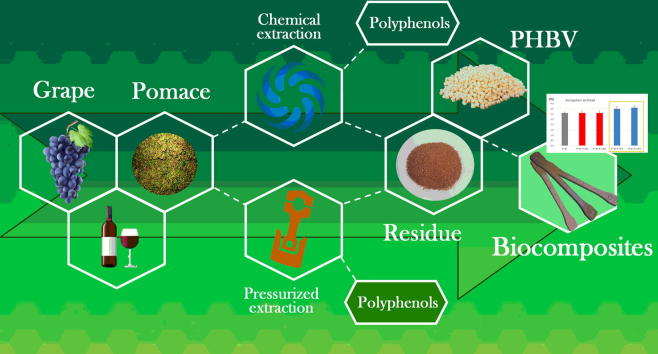
Graphical abstract
Keywords: Biocomposites, Biowaste, Grape pomace, Polyphenols, Solvent-based extraction, Pressurized liquid extraction
Abstract
The paper aims at optimising and validating possible routes toward the full valorisation of grape agrowaste to produce bioactive molecules and new materials. Starting from Merlot red pomace, phenol complex mixtures were successfully extracted by using two different approaches. Extracts obtained by solvent-based (SE) technique contained up to 46.9 gGAeq/kgDW of total phenols. Depending on the used solvent, the prevalence of compounds belonging to different phenol families was achieved. Pressurized liquid extraction (PLE) gave higher total phenol yields (up to 79 gGAeq/kgDW) but a lower range of extracted compounds. All liquid extracts exerted strong antioxidant properties. Moreover, both SE and PLE extraction solid residues were directly exploited (between 5 and 20% w/w) to prepare biocomposite materials by direct mixing via an eco-friendly approach with PHBV polymer. The final composites showed mechanical characteristics similar to PHVB matrix. The use of pomace residues in biocomposites could therefore bring both to the reduction of the cost of the final material, as a lower amount of costly PHBV is used. The present research demonstrated the full valorisation of grape pomace, an agrowaste produced every year in large amounts and having a significant environmental impact.
Introduction
In the European Union (EU) about 55 million tonnes (MT) of both agricultural vegetal waste and forestry wood waste were produced in 2016 (https://ec.europa.eu/eurostat). These residues have a great potential of being transformed into energy or biobased products (e.g. organic fertilizers, feed, biopesticides, bioplastics, etc), or of being valorised as a source of added-value molecules [1], [2], [3].
A case-study of a valuable agro-industrial by-product is grape pomace. Grape is one of the largest fruit crop in the world and its 2017 production reached 74 MT worldwide and 24 MT in EU (www.faostat.org). The vast majority of total produced grape is used in winemaking during which at least 20% of the fruit weight is discarded as pomace [4], [5]. The most relevant pomace management systems are currently one-way flow systems such as landfilling, incineration and composting [4], [6]. In addition a small percentage of grape pomace goes to distillation processes to produce different types of spirits and liquors, or it’s used as fertilizer or animal feed [4], [5], [6]. However, even if these solutions represent a significant pomace exploitation, they have some drawbacks mainly related to the presence of anti-nutritive compounds (such as some organic acids and tannins) that may negatively affect crop yields and animal growth [5]. In the last 20 years, several grape pomace exploitation alternatives were studied, mainly focusing on the extraction and further valorisation of high-value compounds present in this by-product. In fact, during winemaking only a minor part of grape phytochemicals are extracted into the wine, leaving the pomace still rich in phenolic compounds (mainly flavonoids, phenolic acids, stilbenes and anthocyanins), dietary fibres, proteins, lipids and minerals [4], [5], [6], [7]. Many of these compounds exert ascertained positive effects on human health [7] and show a great potential of being applied as ingredients in the food, nutraceutical, pharmaceutical and cosmetic fields. Other components can be exploited as colorants, antioxidant or antimicrobial agents, or in packaging formulations [1], [4], [5], [7], [8].
The recovery of valuable compounds from grape pomace was increasingly investigated and different approaches were taken into consideration following the 5-Stage Universal Recovery Process [1]. The most important issue in a recovery process is to effectively separate the target compounds from the waste matrix by applying a progressive separation procedure from the macroscopic to the macromolecular and then to the micromolecular level. Generally, five distinct stages have been identified: macroscopic pre-treatment; macro- and micro-molecules separation; extraction; isolation and purification; product formation. Each step can be carried out with different conventional or emerging technologies and the main advantage of this strategy is that it can be applied for simultaneous recovery of several ingredients in different streams [1]. In the case of grape pomace, when the target compounds were soluble or weakly bound, such as polyphenols, the most common technique was solid-liquid extraction, mainly based on organic solvents (SE) [4], [6]. The need of more green technologies led the research towards supercritical fluid extraction (SFE) and pressurized liquid extraction (PLE), which can achieve high yields of bioactive compounds from natural sources with the use of food grade and non-toxic solvents [3], [6], [9]. Alternatively, the treatment of grape pomace with cell wall polysaccharide degrading enzyme mixtures also led to the recovery of compounds with targeted bioactivities [10], [11]. Currently, polyphenol-rich red grape pomace extracts were mainly industrially exploited as ingredients in food products [5], but they could also be valorised as additives in polymer formulations, to obtain materials with antioxidant and antibacterial properties [4], [8].
Independently from the technique applied for phenol recovery, an extraction solid residue is always present. In compliance with the zero-waste society and the circular economy concepts, this residue must be further valorised. Actually, its major use is in biogas or compost production [6], but more valuable routes can be explored [12]. In fact, this solid residue is rich in fibres and can be used in the production of new polymeric materials. In particular, the residue without any chemical pre-treatment can be applied as filler to reinforce a bio-polymeric matrix producing 100% bio-based composites which are characterized by a lower cost, with respect to the original matrix, and possibly by improved or comparable properties [13].
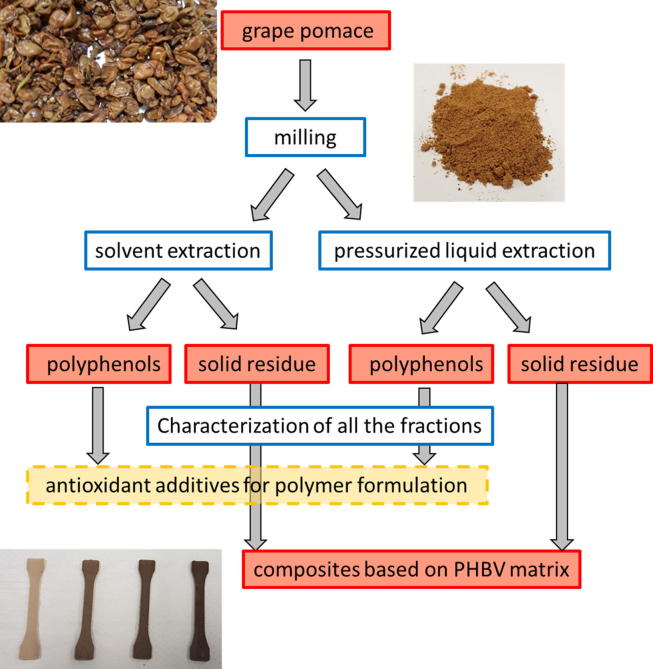
The aim of the present study was to integrate different red pomace (Vitis vinifera L., Merlot cultivar) valorisation cascading approaches (Fig. 1) to develop a zero-waste exploitation route of this winery waste. In particular, two different procedures of polyphenol extraction (SE and PLE) were optimised and the phytochemical complexity and bioactivity of the extracts were compared in view of their application as ingredients in added-value products. In addition, the solid grape pomace extraction residues were used in biocomposites formulations based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), a renewable and biodegradable matrix, to obtain new materials having lower cost and comparable properties. Following this approach, grape pomace agrowaste could be considered as a high value resource which could be converted into sustainable materials and new ingredients fully complying with the circular economy concept.
Fig. 1.
Description of the cascading activities (in blue) developed in the present study and of the materials involved (in red). Other materials (not subject of the present study) can be achieved by exploiting the obtained phenol extracts (example in yellow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Material and methods
Grape pomace
Red grape pomace (Vitis vinifera L., Merlot cultivar) was provided by InnovEn Srl (Verona, Italy) and contained berry skins, seeds, petioles and stalks. Grape was harvested in September 2016 and pomace was collected after pressing and wine fermentation, frozen and stored at −20 °C the same day of wine production. To determine the pomace dry weight (DW), aliquots of 3 g fresh weight (FW) were placed at 80 °C for 48 h and weighed: DW was about 37.1% of the FW.
