Abstract
Liposomes are very useful biocompatible tools used in diverse scientific disciplines, employed for the vehiculation and delivery of lipophilic, ampiphilic or hydrophilic compounds. Liposomes have gained the importance as drug carriers, as the drugs alone have limited targets, higher toxicity and develop resistance when used in higher doses. Conventional liposomes suffer from several drawbacks like encapsulation inefficiencies and partially controlled particle size. The surface chemistry of liposome technology started from simple conventional vesicles to second generation liposomes by modulating their lipid composition and surface with different ligands. Introduction of polyethylene glycol to lipid anchor was the first innovative strategy which increased circulation time, delayed clearance and opsonin resistance. PEGylated liposomes have been found to possess higher drug loading capacity up to 90% or more and some drugs like CPX-1 encapsuled in such liposomes have increased the disease control up to 73% patients suffering from colorectal cancer. The surface of liposomes have been further liganded with small molecules, vitamins, carbohydrates, peptides, proteins, antibodies, aptamers and enzymes. These advanced liposomes exhibit greater solubility, higher stability, long-circulating time and specific drug targeting properties. The immense utility and demand of surface modified liposomes in different areas have led their way to the modern market. In addition to this, the multi-drug carrier approach of targeted liposomes is an innovative method to overcome drug resistance while treating ceratin tumors. Presently, several second-generation liposomal formulations of different anticancer drugs are at various stages of clinical trials. This review article summarizes briefly the preparation of liposomes, strategies of disease targeting and exclusively the surface modifications with different entities and their clinical applications especially as drug delivery system.
Keywords: Liposomes, Ligand-targeted liposomes, Immunoliposome, Aptasome, Enzymosome
Introduction
Liposomes are made up of one or more double-layered phospholipid vesicles that enclose a discrete aqueous space, used in diverse scientific disciplines from mathematics, biophysics, biochemistry to biology, etc. The drug-loaded liposomes have improved their therapeutic index by modifying absorption, decreasing metabolism, enhancing half-life or lessening toxicity (Sakamoto et al. 2010). The liposome characteristics vary greatly with size, charge, lipid composition and preparation methods. Liposomes are biocompatible, non-toxic and biodegradable entities which help to enhance the efficacy, improve the stability and minimize the toxicity of encapsulated drugs (Samad et al. 2007). Liposomes are widely used for enhanced solubility, specific drug targeting, proper delivery and controlled release of various formulations (Yun et al. 2015). Recently, mathematical models by partial differential equations and graphical representations have been proposed to study the liposome-encapsulated drug kinetics, drug release, interation with proteins and tumor cell receptors, internalization through endocytosis and plasma clearance (Chakravarty and Dalal 2018).
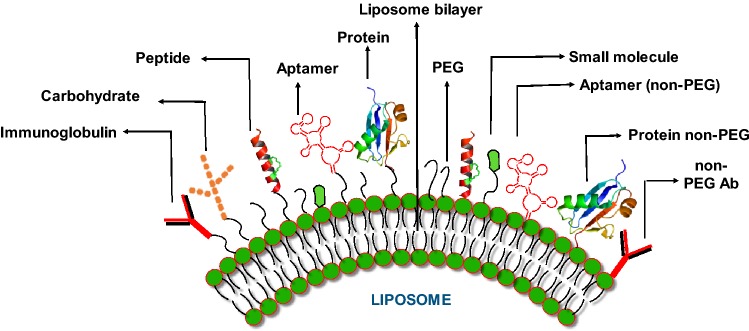
Conventional liposomes (basic forms) are considered as first-generation liposomes, composed of phospholipid bilayer with anionic, cationic or neutral phospholipids and cholesterol enclosing an aqueous space (Riaz et al. 2018). One of the major shortcomings of the these traditional liposomes is short life span during intravenous circulation due to uptake by reticuloendothelial system (RES). Phagocytes with the help of opsonins identify them as foreign bodies and engulf them quickly. Keeping these limitations of liposomes in view, different researchers have engineered the surface of these particles with various substances, thus enhancing the repulsion with serum components (Steichen et al. 2013) (Fig. 1).
Fig. 1.
Modification of liposome surface with different ligands (targeted liposomes). The different modifications can be achieved using small molecules, peptides, carbohydrates, aptamers, proteins/enzymes. These entities can be bonded to liposome phospholipid bilayer directly or through polyethylene glycol
The first innovative modification of liposomes leads to the conjugation of polyethylene glycol (PEG) to lipid anchor, which resulted in significant circulation time, stress stabilization and protection against opsonins. These additional features resulted in delayed clearance from hepatic RES, so greatly enhancing circulation time (Kamaly et al. 2012). The PEGylated liposomes act efficiently as drug carriers and show enhanced accumulation near tumors (Torchilin and Lukyanov 2003). In addition to PEG, the surface modification of liposomes has been performed with suitable ligands like small molecules, vitamins (Sperling and Parak 2010), carbohydrates (Faraasen et al. 2003), polysaccharides (Sihorkar and Vyas 2001), peptides (Torchilin 2005), aptamers (Farokhzad et al. 2006), antibodies (Anabousi et al. 2005) and enzymes based on their application (Nobs et al. 2004).
These additional components have drastically enhanced the applications of liposomes in different fields like drug delivery, cancer detection and therapy. The utilization of supplementary biomolecules on the surface of liposomes works as ligands for the receptors expressed as docking sites on the interested tissues. The targeted delivery of different formulations, common drugs and anticancer agents at the required location, i.e., tumor has revolutionized the field of liposome applications. Targeted liposomes are more specific, tissue oriented, have minimum off-target effects, and are required in lesser quantity as compared to conventional liposomes. Immunoliposomes (antibody coupled liposomes) have been used as the most efficient targeted liposome to bind with their ideal antigens (Salim et al. 2014).
Targeting of ligands on liposomes is usually performed by utilizing three types of reactions as by the formation of amide bond, thioester bond and disulfide bond (Nobs et al. 2004). Liposome functionalization with different specific ligands like small molecules, vitamins, peptides, antibody fragments or whole antibody, aptamers or enzymes enhances the efficiency of targeted delivery of anticancer agents at the requisite site (Riaz et al. 2018).
During 1970s, the liposomes were first used as drug delivery system for humans, which has now gradually improved as a special treatment system, and currently several commercial liposomal formulations are available in the market against several diseases including cancer (Lasic and Papahadjopoulos 1995; Singh et al. 2012). Liposomal formulation of amphotericin B and doxorubicin (Lopez-Berestein et al. 1985; Gabizon et al. 1989) was the first clinical trial vehicle used for human beings. Nowadays, there are different liposomal products available in the market which include antibacterial, antifungal and mostly anticancer formulations. In addition to this, liposomal formulations are available as therapeutic agents to treat eye, skin and respiratory disorders (Adjei 1997).
There are several special liposomal formulations available for the clinical practice which include chemotherapeutics as cytarabine (DepoCyte1) daunorubicin (DaunoXome1), doxorubicin (Doxil1/Caelys1), doxorubicin (Myocet1) use for the treatment of AIDS related Kaposi’s sarcoma, ovarian cancer, lymphomas, multiple myeloma, leukemia, etc. In addition to this, several other liposomal formulations of mitoxantrone, camptothecine, annamycin, cisplatin, 9-nitro-20(S)-camptothecine, lurtotecan, paclitaxel, iritotecan, vincristine, and floxuridine are at various stages of clinical trials (Slingerland et al. 2012).
Here, in this review, we are neither focusing on the structure of the phospholipid bilayer molecules nor on the structure of the liganded molecules. We have evaluated the latest updates on liposome preparation, strategies of its targeting, multidrug delivery and the surface modifications of liposomes with various ligands by consulting competitive literature available.
Liposome preparation
The preparation and size reduction of liposomes by conventional techniques is popular due to its simplicity and least requirements of sophisticated equipment. However, the growing demand and point-of-care applications of liposomes have motivated the improvement of conventional methods to second generation liposomal formulations (Nguyen et al. 2016). The preparation of liposomes basically involves three main steps as: vesicle formation, vesicle volume reduction and its purification. The ordinary liposomes are simply prepared by adding phospholipids to an aqueous medium and these phospholipids organize and form vesicles with one or more bilayers (Lopes et al. 2013; Singh 2018).
Different strategies are employed to prepare liposomes, depending upon the desired formulation. These methods differ on their approach to overcome the hydrophobic nature of lipids when mixed with water. Accordingly, these methods are classified as detergent solubilization, solvent evaporation, mechanical agitation, solvent injection. All these methods involve passive drug loading (Çagdaş et al. 2014).
Liposomes are most commonly prepared by lipid hydration and the organic solvent replacement by an aqueous medium. These methods can be performed either by reverse-phase evaporation or by organic-solvent injection. The lipid hydration is followed by manual stirring or by vortex (Bangham’s method), followed by solvent removal, under reduced pressure or by the method of rotary evaporation, until a thin film is formed. Aqueous medium is used to hydrate the thin film above the phase-transition temperature, which results in multilamellar vesicle (MLV) liposome formation (Bangham et al. 1965; Wagner and Vorauer-Uhl 2011). Furthermore, different strategies like sonication, extrusion, homogenization or microfluidization convert MLV to large unilamellar vesicles (LUV) or small unilamellar vesicles (SUV). The final purified liposome preparation is performed by either centrifugation, dialysis, ultrafiltration or by column chromatography.
In addition to this, several stratagies are further applied to prepared targeted liposomes based on scale of production, liposome lipid structure and charge, drug encapsulation efficiency, physicochemical characteristics of encapsulating drugs and the administrative route (Lasch et al. 2003). Targeted liposome preparation is also performed by checking the nature of ligand attached (small molecule, peptide, protein, aptamer, antibody fragments, whole antibody, etc.), and drug release as long release or triggered release (Storm and Crommelin 1998).
The most common methods of liposome characterization include Bartlet method, thin layer chromatography (TLC), size-exclusion chromatography, high-performance liquid chromatogragphy (HPLC), gas chromatography (GC), nuclear magnetic resonance (P31-NMR), electron microscopy and X-ray diffraction and scattering (Edwards and Baeumner 2006).
Strategies for liposome targeting to disease sites
Different approaches have been adopted for targeting liposomes to the disease sites, especially the tumor (Deshpande et al. 2013). These approaches include passive/spontaneous liposomal targeting and active liposomal targeting.
Passive targeting
The basis for the passive targeting of liposomes at the disease sites and especially at tumor is mainly due to the leaky structure of tumor-associated blood vessels. Depending upon the cancer type, the size of the gaps between the endothelial cells ranges between 100 and 780 nm in tumor capillaries; while in normal endothelium, it is between 5 and 10 nm (Haley and Frenkel 2008). In addition to this, due to enhanced permeability and retention (EPR) effect at the site of tumor, this strategy is effective for targeting liposomes at such sites. Therefore, if liposomes are prepared with permissible size to extravasate in cancer tissue, an ideal passive targeting strategy is achieved (Lehtinen et al. 2012; Kim and Huang 2012).
A good example of passive targeting include the use of Sclareol solid lipid nanoparticles (Sclareol-SLNs), with an average particle size of 88 ± 5 nm, against human lung epithelial cancer cells, which showed sustained drug release even after a period of 48 h as compared to the free drug alone (Paszko and Senge 2012).
