Abstract
Background:
Despite the dramatic efficacy of erlotinib, an EGFR tyrosine kinase inhibitor (TKI), most of non-small cell lung cancer (NSCLC) patients ultimately acquire resistance to this agent. Different studies indicated that miRNA-125a-5p is down-regulated in human lung cancer cells and may function as a tumor suppressor by targeting EGFR. However, the biological function of miRNA-125a-5p in NSCLC resistance to EGFR-TKIs is not fully understood. In this study the effect of miRNA-125a-5p on cell proliferation, apoptosis and sensitivity of the A549 lung cancer cells to erlotinib was investigated.
Methods:
After miRNA-125a-5p transfection, the expression levels of EGFR mRNA were measured by QRT-PCR. Trypan blue assays were performed to evaluate the proliferation of the A549 lung cancer cells. The cytotoxic effects of miRNA-125a-5p and erlotinib, alone and in combination, were determined using MTT assay. Combination index study was performed using the method of Chou-Talalay. Apoptosis was assessed using an ELISA cell death assay kit.
Results:
MiRNA-125a-5p clearly reduced the expression of EGFR mRNA in a time dependent manner, causing marked cell proliferation inhibition and spontaneous apoptosis (p<0.05, relative to control). Pretreatment with miRNA-125a-5p synergistically increased the cytotoxic effect of erlotinib and decreased its IC50. Furthermore, miRNA-125a-5p significantly enhanced the apoptotic effect of erlotinib. Negative control miRNA had no significant effect on biological parameter of the tumor cells.
Conclusions:
Our data suggest that suppression of EGFR by miRNA-125a-5p can effectively trigger apoptosis and overcome EGFR-TKs resistance of lung cancer cells. Therefore, miRNA-125a-5p may be a potential therapeutic adjuvant in patients with lung cancer.
Key Words: Apoptosis, EGFR, Erlotinib, lung cancer, MiRNA, 125a-5p
Introduction
Lung cancer is one of the most common cancers in terms of both incidence and mortality worldwide in men and women (Ma et al., 2016; Ashour Badawy et al., 2018). It is classified into two different groups: small cell lung cancer (SCLC), which account for the 20% of cases, and non-small cell lung cancers (NSCLC), which account for 80% (Garinet et al., 2018; Wu et al., 2019). Intrinsic resistance presents a significant challenge in the treatment of NSCLC and contributes to tumor recurrence and progression (Leonetti et al., 2019; Terlizzi et al., 2019). The epidermal growth factor receptor (EGFR), a member of the ErbB family of receptor tyrosine kinase (RTK), is frequently overexpressed in NSCLC and negatively correlated with poor prognosis (Barr Kumarakulasinghe et al., 2015; Hsu et al., 2019). EGFR signaling triggers intracellular signaling pathways such as the STAT signaling pathway, phosphoinositide 3-kinase (PI3K)/Akt pathway, and the Ras/Raf/MEK/ERK1/2 pathway, which enhances tumor cell proliferation, angiogenesis, invasion, metastasis, and apoptosis resistance (Seshacharyulu et al., 2012; Wang et al., 2013; Barr Kumarakulasinghe et al., 2015; Oronsky et al., 2018; Yang and Tam, 2018). Consequently, the EGFR has emerged as the target of effective cancer therapies.
EGFR tyrosine kinase inhibitors (TKIs), such as gefitinib and erlotinib, developed as therapeutic agents for NSCLC treatment. Despite the therapeutic benefit of EGFR-TKIs, all patients eventually develop resistance to these agents (Antonicelli et al., 2013; Barr Kumarakulasinghe et al., 2015; Ralki et al., 2019; Xia et al., 2019). The poor clinical response of NSCLC to anti-EGFR therapies is due to the primary and secondary resistance of cancer cells to these drugs, which is thought to occur via several mechanisms, including HER-2 amplification, MET amplification, mutation in exon 20 of EGFR (T790M), PI3K mutations, and transformation into small cell lung cancer (Antonicelli et al., 2013; Barr Kumarakulasinghe et al., 2015). However, the additional EGFR-TKIs resistance mechanisms had remained unclear.
MicroRNAs (miRNAs) are abundant class of non-coding 18-25 nucleotide small RNAs, which bind to the 3’-UTR of specific target mRNAs to suppress gene expression, either via inducing translational inhibition or mRNA degradation (Ricciuti et al., 2014; Abu-Duhier et al., 2018; Amri et al., 2019). MiRNAs participate in a variety of biological processes, such as cell cycle progression, proliferation, growth and apoptosis (Zhang et al., 2014; Fatima et al., 2019; Miroshnichenko and Patutina, 2019). Aberrant expression of particular miRNAs is a hallmark of many human tumor types, including NSCLC, and they can act as either oncogenes or as tumor suppressors (Zhao et al., 2013; MacDonagh et al., 2015; Yin et al., 2017; Bharali et al., 2018). For example, miRNA-143 expression is strongly down-regulated in lung cancer cells, causing elevated c-MYC, NUDT1, OCT4 and EGFR expression, increased tumor cell growth, metastasis and migration (Ricciuti et al., 2014; Zhang et al., 2014). In contrast, miRNA-21 is overexpressed in different forms of cancers, including NSCLC, leading to suppression of the PTEN, increased cell growth and invasion (Wang et al., 2014; Zhang et al., 2014). Thus, miRNAs can be served as potentially useful biomarkers for the diagnosis, prognosis and treatment of lung cancer (Markou et al., 2013; Ricciuti et al., 2014; Zhang et al., 2014).
