate, containing several guttules, smooth; β-conidia (7.3–)8.8–12(–16.5) × (2–)2.2–2.7(–3.4) µm, l/w (2.6–)3.3–5.3(–8.9) (n = 104), hyaline, dilute brownish with age, sometimes turning rosy in 3% KOH, oblong to cylindrical, straight or curved or sigmoid, thick-walled in water, smooth, with truncate basal scar and minute guttules to eguttulate.
Culture: Colony on MEA dense, first hyaline to white, with restricted growth, forming brown radial portions mostly submerged in the agar. Odour unpleasant.
Distribution and ecology.
Co-occurring with Melanconis marginalis subsp. europaea in a subalpine area of eastern Tyrol, Austria, Europe, on Alnus alnobetula.
Additional material examined.
Austria, Tirol, Osttirol, Virgental, Prägraten am Großvenediger, Lasörling, Zopatnitzen on path between Wetterkreuz and Berger See, 2100 m a.s.l., on Alnus alnobetula, 26 Oct 2019, H. Voglmayr & C.M. Botoaca (WU 37851; culture D322a (from α-conidia)).
Notes.
As this subspecies differs morphologically only subtly from the other varieties of M. marginalis, we prefer to classify it as a subspecies rather than a separate species. While the ITS sequences of Melanconis marginalis subsp. tirolensis differs from Melanconis marginalis subsp. europaea in only a single base pair, the differences are substantial in all other markers included, particularly tef1 and tub2.
Melanconis pacifica
Jaklitsch & Voglmayr sp. nov.
5533B67C-06C9-547A-84AB-FA8223488A9B
834112
Figure 12.
Melanconis pacifica. Asexual morph a–d conidiomata in face view e conidioma in cross section f conidioma in vertical section g–k conidiophores (g with both conidial types, note annellations in right conidiophore in k) l–r α-conidia s–z β-conidia a–k, n–p, z DAOM 220988 l, m, r–y holotype DAOM 230637 q isotype BPI 748446 g–o, r–z in 3% KOH. Scale bars: 300 µm (a–f), 30 µm (g), 10 µm (h–k), 5 µm (l–z).
Diagnosis.
This species is characterised by its occurrence on Alnus rubra and α-conidia, which are wider and darker than those of M. marginalis and differ by a different shape and absence of a light band from those of M. alni.
Type material.
Holotype. Canada, British Columbia, Sidney, off Jura, on Alnus rubra, 26 May 2000, M.E. Barr 1021A (DAOM 230637; ex-type culture CBS 109744; isotype BPI 748446).
Etymology.
For its occurrence in the Pacific region of western North America.
Description.
Asexual morph: Conidiomata 0.7–2.1 mm diam., visible as dark brown to blackish spots, acervular, subperidermal, scattered, discrete, rarely two confluent, pulvinate to conical, consisting of an erumpent central or eccentric, circular or elliptic to fusoid, flat or convex disc 0.2–1.3 mm diam., whitish, yellowish to reddish-orange when young, becoming concealed by ejected conidia and internally a narrow central or eccentric, whitish to yellowish stromatic column sometimes fraying out laterally and a dark ring-like periphery containing conidia. Conidia becoming discharged through a mostly slit-like rupture of the disc, forming dark brown to black, up to 0.7 mm high masses or tendrils. Conidiophores densely aggregated forming palisades, up to ca. 50 µm long, arising from a yellowish, nearly pseudoparenchymatous tissue of compacted hyphae, either consisting solely of conidiogenous cells or of a stout main axis with few side branches and a terminal whorl of 2–4 more or less vertical conidiogenous cells, hyaline to yellowish. Conidiogenous cells mostly 11–32 × (2–)2.5–3.3(–3.5) μm, annellidic, more or less cylindrical, hyaline, turning brown with age, forming simultaneously two types of conidia on top. Conidia dimorphic, α-conidia (8.8–)10.5–12.5(–15.5) × (5–)6.5–7.7(–8.8) µm, l/w (1.2–)1.4–1.8(–2.7) (n = 615), oval to ellipsoid, dark brown, with a distinct basal abscission scar; β-conidia (6.2–)8.2–12.5(–18.5) × (2–)2.3–3(–3.6) µm, l/w (1.7–)3–4.9(–7.6) (n = 103), oblong to cylindrical, straight or curved, sometimes sigmoid or kidney-shaped to subellipsoid, hyaline, turning dilute brownish with age, typically containing two subterminal groups of minute guttules, with a distinct basal abscission scar.
Culture: Colony on MEA circular, first hyaline, turning white and later brownish in spots or patches, with stellate margin and radial stripes; black conidiomata forming along the stripes. Odour indistinct.
Additional materials examined
(all on/from Alnus rubra). Canada, British Columbia, Sidney, Bazan Bay, 28 May 1995, M.E. Barr (DAOM 220988); Victoria, 26 km N of Campbell River, 50.1262N, 125.3084W, 2 Jan 1989, T.N. Sieber (DAVFP 24981, dried culture PFC-071 only); Caycuse, W shore of Cowichan Lake, 48.8810N, 124.4321W, 24 Oct 1988, T.N. Sieber (DAVFP 24980, dried culture PFC-068 only); Gordon Head, C. Dorworth’s property, 48.4396N, 123.3380W, 4 Jun 1988, C. Dorworth (DAVFP 24973, dried culture PFC-043 only); East Sooke, 48.4377N, 123.7436W, 29 Jun 1948, W.G. Ziller (DAVFP 3092); Nanaimo, DeCourcy Island, 49.0641N, 123.7732W, 1 Jun 1988, C. Dorworth (DAVFP 24974, dried culture PFC-047 only); Parksville, NW Bay, 3.1 km W of M&B office, 49.3238N, 124.1479W, 13 Jul 1988, C. Dorworth (DAVFP 24975, dried culture PFC-050 only); Port Renfrew, Sombrio Beach, 48.5229N, 124.2866W, 4 Nov 1988, C. Dorworth (DAVFP 24977, dried culture PFC-053 only); Revelstoke, Jordan River, gravel pit S of the river, 48.4356N, 124.0140W, 24 Oct 1988, C. Dorworth (DAVFP 24978, dried culture PFC-055 only); ibid., 24 Oct 1988, T.N. Sieber (DAVFP 24979, dried culture PFC-067 only); Sooke, East Sooke Park, Babbington Trail, 48.3485N, 123.6073W, 9 Sep 1988, C. Dorworth (DAVFP 25029, dried culture PFC-054).
