Abstract
MicroRNAs (miRNAs) are small, non-coding RNA molecules that inhibit protein translation from target mRNAs. Accumulating evidence suggests that miRNAs can regulate a broad range of biological pathways, including cell differentiation, apoptosis, and carcinogenesis. With the development of miRNAs, the investigation of miRNA functions has emerged as a hot research field. Due to the intensive farming in recent decades, chickens are easily influenced by various pathogen transmissions, and this has resulted in large economic losses. Recent reports have shown that miRNAs can play critical roles in the regulation of chicken diseases. Therefore, the aim of this review is to briefly discuss the current knowledge regarding the effects of miRNAs on chickens suffering from common viral diseases, mycoplasmosis, necrotic enteritis, and ovarian tumors. Additionally, the detailed targets of miRNAs and their possible functions are also summarized. This review intends to highlight the key role of miRNAs in regard to chickens and presents the possibility of improving chicken disease resistance through the regulation of miRNAs.
Keywords: chicken, miRNA, necrotic enteritis, ovarian tumor, virus disease
Introduction
Poultry rearing is an industry that is superior to other sectors in agriculture, and recently, this industry has undergone tremendous development (Uddin et al., 2010; Landoni and Albarello, 2015). Commercial poultry production is a very intensive animal agricultural system, and one poultry house or barn can contain as many as 100,000 commercial layers or broilers (Landoni and Albarellos, 2015). Given this, mortality of chickens due to various infectious and non-infectious diseases is emerging as the major constraint against profitable poultry production. For example, Marek's disease (MD) and lymphoid leukosis (LL) are both viral diseases that affect chickens to cause tumors and high mortality (Kreager, 1998). Infectious bursal disease (IBD) is one of the most prevalent infectious virus diseases, causing bursal necrosis and immunosuppression that lead to severe damage to the immune system in chickens (Xu et al., 2019). Avian influenza (AI) is also a highly contagious viral disease that is responsible serious animal health crises that result in high fatality rates in poultry worldwide (Paul et al., 2019). Avian mycoplasmosis is another disease that can cause chronic respiratory disease (CRD) in chickens (Ley, 2008), and necrotic enteritis (NE) is a ubiquitous poultry disease caused by Clostridium perfringens that affects the intestines of chickens, resulting in major impacts on performance (Whelan et al., 2018). Given these examples and the prevalence of diseases affecting chickens, it is clear that disease control and prevention at all levels must be a major focus for the poultry industry.
MicroRNAs (miRNAs) are small, non-coding RNAs. In animals, miRNAs are approximately 22 nucleotides in length, and they play vital roles in almost all developmental and pathological pathways (Jansson and Lund, 2012). MiRNAs play an important role in mRNA degradation, where they can bind to the three prime untranslated regions (3′-UTR) of various mRNA transcripts to reduce translation (Bartel, 2004). It has been reported that within a given genome, 20%–30% of genes are regulated by miRNA (Enright et al., 2003), and a single miRNA may target more than 100 mRNAs (Friedman et al., 2009). Therefore, identification of miRNA functions in different organisms is a critical step to facilitate our understanding of genome organization, genome biology, and evolution (Carrington and Ambros, 2003).
In the past ten years, miRNAs have been discovered to possess multiple functions in chickens, including the regulation of development and various growth processes (Bannister et al., 2011; Luo et al., 2014). Additionally, in recent decades researchers have reported that miRNAs play diverse roles in chicken diseases and immune system functions (Ahanda et al., 2009; Hicks et al., 2009; Dinh et al., 2014; Liu et al., 2016). The majority of these studies investigated the possible mRNAs that could be targeted by a given miRNA and the underlying mechanisms responsible for the observed effects. Thus, this review aims to introduce the functions of miRNAs in the context of chickens, and particular focus is placed on the advances related to the impact of miRNAs on various diseases that affect chickens. The identification of distinct miRNA expression patterns and the roles of those dysregulated miRNAs in the context of pathological conditions that affect chickens will broaden our understanding of how miRNAs regulate the chicken immune system and reveal potential therapeutic targets for the treatment of miRNA-related diseases.
MiRNA Biogenesis
The biogenesis of miRNA has been elaborately reviewed in the existing literature (Bushati and Cohen, 2007; Lin and Gregory, 2015). Briefly, miRNA begins from a primary transcript, termed the pri-miRNA, that is typically a long mRNA that is thousands of nucleotides in length and transcribed by RNA polymerase II (only a few miRNAs are transcribed by RNA polymerase III) (Magenta et al., 2013). Then, the pri-miRNA is processed by the nuclear RNase III Drosha-DGCR8 complex into an approximately 65 nucleotide hairpin precursor miRNA (pre-miRNA) (Denli et al., 2004). Next, the pre-miRNAs are exported from the nucleus into the cytoplasm by exportin 5 and further processed by DICER1, an RNase III enzyme that measures from the 5’ and 3’ ends of the pre-miRNA to form the mature ∼22 nucleotide miRNA: miRNA* (the complementary strand of miRNA) duplex (Lin and Gregory, 2015). The miRNA* is typically degraded, while the mature miRNA is incorporated into the RNA-induced silencing complex (RISC) (Bartel, 2004). MiRNAs play an important role in mRNA degradation, as they can bind to the 3′-UTR of mRNA transcripts to reduce translation (Bartel, 2004) (Fig. 1).
Fig. 1.
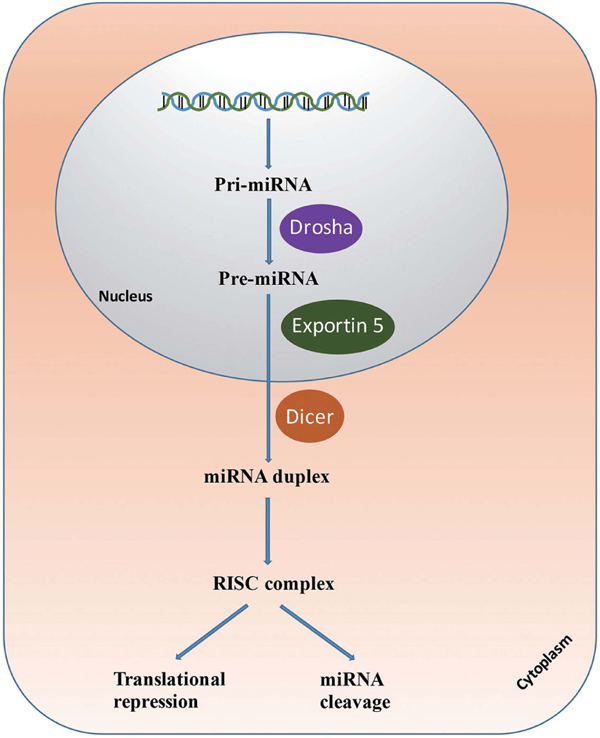
miRNA biogenesis paradigm
MiRNAs Involved in Chicken Virus Diseases
The interactions among miRNAs and viruses are highly complicated. In some instances, viruses may encode their own miRNA to facilitate their pathogenesis (Muylkens et al., 2010). In other instances, however, host miRNAs can directly target viral RNAs during infections or they can regulate various immune responses to inhibit virus replication (Wang et al., 2010; Ingle et al., 2015; Wang et al., 2018).
