Abstract
Background:
Physical exercise may be beneficial to breast cancer (BC) survivors. Here, we systematically summarized the effects of aerobic exercise in BC survivors. We conducted a systematic review of randomized controlled trials (RCTs).
Methods:
We searched PubMed, Web of knowledge, Scopus, Cochrane Central, Virtual Health Library and PEDRO databases for relevant RCTs, comparing aerobic exercise with usual care among BC survivors. Data were extracted and evidence was synthesized narratively.
Results:
Twelve studies were included in this systematic review. Studies reported that aerobic exercise can significantly improve the quality of life in BC survivors. Moreover, aerobic exercise alleviated the symptoms of depression and anxiety. However, current evidence from the included studies showed that there was no significant benefit for aerobic exercise in terms of weight loss. Conclusion: Our study suggests that aerobic exercise is beneficial to BC survivors.
Clinical Relevance:
Aerobic exercise should be recommended in the therapeutic and rehabilitative regimens of BC survivors.
Key Words: Aerobic exercise, breast cancer, physical activity, quality of life
Introduction
Breast cancer (BC) is the second most common cancer worldwide and the most frequent cancer among women (25% of the overall cancer among women) with an increasing incidence in the last decade. The World Health Organization (WHO) ranked BC as the fifth most common cause of cancer mortality worldwide (522,000 deaths in 2012) (Torre et al., 2015). The significant improvements in BC screening, diagnosis, and treatment over the past few decades led to a decline in BC-related death rate, which in turn caused a substantial increase in the number of BC survivors (Siegel et al., 2017).
Physical activity plays a significant role in improving quality of life (QoL), physical, psychological functions, and other health indicators in cancer patients (Fong et al., 2012). The literature showed several benefits for exercise therapy as a supportive care for BC patients and survivors (Brockow et al., 2016; Kim et al., 2009; McNeely et al., 2006a) These beneficial effects vary according to disease stage, type of primary treatment and the lifestyle of patients (Knols et al., 2005)
The survival outcome in BC survivors is likely to have some correlation with exercise; long-term physical activity levels are important for BC prognosis and are associated with improved survival (Bertram et al., 2011). Moreover, previous research showed that exercise improves the QoL and lessens the symptoms of depression and anxiety in BC survivors (Segar et al., 1998). Additionally, it was associated with benefits on muscle strength and body composition (Zhu et al., 2016). Therefore, the American Cancer Society guidelines recommend at least 150 minutes on a weekly basis for exercise practicing. These guidelines also encouraged long-term adherence to daily exercise beyond the initial post-surgical rehabilitation (Rock et al., 2012).
Aerobic exercise is a form of physical activity that depends primarily on the aerobic energy-generating process i.e. it requires free oxygen to meet the demands of aerobic metabolism. It involves running/jogging, swimming, cycling, and walking. Several randomized controlled trials (RCTs) suggested the efficacy of aerobic exercise in reducing cancer-related fatigue among BC survivors (Cantarero-Villanueva et al., 2013; Carter et al., 2016; Vardar Yağlı et al., 2015). Moreover, aerobic physical activity was found effective in improving anthropometric measures as body weight, body composition, and VO2 peak, as well as reducing inflammatory markers (Jones et al., 2013; Matthews et al., 2007; Tizdast et al.,2016)..
The benefits of exercise in general in BC survivors have been assessed in several systematic reviews and meta-analyses (Floyd and Moyer, 2010; Meneses-Echávez et al., 2015; Zhu et al., 2016). However, there is a gap in addressing the effects of specific exercise types in BC rehabilitation program as published trials showed conflicting results, especially in terms of physical activity and QoL outcomes. According to our knowledge, no former systematic review or meta-analysis has assessed the benefits of aerobic exercise specifically in BC survivors. The National Comprehensive Cancer Network (NCCN) reported the need for future research work to identify the effects of specific modes of exercise on specific outcomes as fatigue and QoL in cancer survivors (Schmitz et al., 2010).
We performed this systematic review of the literature to investigate the efficacy of aerobic exercise intervention on physical activity, QoL (primary outcomes), weight, inflammatory markers and sleep among BC survivors by scrutinizing the published trials.
Materials and Methods
We followed the PRISMA statement guidelines (Table S1 of Supplementary material) during the preparation of this review (Moher et al., 2009). This systematic review is registered on the PROSPERO international prospective register of systematic reviews (CRD42017060106).
Literature search strategy
We searched PubMed https://www.ncbi.nlm.nih.gov/pubmed/, Web of knowledge http.apps.webofknowledge.com, Scopus https-www-scopus.com, Cochrane Central https://www.cochranelibrary.com/central, Virtual Health Library (VHL) https://bvsalud.org/en/ and PEDRO https://www.pedro.org.au/ for RCTs that compared Aerobic Exercise with usual care in BC survivors. We used the following search strategy “(breast cancer survivors* OR breast tumor survivors*) AND (Aerobic exercise OR physical fitness OR physical therapy OR rehabilitation)”. No restrictions by publication period were employed.. In addition, we manually scanned the references list of selected articles for relevant studies.
Eligibility criteria and study selection
We included RCTs that met our inclusion criteria, a) Population: BC survivor women, treated with chemotherapy or radiotherapy (stopped at least six months earlier) or patients who have started adjuvant endocrine therapy (antiestrogens, aromatase inhibitors, LHRH agonists, or combinations) not less than 4 months earlier and not scheduled for and not currently undergoing chemo-/radiation therapy, b) Intervention: Studies in which the intervention was aerobic exercise (walking, cycle ergometers, swimming, and stair climbing), not mixed with any other type of exercise, c) Comparator: Studies where the control group received usual care, d) Outcomes: Physical Activity level, QoL, Sleep parameters, weight, as well as inflammatory markers.
We excluded studies with the following criteria: a) patients on active therapy, b) patients with uncontrolled cardiac or vascular disease, c) patients exercising at a regular basis at baseline, and d) non-English articles. The retrieved citations were added to a Microsoft Excel sheet that was distributed to reviewers. Eligibility screening was done through two separate steps: a) titles and abstracts screening, and b) full texts screening. Each study title and/or full-text was screened by two independent reviewers (therefore, each reviewer screened half the retrieved citations) and disagreements were resolved by discussion between all reviewers.
