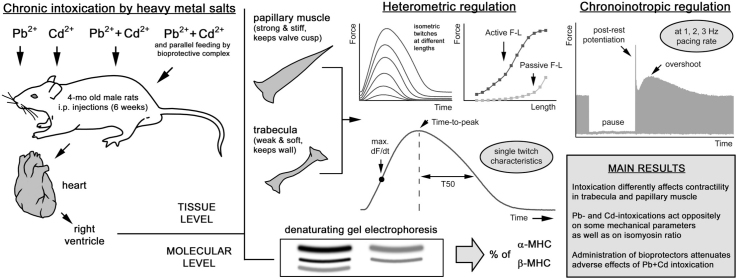
Graphical abstract
Keywords: Cardiotoxicity, Lead, Cadmium, Myocardium contractility, Myosin, Trabecules, Papillary muscles, Bioprotectors
Highlights
-
•
Subchronic intoxications were induced in male rats by repeated IP injections of lead acetate and/or cadmium chloride.
-
•
Right ventricle trabecules and papillary muscle preparations were used for modeling the heart cycle.
-
•
Single twitch parameters in trabecules and papillary muscles respond to the intoxications differently.
-
•
Intoxications with Pb and Cd led to contra-directional effects on some mechanical parameters and the myosin isoform ratio.
-
•
Background administration of bioprotectors attenuated cardio-mechanical effects of Pb + Cd intoxication.
Abstract
Subchronic intoxications induced in male rats by repeated intraperitoneal injections of lead acetate and cadmium chloride, administered either alone or in combination, are shown to affect the biochemical, cytological and morphometric parameters of blood, liver, heart and kidneys. The single twitch parameters of myocardial trabecular and papillary muscle preparations were measured in the isometric regime to identify changes in the heterometric (length-force) and chronoinotropic (frequency-force) contractility regulation systems. Differences in the responses of these systems in trabecules and papillary muscles to the above intoxications are shown. A number of myocardium mechanical characteristics changing in rats under the effect of a combined lead-cadmium intoxication and increased proportion of α-myosin heavy chains were observed to normalize fully or partially if such intoxication was induced against background administration of a proposed bioprotective complex. Based on the experimental results and literature data, some assumptions are suggested concerning the mechanisms of the cardiotoxic effects produced by lead and cadmium.
1. Introduction
Previously we [1,2] demonstrated a number of lead cardiotoxicity effects in vivo estimated by changes in the contractility of multicellular myocardial preparations and in the functional characteristics of contractile proteins in the heart extracted from the body of a rat with moderate subchronic lead intoxication in comparison with respective control values. These effects of lead intoxication were observed for the first time to be attenuated if induced along with oral administration of a combination of bioprotectors.
The objective of our subsequent work in this field was not only to confirm the essential reproducibility of these results but also to compare corresponding effects under similar subchronic intoxications with lead, cadmium and their combination. The practical importance of this endeavor is determined by the fact that combined harmful exposure to these two metals in different quantitative ratios is typical of rather large population groups occupied in copper smelting and/or residing in areas contaminated by copper smelting facilities (e.g. [3]) or by contaminated irrigation [4]. As it is underlined by Hernandes et al. [5] many national and international organizations are currently stressing that studies on chemical mixture toxicity are of primary importance. This problem is one of crucial issues of our own studies as well [6]. Moreover, there is scientific interest in studying the comparative and combined toxicity of lead and cadmium since both of these elements are characterized by multivectorial adverse impacts on the organism, affecting both the same and differing targets, acting, in particular, upon the condition and functioning of the cardio-vascular system directly or indirectly.
The most significant literature data concerning the still debatable issue of lead vaso- and cardio-toxicity in vivo or in vitro were recently reviewed by us in the above-mentioned papers [1,2]. As for the adverse cardiovascular effects of cadmium, they have also been addressed in a number of both epidemiological [[7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]] and experimental [[19], [20], [21], [22], [23], [24], [25]] studies. In particular, special attention has been given to the relationship between elevated blood pressure and increased concentration of cadmium in human blood and urine. This relationship was estimated by Gallagher and Melike [26] as inconclusive based on an overview of eight epidemiological studies.