Active targeting
Keeping in view the various limitations of passively targeted liposomes, the new strategies led to the discovery of actively targeted liposomes. These liposomes are designed to lessen the off-target effects and are prepared by conjugating their surface with targeting moieties. The targeting moieties include small molecules, peptides, aptamers, antibody fragments or whole antibodies (Bryne et al. 2008; Egusquiaguirre et al. 2012). To efficiently aim liposomes at cancer cells, it is a prime requisite to attach the specific targeting moiety/moieties in enough amount to achieve maximum affinity for the tumor cell surface receptors (Egusquiaguirre et al. 2012). Recently, the strategy of tumor-specific active targeting has significantly increased by focusing on different macromolecules overexpressed in different cancer cells as:
-
Targeting cell surface receptors: The approach to target cell surface receptors expressed on cancer cells is the most common strategy to deliver the drugs by liposomes liganded with antibodies or other receptor specific ligands. The different targeting receptors in focus are as:
Targeting folate receptors: Folate receptors are frequently overexpressed in certain lung, ovarian, colon, breast, brain and kidney tumors. Due to the overexpression of such receptors on cancer cells as compared to the normal cells, this receptor has been efficiently used as a targeting ligand for specific drug delivery liposomes against different tumor cells (Low et al. 2007).
Targeting transferrin receptors: Increased expression of transferrin receptors on cancer cells is associated with higher iron demand due to quickly proliferating cancer cells (Ying et al. 2010). This overexpression of transferrin has led to design of transferrin targeted anticancer therapy (Li et al. 2009). Transferin-targeted stealth liposomes containing doxorubicin against liver cancer cells have been found to possess improved intracellular uptake, biodistribution and pharmacokinetic profile and has led to enhanced therapeutic efficacy (Li et al. 2009).
Targeting EGFRs: The epidermal growth factor receptors (EGFRs) mediates repair, growth and differentiation of non-cancerous cells (Lehtinen et al. 2012). Many solid tumors like non-small-cell lung cancer, colorectal cancer, squamous cell carcinoma of kidney, ovary, pancreas and prostate and breast cancer show its overexpression, thus making it a smart target for therapeutic drug delivery (Danhier et al. 2010). In tumor cells, this growth factor mediates several processes like angiogenesis, proliferation and metastasis.
-
Targeting the tumor microenvironment: There are several reasons which indicate that targeting the tumor microenvironment is advantageous as compared to targeting the cell surface receptors. Some of the reasons are: vascular destruction reduces the tumor growth and metastasis, the blockade for the liposome diffusion through the tumor can be overcome directly by tumor vasculature targeting, neovascular endothelial cell phenotypic variation can be curbed, which can prevent the resistance development, and in addition to this, for any cancer type, the tumor vasculature is non-specific (Byrne et al. 2008).
Targeting VEGF: Vascular endothelial growth factor (VEGF) and its receptors (VEGFR) are well known as proangiogenic proteins and are well-defined targets for antiangiogenic therapy (Li et al. 2008; Wicki et al. 2012). Bevacizumab is a monoclonal antibody used as an antihuman VEGF, which has been approved by United States Food and Drug Administration (US-FDA) and works as an anticancer drug. In addition to this drug, different inhibitors of tyrosine kinase receptors like VEGFRs or basic FGFRs have been used as anticancer agents (Katanasaka et al. 2008).
Targeting VCAM: As Vascular cell adhesion molecules (VCAM) are involved in inflammatory complications like cancer, they are good targets for anticancer therapy. Tumor vessels show overexpression of VCAM-1, and thus represents an interesting target for anticancer drug delivery (Kang et al. 2011).
Targeting B-Raf: Recently, an antitumor drug, vemurafenib, has been used for some cancer patients. It acts as a targeting agen for V600EB-Raf protein expressed in melanoma and such patients showed 80% regression during the first 2 months of treatment cycle. However, majority of the patients developed drug resistance, even though initially they were responsive to this drug (Aryal et al. 2011; Khdair et al. 2009).
Targeting matrix metalloproteases: These endopeptidases are involved in remodeling of tissues, tumor invasiveness, apoptosis resistance and metastasis. Inhibitors of MMP family proteins have been used to suppress angiogenesis in tumor-bearing mice (Hatakeyama et al. 2007).
Liposomal formulation with multidrug delivery approach
Drug resistance is a major complication towards a good outcome during a disease treatment. A single liposomal formulation with both hydrophilic and hydrophobic regions, can be designed for loading different types of anti-cancer drugs with synergizing drug ratios. The liposomal formulation delivers simultaneously the drugs with optimizing ratios at the tumor site, and thus used to decrease the resistance development. Two drugs, Plumbagin and Celecoxib, were identified to kill synergistically the melanoma cells as compared to the normal cells. The traditional use of these drugs in combined form was not certified due to its poor bioavailability and the toxicological issues. A nanoliposomal formulation of plumbagin and celecoxib, called Celeplum-777, was prepared against melanoma cells. This formulation is stable and releases the drugs at an ideal ratio to kill the melanoma cells only, inhibiting tumor growth by 72% without any apparent toxicity (Gowda et al. 2017).
Two antitumor drugs (Gemcitabine and Paclitaxel) were investigated to study the multidrug carrier approach of liposomes. The PEGylated liposomes encapsulated with these two drugs were found to possess high drug loading capacity (~ 90% for Gemcitabine and ~ 80% for Paclitaxel) and no destabilizing effect. Treatment of human breast cancer cells (Michigan Cancer Foundation-7 cells) with this formulation showed stronger cytotoxic activity as compared to freed and individual drug liposomal preparation. In addition to this, the formulation possessed a controlled release to these two compounds (Cosco et al. 2011).
Co-encapsulation of two hydrophilic drugs (irinotecan and floxuridine) as a liposomal formulation, also named as CPX-1 was found to be well tolerated during in vivo conditions, improved the pharmacokinetic profiles of these drugs, and increased the disease control in about 73% patients suffering from colorectal cancer (Harasym et al. 2007; Batist et al. 2009). In addition to this, the encapsulated drugs did not show any destabilizing behavior to colloidal structure and showed synergistic action on different cancer cell lines (Tardi et al. 2009).
Liposomes as carriers of prodrugs and conversion to active metabolites
A prodrug is a compound with little or no pharmacological properties, which are converted to active form (active drugs) by specific chemical reactions or enzymes in vivo or by a combination of the two mechanisms (Rautio et al. 2018). Prodrugs are designed to achieve bioavailability, when its active form is poorly absorbed by gastrointestinal (GI) tract (Nofsinger et al. 2014).
Liposomes are very efficient vehicles to carry prodrugs to achieve a proper pharmacokinetic goal of its active form. Lipophilic forms of methotrexate and melphalan as prodrugs have been loaded as unilamellar liposomes to overcome the resistance exhibited by human leukemia cells for initial drugs across the membrane (Kuznetsova et al. 2009). Methotrexate is widely used as an autoimmune and anticancer drug (McGuire 2003). The therapeutic efficacy of this native drug is impeded due to its toxicity and resistance by tumor cells (Pui 1995). The phospholipid derivative of methotrexate, esterified by lysophosphatidylcholine, acts as an active agent against leukemia (CEM/MTX) cells (Kozak et al. 2001).
Irinotecan hydrochloride (CPT-11) is a prodrug which is converted to its active form SN-38 at the tumor site. The active drug possesses efficient antitumor activity when used at proper concentration. PEGylated liposome preparation of CPT-11 enhances its antitumor activity due to increased blood circulation time. The formation of SN-38 within liposomal membranes occurs by incubation with carboxylesterase, present within the liposomes (Sadzuka 2000).
Surface modification of liposomes
Liposomes have been potentially used as drug carriers against different diseases including cancer therapy. To facilitate their use specifically and effectively, it is important to identify the chemistry of targeted cell surface receptors, identification of ligands to be used on liposome surface against these receptors, and delaying of fast clearance of liposomes by RES (Zhao et al. 2008). Here, we are describing the different liposomal surface modifications that address their efficient targeting against different diseases, especially cancer.
Surface modifications of liposomes with vitamins
The employment of vitamins as liposome ligands for efficient and targeted drug delivery has opened a vast field of their applications. Different types of cancer cells overexpress vitamin receptors more as compared to the normal cells; so, the knowledge of receptors for the docking of vitamin-liganded liposomes is crucial. The expression of different vitamins receptors are often raised in malignant phenotypes (Kuldo et al. 2005). Different vitamins used as liposome ligands for receptors on malignant cells are mostly folate, although tocopherol, pyridoxal phosphate or pyridoxine have also been used (Drummond et al. 2000) (Table 1).
Table 1.
Surface-engineered liposomes functionalized with different vitamins for varied clinical and research applications
| S. no | Vitamin | Liposome applications | References |
|---|---|---|---|
| 1 | Biotin | As DNA sensors | Patolsky et al. (2000) |
| Oral delivery of insulin | Zhang et al. (2014) | ||
| Delivery of quantum dots conjugated to epidermal growth factor ligand to target EGFR | Sigot et al. (2010) | ||
| Used for analysis of solutes during capillary electrophoresis | Yang et al. (1998) | ||
| 2 | Nicotinamide | Radiolabelling liposome | Laverman et al. (2000) |
| To study the thermal stability of formaldehyde dehydrogenase | Yoshimoto et al. (2011) | ||
| 3 | Vitamin A | siRNA carrier to ameliorate skin fibrosis | Yamakawa et al. (2018) |
| 4 | Pyridoxal phosphate | To study polarization anisotropy and fluorescence lifetime of liposomes | Greenaway and Ledbetter (1987) |
| 5 | Riboflavin | Riboflavin photolysis stability | Ahmad et al. (2015) |
| 6 | Tocopherol | Lipopolysaccharide induced lung injury | Suntres and Shek (1998) |
| Delivery of docetaxel | Raju et al. (2013) | ||
| Added to liposomes to enhance their antioxidant activity | Fex and Johannesson (1987) | ||
| 7 | Folic acid | Macrophage targeting with ovarian carcinoma | Turk et al. (2004) |
| Oligodeoxynucleotide targeting to cancer cells | Leamon et al. (2003) |
Vitamin E has been used as a liposomal ligand as d-alpha tocopheryl polyethylene glycol succinate (TPGS). It is a water-soluble amphiphilic structure with PEG as hydrophilic portion and tocopherol succinate as lipophilic group. Due to its hydrophilic–lipophilic balance value, TPGS works as an excellent solubilizer, emulsifier and bioavailability enhancer for hydrophobic drugs (Duhem et al. 2014). In addition to this, TPGS amplifies the drug absorption and its cytotoxicity, and slows down the multi-drug resistance (Pérez-Herrero and Fernández-Medarde 2015). Furthermore, to prevent the free radical induced damage of liposomes, vitamin E is used to prevent oxidative stress during its storage (Halliwell et al. 1995).
The tumor cells overexpress folate receptors on their plasma membranes which has a specific affinity with folic acid. The principle used for the conjugation of folate to liposomes is to prepare folate-linked peptides, cholesterol or phospholipids first, before the preparation of tumor specific liposomes. The normal tissues usually lacks the expression of folate receptors on their cells (O’Shannessy et al. 2015). So, this advantage of expression of folic acid receptor only by cancer cells has been utilized as a selective marker for liposomes for specific location and shipment of therapeutics to defective cells (Lu and Low 2002). Different types of chemotherapeutics, imaging agents, genes and antisense oligonucleotides have been delivered to tumor cells utilizing folate–folate receptor binding (Steichen et al. 2013).