Notes.
The description is largely based on DAOM 220988 due to good development of conidiomata. However, we select DAOM 230637 as the holotype, because DNA data are only available for this specimen. Microscopic data of the two specimens are identical. This species is currently only known as an asexual morph. One specimen from Victoria (DAVFP 3092) contains also an immature sexual morph, which corresponds to Melanconis alni superficially. Barr apparently identified her collections as M. marginalis because the latter was, at that time, considered to be the only alnicolous species occurring in North America (Jensen 1984), which also occurs on A. rubra (Sieber et al. 1991). However, the conidia of the latter species are longer, more fusoid, have a larger l/w ratio and are lighter in colour than those of M. pacifica. α-conidia of M. pacifica and M. alni are virtually identical in size. Those of the latter, however, have a different shape, a median light band and a more greyish-brown colour. Remarkably, Wehmeyer (1941) mentioned a collection from the American Pacific region (Oregon) which had conidia resembling Melanconium sphaeroideum, a synonym of M. alni. Sieber et al. (1991) included 10 isolates from Alnus rubra, sampled in British Columbia, that they identified as Melanconium apiocarpum, another synonym of M. alni (see above), based on conidial size and shape. Their measurements and, in particular, their illustration (fig. 2a) of α-conidia fully agree with M. pacifica. The isozyme patterns of Sieber et al. (1991) revealed high similarities, but also diagnostic differences between the isolates from European A. glutinosa and Canadian A. rubra, which is in agreement with the close phylogenetic relationship between M. alni and M. pacifica. Our morphological re-investigations of the isolates of Sieber et al. (1991), which are kept as dried cultures at DAVFP (see specimens cited above), confirmed that they represent M. pacifica.
In DAOM, two additional specimens, labelled Melanconis marginalis collected by Barr in the same area, are extant, DAOM 227727 and DAOM 227345. These specimens, however, do not contain M. pacifica, but the sexual morph of a Diaporthe sp.
Melanconis stilbostoma
(Fr. : Fr.) Tul. & C. Tul., Select. fung. carpol. (Paris) 2: 115 (1863).
22504CDE-F072-5268-B981-F3B1A0C13B67
Figure 13.
Melanconis stilbostoma. a–r Sexual morph a–d pseudostromata with ectostromatic discs in face view e cross section through 2 adjacent pseudostromata f vertical section showing 2 perithecia, ostiolar necks and central column g–k asci l–r ascospores j, k in aqueous Congo Red s–b1 Asexual morph s, t conidiomata in face view u conidioma in cross section v–x conidiophores and conidiogenous cells y–b1 α-conidia v–b1 in 3% KOH a, j, s, v–x iso-epitype WU 31897 = W.J. 1543 b–dWU 31896 e–g, i, k, oWU 38241 h, p, qWU 36779 l–n, a1 WU31899 rWU 37048 tWU 31894 uWU 15266 yM. betulinum B700016529 zM. betulinum B700016528 a1 WU31899 b1WU 35970 = D143. Scale bars: 1 mm (a, b), 300 µm (c, d), 500 µm (e, f, t, u), 15 µm (g–k), 5 µm (l–r, y–b1), 2 mm (s), 10 µm (v–x).
≡ Sphaeria stilbostoma Fr. : Fr., K. svenska Vetensk-Akad. Handl., ser. 3, 39: 102 (1818) (Basionym)
≡ Melanconis stilbostoma (Fr. : Fr.) Tul., Annls Sci. Nat., Bot., sér. 4, 5: 109 (1856). (Nom. inval., Art. 35.1).
?= Melanconium bicolor Nees : Fr., Syst. Pilze (Würzburg): 32 (1816) [1816–17].
= Melanconium betulinum J.C. Schmidt & Kunze, Deutschl. Schwämme, Neunte Lieferung: 3 (1819).
= Melanconium elevatum Corda, Icon. fung. (Prague) 3: 22 (1839).
Type material.
Lectotype. Sweden, without data, Fries, Scleromyc. Suec. no. 145, as Sphaeria stilbostoma (UPS:BOT:F-117590, lectotype here designated; MBT390467)). Epitype, here designated: Austria, Tirol, Prägraten, Umbalfälle, grid square 8939/4, on Betula pendula, 28 Aug 2000, W. Jaklitsch W.J. 1543 (BPI 748447; ex-epitype culture CBS 109778 = A.R. 3501 = ME11; AFTOL-ID 936; MBT390383; iso-epitype WU 31897).
Description.
