Abstract
Purpose
We assessed prognostic implication of bone marrow uptake on baseline F-18 fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) in patients with advanced-stage diffuse large B-cell lymphoma (DLBCL).
Methods
We retrospectively reviewed 140 patients with stage III and IV DLBCL, who underwent baseline F-18 FDG PET/CT at diagnosis. Bone marrow uptake on F-18 FDG PET/CT (BM FDG) was compared with findings on bone marrow biopsy (BMB), and patients were grouped based on these results: BMB-positive and BM FDG-positive (group 1), BMB-positive and BM FDG-negative (group 2), BMB-negative and BM FDG-positive (group 3), and BMB-negative and BM FDG-negative (group 4). The prognostic value of clinicopathologic factors and BM FDG for predicting progression-free survival (PFS) and overall survival (OS) was assessed using a Cox proportional hazards model. Differences in PFS and OS were examined by the Kaplan-Meier method.
Results
BMB was the only significant indicator in predicting PFS, and age, IPI score higher than 3, and BM FDG significantly predicted OS. Group 1 showed inferior PFS than group 2 (median PFS, 7.4 vs. 13.9 months; p = 0.04). In contrast, there was no significant difference either in PFS or OS between group 2 and group 3.
Conclusion
We showed that BM FDG-positive predicted a poorer survival in patients with advanced stage DBLCL. We also found that BMB-negative and BM FDG-positive patients had similar PFS or OS to BMB-positive and BM FDG-negative patients. Further study in a larger population is needed to clarify clinical significance of BM FDG in these patients.
Keywords: Diffuse large B-cell lymphoma, Fluorodeoxyglucose, Bone marrow involvement, Prognosis
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL), accounting 30–40% of newly developed disease. DLBCL is characterized by relatively high incidence of extra-nodal spread [1]. Bone marrow involvement of DLBCL is reported up to 27%, as assessed by unilateral or bilateral iliac crest bone marrow biopsy (BMB) at the time of diagnosis [2, 3].
Accurate assessment of extralymphatic involvement, including bone marrow involvement, is crucial in initial staging workup for DLBCL to optimally treat patients [4]. Bone marrow involvement, an extra-nodal site, is recognized as a poor prognostic indicator, and its significance is reflected in staging and International Prognostic Index (IPI). BMB is currently the gold standard in evaluating bone marrow involvement for staging of DLBCL [5]. However, BMB might show low sensitivity and is an invasive procedure with possible complications such as pain, anxiety, hemorrhage, and infection [6, 7].
F-18 fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) is a noninvasive imaging technique and has been widely used in the initial staging of DLBCL [8, 9]. Compared to CT, FDG PET/CT provides more accurate staging by upstaging or downstaging up to 30% of patients [10]. In assessment of bone marrow involvement, focal bone marrow uptake with or without diffuse bone marrow uptake shown on FDG PET/CT was more sensitive than BMB. However, FDG PET/CT might miss up to 6% of patients with low-volume diffuse bone marrow involvement [11, 12]. Also, there were conflicting reports on prognostic implication of bone marrow involvement assessed by FDG PET/CT: Berthet et al. stated that FDG PET/CT can predict progression-free survival (PFS) but not overall survival (OS) [11], whereas Hong et al. showed that findings on FDG PET/CT were not associated with either PFS or OS [13]. In this study, we assessed prognostic implication of bone marrow uptake on FDG PET/CT (BM FDG) in patients with advanced stage DLBCL and compared with BMB.
Materials and Methods
Patient Population
We retrospectively reviewed the records of all patients who were newly diagnosed with stage III and IV DLBCL and underwent FDG PET/CT between 2011 and 2017 at our institution. Clinical stage was based on routine clinical and laboratory assessments, computed tomography (CT), FDG PET/CT, and bilateral iliac crest BMB. All patients were histologically confirmed as DLBCL by either biopsy or excision. We excluded patients who had a history of other malignancy and aged under 18. Patient data were recorded at the time of first admission, in details as follows: age, sex, Ann Arbor stage, and bone marrow involvement by BMB, IPI score, presence of B symptom, and germinal center B-cell like (GCB) subtypes. The interval between the date of staging FDG PET/CT and BMB was less than a month in all patients. All FDG PET/CT were performed before treatment of DLBCL.
