Abstract
Reactive oxygen species (ROS) constitute a group of highly reactive molecules that have evolved as regulators of important signaling pathways. It is now well accepted that moderate levels of ROS are required for several cellular functions, including gene expression. The production of ROS is elevated in tumor cells as a consequence of increased metabolic rate, gene mutation and relative hypoxia, and excess ROS are quenched by increased antioxidant enzymatic and nonenzymatic pathways in the same cells. Moderate increases of ROS contribute to several pathologic conditions, among which are tumor promotion and progression, as they are involved in different signaling pathways and induce DNA mutation. However, ROS are also able to trigger programmed cell death (PCD). Our review will emphasize the molecular mechanisms useful for the development of therapeutic strategies that are based on modulating ROS levels to treat cancer. Specifically, we will report on the growing data that highlight the role of ROS generated by different metabolic pathways as Trojan horses to eliminate cancer cells.
Subject terms: Health sciences, Cancer therapy, Therapeutics
Cancer: A Trojan horse to kill cancer cells
Highly reactive molecules called reactive oxygen species (ROS), which at low levels are natural regulators of important signaling pathways in cells, might be recruited to act as “Trojan horses” to kill cancer cells. Researchers in Italy led by Bruno Perillo of the Institute of Food Sciences in Avelllino review the growing evidence suggesting that stimulating production of natural ROS species could become useful in treating cancer. Although ROS production is elevated in cancer cells it can also promote a natural process called programmed cell death. This normally regulates cell turnover, but could be selectively activated to target diseased cells. The authors discuss molecular mechanisms underlying the potential anti-cancer activity of various ROS-producing strategies, including drugs and light-stimulated therapies. They expect modifying the production of ROS to have potential for developing new treatments.
Introduction
Reactive oxygen species (ROS) produced in eukaryotic cells through aerobic metabolism have evolved as regulators of important signaling pathways. ROS, previously considered mere byproducts of cellular respiration, are oxygen-containing molecules with high reactivity. They include hydroxyl (HO*) and superoxide (O2*) free radicals and nonradical molecules, such as hydrogen peroxide (H2O2), which is less reactive than the majority of ROS but is able to reach any cellular compartment prior to being converted by peroxiredoxins and glutathione peroxidases into water and oxygen. In fact, H2O2 plays the role of a second messenger in some pathways that involve the transduction of extracellular signals and the control of gene expression, contributing to what is currently defined as redox signaling1.
ROS are produced in mitochondria (mainly via the electron transport chain, where ~1–2% of O2 is reduced to form superoxide anions), peroxisomes (through the β-oxidation of fatty acids) and the endoplasmic reticulum (through the oxidation of proteins). Oxidative phosphorylation in mitochondria involves four electron-transporting complexes and a proton-translocating ATP synthase that direct electrons derived from the initial oxidation of NADPH and FADH2 along a multistep pathway that culminates in protons being pumped outside of mitochondria. ROS are also continuously generated by enzymatic reactions involving cyclooxygenases, NADPH oxidases (NOXs), xanthine oxidases and lipoxygenases and through the iron-catalyzed Fenton reaction; indeed, it should be noted that NOXs have primarily evolved to produce ROS2. Finally, ROS are generated after exposure to physical agents (ultraviolet rays and heat) and after chemotherapy and radiotherapy in cancer.
Tight regulation of ROS levels is crucial for cellular life; in fact, moderate ROS contribute to the control of cell proliferation and differentiation. Therefore, eukaryotic cells benefit from a complex scavenging system based on superoxide dismutases (SODs), located in the cytoplasm, mitochondria and the extracellular matrix; glutathione peroxidase (GPX); glutathione reductase (GR); peroxiredoxin; thioredoxin; and catalase, which convert superoxide anions into water and recycle the antioxidants in the reduced state.
Here, we focus on the molecular mechanisms that support the elaboration of anticancer therapies that modulate the production and scavenging of ROS and, in particular, on the opportunities raised by their ability to induce cell death upon exceeding a threshold level.
Biological outcomes of oxidation by ROS
It has been determined that each cell is exposed to ~1.5 × 105 oxidative hits per day. If, for any reason, ROS production increases or the number of scavenged ROS decreases, then cells experience a condition known as oxidative stress. Oxidative stress has been implicated in the pathophysiology of cancer: in fact, high levels of ROS generated by ongoing aerobic glycolysis followed by pyruvate oxidation in mitochondria (the Warburg effect), increase receptor and oncogene activity, and the stimulation of growth factor-dependent pathways or oxidizing enzymes induce genetic instability3,4. Moreover, excessive intracellular levels of ROS may damage lipids, proteins and DNA, and this ability has been exploited in a series of anticancer strategies, as detailed below.
ROS and lipids
By interacting with lipids, ROS can induce oxidative stress through a feedback loop initiated by the peroxidation of fatty acids, which alters the lipid bilayer of cell membranes and generates free radicals. This process is potentially dangerous to cells, as peroxidation of mitochondrial phospholipids may affect the integrity of permeability transition pores (PTPs) and disaggregate complexes I and III of the respiratory chain, thereby enhancing electron leakage within the mitochondrial intermembrane space5,6. However, free radicals produced by lipid peroxidation are short-lived7.
ROS and cytoplasmic signaling
By interacting with proteins, ROS have an impact on several signaling pathways involved in the control of cell proliferation and apoptosis. The underlying mechanism generally consists of the oxidation of redox-reacting cysteine and/or tyrosine residues located within or near the active site, which creates intraprotein and interprotein bridges that affect protein function8,9. These modifications are reversible and generate a wide array of cellular responses10.
In general, phosphatases are inhibited by ROS11, whereas kinases may be inhibited or activated12. In particular, ROS activate nonreceptor protein kinases belonging to the Src family; small G proteins, such as Ras; and the tyrosine kinase receptors of growth factors13,14, as well as components of the c-Jun N-terminal kinase (JNK) and p38 kinase (p38MAPK) pathways that induce apoptosis15. Specifically, through the formation of disulfide bonds between catalytic cysteines, H2O2 inactivates phosphatase and tensin homolog phosphatase (PTEN) and unlocks the phosphoinositide 3-kinase (PI3-K)-dependent recruitment of its downstream kinases, such as protein kinase B (Akt)16, or oxidizes the redox protein thioredoxin and thus suppresses its inhibitory effect on the p38MAPK signaling cascade17. Intuitively, small increases in ROS would be expected to activate the PI3-K/Akt pathway preferentially, while further increases would be expected to trigger p38MAPK-dependent apoptosis.
ROS also influence the activity of calcium channels; in fact, they induce the release of calcium from cellular stores with the consequent activation of kinases, such as protein kinase C (PKC), thereby playing important roles in the proliferation of cancer cells18.
ROS and nuclear signaling
Most ROS-sensitive pathways transduce cytoplasmic signals to the nucleus, where they influence the activity of transcription factors that control the expression of a wide array of genes. In this regard, to prevent excessive intracellular ROS, cancer cells respond to oxidative stress by inducing the transcription of antioxidant enzymes, highlighting the relevance of an in-depth knowledge of these pathways for use in elaborating therapies that alter ROS levels.
The pivotal redox-sensitive transcription factor is nuclear factor erythroid 2-related factor 2 (Nrf2)19, recognized as the leading transcription factor driving the antioxidant response in cancer cells. Under normal conditions, Nrf2 is degraded through its interaction with Kelch-like ECH-associated protein 1 (Keap1), whereas under oxidative stress conditions, Keap1 is oxidized and Nrf2 is translocated to the nucleus, where it induces the expression of several genes19. Nrf2 controls the production of glutathione (GSH), the leading antioxidant molecule within cells, through the expression of the enzyme that catalyzes the rate-limiting reaction of GSH synthesis, glutamate-cysteine ligase (GCL), and GSH utilization and regeneration20,21. It also controls free Fe(II) homeostasis, upregulating the expression of heme oxygenase HMOX1, which generates free Fe(II) via the breakdown of heme molecules. Since Fe(II) catalyzes the Fenton reaction to produce the free radical OH* from hydrogen peroxide, its upregulation presents a paradox: Nrf2 also boosts the expression of the genes encoding several components of the ferritin complex that detoxifies Fe(II) by converting it to Fe(III) and then stores it22. Notably, high serum concentrations of ferritin have been described in several cancers with a poor prognosis23.