MiRNA-125a-5p was known as tumor suppressor that inhibits the expression of EGFR and downstream genes involved in EGFR signaling pathway, leading to inhibition of invasion and migration of lung cancer cells. Moreover, down-regulation of miRNA-125a-5p has been observed in several types of cancers, including lung cancer (Wang et al., 2009b; Jiang et al., 2010b; Nishida et al., 2011; Zhang et al., 2014; Wang et al., 2015). In this study, we examined the effect of miRNA-125a-5p on EGFR expression, cell proliferation and apoptosis in NSCLC cells. We hypothesized that miRNA-125a-5p would enhance the sensitivity of the NSCLC cells to EGFR-TKIs by EGFR silencing, and evaluated the combination effect of miRNA-125a-5p and erlotinib on A549 cells.
Materials and Methods
Cell culture
Human lung cancer cell line A549 (Pasteur Institute, Tehran, Iran) was maintained in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) that was supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco; Invitrogen; Life Technologies, Germany), 1% antibiotics (100 IU/ml penicillin, 100 µg/ml streptomycin) (Sigma-Aldrich), 1% sodium pyruvate and 2 mM of glutamine at 37oC and 5% CO2.
MiRNA transfection
The miRNA-125a-5p mimics and negative control (NC) miRNA were ordered from Dharmacon (Lafayette, CO, USA). The sequences of miRNAs are as follows: NC miRNA: 5’-UUCUUCGAACGUGUCACGUTT-3’, miRNA-125a-5p: 5’-UCCCUGAGACCCUUUAACCUGUGA-3’. Just before transfection, A549 cells were cultured in RPMI-1640 medium without FBS and antibiotics. Transfection of miRNAs was performed using Lipofectamine™2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Briefly, miRNAs (at a final concentration of 50 nM) and lipofectamine (4 µl/ml of transfection medium) were diluted in Opti-MEM I Reduced Serum Medium (Invitrogen) separately and incubated for 5 min at ambient temperature. Then the diluted miRNAs were mixed with the diluted Lipofectamine and incubated for another 20 min. Following on, the mixtures were added to each well containing cells and medium. After 6 h incubation of the cell culture plates at 37oC in CO2 incubator, complete growth medium was added to a final FBS concentration of 10%, with cells being incubated under the same conditions. After 48 and 48 h, down-regulation of EGFR was assessed by real-time quantitative PCR (qRT-PCR).
QRT-PCR
At 24, 48 and 72 h after transfection, total RNA was isolated from cells by RNA extraction kit (Takara Bio Inc., Kusatsu, Shiga, Japan) according to the manufacturer’s protocol. Complementary DNA (cDNA) was synthesized from 1 µg of total cellular RNA by use of PrimeScript 1st strand cDNA Synthesis Kit (Takara Bio Inc.) and oligo-dT primer according to the manufacturer’s recommendations. Real-time PCR was performed using SYBR Green qPCR MasterMix (Yekta Tajhiz Azma, Tehran, Iran) and a LightCycler 96 System (Roche Diagnostics GmbH, Mannhein, Germany). Each PCR reaction had the following components: 1 µl of RT product, 500 nM each of the forward and reverse primers and 10 µl of SYBR Green qPCR MasterMix. The sequences of primers used for quantitative PCR were as follows: forward, 5’-TTTACAGGAAATCCTGCATGG -3’ ,reverse, 5’- TCACTGCTGACTATGTCCC -3’, for EGFR, and forward, 5’- CTACAATGAGCTGCGTGTG -3’, and reveres, 5’- GTCTCAAACATGATCTGGGTC -3’, for β-actin. The protocol parameters were 95 °C 10 min; 95 °C 10 sec, 57°C 20 sec, 72°C 20 sec, 40 cycles. The relative transcript abundance (the amount of EGFR normalized to the β-actin) was measured using the 2- (∆∆Ct) method (Livak and Schmittgen, 2001).
Cytotoxicity assay
The effect of miRNA-125a-5p on the sensitivity of A549 cell line to erlotinib (Sigma- Aldrich) was evaluated using 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide (MTT) assay. The experiment was divided into eight groups: erlotinib, miRNA-125a-5p mimics, NC miRNA, miRNA-125a-5p mimics and erlotinib, NC miRNA and erlotinib, miRNA blank control, erlotinib blank control and combination blank control. Briefly, cells were seeded at a density of 5×103 cells/well in 96-well culture plates, and then transfected with miRNAs. Six hours after transfection, the cells were exposed to various concentrations of erlotinib (0, 2, 4, 8, 16, 32 and 64 µM). After 24 and 48 h of transfection, the cell cytotoxicity was determined using a cell MTT kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s protocol. The absorbance (A) of the formazan dye was measured on a microplate reader (Awareness Technology, Palm City, FL, USA) at a wavelength 570 nm. The survival rate (SR) was calculated according to the equation as follows: SR (%) = (A Test /A Control) ×100%. IC50 (concentration that produced 50% cytotoxicity) values of the treatments alone or in combination were determined using Prism 6.01 software (GraphPad Software Inc., San Diego, CA, USA).