Patients were treated by 6 cycles of standard R-CHOP therapy for 21 days. Patients were followed up with CT after 3 cycles, and interim FDG PET/CT was imaged after 3 or 4 cycles. At the end of 6 cycles, CT and FDG PET/CT were performed. Disease progression was defined according to the Lugano classification: increase in intensity of FDG uptake from baseline and/or newly developed FDG uptake at interim or end-of-treatment evaluation, which is consistent with lymphoma rather than other etiology [9]. We calculated PFS as from diagnosis to first disease progression and OS as from diagnosis to death from any cause. Outcomes were assessed blindly to avoid measurement bias.
FDG PET/CT Imaging
FDG PET/CT images were acquired using a Discovery STE scanner (GE Healthcare, Milwaukee, WI) or a Biograph TruePoint 40 scanner (Siemens Medical Systems, Knoxville, TN). Patients were instructed to fast for minimum 6 h before imaging, and peripheral blood glucose level was equal to or less than 140 mg/dL prior to the injection of FDG. Approximately 5.5 MBq/kg of FDG was intravenously administrated 1 h before image acquisition. Low-dose CT (Discovery STE, 30 mA, 130 kVp or Biograph TruePoint 40, 36 mA, 120 kVp) was acquired, and then standard PET imaging was performed from the cerebellum to the mid-thigh with acquisition times of 3 min/bed position for the Discovery STE and 2.5 min/bed position for the Biograph TruePoint 40 [14]. PET images were iteratively reconstructed with CT-based attenuation correction.
PET Scan Interpretation
All FDG PET/CT images were assessed independently by two experienced nuclear medicine physicians, who were blinded to BMB, and other clinical parameters. If there were disagreement on FDG PET/CT findings between two physicians, consensus was made. We visually classified BM FDG as positive or negative based on the following definitions: BM FDG-positive was defined as unifocal or multifocal bone marrow uptake, which was greater than background uptake and cannot be explained by a benign etiology [15, 16]. BM FDG-negative was defined as diffuse bone marrow uptake, irrespective of the degree of uptake, or no discernible uptake. The kappa statistic was assessed to measure reproducibility.
Statistical Analysis
We classified patients into four groups based on BMB and BM FDG-positivity: group 1 was defined as BMB-positive and BM FDG-positive, group 2 as BMB-positive and BM FDG-negative, group 3 as BMB-negative and BM FDG-positive, and group 4 as BMB-negative and BM FDG-negative.
SPSS software (version 23.0, IBM Corp., Armonk, NY, USA) was used to perform statistical analyses. Prognostic association of variables was assessed by the Cox proportional hazards regression model in univariate and multivariate analysis. Backward stepwise regression was used in multivariate analysis. Survival curves were obtained by Kaplan-Meier method and compared by log-rank test. We considered p value lower than 0.05 as statistically significant.