MiRNA and Infectious Bursal Disease
Infectious bursal disease (IBD) is an acute, highly contagious disease in young chickens (Pitcovski et al., 2003). Infectious bursal disease virus (IBDV), which destroys B-lymphocyte precursors and causes a high degree of immunosuppression, is considered to be one of the major threats to the poultry industry (Wang et al., 2018). IBDV contains two segments of double-stranded RNA (A and B) (Hirai and Shimakura, 1974, Azad et al., 1985). Segment A possesses two partially overlapping open reading frames (ORFs) (Kibenge et al., 1991), while segment B encodes an RNA-dependent RNA polymerase, viral protein 1 (VP1) (von Einem et al., 2004). Recent studies have revealed that miRNAs may suppress IBDV replication by targeting the viral genome segments and host cellular SOCSs (suppressor of cytokine signaling). SOCSs are considered to be key physiological regulators of the immune system and can negatively regulate type I interferon (IFN) (Fu et al., 2017) that allows host cells to recognize viral dsRNA through the action of TLR3 or RIG-I molecules (Li et al., 2013) to combat viral infection. For example, according to Wang et al., gga-miR-155 could inhibit IBDV replication and enhance the expression of IFN via targeting SOCS1 and TANK (TNF receptor-associated factor family member-associated NF-κB activator) in response to IBDV infection (Table 1, Wang et al., 2018). Additionally, gga-miR-130b could suppress IBDV replication via targeting a specific sequence of IBDV segment A and enhancing the expression of IFN-β by binding to the host SOCS5 (Table 1, Fu et al., 2017). Similarly, gga-miR-454 was found to inhibit IBDV replication by binding to a specific sequence of IBDV segment B and enhancing the expression of IFN-β by targeting cellular SOCS6 (Table 1, Fu et al., 2018). Additionally, gga-miR-21 could target viral genomic RNA and inhibit VP1 translation to lower IBDV replication (Table 1, Wang et al., 2013a).
Table 1. Validated targets of miRNA relevant to the immunity of chickens.
| miRNA | immune function | Tissue/ cell | Targets | Reference |
|---|---|---|---|---|
| gga-miR-155 | inhibits IBDV replication | DF-1 cell | SOCS1, TANK | Wang et al., 2018 |
| gga-miR-130b | inhibits IBDV replication | DF-1 cell | SOCS5, the specific sequence of IBDV segment A | Fu et al., 2017 |
| gga-miR-454 | inhibits IBDV replication | DF-1 cell | SOCS6, the specific sequence of IBDV segment B | Fu et al., 2018 |
| gga-miR-21 | inhibits IBDV replication | DF-1 cell | VP1 of IBDV genome | Wang et al., 2013a |
| gga-miR-9* | promotes IBDV replication | DF-1 cell | IRF2 | Ouyang et al., 2015 |
| gga-miR-2127 | promotes IBDV replication | DF-1 cell | p53 | Ouyang et al., 2017 |
| gga-miR-142-5p | promotes IBDV replication | DT40 cell | chMDA5 | Ouyang et al., 2018 |
| gga-miR-15b | regulates MDV1-induced tumorigenesis | spleen | ATF2 | Tian et al., 2012 |
| gga-miR-26a | suppresses MD lymphoma cell proliferation | MSB-1 cell | NEK6 | Li et al., 2014b |
| gga-miR-181a | suppresses MD lymphoma cell proliferation | MSB-1 cell | MYBL1 | Lian et al., 2015 |
| gg-miR-130a | suppresses MD lymphoma cell proliferation | MSB-1 cell | HOXA3 | Han et al., 2016 |
| gga-miR-219b | suppresses MD lymphoma cell proliferation, migration and invasion | MSB-1 cell | BCL11B | Zhao et al., 2017a |
| MDV1-miR-M4-5p | promotes MDV1-induced tumorigenesis | CEF and DF-1 cells | LTBP1, hnRNPAB | Chi et al., 2015; Dang et al., 2017 |
| MDV1-miR-M4-5p | modulates immune response to MDV infection | DF-1, MDCC-MSB-1 and MDCC-54-O cell | GPM6B, RREB1, c-Myb, MAP3-K7IP2, PU.1, C/EBP | Muylkens et al., 2010 |
| gga-miR-485 | inhibits H5N1 replication and dampens RIG-I-dependent inflammatory response | HEK 293T cell, A549 cell, MCF7 cell | PB1 of H5N1 and RIG-I of host | Ingle et al., 2015 |
| gga-miR-1249 | inhibits H1N1 replication | HEK 293T cell, A549 cell, MDCK cell | PB2 of H1N1 | Wang et al., 2017 |
| gga-miR-375 | suppresses proliferation and promotes apoptosis of cells infected with ALV-J | DF-1 cell | YAP1 | Li et al., 2014c |
| gga-miR-221 and -222 | promotes proliferation and inhibits apoptosis of cells infected with ALV-J | DF-1 cell | BMF | Dai et al., 2015 |
| gga-miR-1650 | inhibits ALV-J replication and infection | DF-1 cell | ALV-J genome | Wang et al., 2013b |
| gga-miR-23b | inhibits ALV-J replication and infection | chicken spleen, DF-1 cell and HD11 cell | IRF1 | Li et al., 2015 |
| gga-miR-34b-5p | promotes ALV-J-infected cell proliferation and ALV-J replication | DF-1 cell | MDA5 | Li et al., 2017 |
| gga-miR-101-3p | suppresses the proliferation of chicken embryonic fibroblast infected with MG | DF-1 cell | EZH2 | Chen et al., 2015 |
| gga-miR-99a | suppresses the proliferation of chicken embryonic fibroblast infected with MG | DF-1 cell | SMARCA5 | Zhao et al., 2017c |
| gga-miR-19a | activates NF-κB signaling pathway, promotes TNF-α expression and enhances cell cycle progression and proliferation of cells infected with MG | DF-1 cell | ZMYND11 | Hu et al., 2016 |
| gga-miR-130b-3p | promotes MG-infected cell proliferation | DF-1 cell | PTEN | Yuan et al., 2018 |
| gga-miR-146c | activates TLR6/MyD88/NF-κB signaling pathway and promotes proliferation by inhibiting apoptosis of cells infected with MG | DF-1 cell | MMP16 | Zhang et al., 2019 |
| gga-miR-1615 | regulates ovarian tumorigenesis | chicken ovary | AvBD-11 | Lim et al., 2013 |
In contrast, gga-miR-9* negatively regulated the host antiviral innate immune response by suppressing type I IFN production by targeting IRF2 (interferon regulatory factor 2), an important factor that functions in IFN antiviral signal transduction pathways, to promote IBDV replication (Table 1, Ouyang et al., 2015). gga-miR-2127 overexpression also promoted IBDV replication and could down-regulate the expression of antiviral innate immunity genes by targeting p53 (Table 1, Ouyang et al., 2017). Additionally, gga-miR-142-5p targeted chMDA5 (chicken melanoma differentiation-associated gene 5), which encodes a protein that recognizes RNA viral infections and initiates an antiviral innate immune response, to promote IBDV replication in an IRF7-dependent pathway (Table 1, Ouyang et al., 2018).
MiRNA and Marek's Disease
Marek's disease (MD) is a type of chicken lymphoma that is caused by Marek's disease virus 1 (MDV1), an α-herpesvirus (Osterrieder et al., 2006), that results in immunosuppression, neurological disorders, and rapid-onset CD4 + T-cell lymphoma (Biggs, 1997). MD was the first tumor disease that could be prevented by vaccination, and it provides an important animal model for the study of viral cancer development and immunity (Osterrieder et al., 2006).
