Abstract
This study was designed to determine the effect of phytase extracted from Aspergillus niger (Natuphos® E) on growth performance, bone mineralization, phosphorous excretion, and meat quality parameters in broilers fed available phosphorous (aP)-deficient diet. In total, 810 one-day-old Indian River broilers were randomly allotted into one of three dietary treatments, with six replicates per treatment. The three dietary treatments were 1) control group (CON: basal diet with sufficient aP), 2) low phytase (LPY: available phosphorus-deficient diet supplemented with 0.01% phytase), and 3) high phytase (HPY: available phosphorus-deficient diet supplemented with 0.02% phytase). Average daily gain and, feed intake, and feed conversion ratio were measured for 35 days. Excreta were collected from each pen on day 35. One broiler from each cage was euthanized to collect visceral organs and tibia samples. Broiler chickens fed LPY and HPY showed improved (P<0.05) growth performance compared to broilers fed CON on day 35. The tibia length of HPY-fed broilers was more than those of broilers fed other diets on day 35 (P<0.05). However, tibia calcium and phosphorous contents in LPY-fed broilers was higher (P<0.05) than in CON and HPY-fed broilers. Tibia length and calcium and phosphorous content showed a positive correlation (P<0.05) with the weight gain of broilers on day 35. Phosphorous level in the excreta of LPY- and HPY-fed broilers was lesser than those of CON broilers on day 35 (P<0.05). Furthermore, HPY-fed broilers showed lower (P<0.05) phosphorous content in the excreta than LPY-fed broilers. LPY- and HPY-fed broilers showed higher (P<0.05) liver weight than the CON broilers. In conclusion, broilers fed aP-deficient diet supplemented with phytase from Aspergillus excreted less phosphorus, which enhanced growth performance and tibia development from time of hatching to day 35 post-hatching.
Keywords: broilers, phosphorous, phytase, tibia, visceral organs
Introduction
Phosphorus (P) is one of the major and expensive macrominerals required for energy metabolism, regulation of several enzymes, and biosynthesis of nucleic acids and cell membranes (Richardson et al., 2009). Furthermore, P is essential for maintaining the skeletal integrity of broilers (Scholey et al., 2018). Better understanding of bone quality in poultry is important as bone breakage and related contaminations increase mortality while reducing efficiency, and carcass quality (Rath et al., 2000). Additionally, weak legs often result in reduced feed intake, thereby affecting weight gain of the poultry (Orban et al., 1999).
The available P (aP) requirement of broiler diets was estimated to be 0.35–0.45% (NRC, 1994). A large portion of P in cereals and oilseeds is bound as phytate, which is unavailable to the broilers due to their limited endogenous phytase level (Morgan et al., 2015). Therefore, poultry diets were formulated with excessive P from inorganic P sources for providing a safety margin (Van der Klis and Versteegh, 1996). Consequently, excess P in the broiler diets is excreted to the environment, which has become a major environmental issue (Sharpley et al., 1994).
The effect of phytase supplementation in the diet of monogastric animals is being extensively investigated (Adeola and Cowieson, 2011). Phytase is a feed additive that dephosphorylates phytate present in broiler diets (Cowieson and Adeola, 2005). Phytase has the ability to increase P availability and utilization in broilers (Nelson et al., 1971). Broilers fed diets that closely mimicked their aP requirement, coupled with phytase, can be beneficial, with limited wastage of nutrients (Waldroup, 1999). Broilers fed phytase-supplemented less aP diet showed improved growth performance and bone ash and mineral utilization (Olukosi et al., 2007). Previous studies have shown that broilers fed diets supplemented with phytase improved P retention by 50–60% (Simons et al., 1990; Kornegay et al., 1996). Pieniazek et al. (2016) reported that super dosing of phytase beyond the industrial level not only increase the P availability, but also the availability of other nutrients such as proteins, lipids, carbohydrates, Ca2+, Cu2+, and Zn2+ as a result of elevated hydrolysis by phytase. Phytase also lowering the global feed industry cost by reducing the cost of nutritional inputs, for example, by replacing inorganic P sources such as dicalcium phosphate (DCP) (Mrigen, 2009). Partial replacement of dietary inorganic phosphorous by phytase was confirmed in layers, whereas the results obtained with broilers were inconsistent (Um et al., 2000).
Therefore, this study aimed to investigate the effect of phytase extracted from Aspergillus niger (as a replacement of dietary inorganic P) on growth performance, bone mineralization, P excretion, and meat quality parameters of broilers from the time of hatching to day 35 post-hatching.
Materials and Methods
Experiment Design and Management
In total, 810 one-day-old Indian River broilers (sex ratio 1:1) were randomly assigned into one of three groups, with six replicate pens per treatment (forty-five broilers per pen). The dietary treatments were as follows: 1) control group (CON: basal diet with sufficient available phosphorus), 2) low phytase (LPY: available phosphorus-deficient diet supplemented with 0.01% phytase), and 3) high phytase (HPY: available phosphorus-deficient diet supplemented with 0.02 % phytase).
