Abstract
Probiotic bacteria are known for their beneficial effects on the intestinal immune function of the host animal. However, their effects on mucosal barrier function in chicks are not completely understood. The aim of this study was to determine the effects of the probiotic bacterium, Lactobacillus reuteri (LR), on the gastrointestinal mucosal barrier function of broiler chicks. One day-old male broiler chicks were orally injected water (300 µL) with or without 1 × 108 cfu of LR (5 mg FINELACT, Asahi Calpis Wellness Co. Ltd.) every morning for 7 days (day 0 to 6). The crop, duodenum, ileum, and cecum were collected on day 7 and were used for histological analysis and RNA extraction. Then, the thickness of the mucosal structures and the number of goblet cells in the digestive tract were assessed using histological analysis. The expression of Mucin 2, factors related to the formation of tight junctions (Claudin1, 5, and 16, ZO2, and JAM2), cytokines (IL-6, CXCLi2, and IL-10), and avian β-defensin 10 (AvBDs) (AvBD2, 10, and 12) in the crop, duodenum, ileum, and cecum were analyzed using real-time polymerase chain reaction (PCR). Results showed that oral administration of LR increased ileal villus height and crypt depth, decreased Claudin16 level in the crop and increased JAM2 level in the crop and ileum, and decreased the expression of AvBD10 in the ileum and cecum and that of AvBD12 in the crop. It did not affect goblet cell number and Mucin 2 expression. These results suggested that LR used in this study may enhance mucosal barrier function by regulating tight junctions in the upper gastrointestinal tract.
Keywords: AvBD, broiler chick, cytokine, digestive tract, probiotics, tight junction
Introduction
The digestive tracts of neonatal chicks are susceptible to infection by pathogenic bacteria via contaminated feed and water, which decreases their growth, and consumption of infected poultry products may cause food-borne human diseases. The intestinal mucosal barrier is composed of various components, such as the mucus layer covering the gastric mucosa, tight junction between gastrointestinal epithelial cells, and innate immune factors, including macrophages, cytokines, and anti-microbial peptides, and adaptive immune factors, including T and B cells and secreted IgA (Awad et al., 2017; Pawlowska and Sobieszczanska, 2017). These mucosal barriers play important roles in protecting the organs against pathogenic invasion and regulating epithelial permeability (Awad et al., 2017; Pawlowska and Sobieszczanska, 2017). However, the lymphoid system, including T cells and B cells of young chicks, is not functionally developed until approximately 2 weeks after hatching (Bar-Shira et al., 2003), although the physical barrier and innate immune system are naturally well-developed in neonatal chicks (Ozden et al., 2010; Terada et al., 2018). Therefore, the mucosal barrier function of especially the mucus, tight junctions, and innate immune system might be important for protection from pathogenic infection during the early phase of development in chicks.
The mucus layer consists of mucin secreted by goblet cells, which is one of the cells on the epithelial surface of the digestive tract (Smirnov et al., 2005; Tsirtsikos et al., 2012). Tight junctions consist of multi-protein complexes, which form not only the paracellular barrier against invading bacteria, but also act as pores that mediate ion permeability (Awad et al., 2017). Claudins, junctional adhesion molecule (JAM), and zona occludens (ZO) are the main proteins that form the tight junction (Gunzel and Yu, 2013; Awad et al., 2017; Guo et al., 2018). Interleukin (IL-) 6 is a proinflammatory cytokine that is produced by macrophages and other cells (Gabay, 2006). CXCLi2 (known as IL-8) is a chemokine, which attracts immunocompetent cells such as macrophages and monocytes (Sick et al., 2000; Poh et al., 2008). IL-10 is an anti-inflammatory cytokine that plays a crucial role in regulating inflammation (Minciullo et al., 2016). Till date, 14 avian β-defensins (AvBD1 to AvBD14), members of a family of anti-microbial peptides, have been identified in chicken (Lynn et al., 2007). According to previous studies, these AvBDs can kill a broad range of microbes such as pathogenic bacteria, fungi, and virus (van Dijk et al., 2008; Cuperus et al., 2013). In our previous studies, several anti-microbial peptides, including AvBDs and cathelicidins, were identified in the mucosal tissue of male and female reproductive organs and in the intestinal mucosa (Mohammed et al., 2015; Yoshimura, 2015; Mohammed et al., 2016). Terada et al. (2018) reported that AvBD expression and AvBD2 localization in the intestine changed dynamically before and after hatching, suggesting that AvBDs are important for host defense in the intestinal mucosa of embryos and neonatal chicks.