Drug combination study
The combination index (CI) analysis based upon the Chou-Talalay method was used to determine the interaction between miRNA-125a-5p and erlotinib (Chou and Talalay, 1984). The data obtained with the MTT assay was converted to Fraction affected (Fa; range 0-1; where Fa = 0 represents 100% cell survival and Fa = 1 represents 0% cell survival) and analyzed with the CompuSyn version 1.0 software (ComboSyn Inc., Paramus, NJ, USA). A CI of < 1, =1 or >1 indicates synergistic, additive and antagonistic effects, respectively.
Cell viability assay
The effect of miRNA-125a-5p on cell proliferation was measured by the trypan blue exclusion assay. A549 cells (5×10 4 cells/well) were treated with miRNA-125a-5p in 24-well cell culture plates and incubated for 5 days. At indicated time points, the cells were harvested and equal volumes of cell suspension and 0.4% trypan blue solution (Merck KGaA, Darmstadt, Germany) were mixed gently. Then, the numbers of viable cells (unstained) were counted microscopically (Nikon Instrument Inc., Melville, NY, USA) using a hemocytometer. The cell viability was expressed as a percentage.
Apoptosis ELISA assay
The A549 cells were seeded at a density of 1×105 cells/well in 12-well plates and then exposed to miRNA-125a-5p mimics or NC miRNA, erlotinib (IC50 doses of 24 and 48 h) and their combination, as described previously. At 24 and 48 h after transfection, cells were collected and apoptosis was assessed using an ELISA cell death detection kit (Roche Diagnostics GmbH) according to the manufacturer’s protocol. This assay measures the amount of nucleosomal formation produced during apoptosis. Briefly, the cell lysates were transferred into the streptlized-coated plate and incubated with a mixture of anti-DNA-peroxidase and anti-histone-biotin. Following color development with 2, 2-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) solution, the absorbances of the samples was measured with an ELISA plate reader at 405 nm (reference wavelength 540 nm). Results were expressed as the fold increase in apoptosis as compared with control group.
Statistical analysis
Quantitative data were presented as mean ± standard deviation (SD). Analysis of variance (ANOVA) followed by Bonferroni’s test were used to determine statistical differences between groups. Value of p less than 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism software.
Results
MiRNA-125a-5p suppressed EGFR mRNA levels in A549 cells
Firstly, we explored the effect of miRNA-125a-5p on EGFR mRNA expression in cancer cells by RT-qPCR. Relative EGFR mRNA expression was calculated in relation to the blank control (set at 100%). Compared with the blank control group, the expression of EGFR mRNA in A549 cells transfected with miRNA-125a-5p was significantly down-regulated (p<0.05; Figure 1). MiRNA-125a-5p reduced the EGFR mRNA level by 82.78%, 68.21% and 55.19% after 24, 48 and 72 h, respectively (p<0.05). Meanwhile, treatment with negative control miRNA had minimal effect on mRNA levels compared with the blank control group (p>0.05; Figure 1).
Figure 1.
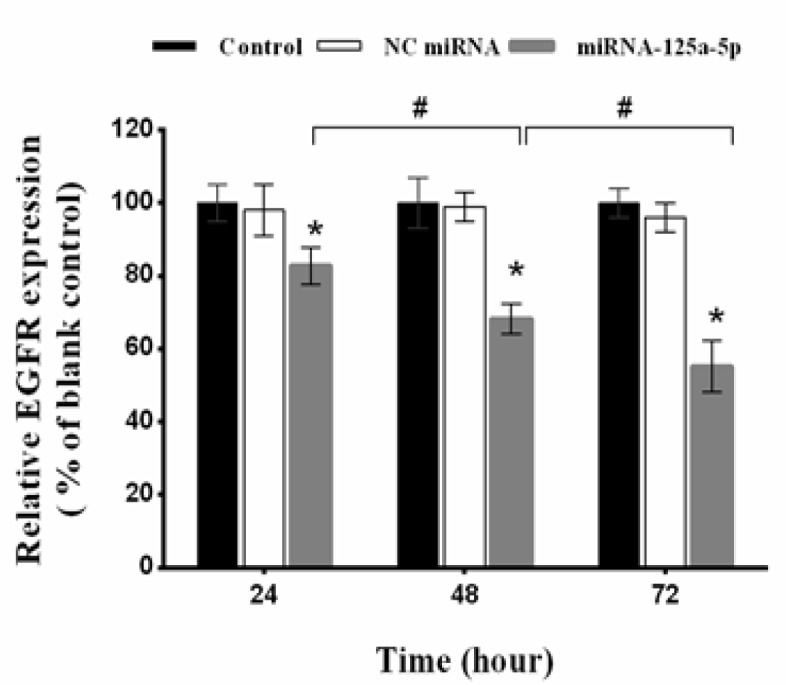
Effect of miRNA-125a-5p on the Expression of EGFR in A549 Cells. The EGFR expression determined by RT-qPCR at 24, 48 and 72 h after transfection of the cells with miRNA-125a-5p and negative control (NC) miRNA. Relative EGFR mRNA expression was measured using the 2- (∆∆Ct) method. The EGFR mRNA decreased clearly at the three time points compared with corresponding blank control and NC miRNA groups (*p<0.05). The results presented are mean±SD of three independent experiments. #p <0.05
MiRNA-125a-5p enhanced the cytotoxic effect of erlotinib in lung cancer cells
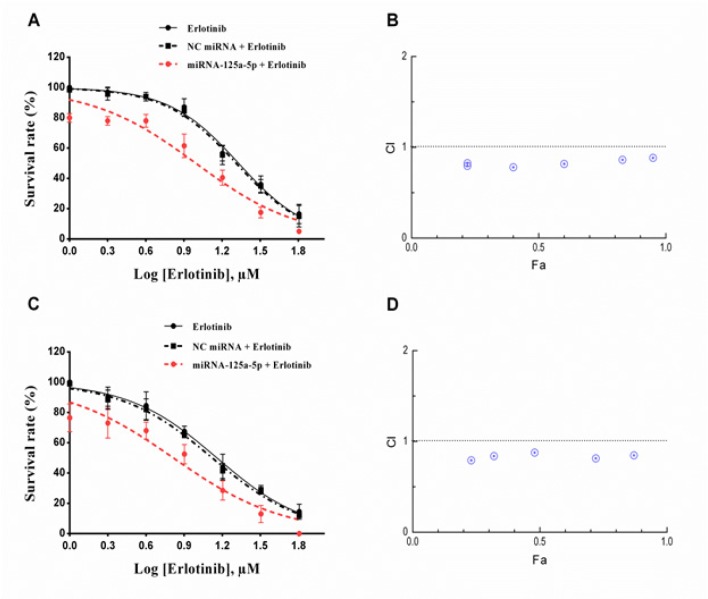