Solvent-based extraction (SE) of phenols
Initial wet Merlot pomace was stored at −20 °C and ground in a kitchen blender before the extraction. Solvent was added to pomace aliquots at a solid/liquid ratio (S/L) of 1:5 (5 gFW, corresponding to 1.85 gDW, +25 mL solvent) or 1:10 (3 gFW, corresponding to 1.11 gDW, +30 mL solvent) and the mixtures were incubated at the specific temperature in a shaking water bath (120 rpm), in a close system to avoid solvent evaporation. At the end of the incubation, liquid extracts were separated from solid residues by centrifugation (5 min, 4500g) and stored at −20 °C. Type and concentration of solvent were selected on the basis of a previous study [14]: 50% (v/v) of aqueous-ethanol (50% EtOH), 50% (v/v) aqueous-acetonitrile (50% AcN) and 75% (v/v) aqueous-acetone (75% acetone). For each solvent, S/L (1:5 or 1:10), incubation temperature (50 °C or 70 °C) and incubation time (1 h, 2 h, 4 h) were screened. Water controls were also performed in order to detect the minimum level of extractable phenols in each testing condition [14].
Pressurized liquid extraction (PLE) of phenols
Different pre-treatments were applied to initial wet pomace. Frozen wet pomace (40.0 ± 0.1 gFW) was either coarse milled in a food mixer (C3, Empire Sweden AB, Bromma, Sweden) for 3 × 15 s at low speed allowing the majority of grape seeds to stay intact (PWC sample), or more fine milled in a knife mill (Grindomix GM 200; Retsch GmbH, Haan, Germany) at 7500 rpm for 5 s crushing the grape seeds into smaller pieces (PWF sample). The coarse milled pomace was dried at 40 °C for 7.5 h in a conventional hot air oven (Garomat 142; Electrolux AB, Stockholm, Sweden) (PD sample) and stored at −40 °C until further use.
In addition, PD was subjected to defatting by supercritical carbon dioxide (SC-CO2) extraction (PDD sample) by means of a laboratory scale SFE-500M1-2-C50 equipment (Waters, Pittsburgh, PA). Briefly, 50 g of the PD sample were loaded into a 500 mL vessel and lipid extraction was performed at 80 °C, 350 bar for 1 h with a CO2 flow rate of 30 g/min [15]. According to the phase diagram of carbon dioxide, under those conditions of temperature and pressure the solvent lies on the supercritical region [16]. The remaining PDD residue was stored at −80 °C until polyphenol extraction. The oil yield, based on the weight reduction of the dried pomace before and after the extraction, was 12.2% (w/w).
Extraction of phenols by PLE was performed on PWC, PWF, PD and PDD pomace samples by using a 50% ethanol-water mixture (v/v) (EtOH-H2O) in combination with CO2 on the basis of previous reports [9] by means of a laboratory scale SFE-500M1-2-C50 equipment (Waters, Pittsburgh, PA). The extraction conditions were 80 °C, 100 bar, 75% EtOH-H2O and 25% CO2, total flow rate of 8 g/min, sample size of 3.2 gDW loaded in a 100 mL extraction vessel. In addition to the effect of pre-treatments, only on PWC samples, the influence of extraction time (30, 40 and 50 min, T30, T40 and T50 samples) and various EtOH-H2O/CO2 mixtures (50/50, 75/25 and 100/0, S50, S75, S100 samples) were studied. The temperature and pressure conditions were set at 80 °C and 100 bar respectively and defined on the basis of equipment stability restrictions and literature [17], [18]. Under the selected conditions of pressure and temperature the solvent mixture was at the subcritical point [19]. Subsequent to the phenol extraction, the solid residues were freeze-dried in an Alpha 1-2 LDplus freeze dryer (Martin Christ, Osterode am Harz, Germany) for 20 h before characterization and composite material preparation.
Characterisation of liquid extracts
Total phenolic content was assessed in all the liquid extracts by the spectrophotometric Folin-Ciocalteu assay [20] and results were used for the selection of the best processes. Phytochemical profiles of best samples and of water extract (as control for SE processes) were further characterised and the most relevant phenolic compound families were quantified by spectrophotometric assays: flavonoids [20], flavanols [21], hydroxycinnamic acids [22] and anthocyanins [11]. Reducing sugar content was also measured [23]. Appropriate dose-response calibration curves were plotted and the results were expressed as g of standard compound equivalents per kg of pomace DW: gallic acid (GA, 0–15 µg) for total phenols, catechin (CAT) for flavonoids (2–14 µg) and flavanols (1–50 µg), ferulic acid (FA, 1–1000 µg) for hydroxycinnamic acids, glucose (GLUC, 50–500 µg) for sugars. Anthocyanin results were converted from absorbance to malvidin-3-glucoside (MALV) equivalents as reported by Considine and Frankish [24]. Total antioxidant activity of the extracts was assessed by ABTS (2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) assay [20] and data were expressed as g of ascorbic acid (AA) equivalents per kg of pomace DW by means of a calibration curve (0–2 µg of AA).
Specific phenols were identified and quantified by HPLC-DAD analyses [25]. Phenols were recovered from samples by means of Strata-X columns (33 mm polymeric reversed phase 60 mg/3 mL, Phenomenex, Bologna, Italy) and analysed in HPLC system (column Gemini C18, 5 µm particles, 110 Å, 250 × 4.6 mm; precolumn SecurityGuard Ea; Phenomenex) equipped with an on-line diode array detector (MD-2010, Plus, Jasco Europe, Cremella, Italy). The adopted HPLC-DAD separation procedure allowed to simultaneously identify and quantify the following compounds: trans-ferulic, caffeic, chlorogenic, p-coumaric, sinapic and trans-cinnamic acids; gallic, protocatechuic, syringic and vanillic acids; catechin, epicatechin, epigallocatechin gallate, epicatechin gallate, epigallocatechin; vanillin, naringenin, quercetin, rutin, myricetin, kaempferol; trans- and cis-resveratrol, trans- and cis-piceid, trans- and cis-resveratroloside, piceatannol.
Solid residue characterization
Thermogravimetric analysis (TGA) of solid residues coming from both SE and PLE was performed using a Perkin Elmer TGA4000 apparatus (Milan, Italy) in nitrogen (gas flow: 40 mL/min) at 10 °C/min heating rate, from 25 °C to 700 °C.
Composite preparation
The solid residues obtained by SE and PLE extractions were dried at 70 °C under vacuum for 24 h, grinded with Ika M20 Mill (Staufen, Germany) and sifted through a sieve (mesh 0.4 mm).
A commercial polyhydroxyalkanoate, PHI 002, supplied from NaturePlast (Ifs, France), was used as matrix for the composites preparation. PHI 002 is a poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) copolyester containing 2 mol% of hydroxyvaleric unit and 98 mol% of hydroxybutyric unit (as determined by 1H NMR analysis). To allow a better mixing, both treated residues and commercial PHBV were again dried under vacuum at 60 °C overnight. Then, to get composites, the residues and the polymer were melt mixed at 200 °C for 5 min in a Brabender mixer, feeding 45–50 g of charge and setting the screw speed at 50 rpm. For each residue, different blends were prepared with an amount of filler in the range of 5–20% (w/w).
Composite characterizations
The initial PHBV and the obtained composites were analysed by 1H NMR, using a Varian Mercury 400 spectrometer (Palo Alto, California). Chemical shifts are downfield from tetramethylsilane and CDCl3 as a solvent. The spectra have been recorded just after dissolution in order to avoid esterification reaction of end groups with trifluoroacetic acid.
To determine molecular weights, the composite samples were dissolved in mixture of CHCl3/1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) 95/5 (v/v) and filtered on Teflon syringe filter with pore size of 0.45 µm to eliminate the insoluble residue. Then, gel permeation chromatography (GPC) measurements were performed at 30 °C on a GPC Knauer Azura (Berlin, Germany) using a PL gel 5 µm Minimixed-C column (Milan, Italy) with chloroform as eluent with a 0.3 mL/min flow; the Refractive Index detector was used and a calibration plot was constructed with monodisperse polystyrene standards.
The TGA were performed using a Perkin Elmer TGA4000 apparatus in nitrogen (gas flow: 40 mL/min) at 10 °C/min heating rate, from 25 °C to 700 °C. The degradation temperature (TD) was calculated as the temperature of the maximum degradation rate, whereas the onset degradation temperature (Tonset) was defined as the initial temperature of degradation, corresponding to the intercept of the tangent drawn at the inflection point of the decomposition step with the horizontal zero-line of the thermogravimetric curve.