The forkhead box O (FOXO) family of transcription factors is activated by JNK after ROS levels are increased and induces the expression of SODs and catalase24. The activation of SODs by a FOXO transcription factor (FOXO4) appears to contradict their antioxidant effect; however, the hydrogen peroxide generated by SODs from O* is the substrate of catalase25.
Another important transcription factor that plays a major role in the control of antioxidant gene expression is p53. In fact, the role of p53 in the control of ROS levels is controversial, as it may promote both oxidant and antioxidant gene expression. Indeed, moderately elevated ROS levels inhibit p53, while higher levels promote its expression. Among the targets of p53 activity are sestrins (sestrin 1 and 2) that induce the activity of peroxiredoxins, increasing the impact of the cellular antioxidant array26. In this way, p53 has a complementary function to that of FOXO transcription factors that induce the expression of sestrin 327. Interestingly, both p53 and FOXO control a distinct set of genes that are not targets of Nrf2 activity, even though all three factors induce HMOX1 expression and, therefore, Fe(II) storage and secretion, that plays a role in breast tumorigenesis, highlighting the role of antioxidants in cancer promotion28.
It is known that a widespread characteristic of tumors is their inability to develop adequate blood vessels with the consequence being relative hypoxia: at moderate levels, ROS induce transcription of HIF1α, the founding member of the family of hypoxia-induced factors, and stabilize the encoded protein, which is normally hydroxylated within less than 5 min, by inhibiting the activity of the iron-dependent prolyl 4-hydroxylase (PHD) involved in its degradation29. As a consequence of HIF1α activation, several genes important for cancer progression, such as VEGF and VEGF receptors, are induced30.
Finally, the DNA-binding ability of some transcription factors is directly influenced by ROS. For example, ROS, via oxidation of thioredoxin, enhance the nuclear localization of both the ataxia-telangiectasia mutated (ATM) serine/threonine kinase, which is involved in DNA damage repair31,32, and redox factor-1 (Ref-1), a multifunctional protein that enables Fos/Jun DNA-binding and is identical to the apurinic/apyrimidinic 1 (APE1) endonuclease33. The latter factor is able to interact with thioredoxin to reduce a specific cysteine (Cys-62) in the Rel-homology domain (RHD) of the NF-kB subunit p50 that had been previously oxidized by ROS34, restoring its ability to interact with specific responsive DNA sequences35,36. These data show that ROS can either activate or suppress the NF-kB signaling involved in the control of several important cellular processes, such as embryogenesis and cell proliferation and death, and the responses to a variety of stress stimuli37.
ROS and chromatin
ROS influence the activity of epigenetic modulators, such as histone deacetylases (HDACs) or DNA methyltransferases (DNMTs) with consequences that are evident in the expression of the target genes38,39. They also oxidize DNA, especially adenine and guanine (8-oxo-A and 8-oxo-G). It has been reported that ~1 in 105 guanines is oxidized in normal cells and that this proportion is increased by 35–50% in transformed cells7. Unrepaired 8-oxo-G is potentially one of the most mutagenic lesions, since it pairs with A, inducing G → T transversions40, and represents a prominent candidate to be a marker of ROS-induced mutagenesis and tumorigenesis41. Oxidized Gs also impact the methylation of DNA, as indicated by reports showing that damaged bases on the DNA nascent strand can suppress the methylation of a cytosine within a distance of one or two base pairs42. However, ROS are able to induce DNA hypermethylation as well, with potential consequences on tumor phenotype when promoter regions of tumor suppressor genes are involved43–45. In addition, 8-oxo-Gs accumulate at telomeres, where they inhibit telomerase and decrease the binding of telomeric proteins, leading to the disruption of telomere length and precluding the maintenance of chromosomal-end capping46.
Finally, ROS also induce mutations in mitochondrial DNA with the potential generation of a feedback loop in which mutations in genes encoding complexes of the ETC may directly affect the efficiency of electron transport. The major sensitivity of mitochondrial DNA to ROS-induced mutagenesis is intuitive, as this DNA is not protected by histones, and mitochondria lack the nucleotide excision repair (NER) enzymatic system.
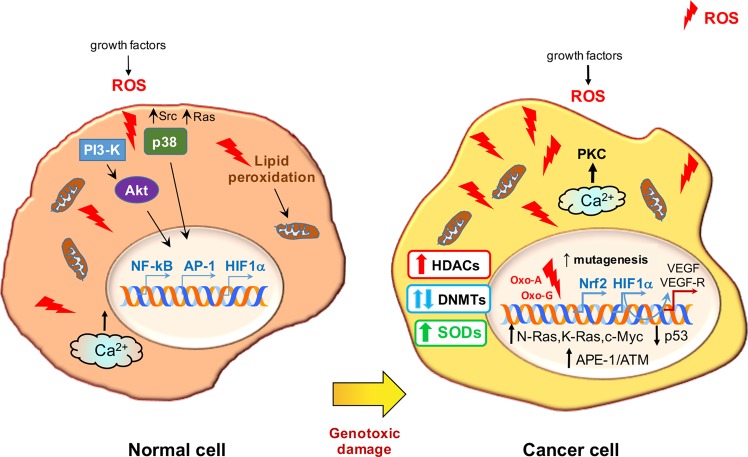
The main consequences of redox signaling and oxidative stress in normal and cancer cells are presented in Fig. 1.
Fig. 1. Redox signaling and oxidative stress in normal and cancer cells.
The major signaling cascades induced by growth factor-stimulated ROS are highlighted on the left. The same pathways influence the cell cycle and affect the activity of transcription factors and genes that play roles in the cellular response to the hypoxic microenvironment. ROS also induce lipid peroxidation with commensurate electron leakage in mitochondria and the release of Ca2+ from intracellular stores. The main consequences of oxidative stress in cancer cells are illustrated on the right. Moderately elevated ROS induce oncogenes and inhibit tumor suppressor genes that, in turn, increase ROS levels. Ca2+ release induces PKC, while the expression of genes involved in the formation of new blood vessels and in the establishment of a boosted antioxidant system is enhanced. ROS also activate HDACs and have a dual effect on DNMTs with important outcomes for the expression of oncogenes and tumor suppressor genes. Oxidized bases trigger mutations and engage DNA repair enzymes.
Oxidative stress promotes cancer and reveals its Achilles heel
Cancer is the second cause of death worldwide and is characterized by several hallmarks47; cell transformation, genome instability, hyperproliferation, immortalization, angiogenesis, epithelial-mesenchymal transition (EMT) and metastasis, which are all influenced in several ways by intracellular ROS48,49.
ROS as double-edged swords in cancer
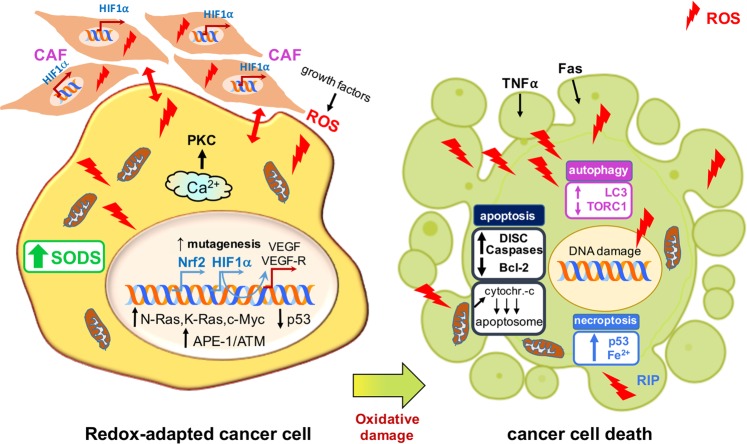
Several noncancer cells associate with tumors: among these, cancer-associated fibroblasts (CAFs), particularly represented in the tumor microenvironment (TME), actively contribute to the regulation of tumor homeostasis, promoting tumor progression and the invasion of cancer cells. CAFs and ROS engage in two-way cross-talk: on the one hand, fibroblasts are targeted by ROS, particularly H2O2, which is able to convert them into active CAFs through the upregulation of HIF1α; on the other hand, CAFs are critical for the increase in ROS levels observed in cancer50,51. CAFs can also promote cancer growth and invasiveness, and both CAFS and ROS are linked through the increases in ROS-generated CAFs to which most cancers respond by increasing the expression of antioxidant genes52–54 (Fig. 2).