In recent decades, numerous studies have been performed to investigate the involvement of miRNAs (both host and MDV miRNAs) in MD tumorigenesis. Through the use of microarrays and qRT-PCR analysis, two clades of chicken miRNAs were found to be increased in splenic tumors and non-tumorous spleen tissues derived from MDV1-infected chickens (Li et al., 2014a). Additionally, chicken lines 63 and 72 are two highly inbred lines of specific-pathogen-free white leghorn chickens that are resistant or susceptible, respectively, to MD tumors. According to Tian et al. (2012), gga-miR-15b, which targets ATF2 (activating transcription factor 2), was reduced in MDV-infected susceptible chicken splenic tumors. To further understand the potential functions of miRNAs in MDV resistance and susceptibility, the expression of ATF2, a protein that can interact with MDV oncogene Meq, was determined. The results of this study revealed that the protein level of ATF2 was significantly increased in infected line 72 chickens compared to that of MDV-free chickens, while its expression was stable in line 63 chicken before and after MDV challenge. Chicken ATF2 was observed to form a heterodimer with c-Jun, the MDV oncogene Meq, and other b-ZIP proteins (Huguier et al., 1998; Levy et al., 2003; Osterrieder et al., 2006). It has been reported that ATF2 exerts tumor suppressor or oncogene activities in different cancers by cooperation with other tumor suppressors or oncogenes (Bhoumik et al., 2004; Bhoumik and Ronai, 2008). As the MDV oncogene Meq was highly expressed in line 72 after MDV infection, ATF2 likely formed a heterodimer with Meq to promote MD development, and in chicken line 63, due to the absence of Meq after MDV exposure, ATF2 may cooperate with c-Jun to facilitate distinct functions, similar to observations in line 72. Taken together, the results of this study suggested that gga-miR-15b may target ATF2 to regulate MD resistance/susceptibility (Tian et al., 2012). Recently, gga-miR-219b was found to be decreased in MDV-induced lymphoma. Additionally, gga-miR-219b was demonstrated to target BCL11B (B-cell chronic lymphocytic/lymphoma 11B), a gene that not only plays an important role in thymocyte development but has also been implicated in lymphoproliferative diseases (Grabarczyk et al., 2007; Gutierrez et al., 2011). The knockdown of BCL11B also reduced the proliferation, migration, and invasion of the Marek's disease tumor cell MSB1. The inhibition of gga-miR-219b was further found to down-regulate the expression of the MDV oncogene Meq; however, BCL11B knockdown significantly induced Meq expression (Table 1, Zhao et al., 2017a). In other studies, gga-miR-26a, -181a, and -130a were similarly reported to inhibit the proliferation of MD lymphoid cells by NEK6 (never in mitosis gene A-related kinase 6), MYBL1 (v-myb myeloblastosis viral oncogene homolog-like 1), and HOXA3 (homeobox A3), respectively (Table 1, Li et al., 2014b; Lian et al., 2015; Han et al., 2016). Additionally, it is known that miRNAs can be packaged into exosomes, a subset of extracellular vesicles that are of 30–150 nm in diameter and secreted by various cell types. Thus, exosome-mediated transport of miRNAs may provide a novel mechanism of intercellular gene regulation. According to Nath Neerukonda et al., serum exosomes were isolated from MDV-infected chickens that were either vaccinated against MD or unvaccinated and bearing MDV-induced tumors. When detecting the expression of various miRNAs within exosomes, researchers observed that exosomes from vaccinated chickens exhibited greater expression of tumor suppressor miRNA (gga-miR-146b) and less expression of oncomiR (gga-miR-21) compared to those obtained from unvaccinated controls. Thus, gga-miR-146b and -21 may serve as serum exosome biomarkers for vaccine-induced protection and for MD tumors, respectively (Nath Neerukonda et al., 2019).
In addition to animals, herpesviruses also encode miRNAs. Early in 2006, Burnside and colleagues found that eight miRNAs were encoded by MDV. Five of these miRNAs flank the Meq oncogene, and three of them map to the latency-associated transcript (LAT) region of the virus genome (Burnside et al., 2006). Later, they also identified an additional seven miRNAs and ‘*’ strands by deep sequencing of MDV1 infected CEF cells (Burnside et al., 2008). Currently, twenty-six MDV-1-encoded miRNAs processed from fourteen pre-miRNAs (MDV1-miR-M31 and MDV1-miR-M1 to MDV1-miR-M13) have been identified (http://micro rna.sanger.ac.uk). All of the MDV-1-encoded miRNAs are focused within three gene clusters, including the Meq cluster, the LAT cluster, and the Mid cluster. The first cluster of MDV1-miRNAs, including MDV1-miR-M2, -M3, -M4, -M5, -M9 and -M12, is antisense to the MDV-1 gene RLORF8 and located adjacent to the Meq oncogene (Luo et al., 2010). MDV1-miR-M4 is a functional ortholog of miR-155 (Zhao et al., 2009). Recently, reports have indicated that MDV1-miR-M4-5p could induce the over-expression of the oncogene c-Myc by targeting LTBP 1 (latent TGF-β binding protein 1) and inactivating the TGF-β signaling pathway during MDV1-infection (Table 1, Chi et al., 2015). Further study revealed that MDV1-miR-M4-5p could also target chicken hnRNPAB (heterogeneous nuclear ribonucleoprotein AB) to promote proliferation of both primary CEF and transformed chicken fibroblast DF-1 cells (Table 1, Dang et al., 2017). Additionally, using viruses modified by reverse genetics of the infectious bacterial artificial chromosome (BAC) clone of the oncogenic RB-1B strain of MDV, Zhao and colleagues demonstrated that the deletion of the six-miRNA cluster 1 from the viral genome abolished the oncogenicity of MDA. This loss of oncogenicity may be due to MDV1-miR-M4, as its deletion or a 2-nucleotide mutation within its seed region was sufficient to inhibit the induction of lymphomas. This role was further confirmed through the rescue of oncogenic phenotype by revertant viruses that expressed either the miR-M4 or the cellular homolog gga-miR-155 (Zhao et al., 2011). Using luciferase reporter assays, Muylkens and colleagues found that MDV1-miR-M4-5p could target the 3′UTR of GPM6B (glycoprotein M6-b), RREB1 (Ras-responsive element-binding protein 1), c-Myb, MAP3-k7ip2 (mitogen-activated protein kinase kinase kinase 7-interacting protein 2), PU.1, and C/EBP (CCAAT-enhancer-binding proteins). Additionally, MDV1-miR-M4-5p specifically inhibited the translation of two viral proteins (UL28 and UL32) that are involved in the cleavage and packaging of herpesvirus DNA (Table 1, Muylkens et al., 2010).
MiRNA and Avian Influenza
Avian influenza, also known as “Bird Flu”, is one of the most severe respiratory viral infectious diseases of this decade (Koparde and Singh, 2011). Avian influenza virus (AIV) is a type A virus of the family Orthomyxoviridae. Although wetland birds such as wild ducks, gulls, and shorebirds are the natural hosts of AIV, this virus can also infect poultry birds, resulting in significant economic losses and an increased threat to public health due to the potential for host jumping from animals to humans (Webby and Webster, 2003). MiRNAs have been found to regulate AIV replication during infection. In chickens, when the miRNA expressions between H5N3 infected and non-infected commercial SPF layer chickens were compared at 4 days post-infection, 73 and 36 miRNAs were differentially expressed in lung and trachea, respectively, and there were more miRNAs highly expressed in non-infected tissues (Wang et al., 2009). In subsequent experiments examining broilers, however, a greater number of miRNAs were highly expressed in infected lungs than in non-infected lungs, indicating that the regulatory mechanism of the host response to AIV infection mediated by miRNAs may be different between layers and broilers (Wang et al., 2012). In another study, 1004 miRNAs were obtained from H9N2-infected and non-infected chicken embryo fibroblasts, and 48 miRNAs were expressed differently between the two groups. These miRNAs were predicted to target immune response-related genes (Peng et al., 2015). In recent years, experiments using mammalian cells have demonstrated that host gga-miR-1249 targets the H5N1 and H1N1 gene PB2 that encodes an RNA polymerase required for viral replication to suppress virus replication (Table 1, Wang et al., 2017). Similarly, gga-miR-485 was found to bind to the H5N1 gene PB1 to inhibit H5N1 replication, and gga-miR-485 could also target the host gene RIG-I (retinoic acid-inducible gene I). Basal amounts of RIG-I protein detect viral nucleic acids in the cytosol and induce type I IFNs. In turn, these IFNs increase the expression of IFN-stimulatory genes, including RIG-I. Thus, gga-miR-485 could suppress the RIG-I-dependent expression of type I and type III IFNs in H5N1-infected cells and dampen the RIG-I-dependent inflammatory response (Table 1, Ingle et al., 2015).