Besides, some experimental studies suggested a direct damaging effect of cadmium on the cardiomyocyte or on the regulatory mechanisms of its contractility [19,20,24,25,[27], [28], [29], [30]]. Thus in a recent study [31], it was found that a direct impact of cadmium on a culture of human cardiomyocytes obtained from stem cells caused reduced cell viability, increased apoptosis, elevated levels of reactive oxygen species, altered action potential, and arrhythmias. However, in contrast to lead cardiotoxicity, there are practically no cadmium cardiotoxicity studies on isolated multicellular myocardial preparations preserving the morpho-functional structure of the myocardium in vivo.
In this paper, we report an analysis of the functional characteristics of isometric contractions studied on just such myocardial preparations (along with estimating the ratio of myosin heavy chain isoforms) from rats sub-chronically treated with cadmium and lead, administered both alone and in combination. The combined intoxication effects were also assessed against the action of mechanistically justified bioprotectors administered in parallel to toxic exposure. This design was dictated not only by understandable practical considerations but also by the fact that the attenuation of myocardial dysfunctions along with the attenuation of the systemic toxicity of these metals could serve an indirect argument in favor of a causal relationship between them.
It should be stressed that not only isolated action of cadmium intoxication on multicellular myocardium preparations, but also the combined lead-cadmium cardiotoxicity in general was studied by us for the first time.
2. Materials and methods
2.1. The general design of the study
This study was carried out on 3,5-4,5 months old outbred white male rats from our own breeding colony with the initial body weight of сa. 220−225 g. The rats were housed in conventional conditions, breathed unfiltered air, and were fed standard balanced food. The experiments were planned and implemented in accordance with the “International guiding principles for biomedical research involving animals” developed by the Council for International Organizations of Medical Sciences (1985) and were approved by the Ethics Committee of the Ekaterinburg Medical Research Center for Prophylaxis and Health Protection in Industrial Workers.
To induce respective subchronic intoxications, lead acetate and/or cadmium chloride solutions were administered to rats by repeated intraperitoneal (IP) injections three times a week up to 18 injections during 6 weeks (either separately or in combination) in one-shot doses equal to 6.01 mg Pb and 0.377 mg Cd per kg body mass. These doses were chosen by pilot trials as causing adverse shifts in several functional and biochemical indices of the organism’s status but not inducing too heavy an intoxication with lethal outcome.
A randomly selected one-half of the rats exposed to the combination of Pb + Cd and of control rats were administered throughout the exposure period a bioprotective complex (BPC) comprising: sodium glutamate; N-acetylcysteine; vitamins А, group B, Е, C and D3; rutin; ω-3 polyunsaturated fatty acids; selenium, iodine, iron, calcium and magnesium supplements; pectin enterosorbent. The mechanistic considerations justifying the choice of these substances as probable bioprotectors against lead and/or cadmium toxicity and their doses and methods of administration are outlined elsewhere [32]. The same paper describes in detail the procedures performed immediately after the end of the exposure period to assess the organ-systemic outcomes of intoxications.
There were 22 rats in every group: 12 were used for toxicological and physiological studies and 10 were used for muscle contractility and myosin heavy chain studies.
2.2. Study of isolated myocardial preparation contractility
A few minutes before sacrificing (by cervical dislocation under ether anesthesia) the rats were injected i.m. heparin (0.2 mL 5000 units) and xylazine muscle relaxant (0.3 mL). Immediately thereafter, the chest was opened up, the spontaneously contracting heart was removed and placed for 15 min into a Petri dish with modified Krebs-Henseleit solution containing 30 mM BDM (2,3-butanedione monoxime) placed on the stage of a binocular microscope at room temperature. Using microsurgical tools under the microscope, we dissected the thin (to avoid hypoxia in the deeper layers of the myocardial preparations) papillary muscle with a piece of the valve and a piece of the mural myocardium, and also the thin mural trabecula.