Results
Patients’ Characteristics
A total of 159 consecutive patients were included in this study. Nineteen out of 159 patients had a history of other malignancy, and no patient was under 18. We further analyzed the remaining 140 (82 males (58.5%), and 58 females (41.4%)) patients. The median age at diagnosis was 64.5 years (range, 22–86 years). Thirty patients (21.4%) were stage III, and 110 patients (78.5%) were stage IV. Forty-four patients (31.4%) were BM FDG-positive, and 96 (68.6%) were BM FDG-negative. Sixty-three patients (45.0%) had IPI score over 3, and 77 (55.0%) had IPI score 3 or less. Seventy-one patients (50.7%) showed B symptom at the time of diagnosis. Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Characteristics | |
|---|---|
| Age, mean ± SD, y | 61.2 ± 14.3 |
| Sex | |
| Male, n (%) | 82 (58.5) |
| Female, n (%) | 58 (41.4) |
| BMB | |
| Negative, n (%) | 90 (64.2) |
| Positive, n (%) | 50 (35.7) |
| BM FDG | |
| Negative, n (%) | 96 (68.5) |
| Positive, n (%) | 44 (31.4) |
| Ann Arbor stage | |
| III, n (%) | 30 (21.4) |
| IV, n (%) | 110 (78.5) |
| IPI score | |
| ≤ 3, n (%) | 77 (55.0) |
| > 3, n (%) | 63 (45.0) |
| GCB | |
| Non-GCB, n (%) | 110 (78.5) |
| GCB, n (%) | 11 (7.8) |
| Unchecked, n (%) | 19 (13.5) |
| B symptom | |
| Negative, n (%) | 69 (49.3) |
| Positive, n (%) | 71 (50.7) |
BMB bone marrow biopsy, BM FDG bone marrow uptake on F-18 fluorodeoxyglucose positron emission tomography/computed tomography, IPI International Prognostic Index, GCB germinal center B-cell like
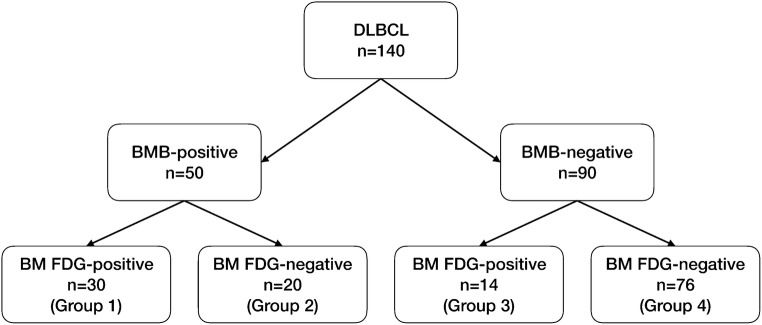
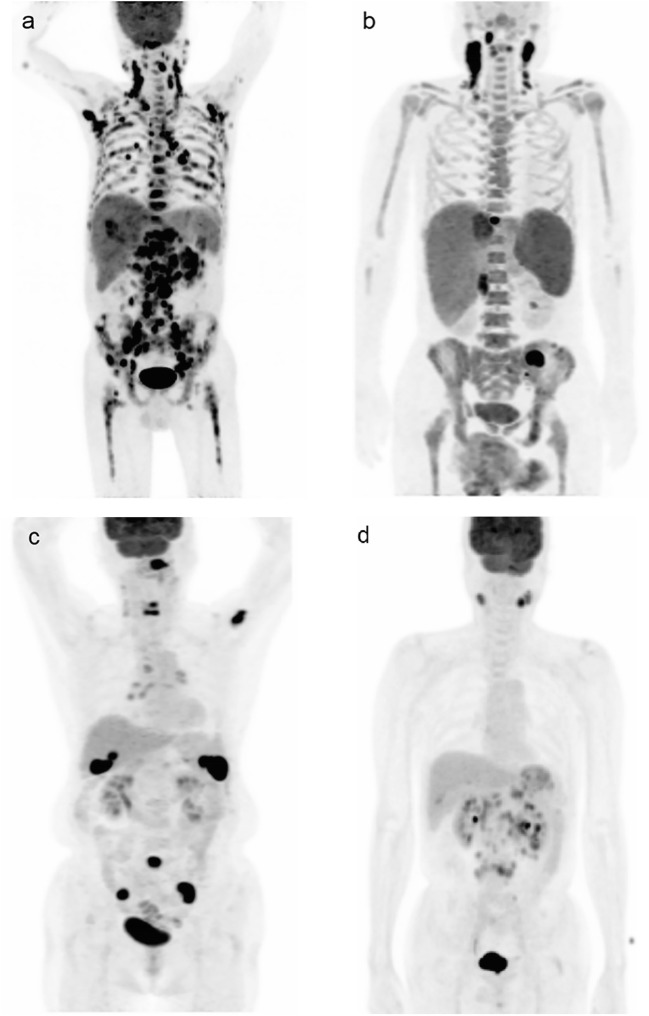
There was good interobserver agreement on the BM FDG, with an agreement rate of 95.7% (κ = 0.90). Of 140 patients, 50 (35.7%) were BMB-positive, and 90 (64.2%) were BMB-negative. In patients with BMB-positive, 30 (60.0%) were BM FDG-positive (group 1), and 20 (40.0%) were BM FDG-negative (group 2). All the group 2 patients showed diffuse bone marrow uptake on PET/CT. And in patients with BMB-negative, 14 (15.6%) were BM FDG-positive (group 3), and 76 (84.4%) were BM FDG-negative (group 4) (Fig. 1). Representative images of each group were shown in Fig. 2.
Fig. 1.
Patient classification based on BMB and BM FDG. Patients were grouped into four groups: BMB-positive and BM FDG-positive (group 1), BMB-positive and BM FDG-negative (group 2), BMB-negative and BM FDG-positive (group 3), and BMB-negative and BM FDG-negative (group 4). DLBCL diffuse large B-cell lymphoma, BMB bone marrow biopsy, BM FDG bone marrow uptake on F-18 fluorodeoxyglucose positron emission tomography/computed tomography
Fig. 2.