Probiotics are live microorganisms that exert health benefits in host animals. In particular, probiotics are recently being used as a replacement ever since the ban on antibiotic growth promoters in the livestock sector (Al-Khalaifah, 2018). Although the mechanisms via which probiotics exert beneficial effects are still unknown, the primary function of probiotics is the improvement of the microbiome in the intestinal tract (Jeong and Kim, 2014; Li et al., 2019). In addition, several effects of probiotics on mucosal barrier function, including regulation of cytokine expression, enhancement of mucus secretion, and improvement of tight junction integrity in the digestive tract of broiler chicks have been reported (Zhang et al., 2016; Gadde et al., 2017; Wang et al., 2018). However, the effects of probiotic bacteria on the mucosal barrier function of the gastrointestinal tract in the early phase of the life cycle of broiler chicks remains unknown.
Thus, the aim of the study was to determine the effects of probiotic bacteria on the gastrointestinal mucosal barrier function of broiler chicks. One day-old broiler chicks were administered viable LR by oral gavage. Subsequently, the thickness of the mucosal structures and number of goblet cells in the digestive tract were estimated. Furthermore, the expression of Mucin 2, factors related to the formation of tight junctions (Claudin1, 5, and 16, ZO2, and JAM2), cytokines (IL-6, CXCLi2, and IL-10), and AvBDs (AvBD2, 10, and 12) in the crop, duodenum, ileum, and cecum were also investigated.
Materials and Methods
Treatment of Birds and Tissue Collection
One day-old male broiler chicks (Chunky broilers) were divided into two groups (control and LR group; n=7). The chicks were maintained under a light schedule of 23 h light/1 h darkness. They were provided with feed (commercial starter diet; Nichiwa Sangyo Co. Ltd., Kobe, Japan) and water ad libitum. The chicks were orally treated with 300 µL, sterilized water with or without 1 × 108 cfu of LR (5 mg FINELACT, Asahi Calpis Wellness Co. Ltd.) once daily for 7 days (days 0 to 6). At day 7, the chicks were euthanized using carbon dioxide, and their crop, duodenum, ileum, and cecum were collected. These tissue samples were processed for paraffin sectioning and total RNA extraction. This study was approved by the Hiroshima University Animal Research Committee (No. C15–16) and was performed in accordance with its guidelines.
Histological Analysis of Mucosal Structure and Localization of Goblet Cells
The digestive tract, including the crop, duodenum, ileum, and cecum, was fixed with 10% (v/v) formalin in phosphate-buffered saline (PBS) and processed for paraffin sectioning. The paraffin sections (4 µm in thickness) were stained with Hansen's hematoxylin and eosin for measuring the thickness of the crop epithelium, villus height, and crypt depth in the duodenum, ileum, and cecum.
For the histochemical localization of mucin polysaccharide, the paraffin sections of the duodenum, ileum, and cecum were stained with Alcian blue (AB). The sections were deparaffinized and immersed in 3% (v/v) acetic acid for 1 min, followed by staining with AB dissolved in 3% (v/v) acetic acid for 1 h. The sections were stained and mounted after the water wash.
Next, the sections were examined under a light microscope connected to an image analysis software (NIS-Elements, Nikon, Tokyo, Japan). The thickness of the stratified squamous epithelium of the crop, villus height, and crypt depth of the duodenum, ileum, and cecum were measured. The number of AB-positive cells (goblet cells) within the epithelial surface, crypt of the ileum, and cecum were also counted. Then, the frequencies of the cells were re-calculated with respect to the total number of cells in 1 × 105 µm2 of tissue. Length analysis was performed in quintuple and cell numbers were counted in triplicate on one section. The average value was used for statistical analysis.
Real Time Polymerase Chain Reaction (PCR) Analysis for Expression of Genes Related to Mucosal Barrier Function
Total RNA was extracted from the mucosa of the crop, duodenum, ileum, and cecum using Sepasol-RNA I Super (Nacalai Tesque Inc., Japan) according to the manufacturer's instructions. The extracted total RNA was dissolved in Tris-ethylenediamine tetraacetic acid (TE buffer) (10 mM Tris-HCl, pH 8.0, with 1 mM EDTA) and stored at −80°C until further use.
The concentration of total RNA in each sample was measured using NanoDrop Lite (Thermo Fisher Scientific Inc. MA, USA). The RNA samples were reverse transcribed using ReverTra Ace® qPCR RT master mix with genomic DNA remover (Toyobo Co. Ltd., Osaka, Japan) on a PTC-100 programmable thermal controller (MJ Research, Waltham, MA, USA), programmed according to the manufacturer's instructions. Real-time PCR was performed using the AriaMX real-time PCR system (Agilent Technologies, Santa Clara, CA, USA) with Brilliant III Ultra-Fast SYBR Green® qPCR master mix (Agilent Technologies). Table 1 shows the primer sequences used for PCR in this study. The cycle parameters for the amplification step of the PCR reaction program were denaturation at 95°C for 5 s and annealing at 56°C (AvBD12), 58°C (Claudin16 and ZO2), 60°C (IL6, CXCLi2, IL-10, Claudinl, and RPS17), 62°C (AvBD2 and Claudin5), 63°C (AvBD10), or 64°C (JAM2) for 10 s and the program was carried out for 50 cycles. The cycle parameters for the melting step were 95°C for 30 s, 65°C for 30 s, and 95°C for 30 s. RNA expression levels were calculated using the relative quantification method and a standard curve for each target gene. The target mRNA expression in each sample was normalized to the expression of the RPS17 house-keeping gene and to the values of mean fold change of gene expression from one standard sample in the control group.