Floor pens (5 m2) were equipped with separate feeders and nipple drinkers. Temperature was maintained at 33±2°C for the first three days and gradually decreased to room temperature (25±2°C) until the end of the experiment. Broilers were allowed ad libitum access to experimental diets and water via nipple drinkers. Broilers were vaccinated with New Castle disease vaccine on day 7 and Gumboro vaccine (Infectious Bursal Disease) on days 10, 17 and 23. All the management practices were conducted according to the guidelines of the Indian River® broiler management handbook (Avigen, 2014) and the experimental procedure was per the specific guidelines for broilers presented in the Guide for the Care and Use of Agricultural Animals in Research and Teaching, 3rd edition (McGlone, 2010).
Experimental Diets
The basal diet was based on corn and soybean meal with added DCP (Table 1) to fulfill the nutritional requirement per the Indian River® nutrition guidelines (Avigen, 2014). The other two experimental diets with aP deficiencies were formulated by removing DCP from the basal diet and supplementing it with 0.01% (LPY) and 0.02% (HPY) phytase (Natuphos® E, BASF, Germany). The phytase activities of the LPY and HPY diets were 500 FTU/kg and 1000 FTU/kg, respectively.
Table 1. Composition (% as-fed basis) of the experimental diets.
| Item | Starter (d 0–21) |
Grower (d 22–35) |
||||
|---|---|---|---|---|---|---|
| CON1 | LPY2 | HPY3 | CON | LPY | HPY | |
| Maize | 44.90 | 45.56 | 45.56 | 45.82 | 46.33 | 46.33 |
| Soybean meal | 29.04 | 28.85 | 28.85 | 32.09 | 32.00 | 32.00 |
| Broken rice | 10.23 | 10.23 | 10.23 | 9.19 | 9.19 | 9.19 |
| Rice polish | 2.97 | 2.97 | 2.97 | 4.00 | 4.00 | 4.00 |
| Vegetable oil | 1.55 | 1.49 | 1.49 | 2.56 | 2.50 | 2.50 |
| DDGS3 | 3.04 | 3.04 | 3.04 | 3.00 | 3.00 | 3.00 |
| Fish meal | 5.05 | 5.05 | 5.05 | — | — | — |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Dicalcium phosphate | 0.82 | — | — | 0.80 | — | — |
| Limestone | 1.27 | 1.67 | 1.66 | 1.38 | 1.79 | 1.78 |
| VIT-Min premix4 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Lysine-HCl | 0.25 | 0.25 | 0.25 | 0.28 | 0.30 | 0.30 |
| DL-Methionine | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 |
| L-Threonine | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Phytase | — | 0.01 | 0.02 | — | 0.01 | 0.02 |
| Calculated values | ||||||
| ME, kcal/kg | 3050 | 3050 | 3050 | 3150 | 3150 | 3150 |
| Crude protein (CP) | 22.50 | 22.50 | 22.50 | 20.50 | 20.50 | 20.50 |
| Available phosphorous | 0.45 | 0.30 | 0.30 | 0.40 | 0.30 | 0.30 |
| Calcium | 1.19 | 1.15 | 1.15 | 1.24 | 1.20 | 1.20 |
| Analyzed values | ||||||
| Crude protein | 22.45 | 22.41 | 22.43 | 20.65 | 20.66 | 20.61 |
| Calcium | 1.17 | 1.14 | 1.12 | 1.22 | 1.20 | 1.19 |
Phosphorous-deficient diet with 0.01% of phytase (500 FTU/kg) without DCP
Phosphorous-deficient diet with 0.02% of phytase (1000 FTU/kg) without DCP
Corn distiller dried grains with soluble
Vitamins: A 7000 IU, D31400 IU, E 20 mg, K 1 mg, B1 1 mg, B2 3 mg, B6 1.5 mg, B12 15 µg, calcium pantothenate 10.7 mg, folic acid 0.2 mg, niacin 12 mg, biotin 30 µg. Minerals: Co 0.2 mg (as cobalt sulphate), Cu 10 mg (as copper sulphate), iodine 0.5 mg (as potassium iodine), iron 60 mg (as ferrous sulphate), Mn 40 mg (as manganous oxide), Se 0.3 mg (as sodium selenite), Zn 100 mg (as zinc oxide).
Measurements
The total body weights of the broilers in each cage and pen and their feed intake were measured on day 35 post-hatching. The total body weights and feed intake were divided by the number of birds in each cage to determine the average daily gain (ADG) and average daily feed intake (ADFI). Average daily gain and ADFI were used to calculate feed conversion ratio (FCR). Thereafter, mortality-corrected FCR was calculated by considering daily mortality records of each pen.
Sample Collection
Excreta samples were collected on day 35 of the experiment. Four broilers from each replicate were selected for collection of excreta samples. The selected broilers were fed pre-analyzed (phosphorous) feed samples for 3 days prior to collection of excreta.
At the end of the experimental period (day 35), the birds were subjected to 10 h feed withdrawal prior to slaughter. Four broilers with the closest median body weight were selected from each treatment. The selected broilers were weighed and sacrificed by cervical dislocation (Houshmand et al., 2011).
Carcass weights and visceral organ weights (gizzard, liver, heart, and whole intestine) were measured from each eviscerated carcass. The carcasses were split into cuts such as breast and thigh, which were weighed to calculate the percentage weight relative to the carcass weight. Visceral organs were weighed using an analytical balance (Model -321 LX, Shanghai, China) and the ratios of the visceral organ weights to body weight were calculated.