Sexual morph: Pseudostromata 1.3–3.6(–4.5) mm diam., scattered to aggregated, slightly or distinctly projecting from bark surface, pulvinate with bluntly conical centre (projecting disc), circular to elliptical in outline; consisting of an ectostromatic disc and perithecia embedded in an entostroma around a central column and often chambers filled with conidia. Ectostromatic discs 0.4–2.4(–2.7) mm diam. or length, fusoid to circular, projecting from the bark surface to 0.5 mm, less commonly 1 mm including pseudostroma, white or yellow, brown when old, flat, concave or convex, often completely filled by tips of ostiolar necks; central column beneath disc brightly white to yellow, consisting of hyaline hyphae and colourless crystals. Ostiolar necks cylindrical, laterally or centrally attached on perithecia, convergent and densely and irregularly or evenly disposed in the disc or around the margin; visible part in the discs (106–)139–231(–283) µm (n = 68) diam., 1–25 per disc, shiny black, convex papillate, discoid with depressed centre or conical to cylindrical and projecting to 300 µm. Entostroma paler than surrounding inner bark, consisting of hyaline to white hyphae and bark cells, sometimes forming white patches. Perithecia (450–)540–700(–780) µm (n = 45) diam., arranged in valsoid configuration around and below central column, globose to subglobose, collapsing upon drying. Peridium pseudoparenchymatous, consisting of a dark brown small-celled outer and a hyaline to brownish, large-celled inner layer. Hamathecium absent at maturity. Asci floating free at maturity, (69–)80–123(–141) × (10–)13–18(–21) µm (n = 64), fusoid to oblong or narrowly clavate, with an apical ring distinct in water and staining in Congo Red but invisible or indistinct in 3% KOH, containing 4–8 biseriate or obliquely uniseriate ascospores. Ascospores (13.7–)16–19(–23) × (4.7–)6.5–8.5(–9.7) µm, l/w (1.9–)2.1–2.7(–3.6) (n = 186), first narrow, fusoid or oblong and with small roundish appendages (1.5–)2–5(–7.3) × (2.2–)3.3–5.5(–6.8) µm, l/w (0.3–)0.5–1.1(–1.7) (n = 60) within asci, later becoming broadly ellipsoid with rounded ends, symmetric or inequilateral, slightly constricted at the central to slightly eccentric septum, hyaline, thick-walled, smooth; appendages fugaceous and absent on released ascospores.
Asexual morph acervular, intermingled with pseudostromata of the sexual morph or developing separately, conspicuous. First white tissue (central column) forming within the bark, becoming surrounded by sterile yellow margin and narrow discs rupturing bark epidermis, followed by the production of conidia in olivaceous to black chambers containing black conidial masses translucent though bark. Conidiomata 0.9–3.2 mm diam., subconical or pulvinate, more or less circular in outline, scattered or crowded.
Covering discs 0.3–1.2 mm long, slit-like to circular, flat to convex, shiny white to yellowish, becoming obscured by dark olivaceous brown to black conidial deposits forming patches to 2.7 mm diam., sometimes confluent to 1 cm; discs and pulvinate or conical columns beneath, consisting of dense textura intricata of hyaline hyphae and numerous colourless crystals, becoming brittle with age. Conidiophores emerging around the central column from a pseudoparenchymatous base, filiform, branched near the base and usually 1–3 fold asymmetrically at higher levels, first hyaline, turning brown from their tips; terminal conidiogenous cells (11.5–)18–33(–42.5) × (2–) 2.5–3.5(–4.5) µm (n = 47), more or less cylindrical, with up to 5 or 6 annellations, densely arranged, repetitive, producing α-conidia. Conidia (10.5–)12.5–15(–17.5) × (6.2–)7.2–8.5(–9.5) µm, l/w (1.3–)1.6–2(–2.7) (n = 260), oval, ellipsoid or subglobose, 1-celled, dark brown, thick-walled, smooth, with a few drops and a small scar. No β-conidia detected.
Culture: Colony on CMD at 16 °C forming irregular white and brown to ochre zones partly covered by aerial hyphae or hyaline, undifferentiated, forming brown spots and irregularly disposed conidiomata; on MEA at room temperature first white, later with broad white and brown zones with undulating margin and conidiomata forming mostly on the outer margin. Odour indistinct to fruity.
Distribution and ecology.
Melanconis stilbostoma occurs frequently on Betula spp. on the northern Hemisphere in Asia, Europe and North America (Barr 1978; Fan et al. 2016, 2018; Kobayashi 1970; Sogonov et al. 2008).
Other material examined.