During the earlier days of folate-conjugated liposome preparation, phospholipids were directly conjugated with folic acid. However, later it was observed that folate receptor expressed on tumor cells did not bind with folate-conjugated liposomes when folic acid was directly bonded to the phospholipids (Drummond et al. 2000). This limitation led to the use of many linkers like polyethylene glycol (PEG) and hydrazine in addition to direct binding of folic acid to cholesterol, protein or other spacers (D’souza and Shegokar 2016). The use of PEG in folate-conjugated liposomes enhanced their efficiency by increasing the solubility and half-life of the drug as well as improved the drug reserve at the tumor spot (Pasut et al. 2008). Folic acid binding with PEG enhanced the liposome retention near the tumor and enhanced its uptake by folate receptor-mediated endocytosis (Kim et al. 2008).
The overall data available which illustrate the use of vitamins other than folate as liposome ligands are few. However, the fame of folate targeting laid a good foundation for other vitamins as ligands for drug delivery by liposome (Kratz et al. 2013). Besides specific targeting, vitamins play some distinct roles in liposome technology. The advantage of non-covalent interaction between biotin and streptavidin has been utilized to prepare biotinylated liposomes for different purposes (Jain and Cheng 2017).
Different vitamins like biotin, nicotinamide, pyridoxal phosphate, pyridoxine, riboflavin have been liganded with liposomes for various diagnostic and therapeutic agents (Table 1).
-
2.
Surface modification of liposomes with carbohydrates
Several carbohydrates have been decorated on liposome surfaces to enhance drug delivery mission and have disclosed great opportunities in clinical field (Chua et al. 1984). It has been a great challenge to point out a ligand that contributes sufficient selectivity for the targeted cells. The binding of glycan ligands expressed on liposomes, by glycan binding protein receptors on different cells, has helped in targeted drug delivery tasks. The mannose has contributed sufficiently as mannosylated liposomes which work as productive drug carriers with no toxicity and very low intrinsic immunogenicity (Kawakami et al. 2000; Tsumoto et al. 2009) (Table 2).
Table 2.
Surface-modified liposomes liganded with different carbohydrates for varied clinical and research purposes
| S. no | Carbohydrate | Application of liposome | References |
|---|---|---|---|
| 1 | Glucose | Tumor study | Luciani et al. (2004) |
| Drug delivery for brain capillary endothelia cells | Xie et al. (2012) | ||
| 2 | Mannose | Efficient formation of giant liposomes | Tsumoto et al. (2009) |
| 3 | Galactose | To study galactose receptors on macrophages and to study the targeted delivery of galactose to hepatocytes | Managit et al. (2005) |
| 4 | Sucrose | Doxirubicin-loaded liposomes for cancer treatment | Song et al. (2009) |
| 5 | Maltose | Doxirubicin transport for cancer treatment | Song et al. (2009) |
| 6 | Lactose | To study liposome size and stability | Zhu et al. (2005) |
| 7 | Oligosaccharides | Therapeutic inhibitor designs | Galustian et al. (2004) |
| 8 | Lectins | Pulmonary drug delivery | Abu-Dahab et al. (2001) |
| 9 | Tomato lectin, wheat germ agglutinin | Oral administration of insulin | Zhang et al. (2005) |
Immunocompetent cells like dendritic cells and macrophages usually express mannose receptors, which are regarded as good recognition sites for liposomes with mannose, fructose and N-acetyl glucosamine. So, mannose-mediated drug transportation systems have been efficiently used for immune cells and this facility has been used to modify immune reactions (Wijagkanalan et al. 2008).
A study was conducted based on liposomes modified with disaccharides (sucrose or maltose) to study the intracellular uptake of anticancer drugs by certain cancer cells. The study showed that disaccharides-liganded liposomes work as excellent tools for drug targeting as malignant cells usually express lectins, the sugar binding proteins. In vitro uptake and cytotoxicity pattern against cancer cells by liposomes liganded with disaccharides were higher than sterically stabilized liposomes (Song et al. 2009).
Doxirubicin-containing liposomes, reshaped with a carbohydrate ligand, have been used for Human Burkitt lymphoma Daudi B-cell lines to find out the targeted delivery of specific chemotherapeutic medicines (Sapra and Allen 2003). These liposomes efficiently dock with B-cell-specific surface protein CD22 (Goldenberg 2001). Sialic acid is a group of diverse acidic monosaccharides, usually found as glycans of glycosphingolipids and glycoproteins, widely found on animal cell membranes. Different modifications of sialic acid diversify its applications for different purposes (Spevak et al. 1993). B-cell surface protein (CD 22) specifically binds with sialic acid and exhibits highly productive endocytosis, leading to rapid internalization of doxirubicin-loaded liposomes (Sun et al. 2016).
Glycoproteins play a major role in different cellular activities like recognition, immune response, inflammation, differentiation (Gordon 2002). Keeping this in view, lectins like concanavalin A have been used as highly specific binding tools for carbohydrates, which are conjugated to liposomal membranes (Abu-Dahab et al. 2001; Vyas et al. 2001).
Lectins have a unique ability to interact with exposed carbohydrate residues on other glycoproteins found on the epithelial cell membranes, so working as efficient bioadhesives. The binding specificity of wheat germ agglutinins liganded on liposomes has been used for transport of some cytotoxic drugs like doxorubicin to tumor cells (Murata et al. 2013). The lectin-mediated drug transport is an alternate to receptor-mediated transport of some ligands like vit. B12 or transferrin. The lectin-mediated liposomes have desired drug release characteristics including antibiotics, antitumor drugs, or plasmid and gene transport (Li et al. 2014).
Insulin has been transported through lectin-liganded liposomes by oral administration and this has led to the idea that peptide and protein drugs can be transported by same idea. Different lectins like Ulex europaeus agglutinin, tomato lectin and wheat germ agglutinin have been conjugated on liposome lipid bilayer (Kuzma 2009). Efficient hypoglycemic activity was found by these lectin-liganded insulin liposomes on diabetic mice as compared to abdominal cavity insulin injection treatment (Zhang et al. 2005; Du et al. 2009).
-
3.
Surface modification of liposomes with peptides, proteins, antibodies and antibody fragments
One of the greatest applications of liposomes is considered to be tumor treatments. The alteration of liposomes with small peptides, antibody fragments, or whole antibodies has been engineered with encouraging strategy against tumor (Sapra and Allen 2003). Different types of human cancer cells like bladder, lung, ovarian and breast cancers express EGFR, which is known as a significant target for therapy. This receptor is also known to be associated with different advanced diseases with poor prognosis (Herbst and Shin 2002) (Table 3).
Table 3.
Liposome surface engineered with antibody fragments and monoclonal antibodies used as targeted delivery of various anti-cancer drugs
| S. no | Antibody/antibody fragment | Liposome loaded drugs for different cancers | References |
|---|---|---|---|
| 1 | Mab CC52 | Liposome carrying 5-fluorodeoxyuridine against Colon cancer | Koning et al. (2002) |
| 2 | Anti B cell lymphoma Mab LL2 | Liposome carrying doxorubicin against Lymphoma | Lundberg et al. (2000) |
| 3 | Anti CD 32 | Liposome carrying oligonucleotides against leukemia | Ma and Wei (1996) |
| 4 | Mab AF 20 | Liposome carrying fluorescent dye used against hepatocarcinoma | Moradpour et al. (1995) |
| 5 | Mab 2C5 | Liposome carrying doxorubicin used against mammary adenocarcinoma and lung carcinoma | Lukyanov et al. (2004) |
| 6 | Anti CD 133 Mab | Liposomes containing bevacizumab against glioblastoma | Shin et al. (2015) |
| 7 | Anti CD 19 and anti CD 37 Mab | Liposome carrying fluorescent dye against leukemia | Yu et al. (2013) |
| 8 | Anti transferrin ScFv antibody fragment | Liposome carrying plasmid DNA against prostate cancer sever cancer cell lines | Xu et al. (2002) |
| 9 | Anti T24 | Liposome carrying pheophorbide a and used against bladder carcinoma | Bergstrom et al. (1994) |
| 10 | Anti FAP Sc Fv antibody | Liposome carrying fluorescent dye against metastatic fibrosarcoma | Tansi et al. (2016) |
| 11 | Anti-ovarian carcinoma Fab’ | Liposome used against ovarian carcinoma | Moradpour et al. (1995) |
| 12 | Fab’ | Liposome with doxorubicin against advanced solid tumors against EGFR receptor | Mamot et al. (2012) |
| 13 | scFv | Liposome carrying P53DNA plasmid against transferin of refractory cancers | Senzer et al. (2013) |
| 14 | scFv | Liposome with transtuzumab against advanced breast cancer | Miller et al. (2016) |
Different approaches have been applied to prepare immunoliposomes, either as whole antibodies directly bound to phospholipids by surface adsorption or through hapten binding. The antibodies are also attached by direct covalent bonding as a whole protein. The antibody can also be bound to liposome surface with the help of linkers like PEG or using the technique of avidin–biotin interaction (Torchilin 2005) (Fig. 2).
Fig. 2.
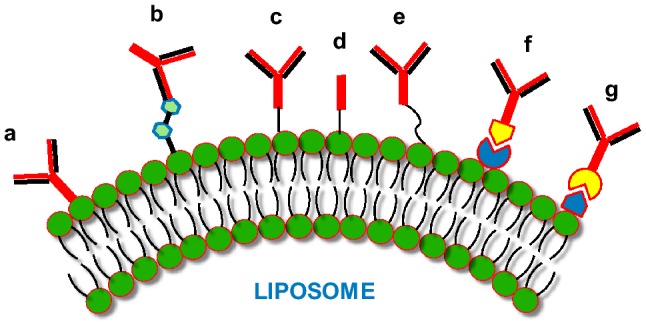
Immunoliposomes: The different antibodies/antibody fragments can be bound to liposome phospholipid surface by employing different procedures. These techniques include: (a) direct adsorption of antibody; (b) hapten binding; (c) binding of whole antibody; (d) covalent binding of Fab’; (e) binding of whole antibody by utilizing PEG spacers; (f) and (g) binding of whole antibody through avidin–biotin with either the biotin or avidin bound to the liposome surface
A novel approach has been applied to engineer and minimize conventional antibodies to form different antibody fragments like Fab, scFv, bispecific antibody, nanobody (Van Driel et al. 2016), bifunctional antibody (Holliger and Hudson 2005), minibody and diabody. These strategies have been applied to form different nanoparticle-antibody fragments and smaller-sized liposomes, which function as important biomedical tools in diagnostic and therapeutic areas (Richards et al. 2017).
The use of immunoliposomes for targeted drug delivery has emerged as an encouraging strategy for drug transportation and delivery for the cells presenting the antigen for the matching antibodies (Zhang et al. 2016). Both non-covalent and covalent strategies have been applied to use monoclonal antibodies or their fragments to be used as ligands on liposomal surface. The important targets with such liganded liposomes have been suggested for the treatment of different diseases like EGFR- and HER2-positive malignancies, neurodegenerative diseases, autoimmune and infectious diseases, cardiovascular and inflammatory disorders. However, immunoliposomes are still struggling for the clinical approval to be used widely (Wang and Thanou 2010).