Sample Preparation and Laboratory Analyses
Excreta and feed samples were dried at 103°C for 4 h in a dry oven (model –GZF6020, Lanphan Ltd. Henan, China) to remove moisture before analyzing the P content. The method described by Quinlan and DeSesa, (1955) was used to determine P content in feed and excreta.
The right tibias of four birds per treatment were removed and stored in a freezer at −18°C for measuring the bone length and, total ash and mineral (Ca, P) content. The tibias were cleaned by removing the adhering tissue. The lengths of the tibia were measured using slide calipers. Subsequently, both the head and shaft portions of the tibia were dried to constant weight at 105°C. The tibia samples were grounded and a known weight (M) was put into an empty crucible (empty crucible weight=M1) and kept in the muffle furnace SX4-10, China) at 550°C for 4 h. Finally, weight of the crucible with remaining sample from the muffle furnace was measured (M2)”.
Ash %=M2−M1/M×100%
Ash was dissolved in concentrated HCl (1:1) for determining mineral content. Bone mineral concentration was determined following the procedure used for estimation of minerals in feeds and feces. Calcium content was determined using a titrimetric method (ISO 6490-1, 1985) and phosphorus content was determined using a spectrometric method (ISO 6491, 1998).
Analysis of Meat Quality Parameter
Meat quality parameters were determined according to Jayasena et al. (2013) and Lakshani et al. (2016).
pH of the Broiler thigh Muscle
Thigh meat samples were minced using a mincing machine (TS-JR12A, Teng Sheng Food Machinery Co., Ltd., Guangdong, China). One gram of minced meat was measured using an analytical balance (Model -321 LX, Shanghai, China). Meat samples were transferred into Falcon tubes (Biologix- model; 10-9152, Ying Xiu Road High-Tech Industrial Development Zone Jinan, Shandong, China). Subsequently, 9 mL distilled water added to the Falcon tubes and mixed well with the meat samples. The meat samples were homogenized using a vortex mixer (XINFU-SK-1, Hebei City Thermal meter instrument factory, Henan, China). The homogenized samples were filtered using Whatman No. 4 filter papers (Whatman International Ltd., Maidstone, UK). Finally, the pH of the filtrate was measured using a pH meter (pH 700, Eutech Instruments, Singapore).
Water Holding Capacity of the Broiler Thigh Muscle
Initially, 2 g thigh meat sample was measured using an analytical balance. The meat samples were wrapped with a round filter paper (Whatman No. 4; Whatman International Ltd.). A standard weight of 10 kg was applied on the meat samples for 5 min, after which, the meat samples were reweighed and the water holding capacity was calculated (Lakshani et al., 2016).
Cooking Loss of the Broiler Thigh Muscle
To analyze cooking loss, 30g thigh meat sample was vacuum-packed, sealed, and placed in a water bath (JONILAB® JNWB001, JOAN Lab Instrument Co. Ltd, Zhejiang Province, China) at 85°C for 30 min. The sample was allowed to cool to room temperature (20–25°C) and the final weight was measured. Cooking loss is equal to the weight loss after cooking and is represented as a percentage of the initial weight (Önenç et al., 2004).
Statistical Analyses
Data were analyzed using the General Linear Model (GLM) method of one-way analysis of variance (ANOVA) of the SPSS software (version 24, Armonk, NY: IBM Corp.). A pen was considered as the experimental unit for growth performance and the selected broilers were used as the experimental units for analyzing tibia characteristics, visceral organ weights, and meat quality parameter. Differences between means were tested using Tukey multiple range test wherever appropriate (P<0.05). Correlation between the tibia characteristics and growth performance was determined using Pearson's correlation test.
Results
The effect of broiler diets with or without phytase on growth performance of broilers from the time of hatching to day 35 post-hatching is summarized in Table 2. Average Daily Gain of broilers fed HPY and LPY was higher (P<0.05) than that of broilers fed CON from the time of hatching to day 35 post-hatching. In the present study, broilers fed LPY showed higher (P<0.05) feed intake than broilers fed CON and HPY from time of hatching to 35 days post-hatching. Furthermore, broilers fed HPY showed lower (P<0.05) feed intake than broilers of the CON group. In addition, broilers fed HPY showed better FCR (P<0.05) than those fed LPY and CON from time of hatching to 35 days post-hatching. These results indicated that, broiler diet supplemented with 500 FTU/kg phytase can replace the DCP supplementation while improving the growth performance of broilers from time of hatching to 35 days post-hatching.
Table 2. Effect of diets supplemented with different amounts of phytase on growth performance of broilers 35 days after hatching1.
| Item | CON | LPY | HPY | SEM2 | P value |
|---|---|---|---|---|---|
| ADG3, g/day | 53.27b | 54.22a | 54.06a | 0.035 | 0.001 |
| ADFI4, g/day | 84.84b | 86.43a | 81.97c | 0.135 | 0.001 |
| FCR5, g/g | 1.59a | 1.59a | 1.52b | 0.003 | 0.001 |
Values are the mean of six replicates per treatment (N=270)
Pooled standard error of mean
CON: control diet (phosphorous-sufficient diet with dicalcium phosphate)
LPY: phosphorous-deficient diet with 0.01% phytase (500 FTU/kg) without dicalcium phosphate
HPY: phosphorous-deficient diet with 0.02% phytase (1000 FTU/kg) without dicalcium phosphate
Average daily gain
Average daily feed intake
Feed conversion ratio
Means in the same row with different superscripts differ significantly (P<0.05).