(all on twigs of Betula pendula except where noted): Austria, Kärnten, Gallizien, near Wildensteiner Wasserfall, grid square 9453/3, 11 Jul 2007, W. Jaklitsch (WU 31896); St. Margareten im Rosental, village area, grid square 9452/4, 27 May 1992, W. Jaklitsch (WU 15266); Trieblach, below Cihuc, grid square 9452/2, 14 Apr 2001, W. Jaklitsch W.J. 1740 (WU 31895, BPI 872036; culture A.R. 3637); Wograda, grid square 9452/3, 27 May 1997, W. Jaklitsch W.J. 1080 (WU 31894); same area and host, 31 May 2000, W. Jaklitsch W.J. 1474 (BPI 871332); Zabrde, grid square 9452/4, 7 Aug 1993, W. Jaklitsch (WU 15191); Niederösterreich, Aspangberg-St. Peter, Außerneuwald, Höllergraben, grid square 8462/1, 24 May 2015, G. Koller (WU 36779); Edlitz, Königsberg, grid square 8562/2, 14 Jul 2007, W. Jaklitsch W.J. 3125 (specimen lost; culture MS = CBS 121894); Friedersbach, S and SO from the village, grid square 7457/2, 19 Aug 2001, W. Jaklitsch W.J. 1775 (BPI 872038; culture A.R. 3725); Neunkirchen, Gloggnitz, Saloder, village area, grid square 8361/2, 10 May 2015, G. Koller (WU 36752); Grimmenstein, between Eben and the Kulmriegel, grid square 8362/4, 14 May 2015, G. Koller (WU 36812); Thaures, grid square 7156/1, 21 Sep 1997, W. Jaklitsch W.J. 1109 (WU 37048); Weidlingbach, grid square 7763/1, 27 Jun 1999, W. Jaklitsch W.J. 1329 (WU 37049); Oberösterreich, Schärding, Raab, Rothmayrberg, Rothmayr, 10 Mar 2012, H. Voglmayr (WU 38241); St. Willibald, Großer Salletwald, at the road B 129 to Peuerbach, grid square 7648/1, 31 Dec 2011, H. Voglmayr (WU 31899); Vienna, Alsergrund, at the hospital AKH, grid square 7764/3, 23 Jul 1993, W. Jaklitsch (WU 15537); Favoriten, Rothneusiedl, grid square 7864/3, 4 Sep 1993, W. Jaklitsch (WU 15758); ibidem, 22 Jan 1994, W. Jaklitsch (WU 15559). Czech Republic, Bohemia, Malonty, Hodonický potok, grid square 7253/3, 25 Sep 2003, W. Jaklitsch W.J. 2427 (WU 31898). Germany, no collection data (type material B 700016528 and B 700016529 of Melanconium betulinum from B). Italy, Sicily, Etna, SW Linguaglossa, near I Due Monti, on Betula aetnensis, 18 Jun 2016, H. Voglmayr & W. Jaklitsch (WU 37047; culture D258). Japan, Nagano, Karuizawa, Mt. Asama, on Betula platyphylla Sukachev var. japonica (Miq.) Hara, 21 Sep 1965, T. Kobayashi (TFM FPH2710; culture MAFF 410225 = M3-9 = ME12). Poland, Narewka, NE Nowa Lewkowo, 27 Jul 2015, H. Voglmayr (WU 35970; culture D143).
Notes.
Melanconis stilbostoma and its basionym Sphaeria stilbostoma (α papula) were mentioned by Tulasne (1856), but the combination was invalid due to the lack of a generic diagnosis; it was, however, validated in Tulasne and Tulasne (1863). According to Ibai Olariaga, who examined the type in UPS, there are 3 scalps of Betula bark containing many clustered perithecia with black ostiolar necks erumpent through a white disc; neither asci nor spores were found, but brown α-conidia are present abundantly. As the type collection was distributed in Fries’ Scleromyceti Sueciae no. 145, we here lectotypify the species with the copy preserved in UPS, which we epitypify with a recent well-developed collection for which a culture and sequence data are available.
Several asexual morph names have been linked with Melanconis stilbostoma: Melanconium bicolor predates Melanconis stilbostoma, but there is no material extant in B, thus it cannot be checked; also Quercus but not Betula was given as host in the protologue. In addition, Melanconis stilbostoma is a well-known and well-defined name for the generic type of Melanconis. The second name is Melanconium betulinum, which is clearly a later synonym upon our examination of type material. Melanconium elevatum is another synonym. We have, however, not seen type material of this taxon, but the description and illustrations in Corda (1839) are conclusive. Melanconis stilbostoma is a very common fungus on birch throughout the northern hemisphere and likely the most conspicuous species of Melanconis due to the shiny white discs of both morphs, contrasting the dark conidial deposits. In older specimens, the latter may have olivaceous tones, but much less conspicuously than with M. larissae. The latter species differs also in a broad light zone present on its conidia. Melanconis stilbostoma was already cultured by Wehmeyer (1926b) on birch twigs from material, whose ascospore measurements were (13–)15–18 × 5–8 µm, corresponding to those of Barr (1978: 12–18.5 × 6.5–8(–9) µm). Wehmeyer (1941) gave (13–)15–19(–23) × (5–)6–7.5(–9) µm for ascospores, which is in accordance with our measurements ((13.7–)16–19(–23) × (4.7–)6.5–8.5(–9.7) µm); Kobayashi (1970) measured 13–25 × 4–7.5 µm, mostly 15–20 × 5–7 µm and Fan et al. (2016) gave (19–)21.5–23.5(–25) × (6–)7–8 μm, which is slightly larger. Wehmeyer (1941) noted for α-conidia from culture and exsiccata mostly 10–16 × 5.5–7.5 µm and 6.5–12 × 2–2.5 for β-conidia in culture; Barr (1978) found only α-conidia and measured 9–16.5 × 5–7.5 µm, which is in accordance with our observations from Europe (see above). Asian authors gave 9–16.5 × 5–7.5 µm (Kobayashi 1970) and (8.5–)9–14.5(–16) × (4.5–)5–6(–6.5) μm (Fan et al. 2016) for α-conidia, but, in some collections, they also found cylindrical to allantoid, unicellular, hyaline β-conidia, 9–11.5 × 1.5–2.5 µm (Kobayashi 1970) or (9–)10–11(–12.5) × (2–)2.5–3 μm (Fan et al. 2016).
Validation of neotypification
Here we also validate the neotypification of Melanconium pterocaryae, the basionym of Juglanconis pterocaryae by Voglmayr et al. (2019), where the new requirement to explicitly state the MBT number in the typification proposal was missing:
Juglanconis pterocaryae
(Kuschke) Voglmayr & Jaklitsch, in Voglmayr, Castlebury & Jaklitsch, Persoonia 38: 150 (2017).
4E86330C-4989-579E-8C94-2AC3A6E4114E
≡ Melanconium pterocaryae Kuschke, Trudy Tiflissk. Bot. Sada 28: 25 (1913) (Basionym).
Typification.
Austria, Oberösterreich, Bad Hall, Kurpark, on corticated twigs of Pterocarya fraxinifolia, 20 Oct 2017, W. Jaklitsch (WU 39981, neotype of Melanconium pterocaryae here proposed; ex neotype culture D272 = CBS 144326; MBT 389379).