Calorimetric analysis was carried out by means of a Perkin Elmer DSC6 calorimeter (Milan, Italy), calibrated with high-purity standards. The thermal treatments were performed under a nitrogen flow as follows: first scan, from 30 to 210 °C at 20 °C/min and 1 min of isotherm at 210 °C; cooling scan, from 210 to 0 °C at 20 °C/min and 1 min of isotherm; second scan, from 0 to 210 °C at 20 °C/min.
Tensile properties of composites were determined on dumbbell-shaped specimens (2 × 5 × 30 mm) obtained by injection moulding (MegaTech Tecnica DueBi injection moulding machine, Ancona, Italy), working between 150 and 165 °C. The tests were carried out by an INSTRON 5966 dynamometer (Turin, Italy) equipped with a 10 kN load cell (test speed 5 mm/min, room temperature 19 ± 1 °C and 70 ± 10% of relative humidity).
Statistical analysis
All the SE and PLE extractions were repeated at least two times and the experimental data were expressed as mean ± SD. All spectrophotometric assay procedures and HPLC-DAD analyses were performed in duplicate in two technical replicates each. The results are expressed as the mean (n = 2) ± SD per kilogram of dry weight (kgDW) or per litre of extract. Statistically significant differences between datasets were analysed by using one-way ANOVA test followed by post-hoc corrected two tail t-student test assuming equal variance (p < 0.05). The composite characterization data are the means of at least five determination for each sample.
Results and discussion
Optimisation of phenol solvent-based extraction (SE) process
SE extractions from red grape pomace (Merlot cultivar) were carried out with the aim of recovering the highest yield of phenolic compounds. Three different solvents (50% ethanol, EtOH; 50% acetonitrile, AcN; 75% acetone) and water control, were assayed and several extraction parameters were optimised (solid/liquid (S/L) ratio, incubation temperature and time) (Table 1). Total phenolic content was quantified via spectrophotometric assay in all the extracts showing that all tested solvents were able to increase phenol extraction in comparison to water control. Higher extraction yields were obtained by using 75% acetone at both S/L ratios, with maximum level of recovered phenols of 50.13 gGAeq/kgDW (S/L 1:10, 50 °C, 2 h) and of 46.90 gGAeq/kgDW (corresponding to 1.86 and 3.48 gGAeq/L respectively) (S/L 1:5, 50 °C, 2 h), followed by 50% AcN and 50% EtOH (Table 1). These data are in agreement with literature reporting that grape phenols, due to their polar nature, were easily solubilised in polar media, such as hydro-alcoholic solutions or organic solvent water mixtures, while single-solvent systems did not provide optimal extraction [4], [26].
Table 1.
Optimization of key parameters for phenol solvent-based extraction (SE). The solid/liquid column indicates the kgDW of used pomace in for 1 L of solvent; the liquid/solid column indicates the amount of solvent for 1 kgDW of pomace. Total phenol quantification results for each liquid extract were expressed as g of gallic acid (GA) equivalent per kg of pomace dry weight (gGAeq/kgDW) and as g of GA equivalent per litre of extract (gGAeq/L). Different letters indicate statistically significant difference (one-way ANOVA followed by post hoc two-tailed Student’s t-test, p < 0.05) among data expressed in the same measure unit. Data are the mean ± SD (n = 2). EtOH, ethanol; AcN, acetonitrile; S/L, solid/liquid ratio.
| Sample | S/L (kgFW/L) | Solid/liquid (kgDW/L) | Liquid/solid (L/kgDW) | Temperature (°C) | Time (h) | Total phenols |
|
|---|---|---|---|---|---|---|---|
| gGAeq/kgDW | gGAeq/L | ||||||
| 50% EtOH | 1:10 | 0.037 | 27.96 | 50 | 1 | 40.43 ± 2.29 a | 1.50 ± 0.08 a |
| 2 | 39.26 ± 9.53 a,b | 1.46 ± 0.35 a,b | |||||
| 4 | 46.63 ± 1.65 b | 1.73 ± 0.06 b | |||||
| 50% AcN | 1:10 | 0.037 | 27.96 | 50 | 1 | 48.61 ± 1.65 b,c | 1.80 ± 0.06 b,c |
| 2 | 46.00 ± 1.52 b | 1.71 ± 0.06 b | |||||
| 4 | 49.15 ± 0.38 c | 1.82 ± 0.01 c | |||||
| 75% Acetone | 1:10 | 0.037 | 27.96 | 50 | 1 | 47.17 ± 0.64 b | 1.75 ± 0.02 b |
| 2 | 50.13 ± 2.54 b,c | 1.86 ± 0.09 b,c | |||||
| 4 | 47.35 ± 3.94 b,c | 1.76 ± 0.15 b,c | |||||
| Water | 1:10 | 0.037 | 27.96 | 50 | 1 | 15.18 ± 0.04 d | 0.56 ± 0.01 d |
| 2 | 15.07 ± 0.57 d | 0.56 ± 0.02 d | |||||
| 4 | 13.58 ± 0.01 e | 0.50 ± 0.01 e | |||||
| 50% EtOH | 1:5 | 0.074 | 13.48 | 50 | 1 | 37.74 ± 0.64 a | 2.80 ± 0.05 f |
| 2 | 37.15 ± 1.84 a | 2.76 ± 0.14 f | |||||
| 4 | 40.03 ± 0.19 a | 2.97 ± 0.01 f | |||||
| 50% AcN | 1:5 | 0.074 | 13.48 | 50 | 1 | 39.35 ± 1.40 a | 2.92 ± 0.10 f |
| 2 | 40.84 ± 1.33 a | 3.03 ± 0.10 f | |||||
| 4 | 40.66 ± 2.60 a | 3.02 ± 0.19 f | |||||
| 75% Acetone | 1:5 | 0.074 | 13.48 | 50 | 1 | 44.61 ± 2.48 a,b | 3.31 ± 0.18 f,g |
| 2 | 46.90 ± 0.13 b | 3.48 ± 0.01 g | |||||
| 4 | 44.83 ± 2.80 a,b | 3.33 ± 0.21 f,g | |||||
| Water | 1:5 | 0.074 | 13.48 | 50 | 1 | 12.04 ± 1.93 e,f | 0.89 ± 0.14 h,i |
| 2 | 12.59 ± 0.88 e | 0.93 ± 0.07 i | |||||
| 4 | 9.47 ± 1.85 f | 0.70 ± 0.14 h | |||||
| 50% EtOH | 1:5 | 0.074 | 13.48 | 70 | 1 | 22.60 ± 0.57 g | 1.68 ± 0.04 j |
| 2 | 25.79 ± 0.01 h | 1.91 ± 0.01 k | |||||
| 4 | 28.84 ± 0.01 i | 2.14 ± 0.01 l | |||||
| 50% AcN | 1:5 | 0.074 | 13.48 | 70 | 1 | 24.80 ± 0.13 h | 1.84 ± 0.01 k |
| 2 | 26.01 ± 0.19 h | 1.93 ± 0.01 k | |||||
| 4 | 27.40 ± 0.89 h,i | 2.03 ± 0.07 k,l | |||||
| 75% Acetone | 1:5 | 0.074 | 13.48 | 70 | 1 | 27.31 ± 2.03 h,i | 2.03 ± 0.15 k,l |
| 2 | 29.83 ± 0.51 i | 2.21 ± 0.04 l | |||||
| 4 | 31.58 ± 1.59 i | 2.34 ± 0.12 l | |||||
| Water | 1:5 | 0.074 | 13.48 | 70 | 1 | 9.87 ± 0.08 f | 0.73 ± 0.01 h |
| 2 | 9.12 ± 2.19 f | 0.68 ± 0.16 h | |||||
| 4 | 8.83 ± 0.97 f | 0.66 ± 0.07 h | |||||
The increase of incubation time (1 h, 2 h, 4 h) in general did not significantly affect phenol recovery (Table 1), as also reported for Sangiovese and Montepulciano cultivar red pomace extraction where extraction times from 2 h to 6 h resulted in phenol yield decrease [11]. Therefore, the intermediate incubation time (2 h) was selected as best condition. Analogously, higher incubation temperature (70 °C with respect to 50 °C, comparing processes with the same S/L and solvent) led to a decrease in phenol yield (e.g. average of −35.3% in 75% acetone extracts, Table 1). This results could be due to thermal-instability of some compounds, to isomerization or polymerization, or to chemical reaction among components into the mixture [7], [26]. Moreover, it was previously observed that thermal processing of grape pomace might increase the extractability of some polyphenols while destroying heat sensitive polyphenols present in grape skin and seeds [7].