To analyze whether down-regulation of EGFR could enhance the sensitivity of the A549 cells to erlotinib, a combination treatment of erlotinib and miRNA-125a was investigated. The results of MTT assay showed that monotreatment with erlotinib induced cell toxicity in a dose-dependent manner. As shown in Figure 2A and 2C, after 24 and 48 h of incubation, miRNA-125a-5p significantly lowered the cell survival rate to 80.30% and 76.51% respectively, relative to the blank control group (p<0.05). Moreover, erlotinib in combination with miRNA-125a-5p further reduced the cell survival rate relative to erlotinib or miRNA-125a-5p alone (p<0.05). Surprisingly, the presence of miRNA-125a-5p caused a clear reduction in the IC50 values of erlotinib from 21.42 µM to 9.87 µM and 14.41 µM to 6.54 µM after 24 and 48 h, respectively (Table 1). Meanwhile, transfection with NC miRNA had an insignificant effect on the sensitivity of the tumor cells relative to the erlotinib treated cells (p>0.05; Figure 2 and Table 1).
Figure 2.
Effect of miRNA-125a-5p in Combination with Erlotinib on Cell Survival. Human A549 cells were treated with miRNA-125a-5p (50 nM) and different concentrations of erlotinib for 24 h (A and B) and 48 h (C and D). Cell survival was determined by the MTT assay as described in the method section. Dose-response curves were plotted using GraphPad Prism 6.01 software. Bars represent mean±SD (n=3). Data from three independent experiments were used to calculate the combination index (CI) according the method of Chou-Talalay. A horizontal dashed marks CI=1
Table 1.
Half Maximal Inhibitory Concentration (IC50) of Erlotinib Alone and in Combination with miRNAs, after 24 and 48 h of Treatment
| Treatment | IC50 |
|
|---|---|---|
| 24 h | 48 h | |
| Erlotinib | 21.42 ± 1.10 | 14.41 ± 2.10 |
| NC miRNA and erlotinib | 20.49 ± 1.17# | 13.25 ± 1.30# |
| miRNA-125a-5p and erlotinib | 9.87 ± 2.15* | 6.54 ± 0.99* |
IC50 values were determined by sigmoidal dose-response (variable slope) model using GraphPad Prism software. Data expressed as the mean±SD (n=3). *p<0.05 relative to the corresponding erlotinib group; #p>0.05 versus corresponding erlotinib.
MiRNA-125a-5p acts synergistically with erlotinib to decrease the cell survival of A549 cells
To further examine whether the decrease in cell survival was the synergistic effect of the miRNA-125a-5p and erlotinib, combination index analysis was performed on MTT assay data using non-constant method of Chou and Talalay. The CI–Fa curves demonstrated a synergism (CI<1) in A549 cells when miRNA-125a-5p (50 nM) combined with erlotinib (2-64 µM) (Figure 2B and 2D). Our data showed that the best mean CI value of 24 h of treatment (CI=0.78) was obtained at 8 µM erlotinib with Fa level of 0.40 (Figure 2B). Moreover, at 2 µM erlotinib with Fa level of 0.23 the best mean CI value for 48 h (CI=0.79) was observed (Figure 2D).
Up-regulation of miRNA-125a-5p inhibited cell proliferation
As down-regulation of miRNA-125a-5p is associated with survival of lung cancer cells; we therefore sought to test whether up-regulation of this miRNA could inhibit the proliferation of A549 cells. The tumor cells were transfected with miRNA-125a-5p and NC miRNA. Then, the cell viability was measured every 24 h for 5 days by trypan blue exclusion assay. The cell proliferation curve showed that miRNA-125a-5p significantly reduced cell viability compared with blank control group in a time dependent way (p<0.05; Figure 3). Twenty-four hours after transfection of miRNA-125a-5p, the cell viability decreased to 84.39% and dropped to 52.61% on day 5. In contrast, no significant alterations in cell proliferation were detected between the NC miRNA and the blank control groups (p>0.05; Figure 3).