Upon dissection, the preparation was placed in one of the two thermostatically controlled baths in a two-channel setup containing modified Krebs-Henseleit solution perfusing a preparation with the help of a peristaltic pump (Masterflex L/S) and bubbled with a mixture of gases (O2 = 95 % and CO2 = 5%) at a temperature of 35 ± 0.1о C. The composition of the solution was as follows: 118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 14.5 mM NaHCO3, 1.2 mM KH2PO4, 2.5 mM CaCl2, 11.1 mM glucose, insulin 5 units/L, pH 7.35.
One of the ends of the preparation was attached to the rod of a force transducer and the other to that of a servomotor so that the attached ventricular mural piece was outside the recorded segment of the preparation. Then, to ensure adaptation to the experimental conditions, the preparations were stimulated for 30−45 min through parallel non-polarizing carbon electrodes with 1−5 ms 2 Hz supra-threshold electric impulses at 35 °C. As soon as contractility reached a steady state, the stretching of the preparation was reduced to the least possible value for which the minimum force could still be recorded, and the respective length was taken as L0. Following this, in each experiment the length of the preparation was increased gradually (commonly in a stepwise manner at 50 μm steps) until force reached a steady state at each length, achieving ultimately the maximum possible length for a given preparation, which was taken as Lmax.
The large and small diameters of the trabecules and papillary muscles were measured at length L0 for computing the cross-sectional area as that of an ellipse. Maximum force development and relaxation rates (dP/dt and dP/dt/Pmax), time to peak force (TTP) and time of decay from peak force to preset contraction amplitude (t50) were measured and analyzed in the course of stretching isolated multicellular preparations of the right ventricular myocardium from L0 to Lmax under 2 Hz constant frequency stimulation. At constant length 0.95 Lmax, we measured the parameters of the first post-rest contraction and the parameters of isometric contractions during the force transient overshoot.
It was shown by Milani-Nejad et al. [33] that the force of the first post-rest twitch at 37 °C increased progressively with the duration of the resting interval and reached a plateau after 30−60 s of rest. In our experiments, we used a 60 s interval.
To ensure the comparability of mechanical characteristics between myocardial preparations from different animals, force was normalized to the cross-sectional area of each preparation, which corresponds to the magnitude of mechanical tension (mN/mm2).
2.3. Determination of α- and β-cardiac myosin heavy chain (MHC) ratios
The ratio of myosin heavy chain isoforms in the rat right ventricular myocardium was determined with denaturating gel electrophoresis (SDS-PAGE) by the method developed by Reiser and Kline [34]. Following the electrophoresis, the gels were Coumassie stained, and, after washing with destaining solution and water, scanned by densitometer (“BioRad”, US), and the percentage ratio of α- and β-MHC was determined in the samples by Image Lab 5.2.1.
2.4. Statistical processing
Mechanical characteristics obtained for the comparison groups do not have a normal distribution (Kolmogorov-Smirnov, Lillieforce tests, Shapiro-Wilk's W test). Therefore, a nonparametric statistical method (Mann-Whitney U test) was used in the analysis of the reliability of differences in the values of parameters. The results are presented as mean ± SEM. The experimental groups with significant differences (p < 0.05) were assigned the following indices: * – Pb vs. Cont; ** – Cd vs. Cont; ^ - Pb + Cd vs. Cont; ^^ - Pb vs. Cd; # - Pb + Cd vs. Pb; ## - Pb + Cd vs. Cd.
3. Results and discussion
3.1. Systemic toxicity characteristics1
All the experimentally recorded functional, biochemical and histo-morphometric indices of the toxic effects produced by lead and/or cadmium on the organ, system and organism levels and the inferences from their mathematical analysis are interesting in their own right and, therefore, they made up the subject-matter of a special publication [32]. Nevertheless, we believe it important to give here an extended summary of that publication in order to give present readers an opportunity to appreciate specific cardiotoxic effects dealt with in this paper against the background of a broader picture of toxicological data.