Table 1. Primer sequences used in PCR analysis.
| Target genes | Forward Primer | Revers Primer | Product size | Accession no. |
|---|---|---|---|---|
| Mucin2 | GCTGATTGTCACTCACGCCTT | ATCTGCCTGAATCACAGGTGC | 442 | NM_001318434.1 |
| Claudin1 | GACTCGCTGCTTAAGCTGGA | AAATCTGGTGTTAACGGGTG | 276 | NM_001013611.2 |
| Claudin5 | GTCCCGCTCTGCTGGTTC | CCCTATCTCCCGCTTCTGG | 84 | NM_204201.1 |
| Claudin16 | TACGCCATTGATGTCTACG | GATAAGAAGCAGCCCAGTG | 125 | XM_426702.4 |
| ZO2 | GAAGCAGAGGTCGTAGTAGG | CTGTCCATAGCCACCATCC | 140 | NM_204918.1 |
| JAM2 | AGCCTCAAATGGGATTGGATT | CATCAACTTGCATTCGCTTCA | 59 | NM_001006257.1 |
| IL-6 | AGAAATCCCTCCTCGCCAAT | AAATAGCGAACGGCCCTCA | 121 | NM_204628.1 |
| CXCLi2 | CTGTCCTGGCCCTCCTCCTGGTT | TGGCGTCAGCTTCACATCTTG | 146 | NM_205498.1 |
| IL-10 | GCTGAGGGTGAAGTTTGAGGAA | GAAGCGCAGCATCTCTGACA | 142 | NM_001004414.2 |
| AvBD2 | GTTCTGTAAAGGAGGGTCCTGCCAC | ACTCTACAACACAAAACATATTGC | 238 | NM_204992.2 |
| AvBD10 | TGGGGCACGCAGTCCACAAC | CATGCCCCAGCACGGCAGAA | 157 | NM_001001609.1 |
| AvBD12 | CCCAGCAGGACCAAAGCAATG | AGTACTTAGCCAGGTATTCC | 157 | NM_001001607.2 |
| RPS17 | AAGCTGCAGGAGGAGGAGAGG | GGTTGGACAGGCTGCCGAAGT | 136 | NM_204217.1 |
Statistical Analysis
Values were expressed as mean±standard error of the mean (SEM). The significant differences in epithelium thickness, villus height, and crypt depth, frequencies of goblet cells, and the mRNA expression levels between the control and LR groups in each tissue were determined using the t-test. Differences were considered significant when the P value was <0.05.
Results
Body Weights and Feed Intake
The body weights of the experimental chicks are shown in Table 2. These did not differ between the control and LR groups on the same day.
Table 2. Body weight (g) of chicks during experimental period.
| day |
||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Control | 43.7±1.0 | 49.1±1.4 | 57.9±2.7 | 77.4±2.6 | 98.0 ±2.9 | 126.1±3.8 | 156.0±6.1 | 179.7±6.9 |
| LR | 42.7±1.4 | 48.9±1.1 | 57.9±1.7 | 76.0±2.3 | 96.86±2.2 | 124.3±2.7 | 156.6±3.6 | 178.0±3.8 |
LR, Lactobacillus reuteri. Values are represented as the mean±SEM (n=7).
Histological Analysis
The mucosal epithelium in the crop was covered with stratified squamous epithelium in both the control and LR group (data not shown). The duodenum and ileum developed tall villi and deep crypts on the basal lamina in both groups (data not shown). The cecum showed short and wide villus and crypt, which were not clearly distinguishable (data not shown). Fig. 1 shows the thickness of crop epithelium, villus height, and crypt depth in the intestine. As the villus was segmented, its length was not measured in the duodenum. Mucosal thickness, villus height, and crypt depth in the ileum of the LR group chicks were significantly more than those in the control group chicks (P<0.05). However, the crypt depth in the duodenum of the LR group chicks was not significantly different compared to that in the control group chicks (Fig. 1b and c). The thickness of stratified squamous epithelium in the crop, and the total villus height and crypt depth in the cecum, did not differ between the control and LR groups (Fig. 1a and 1d).