Fig. 2. The three types of programmed cell death induced by elevated ROS levels in cancer cells.
ROS, in response to death-inducing ligands (TNFα and Fas), enhance the assembly of DISCs and the activation of effector caspases and reduce Bcl-2 activity or, as a consequence of increased permeability of mitochondrial PTPs, stimulate the intracytoplasmic release of cytochrome c, which interacts with Apaf-1 and procaspases and forms the apoptosome (apoptosis). ROS can also inhibit the negative regulators of autophagy (TORC1) and increase the formation of LC3-dependent autophagosomes (autophagy). Finally, high levels of ROS, induced by several receptor-interacting protein kinases (RIPs), increase p53 expression, which increases ROS levels via a mechanism that depends on intracellular iron (ferroptosis).
However, a growing body of evidence supports the view that antioxidant activities are essential for tumorigenesis. It has been recently reported that targets of the Nrf2 gene, such as HMOX1, facilitate cancer development because they counteract the effect of oxidative stress in transformed cells55. Moreover, established oncogenes such as K-RAS and c-MYC, which had been previously demonstrated to induce intracellular ROS56,57, have been recently shown to stabilize Nrf258. In this regard, mutations to NRF2 and its regulator KEAP1 have been found in cancer cells, supporting the supposition that antioxidant genes are pivotal in tumor progression59–62. In fact, it has been found that the breast cancer susceptibility 1 (BRCA1) gene interacts with and induces Nrf2 expression with positive outcomes on cancer cell survival63. Interestingly, estrogen stimulation of breast cancer cells that do not express BRCA1 and, as a result, suffer from high intracellular ROS levels rescues NRF2 transcription, enhancing the survival of these cancer cells64.
Additionally, FOXO transcription factors have recently been implicated in tumorigenesis: in fact, rhabdomyosarcomas present FOXO genes with a high percentage of mutations that render them insensitive to inhibition by AKT signaling65. Moreover, increased intracellular levels of GSH are required for the initiation and progression of various types of cancer, and inhibitors of GR behave as anticancer drugs66, while high levels of NADPH boost the metastatic ability of melanoma cells, and protocols based on depletion of GSH (isothiocyanates and aziridine derivatives that bind GSH) or based on blocking the uptake of a rate-limiting precursor of its synthesis (inhibitors of the cysteine/glutamate antiporter, XCT) greatly impact cancer cell survival67,68. Specifically, sulfasalazine, an XCT inhibitor, appears useful in the treatment of pancreatic and small-cell lung cancer cells69,70, while NOV-002, a glutathione disulfide mimetic that alters the GSSG/GSH ratio and induces oxidative stress, has been favorably used in patients with HER2-negative breast cancer71. In addition, inhibitors of the enzyme glutaminase (GLS) that converts glutamine to glutamate, which is subsequently transformed to GSH via the glutamate–cysteine ligase complex, efficiently induce cancer cell death through dysregulation of their antioxidant system72. As mentioned above, another central player in these redox systems is thioredoxin, which is reduced by NADPH to induce the transfer of electrons for use in DNA synthesis, signal transduction and redox regulation. Interestingly, auranofin, which functions as a thioredoxin inhibitor, has been used with beneficial effects in the treatment of head and neck carcinoma cell lines; prevention of this effect by the ROS scavenger N-acetylcysteine (NAC) confirms the role of ROS in these cancers73.
ROS and apoptosis (type I programmed cell death)
The most common method by which ROS kill transformed cells is the activation of PCD, which is completed within less than 60 min by a family of cysteine-dependent aspartate-directed proteases known as caspases. Triggered by an extrinsic or an intrinsic pathway, caspase-induced PCD culminates with the formation of apoptotic bodies that are eliminated by adjacent phagocytes74. The extrinsic pathway is mediated by binding of death-inducing ligands such as TNFα and Fas ligand that bind to cognate receptors that, in turn, recruit adaptor proteins and pro-caspases, leading to the assembly of the death-inducing signaling complex (DISC) and the activation of effector caspases75. This interaction is competed by the cellular FLICE-inhibitory protein (c-FLIP): ROS have been shown to downregulate the c-FLIP half-life by inducing its ubiquitin-proteasomal degradation, thus enhancing this extrinsic pathway76. However, compelling evidence suggests that, for the majority of ROS-related anticancer drugs, apoptosis depends on the activation of the intrinsic pathway that involves mitochondrial PTPs, the permeability of which is increased with the cytoplasmic release of pro-apoptotic factors such as cytochrome c that forms a complex with apoptotic protease activating factor 1 (Apaf-1) and pro-caspase 9 to build the apoptosome, activating, in turn, effector caspases77–80 (Fig. 2).
In fact, ROS induce the three major components critical for the opening of the PTPs, the voltage-dependent anion-selective channel (VDAC), adenine nucleotide translocase (ANT) and cyclophilin D, via the oxidation of specific cysteines in their active sites81,82. ROS also trigger apoptosis by inactivating or increasing the ubiquitination of the pivotal anti-apoptotic protein Bcl-2 and by decreasing the intracellular levels of Bax and Bad83,84 (Fig. 2).
The induction of apoptosis by elevated ROS levels has been highlighted as the central mechanism responsible for the positive effects of monoclonal antibodies85 and tyrosine kinase inhibitors86, which represent the core of targeted cancer therapy87. Among tyrosine kinase inhibitors, imatinib (a PDGFR inhibitor) and erlotinib (an EGFR inhibitor) induce ROS-dependent apoptosis in melanoma and non-small-cell lung cancer cells, respectively, through disruption of mitochondrial membrane potential upon the stimulation of JNK and p38 phosphorylation88,89, while vemurafenib (a BRAF inhibitor) increases the production of superoxide anions with the commensurate depolarization of the mitochondrial membranes in melanoma cells90. Among the monoclonal antibodies, rituximab (specific to the calcium-channel protein CD20 on the surface of B cells and mature plasma cells) increases ROS and induces apoptosis via the inhibition of Bcl-2 and p38MAPK signaling and is used in the treatment of B cell lymphomas91.
As noted above, chemotherapy and radiotherapy cause an increase in intracellular ROS that can lead to apoptosis92,93 via extrinsic or intrinsic pathways94,95. Many drugs used in anticancer therapy induce oxidative stress. Apoptosis is stimulated by procarbazine, which induces oxidative DNA damage that cannot be repaired by the BER/NER system in Hodgkin’s lymphoma and brain cancers96. Doxorubicin-dependent cytotoxicity is linked to the stimulation of a Fenton reaction that generates hydroxyl radicals successfully used for the treatment of Kaposi’s sarcoma, breast and bladder cancer and acute lymphocytic leukemia97. A course of treatment with arabinocytosine, which hampers DNA replication, followed by anthracyclines to increase ROS, has been shown to drive PCD, with beneficial effects for patients with acute myeloid leukemia (AML)98.
Arsenic trioxide has recently carved out a role in cancer therapy because it can induce electron leakage along the respiratory chain99. It triggers apoptosis in different cancer cells, including those of myeloma, lung cancer, and leukemia100,101. Moreover, 5-fluorouracil, a pyrimidine analog, produces ROS through p53-dependent pathways and induces apoptosis in colon and rectal cancer cells102,103.
ROS-induced apoptosis also explains the beneficial effect of two analogs of nuclear receptor ligands in several types of cancer: 2-methoxyestradiol, a 17β-estradiol metabolite, and N-(4-hydroxyphenyl) retinamide, a synthetic analog of retinoic acid, have been shown to induce PCD in neuroblastoma and lung cancer cells, respectively104,105. Furthermore, platinum-based drugs elevate ROS levels that promote PCD; protocols for the administration of these compounds in combination with inhibitors of poly(ADP-ribose) polymerase (PARP), which is involved in the maintenance of DNA integrity, have been shown to arrest the growth of breast cancer cells, even in BRCA-deficient models106,107. Intuitively, the inhibition of DNA damage repair by PARP may sensitize cancer cells to the oxidative stress induced by platinum-containing drugs.