Figure 3.
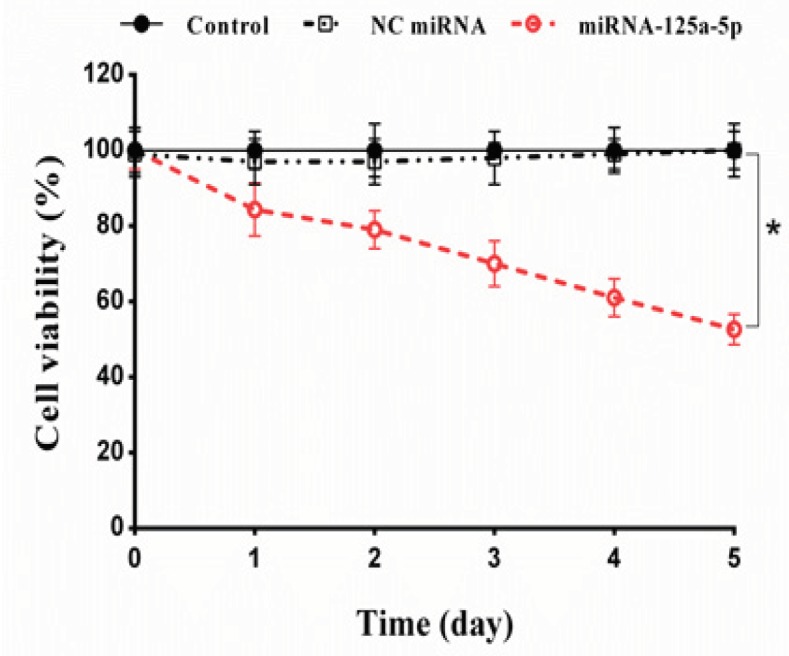
Effect of Down-Regulation of EGFR by miRNA-125a-5p on Lung Cancer Cell Proliferation. The A549 cells were transfected with miRNA-125a-5p and negative control (NC) miRNA and then cell viability was tested by trypan blue assay over a period of 5 days. The results represent mean±SD of three independent experiments. *p<0.05 versus blank control or NC miRNA
MiRNA-125a-5p sensitized lung cancer cells to apoptosis induced by erlotinib
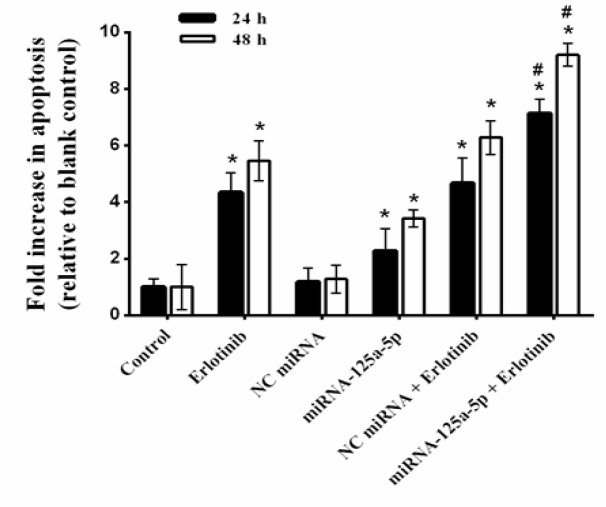
To confirmed investigate whether the sensitizing effect of the miRNA-125a-5p was related to the increase in the extent of apoptosis, the effects of miRNA-125a-5p, erlotinib and their combination on apoptosis, were evaluated using an ELISA-based cell death detection system. As shown in Figure 4, 24 h after transfection of miRNA-125a-5p alone, apoptosis enhanced by 2.27 fold, whereas erlotinib treatment alone caused 4.35 fold increase in apoptosis (p<0.05, compared to the blank control). In contrast, the combination treatment further enhanced apoptosis to 7.14 fold (p<0.05, compared with single agent treatment). Moreover, after 48 h of treatment of A549 cells to miRNA-125a-5p or erlotinib alone, apoptosis increased by 3.43 and 5.46 fold, respectively, compared to the blank control (p<0.05). Also, combination therapy further enhanced apoptosis to 9.21 fold after 48 h (Figure 4; p<0.05, relative to the blank control or monotreatment). On the other hand, treatment with NC miRNA alone or in combination with erlotinib displayed no significant alterations in the extents of apoptosis compared with the blank control or erlotinib monotreatment, respectively. Therefore, these results indicate that the sensitization effect of miRNA-125a-5p is partially attributed to the induction of apoptosis.
Figure 4.