Representative images of all groups. (a) A group 1 patient (BMB-positive and BM FDG-positive) had multifocal increased uptake throughout bone marrow. (b) A group 2 patient (BMB-positive and BM FDG-negative) showed diffuse bone marrow uptake. (c) A group 3 patient (BMB-negative and BM FDG-positive) showed multifocal bone marrow uptake. (d) A group 4 patient (BMB-negative and BM FDG-negative) showed no discernible bone marrow uptake. All images were shown in maximum intensity projection. DLBCL diffuse large B cell lymphoma, BMB bone marrow biopsy, BM FDG bone marrow uptake on F-18 fluorodeoxyglucose positron emission tomography/computed tomography
Patients were followed up for 1 to 98 months (median, 49 months). Disease progression was found in 56 patients (40.0%). Sixty-three patients (35.0%) died during follow-up period. The 1-year PFS rate was 57.1%, and 1-year OS rate was 66.4%.
Factors Influencing Progression-Free Survival and Overall Survival
PFS analysis showed that positive BMB (hazard ratio (HR), 2.311; 95% confidence interval (CI), 1.37–3.89; p = 0.002), positive BM FDG (HR, 2.13; 95% CI, 1.26–3.60; p = 0.005), and presence of B symptom (HR, 1.85; 95% CI, 1.07–3.18; p = 0.03) were significantly associated with PFS in univariate analysis. Sex, age, stage, and IPI score were not predictive of PFS. In multivariate analysis, positive BMB was the only significant indicator in predicting PFS (Table 2). Univariate OS analysis revealed that age (HR, 1.03; 95% CI, 1.01–1.05; p = 0.006), IPI score higher than 3 (HR, 2.07; 95% CI, 1.25–3.43; p = 0.004), positive BMB (HR, 1.67; 95% CI, 1.02–2.75; p = 0.04), and positive BM FDG (HR, 1.80; 95% CI, 1.09–2.97; p = 0.02) were significant. Sex, stage, and presence of B symptom were not predictive of OS. In multivariate analysis, age (HR, 1.03; 95% CI, 1.01–1.05; p = 0.004), IPI score higher than 3 (HR, 1.97; 95% CI, 1.17–3.22; p = 0.001), and BM FDG (HR, 1.88; 95% CI, 1.14–3.10; p = 0.01) were significantly associated with OS (Table 3).
Table 2.
Univariate and multivariate analyses of progression-free survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variable | p value | HR (95% CI) | p value | HR (95% CI) |
| Sex | 0.19 | |||
| Male | 0.70 (0.42–1.19) | |||
| Female | ||||
| Age | 0.58 | 1.01 (0.99–1.03) | ||
| BMB | 0.002 | 0.002 | ||
| Negative | ||||
| Positive | 2.31 (1.37–3.90) | 2.31 (1.37–3.90) | ||
| BM FDG | 0.005 | |||
| Negative | ||||
| Positive | 2.13 (1.26–3.60) | |||
| Ann Arbor stage | 0.06 | |||
| III | ||||
| IV | 1.96 (0.96–4.00) | |||
| IPI score | 0.07 | |||
| ≤3 | ||||
| >3 | 1.63 (0.96–2.75) | |||
| B symptom | 0.03 | |||
| Negative | ||||
| Positive | 1.85 (1.07–3.18) | |||
BMB bone marrow biopsy, BM FDG bone marrow uptake on F-18 fluorodeoxyglucose positron emission tomography/computed tomography, IPI International Prognostic Index, HR hazard ratio, CI confidence interval
Table 3.
Univariate and multivariate analyses of overall survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variable | p value | HR (95% CI) | p value | HR (95% CI) |
| Sex | 0.44 | |||
| Male | 0.82 (0.50–1.35) | |||
| Female | ||||
| Age | 0.006 | 1.03 (1.01–1.05) | 0.004 | 1.03 (1.01–1.05) |
| BMB | 0.04 | |||
| Negative | ||||
| vPositive | 1.67 (1.02–2.75) | |||
| BM FDG | 0.02 | 0.01 | ||
| Negative | ||||
| Positive | 1.80 (1.09–2.97) | 1.88 (1.14–3.10) | ||
| Ann Arbor stage | 0.09 | |||
| III | ||||
| IV | 1.81 (0.92–3.55) | |||
| IPI score | 0.004 | 0.001 | ||
| ≤3 | ||||
| >3 | 2.07 (1.25–3.43) | 1.97 (1.17–3.23) | ||
| B symptom | 0.13 | |||
| Negative | ||||
| Positive | 1.48 (0.90–2.43) | |||
BMB bone marrow biopsy, BM FDG bone marrow uptake on F-18 fluorodeoxyglucose positron emission tomography/computed tomography, IPI International Prognostic Index, HR hazard ratio, CI confidence interval
Group Analysis Based on BM FDG and BMB
The median PFS for each group were as follows: group 1, 7.4 months; group 2, 13.9 months; group 3, 26.3 months; and group 4, 19.6 months. And the median OS were as follows: group 1, 12.1 months; group 2, 26.9 months; group 3, 31.0 months; and group 4, 27.7 months. Group 1 showed worse PFS than group 2 (group 1, median PFS, 7.4 months; group 2, median PFS, 13.9 months; p = 0.04), group 3 (group 1, median PFS, 7.4 months; group 3, median PFS, 26.3 months; p = 0.02), and group 4 (group 1, median PFS, 7.4 months; group 4, median PFS, 19.6 months; p < 0.001). No significant difference in PFS was found between group 2, group 3, and group 4 (Fig. 3a). Group 1 showed inferior OS than group 4 (group 1, median OS, 12.1 months; group 4, median OS, 27.7 months; p = 0.004). No other significant difference in OS was found between each group (Fig. 3b).