MiRNA and Subgroup J Avian Leucosis Virus
Avian leukosis viruses (ALVs) are a group of avian retroviruses that induce tumors in chickens (Liu et al., 2011). Chicken ALVs are classified into six subgroups (A-E and J). ALV-J, which primarily induces myeloid leukosis, causes more serious damage than that caused by the other virus subgroups (Gao et al., 2012). To broaden our understanding of the molecular mechanisms that occur during ALV-J infection, efforts have been made to examine the miRNA expression patterns in chickens that are infected with ALV-J. Through the use of chicken dendritic cells (DC), ALV-J was found to induce apoptosis during the early infection stages. MiRNA sequencing data from ALV-J-infected and -uninfected DCs revealed 122 differentially expressed miRNAs (115 that were up-regulated and 7 exhibiting down-regulation after injection). Through GO analysis, the target genes of these miRNAs, including gga-miR-204, -211, -221 and -6651, were found to be involved in the processes indicated by the ‘antigen processing and presentation of exogenous peptides’ and ‘apoptosis’ GO terms (Liu et al., 2016). Using miRNA microarray analysis, Li and colleagues found that in 10-week-old chickens, 12 miRNAs were differentially expressed within the livers of ALV-J-uninfected and -infected chickens. The expression levels of gga-mir-221, -222, -1456, -1704, -1777, -1790, and -2127 were up-regulated by ALV-J infection, while the expression levels of gga-let-7b, -let-7i, -125b, -375, and -458 were significantly downregulated (Li et al., 2012). Among these differentially expressed miRNAs, gga-miR-375 overexpression was observed to decrease DF-1 cell proliferation and promote DF-1 apoptosis. Through prediction and dual luciferase reporter assays, gga-miR-375 was confirmed to target YAP1 (yes-associated protein 1), an effector of Hippo signaling, that restrains cell proliferation and promotes apoptosis to influence normal cell fate and tumorigenesis (Harvey and Tapon, 2007; Saucedo and Edgar, 2007). Additionally, in vivo assays further indicated that gga-miR-375 was decreased in chicken livers 20 days after ALV-J infection. Simultaneously, the expression levels of cyclin E and DIAP1 (drosophila inhibitor of apoptosis protein 1) were up-regulated after infection. DIAP1 functions in the early embryo to inhibit apoptosis (Yoo et al., 2000). Cyclin E, an important regulator of cell cycle progression, was reported to be increased in the majority of breast tumor tissues and in most leukemia solid tumors (Keyomarsi et al., 1994). Thus, the above findings indicate that gga-miR-375 functions as a tumor suppressor and plays an important role in inhibiting ALV-J tumorigenesis (Table1, Li et al., 2014c). In contrast, in their later study researchers discovered that gga-miR-221 and -222 may be tumor formation-relevant genes that could contribute to tumorigenesis during ALV-J infection by targeting BMF (BCL-2 modifying factor), a gene that exhibits pro-apoptotic function (Table1, Dai et al., 2015).
In addition to control tumorigenesis, chicken miRNAs can also regulate ALV replication directly. The genome of ALV-J is composed of three genes, including gag, pol, and env (Li et al., 2015). Wang and colleagues revealed that gga-miR-1650 could target the 5'UTR of ALV-J, and gga-miR-1650 overexpression could decrease gag protein expression and inhibit ALV-J replication (Table 1, Wang et al., 2013b). The gp85 protein is a viral envelope polypeptide that is encoded by the env gene within the ALV-J genome. As reported, gga-miR-23b expression was up-regulated in ALV-infected chicken spleens and could target IRF1 (interferon regulatory factor 1), a critical regulatory protein of the inflammatory response that functions as a tumor suppressor and is involved in cell cycle progression and apoptosis (Hong et al., 2013). Additionally, gga-miR-23b overexpression resulted in increased gp85 expression, while IRF1 overexpression caused a decrease in gp85 level. These results indicate that gga-miR-23b promoted ALV-J replication by targeting IRF1 and by affecting gp85 expression (Table 1, Li et al., 2015). gga-miR-34b-5p could also target MDA5 (melanoma differentiation-associated gene 5), a gene that encodes a protein that can detect ALV-J infection and trigger the MDA5 signaling pathway, to promote ALV-J-infected cell proliferation. Additionally, MDA5 overexpression significantly decreased env levels and ALV-J virion secretion, while gga-miR-34b-5p overexpression elevated env protein expression after ALV-J infection to induce ALV-J replication (Table 1, Li et al., 2017).
MiRNA Involved in Avian Mycoplasmosis
Mycoplasma, an important prokaryote, can infect humans and a wide range of economically important livestock species (Hu et al., 2016; Nicholas and Ayling, 2016). Mycoplasma gallisepticum (MG) is one of the most important mycoplasmas that is frequently associated with avian CRD (Ley, 2008), which causes severe inflammation in the tracheas and lungs of chickens and turkeys (Davidson et al., 1982; Stipkovits et al., 2012). Recently, several studies have suggested that miRNAs play a key role in MG infection in chicken. By deep sequencing, Zhao and colleagues found that miRNAs are associated with MG infection in chicken lungs at 3 and 10 days post-infection. They found 45 and 68 differentially expressed miRNAs at 3 and 10 days, and these miRNAs targeted 6290 and 7181 genes, respectively. GO, KEGG, miRNA-GO-network, path-net, and gene-net analyses determined that the altered miRNAs regulated a large number of genes that function throughout various signaling pathways including the MAPK pathway, the focal adhesion regulatory pathways, the Wnt pathway, and the JAK/STAT pathway (Zhao et al., 2017b). gga-miR-101-3p was found to be up-regulated in the lungs of MG-infected chicken embryos, and gga-miR-101-3p could target and inhibit the host EZH2 (enhancer of zeste homolog 2), a growth suppressor gene involved in the regulation of cell cycle progression and proliferation, to suppress the proliferation of chicken embryonic fibroblasts by blocking the G1-to-S phase transition (Chen et al., 2015). Additionally, SMARCA5 (SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily A, member 5) is a member of the SWI/SNF family that exhibits ATPase and helicase activities. It has also been reported that SMARCA5 plays an important role in promoting cell proliferation both in vitro and in vivo (Stopka et al., 2000; Gigek et al., 2011). According to a study by Zhao et al., gga-miR-99a was significantly down-regulated in the lungs of MG-infected chicken embryos and could target SMARCA5 to repress the proliferation of DF-1 cells by inhibiting the transition from the G1 phase to the S and G2 phases (Zhao et al., 2017c). Additionally, gga-miR-19a, which targeted the host ZMYND11 (zinc-finger protein, MYND-type containing 11), was found to be up-regulated both in chicken embryonic lungs and in MG-infected DF-1 cells to activate the NF-κB signaling pathway, ultimately promoting TNF-α expression and enhancing cell cycle progression and proliferation to defend against MG infection (Table 1, Hu et al., 2016). gga-miR-130b-3p was also markedly up-regulated in MG-infected DF-1 cells. Luciferase reporter assays confirmed that PTEN (phosphatase and tensin homolog deleted on chromosome ten) was a direct target of gga-miR-130b-3p. As one of the most important tumor suppressors, PTEN is a phosphatase that exhibits both protein and lipid activities, and this protein plays a central role in various cellular functions and in the immune response (Di Cristofano and Pandolfi, 2000). Studies also demonstrated that PTEN exerted its inhibitory effects against cell proliferation by blocking cell cycle progression at the G1 phase by facilitating the down-regulation of cyclins and CDKs proteins (Moon et al., 2004). Thus, the overexpression of gga-miR-130b-3p remarkably promoted MG-infected DF-1 proliferation by binding to PTEN (Table 1, Yuan et al., 2018). Matrix metalloproteins (MMPs), a family of zinc-dependent endopeptidases, are important for a number pathological process such as inflammation, cardiovascular disease, and cancer (Huang et al., 2017; Mali et al., 2017). A recent study revealed that gga-miR-146c was up-regulated in response to MG infection, and MMP16 was validated as the target gene of gga-miR-146c (Zhang et al., 2019). Additionally, using loss- and gain-of-function approaches, Zhang and colleagues also found that gga-miR-146c participated in the activation of the TLR6/MyD88/NF-κB pathway. It was also observed that gga-miR-146c could decrease cell apoptosis and stimulate DF-1 proliferation after MG infection. Taken together, these findings indicated that gga-miR-146c defended against host MG infection by inhibiting MMP16 expression, activating the TLR6/MyD88/NF-κB signaling pathway, and promoting cell proliferation (Table 1, Zhang et al., 2019).