Programmed cell death may also be mediated by the effect of elevated ROS on sphingomyelinase, which generates ceramide from sphingomyelin and binds to death receptors on the cell membrane of cancer cells. Activation of this pathway has been observed after UV irradiation of lymphoma cells108. Moreover, the use of drugs affecting mitochondria, where more than one-half of all ROS are generated, represents a suitable approach to induce oxidative stress and PCD in cancer cells109: gamitrinib, an inhibitor of heat shock protein 90 (HSP90), induces a dramatic collapse of mitochondria in prostate cancer cells110, while ARQ 501 (a quinone derivative) and STA-4783 (a copper chelator) increase ROS through leakage in the electron transport chain and have beneficial effects in patients with solid tumors and pancreatic adenocarcinoma111.
Apoptosis is triggered in cells with excessive endoplasmic reticulum (ER) stress that is induced when the protein folding ability of the ER is overwhelmed or impaired. Recently, several drugs have been designed on the basis of their ability to aggravate ER stress in cancer cells via the induction of oxidative stress. Among these, bortezomib is a proteasome inhibitor that induces ROS and ER stress in head and neck squamous cell carcinoma cells112, and celecoxib, a nonsteroidal anti-inflammatory drug, aggravates ER stress and induces apoptosis by altering the Bax/Bcl-2 ratio and increasing ROS in prostate cancer cells113.
ROS and autophagy (type II programmed cell death)
Recently, an important therapeutic approach to kill cancer cells has been presented by ROS-induced autophagy114. Specifically, it has been reported that H2O2-dependent inactivation of autophagy-related gene-4 (ATG4) increases LC3-associated autophagosomes and that ATM-mediated oxidation of AMP-activated protein kinase (AMPK) inhibits mammalian target of rapamycin 1 (TORC1), a pivotal negative regulator of autophagy115–117 (Fig. 2). Indeed, autophagy, also known as type II programmed cell death, is now considered not only as a cell survival mechanism but also a tumor suppressor mechanism that induces the death of transformed cells118. In this regard, it has been reported that H2O2 induces autophagic cell death in glioma cells after treatment with the polycyclic ammonium ion sanguinarine, which increases electron leakage from mitochondria and induces NOXs119. Rapamycin, administered in combination with inhibitors of HSP90, causes mitochondrial damage with accompanying oxidative stress and autophagy and reduces tumor growth in RAS-dependent tumors120.
ROS and necroptosis (type III programmed cell death)
ROS are also able to induce necrosis, which was originally considered an unregulated form of cell death but is now recognized as type III programmed cell death (necroptosis)121,122. ROS generated after the formation of ceramide or after an increase in energy metabolism induced by several receptor-interacting protein kinases (RIPs), either in the mitochondrial ETC and/or by NOXs, have been reported to enhance necroptosis123–125.
In addition, a very intriguing ROS-related molecular mechanism of tumor suppression by p53 has recently been highlighted; this protein induces a peculiar form of cell death, now called ferroptosis, via an increase in ROS levels that subsequently inhibit the cystine uptake typically mediated by the repression of a key component of the cystine/glutamate antiporter126. Ferroptosis depends on the presence of intracellular iron and is induced by ROS127 (Fig. 2). Therefore, the role of p53 in this context appears to be different from that reported in several studies showing that it decreases the levels of ROS. A plausible explanation of this apparent dichotomy is that p53 promotes cell survival by preventing excessive increases in ROS under moderate oxidative stress, whereas when the oxygen species increase over a threshold level, it switches to becoming a ROS inducer, triggering cell death. On the basis that ferroptosis is considered an oxidation-induced cell death mechanism, several trials with different drugs that elicit this pathway have been conducted128,129. Erastin is a synthetic drug that induces cell death through ferroptosis in tumor cells bearing mutant RAS by increasing intracellular ROS levels and altering the permeability of the outer mitochondrial membrane130,131.
ROS and multidrug resistance
Increased ROS levels are thought to impair the multidrug resistance of cancer cells, which causes cancer development and metastasis during or after chemotherapy132,133. It has been recently shown that efflux pumps in the plasma membrane of cancer cells are crucial for the extracellular efflux of anticancer drugs134. These pumps belong to the adenosine triphosphate (ATP)-binding cassette (ABC) transporter superfamily and are dependent on intracellular ATP stores135. ATP is accumulated by a synthase driven by a proton gradient generated in mitochondria by the NADH-dependent electron transport chain136,137; therefore, one possible way to overcome efficient efflux in cancer drugs is to inhibit ATP synthesis by promoting NADH conversion to NAD through lipid membrane-coated silica carbon nanoparticles that, under near-infrared laser irradiation, target mitochondria and produce ROS with simultaneous consumption of NADH138.
Nuclear ROS: a Trojan horse that induces DNA damage
A new role of ROS related to transcriptional output has been recently highlighted. It is well known that cells follow a strictly scheduled program for differentiation that is based on an orchestrated sequence of gene expression. Because of spatial constraints, genes must engage in a complex unfolding process to become accessible to the transcriptional machinery, which is triggered through posttranslational modifications at the N-terminal tails of core histones. Together, these modifications, induced by coordinated targeting of transcription factors that is currently referred to as epigenetic marks, conform to a precise code with specific time requirements to control whole gene expression139. We have previously shown that estrogen-induced transcription is triggered by LSD1-catalyzed demethylation of lysine 9 in histone H3 (H3K9), which is activated by the binding of liganded estrogen receptor to the enhancers of target genes140. This event is followed by the generation of ROS from the oxidation of FADH2 as induced by the demethylase, with consequent oxidation of nearby guanines (8-oxo-Gs) and recruitment of DNA repair enzymes (among which is APE1) that cause single-strand breaks in DNA and enable looping between the enhencer/promoter and the polyadenylation sites of the target genes, with productive transcription140,141. Intuitively, generation of ROS in this process must be timely and spatially controlled to prevent excessive damage to the DNA: a recent report, in fact, describes a new role for the originally discovered superoxide dismutase, SOD1, that is recruited to the nucleus in response to specific stimuli142. However, it has also been observed that hormone-induced phosphorylation of serine 10 in H3 histone (H3S10) prevents the rapid remethylation of the preceding lysine, serving as the metronome of the process and giving the DNA damage repair system enough time to eliminate the oxidized nucleotides from nearby DNA143. It has been reported that by inhibiting phosphorylation of serine 10 in this pathway, breast cancer cells simultaneously challenged with estradiol show an overproduction of ROS, with increased oxidation of the DNA that overwhelms the repair apparatus and triggers PCD in a great percentage of these cells144 (Fig. 3).
Fig. 3. Role of nuclear ROS in transcription and DNA damage.
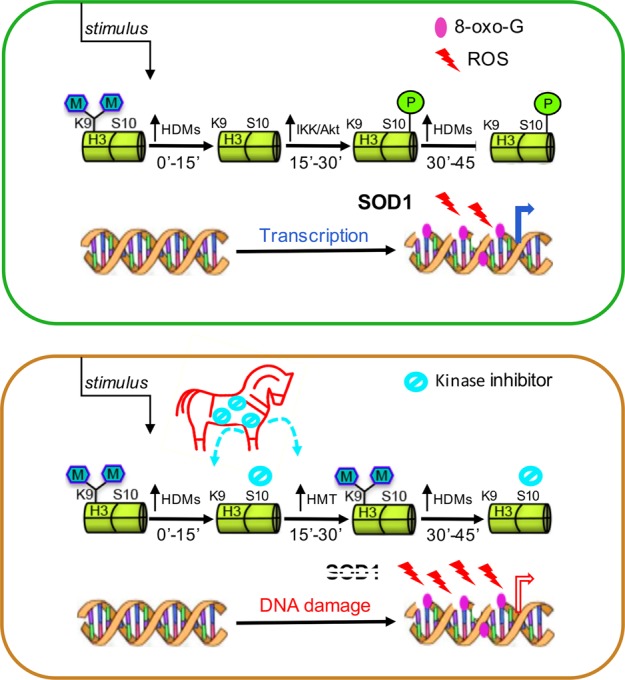
ROS generated during nuclear receptor-induced transcription of target genes by the activity of lysine demethylases on lysine 9 in histone H3 must be controlled to prevent their accumulation. To this end, SOD1 reaches the nuclear space, while phosphorylation of H3S10 inhibits the rapid remethylation of the same lysine. If inhibitors of the H3S10 kinases are introduced as a Trojan horse together with nuclear receptor ligands, remethylation of H3K9 is quick, nuclear ROS accumulate, and unrepaired DNA damage triggers PCD.