Cell Apoptosis of A549 Cells Treated with miRNA-125a-5p and Erlotinib. The cells were treated with miRNA-125a-5p (50 nM), negative control (NC) miRNA (50 nM) and erlotinib (IC50 doses of 24 and 48 h), alone and in combination, and then apoptosis was measured by cell death ELISA. The data are expressed as mean±SD (n=3); *p<0.05 versus blank control or NC miRNA; #p<0.05 versus miRNA-125a-5p or erlotinib
Discussion
Despite intensive advances in the treatment of lung cancer, it is remains an incurable disease. Owing to the occurrence of drug resistance in lung cancer cells, the survival rate still remains at low level (Mac Donagh et al., 2015; Wang et al., 2015). Therefore, development of new strategies for improved therapy is required. Overexpression of EGFR is attributed to the invasion, angiogenesis, proliferation, metastasis, and apoptosis resistance of many tumor cells including lung cancer (Yoshida et al., 2010; Seshacharyulu et al., 2012; Barr Kumarakulasinghe et al., 2015). Despite the therapeutic benefit of EGFR tyrosine kinase inhibitors, the efficacy of these agents is often limited by the development of drug resistance (Yoshida et al., 2010; Seshacharyulu et al., 2012; Antonicelli et al., 2013; Barr Kumarakulasinghe et al., 2015). However, the exact molecular mechanisms of resistance had remained unclear. In this study, we explored the effect of miRNA-125a-5p on EGFR expression, cell proliferation and sensitivity of NSCLC cells to erlotinib.
qRT-PCR revealed that transfection of miRNA-125a-5p markedly reduced EGFR mRNA levels during the 3-day period. These data suggest that miRNA-125a-5p could effectively inhibit the expression of the EGFR, partly by decomposition of the corresponding mRNA. The results of the cell proliferation assay revealed that the up-regulation of miRNA-125a-5p significantly inhibited the proliferation of A549 cells, demonstrating its important role in the growth of lung cancer cells. Moreover, the results of MTT assay showed that pretreatment with miRNA-125a-5p distinctly decreased the IC50 value of erlotinib and subsequently enhanced its cytotoxicity. Combination study results clearly showed a synergistic interaction between miRNA-125a-5p and erlotinib at all concentrations of erlotinib.
To further explore the role of miRNA-125a-5p in the drug resistance of lung cancer cells, we examined the effect of miRNA-125a-5p on erlotinib-induced apoptosis. ELISA cell death assay revealed that erlotinib, alone, caused remarkable apoptosis in lung cancer cells. Of note, ELISA assay indicated that the inhibition of EGFR using miRNA-125a-5p also led to significant apoptosis in the absence of erlotinib. In addition, miRNA-125a-5p, in combination with erlotinib dramatically increased apoptosis level compared with miRNA-125a-5p alone or erlotinib alone. In contrast, neither NC miRNA nor lipofectamine changed the impact on drug sensitivity, which confirms the specific effect of miRNA-125a-5p. These data proposes that up-regulation of miRNA-125a-5p could sensitize the lung cancer cells to erlotinib via suppression of EGFR.
Evidences suggests that dysregulation of miRNAs can be involved in the carcinogenesis and acquisition of resistance in cancer cells (MacDonagh et al., 2015). MiRNA-125a is a tumor suppressor that is transcripted from a gene on chromosome 19. Its tumor suppressive function was firstly confirmed in some types of cancers such as gastric, breast, glioblastoma and lung (Scott et al., 2007; Wang et al., 2009b; Cortez et al., 2010; Nishida et al., 2011; Wang et al., 2015). Previous studies showed that the expression of miRNA-125a -3p, one of the derivatives of miRNA-125a, was reduced in lung cancer cells and NSCLC tissues (Jiang et al., 2010a; LU et al., 2011). As to miRNA-125a-5p, another derivative of miRNA-125a, conflicting results have been observed. Jiang et al., (2010b) showed that the expression of miRNA-125a-5p was lower in lung cancer tissues than in adjacent normal tissues. The results of their study indicated that down-regulation of miRNA-125a-5p enhanced the migration and invasion of the lung tumor cells. Wang et al., (2009a) also found that miRNA-125a-5p is an EGFR-regulated miRNA that may function as a metastatic suppressor. The results of our study are in agreement with these reports and further confirm the negative correlation of miRNA-125a-5p with lung carcinogenesis. Nevertheless, conclusions from other studies which performed on the lung cancer cells are not consistent (Jiang et al., 2010b; LU et al., 2011).
The EGFR expression level has been shown to enhanced in many human malignancies (Yoshida et al., 2010; Barr Kumarakulasinghe et al., 2015). Some previous reports demonstrated that miRNA-146a and miRNA-7 can inhibit the expression of EGFR and increase the sensitivity of the lung tumor cells to EGFR tyrosine kinase inhibitors (Rai et al., 2011; Chen et al., 2013). Another study showed that down-regulation of miRNA-125a-5p is associated with enhanced malignant potential such as tumor invasion, tumor size and poor prognosis in human gastric cancer (Nishida et al., 2011). However, other studies showed that down-regulation of miRNA-125a-5p, leads to an increase in the expression of EGFR and its downstream gene, enhancement of lung tumor cell migration and invasion (Wang et al., 2009b; Zhang et al., 2014; Wang et al., 2015). In this study, we showed that miRNA-125a-5p can inhibit the proliferation of the lung cancer cells and enhance the apoptotic effect of erlotinib by targeting EGFR.
In conclusion, our study results indicate that miRNA-125a-5p inhibits EGFR expression in A549 cells, and that miRNA-125a-5p has the capacity to inhibit A549 growth in vitro. Down-regulation of EGFR by miRNA-125a-5p triggered significant apoptosis and enhanced sensitivity of the lung cancer cells to erlotinib in a synergistic way. Our data propose that the therapeutic delivery of miRNA-125a-5p may inhibit tumor proliferation, induce apoptosis and sensitize lung tumor cells to EGFR tyrosine kinase inhibitors.