Fig. 3.
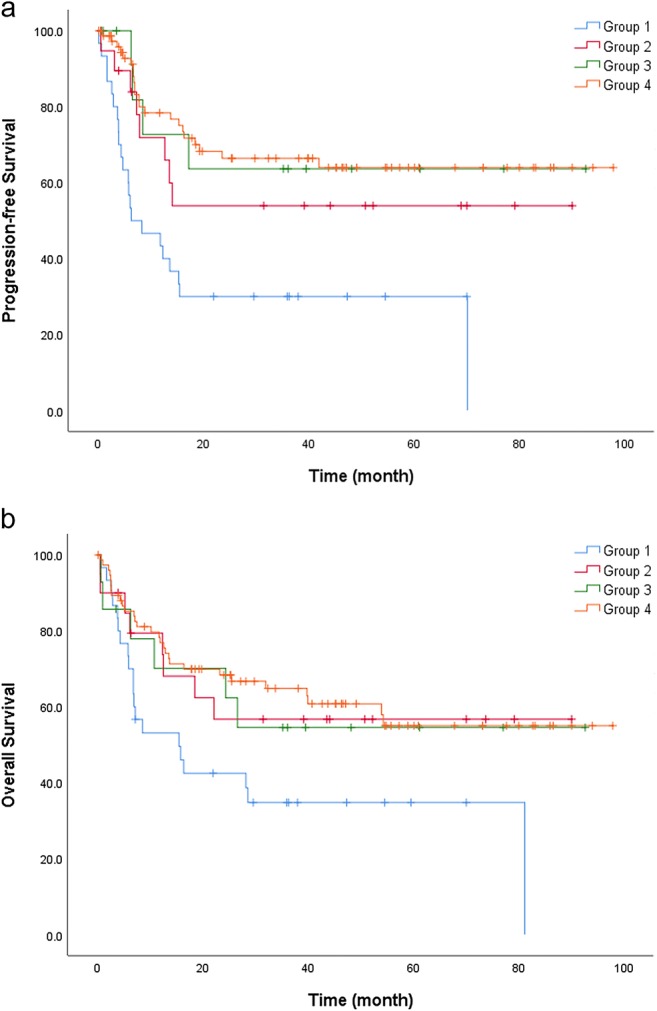
Survival curves of PFS and OS obtained by Kaplan-Meier method. (a) Group 1 (median PFS, 7.4 months) had worse PFS than group 2 (median PFS, 13.9 months; p = 0.04), group 3 (median PFS, 26.3 months; p = 0.02), and group 4 (median PFS, 19.6 months; p < 0.001). Between group 2, group 3, and group 4, no significant difference in PFS was found. (b) Group 1 (median OS, 12.1 months) showed poorer OS than group 4 (27.7 months; p = 0.004). No other significant difference in OS was found between each group. PFS progression-free survival, OS overall survival
Discussion
We found that BMB was the only significant indicator in predicting PFS, and age, IPI score higher than 3, and positive BM FDG significantly associated with OS. BM FDG-positive was associated with PFS in patients with BMB-positive, advanced stage DBLCL: BMB-positive and BM FDG-positive patients showed a worse PFS than BMB-positive and BM FDG-negative patients. We also revealed that BMB-negative and BM FDG-positive patients had similar PFS or OS to BMB-positive and BM FDG-negative patients.