In general, the experimental models that we obtained for isolated and combined subchronic intoxications with lead and cadmium could be characterized as moderately pronounced and thus comparable with severity of occupational human intoxications which are still possible in modern industrial conditions. However, they point to a markedly higher toxicity of cadmium according to the majority of the indices excepting lead-specific disturbances of porphyrin metabolism and signs of anemia. At the same time, statistically significant correlations between the shifts in a number of indices for the organism’s status under lead or cadmium intoxication and the concentration of a respective metal in the blood suggested that not only lead-specific but also a number of integral and non-specific shifts were most likely caused by the toxic impact of both of these metals or one of them. As concerns their combined toxicity directionality, it was found like in many other experiments carried out by our team (see the overview by Minigalieva et al. [35]) to be ambiguous depending on the outcome by which this toxicity is assessed, on this outcome’s level, and on the toxicant dose ratio. It has been also shown that even the additive combined toxicity of metals (including genotoxicity in vivo) can be considerably attenuated by background oral administration of the bioprotective complex (BPC) described in Section 2.1.
However, all the above is only partly relevant to the functional and morphological changes of the cardio-vascular system. Specifically, it has been found that only rats exposed to lead alone or in combination with cadmium had elevated values of systolic, diastolic and mean arterial blood pressure as compared with control ones. In good agreement with the systemic increase in resistance to arterial blood flow (which might be assumed as a probable mechanism of lead-induced hypertension) was the hypertrophy of ventricular cardiomyocytes, whose mean thickness according to the morphometry of histological myocardial preparations was statistically significantly greater than in the control group. On the contrary, in rats exposed to cadmium alone all the three blood pressure indices were lower than in the controls while the thickness of cardiomyocytes (showing some morphological features of apoptosis) was statistically significantly decreased as compared with both control and, especially, lead-exposed animals. Both toxic metals reduced statistically significantly the thickness of the aortal tunica intima. This effect is worthy of attention since the role of the endothelium forming this layer of the vascular wall in maintaining blood pressure homeostasis through the regulation of vasodilatation and vasoconstriction is well known.
As far as we know, this is the first study that has reported a reduced blood pressure under chronic or subchronic experimental cadmium intoxication. However, Puri [36] observed a transient fall of the blood pressure preceding the onset of arterial hypertension in rats induced by a one-shot intravenous injection of cadmium acetate. Literature suggesting increased blood pressure under chronic intoxication with low doses of cadmium is rather scarce. Thus, according to Walker and Moses [37], rats exposed to cadmium added to drinking water at a concentration of 1 mg% developed arterial hypertension only by the 36th week of this exposure. Boscolo and Carmignani [38] did not discover it at all throughout a 9 months long exposure of rabbits consuming water with the cadmium content of 20 μg/mL. Moreover, the meta-analysis of epidemiological data mentioned in the Introduction led Gallagher and Meliker [26] to the conclusion of a possible negative association between the development of hypertension and the level of cadmium in urine.
It may be assumed that the above-mentioned toxic damage to the cardiomyocyte (apoptosis) causes an impairment of the heart’s pumping function thus inducing a decrease in blood pressure. In our experiment, this mechanism prevailed over assumed changes in arterial tonus regulation under exposure to cadmium, and vice versa under exposure to lead. The contra-directional action of lead and cadmium on arterial pressure under combined exposure was confirmed to be true by mathematical analysis. A similar Cd-Pb antagonism was revealed by this method in relation to the diameter of the Malpighian glomeruli as well. This result is of special interest if we assume that arterial hypertension under lead intoxication is, at least partly, of renal origin.
