Abstract
Internal carotid artery (ICA) stenosis including Moyamoya disease needs revascularization when hemodynamic insufficiency is validated. Vascular reserve impairment was the key to find the indication for endarterectomy/bypass surgery in the atherosclerotic ICA stenosis and to determine the indication, treatment effect, and prognosis in Moyamoya diseases. Vascular reserve was quantitatively assessed by 1-day split-dose I-123 IMP basal/acetazolamide SPECT in Japan or by Tc-99m HMPAO SPECT in other countries using qualitative or semi-quantitative method. We summarized the development of 1-day basal/ acetazolamide brain perfusion SPECT for ICA stenosis, both quantitative and qualitative methods, and their methodological issues regarding (1) acquisition protocol; (2) qualitative assessment, either visual or deep learning-based; (3) clinical use for atherosclerotic ICA steno-occlusive diseases and mostly Moyamoya diseases; and (4) their impact on the choice of treatment options. Trials to use CT perfusion or perfusion MRI using contrast materials or arterial spin labeling were briefly discussed in their endeavor to use basal studies alone to replace acetazolamide-challenge SPECT. Theoretical and practical issues imply that basal perfusion evaluation, no matter how much sophisticated, will not disclose vascular reserve. Acetazolamide rarely causes serious adverse reactions but included fatality, and now, we need to monitor patients closely in acetazolamide-challenge studies.
Keywords: Brain perfusion, Vascular reserve, Acetazolamide SPECT, Carotid artery stenosis, Moyamoya disease, Deep learning
Introduction
In internal carotid artery (ICA) stenosis of various causes, bypass surgery, surgical or endovascular revascularization procedures, and intensive medical therapy raised the needs of the evaluation of hemodynamic insufficiency caused by ICA stenosis, extracranial or intracranial. Among these treatment measures, extracranial-intracranial (EC-IC) bypass surgery was introduced decades ago, and a randomized trial published in 1985 made this direct bypass surgery obsolete as it did not reduce consequent strokes [1–3]. After this, investigators tried to find the subgroup of patients who might benefit from bypass surgery by determining hemodynamic consequences of ICA stenosis especially in elderly patients with atherosclerotic ICA stenosis. Recently, another trial about the effect of atherosclerotic carotid artery stenosis surgery (COSS) for stroke prevention again concluded that EC-IC bypass surgery did not reduce stroke compared with medical therapy [2–5]. Intracranial atherosclerosis was separately examined with similar results [6, 7]. These disappointing results did not seem to be due to graft occlusion as grafts were almost always patent [8]. Compared with intensive medical treatment, endarterectomy for extracranial ICA stenosis and stenting as an alternative option improved long-term outcome similarly to each other while their peri-procedural outcome was also the same [9–12]. Investigators are now trying to narrow the surgery-indicated cases with the determination of impaired vascular reserve of ipsilateral hemisphere [10] even in asymptomatic patients [11], which might be disclosed with single photon emission computed tomography (SPECT) after acetazolamide challenge. Prevention of the stroke or reduction of its incidence on long-term follow-up is an issue of concern.
Moyamoya disease (MMD) is a progressive steno-occlusive disease typically involving the intracranial ICAs and cerebral arteries [13]. Japanese epidemiologic studies showed previously childhood preponderance, which recently changed to the highest peak at fifth decade of age, meaning that more and more cases are identified in adults [14, 15]. Presenting symptoms are mostly ischemia (57%) and hemorrhage (21%) or asymptomatic (18%) [15]. In children [16–18] as well as in adults [19], indirect or direct revascularization surgery could improve hemodynamics of the steno-occlusive artery territories and even when asymptomatic cases were operated [19]. In childhood cases, younger children less than 3 years old show aggressive course which requires immediate indirect bypass surgery [17], among which encephaloduroarteriosynangiosis (EDAS) with bifrontal encephalogaleo(periosteal)synangiosis are better than EDAS alone to improve basal perfusion and vascular reserve in the anterior cerebral artery territory [16]. Developing brains of children were considered to respond to indirect bypass actively with new collateral vessel formation. Child patients are usually found with ischemic symptoms or stroke and as their course is aggressive, despite the peri-operative risk, bypass surgery is indicated [17]. In adult cases, if symptomatic, direct superficial temporal artery-middle cerebral artery bypass surgery led to better outcomes than indirect bypass surgery such as EDAS [20–23]. In asymptomatic cases, though hemodynamic instability is worrying, if without current evidence of hemodynamic decompensation, conservative management or observation was recommended as an alternative to surgical treatment while watching regularly if posterior circulation is further involved or vascular reserve comes to be impaired [24].
Atherosclerotic ICA stenosis is a disease affecting the humankind worldwide, and the medical treatment has ever been improved as its pathogenesis is now better understood [6, 10, 25, 26]. Once administered early in the course of ICA stenosis, intensive medical treatment might replace invasive procedures later in the disease course. However, found later, atherosclerotic patients need invasive procedures to reduce the disease burden and to let further intensive medical treatments work well. And again stenting, an endovascular revascularization therapy, accompanied by medical treatments might be able to replace carotid endarterectomy. Currently, though, both stenting and endarterectomy have their own advantages and shortcomings. MMD has just recently been found to have genetic background in genome-wide association studies in RNF213 (p.R4859K) both in Japan [27] and Korea [28]. Prevalence of the polymorphism is biased to East Asia including China [29–32] and Taiwan; however, consequent pathophysiology further to this genetic predisposition is not yet known [27, 28]. Its progressive nature facilitated the adoption of indirect bypass surgery for children based on the cost-benefit aspect [17] despite high peri-operative risks. In adult patients with Moyamoya disease, direct bypass surgery was also tried and well received [20]. Until the fifth decade of age, the brains themselves responded to bypass surgery much better than did those in atherosclerotic intracranial ICA stenosis [28, 33]. Considering that a substantial proportion of adult-onset MMD was misclassified as intracranial ICA stenosis, diagnostic strategy for adult-onset MMD referring to molecular or mechanistic classification was proposed by investigators [28].
In extracranial [1–5] or intracranial [6, 7, 33] atherosclerotic ICA stenosis and in child and adult MMD [16–26, 34], respectively with different reasons, basal/acetazolamide SPECT was proposed to evaluate the hemodynamic significance of already-characterized steno-occlusive lesions in ICA or cerebral arteries [6, 7, 21, 22]. For elderly atherosclerotic patients, once functional impairment due to stenosis on the ipsilateral hemisphere is suspected, basal/acetazolamide SPECT was supposed to disclose the cases that need EC-IC bypass surgery [1, 4, 35], endarterectomy [36], stenting [37], or medical treatments. There is obviously a gray area of finding the indicated patients with failure of medical treatment, which depends on patients’ suffering of a certain period taking the risk of strokes. Finding these patients beforehand to be diverted to surgery instead of observation with medical treatments is an instrumental role of basal/acetazolamide SPECT. This used to be the case with child and adult MMD subjects, as the direct or indirect bypass surgery resulted in favorable adaptive response of ipsilateral hemispheres or cerebral artery territories. The impairment of vascular reserve in the ipsilateral carotid or cerebral artery territories, if discovered, was used as a reliable image biomarker for decision, which was also used to reveal the hemodynamic improvement owing to bypass surgery, to disclose the superiority/non-inferiority of stenting/endarterectomy over intensive medical treatments or between these two, or to disclose plausible longitudinal impairment of vascular reserve heralding the needs of bypass surgery at the right time for the side or the artery of interest.
Here in this review, we summarized the clinical problems associated with ICA stenosis of various causes and the appropriate use of basal/acetazolamide SPECT. Literature was searched to yield the clinical problems in ICA stenosis, theoretical and practical development of test use of basal/acetazolamide SPECT, the optimal use thereof, the alternative imaging or quantification methods of vascular reserve, refinement of methods to offer better assistance for clinical decision regarding whether patients are indicated for observation with serial imaging studies, intensive medical treatments, stenting with medical treatments, endarterectomy with medical treatments and eventually or from the beginning, or for triaging them to direct or indirect bypass surgery. In the end, we added the recent innovative approach regarding how to make a biomarker with basal/acetazolamide SPECT, and how it is to be made possible using artificial intelligence (AI)-aid. This will enlighten how to use AI assistance in image reading of well-established clinically useful images. This can be a precedent of successful use of single machine operator which produces physician-aid score-type biomarker per each territory of cerebral/carotid arteries.
Clinical Problems of ICA Steno-Occlusive Diseases in the Elderly and the Young
Appropriate selection of candidates among elderly atherosclerotic ICA steno-occlusive disease patients for each treatment strategy including EC-IC bypass, endarterectomy, and stenting is still an open question. Medical treatment, which is also called as best or intensive medical treatment, has been improved much during the past several decades [10, 26, 38]. In the literature, at first, investigators proposed that randomized clinical trials were warranted and then several trials revealed that EC-IC bypass for extracranial ICA stenosis [1] and EC-IC bypass surgery for extracranial or intracranial ICA stenosis [3] and direct or indirect bypass surgery for intracranial ICA stenosis [6] did not prove their superiority in efficacy in short-term or long-term prevention of stroke or its related death compared with best medical treatment. In addition, carotid artery endarterectomy was not found to improve the prevention of stroke [9–12], that is to say, the annual risk of stroke of 5 to 8% with medical therapy was not reduced with endarterectomy [4]. Mostly, these surgical procedures were adopted as a remedy of hemodynamic failure, insufficiency, or impairment and for reduction of the next incidents of stroke for symptomatic patients. Positron emission tomography (PET) to measure oxygen consumption and blood flow so as to define the misery perfusion or basal/acetazolamide SPECT to disclose the impaired vascular reserve were used as inclusion criteria in these randomized clinical trials [4]. Acute or emergency cases are sometimes said to be an appropriate indication despite the non-superior preventive effect of these surgical procedures. Ironically, bypass surgery in acute cases is one of the signs predicting poor prognosis. Among rebuttal opinions to the famous clinical trials of COSS or carotid revascularization endarterectomy versus stenting trial (CREST), peri-operative strokes were proposed to be taken care of by better surgical or peri-surgical care of the patients. Neurosurgeons are yet looking for the best candidate patients who are to be proven to benefit from the variety of bypass surgeries for the ICA stenosis of diverse etiologies. Nevertheless, stenting used to be comparable with endarterectomy surgery in terms of safety and cost [39], as well as for prevention of subsequent stroke and other complication either peri-procedural or long-term [10, 12]. In addition to these symptomatic patients with hemodynamic failure, asymptomatic patients were studied for their candidacy for stenting and/or endarterectomy and enjoyed similar benefits from endarterectomy and stenting, a little bit favorable to endarterectomy [9, 11].
MMD patients reveal symptoms which come to be easily recognized by physicians and pediatricians, and thus, early discovery and surgery is the trend. Unlike atherosclerotic elderly subjects, the children or the adults suffer from prominent symptoms and characteristic magnetic resonance imaging (MRI) or MR angiography findings. The determination of hemodynamic failure/insufficiency/impairment is not for the support of diagnosis, but rather PET or SPECT is used to determine hemodynamic functional outcome derived from the intracranial ICA steno-occlusive lesions. As MMD patients are young compared with atherosclerotic ICA stenosis patients, collateral formation is profuse, and thus, fully or partially compensated basal perfusion and vascular reserve is frequently observed. Clinical courses of child MMD patients are well known especially according to the ages [17], and almost all the patients are subjected to hemodynamic evaluation using Tc-99m HMPAO SPECT in other countries including Korea than Japan and I-123 iodoamphetamine (I-123 IMP) SPECT in Japan alone during the last several decades. Poor prognostic factors were looked for in the cohorts of child patients, and the infarction and younger ages predicted the poor outcome while decreased vascular reserve with preserved basal perfusion predicted good outcome [17–19]. In adult MMD subjects, asymptomatic subjects are found on routine screening these days or for other check-ups. Their clinical course was favorable without any fatality in a study [24]. However, investigators recommended close follow-up with basal/acetazolamide SPECT and symptoms considering the fact that in the symptomatic long-term follow up patients, the impaired vascular reserve, as well as posterior cerebral artery involvement, was associated with the future ischemic stroke [22]. In symptomatic adult MMD subjects, a meta-analysis revealed that bypass surgery improved stroke prevention than conservative treatments, while no difference in peri-operative outcomes between direct and indirect surgery and direct bypass showed better angiographic outcomes [20]. Country differences were noted in detail in the reports by Chinese investigators [29–31] or western countries [21]; however, the gross trends were similar to Japanese and Korean reports [13–26].