Data Extraction
Three independent reviewers extracted the relevant data from the included studies, using a preformatted data extraction sheet. Disagreements were resolved by the opinion of a fourth reviewer. The extracted data included: a) baseline characteristics of participants as age and cancer stage, b) general characteristics of included studies as design, sample size, and assessed outcomes, c) characteristics of the intervention as type, intensity, and duration and d) the results of each included study. The summary effect size was difficult to calculate due to the lack of sufficient data for subsequent pooling, as well as lack of the homogeneity needed to make a calculation.
Risk of bias assessment
The risk of bias in included RCTs was assessed according to the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (updated March 2011) using the risk of bias assessment table, provided in the same book (part 2, Chapter 8.5). The Cochrane risk of bias assessment tool includes the following domains: Random sequence generation (selection bias), allocation sequence concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other potential sources of bias. The reviewer judgment is categorized as low risk, high risk, or unclear risk of bias. For risk of bias exclusion across the included studies, we compared the reported outcomes between the studies to exclude reporting of selective outcome.
Results
Literature search results
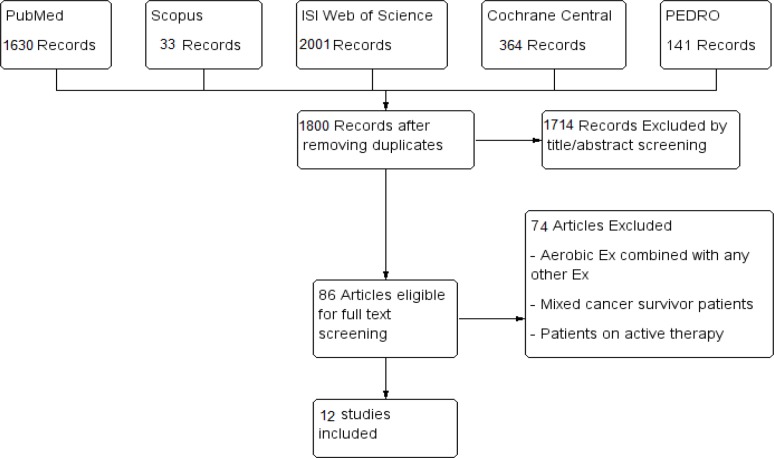
We retrieved 1,800 unique citations. After the initial title and abstract screening, 86 full-text articles were retrieved and screened for eligibility. After full text screening, 12 RCTs (n= 1,120 patients) were included in our systematic review (see PRISMA flow diagram; Figure 1).
Figure 1.
PRISMA Flow Diagram of Screening and Study Selection Process
Characteristic of included studies
The sample size of included studied ranged from 19 to 357 patients, with an average age of 44 to 61 years. Aerobic exercise was the only intervention of interest in this meta-analysis either supervised or home-based training programs. Most of the included studies reported walking as the preferred type of activity among BC survivors. Exercise sessions were of moderate intensity. Irwin et al., (2008), Matthews et al., (2007) and Saarto et al., (2012) assessed physical activity outcome, while Campbell et al., (2017), Murtezani et al., (2014) and Saarto et al., (2012) examined the effect of aerobic exercise on the QoL. Murtezani et al., (2014), Roveda et al., (2016) and Tizdast et al., (2016) investigated the role of aerobic exercise on weight reduction among BC survivors. Two studies assessed the effect of aerobic exercise on inflammatory markers (Fairey et al., 2005; Jones et al., 2013). Only one study reported the effect of aerobic exercise on sleep parameters (Roveda et al., 2016). A summary of the design and main findings of included studies is shown in Table 1, and the baseline characteristics of their participants are shown in Table 2.
Table 1.
Shows a Summary of the Design and Main Findings of Included Studies
| study ID | Population (cancer stage) |
Mean age (SD) Intervention/control |
sample size intervention/control groups |
duration of treatment | Type of Exercise Intervention/control |
intensity | frequency | Outcomes measured | Summary of results |
|---|---|---|---|---|---|---|---|---|---|
| Matthews 2007 | Stage I–III breast cancer | 51.3 (9.0) / 56.9 (12.3) | 22 / 14 | 12 weeks | Walking / Usual care | Moderate | *In the first 4 weeks, three times/week. *5–7 weeks, four times/week *The final 5 weeks, five times/week |
* Overall physical activity level measured by CHAMPS questionnaire (MET-h/week) and the actigraph. *Effect of intervention on physical activity behaviors and body composition measured by (body mass, Fat-free mass , fat mass, body fat). |
* Intervention proved effective in increasing physical activity levels among breast cancer survivors. *No significant changes in body fat and composition. |
| Campbell 2017 | stage I-IIIA breast cancer | 53.2 (7.0) / 51.4 (5.1) | 10/9 | 24 weeks | Walking / usual lifestyle | Moderate-to-vigorous | 150 min/week of aerobic exercise + two 45-minute supervised sessions per week in a research gym + two additional 30 minute unsupervised home sessions | *Self-reported cognitive dysfunction and associated impact on quality of life measured by (FACT-Cog). *Objective neuropsychological tests in cancer survivors based on TMT-A and TMT-B, Stroop test, fMRI. |
No statistically significant improvement in self-reported cognitive function and neuropsychological tests. |
| Roveda 2016 | NR | 55.2 (6.8) / 58.2 (6.4) | 19 / 21 | 3 months | Brisk walking . |
Moderate | 2 sessions of 1-hour brisk walking per week |
*Anthropometric and Body Composition Evaluation *Actigraphic Monitoring of Sleep Patterns *Actigraphic Monitoring of Activity Level and Circadian Rhythm *Armband-Based Monitoring of Energy Expenditure |
aerobic PA has a protective effect against factors promoting sleep disruption. PA improved sleep behavior and reduced sleep worsening of BCS. |
| Fairey 2005 | Stage I-IIIA breast cancer | 59 (5) / 58 (6) | 24 / 28 | 15 weeks | cycle ergometers / no activity | Moderate | 3 times per week | *Effect of PA on CRP * Effect of PA on cardiovascular risk factors (RHR, HRR, SBP, DBP, TC, LDL-C, HDL-C, TG, and TC:HDL-C ratio). |
*PA had borderline significant effect on CRP *PA also had a clinically and statistically significant effect on HRR and clinically but not statistically significant effects on RHR, SBP, DBP, HDL-C, and TG |
| Thomas 2013 | Stage 0–111A breast cancer | 56.5 (9.8) / 55.1 (7.6) | 35 / 30 | 6 months | Walking / usual care | Moderate intensity |
*Three 15-minute sessions (week1) * gradually built up to five 30-minute (Week 5) |
Effect of aerobic exercise on metabolic syndrome prevalence - Waist circumference (cm) - HDL-C (mg/dL) -Triglycerides (mg/dL) - Glucose (mg/dL) - Metabolic syndrome z-score |
*In overweight or obese, physically inactive BCS the adherence to a moderate intensity aerobic exercise was associated with improvements in metabolic syndrome criteria. |
| Murtezani 2014 | 53 ( 11) / 51( 11) | 30/32 | 10 week | Aerobic exercise(treadmills, stationary bicycles, and stairclimbing Machines) / usual care |
Moderate intensity |
three times per week for 10 weeks | *-Anthropometric Changes a) BMI b) % Total body fat *-Quality of life assessment by (FACTB) *12 min walk test. |
Results revealed that 10 week of moderateintensity aerobic exercise program significantly improves QOL and physical functioning in breast cancer survivors | |