Concluding remarks
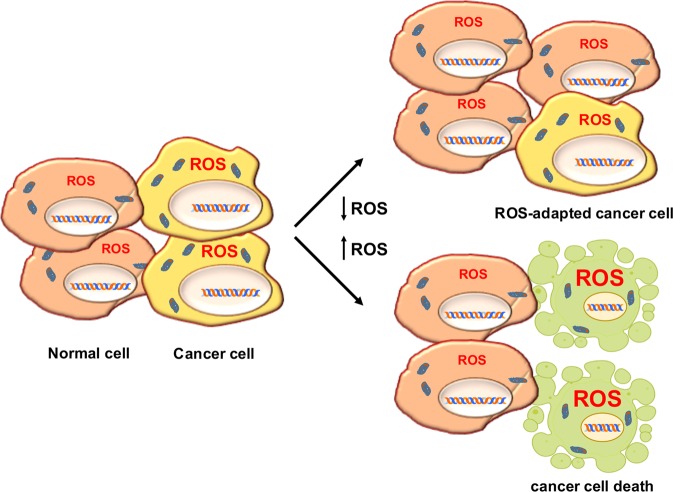
The complex interconnection between ROS levels and cancer is essentially based on accurate fine-tuning between ROS production and scavenging. Cancer initiation and progression leverage slight increases in ROS levels. Therefore, cancer cells thrive on levels of ROS that are moderately higher than those in their normal counterparts, as they have developed increased antioxidant systems. This feature renders cancer cells more sensitive to external stimuli that further increase the production of ROS145–147, and, as schematically summarized in Table 1, an increasing number of therapeutic strategies are being developed to elevate ROS levels to overwhelm the redox adaptation of the same cells, inducing oxidative stress incompatible with cellular life148–151 (Fig. 4).
Table 1.
List of anticancer drugs according to their effect on intracellular ROS, and types of cancer where are used.
| Name | Mechanism to increase ROS | Cancers treated | Ref. |
|---|---|---|---|
| Sulfasalazine | XCT inhibitor | Pancreatic and small-cell lung cancer | 69,70 |
| NOV-002 | GSSG mimetic | HER2-negative breast cancer | 71 |
| Imatinib (PDGFR inhibitor) | Loss of mitochondrial membrane potential | Melanoma | 88 |
| Erlotinib (EGFR inhibitor) | Loss of mitochondrial membrane potential | Non-small-cell lung cancer | 89 |
| Vemurafenib (BRAF inhibitor) | Depolarization of mitochondrial membrane | Melanoma | 90 |
| Rituximab (anti-CD20) | Inhibition of Bcl-2 via mitochondrial ROS | B-cell lymphoma | 91 |
| Procarbazine | Oxidized, generates ROS | Hodgkin’s lymphomas, brain cancer | 96 |
| Doxorubicin | Fenton’s reaction and electron leakage | Kaposi’s sarc, breast and bladder cancer, ALL | 97 |
| Arsenic trioxide | Electron leakage | Myeloma, lung cancer and leukemia | 100,101 |
| 5-fluorouracil | P53-dependent ROS | Colon and rectal cancer | 102,103 |
| 2-methoxyestradiol | Loss of mitochondrial membrane potential | Neuroblastoma | 104 |
| N-(4-hydroxyphenyl retinamide | Mitochondrial damage | Lung cancer | 105 |
| Platinum drugs | ROS-dependent DNA damage | Breast cancer (in ccombination with PARP inhibitors) | 106,107 |
| Gamitrinib | Mitochondrial collapse | Prostate cancer | 110 |
| ARQ 501 and STA-4783 | Leakage of electron transport | Pancreatic adenocarcinoma and solid tumors | 111 |
| Bortezomib | ROS due to ER stress | Head and neck squamous cell carcinoma | 112 |
| Celecoxib | ROS after ER stress | Prostate cancer | 113 |
| Sanguinarine | Electron leakage and induction of NOXs | Glioma | 119 |
| Rapamycin | ROS from ER stress | RAS-driven tumors | 120 |
Fig. 4. The two possible ROS-related anticancer therapeutic strategies.
The first approach is based on lowering ROS levels to counteract their role in cellular transformation; it is aimed at reducing the number of transformed cells by depriving them of fuel (represented in the upper right side of the figure as a lower proportion of transformed cells with respect to that of normal cells). The second approach is based on the consideration that cancer cells, with an antioxidant system already triggered, are more sensitive than their normal counterparts to further increases in ROS and are unable to achieve redox balance. Therefore, by inducing ROS under these metabolic conditions, a high percentage of the cells undergo death (represented in the lower right side of the figure, where transformed cells are depicted as apoptotic).
Specifically, cellular responses to ROS must be imagined as the integration of multiple levels in which, in addition to their nature and relative concentration, their location plays an important role. In fact, mitochondrial ROS have been reported to essentially promote cell death, while NOX-generated ROS have been associated with the promotion of cell proliferation and migration152. Furthermore, in contrast to the mechanism of sister pathways, redox signaling is based on migrating electrons, and therefore, the signaling in this pathway is much more diffuse.
In reference to the nature of ROS behavior as a double-edged sword, even though several studies have documented the benefits of antioxidant drugs for cancer therapies, none has been supported by solid trials performed on a large scale153,154. In contrast, the most recent studies have shown an increase in tumor development and metastasis in mouse models treated with vitamin E155 (an opposite result of that in which high doses of vitamin C increase ROS levels to induce the death of colon cancer cells bearing KRAS and BRAF mutations)156. In addition, it has been shown that the administration of antioxidants, such as N-acetylcysteine, accelerates the progression of lung cancers and melanomas146 and that increasing the expression of the antioxidant-encoding Nrf2 gene enhances the growth of lung tumors157–160.
In fact, and in contrast to the previous view, the results of many studies support a scenario in which the inhibition of antioxidant enzymes ensures the death of cancer cells, especially when this approach is used in combination with treatments that increase ROS. This approach is an alternative to the traditional strategy of targeting oncogenes and tumor suppressor genes, a strategy that appears complicated because of the high number of genes involved and their ability to drive compensatory pathways161.
Interestingly, increased ROS-induced apoptosis has been reported in cancer cells after depletion of ATP derived from the manipulation of glycolytic enzymes, chemotherapy or radiation therapy; these data highlight the potential eminent role of ROS modulation in anticancer combinatorial therapies162,163.
Finally, the most recent ROS-inducing drugs have addressed the pivotal goal of therapists: cancer selectivity. In this regard, good results have been reached through photodynamic therapy, which is based on the generation of ROS after stimulation of a photosensitizer by light: cancer cells under treatment internalize porphyrin precursor molecules to induce the formation of ROS that lead to photooxidative stress and cancer-specific cell death164,165. In fact, although more studies are required to increase the selectivity of these anticancer ROS-related drugs, the common mechanisms elicited by oncogenes to promote the adaptation to a large set of stress conditions are being revealed in more depth every day, and in a high percentage, they concern the redox balance.
In conclusion, we expect that targeting ROS will represent fruitful ground for future molecular anticancer strategies.
Acknowledgements
This study was supported by Italian Ministry of University and Scientific Research [P.R.I.N. 2015B7M39T_003 and P.R.I.N. 2017EKMFTN_002 to G.C.]; P.O.R-Regione Calabria [Progetto “Razionale” to A.M.]. Giovanni Galasso is supported by a fellowship of P.R.I.N. (P.R.I.N. 2010NFEB9L). Pia Giovannelli is supported by VALERE (Vanvitelli per la Ricerca) Program. Marzia Di Donato is supported by a fellowship of ‘Fondazione Umberto Veronesi’ [FUV Postdoctoral fellowship-2019].