Discussion
Circumscription of the genus Melanconis, morphology and delimitation from morphologically similar genera
As already mentioned in the Introduction, the genus Melanconis historically has been considered a large, heterogeneous genus. Many species were removed to other genera in the past on morphological grounds or due to different associated asexual morphs: Chapeckia (Barr 1978), Caudospora (Starbäck 1889), Hapalocystis (Fuckel 1863), Macrodiaporthe (Petrak 1920), Massariovalsa (Saccardo 1882; Barr 1978), Phaeodiaporthe (Petrak 1920), Pseudovalsa (Ces and De Not 1863) and Pseudovalsella (Höhnel 1918). Only recently, species were relegated to other genera and families based on molecular phylogenetic analyses: Alnecium (Voglmayr and Jaklitsch 2014), Caudospora (Voglmayr and Mehrabi 2018), Coryneum/Pseudovalsa (De Silva et al. 2009), Hapalocystis (Jaklitsch and Voglmayr 2004), Juglanconis (Voglmayr et al. 2017, 2019), Lamproconium (Norphanphoun et al. 2016), Melanconiella (Voglmayr et al. 2012), Phaeodiaporthe (Voglmayr and Jaklitsch 2014), Stilbospora/Prosthecium (Voglmayr and Jaklitsch 2008, 2014).
All melanconis-like species form their fructifications in bark and lack black zones, which delimit the pseudostromata from surrounding bark tissue in genera like Diaporthe. The sexual morph in Melanconis sensu stricto is characterised by distinctly projecting white to yellowish ectostromatic discs, which continue as stromatic central columns downwards, by entostroma, which is optically scarcely different from internal bark tissue, by long cylindrical ostiolar necks, which converge in the disc, by hyaline bicellular ascospores with or without appendages, by absence of paraphyses at maturity and asci, which have an apical ring and are released from the subhymenium at maturity. Conidiomata of the asexual morph are acervular. They commonly produce two types of conidia, melanconium-like brown α-conidia and narrow hyaline to brownish β-conidia. Species of Dendrostoma in the Erythrogloeaceae (Jaklitsch and Voglmayr 2019; Jiang et al. 2019) also produce two types of conidia on the same conidiophores, but both are hyaline. Acervuli of Melanconis, however, particularly in M. marginalis, form chambers, in which first β-conidia are produced. Such chambers are still present when α-conidia are produced, but in the latest stages of maturation, the entire fertile region around the central column is filled with α-conidia and appears as a single locule. In species of the morphologically most similar genera Melanconiella (Voglmayr et al. 2012) and Juglanconis (Voglmayr et al. 2017, 2019), pseudostromata are less conspicuous and project to a lesser degree from the bark surface than in Melanconis. The central column in Melanconiella is usually grey, dull yellow to greenish, only rarely white and often poorly developed and ascospores may be hyaline or brown. The most striking difference between Melanconis and Melanconiella lies in the asexual morph. In Melanconis, each species produces α- and β-conidia in the same conidiomata, whereas each species of Melanconiella only produces a single type of conidia, either brown melanconium-like (corresponding to α-conidia) or hyaline discosporina-like conidia (corresponding to β-conidia). Species of Juglanconis only produce melanconium-like conidia, which have a gelatinous sheath (also present in a few Melanconiella spp.) and differ from the other genera by the presence of verrucae on the inner surface of the conidial wall.
Molecular phylogeny, species numbers, concept and delimitation
In Melanconiella, 15 species have been recognised (Voglmayr et al. 2012; Fan et al. 2018) and five in Juglanconis (Voglmayr et al. 2017, 2019). Fan et al. (2016, 2018) included five species of Melanconis sensu stricto in their phylogenetic trees. Here we add three species, of which two are new. While all betulicolous species, except for the basal M. betulae, formed a highly supported clade, those on Alnus were scattered in between, so no general evolutionary pattern in host association could be revealed. Remarkably, within species, a commonly high genetic divergence and variability was observed (e.g. within M. groenlandica, M. itoana, M. marginalis and M. stilbostoma; see Fig. 1), contrary to Melanconiella and Juglanconis, where the species clades were genetically rather homogeneous (Voglmayr et al. 2012, 2017, 2019; Fan et al. 2018). This may, in part, be attributed to the wider geographic distribution and host range of these Melanconis species, but it may also indicate that they are within the process of evolutionary radiation and speciation. Although the species concept in Melanconis is primarily based on phylogenetic analyses, we consider morphological and ecological evidence as important criteria for taxonomic conclusions. The taxa on Betula spp. may be more or less easily distinguished by differences in the morphology of α-conidia and by ecology: α-conidia of M. larissae have a large light-coloured zone, those of M. itoana have a l/w ratio of > 3 and those of M. betulae and M. groenlandica, as given by the respective authors, are shorter than those of the other species, albeit similar. However, the latter two species occur on different host species: M. betulae on Betula albosinensis, M. groenlandica on Betula maximowicziana, B. nana and B. papyrifera.
Taxa on Alnus spp. may pose difficulties in differentiation. Ascospores of M. alni and M. marginalis differ in shape, size and particularly in appendages from each other. Nonetheless, all features are overlapping and, for example, ascospore appendages of M. alni are not always long and pointed, particularly in old fungarium specimens, but show some similarities with those of M. marginalis. In such cases, it is important to have the asexual morph in order to study its conidia, which are strikingly different from those of M. marginalis. The same applies to Melanconis accessions from the western North American Alnus rubra, where the co-occurring M. pacifica and M. marginalis can be reliably distinguished by their conidia (see, for example, also fig. 2 in Sieber et al. 1991).