In the second-lead ECG, both metals when administered alone provoked some lengthening of all inter-wave intervals corresponding to a reduced heart rate, while under combined exposure it was the QT interval only that was elongated statistically significantly. In all toxically exposed groups, the baseline was somewhat lowered (statistically significantly under combined exposure), which can point to some damage to the myocardium or, at least, metabolic disturbances in it. It may be assumed that diffuse metabolic (ion-exchange and/or energy) disturbances are not only, and even not so much, a direct consequence of the metals’ cardiotoxicity as, in the case of lead, a secondary result of the above-mentioned cardiomyocyte hypertrophy. Neither can we rule out, however, the association of the revealed ECG-phenomena with toxic damage (e.g. with partial apoptosis) to cardiomyocytes. The type of the combined action of lead and cadmium on ECG judging by the majority of the indices may be regarded as additivity, and as contra-directionality judging by the amplitude of the P wave. Five ECG indices in rats under combined exposure with the administration of the BPC were statistically significantly different from the corresponding indices under similar exposure without the BPC. Of significant importance is normalization of the isoelectric line voltage, i.e. the alleviation of the main adverse effect of intoxication as per the ECG amplitude indices. Noteworthy is also the fact that the voltage of the P and T waves, which was elevated a little under exposure to the lead-cadmium combination without the BPC, was found to be close to the control values under a similar exposure with the BPC.
3.2. The results of gel electrophoresis of rat myocardium
The electrophoretic study revealed a higher expression V3 myosin isoform, which is a homodimer of β-MHC, in the rat myocardium after exposure to lead compared with the rat myocardium in the control group (Fig. 1, Table 1).
Fig. 1.
Representative examples of electrophoregrams showing the location of α- and β-MHC recovered from the myocardia of experimental rats. Left to right: control, group after exposure to lead, after exposure to cadmium, after combined exposure to lead and cadmium, and after exposure to lead and cadmium against oral BPC administration.
Table 1.
Some characteristics of myocardium in controls and in rats exposed to lead-cadmium combination with and without bioprotection (x ± s.e.).
| Myocardium characteristics | Group of rats |
||
|---|---|---|---|
| Controls | Exposed to Cd + Pb | Exposed to Cd + Pb along with BPC | |
| Expression of α-MHC, % | 80 ± 6 | 90 ± 7 c | 78 ± 5 * |
| Expression of β-MHC, % | 20 ± 5 | 10 ± 3 c | 22 ± 5 * |
| Active tension of isolated multicellular myocardium preparations at working length corresponding to 0.95 Lmax, mN/mm2 | 53 ± 3 | 40 ± 3 c | 47 ± 4 * |
| Passive tension of isolated multicellular myocardium preparations at working length corresponding to 0.95 Lmax, mN/mm2 | 2.0 ± 0.3 | 6.5 ± 0.5 c | 2.5 ± 0.3 * |
Note: c – statistically significant difference from the control group, * - statistically significant difference from the group exposed to Cd + Pb.
The replacement of a considerable proportion of fast V1 myosin isoforms (α-α-homodimers of MHC) with slower ones (V3) along with the increase in cardiomyocyte thickness (mentioned in the Section 3.1) may point to the presence of myocardial hypertrophy in rats with subchronic lead intoxication since it has been repeatedly shown that myocardium hypertrophy is accompanied by a shift in the isomyosin ratio towards the slower V3 [39,40]. On the contrary, cadmium exposure caused enhanced expression of the V1 fast cardiac myosin isoform, while combined exposure also increased the V1 isoform content compared with the control. Combined exposure with the ВPC led again to a shift in the expression towards slower V3 myosin isoforms. Interestingly, a similar shift towards the expression of V1 myosin isoforms had been observed in animals with hyperthyreosis [41]. On the other hand, it is known that one of the likely consequences of cadmium’s toxic effect is hyperthyreosis [42].