The effect of broiler diets with or without phytase on tibia measurement of broilers from time of hatching till day 35 is summarized in Table 3. The tibia length of broilers fed HPY and LPY was higher (P<0.05) than those of broilers fed CON. Furthermore, broilers fed HPY had longer (P<0.05) tibia than those of broilers fed LPY on day 35. The tibia ash content on day 35 did not vary significantly between broilers fed different diets. However, broilers fed LPY showed higher (P<0.05) P and Ca content in the tibia than broilers fed HPY and CON. Interestingly, broilers fed CON showed lower (P<0.05) tibial Ca and P content than broilers fed HPY. Table 4 shows the correlation between tibia measurement and growth performance. A positive high (P<0.05) correlation was observed between the ADG and tibial Ca and P content in the broilers. The length of the tibia correlated negatively (P<0.05) with the FCR of the broilers on day 35.
Table 3. Effect of diets supplemented with different amounts of phytase on tibia parameters of broilers 35 days after hatching1.
| Item | CON | LPY | HPY | SEM2 | P value |
|---|---|---|---|---|---|
| Tibia bone length, cm | 7.35c | 8.13b | 8.67a | 0.014 | 0.001 |
| Tibia ash, % | 38.96 | 39.38 | 39.63 | 2.930 | 0.987 |
| Tibia Ca, % | 7.23b | 10.44a | 8.17ab | 0.580 | 0.010 |
| Tibia P, % | 3.55c | 4.91a | 4.39b | 0.060 | 0.001 |
Values are the mean of six replicates per treatment (N=270)
Pooled standard error of mean
CON: control diet (phosphorous-sufficient diet with dicalcium phosphate)
LPY: phosphorous-deficient diet with 0.01% phytase (500 FTU/kg) without dicalcium phosphate
HPY: phosphorous-deficient diet with 0.02% phytase (1000 FTU/kg) without dicalcium phosphate
Means in the same row with different superscripts differ significantly (P<0.05).
Table 4. Correlation (r) amongtibia parameters and growth parameters of broilers 35 days after hatching.
| Item | ADG1 | ADFI2 | FCR3 |
|---|---|---|---|
| Tibia ash % | 0.078 | −0.058 | −0.086 |
| Tibia Ca % | 0.626* | 0.433 | 0.197 |
| Tibia P % | 0.933** | 0.223 | −0.122 |
| Tibia length (cm) | 0.643* | −0.562 | −0.768** |
Average daily gain
Average daily feed intake
Feed conversion ratio
Correlation is significant at the 0.01 level (2-tailed)
Correlation is significant at the 0.05 level (2-tailed)
The effect of different dietary treatments on carcass quality is summarized in Table 5. None of the experimental diets significantly affected the carcass, thigh muscle, and breast muscle weight percentages on day 35.
Table 5. Effect of diets supplemented with different amounts of phytase on carcass parameters of broilers 35 days after hatching1.
| Item | CON | LPY | HPY | SEM2 | P value |
|---|---|---|---|---|---|
| Carcass weight, % | 74.34 | 71.91 | 71.06 | 1.330 | 0.792 |
| Breast muscle weight, % | 26.21 | 24.98 | 25.37 | 0.500 | 0.250 |
| Thigh muscle weight, % | 5.49 | 5.97 | 5.75 | 1.560 | 0.127 |
Values are the mean of six replicates per treatment (N=270)
Pooled standard error of mean
CON: control diet (phosphorous-sufficient diet with dicalcium phosphate)
LPY: phosphorous-deficient diet with 0.01% phytase (500 FTU/kg) without dicalcium phosphate
HPY: phosphorous-deficient diet with 0.02% phytase (1000 FTU/kg) without dicalcium phosphate
Means in the same row with different superscripts differ significantly (P<0.05).
The effect of different diets on the weight of visceral organs after 35 days is shown in Table 6. In this study, the liver weight of broilers fed diets supplemented with phytase was higher (P<0.05) than those of broilers fed CON. Furthermore, the heart weight of broilers fed LPY was lower (P<0.05) than those of broilers fed HPY and CON on day 35. In addition, the weights of gizzard and small intestine did not differ significantly among broilers fed different diets.
Table 6. Effect of diets supplemented with different amounts of phytase on relative weights of visceral organs of broilers 35 days after hatching1.
| Item | CON | LPY | HPY | SEM2 | Pvalue |
|---|---|---|---|---|---|
| Liver, % | 2.20b | 2.24b | 3.27a | 0.100 | 0.001 |
| Heart, % | 0.52a | 0.39b | 0.51a | 0.020 | 0.003 |
| Gizzard, % | 1.02 | 0.97 | 1.06 | 0.050 | 0.462 |
| Small intestine, % | 5.12 | 4.84 | 4.75 | 0.210 | 0.367 |
Values are the mean of six replicates per treatment (N=270)
Pooled standard error of mean
CON: control diet (phosphorous-sufficient diet with dicalcium phosphate)
LPY: phosphorous-deficient diet with 0.01% phytase (500 FTU/kg) without dicalcium phosphate
HPY: phosphorous-deficient diet with 0.02% phytase (1000 FTU/kg) without dicalcium phosphate
Means in the same row with different superscripts differ significantly (P<0.05).