In line with our results, previous studies have shown that BM FDG better predicted bone marrow involvement of DLBCL than BMB and combined stratification using BMB and BM FDG results in better prognostication in patients with DLBCL [11, 17]. These studies suggested that BM FDG-positive could predict survival equivalent to BMB-positive: either positive BM FDG or positive BMB was associated with poorer survival in patients with DLBCL. In contrast, some reports suggested that BM FDG was less sensitive in assessing bone marrow involvement of DLBCL, and BM FDG-positive alone was not sufficient to predict a worse prognosis [18–20].
The discordance may stem from differences in patient population and criteria for BM FDG. The incidence of bone marrow involvement of DLBCL by BMB was reported as 11–17% [4, 21, 22] and 3.6% in early stage (stage I and II) DLBCL [23]. We included patients with advanced stage (stage III and IV) DLBCL, who assumed to have a high incidence of bone marrow involvement, to compare bone marrow involvement by BM FDG and BMB. As other studies included patients with lymphoma other than DLBCL, or patients with early stage (stage I and II) DLBCL, this may have caused discordant results. Also, currently there is no consensus on the criteria interpreting BM FDG [24]. We defined BM FDG-positive as unifocal or multifocal bone marrow uptake, which is identifiable from background uptake, irrespective of the degree of uptake relative to that of the liver. However, other studies defined BM FDG-positive as bone marrow uptake higher than that of the liver [17, 19, 20], bone marrow SUV equal to or higher than 4 [18], or same as our criteria [11].
In this study, we considered diffuse FDG uptake in bone marrow as negative for lymphomatous involvement, and 20 patients who were BMB-positive were classified as BM FDG-negative. In patients with DLBCL, diffuse bone marrow uptake on FDG PET usually indicates reactive changes in bone marrow. Sometimes diffuse bone marrow uptake may represent low-volume infiltration by lymphoma [12, 19, 25]. Thus, defining diffuse uptake in bone marrow as negative for lymphomatous involvement may lead to an increased false negative rate on FDG PET. However, lower tumor burden in bone marrow is less likely impact on prognosis as shown in our study [3, 4].
FDG PET/CT is sensitive in detection of bone marrow involvement of lymphoma. In patient with Hodgkin lymphoma, a BMB is no longer recommended for routine evaluation if a PET/CT is acquired [9]. In contrast, FDG PET/CT is less sensitive (71–86%) for detection of bone marrow involvement in patients with DLBCL [12, 19, 25], and diffuse bone marrow uptake can be seen in patient with positive BMB [26]. This renders interpretation of BM FDG often difficult. We defined that BM FDG as focal increased uptake and showed that upstaging by FDG PET/CT might provide better patient prognostication. As an increased rate of cellular glycolysis reflects more aggressive tumor biology, FDG PET/CT can show prognostic value not available from conventional imaging modalities [27]. However, currently whether upstaging by FDG PET/CT improves survival or induces overtreatment is unknown. Also, it is of note that as shown by our study, associations between BMB and PFS and between BM FDG and OS in patients with advanced DLBCL could suggest that BMB and BM FDG were not superior to each other in predicting survival, and patients could be better stratified by combining the results of BMB and BM FDG [12, 28].
There are some limitations in this study. First, as a retrospective study, inherent selection bias may have affected result. We also found that BM FDG-positive was not considered over BMB in patient management. Thus, clinical impact of BM FDG in patient management needs to be addressed in a future study. Second, we did not evaluate quantitative measures of BM FDG. In this study, we dichotomously classified BM FDG into either positive or negative. However, the degree of lymphomatous bone marrow involvement in BM FDG-positive patients could be different, and this heterogeneity in tumoral burden might affect survival. In this regard, quantitative analysis may provide better stratification than qualitative visual analysis. Third, because no consensus was made on criteria for BM FDG, comparison between different criteria needs to be addressed. Fourth, other prognostic factors such as GCB subtype were not included in the analysis because not all patients underwent gene expression profiling. Further study in a larger population is needed to clarify clinical significance of BM FDG in these patients.
Conclusion
We showed that BM FDG-positivity was a significant indicator in predicting PFS and OS in patients with advanced stage DLBCL. BM FDG-positivity predicted a poorer survival in patients with BMB-positive, advanced stage DBLCL. We also found that BMB-negative and BM FDG-positive patients had similar PFS or OS to BMB-positive and BM FDG-negative patients. To address potential implications of FDG PET/CT on treatment intensification or follow-up in patients with DLBCL, further study in a larger population is warranted.
Acknowledgments
We thank the members of the Department of Nuclear Medicine, Severance Hospital for their support and helpful comments.