Two different S/L ratios were assayed: 1:5 and 1:10 (kgFW: L) (Table 1). When phenol contents were expressed per L there resulted higher in S/L 1:5 as the use of a lower solvent volume led to more concentrated extracts. On the other hand, considering the yield expressed per kgDW, S/L 1:10 led to a slightly higher phenol recovery (on average, +6.1% in 75% acetone extractions and up to +19.0% in 50% AcN) (Table 1). Nonetheless, these small yield increases seemed not enough consistent to justify the use at industrial level of a double volume of solvent for kg of pomace, and therefore 1:5 S/L ratio was selected for further experiments.
Finally, the selected SE process conditions for phenolic compounds extraction from Merlot pomace were: 1:5 S/L ratio, 2 h incubation at 50 °C. The best extracts obtained with the three solvents and water control were further characterized.
Optimisation of phenol pressurized liquid extraction (PLE) process
PLE extractions were carried out on the same Merlot pomace aiming at recovering the highest yield of phenolic compounds using food grade and non-toxic solvents. In that perspective several critical process parameters such as extraction time, mobile phase composition and initial pomace pre-treatment were optimised, and the total phenolic content of the extracts was determined via spectrophotometric assay (Table 2).
Table 2.
Optimization of key parameters for phenol pressurized liquid extraction (PLE). All extractions were performed at 80 °C temperature and at 100 bar pressure. The solid/liquid column indicates the kgDW of used pomace in for 1 L of solvent; the liquid/solid column indicates the amount of solvent for 1 kgDW of pomace. Total phenol quantification results of each extract were expressed as g of gallic acid (GA) equivalent per kg of pomace dry weight (gGAeq/kgDW) and as g of GA equivalent per litre of extract (gGAeq/L). Different letters indicate statistically significant difference (one-way ANOVA followed by post hoc two-tailed Student’s t-test, p < 0.05) among data expressed in the same measure unit. The extended sample code indicates the type of pre-treatment, the extraction time and the mobile phase composition. Data are the mean ± SD (n = 2). EtOH-H2O, 50% ethanol/water mixture; PWC, wet coarse milled pomace; PWF, wet fine milled pomace; PDD, defatted dried coarse milled pomace; PD, dried coarse milled pomace.
| Sample (Extended sample code) | Solid/liquid (kgDW/L) | Liquid/solid (L/kgDW) | Pre-treatment | Time (min) | EtOH-H2O /CO2 ratio | Total phenols |
|
|---|---|---|---|---|---|---|---|
| gGAeq/kgDW | gGAeq/L | ||||||
| T30 (PWC-T30-S75) | 0.023 | 43.41 ± 6.67 | PWC | 30 | 75% | 61.91 ± 6.51 a | 1.44 ± 0.07 a |
| T40 (PWC-T40-S75) | 0.016 | 63.47 ± 5.67 | PWC | 40 | 75% | 70.67 ± 3.86 b | 1.13 ± 0.04 b |
| T50 (PWC-T50-S75) | 0.012 | 83.21 ± 5.34 | PWC | 50 | 75% | 78.85 ± 7.33 b | 0.94 ± 0.02 b |
| S50 (PWC-T30-S50) | 0.051 | 19.48 ± 0.00 | PWC | 30 | 50% | 48.46 ± 11.42 a,c | 2.47 ± 0.58 a,c |
| S100 (PWC-T30-S100) | 0.019 | 52.75 ± 5.71 | PWC | 30 | 100% | 67.72 ± 5.37 a,b | 1.31 ± 0.04 a,b |
| PWF (PWF-T30-S75) | 0.028 | 35.12 ± 0.11 | PWF | 30 | 75% | 70.55 ± 0.61 b | 2.01 ± 0.07 b |
| PD (PD-T30-S75) | 0.028 | 35.96 ± 0.52 | PD | 30 | 75% | 72.18 ± 7.33 a,b | 2.30 ± 0.41 a,b |
| PDD (PDD-T30-S75) | 0.027 | 37.04 ± 0.22 | PDD | 30 | 75% | 67.36 ± 2.21 a,b | 1.76 ± 0.06 a,b |
Preliminarily and only on PWC (wet coarse milled pomace) pre-treated sample, extraction times ranging from 30 to 50 min (PWC samples T30, T40, T50, Table 2) were tested. When data were expressed per L, the maximum content of total phenols was detected after 30 min (1.44 gGAeq/L), to decrease progressively up to 50 min (0.94 gGAeq/L). Opposite trend was observed considering the phenol yield expressed per kgDW of initial pomace with maximum levels detected in the 50 min extract (78.85 gGAeq/kgDW, sample T50, Table 2). T50 sample resulted 1.7-times higher than the best samples obtained via SE extraction (75% acetone, 1:5 S/L ratio, 2 h at 50 °C; 46.90 gGAeq/kgDW, Table 1) even though the amount of solvent used for 1 kgDW of pomace was 6.2-times higher than in SE (83.21 L in PLE and 13.48 L in SE; Table 1, Table 2).
Preliminary tests also aimed at optimising, the composition of the mobile phase with different EtOH-H2O/CO2 mixtures assayed: 50/50, 75/25 and 100/0 (v/v) (samples S50, S75, S100, Table 2). Data reported as kgDW showed that the progressive increase of the EtOH-H2O content in the mobile phase facilitated the extraction process and led to slightly higher total phenolic yields (up to 67.72 gGAeq/kgDW in S100 sample, Table 2). The use of EtOH-H2O mixture for the PLE of grape pomace phenols was previously reported showing that 80% of aqueous ethanol solution led to the highest amount of extracted flavonoids when compared with 0% and 40% content [18].
In addition, the influence of different pre-treatments of red grape pomace was evaluated. Initially two different forms of wet raw material pre-milling were tested: coarse milling (PWC samples) with higher particle size large presence of intact seeds, and fine (PWF samples) with smaller particles and ground seeds. After extraction (30 min, 75% EtOH-H2O solvent), PWF sample showed a 14% increase in phenol yield with respect to PWC (Table 2). Grape seeds are reported to contain high amounts of phenolic compounds [27], but when comparing PWC and PWF samples under the tested PLE conditions, the fragmented seeds in PWF seemed not to provide the extract with higher amounts of phenolics. One possible reason might be that the total phenol content from the seeds is much lower due to the lower seed volume (20–26%) present in the total pomace [5], [28]. It has been reported that by minimizing the particle size of the starting material, it is possible to improve the extraction of phenolic compounds by PLE even though the relation between the particle size and the extraction yield is not always linear and depends from the type of starting material [29].
PLE following optimised conditions, was also performed by using dried PWC, which were or not subjected to a defatting step (PDD and PD samples respectively). Phenol extraction yields were not significantly different between PDD and PD, being also similar to those of PWF (Table 2). Similarly to the present results, a lower amount of polyphenols was previously recovered from Sangiovese and Montepulciano dried pomace when compared to fresh pomace, most probably as consequence of degradation caused by high temperature drying process [11]. The defatting step mainly removes from the seeds part of the oil which also contains portions of hydrophobic polyphenols that most probably were not detected by Folin-Ciocalteau assay that is performed in water conditions.
Overall the four best extracts (namely T50, S100, PWF and PD) were selected on the basis of their total phenol content expressed per kgDW (Table 2), for further characterization.
Spectrophotometrical characterisation of solvent-based (SE) phenolic extracts
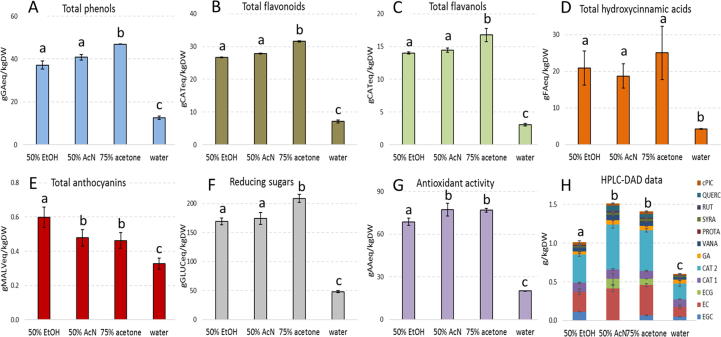
Phytochemical profiles of the three best SE extracts and the water control were characterised by spectrophotometric assays in terms of the most relevant phenolic compound family contents (Fig. 2A–G).
