Abstract
Precision allergy molecular diagnostic applications (PAMD@) is increasingly entering routine care. Currently, more than 130 allergenic molecules from more than 50 allergy sources are commercially available for in vitro specific immunoglobulin E (sIgE) testing. Since the last publication of this consensus document, a great deal of new information has become available regarding this topic, with over 100 publications in the last year alone. It thus seems quite reasonable to publish an update. It is imperative that clinicians and immunologists specifically trained in allergology keep abreast of the new and rapidly evolving evidence available for PAMD@.
PAMD@ may initially appear complex to interpret; however, with increasing experience, the information gained provides relevant information for the allergist. This is especially true for food allergy, Hymenoptera allergy, and for the selection of allergen immunotherapy. Nevertheless, all sIgE tests, including PAMD@, should be evaluated within the framework of a patient's clinical history, because allergen sensitization does not necessarily imply clinical relevant allergies.
Keywords: Diagnosis; Molecular allergy; Cross reactivity; Panallergen; Specific IgE, PAMD@
Introduction
In the late 1960s, the discovery of the immunoglobulin (IgE) antibody provided a specific biomarker that could be used to identify allergic diseases triggered by environmental allergens (generally proteins). Traditional IgE antibody tests such as skin prick tests (SPTs) and in vitro specific IgE (sIgE) tests are based on crude extracts composed of allergenic and non-allergenic molecules obtained from an allergenic source. With the application of DNA technology in the late 1980s, allergenic molecules were characterized and cloned to resolve the determinants of various allergic diseases.1, 2, 3, 4 The availability of allergenic molecules in the last decade has ushered in a new phase of diagnostics,5 now termed precision allergy molecular diagnostic applications (PAMD@), allowing improved management of allergic diseases.6 In previous years, this diagnostic strategy has been termed component-resolved diagnostics (CRD), molecular-based allergy diagnostics (MBAD), or molecular allergy diagnostics (MAD). A multitude of articles regarding PAMD@ have been published that reinforce the utility of adding this testing method to the care of the allergic patient.1 Thus, it appears useful to provide an update to the WAO — ARIA — GA2LEN consensus document on PAMD@ which was published in 2013.2
Nomenclature
Today, many of the most common allergenic molecules have been cloned or purified, have had their 3-dimensional structures elucidated, and can be consistently produced.7 Because of the growing number of allergens identified, a systematic allergen nomenclature, approved by the World Health Organization and International Union of Immunological Species (WHO/IUIS) Allergen Nomenclature Subcommittee, has been established.8 The subcommittee has been charged with developing and maintaining not only the systematic nomenclature developed for allergenic molecules but also a comprehensive database of known allergenic proteins, which can be accessed at http://www.allergen.org. Allergenic molecules are named using their Latin binomial name (genus and species). A detailed description of the terminology has been recently published.9 For example, allergens that begin with Phl p are from Phleum pratense (Timothy grass). A number is added to the name to distinguish the various allergens from the same species (e.g., Phl p 1, Phl p 2). The numbers are assigned to the allergens in the order of their identification. Allergenic molecules are classified into protein families according to their structure and biological function.10, 11, 12, 13 Many different molecules share common epitopes (antibody binding sites), and the same IgE antibody can bind and induce an immune response to allergenic molecules with similar structures from various allergen sources. These cross-reactive allergens give valuable information regarding sensitization to several different sources. In contrast, some molecules are unique markers for specific allergen sources, allowing for the identification of the primary sensitizer. Table 1 lists the components belonging to the most frequent allergen families and their availability on 3 different multiplex tests.
Table 1.
Components belonging to the 8 most common allergen families in ISAC, ALEX, and Euroline 334
| Family | Species | Allergen | Multiplex text |
||
|---|---|---|---|---|---|
| ISAC | ALEX | Euroline | |||
| Bet v 1–related protein (PR-10) | Actinidia deliciosa (green kiwi) | Act d 8 | X | ||
| Alnus glutinosa (alder) | Aln g 1 | X | X | ||
| Apium graveolens (celery) | Api g 1 | X | X | ||
| Arachis hypogaea (peanut) | Ara h 8 | X | X | ||
| Betula pendula (white birch) | Bet v 1 | X | X | X | |
| Corylus avellana (hazel) | Cor a 1 | X | X | ||
| Glycine max (soybean) | Gly m 4 | X | X | ||
| Daucus carota (carrot) | Dau c 1 | X | |||
| Malus domestica (apple) | Mal d 1 | X | X | ||
| Prunus persica (peach) | Pru p 1 | X | |||
| Venom group 5 allergen family | Polistes dominulus (European paper wasp) | Pol d 5 | X | X | X |
| Vespula vulgaris (yellow jacket) | Ves v 5 | X | X | X | |
| Cupin Superfamily | Anacardium occidentale (cashew) | Ana o 2 | X | ||
| A. hypogaea (peanut) | Ara h 1 | X | X | X | |
| Ara h 3 | X | X | X | ||
| Corylus avellana (hazel) | Cor a 9 | X | X | ||
| Cor a 11 | X | ||||
| G. max (soybean) | Gly m 5 | X | X | ||
| Gly m 6 | X | X | |||
| Juglans regia (English walnut) | Jug r 2 | X | X | ||
| EF hand domain (Ca++ binding proteins) | B. pendula (white birch) | Bet v 4 | X | ||
| A. glutinosa (alder) | Aln g 4 | X | |||
| Gadus callarias (Baltic cod) | Gad c 1 | X | |||
| Gadus morhua (Baltic cod) | Gad m 1 | X | |||
| Penaeus monodon (black tiger shrimp) | Pen m 4 | X | |||
| Cyprinus carpio (European carp) | Cyp c 1 | X | X | ||
| Phleum pratense (Timothy grass) | Phl p 7 | X | X | X | |
| Expansin, C-terminal domain | Cynodon dactylon (Bermuda grass) | Cyn d 1 | X | ||
| P. pratense (Timothy grass) | Phl p 1 | X | X | X | |
| Phl p 2 | X | X | |||
| Lipocalin | Blatella germanica (cockroach) | Bla g 4 | X | ||
| Bos domesticus (domestic cattle) | Bos d 2 | X | |||
| Bos d 5 | X | X | X | ||
| Canis familiaris (domestic dog) | Can f 1 | X | X | ||
| Can f 2 | X | X | |||
| Equus caballus (horse) | Equ c 1 | X | X | ||
| Felis domesticus (domestic cat) | Fel d 4 | X | X | ||
| Mus musculus (mouse) | Mus m 1 | X | X | ||
| Profilin | B. pendula (white birch) | Bet v 2 | X | X | |
| Hevea brasiliensis (Para rubber tree) | Hev b 8 | X | X | ||
| Mercurialis annua (annual mercury) | Mer a 1 | X | |||
| P. pratense (Timothy grass) | Phl p 12 | X | X | X | |
| Phoenix dactylifera (date palm) | Pho d 2 | X | |||
| Olea europaea (olive) | Ole e 2 | X | |||
| Prolamin superfamily | A. occidentale (cashew) | Ana o 3 | X | ||
| A. hypogaea (peanut) | Ara h 2 | X | X | X | |
| Ara h 6 | X | X | X | ||
| Ara h 9 | X | X | X | ||
| Artemisia vulgaris (mugwort) | Art v 3 | X | X | ||
| Bertholletia excelsa (Brazil nut) | Ber e 1 | X | X | ||
| Brassica /Sinapis spp. | Sin a 1 | X | |||
| Corylus avellana (hazel) | Cor a 8 | X | X | ||
| Cor a 14 | X | ||||
| Fagopyrum esculentum (buckwheat) | Fag e 2 | X | X | ||
| G. max (soybean) | Gly m 8 | X | |||
| J. regia (Englich walnut) | Jug r 1 | X | X | ||
| Macadamia integrifolia (macadamia) | Mac i 2S | X | |||
| Papaver somniferum (poppy) | Pap s 2S | X | |||
| Sesamum indicum (sesame) | Ses i 1 | X | |||
New concepts regarding the mechanisms of action of allergens
Allergens induce sIgE sensitization of mast cells and trigger allergic inflammation upon re-exposure. The availability of natural purified (n) or recombinant (r) allergens has helped to improve our understanding of the mechanisms leading to this phenomenon, which vary depending on several ecological, biological, and structural characteristics of the allergenic molecules.14 In addition to the production of sIgE and the IgE binding associated with Th2 immunity, allergens may also act by promoting tissue inflammation directly because of their enzymatic or other (still unknown) biological properties.
The induction of sIgE and sensitization are not straightforward processes. For example, because aeroallergens are transported in particles (e.g., the feces of mites) and mostly interact with respiratory mucosae, the immune response results from stimulation by several components in addition to allergens.13 This means that natural exposure sometimes does not result in important sensitization (and probably a greater IgG response than IgE response) if Th1-promoting components are inhaled simultaneously and overcome the effect of allergenic molecules, or if interleukin (IL)-10–mediated tolerance is induced by the presence of bacteria.15 In addition, the immune response to allergens starts with the activation of innate immune receptors, which can also modulate the strength of the Th2 response.16, 17, 18 Here again, we can infer that there are several pathways, some of them antagonistic, to the production of sIgE and clinically relevant sensitization.
These pathways involve various types of cells, including the recently discovered innate lymphoid cells (ILC). Because several members of this cell population have been described, more data have to be obtained before a clear role for each member during the allergic response can be defined. The participation of ILC type 2 (ILC2) seems to be influenced by the type of allergen; for example, ILC2 cells are expanded more in allergic rhinitis induced by house dust mite (HDM) than in that induced by mugwort.19 Also, in parasite-infected mice, HDM can induce the production of IL-13 and mediation of conventional type 2 inflammatory responses independently of T-cell receptor stimulation.20 ILC2 may act to intensify the specific Th2 response or to provide, together with other ILC types, pro-inflammatory cytokines in reactions not mediated by IgE. Activation of ILC2 is mediated by IL-25, thymic stromal lymphopoietin, and particularly IL-33 (alarmin), which is produced by stimulated epithelial cells after exposure to allergens, infectious organisms, and pollutants.21
An example of the pro-inflammatory properties of allergens that are not mediated by IgE includes the proteolytic activity of Der p 1, which directly activates epithelial cells to induce the production of pro-inflammatory cytokines.22 Also, the non-proteolytic components of Der p 2 induce inflammation by other IgE-independent mechanisms.23, 24, 25, 26, 27, 28, 29 These characteristics could influence the clinical impact of each allergen but may not correlate with the frequency of IgE binding. This could be the case for Der p 13 18; Der p 18 30,31; Der p 7 31; Der p 5 32; other proteases such as Der p 3, Der p 6, and Der p 9 33; and other lipid-binding proteins such as Der p 21 and Blo t 13. Therefore, in some cases, the IgE binding detected by PAMD@ might be considered a proxy for more crucial allergenic properties.
An interesting mechanism of action of HDM is that it induces epigenetic changes in immune cells and epithelial/bronchial muscle cells. Recent studies revealed that modifications to DNA methylation in B cells might influence the susceptibility to mite sensitization,34 and hypomethylation of the IL-13 gene is associated with an increased risk of allergic rhinitis due to HDM sensitization.35 Also, HDM can induce epigenetic modifications in experimental airway inflammation in mice, changing the methylation pattern of important genes such as pde4 d36 and tgfb1.37 Also, HDM induces the same epigenetic modifications as diesel exhaust in an ex vivo model of inflammation in human bronchial epithelial cells.38 These studies suggest that HDM also induces IgE-mediated bronchial inflammation by altering the epigenetic patterns of cells involved in bronchial homeostasis.
Another important point on the mechanisms of action of allergens is the role of IgG and its subclasses, which in turn also has 2 aspects. One is the involvement of IgG as an effector mechanism in the pathogenesis of some allergic reactions, such as food allergy. Although there is some evidence that IgG participates in food allergy not mediated by IgE,39 the evaluation of serum-specific IgG and IgG4 has no proven predictive value in food allergy diagnosis.40,41 More studies are therefore needed to define specific IgG as a marker of food allergy.42,43 The second aspect is the potential clinical impact of the IgG/IgE ratio, an interesting and traditional theme that has been revived in recent years, probably because of the availability of purified components for PAMD@. There is important evidence suggesting that, in addition to what is observed during allergen immunotherapy (AIT), a high IgG/IgE ratio is associated with fewer allergic symptoms.44, 45, 46 In particular, IgG4 seems to have a direct role in the induction of tolerance.47 This area of research should provide useful information regarding the inception, evolution, and diagnosis of allergic diseases, but there is currently no standardized way to apply these findings to PAMD@.
The usefulness of PAMD@ for management of allergic diseases: a bird's eye view
PAMD@ is increasingly entering routine care and improves management of allergy. This is particularly evident for food allergy.3,4,6,7,11, 12, 13 Knowing which allergenic molecules the patient is sensitized to can help to determine the likelihood of local versus systemic reactions and to predict the persistence of clinical symptoms. For example, some allergens, such as storage proteins in peanuts (e.g., Ara h 2) and nuts (e.g., Cor a 9), are associated with severe reactions, whereas sensitization to other allergens is usually associated with less severe reactions. Another critical aspect, difficult to elucidate using traditional tests, is the stability of the allergen. Allergens that are stable to heat and digestion (e.g., Ara h 2 from peanut) are more likely to cause severe clinical reactions, whereas heat- and digestion-labile molecules (e.g., Ara h 8 from peanut) are more likely to cause milder, local reactions (such as pruritus) or to be tolerated. Similarly, identifying whether the sensitization is primary and likely to be clinically relevant or due to clinically irrelevant cross-sensitization helps to evaluate the likelihood of reaction on exposure to different allergen sources. A recent meta-analysis determined that molecular diagnostics are particularly useful for food allergy.48
PAMD@ may also be helpful in the assessment of idiopathic anaphylaxis. If positive, it may orient the allergist to the triggering allergen; if negative, a non-IgE-mediated mechanism underlying the anaphylaxis (such as mast cell disorders) should be considered.3,49 It is also useful in cases of Hymenoptera venom allergy, with a recent study demonstrating that PAMD@ can identify a subgroup of patients likely to fail on honey bee venom (HBV) immunotherapy.18,19,50 PAMD@ may also improve the selection of both patients and specific allergens for AIT for inhalant allergies (e.g., for pollen),10,16,17 with a recent study demonstrating that the added precision PAMD@ brought to the prescribing of immunotherapy reduced overall costs.51 All of these topics are described in greater detail in this revised WAO - ARIA - GA2LEN consensus document.
In vitro diagnostics
Singleplex and multiplex measurement arrays
An ever-increasing number of studies focusing on different allergenic molecules or allergic diseases are rapidly being published. However, the search for additional clinically relevant molecules is ongoing and needed to improve the positive predictive value of allergy diagnostic testing. Both the scientific and industrial communities have been involved in developing new reagents and new diagnostic tools in the past few years. At present, the presence of IgE antibodies against allergenic molecules may be determined using a singleplex (one assay per sample) or multiplex (multiple assays per sample) measurement platform. A singleplex platform allows the clinician to select those allergenic molecules necessary for an accurate diagnosis as determined by the clinical history of the patient.52 The multiplex approach allows for the IgE response to a broad array of preselected allergens on a chip to be characterized independently of the clinical history. Notably, analysis by multiplex assay may be even possible with a dried blood spot, which can be easily transported.53
There are several commercially available immuno-solid-phase multiplex allergen arrays: the Thermo Fisher ImmunoCAP ISAC (Immuno-solid-phase Allergen Chip), which contains 112 allergens from 51 allergen sources12; the new ImmunoCAP ISAC 112i, with 112 components from 48 allergen sources, where some components have been removed (Hymenoptera components, Pla a 2, Jug r 2) and some others were added (Ana o 3, Can f 4, Can f 6, Cor a 14, Der p 23 and alpha-Gal); the MADx Allergen Explorer (ALEX; containing 282 allergens: 156 extracts and 126 components)54; and the Euroline microstrips.55 The large number of extracts/allergens from multiple classes of allergenic sources (Table 2) provides extensive and detailed information about a patient's sensitization profile.54,56 Other diagnostic tools based on allergen arrays are under development, and new tools will likely be available in the near future.
Table 2.
List of Components available for the Molecular Allergy Diagnosis
| Common components available on the market. Components are in in alphabetic order, as of June 2019, from different sources (Thermofisher, MADx, Hycor, Euroline, Siemens) |
| Act d 1, Act d 2, Act d 5, Act d 8, Alkalase, Alt a 1, Alt a 1, Alt a 6, Amb a 1, Amb a 4, Ana c 2, Ana o 2, Ana o 3, Ani s 1, Ani s 3, Api g 1., Api g 2, Api g 6, Api m 1, Api m 4, Api m 10, Api m 2, Api m 3, Api m 5, Ara h 1, Ara h 2, Ara h 3, Ara h 6, Ara h 8, Ara h 9, Art v 1, Art v 3, Asp f 1, Asp f 2, Asp f 3, Asp f 4, Asp f 6, Asp o 21, Asp r 1, Ber e 1, Bet v 1, Bet v 2, Bet v 4, Bet v 6, Bla g 1, Bla g 2, Bla g 4, Bla g 5, Bla g 7, Blo t 5, Bos d 2, Bos d 4, Bos d 5, Bos d 6, Bos d 8, Bos d Lactoferrin, Can f 1, Can f 2, Can f 3, Can f 4, Can f 5, Can f 6, Car p 1, Che a 1, Cla h 8, Cor a 1, Cor a 11, Cor a 14, Cor a 8, Cor a 9, Cry j 1, Cup a 1, Cyn d 1, Cyp c 1, Dau c 1, Der f 1, Der f 2, Der p 1, Der p 10, Der p 11, Der p 2, Der p 23, Der p 5, Der p 7, Equ c 1, Equ c 3, Fag e 2, Fel d 1, Fel d 2, Fel d 4, Fel d 7, Fra e 1, Gad m 1, Gad c 1, Gal d 1, Gal d 2, Gal d 3, Gal d 4, Gal d 5, Gal-alpha, gliadin, Gly d 2, Gly m 4, Gly m 5, Gly m 6, Gly m 8, Hev b 1, Hev b 11, Hev b 3, Hev b 5, Hev b 6., Hev b 8, Hom s LF, Jug r 1, Jug r 2, Jug r 3, Lep d 2, Lol p 1, Mac i 2S Albumin, Mal d 1, Mal d 2, Mal d 3, Mal d 4, Maxatase, Mala s 1, Mala s 11, Mala s 5, Mala s 6, Mala s 9, Mer a 1, Mus m 1, MUXF3, Ole e 1, Ole e 2, Ole e 7, Ole e 9, Pap s 2S Albumin, Par j 2, Pen a 1, Pen m 1, Pen m 2, Pen m 4, Per a 7, Phl p 1, Phl p 11, Phl p 12, Phl p 2, Phl p 4, Phl p 5, Phl p 6, Phl p 7, Pho d 2, Pla a 1, Pla a 2, Pla a 3, Pla l 1, Pol d 5, Pru av 1, Pru av 3, Pru av 4, Pru p 1, Pru p 3, Pru p 4, Sal k 1, Savinase, Ses i 1, Sin a 1, Sola l 6, Sus s Pepsin, Sus s PSA, Tri a gliadin, Tri a 14, Tri a 19, Tri a aA_TI, Ves v 1, Ves v 5, Vit v 1. |
| Components only available in the Thermofisher ImmunoCAP system (including ISAC) |
| Act d 8, Alkalase, Ana o 2, Api m 3, Api m 4, Api m 5, Asp f 1, Asp f 2, Asp o 21, Bet v 4, Bla g 7, Blo t 5, Bos d lactoferrin, Can f 4, Can f 5, Can f 6, Car p 1, Cry j 1, Cyn d 1, Equ c 3, Fel d 7, Gad c 1, Gal-alpha, gliadin f9, Jug r 3, Lep d 2, Maxatase, Mer a 1, MUXF3, Ole e 7, Ole e 9, Pen a 1, Pen m 2, Pen m 4, Phl p 11, Phl p 4, Pla a 2, Pla a 3, Pru p 1, Pru p 4, Sal k 1, Savinase, Sus s Pepsin, Sus s PSA, Tri a 14, Tri a 19.0101, Tri a aA_TI, Ves v 1 |
| Components only available in the MADx ALEX |
| Act d 10, Amb a 4, Api g 2, Api g 6, Bla g 4, Bos d 2, Cor a 11, Dau c 1, Der p 11, Der p 5, Der p 7, Fra e 1, Gad m 1, Gly d 2, Gly m 8, Hom s LF, Lol p 1, Mac i 2S Albumin, Mal d 2, Mala s 1, Mala s 11, Mala s 5, Mala s 6, Mala s 9, Ole e 2, Pap s 2S Albumin, Per a 7, Pho d 2, Sin a 1, Sola l 6, Tri a gliadin, Vit v 1 |
Multiplex assays are especially suited for use in patients with complex sensitization patterns or symptoms. The multiplex technology is a consolidated PAMD@ approach for improved diagnosis, prognosis, and selection of patients for AIT. Although they are commercial products, they have been the mainstay of many studies. In recent years, an increasing number of reagents have been made available for singleplex assays, but multiplex assays have been the object of both research and development by the diagnostic industry, and they contain many more components than are available for singleplex assays. These new features are discussed in more detail in the sections below, together with the available informatics support and criteria for interpreting allergen arrays and supporting the diagnosis.
Precision medicine was launched at a worldwide level by the initiative of then US President Barack Obama in 2015. Before that, precision (or personalized) medicine was more a dream than a reality. Precision or personalized medicine is a discipline that identifies specific biomarkers of a given disease in a given patient (the so-called “endotype”) that are based on the patient's characteristics, evaluated in real time, and may impact the therapeutic approach. Precision or personalized medicine is expected to deeply affect all medical procedures in the near future,57 including more-appropriate selection of patients and treatments and more-appropriate allocation of resources and interventions in general. The increasing availability of biological drugs, bio-engineering, and genetic and “omics” tools have already started to affect the decisional processes in medicine.
Allergy represents an excellent paradigm for precision medicine because, in many cases, the patient can be well characterized by available clinical features, diagnostic tests, and biomarkers. In addition, many of the underlying mechanisms are known in detail even if others are still being investigated. In this context, the introduction and availability of PAMD@ represent a real advance in the description of the patient's IgE repertoire. At present, the therapeutic strategy based on standard drugs (such as inhaled corticosteroids, bronchodilators, and antihistamines) has not substantially changed, but the detailed definition of the sensitization profile has allowed the use of AIT to be refined.58, 59, 60 In this context, AIT still represents an etiology-based treatment when the clinical aspects and the diagnostic procedures are well standardized, but PAMD@ allows its prescription to be better focused, and to achieve the best results, reducing costs. The impact of PAMD@ on the use and prescription of AIT will be discussed below.
In summary, with increasing experience, PAMD@ is generally straightforward to interpret and can provide relevant, additional information for the allergist. However, the clinical utility of many of the allergenic molecules needs further investigation. Because of the speed at which new PAMD@ data are becoming available, clinicians are required to keep pace with a large amount of novel information. This WAO - ARIA - GA2LEN consensus update document on PAMD@ provides a practical guide to the indication, determination, and interpretation of PAMD@. It is designed for clinicians specifically trained in allergology but can also be a good starting point for new users.
Available diagnostic tools
In the last year, the multiplex approach to in vitro PAMD@ has greatly improved. The ISAC 103 version was described in the original consensus statement,2 and an improved version of ISAC, based on 112 different components from 51 allergen sources, was released in 2011. The new assay has been shown to be repeatable (intra-assay assessment), reproducible (interassay assessment), and suitable for supporting a multiplex allergy diagnosis.61 A deeper analysis of ISAC 112 characteristics also demonstrated that it is a highly reproducible and accurate method that may be considered as a single analyte assay given the EN ISO 15189 accreditation procedure.62 Results obtained by ISAC 112 also correlate well with singleplex ImmunoCAP results.63 Recently, the new ISAC 112i was introduced.
Despite the success of the ISAC assays, some partially unexpected cross-binding between its components have been identified.64 For example, nPhl p 4, a highly glycosylated protein, can bind to IgE specific for cross-reactive carbohydrate determinants (CCDs), and IgE to the native walnut vicilin-like protein nJug r 2 can also be raised in patients sensitized to CCDs.64 For this reason, the real clinical significance of a positive nJug r 2 result must be carefully evaluated in the context of the results of other components and clinical findings.
Allergen microarrays have also been used to evaluate the presence of sIgE in fluids other than serum or plasma. Leonardi et al.65 recently showed that in vernal keratoconjunctivitis, ISAC can detect the presence of sIgE to grass, tree, mites, animals, and food allergens in tears. What was particularly interesting was that, in some patients, sIgE were absent in serum but detectable in tears. The presence of sIgE only in tears of patients with symptoms only in the eyes supports the idea that tissues can be locally sensitized. Using an innovative approach,66 Valenta and co-workers analyzed the presence of IgE in samples of breast milk. This use of microarray technology for 2 alternative specimen types, tears and breast milk, opens new fields for research and in clinics.
PAMD@ is useful for identifying sensitivities to many, but not all, allergens. For example, D'Amelio et al.67 examined whether the performance of ImmunoCAP ISAC 112 is sufficient to diagnose peach and apple allergies. They conclude that although the sensitivity of the peach components in ISAC could be improved, it is adequate in Italy. The same authors68 concluded that the diagnostic performance of ISAC was adequate for hazelnut and walnut allergy but not for peanut allergy. Finally, in a different situation,69 even if standard ImmunoCAP have, for apple and peach, a wider number of available components (in particular Mad d 3, a lipid transfer protein [LTP]), the evaluation of Pru p 3 (largely homologous to Mal d 3) may support the identification of an apple sensitivity even if “the presence of sIgE against Pru p 3 in LTP sensitized patients can be due to cross-sensitization and should therefore not be used to predict clinical symptoms".