Fig. 1.
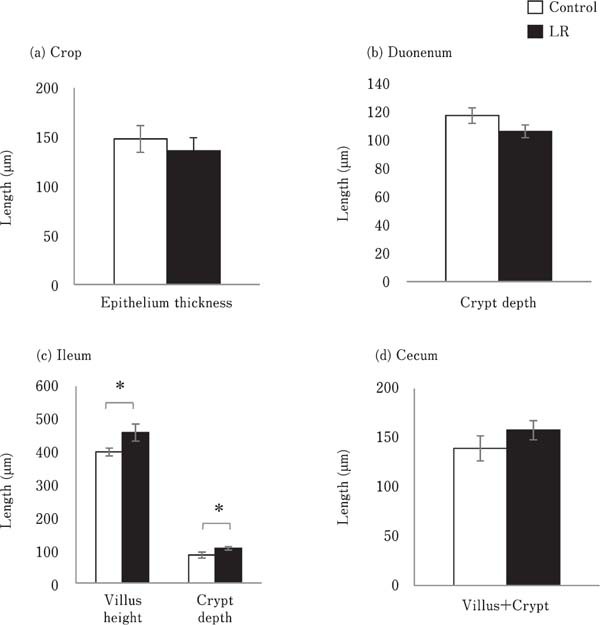
Effects of oral administration of Lactobacillus reuteri (LR) on the thickness of the mucosal structure in the (a) crop, (b) duodenum, (c) ileum, and (d) cecum. The thickness of stratified squamous epithelium in the crop, crypt depth in the duodenum, villus height and crypt depth in the ileum, and total length of villus and crypt in the cecum were measured. Values represent mean±standard error of the mean (SEM) (n=7). □=control, ■=LR groups. *P<0.05.
Goblet cells were observed on the epithelial cell layer of the villus and crypt in the duodenum, ileum, and cecum (data not shown). No significant differences in the number of goblet cells on the villus epithelium and intestinal glands were observed between the control and LR groups (Fig. 2a and 2b).
Fig. 2.
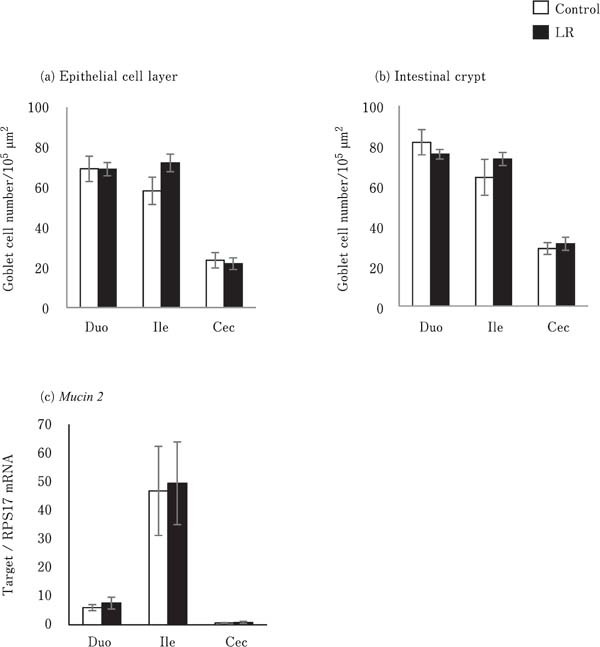
Effects of oral administration of Lactobacillus reuteri (LR) on mucus production in the intestinal mucosa. The number of goblet cells in the (a) epithelial cell layer and (b) intestinal gland were counted. Values represent mean±SEM (n=7) of the number of goblet cells per unit area of epithelial cell area (1 × 105 µm2). Mucin 2 mRNA expression was analyzed in the intestinal mucosa (c). Values represent mean± SEM (n=7) of fold change in target gene expression compared to a standard sample of the cecum in the control group. □=control, ■=LR groups.
Expression of Mucosal Barrier Function-related Factors
The expression of Mucin 2 did not differ between the LR group and control group (Fig. 2c). The expression of Claudin1, Claudin5, and ZO2 in the crop, duodenum, ileum, and cecum did not vary significantly between the control and LR groups (Fig. 3a, 3b, and 3d). In contrast, the expression of Claudin16 in the crop in the LR group chicks was significantly lower than that in the control group chicks (P<0.05; Fig. 3c). However, JAM2 expression in the crop and duodenum of the LR group chicks was significantly higher than that in the control group chicks (P<0.05; Fig. 3e).
Fig. 3.