The effect of different dietary treatments on meat quality parameters of broilers is shown in Table 7. Meat quality parameters (water holding capacity, cooking loss, pH and color) of the thigh muscle of broilers did not differ significantly between the experimental groups on day 35.
Table 7. Effect of diets supplemented with different amounts of phytase on meat quality parameters of broilers 35 days after hatching1.
| Item | CON | LPY | HPY | SEM2 | P value |
|---|---|---|---|---|---|
| WHC3, % | 77.41 | 75.81 | 72.32 | 1.680 | 0.146 |
| CL4, % | 28.26 | 28.01 | 26.96 | 0.500 | 0.209 |
| pH | 6.22 | 6.16 | 6.35 | 0.080 | 0.333 |
| Surface color (L*) | 58.61 | 61.54 | 61.04 | 0.610 | 0.271 |
| Surface color (a*) | 11.89 | 10.45 | 11.66 | 0.640 | 0.283 |
| Surface color (b*) | 11.61 | 12.73 | 12.15 | 0.760 | 0.597 |
Values are the mean of six replicates per treatment (N=270)
Pooled standard error of mean
CON: control diet (phosphorous-sufficient diet with dicalcium phosphate)
LPY: phosphorous-deficient diet with 0.01% phytase (500 FTU/kg) without dicalcium phosphate
HPY: phosphorous-deficient diet with 0.02% phytase (1000 FTU/kg) without dicalcium phosphate
Water Holding capacity
Cooking loss
L*-Lightness, a*- Redness, b*- Yellowness (meat color) a, b Means in the same row with different superscripts differ significantly (P<0.05)
The outcome of broilers fed different diet on the ratio of feed P to excreta P levels on day 35 is shown in Fig. 1. Results showed that the broilers fed HPY attained the least (P<0.05) fecal P content, which was proportionate to the feed P content, compared to broilers fed CON and LPY on day 35. In contrast, broilers fed CON showed the highest (P<0.05) P excretion, which was proportionate to the feed P content on day 35.
Fig. 1.
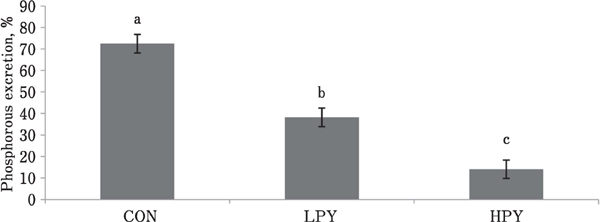
Effect of diets supplemented with different amounts of phytase on phosphorous content in excreta that is proportional to the phosphorous content in feed of broilers on day 35 after hatching. Values are the mean of six replicates per treatment (N=270).
CON: control diet (phosphorous-sufficient diet with dicalcium phosphate)
LPY: phosphorous-deficient diet with 0.01% phytase (500 FTU/kg) without dicalcium phosphate
HPY: phosphorous-deficient diet with 0.02% phytase (1000 FTU/kg) without dicalcium phosphate
a, b Means represented by the bars with different superscripts differ significantly (P<0.05).
Discussion
Reduction of inorganic P usage in broiler diets using microbial phytase has been extensively debated (Scholey et al., 2018). Furthermore, previous studies have shown that microbial phytase supplementation in P-deficient diets affect growth performance and bone mineralization of broilers (Anjum et al., 2018; Scholey et al., 2018). Waldroup, (1999) noted that broilers fed P-deficient diet supplemented with phytase excreted less P while performance of these broilers was improved. However, these studies did not focus on the effect of phytase on broiler meat quality, visceral organs weight, and growth performance when inorganic P sources were completely eliminated from broiler diet. Therefore, the present study aimed to determine the feasibility of using phytase in P-deficient diet for obtaining better growth performance, tibia characteristics, and meat quality by improving P utilization.
Interestingly, improved growth performance was observed in broilers fed phytase-supplemented diet. This is in agreement with the results of Viveros et al. (2002) who showed that the growth performance of broilers fed P-deficient diet supplemented with 500 FTU/kg phytase was better than that of broilers fed P-sufficient diet on day 42. Furthermore, many previous studies (Waldroup et al., 2000, Zyla et al., 2000) have reported that the ADG of broilers fed P-deficient diet supplemented with phytase was higher than that of broilers fed P-deficient diet without phytase. Improved growth performance was noted when phytase was added to the P-deficient diets, which might be due to increased supply of P during growth (Onyango et al., 2004). Furthermore, Nagata et al. (2011) has reported that phytase can improve growth performance by supplying better nutrients (mineral, protein) and promoting energy utilization in Cobb broilers for 42 days. In summary, 500 FTU/kg improved ADG, whereas 1000 FTU/kg amended the FCR of broilers on day 35.
A recent study indicated that phytase enhanced the length of the tibia. Qian et al. (1996) mentioned that broilers fed P-deficient diet had shorter tibia on day 21, which clearly indicates the importance of P regarding tibia length. Furthermore, Qian et al. (1997) observed that broilers (Peterson × Arbor Acres) fed P-deficient diet with 1050 FTU/kg phytase possessed long tibia on day 21. This indicated that the young broilers required high P levels for improving tibia length. Our results showed that longer tibias were obtained when broilers were fed diet supplemented 500 FTU/kg phytase along with a P-deficient diet on day 35.