In the period that ISAC 103 and ISAC 112 have been used in clinics, other strategies for multiplex molecular diagnostics have been developed. The MeDALL group developed a novel microarray adding more than 70 new components to the standard panel of ISAC 112.70 These new components are allergens from peanut, nuts (almond, cashew, and pistachio), cow's milk, wheat, olive pollen, mites, dogs, insect venom, Staphylococcus aureus toxins, and maltose binding protein. The clinical features of the MeDALL microarray were evaluated during the so-called allergen march from childhood to adolescence.71 The prevalence of allergic sensitization increased from age 10–16 years and was similar by SPT and ImmunoCAP and significantly higher with the MeDALL chip at age 10. All 3 tests were comparable for identification of allergic sensitization among children with current rhinitis or asthma.
A different approach has been developed by an English/Swedish company that designed and further implemented a microarray, the Microtest system, where whole extracts are spotted in addition to single-allergen components.72 This combination of extracted allergens and recombinant components was tested against 3 other allergy test methods (SPT, ImmunoCAP, and ISAC 112) in a pairwise fashion for each component. The methods produced concordant results 81%–88% of the time, with correlation coefficients for the most prevalent allergens (cat, dog, mite, Timothy, birch, and peanut) ranging from 0.73 to 0.95, although results of the different tests were discordant in some patients. Thus, the Microtest system provides another alternative for testing common allergens.
More recently, ALEX (Allergen Explorer) was developed by MADx in Vienna, Austria. ALEX is an array of allergens spotted on a solid phase by the use of nanoparticles. ALEX contains 282 reagents (156 allergen extracts and 126 recombinant or highly purified molecules). Thus, this chip, like the Microtest, contains second-level diagnostics (represented by extract allergens) and third-level diagnostics (represented by single molecules) are available, which is remarkable, and the results from ALEX correlate well with those from ISAC.54,73 This microarray allows the measurement of an IgE profile including “whole” allergens and recombinant or purified allergen proteins in a single chip. Thus, 2 characteristics are specific of this assay: the first is that it can be a suitable method for the bottom-up strategy of allergy diagnosis, which tests isolated allergens before whole extracts.74 The second is that it provides an extended IgE profile in line with the indications of a precision medicine approach.59 This approach requires the most accurate definition of the patient's phenotype to identify the patient's endotype75 and to provide an accurate diagnosis and appropriate treatment. In consideration of the very large number of allergens and components and the significant complexity of the interpretation of the results (at least for non-professional molecular allergists), ALEX has been associated with a new version of the expert system Allergenius, originally developed for the interpretation of ISAC results.74,76 Perhaps the greatest value of ALEX is the provision of a CCD inhibitor, which reduces clinically irrelevant cross-sensitization and has a direct impact on primary sensitization information.54
Another group of tests are represented by the multiple allergen simultaneous tests (MAST) immunoblots, such as Euroline, which is a commercially available assay for PAMD@ based on the immunoblot technique. A sensitization profile can be generated from the simultaneous determination of sIgE to different allergen components and extracts. Notably, different IgE profiles are available. Substantial agreement between MAST and ImmunoCAP was found for inhalant, food, and venom allergens, and it thus represents a valid diagnostic alternative.55
Finally, epitope mapping will be the real future, provided that a large number of clinical and experimental data become available. Indeed, peptides are recognized by humans in an HLA-restricted manner. For this reason, “dominant peptides” can be identified, but many other “individual peptides” should also be considered. A panel of partially overlapping peptides fully covering the whole allergenic protein could be used for highly represented allergens and has meaning only if sIgE have been previously observed in a more classic allergen-component assay. Keeping this consideration in mind, many groups have published extremely exciting results that clearly show that both diagnostic and prognostic indications can be derived from the analysis of the IgE profile directed to peptides of different allergen sources.77, 78, 79, 80, 81, 82, 83 Of course, the need for large epidemiological studies will be necessary to define rules that can be applied to different populations of patients. However, the possibility of dissecting the immune response mediated by IgE will be included in the fourth diagnostic level of allergic diseases, such as the basophil activation test, in the near future. In this context, it is clear that the diagnostic process in complex or highly complex patients will need to be managed by highly skilled groups with a specialized laboratory and advanced informatics tools.
Patients most likely to benefit from PAMD@
There is a general consensus that patients with multiple sensitizations will likely benefit from PAMD@. This includes patients with respiratory sensitization to a large number of allergen families and patients with pollen-food or inhalant food syndromes. Another relevant application of PAMD@ is food allergy and the food protein-induced enterocolitis syndrome,84, 85, 86 because it is now possible to determine the individual pattern of IgE sensitization by analyzing single allergenic molecules instead of complex allergenic extracts.
Allergens commonly used for PAMD@ can be either recombinant or purified native, and serum sIgE levels can be detected through singleplex assays or in multiplex arrays. In order to choose the test correctly, the specialist should take several factors into consideration: the target of the PAMD@ (e.g., immunotherapy for respiratory diseases, latex allergy, food allergy, cross-reactions between food and inhalant allergens), the complexity of the clinical case, and the availability in each country of the molecular diagnostic tools. Complex cases, such as multiple sensitizations to respiratory and food allergens and idiopathic anaphylaxis, are preferably studied with multiplex assays.87 Multiplex assays performed early in life seem to be also useful for predicting the risk of developing allergic symptoms in later life.88, 89, 90
Clinicians should always be adequately trained, familiar with the main allergen protein families, and aware of the characteristics of the PAMD@ assays. In particular, the multiplex tests can produce complex results, and the clinician should therefore be familiar with the results each type of assay might produce. In general, educational programs for both the use and interpretation of PAMD@ tests should be implemented.76 Multiplex platforms allow for testing of more than 100 molecules simultaneously (Table 2) in a very low quantity of serum and without interference from high total IgE levels, but they are less sensitive and less appropriate for monitoring sensitization than their singleplex counterparts.2 However, when the detection of more than 12 or 13 sIgE is needed, it has been suggested that the multiplex assay is more cost-effective than the singleplex diagnostic approach and is therefore preferred.91
Risk assessment and PAMD@
PAMD@ can increase the accuracy of an allergy diagnosis in certain circumstances.3 In allergic patients, a molecular approach is suitable for the following:
-
•
assessing the risk of potential allergic reactions, which depend on the individual allergic (clinical) sensitization profile;
-
•
evaluating whether unknown potential triggering factors are present (i.e., the presence of sIgE versus allergenic molecules correlated with high risk for allergic reactions).
In particular, in the case of polysensitization, PAMD@ makes it possible to distinguish between primary and cross-sensitization, leading to a significant improvement in patient management. For example, appropriate avoidance diets can be recommended when the correct food allergen is identified; conversely, by identifying cross-sensitivities PAMD@ can prevent needless food avoidance or prescriptions for self-injectable adrenaline. Cross-sensitization between aeroallergens and food allergens is very common. In some cases, the presence of a respiratory allergy to an allergen with a shared epitope to food may lead to a clinically relevant cross-reactivities. Pollen sensitization may lead to “pollen-food syndromes”, such as birch-apple or celery-mugwort-spice syndrome. Nonpollen aeroallergens cross-reacting with foods include mite-shrimp syndrome. This topic has been covered in an extensive review92 that describes cross-reactivities between different combinations of food and inhalant allergens and which could be an important tool for the interpretation of multiple sensitivities detected with PAMD@ (either multiplex or singleplex). The fundamental work of Valenta et al. is another useful guide for understanding the molecular mechanisms underlying allergic sensitization and cross-reactivities of food allergens.93 If patients have sIgE to natural allergens but no clinical manifestations after exposure to the allergen, the presence of IgE directed to CCDs should be investigated; in 10–20% of patients with pollen sensitivity, sIgE against carbohydrate epitopes called N-glycans can be detected.94
The presence of serum sIgE directed towards proteins that are stable to heat and acidic conditions (such as LTPs, storage proteins, gliadins, tropomyosins, parvalbumins, caseins, and ovomucoid) is generally associated with a higher risk of systemic or severe reactions. In contrast, a pattern of sensitization to gastro- and heat-labile proteins (families such as profilins, PR-10, thaumatins, ovalbumin, and lactalbumin) is generally correlated with a lower risk of severe allergic manifestations; an exception is Gly m 4, a member of the PR-10 family, which is a potential marker for severe reactions in patients consuming large quantities of soy drinks and sensitized to Bet v1, the major allergen of birch.95 There are other exceptions to the classical rule of “stable allergens–severe reactions and labile allergens–mild reactions”. Indeed, anaphylactic reactions to foods have occurred in patients monosensitized to PR-10 allergens96 or profilins,97 and it is well known that clinical expression of LTPs varies from asymptomatic to anaphylactic reactions.
A molecular approach allows patients (e.g., those with occupational latex sensitization) to be stratified according to the risk of reactions as a secondary prevention strategy, but it is important to remember that the severity of allergic reactions also depends on other factors, such as the route of allergen exposure and the presence of cofactors, such as exercise or concomitant drug consumption. Interestingly, the geographic area can also influence the risk of allergic manifestations: in the Mediterranean area, for instance, Pru p 3 is one of the most common triggers for anaphylaxis and severe reactions. A cohort of 133 Italian patients allergic to peach with positive SPT to whole peach extract and sIgE against Pru p 3 has been studied.98 The population recruited from the northeastern part of the country, where sensitization to PR-10 and profilin is more common than in the south, also had sIgE to Pru p 1 (42.8%) and Pru p 4 (12.7%), whereas no patients from the south were sensitized to PR-10 or profilin. In the southern population, the levels of sIgE to Pru p 3 were significantly different in symptomatic patients and asymptomatic patients, and in subjects with mild systemic reactions and subjects with severe systemic reactions. In contrast, in the northeastern population, no differences were found in the levels of sIgE to Pru p 3 between these groups. Also, in the southern population, the severity scores of the clinical reactions and the levels of sIgE to Pru p 3 were statistically correlated, whereas in the northeastern population, the correlation was not statistically significant due to the low number of patients with severe allergic reactions. Another important finding of this study was the determination of cutoff levels for sIgE to Pru p 3 that allowed discriminating asymptomatic from symptomatic patients (2.87 kUA/L for the southern Italian population and 2.69 kUA/L for the northeastern population). Lower levels of sIgE to Pru p 3 and the co-sensitization to Pru p1 and/or Pru p 4 could explain the lower risk of severe reactions in the northeastern patients than in the southern patients, who were monosensitized and presented with higher levels of sIgE to Pru p 3, as if the simultaneous positivity to Pru p 1 and/or Pru p 4 can play a sort of ‘protective’ role against the development of severe symptoms induced by Pru p 3.99 Geographic differences are extremely relevant, and the future work of the PAMD@ committee will attempt to define a geographic map of allergen sensitization for the whole world.
The role of PAMD@ in food allergy risk assessment is relevant in pediatric patients, where it is now possible to map the sensitization profile in a low quantity of serum and, in some cases, to avoid oral food challenges that are costly, time-consuming, and not free from risk.100 An interesting review recently reported the cutoff values of sIgE levels for stratifying the risk of reactions to food (peanuts and tree nuts, cow's milk, egg, wheat, fish and seafood, fruits, and vegetables) in pediatric populations.101 Nevertheless, the results probably depend on the in vitro method used and comorbidities, and further large-scale studies are required before these cutoff values can be officially validated.101 Another paper extensively discussed the role of specific allergenic molecules in the development and persistence of egg allergy; for instance, the presence of elevated sIgE levels against ovomucoid (Gal d 1) seems to predict the persistence of allergy over time102,103 and the inability to tolerate even extensively cooked eggs. Also, the evaluation of protein-specific sIgE levels and IgE/IgG4 ratios seems to be more helpful than the SPT in predicting tolerance to baked egg. In general, children sensitized to sequential epitopes are less likely to resolve their egg allergy than those presenting with sIgE to conformational epitopes. Nevertheless, even though PAMD@ represents a promising diagnostic tool, larger studies (including challenges with cooked egg) are needed to confirm its clinical utility; furthermore, the possibility of performing PAMD@ does not exclude the need for oral provocation tests in many patients104 because sIgE levels do not significantly predict the severity of allergic reactions or the outcome of oral provocation tests with the culprit food; that is, sIgE represents sensitization but not necessarily allergy. The presence of serum sIgE against food allergens in the absence of a significant clinical history is a common finding. In this case, the patient should be followed carefully for possible allergic manifestations, including reactions to other cross-reacting foods, but avoiding the suspected trigger only because of a positive sIgE result is not recommended, particularly during childhood when avoidance can lead to failure to thrive. An elimination diet, especially when the suspected food allergy could be due to cross-reactions, should be required only when a clear history is present or an oral food challenge is positive.
PAMD@ is also useful in cases of anaphylaxis, especially idiopathic anaphylaxis, food-dependent exercise-induced anaphylaxis, and red-meat anaphylaxis.105 Multiplex testing was used to identify potential causes of idiopathic anaphylaxis.106 The test identified 203 sensitizations in 22 (20%) of the 110 patients examined. Of these, 35 were considered to be plausible causes of the anaphylaxis, and the newly identified triggers were confirmed in 11 of the 22 patients. Omega-5-gliadin and shrimp allergens represented 45% of the previously unrecognized sensitizations, followed by seed storage proteins, nonspecific LTP (nsLTP), and latex allergens.
Specialists should keep in mind that some allergenic molecules are poorly represented in allergen extracts, and the predictive value of first-level diagnostic tests (SPT, sIgE detection) may not allow a proper diagnosis. For example, a proper investigation of serum sIgE directed against proteins belonging to the 2S-albumin family of seed storage proteins is quite necessary for risk assessment.101 Molecules such as Ara h 2 and 6, Cor a 14, Ana o 3, Ber e 1, and Jug r 1 are significantly predictive of allergic reactions to peanut, hazel, cashew, Brazil nut, and walnut, respectively. Co-sensitization to Ara h 2 and Ara h 6 is associated with severe reactions to peanut. Recent data indicate that Ses i 1 may represent the best marker for sesame allergy.7
A recent review has been published regarding the relevance of lipophilic molecular allergens in diagnosis of food allergy.107 In fact, some severe allergic reactions are caused by lipophilic molecules that are not contained in the allergenic extracts for in vivo tests; among them are the oleosins, which are insoluble in saline or aqueous solutions. At the time this guideline was written, only oleosins of peanut (Ara h 10, Ara h 11), sesame (Ses i 4 and Ses i 5), and hazelnut (Cor a 12 and Cor a 13) had been registered as allergens. Furthermore, serum IgE specific for a group of molecular allergens, such as Jug r 2 (vicilin-like protein), Ana o 2 (legumin-like protein), Ses i 1 (2S albumin storage protein), Pen m 2 (arginine kinase), and Pen m 4 (sarcoplasmic calcium-binding protein), are identifiable only through multiplex arrays and are not available in singleplex.
In the case of food-dependent exercise-induced anaphylaxis, PAMD@ makes it possible to identify the pattern of allergic sensitization and then to avoid the trigger allergen(s) and other possible cofactors (such as nonsteroidal anti-inflammatory drugs or alcohol). Wheat-dependent exercise-induced anaphylaxis (WDEIA), for instance, is classically associated with allergic sensitization to omega-5-gliadin (Tri a 19)108; nevertheless, patients reporting symptoms suggestive for WDEIA should be tested also for nsLTP, such as Pru p 3 and Tri a 14, because sensitization to the LTP family has been reported to be very common in cofactor-exacerbated allergic reactions in the Mediterranean basin.109,110 In another study, 64 (78%) of 82 patients with food-dependent exercise-induced anaphylaxis were positive to Pru p 3, indicating that LTPs are the most frequent sensitizer in Italian subjects.111
Of recent interest is the use of PAMD@ in the diagnosis of anaphylaxis occurring after ingestion of red meat (e.g., beef, pork, and lamb), despite tolerance to other meats like chicken or turkey. Tick bites from Ixodes ricinus in Europe and Amblyomma americanum in the US are considered the main determinants for the IgE responses against a mammalian oligosaccharide epitope called galactose-α-1,3-galactose (alpha-gal).112 IgE to alpha-gal has been associated with 2 distinct forms of anaphylaxis: i) delayed-onset anaphylaxis, which occurs 3–6 h after ingestion of mammalian food products (e.g., beef or pork); and ii) immediate-onset anaphylaxis during the first exposure to intravenous cetuximab, a monoclonal antibody for the treatment of metastatic colorectal cancer.113 sIgE levels seem to decrease over time, and some patients can tolerate red meat again after about 1–2 years if no additional tick bites occur.114
PAMD@ in selected sensitizations
Respiratory allergens
Data have been published recently related to the molecular profiles associated with the development of allergic asthma.90,115,116 In a Korean study of 168 patients with allergic rhinitis, sIgE to molecular HDM allergens obtained with ISAC correlated with the clinical diagnosis and was 78.9% comparable to ImmunoCAP specificity, although the concordance was lower for Alternaria, birch, and mugwort.117 In a study from Singapore, 135 atopic subjects presenting mostly with allergic rhinitis underwent multiplex testing. A strong association was found between allergic rhinitis and HDM sensitization, whereas asthma and atopic dermatitis were not correlated with sensitization to any allergen, prompting the conclusion that the microarray should probably be adjusted for regional allergens.118 A Bavarian study with 86 patients showed that the IgE reactivities to major allergens had direct clinical relevance, whereas pan-allergens alone did not lead to clinical symptoms.119
The often complex patterns of multisensitizations, which include IgE to cross-binding epitopes, may prompt the development of more sophisticated analysis tools. A UK-based group used latent variable modeling to study the patterns of sensitivities on ISAC in 461 children.116 Allergens clustered into 3 groups of allergenic components, which allowed the authors to correlate different clinical patterns with these clusters of IgE sensitivity. Because multiplex diagnostics are complex, it is likely that such models will be needed to understand the clinical implications of the results.116
The specific molecular sensitization pattern of IgE, combined with the semiquantitative level determination, may predict the risk for allergic rhinitis and asthma, as well as the risk of accelerated and severe clinical reactivity for Hymenoptera venom,120 food allergy, exercise-induced food allergy, red-meat delayed anaphylaxis, latex allergy, or Anisakis allergy in the individual patient.49 Equally important is the fact that clinically relevant sensitizations can be differentiated from non-relevant sensitizations to CCDs and profilins, which translate to practical, relevant recommendations for patients.121,122 Along these lines, it has been observed that co-sensitization to profilins is associated with a lower occurrence of systemic reactions to nsLTP or storage proteins.123
House dust mites
The presence of IgE to both Der p 2 and Der p 1 has highly significant predictive value for immediate-type asthma.124 In a birth cohort study of 1184 subjects in Italy,125 a combined sensitization to Der p 1 and Der p 2 represented the highest risk factor for asthma development, independent of age. Others have reported the specific impact of Der p 2 and Der f 2 allergens on severe asthma.126 Cysteine proteases, such as Der p 1 and papain, may have a percutaneous sensitization capacity, in addition to the described capacity to disrupt bronchial epithelial barriers.127 Der p 23 is a major allergen associated with asthma in both pediatric and adult populations.89,128 The recently identified Der p 11 (a non-fecal allergen from Dermatophagoides pteronyssinus) seems to be a useful serological marker for the identification of a subgroup of HDM-allergic patients suffering from atopic dermatitis.129 The development of HDM allergen sensitization during life (the so-called allergen march) has been studied at a molecular level with their relationships with symptoms.89
Dermatophagoides components cross-react with components of other HDM, in particular those from Blomia tropicalis. In this context, a positive IgE to Blomia components is specific in tropical environments, whereas it is related to a cross-reaction in temperate climates.
The cross-reactive capacity of mite allergens was analyzed in 200 Chinese patients with HDM allergy by PAMD@, showing that IgE to Der p 10 correlated with cross-sensitization to shrimp, moth, and cockroach allergens (Pen m 1, Ani s 3, and Bla g 7).130 A clinical risk assessment of the apparent cross-reactivity revealed that higher levels of IgE to rPen a 1 and rDer p 10 correlated with positive responses in double-blind placebo-controlled food challenges (DBPCFC) with shrimp. Furthermore, diagnosed asthma to HDM and IgE to nDer p 1, 2, and 10 predicted a higher risk for clinically relevant shrimp allergy.131
Tropical climates, although scarcely studied, are a suitable environment for the development of mite sensitization; indeed, mites are the most prevalent allergen source in the tropics, followed by pets and cockroaches,132 whereas pollen and molds are less relevant than in North America and Europe. In tropical places where helminth infections are prevalent, cross-reactivity between mites and tropomyosins from the nematode Ascaris lumbricoides are associated with asthma symptoms.128,133,134 In tropical climates, Blo t 5 from B. tropicalis has been associated with severe allergic dermatitis.135 Recently, a clear relationship has been shown between sensitization to HDM components and the outcome of AIT with the relevant components.136
Several cases of anaphylaxis after oral ingestion of mites have been reported in patients sensitized to mites who ingested contaminated food (pancake syndrome). The species identified were Suidasia spp., D. pteronyssinus, Aleuroglyphus ovatus, Lepidoglyphus destructor, and B. tropicalis.137 Of note, group 2 mite allergens (NPC2), which are heat resistant, have been suggested to be responsible for (severe) symptoms of oral mite anaphylaxis.137
Pollens
Regional and climate differences determine pollen counts and species. Birch pollen is a dominant pollen source in much of Europe, with Bet v 1 as the single major allergen that is responsible for cross-sensitizations and cross-reactivities with pollen from other species and plant-derived food. The higher the IgE levels to Bet v 1, the higher the risk for cross-reactivities in a hierarchical clustering of Bet v 1 > Mal d 1 > Cor a 1.04 > Ara h 8 > Pru p 1 > Aln g 1 > Api g 1 > Act d 8 > Gly m 4, such that if IgE was present to the allergens lower in the hierarchy it was also present to those allergens higher in the list.88 Additionally, high levels of IgE to Bet v 1 correlate with the likelihood of allergic rhinitis persisting beyond the age of 16 years. Interesting associations between clinical presentation and specific PR-10 sensitization profiles were observed in a birch-free Mediterranean area: Bet v 1, Cor a 1.0101, and Aln g 1 reactivity are associated with respiratory symptoms, whereas Ara h 8, Cor a 1.0401, Gly m 4, Mal d 1, and Pru p 1 are selectively linked to the occurrence of oral allergic syndrome.138
In Brazil, IgE profiles typical of grass pollen sensitization were found.139 In Iran, IgE in 202 adult asthmatic patients was directed against the grass pollen allergens Phl p 1 and 5, but even more to Salsola kali (Sal k 1), which is an important pollen source in this area.140 However, the authors did not find a correlation between IgE levels and asthma symptoms.
The development of IgE to grass pollen and mite during childhood was studied in a component-based fashion in the birth cohort of 1184 subjects in Italy mentioned above. Whereas an early onset of sensitization was associated with decreased lung function and asthma, the late-onset type was predictive for allergic rhinitis.125 In the case of grass pollen sensitization, the timing of IgE development (early vs. late) was rather decisive for the clinical course, whereas in mite allergy, the presence of IgE to Der p 1 or 2, or combined sensitization, had a higher predictive value. Another extensive study on the development of grass pollen allergy has been published in the past.141 Savi et al. found that in 140 patients with allergic rhinitis or asthma caused by sensitization to grass pollen, the only correlation between allergens and symptoms was that low levels of Phl p 5 IgE were correlated with an absence of asthma.142 Similarly, Bokanovic et al. did not find a correlation of IgE levels to any of the tested grass pollen molecules, nor to symptom severity, and suggested that Phl p 1 is sufficient as a marker for sensitization without clinical relevance.143 Interesting work on mugwort sensitization comes from China: Art v 1 and Art an 7 were predominant in a patient cohort in the northern part of the country that suffered more often from asthma than a cohort in the Southwest, where Art v 3 was prominent in addition to Art v 1 and Art an 7. However, potential confounding environmental factors were not addressed.20,144
Vernal conjunctivitis usually occurs on a background of a family history of allergies, most often in young boys. Armentia et al.145 showed that PAMD@ could be used to diagnose allergy and to introduce precise AIT, which was successful in reducing eye diseases after 1 year in 13 of the 25 patients examined.
Pet dander
Animals are the leading cause of allergic asthma after mites and pollens,146 with some geographic exceptions. In Europe, about 26% of adults evaluated for suspected allergy to inhalant allergens are sensitized to cats and about 27% are sensitized to dogs.147 In the US, 12.1% are sensitized to cats and 11.8% are sensitized to dogs.148 Although there are possible differences between breeds, all dogs produce allergenic proteins found in the epithelium, dander, lingual glands, prostate, and parotid glands.149 The major dog allergens are Can f 1 and Can f5 149; Can f 5, a prostatic kallikrein, is present only in male dogs that have not been neutered.150 IgE to Can f1 and Can f 5 are highly predictive of dog allergy, although other allergens such as Can f 4 and Can f 6 may be clinically significant. The major cat allergens are Fel d 1 and Fel d 4; the sources are salivary, sebaceous, and perianal glands.150 Fel d 1 is associated with hormone production and acts as a uteroglobin; it is found mainly in saliva, sebaceous glands of the skin, and the urine of male cats. Fel d 1 is carried in very small airborne particles <5 μm in diameter; this likely renders it able to reach small bronchioles and can explain why it is difficult to remove from the house.