Hemodynamic Evaluation Using Basal/Acetazolamide SPECT in ICA Stenosis
Pathophysiology of Cerebral Ischemia Revealed on SPECT
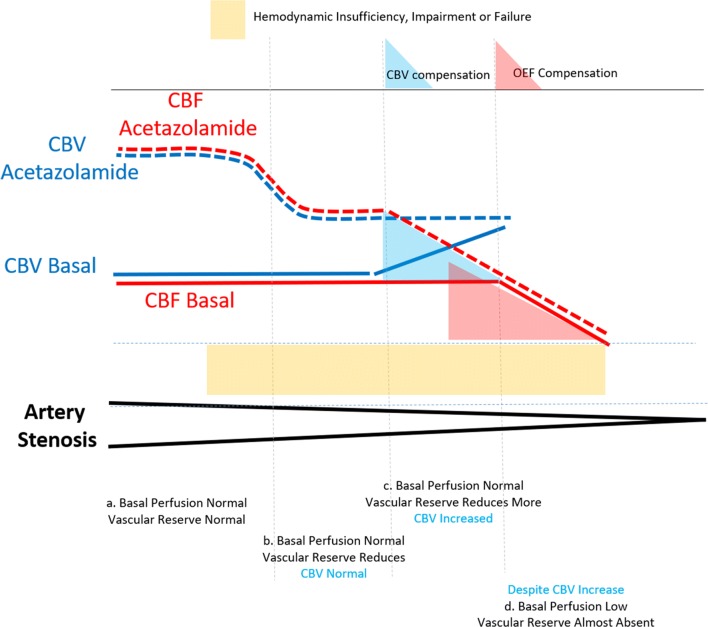
In carotid or cerebral artery stenosis, basal perfusion can be normal owing to the compensation of brain vessels resulting in the increase of cerebral blood volume (CBV) [40, 41] (Fig. 1). The CBV and consequently brain perfusion (cerebral blood flow) is also increased regionally during task-related brain activation [42] or globally by increased CO2 pressure [40] or acetazolamide challenges [43]. Acetazolamide is known to inhibit carbonic anhydrase, ubiquitous in tissues and blood, which means if acetazolamide is injected, CO2 is not converted to bicarbonate ion resulting in increased CO2 pressure just mimicking ventilator supply of the air with more amount of CO2. When extracranial or intracranial ICA or middle or anterior cerebral arteries undergo progressive stenosis, at an early stage in the compensated state, cerebral blood flow (CBF) is complimented by capillary recruitment/vasodilatation with a resultant increase in CBV [40–43]. This is called cerebral vascular reserve. Vasodilatory challenge by acetazolamide will disclose the difference between completely normal, i.e., preserved vascular reserve, and the spectrum of decrease in vascular reserve according to the degree of artery stenosis. In these cases, basal perfusion might look normal like the remote non-stenosed vessel territories; instead, if vascular reserve is not sufficient, then acetazolamide challenge discloses the difference between the affected areas with insufficient reserve and the remote areas with normal reserve.
Fig. 1.
Hemodynamic consequences on cerebral blood flow (CBF) and vascular reserve of carotid or cerebral artery stenosis according to the degree of artery stenosis on basal states and after acetazolamide challenges. When artery stenosis is mild or collaterals can compensate the shortage of inflow, CBV is at basal state (5 mL/100 g) and CBF is normal (5 mL/min/100 g) and also CBF increase is as expected (50% increase) on acetazolamide challenge (a). When stenosis has progressed, basal perfusion is normal but vascular reserve is reduced (for example 20% instead of 50%). Basal perfusion is still normal without increase of CBV (b). When stenosis has progressed further, owing to the compensating CBV increase, basal perfusion is preserved but vascular reserve reduces more (c). Here, CBV is increased on basal state, and after acetazolamide challenge, reserve is not sufficient and delta increase is smaller and might call for the help of compensation of OEF (c). When stenosis becomes critical, basal perfusion starts to reduce despite compensation by increased CBV. Vascular reserve becomes nearly absent and on acetazolamide challenge, CBF does not increase or sometimes decrease (steal phenomenon) (d). On the brink of critical state, any incident can make an event known to be ischemic stroke. At this phase, there is no autoregulation or compensation and cascade process kicks in (infarction)
There should be a threshold that the vascular reserve looks impaired compared with areas supplied by non-stenosed vessels. In other words, if there were found a decrease in vascular reserve, i.e., decrease in the areas supplied by stenosed vessels on acetazolamide-challenged SPECT, the stenosis of vessel of interest has consumed the compensatory capacity for its territory leading to the current state of suffering from shortage or insufficiency of CBF, which is needed for the brain functions of full capacity. Anatomical percentage of stenosis of lumen is known not to be one-to-one matching with the functional consequences of stenosis [44, 45], partly because the current stenosis of the same degree differs in its establishment of time to make it, length of the stenosed area along the vessels, eccentricity of the atherosclerotic plaques, vulnerability of the plaques, and compensatory collateral formation, which is often highly proliferative in child MMD patients, moderately in adult MMD and not in others. Fluid dynamics was also supposed to act on the crooked or rather straight nature of the MMD subjects [46].
Then, if the stenosis has progressed further to decrease perfusion pressure and exhausting compensatory CBV increase, basal perfusion will be reduced too (Fig. 1). However, basal perfusion can also be lowered by other mechanisms such as deafferentation, a.k.a. diaschisis. Basal perfusion and its reduction might not as well represent the decompensated state, and thus, the acetazolamide-challenged perfusion can be normal (increased like the non-stenosed vessel territory) or compromised. Once a sufficient increase of perfusion was observed after acetazolamide challenge, we conclude that the apparent decrease of basal perfusion is not with insufficient vascular reserve. We also easily speculate that mean transit time will be normal in these cases. On the contrary, once perfusion increase was impaired after acetazolamide challenge, the response will lead us to suspect misery perfusion [41, 47]. Misery perfusion is different from normal perfusion accompanied by the impaired vascular reserve in that CBF reduction accompanies that increased oxygen extraction in misery perfusion. Here comes the refined definition of misery perfusion which was made possible by using “gas: PET. Gas PET was probably named as gas PET because O-15 CO is used for measuring CBV (CO is binding hemoglobin and thus RBC), O-15 CO2 is used for measuring CBF (CO2 ← → H2O, where O-15 oxygen atom is interchangeable), and O-15 O2 is used for oxygen extraction fraction (OEF) and consequently oxygen consumption (cerebral metabolic rate of oxygen (CMRO2)). By definition, misery perfusion is accompanied by decreased CBF, increased CBV (exhausted at maximum according to the assumption), and increased OEF but maintained CMRO2. Maintained CMRO2 with decreased CBF is equivalent to OEF increase. This has always made a great cartoon that showed autoregulatory and compensatory power of brain in its frustrating situation receiving blood supply from (progressive) steno-occlusive ICA or cerebral arteries and their collaterals [41]. What we need is to find the patients at least at this phase that CBF starts to decrease despite the increase of CBV and/or OEF, as this phase was supposed to be the optimal time of intervention before no further progress to the state of no help from any medical or surgical interventions. Important is that especially in MMD, late Suzuki grade is not always without collaterals and the hemodynamic state was so variable according to the patients’ collaterals and pathologic courses of intracranial ICA obstruction. Thus, hemodynamic insufficiency or vascular reserve was proposed to be evaluated with SPECT or even PET. With the same degree of stenosis or occlusion, aggressive/indolent course of each individual patient and formation of collaterals make really diverse status of vascular reserve. We can say that until acetazolamide challenge is done, nothing is easily and correctly determined by anatomy of artery of interest (ICA or cerebral arteries) alone.
According to the earlier reports by Rogg and colleagues [47] and by Kuroda and colleagues [48], the pattern of basal perfusion and vascular reserve was classified (Table 1). These investigators used Xe CT and Xe-133 SPECT, respectively. According to Rogg [47], type I was normal basal perfusion and normal preserved vascular reserve, type II was low basal perfusion and preserved vascular reserve after acetazolamide challenge, and type III was normal or low basal perfusion with reduced to absent vascular reserve after acetazolamide challenge. According to Kuroda, type 1 was normal basal perfusion and normal preserved vascular reserve (Rogg’s type I), type 2 was normal basal perfusion and reduced vascular reserve (Rogg’s type III), type 3 was low basal perfusion and reduced vascular reserve (Rogg’s type III), and type 4 would have been low basal perfusion and preserved reserve (Rogg’s type II). Now, we can recognize that both reports just classified all the phenomena we are going to confront with basal/acetazolamide SPECT. In the previous paragraph, I explained the Rogg’s type II (Kuroda’s type 4) as diaschisis; these can now be considered good not needing any intervention as vascular reserve is preserved. Attention should be given to Rogg’s type III and Kuroda’s type 2, as this is the most important one to find and if without acetazolamide challenge, we would have missed. In clinical routine, this type of normal basal perfusion and reduced vascular reserve are so prevalent. Once basal perfusion starts to reduce, there rose the chances to progress further or if left without any treatment, patients would go the natural disease course while suffering from a new stroke episode. On basal/acetazolamide SPECT, cerebral ischemia will be evaluated as preserved or reduced vascular reserve to acetazolamide challenge. Once basal perfusion begins to reduce (Kuroda’s type 3), CT perfusion or MR perfusion studies or arterial spin labeling MR might be able to find the basal hemodynamic failure. This will be discussed further in another chapter at the end.
Table 1.
Classification of Basal Perfusion and Vascular Reserve on Basal/ Acetazolamide CT or SPECT (summarized from References 47 and 48)
| Xe-CT (Rogg et al. AJR Am J Roentgenol. 1989.) [47] | |||
|---|---|---|---|
| Acetazolimide | |||
| Normal | Reduced | ||
| Basal | Normal | Type I | Type III |
| Low | Type II | Type III | |
| Xe-133 SPECT (Kuroda et al. Neurosurgery. 1993.) [48] | |||
| Acetazolimide | |||
| Normal | Reduced | ||
| Basal | Normal | Type 1 | Type 2 |
| Low | Type 4 | Type 3 | |
Methodological Issues of Basal/Acetazolamide SPECT
Radiopharmaceuticals
Currently, three CBF tracers are used for SPECT imaging in routine clinical practice: Tc-99m hexamethyl-propyleneamine-oxime (HMPAO), Tc-99m ethyl-cysteinate dimer (ECD), and I-123-labeled N-isopropyl-p-iodoamphetamine (IMP). They are lipophilic agents that readily cross the blood-brain barrier and have high first-pass extraction fraction. However, their mechanisms of retention in the brain are diverse. Accumulation of Tc-99m HMPAO is by the action of glutathione and other redox activities for its conversion to the hydrophilic form after being transported by a trans-cellular transport system when the oxido-reductive state in the extracellular milieu keep the lipophilic form of Tc-99m HMPAO from being converted to hydrophilic form in extracellular milieu [49]. Intracellular Tc-99m ECD is retained by de-esterification to its hydrophilic acid derivative catalyzed by the intracellular esterase enzymes [50]. The I-123 IMP is retained by binding to various amine receptors in viable brain synaptosomes [51]. I-123 IMP has an extraction fraction higher than Tc-99m HMPAO and Tc-99m ECD. I-123 IMP is exclusively available in Japan. Tc-99m labeled tracers produce images with better quality as they are given 10 times higher amount reflecting the half-life differences between Tc-99m and I-123.
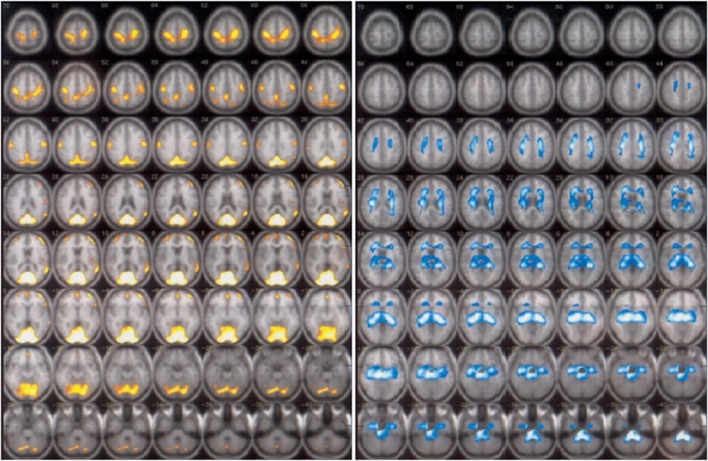
Uptake patterns of Tc-99m HMPAO and Tc-99m ECD are different in the healthy controls [52] and in patients with and without acute or subacute stroke [53, 54]. In the healthy subjects, Tc-99m HMPAO uptake was higher relative to ECD at cerebellum, medial temporal lobes, thalami, and brain stem, and Tc-99m ECD uptake was relatively higher at occipital, supratemporal, and parietal lobes. This was speculated to be due to the differences of concentration of glutathione/red-ox activities for reduction of Tc-99m HMPAO, of esterase for de-esterification of Tc-99m ECD, or both [52–54]. In non-cortical stroke patients, sequential split-dose study of Tc-99m HMPAO and Tc-99m ECD or reverse revealed similar difference in uptake pattern. Tc-99m HMPAO uptake was higher in medial temporal lobes, thalami, and cerebellum, while Tc-99m ECD uptake was higher in frontal, parietal, and occipital lobes and other areas [53] (Fig. 2).
Fig. 2.
Areas of higher Tc-99m ECD uptake than Tc-99m HMPAO uptake (left) and higher Tc-99m HMPAO uptake than Tc-99m ECD uptake (right). SPECT images are displayed on MRI brain template. (from Ref[53]by the permission of EJNMMI)
More importantly, Tc-99m ECD was not taken up in luxury perfusion of subacute infarction, and even proposed as viability agent unlike hyperfixating Tc-99m HMPAO in these areas [54] heralding the use of Tc-99m HMPAO in hyperperfusion syndrome after bypass surgery.