In the present study, dietary treatments did not significantly alter the tibia ash content from the time of hatching to day 35 post-hatching. In agreement with this observation, Onyango et al. (2004) and Sebastian et al. (1996) demonstrated that broilers (Ross 308) fed P-sufficient diet showed similar tibia ash content along with broilers fed P-deficient diet supplemented with 500 FTU/kg on days 21 and 35, respectively. These results indicated that broilers fed P-deficient diet supplemented with 500 FTP/kg phytase completed bone mineralization on day 35. In contrast, tibia Ca and P content increased in broilers fed phytase-supplemented diet. Mondal et al. (2007) demonstrated that the tibia P and Ca levels were higher in broilers (Cobb) fed P-deficient diet supplemented with 500 FTU/kg phytase than in those fed P-sufficient diet on day 42. Interestingly, Sebastian et al. (1996) observed that the Ca and P levels in tibia ash were higher in broilers fed diet supplemented with phytase instead of shaft of tibia, indicating that the head is the most active part in tibia. These results suggested that the availability of Ca and P from the phytase-mineral complex in feed stuffs was increased in broilers fed phytase-supplemented diet, which supported bone mineralization.
Dietary phytase did not alter the carcass, breast muscle, and thigh muscle yields of the broilers on day 35. In agreement with this observation, several previous studies (Angel et al., 2006; Nourmohammadi et al., 2010) have shown that broilers fed P-deficient diet supplemented with up to 1000 FTU/kg phytase did not affect carcass parameters on day 35. In contrast, Ravindran et al. (2001) suggested that incorporation of phytase into the diet may liberate the lysine from the phytase-protein complex. Lysine is one of the most important amino acids involved in accretion of breast muscle (Nourmohammadi et al., 2010). The contrasting results obtained might be probably due to differences in the amino acid composition of the basal diet between the present and previous studies.
In the present study, the liver weight of broilers fed phytase diet was higher than those of broilers that did not receive phytase. The study of Akyurek et al. (2011) observed that Ross broilers fed P-deficient diet supplemented with 500 FTU/kg phytase showed higher liver weight than broilers fed an adequate P diet on day 21. Higher liver weight is associated with increased storage of glycogen in hepatic tissues (Nourmohammadi et al., 2010). Therefore, phytase may increase glycogenesis by enhancing the influx of glucose 6-phosphate in to the glycogen synthesis pathway while simultaneously inhibiting glycolysis (Fushimi et al., 2001).
Dietary supplementation with phytase did not affect the meat quality parameters of broilers on day 35 in this study. Similarly, Han et al. (2009) and Attia, (2003) reported that broilers fed a diet with 500 FTU/kg phytase did not display a significant effect on meat quality parameters (water holding capacity, pH, cooking loss, and meat color) compared to those fed phytase-minus diet.
Anjgel et al. (2006) also observed reduction in litter P content in broilers fed a diet containing 600 FTU/kg phytase compared to that in broilers fed a diet without phytase at 49 days of age. Corn-soybean diets are rich in phytic acid, which is not available to non-ruminant animals. The ability of poultry and pigs to utilize phytate P is poor (Wu et al., 2003; Ravindran et al., 2006) due to insufficient quantities or lack of intestinal phytase. As a result, large amounts of P are excreted in feces, causing environmental hazard, especially in areas of intense livestock operations. Simons et al. (1990) and Um et al. (2000) reported that supplementation of phytase may lower P excretion by more than 40%, thereby improving P availability to the bird and increasing the retention of Ca and P (Lim et al., 2001). These observations support the findings of our study.
In conclusion, fecal P excretion, along with Ca and P retention in the tibia, was improved in broilers fed P-deficient diet (available P: 0.30%) supplemented with up to 500 FTU/kg phytase, which enhanced the growth performance from the time of hatching to day 35 post-hatch.
Acknowledgements
The authors acknowledge the support provided by New Hope Lanka Ltd., Sri Lanka, BSAF, Germany, and the Lankem Ceylon PLC.