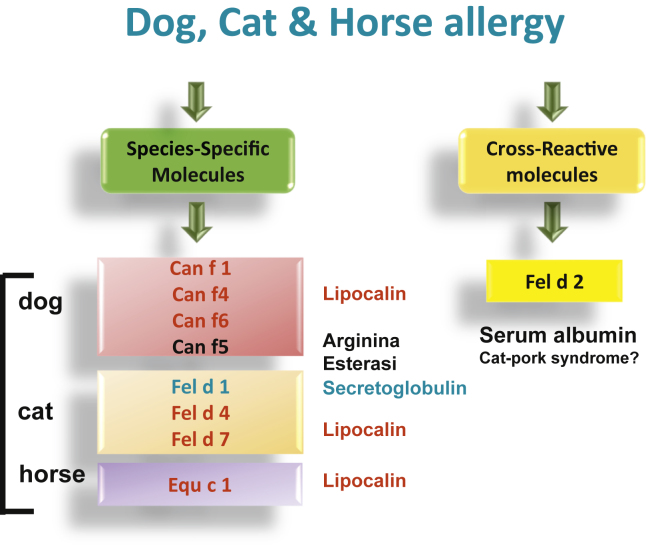
The homology between different dog and cat allergens such as albumins and lipocalins explains the cross-sensitization between them and allergens from other mammals and the presence of simultaneous sensitization to dogs, cats, and other mammals regardless of whether there is or is not direct exposure to all of them.151 Some of these antigens cause clinically relevant cross-reactivity between different animals (Fig. 1). The most significant cross-binding patterns between allergens of cats, dogs, and other mammals are with lipocalins and Can f 5. Lipocalins have amino acid sequences with up to 60% identity with Can f 6 (dog), Equ c 1 (horse), Fel d 4 (cat), Ory c 4 (rabbit), and Mus m 1 (mouse). Can f 5 shows a certain homology with prostate-specific antigen, which is also in the kallikrein family. It has been speculated that prior sensitization to Can f 5 from dogs could be associated with a greater propensity for developing allergic reactions to human seminal fluid.152 Finally, Can f 7, an NPC2-like protein that is homologous with the NPC2 components of HDM, has been identified in dogs.153
Fig. 1.
Relevant molecules involved in the identification of cat, dog and horse sensitization
Exposure before and around birth to dog dander or to dust from cow barns is regarded as protective against allergies and asthma. However, if sensitization does occur, multiplexing could be helpful to identify the extent of risk for the individual patient to define avoidance or AIT strategies,154 and to identify other animals that may be a source of clinically relevant cross-reacting allergens despite no prior exposure. Sensitization to Can f 1 and Fel d1 and polysensitization to cat and dog allergens during childhood have been associated with the development of subsequent allergy to cats and dogs.155 In 259 children sensitized to cats, co-sensitization to Fel d 1 and Fel d 4 was associated with a risk for asthma; for dog sensitization, IgE to Can f 5, 1, and 2 were the most significant risk factors.156 In contrast, IgE to serum albumins was rare and not clinically relevant. In 304 children, early sensitization to pet allergens (lipocalins, secretoglobins) preceded a risk for respiratory sensitization, followed by asthma and by meat and wheat allergy.157 Sensitivity to the dog allergens Can f 4 and Can f 6 were associated with positive nasal provocation in children with allergic rhinitis.158 The authors did not, however, regard monosensitization to Can f 5 as an indicator of a dog allergy.
In contrast to its usefulness in determining dog allergy, PAMD@ has limited effectiveness for cat allergy. Its perfomance in determining sIgE is similar for complete cat extract and Fel d 1,159 a predictive marker of allergy to cats.
Sensitization to certain allergens seems to be associated with the severity and persistence of clinical symptoms, and sensitization to more than 1 allergen or sensitization to albumins seems to be associated with more-severe respiratory symptoms.148,159 In patients allergic to cats, the main cross-reacting food allergy syndrome is the pork-cat syndrome due the cross-binding of Fel d 2 with other albumins from mammals; it can lead to immediate anaphylactic reactions after consuming raw or undercooked pork. As noted above in the section on meat allergy, alpha-gal is also present on cat IgA Fel d 5 and IgM Fel d 6.160
Cockroach
SIgE to Per a 2 has been found in patients with persistent asthma and is a potential marker for more-severe airway disease. PAMD@ could help to detect genuine sensitization to mite and cockroach allergens.161 Two cockroach allergens, Per a 11 (alpha-amylase) and Per a 12 (chitinase), have been identified by serological and in vitro investigations and SPT in 47 cockroach-sensitized patients.162
Fungal allergens
Tanimoto et al. aimed to differentiate between allergic bronchopulmonary aspergillosis (ABPA) and Aspergillus fumigatus–sensitized asthmatic patients without ABPA.163 ImmunoCAP showed that ABPA patients (n = 53) had significantly higher levels of IgE to Asp f 1 and Asp f 2 than patients with asthma (n = 253). The result was complicated by comorbid atopic dermatitis, where high levels of anti–Asp f 6 IgE levels were found. Interestingly, Asp f 13, a serine protease, exerts innate adjuvant effects by disrupting fixation of smooth muscles to the extracellular matrix in the bronchi,164 but the risk of a clinical reaction has not yet been defined. In a meta-analysis of 26 studies on Aspergillus, IgE to Asp f 1 and Asp f 3 was most specific for ABPA.165 A combination of several molecules was suggested to help with diagnosis, but the clinical utility was not assessed.
Food allergens
Multiplex testing provides a major advantage for diagnosing food allergy, whereas IgE binding to single molecules may help to predict the severity of symptoms upon allergy exposure according to the stability of the allergen. This prediction is based on the classification of food allergens according to whether they only elicit local symptoms in the mouth and are then digested, or they survive gastrointestinal digestion and, via effective mucosal adsorption, lead to systemic symptoms.166
This classification, however, is only a prediction, because cofactors like age, hormonal status, the capacity for gastric digestion, drugs, infections, menses, alcohol, and exercise may accelerate the allergen entry into the body, thereby lowering the threshold for allergic reactivity and increasing the likelihood of systemic reactions.167
In 86 patients with food allergies, a prescription for epinephrine was taken as a measure of the clinically predicted risk for severe reactions.168 The presence of a prescription was in fair agreement with the predictive value of multiplex testing. However, the authors found 3 problems with the microarray that need to be addressed: poor sensitivity, some discrepancies between the clinical and serological risk assessments, and the absence of some causative allergens from the microarray. For instance, LTPs from tomato are not yet included in the multiplex test, but studies indicate their importance in clinically relevant sensitization, particularly in the Mediterranean area.169
A European consensus paper focused on food-inhalant cross-reactivity noted that respiratory sensitizations may lead to clinically relevant, even severe, reactions to cross-reactive food.41 This is a noncanonical view, because pollen-associated foods are usually regarded as labile and less potent, despite reports of anaphylactic reactions to foods in patients monosensitized to PR-10 allergens96 or profilins.97 There is accumulating evidence that pollen also contains LTPs and nsLTPs that may elicit cross-reactivity to foods that are associated with a higher anaphylaxis risk.170 An Italian study of 568 patients170 found that hierarchical patterns and cluster relationships predicted a higher risk for systemic food-associated reactions when a subject had IgE directed against more than 5 different nsLTPs, including peach Pru p 3 and walnut Jug r 3. Sensitization to mugwort Art v 3 and plantain Pla a 3 was associated with an increased likelihood of respiratory symptoms. Finally, Ole e 7 reactivity in olive tree–sensitized subjects is associated with the presence of adverse reactions to food and not with respiratory symptoms.171
IgE to LTPs or storage proteins of a specific plant food did not correlate with clinical reactivity in the majority of 130 Spanish children with food-associated anaphylaxis to mostly peach, walnut, peanut, and hazelnut,172 calling into question the predictive value of PAMD@ for food allergy to LTPs and seed storage proteins. These data were in line with an earlier study173 that found no correlation between sensitization to the LTP Pru p 3, as measured with component-resolved SPT, and the severity of clinical reactivity. Therefore, more studies need to be performed that examine the correlation between the multiplex IgE results, the level of sensitization, and the clinical picture. However, considering the risk of in vivo tests for diagnosing patients with a previous systemic reaction, multiplex IgE testing is an interesting alternative tool to examine the IgE profile and level of sensitization in a semiquantitative manner in settings when SPT cannot be applied.122 Of course, cofactors cannot be considered or predicted. Likely, clustering algorithms like those recently developed for allergic rhinitis and asthma116 are needed for improving the accuracy of risk evaluation in food allergy by PAMD@, but overall, clinical studies on multiplex IgE testing indicate good reliability for risk assessment.174 The highest diagnostic accuracy is achieved with Bos d 4 for cow's milk, Gal d 1 for heated or raw hen's egg, Ara h 6 for peanut, Cor a 14 for hazelnut, and Lit v 1 for shrimp.48 In addition PAMD@ may provide an economic benefit, especially in multisensitized patients.175
Milk
In milk allergy, desensitization strategies are important both for the management of the disease and for nutrition in children. Kuitunen et al. treated 76 milk-allergic children with oral immune therapy (OIT). Children with high levels of IgE to α-lactalbumin, β-lactoglobulin, and casein reached a lower maintenance dose than the target dose.176 In patients who did respond, IgG4 was produced against these same molecules and lactoferrin during OIT. The authors suggested that the use of PAMD@ before OIT could improve the selection of children for whom OIT would probably succeed176; that is, these molecules represent biomarkers.177 On the other hand, those children with the highest IgE have the highest need for desensitization. For example, children with higher levels of IgE to cow's milk allergens in PAMD@ had a higher risk for persisting milk allergy.178
Using PAMD@, the IgE/IgG4 ratio and the sIgE sensitization pattern allowed the discrimination of patients with cow's milk allergy who tolerate cooked milk.179 However, further PAMD@ studies are needed to predict tolerance, with the hope of being able to reduce the need for food challenges.180
Egg
Sato et al. reported that ovomucoid is a diagnostic marker for egg allergy.177 However, this may differ with age: Kim et al. divided 27 children with convincing egg-related, immediate-type symptoms into 3 age groups and analyzed their IgE reactivity to ovomucoid (Gal d 1), ovalbumin (Gal d 2), and ovotransferrin (Gal d 3) by enzyme-linked immunosorbent assay or immunoblot.181 The study showed that the IgE against Gal d 2 developed in the first year of life, in the second year the IgE changed to be predominantly against Gal d 1, and after 24 months an additional Gal d 3 response developed. Hence, there appears to be an age-dependent effect on IgE evolution to egg allergens in small children. Dang et al. calculated the risk for clinically relevant, persistent egg allergy in the HealthNuts cohort: IgE to Gal d 1 elevated the risk 2- to 3-fold, and IgE to Gal d 1, 2, 3, or 5 elevated the risk up to 4-fold.182,183 Accordingly, Gal d 1 and 2 are associated with a higher risk for egg anaphylaxis.183
Peanut
Among the food allergies, multiplex testing is probably the most advanced for peanut allergy, where it has found its place in risk assessment. Based on a retrospective serological examination of 89 Belgian patients with peanut allergy, Ara h 1, 2, and 3 were proposed as markers for sensitization and were associated with clinical reports of more-severe reactions, whereas IgE to Ara h 8 was a sign of cross-sensitization by pollen and was associated with oral allergy syndrome rather than severe reactivity.184 This was supported by Giovanni et al., who found that IgE to Ara h 1 and 2 was associated with anaphylaxis, whereas Ara h 8 produced only weak clinical reactivity.185 In Austria, sensitization to Ara h 2 and 6 was more common than to Ara h 8, 3, and 9,186 although the diagnostic sensitivity was good for each.
A double-blind, placebo-controlled study in 102 patients provided evidence that the storage proteins Ara h 2 and Ara h 6 are the best predictors of severe systemic reactions to peanuts that require treatment with epinephrine.187 The authors concluded that multiplex IgE testing could replace provocation for diagnosis of severe peanut allergy. The EuroPrevall team stated that Ara h 2 might be regarded as the single major allergen,188 whereas peanut-tolerant individuals had more IgE to Ara h 8 and 9. However, in an Asian cohort of 40 patients tolerant patients had IgE to neither Ara h 2 nor Ara h 9. A ratio of Ara h 2 IgE to peanut IgE above 0.6 was suggested to be predictive of severe reactions,189 although different methods can result in different cut-off values.
Interestingly, IgE to CCDs was exclusively found in tolerant patients.189 This phenomenon was also seen in a Ghanaian cross-sectional study of 1604 children, with adverse reactions in 17% of them, mostly associated with a sIgE response. A co-infection with Schistosoma haematobium was revealed as the sensitizer to CCDs, resulting in peanut-cross-reactive IgE without clinical implications.190 A PAMD@ result including CCDs may thus be an indicator of tolerance. In contrast, sensitization to the defensins Ara h 12 and Ara h 13 in the lipophilic peanut fraction was associated with more-severe reactions.178
Alternative efforts include determining IgE, IgG, and IgG4 in the same sample. Lower levels of IgE to Ara h 2 predicted clinically relevant tolerance, in contrast to IgG and IgG4, which failed to discriminate.191
Soy
Because soy is related to peanut, it also represents a source of dangerous allergens. Notably, in addition to Gly m 5 and 6, the Bet v 1 homolog Gly m 4 harbors high allergenic potency due to its heat stability. In a multiplex study with sera from 20 soy-allergic patients, IgE to Gly m 4 predicted severe systemic reactions.95 Soy is important in global nutrition, and its allergenicity is best evaluated at a molecular level. Lu et al.192 identified β-conglycinin (Gly m 5), glycinin (Gly m 6), and soybean Kunitz trypsin inhibitor as major IgE binders in the sera of patients with clinical symptoms of soy allergy; in contrast, in sensitized-only subjects, IgE was bound by soybean agglutinin, seed biotinylated protein, late embryogenesis protein, and sucrose-binding protein. Gly m 8 (a 2S albumin) is also a relevant allergen in both children and adults.193,194
Hazelnut
In the EuroPrevall setting, hazelnut sensitization across Europe is dominated by IgE to Cor a 1,195 a labile Bet v 1 homolog that cross-reacts with birch pollen but is less dangerous. There were, however, a few geographic exceptions. For example, in Athens the storage protein Cor a 8 was a frequent sensitizer, and this sensitization was associated with food allergy to other nuts and rosacea fruits, and with an allergy to pollen from the goosefoot weed, plane tree, and mugwort. Increasing evidence supports the LTP Cor a 9 and the storage proteins Cor a 14 as causing severe hazelnut-associated symptoms and the more-severe type of cross-reactivity symptoms. In the Eastern Mediterranean area, IgE to Cor a 14 was identified in 56 children with clinically relevant sensitization.196 This is in agreement with a Dutch study of 161 hazelnut-sensitized patients that confirmed the predictive value of Cor a 9 and 14 sensitization by DBPCFC, where 13% of children and 49% of adults could be identified objectively.197 Importantly, the levels of IgE to Cor a 9 and 14 directly correlated with the reactivity level of 40 hazelnut-allergic children in DBPCFC.198
Moreover, IgE cross-binding between rCor a 9, rCor a 14, and rJug r 1 indicated the potential clinical implications for patients sensitized to one of these nuts.199 Two studies using PAMD@ identified sIgE to Cor a 9 and Cor a 14 as being associated with a history of anaphylaxis in response to hazelnut.185,200 In contrast, Cor a 1 was associated with mild reactions. When symptoms like atopic dermatitis and pollen allergy were combined with PAMD@, the highest risks for symptoms were found when IgE to both Cor a 14 and walnut were present. Another study found that PAMD@ allowed for more accuracy in predicting severe hazelnut-associated symptoms in patients with the pollen-food syndrome, but was poor in peanut allergy diagnosis.201
Walnut
As noted above, 2S seed storage proteins were recently identified as eliciting cross-reactivity between walnut and hazelnut.199 Giovannini et al. showed that the presence of Jug r 1, Jug r 2, or Jug r 3 sIgE was associated with preceding anaphylaxis,185 indicating that sensitivity to these allergens should be considered a risk factor for anaphylaxis.
Wheat
ImmunoCAP and immunoblot were used for PAMD@ in sera from children with a suspected wheat allergy.202 Of these children, 44 were reactive and 62 nonreactive to the food challenge. Although the authors confirmed α-, β-, γ-, and ω-gliadins and low-molecular-weight glutenin as major allergens in terms of IgE binding, these allergens were not able to discriminate the tolerant from the challenge-positive children. In contrast, a Scandinavian study found that these same compounds, and the dimeric alpha-amylase inhibitor AAI 0.19 (Tri a 28), were clinically relevant antigens that helped to identify reactive patients among 108 children with a suspected wheat allergy.203 Higher levels of sIgE to wheat or ω-5 gliadins also predicted anaphylaxis to wheat in Japanese infants204 and likely predict reactions to food challenges.205 This is significantly associated with WDEIA.206
Tri tu 14 (from durum wheat) shares about 50% amino acid identity with Tri a 14 (from common wheat), leading to a potential danger for cross-reactivity in WDEIA. Further in vitro cross-binding was found to Pru p 3 from peach.207
Wheat allergy represents not only a food allergy but also a relevant occupational allergy in bakers' asthma. When 101 bakers (40 German, 37 Dutch, and 24 Spanish) with wheat flour allergy were tested by CAP-FEIA combined with inhibition by wheat or rye flour and grass pollen extract, Tri a 27 and Tri a 28 were found to be the most important IgE binders.208 The highest diagnostic accuracy, however, was achieved with a combination of 5 allergens, which allowed discrimination of occupational sensitization from exposure to homologous pollen.
Buckwheat
Buckwheat (Fagopyrum esculentum and F. tataricum) is not in the same family (Poaceae) as wheat and is used as an alternative to wheat. However, it also poses a risk for food-induced anaphylaxis. Buckwheat allergy is predicted by concomitant sensitization to legumin, Fag e 2, and Fag e 5.209 IgE to Fag e 3 correlates with a risk for anaphylaxis.210 Interestingly, horses may also develop IgE to buckwheat from their diet.211
Rosaceae fruits
Peach allergy in northern and southern Italy differs in terms of the molecular allergens relevant for IgE binding. In the north, pollen-derived PR-10 allergens are the primary sensitizers, and anti–Pru p 1 sensitization produces weaker symptoms. In the south, the nsLTP Pru p 3 was long regarded as the major sensitizer as well as an elicitor of allergic reactions. In a study on 133 Pru p 3–positive patients, a south-north gradient in sensitization patterns to peach molecules was reported.98 Additionally, the authors found a significant correlation between the levels of IgE and symptom severity and were able to differentiate symptomatic from non-symptomatic patients. A suspected cross-sensitizing capacity of cypress to peach could not be confirmed.98,212 A recent study changed this paradigm by reporting that Pru p 3 sensitization is a marker for severe reactions in central Europe as well as southern Europe.213 Recently, a new cross-reactive pan-allergen has been characterized that belongs to the gibberellin-regulated protein (GRP) family. BP14 from cypress pollen, Pru p 7 from peach, and Pun g 7 from pomegranate are some representative GRP molecules.214, 215, 216, 217
Kiwi
Among 59 people in Sweden with peanut allergy, 39% also reported symptoms to kiwi, likely elicited by cross-reactivity between the 11S and 7S globulins present in both kiwi and peanut, and co-sensitization to the kiwi 2S albumin Act d 13.218 Additional reactivities to nuts and legumes other than peanuts were also reported.218
Fish
Parvalbumin is the most frequent cause of fish allergy and has been implicated in cross-reactivity among fish species. Parvalbumin is heat stable, so cooking is not expected to affect reactions to it in sensitized patients.74 Kuehn et al. identified 2 additional allergens: beta-enolase and fructose-bisphosphate aldolase.219 Among 62 patients, 60% showed IgE to these new allergens. Their specific implications for risk assessment and cross-reactivities still need to be evaluated, but recent papers support the high predictive value of PAMD@ for risk assessment in patients with fish and shellfish allergies.220
Shellfish
To identify the clinical relevance of sensitization to shrimp allergens, IgE to recombinant shellfish antigens and IgE and IgG4 to peptides were determined in 86 patients with a positive SPT to shrimp; a group co-sensitized to HDM or cockroach were used as controls.221 All subjects underwent DBPCFC. Positive challenges to shrimp were more frequently associated with IgE to tropomyosin and, especially, to sarcoplasmic calcium-binding proteins. The control subjects only showed positive challenges with arginine kinase and hemocyanin. IgE to HDM Der p 10 is associated with clinically relevant cross-reactive syndromes to shrimp,130,131 as discussed in more detail in the Mite section below. However, high shrimp-sIgE levels and high IgE to Der p 10 were revealed as specific risk markers for genuine shrimp allergy that is independent of HDM sensitization.222 Recently, several other molecules associated with IgE-mediated reactivity to crustaceans have been isolated in allergic patients.223 Arginine kinase, sarcoplasmic calcium-binding protein, myosin light chain, myosin heavy chain, troponin C, glyceraldehyde-3-phosphate dehydrogenase, triosin phosphate isomerase, enolase, hemocyanin, Ca++ ATPase of smooth endoplasmic reticulum, and pyruvate kinase are the most relevant.224
Meat
The oligosaccharide antigen alpha-gal has been identified as the causative allergen in an unusual delayed-type yet IgE-mediated allergy to meat.49 Alpha-gal epitopes were detected on porcine peptidases and on the angiotensin-I-converting enzyme and aminopeptidase N from pork kidney. A positive basophil degranulation assay with IgE using serum samples from 59 meat-allergic patients associated these epitopes with a delayed type of anaphylaxis.225 Alpha-gal–containing allergens from beef can be either heat labile (triosephosphate isomerase, carbonic anhydrase 3, lactate dehydrogenase) or heat stable (creatine kinase M-type, aspartate aminotransferase, beta-enolase and alpha-enolase).226 The numbers of reported cases have continued to increase and are now reported from sites across the world and are closely associated with tick bites.113 Salivary prostaglandin E2 from ticks may serve as an adjuvant, increasing sIgE to alpha-gal.227 Tick feeding induces the production of alpha-gal–containing proteins in tick salivary glands, even after feeding on human blood not containing alpha-gal, implying that the alpha-gal–containing proteins are inherent tick proteins, not transferred via a prior blood meal.228 Feeding causes increased tick galactosyltransferase expression in the midgut and increased alpha-gal levels throughout the tick.229 Alpha-gal is also present also on cat IgA Fel d 5 and IgM Fel d 6; however, cross-sensitization between cat antigens and alpha-gal is not clinically relevant.160 Alpha-gal is not the only meat allergy. For example, although rare, poultry allergy has been observed, both as a primary allergy and as secondary sensitization associated with Gal d 5 sensitization.230 In addition, a rare cat-pork syndrome related to sensitization to Fel d 2 has been described.231
Latex
PAMD@ has been applied to occupational allergies, including natural rubber latex allergy.232 While Hev b 1 and Hev b 3 are associated with multiple surgeries and severe perioperative anaphylaxis, Hev b 5 and Hev b 6.02 are associated with glove usage and urticaria, angioedema, rhinoconjunctivitis, and asthma. Sensitization to latex compounds was analyzed in 82 patients with occupational allergy to latex and affirmed by specific inhalation test, showing that especially high IgE levels to both rHev b 5 and rHev b 6.01 or 6.02 significantly predicts a bronchial response.233 When patients identified as sensitized by microarray and symptomatic patients were tested on Hev b 1, 3, 5, 6, 7, 8, 9, 10, and 11, patients sensitized to only Hev b 8 were likely to be asymptomatic.
About half of patients sensitized to latex demonstrate allergic symptoms related to food intake, particularly with fresh fruits, in a condition known as the latex-fruit syndrome. Avocado, banana, chestnut, and kiwi are the most frequent inducers. Hev b 2, Hev b 6.02, Hev b 7, Hev b 8, and Hev b 12 have been described as the responsible components.74
Parasite allergy
Although the natural function of IgE has been proposed to be a defense against parasites, high parasite IgE levels may be associated with asthma and allergy. Therefore, allergens from worms may be useful for diagnosis. A study on Ascaris allergy (versus mite allergy) in the tropics investigated IgE to Asc s 1 (ABA-1), Asc l 3 (tropomyosin), and glutathione transferase.133 Even though the tropomyosins Asc l 3, Blo t 10, and Der p10 are highly homologous, sensitization to Ascaris turned out to be independent of co-sensitization to the HDM tropomyosins. However, high levels of IgE to species-specific Blo t 5 and Asv s 1 and to the tropomyosins Asc l 3, Blo t 10, and Der p10 predicted more-severe asthma.134
A major new allergen, Ani s 11-like protein, was found in the parasite Anisakis. This allergen is greatly resistant to in vitro digestion and heat,234 which may be important when the allergen is encountered orally. Ani s 11-like protein was recognized by 78% of 37 tested Anisakis-allergic patients. In 2 groups of Italian and Spanish patients, one with Anisakis allergy (n = 32) and the other asymptomatic (n = 77), the prognostic value IgE to nAni s 4, rAni s 1, rAni s 5, rAni s 9, and rAni s 10 was only moderate.235
Hymenoptera and insects
PAMD@ is helpful to discriminate between true allergy to different venoms and cross-sensitization, allowing physicians to correctly identify the risk and to optimize venom selection for immunotherapy.5,74 PAMD@ is also useful in identifying patients with Hymenoptera venom-induced anaphylaxis who have negative test results to whole-venom extracts.236,237 PAMD@ with CCD-free allergens and CCD markers is always preferable. PAMD@ can also discriminate between primary sensitization and cross-sensitization in patients who have positive responses to more than one whole extract, allowing the specialist to choose the most suitable venom for venom immunotherapy (VIT) and to avoid treatment with double VIT.
More work has to be carried out to assess whether PAMD@ may detect biomarkers for VIT efficacy, as well as the risk of side effects and relapse after stopping the treatment.
Diagnosis of venom allergy can be difficult because patients who have not identified the stinging Hymenoptera may be sensitized to multiple venoms. Double positivity to the venoms of Apis mellifera and Vespula spp. is found in 25–40% of cases and may be due to true double sensitization or to cross-binding between epitopes present in both venoms (hyaluronidase Api m 2 in bee and Ves v 2 in wasp, dipeptidyl peptidase IV Api m 5 and Ves v 3, or vitellogenin Api m 12 and Ves v 6) or to CCD.238,239
Api m 1 (phospholipase) and Api m4 (melittin) are the most relevant allergens of bee venoms. Other HBV allergens are contained in the venom in lower quantities: rApi m 2 (hyaluronidase), rApi m 3 (acid phosphatase), rApi m 5 (dipeptidyl peptidase V), and rApi m 10 (icarapin). Despite the low abundance of these allergens,240 patients allergic to bee venom often have a broad sensitization profile to them. The combination of 2 allergens (Api m 1 and Api m10 10) allows diagnosis in 86.8% of cases, and a combination of 6 allergens (Api m 1-2-3-4-5-10) has a sensitivity of 94.4%.