Acquisition Protocol 1-Day Sequential Acquisition
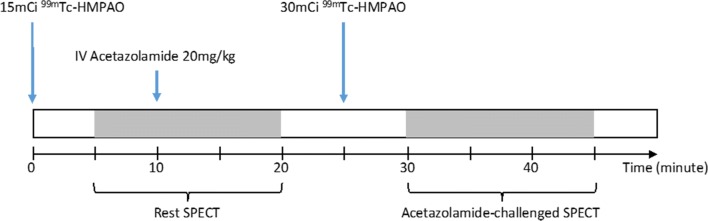
Two protocols have been used for CBF SPECT imaging in clinical settings. The 2-day protocol was recommended by EANM and SNM guidelines [55, 56]. This was published in 2009 and was not revised since then. Before the publication of guidelines, however, there were reports to propose the use of 1-day protocol. This 1-day protocol was validated, simplified, analyzed for error, tested for reproducibility, both intra-institutional and inter-institutional, and standardized to yield clinically applicable software [57–63]. All these were done in Japan using I-123 IMP, and recently, multicenter evaluation was reported with standardized protocol in 12 institutions [62]. Unlike the misleading explanation in the guidelines saying that 1-day protocol using split-dose require sophisticated analysis and data processing, it is not anymore true and rather the 2-day protocol renders the burden to register two separate images for comparison and if co-registration is not done, visual interpretation becomes difficult and sometimes misleading. One-day protocol with subtraction makes the reading physicians confident without concerns of mis-registration due to patients’ motion during acquisition. Patients’ convenience is also obviously guaranteed. One-day protocol for the rest and acetazolamide-challenged brain perfusion SPECT studies is depicted in Fig. 3.
Fig. 3.
One-day sequential acquisition protocol for basal and acetazolamide-challenged Tc-99m HMPAO HMPAO brain perfusion SPECT imaging. Acetazolamide-challenged SPECT images are acquired by the subtraction of basal SPECT images from the second SPECT images
The only concern of quantification of basal perfusion, acetazolamide-challenged perfusion, and vascular reserve with I-123 IMP was the need to sample the arterial blood at 10 min after the injection [64, 65]. Recently, venous sampling was tried to replace the arterial sampling [66]; however, blood is still needed to be put into the dual-table autoradiographic method of CBF quantification for acetazolamide studies [61]. Another problem of I-123 IMP was the longer half-life of I-123 and thus smaller injection dose of I-123 IMP. Lower photon flux made the SPECT images noisy and with more smoothing, images came to be blurred with lower resolution. Despite the use of triple-head camera for better delineation of artery territory, especially anterior cerebral artery, voxel-wise determination of areas showing reduced vascular reserve on the parametric vascular reserve map was not easy with current I-123 IMP SPECT [60, 61, 64, 65]. Split-dose Tc-99m HMPAO basal/acetazolamide SPECT and also split-dose Tc-99m ECD SPECT were also reported to yield correct basal perfusion, acetazolamide-challenged perfusion, and vascular reserve images [67, 68]. Parametric mapping with the Tc-99m HMPAO was feasible, and the anterior cerebral artery territory was clearly delineated. To get the optimal signal-noise ratios for both studies, 15mCi and 30mCi of Tc-99m HMPAO are used for the first and the second studies, respectively [57] (Fig. 3). Subtraction of the first SPECT images from the second SPECT is necessary to remove residual counts from the first study, and this propagates noise. Twice [57] and not 1.33 times [67] the amount of Tc-99m HMPAO is required for the second study of acetazolamide challenge, and only then, the subtracted image representing acetazolamide challenge yields the similar signal to noise ratio to the first study of basal perfusion. It is imperative for the patients to remain still during the entire procedure of 30 or more minutes in a SPECT camera with triple detectors and high-resolution fan-beam collimators. Dual-detector SPECT camera is available in almost every institution worldwide, but it takes longer time (50 or more minutes) and requires zooming due to shoulders of the patients, producing low-resolution images.
Side Effects and Safety of Acetazolamide Challenge
Acetazolamide is a carbonic anhydrase inhibitor, which has been used to treat glaucoma, acute motion sickness, and congestive heart failure. It has a cerebral vasodilatation effect that increases blood flow to the normal brain regions that allows identification of areas of reduced vascular reserve. For acetazolamide-challenged imaging, EANM recommended dose is 14 mg/kg for children and 1 g for adults by slow intravenous administration. The contraindications include allergy to sulfa drugs and acute stroke or recent intracranial hemorrhage [55].
Mortalities due to acute pulmonary edema after acetazolamide-challenged imaging have been reported with total eight incidences in Japan after 20 years of use of annual 20,000 to 30,000 procedures [69]. Multiple causes were proposed for these incidences such as anaphylaxis and acute pulmonary edema probably due to cardiogenic or metabolic acidosis [70–72]. This raised concern in Japan, so that in 2016, Japanese societies who are stakeholders of acetazolamide studies published a guideline related with the use of acetazolamide-challenged tests and emphasized the discreet selection of patients for acetazolamide challenge tests considering cost and benefit and recommended monitoring patients for their respiratory and circulatory sufficiency in high-risk patients and receiving informed consent in advance [69]. Afterward, there was a report of extensive brain infarction after acetazolamide stress in a teenager with MMD unprecedented in the previously reported cases [73]. There, they recommended that acetazolamide should be given under close monitoring or even abstained in the patients who are concerned to show steal phenomenon like in acute stroke or even in chronic stenosis [74], in the case of reversible pontine ischemia [75] or in patients with probable steal phenomena [76–78] considering their clinical courses or when the patients have other concomitant risk factors.
Quantitative Assessment Using Tracer Kinetic Analysis
Quantitative assessment of basal CBF using I-123 IMP SPECT was established two decades ago by Iida [64, 65]. Fortunately, unlike many innovations developed for possible clinical use but not in use in clinical routines, this method was well received by his domestic colleagues despite arterial sampling at a single point of 10 min [58–60] for clinical researches and clinical trials, eventually to have been used popularly for measuring basal perfusion and vascular reserve with expanded development of 1-day split-dose basal/acetazolamide I-123 IMP SPECT [61]. In the literature, this method seemed to be used by neurosurgeons for the selection of bypass surgery candidates, prediction of the natural course with best medical treatment, and elucidating post-treatment improvement of hemodynamic insufficiency. What remains to be expected is using it as a clinical routine in every institution in everyday routine. To achieve this goal of using I-123 IMP SPECT, handy software was warranted and is now available as QSPECT [62] and both basic principle and reproducibility of quantification method have been well understood by the clinical community [60, 63] in Japan. The only problem is that I-IMP is not available in countries other than Japan. Iida called this approach as autoradiography method, and we have several further jargons such as IMPARG (iodoamphetamine autoradiography) or DTARG (dual table autoradiography) [61] for this line of software for basal perfusion and vascular reserve quantification.
Autoradiography method, also known as Gjedde Patlak plot, assumes that radiopharmaceuticals behave like C-14 deoxyglucose of classical in vitro study. F-18 fluorodeoxyglucose followed this example for in vivo imaging, and we assume that once taken up in the cells, this kind of radiopharmaceutical is not washed out. Tc-99m HMPAO and Tc-99m ECD were found to fix in the cells after having been taken up. I-123 IMP washes out with the half-life of 5 min [51] but was modeled to behave as an irreversible agent. Iida [64] tested this hypothesis in his modeling and confirmed that two-compartment model (blood vessel and brain tissue) and three-compartment model (blood vessel and extracellular tissue and intracellular compartments) yielded the same CBF as the same values of obtained K1/k2 and K1*k2/(k2 + k3) while fixing volume of distribution Vd or 30 mL/mL. Of course, this modeling and consequent measurement was validated with O-15 H2O PET, dynamic acquisition with arterial blood sampling, and nonlinear least square fitting methods for basal perfusion SPECT [65]. Later on, acetazolamide-challenged SPECT was added to this quantification and dual-table autoradiography method was introduced by his group again [61]. For this add-up, O-15 H2O PET was again used as the gold standard, going further to confirm the test-retest reproducibility without acetazolamide challenge and also triple acquisition and noise control for subtraction procedures [61]. Other groups tested reliability based on sensitivity analysis and the possibility of using venous blood sample at 10 min and 40 min instead of arterial sampling during basal/acetazolamide SPECT, taking the efforts to make the cumbersome blood sampling procedures simple [66].
Thus, the tracer kinetic for perfusion studies is based on the three-compartment modeling of Iida, with the net transport rate given by this equation: Ki = K1* k3 / (k2 + k3). This means k4 is null or disregarded. Tc-99m HMPAO, once converted to hydrophilic form inside the cells by redox activity/glutathione, does not diffuse back (k4) and Tc-99m ECD, once converted by esterase, does not leave the cells. As observed, Tc-99m HMPAO has initial back-diffusion and Tc-99m ECD takes longer time to reach the plateau of uptake in the cells proportional to the CBF. As is expected, autoradiography methods were developed using Tc-99m HMPAO with dynamic arterial blood sampling [79, 80] or Tc-99m ECD with image-derived input function (aortic arch) [81]. Parametric images of CBF using I-123 IMP were acquired, and database revealed the age-related decline of CBF even after partial volume correction using MRI [82]. In a simplified version of autoradiography method without input function, by setting normal voxel have 50 mL/min/100 g, Tc-99m HMPAO and Tc-99m ECD SPECT yielded comparable CBF over the brain areas compared with I-123 IMP SPECT [83].
Tc-99m HMPAO has higher first-pass extraction, and its back-diffusion (k2) is three times higher than Tc-99m ECD; however, unlike the initial report saying that HMPAO has lower retention fraction [79, 80], Ki values of Tc-99m HMPAO and Tc-99m ECD were similar (0.32 and 0.35 mL/min/g, respectively) in the gray matter [80]. Final brain uptake was 3.5 to 7.0% in Tc-99m HMPAO SPECT and 4.8 to 6.5% in Tc-99m ECD SPECT according to the package inserts by GE and Lantheus, respectively [84, 85]. In vitro stability of short period of 30 min of Tc-99m HMPAO was complemented by adding CoCl2 supplied concomitantly by the supplier [84]. The difference between Tc-99m HMPAO and Tc-99m ECD resides in regional brain areas that Tc-99m HMPAO is higher in mediotemporal lobe, and Tc-99m ECD is higher in occipital lobe [52, 53]. As Tc-99m ECD uptake is low in luxury perfusion in subacute cerebral infarction, and this was supposed to be due to impaired esterase activities of hyperperfused areas [54]. Thus, mechanistically Tc-99m HMPAO seemed to be the preferred agent for examining CBF itself. Animal studies elucidated the differential utility of Tc-99m HMPAO and Tc-99m ECD [86–89]. In rats, extraction fraction ofTc-99m ECD was lower than that of Tc-99m HMPAO and decreased as blood flow increased [86]. Expectedly, the linearity of uptake by I-123 IMP was much better than Tc-99m HMPAO, which was also better than Tc-99m ECD [86]. In other words, there was an inverse relationship between extraction and CBF in Tc-99m ECD SPECT, but less inverse in Tc-99m HMPAO and almost no decrease in I-123 IMP SPECT [86]. Blood flow quantified in rats using acetazolamide-challenged Tc-99m HMPAO was almost equivalent to that by I-123 IMP studies [88]. In primates, while I-123 IMP was the best among SPECT agents especially in high flow states by acetazolamide, Tc-99m HMPAO matched I-123 IMP but Tc-99m ECD did not reflect the increase properly [89]. Interestingly, calcium-channel blocker-induced high CBF was not represented by Tc-99m ECD and I-123 IMP but was only by Tc-99 m HMPAO [89].
In summary, Tc-99m HMPAO SPECT is the preferred choice for basal/acetazolamide brain perfusion SPECT to disclose basal perfusion and vascular reserve. This was also the case with ictal brain perfusion SPECT which had been used to reveal ictal hyperperfused areas [90]. The higher sensitivity of Tc-99m HMPAO ictal SPECT was simply because Tc-99m HMPAO uptake was much higher than Tc-99 m ECD uptake in epileptogenic zones. Better performance is also expected with Tc-99m HMPAO for detecting areas of interest in hyperperfusion syndrome after bypass surgery in MMD patients [91]. As a standard, if we refer to the results obtained on basal/acetazolamide brain perfusion SPECT in humans, CBF of normal human cortical brain is approximately 50 mL/g/min [83] and can be increased by up to 50% after acetazolamide infusion [92].
Image Display and Qualitative Assessment
Brain perfusion SPECT images are usually displayed as pseudo-color images converted from grayscale images to improve the diagnostic performance of the interpreters [93]. EANM guideline recommends the use of continuous color tables [55]. Visual assessment of the brain perfusion SPECT is generally used in daily clinical practice with good agreement among the interpreters [94]. On the perfusion SPECT images, the cerebellum is often taken as the reference region because it is least involved in MMD. To evaluate vascular reserve, both rest and stress brain perfusion SPECT images are displayed side-by-side and the intensity of cerebellum of the two image sets are adjusted to the same level in case of Tc-99m HMPAO SPECT. Recently, the findings of the acetazolamide-challenged SPECT and O-15 H2O PET were compared in clinical setting [95, 96]. O-15 H2O PET was favored in both reports, and this is an evidence of great improvement of O-15 H2O PET as O-15 H2O PET images were traditionally of very poor quality due to very short half-time of O-15. Low resolution and reading visually the SPECT images might have resulted in low sensitivity in the report by Acker and colleagues [96], though dual-head SPECT camera with smoothing would have degraded resolution further and lowered the sensitivity to find the areas with reduced vascular reserve. We suspect that the totally different findings between two studies in some cases might represent rapidly changing hemodynamic status of that individual and thus cannot be compared to reveal the differential efficacy as both studies are 4 weeks apart [96].