3.3. Myocardial contractility
3.3.1. Thickness of the myocardial preparations
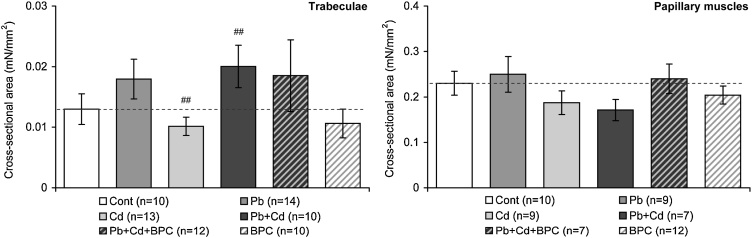
The cross-sectional area of the trabecules dissected for the experimental modeling of myocardial contractility ex vivo was in the control group equal to 0.071 ± 0.013 mm2, which is one-third of that (0.214 ± 0.025 mm2) of the pаpillary muscle preparations. The cross-sectional area of the trabecular and papillary muscle preparations from lead-exposed animals was found to be somewhat larger (0.100 ± 0.017 mm2 and 0.25 ± 0.04 mm2, respectively) than that from rats exposed to cadmium (0.063 ± 0.009 mm2 and 0.187 ± 0.026 mm2, respectively) and from control animals (see above). A comparison of the same groups by the normalized (i.e. referred to the control) cross-sectional area of respective preparations confirms the regular character of increase in this index under lead intoxication and decrease under exposure to cadmium (Fig. 2). As for the group of rats exposed to the lead-cadmium combination, the trabecular preparations from this group show an explicit prevalence of lead effects while the papillary muscle preparations - the prevalence of cadmium effects, but in both cases the action of the bioprotectors manifested itself in a shift in the index under consideration towards the control value.
Fig. 2.
The ratio of the cross-sectional area of trabecular (left panel) and papillary muscle (right panel) preparations from the right ventricle of rats to the respective value for the control group (CONT) under exposure to lead, cadmium, their combination, and the same combination along with background administration of the bioprotective complex (BPC) or BPC alone. The columns with bars show mean values and standard errors of the mean. The differences are significant (p < 0.05) between the groups: ## - Cd vs. Pb + Cd.
Note that these inter-group differences generally have the same sign as those obtained on histological cardiac preparations in morphometric indices, namely clearly visible hypertrophy of cardiomyocytes under lead intoxication and apoptosis under cadmium one as mentioned in Section 3.1 and presented in detail by Klinova et al. [32]. This suggests that whereas one of the direct causes of the inter-group biomechanical differences considered below is likely to be geometric differences between muscle preparations from respective groups, these geometric differences themselves are most likely associated with real effects of toxic exposure rather than with small-sample randomness.
3.3.2. Estimation of the effect of intoxication on the length-passive tension relationship
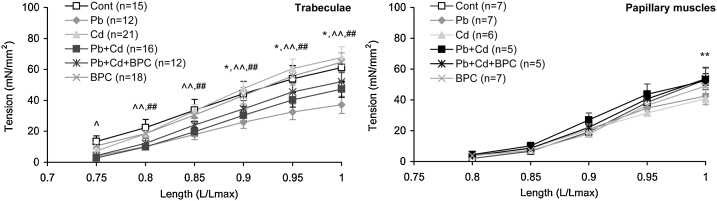
With an increase in the initial extent of cardiac muscle stretching, growth in the active force developed by it in accordance with the Frank-Starling law is commonly accompanied by a non-linear growth in mechanical tension at rest (diastolic). With regard to the various levels of the myocardial tissue biological organization, such non-linear growth is due, mainly, to the stretching of various elastic morphological inter-cellular and intra-cellular components. In particular, the main contribution to passive tension in cardiomyocytes comes from titin [43,44].