Immunoliposomes targeted against EGFR have been efficient in delivery of doxorubicin against tumor cells; so, better anti-tumor results have been obtained when working on animal xenograft models (Mamot et al. 2003). Besides antibody fragments or whole antibodies, the peptide ligands have also been observed to possess specific interaction with receptors present on tumor neovasculature. This interaction was utilized for direct intervention of chemotherapeutics, gene constructs or other proteins (Das et al. 2009; Torchilin and Lukyanov 2003).
On the basis of comparative sequence and structural analysis of proteins and phage display screening, different attempts have been tried to search for effective ligands for many targets on cancer cells (Wu et al. 2016). Based on these trials, the screening of a novel peptide (GE11) for EGFR was reported. EGFR over-expressing cancer cells showed promising results for gene transfection (Song et al. 2008).
The protein data bank of EGFR is well known, and thus provided a straightforward template for computer-assisted design (CAD) for a novel ligand suitable for cancer therapy (Feiner 2019). Recently, a growth factor antagonist named as antagonist G (H-Arg-D-Trp-NmePhe-D-Trp-Leu-Met-NH2) was used on liposomes (Moreira et al. 2001). This antagonist G is a broad-range molecule which blocks competitively the action of multiple neuropeptides like vasopressin, bradykinin, gastrin-releasing peptide) by binding with different receptors through the residues D-Trp-NmePhe-D-Trp-Leu, which is found on small cell lung cancer cell surface (Arai et al. 2015).
-
4
Surface modification of liposomes with aptamers
Aptamers are short single-stranded DNA or RNA oligonucleotides (usually 25–90 nucleotide bases) which can bind through unique three-dimensional pattern with corresponding targets like proteins and cells (Płotka-Wasylka et al. 2016). Aptamers have been revealed as superior and fastest emerging new tools to target cancer biomarkers quite efficiently and are employed as effective ligands for drug delivery and anti-cancer therapy (Table 4). These ligands usually bind with affinities in nanomolar–picomolar range to target molecules (Dreaden et al. 2012; Yüce et al. 2015).
Table 4.
Liposome surface liganded with aptamers used as targeted delivery for several cancers
| S. no | Aptamer | Application of liposome | References |
|---|---|---|---|
| 1 | NCL-Aptamer | Cisplatin encapsulated liposome as chemotherapeutic agent against broad range of cancer | Cao et al. (2009) |
| 2 | sgc8 aptamer | Liposomes for leukemia CEM-CCRF cells | áO’Donoghue (2010) |
| 3 | NX 1838 | Aptamer having specific binds with VEGF on cancer cells | Brody and Gold (2000) |
| 4 | Anti-CD44 | Selective targeting of cancer cells | Alshaer et al. (2014) |
| 5 | DAG-NX213 | Specificity for VEGF, the inducer of angiogenesis | Yang et al. (2011) |
| 6 | AS1411 | Cytotoxicity to MCF-7 breast cancer cells | Xing et al. (2013) |
| 7 | Macugen | Treatment of macular age-related macular degeneration | Sun et al. (2014) |
| 8 | BOCK | Used to recognize distinct binding sites on thrombin | Jung et al. (2010) |
| 9 | TASSET | Used to recognize distinct binding sites on target protein | Jung et al. (2010) |
| 10 | xPSM-A9 | Used against prostate-specific membrane antigen expressed on prostate cancer cells | Baek et al. (2014) |
| 11 | IL-4R⍺ | Suppression of tumor growth by targeting tumor microenvironment | Liu et al. (2017) |
Aptamers are created by systematic evolution of ligands by exponential enrichment (SELEX) technique, which specifically bind with broad range of targets like cells, viruses, proteins, and small molecules (Daniels et al. 2003). For the targeted delivery of anticancer drugs, the nucleic acid aptamers have won the race as compared to antibodies. There are several reasons for aptamers to be efficient ligands as they are resistant to organic solvent, resistant to variation in temperature and pH and can have mass production through chemical synthesis. Besides this, aptamers are resistant to denaturation–renaturation cycles and are less immunogenic than antibodies (Niemeyer 2001; Shah et al. 2014; Yu et al. 2012).
Aptamer-liganded liposomes are most efficient drug-delivery systems. The US-FDA has approved a number of liposome-based therapies in different clinics for disease treatments (Cao et al. 2009). Aptamers have also been chemically modified with varied functional groups on either ends to enhance site-specific conjugation. Nucleic acid aptamers exhibit swift penetration to target cells and good serum retention due to better stability (Lee et al. 2016). Tumor growth and metastasis are contributed by some immunosuppressive cells like tumor-associated macrophages, myeloid-derived suppressor cells, and tumor-resident regulatory T cells (Fujimura et al. 2010). Targeting these immunosuppressive cells can improve the efficacy of cancer therapy.
The use of an IL-4Ra RNA aptamer blocks human IL-4 receptor and also suppresses myeloid-derived suppressor cells (Liu et al. 2017). CpG oligodeoxynucleotide-10 displays exceptional anti-tumor activity as aptamer (IL-4Ra)-liganded liposome selectively targets IL-4Ra receptor expressed on CT26 carcinoma cells, which expresses good level of IL-4Ra receptor. The efficient uptake of CpG by tumor cells noticeably inhibit in vivo CT26 tumor growth and this cancer-targeting aptamer delivery by liposomes may support to be an efficient approach for successful victory against immunosuppression and facilitating immunotherapy (Andon et al. 2017).
-
5.
Surface modification of liposomes with enzymes
Liposomes liganded with enzymes (enzymosomes) are emerging as a novel drug transportation and delivery system for site-specific action. The enzymosomes avail the same peculiar nature of an enzyme, binding to its specific substrate and catalyzing the normal product formation (Petrak 2005). The enzyme is covalently bound by physical adsorption, acetylation, direct conjugation, etc. to the surface of liposome to form the enzymosome. Enzymosomes prove to be efficient drug transportation and release vehicles and minimize unwanted side effects of some traditional treatment ways (Shefrin et al. 2017). They show efficient long duration treatment for some diseases and are promising substitutes of conventional management of anti-platelet activities, gout etc.
The covalent bonding of the enzymes with the liposome surface attributes the biological activity and conformational structural stability of the enzyme as reported with d-amino acid oxidase and catalase (Shefrin et al. 2017). Most commonly used enzymes used for the preparation of enzymosomes to enhance the potential of anti-tumor drugs include alkaline phosphatase, β-glucosidase, β-lactamase, and carboxypeptidase (Table 5).
Table 5.
Liposome surface engineered with different enzymes to study different biochemical activities
| S. no | Enzymosome | Liposome application | References |
|---|---|---|---|
| 1 | Brain glutamate-decarboxylase | To study the comparison of pyridoxal phosphate (PLP) activation and inhibition kinetics by PLP oxime-o-acetic acid between free and liposome bound form | Covarrubias and Tapia (1980) |
| 2 | SOD | To study retention, efficiency, enzymatic activity, incorporation, protein–lipid ratio and zeta potential between conventional and long circulating liposome (LCL) | Gaspar et al. (2003) |
| 3 | SOD | SOD-liposome and SOD-enzymosome were used to compare their therapeutic activities on different animal models for oxidative stress and liver ischemia, | Corvo et al. (2015) |
| 4 | Catalase | To study controlled oxidation of glucose by encapsulated glucose oxidase with decomposition of H2O2 | Yoshimoto et al. (2010) |
| 5 | Catalase–sSOD | Stability studies including half-life and activity | Skólmowska and Kmieć (2011) |
| 6 | C. utilis Uricase | Comparison between in vitro uricolytic activity of free and PEGylated uricase and in vivo uricase studies of its enzymosome in animal models | Tan et al. (2012) |
| 7 | NTP Dase-1 | To study the inhibition of platelet activation induced by ADP, collagen or thrombin | Chaikof et al. (2006) |
| 8 | β-glucuronidase | Immunoliposome directed against human ovarian cancer cells to study anti-tumor activity | Fonseca et al. (1999) |
| 9 | Carboxypeptidase | To study membrane targeting and biological functions | Mima et al. (2006) |
| 10 | Carbonic anhydrase | to study protein membrane interaction and refolding of denatured enzyme | Yoshimoto et al. (1998) |
| 11 | Glucose oxidase | To study the sensitivity of liposomes for glucose concentration | Jo et al. (2008) |
Carbonic anhydrase molecules have been encapsulated inside the liposomes for checking the permeability of some substrates including CO2 through lipid bilayer (Renggli et al. 2011). This approach has also been applied to study the liposomal reaction kinetics (Ramundo‐Orlando et al. 2007). A novel enzymosome containing uricase and catalase has been prepared recently against anti-hyperuricemia complication, and the use of these enzymosomes has proved to be much efficient than conventional uricase application and traditional chemotherapy (Zhou et al. 2016). In addition to this, these enzymosomes showed higher acid–base, thermal, hypothermal stability and much lowering effects of uric acid (Zhou et al. 2016).
To enhance the utility of superoxide dismutase (SOD), many attempts were made including covalent attachment with albumin, PEG, dextran and encapsulation within liposomes, but it was not quite efficient in its activity in different organs. In comparison with all these different methods of SOD vehiculation, SOD surface liganded liposomes have been found to possess enhanced incorporation efficiency and half-life (Bansal et al. 2011; Corvo et al. 1999). The covalent-liganded form of SOD on the liposome surface is assumed to be more advantageous rather than its encapsulated form, as its release at the inflammation site may not be required to achieve its therapeutic activity (Bansal et al. 2011).
Even though enzymosomes are emerging as novel drug delivery systems, there are some drawbacks with this type of liposome as well. The production cost of enzymosomes is usually higher, and the constituent phospholipids may undergo hydrolysis or oxidation. In some conditions, low solubility and shorter half-life curtail the bioavailability (Bansal et al. 2012).
Conclusion
Development of nanoscale drug transport and delivery setup with liganded liposomes provides an efficient solution to improve disease therapy. Liposomes have played a great role in drug targeting against cancer tissue. Drug distribution by liganded liposome has improved the therapeutics as compared to the free drugs. Free drugs are inefficient in targeting tumors as reticuloendothelial system remarkably reduces its peak levels. Long circulating liposomal formulations, including PEGylated liposomes, are engineered to survive identification and removal by reticuloendothelial system. Liposomal formulation of Doxorubicin, encapsulated in PEGylated liposomes (Doxil), was the first anti-cancer drug, approved by US-FDA. The efficiency of liposome targeting has been drastically improved by surface modification with vitamins, carbohydrates, aptamers and monoclonal antibodies or their fragments directed against cancer-associated antigens. These modified conventional liposomes are efficient in carrying anti-angiogenesis drugs and antisense oligonucleotides (aptamers) for delivery with improved accumulation and prolonged exposure near tumors. Nowadays, there are different liposomal products available in the market which include antibacterial, antifungal and mostly anticancer formulations. In addition to this, liposomal formulations are available as therapeutic agents to treat eye, skin and respiratory disorders. A novel multidrug carrier approach of liposomes like (Gemcitabine and Paclitaxel) has been discovered and treatment of some human diseases with such formulations has shown stronger cytotoxic activity as compared to individual free or liposomal preparations. The future engineered liposomal therapeutics based on different surface modifications are emerging as new pharmacological tools. The technology of surface modification of liposomes will include much better and specific ligands with improved target delivery of specific drugs against different diseases.