MiRNAs Involved in Chicken Necrotic Enteritis
Necrotic enteritis (NE), commonly caused by Clostridium perfringens, is an acute clostridial disease that causes weight depression, loss of appetite, and sudden death (Hermans and Morgan, 2007). NE has re-emerged as a significant problem as a result of restrictions on the use of antibiotics in the poultry industry (Williams, 2005). In 2014, Dinh and colleagues investigated the effects of miRNAs on NE induced by Eimeria maxima and Clostridium perfringens in two genetically disparate chicken lines (Marek's disease resistant line 63 and Marek's disease-susceptible line 72). Their results demonstrated that miR-215, -217, -194, -200a, -200b, -216a, -216b, and -429 were highly expressed in intestine tissues derived from line 72 and that miR-1782 and -499 were down-regulated in these tissues. In spleen tissues, miR-34b and -1684 were the most up-regulated miRNAs in line 63. Additionally, the immune-related target genes (CXCR5, BCL2, GJA1, TCF12 and TAB3) of these miRNAs were differentially expressed between the two chicken lines, and they were suppressed in the Marek's disease-susceptible chicken line (Dinh et al., 2014). Later, using the same chicken lines, Truong and colleagues explored the effects of TGF-β and miRNAs on NE. Members of the TGF-β family that is composed of TGF-β, BMPs, and SMADs function in the immune response (Escalona et al., 2012). According to their results, TGF-β1-3, BMP1-7, and SMAD1-9 were differentially expressed between the two chicken lines relative to their respective controls. Also, six miRNAs (ggalet-7c, -199, -200a, -200b, -429, and -499) were predicted and validated as the targets of BMP7 (Truong et al., 2017a), indicating that the modulation of the TGF-β signaling pathway through BMP7 and miRNAs may play a role in the complex regulation of signaling pathways involved in the spleens of two NE-induced chicken lines. Additionally, the JAK-STAT signaling pathway is activated by over 40 cytokines and growth factors. This pathway is involved in cell differentiation, proliferation, apoptosis, and the progression of several diseases through its ability to regulate gene expression. In the spleens of chickens from line 63 and 72, 116 JAK-STAT pathway genes were found to be differentially expressed, and 63 mature miRNAs that variably target JAK-STAT pathway genes were also differentially expressed in the spleens of chickens from both lines (Truong et al., 2017b).
MiRNAs Involved in Chicken Ovarian Tumor
The laying hen is the only non-human animal that spontaneously develops ovarian cancer at a high prevalence rate, and this animal can be used as a model for ovary development and tumorigenesis (Johnson and Giles, 2013). Kang and colleagues found that in ovaries isolated from sexually mature (162-d) and sexually immature (42-d) chickens, 93 miRNAs were significantly differentially expressed, and these included gga-miR-1a, 21, 26a, 137, and 375 (Kang et al., 2013). Additionally, in recent years, miRNA has been found to regulate ovarian tumorigenesis. Avian beta-defensin (AvBD) proteins are members of beta-defensin subfamily and can protect against various microorganisms (van Dijk et al., 2008). In a study by Lim et al., gga-miR-1615 could influence AvBD-11 expression via its 3'-UTR, and AvBD-11 was most abundant in the glandular epithelium of endometrioid-type ovarian tumors, but not normal ovaries of laying hens, suggesting that AvBD-11 and gga-miR-1615 may be useful as biomarkers for the diagnosis of ovarian cancer (Table 1, Lim et al., 2013).
Conclusions
Our knowledge regarding the functions of miRNAs in chickens has expanded in the past several decades. Current studies provide promising evidence concerning the ability of miRNAs to regulate immune function and diseases in chickens. Compared to the number of human studies, however, very few studies have been performed in farm animal to determine the mechanisms underlying the function of miRNAs. In these studies, many were limited to the identification of new and differentially expressed miRNAs or to the prediction of possible targets, and only a small number of these studies further validated the function of these miRNAs and the predicted targets experimentally. Recently, new mechanisms of miRNA action have been discovered, including the ability of these molecules to spread throughout the body when transported within exosomes and to act as protein ligands. Thus, continued mechanistic studies are required to more fully assess the impact of these miRNAs on the biological processes in chickens. Progress in the study of the roles and mechanisms of miRNAs in chickens will provide new ideas and targets for the improvement of chicken growth and the treatment of chicken diseases.
Acknowledgments
This work was supported by the Doctoral Science Research Startup Funding of Qingdao Agricultural University (663/1119042). The author thanks Mr. Chundong Dong for the helpful assistance.
References
- Ahanda MLE, Ruby T, Wittzell H, Bed'Hom B, Chausse AM, Morin V, Oudin A, Chevalier C, Young JR, Zoorob R. Non-coding RNAs revealed during identification of genes involved in chicken immune responses. Immunogenetics, 61: 55-70. 2009. [DOI] [PubMed] [Google Scholar]
- Azad AA, Barrett SA, Fahey KJ. The characterization and molecular cloning of the double-stranded RNA genome of an Australian strain of infectious bursal disease virus. Virology, 143: 35-44. 1985. [DOI] [PubMed] [Google Scholar]
- Bannister SC, Smith CA, Roeszler KN, Doran TJ, Sinclair AH, Tizard MLV. Manipulation of estrogen synthesis alters MIR 202* expression in embryonic chicken gonads. Biology of Reproduction, 85: 22-30. 2011. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell, 116: 281-297. 2004. [DOI] [PubMed] [Google Scholar]
- Bhoumik A, Gangi L, Ronai Z. Inhibition of melanoma growth and metastasis by ATF2-derived peptides. Cancer Research, 64 8222-8230. 2004. [DOI] [PubMed] [Google Scholar]
- Bhoumik A, Ronai Z. ATF2: a transcription factor that elicits oncogenic or tumor suppressor activities. Cell Cycle, 7: 2341-2345. 2008. [DOI] [PubMed] [Google Scholar]
- Biggs PM. The Leeuwenhoek Lecture. Marek's disease herpesvirus: oncogenesis and prevention. Philosophical Transactions of The Royal Society B-Biological Sciences, 352: 1951-1962. 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnside J, Bernberg E, Anderson A, Lu C, Meyers BC, Green PJ, Jain N, Isaacs G, Morgan RW. Marek's disease virus encodes MicroRNAs that map to meq and the latency-associated transcript. Journal of Virology, 80: 8778-8786. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnside J, Ouyang M, Anderson A, Bernberg E, Lu C, Meyers BC, Green PJ, Jain N, Isaacs G, Morgan RW. Deep sequencing of chicken microRNAs. BMC Genomics, 9: 185 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushati N, Cohen SM. microRNA functions. Annual Review of Cell and Development Biology, 23: 175 2007. [DOI] [PubMed] [Google Scholar]
- Carrington JC, Ambros V. Role of microRNAs in plant and animal development. Science, 301: 336-338. 2003. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang Z, Bi D, Hou Y, Zhao Y, Sun J, Peng X. gga-miR-101-3p plays a key role in Mycoplasma gallisepticum (HS Strain) infection of chicken. International Journal of Molecular Sciences, 16: 28669-28682. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi J, Teng M, Yu Z, Xu H, Su J, Zhao P, Xing G, Liang H, Deng R, Qu L, Zhang G, Luo J. Marek's disease virus-encoded analog of microRNA-155 activates the oncogene c-Myc by targeting LTBP1 and suppressing the TGF-β signaling pathway. Virology, 476: 72-84. 2015. [DOI] [PubMed] [Google Scholar]