Patients with allergy to venom from Vespula spp. are sensitized mainly to Ves v 1 and Ves v 5. The presence of sIgE to the combination of these 2 recombinant allergens allows the identification of >95% of patients allergic to Vespula.241,242 A combination of rApi m 1, rApi m 10, Ves v 1, and rVes v 5 was found to be useful for identification of the culprit venom in patients allergic to bee and wasp.243
Yellow jacket venom (YJV) also contains the dipeptidyl peptidase Ves v 3, which shows high homology to Api m 5 and induces sensitization in 50–62% of YJV-allergic patients. The hyaluronidase Ves v 2 is reported in 5–20% of the YJV-allergic patients and seems to be of limited relevance in the sensitization. Aedes communis reactivity has been associated with bee venom hypersensitivity in a large group of Italian individuals reporting immediate reactions after mosquito bites.244
The allergen composition of Polistes dominulus venom (PDV) is very similar to that of YJV, and the most important allergens are Pol d 1 (phospholipase A1), Pol d 3 (dipeptidyl peptidase IV), and Pol d 5 (antigen 5). Pol d 3 is a very good reagent, but it cross-binds with both Apis and Vespula dipeptidyl peptidase IV.245 PDV also contains a serine protease (Pol d 4) and a hyaluronidase (Pol d 2), for which no data about clinical relevance are available so far.245
Cross-sensitization between allergens of 2 species often induces diagnostic difficulties.246, 247, 248 In cases of difficult interpretation between Vespula and Polistes sensitizations, the use of Ves v 5 and Pol d 5 in the diagnostic panel seems to be helpful, provided that the difference of sIgE levels between the 2 molecules is particularly large, with the value of one at least double that of the other.249, 250, 251, 252 In southern Europe, Vespula-Polistes double sensitization is more frequent than Vespula-Apis double sensitization.253
IgE to CCD can also explain multiple positive in vitro results, and serum determination for CCD (bromelain or MUXF3) allows greater diagnostic accuracy.254 Of note, ImmunoCAP cellulose displays CCD epitopes that may cause false-positive test results in patients with elevated anti-CCD IgE antibody levels, even when recombinant molecules are evaluated.255
Only 83.4% of patients with systemic reactions to YJV could be diagnosed by using the conventional YJV ImmunoCAP, whereas sensitization was confirmed in 96% of patients using the individual allergens Ves v 1 and Ves v 5.256 Of the patients with negative ImmunoCAP results, only one tested positive for Ves v 1, but 84.4% showed a positive test for Ves v 5, suggesting a shortage of available Ves v 5 epitopes in the whole-venom preparation despite the abundance of this allergen in YJV. Since 2012, the YJV ImmunoCAP has been spiked with recombinant Ves v 5, increasing the diagnostic sensitivity to 96.8%.257
In HBV, only 1 of 5 relevant allergens (Api m 1) is present in the venom in substantial amounts, and only trace amounts of the others are detectable. Moreover, an intrinsic instability and rapid degradation of the important major allergen Api m 10 has been reported.253,258 (Fig. 2).
Fig. 2.
A practical algorithm emplying allergen molecules for the identification of sensitization to hymenoptera. HBV: Honey bee venom; VYJV = Yallow Jacket venoms; PDV: polisted dominulus venom; CCD: Cross-reactive Carbohydrate Determinants
Mastocytosis and elevated tryptase indicate a higher risk for venom-induced anaphylaxis due to lowered allergen thresholds. When 53 patients were analyzed for IgE to Ves v 1, Ves v 5, Api m 1 to 4, and Api m 10, it was necessary to reduce the threshold of IgE detection to 0.1 kUA/L in the Immulite assay in order to enhance the sensitivity of the assay and allow diagnosis of more patients in this risk group.259 In wasp venom–sensitized patients, sIgE to rVes v 1 together with rVes v 5 facilitated the correct diagnosis in 94% of 148 patients.250 In the 13 patients investigated, phospholipase was a major allergen, in addition to Ves v 1 and 5, and rPol d 1 and 5.260 Interestingly, treatment with one allergen resulted in a reduction of IgE to the second in cases of double sensitization. Api m 4 was found to be a useful marker to identify bee venom allergy,50,261 and sensitization to Api m 10 poses a risk for failure of bee venom AIT. Consistent with this, Api m 10 was underrepresented in 3 of 5 therapeutic HBV commercial preparations.50
Of note, the Sol i 1 allergen from fire ant (Solenopsis invicta) venom has homology with yellow jacket phospholipase.262 Also, the Asian needle ant (Pachycondyla chinensis) is a cause of anaphylaxis. Jeong et al. recently recombinantly expressed Pac c 3 from needle ants and showed its cross-binding with Ves v 5 by ImmunoCAP.263
Interpretation of PAMD@ tests
The interpretation of PAMD@ tests may be challenging, even for the experienced and trained allergist. Some important premises should be taken into account when interpreting these results.3
-
1
Measurement of serum sIgE to individual allergens detects sensitization, NOT allergy.
Identification of sIgE, either bound to mast cells (SPT) or in serum, detects sensitization, a condition necessary but not sufficient to make the definitive diagnosis of IgE-mediated allergy. Whether the sensitizing agent is responsible for the symptoms (allergy) should be evaluated with a careful clinical history and, when necessary, by challenge tests. A raised allergen sIgE response in the absence of a history of allergic symptoms or in the context of a negative provocation test should be considered clinically irrelevant.264
-
2
PAMD@ and “traditional” tests are complementary
Molecular allergen IgE assays should not be interpreted as an alternative to allergen extract–based assays, but rather as complementary.265 The interpretation of discrepancies between the 2 approaches has been comprehensively addressed in recent publications (Table 3).121,122,266
Table 3.
Interpretation of non-concordance between allergen extract and allergen molecular IgE assay results
| A. Positive specific sIgE to whole-allergen extracts but negative to its relevant components |
| Possible explanations: |
|
| B. Positive specific IgE to molecules but not to the relevant whole-allergen extract |
| Possible explanations: |
|
When sIgE to the allergen extract is present, but sIgE to its individual molecules is negative, the clinician should consider the possibility that the extract's molecules that are responsible for the sensitization are not included in the molecular assay (see guidance in the Informatics section below). Analytical sensitivity should also be considered in cases of discrepancy. Note that singleplex methods are preferred over multiplex assays in the case of low serum total IgE or sIgE values between 0.1 and 1 kUA/L.12 However, the presence of contaminants from other sources (e.g., HDM molecules within dog extracts) can also affect the reliability of the test.267
In cases when sIgE to an allergen extract is present but to its genuine components are negative, sensitization to minor allergenic molecules or CCD determinants responsible for cross-sensitization should be ruled out.
The specialist has to be aware that not all allergens of a given allergenic source are available for sIgE tests or have been characterized. The list of important allergenic molecules cloned or purified and introduced for diagnostic purposes, although rapidly increasing, is still incomplete.74 New relevant allergenic molecules, such as Der p 23 for D. pteronyssinus268 and Pru p 7 269 for peach have been discovered and made available as diagnostic tests only recently.
Conversely, when no sIgE is detected to an allergen extract, but sensitization to any of its individual molecules are present, one should consider that sensitizing components are missing or in low abundance in the extract. Sometimes these discrepancies are quantitative, and sIgE levels to the allergen extract are lower than for the individual allergens when components are in low abundance in the extract.
-
3
Interpretation of the results based upon the source of the allergen components
Although recombinant allergens expressed in E. coli lack glycosylation, the natural purified allergens have the same sugars as their natural counterparts.270 Highly glycosylated allergens induce the production of sIgE against the sugar moiety (CCD), which can be responsible for cross-reactivity. Six highly glycosylated allergens are in their natural purified form in the ImmunoCAP ISAC: walnut nJug r 2, Bermuda grass nCyn d 1, Timothy grass nPhl p 4, Japanese cedar nCry j 1, Arizona cypress nCup a 1, and plane nPla l 2.271 It is not possible to determine whether IgE to these 6 allergen components is directed to the protein or the carbohydrate side chain, so ruling out the presence of sIgE against CCD is important, especially when other markers of genuine sensitization to the same source are lacking.64 Of note, the CCD marker MUXF3 in ISAC seems to be less sensitive than the same test on the standard ImmunoCAP assay, and points to the need for a better marker of CCD sensitization.76 The ALEX microarray added a CCD inhibitor to help overcome this problem.
-
4
Interpretation based upon local molecular profiles
Patterns of sensitization vary depending on the geographical area. The knowledge of local molecular epidemiology is essential for guiding allergists in choosing the components to test in their population and to interpret the results properly. For example, Ole e 1 would be a marker of genuine sensitization to olive pollen in southern Spain but a marker of genuine sensitization to the ash tree in northern France. Interestingly, it has been shown that human changes to the local environment can change allergen-specific sensitization profiles.272
-
5
Interpretation of unexpected results
The generation of an extensive IgE sensitization profile is both an advantage of multiplex analysis and also one of its main pitfalls, because detecting IgE to unexpected allergens may sometimes confuse the clinician if there is no suggestive clinical history available before the test. This may be the case with insect venom allergy.273 Due to the high prevalence of insect venom sensitization, which occurs in up to 15% of the population, nonspecific screening would generate an abundance of clinically irrelevant results and serve to unsettle patients and their physicians. No indications are currently available on how to effectively manage these cases, but it seems reasonable to act in the same way as with other clinically irrelevant sensitizations to food or respiratory allergens detected with “traditional methods”, such as SPT, that do not need any intervention: follow the patient to detect possible future reactions.
A positive aspect is that the detection of silent sensitivities may give the allergist the chance to investigate other hypersensitivities and to alert the patient of possible risks.274 In the case of sensitization to allergens responsible for food-pollen cross-reactive syndromes, the clinician should re-interrogate the patient for symptoms upon consumption of foods containing those allergens, but sensitization itself should not drive avoidance measures.275 Importantly, an elimination diet should be recommended only if food allergy due to cross-reactions is based on a clear history or a clinical observation after oral provocation tests.41
-
6
Interpretation of low- or high-risk markers and component combinations that are related to different risk phenotypes
Generally, allergens resistant to heat and digestion, like seed storage proteins or LTPs, trigger more-severe allergic reactions and have been proposed as markers for severe reactions. Again, the specific relevance of each marker of severity will vary according to local molecular profiles. In contrast, Bet v 1 homologs and profilins are labile allergens that typically induce local symptoms such as oral allergy syndrome and have been proposed as markers of mild reactions. However, the clinician needs to be aware that there may be exceptions to this rule in situations when large quantities of allergens are consumed, cofactors are present, or in regions with a large quantity of pollen exposure. Examples of this are severe anaphylactic reactions reported in patients monosensitized to Bet v1 homologs when drinking apple juice after performing exercise96 or severe reactions in patients monosensitized to profilin in areas with significant overexposure to grass pollen.97 It has also been reported that patients with high Bet v 1–specific IgE levels frequently suffer from oral allergy syndrome.276
In addition, component combinations can define phenotypes with different clinical expression. It has recently been reported in an Italian cohort that sensitization to more than 5 nsLTPs out of the 8 present in ImmunoCAP ISAC is related to a higher incidence of food-induced systemic reactions, whereas sensitization to PR-10 or profilin pan-allergens is associated with milder symptoms.170 According to this, the assessment of IgE sensitization to 3 key allergens—Bet v 1 homologs, LTPs, and profilins—is of paramount importance for the interpretation of PAMD@ to fruits and vegetables, especially in the Mediterranean area.277
Informatics support for PAMD@
IgE results, whether to whole extracts or to components, can be classified as primary or caused by cross-sensitization. Some allergens are predominantly primary, whereas others are predominantly encountered as pan-allergens that cause cross-sensitization. IgE sensitization is not synonymous with allergy, and significantly elevated IgE levels may be encountered in people who do not have clinical allergy. Such “innocent” sensitization is common. Some allergens are almost always clinically relevant (e.g., Ves V 5), and some are almost always clinically irrelevant (e.g., CCD), but many allergens may be associated with high levels of IgE with or without clinical reactivity. It is thus imperative that the interpretation of laboratory results to multiple allergens endeavors to distinguish between clinically irrelevant cross-sensitization and clinically relevant cross-reactivity.
Multiplex diagnosis based on allergen microarrays consists of the evaluation of more than 100 different components belonging to inhalant, food, latex, and Hymenoptera allergen sources. The number of interactions between the different components can be extremely high, and for this reason, the interpretation of allergen microarray results can sometimes be complex. In the 112-component version, ImmunoCAP ISAC producers improved the interpretation of component results by the use of Xplain, specific software that arranges the components into different families and adds relevant information for the interpretation of the results. The other commercially available allergen array, ALEX by MADx, offers a link to a dedicated expert system.76 The support of artificial intelligence tools has allowed new opportunities for interpretation and introduced new concepts to the diagnostic approach. Indeed, allergen arrays seem to be redundant to some extent; for example, the number of profilins and LTPs seem to be higher than needed. Sensitization to many homologous components may be more indicative of clinically relevant allergy than sensitization to only a few. Along this line, the empirical rule can be used that if the number of positive components is >40% of the total number of components of a given family, the patient can be considered sensitized to the whole family of homologous molecules. Also, expert systems can identify the primary sensitizer in a family of homologous components as the member with the highest IgE score. In the case of ISAC, where only components are present, an expert system can have other routines implemented to address discrepancies between the results of SPT (or sIgE) with an extracted allergen and the ISAC results, such as a positive SPT result but a negative result for specific components derived from that allergen. The expert system can evaluate whether components that belong to other allergen sources but are well known to cross-bind to components in the whole allergen are also positive. So, for example, if Ambrosia a. is positive in SPT but Amb a 1 is negative, other cross-binding components should be evaluated, such as profilins, PR-10, and calcium-binding proteins, which are all well represented in Ambrosia but are not present in ISAC. If at least one of these cross-binding components is positive, the discrepancy is considered less relevant. By using this approach, the number of apparent discrepancies is reduced significantly. Expert systems have the capacity to evaluate the ratio between primary and cross-binding components or pan-allergens.278 At least for inhalants, patients who are sensitized to primary components respond better to AIT than people sensitized to cross-binding components, at least in retrospective studies.279,280 In the case of ALEX, the presence of both whole-allergen extracts and components on the same array, and the larger number of allergens available, reduces the risk of discrepancies.
Considering the intrinsic complexity of allergen arrays, mathematical and statistical support for the interpretation of data from allergen microarrays seem to be particularly relevant now. For example, in a specific environment represented by Hymenoptera sensitization of horses, Marti et al.281 described the use of advanced statistical methods to identify relevant sensitizations and to validate the experimental approach. These techniques are particularly efficient when variables are more frequent than samples, allowing the description of the features of the microarrays in a trustworthy manner. In another approach, Prosperi et al.282 used machine learning to link the results of an allergen microarray to clinical symptoms. After validation, the mathematics used demonstrated a reasonable discrimination ability for asthma, rhinoconjunctivitis, and wheeze but not for eczema, perhaps due to patients with atopic dermatitis having multiple clinically irrelevant sensitizations. The results of these machine learning experiments will be extremely useful to allergists and also to the laboratories in charge of singleplex and multiplex diagnostics. The identification of certain patterns of sIgE positivity will automatically validate the results of the assay if some clinical information about the patient is known.
A similar approach was followed by Simpson et al.116 By using the latent variable modeling statistical approach, they identified 3 different patterns of sensitization to multiple allergens and clinical symptoms at age 11. The pattern associated with plant proteins was found in patients suffering from hay fever, and that associated with mite components was found in patients with asthma and hay fever. The third pattern, represented by polysensitized patients, was found in asthmatic subjects. The authors noted that eczema was not associated with a given pattern, but this seems reasonable because sIgE are rarely positive in patients with allergic skin diseases.
Molecular diagnosis and allergen immunotherapy
PAMD@ represents a useful tool to distinguish clinically relevant and/or primary sensitizations from cross-sensitization in polysensitized patients in cases where traditional diagnostic tests and clinical history are unable to identify the relevant allergen(s) that should be used for AIT. AIT is an expensive and time-consuming treatment (3–5 years) and requires strict adherence. It involves the administration, either subcutaneously or sublingually, of an extract of the allergen(s) responsible for clinical symptoms; this induces tolerance and decreases symptoms and the need for drug intake after allergen exposure.2,283,284 Tolerance is achieved through complex immune modifications involving both humoral and cell-mediated immunity.285, 286, 287
AIT is, by definition, “allergen-specific”, and it modifies the immune response against the allergen for which the desensitization is performed. As a consequence, a precise etiological diagnosis is required for the prescription of AIT, and the sensitizing allergen must be unequivocally identified. Usually, a detailed clinical history and the standard extract-based IgE testing (SPT and/or in vitro sIgE) is sufficient to identify the relevant allergen(s).267,288,289 This is especially true for allergy to plants with well-defined pollen seasons that possibly do not overlap, so that symptoms can be readily linked to the season. However, the complexity of diagnosis increases when a patient displays polysensitization on the traditional diagnostic tests (based on allergen extracts) and the clinical history and history of allergen exposure do not help in clearly identifying the relevant allergen(s). This may occur in a relatively high proportion of patients.290,291 In such cases in the United States, the vaccine for AIT is prepared by mixing all of the allergens a patient tests positive for.292 Mixing numerous allergens appears to achieve good clinical efficacy; however, there may be an inability to identify the responsible allergen in the case of adverse events.293
Certain structural or enzymatic proteins (e.g., profilins, polcalcins, LTPs, PR10, tropomyosins) are highly conserved across a wide variety of species. For instance, a patient who is primarily sensitized to grasses may also test positive for birch with SPT. This cross-sensitization occurs because the birch extract used in SPT contains profilins (e.g., Bet v 2), which are largely similar to the allergens in grasses (e.g., Phl p 12). Indeed, the use of recombinant or purified allergens allows the discrimination of primary and/or clinically relevant sensitizations and cross-sensitization. In the example mentioned above, a patient with sIgE antibodies against Phl p 1 and Phl p 5, the major allergens in grass, but no sIgE to Bet v 1, the major allergen in birch, is truly sensitized to grass, and not to birch. If sIgE antibodies to Phl p 12 (a profilin) were also detected, profilin sensitization would probably be responsible for the positive SPT result obtained with birch extract, which contains profilins as well. Thus, using the knowledge gained through the identification of allergens, AIT would be prescribed for grass only.
Similarly, if a patient is sensitized to a whole HDM extract, but their IgE antibodies are specifically directed against Der p 10 (tropomyosin) and not to Der p 1, 2/Der f 1, 2, AIT for mites should not be prescribed, because mite extracts mainly contain Der p 1, 2/Der f 1, 2 and have variable or low amounts of Der p 10. Moreover, it has been shown that patients sensitized to important allergens besides Der p 1 and Der p 2, such as Der p 5, Der p 7, Der p 21, and Der p 23 do not benefit from AIT when these allergens are not present in an immunogenic form in the vaccine.136 PAMD@ can also improve the selection of patients for Hymenoptera venom AIT.243 Sensitization to the major allergens of the honey bee (Api m 1) and yellow jackets (Ves v 5/Ves v 1) may help discriminate between true double bee and wasp sensitization and cross-sensitization due to CCDs.294
Most commercial allergen extracts used in AIT are well standardized for major allergens but contain only minimal or variable amounts of minor allergens.295,296 Successful AIT is dose-dependent; thus, it can be hypothesized that its therapeutic success might be associated with the concentration of the allergens the patient is sensitized to, and patients with sensitization to minor allergens alone may not benefit from AIT. In HBV, Api m 1 is the only major allergen that is present in substantial amounts, and all other relevant allergens make up only 0.6%–2% of the venom dry weight.253 In contrast, in YJV, the 2 most relevant allergens, Ves v 1 and Ves v 5, are present in relatively high and nearly equimolar amounts, which implies that the differences in the allergen content between the 2 venoms might be one reason for the higher success rate of VIT with YJV than with HBV. A multicenter study demonstrated that a predominant sensitization to Api m 10 (>50% of sIgE to HBV) represents a relevant risk factor for treatment failure.50 Another study reported that patients receiving a 2-year course of AIT with either birch or grass pollen had a much more favorable outcome with AIT if sensitization to the marker allergens of birch or grass pollen were detected, as compared with patients sensitized to only minor or homologous allergens.279 Therefore, it is necessary to have accurate methods to investigate the exact molecular composition of the different AIT extracts for a “tailored therapy” based on the patient's sensitization profile.
The literature concerning the practical role of PAMD@ in prescribing AIT is rapidly increasing since this was envisaged about 10 years ago.297 In a cross-sectional study involving more than 500 Italian polysensitized patients, the number of AIT prescriptions more than doubled after use of the ISAC assay relative to the number of prescriptions after standard diagnostic tests (although the specific allergens were not reported).91 Two Spanish studies evaluated the role of PAMD@ in AIT prescription in patients with seasonal symptoms who proved positive to grass and pollens. The first10 involved 175 patients, and after the molecular test, the prescription of AIT was changed in about 50% of them. The second study298 had a similar design but involved more than 1200 patients with allergic rhinitis who had positive SPT to olive and grass. Again, the AIT prescription was changed in more than 50% of cases because the identification of cross-sensitization allowed more targeted treatment.
Several other studies have reported similar findings that about half of AIT prescriptions change after PAMD@ data are available. Sastre et al.299 analyzed the AIT prescriptions for 141 pollen-sensitized patients. The prescriptions before and after PAMD@ agreed in only 46% of patients, with the best agreements reached for olive, grass, and cypress. In a prospective study with 476 patients with polysensitization to inhaled and food allergens,175 the molecular diagnostic approach changed the prescription of AIT in about 50% of patients, which also decreased the cost of AIT. Martinez-Canavate Burgos et al. observed in 281 children with double sensitization to olive and grass,300 and Del-Rio Camacho et al.301 observed in 70 children with various sensitizations, that the AIT prescription was changed in more than 50% after PAMD@; Del-Rio Camacho et al. also noted that the proportion of AIT prescriptions for a single allergen increased from 18% to 51%. Similarly, the use of PAMD@ changed prescriptions in 42%–48% of 651 children in an Italian study.302 The question has also been raised of whether AIT based on molecular diagnosis can be started earlier in life.141
Although the rationale for prescribing AIT based on information about the specific components responsible for the sensitization is to increase the efficacy of the AIT, this specific issue has not been prospectively addressed to date. Two retrospective post-hoc studies on HDM-AIT efficacy based on PAMD@ have reached discordant conclusions. Tavar et al.303 found no association between the clinical efficacy of AIT based on HDM and sensitization to mite allergens, whereas Chen et al. concluded that the use of PAMD@ to select patients with HDM allergy for AIT may enhance treatment success.136 Thus, prospective well-designed studies are needed to assess the impact of PAMD@ in allergen immunotherapy efficacy.
The molecular sensitization profile could also affect the safety of AIT. In a recent study involving 200 patients receiving AIT by subcutaneous injection, the occurrence of adverse events was greater in those patients sensitized to Phl p 1 and Phl p 5 (and Phl p 12) than in patients sensitized to Phl p 1 only.304,305 During the up-dosing phase, VIT with HBV is less safe than VIT with vespid venom.306 A study with a very small population (n = 31) showed that Api m 4 sensitization might be a useful marker to identify a particular phenotype of patients with HBV allergy with a higher risk of systemic reactions during the up-dosing phase of immunotherapy.261 No data are available on the correlation between sensitization profiles to vespid allergens and the severity of the disease, or to side effects or therapeutic outcome of VIT. However, Api m 10 sensitization has been linked to the risk of therapeutic failure.252
In principle, a detailed identification of molecules against which IgE antibodies are directed would allow “tailored” AIT based only on allergens with a documented IgE response. In practice, this does not seem feasible. First, the number of possible combinations of sensitization profiles is too large when single allergenic sources are also considered307; second, recombinant vaccines do not always perform better than traditional allergen extracts308; and third, each single recombinant/purified allergen would need to be individually tested and registered, which carries a substantial financial burden for manufacturers. Thus, the reality of patient-tailored AIT is still a distant prospect.309 However, molecular-based algorithms have been proposed that would optimize the prescription.310, 311, 312 These algorithms all incorporate the idea that detecting sensitization to genuine components is essential.313
Although AIT for pollen-associated food allergy has shown beneficial effects in some studies, particularly for oral symptoms, other clinical trials have not shown similar outcomes; thus AIT cannot be recommended as a treatment in these cases and should be considered only when respiratory symptoms are present.314, 315, 316, 317, 318 However, there are some fascinating potential applications in the field of immunotherapy: a research group in Vienna has recently generated mimotopes using a monoclonal antibody (BIP3) recognizing high-molecular-weight glycoproteins in birch and mugwort pollens, celery, and Apiaceae spices (anise, fennel, coriander, and cumin), which are responsible for the “celery-mugwort-birch-spice syndrome”. These mimotopes mimic the BIP3 epitope relevant to Api g 5 (the celery allergen mainly responsible for the syndrome) and, because of their good immunogenicity, they could potentially be used as Api g 5 surrogates for hyposensitization.319
Another interesting and innovative use of the allergen microarray is the monitoring of AIT. Indeed, it has been observed320 that allergen microarrays are useful for monitoring the development of allergen-specific IgG responses during AIT, both against the allergen present in the AIT vaccine and against cross-reactive allergens. This application of the technique may finally offer a general-purpose tool for monitoring the immunological effects of AIT, resulting in better control of the treatment and a better understanding of therapeutically positive and negative results. For example, when IgE and IgG to peanut Ara h 1, Ara h 2, Ara h 3, Ara h 8, and Ara h 9 were investigated in a multiplex analysis in 33 patients undergoing peanut sublingual immunotherapy,321 successful desensitization was associated with significantly lower IgE levels to Ara h 2 and 3. PAMD@ may thus be used to monitor immunotherapeutic strategies in food allergy and has great potential to specifically identify those allergens that are most relevant for AIT of the individual patient. This strategy is fully in line with the concept of precision medicine in allergy.322 These data were further supported by an article323 that demonstrated that pretreatment sIgE to allergen components appears to determine the induction of IgG4 in the up-dosing phase. Induced IgG4 seems to suppress IgE levels on ISAC, resulting in a marked decrease in ISAC-measured sIgE levels after up-dosing of subcutaneous immunotherapy. The authors conclude that the decrease in ISAC IgE levels can be used to monitor the blocking effect of non-IgE antibodies induced by allergen-specific immunotherapy.