The situation is particularly complex within M. marginalis, which splits up into four subclades in our phylogenetic analyses. Morphology amongst those subclades is very similar, measurements are heavily overlapping and only subtle differences or tendencies are recognisable. In addition to the lack of distinctive morphological characters, there is also a substantial amount of genetic variation within the two of the four subclades, for which several accessions are available, particularly within M. marginalis sensu stricto, which will certainly increase if more accessions from additional geographic areas and Alnus species and subspecies are added. Only a small part of the distribution area of M. marginalis is yet sampled. We, therefore, do not think that these subclades should be interpreted as different species, but as a single variable species. Acknowledging the geographical and genetic differentiaton, we decided to classify them as subspecies that may be within the process of speciation. Vicariant speciation may be the reason for splitting of the M. marginalis clade into two main clades, but the residual two clades that are only based on a single and two specimens, were gathered within a small restricted region in Austria and northern Italy. The internal structure of the whole clade may therefore change, in particular, if isolates from additional specimens collected in western and central Russia were added to the phylogenetic analyses and if sequences of all phylogenetic markers of Melanconis marginalis subsp. italica were included.
Misidentification of M. alni and M. marginalis is also prominent in GenBank sequences that were used in all published phylogenetic analyses including these species, resulting in an interchanged application of the names. Based on, as we now know, incorrect assumptions purported in the literature (e.g. Wehmeyer 1941) that M. marginalis is a North American and M. alni a European species, Central European accessions of M. marginalis were misidentified as M. alni. Vice versa, M.E. Barr misidentified her Canadian isolate from Alnus rubra, that is closely related to M. alni and here described as M. pacifica, as M. marginalis. Therefore, all sequences currently deposited in GenBank as M. alni actually represent M. marginalis, while those of M. marginalis belong to M. pacifica.
Hosts
While Juglanconis is confined to the Juglandaceae, subtribus Juglandinae (Voglmayr et al. 2017, 2019), both Melanconiella and Melanconis occur on the Betulaceae. So far, species of Melanconiella primarily occur on the subfamily Coryloideae with the exception of M. betulae and M. decorahensis, which inhabit Betula (Voglmayr et al. 2012; Fan et al. 2018). In contrast, Melanconis is confined to Alnus and Betula, the sole genera of the subfamily Betuloideae. While all known Melanconis species are highly host specific on the generic level (i.e. no Melanconis species occurs on Alnus as well as Betula hosts), host specificity is less expressed and variable concerning their host species range. In addition, the same host species is commonly used by more than one Melanconis species. For instance, the widely distributed M. stilbostoma has been recorded from various species of Betula, which is likewise true for M. groenlandica (for confirmed hosts, see Table 1). Conversely, M. betulae is so far only known from a single host, B. albosinensis, which, however, is also host for M. itoana (Fan et al. 2016, 2018). For Melanconis species on Alnus, M. alni and M. marginalis show some host specificity but are not strictly host specific; while A. glutinosa and A. alnobetula are apparently only colonised by M. alni and M. marginalis, respectively, both species occur on A. incana. Melanconis pacifica, here described as a new species, seems to be host specific on A. rubra, which, however, also harbours M. marginalis. Therefore, the host species are of limited use for species identification and additional investigations are required to elucidate the host range of the various Melanconis species.
Supplementary Material
Acknowledgments
We thank David Malloch for fresh material of Melanconis marginalis subsp. marginalis, Amy Rossman for providing Melanconis cultures, Ibai Olariaga for studying and Åsa Kruys for providing information on the type of Sphaeria stilbostoma in UPS, Paul Kirk for nomenclatural information, the curators of B, BPI, DAOM, DAVFP, NYS, PC and TFM for loan of herbarium material and Irmgard Greilhuber and Walter Till (WU) for incorporating the specimens in the herbarium. The financial support by the Austrian Science Fund (FWF; project P27645-B16) to HV is gratefully acknowledged.
Citation
Jaklitsch WM, Voglmayr H (2020) The genus Melanconis (Diaporthales). MycoKeys 63: 69–117. https://doi.org/10.3897/mycokeys.63.49054
References
- Barr ME. (1978) The Diaporthales of North America. Mycologia Memoirs 7: 1–232. [Google Scholar]
- Bohn M. (1993) Myrothecium groenlandicum sp. nov., a presumed endophytic fungus of Betula nana. Mycotaxon 46: 335–341. [Google Scholar]
- Carbone I, Kohn LM. (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. 10.1080/00275514.1999.12061051 [DOI] [Google Scholar]
- Castlebury LA, Rossman AY, Jaklitsch WM, Vasilyeva LN. (2002) A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94: 1017–1031. 10.1080/15572536.2003.11833157 [DOI] [PubMed] [Google Scholar]
- Cesati V de, Notaris G de. (1863) Schema di classificazione degli sferiacei Italici aschigeri piu o meno appartenenti al genere Sphaeria nell’antico significato attribuitogli de Persoon. Commentario della Società Crittogamologica Italiana 1(4): 177–240. [Google Scholar]
- Corda ACJ. (1839) Icones Fungorum hucusque Cognitorum 3: i–vi, 1–55, plates 1–9. J.G. Calve, Prague.
- De Hoog GS, Gerrits van den Ende AHG. (1998) Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 41: 183–189. 10.1111/j.1439-0507.1998.tb00321.x [DOI] [PubMed] [Google Scholar]
- De Silva H, Castlebury LA, Green S, Stone JK. (2009) Characterisation and phylogenetic relationships of Anisogramma virgultorum and A. anomala in the Diaporthales (Ascomycota). Mycological Research 113: 73–81. 10.1016/j.mycres.2008.08.008 [DOI] [PubMed] [Google Scholar]
- Fan XL, Du Z, Liang Y, Tian CM. (2016) Melanconis (Melanconidaceae) associated with Betula spp. in China. Mycological Progress 15: 40. 10.1007/s11557-016-1163-2 [DOI]
- Fan XL, Du Z, Bezerra JDP, Tian CM. (2018) Taxonomic circumscription of melanconis-like fungi causing canker disease in China. MycoKeys 42: 89–124. 10.3897/mycokeys.42.29634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuckel L. (1863) Fungi rhenani exsiccati, fasc. 6: no. 585 (in sched.)