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Forman HJ, Ursini F, Maiorino M. An overview of mechanisms of redox signaling. J. Mol. Cell. Cardiol. 2014;73:2–9. doi: 10.1016/j.yjmcc.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 3.Vander Heiden M, Cantley L, Thompson C. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paradies G, et al. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circulation Res. 2004;94:53–59. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- 6.Petrosillo G, Ruggiero FM, Di Venosa N, Paradies G. Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: role of reactive oxygen species and cardiolipin. FASEB J. 2003;17:714–716. doi: 10.1096/fj.02-0729fje. [DOI] [PubMed] [Google Scholar]
- 7.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houee-Levin C, et al. Exploring oxidative modifications of tyrosine: an update on mechanisms of formation, advances in analysis and biological consequences. Free Radic. Res. 2015;49:347–373. doi: 10.3109/10715762.2015.1007968. [DOI] [PubMed] [Google Scholar]
- 10.Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J. Biol. Chem. 1995;270:30334–30338. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 11.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 12.Kamata H, et al. Reactive oxygen species promote TNF alpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 13.Esposito F, et al. Protein kinase B activation by reactive oxygen species is independent on tyrosine kinase receptor phosphorylation and required Src activity. J. Biol. Chem. 2003;278:20828–20834. doi: 10.1074/jbc.M211841200. [DOI] [PubMed] [Google Scholar]
- 14.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 15.Corcoran A, Cotter TG. Redox regulation of protein kinases. FEBS J. 2013;280:1944–1965. doi: 10.1111/febs.12224. [DOI] [PubMed] [Google Scholar]
- 16.Kwon J, et al. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl Acad. Sci. USA. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latimer, H. R. & Veal E. A. Peroxiredoxins in regulation of MAPK signalling pathways; sensors and barriers to signal transduction. 10.14348/molcells.2016.2327 (2016). [DOI] [PMC free article] [PubMed]
- 18.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Rad. Biol. Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 19.Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16:123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 20.Meister A. Selective modification of glutathione metabolism. Science. 1983;220:472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- 21.Thimmulappa RK, et al. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 22.Orino K, et al. Ferritin and the response to oxidative stress. Biochem. J. 2001;357:241–247. doi: 10.1042/0264-6021:3570241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinberg ED. The role of iron in cancer. Eur. J. Cancer Prev. 1996;5:19–36. [PubMed] [Google Scholar]
- 24.Essers MA, et al. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23:4802–4812. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putker M, et al. Redox-dependent control of FOXO/DAF-16 by transportin-1. Mol. Cell. 2013;49:730–742. doi: 10.1016/j.molcel.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Melnik BC. p53: key conductor of all anti-acne therapies. J. Transl. Med. 2017 doi: 10.1186/s12967-1297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhee Sue Goo, Bae Soo Han. The antioxidant function of sestrins is mediated by promotion of autophagic degradation of Keap1 and Nrf2 activation and by inhibition of mTORC1. Free Radical Biology and Medicine. 2015;88:205–211. doi: 10.1016/j.freeradbiomed.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Pinnix ZK, et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci. Transl. Med. 2010;2:43ra56. doi: 10.1126/scisignal.3001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abid MR, Spokes KC, Shih SC, Aird WC. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J. Biol. Chem. 2007;282:35373–35385. doi: 10.1074/jbc.M702175200. [DOI] [PubMed] [Google Scholar]
- 31.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 32.Svegliati S., Marrone G., Pezone A., Spadoni T., Grieco A., Moroncini G., Grieco D., Vinciguerra M., Agnese S., Jungel A., Distler O., Musti A. M., Gabrielli A., Avvedimento E. V. Oxidative DNA damage induces the ATM-mediated transcriptional suppression of the Wnt inhibitor WIF-1 in systemic sclerosis and fibrosis. Science Signaling. 2014;7(341):ra84–ra84. doi: 10.1126/scisignal.2004592. [DOI] [PubMed] [Google Scholar]
- 33.Tell G, et al. An ‘environment to nucleus’ signaling system operates in B lymphocytes: redox status modulates BSAP/Pax-5 activation through Ref-1 nuclear translocation. Nucleic Acids Res. 2000;28:1099–1105. doi: 10.1093/nar/28.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ando K, et al. A new APE1/Ref-1-dependent pathway leading to reduction of NF-kappaB and AP-1, and activation of their DNA-binding activity. Nucleic Acids Res. 2008;36:4327–4336. doi: 10.1093/nar/gkn416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toledano MB, Leonard WJ. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc. Natl Acad. Sci. USA. 1991;88:4328–4332. doi: 10.1073/pnas.88.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matthews JR, Kaszubska W, Turcatti G, Wells TN, Hai RT. Role of cysteine62 in DNA recognition by the p50 subunit of NF-kappa B. Nucleic Acids Res. 1993;21:1727–1734. doi: 10.1093/nar/21.8.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lingappan K. NF-kB in oxidative stress. Curr. Opin. Toxicol. 2018;7:81–86. doi: 10.1016/j.cotox.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsushima S, et al. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ. Res. 2013;112:651–663. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ushijima T. Detection and interpretation of altered methylation patterns in cancer cells. Nat. Rev. Cancer. 2005;5:223–231. doi: 10.1038/nrc1571. [DOI] [PubMed] [Google Scholar]
- 40.Grollman AP, Moriya M. Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet. 1993;9:246–249. doi: 10.1016/0168-9525(93)90089-z. [DOI] [PubMed] [Google Scholar]
- 41.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 42.Turk PW, Laayoun A, Smith SS, Weitzman SA. DNA adduct 8-hyroxyl-2’-deoxyguanosine (8-hydroxyguanine) affects function of human DNA methyltransferase. Carcinogenesis. 1995;16:1253–1255. doi: 10.1093/carcin/16.5.1253. [DOI] [PubMed] [Google Scholar]
- 43.Pezone, A. et al. High-coverage methylation data of a gene model before and after DNA damage and homologous repair. Sci. Data10.1038/sdata.2017.43 (2017)- [DOI] [PMC free article] [PubMed]
- 44.Russo, G. et al. DNA damage and repair modify DNA methylation and chromatin domain of the targeted locus: mechanism of allele methylation polymorphism. Sci. Rep. 6, 33222 (2016). [DOI] [PMC free article] [PubMed]
- 45.Morano A, et al. Targeted DNA methylation by homology-directed repair in mammalian cells. Transcription reshapes methylation on the repair gene. Nucleic Acids Res. 2014;42:804–821. doi: 10.1093/nar/gkt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Opresko PL, Fan J, Danzy S, Wilson DM, 3rd, Bohr VA. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005;33:1230–1239. doi: 10.1093/nar/gki273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Radisky DC, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishikawa M. Reactive oxygen species in tumor metastasis. Cancer Lett. 2008;266:53–59. doi: 10.1016/j.canlet.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 50.Chan JS, et al. Cancer-associated fibroblasts enact field cancerization by promoting extratumoral oxidative stress. Cell Death Dis. 2017;8:e2562. doi: 10.1038/cddis.2016.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toullec A, et al. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol. Med. 2010;2:211–230. doi: 10.1002/emmm.201000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat. Rev. Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 54.Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signaling. Nat. Rev. Mol. Cell. Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 55.Diehn M, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Irani K, et al. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 57.Vafa O, et al. C-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol. Cell. 2002;9:1031–1044. doi: 10.1016/s1097-2765(02)00520-8. [DOI] [PubMed] [Google Scholar]
- 58.De Nicola GM, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shibata T, et al. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc. Natl Acad. Sci. USA. 2008;105:13568–13573. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh A, et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Menegon S, Columbano A, Giordano S. The dual roles of NRF2 in cancer. Trends Mol. Med. 2016;22:578–593. doi: 10.1016/j.molmed.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Lignitto L, et al. Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of Bach1. Cell. 2019;178:316–329. doi: 10.1016/j.cell.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bae I, et al. BRCA1 induces antioxidant gene expression and resistance to oxidative stress. Cancer Res. 2004;64:7893–7909. doi: 10.1158/0008-5472.CAN-04-1119. [DOI] [PubMed] [Google Scholar]