In conclusion, PAMD@ is certainly of help in better refining the AIT prescriptions in individual patients, especially when polysensitization patterns from standard diagnostic tests are difficult to interpret.312,324, 325, 326, 327 The availability of multiplex IgE tests remains limited and requires a specialist approach. They are therefore currently used as a third-line diagnosis focused on polysensitized patients and patients with pollen-food syndromes.
Unmet needs
Advances in the identification of allergens at a molecular level are indisputable and are a huge step forward in both the in vitro and the in vivo diagnosis of allergy. By identifying specific allergens that are associated with different risk profiles, PAMD@ can help avoid exposing patients with limited oral allergy to treatment or risking a near-fatal anaphylactic reaction when challenging a patient to confirm a diagnosis. However, some points need to be elucidated:
-
1.
It must be clearly stated when PAMD@ should be used in clinical settings or research.
Some major allergens are region-specific, and different populations demonstrate different sensitizations, particularly for pollens.328 Population studies characterizing the sensitizations representative of each region are needed. Additionally, multicenter studies would be extremely helpful to reinforce the role of major allergens in coincident clinical pictures and corresponding cofactors.
PAMD@ is emerging as a useful tool for determining the variation in the concentrations of major allergens in different samples and extracts.329
-
2.
Training on the usefulness of PAMD@ should be widely available to allergists, and multiplex PAMD@ should be made more available.
Clear indications for the use of PAMD@ are suspected idiopathic or delayed-onset anaphylaxis, polysensitization when prescribing immunotherapy, pollen-food allergy, latex allergy, and Hymenoptera venom allergy.3,5 With regard to venom allergies, PAMD@ allows the identification of allergy specific to Vespula or Polistes when both are positive by either SPT or in vitro sIgE; it also allows the cause to be identified in at least 20% of cases of idiopathic anaphylaxis.
PAMD@ is also useful for providing information on primary sensitization or cross-reactivity, as well as risk assessment or potential cofactors. However, the limited availability of the assay has restricted its use and recognition, particularly in developing regions where the low-income or uninsured population cannot afford the cost of multiplex PAMD@.
-
3.
PAMD@ must be cost-effective.
PAMD@ is beneficial for the proper selection of children to undergo immunotherapy302 and of pollen-sensitized patients of all ages to receive immunotherapy prescriptions.312 The ability to determine the contributions of major versus minor allergens affects both effectiveness (AIT is more effective against major allergens) and potential adverse reactions.
Different technologies, such as single-component assays and microarrays,4 as well as in vitro and in vivo tests, must be compared to elucidate the most convenient and cost-effective choice of a diagnostic test.
A cost-benefit evaluation in the Netherlands compared PAMD@ with SPT for the diagnosis and treatment of patients with allergic rhinitis, demonstrating that diagnosis through PAMD@ increased the number of patients who fully responded to immunotherapy and reduced the cost per patient and per quality-adjusted life year.330
At present, most scientific guidelines recommend clinical evaluation and SPT as the first level of allergy diagnosis. However, other approaches have been recommended, especially in areas where concern exists about the preparation of SPT reagents in accordance with Good Manufacturing Procedures. The classic top-down method is currently widely practiced, but PAMD@ allows the use of a bottom-up approach where multiple component allergens are tested before (or instead of) testing smaller numbers of multiple-protein allergen extracts.
Presently, reagents are available for the evaluation of almost 300 different allergens on a single chip. Whether all of these results are useful in all allergic patients may be debated, but in complex patients (such as those who are polysensitized or have a food allergy), the societal cost of evaluating hundreds of allergens could significantly overcome the cost of a single multiplex allergen chip. Medical innovations and developments should include the possibility of providing these advances to everyone at an affordable price.
-
4.
PAMD@ should become part of personalized medicine.
Proper identification of the individual or cross-reactive components causing or inducing allergy is essential for providing personalized care, and for successful treatment and prevention. Physicians must have knowledge of PAMD@ to discriminate individual phenotypes and endotypes, which is essential for the appropriate management of allergy. Physicians should also help patients to understand their specific situations and participate in treatment decisions.331
PAMD@ has begun to be used worldwide. Its sensitivity and specificity have been demonstrated in clinical studies, which has triggered the desire to understand the context between the IgE profile and the risk for clinical reactivity, from allergic rhinitis and asthma to anaphylaxis.5,74 It is accepted as a first-line diagnostic tool and helps to reduce the time to diagnosis, while having the same precision as the conservative SPT-first approach.332 Overall, debate in the allergy community reveals that the initial skepticism regarding PAMD@121 has moved towards the possibility that it could replace SPT in the future.122
The path forward for PAMD@ must include education regarding its clinical utility, how to interpret results to improve the identification of clinically relevant primary and cross-reactivity rather than cross-sensitization, and its cost-effectiveness. Greater knowledge will help to make it more widely available.
Tables
Definitions and concepts:
-
•
Allergen extract: the part of allergen sources that is soluble in water or other specific solvents. Allergen extracts from different sources and different batches may vary, and the allergen contents can be both qualitatively and quantitatively different. Many proteins or other kinds of molecules without allergenic properties are contained in an allergen extract. The main problem is the presence of multiple allergens in the mixture, some which may be clinically relevant and others of which may be irrelevant, causing cross-sensitization patterns in subjects with sensitization to common components.
-
•
Allergen source: the raw material from which the allergen extract is obtained, such as pollens, animal furs, or cultures of molds. Allergen sources vary from producers and over time. Significant batch-to-batch heterogeneity has also been observed. Thus, standardization of allergen sources, and of allergen extracts, is needed.
-
•
Allergen: The molecule that expresses epitopes recognized by an sIgE.
-
•
Allergy: The presence of sensitization to one or more allergens and the presence of clinical symptoms that can be associated with that sensitization. Laboratory tests can only identify sensitization, not an allergy. The diagnosis of allergy is the responsibility of the allergist.
-
•
Basophil activation test (BAT): The test can be based on different techniques. The most frequently used are based on flow cytometry and the detection of molecules secreted from activated basophils by the use of monoclonal antibodies conjugated with a fluorochrome. The activation of basophils is, within certain limits, dose-dependent, and suggests sensitization of the patient to an allergen. Of note, basophils react not only to allergens recognized by sIgE on the surface of the cell but also to other molecules, such as drugs.
-
•
Bottom-up diagnostic approach. An approach that starts with the collection of the patient's history, followed by testing with multiplex methods to obtain a virtually complete analysis of the IgE profile. Additional tests, if necessary, are based on the information obtained.
-
•
Component: see Allergen.
-
•
Cross-binding: The ability of IgE to bind to allergens with significant sequence homology.
-
•
Cross-reactivity: Allergy caused by an allergen to which an individual is sensitized via cross-binding to the allergen which caused the primary sensitization.
-
•
Cross-reactive carbohydrate determinants (CCDs). CCDs are protein-linked carbohydrate structures. CCDs with wide homogeneity to many allergens are considered pan-allergens. CCD can be found only in natural allergens and not in recombinant molecules produced in E. coli. In patients sensitized to CCD, sIgE tests on allergen extracts may show false-positive results. The addition of a polysaccharide to the dilution buffer helps to reduce this cross-binding effect.
-
•
Cross-sensitization: Sensitization caused not though primary exposure, but due to cross-binding of IgE to allergens with significant sequence homology. Cross-sensitization may be clinically irrelevant. If it causes symptoms, it may be referred to as cross-reactivity.
-
•
Geographic distribution of allergens: Different inhalant and food allergens are detectable in different countries due to differences in the environment. For example, the weed Parietaria judaica is frequent in the Mediterranean region but is virtually absent in other areas.
-
•
Geographic distribution of sensitization: Because allergenic sources vary in different regions, the sensitization profile in these regions can also be heterogeneous. For example, although birch is a frequent allergen in northern Europe, the frequency of sensitization to birch in Mediterranean patients is probably related to the presence of trees with allergens homologous to those of birch. Sensitization to Blomia t is primary in equatorial regions, whereas in northern regions it is mainly caused by cross-binding with Dermatophagoides spp. allergens, which may or may not cause clinically relevant symptoms on exposure.
-
•
Isoforms: Molecules with homology between 60% and 95%. In nature, allergens are a mixture of molecular isoforms (homology >60%) and variants (homology >95%). In diagnostics, in general, the most represented isoforms are used. In recombinant molecules, increased heterogeneity is related to the absence of the post-translational modifications occurring in nature.
-
•Levels of allergy diagnostics:
-
oFirst level: Also called first line. In established practice, this is represented by the patient's clinical history and skin prick tests, Phadiatop, or similar tests.
-
oSecond level: Generally represented by the analysis of the patient's sensitization by sIgE assays performed on extracts from whole-allergen sources. At this level, distinguishing primary and cross-sensitization is not possible.
-
oThird level: This level is characterized by the use of either recombinant or purified natural allergens to detect the presence of IgE directed to primary, cross-binding, or pan-allergens. The third level can be based on singleplex or multiplex technology. The former is based on single allergens specifically ordered by the allergist. The latter is based on allergen arrays, where more than 100 allergens are spotted on an immunosorbent surface, and the presence of IgE to all are assayed in a single run.
-
oFourth level: This level includes western blot analysis of allergen recognized by IgE following separation on a gradient, basophil activation test, and CAP inhibition. These assays can only be performed by specialized laboratories, and certain of these procedures have not been standardized, bringing the relevance of their results in the diagnostic process into question.
-
o
-
•
Major allergens: Initially, these were defined as highly purified allergens that induced immediate skin test responses in >90% of allergic individuals. Today, in the IUIS Allergen Nomenclature, a major allergen is generally regarded as one to which >50% of allergic patients react.
-
•
Minor allergen: Initially defined as an allergen that produces a positive skin test in <20% of patients.
-
•
Molecular allergen nomenclature: A code to describe molecular allergens. As an example, take rBet v 1.0101. The first letter (r or n) is for recombinant or natural. The first 3 letters are the beginning of the genus name; here Bet for Betula. The single letter is for the species name (v for verucosa). The first number is a sequential number initially given in order of discovery, but the numbers were subsequently associated with protein families. The first 2 digits after the dot designate isoallergens. The third and fourth digits distinguish different variants of an isoallergen. (from Update of the WHO/IUIS Allergen Nomenclature Database based on analysis of allergen sequences, 2014)
-
•
Molecular allergen: see allergen.
-
•
Pan-allergens (see also cross-binding allergens): Groups of proteins that are involved in the general life processes of plants and animals and are therefore widely distributed in nature.333 Homology in amino acid sequence and high homogeneity in structure (folding) and functions results in the presence of epitopes that are shared by many different organisms, even among species that are not closely related. According to Hauser et al.,333 profilin is the only real pan-allergen; polcalcins, PR-10, nsLTP, and related molecules are defined as eurallergens (wide); expansin, pectate-lyase, thaumatins, and cupins are defined as stenallergens (tight); and alpha-amylase, 2S albumin, protein kinase, and similar proteins are defined as monallergens, being restricted to a single family of plants.
-
•
Pathogenic allergens: Allergens that are positive in patients with clinical symptoms.
-
•
Personalized medicine: A modern approach that sorts patients by their endotypes (for example, rhinitis, asthma, severe asthma) starting from precision medicine data offering a clear and exhaustive picture of the patients.
-
•
Potentially harmful allergens: Allergens that may cause severe systemic reactions in sensitized patients, such as 2S albumins, 7S, and 11S globulins, nonspecific LTPs, tropomyosins, and parvalbumins.
-
•
Precision medicine: A modern approach based on a patient's genotype or endotype. Patients are classified according to objective parameters from laboratory assays and not, for example, by symptoms.
-
•
Primary sensitization: Sensitization caused by the individual allergen itself rather than through cross-sensitization to a homologous allergen.
-
•
Purified natural allergen: The single allergen isolated from an allergen extract by either chemical or physical methods such as high-performance liquid chromatography, gel filtration, or immune absorption. The heterogeneity of these highly purified allergens is reduced, and they can be used in in vitro diagnostics. Of note, many purified allergen extracts contain CCD.
-
•
Purified natural component: See Purified natural allergen.
-
•
Recombinant allergen: Allergens produced through genetic engineering and often expressed in E. coli. Allergens expressed in E. coli do not have post-translational modifications such as glycosylation, although recombinant allergens produced in eukaryotic cells may have post-translational modifications.
-
•
Recombinant component: see Recombinant allergen.
-
•
Sensitization: The presence of sIgE to one or more allergens in serum tests. In skin prick tests, sensitization is the presence of a cutaneous reaction in the presence of a given allergen. In PAMD@, sIgE sensitization only includes serum IgE and not IgE bound to mast cells' high-affinity receptor, which can be qualitatively assessed by SPT. Sensitization cannot be considered an allergy.
-
•
SIgE: Discovered in the late 1960s, IgE is the antibody secreted in sensitized patients and specific for a given allergen. The detection of these antibodies suggests that the patient is sensitized to the allergen. The presence of signs and symptoms compatible with the IgE profile allow the allergist to identify the patient as allergic.
-
•
Top-down diagnostic approach: The classic approach. The diagnostic procedure starts with the patient's history and a skin prick test. If more-detailed results are needed, extracts of the allergen sources are assayed for sIgE. If still more information is required, an sIgE singleplex or multiplex approach is used. Finally, in selected cases, other assays, such as BAT or CAP inhibition are carried out. Contrasts with the bottom-up approach, which uses allergen components before extracts.
-
•
Total IgE: The concentration of IgE circulating in the serum.
-
•
Virtually innocuous allergens: Allergens that do not generally cause a severe or systemic reaction in sensitized patients, although exceptions are possible. Includes profilins, polcalcins, PR-10, and CCD.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Competing interests
R Valenta discloses research grants from Viravaxx, Vienna, Austria and serves as a consultant for this company. E Jensen-Jarolim discloses she is a shareholder and inventor on EP 2894478, with Biomedical International R + D GmbH Vienna; has received a grant and lecture honoraria from Bencard GmbH Germany; has received lecture honoraria from Sanofi GmbH, Roxall, Meda, and Novartis; and is a joint Editor-in-Chief of this journal. All other authors have declared they have no competing interests related to this work.
Authors’ contributions
IJA, GM, and GWC initiated the update. All Steering Committee Authors developed the draft and contributed equally. All Review Panel Members provided thorough critical review. All authors approved the final version.
Acknowledgements
The authors extend their appreciation to Naomi Ruff, PhD, for her contribution as editor of the document. The World Allergy Organization thanks Thermo Fisher Scientific for supporting this project, in part, by a medical educational grant. The supporter did not participate in document development.
Footnotes
Full list of author information is available at the end of the article
Contributor Information
Steering Committee Authors:
Ignacio J. Ansotegui, Giovanni Melioli, Giorgio Walter Canonica, R. Maximiliano Gómez, Erika Jensen-Jarolim, Motohiro Ebisawa, Olga Luengo, Luis Caraballo, Giovanni Passalacqua, Lars K. Poulsen, Eleonora Savi, Torsten Zuberbier, Elisa Villa, and John Oppenheimer
Review Panel Members:
Riccardo Asero, Jonathan Bernstein, Jean Bousquet, Victoria Cardona, Lindo Cox, Pascal Demoly, Fatima Ferreira, Pedro Giavina Bianchi, Sandra Gonzalez Diaz, Thilo Jakob, Luciana Kase Tanno, Jorg Kleine-Tebbe, Michael Levin, Bryan Martin, Paolo Maria Matricardi, Olga Patricia Monge Ortega, Mario Morais Almeida, Carlos Nunes, José Antonio Ortega Martell, Harald Renz, Nelson Rosário Filho, Philip Rouadi, Alessia Ruiba, Hugh Sampson, Mario Sánchez Borges, Enrico Scala, Peter Schmid-Grendelmeier, Gian-Enrico Senna, Juan Carlos Sisul, Mimi L.K. Tang, Rudolf Valenta, Marianne van Hage, Gary W.K. Wong, and Anahí Yáñez
References
- 1.Fiocchi A. Setting the stage of innovations in allergy globally. World Allergy Organ J. 2014;7:5. doi: 10.1186/1939-4551-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canonica G.W., Ansotegui I.J., Pawankar R. Wao - ARIA - GA(2)LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J. 2013;6:17. doi: 10.1186/1939-4551-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luengo O., Cardona V. Component resolved diagnosis: when should it be used? Clin Transl Allergy. 2014;4:28. doi: 10.1186/2045-7022-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tallar M.T., Grayson M.H. Component-resolved allergen testing: the new frontier. World J Transl Med. 2015;4:44–50. [Google Scholar]
- 5.Kleine-Tebbe J., Jakob T. Springer International Publishing; 2017. Molecular Allergy Diagnostics. [Google Scholar]
- 6.Van Gasse A.L., Mangodt E.A., Faber M., Sabato V., Bridts C.H., Ebo D.G. Molecular allergy diagnosis: status anno 2015. Clin Chim Acta. 2015;444:54–61. doi: 10.1016/j.cca.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Maruyama N., Nakagawa T., Ito K. Measurement of specific IgE antibodies to Ses i 1 improves the diagnosis of sesame allergy. Clin Exp Allergy. 2016;46:163–171. doi: 10.1111/cea.12626. [DOI] [PubMed] [Google Scholar]
- 8.Pomes A., Davies J.M., Gadermaier G. WHO/IUIS allergen nomenclature: providing a common language. Mol Immunol. 2018;100:3–13. doi: 10.1016/j.molimm.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molecular Allergy Diagnostics. 1 ed. Springer International Publishing; 2017. [Google Scholar]
- 10.Letran A., Espinazo M., Moreno F. Measurement of IgE to pollen allergen components is helpful in selecting patients for immunotherapy. Ann Allergy Asthma Immunol. 2013;111:295–297. doi: 10.1016/j.anai.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton R.G., Oppenheimer J. Serological IgE analyses in the diagnostic algorithm for allergic disease. J Allergy Clin Immunol Pract. 2015;3:833–840. doi: 10.1016/j.jaip.2015.08.016. quiz 841-832. [DOI] [PubMed] [Google Scholar]
- 12.Jakob T., Forstenlechner P., Matricardi P., Kleine-Tebbe J. Molecular allergy diagnostics using multiplex assays: methodological and practical considerations for use in research and clinical routine: Part 21 of the Series Molecular Allergology. Allergo J Int. 2015;24:320–332. doi: 10.1007/s40629-015-0087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodfolk J.A., Commins S.P., Schuyler A.J., Erwin E.A., Platts-Mills T.A. Allergens, sources, particles, and molecules: why do we make IgE responses? Allergol Int. 2015;64:295–303. doi: 10.1016/j.alit.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valenta R., Karaulov A., Niederberger V. Molecular aspects of allergens and allergy. Adv Immunol. 2018;138:195–256. doi: 10.1016/bs.ai.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Schulke S. Induction of interleukin-10 producing dendritic cells as a tool to suppress allergen-specific T helper 2 responses. Front Immunol. 2018;9:455. doi: 10.3389/fimmu.2018.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J.Y. The innate immune response in house dust mite-induced allergic inflammation. Allergy Asthma Immunol Res. 2013;5:68–74. doi: 10.4168/aair.2013.5.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacquet A. Innate immune responses in house dust mite allergy. ISRN Allergy. 2013;2013:735031. doi: 10.1155/2013/735031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satitsuksanoa P., Kennedy M., Gilis D. The minor house dust mite allergen Der p 13 is a fatty acid-binding protein and an activator of a TLR2-mediated innate immune response. Allergy. 2016;71:1425–1434. doi: 10.1111/all.12899. [DOI] [PubMed] [Google Scholar]
- 19.Fan D., Wang X., Wang M. Allergen-dependent differences in ILC2s frequencies in patients with allergic rhinitis. Allergy Asthma Immunol Res. 2016;8:216–222. doi: 10.4168/aair.2016.8.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo L., Huang Y., Chen X., Hu-Li J., Urban J.F., Jr., Paul W.E. Innate immunological function of TH2 cells in vivo. Nat Immunol. 2015;16:1051–1059. doi: 10.1038/ni.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peebles R.S., Jr. At the bedside: the emergence of group 2 innate lymphoid cells in human disease. J Leukoc Biol. 2015;97:469–475. doi: 10.1189/jlb.3BT0814-383R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambrecht B.N., Hammad H. Allergens and the airway epithelium response: gateway to allergic sensitization. J Allergy Clin Immunol. 2014;134:499–507. doi: 10.1016/j.jaci.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 23.Osterlund C., Gronlund H., Polovic N., Sundstrom S., Gafvelin G., Bucht A. The non-proteolytic house dust mite allergen Der p 2 induce NF-kappaB and MAPK dependent activation of bronchial epithelial cells. Clin Exp Allergy. 2009;39:1199–1208. doi: 10.1111/j.1365-2222.2009.03284.x. [DOI] [PubMed] [Google Scholar]
- 24.Chiou Y.L., Lin C.Y. Der p2 activates airway smooth muscle cells in a TLR2/MyD88-dependent manner to induce an inflammatory response. J Cell Physiol. 2009;220:311–318. doi: 10.1002/jcp.21764. [DOI] [PubMed] [Google Scholar]
- 25.Ye Y.L., Wu H.T., Lin C.F. Dermatophagoides pteronyssinus 2 regulates nerve growth factor release to induce airway inflammation via a reactive oxygen species-dependent pathway. Am J Physiol Lung Cell Mol Physiol. 2011;300:L216–L224. doi: 10.1152/ajplung.00165.2010. [DOI] [PubMed] [Google Scholar]
- 26.Yu S.J., Liao E.C., Sheu M.L., Chang D.T., Tsai J.J. Cell-penetrating peptide derived from human eosinophil cationic protein inhibits mite allergen Der p 2 induced inflammasome activation. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin S.C., Liao E.C., Chiu C.L., Chang C.Y., Tsai J.J. Der p2 internalization by epithelium synergistically augments toll-like receptor-mediated proinflammatory signaling. Allergy Asthma Immunol Res. 2015;7:393–403. doi: 10.4168/aair.2015.7.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trompette A., Divanovic S., Visintin A. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W.C., Tsai J.J., Kuo C.Y., Chen H.M., Kao S.H. Non-proteolytic house dust mite allergen, Der p 2, upregulated expression of tight junction molecule claudin-2 associated with Akt/GSK-3beta/beta-catenin signaling pathway. J Cell Biochem. 2011;112:1544–1551. doi: 10.1002/jcb.23067. [DOI] [PubMed] [Google Scholar]