- Hall TA. (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Höhnel F von. (1918) Mycologische Fragmente. Annales Mycologici 16: 35–174. [Google Scholar]
- Jaap O. (1917) Weitere Beiträge zur Pilzflora der Schweiz. Annales Mycologici 15: 97–124. [Google Scholar]
- Jaklitsch WM. (2009) European species of Hypocrea – Part I. The green-spored species. Studies in Mycology 63: 1–91. 10.3114/sim.2009.63.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Baral HO, Lücking R, Lumbsch HT, Frey W. (2016) Syllabus of plant families – A. Engler’s Syllabus der Pflanzenfamilien Part 1/2: Ascomycota 13th edn. Borntraeger, Berlin.
- Jaklitsch WM, Komon M, Kubicek CP, Druzhinina IS. (2005) Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia 97: 1365–1378. 10.1080/15572536.2006.11832743 [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Stadler M, Voglmayr H. (2012) Blue pigment in Hypocrea caerulescens sp. nov. and two additional new species in sect. Trichoderma. Mycologia 104: 925–941. 10.3852/11-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Voglmayr H. (2004) Hapalocystis occidentalis – a new species of Diaporthales from North America and a key to the species of Hapalocystis. Studies in Mycology 50: 229–234. [Google Scholar]
- Jaklitsch WM, Voglmayr H. (2019) European species of Dendrostoma (Diaporthales). MycoKeys 59: 1–26. 10.3897/mycokeys.59.37966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JD. (1984) Melanconis marginalis from Northern Idaho. Mycotaxon 20: 275–281. [Google Scholar]
- Jiang N, Fan X-L, Crous PW, Tian C-M. (2019) Species of Dendrostoma (Erythrogloeaceae, Diaporthales) associated with chestnut and oak canker diseases in China. MycoKeys 48: 67–96. 10.3897/mycokeys.48.31715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T. (1968) Notes on Japanese species of the genus Melanconium. Transactions of the Mycological Society of Japan 9: 1–11. [Google Scholar]
- Kobayashi T. (1970) Taxonomic studies of Japanese Diaporthaceae with special reference to their life-histories. Bulletin of the Government Forest Experimental Station Meguro 226: 1–242. [Google Scholar]
- Link HF. (1825) Melanconium. In: Willdenow CF (Ed.) Caroli a Linné Species plantarum: exhibentes plantas rite cognitas, ad genera relatas, cum differentiis specificis, nominibus trivialibus, synonymis selectis, locis natalibus, secundum systema sexuale digestas, editio 4, 6(2): 89–93.
- Kowalski T, Kehr RD. (1992) Endophytic fungal colonization of branch bases in several forest tree species. Sydowia 44: 137–168. [Google Scholar]
- Liu YL, Whelen S, Hall BD. (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Müller K. (2004) PRAP – computation of Bremer support for large data sets. Molecular Phylogenetics and Evolution 31: 780–782. 10.1016/j.ympev.2003.12.006 [DOI] [PubMed] [Google Scholar]
- Norphanphoun C, Hongsanan S, Doilom M, Bhat DJ, Wen TC, Senanayake IC, Bulgakov TS, Hyde KD. (2016) Lamproconiaceae fam. nov. to accommodate Lamproconium desmazieri. Phytotaxa 270: 89–102. 10.11646/phytotaxa.270.2.2 [DOI] [Google Scholar]
- O’Donnell K, Cigelnik E. (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116. 10.1006/mpev.1996.0376 [DOI] [PubMed] [Google Scholar]
- Petrak F. (1920) [1919] Mykologische Notizen. I. Annales Mycologici 17: 59–100. [Google Scholar]
- Petrak F. (1941) Mykologische Notizen. Annales Mycologici 39: 251–349. [Google Scholar]
- Petrini O, Fisher PJ. (1988) A comparative study of fungal endophytes in xylem and whole stem of Pinus sylvestris and Fagus sylvatica. Transactions of the British Mycological Society 91: 233–238. 10.1016/S0007-1536(88)80210-9 [DOI] [Google Scholar]
- Podlahová R. (1973) Über einige Pyrenomyceten auf Alnus viridis (Chaix) Lam. et DC. aus Südböhmen. Ceska Mykologie 27: 84–97. [Google Scholar]
- Rossman AY, Adams GC, Cannon PF, Castlebury LA, Crous PW, Gryzenhout M, Jaklitsch WM, Mejia LC, Stoykov D, Udayanga D, Voglmayr H, Walker DM. (2015) Recommendations of generic names in Diaporthales competing for protection or use. IMA Fungus 6: 145–154. 10.5598/imafungus.2015.06.01.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccardo PA. (1882) Fungi boreali-Americani. Michelia 2(8): 564–582. [Google Scholar]
- Senanayake IC, Crous PW, Groenewald JZ, Maharachchikumbura SSN, Jeewon R, Phillips AJL, Bhat JD, Perera RH, Li QR, Li WJ, Tangthirasunun N, Norphanphoun C, Karunarathna SC, Camporesi E, Manawasighe IS, Al-Sadi AM, Hyde KD. (2017) Families of Diaporthales based on morphological and phylogenetic evidence. Studies in Mycology 86: 217–296. 10.1016/j.simyco.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senanayake IC, Jeewon R, Chomnunti P, Wanasinghe DN, Norphanphoun C, Karunarathna A, Pem D, Perera RH, Camporesi E, McKenzie EHC, Hyde KD, Karunarathna SC. (2018) Taxonomic circumscription of Diaporthales based on multigene phylogeny and morphology. Fungal Diversity 93: 241–443. 10.1007/s13225-018-0410-z [DOI] [Google Scholar]