- 64.Gorrini C, et al. BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. J., Exp. Med. 2013;210:1529–1544. doi: 10.1084/jem.20121337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olanich ME, Barr FG. A call to ARMS: targeting the PAX3-FOXO1 gene in alveolar rhabdomyosarcoma. Expert Opin. Ther. Targets. 2013;17:607–623. doi: 10.1517/14728222.2013.772136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris IS, et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 2015;27:211–222. doi: 10.1016/j.ccell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 67.Sayin VI, et al. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014;6:221ra215. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- 68.Lo M, Wang YZ, Gout PW. The x(c)-cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J. Cell Physiol. 2008;215:593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- 69.Lo M, Ling V, Low C, Wang YZ, Gout PW. Potential use of the anti-inflammatory drug, sulfasalazine, for targets therapy of pancreatic cancer. Curr. Oncol. 2010;17:9–16. doi: 10.3747/co.v17i3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guan J, et al. The xc-cystine/glutamate antiporter as a potential therapeutic target for small-cell lung cancer: use of sulfasalazine. Cancer Chemoter. Pharmacol. 2009;64:463–472. doi: 10.1007/s00280-008-0894-4. [DOI] [PubMed] [Google Scholar]
- 71.Townsend DM, et al. NOV-002, a glutathione disulfide mimetic, as a modulator of cellular redox balance. Cancer Res. 2008;68:2870–2877. doi: 10.1158/0008-5472.CAN-07-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang JB, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18:207–219. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sobhakumari A, et al. Susceptibility of human head and neck cancer cells to combined inhibition of glutathione and thioredoxin metabolism. PLoS ONE. 2012;7:e48175. doi: 10.1371/journal.pone.0048175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000;29:323–333. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 75.Wang L, et al. The Fas death signaling pathway connecting reactive oxygen species generation and FLICE inhibitory protein down-regulation. J. Immunol. 2008;180:3072–3080. doi: 10.4049/jimmunol.180.5.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilkie-Grantham RP, Matsuzawa S, Reed JC. Novel phosphorylation and ubiquitination sites regulate reactive oxygen species-dependent degradation of anti-apoptotic c-FLIP protein. J. Biol. Chem. 2013;288:12777–12790. doi: 10.1074/jbc.M112.431320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stennicke HR, et al. Caspase 9 can be activated without proteolytic processing. J. Biol. Chem. 1999;274:8359–8362. doi: 10.1074/jbc.274.13.8359. [DOI] [PubMed] [Google Scholar]
- 78.Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp. Cell Res. 2000;256:42–49. doi: 10.1006/excr.2000.4838. [DOI] [PubMed] [Google Scholar]
- 79.Kagan VE, et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 80.Zuo Y, et al. Oxidative modification of caspase-9 facilitates its activation via disulfide-mediated interaction with Apaf-1. Cell Res. 2009;19:449–457. doi: 10.1038/cr.2009.19. [DOI] [PubMed] [Google Scholar]
- 81.Madesh M, Hajnoczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J. Cell Biol. 2001;155:1003–1015. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McStay GP, Clarke SJ, Halestrap AP. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem. L. 2002;367:541–548. doi: 10.1042/BJ20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luanpitpong S, et al. Regulation of apoptosis by Bcl-2 cysteine oxidation in human lung epithelial cells. Mol. Biol. Cell. 2013;24:858–869. doi: 10.1091/mbc.E12-10-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li D, Ueta E, Kimura T, Yamamoto T, Osaki T. Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. 2004;95:644–650. doi: 10.1111/j.1349-7006.2004.tb03323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dimitrov DS, Marks DJ. Therapeutic antibodies current state and future trends-is a paradigm change coming soon? Methods Mol. Biol. 2009;525:1–27. doi: 10.1007/978-1-59745-554-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hartmann JT, Haap M, Kopp HG, Lipp HP. Tyrosine kinase inhibitors- a review on pharmacology, metabolism and side effects. Curr. Drug Metab. 2009;10:470–481. doi: 10.2174/138920009788897975. [DOI] [PubMed] [Google Scholar]
- 87.Teppo Hanna-Riikka, Soini Ylermi, Karihtala Peeter. Reactive Oxygen Species-Mediated Mechanisms of Action of Targeted Cancer Therapy. Oxidative Medicine and Cellular Longevity. 2017;2017:1–11. doi: 10.1155/2017/1485283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang SP, Shen SC, Lee WR, Yang LL, Chen C. Imatinib mesylate induction of ROS-dependent apoptosis in melanoma B16F0 cells. J. Dermatol. Sci. 2011;62:183–191. doi: 10.1016/j.jdermsci.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 89.Shan F, Shao Z, Jiang S, Cheng Z. Erlotinib induces the human non-small-cell lung cancer cells apoptosis via activating ROS-dependent JNK pathways. Cancer Med. 2016;5:3166–3175. doi: 10.1002/cam4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bauer, D., Werth, F., Nguyen, H. A., Kiecker, F. & Eberle, J. Critical role of reactive oxygen species (ROS) for synergistic enhancement of apoptosis by vemurafenib and the potassium channel inhibitor TRAM-34 in melanoma cells. Cell Death Dis. 8, e2594 (2017). [DOI] [PMC free article] [PubMed]
- 91.Alas S, Ng CP, Bonavida B. Rituximab modifies the cisplatin-mitochondrial signaling pathway, resulting in apoptosis in cisplatin-resistant non-Hodgkin’s lymphoma. Clin. Cancer Res. 2002;8:836–845. [PubMed] [Google Scholar]
- 92.Shen B, He PJ, Shao CL. Norcantharidin induced DU145 cell apoptosis through ROS-mediated mitochondrial dysfunction and energy depletion. PLoS One. 2013;8:e84610. doi: 10.1371/journal.pone.0084610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brenneisen, P. & Reichert, A. S. Nanotherapy and reactive oxygen species (ROS) in cancer: a novel perspective. Antioxidants7, E31 (2018). [DOI] [PMC free article] [PubMed]
- 94.Qu W, et al. Bisphenol A suppresses proliferation and induces apoptosis in colonic epithelial cells through mitochondrial and MAPK/AKT pathways. Life Sci. 2018;208:167–174. doi: 10.1016/j.lfs.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 95.Oliveira MS, et al. A novel platinum complex containing a piplartine derivative exhibits enhanced cytotoxicity, causes oxidative stress and triggers apoptotic cell death by ERK/p38 pathway in human acute promyelocytic leukemia HL-60 cells. Redox Biol. 2019;20:182–194. doi: 10.1016/j.redox.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Renschler MF. The emerging role of reactive oxygen species in cancer therapy. Eur. J. Cancer. 2004;40:1934–1940. doi: 10.1016/j.ejca.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 97.Kotamraju S, Chitambar CR, Kalivendi SV, Joseph J, Kalyanaraman B. Transferrin receptor-dependent iron uptake is responsible for doxorubicin-mediated apoptosis in endothelial cells: role of oxidant-induced iron signaling in apoptosis. J. Biol. Chem. 2002;277:17179–17187. doi: 10.1074/jbc.M111604200. [DOI] [PubMed] [Google Scholar]
- 98.Prieto-Bermejo, R., Romo-Gonzalez, M., Perez-Fernandez, A., Ijurko, C. & Hernandez-Hernandez, A. Reactive oxygen species in hematopoiesis: leukemic cells take a walk on the wild side. J. Exp. Clin. Cancer Res. 10.1186/s13046-018-0797-0 (2018). [DOI] [PMC free article] [PubMed]
- 99.Pelicano H, et al. Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J. Biol. Chem. 2003;278:37832–37839. doi: 10.1074/jbc.M301546200. [DOI] [PubMed] [Google Scholar]
- 100.Miller WH, et al. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–3903. [PubMed] [Google Scholar]
- 101.Yi J, et al. The inherent cellular level of reactive oxygen species: one of the mechanisms determining the apoptotic susceptibility of leukemic cells to arsenic trioxide. Apoptosis. 2002;7:209–215. doi: 10.1023/a:1015331229263. [DOI] [PubMed] [Google Scholar]
- 102.Hwang PM, et al. Ferrodoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat. Med. 2001;7:1111–1117. doi: 10.1038/nm1001-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Q, et al. Involvement of reactive oxygen species in 2-methoxyestradiol-induced apoptosis in human neuroblastoma cells. Cancer Lett. 2011;313:201–210. doi: 10.1016/j.canlet.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lai WL, Wong NS. ROS mediates 4-HPR-induced posttranscriptional expression of the Gadd153 gene. Free Radic. Biol. Med. 2005;38:1585–1593. doi: 10.1016/j.freeradbiomed.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 106.Berndtsson M, et al. Acute apoptosis by cisplatin requires induction of reactive oxygen species but is not associated with damage to nuclear DNA. Int. J. Cancer. 2007;120:175–180. doi: 10.1002/ijc.22132. [DOI] [PubMed] [Google Scholar]