- 30.Resch Y., Blatt K., Malkus U. Molecular, structural and immunological characterization of Der p 18, a chitinase-like house dust mite allergen. PLoS One. 2016;11 doi: 10.1371/journal.pone.0160641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mueller G.A., Edwards L.L., Aloor J.J. The structure of the dust mite allergen Der p 7 reveals similarities to innate immune proteins. J Allergy Clin Immunol. 2010;125:909–917 e904. doi: 10.1016/j.jaci.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pulsawat P., Soongrung T., Satitsuksanoa P. The house dust mite allergen Der p 5 binds lipid ligands and stimulates airway epithelial cells through a TLR2-dependent pathway. Clin Exp Allergy. 2019;49:378–390. doi: 10.1111/cea.13278. [DOI] [PubMed] [Google Scholar]
- 33.Chevigne A., Jacquet A. Emerging roles of the protease allergen Der p 1 in house dust mite-induced airway inflammation. J Allergy Clin Immunol. 2018;142:398–400. doi: 10.1016/j.jaci.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 34.Pascual M., Suzuki M., Isidoro-Garcia M. Epigenetic changes in B lymphocytes associated with house dust mite allergic asthma. Epigenetics. 2011;6:1131–1137. doi: 10.4161/epi.6.9.16061. [DOI] [PubMed] [Google Scholar]
- 35.Li J.Y., Zhang Y., Lin X.P. Association between DNA hypomethylation at IL13 gene and allergic rhinitis in house dust mite-sensitized subjects. Clin Exp Allergy. 2016;46:298–307. doi: 10.1111/cea.12647. [DOI] [PubMed] [Google Scholar]
- 36.Shang Y., Das S., Rabold R., Sham J.S., Mitzner W., Tang W.Y. Epigenetic alterations by DNA methylation in house dust mite-induced airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2013;49:279–287. doi: 10.1165/rcmb.2012-0403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng R.Y., Shang Y., Limjunyawong N. Alterations of the lung methylome in allergic airway hyper-responsiveness. Environ Mol Mutagen. 2014;55:244–255. doi: 10.1002/em.21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X., Chen X., Weirauch M.T. Diesel exhaust and house dust mite allergen lead to common changes in the airway methylome and hydroxymethylome. Environ Epigenet. 2018;4:dvy020. doi: 10.1093/eep/dvy020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu F., Lin L.R., Zhang H.L. Laboratorial characteristics of patients with diarrhoea suffering from egg white allergy. Allergol Immunopathol. 2014;42:180–185. doi: 10.1016/j.aller.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Stapel S.O., Asero R., Ballmer-Weber B.K. Testing for IgG4 against foods is not recommended as a diagnostic tool: EAACI Task Force Report. Allergy. 2008;63:793–796. doi: 10.1111/j.1398-9995.2008.01705.x. [DOI] [PubMed] [Google Scholar]
- 41.Werfel T., Asero R., Ballmer-Weber B.K. Position paper of the EAACI: food allergy due to immunological cross-reactions with common inhalant allergens. Allergy. 2015;70:1079–1090. doi: 10.1111/all.12666. [DOI] [PubMed] [Google Scholar]
- 42.Antico A., Pagani M., Vescovi P.P., Bonadonna P., Senna G. Food-specific IgG4 lack diagnostic value in adult patients with chronic urticaria and other suspected allergy skin symptoms. Int Arch Allergy Immunol. 2011;155:52–56. doi: 10.1159/000318736. [DOI] [PubMed] [Google Scholar]
- 43.Lucas Moreno J.M. Diarrhoea due to allergy to egg: is there a role for specific IgG? Allergol Immunopathol. 2014;42:177–179. doi: 10.1016/j.aller.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Santos A.F., James L.K., Bahnson H.T. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol. 2015;135:1249–1256. doi: 10.1016/j.jaci.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hofmaier S., Hatzler L., Rohrbach A. Default" versus "pre-atopic" IgG responses to foodborne and airborne pathogenesis-related group 10 protein molecules in birch-sensitized and nonatopic children. J Allergy Clin Immunol. 2015;135:1367–1374. doi: 10.1016/j.jaci.2014.09.048. e1361-1368. [DOI] [PubMed] [Google Scholar]
- 46.Holt P.G., Strickland D., Bosco A. Distinguishing benign from pathologic TH2 immunity in atopic children. J Allergy Clin Immunol. 2016;137:379–387. doi: 10.1016/j.jaci.2015.08.044. [DOI] [PubMed] [Google Scholar]
- 47.Bianchini R., Roth-Walter F., Ohradanova-Repic A. IgG4 drives M2a macrophages to a regulatory M2b-like phenotype: potential implication in immune tolerance. Allergy. 2019;74:483–494. doi: 10.1111/all.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flores Kim J., McCleary N., Nwaru B.I., Stoddart A., Sheikh A. Diagnostic accuracy, risk assessment, and cost-effectiveness of component-resolved diagnostics for food allergy: a systematic review. Allergy. 2018;73:1609–1621. doi: 10.1111/all.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cardona V., Ansotegui I.J. Component-resolved diagnosis in anaphylaxis. Curr Opin Allergy Clin Immunol. 2016;16:244–249. doi: 10.1097/ACI.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 50.Frick M., Fischer J., Helbling A. Predominant Api m 10 sensitization as risk factor for treatment failure in honey bee venom immunotherapy. J Allergy Clin Immunol. 2016;138:1663–1671 e1669. doi: 10.1016/j.jaci.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 51.Saltabayeva U., Garib V., Morenko M. Greater real-life diagnostic efficacy of allergen molecule-based diagnosis for prescription of immunotherapy in an area with multiple pollen exposure. Int Arch Allergy Immunol. 2017;173:93–98. doi: 10.1159/000477442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Hage M., Hamsten C., Valenta R. ImmunoCAP assays: pros and cons in allergology. J Allergy Clin Immunol. 2017;140:974–977. doi: 10.1016/j.jaci.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Garib V., Rigler E., Gastager F. Determination of IgE and IgG reactivity to more than 170 allergen molecules in paper-dried blood spots. J Allergy Clin Immunol. 2019;143:437–440. doi: 10.1016/j.jaci.2018.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heffler E., Puggioni F., Peveri S., Montagni M., Canonica G.W., Melioli G. Extended IgE profile based on an allergen macroarray: a novel tool for precision medicine in allergy diagnosis. World Allergy Organ J. 2018;11:7. doi: 10.1186/s40413-018-0186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Fraia M., Arasi S., Castelli S. A new molecular multiplex IgE assay for the diagnosis of pollen allergy in Mediterranean countries: a validation study. Clin Exp Allergy. 2019;49:341–349. doi: 10.1111/cea.13264. [DOI] [PubMed] [Google Scholar]
- 56.Melioli G., Bonifazi F., Bonini S. The ImmunoCAP ISAC molecular allergology approach in adult multi-sensitized Italian patients with respiratory symptoms. Clin Biochem. 2011;44:1005–1011. doi: 10.1016/j.clinbiochem.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Hamburg M.A., Collins F.S. The path to personalized medicine. N Engl J Med. 2010;363:301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 58.Passalacqua G., Canonica G.W. AIT (allergen immunotherapy): a model for the "precision medicine. Clin Mol Allergy. 2015;13:24. doi: 10.1186/s12948-015-0028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riccio A.M., De Ferrari L., Chiappori A. Molecular diagnosis and precision medicine in allergy management. Clin Chem Lab Med. 2016;54:1705–1714. doi: 10.1515/cclm-2016-0007. [DOI] [PubMed] [Google Scholar]
- 60.Kleine-Tebbe J., Matricardi P.M., Hamilton R.G. Allergy work-up including component-resolved diagnosis: how to make allergen-specific immunotherapy more specific. Immunol Allergy Clin N AM. 2016;36:191–203. doi: 10.1016/j.iac.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 61.Martinez-Aranguren R., Lizaso M.T., Goikoetxea M.J. Is the determination of specific IgE against components using ISAC 112 a reproducible technique? PLoS One. 2014;9 doi: 10.1371/journal.pone.0088394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lambert C., Sarrat A., Bienvenu F. The importance of EN ISO 15189 accreditation of allergen-specific IgE determination for reliable in vitro allergy diagnosis. Allergy. 2015;70:180–186. doi: 10.1111/all.12546. [DOI] [PubMed] [Google Scholar]
- 63.Huss-Marp J., Gutermuth J., Schaffner I. Comparison of molecular and extract-based allergy diagnostics with multiplex and singleplex analysis. Allergo J Int. 2015;24:46–53. doi: 10.1007/s40629-015-0046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Villalta D., Conte M., Asero R., Da Re M., Stella S., Martelli P. Isolated IgE reactivity to native walnut vicilin-like protein (nJug r 2) on ISAC microarray is due to cross-reactive carbohydrate epitopes. Clin Chem Lab Med. 2013;51:1991–1995. doi: 10.1515/cclm-2013-0027. [DOI] [PubMed] [Google Scholar]
- 65.Leonardi A., Borghesan F., Faggian D., Plebani M. Microarray-based IgE detection in tears of patients with vernal keratoconjunctivitis. Pediatr Allergy Immunol. 2015;26:641–645. doi: 10.1111/pai.12450. [DOI] [PubMed] [Google Scholar]
- 66.Hochwallner H., Alm J., Lupinek C. Transmission of allergen-specific IgG and IgE from maternal blood into breast milk visualized with microarray technology. J Allergy Clin Immunol. 2014;134:1213–1215. doi: 10.1016/j.jaci.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D'Amelio C.M., Goikoetxea M.J., Martinez-Aranguren R. Is the performance of ImmunoCAP ISAC 112 sufficient to diagnose peach and apple allergies? Ann Allergy Asthma Immunol. 2016;116:162–163. doi: 10.1016/j.anai.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Goikoetxea M.J., D'Amelio C.M., Martinez-Aranguren R. Is microarray analysis really useful and sufficient to diagnose nut allergy in the mediterranean area? J Investig Allergol Clin Immunol. 2016;26:31–39. [PubMed] [Google Scholar]
- 69.Javaloyes G., Goikoetxea M.J., Garcia Nunez I. Pru p 3 acts as a strong sensitizer for peanut allergy in Spain. J Allergy Clin Immunol. 2012;130:1432–1434 e1433. doi: 10.1016/j.jaci.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 70.Lupinek C., Wollmann E., Baar A. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. 2014;66:106–119. doi: 10.1016/j.ymeth.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skrindo I., Lupinek C., Valenta R. The use of the MeDALL-chip to assess IgE sensitization: a new diagnostic tool for allergic disease? Pediatr Allergy Immunol. 2015;26:239–246. doi: 10.1111/pai.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams P., Onell A., Baldracchini F., Hui V., Jolles S., El-Shanawany T. Evaluation of a novel automated allergy microarray platform compared with three other allergy test methods. Clin Exp Immunol. 2016;184:1–10. doi: 10.1111/cei.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buzzulini F., Da Re M., Scala E. Evaluation of a new multiplex assay for allergy diagnosis. Clin Chim Acta. 2019;493:73–78. doi: 10.1016/j.cca.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 74.Matricardi P.M., Kleine-Tebbe J., Hoffmann H.J. EAACI molecular allergology user's guide. Pediatr Allergy Immunol. 2016;27(Suppl 23):1–250. doi: 10.1111/pai.12563. [DOI] [PubMed] [Google Scholar]
- 75.Canonica G.W., Ferrando M., Baiardini I. Asthma: personalized and precision medicine. Curr Opin Allergy Clin Immunol. 2018;18:51–58. doi: 10.1097/ACI.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 76.Melioli G., Spenser C., Reggiardo G. Allergenius, an expert system for the interpretation of allergen microarray results. World Allergy Organ J. 2014;7:15. doi: 10.1186/1939-4551-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuhne Y., Reese G., Ballmer-Weber B.K. A novel multipeptide microarray for the specific and sensitive mapping of linear IgE-binding epitopes of food allergens. Int Arch Allergy Immunol. 2015;166:213–224. doi: 10.1159/000381344. [DOI] [PubMed] [Google Scholar]
- 78.Savilahti E.M., Kuitunen M., Valori M. Use of IgE and IgG4 epitope binding to predict the outcome of oral immunotherapy in cow's milk allergy. Pediatr Allergy Immunol. 2014;25:227–235. doi: 10.1111/pai.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lisson M., Erhardt G. Mapping of epitopes occurring in bovine alpha(s1)-Casein variants by peptide microarray immunoassay. Methods Mol Biol. 2016;1352:279–296. doi: 10.1007/978-1-4939-3037-1_21. [DOI] [PubMed] [Google Scholar]
- 80.De-Simone S.G., Napoleao-Pego P., De-Simone T.S. Spot synthesis: an optimized microarray to detect IgE epitopes. Methods Mol Biol. 2016;1352:263–277. doi: 10.1007/978-1-4939-3037-1_20. [DOI] [PubMed] [Google Scholar]
- 81.Perez-Gordo M., Pastor-Vargas C., Lin J. Epitope mapping of the major allergen from Atlantic cod in Spanish population reveals different IgE-binding patterns. Mol Nutr Food Res. 2013;57:1283–1290. doi: 10.1002/mnfr.201200332. [DOI] [PubMed] [Google Scholar]
- 82.Martinez-Botas J., Cerecedo I., Zamora J. Mapping of the IgE and IgG4 sequential epitopes of ovomucoid with a peptide microarray immunoassay. Int Arch Allergy Immunol. 2013;161:11–20. doi: 10.1159/000343040. [DOI] [PubMed] [Google Scholar]
- 83.Gimenez G., Benede S., Lin J. IgE epitope mapping using peptide microarray immunoassay. Methods Mol Biol. 2016;1352:251–261. doi: 10.1007/978-1-4939-3037-1_19. [DOI] [PubMed] [Google Scholar]
- 84.Mehr S., Lee E., Hsu P. Innate immune activation occurs in acute food protein-induced enterocolitis syndrome reactions. J Allergy Clin Immunol. 2019;144:600–602 e602. doi: 10.1016/j.jaci.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 85.Nowak-Wegrzyn A., Warren C.M., Brown-Whitehorn T., Cianferoni A., Schultz-Matney F., Gupta R.S. Food protein-induced enterocolitis syndrome in the US population-based study. J Allergy Clin Immunol. 2019;144(4):1128–1130. doi: 10.1016/j.jaci.2019.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feuille E., Nowak-Wegrzyn A. Recognizing and Treating Food Protein-Induced Enterocolitis Syndrome. Allergy. 2019;74(10):2019–2022. doi: 10.1111/all.13857. [DOI] [PubMed] [Google Scholar]
- 87.Fedenko E., Elisyutina O., Shtyrbul O. Microarray-based IgE serology improves management of severe atopic dermatitis in two children. Pediatr Allergy Immunol. 2016;27:645–649. doi: 10.1111/pai.12572. [DOI] [PubMed] [Google Scholar]
- 88.Westman M., Lupinek C., Bousquet J. Early childhood IgE reactivity to pathogenesis-related class 10 proteins predicts allergic rhinitis in adolescence. J Allergy Clin Immunol. 2015;135:1199–1206 e1191-1111. doi: 10.1016/j.jaci.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Posa D., Perna S., Resch Y. Evolution and predictive value of IgE responses toward a comprehensive panel of house dust mite allergens during the first 2 decades of life. J Allergy Clin Immunol. 2017;139:541–549 e548. doi: 10.1016/j.jaci.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 90.Wickman M., Lupinek C., Andersson N. Detection of IgE reactivity to a handful of allergen molecules in early childhood predicts respiratory allergy in adolescence. EBioMedicine. 2017;26:91–99. doi: 10.1016/j.ebiom.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Passalacqua G., Melioli G., Bonifazi F. The additional values of microarray allergen assay in the management of polysensitized patients with respiratory allergy. Allergy. 2013;68:1029–1033. doi: 10.1111/all.12194. [DOI] [PubMed] [Google Scholar]
- 92.Popescu F.D. Cross-reactivity between aeroallergens and food allergens. World J Methodol. 2015;5:31–50. doi: 10.5662/wjm.v5.i2.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Valenta R., Hochwallner H., Linhart B., Pahr S. Food allergies: the basics. Gastroenterology. 2015;148:1120–1131 e1124. doi: 10.1053/j.gastro.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fotisch K., Vieths S. N- and O-linked oligosaccharides of allergenic glycoproteins. Glycoconj J. 2001;18:373–390. doi: 10.1023/a:1014860030380. [DOI] [PubMed] [Google Scholar]
- 95.Berneder M., Bublin M., Hoffmann-Sommergruber K., Hawranek T., Lang R. Allergen chip diagnosis for soy-allergic patients: Gly m 4 as a marker for severe food-allergic reactions to soy. Int Arch Allergy Immunol. 2013;161:229–233. doi: 10.1159/000345970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roseler S., Balakirski G., Plange J. [Anaphylaxis to PR-10 proteins (Bet v1 homologues)] Hautarzt. 2013;64:890–892. doi: 10.1007/s00105-013-2683-1. [DOI] [PubMed] [Google Scholar]
- 97.Alvarado M.I., Jimeno L., De La Torre F. Profilin as a severe food allergen in allergic patients overexposed to grass pollen. Allergy. 2014;69:1610–1616. doi: 10.1111/all.12509. [DOI] [PubMed] [Google Scholar]
- 98.Uasuf C.G., Villalta D., Conte M.E. Different co-sensitizations could determine different risk assessment in peach allergy? Evaluation of an anaphylactic biomarker in Pru p 3 positive patients. Clin Mol Allergy. 2015;13:30. doi: 10.1186/s12948-015-0035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pastorello E.A., Farioli L., Pravettoni V. Pru p 3-sensitised Italian peach-allergic patients are less likely to develop severe symptoms when also presenting IgE antibodies to Pru p 1 and Pru p 4. Int Arch Allergy Immunol. 2011;156:362–372. doi: 10.1159/000324440. [DOI] [PubMed] [Google Scholar]
- 100.Van Hoeyveld E.D.S.L. Diagnosis of food allergy Part 2. The use of allergen components for in vitro diagnosis of food allergy in children. Tijdschrift van de Belgische Kinderarts. 2014;16:112–115. [Google Scholar]
- 101.Borres M.P., Maruyama N., Sato S., Ebisawa M. Recent advances in component resolved diagnosis in food allergy. Allergol Int. 2016;65:378–387. doi: 10.1016/j.alit.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 102.Ando H., Moverare R., Kondo Y. Utility of ovomucoid-specific IgE concentrations in predicting symptomatic egg allergy. J Allergy Clin Immunol. 2008;122:583–588. doi: 10.1016/j.jaci.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 103.Ohtani K., Sato S., Syukuya A. Natural history of immediate-type hen's egg allergy in Japanese children. Allergol Int. 2016;65:153–157. doi: 10.1016/j.alit.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 104.Chokshi N.Y., Sicherer S.H. Molecular diagnosis of egg allergy: an update. Expert Rev Mol Diagn. 2015;15:895–906. doi: 10.1586/14737159.2015.1041927. [DOI] [PubMed] [Google Scholar]
- 105.Pattanaik D., Lieberman P., Lieberman J., Pongdee T., Keene A.T. The changing face of anaphylaxis in adults and adolescents. Ann Allergy Asthma Immunol. 2018;121:594–597. doi: 10.1016/j.anai.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 106.Heaps A., Carter S., Selwood C. The utility of the ISAC allergen array in the investigation of idiopathic anaphylaxis. Clin Exp Immunol. 2014;177:483–490. doi: 10.1111/cei.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jappe U., Schwager C. Relevance of lipophilic allergens in food allergy diagnosis. Curr Allergy Asthma Rep. 2017;17:61. doi: 10.1007/s11882-017-0731-0. [DOI] [PubMed] [Google Scholar]
- 108.Matsuo H., Dahlstrom J., Tanaka A. Sensitivity and specificity of recombinant omega-5 gliadin-specific IgE measurement for the diagnosis of wheat-dependent exercise-induced anaphylaxis. Allergy. 2008;63:233–236. doi: 10.1111/j.1398-9995.2007.01504.x. [DOI] [PubMed] [Google Scholar]
- 109.Pastorello E.A., Farioli L., Stafylaraki C. Wheat-dependent exercise-induced anaphylaxis caused by a lipid transfer protein and not by omega-5 gliadin. Ann Allergy Asthma Immunol. 2014;112:386–387 e381. doi: 10.1016/j.anai.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 110.Cardona V., Luengo O., Garriga T. Co-factor-enhanced food allergy. Allergy. 2012;67:1316–1318. doi: 10.1111/j.1398-9995.2012.02877.x.. [DOI] [PubMed] [Google Scholar]
- 111.Romano A., Scala E., Rumi G. Lipid transfer proteins: the most frequent sensitizer in Italian subjects with food-dependent exercise-induced anaphylaxis. Clin Exp Allergy. 2012;42:1643–1653. doi: 10.1111/cea.12011. [DOI] [PubMed] [Google Scholar]
- 112.Apostolovic D., Mihailovic J., Commins S.P. Allergenomics of the tick Ixodes ricinus reveals important alpha-Gal-carrying IgE-binding proteins in red meat allergy. Allergy. 2020;75(1):217–220. doi: 10.1111/all.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Levin M., Apostolovic D., Biedermann T. Galactose alpha-1,3-galactose phenotypes: lessons from various patient populations. Ann Allergy Asthma Immunol. 2019;122:598–602. doi: 10.1016/j.anai.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wilson J.M., Platts-Mills T.A.E. Meat allergy and allergens. Mol Immunol. 2018;100:107–112. doi: 10.1016/j.molimm.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Karaulov A.V., Garib V., Garib F., Valenta R. Protein biomarkers in asthma. Int Arch Allergy Immunol. 2018;175:189–208. doi: 10.1159/000486856. [DOI] [PubMed] [Google Scholar]
- 116.Simpson A., Lazic N., Belgrave D.C. Patterns of IgE responses to multiple allergen components and clinical symptoms at age 11 years. J Allergy Clin Immunol. 2015;136:1224–1231. doi: 10.1016/j.jaci.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jung J.H., Kang I.G., Kim S.T. Comparison of component-resolved diagnosis by using allergen microarray with the conventional tests in allergic rhinitis patients: the first using in korea. Clin Exp Otorhinolaryngol. 2015;8:385–389. doi: 10.3342/ceo.2015.8.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Santosa A., Andiappan A.K., Rotzschke O. Evaluation of the applicability of the Immuno-solid-phase allergen chip (ISAC) assay in atopic patients in Singapore. Clin Transl Allergy. 2015;5:9. doi: 10.1186/s13601-015-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.San Nicolo M., Braun T., Eder K., Berghaus A., Groger M. Clinical relevance of IgE to profilin and/or polcalcin in pollen-sensitized patients. Int Arch Allergy Immunol. 2016;169:101–107. doi: 10.1159/000444279. [DOI] [PubMed] [Google Scholar]
- 120.Jakob T., Rafei-Shamsabadi D., Spillner E., Muller S. Diagnostics in Hymenoptera venom allergy: current concepts and developments with special focus on molecular allergy diagnostics. Allergo J Int. 2017;26:93–105. doi: 10.1007/s40629-017-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Larenas-Linnemann D., Luna-Pech J.A., Mosges R. Debates in Allergy Medicine: allergy skin testing cannot be replaced by molecular diagnosis in the near future. World Allergy Organ J. 2017;10:32. doi: 10.1186/s40413-017-0164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jensen-Jarolim E., Jensen A.N., Canonica G.W. Debates in allergy medicine: molecular allergy diagnosis with ISAC will replace screenings by skin prick test in the future. World Allergy Organ J. 2017;10:33. doi: 10.1186/s40413-017-0162-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Scala E., Abeni D., Guerra E.C. Cosensitization to profilin is associated with less severe reactions to foods in nsLTPs and storage proteins reactors and with less severe respiratory allergy. Allergy. 2018;73:1921–1923. doi: 10.1111/all.13501. [DOI] [PubMed] [Google Scholar]
- 124.Minami T., Fukutomi Y., Lidholm J. IgE Abs to Der p 1 and Der p 2 as diagnostic markers of house dust mite allergy as defined by a bronchoprovocation test. Allergol Int. 2015;64:90–95. doi: 10.1016/j.alit.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 125.Custovic A., Sonntag H.J., Buchan I.E., Belgrave D., Simpson A., Prosperi M.C. Evolution pathways of IgE responses to grass and mite allergens throughout childhood. J Allergy Clin Immunol. 2015;136:1645–1652. doi: 10.1016/j.jaci.2015.03.041. e1641-1648. [DOI] [PubMed] [Google Scholar]
- 126.Sylvestre L., Jegu J., Metz-Favre C., Barnig C., Qi S., de Blay F. Component-based allergen-microarray: der p 2 and der f 2 dust mite sensitization is more common in patients with severe asthma. J Investig Allergol Clin Immunol. 2016;26:141–143. doi: 10.18176/jiaci.0035. [DOI] [PubMed] [Google Scholar]
- 127.Stremnitzer C., Manzano-Szalai K., Willensdorfer A. Papain degrades tight junction proteins of human keratinocytes in vitro and sensitizes C57bl/6 mice via the skin independent of its enzymatic activity or TLR4 activation. J Investig Dermatol. 2015;135:1790–1800. doi: 10.1038/jid.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Celi G., Brusca I., Scala E. House dust mite allergy in Italy-Diagnostic and clinical relevance of Der p 23 (and of minor allergens): a real-life, multicenter study. Allergy. 2019;74(9):1787–1789. doi: 10.1111/all.13776. [DOI] [PubMed] [Google Scholar]
- 129.Banerjee S., Resch Y., Chen K.W. Der p 11 is a major allergen for house dust mite-allergic patients suffering from atopic dermatitis. J Investig Dermatol. 2015;135:102–109. doi: 10.1038/jid.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zeng G., Luo W., Zheng P. Component-resolved diagnostic study of Dermatophagoides pteronyssinus major allergen molecules in a southern Chinese cohort. J Investig Allergol Clin Immunol. 2015;25:343–351. [PubMed] [Google Scholar]
- 131.Farioli L., Losappio L.M., Giuffrida M.G. Mite-induced asthma and IgE levels to shrimp, mite, tropomyosin, arginine kinase, and der p 10 are the most relevant risk factors for challenge-proven shrimp allergy. Int Arch Allergy Immunol. 2017;174:133–143. doi: 10.1159/000481985. [DOI] [PubMed] [Google Scholar]
- 132.Sanchez-Borges M., Capriles-Hulett A., Torres J. Diagnosis of allergic sensitization in patients with allergic rhinitis and asthma in a tropical environment. Rev Alerg Mex. 2019;66:44–54. doi: 10.29262/ram.v66i1.570. [DOI] [PubMed] [Google Scholar]
- 133.Ahumada V., Garcia E., Dennis R. IgE responses to Ascaris and mite tropomyosins are risk factors for asthma. Clin Exp Allergy. 2015;45:1189–1200. doi: 10.1111/cea.12513. [DOI] [PubMed] [Google Scholar]