References
- Adeola O, Cowieson AJ. Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. Journal of Animal Science, 89: 3189-3218. 2011. [DOI] [PubMed] [Google Scholar]
- Akyurek H, Ozduven ML, Okur AA. The effect of supplementing an organic acid blend and/or microbial phytase to a corn-soybean based diet fed to broiler chickens. African Journal of Agricultural Research 6: 642-649. 2011. [Google Scholar]
- Angel R, Saylor WW, Mitchell AD, Powers W, Applegate TJ. Effect of dietary phosphorus, phytase, and 25-hydroxycholecalciferol on broiler chicken bone mineralization, litter phosphorus, and processing yields. Poultry science, 85: 1200-1211. 2006. [DOI] [PubMed] [Google Scholar]
- Anjum MI, Javaid S, Nadeem MA. Effect of supplementing microbial phytase on broiler chicks fed low di-calcium phosphate diets. Pakistan Journal of Zoology, 50: 347-351. 2018. [Google Scholar]
- Attia YA. Performance, carcass characteristics, meat quality and plasma constituents of meat type drakes fed diets containing different levels of lysine with or without a microbial phytase. Archives of Animal Nutrition, 57: 39-48. 2003. [DOI] [PubMed] [Google Scholar]
- Avigen. Indian River®: Broiler Management Handbook. Aviagen Inc., Huntsville, AL: 2014. [Google Scholar]
- Cowieson AJ, Adeola O. Carbohydrases, protease, and phytase have an additive beneficial effect in nutritionally marginal diets for broiler chicks. Poultry Science, 84: 1860-1867. 2005. [DOI] [PubMed] [Google Scholar]
- Fushimi T, Tayama K, Fukaya M, Kitakoshi K, Nakai N, Tsukamoto Y, Sato Y. Acetic acid feeding enhances glycogen repletion in liver and skeletal muscle of rats. Journal of nutrition, 131: 1973-1977. 2001. [DOI] [PubMed] [Google Scholar]
- Han JC, Yang XD, Zhang LM, Li WL, Zhang T, Zhang ZY, Yao JH. Effects of 1α-hydroxycholecalciferol and phytase on growth performance, tibia parameter and meat quality of 1-to 21-d-old broilers. Asian-Australasian Journal of Animal Science, 22: 857-864. 2009. [Google Scholar]
- Houshmand M, Azhar K, Zulkifli I, Bejo MH, Meimandipour A, Kamyab A. Effects of non-antibiotic feed additives on performance, tibial dyschondroplasia incidence and tibia characteristics of broilers fed low-calcium diets. Journal of Animal Physiology and Animal Nutrition, 95: 351-358. 2011. [DOI] [PubMed] [Google Scholar]
- Jayasena DD, Jung S, Kim HJ, Bae YS, Yong HI, Lee JH, Kim JG, Jo C. Comparison of quality traits of meat from Korean native chickens and broilers used in two different traditional Korean cuisines. Asian-Australasian Journal of Animal Sciences, 26: 1038 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornegay ET, Denbow DM, Yi Z, Ravindran V. Response of broilers to graded levels of microbial phytase added to maize–soyabean-meal-based diets containing three levels of nonphytate phosphorus. British Journal of Nutrition. 75: 839-52. 1996. [DOI] [PubMed] [Google Scholar]
- Lakshani P, Jayasena DD, Jo C. Comparison of quality traits of breast meat from commercial broilers and spent hens in Sri Lanka. Korean Journal of Poultry Science. 43: 55-61. 2016. [Google Scholar]
- Lim HS, Namkung H, Um JS, Kang KR, Kim BS, Paik IK. The effects of phytase supplementation on the performance of broiler chickens fed diets with different levels of non-phytate phosphorus. Asian-Australasian Journal of Animal Sciences. 14: 250-257. 2001. [Google Scholar]
- McGlone J. Guide for the care and use of agricultural animals in research and teaching. 3rd ed. Federation of Animal Science Societies; Chapaign: 2010 [Google Scholar]
- Mondal MK, Panda S, Biswas P. Effect of microbial phytase in soybean meal based broiler diets containing low phosphorous, International Journal of Poultry Science, 6: 201-206. 2007. [Google Scholar]
- Morgan NK, Walk CL, Bedford MR, Burton EJ. Contribution of intestinal- and cereal-derived phytase activity on phytate degradation in young broilers. Poultry Science. 94: 1577-1583. 2015. [DOI] [PubMed] [Google Scholar]
- Mrigen D. Feed cost savings via the phytase matrix. Poultry International, 48: 18-19. 2009. [Google Scholar]
- Nagata A, Rodrigues PB, Alvarenga RR. Carcass characteristics of broilers at 42 days receiving diets with phytase in different energy and crude protein levels, Ciênc Agrotec Lavras, 35: 575-581. 2011. [Google Scholar]
- National Research Council. Nutrient Requirements of Poultry Ninth Revised Edition National Academy Press; Washington DC: 1994. [Google Scholar]
- Nelson TS, Shieh TR, Wodzinski RJ, Ware JH. Effect of supplemental phytase on the utilization of phytate phosphorus by chicks. Journal of Nutrition, 101: 1289-1294. 1971. [DOI] [PubMed] [Google Scholar]
- Nourmohammadi R, Hosseini SM, Farhangfar H. Influence of citric acid and microbial phytase on growth performance and carcass characteristics of broiler chickens. American Journal of Animal and Veterinary Sciences, 5: 282-288. 2010. [Google Scholar]
- Olukosi O, Cowieson AAJ, Adeola O. Age-related influence of a cocktail xylanase, amylase, and protease or phytase individually or in Combination in broilers. Poultry science. 86: 77-86. 2007. [DOI] [PubMed] [Google Scholar]