Author contributions
AAK and KSA have contributed in concept, design, diagrams and information collection of the work. SAA and AAA has reviewed it thoroughly and AHR has contribution in reviewing and final approval of the work.
Compliance with ethical standards
Conflict of interest
All the authors state that there is no conflict of interest.
References
- Abu-Dahab R, Schafer UF, Lehr C-M. Lectin-functionalized liposomes for pulmonary drug delivery: effect of nebulization on stability and bioadhesion. Eur J Pharm Sci. 2001;14:37–46. doi: 10.1016/s0928-0987(01)00147-6. [DOI] [PubMed] [Google Scholar]
- Adjei A, editor. Inhalation delivery of therapeutic peptides and proteins. London: Informa Health Care; 1997. [Google Scholar]
- Ahmad I, Arsalan A, Ali SA, Sheraz MA, Ahmed S, Anwar Z, Munir I, Shah MR. Formulation and stabilization of riboflavin in liposomal preparations. J Photochem Photobiol B Biol. 2015;153:358–366. doi: 10.1016/j.jphotobiol.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Alshaer W, Hillaireau H, Vergnaud J, Ismail S, Fattal E. Functionalizing liposomes with anti-CD44 aptamer for selective targeting of cancer cells. Bioconjug Chem. 2014;26:1307–1313. doi: 10.1021/bc5004313. [DOI] [PubMed] [Google Scholar]
- Anabousi S, Laue M, Lehr C-M, Bakowsky U, Ehrhardt C. Assessing transferrin modification of liposomes by atomic force microscopy and transmission electron microscopy. Eur J Pharm Biopharm. 2005;60:295–303. doi: 10.1016/j.ejpb.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Andon FT, Digifico E, Maeda A, Erreni M, Mantovani A, Alonso MJ, Allavena P (2017) Targeting tumor associated macrophages: the new challenge for nanomedicine. In: Seminars in immunology, vol 34. Academic Press, pp 103–113 [DOI] [PubMed]
- áO’Donoghue MB. A liposome-based nanostructure for aptamer directed delivery. Chem Commun. 2010;46:249–251. doi: 10.1039/b916911c. [DOI] [PubMed] [Google Scholar]
- Arai K, Kashiwazaki A, Fujiwara Y, Tsuchiya H, Sakai N, Shibata K, Koshimizu T-A. Pharmacological lineage analysis revealed the binding affinity of broad-spectrum substance P antagonists to receptors for gonadotropin-releasing peptide. Eur J Pharmacol. 2015;749:98–106. doi: 10.1016/j.ejphar.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Aryal S, Hu CM, Zhang L. Polymeric nanoparticles with precise ratio-metric control over drug loading for combination therapy. Mol Pharm. 2011;8:1401–1407. doi: 10.1021/mp200243k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek SE, Lee KH, Park YS, Oh D-K, Oh S, Kim K-S, Kim D-E. RNA aptamer-conjugated liposome as an efficient anticancer drug delivery vehicle targeting cancer cells in vivo. J Control Release. 2014;196:234–242. doi: 10.1016/j.jconrel.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev Res. 2011;4:1158–1171. doi: 10.1158/1940-6207.CAPR-10-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal S, Kashyap CP, Aggarwal G, Harikumar S. A comparative review on vesicular drug delivery system and stability issues. Int J Res Pharm Chem. 2012;2:704–713. [Google Scholar]
- Batist G, Gelmon KA, Chi KN, Miller WH, Chia SK, Mayer LD, Swenson CE, Janoff AS, Louie AC. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin Cancer Res. 2009;15(2):692–700. doi: 10.1158/1078-0432.CCR-08-0515. [DOI] [PubMed] [Google Scholar]
- Bergstrom LC, Vucenik I, Hagen IK, Chernomorsky SA, Poretz RD. In vitro photocytotoxicity of lysosomotropic immunoliposomes containing pheophorbide a with human bladder carcinoma cells. J Photochem Photobiol B Biol. 1994;24:17–23. doi: 10.1016/1011-1344(94)07008-3. [DOI] [PubMed] [Google Scholar]
- Brody EN, Gold L. Aptamers as therapeutic and diagnostic agents. Rev Mol Biotechnol. 2000;74:5–13. doi: 10.1016/s1389-0352(99)00004-5. [DOI] [PubMed] [Google Scholar]
- Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Del Rev. 2008;60:1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Çagdaş M, Sezer AD, Bucak S (2014) Liposomes as potential drug carrier systems for drug delivery. Application of nanotechnology in drug delivery, pp1–100
- Cao Z, Tong R, Mishra A, Xu W, Wong GC, Cheng J, Lu Y. Reversible cell-specific drug delivery with aptamer-functionalized liposomes. Angew Chem Int Ed. 2009;48:6494–6498. doi: 10.1002/anie.200901452. [DOI] [PubMed] [Google Scholar]
- Chaikof E, Haller C, Cui W, Wen J, Robson S. CD39 enzymosomes inhibit platelet activation in vitro and in vivo. J Surg Res. 2006;130:234–235. [Google Scholar]
- Chakravarty K, Dalal C. Mathematical modelling of liposomal drug release to tumor. Math Biosci. 2018;306:82–96. doi: 10.1016/j.mbs.2018.10.012. [DOI] [PubMed] [Google Scholar]
- Chua MM, Fan ST, Karush F. Attachment of immunoglobulin to liposomal membrane via protein carbohydrate. Biochimica et Biophysica Acta (BBA) Gen Subj. 1984;800(3):291–300. doi: 10.1016/0304-4165(84)90408-2. [DOI] [PubMed] [Google Scholar]
- Corvo MLS, Boerman OC, Oyen WJ, Van Bloois L, Cruz MEM, Crommelin DJ, Storm G. Intravenous administration of superoxide dismutase entrapped in long circulating liposomes: II. In vivo fate in a rat model of adjuvant arthritis. Biochimica et Biophysica Acta (BBA) Biomembr. 1999;1419:325–334. doi: 10.1016/s0005-2736(99)00081-4. [DOI] [PubMed] [Google Scholar]
- Corvo ML, Marinho HS, Marcelino P, Lopes RM, Vale CA, Marques CR, Martins LC, Laverman P, Storm G, Martins MBA. Superoxide dismutase enzymosomes: carrier capacity optimization, in vivo behaviour and therapeutic activity. Pharm Res. 2015;32:91–102. doi: 10.1007/s11095-014-1447-7. [DOI] [PubMed] [Google Scholar]
- Cosco D, Paolino D, Maiuolo J, Russo D, Fresta M. Liposomes as multicompartmental carriers for multidrug delivery in anticancer chemotherapy. Drug Deliv Transl Res. 2011;1:66–75. doi: 10.1007/s13346-010-0007-x. [DOI] [PubMed] [Google Scholar]
- Covarrubias M, Tapia R. Brain glutamate decarboxylase: properties of its calcium-dependent binding to liposomes and kinetics of the bound and the free enzyme. J Neurochem. 1980;34:1682–1688. doi: 10.1111/j.1471-4159.1980.tb11261.x. [DOI] [PubMed] [Google Scholar]
- D’souza AA, Shegokar R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv. 2016;13:1257–1275. doi: 10.1080/17425247.2016.1182485. [DOI] [PubMed] [Google Scholar]
- Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anticancer drug delivery. J Control Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- Daniels DA, Chen H, Hicke BJ, Swiderek KM, Gold L. A tenascin-C aptamer identified by tumor cell SELEX: systematic evolution of ligands by exponential enrichment. Proc Natl Acad Sci. 2003;100:15416–15421. doi: 10.1073/pnas.2136683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Mohanty C, Sahoo SK. Ligand-based targeted therapy for cancer tissue. Expert Opin Drug Deliv. 2009;6:285–304. doi: 10.1517/17425240902780166. [DOI] [PubMed] [Google Scholar]
- De Lopes SCA, dos Santos GC, Rocha TGR, dos Santos FD, Leite EA, Oliveira MC. Liposomes as carriers of anticancer drugs. In: Rangel L, editor. Cancer treatment—conventional and innovative approaches. Rijeka: InTech; 2013. [Google Scholar]
- Deshpande PP, Biswas S, Torchilin VP. Current trends in the use of liposomes for tumor targeting. Nanomedicine (London, England) 2013;8(9):1509–1528. doi: 10.2217/nnm.13.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev. 2012;41:2740–2779. doi: 10.1039/c1cs15237h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DC, Hong K, Park JW, Benz CC, Kirpgtin DB. Liposome targeting to tumors using vitamin and growth factor receptors. Vitam Horm. 2000;60:285–332. doi: 10.1016/s0083-6729(00)60022-5. [DOI] [PubMed] [Google Scholar]
- Du J, Lu WL, Ying X, Liu Y, Du P, Tian W, Men Y, Guo J, Zhang Y, Li RJ, Zhou J. Dual-targeting topotecan liposomes modified with tamoxifen and wheat germ agglutinin significantly improve drug transport across the blood–brain barrier and survival of brain tumor-bearing animals. Mol Pharm. 2009;6(3):905–917. doi: 10.1021/mp800218q. [DOI] [PubMed] [Google Scholar]
- Duhem N, Danhier F, Préat V. Vitamin E-based nanomedicines for anti-cancer drug delivery. J Control Release. 2014;182:33–44. doi: 10.1016/j.jconrel.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Edwards KA, Baeumner AJ. Analysis of liposomes. Talanta. 2006;68:1432–1441. doi: 10.1016/j.talanta.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Egusquiaguirre SP, Igartua M, Hernández RM, Pedraz JL. Nanoparticle delivery systems for cancer therapy: advances in clinical and preclinical research. Clin Transl Oncol. 2012;14:83–93. doi: 10.1007/s12094-012-0766-6. [DOI] [PubMed] [Google Scholar]
- Faraasen S, Vörös J, Csúcs G, Textor M, Merkle HP, Walter E. Ligand-specific targeting of microspheres to phagocytes by surface modification with poly (l-lysine)-grafted poly (ethylene glycol) conjugate. Pharm Res. 2003;20:237–246. doi: 10.1023/a:1022366921298. [DOI] [PubMed] [Google Scholar]
- Farokhzad OC, Karp JM, Langer R. Nanoparticle–aptamer bioconjugates for cancer targeting. Expert Opin Drug Deliv. 2006;3:311–324. doi: 10.1517/17425247.3.3.311. [DOI] [PubMed] [Google Scholar]
- Feiner R (2019) Design and characterization of EGFR-specific peptides and re-targeted rAAVs for tumor therapy. Dissertation, pp 1–91
- Fex G, Johannesson G. Studies of the spontaneous transfer of retinol from the retinol: retinol-binding protein complex to unilamellar liposomes. Biochimica et Biophysica Acta (BBA) Biomembr. 1987;901:255–264. doi: 10.1016/0005-2736(87)90122-2. [DOI] [PubMed] [Google Scholar]
- Fonseca M, Haisma H, Klaassen S, Vingerhoeds M, Storm G. Design of immuno-enzymosomes with maximum enzyme targeting capability: effect of the enzyme density on the enzyme targeting capability and cell binding properties. Biochimica et Biophysica Acta (BBA) Biomembr. 1999;1419:272–282. doi: 10.1016/s0005-2736(99)00073-5. [DOI] [PubMed] [Google Scholar]