Fig. 2.
Total amounts of phenols (A), flavonoids (B), flavanols (C), hydroxycinnamic acids (D), anthocyanins (E) reducing sugars (F), antioxidant activity (G) and specific bioactive compounds (H) detected in selected solvent-based (SE) extracts. Results are expressed as g of standard compound equivalent per kg of pomace dry weight (g eq/kgDW). Different letters indicate a statistically significant difference (oneway ANOVA followed by a post hoc two-tailed Student’s t-test, p < 0.05) between the same type of data. Data are the mean ± SD (n = 2). AA, ascorbic acid; AcN, acetonitrile; CAT, catechin; cPIC, cis-piceid; EC, epicatechin; ECG epicatechin gallate; EGC, epigallocatechin; EtOH, ethanol; FA, ferulic acid; GA, gallic acid; GLUC, glucose; MALV, malvidin; PROTA, protocatechuic acid; QUERC, quercetin; RUT, rutin; SYRA, syringic acid; VANA, vanillic acid.
The three tested solvents were able to increase, in comparison to water control, the extraction of total phenols, flavonoids, flavanols, hydroxycinnamic acids and, to a lesser extent, of anthocyanins. Overall, flavonoids, flavanols and hydroxycinnamic acids were more concentrated in 75% acetone extract, anthocyanins in 50% EtOH sample, while 50% AcN released an intermediate amount of these compounds (Fig. 2).
Total extracted phenols ranged between 37.2 (50% EtOH) and 46.9 (75% acetone) gGAeq/kgDW (Fig. 2A), in agreement or higher than Merlot pomace published results obtained with different extraction procedures: around 30 gGAeq/kgDW (40% EtOH, 24 h at 25 °C, S/L 1:50) [30], 32.7 gGAeq/kgDW (70% EtOH, 20 min at 30 °C in ultrasonic unit, S/L 1:8) [31], 23.9 to 41.0 gGAeq/kgDW (70% EtOH/0.1% HCl/29.9% water, 60 min in ultrasonic unit, S/L 1:4, different pomace drying methods) [32]. Similar level of total phenols were also quantified in red winery sludge extracted with methanol [2] (1.97 g/L compared with averages of 1.56 and 2.84 gGA/L in the present ethanol extracts, S/L 1:10 and 1:5 respectively). The maximum amount of recovered flavonoids was 31.6 gCATeq/kgDW (Fig. 2B, 75% acetone), almost twice with respect to data reported by Ribeiro [30] (17.5 gCATeq/kgDW), while in case of flavanols the maximum content was again detected in 75% acetone extracts (16.8 gCATeq/kgDW, Fig. 2C) and was about 4-times lower than that reported in Merlot pomace [32]. Total hydroxycinnamic acids were for the first time quantified via a spectrophotometric assay in Merlot pomace extracts (Fig. 2D) as only HPLC data of single compounds are present in literature. Their release was largely increased by the solvents with respect to water (up to 5.8-times with 75% acetone, 25.0 gFAeq/kgDW). Conversely to what observed for the other compounds, anthocyanins were mainly extracted by 50% EtOH (up to 1.8-times respect to water control), followed by 50% AcN and 75% acetone (Fig. 2E). The anthocyanin levels (between 0.46 and 0.60 gMALVeq/kgDW, Fig. 2E) were comparable with Merlot pomace literature data (0.55 gMALVeq/kgDW [32] or 0.76 g Cya-3-glu eq/kgDW [30]).
The extracts were also quantified for the content of reducing sugars with 75% acetone being the most efficient in their solubilisation (209 gGLUC/kgDW, 4.3-times higher than water control) (Fig. 2F), with levels on average 10-times higher than previous data of Merlot pomace [30], [32] but of the same order of magnitude of Cabernet Sauvignon pomace extracts (307 gGLUC/kgDW) [30]. Differences among sugar amounts in pomace obtained from the same grape cultivar can be ascribed to use of different the time and conditions of the alcoholic fermentation step during winemaking.
SE extracts exerted an antioxidant capacity (Fig. 2G) which correlated with their phytochemical profiles (total phenol, phenolic family and sugar contents; Fig. 2A–F). In fact, the most active extracts were 75% acetone and 50% AcN samples, with average antioxidant activity levels of 77 gAAeq/kgDW. The present data (Fig. 2G) seemed to be coherent with Merlot pomace literature (e.g. 68.6 g Trolox eq/kgDW, measured by Lingua et al. [33] with ABTS assay) even though antioxidant activity was assayed by using many methodologies and results were expressed in different units.
With the exception of flavonoids, almost all the analysed molecule families were recovered at levels generally higher or of the same order of magnitude of those previously reported on Merlot pomace SE extracts [30], [31], [32], [33]. However, the present one-step SE process resulted to be simpler and/or faster with respect to methodologies previously applied.
Spectrophotometrical characterisation of pressurized liquid (PLE) phenol extracts
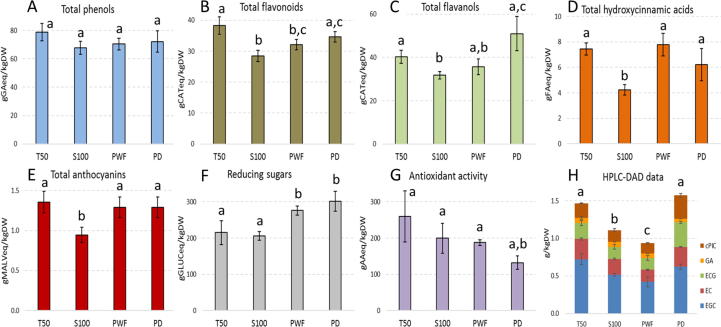
Similarly to SE samples, the four best PLE extracts (T50, S100, PWF, PD) were further characterised for their phytochemical profile, reducing sugar content and antioxidant activity by means of spectrophotometric assays (Fig. 3A–G).
Fig. 3.
Total amounts of phenols (A), flavonoids (B), flavanols (C), hydroxycinnamic acids (D), anthocyanins (E) and reducing sugars (F), antioxidant activity (G) and specific bioactive compounds (H) detected in selected pressurized liquid extracts. Results are expressed as g of standard compound equivalent per kg of pomace dry weight (g eq/kgDW). Different letters indicate a statistically significant difference (oneway ANOVA followed by a post hoc two-tailed Student’s t-test, p < 0.05) between the same type of data. Data are the mean ± SD (n = 2). Samples: T50, wet coarse milled pomace, 50 min extraction, 75% EtOH-H2O /CO2 ratio; S100, wet coarse milled pomace, 30 min extraction, 100% EtOH-H2O /CO2 ratio; PWF, wet fine milled pomace, 30 min extraction, 75% EtOH-H2O /CO2 ratio; PD, dried coarse milled pomace, 30 min extraction, 75% EtOH-H2O /CO2 ratio. AA, ascorbic acid; CAT, catechin; cPIC, cis-piceid; EC, epicatechin; ECG epicatechin gallate; EGC, epigallocatechin; FA, ferulic acid; GA, gallic acid; GLUC, glucose; MALV, malvidin.
All the extracts contained similar levels of total phenols (on average 72.3 gGAeq/kgDW; Fig. 3A), while specific compound families were differently released by the treatments (Fig. 3B–E).
The T50 extract showed an average 5.2% higher flavonoid amount if compared with the other selected samples (Fig. 3B) while the highest flavanol yields were detected in PD sample (Fig. 3C). When the PLE was carried out in the presence with 100:0 of EtOH-H2O:CO2 (S100), lower contents of flavonoids, flavanols, hydroxycinnamic acids and anthocyanins were observed (Fig. 3B–E). The lowest antioxidant activity was detected in PD (Fig. 3G) in accordance with previous data that showed the negative impact of pomace drying on the total phenol content as well as on antioxidant activity, mostly due to phenol complexation and/or degradation caused by the high temperature [11], [34]. The type of pre-treatment was the parameter mostly affecting the release of reducing sugars, with levels in PWF and PD samples 37.1% higher than in T50 and S100 (Fig. 3F).