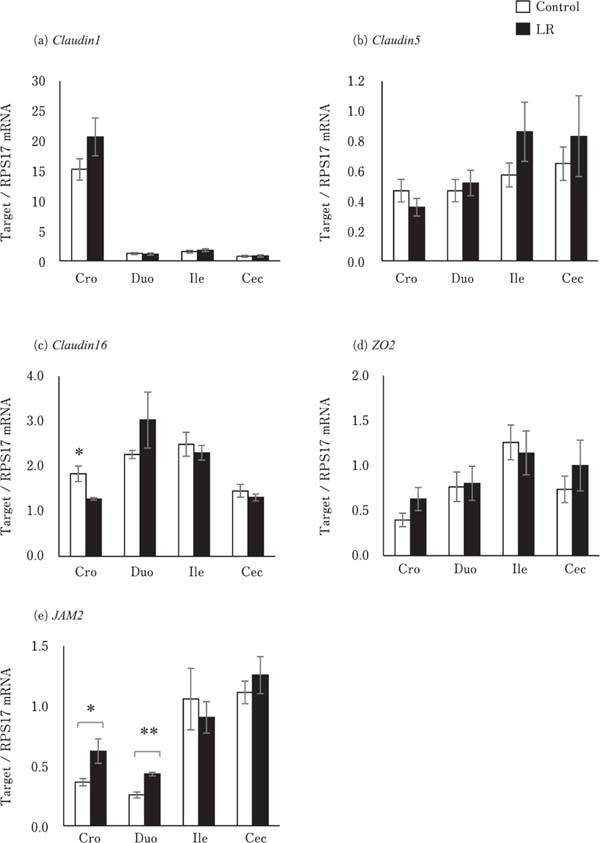
Effects of oral administration of Lactobacillus reuteri (LR) on the mRNA levels of tight junction-related genes in the digestive tract. Values represent mean± SEM (n=7) of fold change in the target gene expression compared to a standard sample of the cecum in the control group. □=control, ■=LR groups. *P<0.05 and **P<0.01.
Expression of Immune Function-related Factors
The expression levels of pro-inflammatory and anti-inflammatory cytokines, including IL-6, CXCLi2, and IL-10, were not significantly different between the control and LR groups in the different segments of the digestive tract (Fig. 4). The expression of AvBD10 in the ileum and cecum and that of AvBD12 in the crop of the LR group chicks were significantly lower than that of the control group chicks (P<0.05). However, they did not differ in the other segments of the digestive tract (Fig. 5b and c). AvBD2 expression level in all the segments of the digestive tract were similar between the control and LR groups (Fig. 5a).
Fig. 4.
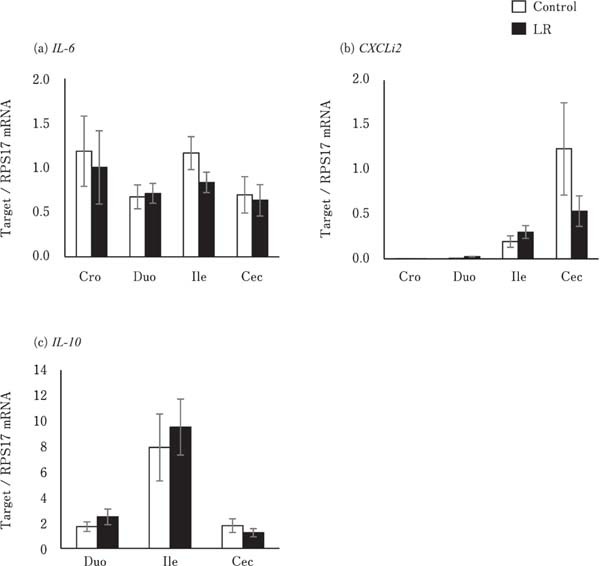
Effects of oral administration of Lactobacillus reuteri (LR) on the mRNA levels of cytokine-encoding genes in the digestive tract. Interleukin-10 (IL-10) is not expressed in the crop. Values represent mean±SEM (n=7) of fold change in the target gene expression compared to a standard sample of the cecum in the control group. □= control, ■=LR groups.
Fig. 5.
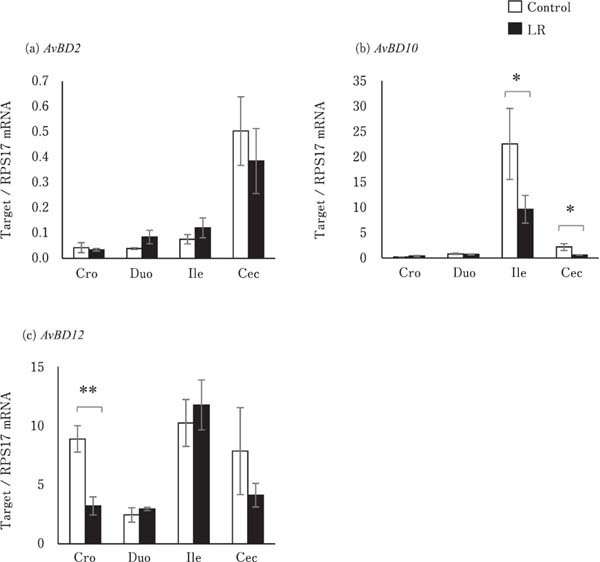
Effects of oral administration of Lactobacillus reuteri (LR) on the mRNA levels of avian β defensin (AvBD) in the digestive tract. Values represent mean±SEM (n=7) of fold change in the target gene expression compared to a standard sample of the cecum in the control group. □=control, ■=LR groups. *P<0.05 and **P<0.01.