- Dang L, Teng M, Li H, Ma S, Lu Q, Hao H, Zhao D, Zhou E, Zhang G, Luo J. Marek's disease virus type 1 encoded analog of miR-155 promotes proliferation of chicken embryo fibroblast and DF-1 cells by targeting hnRNPAB. Veterinary Microbiology, 207: 210-218. 2017. [DOI] [PubMed] [Google Scholar]
- Dai Z, Ji J, Yan Y, Lin W, Li H, Chen F, Liu Y, Chen W, Bi Y, Xie Q. Role of gga-miR-221 and gga-miR-222 during tumour formation in chickens infected by subgroup J avian leukosis virus. Viruses, 7: 6538-6551. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson W, Nettles V, Couvillion C, Yoder HW., Jr. Infectious sinusitis in wild turkeys. Avian Diseases, 26: 402-405. 1982. [PubMed] [Google Scholar]
- Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature, 432: 231-235. 2004. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell, 100: 387-390. 2000. [DOI] [PubMed] [Google Scholar]
- Dinh H, Hong YH, Lillehoj HS. Modulation of microRNAs in two genetically disparate chicken lines showing different necrotic enteritis disease susceptibility. Veterinary Immunology and Immunopathology, 159: 74-82. 2014. [DOI] [PubMed] [Google Scholar]
- Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biology, 5: R1 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalona R, Diaz V, Pedernera E, Mendez C. Transforming growth factor beta mRNA and protein expression in the ovary of the chicken embryo. Growth Factors, 30: 297-303. 2012. [DOI] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Research, 19: 92-105. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Wang B, Chen X, He Z, Wang Y, Li X, Cao H, Zheng S. gga-miR-130b suppresses infectious bursal disease virus replication via targeting the viral genome and cellular SOCS5. Journal of Virology, 92: e01646-17. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Wang B, Chen X, He Z, Wang Y, Li X, Cao H, Zheng S. gga-miR-454 suppresses infectious bursal disease virus (IBDV) replication via directly targeting IBDV genomic segment B and cellular Suppressors of Cytokine Signaling 6 (SOCS6). Virus Research, 252: 29-40. 2018. [DOI] [PubMed] [Google Scholar]
- Gao Y, Yun B, Qin L, Pan W, Qu Y, Liu Z, Wang Y, Qi X, Gao H, Wang X. Molecular epidemiology of avian leukosis virus subgroup J in layer ocks in China. Journal of Clinical Microbiology, 50: 953-960. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigek CO, Lisboa LC, Leal MF, Silva PN, Lima EM, Khayat AS, Assumpcao PP, Burbano RR, Smith Mde A. SMARCA5 methylation and expression in gastric cancer. Cancer Investigation, 29: 162-166. 2011. [DOI] [PubMed] [Google Scholar]
- Grabarczyk P, Przybylski gK, Depke M, Volker U, Bahr J, Assmus K, Broker BM, Walther R, Schmidt CA. Inhibition of BCL11B expression leads to apoptosis of malignant but not normal mature T cells. Oncogene, 26: 3797-3810. 2007. [DOI] [PubMed] [Google Scholar]
- Gutierrez A, Kentsis A, Sanda T, Holmfeldt L, Chen SC, Zhang J, Protopopov A, Chin L, Dahlberg SE, Neuberg DS, et al. The BCL11B tumor suppressor is mutated across the major molecular subtypes of T-cell acute lymphoblastic leukemia. Blood, 118: 4169-4173. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Lian L, Li X, Zhao C, Qu L, Liu C, Song J, Yang N. Chicken gga-miR-130a targets HOXA3 and MDFIC and inhibits Marek's disease lymphoma cell proliferation and migration. Molecular Biology Reports, 43: 667-676. 2016. [DOI] [PubMed] [Google Scholar]
- Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nature Reviews Cancer, 7: 182-191. 2007. [DOI] [PubMed] [Google Scholar]
- Hermans PG, Morgan KL. Prevalence and associated risk factors of necrotic enteritis on broiler farms in the United Kingdom; a cross-sectional survey. Avian Pathology, 36: 43-51. 2007. [DOI] [PubMed] [Google Scholar]
- Hirai K, Shimakura S. Structure of infectious bursal disease virus. Journal of Virology, 14: 957-964. 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks JA, Tembhurne P, Liu HC. MicroRNA expression in chicken embryos. Poultry Science, 87: 2335-2343. 2008. [DOI] [PubMed] [Google Scholar]
- Hicks JA, Tembhurne PA, Liu HC. Identification of microRNA in the developing chick immune organs. Immunogenetics, 61: 231-240. 2009. [DOI] [PubMed] [Google Scholar]
- Hong S, Kim HY, Kim J, Ha HT, Kim YM, Bae E, Kim TH, Lee KC, Kim SJ. Smad7 protein induces interferon regulatory factor 1-dependent transcriptional activation of caspase 8 to restore tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis. Journal of Biological Chemistry, 288: 3560-3570. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Zhao Y, Wang Z, Hou Y, Bi D, Sun J, Peng X. Chicken gga-miR-19a targets ZMYND11 and plays an important role in host defense against Mycoplasma gallisepticum (HS Strain) infection. Frontiers in Cellular and Infection Microbiology, 6: 102 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguier S, Baguet J, Perez S, van Dam H, Castellazzi M. Transcription factor ATF2 cooperates with v-Jun to promote growth factor-independent proliferation in vitro and tumor formation in vivo. Molecular and Cellular Biology, 18: 7020-7029. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BY, Hu P, Zhang DD, Jiang GM, Liu SY, Xu Y, Wu YF, Xia X, Wang Y. C-type natriuretic peptide suppresses mesangial proliferation and matrix expression via a MMPs/TIMPs-independent pathway in vitro. Journal of Receptor and Signal Transduction Research. 37: 355-364. 2017. [DOI] [PubMed] [Google Scholar]
- Ingle H, Kumar S, Raut AA, Mishara A, Kulkarni DD, Kameyama T, Takaoka A, Akira S, Kumar H. The microRNA miR-485 targets host and influenza virus transcripts to regulate antiviral immunity and restrict viral replication. Science Signaling, 8: ra126 2015. [DOI] [PubMed] [Google Scholar]
- Jansson MD, Lund AH. MicroRNA and cancer. Molecular Oncology, 6: 590-610. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PA, Giles JR. The hen as a model of ovarian cancer. Nature Reviews Cancer, 13: 432-436. 2013. [DOI] [PubMed] [Google Scholar]
- Kang L, Cui X, Zhang Y, Yang C, Jiang Y. Identification of miRNAs associated with sexual maturity in chicken ovary by Illumina small RNA deep sequencing. BMC Genomics, 14: 352 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyomarsi K, O'Leary N, Molnar G, Lees E, Fingert HJ, Pardee AB. Cyclin E, a potential prognostic marker for breast cancer. Cancer Res, 54: 380-385. 1994. [PubMed] [Google Scholar]
- Kibenge FS, McKenna PK, Dybing JK. Genome cloning and analysis of the large RNA segment (segment A) of a naturally avirulent serotype 2 infectious bursal disease virus. Virology, 184: 437-440. 1991. [DOI] [PubMed] [Google Scholar]
- Koparde P, Singh S. Avian influenza and micro RNA: Role of bioinformatics. Journal of Bioinformatcs and Sequence Analysis, 2: 12-22. 2011. [Google Scholar]
- Kreager KS. Chicken industry strategies for control of tumor virus infections. Poultry Science, 77: 1213-1216. 1998. [DOI] [PubMed] [Google Scholar]
- Landoni MF, Albarellos G. The use of antimicrobial agents in broiler chickens. The Veterinary Journal, 205: 21-27. 2015. [DOI] [PubMed] [Google Scholar]
- Ley DH. Mycoplasma gallisepticum infection. Diseases of Poultry. 2008. [Google Scholar]