- Fujimura T, Mahnke K, Enk AH. Myeloid derived suppressor cells and their role in tolerance induction in cancer. J Dermatol Sci. 2010;59:1–6. doi: 10.1016/j.jdermsci.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Gabizon A, Peretz T, Sulkes A, Amselem S, Ben-Yosef R, Ben-Baruch N, Catane R, Biran S, Barenholz Y. Systemic administration of doxorubicin-containing liposomes in cancer patients: a phase I study. Eur J Cancer Clin Oncol. 1989;25(12):1795–1803. doi: 10.1016/0277-5379(89)90350-7. [DOI] [PubMed] [Google Scholar]
- Galustian C, Park CG, Chai W, Kiso M, Bruening SA, Kang YS, Steinman RM, Feizi T. High and low affinity carbohydrate ligands revealed for murine SIGN-R1 by carbohydrate array and cell binding approaches, and differing specificities for SIGN-R3 and langerin. Int Immunol. 2004;16:853–866. doi: 10.1093/intimm/dxh089. [DOI] [PubMed] [Google Scholar]
- Gaspar M, Martins M, Corvo M, Cruz M. Design and characterization of enzymosomes with surface-exposed superoxide dismutase. Biochimica et Biophysica Acta (BBA) Biomembr. 2003;1609:211–217. doi: 10.1016/s0005-2736(02)00702-2. [DOI] [PubMed] [Google Scholar]
- Goldenberg DM (2001) Immunotherapy of B-cell malignancies using anti-CD22 antibodies Immunomedics Inc. U.S. Patent 6,306,393
- Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- Gowda R, Kardos G, Sharma A, Singh S, Robertson GP. Nanoparticle-based celecoxib and plumbagin for the synergistic treatment of melanoma. Mol Cancer Ther. 2017;16:440–452. doi: 10.1158/1535-7163.MCT-16-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenaway FT, Ledbetter JW. Fluorescence lifetime and polarization anisotropy studies of membrane surfaces with, pyridoxal 5′-phosphate. Biophys Chem. 1987;28:265–271. doi: 10.1016/0301-4622(87)80097-2. [DOI] [PubMed] [Google Scholar]
- Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol Oncol. 2008;26:57–64. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Murcia MA, Chirico S, Aruoma OI. Free radicals and antioxidants in food and in vivo: what they do and how they work. Crit Rev Food Sci Nutr. 1995;35:7–20. doi: 10.1080/10408399509527682. [DOI] [PubMed] [Google Scholar]
- Harasym TO, Tardi PG, Johnstone SA, Bally MB, Janoff AS, Mayer LD. Fixed drug ratio liposome formulations of combination cancer therapeutics. Liposome Technol. 2007;3:25–46. [Google Scholar]
- Hatakeyama H, Akita H, Ishida E, et al. Tumor targeting of doxorubicin by anti-MT1-MMP antibody-modified PEG liposomes. Int J Pharm. 2007;342:194–200. doi: 10.1016/j.ijpharm.2007.04.037. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer. 2002;94:1593–1611. doi: 10.1002/cncr.10372. [DOI] [PubMed] [Google Scholar]
- Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23(9):1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- Jain A, Cheng K. The principles and applications of avidin-based nanoparticles in drug delivery and diagnosis. J Control Release. 2017;245:27–40. doi: 10.1016/j.jconrel.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S-M, Lee HY, Kim J-C. Glucose-sensitive liposomes incorporating hydrophobically modified glucose oxidase. Lipids. 2008;43:937–943. doi: 10.1007/s11745-008-3223-0. [DOI] [PubMed] [Google Scholar]
- Jung YK, Kim TW, Park HG, Soh HT. Specific colorimetric detection of proteins using bidentate aptamer-conjugated polydiacetylene (PDA) liposomes. Adv Funct Mater. 2010;20:3092–3097. [Google Scholar]
- Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DI, Lee S, Lee JT, et al. Preparation and in vitro evaluation of anti-VCAM-1-Fab′-conjugated liposomes for the targeted delivery of the poorly water-soluble drug celecoxib. J Microencapsul. 2011;28:220–227. doi: 10.3109/02652048.2011.552989. [DOI] [PubMed] [Google Scholar]
- Katanasaka Y, Ida T, Asai T, Maeda N, Oku N. Effective delivery of an angiogenesis inhibitor by neovessel-targeted liposomes. Int J Pharm. 2008;360:219–224. doi: 10.1016/j.ijpharm.2008.04.046. [DOI] [PubMed] [Google Scholar]
- Kawakami S, Sato A, Nishikawa M, Yamashita F, Hashida M. Mannose receptor-mediated gene transfer into macrophages using novel mannosylated cationic liposomes. Gene Ther. 2000;7(4):292–299. doi: 10.1038/sj.gt.3301089. [DOI] [PubMed] [Google Scholar]
- Khdair A, Handa H, Mao G, Panyam J. Nanoparticle-mediated combination chemotherapy and photodynamic therapy overcomes tumor drug resistance in vitro. Eur J Pharm Biopharm. 2009;71:214–222. doi: 10.1016/j.ejpb.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Kim SK, Huang L. Nanoparticle delivery of a peptide targeting EGFR signaling. J Control Release. 2012;157:279–286. doi: 10.1016/j.jconrel.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-H, Kim J-K, Lim S-J, Park J-S, Lee M-K, Kim C-K. Folate-tethered emulsion for the target delivery of retinoids to cancer cells. Eur J Pharm Biopharm. 2008;68:618–625. doi: 10.1016/j.ejpb.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Koning GA, Kamps JA, Scherphof GL. Efficient intracellular delivery of 5-fluorodeoxyuridine into colon cancer cells by targeted immunoliposomes. Cancer Detect Prev. 2002;26:299–307. doi: 10.1016/s0361-090x(02)00087-9. [DOI] [PubMed] [Google Scholar]
- Kozak A, Shapiro I, Vinnikova M, Ershov L, Senderikhin A, Ayalon O (2001) Phospholipid prodrugs of anti-proliferative drugs. International Patent Publication (PCT) WO/2001/19320
- Kratz F, Senter P, Steinhagen H. Drug delivery in oncology: from basic research to cancer therapy. New York: Wiley; 2013. [Google Scholar]
- Kuldo JM, Ogawara KI, Werner N, Ásgeirsdóttir SA, Kamps JA, Kok RJ, Molema G. Molecular pathways of endothelial cell activation for (targeted) pharmacological intervention of chronic inflammatory diseases. Curr Vasc Pharmacol. 2005;3:11–39. doi: 10.2174/1570161052773898. [DOI] [PubMed] [Google Scholar]
- Kuzma JN. Ingestion of wheat germ in healthy subjects does not acutely elevate plasma wheat germ agglutinin concentrations. Fort Collins: Colorado State University, Libraries; 2009. [Google Scholar]
- Kuznetsova N, Kandyba A, Vostrov I, Kadykov V, Gaenko G, Molotkovsky J, Vodovozova E. Liposomes loaded with lipophilic prodrugs of methotrexate and melphalan as convenient drug delivery vehicles. J Drug Deliv Sci Technol. 2009;19:51–59. [Google Scholar]
- Lasch J, Weissing V, Brandl M. Preparation of liposomes. In: Torchilin VP, Weissig V, editors. Liposomes: a pratical approach. 2. New York: Oxford Universty Press; 2003. pp. 3–27. [Google Scholar]
- Lasic DD, Papahadjopoulos D. Liposomes revisited. Science. 1995;267(5202):1275–1277. doi: 10.1126/science.7871422. [DOI] [PubMed] [Google Scholar]
- Laverman P, Bloois LV, Boerman OC, Oyen WJ, Corstens FH, Storm G. Lyophilization of Tc-99m-HYNIC labeled PEG-liposomes. J Liposome Res. 2000;10:117–129. [Google Scholar]
- Leamon CP, Cooper SR, Hardee GE. Folate-liposome-mediated antisense oligodeoxynucleotide targeting to cancer cells: evaluation in vitro and in vivo. Bioconj Chem. 2003;14:738–747. doi: 10.1021/bc020089t. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Lee NK, Kim I-S. Bioengineered protein-based nanocage for drug delivery. Adv Drug Deliv Rev. 2016;106:157–171. doi: 10.1016/j.addr.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Lehtinen J, Raki M, Bergstrom KA, et al. Pre-targeting and direct immunotargeting of liposomal drug carriers to ovarian carcinoma. PLoS ONE. 2012;7:e41410. doi: 10.1371/journal.pone.0041410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SD, Chono S, Huang L. Efficient oncogene silencing and metastasis inhibition via systemic delivery of siRNA. Mol Ther. 2008;16:942–946. doi: 10.1038/mt.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XM, Ding LY, Xu Y, Wang Y, Ping QN. Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int J Pharm. 2009;373:116–123. doi: 10.1016/j.ijpharm.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Li XT, Ju RJ, Li XY, Zeng F, Shi JF, Liu L, Zhang CX, Sun MG, Lou JN, Lu WL. Multifunctional targeting daunorubicin plus quinacrine liposomes, modified by wheat germ agglutinin and tamoxifen, for treating brain glioma and glioma stem cells. Oncotarget. 2014;5(15):6497. doi: 10.18632/oncotarget.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-J, Dou X-Q, Wang F, Zhang J, Wang X-L, Xu G-L, Xiang S-S, Gao X, Fu J, Song H-F. IL-4Rα aptamer-liposome-CpG oligodeoxynucleotides suppress tumour growth by targeting the tumour microenvironment. J Drug Target. 2017;25:275–283. doi: 10.1080/1061186X.2016.1258569. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G, Fainstein V, Hopfer R, Mehta K, Sullivan MP, Keating M, Rosenblum MG, Mehta R, Luna M, Hersh EM, Reuben J. Liposomal amphotericin B for the treatment of systemic fungal infections in patients with cancer: a preliminary study. J Infect Dis. 1985;151(4):704–710. doi: 10.1093/infdis/151.4.704. [DOI] [PubMed] [Google Scholar]
- Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res. 2007;41:120–129. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- Lu Y, Low PS. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv Drug Deliv Rev. 2002;54:675–693. doi: 10.1016/s0169-409x(02)00042-x. [DOI] [PubMed] [Google Scholar]
- Luciani A, Olivier J-C, Clement O, Siauve N, Brillet P-Y, Bessoud B, Gazeau F, Uchegbu IF, Kahn E, Frija G. Glucose-receptor MR imaging of tumors: study in mice with PEGylated paramagnetic niosomes. Radiology. 2004;231:135–142. doi: 10.1148/radiol.2311021559. [DOI] [PubMed] [Google Scholar]
- Lukyanov AN, Elbayoumi TA, Chakilam AR, Torchilin VP. Tumor-targeted liposomes: doxorubicin-loaded long-circulating liposomes modified with anti-cancer antibody. J Control Release. 2004;100:135–144. doi: 10.1016/j.jconrel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Lundberg BB, Griffiths G, Hansen HJ. Specific binding of sterically stabilized anti-B-cell immunoliposomes and cytotoxicity of entrapped doxorubicin. Int J Pharm. 2000;205:101–108. doi: 10.1016/s0378-5173(00)00492-0. [DOI] [PubMed] [Google Scholar]
- Ma DD, Wei A-Q. Enhanced delivery of synthetic oligonucleotides to human leukaemic cells by liposomes and immunoliposomes. Leuk Res. 1996;20:925–930. doi: 10.1016/s0145-2126(96)00062-8. [DOI] [PubMed] [Google Scholar]