| study ID | Population (cancer stage) |
Mean age (SD) Intervention/control |
sample size intervention/control groups |
duration of treatment | Type of Exercise Intervention/control |
intensity | frequency | Outcomes measured | Summary of results |
| Carter 2016 | DCIS or stage I-IIIA breast cancer | 55(8) for all participants | 76/76 | 3 months | treadmill walking/usual care | Vigrous-Moderate intensity |
-goal of meeting ≥150 min of moderate-intensityexercise -less than 30 min of vigorous or 60 min of self-reported moderate- intensity physical activity per week. |
-baseline and immediately postintervention (M3) on measures of physical activity (accelerometry), graded walk test, and average fatigue over the previous 7 days. -calculating Pressure product rate (PPR) |
Lower rate-pressure product during submaximal walking was significantly associated with reduced fatigue in BCS. for Cancer Survivors Exercise/physical activity training programs that lower the physiological strain during submaximal walking may produce the largest improvements in reported fatigue. |
| Saarto 2012 | Histologically proven invasive breast cancer T1-4,N0-3,M0 | Mean age (range) 52.3(36-68)/52.4(35-68) |
263/237 | 12 months | supervised and home training -supervised training: endurance training such as walking, Nordic walking or aerobic training, jumps and leaps. In addition, brisk endurance training (walking, Cycling, swimming etc.) |
progressive vigorous exercise training |
-supervised training: once a week 60-minute aerobics and circuit training |
Changes in quality of life (QoL) during the intervention measured by the * EORTC QLQ-C30 and *BR-23 *FACIT-F BDI questionnaires |
*The amount of physical activity increased from baseline to 12 months *Neuromuscular performance improved significantly in the training group *No significant effect of the intervention was observed on cardiorespiratory fitness. *No significant difference was found between the exercise and control groups in changes of QoL during the intervention |
| Segar 1998 | Any type of breast cancer surgery | 47.5(7.1)/61.8(8.1) | 16/8 | 10 weeks | Exercise group / Exercise plus Behavior / control group ((study participant choose her favorite and most conveient type of aerobic exercise)) |
=< 60 of age predicted maximum heart rate | a minimum 30 minutes/seesion, 4 days/week |
*Exercise adherence *Depression (Beck depresion Inventory) *Anxiety(spellberger stat trait Anxiety inventory) *Rosenberg selfesteem inventory |
*women who exercised had signficantly less depression and state and anxiety over time compared to control *after crossover,the control group demonstrated comperable improvement in both depressive and state anxiety scores, self esteem didn`t changed significantly *subjects wh recieved exercise recommendation from their physcian exercised significantly more than subjects who recieved no recommendation |
| Jones 2012 | stage0 to IIIA | 56.4 (9.6)/ 55.4(7.6) | 36/32 | 6 months | Aerobic exercise/ usual care | moderate- intensity aerobic exercise | 150 minutes over 3 weeks | 1-Arthopometric Changes c) BMI d) % Total body fat 2- Changes in inflammatory markers Levels. |
Results revealed that the moderate-intensity aerobic exercise intervention did not significantly alter concentrations of IL-6, CRP, or TNF-a. |
| study ID | Population (cancer stage) |
Mean age (SD) Intervention/control |
sample size intervention/control groups |
duration of treatment | Type of Exercise Intervention/control |
intensity | frequency | Outcomes measured | Summary of results |
| Tizdast 2016 | stage I-III breast cancer The Yale Exercise and Survivorship Study. |
44.1(4.6) for all participants | continous exercise grpup(n=9), interval exercise group (n=9), control group(n=9) | 8 weeks | Continuous exercise group/ Interval exercise group/ control group (planned treadmill exercise)) |
varied according to trainng schedule | three times per week | *Body weight, *body mass index, *adipose tissue, body fat percentage *Waist to Hip Ratio(WHR) |
*The highest reduction in weight, body fat percentage and adipose tissue and the highest increase in VO2 peak occurred in the interval exercise group. *The waist-hip ratio reduced equally in both exercise groups *The anthropometric variables, body fat percentage and adipose tissue decreased significantly in both exercise groups but the differencebetween the two exercise groups was not significant |
| Irwin 2008 | stage 0 (in situ) to stage IIIA breast cancer | 56.5 ( 9.5) /55.1 ( 7.7 ) | 37/38 | 6 months | Moderate To vigorous intensity |
150 min/wk. of supervised gym-based and home-based aerobic exercise. 44 min/wk. among usual-care to maintain current physical activity level. |
1-Arthopometric Changes a) BMI b) % Total body fat 2- Satisfaction and competence questionnaire. |
Women in the exercise-intervention group increased their average pedometer steps by 1621 steps per day compared with a decrease of 60 steps per day among women in the usual-care group (P <.01). | |
| Irwin 2009 (1) | stage 0 to IIIA breast cancer | 56.4 ( 9.5) /55.6 (7.7) | 37/38 | 6 months | moderate-intensity aerobic exercise | 150 minutes per week 30 minutes on 5 days |
1-Arthopometric Changes c) BMI d) % Total body fat 2- Changes in insulin, IGF-1, IGFBP3 Levels. |
Moderate-intensity aerobic exercise, per week, used in the study is proved to be tolerated in breast cancer survivors and efficacious in decreasing levels of IGF-I and IGFBP-3. | |
| Irwin 2009 (2) | Stage 0–IIIA | 56.5 (9.5)/ 55.1( 7.7) | 37/38 | 6 months | Moderate intensity aerobic exercise |
150 min/week . |
1-Arthopometric Changes g) BMI h) % Total body fat 2-Changes in inflammatory markers Levels. (BMD) |
Aerobic exercise, such as brisk walking, was associated with favorable changes in body fat and LBM, and maintenance of BMD in postmenopausal breast cancer survivors |
|
| Latka 2009 | stage 0 to stage IIIA breast cancer | 56.5(9.5)/NR | 37/38 | 6 months | Moderate | exercise goal was 30 min of exercise 5 days/week for 6 months. *(Participants wereasked to perform three 15-minsessions during Week1, building to five 30min-sessions by Week5) |
*BMI *waist circumference *FACT-B Breast Cancer subscale *Transtheoretical Model Stage |
*Women with higherBMI, larger waist circumference, fewer minutes of physical activity at baseline associated with breast cancer as measured by the FACT-B Breast Cancer subscale score had a lower adherence to the exercise prescription. *Specifically, a lower BMI and a higher degree of readiness to change physical activity behavior were associated with better adherence. |
Table 2.