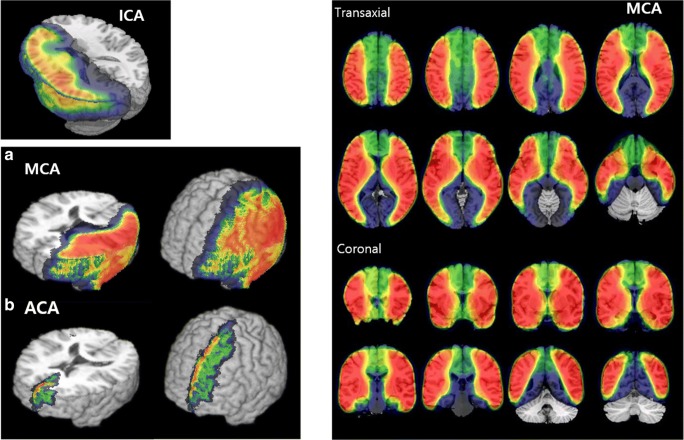
As SPECT images are without anatomical information on its own, there were efforts to transform, normalize, and co-register the images to a standard brain template (e.g., the Talairach atlas or MNI Average Brain Model). Age-, gender-, and population-specific brain templates are available and especially for arterial territories [97]. Population probabilistic maps for arterial territories were also made by the population-based atlas using the images acquired after direct injection of Tc-99m HMPAO or Tc-99m ECD into the ICA or middle cerebral arteries in human subjects [98, 99] (Fig. 4). The highlighted areas indicated the probability of those areas or voxels belonging to the territories supplied by specific arteries such as ICA and middle cerebral artery. Posterior cerebral artery territories could be calculated by subtraction of ICA territory map from hemispheric map and anterior cerebral artery territories by subtraction of middle cerebral artery territory map from ICA map [98, 99]. The deviation of basal perfusion and acetazolamide-challenged perfusion of every voxel from the norms of normal controls were measured, whose number of voxels below normal ranges were used to compare postoperative changes of basal and acetazolamide-challenged perfusion [100]. The counts of certain artery territory represented by the above maps at basal and acetazolamide-challenged perfusion were used to calculate cerebrovascular reserve index (CVRI) by CVRI = [Cacetazolamide/Cbasal − 1] × 100, where Cacetazolamide and Cbasal are the counts on acetazolamide-challenged and basal SPECT images respectively [94, 100–102]. Other semi-quantitative measures based on ROIs drawn manually and their ROI/cerebellum ratios or voxel-based quantitative mapping methods using counts were also tried [94, 100–104].
Fig. 4.
Delineation of brain territories supplied by internal carotid arteries, ICA (left panel), and middle cerebral arteries, MCA (right panels), using the statistical probabilistic anatomic mapping (SPAM). Anterior cerebral artery, ACA territories were subtracted one of MCA territorial probabilistic map from ICA map. Abbreviations: ICA, internal carotid artery; MCA, middle cerebral artery. (Left from Ref [98], by the permission of Neuroimage, Right from ref [99], by the permission of JNM)
Comparing Brain Perfusion SPECT to Other Techniques: Angiography, PET, Perfusion CT, Contrast-Enhanced MR, and Arterial Spin Labeling (ASL) MRI
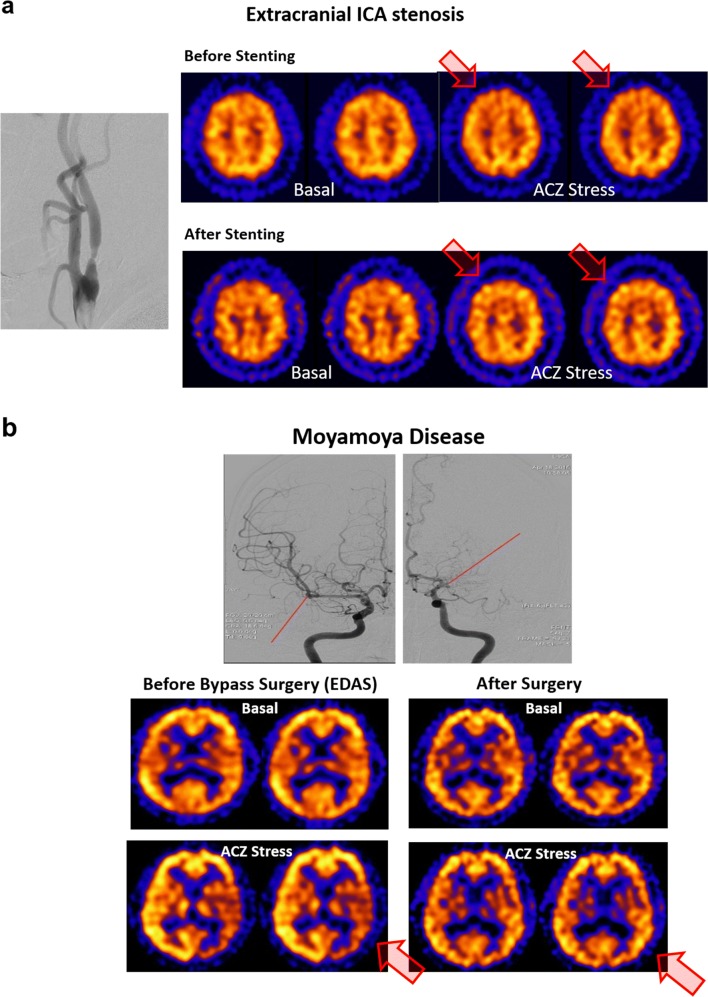
Contrast angiography is the gold standard in the diagnosis of extracranial or intracranial ICA steno-occlusive diseases including MMD. MR angiography was proposed to replace invasive contrast angiography, however, sluggish flow of stenotic lesions used to let overestimate the degree of stenosis. Nevertheless, MR angiography is used for non-invasive preliminary study. Basal/acetazolamide SPECT studies were compared with MR angiography and revealed that this basal/acetazolamide SPECT disclose hemodynamic significance of known stenosis on MR angiography [45]. Degree of hemodynamic impairment due to the stenosis found on X-ray contrast angiography was also moderately correlated with basal perfusion and vascular reserve disclosed by basal/acetazolamide SPECT [44, 105]. Basal perfusion and vascular reserve were always the additional information easily obtained from basal/acetazolamide brain perfusion SPECT (Fig. 5).
Fig. 5.
Examples of extracranial ICA stenosis and Moyamoya disease before and after treatments with basal/acetazolamide SPECT. a Tight stenosis on the right internal carotid artery was accompanied by mildly reduced vascular reserve in the right frontal lobe. Reduction ranged from medial frontal to anterior dorsal frontal areas implying ACA and anterior MCA territories. It completely recovered weeks after stenting. b Despite intracranial stenosis on both ICA with basal collaterals, typical of Moyamoya disease, right hemispheric basal perfusion and vascular reserve was normal and left fronto-temporo-parietal lobes showed reduction of basal perfusion and vascular reserve. Affected territory was mainly MCA territory and after left EDAS surgery, both basal perfusion and vascular reserve recovered to normal
As is explained above, O-15 H2O PET is the gold standard method to quantify absolute amount of basal perfusion, acetazolamide-challenged perfusion, and the percent increase of perfusion as vascular reserve [106]. It also serves as a reference for evaluating new techniques. This is true though I-123 IMP SPECT with single arterial blood sampling and autoradiography method [64, 65] could yield basal perfusion and also once dual table method was adopted to this autoradiography method [61], basal perfusion and vascular reserve could be measured by basal/acetazolamide SPECT [106]. Other SPECT studies, quantification methods of Tc-99m HMPAO or Tc-99m ECD SPECT were proposed but not yet standardized for clinical use [67, 68]. Basal perfusion, acetazolamide-challenged perfusion, and consequently measured vascular reserve on O-15 H2O PET or I-123 IMP SPECT can now be considered to be the ground truth for the validation of other new technologies developed later.
Recently developed competing technologies to measure basal perfusion and vascular reserve are contrast-enhanced CT perfusion, contrast (or susceptibility)-enhanced perfusion MR, and arterial spin labeling (ASL) MR studies [107]. Among these techniques, ASL MR is the most promising and still on the fast wave of development and improvement [108–110] as ASL MR does not require contrast media but use arterial spin labeling of tissue water of the neck which includes internal carotid artery [107]. Before decay of labeled spin of input arterial blood to the brain, brain tissue perfusion is modeled and measured to yield basal perfusion and acetazolamide-challenged perfusion. Vascular reserve was also calculated and even vascular reserve map was drawn using those measures of basal perfusion and vascular reserve [111–113]. Basal perfusion and vascular reserve measured using I-123 IMP SPECT [108–110] or Tc-99m HMPAO [111–113] basal/acetazolamide SPECT were used as ground truth to draw receiver-operating characteristics (ROC) curves for these studies. Area under ROC curves were around 0.82 or higher [111]. Quantification was tried; however, the CBF values have tended to underestimate the CBF which was suggested to be due to prolonged arrival time of spin-labeled blood [107]. This seems to be exactly the case in MMD as these patients have profuse collateral pathways to deliver spin-labeled blood to brain tissues. Errors due to labeled blood residing in the blood vessels not being extracted by brain parenchyma make another challenge. As yet, due to the lower signal acquired from various ASL protocols/packages, image quality is not so excellent. Improving signal-to-noise ratio, improving acquisition protocols, models, and correct calculation of regional voxel-wise CBF continue to be the subjects of active research in ASL MR.
Contrast-enhanced perfusion MRI mostly using Gd-DTPA and analogous agents are using the same technology as PET or SPECT to derive arterial input function and tissue function. Perfusion MRI had been tried for a long time now, and image quality is yet to be improved further. Quantified values of CBF are still inaccurate and imprecise; furthermore, parametric images lack voxel-level details [114–119]. As well as contrast-enhanced perfusion MRI, contrast-enhanced perfusion CT using iodinated contrast agents has been developed to yield CBV, CBF, and mean transit time images [120–124]. In the model of perfusion MRI and perfusion CT, measured CBV was used to yield mean transit time (MTT), and considering the autoregulation of brain using capillary bed and CBV compensation of basal perfusion, in theory, MTT (=CBV/CBF) will be a good indicator to find whether brain areas of interest are compensated or not. And thus, using experimental evidence, some reported that MTT images were sufficient to replace vascular reserve studies, which yielded basal perfusion and vascular reserve images of basal/acetazolamide-challenged Xe-133 SPECT, Tc-99m HMPAO or ECD SPECT or O-15 H2O PET [115–119]. However, with contrast-enhanced MR perfusion studies or perfusion CT at basal states, acetazolamide-challenged increase of perfusion could not be exactly predicted. Correlation of MTT (of basal perfusion on contrast-enhanced perfusion MR or perfusion CT) and vascular reserve (on acetazolamide-challenged SPECT of PET), if any, is just the correlation and represents only the fact that brain areas with impaired vascular reserve at acetazolamide challenge tended to compensate the perfusion pressure reduction by increasing CBV (and prolonging MTT). Whether the correlation between vascular reserve and basal MTT or CBV let us use basal imaging instead of acetazolamide-challenge studies, if ever, remains to be validated.
Clinical Use of Basal SPECT for Peri-Operative Care
Bypass surgery is effective definitely in reducing ipsilateral stroke among MMD patients [13, 19] and repeatedly validated in large observational studies globally [16–32]. In the case of MMD, direct or indirect bypass surgery is necessary for most pediatric patients even despite peri-operative mortalities in the very young subjects due to their aggressive nature [17, 19]. In adult MMD subjects, direct bypass surgery is done with almost perfect graft patency and indirect EDAS is also sure to improve vascular reserve and basal perfusion months after the operation. Problem mostly resides in peri-operative care because hyperperfusion syndrome [91, 104, 125–129] occurs and, if identified, can be treated without any sequelae. Interestingly, on postoperative basal SPECT, increased perfusion is observed in half to two-thirds of MMD or non-Moyamoya ICA stenosis patients ranging from small to large areas [129].
For therapeutic aspects, this was important, though not all the patients having hyperperfused areas show hyperperfusion syndrome, that the close monitoring of blood pressure and management helped the improvement of syndrome, instead of being distracted by the possible graft problems or the remaining ischemia. Hyperperfused areas could be delineated better by Tc-99m HMPAO SPECT than Tc-99m ECD SPECT, which should be correlated with the possibility that these patients might have higher chance of symptomatic presentation. Obviously, preoperative reduction of vascular reserve predisposes to postoperative hyperperfusion and hyperperfusion syndrome. Other biochemical factors or intraoperative conditions were investigated in addition to preoperative impairment of vascular reserve [125–128].