- 107.Rottenberg S, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl. Acad. Sci. USA. 2008;105:17079–17084. doi: 10.1073/pnas.0806092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Charruyer A, et al. UV-C light induces raft-associated acid sphingomyelinase and JNK activation and translocation independently on a nuclear signal. J. Biol. Chem. 2005;280:19196–19204. doi: 10.1074/jbc.M412867200. [DOI] [PubMed] [Google Scholar]
- 109.Caino MC, Altieri DC. Molecular pathways: mitochondrial reprogramming in tumor progression and therapy. Clin. Cancer Res. 2016;22:540–545. doi: 10.1158/1078-0432.CCR-15-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kang BH, et al. Preclinical characterization of mitochondria-targeted small molecule hsp90 inhibitors, gamitrinibs, in advanced prostate cancer. Clin. Cancer Res. 2010;16:4779–4788. doi: 10.1158/1078-0432.CCR-10-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sborov DW, Haverkos BM, Harris PJ. Investigational cancer drugs targeting cell metabolism in clinical development. Expert Opin. Investig. Drugs. 2015;24:79–94. doi: 10.1517/13543784.2015.960077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fribley A, Zeng Q, Wang CY. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol. Cell Biol. 2004;24:9695–9704. doi: 10.1128/MCB.24.22.9695-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu J, et al. Using cyclooxygenase-2 inhibitors as molecular platforms to develop a new class of apoptosis-inducing agents. J. Natl Cancer Inst. 2002;94:1745–1757. doi: 10.1093/jnci/94.23.1745. [DOI] [PubMed] [Google Scholar]
- 114.Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem. Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 115.Poillet-Perez L, Despouy G, Delage-Mourroux R, Boyer-Guittaut M. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol. 2015;4:184–192. doi: 10.1016/j.redox.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scherz-Shouval R, et al. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alexander A, et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc. Natl Acad. Sci. USA. 2010;107:4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li L, Ishdorj G, Gibson SB. Reactive oxygen species regulation of autophagy in cancer: implications for cancer treatment. Free Radi. Biol. Med. 2012;53:1399–1410. doi: 10.1016/j.freeradbiomed.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 120.De Raedt T, et al. Exploiting cancer cell vulnerabilities to develop a combination therapy for Ras-driven tumors. Cancer Cell. 2011;20:400–413. doi: 10.1016/j.ccr.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Galluzzi L, Kroemer G. Necroptosis: a specialized pathway of programmed necrosis. Cell. 2008;135:1161–1163. doi: 10.1016/j.cell.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 122.Vandenabeele P, Galluzzim L, Vandenm Berghem T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 123.Kim YS, Morgan MJ, Chocksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol. Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 124.Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 125.Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 126.Jiang L, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dixon SJ, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Louandre C, et al. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int. J. Cancer. 2013;133:1732–1742. doi: 10.1002/ijc.28159. [DOI] [PubMed] [Google Scholar]
- 129.Shaw AT, et al. Selective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stress. Proc. Natl Acad. Sci. USA. 2011;108:8773–8778. doi: 10.1073/pnas.1105941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agentsw using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285–296. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 131.Yagoda N, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 133.Steeg PS. Targeting metastasis. Nat. Rev. Cancer. 2016;16:201–218. doi: 10.1038/nrc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat. Rev. Cancer. 2010;10:147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 135.Gottesman MM. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 136.Zhang Y, Marcillat O, Giulivi C, Ernster L, Davies K. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J. Biol. Chem. 1990;265:16330–16336. [PubMed] [Google Scholar]
- 137.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 138.Wang, H. et al. Targeted production of reactive oxygen species in mitochondria to overcome cancer drug resistance. Nat. Commun. 10.1038/s41467-018-02915-8 (2018). [DOI] [PMC free article] [PubMed]
- 139.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 140.Perillo B, et al. DNA oxidation as triggered by H3K9me2 demethylation drives estrogen-induced gene expression. Science. 2008;319:202–206. doi: 10.1126/science.1147674. [DOI] [PubMed] [Google Scholar]
- 141.Abbondanza C, et al. Highlighting chromoosome loops in DNA-picked chromatin (DPC) Epigenetics. 2011;6:979–986. doi: 10.4161/epi.6.8.16060. [DOI] [PubMed] [Google Scholar]
- 142.Tsang, C. K., Liu, Y., Thomas, J., Zhang, Y. & Zheng, X. F. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat. Commun. 10.1038/ncomms4446 (2014). [DOI] [PMC free article] [PubMed]
- 143.Perillo B, et al. Phosphorylation of H3 serine 10 by IKKα governs cyclical production of ROS in estrogen-induced transcription and ensures DNA wholeness. Cell Death Differ. 2014;21:1503. doi: 10.1038/cdd.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Perillo B, et al. Nuclear receptor-induced transcription is driven by spatially and timely restricted waves of ROS. The role of Akt, IKKα, and DNA damage repair enzymes. Nucleus. 2014;5:482–491. doi: 10.4161/nucl.36274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Braumuller H, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–365. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 146.Kodama R, et al. ROS-generating oxidases Nox1 and Nox4 contribute to oncogenic Ras-induced premature senescence. Genes Cells. 2013;18:32–41. doi: 10.1111/gtc.12015. [DOI] [PubMed] [Google Scholar]
- 147.Hodny, Z., Reinis, M., Hubackova, S., Vasicova, P. & Bartek, J. Interferon gamma/NADPH oxidase defense system in immunity and cancer. Oncoimmunology5, e1080416 (2015). [DOI] [PMC free article] [PubMed]
- 148.Trachootam D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 149.Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J. Pharm. Sci. 2007;96:2181–2196. doi: 10.1002/jps.20874. [DOI] [PubMed] [Google Scholar]
- 150.Wang J, Yi J. Cancer cell killing via ROS: to increase or decrease, that is the question. Cancer Biol. Ther. 2008;7:1875–1884. doi: 10.4161/cbt.7.12.7067. [DOI] [PubMed] [Google Scholar]
- 151.Halliwell B. The antioxidant paradox. Lancet. 2000;355:1179–1180. doi: 10.1016/S0140-6736(00)02075-4. [DOI] [PubMed] [Google Scholar]
- 152.Cheung EC, et al. TIGAR is required for efficient intestinal regeneration and tumorigenesis. Dev. Cell. 2013;25:463–477. doi: 10.1016/j.devcel.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Klein EA, et al. Vitamin E and the risk of prostate cancer: The selenium and vitamin E cancer prevention trial (SELECT) J. Am. Med. Assoc. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Omenn GS, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 155.Le Gal K, et al. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 2015;7:308re8. doi: 10.1126/scitranslmed.aad3740. [DOI] [PubMed] [Google Scholar]
- 156.Yun J, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350:1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang H, et al. NRF2 activation by antioxidant antidiabetic agents accelerates tumor metastasis. Sci. Transl. Med. 2016;8:334ra51. doi: 10.1126/scitranslmed.aad6095. [DOI] [PubMed] [Google Scholar]
- 158.Jeong Y, et al. Role of KEAP1/NRF2 and TP53 mutations in lung squamous cell carcinoma development and radiation resistance. Cancer Discov. 2017;7:86–101. doi: 10.1158/2159-8290.CD-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Romero R, et al. Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat. Med. 2017;23:1362–1368. doi: 10.1038/nm.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wiel C, et al. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell. 2019;178:330–345. doi: 10.1016/j.cell.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 161.Misale S, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–536. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Panieri E, Santoro MM. ROS homeostasis and metabolism: a dangerous liaison in cancer cells. Cell Death Dis. 2016;7:e2253. doi: 10.1038/cddis.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim J, Kim J, Bae J-S. ROS homeostasis and metabolism: a critical liaison for cancer therapy. Exp. Mol. Med. 2016;48:e269. doi: 10.1038/emm.2016.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Buytaert E, Dewaele M, Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Biophys. Acta Rev. Cancer. 2007;1776:86–107. doi: 10.1016/j.bbcan.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 165.Sies H, Berndt C, Jones DP. Oxidative stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]