- 134.Buendia E., Zakzuk J., Mercado D., Alvarez A., Caraballo L. The IgE response to Ascaris molecular components is associated with clinical indicators of asthma severity. World Allergy Organ J. 2015;8:8. doi: 10.1186/s40413-015-0058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Camargo Lopes de Oliveira L., Pierotti F.F., Mallozi M. rBlo t 5 is a potential contributor to the severity of atopic dermatitis in a Brazilian population. Pediatr Allergy Immunol. 2019;30:575–579. doi: 10.1111/pai.13050. [DOI] [PubMed] [Google Scholar]
- 136.Chen K.W., Zieglmayer P., Zieglmayer R. Selection of house dust mite-allergic patients by molecular diagnosis may enhance success of specific immunotherapy. J Allergy Clin Immunol. 2019;143:1248–1252 e1212. doi: 10.1016/j.jaci.2018.10.048. [DOI] [PubMed] [Google Scholar]
- 137.Sanchez-Borges M., Fernandez-Caldas E. Hidden allergens and oral mite anaphylaxis: the pancake syndrome revisited. Curr Opin Allergy Clin Immunol. 2015;15:337–343. doi: 10.1097/ACI.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 138.Scala E., Abeni D., Cecchi L. Molecular recognition profiles and clinical patterns of PR-10 sensitization in a birch-free mediterranean area. Int Arch Allergy Immunol. 2017;173:138–146. doi: 10.1159/000477565. [DOI] [PubMed] [Google Scholar]
- 139.Moreira P.F., Gangl K., Vieira Fde A. Allergen microarray indicates pooideae sensitization in Brazilian grass pollen allergic patients. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Teifoori F., Shams-Ghahfarokhi M., Postigo I. Identification of the main allergen sensitizers in an Iran asthmatic population by molecular diagnosis. Allergy Asthma Clin Immunol. 2014;10:41. doi: 10.1186/1710-1492-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hatzler L., Panetta V., Lau S. Molecular spreading and predictive value of preclinical IgE response to Phleum pratense in children with hay fever. J Allergy Clin Immunol. 2012;130:894–901 e895. doi: 10.1016/j.jaci.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 142.Savi E., Peveri S., Incorvaia C. Association between a low IgE response to Phl p 5 and absence of asthma in patients with grass pollen allergy. Clin Mol Allergy. 2013;11:3. doi: 10.1186/1476-7961-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bokanovic D., Aberer W., Hemmer W. Determination of sIgE to rPhl p 1 is sufficient to diagnose grass pollen allergy. Allergy. 2013;68:1403–1409. doi: 10.1111/all.12263. [DOI] [PubMed] [Google Scholar]
- 144.Gao Z., Fu W.Y., Sun Y. Artemisia pollen allergy in China: component-resolved diagnosis reveals allergic asthma patients have significant multiple allergen sensitization. Allergy. 2019;74:284–293. doi: 10.1111/all.13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Armentia A., Iglesias B., Iglesias D. Component-resolved diagnostics in vernal conjunctivitis. Ann Allergy Asthma Immunol. 2015;115:446–450. doi: 10.1016/j.anai.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 146.Quirce S. Asthma in alergologica-2005. J Investig Allergol Clin Immunol. 2009;19(Suppl 2):14–20. [PubMed] [Google Scholar]
- 147.Heinzerling L.M., Burbach G.J., Edenharter G. GA(2)LEN skin test study I: GA(2)LEN harmonization of skin prick testing: novel sensitization patterns for inhalant allergens in Europe. Allergy. 2009;64:1498–1506. doi: 10.1111/j.1398-9995.2009.02093.x. [DOI] [PubMed] [Google Scholar]
- 148.Konradsen J.R., Fujisawa T., van Hage M. Allergy to furry animals: new insights, diagnostic approaches, and challenges. J Allergy Clin Immunol. 2015;135:616–625. doi: 10.1016/j.jaci.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 149.Smith D.M., Coop C.A. Dog allergen immunotherapy: past, present, and future. Ann Allergy Asthma Immunol. 2016;116:188–193. doi: 10.1016/j.anai.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 150.Portnoy J., Kennedy K., Sublett J. Environmental assessment and exposure control: a practice parameter--furry animals. Ann Allergy Asthma Immunol. 2012;108:223 e221–215. doi: 10.1016/j.anai.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cabanas R., Lopez-Serrano M.C., Carreira J. Importance of albumin in cross-reactivity among cat, dog and horse allergens. J Investig Allergol Clin Immunol. 2000;10:71–77. [PubMed] [Google Scholar]
- 152.Basagana M., Bartolome B., Pastor C. Allergy to human seminal fluid: cross-reactivity with dog dander. J Allergy Clin Immunol. 2008;121:233–239. doi: 10.1016/j.jaci.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 153.Khurana T., Newman-Lindsay S., Young P.R., Slater J.E. The NPC2 protein: a novel dog allergen. Ann Allergy Asthma Immunol. 2016;116:440–446 e442. doi: 10.1016/j.anai.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 154.Liccardi G., Bilo M.B., Manzi F., Piccolo A., Di Maro E., Salzillo A. What could be the role of molecular-based allergy diagnostics in detecting the risk of developing allergic sensitization to furry animals? Eur Ann Allergy Clin Immunol. 2015;47:163–167. [PubMed] [Google Scholar]
- 155.Asarnoj A., Hamsten C., Waden K. Sensitization to cat and dog allergen molecules in childhood and prediction of symptoms of cat and dog allergy in adolescence: a BAMSE/MeDALL study. J Allergy Clin Immunol. 2016;137:813–821 e817. doi: 10.1016/j.jaci.2015.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bjerg A., Winberg A., Berthold M., Mattsson L., Borres M.P., Ronmark E. A population-based study of animal component sensitization, asthma, and rhinitis in schoolchildren. Pediatr Allergy Immunol. 2015;26:557–563. doi: 10.1111/pai.12422. [DOI] [PubMed] [Google Scholar]
- 157.Nagao M., Borres M.P., Sugimoto M. Sensitization to secretoglobin and lipocalins in a group of young children with risk of developing respiratory allergy. Clin Mol Allergy. 2017;15:4. doi: 10.1186/s12948-017-0061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kack U., Asarnoj A., Gronlund H. Molecular allergy diagnostics refine characterization of children sensitized to dog dander. J Allergy Clin Immunol. 2018;142:1113–1120 e1119. doi: 10.1016/j.jaci.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 159.Uriarte S.A., Sastre J. Clinical relevance of molecular diagnosis in pet allergy. Allergy. 2016;71:1066–1068. doi: 10.1111/all.12917. [DOI] [PubMed] [Google Scholar]
- 160.Adedoyin J., Gronlund H., Oman H., Johansson S.G., van Hage M. Cat IgA, representative of new carbohydrate cross-reactive allergens. J Allergy Clin Immunol. 2007;119:640–645. doi: 10.1016/j.jaci.2006.11.637. [DOI] [PubMed] [Google Scholar]
- 161.Barbosa M.C., Santos A.B., Ferriani V.P., Pomes A., Chapman M.D., Arruda L.K. Efficacy of recombinant allergens for diagnosis of cockroach allergy in patients with asthma and/or rhinitis. Int Arch Allergy Immunol. 2013;161:213–219. doi: 10.1159/000346318. [DOI] [PubMed] [Google Scholar]
- 162.Fang Y., Long C., Bai X. Two new types of allergens from the cockroach, Periplaneta americana. Allergy. 2015;70:1674–1678. doi: 10.1111/all.12766. [DOI] [PubMed] [Google Scholar]
- 163.Tanimoto H., Fukutomi Y., Yasueda H. Molecular-based allergy diagnosis of allergic bronchopulmonary aspergillosis in Aspergillus fumigatus-sensitized Japanese patients. Clin Exp Allergy. 2015;45:1790–1800. doi: 10.1111/cea.12590. [DOI] [PubMed] [Google Scholar]
- 164.Balenga N.A., Klichinsky M., Xie Z. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat Commun. 2015;6:6763. doi: 10.1038/ncomms7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Muthu V., Sehgal I.S., Dhooria S., Aggarwal A.N., Agarwal R. Utility of recombinant Aspergillus fumigatus antigens in the diagnosis of allergic bronchopulmonary aspergillosis: a systematic review and diagnostic test accuracy meta-analysis. Clin Exp Allergy. 2018;48:1107–1136. doi: 10.1111/cea.13216. [DOI] [PubMed] [Google Scholar]
- 166.Pali-Scholl I., Untersmayr E., Klems M., Jensen-Jarolim E. The effect of digestion and digestibility on allergenicity of food. Nutrients. 2018;10 doi: 10.3390/nu10091129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Simons F.E., Ardusso L.R., Bilo M.B. 2012 update: world allergy organization guidelines for the assessment and management of anaphylaxis. Curr Opin Allergy Clin Immunol. 2012;12:389–399. doi: 10.1097/ACI.0b013e328355b7e4. [DOI] [PubMed] [Google Scholar]
- 168.Antonicelli L., Massaccesi C., Braschi M.C., Cinti B., Bilo M.B., Bonifazi F. Component resolved diagnosis in real life: the risk assessment of food allergy using microarray-based immunoassay. Eur Ann Allergy Clin Immunol. 2014;46:30–34. [PubMed] [Google Scholar]
- 169.Lopez-Matas M.A., Larramendi C.H., Huertas A.J. Tomato nsLTP as an “in vivo" diagnostic tool: sensitization in a Mediterranean population. J Investig Allergol Clin Immunol. 2015;25:196–204. [PubMed] [Google Scholar]
- 170.Scala E., Till S.J., Asero R. Lipid transfer protein sensitization: reactivity profiles and clinical risk assessment in an Italian cohort. Allergy. 2015;70:933–943. doi: 10.1111/all.12635. [DOI] [PubMed] [Google Scholar]
- 171.Scala E., Abeni D., Pomponi D. Ole e 1, Ole e 7, and Ole e 9: identifying distinct clinical subsets of olive tree-allergic patients. J Allergy Clin Immunol. 2016;137:629–631 e623. doi: 10.1016/j.jaci.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 172.Pascal M., Vazquez-Ortiz M., Folque M.M. Asymptomatic LTP sensitisation is common in plant-food allergic children from the Northeast of Spain. Allergol Immunopathol. 2016;44:351–358. doi: 10.1016/j.aller.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 173.Vieira T., Cunha L., Neves E., Falcao H. Diagnostic usefulness of component-resolved diagnosis by skin prick tests and specific IgE to single allergen components in children with allergy to fruits and vegetables. Allergol Immunopathol. 2014;42:127–135. doi: 10.1016/j.aller.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 174.Patelis A., Borres M.P., Kober A., Berthold M. Multiplex component-based allergen microarray in recent clinical studies. Clin Exp Allergy. 2016;46:1022–1032. doi: 10.1111/cea.12761. [DOI] [PubMed] [Google Scholar]
- 175.Peveri S., Pattini S., Costantino M.T. Molecular diagnostics improves diagnosis and treatment of respiratory allergy and food allergy with economic optimization and cost saving. Allergol Immunopathol (Madr) 2019;47(1):64–72. doi: 10.1016/j.aller.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 176.Kuitunen M., Englund H., Remes S. High IgE levels to alpha-lactalbumin, beta-lactoglobulin and casein predict less successful cow's milk oral immunotherapy. Allergy. 2015;70:955–962. doi: 10.1111/all.12647. [DOI] [PubMed] [Google Scholar]
- 177.Sato S., Yanagida N., Ohtani K., Koike Y., Ebisawa M. A review of biomarkers for predicting clinical reactivity to foods with a focus on specific immunoglobulin E antibodies. Curr Opin Allergy Clin Immunol. 2015;15:250–258. doi: 10.1097/ACI.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 178.Petersen T.H., Mortz C.G., Bindslev-Jensen C., Eller E. Cow's milk allergic children-Can component-resolved diagnostics predict duration and severity? Pediatr Allergy Immunol. 2018;29:194–199. doi: 10.1111/pai.12854. [DOI] [PubMed] [Google Scholar]
- 179.Bartuzi Z., Cocco R.R., Muraro A., Nowak-Wegrzyn A. Contribution of molecular allergen analysis in diagnosis of milk allergy. Curr Allergy Asthma Rep. 2017;17:46. doi: 10.1007/s11882-017-0716-z. [DOI] [PubMed] [Google Scholar]
- 180.Agyemang A., Saf S., Sifers T. Utilizing boiled milk sIgE as a predictor of baked milk tolerance in cow's milk allergic children. J Allergy Clin Immunol Pract. 2019;7(6):2049–2051. doi: 10.1016/j.jaip.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 181.Kim J., Lee J., Park M.R., Han Y., Shin M., Ahn K. Special consideration is required for the component-resolved diagnosis of egg allergy in infants. Ann Allergy Asthma Immunol. 2014;112:53–57. doi: 10.1016/j.anai.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 182.Dang T.D., Peters R.L., Koplin J.J. Egg allergen specific IgE diversity predicts resolution of egg allergy in the population cohort HealthNuts. Allergy. 2019;74(2):318–326. doi: 10.1111/all.13572. [DOI] [PubMed] [Google Scholar]
- 183.Petrosino M.I., Scaparrotta A., Marcovecchio M.L. Usefulness of molecular diagnosis in egg allergic children. Arch Med Sci. 2018;14:132–137. doi: 10.5114/aoms.2016.58796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Faber M.A., Donne I., Herrebosch E. Sensitization profiles to peanut allergens in Belgium; cracking the code in infants, children and adults. Acta Clin Belg. 2016;71:32–37. doi: 10.1080/17843286.2015.1109170. [DOI] [PubMed] [Google Scholar]
- 185.Giovannini M., Comberiati P., Piazza M. Retrospective definition of reaction risk in Italian children with peanut, hazelnut and walnut allergy through component-resolved diagnosis. Allergol Immunopathol. 2019;47:73–78. doi: 10.1016/j.aller.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 186.Ackerbauer D., Bublin M., Radauer C. Component-resolved IgE profiles in Austrian patients with a convincing history of peanut allergy. Int Arch Allergy Immunol. 2015;166:13–24. doi: 10.1159/000371422. [DOI] [PubMed] [Google Scholar]
- 187.Kukkonen A.K., Pelkonen A.S., Makinen-Kiljunen S., Voutilainen H., Makela M.J. Ara h 2 and Ara 6 are the best predictors of severe peanut allergy: a double-blind placebo-controlled study. Allergy. 2015;70:1239–1245. doi: 10.1111/all.12671. [DOI] [PubMed] [Google Scholar]
- 188.Ballmer-Weber B.K., Lidholm J., Fernandez-Rivas M. IgE recognition patterns in peanut allergy are age dependent: perspectives of the EuroPrevall study. Allergy. 2015;70:391–407. doi: 10.1111/all.12574. [DOI] [PubMed] [Google Scholar]
- 189.Suratannon N., Ngamphaiboon J., Wongpiyabovorn J., Puripokai P., Chatchatee P. Component-resolved diagnostics for the evaluation of peanut allergy in a low-prevalence area. Pediatr Allergy Immunol. 2013;24:665–670. doi: 10.1111/pai.12125. [DOI] [PubMed] [Google Scholar]
- 190.Amoah A.S., Obeng B.B., Larbi I.A. Peanut-specific IgE antibodies in asymptomatic Ghanaian children possibly caused by carbohydrate determinant cross-reactivity. J Allergy Clin Immunol. 2013;132:639–647. doi: 10.1016/j.jaci.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lopes de Oliveira L.C., Aderhold M., Brill M. The value of specific IgE to peanut and its component Ara h 2 in the diagnosis of peanut allergy. J Allergy Clin Immunol Pract. 2013;1:394–398. doi: 10.1016/j.jaip.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 192.Lu M., Jin Y., Cerny R., Ballmer-Weber B., Goodman R.E. Combining 2-DE immunoblots and mass spectrometry to identify putative soybean (Glycine max) allergens. Food Chem Toxicol. 2018;116:207–215. doi: 10.1016/j.fct.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 193.Ebisawa M., Brostedt P., Sjolander S., Sato S., Borres M.P., Ito K. Gly m 2S albumin is a major allergen with a high diagnostic value in soybean-allergic children. J Allergy Clin Immunol. 2013;132:976–978. doi: 10.1016/j.jaci.2013.04.028. e971-975. [DOI] [PubMed] [Google Scholar]
- 194.Klemans R.J., Knol E.F., Michelsen-Huisman A. Components in soy allergy diagnostics: Gly m 2S albumin has the best diagnostic value in adults. Allergy. 2013;68:1396–1402. doi: 10.1111/all.12259. [DOI] [PubMed] [Google Scholar]
- 195.Datema M.R., Zuidmeer-Jongejan L., Asero R. Hazelnut allergy across Europe dissected molecularly: a EuroPrevall outpatient clinic survey. J Allergy Clin Immunol. 2015;136:382–391. doi: 10.1016/j.jaci.2014.12.1949. [DOI] [PubMed] [Google Scholar]
- 196.Buyuktiryaki B., Cavkaytar O., Sahiner U.M. Cor a 14, hazelnut-specific IgE, and SPT as a reliable tool in hazelnut allergy diagnosis in eastern mediterranean children. J Allergy Clin Immunol Pract. 2016;4:265–272 e263. doi: 10.1016/j.jaip.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 197.Masthoff L.J., Mattsson L., Zuidmeer-Jongejan L. Sensitization to Cor a 9 and Cor a 14 is highly specific for a hazelnut allergy with objective symptoms in Dutch children and adults. J Allergy Clin Immunol. 2013;132:393–399. doi: 10.1016/j.jaci.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 198.Brandstrom J., Nopp A., Johansson S.G. Basophil allergen threshold sensitivity and component-resolved diagnostics improve hazelnut allergy diagnosis. Clin Exp Allergy. 2015;45:1412–1418. doi: 10.1111/cea.12515. [DOI] [PubMed] [Google Scholar]
- 199.Villalta D., Scala E., Mistrello G., Amato S., Asero R. Evidence of cross-reactivity between different seed storage proteins from hazelnut (corylus avellana) and walnut (juglans regia) using recombinant allergen proteins. Int Arch Allergy Immunol. 2018:1–4. doi: 10.1159/000492399. [DOI] [PubMed] [Google Scholar]
- 200.Datema M.R., van Ree R., Asero R. Component-resolved diagnosis and beyond: multivariable regression models to predict severity of hazelnut allergy. Allergy. 2018;73:549–559. doi: 10.1111/all.13328. [DOI] [PubMed] [Google Scholar]
- 201.Beck S.C., Huissoon A.P., Collins D., Richter A.G., Krishna M.T. The concordance between component tests and clinical history in British adults with suspected pollen-food syndrome to peanut and hazelnut. J Clin Pathol. 2018;71:239–245. doi: 10.1136/jclinpath-2017-204573. [DOI] [PubMed] [Google Scholar]
- 202.Sievers S., Rawel H.M., Ringel K.P., Niggemann B., Beyer K. Wheat protein recognition pattern in tolerant and allergic children. Pediatr Allergy Immunol. 2016;27:147–155. doi: 10.1111/pai.12502. [DOI] [PubMed] [Google Scholar]
- 203.Makela M.J., Eriksson C., Kotaniemi-Syrjanen A. Wheat allergy in children - new tools for diagnostics. Clin Exp Allergy. 2014;44:1420–1430. doi: 10.1111/cea.12393. [DOI] [PubMed] [Google Scholar]
- 204.Koike Y., Yanagida N., Sato S. Predictors of persistent wheat allergy in children: a retrospective cohort study. Int Arch Allergy Immunol. 2018;176:249–254. doi: 10.1159/000489337. [DOI] [PubMed] [Google Scholar]
- 205.Yanagida N., Sato S., Takahashi K. Increasing specific immunoglobulin E levels correlate with the risk of anaphylaxis during an oral food challenge. Pediatr Allergy Immunol. 2018;29:417–424. doi: 10.1111/pai.12896. [DOI] [PubMed] [Google Scholar]
- 206.Kennard L., Thomas I., Rutkowski K. A multicenter evaluation of diagnosis and management of omega-5 gliadin allergy (also known as wheat-dependent exercise-induced anaphylaxis) in 132 adults. J Allergy Clin Immunol Pract. 2018;6:1892–1897. doi: 10.1016/j.jaip.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 207.Safi H., Wangorsch A., Lidholm J. Identification and molecular characterization of allergenic non-specific lipid-transfer protein from durum wheat (Triticum turgidum) Clin Exp Allergy. 2019;49:120–129. doi: 10.1111/cea.13271. [DOI] [PubMed] [Google Scholar]
- 208.Sander I., Rihs H.P., Doekes G. Component-resolved diagnosis of baker's allergy based on specific IgE to recombinant wheat flour proteins. J Allergy Clin Immunol. 2015;135:1529–1537. doi: 10.1016/j.jaci.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 209.Geiselhart S., Nagl C., Dubiela P. Concomitant sensitization to legumin, Fag e 2 and Fag e 5 predicts buckwheat allergy. Clin Exp Allergy. 2018;48:217–224. doi: 10.1111/cea.13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yanagida N., Sato S., Maruyama N. Specific IgE for Fag e 3 Predicts Oral Buckwheat Food Challenge Test Results and Anaphylaxis: a Pilot Study. Int Arch Allergy Immunol. 2018;176:8–14. doi: 10.1159/000487135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Einhorn L., Hofstetter G., Brandt S. Molecular allergen profiling in horses by microarray reveals Fag e 2 from buckwheat as a frequent sensitizer. Allergy. 2018;73:1436–1446. doi: 10.1111/all.13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Caimmi D., Barber D., Hoffmann-Sommergruber K. Understanding the molecular sensitization for Cypress pollen and peach in the Languedoc-Roussillon area. Allergy. 2013;68:249–251. doi: 10.1111/all.12073. [DOI] [PubMed] [Google Scholar]
- 213.Mothes-Luksch N., Raith M., Stingl G. Pru p 3, a marker allergen for lipid transfer protein sensitization also in Central Europe. Allergy. 2017;72:1415–1418. doi: 10.1111/all.13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Inomata N., Miyakawa M., Aihara M. High prevalence of sensitization to gibberellin-regulated protein (peamaclein) in fruit allergies with negative immunoglobulin E reactivity to Bet v 1 homologs and profilin: clinical pattern, causative fruits and cofactor effect of gibberellin-regulated protein allergy. J Dermatol. 2017;44:735–741. doi: 10.1111/1346-8138.13795. [DOI] [PubMed] [Google Scholar]
- 215.Klingebiel C., Chantran Y., Arif-Lusson R. Pru p 7 sensitization is a predominant cause of severe, cypress pollen-associated peach allergy. Clin Exp Allergy. 2019;49:526–536. doi: 10.1111/cea.13345. [DOI] [PubMed] [Google Scholar]
- 216.Poncet P., Aizawa T., Senechal H. The subtype of Cupressaceae pollinosis associated with Pru p 7 sensitization is characterized by a sensitization to a cross-reactive gibberellin-regulated protein in cypress pollen: BP14. Clin Exp Allergy. 2019;49:1163–1166. doi: 10.1111/cea.13418. [DOI] [PubMed] [Google Scholar]
- 217.Senechal H., Santrucek J., Melcova M. A new allergen family involved in pollen food-associated syndrome: snakin/gibberellin-regulated proteins. J Allergy Clin Immunol. 2018;141:411–414 e414. doi: 10.1016/j.jaci.2017.06.041. [DOI] [PubMed] [Google Scholar]
- 218.van Odijk J., Sjolander S., Brostedt P., Borres M.P., Englund H. High frequency of IgE sensitization towards kiwi seed storage proteins among peanut allergic individuals also reporting allergy to kiwi. Clin Mol Allergy. 2017;15:18. doi: 10.1186/s12948-017-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kuehn A., Hilger C., Lehners-Weber C. Identification of enolases and aldolases as important fish allergens in cod, salmon and tuna: component resolved diagnosis using parvalbumin and the new allergens. Clin Exp Allergy. 2013;43:811–822. doi: 10.1111/cea.12117. [DOI] [PubMed] [Google Scholar]
- 220.Tong W.S., Yuen A.W., Wai C.Y., Leung N.Y., Chu K.H., Leung P.S. Diagnosis of fish and shellfish allergies. J Asthma Allergy. 2018;11:247–260. doi: 10.2147/JAA.S142476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pascal M., Grishina G., Yang A.C. Molecular diagnosis of shrimp allergy: efficiency of several allergens to predict clinical reactivity. J Allergy Clin Immunol Pract. 2015;3:521–529 e510. doi: 10.1016/j.jaip.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 222.Tuano K.T.S., Anvari S., Hanson I.C. Improved diagnostic clarity in shrimp allergic non-dust-mite sensitized patients. Allergy Asthma Proc. 2018;39:377–383. doi: 10.2500/aap.2018.39.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lopata A.L., Kleine-Tebbe J., Kamath S.D. Allergens and molecular diagnostics of shellfish allergy: Part 22 of the Series Molecular Allergology. Allergo J Int. 2016;25:210–218. doi: 10.1007/s40629-016-0124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ruethers T., Taki A.C., Johnston E.B. Seafood allergy: a comprehensive review of fish and shellfish allergens. Mol Immunol. 2018;100:28–57. doi: 10.1016/j.molimm.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 225.Hilger C., Fischer J., Swiontek K. Two galactose-alpha-1,3-galactose carrying peptidases from pork kidney mediate anaphylactogenic responses in delayed meat allergy. Allergy. 2016;71:711–719. doi: 10.1111/all.12835. [DOI] [PubMed] [Google Scholar]
- 226.Apostolovic D., Tran T.A., Hamsten C., Starkhammar M., Cirkovic Velickovic T., van Hage M. Immunoproteomics of processed beef proteins reveal novel galactose-alpha-1,3-galactose-containing allergens. Allergy. 2014;69:1308–1315. doi: 10.1111/all.12462. [DOI] [PubMed] [Google Scholar]
- 227.Cabezas-Cruz A., Mateos-Hernandez L., Chmelar J., Villar M., de la Fuente J. Salivary prostaglandin E2: role in tick-induced allergy to red meat. Trends Parasitol. 2017;33:495–498. doi: 10.1016/j.pt.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 228.Crispell G., Commins S.P., Archer-Hartman S.A. Discovery of alpha-gal-containing antigens in north American tick species believed to induce red meat allergy. Front Immunol. 2019;10:1056. doi: 10.3389/fimmu.2019.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cabezas-Cruz A., Espinosa P.J., Alberdi P. Tick galactosyltransferases are involved in alpha-Gal synthesis and play a role during Anaplasma phagocytophilum infection and Ixodes scapularis tick vector development. Sci Rep. 2018;8:14224. doi: 10.1038/s41598-018-32664-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hemmer W., Klug C., Swoboda I. Update on the bird-egg syndrome and genuine poultry meat allergy. Allergo J Int. 2016;25:68–75. doi: 10.1007/s40629-016-0108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Savi E., Rossi A., Incorvaia C. Cat-pork syndrome: a case report with a thee years follow-up. Eur Ann Allergy Clin Immunol. 2006;38:366–368. [PubMed] [Google Scholar]
- 232.Raulf M. Allergen component analysis as a tool in the diagnosis of occupational allergy. Curr Opin Allergy Clin Immunol. 2016;16:93–100. doi: 10.1097/ACI.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 233.Vandenplas O., Froidure A., Meurer U. The role of allergen components for the diagnosis of latex-induced occupational asthma. Allergy. 2016;71:840–849. doi: 10.1111/all.12872. [DOI] [PubMed] [Google Scholar]
- 234.Carballeda-Sangiao N., Rodriguez-Mahillo A.I., Careche M. Ani s 11-like protein is a pepsin- and heat-resistant major allergen of Anisakis spp. and a valuable tool for Anisakis allergy component-resolved diagnosis. Int Arch Allergy Immunol. 2016;169:108–112. doi: 10.1159/000444981. [DOI] [PubMed] [Google Scholar]