Shows Baseline-Characteristics of Included Studies Participants
| study | Study arm | Sample Size Experimental/ control |
Age mean (SD) |
Disease stage (%) In situ Stage I Stage II Stage IIIA |
BMI mean (SD) |
Weight (kg) mean (SD) |
Menopausal status *Pre-menopausal *Post-menopausal |
Time since diagnosis | Ethnicity (%) | Pedometer average, steps/d | Physical activity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Matthews 2007 | Experimental | 22 | 51.3 (9) | Stage I (59%) Stage II /III (18%) Not available (23%) |
28.3 (4.9) | 74.9(14.5) | Post-menopausal | 0.9 (0.7 – 1) |
White 82% African American/other 18% |
7,409.4 (2,791.1) | *By CHAMPS unit of MET-h/week: Total activity 32.7 (24.0) |
| control | 14 | 56.9 (12.3) | Stage I (64%) Stage II/III (21%) Not available (14%) |
29.9 (7.1) | 78.9(20.3) | Post-menopausal | 0.7 (0.6 – 1) |
White86% African American/other 14% |
5,939.00 -2,203.50 |
*By CHAMPS unit of MET-h/week: Total activity 22.2 (20.7) |
|
| Campbell 2017 | Experimental | 10 | 53.2 (7) | Stage I: 0 Stage II: 10(100) Stage III:0 |
26.3 (5.7) | 71.1 (15.9) | Post-menopausal | NR | NR | NR | (MET-hr./wk.) 14.1 (12.0) |
| control | 9 | 51.4 (5.1) | Stage I: 0 Stage II: 7(78) Stage III: 2(22) |
26.1 (5.5) | 66.6 (16.7) | Post-menopausal | NR | NR | NR | (MET-hr./wk.) 11.2 (7.0) |
|
| Roveda 2016 | Experimental | 19 | 55.2 (6.8) | NR | 25.4 (3.8) | 67.1 (11.9) | Pre & post-menopausal | NR | NR | 9790.9 ± 2817.2 | physical activity level 1.49 ( 0.10) |
| control | 21 | 58.2 (6.4) | NR | 24.6 (3.4) | 64.5 (9.6) | Pre & post-menopausal | NR | NR | 11001.6 ± 3702.2 | physical activity level 1.56 ( 0.14) |
|
| Fairey 2005 | Experimental | 24 | 59 (5) | Stage I (42%) Stage IIa (25%) Stage IIb (25%) Stage IIIa (8%) |
29.4 (7.4) | 78.1 (20.4) | Post-menopausal | NR | NR | NR | fitness described in baseline values for peak oxygen consumption (ml/kg/min 18.6 (3.9) |
| control | 28 | 58 (6) | Stage I (39%) Stage IIa (39%) Stage IIb (18%) Stage IIIa -4% |
29.1 (6.1) | 79.4 (16.4) | Post-menopausal | NR | NR | NR | cardiopulmonary fitness described in baseline values for peak oxygen consumption (ml/kg/min) 18.8 (3.8) |
|
| Thomas 2013 | Experimental | 35 | 56.5 (9.8) | In situ (11%) Stage I (54%) Stage II (26%) Stage IIIA -9% |
30.8 (5.9) | 82.1 (16.5) | Post-menopausal | 3.6 (2.2) | White 83% African American 17% Asian/Pacific Islander 0% |
NR | Physical activity questionnaire 13.0 (24.0) |
| control | 30 | 55.1 (7.6) | In situ (10%) Stage I (27%) Stage II (47%) Stage IIIA (17%) |
29.4 (7.4) | 77.2 (20.4) | Post-menopausal | 3.3 (2.6) | White 90% African American 7% Asian/Pacific Islander 3% |
NR | Physical activity questionnaire 12.0 (20.0) |
|
| Study arm | Sample Size Experimental/ control |
Age mean (SD) |
Disease stage (%) In situ Stage I Stage II Stage IIIA |
BMI mean (SD) |
Weight (kg) mean (SD) |
Menopausal status *Pre-menopausal *Post-menopausal |
Time since diagnosis | Ethnicity (%) | Pedometer average, steps/d | Physical activity | |
| Experimental | 37 | 56.5 (9.5) | In situ -11% Stage I -54% Stage II -27% Stage IIIA -8% |
30.4 (6.0) | NR | Post-menopausal | 3.6 (2.2) | Non-Hispanic white 84% African American 16% Asian/Pacific Islander 0% |
5145 (2,312) | -Physical Activity Questionnaire 13.0 (24.0) -Daily Activity Log, min/wk. recreational exercise 30.0 (41.1) |
|
| control | 38 | 55.1 (7.7) | In situ -11% Stage I -27% Stage II -46% Stage IIIA -16% |
30.1 (7.4) | NR | Post-menopausal | 3.3 (2.6) | Non-Hispanic white 84% African American 11% Asian/Pacific Islander 3% |