- Levy A.M, Izumiya Y, Brunovskis P, Xia L, Parcells MS, Reddy SM, Lee L, Chen HW, Kung HJ. Characterization of the chromosomal binding sites and dimerization partners of the viral oncoprotein Meq in Marek's disease virus-transformed T cells, Journal of Virology, 77: 12841-12851. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Li Y, Zheng H, Zheng Y, Liu C. Distinct expression pattern of miRNAs in Marek's disease virus infected-chicken splenic tumors and non-tumorous spleen tissues. Research in Veterinary Science, 97: 156-161. 2014. a. [DOI] [PubMed] [Google Scholar]
- Li X, Lian L, Zhang D, Qu L, Yang N. gga-miR-26a targets NEK6 and suppresses Marek's disease lymphoma cell proliferation. Poultry Science, 93: 1097-1105. 2014. b. [DOI] [PubMed] [Google Scholar]
- Li H, Shang H, Shu D, Zhang H, Ji J, Sun B, Li H, Xie Q. gga-miR-375 plays a key role in tumorigenesis post subgroup J avian leukosis virus infection. PLoS ONE, 9 e90878 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang Y, Li X, Li X, Cao H, Zheng SJ. Critical roles of glucocorticoid-induced leucine zipper in infectious bursal disease virus (IBDV)-induced suppression of type I interferon expression and enhancement of IBDV growth in host cells via interaction with VP4. Journal of Virology, 87: 1221-1231. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ji J, Xie Q, Shang H, Zhang H, Xin X, Chen F, Sun B, Xue C, Ma J, et al. Aberrant expression of liver microRNA in chickens infected with subgroup J avian leukosis virus. Virus Research, 169: 268-271. 2012. [DOI] [PubMed] [Google Scholar]
- Li Z, Chen B, Feng M, Ouyang H, Zheng M, Ye Q, Nie Q, Zhang X. MicroRNA-23b promotes avian leukosis virus subgroup J (ALV-J) replication by targeting IRF1. Scientific Reports, 5: 10294 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Luo Q, Xu H, Zheng M, Abdalla BA, Feng M, Cai B, Zhang X, Nie Q, Zhang X. MiR-34b-5p suppresses melanoma differentiation-associated gene 5 (MDA5) signaling pathway to promote avian leukosis virus subgroup J (ALV-J)-infected cells proliferaction and ALV-J replication. Frontiers in Cellular Infection Microbiology, 7: 17 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian L, Li X, Zhao C, Han B, Qu L, Song J, Liu C, Yang N. Chicken gga-miR-181a targets MYBL1 and shows an inhibitory effect on proliferation of Marek's disease virus-transformed lymphoid cell line. Poultry Science, 94: 2616-2621. 2015. [DOI] [PubMed] [Google Scholar]
- Lin S, Gregory RI: MicroRNA biogenesis pathways in cancer. Nature Review Cancer, 15: 321 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim W, Jeong W, Kim J, Yoshimura Y, Bazer FW, Han JY, Song G. Expression and regulation of beta-defensin 11 in the oviduct in response to estrogen and in ovarian tumors of chickens. Molecular and Cellular Endocrinology, 366: 1-8. 2013. [DOI] [PubMed] [Google Scholar]
- Liu C, Zheng S, Wang Y, Jing L, Gao H, Gao Y, Qi X, Qin L, Pan W, Wang X. Detection and molecular characterization of recombinant avian leukosis viruses in commercial egg-type chickens in China. Avian Pathology 40: 269-275. 2011. [DOI] [PubMed] [Google Scholar]
- Liu D, Dai M, Zhang X, Cao W, Liao M. Subgroup J avian leukosis virus infection of chicken dendritic cells induces apoptosis via the aberrant expression of microRNAs. Scientific Reports, 6: 20188 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Wu H, Ye Y, Li Z, Hao S, Kong L, Zheng X, Lin S, Nie Q, Zhang X. The transient expression of miR-203 and its inhibiting effects on skeletal muscle cell proliferation and differentiation. Cell Death & Disease, 5: e1347 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Man T, Fan J, Wang F, Zhou L, Deng R, Zhang G. Marek's disease virus-encoded microRNAs: genomics, expression and function. Science China-Life Sciences, 53: 1490-1490. 2010. [DOI] [PubMed] [Google Scholar]
- Magenta A, Greco S, Gaetano C, Martelli F: Oxidative stress and microRNAs in vascular diseases. International Journal of Molecular Science, 14: 17319-17346. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali AV, Joshi AA, Hegde MV, Kadam SS. Enterolactone suppresses proliferation, migration and metastasis of MDAMB-231 breast cancer cells through inhibition of uPA induced plasmin activation and MMPs-mediated ECM remodeling. Asian Pacific Journal of Cancer Prevention, 18: 905-915. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SK, Kim HM, Kim CH. PTEN induces G1 cell cycle arrest and inhibits MMP-9 expression via the regulation of NFkappab and AP-1 in vascular smooth muscle cells. Archives of Biochemistry and Biophysics, 421: 267-276. 2004. [DOI] [PubMed] [Google Scholar]
- Muylkens B, Coupeau D, Dambrine G, Trapp S, Rasschaert D. Marek's disease virus microRNA designated Mdv1-pre-miRM4 targets both cellular and viral genes. Archives of Virology, 155: 1823-1837. 2010. [DOI] [PubMed] [Google Scholar]
- Nicholas R, Ayling R. Mycoplasma in cattle. Veterinary Research, 178: 478-479. 2016. [DOI] [PubMed] [Google Scholar]
- Nath Neerukonda S, Egan NA, Patria J, Assakhi I, Tavlarides-Hontz P, Modla S, Munoz ER, Hudson MB, Parcells MS. Comparison of exosomes purified via ultracentrifugation (UC) and total exosome isolation (TEI) reagent from the serum of Marek's disease virus (MDV)-vaccinated and tumor-bearing chickens. Journal of Virological Methods, 263: 1-9. 2019. [DOI] [PubMed] [Google Scholar]
- Osterrieder N, Kamil JP, Schumacher D, Tischer BK, Trapp S. Marek's disease virus: from miasma to model. Nature Reviews Microbiology, 4: 283-294. 2006. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Wang Y, Du X, Liu H, Zhang H. gga-miR-9* inhibits IFN production in antiviral innate immunity by targeting interferon regulatory factor 2 to promote IBDV replication. Veterinary Microbiology, 178: 41-49. 2015. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Wang Y, Meng K, Pan Q, Wang X, Xia X, Zhu Y, Bi Z, Zhang H, Luo K. gga-miR-2127 downregulates the translation of chicken p53 and attenuates chp53-mediated innate immune response against IBDV infection. Veterinary Microbiology, 198: 34-42. 2017. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Qian J, Pan Q, Wang J, Xia X, Wang X, Zhu Y, Wang Y. gga-miR-142-5p attenuates IRF7 signaling and promotes replication of IBDV by directly targeting the chMDA5's 3′ untranslated region. Veterinary Microbiology, 221: 74-80. 2018. [DOI] [PubMed] [Google Scholar]
- Paul MC, Vergne T, Mulatti P, Tiensin T, Iglesias I. Editorial: Epidemiology of avian influenza viruses. Frontiers in Veterinary Science, 6: 150 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Gao Q, Zhou L, Chen Z, Lu S, Huang H, Zhan C, Xiang M. MicroRNAs in avian influenza virus H9N2-infected and non-infected chicken embryo fibroblasts. Genetics and Molecular Research, 14: 9081-9091. 2015. [DOI] [PubMed] [Google Scholar]
- Pitcovski J, Gutter B, Gallili G, Goldway M, Perelman B, Gross G, Krispel S, Barbakov M, Michael A. Development and large-scale use of recombinant VP2 vaccine for the prevention of infectious bursal disease of chickens. Vaccine, 21: 4736-4743. 2003. [DOI] [PubMed] [Google Scholar]
- Rathjen T, Pais H, Sweetman D, Moulton V, Munsterberg A, Dalmay T. High throughput sequencing of microRNAs in chicken somites. Febs Letters, 583: 1422-1426. 2009. [DOI] [PubMed] [Google Scholar]
- Saucedo LJ, Edgar BA. Filling out the Hippo pathway. Nature Reviews Molecular Cell Biology, 8: 613-621. 2007. [DOI] [PubMed] [Google Scholar]