Discussion
In this study, we demonstrated the effects of oral administration of a probiotic bacterium, Lactobacillus reuteri, on the gastrointestinal mucosal barrier function of broiler chicks. The significant observations of this study were that oral administration of LR increased ileal villus height and crypt depth, but not goblet cell number and Mucin 2 expression, decreased Claudin16 expression in the crop and increased JAM2 expression in the crop and ileum, and decreased AvBD10 expression in the ileum and cecum and AvBD12 expression in the crop.
Smirnov et al. (2005) reported that dietary probiotics, including L. acidophilus, L. casei, Bifidobacterium bifidum, and Enterococcus faecium promoted the development of larger goblet cells in the small intestine, and increased mucin mRNA expression and the concentration of mucin glycoprotein in the ileum. However, our results showed that oral administration of LR did not change the number of goblet cells in the villus epithelial layer and intestinal gland, and Mucin 2 mRNA expression. Hence, we reasoned that the effects of probiotic bacteria on mucus production vary with bacterial strains, feeding method, and schedule. Furthermore, the villus height and crypt depth in the ileum of the LR group birds were higher than those in the control group. Reports show that feeds supplemented with L. salivarius and L. reuteri increased villus height in the duodenum of broiler chicks (Awad et al., 2010). Other strains of probiotic bacteria such as Bacillus and Enterococcus also improved villus height and the ratio of villus height to crypt depth in the small intestine of chicks (Huang et al., 2019; Li et al., 2019). Increase in villus height is indicative of increase in digestive and absorptive capacity, whereas increase in crypt depth is indicative of increase in cell proliferation in the intestinal mucosa (Pluske et al., 1997). Therefore, improvement in nutrient digestion and absorption in the intestinal tract may be a common function of various probiotic bacterial strains. Body weight did not vary between the control and LR group in this study. Thus, the development of ileal mucosal structure may not be facilitated by increase in feed intake, but by direct or indirect effects of LR (for example, LR component, metabolites, or others).
LR administration did not change the expression of Claudin1 and 5, and ZO2 in the crop, small intestine, and cecum, but reduced that of Claudin16 in the crop and increased that of JAM2 in the crop and duodenum. Claudin1 and 5 are members of the physical barrier-forming claudin family that decrease gut permeability; however, Claudin16 is a pore-forming claudin that increases gut permeability (Gunzel and Yu, 2013). Therefore, the decrease in Claudin 16 levels observed in this study may enhance intestinal barrier function. JAM2 is a component of the tight junction, which controls intestinal permeability and provides protection from bacterial invasion (Luissint et al., 2014; Awad et al., 2017). In addition, the JAM family is involved in attracting leukocytes under inflammatory conditions (Luissint et al., 2014). In this study, inflammation in the intestinal mucosa was negligible in chicks of the LR group, as their intestinal mucosa did not show histological signs of mucosal inflammation and showed no significant difference in proinflammatory and anti-inflammatory cytokine and mRNA levels between the control and LR groups. Thus, oral administration of LR may enhance the function of tight junctions against bacterial invasion in the upper digestive tract via increase in the expression of JAM2 and decrease in Claudin16 expression. In addition, increase in JAM2 expression may result in a leukocyte-rich condition in the mucosa, which is effective against bacterial infections. Yang et al. (2019) reported that consumption of encapsulated organic acids and essential oils increased the butyric acid and acetic acid concentration in the ileal digesta, and Claudin5 expression in the ileum of broiler chicks. We assumed that the organic acid produced by LR affected Claudin16 and JAM2 expression in this study. However, further studies are necessary to confirm this hypothesis.