As already observed for SE samples (Fig. 2), also PLE liquid extracts were complex mixtures of different phenols. In general, PLE seemed to be able to extract higher amount of phenols and reducing sugars with respect to SE (on average between 1.2-times for flavonoids and 2.6-times for flavanols), with the only exception of hydroxycinnamic acids that were on average 3.3-times more present in solvent extracts (Fig. 2, Fig. 3). As consequence of the higher phenol content, PLE samples also showed on average 2.6-fold higher antioxidant capacity than SE extracts (Fig. 2G and 3G). Respect to PLE, SE samples were richer in hydroxycinnamic acids, but these compounds exert a generally lower an antioxidant activity compared other types of phenols (e.g. flavonoids) [35].
By comparing data of 50% EtOH SE (Fig. 2) and S100 PLE (Fig. 3) extractions performed with the use of the same solvent mixture, it can be assumed that the different sample compositions were mainly influenced by process factors such as temperature and pressure, rather than by the solvent. This fact is in accordance with the literature where, when compared to other methodologies, PLE gave higher phenol contents from plant-based materials [36], [37]. PLE in addition to solvent extraction effects combines pressure increase at elevated temperature. As a result, the diffusivity of a solvent could be increased facilitating its penetration through the plant matrix so disrupting the strong solute-matrix interactions and increasing the final extraction yield [38].
Chromatographic analysis of phenolic extracts
HPLC-DAD analyses (Figs. 2H and 3H) confirmed that all the extracts were very complex mixtures and a larger spectrum of phytochemicals was identified in SE (Fig. 2H) respect to PLE samples (Fig. 3H). Several potentially bioactive and exploitable compounds were quantified.
Most of the detected and more abundant compounds belonged to the flavanol family (CAT, EC, EGC, ECG) (e.g. 0.583 g/kgDW of CAT2 in 50% AcN SE extracts; 0.775 g/kgDW of EGC in T50 PLE sample). Moreover, many unidentified chromatographic peaks showed catechin characteristic spectrum with different retention times, suggesting to be flavanol derivatives. Flavanols are one of the most abundant classes of grape phenolics, based on catechin chemical structure and including monomers, dimers and oligomers and gallate forms. They are extremely important for wine sensorial characteristics and exert several healthy bioactivities [4], [7]. CAT, EC and ECG were previously reported in Merlot pomace extracts [30], [33], [39], while EGC was here detected for the first time in this type of pomace (Figs. 2H and 3H).
Quercetin (QUERC) and rutin (RUT) were measured in SE extracts, respectively up to 0.067 and 0.019 g/kgDW in 50% AcN sample (Fig. 2H). QUERC is one of the most abundant dietary flavonoids and it is the aglycone form of a number of other flavonoid glycosides, such as RUT [7]. QUERC and its derivatives were previously reported in Merlot pomace extracts [30], [33], [39].
Stilbenes are common in grape and include resveratrol and its glycosides or dimers [7]. Among these, only cis-piceid (cPIC) was present in all samples at an average concentration of 0.034 g/kgDW in SE and of 0.162 g/kgDW in PLE (Figs. 2H and 3H). cPIC was never reported in Merlot pomace extracts. Few reports quantified free resveratrol in Merlot pomace [30], [39], while a recent study ascribed its absence to the high transfer ratio to wine [33]. Similarly, also for the present results it can be suggested a differential extraction occurring during winemaking alcoholic fermentation for resveratrol compared to its glycoside derivatives (such as piceid). This difference might therefore explain the absence of free resveratrol in both SE and PLE samples.
Other compounds identified in SE extracts were gallic (GA), syringic (SYRA), protocatechuic (PROTA) and vanillic (VANA) acids, all belonging to the phenolic acids class, while only GA was found in PLE samples (Figs. 2H and 3H).
Composite formulation and characterization
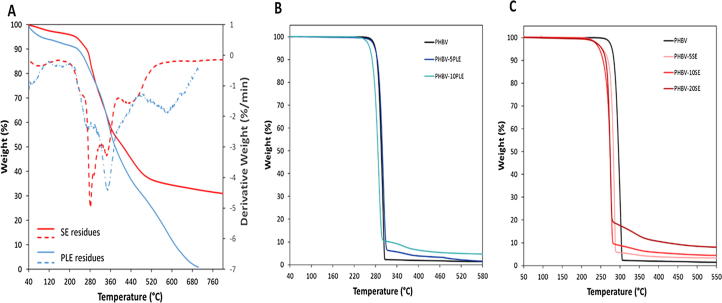
In order to fully valorise Merlot grape pomace, solid extraction residues obtained after best SE (75% acetone) and PLE (T50) processes were used as a filler for biocomposites preparation. The residues were firstly characterized by means of thermogravimetric analysis (TGA) (Fig. 4A). After an initial moisture loss, the main degradation processes took place at temperatures above 200 °C for both type of residues (Fig. 4A, solid lines). In addition the samples showed a different chemical composition (Fig. 4A, dotted lines), with SE residue presenting a maximum degradation at 285 °C, probably due to the decomposition of hemicellulose the most unstable fraction of lignocellulose biomass [40], [41]. On the other hand, beside the mentioned hemicellulose degradation, PLE residue showed a derivative minimum at almost 350 °C, corresponding to the cellulose decomposition. At higher temperatures, the residual part of the samples, mainly composed of lignin [40], [41], remained almost constant only in SE sample showing no sign of degradation which were instead detectable in PLE (Fig. 4A). Therefore, on the basis of TGA data, the two solid residues seemed to be composed by hemicellulose/cellulose fractions in different ratios, probably due to the different extraction conditions applied (in SE solvent and medium temperature; in PLE solvent, pressure increase and high temperature) which may have had effect also on cellulose and hemicellulose availability, degradation or modification.
Fig. 4.
Thermogravimetric (TGA) curves (solid lines, left axis) and related derivative curves (dotted lines, right axis) of SE and PLE extraction solid residues (A) and of PHBV polymer and bio-composites based on SE (B) or PLE (C) extraction residues.
Literature describes the preparation of composites based on PHB and various residues by using a solvent-assisted method [42], [43]. Instead, in the present study a green solvent-less process consisting in a simple and rapid mixing of the PHBV polymer and of the 5–20% (w/w) residue at 200 °C, was carried out. This temperature allowed PHBV to rapidly melt preventing at the same time fibre degradation, as indicated by the TGA analyses. A first reference sample, containing just PHBV, was prepared. The thermal and mechanical characteristics of obtained biocomposites and of PHBV control, are summarized in Table 3. The results of TGA analyses showed that PHBV homopolymer degradation occurred in a single step and was completed just above 300 °C (Fig. 4B and C). Instead, all composites presented a residual char the amount of which increased with the grape filler content. Since no changes in the curve slopes were evident, the degradation mechanism seemed to be independent from the filler presence [42]. Considering both the temperature of initial decomposition (Tonset) and the temperature of maximum degradation rate (TD), a high thermal stability was generally observed for all composites. In comparison to the polymer control, the PHBV-5PLE composite showed the same Tonset while in PHBV-10PLE the initial degradation started at 10 °C lower than that of PHBV indicating of possible positive effect of the content of PLE residue on the composites thermal stability. Instead, a different behaviour was recorded for SE composites with both Tonset and TD decreasing with the filler content increment. This trend was in accordance with the TGA data (Fig. 4A), showing a high content of hemicellulose in the SE residue, and also with the gel permeation chromatography (GPC) data (Table 3) where the macromolecular weight of PHBV matrix was generally unchanged for the composites including with PLE residue, whereas it decreased from Mn = 62800 to 54400, after addition of the SE residue. The reduction of PHBV molecular weight in the presence of increasing SE residue content could be due to a faster hydrolysis of PHBV chains induced by the filler degradation products as previously observed [44]. On the basis of differential scanning calorimetry (DSC) data (Table 3), PHBV resulted to be a semi-crystalline polymer characterized by a noteworthy crystallinity. The PHBV crystallization and melting enthalpies were high (ΔHc = 73 J/g; ΔHm = 78 and 82 J/g in the first and in the second heating scan, respectively; Table 3), moreover, the polymer showed a high crystallization capability and completely crystallized during the cooling step. The addition of the SE residues into the PHBV matrix entailed a slight decrement in the crystallization temperatures (Tc) during the cooling scan and Tc went from 114 °C for PHBV to 108 °C for the sample containing SE 20% (w/w). A similar trend was recorded for materials obtained with PLE samples, indicating that these residues acted as physical hindrances to the chain mobility, slightly slowing down the crystallization process. All PLE composites completely crystallized during the cooling step and the subsequent melting was very similar to the PHBV control. The composites’ enthalpies of crystallization and melting decreased according to their composition (Table 3). A reduction in the crystallization rate, even exiguous, is however desirable when it is necessary to enlarge the processing window for highly crystalline materials (like PHBV). The remarkable crystallinity of PHBV affects its mechanical properties indeed making this polymer extremely brittle.