Acknowledgements
This study was supported by a grant for scientic research from the Molecular and Medicine Research Center, Arak University of Medical Sciences (Grant No. 2215). JA performed the experiments, analyzed the data and wrote the paper; NM performed the experiments and wrote the paper; HK designed the study, analyzed the data, discussed the results and commented on the manuscript. We thank Dr. Maryam Baazm and staffs of the Anatomy Department for their technical assistance.
References
- Abu-Duhier FM, Javid J, Sughayer MA, et al. Clinical significance of circulatory miRNA-21 as an efficient non-invasive biomarker for the screening of lung cancer patients. Asian Pac J Cancer Prev. 2018;19:2607–11. doi: 10.22034/APJCP.2018.19.9.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri J, Molaee N, Baazm M, et al. Targeting epidermal growth factor receptor by MiRNA-145 inhibits cell growth and sensitizes NSCLC cells to Erlotinib. Asian Pac J Cancer Prev. 2019;20:2781–7. doi: 10.31557/APJCP.2019.20.9.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonicelli A, Cafarotti S, Indini A, et al. EGFR-targeted therapy for non-small cell lung cancer: focus on EGFR oncogenic mutation. Int J Med Sci. 2013;10 doi: 10.7150/ijms.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour Badawy A, Khedr G, Omar A, et al. Site of metastases as prognostic factors in unselected population of stage IV non-small cell lung cancer. Asian Pac J Cancer Prev. 2018;19:1907–10. doi: 10.22034/APJCP.2018.19.7.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr Kumarakulasinghe N, Zanwijk Nv, Soo RA. Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC) Respirology. 2015;20:370–8. doi: 10.1111/resp.12490. [DOI] [PubMed] [Google Scholar]
- Bharali D, Jebur HB, Baishya D, et al. Expression analysis of serum microRNA-34a and microRNA-183 in Hepatocellular Carcinoma. Asian Pac J Cancer Prev. 2018;19:2561–8. doi: 10.22034/APJCP.2018.19.9.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Umelo IA, Lv S, et al. miR-146a inhibits cell growth, cell migration and induces apoptosis in non-small cell lung cancer cells. PLoS One. 2013;8:e60317. doi: 10.1371/journal.pone.0060317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Cortez MA, Nicoloso MS, Shimizu M, et al. miR-29b and miR-125a regulate podoplanin and suppress invasion in glioblastoma. Genes Chromosomes Cancer. 2010;49:981–90. doi: 10.1002/gcc.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima N, Srivastava AN, Nigam J, et al. Low expression of MicroRNA335-5p is associated with malignant behavior of gallbladder cancer: A clinicopathological study. Asian Pac J Cancer Prev. 2019;20:1895–900. doi: 10.31557/APJCP.2019.20.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garinet S, Laurent-Puig P, Blons H, et al. Current and future molecular testing in NSCLC, What can we expect from new sequencing technologies? J Clin Med. 2018;7:1–23. doi: 10.3390/jcm7060144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PC, Jablons DM, Yang CT, et al. Epidermal growth factor receptor (EGFR) pathway, yes-associated protein (YAP) and the regulation of programmed death-ligand 1 (PD-L1) in non-small cell lung cancer (NSCLC) Int J Mol Sci. 2019;20:3821. doi: 10.3390/ijms20153821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Huang Q, Zhang S, et al. Hsa-miR-125a-3p and hsa-miR-125a-5p are downregulated in non-small cell lung cancer and have inverse effects on invasion and migration of lung cancer cells. BMC Cancer. 2010a;10:1471–2407. doi: 10.1186/1471-2407-10-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Huang Q, Zhang S, et al. Hsa-miR-125a-3p and hsa-miR-125a-5p are downregulated in non-small cell lung cancer and have inverse effects on invasion and migration of lung cancer cells. BMC Cancer. 2010b;10:318. doi: 10.1186/1471-2407-10-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti A, Assaraf YG, Veltsista PD, et al. MicroRNAs as a drug resistance mechanism to targeted therapies in EGFR-mutated NSCLC: Current implications and future directions. Drug Resist Updat. 2019;42:1–11. doi: 10.1016/j.drup.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu W, Li S, Liu B, et al. Screening of metastasis-related MicroRNAs in the large-cell lung cancer cell lines with different metastastic potentials. Chin J Lung Cancer. 2011;14:835–40. doi: 10.3779/j.issn.1009-3419.2011.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Zhao Z, Wu K, et al. MCL-1 is the key target of adjuvant chemotherapy to reverse the cisplatin-resistance in NSCLC. Gene. 2016;587:147–54. doi: 10.1016/j.gene.2016.04.054. [DOI] [PubMed] [Google Scholar]