- Mamot C, Drummond DC, Hong K, Kirpotin DB, Park JW. Liposome-based approaches to overcome anticancer drug resistance. Drug Resist Updates. 2003;6:271–279. doi: 10.1016/s1368-7646(03)00082-7. [DOI] [PubMed] [Google Scholar]
- Mamot C, Ritschard R, Wicki A, Stehle G, Dieterle T, Bubendorf L, Hilker C, Deuster S, Herrmann R, Rochlitz C. Tolerability, safety, pharmacokinetics, and efficacy of doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid tumours: a phase 1 dose-escalation study. Lancet Oncol. 2012;13(12):1234–1241. doi: 10.1016/S1470-2045(12)70476-X. [DOI] [PubMed] [Google Scholar]
- Managit C, Kawakami S, Yamashita F, Hashida M. Effect of galactose density on asialoglycoprotein receptor-mediated uptake of galactosylated liposomes. J Pharm Sci. 2005;94:2266–2275. doi: 10.1002/jps.20443. [DOI] [PubMed] [Google Scholar]
- McGuire JJ. Anticancer antifolates: current status and future directions. Curr Pharm Des. 2003;9:2593–2613. doi: 10.2174/1381612033453712. [DOI] [PubMed] [Google Scholar]
- Miller K, Cortes J, Hurvitz SA, Krop IE, Tripathy D, Verma S, Riahi K, Reynolds JG, Wickham TJ, Molnar I, Yardley DA. HERMIONE: a randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician’s choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer. 2016;16(1):352. doi: 10.1186/s12885-016-2385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima J, Fukada H, Nagayama M, Ueda M. Specific membrane binding of the carboxypeptidase Y inhibitor IC, a phosphatidylethanolamine-binding protein family member. FEBS J. 2006;273:5374–5383. doi: 10.1111/j.1742-4658.2006.05530.x. [DOI] [PubMed] [Google Scholar]
- Moradpour D, Compagnon B, Wilson BE, Nicolau C, Wands JR. Specific targeting of human hepatocellular carcinoma cells by immunoliposomes in vitro. Hepatology. 1995;22:1527–1537. [PubMed] [Google Scholar]
- Moreira JN, Hansen CB, Gaspar R, Allen TM. A growth factor antagonist as a targeting agent for sterically stabilized liposomes in human small cell lung cancer. Biochimica et Biophysica Acta (BBA) Biomembr. 2001;1514:303–317. doi: 10.1016/s0005-2736(01)00386-8. [DOI] [PubMed] [Google Scholar]
- Murata M, Yonamine T, Tanaka S, Tahara K, Tozuka Y, Takeuchi H. Surface modification of liposomes using polymer-wheat germ agglutinin conjugates to improve the absorption of peptide drugs by pulmonary administration. J Pharm Sci. 2013;102(4):1281–1289. doi: 10.1002/jps.23463. [DOI] [PubMed] [Google Scholar]
- Nguyen TX, Huang L, Gauthier M, Yang G, Wang Q. Recent advances in liposome surface modification for oral drug delivery. Nanomedicine. 2016;11(9):1169–1185. doi: 10.2217/nnm.16.9. [DOI] [PubMed] [Google Scholar]
- Niemeyer CM. Nanoparticles, proteins, and nucleic acids: biotechnology meets materials science. Angew Chem Int Ed. 2001;40:4128–4158. doi: 10.1002/1521-3773(20011119)40:22<4128::AID-ANIE4128>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Nobs L, Buchegger F, Gurny R, Allémann E. Current methods for attaching targeting ligands to liposomes and nanoparticles. J Pharm Sci. 2004;93:1980–1992. doi: 10.1002/jps.20098. [DOI] [PubMed] [Google Scholar]
- Nofsinger R, Clas SD, Sanchez RI, Walji A, Manser K, Nissley B, Balsells J, Nair A, Dang Q, Bennett DJ, Hafey M. Design of prodrugs to enhance colonic absorption by increasing lipophilicity and blocking ionization. Pharmaceuticals. 2014;7(2):207–219. doi: 10.3390/ph7020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shannessy DJ, Somers EB, Wang LC, Wang H, Hsu R. Expression of folate receptors alpha and beta in normal and cancerous gynecologic tissues: correlation of expression of the beta isoform with macrophage markers. J Ovarian Res. 2015;8:29. doi: 10.1186/s13048-015-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasut G, Canal F, Dalla Via L, Arpicco S, Veronese FM, Schiavon O. Antitumoral activity of PEG-gemcitabine prodrugs targeted by folic acid. J Control Release. 2008;127:239–248. doi: 10.1016/j.jconrel.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Paszko E, Senge M. Immunoliposomes. Curr Med Chem. 2012;19:5239–5277. doi: 10.2174/092986712803833362. [DOI] [PubMed] [Google Scholar]
- Patolsky F, Lichtenstein A, Willner I. Amplified microgravimetric quartz-crystal-microbalance assay of DNA using oligonucleotide-functionalized liposomes or biotinylated liposomes. J Am Chem Soc. 2000;122:418–419. [Google Scholar]
- Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. doi: 10.1016/j.ejpb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Petrak K. Essential properties of drug-targeting delivery systems. Drug Discov Today. 2005;10:1667–1673. doi: 10.1016/S1359-6446(05)03698-6. [DOI] [PubMed] [Google Scholar]
- Płotka-Wasylka J, Szczepańska N, de la Guardia M, Namieśnik J. Modern trends in solid phase extraction: new sorbent media. TrAC Trends Anal Chem. 2016;77:23–43. [Google Scholar]
- Pui C-H. Childhood leukemias. N Engl J Med. 1995;332:1618–1630. doi: 10.1056/NEJM199506153322407. [DOI] [PubMed] [Google Scholar]
- Raju A, Muthu MS, Feng S-S. Trastuzumab-conjugated vitamin E TPGS liposomes for sustained and targeted delivery of docetaxel. Expert Opin Drug Deliv. 2013;10:747–760. doi: 10.1517/17425247.2013.777425. [DOI] [PubMed] [Google Scholar]
- Ramundo-Orlando A, Gallerano GP, Stano P, Doria A, Giovenale E, Messina G, Cappelli M, D'Arienzo M, Spassovsky I. Permeability changes induced by 130 GHz pulsed radiation on cationic liposomes loaded with carbonic anhydrase. Bioelectromagn J Bioelectromagn Soc Soc Phys Regul Biol Med Eur Bioelectromagn Assoc. 2007;28:587–598. doi: 10.1002/bem.20343. [DOI] [PubMed] [Google Scholar]
- Rautio J, Meanwell NA, Di L, Hageman MJ. The expanding role of prodrugs in contemporary drug design and development. Nat Rev Drug Discov. 2018;17(8):559. doi: 10.1038/nrd.2018.46. [DOI] [PubMed] [Google Scholar]
- Renggli K, Baumann P, Langowska K, Onaca O, Bruns N, Meier W. Selective and responsive nanoreactors. Adv Funct Mater. 2011;21:1241–1259. [Google Scholar]
- Riaz MK, Riaz MA, Zhang X, Lin C, Wong KH, Chen X, Zhang G, Lu A, Yang Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: a review. Int J Mol Sci. 2018;9:19. doi: 10.3390/ijms19010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards DA, Maruani A, Chudasama V. Antibody fragments as nanoparticle targeting ligands: a step in the right direction. Chem Sci. 2017;8(1):63–77. doi: 10.1039/c6sc02403c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadzuka Y. Effective prodrug liposome and conversion to active metabolite. Curr Drug Metab. 2000;1:31–48. doi: 10.2174/1389200003339225. [DOI] [PubMed] [Google Scholar]
- Sakamoto JH, van de Ven AL, Godin B, Blanco E, Serda RE, Grattoni A, Ziemys A, Bouamrani A, Hu T, Ranganathan SI. Enabling individualized therapy through nanotechnology. Pharmacol Res. 2010;62:57–89. doi: 10.1016/j.phrs.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim M, Minamikawa H, Sugimura A, Hashim R. Amphiphilic designer nano-carriers for controlled release: from drug delivery to diagnostics. MedChemComm. 2014;5:1602–1618. [Google Scholar]
- Samad A, Sultana Y, Aqil M. Liposomal drug delivery systems: an update review. Curr Drug Deliv. 2007;4:297–305. doi: 10.2174/156720107782151269. [DOI] [PubMed] [Google Scholar]
- Sapra P, Allen T. Ligand-targeted liposomal anticancer drugs. Progress Lipid Res. 2003;42:439–462. doi: 10.1016/s0163-7827(03)00032-8. [DOI] [PubMed] [Google Scholar]
- Senzer N, Nemunaitis J, Nemunaitis D, Bedell C, Edelman G, Barve M, Nunan R, Pirollo KF, Rait A, Chang EH. Phase I study of a systemically delivered p53 nanoparticle in advanced solid tumors. Mol Ther. 2013;21(5):1096–1103. doi: 10.1038/mt.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MAA, He N, Li Z, Ali Z, Zhang L. Nanoparticles for DNA vaccine delivery. J Biomed Nanotechnol. 2014;10:2332–2349. doi: 10.1166/jbn.2014.1981. [DOI] [PubMed] [Google Scholar]
- Shefrin S, Sreelaxmi C, Vishnu V, Sreeja C. Enzymosomes: a rising effectual tool for targeted drug delivery system. Int J Appl Pharm. 2017;9:1–9. [Google Scholar]
- Shin DH, Lee S-J, Kim JS, Ryu J-H, Kim J-S. Synergistic effect of immunoliposomal gemcitabine and bevacizumab in glioblastoma stem cell-targeted therapy. J Biomed Nanotechnol. 2015;11:1989–2002. doi: 10.1166/jbn.2015.2146. [DOI] [PubMed] [Google Scholar]
- Sigot V, Arndt-Jovin DJ, Jovin TM. Targeted cellular delivery of quantum dots loaded on and in biotinylated liposomes. Bioconj Chem. 2010;21:1465–1472. doi: 10.1021/bc100054c. [DOI] [PubMed] [Google Scholar]
- Sihorkar V, Vyas S. Potential of polysaccharide anchored liposomes in drug delivery, targeting and immunization. J Pharm Pharm Sci. 2001;4:138–158. [PubMed] [Google Scholar]
- Singh S. Liposome encapsulation of doxorubicin and celecoxib in combination inhibits progression of human skin cancer cells. Int J Nanomed. 2018;13:11. doi: 10.2147/IJN.S124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Sharma A, Robertson GP. Realizing the clinical potential of cancer nanotechnology by minimizing toxicologic and targeted delivery concerns. Cancer Res. 2012;72(22):5663–5668. doi: 10.1158/0008-5472.CAN-12-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skólmowska M, Kmieć M. Enzymosomy antyoksydacyjne—właściwości i zastosowanie antioxidant enzymosomes—properties and application. Postepy Hig Med Dosw (Online) 2011;65:640–644. doi: 10.5604/17322693.962163. [DOI] [PubMed] [Google Scholar]
- Slingerland M, Guchelaar HJ, Gelderblom H. Liposomal drug formulations in cancer therapy: 15 years along the road. Drug Discov Today. 2012;17(3–4):160–166. doi: 10.1016/j.drudis.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Song S, Liu D, Peng J, Sun Y, Li Z, Gu J-R, Xu Y. Peptide ligand-mediated liposome distribution and targeting to EGFR expressing tumor in vivo. Int J Pharm. 2008;363:155–161. doi: 10.1016/j.ijpharm.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Song CK, Jung SH, Kim D-D, Jeong K-S, Shin BC, Seong H. Disaccharide-modified liposomes and their in vitro intracellular uptake. Int J Pharm. 2009;380:161–169. doi: 10.1016/j.ijpharm.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Parak WJ. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos Trans R Soc A Math Phys Eng Sci. 2010;368:1333–1383. doi: 10.1098/rsta.2009.0273. [DOI] [PubMed] [Google Scholar]