Table 3.
Thermal and mechanical characteristics of final bio-composites and PHBV. The sample code indicates the type of polymer (PHBV), the percentage and type of used residues according to the extraction (SE or PLE). Tonset, temperature of initial decomposition; TD, temperature of the maximum degradation rate; Mn, number average molecular weight; Mw, weight average molecular weight; PD, polydispersity; Tm, melting temperature; ΔHm, melting enthalpy; Tc, crystallization temperature; ΔHc, crystallization enthalpy; E, elastic modulus; σ, tensile strength; ε, elongation at break.
| Sample | Tonset (°C)a | TD (°C)a | Mn · 10-3b | Mw · 10-3b | PDb | Tm (°C)c | ΔHm (J/g)c | Tc (°C)d | ΔHc (J/g)d | Tm (°C)e | ΔHm (J/g)e | E (MPa)f | σ (MPa)f | ε (%)f |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHBV | 288 | 302 | 62.8 | 129 | 2.05 | 172 | 78 | 114 | 73 | 168 | 82 | 1728 ± 50 | 33.2 ± 1.0 | 3.1 ± 0.1 |
| PHBV-5SE | 277 | 286 | 55.5 | 115 | 2.08 | 170 | 72 | 112 | 68 | 168 | 77 | 1640 ± 58 | 30.1 ± 1.5 | 3.1 ± 0.2 |
| PHBV-10SE | 267 | 277 | 57.0 | 117 | 2.05 | 169 | 74 | 110 | 65 | 168 | 74 | 1564 ± 50 | 27.0 ± 0.6 | 3.1 ± 0.2 |
| PHBV-20SE | 268 | 276 | 54.4 | 112 | 2.06 | 170 | 64 | 108 | 57 | 168 | 66 | 1631 ± 84 | 23.8 ± 1.6 | 2.5 ± 0.1 |
| PHBV-5PLE | 289 | 302 | 59.2 | 121 | 2.04 | 171 | 77 | 112 | 71 | 169 | 82 | 1491 ± 38 | 28.8 ± 1.1 | 3.5 ± 0.2 |
| PHBV-10PLE | 276 | 290 | 63.0 | 132 | 2.09 | 171 | 73 | 110 | 66 | 169 | 76 | 1373 ± 15 | 24.7 ± 0.3 | 3.6 ± 0.2 |
Determined by thermogravimetric analysis (TGA) under N2 flux, by heating at 10 °C/min.
Determined by gel permeation chromatography (GPC).
Determined by differential scanning calorimetry (DSC) during the first heating scan.
Determined by DSC during the cooling scan.
Determined by DSC during the second heating scan.
Determined by INSTRON dynamometer.
The effect of the addition of grape pomace residues on mechanical properties of PHBV was investigated by tensile tests. Table 3 reports the values of the elastic modulus (E), tensile strength (σ) and elongation at break (ε) of all the investigated materials. The samples composites containing the SE residues showed a progressive moderate decrease in all the values with the increase of the filler amount, indicating the occurrence of a weak adhesion and poor impregnation between the filler and the matrix. The low compatibility between the two phases was probably the reason for a poor stress-transfer capability, thus less energy was required to initiate the fracture propagation. Similar results were previously reported in literature [45], [46]. In the case of the PLE composites, an increment of the elongation at break could be observed, suggesting that the filler was able to create a better anchoring to the matrix, resulting in a moderately more ductile material respect to the pure PHBV. The better interaction between matrix and residue can be justified by considering that the PLE residue seemed to be richer or to have a higher availability of the cellulose fraction with respect to the SE one, therefore resulting more polar and able to easily interact with PHBV.
In conclusion, the data indicated that composites obtained with the addition of 10–20% (w/w) of Merlot pomace solid residues showed similar or no worse mechanical and thermal properties respect to PHBV matrix alone.
Conclusions
The present paper aimed at developing and validating sustainable routes towards a full valorisation of Merlot grape pomace, demonstrating that it could represent a valuable feedstock for the production of bioactive molecules and new materials. Solvent-based (SE) and pressurized liquid (PLE) extractions were successfully applied and optimised for phenolic compounds recovery. In both cases, liquid extracts were complex mixtures of phenols, containing several valuable compound families and specific bioactive phytochemicals, and exerted antioxidant activity. The validated SE processes were one-step procedures requiring basic equipment and showing potentiality of being easily scaled-up in chemical or extraction industrial pilot plants, while PLE utilised food grade and non-toxic solvents while, on the other hand, requiring more sophisticated equipment and larger solvent volumes. The pomace extraction solid residues were mixed with PHBV polymer at high temperature, using an eco-friendly approach not involving solvents and other additives. Interestingly the final properties of the PHBV matrix were not worsened by a high content of the fibrous residues (up to 20% w/w). The use of pomace residues in biocomposites could therefore bring both to the reduction of the cost of the final material, as a lower amount of costly PHBV is used, as well as to the full valorisation of the main grape agrowaste. In conclusion, the present paper demonstrated that Merlot pomace can be both valorised as phenolic extract and as fibrous residue and exploited in the food, nutraceutical, cosmetic and packaging/material fields.
Compliance with Ethics Requirements
This article does not contain any study with human or animal subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the NoAW project (“Innovative approaches to turn agricultural waste into ecological and economic assets”), founded by the European Union Horizon 2020 research and innovation programme under the grant agreement No 688338.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Galanakis C.M. Recovery of high added-value components from food wastes: conventional, emerging technologies and commercialized applications. Trends Food Sci Technol. 2012;26(2):68–87. [Google Scholar]
- 2.Galanakis C.M. Emerging technologies for the production of nutraceuticals from agricultural by-products: a viewpoint of opportunities and challenges. Food Bioprod Process. 2013;91(C4):575–579. [Google Scholar]
- 3.Galanakis C.M., Markouli E., Gekas V. Recovery and fractionation of different phenolic classes from winery sludge using ultrafiltration. Sep Purif Technol. 2013;107:245–251. [Google Scholar]
- 4.Beres C., Costa G.N.S., Cabezudo I., da Silva-James N.K., Teles A.S.C., Cruz A.P.G. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manage. 2017;68:581–594. doi: 10.1016/j.wasman.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Lomillo J., Gonzalez-SanJosè M.L. Applications of wine pomace in the food industry: approaches and functions. Compr Rev Food Sci Food Saf. 2017;16(1):3–22. doi: 10.1111/1541-4337.12238. [DOI] [PubMed] [Google Scholar]
- 6.Muhlack R.A., Potumarthi R., Jeffery D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manage. 2018;72:99–118. doi: 10.1016/j.wasman.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Yu J.M., Ahmedna M. Functional components of grape pomace: their composition, biological properties and potential applications. Int J Food Sci Technol. 2013;48(2):221–237. [Google Scholar]
- 8.Panzella L., Napolitano A. Natural phenol polymers: recent advances in food and health applications. Antioxidants-Basel. 2017;6(2) doi: 10.3390/antiox6020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otero-Pareja M.J., Casas L., Fernandez-Ponce M.T., Mantell C., de la Ossa E.J.M. Green extraction of antioxidants from different varieties of red grape pomace. Molecules. 2015;20(6):9686–9702. doi: 10.3390/molecules20069686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferri M., Rondini G., Calabretta M.M., Michelini E., Vallini V., Fava F. White grape pomace extracts, obtained by a sequential enzymatic plus ethanol-based extraction, exert antioxidant, anti-tyrosinase and anti-inflammatory activities. New Biotechnol. 2017;39:51–58. doi: 10.1016/j.nbt.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Ferri M., Bin S., Vallini V., Fava F., Michelini E., Roda A. Recovery of polyphenols from red grape pomace and assessment of their antioxidant and anti-cholesterol activities. New Biotechnol. 2016;33(3):338–344. doi: 10.1016/j.nbt.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Totaro G., Sisti L., Vannini M., Marchese P., Tassoni A., Lenucci M.S. A new route of valorization of rice endosperm by-product: Production of polymeric biocomposites. Compos Part B-Eng. 2018;139:195–202. [Google Scholar]
- 13.Nanni A., Messori M. A comparative study of different winemaking by-products derived additives on oxidation stability, mechanical and thermal proprieties of polypropylene. Polym Degrad Stabil. 2018;149:9–18. [Google Scholar]
- 14.Tassoni A., Ferri M. Winery by-products: pomace as source of high value phenols. In: Vilarinho C., Castro F., Goncalves M., Fernando A.L., editors. Wastes: Solutions, Treatments and Opportunities. CRC Press Taylor & Francis Group; Boca Raton, USA: 2019. pp. 440–444. [Google Scholar]
- 15.Gustinelli G, Eliasson L, Svelander C, Andlid T, Lundin L, Ahrné L, et al. Supercritical fluid extraction of berry seeds: chemical composition and antioxidant activity. J Food Qual 2018; Volume 2018: Article ID 6046074.