Concept Using Basal Cerebral Blood Volume/Mean Transit Time Instead of Measurement of Vascular Reserve
There has been a huge effort to simplify the imaging methods to find the brain tissues at risk in ICA stenosis. Around 1980, PET with O-15 H2O, O-15 CO, and O-15 O2 elucidated the autoregulation of brain in response to decreased perfusion pressure by images and quantified parameters of CBF, CBV, and oxygen extraction fraction (OEF) [130] (Fig. 1). Using PET, multicenter trial yielded quite excellent reproducibility [106]. CMRO2 was the calculated parameter based on CBF, OEF, and oxygen contents of the blood. In ICA steno-occlusive diseases, OEF and CBV were increased owing to autoregulation and its derangement was associated with the future development of strokes. Thus, if autoregulation is maintained, though OEF is increased, once CBV is normal, then patients are not at risk of strokes. Cerebral vasodilation (increased CBV) was considered to be compensatory to the reduced perfusion pressure due to stenosis; however, it might also be the evidence of decompensation representing the failure in maintaining the vascular tones [41]. Now, CBV increase might be either the evidence of desirable compensating mechanism or exhaustion of compensation in miserable condition. Investigators normalized the degree of CBV increase by the accompanying CBF and yielded MTT was produced. In this way, accuracy of MTT depended on the accuracy of two measurements, CBV and CBF. By the way, accuracy of CBF measurement depends heavily on the determination of correct arterial input function on the images of perfusion CT, contrast-enhanced MRI, and even ASL MRI [107]. PET or SPECT originally used input function from sampled arterial blood. When image-derived input was used in CT or MRI studies, especially narrowed extracranial-intracranial vessels cannot be easily used as input, either manually or automatically drawn in ICA stenosis patients, which caused much errors. These technical difficulties resulted in overestimated CBF for affected arterial territories. Then, MTT is erroneously produced to yield smaller numbers than real. Independent measurement of MTT might be a solution to this problem. Another problem related with CBV measurement is explained in the following paragraph.
On activation, brain regions are recruiting their capacity of CBF reserve to their regions relevant to the tasks that the subjects are doing by increasing 10 to 40% (20% on average) of basal CBF [42, 131]. This has been supposed to be caused by the increase of similar amount of regional CBV. On CO2 inhalation activation O-15 CO PET, CBV was found to be 5 mL/100 g of brain tissue and increase 10% over basal CBV during hypercapnia was reported globally over the brain [40, 41] (Fig. 1). On a recent study, around 5 mL/100 g of CBV was again observed at basal state and in the tissues supplied by stenosed vessels, 10 to 20% increase of CBV was also reported according to the degree of stenosis [116]. CBF was preserved or decreased (10%), and accordingly, MTT was prolonged around 20 to 40% increase. Combining all these data, on activation, regional brain tissues enjoy 20% increase in CBF with 10% increase in CBV on steno-occlusive states, and thus, stenosis-related territory mobilizes 10 to 20% increase of CBV to maintain its desired CBF (Fig. 1). MTT is prolonged to support the CBF as much as possible. On acetazolamide challenge, CBF is known to increase up to 50% of basal perfusion on many reports using O-15 H2O PET or I-123 IMP SPECT. This global increase of CBF should be considered as the brain’s response to pharmacologic acetazolamide challenge mimicking severe global hypercapnia. This pharmacologic challenge had better be considered as testing the limit of vascular reserve of the entire brain (Fig. 1), which exceeds regionally activated on brain activation or compensatory vascular reserve in the stenosis-affected brain areas.
Thus, in practicality, this test of brain’s full capacity using SPECT or PET easily discloses the areas of insufficient vascular reserve (despite compensated response assisted by collaterals) in that the enhanced perfusion pressure difference across the stenosis, which had been called upon as the compensatory mechanism, will be neutralized and also the collaterals’ assistance shut down. This is because pressure difference over stenotic areas and the collateral vessels are dilated resulting in loss of the pressure difference between supplier-recipient vessels. Steal phenomenon can also occur easily in this situation even causing rare side effects explained in side-effect chapter and cited above. Basal-alone studies of evaluating CBV and MTT has the inherent shortcoming not to be able to disclose this capacity of brain’s vascular reserve and thus acetazolamide-challenged CBF will make an additional discovery which does not fit into the definition of misery perfusion. Misery perfusion was originally defined as the area which is suffering from exhausted CBV, i.e., vasodilatory capacity (and also prolonged MTT) with maintained CBF or decreased CBF. All these compensatory activities with the OEF increase enable to maintain CMRO2 of the affected tissue. This made a report [95] confusing that basal/acetazolamide SPECT had yielded many false positives when the findings of SPECT of preserved basal perfusion and decreased vascular reserve (positive test for basal/acetazolamide SPECT) were compared with the definition of misery perfusion and thus not fitting into the category of misery perfusion (negative in truth and thus false positive result). We suspect that this report by Setta and colleagues [95] is an eloquent example of the benefit of acetazolamide-challenged SPECT which enables us to find the patients with hemodynamically significant impairment of vascular reserve with normal basal perfusion (with robust non-exhaustive increase of CBV) with impaired vascular reserve. These patients are good candidates for further treatments, whatever they are named as misery perfusion, impaired vascular reserve, or hemodynamic insufficiency/impairment, i.e., treatments by stenting, endarterectomy, bypass surgery, or best medical treatment.
Qualitative Visual Assessment Aided by Big Data-Deep Learning-Yielded Scores
During the last 25 years, basal/acetazolamide SPECT was adopted to Korea, and several tens of thousands cases are being performed consuming 48,000 acetazolamide vials per year in 2018. Since the introduction, triple-head SPECT was essential for achieving the best SPECT images for easier visual assessment. In the meantime, there had been changes of SPECT-producing companies, and less and less companies supplied triple-head SPECT until finally, only one remained. To replace triple-head SPECT with more popular dual-head SPECT in our institution, many solutions were tried including optimizing zoom-factor, changing collimator to increase photon flux, or combining adjusted collimator-zoom-reconstruction software matching. However, shoulder prevented the detectors from coming close to the head and this distance problem could not be overcome with any combination of acquisition-reconstruction protocols with dual-head SPECT camera. We were fortunate to have triple-head SPECT camera and also having invested small talents to write the in-house software to display with the best color stripes to convert grayscale images to pseudo-color, and finally, it was well adapted to the clinical routines and the training of the new nuclear medicine physicians. About half of the faculty had become the best experts in reading basal/subtracted acetazolamide-challenged brain perfusion SPECT from very young child patients to elderly patients, which were all acquired using 1-day sequential acquisition and subtraction (of the first image from the second image) protocol. Last 10 years, we enjoyed a similar quality of basal/acetazolamide SPECT images and correlative imaging with contrast angiography as well as structural MRIs and MR angiography. During these 10 years, we performed 7800 basal/SPECT studies, all in digital, and uploaded them in the hospital PACS system. These images were acquired from 4000 or more subjects mostly having the ICA or cerebral artery stenosis, who had angiography and many of them were operated with direct and indirect bypass surgery. Repeated imaging was done one to several times as follow up studies, and all these images became a big data.
Using this big data or basal/acetazolamide SPECT, we developed a physician-aid machine learning program having the capability of a single competent operator which can read the images by itself and designate that the basal perfusion of either an artery territory or combined territories and vascular reserve thereof are normal or reduced [132]. For this work, foremost, we needed to develop another deep learning algorithm to read all these five to seven human expert operators’ reports on their clinical routines during the last 10 years and interpret them as labels. Using 500 cases manually labeled by one of the authors, a deep learning algorithm, called long short-term memory (LSTM) network, could extract structured labels from the text reports, and with test cases of 100, area under ROC curves were perfect for interpreting these unstructured text reports to extract label. With this, we could have labels for the entire 7345 cases. A deep three-dimensional convolutional neural network (3D CNN) was adopted to read the entire 7345 images and using 500 cases as test group, area under ROC curves were around 0.85 for designating each artery with compromised basal perfusion or impaired vascular reserve. 3D CNN yielded scores ranging from 0 to 1 for differentiating normal from abnormal perfusion and reserve. Especially basal perfusion could have the scores ranging similarly to vascular reserve and thus raise hope to behave as physician-aid in further prospective studies or clinical routines in the future. While we already divided the cerebral artery territories according to probabilistic atlas for major cerebral artery territories and single-operator deep learning algorithm were trained by these labels by the collective human experts, we believe that this algorithm and its product scores might become an example of transfer learning, if it is proven to be successful in reading the images from other institutions or explainable artificial intelligence (XAI) if it determines which areas were the evidence of determination.
Conclusion
Considering the failure to prove the efficacy of protective efficacy of bypass surgery or endarterectomy against future ipsilateral stroke and consequent mortality, for atherosclerotic ICA stenosis in the elderly, basal/acetazolamide SPECT can help find the subgroup of bypass-indicated patients. However, in the symptomatic patients with ICA steno-occlusive diseases receiving stent or medical treatment, basal/acetazolamide SPECT can help determine vascular reserve exactly and be used as a predictive biomarker for future stroke or the needs of considering a change of the treatment modality, i.e., to surgery. In MMD, basal/acetazolamide SPECT, either quantitative or qualitative, either I-123 IMP or Tc-99m HMPAO, worked well to help clinical decision for various purposes. This SPECT was used for comparing the performances of direct and indirect EC-IC bypass surgery in adult MMD patients and also offered the follow-up tool in asymptomatic adult MMD patients, too.
Despite the introduction of new technologies of measuring cerebrovascular autoregulation based on CT or MRI at basal states, acetazolamide-challenged ASL MRI was proposed to match the acetazolamide-challenged Tc-99m HMPAO SPECT. Theoretically or based on collected data in the literature, vascular reserve documented on basal/acetazolamide brain perfusion SPECT is continuously valuable for determining basal perfusion and vascular reserve. Considering a huge individual variation of cerebral vessel anatomy and variable collateral formation especially in MMD patients, the help of deep learning-based algorithm and its product biomarker might act as a physician-aid to be given to the physicians trying to determine subtle changes of basal perfusion and vascular reserve.
Compliance with Ethical Standards
Conflict of Interest
Teck Huat Wong, Qaid Ahmed Shagera, Hyun Gee Ryoo, Seunggyun Ha, and Dong Soo Lee declare no conflict of interest.
Ethical Statement
This work does not contain any studies with human participants or animals performed by any of the authors. For this type of study formal consent is not required.
Informed Consent
Not Applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Teck Huat Wong and Qaid Ahmed Shagera contributed equally to this work.