All experimental birds expressed AvBD2, 10, and 12 in the crop, duodenum, ileum, and cecum. AvBD2, 10, and 12 have been reported to exert antimicrobial activity against pathogenic bacteria such as Escherichia coli, Salmonella typhimurium, and Staphylococcus aureus (Cuperus et al., 2013; Yacoub et al., 2015; Yang et al., 2016). Thus, AvBDs are involved in elimination of pathogenic bacteria in the digestive tract. In this study, birds in the LR group showed reduction in the expression of AvBD10 in the ileum and cecum, and that of AvBD12 in the crop. We have previously reported that probiotic bacteria, including Streptococcus faecalis, Clostridium butyricum, and B. mesentericus lowered AvBD12 protein level in the proventriculus of broiler chicks (Mohammed et al., 2015). Akbari et al. (2008) reported that probiotic feeding did not change the expression of AvBDs in the cecal tonsil, but Salmonella infection did. However, costimulations such as probiotic feeding and Salmonella infection did not increase AvBD expression. In contrast, the mRNA levels of genes encoding cathelicidins, members of an anti-microbial peptide family, were not affected by mono-stimulation such as probiotic feeding or lipopolysaccharide (LPS) exposure, but were increased by their co-stimulation (Mohammed et al., 2016). Therefore, intake of probiotic bacteria might modulate the immune response of antimicrobial peptides against antigen stimulation in the mucosa of the digestive tract. However, the underlying probiotic mechanism is still unknown.
In conclusion, broiler chicks normally form the mucosal barrier, which involves mucus production, tight junctions, cytokines, and AvBD production in the digestive tract. L. reuteri used in this study may enhance the function of tight junctions by increasing JAM2 expression and decreasing Claudin16 expression in the upper gastrointestinal tract of broiler chicks. Thus, oral administration of LR may be an effective approach for enhancing mucosal barrier function and protecting against pathogen infection in newly hatched chicks.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (No. 17H03904) and by a grant from the Mishima Kaiun Memorial Foundation (Tokyo, Japan) to YY. We thank Editage (www.editage.jp) for English language editing.
References
- Akbari MR, Haghighi HR, Chambers JR, Brisbin J, Read LR, Sharif S. Expression of antimicrobial peptides in cecal tonsils of chickens treated with probiotics and infected with Salmonella enterica serovar typhimurium. Clinical and Vaccine Immunology, 15: 1689-1693. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khalaifah HS. Benefits of probiotics and/or prebiotics for antibiotic-reduced poultry. Poultry Science, 97: 3807-3815. 2018. [DOI] [PubMed] [Google Scholar]
- Awad WA, Ghareeb K, Bohm J. Effect of addition of a probiotic micro-organism to broiler diet on intestinal mucosal architecture and electrophysiological parameters. Journal of Animal Physiology and Animal Nutrition, 94: 486-494. 2010. [DOI] [PubMed] [Google Scholar]
- Awad WA, Hess C, Hess M. Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens. Toxins, 9: E60 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shira E, Sklan D, Friedman A. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Developmental and Comparative Immunology, 27: 147-157. 2003. [DOI] [PubMed] [Google Scholar]
- Cuperus T, Coorens M, van Dijk A, Haagsman HP. Avian host defense peptides. Developmental and Comparative Immunology, 41: 352-369. 2013. [DOI] [PubMed] [Google Scholar]
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Research and Therapy, 8 Suppl 2: S3 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde U, Oh ST, Lee YS, Davis E, Zimmerman N, Rehberger T, Lillehoj HS. The Effects of Direct-fed Microbial Supplementation, as an Alternative to Antibiotics, on Growth Performance, Intestinal Immune Status, and Epithelial Barrier Gene Expression in Broiler Chickens. Probiotics and Antimicrobial Proteins, 9: 397-405. 2017. [DOI] [PubMed] [Google Scholar]
- Gunzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiological Reviews, 93: 525-569. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wang P, Liu ZH, Ye P. Analysis of differential expression of tight junction proteins in cultured oral epithelial cells altered by Porphyromonas gingivalis, Porphyromonas gingivalis lipopolysaccharide, and extracellular adenosine triphosphate. International Journal of Oral Science, 10: e8 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Luo L, Zhang Y, Wang Z, Xia Z. Effects of the Dietary Probiotic, Enterococcus faecium NCIMB11181, on the Intestinal Barrier and System Immune Status in Escherichia coli O78-Challenged Broiler Chickens. Probiotics and Antimicrobial Proteins, 11: 946-956. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JS, Kim IH. Effect of Bacillus subtilis C-3102 spores as a probiotic feed supplement on growth performance, noxious gas emission, and intestinal microflora in broilers. Poultry Science, 93: 3097-3103. 2014. [DOI] [PubMed] [Google Scholar]