- 16.Witkowski A., Majkut M., Rulik S. Analysis of pipeline transportation systems for carbon dioxide sequestration. Arch Thermodyn. 2014;35(1):117–140. [Google Scholar]
- 17.Cardoso L.C., Serrano C.M., Quintero E.T., López C.P., Antezana R.M. Martínez de la Ossa EJ. High pressure extraction of antioxidants from Solanum stenotomun peel. Molecules. 2013;18:3137–3151. doi: 10.3390/molecules18033137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivas K., King J.W., Monrad J.K., Howard L.R., Zhang D. Pressurized solvent extraction of flavonoids from grape pomace utilizing organic acid additives. Ital J Food Sci. 2011;23(1):90–105. [Google Scholar]
- 19.Mantell C., Rodriguez M., de la Ossa E.M. A Screening analysis of the high-pressure extraction of anthocyanins from red grape pomace with carbon dioxide and cosolvent. Eng Life Sci. 2003;3(1):38–42. [Google Scholar]
- 20.Ferri M., Gianotti A., Tassoni A. Optimisation of assay conditions for the determination of antioxidant capacity and polyphenols in cereal food components. J Food Comp Anal. 2013;30(2):94–101. [Google Scholar]
- 21.McMurrough I., McDowell J. Chromatographic separation and automated analysis of flavanols. Anal Biochem. 1978;91(1):92–100. doi: 10.1016/0003-2697(78)90819-9. [DOI] [PubMed] [Google Scholar]
- 22.Bival Štefan M., Vuković Rodríguez J., Blažeković B., Kindl M., Vladimir-Knežević S. Total hydroxycinnamic acids assay: prevalidation and application on Lamiaceae species. Food Anal Methods. 2014;7:326–336. [Google Scholar]
- 23.Bailey M., Biely P., Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J Biotech. 1992;23:257–270. [Google Scholar]
- 24.Considine J.A., Frankish E. Academic Press. Elsevier; Cambridge, USA: 2014. A complete guide to quality in small-scale wine making. [Google Scholar]
- 25.Ferri M., Tassoni A., Franceschetti M., Righetti L., Naldrett M.J., Bagni N. Chitosan treatment induces changes of protein expression profile and stilbene distribution in Vitis vinifera cell suspensions. Proteomics. 2009;9(3):610–624. doi: 10.1002/pmic.200800386. [DOI] [PubMed] [Google Scholar]
- 26.Fontana A.R., Antoniolli A., Bottini R. Grape pomace as a sustainable source of bioactive compounds: extraction, characterization and biotechnological applications of phenolics. J Agric Food Chem. 2013;61:8987–9003. doi: 10.1021/jf402586f. [DOI] [PubMed] [Google Scholar]
- 27.Xia E.-Q., Deng G.-F., Guo Y.-H., Li H.-B. Biological activities of polyphenols from grapes. Int J Mol Sci. 2010;11:622–646. doi: 10.3390/ijms11020622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mourtzinos I, Goula A. Polyphenols in agricultural byproducts and food waste. In: Watson R, editor. Polyphenols in Plants - Isolation, Purification and Extract Preparation. 2nd Edition ed. London, UK: Academic Press, Elsevier; 2019. p. 23–38.
- 29.Luthria D.L. Influence of experimental conditions on the extraction of phenolic compounds from parsley (Petroselinum crispum) flakes using a pressurized liquid extractor. Food Chem. 2008;107(2):745–752. [Google Scholar]
- 30.Ribeiro L.F., Ribani R.H., Francisco T.M.G., Soares A.A., Pontarolo R., Haminiuk C.W.I. Profile of bioactive compounds from grape pomace (Vitis vinifera and Vitis labrusca) by spectrophotometric, chromatographic and spectral analyses. J Chromatogr B. 2015;1007:72–80. doi: 10.1016/j.jchromb.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Sporin M., Avbelj M., Kovac B., Smole Mozina S. Quality characteristics of wheat flour dough and bread containing grape pomace flour. Food Sci Technol Int. 2018;24(3):251–263. doi: 10.1177/1082013217745398. [DOI] [PubMed] [Google Scholar]
- 32.Tseng A., Zhao Y. Effect of different drying methods and storage time on the retention of bioactive compounds and antibacterial activity of wine grape pomace (Pinot Noir and Merlot) J Food Sci. 2012;77(9):H192–H201. doi: 10.1111/j.1750-3841.2012.02840.x. [DOI] [PubMed] [Google Scholar]
- 33.Lingua M.S., Fabani M.P., Wunderlin D.A., Baroni M.V. From grape to wine: changes in phenolic composition and its influence on antioxidant activity. Food Chem. 2016;208:228–238. doi: 10.1016/j.foodchem.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Sólyom K., Sola R., Cocero M.J., Mato R.B. Thermal degradation of grape marc polyphenols. Food Chem. 2014;159:361–366. doi: 10.1016/j.foodchem.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 35.Teixeira J, Gaspar A, Garrido EM, Garrido J, Borges F. Hydroxycinnamic acid antioxidants: an electrochemical overview. Biomed Res Int 2013; Volume 2013: Article ID 251754. [DOI] [PMC free article] [PubMed]
- 36.Suwal S., Marciniak A. Technologies for the extraction, separation and purification of polyphenols - A review. Nepal J Biotechnol. 2018;6(1):74–91. [Google Scholar]
- 37.Naczk M., Shahidi F. Extraction and analysis of phenolics in food. J Chromatogr A. 2004;1054(1–2):95–111. [PubMed] [Google Scholar]
- 38.Teo C.C., Tan S.N., Yong J.W.H., Hew C.S., Ong E.S. Pressurized hot water extraction (PHWE) J Chromatogr A. 2010;1217(16):2484–2494. doi: 10.1016/j.chroma.2009.12.050. [DOI] [PubMed] [Google Scholar]
- 39.Kadouh H.C., Sun S., Zhu W.J., Zhou K.Q. alpha-Glucosidase inhibiting activity and bioactive compounds of six red wine grape pomace extracts. J Funct Foods. 2016;26:577–584. doi: 10.1016/j.jff.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orfao J.J.M., Antunes F.J.A., Figueiredo J.L. Pyrolysis kinetics of lignocellulosic materials - three independent reactions model. Fuel. 1999;78(3):349–358. [Google Scholar]
- 41.Amutio M., Lopez G., Aguado R., Artetxe M., Bilbao J., Olazar M. Kinetic study of lignocellulosic biomass oxidative pyrolysis. Fuel. 2012;95(1):305–311. [Google Scholar]
- 42.Angelini S., Cerruti P., Immirzi B., Scarinzi G., Malinconico M. Acid-insoluble lignin and holocellulose from a lignocellulosic biowaste: Bio-fillers in poly(3-hydroxybutyrate) Eur Polym J. 2016;76:63–76. [Google Scholar]
- 43.Feng P., Kong Y., Yu L., Li Y., Gao C.D., Peng S.P. Molybdenum disulfide nanosheets embedded with nanodiamond particles: co-dispersion nanostructures as reinforcements for polymer scaffolds. Appl Mater Today. 2019;17:216–226. [Google Scholar]
- 44.Berthet M.A., Angellier-Coussy H., Chea V., Guillard V., Gastaldi E., Gontard N. Sustainable food packaging: valorising wheat straw fibres for tuning PHBV-based composites properties. Compos Part A-Appl Sci. 2015;72:139–147. [Google Scholar]
- 45.Saccani A., Sisti L., Manzi S., Fiorini M. PLA composites formulated recycling residuals of the winery industry. Polym Compos. 2019;40(4):1378–1383. [Google Scholar]
- 46.Gultekin M., Cayli G., Esen H. Utilization of renewable filler from lichen in low-density polyethylene. Polym Compos. 2017;38(2):389–395. [Google Scholar]