Compliance with Ethical Standards
Conflict of Interest
Jiyoung Wang, Dongwoo Kim, Won Jun Kang, and Hojin Cho declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee.
Informed Consent
The institutional review board of our institute approved this retrospective study, and the requirement to obtain informed consent was waived.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morra E, Lazzarino M, Castello A, Inverardi D, Coci A, Pagnucco G, et al. Bone marrow and blood involvement by non-Hodgkin’s lymphoma: a study of clinicopathologic correlations and prognostic significance in relationship to the working formulation. Eur J Haematol. 1989;42:445–453. doi: 10.1111/j.1600-0609.1989.tb01469.x. [DOI] [PubMed] [Google Scholar]
- 2.Conlan MG, Bast M, Armitage JO, Weisenburger DD. Bone marrow involvement by non-Hodgkin’s lymphoma: the clinical significance of morphologic discordance between the lymph node and bone marrow. Nebraska lymphoma study group. J Clin Oncol. 1990;8:1163–1172. doi: 10.1200/JCO.1990.8.7.1163. [DOI] [PubMed] [Google Scholar]
- 3.Campbell J, Seymour JF, Matthews J, Wolf M, Stone J, Juneja S. The prognostic impact of bone marrow involvement in patients with diffuse large cell lymphoma varies according to the degree of infiltration and presence of discordant marrow involvement. Eur J Haematol. 2006;76:473–480. doi: 10.1111/j.1600-0609.2006.00644.x. [DOI] [PubMed] [Google Scholar]
- 4.Sehn LH, Scott DW, Chhanabhai M, Berry B, Ruskova A, Berkahn L, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. 2011;29:1452–1457. doi: 10.1200/JCO.2010.33.3419. [DOI] [PubMed] [Google Scholar]
- 5.Tilly H, Gomes da Silva M, Vitolo U, Jack A, Meignan M, Lopez-Guillermo A, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v116–v125. doi: 10.1093/annonc/mdv304. [DOI] [PubMed] [Google Scholar]
- 6.Bain BJ. Morbidity associated with bone marrow aspiration and trephine biopsy - a review of UK data for 2004. Haematologica. 2006;91:1293–1294. [PubMed] [Google Scholar]
- 7.Brunetti GA, Tendas A, Meloni E, Mancini D, Maggiore P, Scaramucci L, et al. Pain and anxiety associated with bone marrow aspiration and biopsy: a prospective study on 152 Italian patients with hematological malignancies. Ann Hematol. 2011;90:1233–1235. doi: 10.1007/s00277-011-1166-7. [DOI] [PubMed] [Google Scholar]
- 8.Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Mueller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol. 2014;32:3048–3058. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheson BD. Role of functional imaging in the management of lymphoma. J Clin Oncol. 2011;29:1844–1854. doi: 10.1200/JCO.2010.32.5225. [DOI] [PubMed] [Google Scholar]
- 11.Berthet L, Cochet A, Kanoun S, Berriolo-Riedinger A, Humbert O, Toubeau M, et al. In newly diagnosed diffuse large B-cell lymphoma, determination of bone marrow involvement with 18F-FDG PET/CT provides better diagnostic performance and prognostic stratification than does biopsy. J Nucl Med. 2013;54:1244–1250. doi: 10.2967/jnumed.112.114710. [DOI] [PubMed] [Google Scholar]
- 12.Khan AB, Barrington SF, Mikhaeel NG, Hunt AA, Cameron L, Morris T, et al. PET-CT staging of DLBCL accurately identifies and provides new insight into the clinical significance of bone marrow involvement. Blood. 2013;122:61–67. doi: 10.1182/blood-2012-12-473389. [DOI] [PubMed] [Google Scholar]
- 13.Hong J, Lee Y, Park Y, Kim SG, Hwang KH, Park SH, et al. Role of FDG-PET/CT in detecting lymphomatous bone marrow involvement in patients with newly diagnosed diffuse large B-cell lymphoma. Ann Hematol. 2012;91:687–695. doi: 10.1007/s00277-011-1353-6. [DOI] [PubMed] [Google Scholar]
- 14.Beyer T, Antoch G, Muller S, Egelhof T, Freudenberg LS, Debatin J, et al. Acquisition protocol considerations for combined PET/CT imaging. J Nucl Med. 2004;45(Suppl 1):25S–35S. [PubMed] [Google Scholar]