References
- 1.Garrett MC, Komotar RJ, Starke RM, Merkow MB, Otten ML, Sciacca RR, et al. The efficacy of direct extracranial-intracranial bypass in the treatment of symptomatic hemodynamic failure secondary to athero-occlusive disease: a systematic review. Clin Neurol Neurosurg. 2009;111:319–326. doi: 10.1016/j.clineuro.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Clarke WR, Grubb RL, Jr, Videen TO. Adams HP, Jr, Derdeyn CP, et al. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the carotid occlusion surgery study randomized trial. JAMA. 2011;306:1983–1992. doi: 10.1001/jama.2011.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds MR, Derdeyn CP, Grubb RL, Jr, Powers WJ, Zipfel GJ. Extracranial-intracranial bypass for ischemic cerebrovascular disease: what have we learned from the carotid occlusion surgery study? Neurosurg Focus. 2014;36:E9. doi: 10.3171/2013.10.FOCUS13427. [DOI] [PubMed] [Google Scholar]
- 4.Hage ZA, Behbahani M, Amin-Hanjani S, Charbel FT. Carotid bypass for carotid occlusion. Curr Atheroscler Rep. 2015;17:36. doi: 10.1007/s11883-015-0517-6. [DOI] [PubMed] [Google Scholar]
- 5.White TG, Abou-Al-Shaar H, Park J, Katz J, Langer DJ, Dehdashti AR. Cerebral revascularization after the carotid occlusion surgery study: what candidates remain, and can we do better? Neurosurg Focus. 2019;46:E3. doi: 10.3171/2018.11.FOCUS18536. [DOI] [PubMed] [Google Scholar]
- 6.Ilyas A, Chen CJ, Ironside N, Buell TJ, Chagoya G, Schmalz PG, et al. Medical management versus surgical bypass for symptomatic intracranial atherosclerotic disease: a systematic review. World Neurosurg. 2019;129:62–71. doi: 10.1016/j.wneu.2019.05.223. [DOI] [PubMed] [Google Scholar]
- 7.Gunawardena Manuri, Rogers Jeffrey M., Stoodley Marcus A., Morgan Michael K. Revascularization surgery for symptomatic non-moyamoya intracranial arterial stenosis or occlusion. Journal of Neurosurgery. 2020;132(2):415–420. doi: 10.3171/2018.9.JNS181075. [DOI] [PubMed] [Google Scholar]
- 8.Matano F, Murai Y, Tateyama K, Tamaki T, Mizunari T, Matsukawa H, et al. Long-term patency of superficial temporal artery to middle cerebral artery bypass for cerebral atherosclerotic disease: factors determining the bypass patent. Neurosurg Rev. 2016;39:655–661. doi: 10.1007/s10143-016-0736-5. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfield K, Matsumura JS, Chaturvedi S, Riles T, Ansel GM, Metzger DC, et al. Randomized trial of stent versus surgery for asymptomatic carotid stenosis. N Engl J Med. 2016;374:1011–1020. doi: 10.1056/NEJMoa1515706. [DOI] [PubMed] [Google Scholar]
- 10.Noiphithak R, Liengudom A. Recent update on carotid endarterectomy versus carotid artery stenting. Cerebrovasc Dis. 2017;43:68–75. doi: 10.1159/000453282. [DOI] [PubMed] [Google Scholar]
- 11.Moresoli P, Habib B, Reynier P, Secrest MH, Eisenberg MJ, Filion KB. Carotid stenting versus endarterectomy for asymptomatic carotid artery stenosis: a systematic review and meta-analysis. Stroke. 2017;48:2150–2157. doi: 10.1161/STROKEAHA.117.016824. [DOI] [PubMed] [Google Scholar]
- 12.Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W, et al. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med. 2016;374:1021–1031. doi: 10.1056/NEJMoa1505215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto N, Tominaga T, Miyamoto S, Nagata I, Houkin K, Suzuki N, et al. Guidelines for diagnosis and treatment of Moyamoya disease (spontaneous occlusion of the circle of Willis) Neurol Med Chir (Tokyo) 2012;52:245–266. doi: 10.2176/nmc.52.245. [DOI] [PubMed] [Google Scholar]
- 14.Wakai K, Tamakoshi A, Ikezaki K, Fukui M, Kawamura T, Aoki R, et al. Epidemiological features of Moyamoya disease in Japan: findings from a nationwide survey. Clin Neurol Neurosurg. 1997;99:S1–S5. doi: 10.1016/s0303-8467(97)00031-0. [DOI] [PubMed] [Google Scholar]
- 15.Baba T, Houkin K, Kuroda S. Novel epidemiological features of Moyamoya disease. J Neurol Neurosurg Psychiatry. 2008;79:900–904. doi: 10.1136/jnnp.2007.130666. [DOI] [PubMed] [Google Scholar]
- 16.Kim SK, Wang KC, Kim IO, Lee DS, Cho BK. Combined encephaloduroarteriosynangiosis and bifrontal encephalogaleo(periosteal)synangiosis in pediatric Moyamoya disease. Neurosurgery. 2002;50:88–96. doi: 10.1097/00006123-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Kim S-K, Seol HJ, Cho B-K, Hwang Y-S, Lee DS, Wang K-C. Moyamoya disease among young patients: its aggressive clinical course and the role of active surgical treatment. Neurosurgery. 2004;54:840–846. doi: 10.1227/01.neu.0000114140.41509.14. [DOI] [PubMed] [Google Scholar]
- 18.Kim SK, Cho BK, Phi JH, et al. Pediatric Moyamoya disease: an analysis of 410 consecutive cases. Ann Neurol. 2010;68:92–101. doi: 10.1002/ana.21981. [DOI] [PubMed] [Google Scholar]
- 19.Kim T, Oh CW, Bang JS, Kim JE, Cho WS. Moyamoya disease: treatment and outcomes. J Stroke. 2016;18:21–30. doi: 10.5853/jos.2015.01739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeon JP, Kim JE, Cho WS, Bang JS, Son YJ, Oh CW. Meta-analysis of the surgical outcomes of symptomatic Moyamoya disease in adults. J Neurosurg. 2018;128:793–799. doi: 10.3171/2016.11.JNS161688. [DOI] [PubMed] [Google Scholar]
- 21.Han JS, Abou-Hamden A, Mandell DM, et al. Impact of extracranial-intracranial bypass on cerebrovascular reactivity and clinical outcome in patients with symptomatic Moyamoya vasculopathy. Stroke. 2011;42:3047–3054. doi: 10.1161/STROKEAHA.111.615955. [DOI] [PubMed] [Google Scholar]
- 22.Noh HJ, Kim SJ, Kim JS, Hong SC, Kim KH, Jun P, et al. Long term outcome and predictors of ischemic stroke recurrence in adult Moyamoya disease. J Neurol Sci. 2015;359:381–388. doi: 10.1016/j.jns.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Kim T, Oh CW, Kwon OK, Hwang G, Kim JE, Kang HS, et al. Stroke prevention by direct revascularization for patients with adult-onset Moyamoya disease presenting with ischemia. J Neurosurg. 2016;124:1788–1793. doi: 10.3171/2015.6.JNS151105. [DOI] [PubMed] [Google Scholar]
- 24.Jo KI, Yeon JY, Hong SC, Kim JS. Clinical course of asymptomatic adult Moyamoya disease. Cerebrovasc Dis. 2014;37:94–101. doi: 10.1159/000356350. [DOI] [PubMed] [Google Scholar]
- 25.Miyoshi K, Chida K, Kobayashi M, Kubo Y, Yoshida K, Terasaki K, et al. Two-year clinical, cerebral hemodynamic, and cognitive outcomes of adult patients undergoing medication alone for symptomatically ischemic Moyamoya disease without cerebral misery perfusion: a prospective cohort study. Neurosurgery. 2019;84:1233–1241. doi: 10.1093/neuros/nyy234. [DOI] [PubMed] [Google Scholar]
- 26.Chiba T, Setta K, Shimada Y, Yoshida J, Fujimoto K, Tsutsui S, et al. Comparison of effects between clopidogrel and cilostazol on cerebral perfusion in nonsurgical adult patients with symptomatically ischemic Moyamoya disease: subanalysis of a prospective cohort. J Stroke Cerebrovasc Dis. 2018;27:3373–3379. doi: 10.1016/j.jstrokecerebrovasdis.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 27.Kamada F, Aoki Y, Narisawa A, Abe Y, Komatsuzaki S, Kikuchi A, et al. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J Hum Genet. 2011;56:34–40. doi: 10.1038/jhg.2010.132. [DOI] [PubMed] [Google Scholar]
- 28.Bang OY, Ryoo S, Kim SJ, Yoon CH, Cha J, Yeon JY, et al. Adult Moyamoya disease: a burden of intracranial stenosis in east Asians? PLoS One. 2015;10:e0130663. doi: 10.1371/journal.pone.0130663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan L, Bao XY, Yang WZ, Shi WC, Li DS, Zhang ZS, et al. Moyamoya disease in China: its clinical features and outcomes. Stroke. 2012;43:56–60. doi: 10.1161/STROKEAHA.111.621300. [DOI] [PubMed] [Google Scholar]
- 30.Bao XY, Duan L, Li DS, Yang WZ, Sun WJ, Zhang ZS, et al. Clinical features, surgical treatment and long-term outcome in adult patients with Moyamoya disease in China. Cerebrovasc Dis. 2012;34:305–313. doi: 10.1159/000343225. [DOI] [PubMed] [Google Scholar]
- 31.Bao XY, Duan L, Yang WZ, Li DS, Sun WJ, Zhang ZS, et al. Clinical features, surgical treatment, and long-term outcome in pediatric patients with Moyamoya disease in China. Cerebrovasc Dis. 2015;39:75–81. doi: 10.1159/000369524. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Zhang D, Wang S, Zhao YL, Teo M, Wang R, et al. Clinical features and long-term outcomes of Moyamoya disease: a single-center experience with 528 cases in China. J Neurosurg. 2015;122:392–399. doi: 10.3171/2014.10.JNS132369. [DOI] [PubMed] [Google Scholar]
- 33.Bang OY. Intracranial atherosclerosis: current understanding and perspectives. J Stroke. 2014;16:27–35. doi: 10.5853/jos.2014.16.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyamoto S, Yoshimoto T, Hashimoto N, Okada Y, Tsuji I, Tominaga T, et al. Effects of extracranial–intracranial bypass for patients with hemorrhagic Moyamoya disease: results of the Japan adult Moyamoya trial. Stroke. 2014;45:1415–1421. doi: 10.1161/STROKEAHA.113.004386. [DOI] [PubMed] [Google Scholar]
- 35.Low SW, Teo K, Lwin S, Yeo LL, Paliwal PR, Ahmad A, et al. Improvement in cerebral hemodynamic parameters and outcomes after superficial temporal artery-middle cerebral artery bypass in patients with severe stenoocclusive disease of the intracranial internal carotid or middle cerebral arteries. J Neurosurg. 2015;123:662–669. doi: 10.3171/2014.11.JNS141553. [DOI] [PubMed] [Google Scholar]
- 36.Kim JS, Moon DH, Kim GE, Cho YP, Kim JS, Ryu JS, et al. Acetazolamide stress brain-perfusion SPECT predicts the need for carotid shunting during carotid endarterectomy. J Nucl Med. 2000;41:1836–1841. [PubMed] [Google Scholar]
- 37.Oka F, Ishihara H, Kato S, Higashi M, Suzuki M. Cerebral hemodynamic benefits after contralateral carotid artery stenting in patients with internal carotid artery occlusion. AJNR Am J Neuroradiol. 2013;34:616–621. doi: 10.3174/ajnr.A3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuroda S, Houkin K, Kamiyama H, Mitsumori K, Iwasaki Y, Abe H. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it? Stroke. 2001;32:2110–2116. doi: 10.1161/hs0901.095692. [DOI] [PubMed] [Google Scholar]
- 39.de Vries EE, Baldew VGM, den Ruijter HM, de Borst GJ. Meta-analysis of the costs of carotid artery stenting and carotid endarterectomy. Br J Surg. 2017;104:1284–1292. doi: 10.1002/bjs.10649. [DOI] [PubMed] [Google Scholar]
- 40.Greenberg JH, Alavi A, Reivich M, Kuhl D, Uzzell B. Local cerebral blood volume response to carbon dioxide in man. Circ Res. 1978;43:324–331. doi: 10.1161/01.res.43.2.324. [DOI] [PubMed] [Google Scholar]
- 41.Derdeyn CP, Videen TO. Yundt KD, Fritsch SM, Carpenter DA, Grubb RL, et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain. 2002;125:595–607. doi: 10.1093/brain/awf047. [DOI] [PubMed] [Google Scholar]
- 42.Halber M, Herholz K, Wienhard K, Pawlik G, Heiss WD. Performance of a randomization test for single-subject (15)O-water PET activation studies. J Cereb Blood Flow Metab. 1997;17:1033–1039. doi: 10.1097/00004647-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Ogasawara K, Ito H, Sasoh M, Okuguchi T, Kobayashi M, Yukawa H, et al. Quantitative measurement of regional cerebrovascular reactivity to acetazolamide using 123I-N-isopropyl-p-iodoamphetamine autoradiography with SPECT: validation study using H215O with PET. J Nucl Med. 2003;44:520–525. [PubMed] [Google Scholar]
- 44.Ozgur HT, Walsh TK, Masaryk A, Seeger JF, Williams W, Krupinski E, et al. Correlation of cerebrovascular reserve as measured by acetazolamide-challenged SPECT with angiographic flow patterns and intra-or extracranial arterial stenosis. AJNR Am J Neuroradiol. 2001;22:928–936. [PMC free article] [PubMed] [Google Scholar]