5342 (2,744) | -Physical Activity Questionnaire 12.0 (20.0) -Daily Activity Log, min/wk. recreational exercise 11.3 (24.8) |
|
| Experimental | 37 | 56.5 (9.5) | In situ -11% Stage I -54% Stage II -27% Stage IIIA -8% |
30.57 (5.95) | 81.28 (16.98) | Post-menopausal | 3.6 (2.2) | Non-Hispanic white 84% African American 16% Asian/Pacific Islander 0% |
5.145 (2.312) | -Physical Activity Questionnaire 13.0 (24.0) Daily activity log (min/week- recreational exercise) 30.0 (41.1) |
|
| control | 38 | 55.1 (7.7) | In situ -11% Stage I -27% Stage II -46% Stage IIIA -16% |
29.74 (7.27) | 78.4 (20.01) | Post-menopausal | 3.3 (2.6) | Non-Hispanic white 84% African American 11% Asian/Pacific Islander 3% |
5.342 (2.744) | -Physical Activity Questionnaire 12.0 (20.0) -Daily activity log (min/wk. recreational exercise) 30.0 (41.1) |
|
| Experimental | 36 | 56.4 (9.5) | In situ -11% Stage I -56% Stage II -25% Stage IIIA -8% |
30.4 (6.0) | 81.0 (16.8) | Post-menopausal | 3.6 (2.2) | Non-Hispanic white 83% African American 17% Asian/Pacific Islander 0% |
5,083 (2,312) | -Physical Activity Questionnaire 13.0 (24.0) -Daily activity log (min/wk. recreational exercise) 30.0 (41.1) |
|
| control | 32 | 55.6 (7.7) | In situ -13% Stage I -25% Stage II -44% Stage IIIA -19% |
30.1 (7.4) | 79.3 (21.3) | Post-menopausal | 3.3 (2.6) | Non-Hispanic white 90% African American 7% Asian/Pacific Islander 3% |
5,624 (2,744) | -Physical Activity questionnaire 12.0 (20.0) - Daily activity log (min/wk. recreational exercise) 11.3 (24.8) |
|
| Experimental | 36 | 56.4 (9.6) | In situ -13% Stage I -25% Stage II -44% Stage IIIA -19% |
30.6 (6.0) | 81.3 (17.0) | Post-menopausal | 3.5 (2.1%) | Non-Hispanic white 83% African American 17% Asian/Pacific Islander 0% Unknown 0% |
5083 (2,313) | -Physical activity questionnaire 30.3 (41.4) |
|
| control | 31 | 55.4 (7.6) | In situ -11% Stage I -56% Stage II -25% Stage III -8% |
29.4 (7.3) | 77.3 (20.0) | Post-menopausal | 3.1 (2.4%) | Non-Hispanic white 87% African American 6% Asian/Pacific Islander 3% Unknown 3% |
5661 (2,740) | -Physical activity questionnaire 11.9 (31.3) |
|
| Study arm | Sample Size Experimental/ control |
Age mean (SD) |
Disease stage (%) In situ Stage I Stage II Stage IIIA |
BMI mean (SD) |
Weight (kg) mean (SD) |
Menopausal status *Pre-menopausal *Post-menopausal |
Time since diagnosis | Ethnicity (%) | Pedometer average, steps/d | Physical activity | |
| Experimental | 30 | 53 (11) | Stage I (33%) Stage IIa (37%) Stage IIb (20%) Stage IIIa -10% |
25.9 (2.8) | 74.0 (9.4) | NR | NR | NR | NR | physical performance assessment by 12min Walk Test (12MWT) 799.6 (81) |
|
| Control | 32 | 51 (11) | Stage I (47%) Stage IIa (25%) Stage IIb (22%) Stage IIIa -6% |
26.0 (3.3) | 73.0 (10.5) | NR | NR | NR | NR | -physical performance assessment by 12min Walk Test (12MWT) 814.4 (88.5) |
|
| Experimental | 37 | 56.5 (9.5) | In Situ 11% Stage I 54% Stage II 27% Stage IIIA 8% |
30.4 (6.0) | NR | Post-menopausal | 3.6 (2.2) | Non-Hispanic white 84% African American 16% Asian/Pacific Islander 0% |
5.145 (2.312) | Physical Activity Questionnaire- 13.0 (24.0) -Daily Activity Log2 30.0 (41.1) |
|
| control | 38 | 55.1 (7.7) | In situ 11% Stage I 27% Stage II 46% Stage IIIA 16% |
30.1 (7.4) | NR | Post-menopausal | 3.3 (2.6) | Non-Hispanic white 84% African American 11% Asian/Pacific Islander 3% |
5.342 (2,744) | -Physical Activity Questionnaire 12.0 (20.0) |
|
| Experimental | 76 | 55 (8) | NR | 29.8 (6.2) | 79.5 (17.1) | Pre & post-menopausal | 54.2 (54.6) | NR | NR | -Physical activity measured by accelerometer (GT3X, Actigraph, Pensacola, FL) |