- Schexnailder R, Griffith M. Liver fat and egg production of laying hens as influenced by choline and other nutrients. Poultry Science, 52: 1188-1194. 1973. [DOI] [PubMed] [Google Scholar]
- Shao F, Wang X, Yu J, Jiang H, Zhu B, Gu Z. Expression of miR-33 from an SREBF2 intron targets the FTO gene in the chicken. Plos One, 9: e91236 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipkovits L, Egyed L, Palfi V, Beres A, Pitlik E, Somogyi M, Szathmary S, Denes B. Effect of low-pathogenicity influenza virus H3N8 infection on Mycoplasma gallisepticum infection of chickens. Avian Pathology, 41: 51-57. 2012. [DOI] [PubMed] [Google Scholar]
- Stopka T, Zakova D, Fuchs O, Kubrova O, Blafkova J, Jelinek J, Necas E, Zivny J. Chromatin remodeling gene SMARCA5 is dysregulated in primitive hematopoietic cells of acute leukemia. Leukemia, 14: 1247-1252. 2000. [DOI] [PubMed] [Google Scholar]
- Tian F, Luo J, Zhang H, Chang S, Song J. MiRNA expression signatures induced by Marek's disease virus infection in chickens. Genomics, 99: 152-159. 2012. [DOI] [PubMed] [Google Scholar]
- Truong AD, Hong Y, Lee J, Lee K, Lillehoj HS, Hong YH. TGF-β signaling and miRNAs targeting for BMP7 in the spleen of two necrotic enteritis-afflicted chicken lines. Korean Journal of Poultry Science, 44: 211-223. 2017. a. [Google Scholar]
- Truong AD, Rengaraj D, Hong Y, Hong GT, Hong YH, Lillehoj HS. Differentially expressed JAK-STAT signaling pathway genes and target microRNAs in the spleen of necrotic enteritis-afflicted chicken lines. Research in Veterinary Science, 115: 235-243. 2017. b. [DOI] [PubMed] [Google Scholar]
- Uddin MB, Ahmed SSU, Hassan MM, Khan SA, Mamun MA. Prevalence of poultry diseases at Narsingdi, Banglandesh. International Journal of Bioresearch, 1: 9-13. 2010. [Google Scholar]
- van Dijk A, Veldhuizen EJ, Haagsman HP. Avian defensins. Veterinary Immunology and Immunopathology, 124: 1-18. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Einem UI, Gorbalenya AE, Schirrmeier H, Behrens SE, Letzel T, Mundt E. VP1 of infectious bursal disease virus is an RNA-dependent RNA polymerase. Journal of General Virology, 85: 2221-2229. 2004. [DOI] [PubMed] [Google Scholar]
- Wang Y, Brahmakshatriya V, Lupiani B, Reddy SM, Sobiam B, Benham AL, Gunaratne P, Liu H, Trakooljul N, Ing N, et al. Integrated analysis of microRNA expression and mRNA transcriptome in lungs of avian influenza virus infected broilers. BMC Genomics, 13: 278 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Fu M, Liu Y, Wang Y, Li X, Cao H, Zheng SJ. gga-miR-155 enhances type I interferon expression and suppresses infectious burse disease cirus replication via targeting SOCS1 and TANK. Frontiers in Cellular and Infection Microbiology, 8: 55 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Hou J, Lin L, Wang C, Liu X, Li D, Ma F, Wang Z, Cao X. Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. The Journal of Immunology, 185: 6226-6233. 2010. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ouyang W, Pan Q, Wang X, Xia X, Bi Z, Wang Y, Wang X. Overexpression of microRNA gga-miR-21 in chicken fibroblasts suppresses replication of infectious bursal disease virus through inhibiting VP1 translation. Antivirus Research, 100: 196-201. 2013. a. [DOI] [PubMed] [Google Scholar]
- Wang Q, Ji X, Gao Y, Qi X, Wang X, Wang Y, Qin L, Gao H, Wang X. Overexpression of microRNA gga-miR-1650 decreases the replication of avian leukosis virus subgroup J in infected cells. Journal of General Virology, 94: 2287-2296. 2013. b. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang Y, Lu J, Xia B, Yang Z, Zhu X, Zhou X, Huang P. The highly pathogenic H5N1 influenza A virus downregulated several cellular MicroRNAs which target viral genome. Journal of Cellular and Molecular Medicine, 21: 3076-3086. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Brahmakshatriya V, Zhu H, Lupiani B, Reddy SM, Yoon B, Gunaratne PH, Kim JH, Chen R, Wang J, Zhou H. Identification of differentially expressed miRNAs in chicken lung and trachea with avian influenza virus infection by a deep sequencing approach. BMC Genomics, 10: 512 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webby RJ, Webster RG. Are we ready for pandemic influenza? Science, 302: 1519-1522. 2003. [DOI] [PubMed] [Google Scholar]
- Whelan RA, Doranalli K, Rinttila T, Vienola K, Jurgens G, Apajalahti J. The impact of Bacillus subtilis DSM 32315 on the pathology, performance, and intestinal microbiome of broiler chickens in a necrotic enteritis challenge. Poultry Science, pey500. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RB. Intercurrent coccidiosis and necrotic enteritis of chickens: Rational, integrated disease management by maintenance of gut integrity. Avian Pathology, 34: 159-180. 2005. [DOI] [PubMed] [Google Scholar]
- Xu Z, Yu Y, Liu Y, Ou C, Zhang Y, Liu T, Wang Q, Ma J. Differential expression of pro-inflammatory and anti-inflammatory genes of layer chicken bursa after experimental infection with infectious bursal disease virus. Poultry Science, pez312. 2019. [DOI] [PubMed] [Google Scholar]
- Yoder HW., Jr. Mycoplasma gallisepticum infection. Diseases of Poultry, 9: 198-212. 1991. [Google Scholar]
- Yoo SJ, Huh JR, Muro I, Yu H, Wang L, Wang SL, Renny Feldman EM, Clem RJ, Muller HJ, Hay BA. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nature Cell Biology, 4: 416-424. 2002. [DOI] [PubMed] [Google Scholar]
- Yuan Bo, Zou M, Zhao Y, Zhang K, Sun Y, Peng X. Upregulation of miR-130b-3p activates the PTEN/PI3K/AKT/NF-κB pathway to defense against Mycoplasma gallisepticum (HS strain) infection of chicken. International Journal of Molecular Sciences, 19: 2172 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon MC. Epidemiology and pathogenesis of influenza. Journal of Antimicrobial Chemotherapy, 44: 3-9. 1999. [DOI] [PubMed] [Google Scholar]
- Zhao C, Li X, Han B, You Z, Qu L, Liu C, Song J, Lian L, Yang N. Gga-miR-219b targeting BCL11B suppresses proliferation, migration and invasion of Marek's disease tumor cell MSB1. Scientific Reports, 7: 4247 2017. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Hou Y, Zhang K, Yuan B, Peng X. Identification of differentially expressed miRNAs through high-throughput sequencing in the chicken lung in response to Mycoplasma gallisepticum HS. Comparative Biochemistry and Physiology-Part D, 22: 146-156. 2017. b. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wang Z, Hou Y, Zhang K, Peng X. gga-miR-99a targets SMARCA5 to regulate Mycoplasma gallisepticum (HS strain) infection by depressing cell proliferation in chicken. Gene, 627: 239-247. 2017. c. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yao Y, Xu H, Lambeth L, Smith LP, Kgosana L, Wang X, Nair V. A functional microRNA-155 ortholog encoded by the oncogenic Marek's disease virus. Journal of Virology, 83: 489-492. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Xu H, Yao Y, Smith LP, Lydia K, Green J, Petherbridge L, Baigent SJ, Nair V. Critical role of the virus-encoded microRNA-155 ortholog in the induction of Marek's disease lymphomas. Plos Pathogens, 7: e1001305 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Han Y, Wang Z, Zhao Y, Fu Y, Peng X. gga-miR-146c Activates TLR6/MyD88/NF-κB Pathway through Targeting MMP16 to Prevent Mycoplasma Gallisepticum (HS Strain) Infection in Chickens. Cells, 8: 501 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