- 15.Spaepen K, Stroobants S, Dupont P, Vandenberghe P, Thomas J, de Groot T, et al. Early restaging positron emission tomography with ( 18)F-fluorodeoxyglucose predicts outcome in patients with aggressive non-Hodgkin’s lymphoma. Ann Oncol. 2002;13:1356–1363. doi: 10.1093/annonc/mdf256. [DOI] [PubMed] [Google Scholar]
- 16.Barrington SF, Kluge R. FDG PET for therapy monitoring in Hodgkin and non-Hodgkin lymphomas. Eur J Nucl Med Mol Imaging. 2017;44:97–110. doi: 10.1007/s00259-017-3690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortes-Romera M, Sabate-Llobera A, Mercadal-Vilchez S, Climent-Esteller F, Serrano-Maestro A, Gamez-Cenzano C, et al. Bone marrow evaluation in initial staging of lymphoma: 18F-FDG PET/CT versus bone marrow biopsy. Clin Nucl Med. 2014;39:e46–e52. doi: 10.1097/RLU.0b013e31828e9504. [DOI] [PubMed] [Google Scholar]
- 18.Chen-Liang TH, Martin-Santos T, Jerez A, Senent L, Orero MT, Remigia MJ, et al. The role of bone marrow biopsy and FDG-PET/CT in identifying bone marrow infiltration in the initial diagnosis of high grade non-Hodgkin B-cell lymphoma and Hodgkin lymphoma. Accuracy in a multicenter series of 372 patients. Am J Hematol. 2015;90:686–690. doi: 10.1002/ajh.24044. [DOI] [PubMed] [Google Scholar]
- 19.Cerci JJ, Gyorke T, Fanti S, Paez D, Meneghetti JC, Redondo F, et al. Combined PET and biopsy evidence of marrow involvement improves prognostic prediction in diffuse large B-cell lymphoma. J Nucl Med. 2014;55:1591–1597. doi: 10.2967/jnumed.113.134486. [DOI] [PubMed] [Google Scholar]
- 20.Adams HJ, Kwee TC, Fijnheer R, Dubois SV, Nievelstein RA, de Klerk JM. Bone marrow 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography cannot replace bone marrow biopsy in diffuse large B-cell lymphoma. Am J Hematol. 2014;89:726–731. doi: 10.1002/ajh.23730. [DOI] [PubMed] [Google Scholar]
- 21.Chung R, Lai R, Wei P, Lee J, Hanson J, Belch AR, et al. Concordant but not discordant bone marrow involvement in diffuse large B-cell lymphoma predicts a poor clinical outcome independent of the international prognostic index. Blood. 2007;110:1278–1282. doi: 10.1182/blood-2007-01-070300. [DOI] [PubMed] [Google Scholar]
- 22.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–18. [PubMed]
- 23.Lim ST, Tao M, Cheung YB, Rajan S, Mann B. Can patients with early-stage diffuse large B-cell lymphoma be treated without bone marrow biopsy? Ann Oncol. 2005;16:215–218. doi: 10.1093/annonc/mdi050. [DOI] [PubMed] [Google Scholar]
- 24.Adams HJ, Nievelstein RA, Kwee TC. Opportunities and limitations of bone marrow biopsy and bone marrow FDG-PET in lymphoma. Blood Rev. 2015;29:417–425. doi: 10.1016/j.blre.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Alzahrani M, El-Galaly TC, Hutchings M, Hansen JW, Loft A, Johnsen HE, et al. The value of routine bone marrow biopsy in patients with diffuse large B-cell lymphoma staged with PET/CT: a Danish-Canadian study. Ann Oncol. 2016;27:1095–1099. doi: 10.1093/annonc/mdw137. [DOI] [PubMed] [Google Scholar]
- 26.Adams HJ, Kwee TC, Fijnheer R, Dubois SV, Nievelstein RA, de Klerk JM. Diffusely increased bone marrow FDG uptake in recently untreated lymphoma: incidence and relevance. Eur J Haematol. 2015;95:83–89. doi: 10.1111/ejh.12483. [DOI] [PubMed] [Google Scholar]
- 27.Kwee TC, Kwee RM, Nievelstein RA. Imaging in staging of malignant lymphoma: a systematic review. Blood. 2008;111:504–516. doi: 10.1182/blood-2007-07-101899. [DOI] [PubMed] [Google Scholar]
- 28.Adams HJ, Kwee TC, de Keizer B, Fijnheer R, de Klerk JM, Nievelstein RA. FDG PET/CT for the detection of bone marrow involvement in diffuse large B-cell lymphoma: systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2014;41:565–574. doi: 10.1007/s00259-013-2623-4. [DOI] [PubMed] [Google Scholar]