|
| control | 76 | 55 (8) | NR | 29.8 (6.2) | 79.5 (17.1) | Pre & post-menopausal | 54.2 (54.6) | NR | NR | -Physical activity measured by accelerometer (GT3X, Actigraph, Pensacola, FL) |
|
| Experimental | 263 | MEAN (range) 52.3 (36-68) |
NR | NR | NR | Pre & post-menopausal | NR | NR | NR | *Cardiorespiratory fitness was tested by a 2-km walk test 18.6 (2.0) *Mean self-reported physical activity, MET-hr./wk. 27.40 (16.54) |
|
| control | 237 | MEAN (range) 52.4 (35-68) |
NR | NR | NR | Pre & post-menopausal | NR | NR | NR | *Cardiorespiratory fitness was tested by a 2-km walk test 18.4 (1.8) *Mean self-reported physical activity, MET-h/wk. 26.37 (15.69) |
|
| Study arm | Sample Size Experimental/ control |
Age mean (SD) |
Disease stage (%) In situ Stage I Stage II Stage IIIA |
BMI mean (SD) |
Weight (kg) mean (SD) |
Menopausal status *Pre-menopausal *Post-menopausal |
Time since diagnosis | Ethnicity (%) | Pedometer average, steps/d | Physical activity | |
| Experimental | 16 | 47.5 (7.1) | NR | NR | NR | NR | NR | white 70% Black 12% Asian 12% Native American 6% |
NR | NR | |
| control | 8 | 61.8 (8.1) | NR | NR | NR | NR | NR | white 88% Black 12% Asian 0% Native American 00% |
NR | NR | |
| Continuous | 9 | 44.1 (4.6) | NR | 30 (3.4) | 75.4 (10.9) | Post-menopausal | NR | NR | NR | NR | |
| Interval | 8 | 44.1 (4.6) | NR | 29.9 (2.8) | 75.4 (6.6) | Post-menopausal | NR | NR | NR | NR | |
| control | 6 | 44.1 (4.6) | NR | 30.9 (4.07) | 81.7 (12.3) | Post-menopausal | NR | NR | NR | NR |
Risk of Bias assessment results
We used the Cochrane risk of bias assessment tool to assess the bias of included studies. All included studies were of low risk in terms of selective reporting and attrition bias. Only five studies were of low risk of bias in terms of allocation concealment (Carter et al., 2016; Irwin et al., 2008; Murtezani et al., 2014; Roveda et al., 2016; Saarto et al., 2012), while three studies were of low risk of bias in terms of blinding the participants/personnel (Campbell et al., 2017; Fairey et al., 2005; Jones et al., 2013). The summary of risk of bias assessment is shown in Figure 2, and the reasons for authors’ judgments are shown in Table S2 of Supplementary material.
Figure 2.
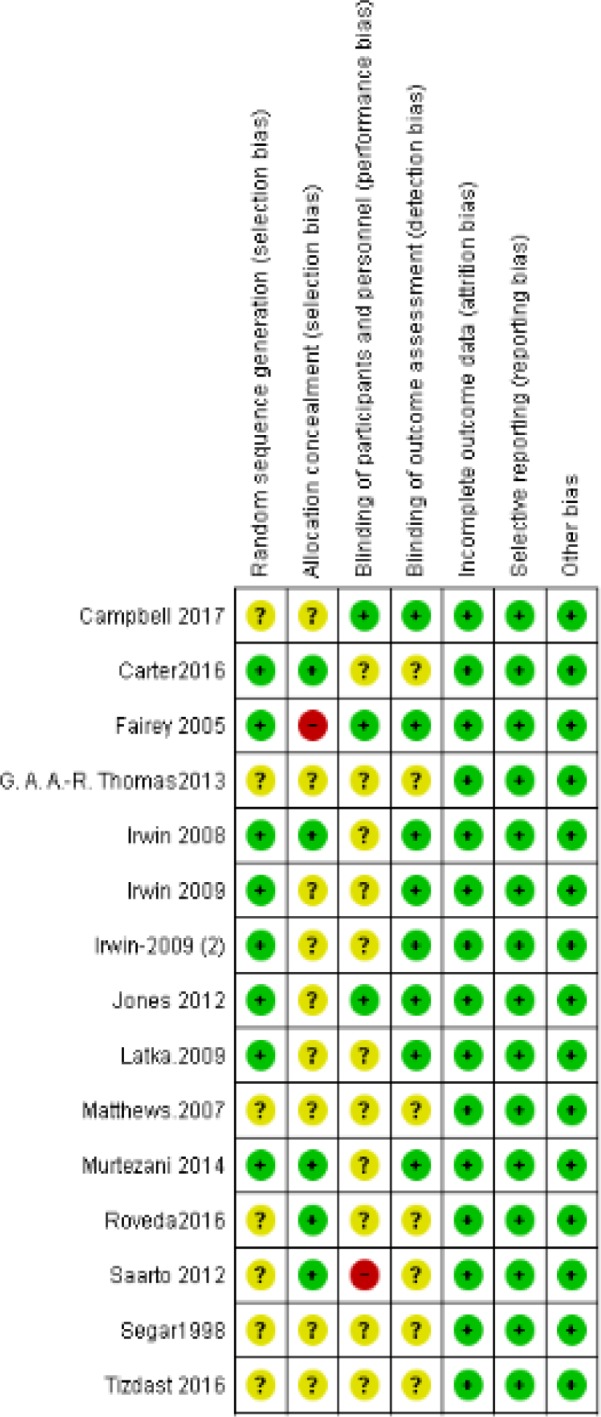
Risk of Bias Summary for Included Studies
Physical Activity
Mathews et al., (2007) reported higher activity levels in the intervention group compared to usual care group (p ≤0.04). Moreover, Irwin et al., (2008) reported that in the 7-day physical activity log, the exercise group increased from moderate to vigorous intensity reactional activity (129 min per week, p <0.001), compared to a smaller increase in the usual care group (45 min per week). However, Saarto et al., (2012) reported an insignificant difference between the exercise and control groups (P=0.97). They detected a significant linear trend between higher physical activity, improved QoL, and recovery from fatigue.
Quality of life
Saarto et al., (2012) measured the QoL using the EORTC-QIQ-C30 or BR-23 forms. They reported no significant difference after 12 months in QoL between exercise and control groups. Campbell et al., (2017) used the FACT-COG tool to measure the cognitive symptoms impacting the QoL, but they found no statistically significant improvement. Murtezani et al., (2014) used the FACT B score to measure the overall QoL after 10 weeks of intervention. They reported that the FACT B score was increased in the intervention group and was decreased in controls (P = 0.003).