- MacDonagh L, Gray SG, Finn SP, et al. The emerging role of microRNAs in resistance to lung cancer treatments. Cancer Treat Rev. 2015;41:160–9. doi: 10.1016/j.ctrv.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Markou A, Sourvinou I, Vorkas P, et al. Clinical evaluation of microRNA expression profiling in non small cell lung cancer. Lung Cancer. 2013;81:388–96. doi: 10.1016/j.lungcan.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Miroshnichenko S, Patutina O. Enhanced inhibition of tumorigenesis using combinations of miRNA-targeted therapeutics. Front Pharmacol. 2019;10:488. doi: 10.3389/fphar.2019.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida N, Mimori K, Fabbri M, et al. MicroRNA-125a-5p is an independent prognostic factor in gastric cancer, and inhibits the proliferation of human gastric cancer cells in combination with trastuzumab. Clin Cancer Res. 2011:2132. doi: 10.1158/1078-0432.CCR-10-2132. [DOI] [PubMed] [Google Scholar]
- Oronsky B, Ma P, Reid TR, et al. Navigating the “No Man’s Land” of TKI-Failed EGFR-mutated non-small cell lung cancer (NSCLC): A review. Neoplasia. 2018;20:92–8. doi: 10.1016/j.neo.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai K, Takigawa N, Ito S, et al. Liposomal delivery of microRNA-7-expressing plasmid overcomes epidermal growth factor receptor-tyrosine kinase inhibitor-resistance in lung cancer cells. Mol Cancer Ther. 2011:0220. doi: 10.1158/1535-7163.MCT-11-0220. [DOI] [PubMed] [Google Scholar]
- Ralki M, Maes B, Pat K, et al. Triple trouble: A case of multiple resistance mechanisms after first generation EGFR-TKI in NSCLC. Case Rep Oncol. 2019;12:625–30. doi: 10.1159/000502214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciuti B, Mecca C, Crinò L, et al. Non-coding RNAs in lung cancer. Oncoscience. 2014;1:674. doi: 10.18632/oncoscience.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GK, Goga A, Bhaumik D, et al. Coordinate suppression of ERBB2 and ERBB3 by enforced expression of micro-RNA miR-125a or miR-125b. J Biol Chem. 2007;282:1479–86. doi: 10.1074/jbc.M609383200. [DOI] [PubMed] [Google Scholar]
- Seshacharyulu P, Ponnusamy MP, Haridas D, et al. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:15–31. doi: 10.1517/14728222.2011.648617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlizzi M, Colarusso C, Pinto A, et al. Drug resistance in non-small cell lung Cancer (NSCLC): Impact of genetic and non-genetic alterations on therapeutic regimen and responsiveness. Pharmacol Ther. 2019;202:140–8. doi: 10.1016/j.pharmthera.2019.06.005. [DOI] [PubMed] [Google Scholar]
- Wang F, Chan LW, Law HK, et al. Exploring microRNA-mediated alteration of EGFR signaling pathway in non-small cell lung cancer using an mRNA: miRNA regression model supported by target prediction databases. Genomics. 2014;104:504–11. doi: 10.1016/j.ygeno.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Wang G, Mao W, Zheng S, et al. Epidermal growth factor receptor-regulated miR-125a-5p--a metastatic inhibitor of lung cancer. FEBS J. 2009a;276:5571–8. doi: 10.1111/j.1742-4658.2009.07238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Mao W, Zheng S, et al. Epidermal growth factor receptor-regulated miR-125a-5p–a metastatic inhibitor of lung cancer. FEBS J. 2009b;276:5571–8. doi: 10.1111/j.1742-4658.2009.07238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Park JO, Zhang M. Treatment of glioblastoma multiforme using a combination of small interfering RNA targeting epidermal growth factor receptor and beta-catenin. J Gene Med. 2013;15:42–50. doi: 10.1002/jgm.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R-J, Zheng Y-H, Wang P, et al. Serum miR-125a-5p, miR-145 and miR-146a as diagnostic biomarkers in non-small cell lung cancer. Int J Clin Exp Pathol. 2015;8:765. [PMC free article] [PubMed] [Google Scholar]
- Wu KL, Tsai YM, Lien CT, et al. The roles of MicroRNA in lung cancer. Int J Mol Sci. 2019;20:1611. doi: 10.3390/ijms20071611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Liu Y, Liao Y, et al. Synergistic effects of gefitinib and thalidomide treatment on EGFR-TKI-sensitive and -resistant NSCLC. Eur J Pharmacol. 2019;856:172409. doi: 10.1016/j.ejphar.2019.172409. [DOI] [PubMed] [Google Scholar]
- Yang Z, Tam KY. Combination strategies using EGFR-TKi in NSCLC therapy: Learning from the Gap between pre-clinical results and clinical outcomes. Int J Biol Sci. 2018;14:204–16. doi: 10.7150/ijbs.22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Xu M, Li P. miRNA-221 acts as an oncogenic role by directly targeting TIMP2 in non-small-cell lung carcinoma. Gene. 2017;620:46–53. doi: 10.1016/j.gene.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Zhang G, Haura EB. Targeting epidermal growth factor receptor: central signaling kinase in lung cancer. Biochem Pharmacol. 2010;80:613–23. doi: 10.1016/j.bcp.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yang Q, Wang S. MicroRNAs: a new key in lung cancer. Cancer Chem Pharmacol. 2014;74:1105–11. doi: 10.1007/s00280-014-2559-9. [DOI] [PubMed] [Google Scholar]
- Zhao C, Xu Y, Zhang Y, et al. Downregulation of miR-145 contributes to lung adenocarcinoma cell growth to form brain metastases. Oncol Rep. 2013;30:2027–34. doi: 10.3892/or.2013.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]