- 235.Caballero M.L., Asero R., Antonicelli L. Anisakis allergy component-resolved diagnosis: clinical and immunologic differences between patients from Italy and Spain. Int Arch Allergy Immunol. 2013;162:39–44. doi: 10.1159/000351056. [DOI] [PubMed] [Google Scholar]
- 236.Rafei-Shamsabadi D., Muller S., Pfutzner W., Spillner E., Rueff F., Jakob T. Recombinant allergens rarely allow identification of Hymenoptera venom-allergic patients with negative specific IgE to whole venom preparations. J Allergy Clin Immunol. 2014;134:493–494. doi: 10.1016/j.jaci.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 237.Cifuentes L., Vosseler S., Blank S. Identification of Hymenoptera venom-allergic patients with negative specific IgE to venom extract by using recombinant allergens. J Allergy Clin Immunol. 2014;133:909–910. doi: 10.1016/j.jaci.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 238.Muller U., Schmid-Grendelmeier P., Hausmann O., Helbling A. IgE to recombinant allergens Api m 1, Ves v 1, and Ves v 5 distinguish double sensitization from crossreaction in venom allergy. Allergy. 2012;67:1069–1073. doi: 10.1111/j.1398-9995.2012.02847.x. [DOI] [PubMed] [Google Scholar]
- 239.Mittermann I., Zidarn M., Silar M. Recombinant allergen-based IgE testing to distinguish bee and wasp allergy. J Allergy Clin Immunol. 2010;125:1300–1307 e1303. doi: 10.1016/j.jaci.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 240.Schiener M., Eberlein B., Moreno-Aguilar C. Application of recombinant antigen 5 allergens from seven allergy-relevant Hymenoptera species in diagnostics. Allergy. 2017;72:98–108. doi: 10.1111/all.13000. [DOI] [PubMed] [Google Scholar]
- 241.Muller U.R., Johansen N., Petersen A.B., Fromberg-Nielsen J., Haeberli G. Hymenoptera venom allergy: analysis of double positivity to honey bee and Vespula venom by estimation of IgE antibodies to species-specific major allergens Api m1 and Ves v5. Allergy. 2009;64:543–548. doi: 10.1111/j.1398-9995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 242.Leimgruber A., Lantin J.P., Frei P.C. Comparison of two in vitro assays, RAST and CAP, when applied to the diagnosis of anaphylactic reactions to honeybee or yellow jacket venoms. Correlation with history and skin tests. Allergy. 1993;48:415–420. doi: 10.1111/j.1398-9995.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 243.Gattinger P., Lupinek C., Kalogiros L. The culprit insect but not severity of allergic reactions to bee and wasp venom can be determined by molecular diagnosis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0199250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Scala E., Pirrotta L., Uasuf C.G. Aedes communis reactivity is associated with bee venom hypersensitivity: an in vitro and in vivo study. Int Arch Allergy Immunol. 2018;176:101–105. doi: 10.1159/000488866. [DOI] [PubMed] [Google Scholar]
- 245.Schiener M., Hilger C., Eberlein B. The high molecular weight dipeptidyl peptidase IV Pol d 3 is a major allergen of Polistes dominula venom. Sci Rep. 2018;8:1318. doi: 10.1038/s41598-018-19666-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kohler J., Blank S., Muller S. Component resolution reveals additional major allergens in patients with honeybee venom allergy. J Allergy Clin Immunol. 2014;133:1383–1389. doi: 10.1016/j.jaci.2013.10.060. 1389 e1381-1386. [DOI] [PubMed] [Google Scholar]
- 247.Monsalve R.I., Vega A., Marques L. Component-resolved diagnosis of vespid venom-allergic individuals: phospholipases and antigen 5s are necessary to identify Vespula or Polistes sensitization. Allergy. 2012;67:528–536. doi: 10.1111/j.1398-9995.2011.02781.x. [DOI] [PubMed] [Google Scholar]
- 248.Sturm G.J., Bilo M.B., Bonadonna P. Ves v 5 can establish the diagnosis in patients without detectable specific IgE to wasp venom and a possible north-south difference in Api m 1 sensitization in Europe. J Allergy Clin Immunol. 2012;130:817. doi: 10.1016/j.jaci.2012.05.047. author reply 818-819. [DOI] [PubMed] [Google Scholar]
- 249.Savi E., Peveri S., Makri E., Pravettoni V., Incorvaia C. Comparing the ability of molecular diagnosis and CAP-inhibition in identifying the really causative venom in patients with positive tests to Vespula and Polistes species. Clin Mol Allergy. 2016;14:3. doi: 10.1186/s12948-016-0040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ebo D.G., Faber M., Sabato V., Leysen J., Bridts C.H., De Clerck L.S. Component-resolved diagnosis of wasp (yellow jacket) venom allergy. Clin Exp Allergy. 2013;43:255–261. doi: 10.1111/cea.12057. [DOI] [PubMed] [Google Scholar]
- 251.Jin C., Focke M., Leonard R., Jarisch R., Altmann F., Hemmer W. Reassessing the role of hyaluronidase in yellow jacket venom allergy. J Allergy Clin Immunol. 2010;125:184–190 e181. doi: 10.1016/j.jaci.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 252.Blank S., Bilo M.B., Ollert M. Component-resolved diagnostics to direct in venom immunotherapy: important steps towards precision medicine. Clin Exp Allergy. 2018;48:354–364. doi: 10.1111/cea.13090. [DOI] [PubMed] [Google Scholar]
- 253.Blank S., Seismann H., Michel Y. Api m 10, a genuine A. mellifera venom allergen, is clinically relevant but underrepresented in therapeutic extracts. Allergy. 2011;66:1322–1329. doi: 10.1111/j.1398-9995.2011.02667.x. [DOI] [PubMed] [Google Scholar]
- 254.Blank S., Neu C., Hasche D., Bantleon F.I., Jakob T., Spillner E. Polistes species venom is devoid of carbohydrate-based cross-reactivity and allows interference-free diagnostics. J Allergy Clin Immunol. 2013;131:1239–1242. doi: 10.1016/j.jaci.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 255.Hemmer W., Altmann F., Holzweber F., Gruber C., Wantke F., Wohrl S. ImmunoCAP cellulose displays cross-reactive carbohydrate determinant (CCD) epitopes and can cause false-positive test results in patients with high anti-CCD IgE antibody levels. J Allergy Clin Immunol. 2018;141:372–381 e373. doi: 10.1016/j.jaci.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 256.Vos B., Kohler J., Muller S., Stretz E., Rueff F., Jakob T. Spiking venom with rVes v 5 improves sensitivity of IgE detection in patients with allergy to Vespula venom. J Allergy Clin Immunol. 2013;131:1225–1227. doi: 10.1016/j.jaci.2012.07.041. 1227 e1221. [DOI] [PubMed] [Google Scholar]
- 257.Bokanovic D., Schwarz I., Wutte N., Komericki P., Aberer W., Sturm G.J. Specificity of conventional and Ves v 5-spiked venom decreases with increasing total IgE. J Allergy Clin Immunol. 2014;134:739–741. doi: 10.1016/j.jaci.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 258.Blank S., Etzold S., Darsow U. Component-resolved evaluation of the content of major allergens in therapeutic extracts for specific immunotherapy of honeybee venom allergy. Hum Vaccines Immunother. 2017;13:2482–2489. doi: 10.1080/21645515.2017.1323603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Michel J., Brockow K., Darsow U. Added sensitivity of component-resolved diagnosis in hymenoptera venom-allergic patients with elevated serum tryptase and/or mastocytosis. Allergy. 2016;71:651–660. doi: 10.1111/all.12850. [DOI] [PubMed] [Google Scholar]
- 260.Galindo-Bonilla P.A., Galan-Nieto A., Alfaya-Arias T., Garcia-Rodriguez C., de la Roca-Pinzon F., Feo-Brito F. Component-resolved diagnosis in vespid venom-allergic individuals. Allergol Immunopathol. 2015;43:398–402. doi: 10.1016/j.aller.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 261.Ruiz B., Serrano P., Moreno C. IgE-api m 4 is useful for identifying a particular phenotype of bee venom allergy. J Investig Allergol Clin Immunol. 2016;26:355–361. doi: 10.18176/jiaci.0053. [DOI] [PubMed] [Google Scholar]
- 262.Potiwat R., Sitcharungsi R. Ant allergens and hypersensitivity reactions in response to ant stings. Asian Pac J Allergy Immunol. 2015;33:267–275. [PubMed] [Google Scholar]
- 263.Jeong K.Y., Yi M.H., Son M. IgE reactivity of recombinant pac c 3 from the asian needle ant (pachycondyla chinensis) Int Arch Allergy Immunol. 2016;169:93–100. doi: 10.1159/000444364. [DOI] [PubMed] [Google Scholar]
- 264.Hamilton R.G., Kleine-Tebbe J. Molecular allergy diagnostics: analytical features that support clinical decisions. Curr Allergy Asthma Rep. 2015;15:57. doi: 10.1007/s11882-015-0556-7. [DOI] [PubMed] [Google Scholar]
- 265.Aalberse R.C., Aalberse J.A. Molecular allergen-specific IgE assays as a complement to allergen extract-based sensitization assessment. J Allergy Clin Immunol Pract. 2015;3:863–869. doi: 10.1016/j.jaip.2015.09.013. quiz 870. [DOI] [PubMed] [Google Scholar]
- 266.Matricardi P.M., Kleine-Tebbe J. Molecular allergology between precision medicine and the choosing wisely initiative. Clin Exp Allergy. 2016;46:664–667. doi: 10.1111/cea.12679. [DOI] [PubMed] [Google Scholar]
- 267.Valenta R., Karaulov A., Niederberger V. Allergen extracts for in vivo diagnosis and treatment of allergy: is there a future? J Allergy Clin Immunol Pract. 2018;6 doi: 10.1016/j.jaip.2018.08.032. 1845-1855 e1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Weghofer M., Grote M., Resch Y. Identification of Der p 23, a peritrophin-like protein, as a new major Dermatophagoides pteronyssinus allergen associated with the peritrophic matrix of mite fecal pellets. J Immunol. 2013;190:3059–3067. doi: 10.4049/jimmunol.1202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Tuppo L., Alessandri C., Pomponi D. Peamaclein--a new peach allergenic protein: similarities, differences and misleading features compared to Pru p 3. Clin Exp Allergy. 2013;43:128–140. doi: 10.1111/cea.12028. [DOI] [PubMed] [Google Scholar]
- 270.Curin M., Garib V., Valenta R. Single recombinant and purified major allergens and peptides: how they are made and how they change allergy diagnosis and treatment. Ann Allergy Asthma Immunol. 2017;119:201–209. doi: 10.1016/j.anai.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Holzweber F., Svehla E., Fellner W. Inhibition of IgE binding to cross-reactive carbohydrate determinants enhances diagnostic selectivity. Allergy. 2013;68:1269–1277. doi: 10.1111/all.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Garib V., Wollmann E., Djambekova G. Possible effect of landscape design on IgE recognition profiles of two generations revealed with micro-arrayed allergens. Allergy. 2017;72:1579–1582. doi: 10.1111/all.13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Incorvaia C., Mauro M., Ridolo E., Makri E., Montagni M., Ciprandi G. A pitfall to avoid when using an allergen microarray: the incidental detection of IgE to unexpected allergens. J Allergy Clin Immunol Pract. 2015;3:879–882. doi: 10.1016/j.jaip.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 274.Macchia D., Melioli G., Pravettoni V. Erratum to: guidelines for the use and interpretation of diagnostic methods in adult food allergy. Clin Mol Allergy. 2015;13:31. doi: 10.1186/s12948-015-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Rosario N.A.L. Does sensitization to food allergens in patients with rhinitis mean food allergy? J Allergy Ther. 2014;5:2. [Google Scholar]
- 276.Elisyutina O., Fedenko E., Campana R. Bet v 1-specific IgE levels and PR-10 reactivity discriminate silent sensitization from phenotypes of birch allergy. Allergy. 2019;74(12):2525–2528. doi: 10.1111/all.13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Fernandez-Rivas M. Fruit and vegetable allergy. Chem Immunol Allergy. 2015;101:162–170. doi: 10.1159/000375469. [DOI] [PubMed] [Google Scholar]
- 278.Melioli G., Passalacqua G., Canonica G.W., Baena-Cagnani C.E., Matricardi P. Component-resolved diagnosis in pediatric allergic rhinoconjunctivitis and asthma. Curr Opin Allergy Clin Immunol. 2013;13:446–451. doi: 10.1097/ACI.0b013e32836274d8. [DOI] [PubMed] [Google Scholar]
- 279.Schmid-Grendelmeier P. [Recombinant allergens. For routine use or still only science?] Hautarzt. 2010;61:946–953. doi: 10.1007/s00105-010-1967-y. [DOI] [PubMed] [Google Scholar]
- 280.Schmid-Grendelmeier P. [Pollen allergy and immunotherapy] Ther Umsch. 2012;69:239–248. doi: 10.1024/0040-5930/a000280. [DOI] [PubMed] [Google Scholar]
- 281.Marti E., Wang X., Jambari N.N. Novel in vitro diagnosis of equine allergies using a protein array and mathematical modelling approach: a proof of concept using insect bite hypersensitivity. Vet Immunol Immunopathol. 2015;167:171–177. doi: 10.1016/j.vetimm.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 282.Prosperi M.C., Belgrave D., Buchan I., Simpson A., Custovic A. Challenges in interpreting allergen microarrays in relation to clinical symptoms: a machine learning approach. Pediatr Allergy Immunol. 2014;25:71–79. doi: 10.1111/pai.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Jutel M., Agache I., Bonini S. International consensus on allergen immunotherapy II: mechanisms, standardization, and pharmacoeconomics. J Allergy Clin Immunol. 2016;137:358–368. doi: 10.1016/j.jaci.2015.12.1300. [DOI] [PubMed] [Google Scholar]
- 284.Passalacqua G., Canonica G.W. Allergen immunotherapy: history and future developments. Immunol Allergy Clin N AM. 2016;36:1–12. doi: 10.1016/j.iac.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 285.Jutel M., Akdis C.A. Immunological mechanisms of allergen-specific immunotherapy. Allergy. 2011;66:725–732. doi: 10.1111/j.1398-9995.2011.02589.x. [DOI] [PubMed] [Google Scholar]
- 286.Wawrzyniak P., Akdis C.A., Finkelman F.D., Rothenberg M.E. Advances and highlights in mechanisms of allergic disease in 2015. J Allergy Clin Immunol. 2016;137:1681–1696. doi: 10.1016/j.jaci.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 287.Berings M., Karaaslan C., Altunbulakli C. Advances and highlights in allergen immunotherapy: on the way to sustained clinical and immunologic tolerance. J Allergy Clin Immunol. 2017;140:1250–1267. doi: 10.1016/j.jaci.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 288.Bousquet J., Khaltaev N., Cruz A.A. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the world Health organization, GA(2)len and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 289.Bousquet J., Heinzerling L., Bachert C. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012;67:18–24. doi: 10.1111/j.1398-9995.2011.02728.x. [DOI] [PubMed] [Google Scholar]
- 290.Bousquet P.J., Castelli C., Daures J.P. Assessment of allergen sensitization in a general population-based survey (European Community Respiratory Health Survey I) Ann Epidemiol. 2010;20:797–803. doi: 10.1016/j.annepidem.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 291.Arbes S.J., Jr., Gergen P.J., Elliott L., Zeldin D.C. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–383. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 292.Cox L., Jacobsen L. Comparison of allergen immunotherapy practice patterns in the United States and Europe. Ann Allergy Asthma Immunol. 2009;103:451–459. doi: 10.1016/S1081-1206(10)60259-1. quiz 459-461, 495. [DOI] [PubMed] [Google Scholar]
- 293.Nelson H.S. Specific immunotherapy with allergen mixes: what is the evidence? Curr Opin Allergy Clin Immunol. 2009;9:549–553. doi: 10.1097/ACI.0b013e328330ee69. [DOI] [PubMed] [Google Scholar]
- 294.Jakob T., Muller U., Helbling A., Spillner E. Component resolved diagnostics for hymenoptera venom allergy. Curr Opin Allergy Clin Immunol. 2017;17:363–372. doi: 10.1097/ACI.0000000000000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Focke M., Marth K., Flicker S., Valenta R. Heterogeneity of commercial timothy grass pollen extracts. Clin Exp Allergy. 2008;38:1400–1408. doi: 10.1111/j.1365-2222.2008.03031.x. [DOI] [PubMed] [Google Scholar]
- 296.Focke M., Marth K., Valenta R. Molecular composition and biological activity of commercial birch pollen allergen extracts. Eur J Clin Investig. 2009;39:429–436. doi: 10.1111/j.1365-2362.2009.02109.x. [DOI] [PubMed] [Google Scholar]
- 297.Valenta R., Twaroch T., Swoboda I. Component-resolved diagnosis to optimize allergen-specific immunotherapy in the Mediterranean area. J Investig Allergol Clin Immunol. 2007;17(Suppl 1):36–40. [PubMed] [Google Scholar]
- 298.Moreno C., Justicia J.L., Quiralte J. Olive, grass or both? Molecular diagnosis for the allergen immunotherapy selection in polysensitized pollinic patients. Allergy. 2014;69:1357–1363. doi: 10.1111/all.12474. [DOI] [PubMed] [Google Scholar]
- 299.Sastre J., Landivar M.E., Ruiz-Garcia M., Andregnette-Rosigno M.V., Mahillo I. How molecular diagnosis can change allergen-specific immunotherapy prescription in a complex pollen area. Allergy. 2012;67:709–711. doi: 10.1111/j.1398-9995.2012.02808.x. [DOI] [PubMed] [Google Scholar]
- 300.Martinez-Canavate Burgos A., Torres-Borrego J., Molina Teran A.B. Molecular sensitization patterns and influence of molecular diagnosis in immunotherapy prescription in children sensitized to both grass and olive pollen. Pediatr Allergy Immunol. 2018;29:369–374. doi: 10.1111/pai.12866. [DOI] [PubMed] [Google Scholar]
- 301.Del-Rio Camacho G., Montes Arjona A.M., Fernandez-Cantalejo Padial J., Rodriguez Catalan J. How molecular diagnosis may modify immunotherapy prescription in multi-sensitized pollen-allergic children. Allergol Immunopathol. 2018;46:552–556. doi: 10.1016/j.aller.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 302.Stringari G., Tripodi S., Caffarelli C. The effect of component-resolved diagnosis on specific immunotherapy prescription in children with hay fever. J Allergy Clin Immunol. 2014;134:75–81. doi: 10.1016/j.jaci.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 303.Arroabarren E., Echechipia S., Galbete A., Lizaso M.T., Olaguibel J.M., Tabar A.I. Association between component-resolved diagnosis of house dust mite allergy and efficacy and safety of specific immunotherapy. J Investig Allergol Clin Immunol. 2019;29:164–167. doi: 10.18176/jiaci.0359. [DOI] [PubMed] [Google Scholar]
- 304.Sastre J., Rodriguez F., Campo P., Laffond E., Marin A., Alonso M.D. Adverse reactions to immunotherapy are associated with different patterns of sensitization to grass allergens. Allergy. 2015;70:598–600. doi: 10.1111/all.12575. [DOI] [PubMed] [Google Scholar]
- 305.Nolte M., Barber D., Maloney J. Timothy specific IgE levels are associated with efficacy and safety of timothy grass sublingual immunotherapy tablet. Ann Allergy Asthma Immunol. 2015;115:509–515 e502. doi: 10.1016/j.anai.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 306.Sturm G.J., Varga E.M., Roberts G. EAACI guidelines on allergen immunotherapy: hymenoptera venom allergy. Allergy. 2018;73:744–764. doi: 10.1111/all.13262. [DOI] [PubMed] [Google Scholar]
- 307.Tripodi S., Frediani T., Lucarelli S. Molecular profiles of IgE to Phleum pratense in children with grass pollen allergy: implications for specific immunotherapy. J Allergy Clin Immunol. 2012;129:834–839 e838. doi: 10.1016/j.jaci.2011.10.045. [DOI] [PubMed] [Google Scholar]
- 308.Pauli G., Larsen T.H., Rak S. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–960. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 309.Cromwell O., Hafner D., Nandy A. Recombinant allergens for specific immunotherapy. J Allergy Clin Immunol. 2011;127:865–872. doi: 10.1016/j.jaci.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 310.Douladiris N., Savvatianos S., Roumpedaki I., Skevaki C., Mitsias D., Papadopoulos N.G. A molecular diagnostic algorithm to guide pollen immunotherapy in southern Europe: towards component-resolved management of allergic diseases. Int Arch Allergy Immunol. 2013;162:163–172. doi: 10.1159/000353113. [DOI] [PubMed] [Google Scholar]
- 311.Sastre J. Molecular diagnosis and immunotherapy. Curr Opin Allergy Clin Immunol. 2013;13:646–650. doi: 10.1097/ACI.0b013e328364f4c6. [DOI] [PubMed] [Google Scholar]
- 312.Sastre J., Sastre-Ibanez M. Molecular diagnosis and immunotherapy. Curr Opin Allergy Clin Immunol. 2016;16:565–570. doi: 10.1097/ACI.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 313.Matricardi P.M., Dramburg S., Potapova E., Skevaki C., Renz H. Molecular diagnosis for allergen immunotherapy. J Allergy Clin Immunol. 2019;143:831–843. doi: 10.1016/j.jaci.2018.12.1021. [DOI] [PubMed] [Google Scholar]
- 314.Bolhaar S.T., Tiemessen M.M., Zuidmeer L. Efficacy of birch-pollen immunotherapy on cross-reactive food allergy confirmed by skin tests and double-blind food challenges. Clin Exp Allergy. 2004;34:761–769. doi: 10.1111/j.1365-2222.2004.1939.x. [DOI] [PubMed] [Google Scholar]
- 315.Mauro M., Russello M., Incorvaia C. Birch-apple syndrome treated with birch pollen immunotherapy. Int Arch Allergy Immunol. 2011;156:416–422. doi: 10.1159/000323909. [DOI] [PubMed] [Google Scholar]
- 316.Bucher X., Pichler W.J., Dahinden C.A., Helbling A. Effect of tree pollen specific, subcutaneous immunotherapy on the oral allergy syndrome to apple and hazelnut. Allergy. 2004;59:1272–1276. doi: 10.1111/j.1398-9995.2004.00626.x. [DOI] [PubMed] [Google Scholar]
- 317.van Hoffen E., Peeters K.A., van Neerven R.J. Effect of birch pollen-specific immunotherapy on birch pollen-related hazelnut allergy. J Allergy Clin Immunol. 2011;127:100–101. doi: 10.1016/j.jaci.2010.08.021. 101 e101-103. [DOI] [PubMed] [Google Scholar]
- 318.Asero R. Effects of birch pollen-specific immunotherapy on apple allergy in birch pollen-hypersensitive patients. Clin Exp Allergy. 1998;28:1368–1373. doi: 10.1046/j.1365-2222.1998.00399.x. [DOI] [PubMed] [Google Scholar]
- 319.Lukschal A., Wallmann J., Bublin M. Mimotopes for Api g 5, a relevant cross-reactive allergen, in the celery-mugwort-birch-spice syndrome. Allergy Asthma Immunol Res. 2016;8:124–131. doi: 10.4168/aair.2016.8.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Wollmann E., Lupinek C., Kundi M., Selb R., Niederberger V., Valenta R. Reduction in allergen-specific IgE binding as measured by microarray: a possible surrogate marker for effects of specific immunotherapy. J Allergy Clin Immunol. 2015;136:806–809 e807. doi: 10.1016/j.jaci.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Burk C.M., Kulis M., Leung N., Kim E.H., Burks A.W., Vickery B.P. Utility of component analyses in subjects undergoing sublingual immunotherapy for peanut allergy. Clin Exp Allergy. 2016;46:347–353. doi: 10.1111/cea.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Canonica G.W., Bachert C., Hellings P. Allergen immunotherapy (AIT): a prototype of precision medicine. World Allergy Organ J. 2015;8:31. doi: 10.1186/s40413-015-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Schmid J.M., Wurtzen P.A., Dahl R., Hoffmann H.J. Pretreatment IgE sensitization patterns determine the molecular profile of the IgG4 response during updosing of subcutaneous immunotherapy with timothy grass pollen extract. J Allergy Clin Immunol. 2016;137:562–570. doi: 10.1016/j.jaci.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 324.Sindher S.B., Long A., Acharya S., Sampath V., Nadeau K.C. The use of biomarkers to predict aero-allergen and food immunotherapy responses. Clin Rev Allergy Immunol. 2018;55:190–204. doi: 10.1007/s12016-018-8678-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Passalacqua G., Bagnasco D., Ferrando M., Heffler E., Puggioni F., Canonica G.W. Current insights in allergen immunotherapy. Ann Allergy Asthma Immunol. 2018;120:152–154. doi: 10.1016/j.anai.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 326.Roberts G., Pfaar O., Akdis C.A. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73:765–798. doi: 10.1111/all.13317. [DOI] [PubMed] [Google Scholar]
- 327.Melioli G., Savi E., Crivellaro M.A., Passalacqua G. Potential of molecular based diagnostics and its impact on allergen immunotherapy. Asthma Res Pract. 2016;2:9. doi: 10.1186/s40733-016-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Barber D., de la Torre F., Feo F. Understanding patient sensitization profiles in complex pollen areas: a molecular epidemiological study. Allergy. 2008;63:1550–1558. doi: 10.1111/j.1398-9995.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- 329.Curin M., Reininger R., Swoboda I., Focke M., Valenta R., Spitzauer S. Skin prick test extracts for dog allergy diagnosis show considerable variations regarding the content of major and minor dog allergens. Int Arch Allergy Immunol. 2011;154:258–263. doi: 10.1159/000321113. [DOI] [PubMed] [Google Scholar]
- 330.Lepage-Nefkens I., Van der Maas M., Rijnen M., Hermansson L. A cost-effectiveness model evaluating component-resolved diagnosis (CRD) versus standard testing method (skin prick testing (SPT)) in the diagnosis and treatment of allergic rhinitis in The Netherlands. Value Health. 2015;18:A359. [Google Scholar]
- 331.Gomez R.M., Ansotegui I., Canonica G.W. Will precision medicine be available for all patients in the near future? Curr Opin Allergy Clin Immunol. 2019;19(1):75–80. doi: 10.1097/ACI.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 332.Mothes-Luksch N., Jordakieva G., Hinterholzl L. Allergy diagnosis from symptoms to molecules, or from molecules to symptoms: a comparative clinical study. World Allergy Organ J. 2018;11:22. doi: 10.1186/s40413-018-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Hauser M., Roulias A., Ferreira F., Egger M. Panallergens and their impact on the allergic patient. Allergy Asthma Clin Immunol. 2010;6:1. doi: 10.1186/1710-1492-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Heffler E., Puggioni F., Descalzi D., Racca F., Canonica G.W., Melioli G. Microarray immunodiagnostics for aeroallergens. Curr Allergy Asthma Rep. 2019;19:10. doi: 10.1007/s11882-019-0832-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.