Weight
Tizdast et al., (2016) reported that higher weight reduction was achieved in the interval exercise group (P=0.012). Murtezani et al., (2014) and Fairy et al., (2005) did not observe any significant difference between the exercise and control groups in terms of weight loss (95% CI,-4.8 to 2.25 Kg; P=0.47 and 95% CI,-1.6 to 0.6 Kg; P=0.39, respectively). On the contrary, Roveda et al., (2016) found that the control group had a significant weight reduction after 3 months of physical activity (P= 0.01).
Inflammatory markers
Two studies examined the effects of aerobic exercise on inflammatory makers in BC survivors. Fairy et al., (2005) reported that C-reactive protein (CRP) decreased by 139 mg/l in the exercise group, while it increased by 0.1 mg/l in the control group. However, this change was not significant (95% CI,-3.09 to 0.1 mg/l; P=0.66) Moreover, Jones et al. (2013) reported an insignificant difference between randomization groups regarding serum concentrations of interleukin (IL)-6, CRP, and tumor necrosis factor (TNF-α).
Sleep parameters
Only one study reported the effects of exercise on sleep parameters for BC survivors. Roveda et al., (2016) found sleep efficiency (SE) to be significantly decreased in the control group, it but remained unchanged in the exercise group (P=0.001). The mean activity score (MAS) was significantly increased in the control group, but it remained unchanged in the exercise group (p <0.001). The authors reported a significant decrease (P=0.03) in the actual sleep time (AST) and a significant increase (P=0.02) in the movement fragmentation index (MFI) after aerobic exercise intervention, compared to baseline scores.
Discussion
This systematic review summarized the evidence on the effects of aerobic exercise interventions (especially walking as the most frequently investigated approach) in BC survivor women. The results showed that aerobic exercise was superior to usual care in enhancing the physical activity, QoL, and sleep parameters. However, weight and inflammatory markers did not show any remarkable difference between the two groups.
Moderate intensity aerobic exercise is associated with increased short-term physical activity levels among BC survivors, especially in patients who did not follow regular exercise before (Irwin et al., 2008; Matthews et al., 2007). However, Saarto et al., (2012) reported no significant difference between the exercise and control groups. This is explained by the considerable motivation and healthy lifestyle in the control group patients, which exerted a ceiling effect on the ability to detect the exercise benefits.
The observed increase in physical activity levels significally correlates with QoL improvement. The results of Murtezani et al., (2014) confirmed the causative relationship between moderate intensity aerobic exercise and QoL improvement. These results are supported by previous studies on the efficacy of aerobic exercise in improving the overall QoL (Burnham., 2002; McNeely et al., 2006). This improvement may be due to reducing anxiety and increasing energy in addition to counteracting some negative effects of cancer treatment (Jones et al., 2013; Meneses-Echávez et al., 2015; Murtezani et al., 2014).
Campbell et al., (2017) found that aerobic exercise ameliorated the resulting cognitive impairment from the psychological burden and cancer therapies. The results of this study come in accordance with a previous meta-analysis of 33 RCTs, which investigated the effects of exercise in general regarding the psychological outcomes, body composition and physical fuctions (Zhu et al., 2016). It concluded that exercise was beneficial for BC survivors because it improved the QoL, body configuration, and muscle strength, decreased serum levels of insulin, IGF-II, and IGFBP-1, and alleviated depression and anxiety.
Roveda et al., (2016) examined the effects of exercise on the sleep pattern. They reported significant improvement of sleep disturbance among BC survivors. Another study conducted by Thomas et al., (2013) revealed positive effects for aerobic exercise on reversing the metabolic syndrome in sedentry BC survivors. Another study examined the effect of aerobic exercise in untrained women in a meta-analysis framework. It included different age groups and exercise intensities and emphasized the golden role of early exercise intervention in improving the cardiovascular outcomes in sedentary women (Zhang et al., 2017).
Strengths of the study
We followed the PRISMA statement during performance and reporting of this review and conducted all steps in accordance to the cochrane handbook for systematic reviews of interventions. Reasons for patient discontinuation were appropriately addressed. Moreover, study investigators used as intention to treat approach so there would not be attrition bias. Studies included in this systematic review were low to moderate risk of bais according to Cochrane ROB assessment tool.
Limitations of the study
We selectively chose studies that investigated the benefits of aerobic exercise in BC survivors. We found scarce literature covering this pattern of exercise with diversity in the measured outcomes, intervention (type, intensity, and duration), and the used assessment methods. Therefor, the extracted data were not sufficient for subsequent pooled analysis. Future studies are recommended to study the effects of aerobic exercise in BC survivors for longer durations with including much important outcomes, such as inflammatory markers, sleep pattern, and other cancer contol measures.
Clinical Implications
The review findings supports that aerobic exercise intervention is beneficial to BC survivor women. It improves fatigue associated with BC by improving levels of physical activity, as well as QoL. It also enhances the stability of sleep among BC survivors (Matthews et al., 2007; Murtezani et al., 2014; Roveda et al., 2016).
Key Practice Points
* Aerobic exercise should be recommended in the rehbailitation regimen of BC survivors.
* The intensity and duration of exercise should be personalized to each patient and further research is needed in this regard.
In conclusion, despite the aforementioned limitations, there is evidence that aerobic exercise (mainly in the form of walking) was associated with beneficial outcomes in BC survivor women; It improved the Qol and alleviated the symptoms of depression and anxiety. Moreover, it enhanced the physical functioning and sleep pattern.
Abbreviations
BC: Breast Cancer, CRP: C - reactive protein, QoL: Quality of Life.