- Spevak W, Nagy JO, Charych DH, Schaefer ME, Gilbert JH, Bednarski MD. Polymerized liposomes containing C-glycosides of sialic acid: potent inhibitors of influenza virus in vitro infectivity. J Am Chem Soc. 1993;115(3):1146–1147. [Google Scholar]
- Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur J Pharm Sci. 2013;48:416–427. doi: 10.1016/j.ejps.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm G, Crommelin DJ. Liposomes: quo vadis? Pharm Sci Technol Today. 1998;1(1):19–31. [Google Scholar]
- Sun H, Zhu X, Lu PY, Rosato RR, Tan W, Zu Y. Oligonucleotide aptamers: new tools for targeted cancer therapy. Mol Ther Nucleic Acids. 2014;3:e182. doi: 10.1038/mtna.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Song Y, Lu M, Lin X, Liu Y, Zhou S, Su Y, Deng Y. Evaluation of the antitumor effect of dexamethasone palmitate and doxorubicin co-loaded liposomes modified with a sialic acid–octadecylamine conjugate. Eur J Pharm Sci. 2016;93:177–183. doi: 10.1016/j.ejps.2016.08.029. [DOI] [PubMed] [Google Scholar]
- Suntres ZE, Shek PN. Prophylaxis against lipopolysaccharide-induced acute lung injury by alpha-tocopherol liposomes. Crit Care Med. 1998;26:723–729. doi: 10.1097/00003246-199804000-00023. [DOI] [PubMed] [Google Scholar]
- Tan Q-Y, Zhang J-Q, Wang N, Yang H, Li X, Xiong H-R, Wu J-Y, Zhao C-J, Wang H, Yin H-F. Improved biological properties and hypouricemic effects of uricase from Candida utilis loaded in novel alkaline enzymosomes. Int J Nanomed. 2012;7:3929. doi: 10.2147/IJN.S33835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansi FL, Rüger R, Böhm C, Kontermann RE, Teichgraeber UK, Fahr A, Hilger I. Potential of activatable FAP-targeting immunoliposomes in intraoperative imaging of spontaneous metastases. Biomaterials. 2016;88:70–82. doi: 10.1016/j.biomaterials.2016.02.028. [DOI] [PubMed] [Google Scholar]
- Tardi P, Johnstone S, Harasym N, Xie S, Harasym T, Zisman N, Harvie P, Bermudes D, Mayer L. In vivo maintenance of synergistic cytarabine: daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33(1):129–139. doi: 10.1016/j.leukres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- Torchilin VP, Lukyanov AN. Peptide and protein drug delivery to and into tumors: challenges and solutions. Drug Discov Today. 2003;8:259–266. doi: 10.1016/s1359-6446(03)02623-0. [DOI] [PubMed] [Google Scholar]
- Tsumoto K, Matsuo H, Tomita M, Yoshimura T. Efficient formation of giant liposomes through the gentle hydration of phosphatidylcholine films doped with sugar. Colloids Surf B Biointerfaces. 2009;68:98–105. doi: 10.1016/j.colsurfb.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Turk MJ, Waters DJ, Low PS. Folate-conjugated liposomes preferentially target macrophages associated with ovarian carcinoma. Cancer Lett. 2004;213:165–172. doi: 10.1016/j.canlet.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Van Driel PB, Boonstra MC, Slooter MD, Heukers R, Stammes MA, Snoeks TJ, De Bruijn HS, Van Diest PJ, Vahrmeijer AL, en Henegouwen PMVB, Van De Velde CJ. EGFR targeted nanobody–photosensitizer conjugates for photodynamic therapy in a pre-clinical model of head and neck cancer. J Control Release. 2016;229:93–105. doi: 10.1016/j.jconrel.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas SP, Sihorkar V, Dubey PK. Preparation, characterization and in vitro antimicrobial activity of metronidazole bearing lectinized liposomes for intra-periodontal pocket delivery. Die Pharm. 2001;56(7):554–560. [PubMed] [Google Scholar]
- Wagner A, Vorauer-Uhl K. Liposome technology for industrial purposes. J Drug Deliv. 2011;2011:1–9. doi: 10.1155/2011/591325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62:90–99. doi: 10.1016/j.phrs.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Wicki A, Rochlitz C, Orleth A, et al. Targeting tumor-associated endothelial cells: anti-VEGFR2 immunoliposomes mediate tumor vessel disruption and inhibit tumor growth. Clin Cancer Res. 2012;18:454–464. doi: 10.1158/1078-0432.CCR-11-1102. [DOI] [PubMed] [Google Scholar]
- Wijagkanalan W, Kawakami S, Takenaga M, Igarashi R, Yamashita F, Hashida M. Efficient targeting to alveolar macrophages by intratracheal administration of mannosylated liposomes in rats. J Control Release. 2008;125(2):121–130. doi: 10.1016/j.jconrel.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Wu C-H, Liu I-J, Lu R-M, Wu H-C. Advancement and applications of peptide phage display technology in biomedical science. J Biomed Sci. 2016;23:8. doi: 10.1186/s12929-016-0223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Yao N, Qin Y, Zhang Q, Chen H, Yuan M, Tang J, Li X, Fan W, Zhang Q. Investigation of glucose-modified liposomes using polyethylene glycols with different chain lengths as the linkers for brain targeting. Int J Nanomed. 2012;7:163. doi: 10.2147/IJN.S23771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing H, Tang L, Yang X, Hwang K, Wang W, Yin Q, Wong NY, Dobrucki LW, Yasui N, Katzenellenbogen JA. Selective delivery of an anticancer drug with aptamer-functionalized liposomes to breast cancer cells in vitro and in vivo. J Mater Chem B. 2013;1:5288–5297. doi: 10.1039/C3TB20412J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Huang C-C, Huang W, Tang W-H, Rait A, Yin YZ, Cruz I, Xiang L-M, Pirollo KF, Chang EH. Systemic tumor-targeted gene delivery by anti-transferrin receptor scFv-immunoliposomes 1 this work was supported in part by National Cancer Institute Grant R01 CA45158 (to EC), National Cancer Institute Small Business Technology Transfer Phase I Grant R41 CA80449 (to EC), and a grant from SynerGene Therapeutics, Inc. 1. Mol Cancer Ther. 2002;1:337–346. [PubMed] [Google Scholar]
- Yamakawa T, Ohigashi H, Hashimoto D, Hayase E, Takahashi S, Miyazaki M, Minomi K, Onozawa M, Niitsu Y, Teshima T. Vitamin A—coupled liposomes containing siRNA against HSP47 ameliorate skin fibrosis in chronic graft-versus-host disease. Blood. 2018;131:1476–1485. doi: 10.1182/blood-2017-04-779934. [DOI] [PubMed] [Google Scholar]
- Yang Q, Liu X-Y, Miyake J, Toyotama H. Self-assembly and immobilization of liposomes in fused-silica capillary by avidin–biotin binding. Supramol Sci. 1998;5:769–772. [Google Scholar]
- Yang L, Zhang X, Ye M, Jiang J, Yang R, Fu T, Chen Y, Wang K, Liu C, Tan W. Aptamer-conjugated nanomaterials and their applications. Adv Drug Deliv Rev. 2011;63:1361–1370. doi: 10.1016/j.addr.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying X, Wen H, Lu WL, et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Control Release. 2010;141:183–192. doi: 10.1016/j.jconrel.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M, Kuboi R, Yang Q, Miyake J. Immobilized liposome chromatography for studies of protein–membrane interactions and refolding of denatured bovine carbonic anhydrase. J Chromatogr B Biomed Sci Appl. 1998;712:59–71. doi: 10.1016/s0378-4347(98)00157-1. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M, Takaki N, Yamasaki M. Catalase-conjugated liposomes encapsulating glucose oxidase for controlled oxidation of glucose with decomposition of hydrogen peroxide produced. Colloids Surf B Biointerfaces. 2010;79:403–408. doi: 10.1016/j.colsurfb.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M, Yamashita T, Kinoshita S. Thermal stabilization of formaldehyde dehydrogenase by encapsulation in liposomes with nicotinamide adenine dinucleotide. Enzyme Microb Technol. 2011;49:209–214. doi: 10.1016/j.enzmictec.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Yu MK, Park J, Jon S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics. 2012;2:3. doi: 10.7150/thno.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Mao Y, Yuan Y, Yue C, Wang X, Mo X, Jarjoura D, Paulaitis ME, Lee RJ, Byrd JC. Targeted drug delivery and cross-linking induced apoptosis with anti-CD37 based dual-ligand immunoliposomes in B chronic lymphocytic leukemia cells. Biomaterials. 2013;34:6185–6193. doi: 10.1016/j.biomaterials.2013.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yüce M, Ullah N, Budak H. Trends in aptamer selection methods and applications. Analyst. 2015;140:5379–5399. doi: 10.1039/c5an00954e. [DOI] [PubMed] [Google Scholar]
- Yun YH, Lee BK, Park K. Controlled drug delivery: historical perspective for the next generation. J Control Release. 2015;219:2–7. doi: 10.1016/j.jconrel.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Ping QN, Huang GH, Xu WF. Investigation of lectin-modified insulin liposomes as carriers for oral administration. Int J Pharm. 2005;294:247–259. doi: 10.1016/j.ijpharm.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Zhang X, Qi J, Lu Y, He W, Li X, Wu W. Biotinylated liposomes as potential carriers for the oral delivery of insulin. Nanomed Nanotechnol Biol Med. 2014;10:167–176. doi: 10.1016/j.nano.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ng HLH, Lu A, Lin C, Zhou L, Lin G, Zhang Y, Yang Z, Zhang H. Drug delivery system targeting advanced hepatocellular carcinoma: current and future. Nanomed Nanotechnol Biol Med. 2016;12:853–869. doi: 10.1016/j.nano.2015.12.381. [DOI] [PubMed] [Google Scholar]
- Zhao X, Li H, Lee RJ. Targeted drug delivery via folate receptors. Expert Opin Drug Deliv. 2008;5:309–319. doi: 10.1517/17425247.5.3.309. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhang M, He D, Hu X, Xiong H, Wu J, Zhu B, Zhang J. Uricase alkaline enzymosomes with enhanced stabilities and anti-hyperuricemia effects induced by favorable microenvironmental changes. Sci Rep. 2016;6:20136. doi: 10.1038/srep20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yan F, Guo Z, Marchant RE. Surface modification of liposomes by saccharides: vesicle size and stability of lactosyl liposomes studied by photon correlation spectroscopy. J Colloid Interface Sci. 2005;289:542–550. doi: 10.1016/j.jcis.2005.03.088. [DOI] [PubMed] [Google Scholar]