- Li CL, Wang J, Zhang HJ, Wu SG, Hui QR, Yang CB, Fang RJ, Qi GH. Intestinal Morphologic and Microbiota Responses to Dietary Bacillus spp. in a Broiler Chicken Model. Frontiers in Physiology, 9: 1968 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luissint AC, Nusrat A, Parkos CA. JAM-related proteins in mucosal homeostasis and inflammation. Seminars in Immunopathology, 36: 211-226. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn DJ, Higgs R, Lloyd AT, O'Farrelly C, Herve-Grepinet V, Nys Y, Brinkman FS, Yu PL, Soulier A, Kaiser P, Zhang G, Lehrer RI. Avian beta-defensin nomenclature: a community proposed update. Immunology Letters, 110: 86-89. 2007. [DOI] [PubMed] [Google Scholar]
- Minciullo PL, Catalano A, Mandraffino G, Casciaro M, Crucitti A, Maltese G, Morabito N, Lasco A, Gangemi S, Basile G. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Archivum Immunologiae et Therapiae Experimentalis, 64: 111-126. 2016. [DOI] [PubMed] [Google Scholar]
- Mohammed ESI, Isobe N, Yoshimura Y. Effects of Probiotics on the Expression of Cathelicidins in Response to Stimulation by Salmonella Minnesota Lipopolysaccharides in the Proventriculus and Cecum of Broiler Chicks. Journal of Poultry Science, 53: 298-304. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed ESI, Okazaki A, Isobe N, Yoshimura Y. Effects of Probiotics on the Expression and Localization of Avian β-defensins in the Proventriculus of Broiler Chicks. Journal of Poultry Science, 52: 57-67. 2015. [Google Scholar]
- Ozden O, Black BL, Ashwell CM, Tipsmark CK, Borski RJ, Grubb BJ. Developmental profile of claudin-3, -5, and -16 proteins in the epithelium of chick intestine. Anatomical Record, 293: 1175-1183. 2010. [DOI] [PubMed] [Google Scholar]
- Pawlowska B, Sobieszczanska BM. Intestinal epithelial barrier: The target for pathogenic Escherichia coli. Advances in Clinical and Experimental Medicine, 26: 1437-1445. 2017. [DOI] [PubMed] [Google Scholar]
- Pluske JR, Hampson DJ, Williams IH. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livestock Production Science, 51: 215-236. 1997. [Google Scholar]
- Poh TY, Pease J, Young JR, Bumstead N, Kaiser P. Reevaluation of chicken CXCR1 determines the true gene structure: CXCLi1 (K60) and CXCLi2 (CAF/interleukin-8) are ligands for this receptor. Journal of Biological Chemistry, 283: 16408-16415. 2008. [DOI] [PubMed] [Google Scholar]
- Sick C, Schneider K, Staeheli P, Weining KC. Novel chicken CXC and CC chemokines. Cytokine, 12: 181-186. 2000. [DOI] [PubMed] [Google Scholar]
- Smirnov A, Perez R, Amit-Romach E, Sklan D, Uni Z. Mucin dynamics and microbial populations in chicken small intestine are changed by dietary probiotic and antibiotic growth promoter supplementation. Journal of Nutrition, 135: 187-192. 2005. [DOI] [PubMed] [Google Scholar]
- Terada T, Nii T, Isobe N, Yoshimura Y. Changes in the Expression of Avian beta-defensins (AvBDs) and Proinflammatory Cytokines and Localization of AvBD2 in the Intestine of Broiler Embryos and Chicks during Growth. Journal of Poultry Science, 55: 280-287. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirtsikos P, Fegeros K, Balaskas C, Kominakis A, Mountzouris KC. Dietary probiotic inclusion level modulates intestinal mucin composition and mucosal morphology in broilers. Poultry Science, 91: 1860-1868. 2012. [DOI] [PubMed] [Google Scholar]
- van Dijk A, Veldhuizen EJ, Haagsman HP. Avian defensins. Veterinary Immunology and Immunopathology, 124: 1-18. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li L, Lv Y, Chen Q, Feng J, Zhao X. Lactobacillus plantarum Restores Intestinal Permeability Disrupted by Salmonella Infection in Newly-hatched Chicks. Scientific Reports, 8: 2229 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub HA, Elazzazy AM, Abuzinadah OA, Al-Hejin AM, Mahmoud MM, Harakeh SM. Antimicrobial activities of chicken beta-defensin (4 and 10) peptides against pathogenic bacteria and fungi. Frontiers in Cellular and Infection Microbiology, 5: 36 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhang C, Zhang X, Zhang MZ, Rottinghaus GE, Zhang S. Structure-function analysis of Avian beta-defensin-6 and beta-defensin-12: role of charge and disulfide bridges. BMC Microbiology, 16: 210 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Liu Y, Yan F, Yang C. Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens. Poultry Science, 98: 2858-2865. 2019. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y. Avian beta-defensins expression for the innate immune system in hen reproductive organs. Poultry Science, 94: 804-809. 2015. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhan X, Zeng X, Zhou L, Cao G, Chen A, Yang C. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. Journal of Animal Science and Biotechnology, 7: 3 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