- Önenç A, Serdaroğlu M, Abdraimov K. Effect of various additives to marinating baths on some properties of cattle meat. European Food Research and Technology, 218: 114-117. 2004. [Google Scholar]
- Onyango EM, Bedford MR, Adeola O. The yeast production system in which Escherichia coli phytase is expressed may affect growth performance, bone ash, and nutrient use in broiler chicks. Poultry Science, 83: 421-427. 2004. [DOI] [PubMed] [Google Scholar]
- Orban JI, Adeola O, Stroshine R. Microbial phytase in finisher diets of White Pekin ducks: Effects on growth performance, plasma phosphorus concentration, and leg bone characteristics. Poultry Science, 78: 366-377. 1999. [DOI] [PubMed] [Google Scholar]
- Pieniazek J, Smith KA, Williams MP, Manangi MK, Vazquez-Anon M, Solbak A, Miller M, Lee JT. Evaluation of increasing levels of a microbial phytase in phosphorus deficient broiler diets via live broiler performance, tibia bone ash, apparent metabolizable energy, and amino acid digestibility. Poultry Science, 96: 370-382. 2016. [DOI] [PubMed] [Google Scholar]
- Qian H, Kornegay ET, Denbow DM. Utilization of phytate phosphorus and calcium as influenced by microbial phytase, choleccalciferol, and the calcium: total phospphorus ratio in broiler diets. Poultry Science, 76: 37-46. 1997. [DOI] [PubMed] [Google Scholar]
- Qian H, Veit HP, Kornegay ET, Ravindran V, Denbow DM. Effects of supplemental phytase and phosphorus on histological and other tibial bone characteristics and performances of broilers fed semi-purified diets. Poultry Science. 75: 618-626. 1996. [DOI] [PubMed] [Google Scholar]
- Quinlan KP, DeSesa MA. Spectrophotometric determination of phosphorus as molybdovanadophosphoric acid. Analytical Chemistry, 27: 1626-1629. 1955. [Google Scholar]
- Rath NC, Huff GR, Huff WE, Balog JM. Factors regulating bone maturity and strength in poultry. Poultry Science, 79: 1024-1032. 2000. [DOI] [PubMed] [Google Scholar]
- Ravindran V, Bryden WL, Kornegay ET. Phytates: Occurrence, bioavailability and implications in poultry nutrition. Poultry and Avian Biology Reviews, 6: 125-143. 2006. [Google Scholar]
- Ravindran V, Selle PH, Ravindran G, Morel PCM, Kies AK. Microbial phytase improves performance, apparent metabolizable energy and ileal amino acid digestibility of broilers fed a lysine-deficient diet. Poultry Science, 80: 338-344. 2001. [DOI] [PubMed] [Google Scholar]
- Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant and Soil, 321: 305-339. 2009. [Google Scholar]
- Scholey DV, Morgan NK, Riemensperger A, Hardy R, Burton EJ. Effect of supplementation of phytase to diets low in inorganic phosphorus on growth performance and mineralization of broilers. Poultry Science, 97: 2435-2440. 2018. [DOI] [PubMed] [Google Scholar]
- Sebastian S, Touchburn SP, Chavez ER, Lague PC. The effects of supplemental microbial phytase on the performance and utilization of dietary calcium, phosphorus, copper, and zinc in broiler chickens fed corn-soybean diets. Poultry Science, 75: 729-736. 1996. [DOI] [PubMed] [Google Scholar]
- Sharpley AN, Chapra SC, Wedepohl R, Sims JT, Daniel TC, Reddy KR. Managing agricultural phosphorus for protection of surface waters: Issues and options. Journal of Environmental Quality. 23: 437-451. 1994. [Google Scholar]
- Simons PC, Versteegh HA, Jongbloed A, Kemme PA, Slump P, Bos KD, Wolters MG, Beudeker RF, Verschoor GJ. Improvement of phosphorus availability by microbial phytase in broilers and pigs. British Journal of Nutrition, 64: 525-540. 1990. [DOI] [PubMed] [Google Scholar]
- Van der Klis JD, Versteegh HAJ. Phosphorus nutrition of poultry. 1996. [Google Scholar]
- Viveros A, Brenes A, Arija I, Centeno C. Effects of microbial phytase supplementation on mineral utilization and serum enzyme activities in broiler chicks fed different levels of phosphorus. Poultry Science, 81: 1172-1183. 2002. [DOI] [PubMed] [Google Scholar]
- Um JS, Lim HS, Ahn HS, Paik HS. Effects of microbial phytase supplementation to low phosphorus diets on the performance and utilization of nutrients in broiler chickens. Asian Australasian Journal of Animal Sciences. 13: 824-829. 2000. [Google Scholar]
- Waldroup P. Nutritional approaches to reducing phosphorus excretion by poultry. Poultry Science, 78: 683-691. 1999. [DOI] [PubMed] [Google Scholar]
- Waldroup PW, Kersey JH, Saleh EA, Fritts CA, Yan F, Stilborn HL, Crum RC, Jr, Raboy V. Nonphytate phosphorus requirement and phosphorus excretion of broiler chicks fed diets composed of normal or high available phosphate corn with and without microbial phytase. Poultry Science, 79: 1451-1459. 2000. [DOI] [PubMed] [Google Scholar]
- Wu YB, Pierce J, Hendriks WH, Ravindran V. Compparison of in vitro nutrient release by three enzyme preparations in wheatand maize-based diets. Proceeding of Australian Poultry Science Symposium, 15: 114-118. 2003. [Google Scholar]
- Zyla K, Wikiera A, Koreleski J, Swiatkiewicz S, Piironen J, Ledoux DR. Comparison of the efficacies of a novel Aspergillus niger mycelium with separate and combined effectiveness of phytase, acid phosphatase, and pectinase in dephosphorylation of wheat-based feeds fed to growing broilers. Poultry Science, 79: 1434-43. 2000. [DOI] [PubMed] [Google Scholar]