- 45.Seo HJ, Pagsisihan JR, Paeng JC, Choi SH, Cheon GJ, Chung JK, et al. Hemodynamic significance of internal carotid or middle cerebral artery stenosis detected on magnetic resonance angiography. Yonsei Med J. 2015;56:1686–1693. doi: 10.3349/ymj.2015.56.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim T, Bang JS, Kwon OK, Hwang G, Kim JE, Kang HS, et al. Morphology and related hemodynamics of the internal carotid arteries of Moyamoya patients. Acta Neurochir (Wien) 2015;157:755–761. doi: 10.1007/s00701-015-2367-y. [DOI] [PubMed] [Google Scholar]
- 47.Rogg J, Rutigliano M, Yonas H, Johnson DW, Pentheny S, Latchaw RE. The acetazolamide challenge: imaging techniques designed to evaluate cerebral blood flow reserve. AJR Am J Roentgenol. 1989;153:605–612. doi: 10.2214/ajr.153.3.605. [DOI] [PubMed] [Google Scholar]
- 48.Kuroda S, Kamiyama H, Abe H, Houkin K, Isobe M, Mitsumori K. Acetazolamide test in detecting reduced cerebral perfusion reserve and predicting long-term prognosis in patients with internal carotid artery occlusion. Neurosurgery. 1993;32:912–918. doi: 10.1227/00006123-199306000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Jacquier-Sarlin MR, Polla BS, Slosman DO. Oxido-reductive state: the major determinant for cellular retention of technetium-99m-HMPAO. J Nucl Med. 1996;37:1413–1416. [PubMed] [Google Scholar]
- 50.Jacquier-Sarlin MR, Polla BS, Slosman DO. Cellular basis of ECD brain retention. J Nucl Med. 1996;37:1694–1697. [PubMed] [Google Scholar]
- 51.Winchell HS, Horst WD, Braun L, Oldendorf WH, Hattner R, Parker H. N-isopropyl-[123I] p-iodoamphetamine: single-pass brain uptake and washout; binding to brain synaptosomes; and localization in dog and monkey brain. J Nucl Med. 1980;21:947–952. [PubMed] [Google Scholar]
- 52.Asenbaum S, Brücke T, Pirker W, Pietrzyk U, Podreka I. Imaging of cerebral blood flow with technetium-99m-HMPAO and technetium-99m-ECD: a comparison. J Nucl Med. 1998;39:613–618. [PubMed] [Google Scholar]
- 53.Hyun IY, Lee JS, Rha JH, Lee IK, Ha CK, Lee DS. Different uptake of 99mTc-ECD and 99mTc-HMPAO in the same brains: analysis by statistical parametric mapping. Eur J Nucl Med. 2001;28:191–197. [PubMed] [Google Scholar]
- 54.Shishido F, Uemura K, Inugami A, Ogawa T, Fujita H, Shimosegawa E, et al. Discrepant 99mTc-ECD images of CBF in patients with subacute cerebral infarction: a comparison of CBF, CMRO2 and 99mTc-HMPAO imaging. Ann Nucl Med. 1995;9:161–166. doi: 10.1007/BF03165046. [DOI] [PubMed] [Google Scholar]
- 55.Kapucu OL, Nobili F, Varrone A, Booij J, Vander Borght T, Nagren K, et al. EANM procedure guideline for brain perfusion SPECT using 99mTc-labelled radiopharmaceuticals, version 2. Eur J Nucl Med Mol Imaging. 2009;36:2093–2102. doi: 10.1007/s00259-009-1266-y. [DOI] [PubMed] [Google Scholar]
- 56.Juni JE, Waxman AD, Devous MD, Sr, Tikofsky RS, Ichise M, Van Heertum RL, et al. Procedure guideline for brain perfusion SPECT using (99m)Tc radiopharmaceuticals 3.0. J Nucl Med Technol. 2009;37:191–195. doi: 10.2967/jnmt.109.067850. [DOI] [PubMed] [Google Scholar]
- 57.Lee DS, Lee TH, Kim KM, Chung JK, Lee MC, Koh CS. Optimization of subtraction brain perfusion SPECT with basal/acetazolamide consecutive acquisition. Korean J Nucl Med. 1997;31:330–338. [Google Scholar]
- 58.Saito N, Nakagawara J, Nakamura H, Teramoto A. Assessment of cerebral hemodynamics in childhood Moyamoya disease using a quantitative and a semiquantitative IMP-SPECT study. Ann Nucl Med. 2004;18:323–331. doi: 10.1007/BF02984471. [DOI] [PubMed] [Google Scholar]
- 59.Ito H, Inoue K, Goto R, Kinomura S, Sato T, Kaneta T, et al. Error analysis of measured cerebral vascular response to acetazolamide stress by I-123-IMP autoradiographic method with single photon emission computed tomography: errors due to distribution volume of I-123-IMP. Ann Nucl Med. 2004;18:221–226. doi: 10.1007/BF02985003. [DOI] [PubMed] [Google Scholar]
- 60.Yamauchi M, Imabayashi E, Matsuda H, Nakagawara J, Takahashi M, Shimosegawa E, et al. Quantitative assessment of rest and acetazolamide CBF using quantitative SPECT reconstruction and sequential administration of (123)I-iodoamphetamine: comparison among data acquired at three institutions. Ann Nucl Med. 2014;28:836–850. doi: 10.1007/s12149-014-0879-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim KM, Watabe H, Hayashi T, Hayashida K, Katafuchi T, Enomoto N, et al. Quantitative mapping of basal and vasareactive cerebral blood flow using split-dose 123I-iodoamphetamine and single photon emission computed tomography. Neuroimage. 2006;33:1126–1135. doi: 10.1016/j.neuroimage.2006.06.064. [DOI] [PubMed] [Google Scholar]
- 62.Iida H, Nakagawara J, Hayashida K, Fukushima K, Watabe H, Koshino K, et al. Multicenter evaluation of a standardized protocol for rest and acetazolamide cerebral blood flow assessment using a quantitative SPECT reconstruction program and split-dose 123I-iodoamphetamine. J Nucl Med. 2010;51:1624–1631. doi: 10.2967/jnumed.110.078352. [DOI] [PubMed] [Google Scholar]
- 63.Yoneda H, Shirao S, Koizumi H, Oka F, Ishihara H, Ichiro K, et al. Reproducibility of cerebral blood flow assessment using a quantitative SPECT reconstruction program and split-dose 123I-iodoamphetamine in institutions with different γ-cameras and collimators. J Cereb Blood Flow Metab. 2012;32:1757–1764. doi: 10.1038/jcbfm.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iida H, Itoh H, Bloomfield PM, Munaka M, Higano S, Murakami M, et al. A method to quantitate cerebral blood flow using a rotating gamma camera and iodine-123 iodoamphetamine with one blood sampling. Eur J Nucl Med. 1994;21:1072–1084. doi: 10.1007/BF00181062. [DOI] [PubMed] [Google Scholar]
- 65.Iida H, Itoh H, Nakazawa M, Hatazawa J, Nishimura H, Onishi Y, et al. Quantitative mapping of regional cerebral blood flow using iodine-123-IMP and SPECT. J Nucl Med. 1994;35:2019–2030. [PubMed] [Google Scholar]
- 66.Inoue T, Fujimura M, Shimizu H, Takahashi Y, Tominaga T. Quantitative assessment of cerebral hemodynamics using single photon emission computed tomography with venous blood sampling. Clin Neurol Neurosurg. 2013;115:684–689. doi: 10.1016/j.clineuro.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 67.Oku N, Matsumoto M, Hashikawa K, Moriwaki H, Okazaki Y, Seike Y, et al. Carbon dioxide reactivity by consecutive technetium-99m-HMPAO SPECT in patients with a chronically obstructed major cerebral artery. J Nucl Med. 1994;35:32–40. [PubMed] [Google Scholar]
- 68.Hattori N, Yonekura Y, Tanaka F, Fujita T, Wang J, Ishizu K, et al. One-day protocol for cerebral perfusion reserve with acetazolamide. J Nucl Med. 1996;37:2057–2061. [PubMed] [Google Scholar]
- 69.Mugikura S, Fujimura M, Takahashi S. Implications of off-label use of acetazolamide in the management of Moyamoya disease in Japan. Radiology. 2016;279:652–653. doi: 10.1148/radiol.2016152305. [DOI] [PubMed] [Google Scholar]
- 70.Vogiatzis I, Koulouris E, Sidiropoulos A, Giannakoulas C. Acute pulmonary edema after a single oral dose of acetazolamide. Hippokratia. 2013;17:177–179. [PMC free article] [PubMed] [Google Scholar]
- 71.Zimmermann S, Achenbach S, Wolf M, Janka R, Marwan M, Mahler V. Recurrent shock and pulmonary edema due to acetazolamide medication after cataract surgery. Heart Lung. 2014;43:124–126. doi: 10.1016/j.hrtlng.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 72.Ono Y, Morifusa M, Ikeda S, Kunishige C, Tohma Y. A case of non-cardiogenic pulmonary edema provoked by intravenous acetazolamide. Acute Med Surg. 2017;4:349–352. doi: 10.1002/ams2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chong SJ, Park JD, Chae JH, Cheon JE, Kim SK, Phi JH, et al. Extensive brain infarction involving deep structures during an acetazolamide-challenged single-photon emission computed tomography scan in a patient with Moyamoya disease. Childs Nerv Syst. 2017;33:2029–2033. doi: 10.1007/s00381-017-3512-0. [DOI] [PubMed] [Google Scholar]
- 74.Kuwabara Y, Ichiya Y, Sasaki M, Yoshida T, Masuda K. Time dependency of the acetazolamide effect on cerebral hemodynamics in patients with chronic occlusive cerebral arteries early steal phenomenon demonstrated by [15O] H2O positron emission tomography. Stroke. 1995;26:1825–1829. doi: 10.1161/01.str.26.10.1825. [DOI] [PubMed] [Google Scholar]
- 75.Komiyama M, Nishikawa M, Yasui T, Sakamoto H. Reversible pontine ischemia caused by acetazolamide challenge. AJNR Am J Neuroradiol. 1997;18:1782–1784. [PMC free article] [PubMed] [Google Scholar]
- 76.Oshima H, Katayama Y, Hirayama T. Intracerebral steal phenomenon associated with global hyperemia in Moyamoya disease during revascularization surgery. J Neurosurg. 2000;92:949–954. doi: 10.3171/jns.2000.92.6.0949. [DOI] [PubMed] [Google Scholar]
- 77.Saito H, Ogasawara K, Suzuki T, Kuroda H, Kobayashi M, Yoshida K, et al. Adverse effects of intravenous acetazolamide administration for evaluation of cerebrovascular reactivity using brain perfusion single-photon emission computed tomography in patients with major cerebral artery steno-occlusive diseases. Neurol Med Chir (Tokyo) 2011;51:479–483. doi: 10.2176/nmc.51.479. [DOI] [PubMed] [Google Scholar]
- 78.Sato K, Yamada M, Oka H, Fujii K. Cerebral infarction after acetazolamide-challenged single-photon emission computed tomography in a patient with adult-onset Moyamoya disease accompanied by several risk factors. Jpn J Cereb Blood Flow Metab. 2013;24:21–24. [Google Scholar]
- 79.Murase K, Tanada S, Fujita H, Sakaki S, Hamamoto K. Kinetic behavior of technetium-99m-HMPAO in the human brain and quantification of cerebral blood flow using dynamic SPECT. J Nucl Med. 1992;33:135–143. [PubMed] [Google Scholar]
- 80.Pupi A, Castagnoli A, De Cristofaro MT, Bacciottini L, Petti AR. Quantitative comparison between 99mTc-HMPAO and 99mTc-ECD: measurement of arterial input and brain retention. Eur J Nucl Med. 1994;21:124–130. doi: 10.1007/BF00175759. [DOI] [PubMed] [Google Scholar]
- 81.Van Laere K, Dumont F, Koole M, Dierckx R. Non-invasive methods for absolute cerebral blood flow measurement using 99mTc-ECD: a study in healthy volunteers. Eur J Nucl Med. 2001;28:862–872. doi: 10.1007/s002590100559. [DOI] [PubMed] [Google Scholar]
- 82.Inoue K, Ito H, Shidahara M, Goto R, Kinomura S, Sato K, et al. Database of normal human cerebral blood flow measured by SPECT: II. Quantification of I-123-IMP studies with ARG method and effects of partial volume correction. Ann Nucl Med. 2006;20:139–146. doi: 10.1007/BF02985626. [DOI] [PubMed] [Google Scholar]
- 83.Ito H, Inoue K, Goto R, Kinomura S, Taki Y, Okada K, et al. Database of normal human cerebral blood flow measured by SPECT: I. comparison between I-123-IMP, Tc-99m-HMPAO, and Tc-99m-ECD as referred with O-15 labeled water PET and voxel-based morphometry. Ann Nucl Med. 2006;20:131–138. doi: 10.1007/BF02985625. [DOI] [PubMed] [Google Scholar]
- 84.Riberich RC, CERETEC- technetium tc-99m exametazime and cobaltous chloride. In: Technetium-99m exametazime (HMPAO). Medi-Physics, Inc. dba GE Healthcare. 2018. http://www.radiopharmaceuticals.info/uploads/7/6/8/7/76874929/ceretec_package_insert_sep_2018.pdf. Accessed 01 Sep 2019.
- 85.Riberich RC, NEUROLITE- bicisate dihydrochloride. In: Technetium-99m bicisate (ECD). Lantheus Medical Imaging, Inc. 2015. http://www.radiopharmaceuticals.info/uploads/7/6/8/7/76874929/neurolite_pi_2015.pdf. Accessed 01 Sep 2019.