- Sieber TN, Sieber-Canavesi F, Petrini O, Ekramoddoullah AKM, Dorworth CE. (1991) Characterization of Canadian and European Melanconium from some Alnus species by morphological, cultural and biochemical studies. Canadian Journal of Botany 69: 2170–2176. 10.1139/b91-272 [DOI] [Google Scholar]
- Silvestro D, Michalak I. (2012) raxmlGUI: a graphical front-end for RAxML. Organisms Diversity & Evolution 12: 335–337. 10.1007/s13127-011-0056-0 [DOI] [Google Scholar]
- Sogonov MV, Castlebury LA, Rossman AY, et al. (2008) Leaf-inhabiting genera of the Gnomoniaceae, Diaporthales. Studies in Mycology 62: 1–79. 10.3114/sim.2008.62.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis E. (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- Starbäck K. (1889) Ascomyceter från Öland och Östergötland. Bihang till Kungliga Svenska Vetenskaps-Akademiens Handlingar 15: 1–28. [Google Scholar]
- Sutton BC. (1964) Melanconium Link ex Fries. Persoonia 3: 193–198. [Google Scholar]
- Swofford DL. (2002) PAUP* 4.0b10: phylogenetic analysis using parsimony (*and other methods). Sinauer Associates, Sunderland, Massachusetts.
- Szász E. (1966) Ciuperci parazite şi saprofite pe Alnus viridis (Chaix) Lam. et DC. Contribuţii Botanice Cluj 2: 27–34. [Google Scholar]
- Thiers B. (2019) Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/
- Tulasne LR. (1856) Note sur l`appareil reproducteur multiple des Hypoxylées (DC.) ou Pyrénomycètes (Fr.). Annales des Sciences Naturelles, Botanique, sér. 4, 5: 107–118.
- Tulasne LR, Tulasne C. (1863) Selecta Fungorum Carpologia: Xylariei-Valsei-Sphaeriei. 2. Imperial Typograph, Paris.
- Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber WH, Li DZ, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF. (2018) International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Koeltz Botanical Books, Glashütten. 10.12705/Code.2018 [DOI]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. 10.1128/JB.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Akulov OY, Jaklitsch WM. (2016) Reassessment of Allantonectria, phylogenetic position of Thyronectroidea, and Thyronectria caraganae sp. nov. Mycological Progress 15: 921. 10.1007/s11557-016-1218-4 [DOI] [PMC free article] [PubMed]
- Voglmayr H, Castlebury LA, Jaklitsch W. (2017) Juglanconis gen. nov. on Juglandaceae, and the new family Juglanconidaceae (Diaporthales). Persoonia 38: 136–155. 10.3767/003158517X694768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. (2008) Prosthecium species with Stegonsporium anamorphs on Acer. Mycological Research 112: 885–905. 10.1016/j.mycres.2008.01.020 [DOI] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. (2011) Molecular data reveal high host specificity in the phylogenetically isolated genus Massaria (Ascomycota, Massariaceae). Fungal Diversity 46: 133–170. 10.1007/s13225-010-0078-5 [DOI] [Google Scholar]
- Voglmayr H, Jaklitsch WM. (2014) Stilbosporaceae resurrected: generic reclassification and speciation. Persoonia 33: 61–82. 10.3767/003158514X684212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. (2017) Corynespora, Exosporium and Helminthosporium revisited – new species and generic reclassification. Studies in Mycology 87: 43–76. 10.1016/j.simyco.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM, Mohammadi H, Kazemzadeh Chakusary M. (2019) The genus Juglanconis (Diaporthales) on Pterocarya. Mycological Progress 18: 425–437. 10.1007/s11557-018-01464-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Mehrabi M. (2018) Molecular phylogeny and a new Iranian species of Caudospora (Sydowiellaceae, Diaporthales). Sydowia 70: 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Rossman AY, Castlebury LA, Jaklitsch WM. (2012) Multigene phylogeny and taxonomy of the genus Melanconiella (Diaporthales). Fungal Diversity 57: 1–44. 10.1007/s13225-012-0175-8 [DOI] [Google Scholar]
- Walker DM, Castlebury LA, Rossman AY, White Jr JF. (2012) New molecular markers for fungal phylogenetics: two genes for species-level systematics in the Sordariomycetes (Ascomycota). Molecular Phylogenetics and Evolution 64: 500–512. 10.1016/j.ympev.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Wehmeyer LE. (1926a) Cultural life-histories of Diaporthe. I. Papers from the Michigan Academy of Science, Arts and Letters 6: 377–396. [Google Scholar]
- Wehmeyer LE. (1926b) Cultural life histories of Melanconis and Pseudovalsa. Mycologia 18: 257–273. 10.1080/00275514.1926.12020516 [DOI] [Google Scholar]
- Wehmeyer LE. (1941) A Revision of Melanconis, Pseudovalsa, Prosthecium, and Titania. University of Michigan Studies, Scientific Series 14: 1–161. [repr. Bibliotheca Mycologica 41 (1973), Cramer, Lehre] [Google Scholar]
- Werle E, Schneider C, Renner M, Völker M, Fiehn W. (1994) Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Research 22: 4354–4355. 10.1093/nar/22.20.4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. (Eds) PCR protocols: A guide to methods and applications: 315–322. Academic Press, San Diego. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.