References
- Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Med Sci Sports Exerc. 2002;34:1863–7. doi: 10.1097/00005768-200212000-00001. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Kam JWY, Neil-Sztramko SE, et al. Effect of aerobic exercise on cancer-associated cognitive impairment: A proof-of-concept RCT. Psychooncology. 2017 doi: 10.1002/pon.4370. https://doi.org/10.1002/pon.4370. [DOI] [PubMed] [Google Scholar]
- Cantarero-Villanueva I, Fernandez-Lao C, Cuesta-Vargas AI, et al. The effectiveness of a deep water aquatic exercise program in cancer-related fatigue in breast cancer survivors: A randomized controlled trial. Arch Phys Med Rehab. 2013;94:221–30. doi: 10.1016/j.apmr.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Carter SJ, Hunter GR, McAuley E, et al. Lower rate-pressure product during submaximal walking: a link to fatigue improvement following a physical activity intervention among breast cancer survivors. J Cancer Surviv. 2016;10:927–34. doi: 10.1007/s11764-016-0539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JM, Loeb SJ, Smith CA, et al. The primary care nurse practitioner and cancer survivorship care. J Am Acad Nurse Pract. 2010;22:394–402. doi: 10.1111/j.1745-7599.2010.00528.x. [DOI] [PubMed] [Google Scholar]
- Dieli-Conwright CM, Lee K, Kiwata JL, et al. Reducing the risk of breast cancer Recurrence: an Evaluation of the Effects and Mechanisms of diet and exercise. Curr Breast Cancer Rep. 2016;8:139–50. doi: 10.1007/s12609-016-0218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairey AS, Courneya KS, Field CJ, et al. Effect of exercise training on C-reactive protein in postmenopausal breast cancer survivors: A randomized controlled trial. Brain Behav Immun. 2005;19:381–8. doi: 10.1016/j.bbi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Floyd A, Moyer A. Group versus individual exercise interventions for women with breast cancer: A meta-analysis. Health Psychol Rev. 2010;4:22–41. doi: 10.1080/17437190903384291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong DY, Ho JW, Hui BP, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:70. doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Alston J. Exercise and the breast cancer survivor: The role of the nurse practitioner. Clin J Oncol Nurs. 2015;19:98–102. doi: 10.1188/15.CJON.E98-E102. [DOI] [PubMed] [Google Scholar]
- Irwin ML, Cadmus L, Alvarez-Reeves M, et al. Recruiting and retaining breast cancer survivors into a randomized controlled exercise trial: The Yale Exercise and Survivorship Study. Cancer. 2008;112:2593–2606. doi: 10.1002/cncr.23446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LW, Alfano CM. Exercise-oncology research: Past, present, and future. Acta Oncol. 2013;52:195–215. doi: 10.3109/0284186X.2012.742564. [DOI] [PubMed] [Google Scholar]
- Jones SB, Thomas GA, Hesselsweet SD, et al. Effect of exercise on markers of inflammation in breast cancer survivors: The yale exercise and survivorship study. Cancer Prev Res. 2013;6:109–118. doi: 10.1158/1940-6207.CAPR-12-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CJ, Kang DH, Park JW. A meta-analysis of aerobic exercise interventions for women with breast cancer. West J Nurs Res. 2009;31:437–61. doi: 10.1177/0193945908328473. [DOI] [PubMed] [Google Scholar]
- Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: A systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005;23:3830–42. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- Ligibel J. Lifestyle factors in cancer survivorship. J Clin Oncol. 2012;30:3697–704. doi: 10.1200/JCO.2012.42.0638. [DOI] [PubMed] [Google Scholar]
- Matthews CE, Wilcox S, Hanby CL, et al. Evaluation of a 12-week home-based walking intervention for breast cancer survivors. Support Care Cancer. 2007;15:203–11. doi: 10.1007/s00520-006-0122-x. [DOI] [PubMed] [Google Scholar]
- McNeely ML, Campbell KL, Rowe BH, et al. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006a;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely ML, Campbell KL, Rowe BH, et al. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006b;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses-Echávez JF, González-Jiménez E, Ramírez-Vélez R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: A systematic review and meta-analysis. BMC Cancer. 2015;15:1–13. doi: 10.1186/s12885-015-1069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009 https://doi.org/10.1371/journal.pmed.1000097. [PMC free article] [PubMed] [Google Scholar]
- Murtezani A, Ibraimi Z, Bakalli A, et al. The effect of aerobic exercise on quality of life among breast cancer survivors: A randomized controlled trial. J Cancer Res Ther. 2014;10:658–64. doi: 10.4103/0973-1482.137985. [DOI] [PubMed] [Google Scholar]
- Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243–74. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- Roveda E, Vitale JA, Bruno E, et al. Protective effect of Aerobic physical activity on sleep behavior in breast cancer survivors. Integr Cancer Ther. 2016:1534735416651719. doi: 10.1177/1534735416651719. https://doi.org/10.1177/1534735416651719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarto T, Penttinen HM, Sievänen H, et al. Effectiveness of a 12-month exercise program on physical performance and quality of life of breast cancer survivors. Anticancer Res. 2012;32:3875–84. [PubMed] [Google Scholar]
- Schmitz KH, Courneya KS, Matthews C, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–26. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- Segar ML, Katch VL, Roth RS, et al. The effect of aerobic exercise on self-esteem and depressive and anxiety symptoms among breast cancer survivors. Oncol Nurs Forum. 1998;25:107. [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- Thomas GA, Alvarez-Reeves M, Lu L, Yu H, Irwin ML. Effect of exercise on metabolic syndrome variables in breast cancer survivors. Int J Endocrinol. 2013 doi: 10.1155/2013/168797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizdast N, Ghazalian F, Gholami M. The effect of exercise type on inflammatory markers in obese survivors with breast cancer: Randomized Control Trial. Health Scope. 2016;5 https://doi.org/10.17795/jhealthscope-33421. [Google Scholar]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Vardar Yağlı N, Şener G, Arıkan H, et al. Do yoga and aerobic exercise training have impact on functional capacity, fatigue, peripheral muscle strength, and quality of life in breast cancer survivors? Integr Cancer Ther. 2015;14:125–32. doi: 10.1177/1534735414565699. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu L, Zhang X, et al. Effects of different durations of aerobic exercise intervention on the cardiovascular health in untrained women: a meta-analysis and meta-regression. J Sport Med Phys Fit. 2017 doi: 10.23736/S0022-4707.17.07029-3. https://doi.org/10.23736/S0022-4707.17.07029-3. [DOI] [PubMed] [Google Scholar]
- Zhu G, Zhang X, Wang Y, et al. Effects of exercise intervention in breast cancer survivors: A meta-analysis of 33 randomized controlled trails. Onco Targets Ther. 2016;9:2153–68. doi: 10.2147/OTT.S97864. [DOI] [PMC free article] [PubMed] [Google Scholar]