- 86.Di Rocco RJ, Silva DA, Kuczynski BL, Narra RK, Ramalingam K, Jurisson S, et al. The single-pass cerebral extraction and capillary permeability-surface area product of several putative cerebral blood flow imaging agents. J Nucl Med. 1993;34:641–648. [PubMed] [Google Scholar]
- 87.Andersen AR, Friberg H, Knudsen KB, Barry DI, Paulson OB, Schmidt JF, et al. Extraction of [99mTc]-d,l-HM-PAO across the blood-brain barrier. J Cereb Blood Flow Metab. 1988;8:S44–S51. doi: 10.1038/jcbfm.1988.32. [DOI] [PubMed] [Google Scholar]
- 88.Suzuki C, Kimura S, Kosugi M, Magata Y. Quantitation of rat cerebral blood flow using (99m)Tc-HMPAO. Nucl Med Biol. 2017;47:19–22. doi: 10.1016/j.nucmedbio.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 89.Dormehl IC, Oliver DW, Langen KJ, Hugo N, Croft SA. Technetium-99m-HMPAO, technetium-99m-ECD and iodine-123-IMP cerebral blood flow measurements with pharmacological interventions in primates. J Nucl Med. 1997;38:1897–1901. [PubMed] [Google Scholar]
- 90.Lee DS, Lee SK, Kim YK, Lee JS, Cheon GJ, Kang KW, Kim ES, Chung JK, Lee MC. Superiority of HMPAO ictal SPECT to ECD ictal SPECT in localizing the epileptogenic zone. Epilepsia. 2002;43:263–269. doi: 10.1046/j.1528-1157.2002.23001.x. [DOI] [PubMed] [Google Scholar]
- 91.Teo K, Choy DK, Lwin S, Ning C, Yeo TT, Shen L, et al. Cerebral hyperperfusion syndrome after superficial temporal artery-middle cerebral artery bypass for severe intracranial steno-occlusive disease: a case control study. Neurosurgery. 2013;72:936–942. doi: 10.1227/NEU.0b013e31828bb8b3. [DOI] [PubMed] [Google Scholar]
- 92.Hashikawa K, Matsumoto M, Moriwaki H, Oku N, Okazaki Y, Uehara T, et al. Split dose iodine-123-IMP SPECT: sequential quantitative regional cerebral blood flow change with pharmacological intervention. J Nucl Med. 1994;35:1226–1233. [PubMed] [Google Scholar]
- 93.Zabala-Travers S, Choi M, Cheng W-C, Badano A. Effect of color visualization and display hardware on the visual assessment of pseudocolor medical images. Med Phys. 2015;42:2942–2954. doi: 10.1118/1.4921125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choi H, Yoo MY, Cheon GJ, Kang KW, Chung JK, Lee DS. Parametric cerebrovascular reserve images using acetazolamide (99m)Tc-HMPAO SPECT: a feasibility study of quantitative assessment. Nucl Med Mol Imaging. 2013;47:188–195. doi: 10.1007/s13139-013-0214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Setta K, Kojima D, Shimada Y, Yoshida J, Oshida S, Fujimoto K, et al. Accuracy of brain perfusion single-photon emission computed tomography for detecting misery perfusion in adult patients with symptomatic ischemic Moyamoya disease. Ann Nucl Med. 2018;32:611–619. doi: 10.1007/s12149-018-1283-7. [DOI] [PubMed] [Google Scholar]
- 96.Acker G, Lange C, Schatka I, Pfeifer A, Czabanka MA, Vajkoczy P, et al. Brain perfusion imaging under acetazolamide challenge for detection of impaired cerebrovascular reserve capacity: positive findings with (15)O-water PET in patients with negative (99m)Tc-HMPAO SPECT findings. J Nucl Med. 2018;59:294–298. doi: 10.2967/jnumed.117.195818. [DOI] [PubMed] [Google Scholar]
- 97.Lee JS, Lee DS, Kim J, Kim YK, Kang E, Kang H, et al. Development of Korean standard brain templates. J Korean Med Sci. 2005;20:483–488. doi: 10.3346/jkms.2005.20.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee JS, Lee DS, Kim YK, Kim J, Lee HY, Lee SK, et al. Probabilistic map of blood flow distribution in the brain from the internal carotid artery. Neuroimage. 2004;23:1422–1431. doi: 10.1016/j.neuroimage.2004.07.057. [DOI] [PubMed] [Google Scholar]
- 99.Kim SJ, Kim IJ, Kim YK, Lee TH, Lee JS, Jun S, et al. Probabilistic anatomic mapping of cerebral blood flow distribution of the middle cerebral artery. J Nucl Med. 2008;49:39–43. doi: 10.2967/jnumed.107.045724. [DOI] [PubMed] [Google Scholar]
- 100.Lee HY, Paeng JC, Lee DS, Lee JS, Oh CW, Cho MJ, et al. Efficacy assessment of cerebral arterial bypass surgery using statistical parametric mapping and probabilistic brain atlas on basal/acetazolamide brain perfusion SPECT. J Nucl Med. 2004;45:202–206. [PubMed] [Google Scholar]
- 101.So Y, Lee HY, Kim SK, Lee JS, Wang KC, Cho BK, et al. Prediction of the clinical outcome of pediatric Moyamoya disease with postoperative basal/acetazolamide stress brain perfusion SPECT after revascularization surgery. Stroke. 2005;36:1485–1489. doi: 10.1161/01.STR.0000170709.95185.b1. [DOI] [PubMed] [Google Scholar]
- 102.Lee TH, Kim SJ, Kim IJ, Kim YK, Kim DS, Park KP. Statistical parametric mapping and statistical probabilistic anatomical mapping analyses of basal/acetazolamide Tc-99m ECD brain SPECT for efficacy assessment of endovascular stent placement for middle cerebral artery stenosis. Neuroradiology. 2007;49:289–298. doi: 10.1007/s00234-006-0188-7. [DOI] [PubMed] [Google Scholar]
- 103.Song YS, Oh SW, Kim YK, Kim SK, Wang KC, Lee DS. Hemodynamic improvement of anterior cerebral artery territory perfusion induced by bifrontal encephalo(periosteal) synangiosis in pediatric patients with Moyamoya disease: a study with brain perfusion SPECT. Ann Nucl Med. 2012;26:47–57. doi: 10.1007/s12149-011-0541-8. [DOI] [PubMed] [Google Scholar]
- 104.Hosoda K, Kawaguchi T, Ishii K, Minoshima S, Shibata Y, Iwakura M, et al. Prediction of hyperperfusion after carotid endarterectomy by brain SPECT analysis with semiquantitative statistical mapping method. Stroke. 2003;34:1187–1193. doi: 10.1161/01.STR.0000068781.31429.BE. [DOI] [PubMed] [Google Scholar]
- 105.Tomura N, Otani T, Koga M, Ishiyama K. Correlation between severity of carotid stenosis and vascular reserve measured by acetazolamide brain perfusion single photon emission computed tomography. J Stroke Cerebrovasc Dis. 2013;22:166–170. doi: 10.1016/j.jstrokecerebrovasdis.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 106.Ito H, Kanno I, Kato C, Sasaki T, Ishii K, Ouchi Y. Database of normal human cerebral blood flow, cerebral blood volume, cerebral oxygen extraction fraction and cerebral metabolic rate of oxygen measured by positron emission tomography with 15O-labelled carbon dioxide or water, carbon monoxide and oxygen: a multicentre study in Japan. Eur J Nucl Med Mol Imaging. 2004;31:635–643. doi: 10.1007/s00259-003-1430-8. [DOI] [PubMed] [Google Scholar]
- 107.Lanzman B, Heit JJ. Advanced MRI measures of cerebral perfusion and their clinical applications. Top Magn Reson Imaging. 2017;26:83–90. doi: 10.1097/RMR.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 108.Noguchi T, Kawashima M, Irie H, Ootsuka T, Nishihara M, Matsushima T, et al. Arterial spin-labeling MR imaging in Moyamoya disease compared with SPECT imaging. Eur J Radiol. 2011;80:e557–e562. doi: 10.1016/j.ejrad.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 109.Uchihashi Y, Hosoda K, Zimine I, Fujita A, Fujii M, Sugimura K, et al. Clinical application of arterial spin-labeling MR imaging in patients with carotid stenosis: quantitative comparative study with single-photon emission CT. AJNR Am J Neuroradiol. 2011;32:1545–1551. doi: 10.3174/ajnr.A2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Noguchi T, Kawashima M, Nishihara M, Egashira Y, Azama S, Irie H. Noninvasive method for mapping CVR in Moyamoya disease using ASL-MRI. Eur J Radiol. 2015;84:1137–1143. doi: 10.1016/j.ejrad.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 111.Yun TJ, Paeng JC, Sohn CH, Kim JE, Kang HS, Yoon BW, et al. Monitoring cerebrovascular reactivity through the use of arterial spin labeling in patients with Moyamoya disease. Radiology. 2016;278:205–213. doi: 10.1148/radiol.2015141865. [DOI] [PubMed] [Google Scholar]
- 112.Lee S, Yun TJ, Yoo RE, Yoon BW, Kang KM, Choi SH, et al. Monitoring cerebral perfusion changes after revascularization in patients with Moyamoya disease by using arterial spin-labeling MR imaging. Radiology. 2018;288:565–572. doi: 10.1148/radiol.2018170509. [DOI] [PubMed] [Google Scholar]
- 113.Yoo RE, Yun TJ, Yoo DH, Cho YD, Kang HS, Yoon BW, et al. Monitoring cerebral blood flow change through use of arterial spin labelling in acute ischaemic stroke patients after intra-arterial thrombectomy. Eur Radiol. 2018;28:3276–3284. doi: 10.1007/s00330-018-5319-0. [DOI] [PubMed] [Google Scholar]
- 114.Petrella JR, DeCarli C, Dagli M, Grandin CB, Duyn JH, Frank JA, et al. Age-related vasodilatory response to acetazolamide challenge in healthy adults: a dynamic contrast-enhanced MR study. AJNR Am J Neuroradiol. 1998;19:39–44. [PMC free article] [PubMed] [Google Scholar]
- 115.Kikuchi K, Murase K, Miki H, Kikuchi T, Sugawara Y, Mochizuki T, et al. Measurement of cerebral hemodynamics with perfusion-weighted MR imaging: comparison with pre-and post-acetazolamide 133Xe-SPECT in occlusive carotid disease. AJNR Am J Neuroradiol. 2001;22:248–254. [PMC free article] [PubMed] [Google Scholar]
- 116.Kaneko K, Kuwabara Y, Mihara F, Yoshiura T, Nakagawa M, Tanaka A, et al. Validation of the CBF, CBV, and MTT values by perfusion MRI in chronic occlusive cerebrovascular disease: a comparison with 15O-PET. Acad Radiol. 2004;11:489–497. doi: 10.1016/S1076-6332(03)00722-0. [DOI] [PubMed] [Google Scholar]
- 117.Grandin CB, Bol A, Smith AM, Michel C, Cosnard G. Absolute CBF and CBV measurements by MRI bolus tracking before and after acetazolamide challenge: repeatability and comparison with PET in humans. Neuroimage. 2005;26:525–535. doi: 10.1016/j.neuroimage.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 118.Ma J, Mehrkens JH, Holtmannspoetter M, Linke R, Schmid-Elsaesser R, Steiger HJ, et al. Perfusion MRI before and after acetazolamide administration for assessment of cerebrovascular reserve capacity in patients with symptomatic internal carotid artery (ICA) occlusion: comparison with 99mTc-ECD SPECT. Neuroradiology. 2007;49:317–326. doi: 10.1007/s00234-006-0193-x. [DOI] [PubMed] [Google Scholar]
- 119.Kim HJ, Kim TW, Ryu SY, Yang PS, Kwon MJ, Kim JC, et al. Acetazolamide-challenged perfusion magnetic resonance imaging for assessment of cerebrovascular reserve capacity in patients with symptomatic middle cerebral artery stenosis: comparison with technetium-99m-hexamethylpropyleneamine oxime single-photon emission computed tomography. Clin Imaging. 2011;35:413–420. doi: 10.1016/j.clinimag.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 120.Chen A, Shyr MH, Chen TY, Lai HY, Lin CC, Yen PS. Dynamic CT perfusion imaging with acetazolamide challenge for evaluation of patients with unilateral cerebrovascular steno-occlusive disease. AJNR Am J Neuroradiol. 2006;27:1876–1881. [PMC free article] [PubMed] [Google Scholar]
- 121.Smith LM, Elkins JS, Dillon WP, Schaeffer S, Wintermark M. Perfusion-CT assessment of the cerebrovascular reserve: a revisit to the acetazolamide challenges. J Neuroradiol. 2008;35:157–164. doi: 10.1016/j.neurad.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 122.Kim E, Sohn CH, Na DG, Kim JE, Chang KH, Kim JH, et al. Perfusion computed tomography evaluation of cerebral hemodynamic impairment in patients with unilateral chronic steno-occlusive disease: a comparison with the acetazolamide challenge 99mTc-hexamethylpropyleneamine oxime single-photon emission computed tomography. J Comput Assist Tomogr. 2009;33:546–551. doi: 10.1097/RCT.0b013e318188887d. [DOI] [PubMed] [Google Scholar]
- 123.Park JC, Kim JE, Kang HS, Sohn CH, Lee DS, Oh CW, et al. CT perfusion with angiography as a substitute for both conventional digital subtraction angiography and acetazolamide-challenged SPECT in the follow-up of postbypass patients. Cerebrovasc Dis. 2010;30:547–555. doi: 10.1159/000319026. [DOI] [PubMed] [Google Scholar]
- 124.Hashimoto A, Mikami T, Komatsu K, Noshiro S, Hirano T, Wanibuchi M, et al. Assessment of hemodynamic compromise using computed tomography perfusion in combination with (123)I-IMP single-photon emission computed tomography without acetazolamide challenge test. J Stroke Cerebrovasc Dis. 2017;26:627–635. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 125.Ogasawara K, Yukawa H, Kobayashi M, Mikami C, Konno H, Terasaki K, et al. Prediction and monitoring of cerebral hyperperfusion after carotid endarterectomy by using single-photon emission computerized tomography scanning. J Neurosurg. 2003;99:504–510. doi: 10.3171/jns.2003.99.3.0504. [DOI] [PubMed] [Google Scholar]
- 126.Kaku Y, Yoshimura SI, Kokuzawa J. Factors predictive of cerebral hyperperfusion after carotid angioplasty and stent placement. AJNR Am J Neuroradiol. 2004;25:1403–1408. [PMC free article] [PubMed] [Google Scholar]
- 127.Komoribayashi N, Ogasawara K, Kobayashi M, Saitoh H, Terasaki K, Inoue T, Ogawa A. Cerebral hyperperfusion after carotid endarterectomy is associated with preoperative hemodynamic impairment and intraoperative cerebral ischemia. J Cereb Blood Flow Metab. 2006;26(7):878–884. doi: 10.1038/sj.jcbfm.9600244. [DOI] [PubMed] [Google Scholar]
- 128.Suga Y, Ogasawara K, Saito H, Komoribayashi N, Kobayashi M, Inoue T, et al. Preoperative cerebral hemodynamic impairment and reactive oxygen species produced during carotid endarterectomy correlate with development of postoperative cerebral hyperperfusion. Stroke. 2007;38:2712–2717. doi: 10.1161/STROKEAHA.107.483495. [DOI] [PubMed] [Google Scholar]
- 129.Fujimura M, Shimizu H, Inoue T, Mugikura S, Saito A, Tominaga T. Significance of focal cerebral hyperperfusion as a cause of transient neurologic deterioration after extracranial-intracranial bypass for Moyamoya disease: comparative study with non-Moyamoya patients using n-isopropyl-p-[ 123I]iodoamphetamine single-pho. Neurosurgery. 2011;68:957–965. doi: 10.1227/NEU.0b013e318208f1da. [DOI] [PubMed] [Google Scholar]
- 130.Mintun MA, Raichle ME, Martin WR, Herscovitch P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J Nucl Med. 1984;25:177–187. [PubMed] [Google Scholar]
- 131.Hiura M, Nariai T, Ishii K, Sakata M, Oda K, Toyohara J, et al. Changes in cerebral blood flow during steady-state cycling exercise: a study using oxygen-15-labeled water with PET. J Cereb Blood Flow Metab. 2014;34:389–396. doi: 10.1038/jcbfm.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ryoo HG, Choi H, Wong TH, Kang SK, Lee JS, Lee DS. Development of a deep learning-based interpretation model for brain perfusion SPECT leveraging unstructured reading reports. J Nucl Med. 2019;60(supplement 1):402. doi: 10.1007/s00259-019-04670-4. [DOI] [PubMed] [Google Scholar]