We found that the magnitude of passive tension characterizing stiffness increased with stretching myocardial preparations of both types to the maximal value of 15 mN/mm2 in all groups of animals (Fig. 3). No additional increase in the passive tension was discovered in the trabecules from the hearts of rats exposed to lead or to lead-cadmium combination (both with and without bioprotection) and in papillary muscles exposed to the metal combination with bioprotection compared with the preparations from the control animals. On the contrary, the dominant tendency for rats exposed to cadmium alone (and to lead-cadmium combination for papillary muscles) was the increasing of passive tension for all stretching lengths. Note that whereas for the papillary muscles from the group of combined exposure without bioprotection this effect was maximal and statistically significant, it was completely absent under a similar combined toxic exposure with BPC administration (as well as in the trabecular preparations).
Fig. 3.
Length-passive tension relationship in trabecular (left panel) and papillary muscle (right panel) preparations from the right ventricle of rats from all experimental groups. The points with bars show mean values and standard errors of the mean. The differences are significant (p < 0.05) between the groups: ** - Cd vs. Cont; ^ - Pb + Cd vs. Cont; ^^ - Pb vs. Cd; ## - Pb + Cd vs. Cd.
When the myocardium is stretched within physiological length range, growth in passive mechanical tension is due, mainly, to the stretching of the «molecular spring» of the gigantic titin protein (from 3000 to 3700 kDa), which largely determines the stiffness of the myocardium [45]. The data of other authors on the contents of different titin isoforms in papillary muscles and trabecules, stiff N2B and more compliant N2BA, suggest that changes in passive stiffness under intoxication with heavy metal salts may be due to the phosphorylation of titin by protein kinase А (PКА) [46,47]. This was demonstrated for rat right ventricular trabecular preparations by Fukuda et al. [48].
Oxidative stress also modulates titin stiffness considerably, reducing it in some domains of this giant protein and increasing it in others depending on the structure of the corresponding domains. The overall effect of these changes has not yet been fully understood [44,49]. Since one of the well-known primary mechanisms of the toxic effect produced by heavy metals is oxidative stress, we cannot rule out its contribution, too, to the changes in passive stiffness that we observed.
3.3.3. Estimation of the effect of intoxication on the length-active tension relationship
As is well-known, active myocardial contraction force depends on the initial sarcomere length [50,51]. We therefore were keenly interested in comparing this relationship in isolated myocardial preparations obtained from control rats and from rats exposed to different variants of lead and cadmium intoxication.
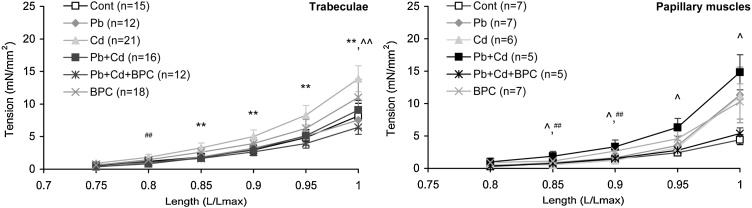
It follows from data illustrated by Fig. 4 that, as the length of the trabecular or papillary muscle preparations is increased, the isometric contraction force grows monotonically in all groups, with a greater inter-group variance of means values in the trabecular preparations in comparison with papillary muscles. Only the trabecular preparations demonstrate statistically significant differences between the mean values of force developed at all lengths exceeding 0.75 Lmax both under lead and cadmium intoxication, and only under lead intoxication were these values significantly lower than in the control group, while under cadmium intoxication they did not differ from the control ones.
Fig. 4.
Length-active tension relationship in isolated trabecular (left panel) and papillary muscle (right panel) preparations from the right ventricle of male rats in all experimental groups. The points with bars show mean values and standard errors of the mean. The differences are significant (p < 0.05) between the groups: * - Pb vs. Cont, ** - Cd vs. Cont, ^ - Pb + Cd vs. Cont; ^^ - Pb vs. Cd; ## - Pb + Cd vs. Cd.
As can be seen from the same Fig. 4, under combined intoxication with Pb + Cd the inhibiting effect of lead is prevalent, but it is less expressed than under the action of lead alone. At the same time, with bioprotection the same type of intoxication (Pb + Cd + BPC) provided a length-active tension relationship shifted a little towards the control value.
All corresponding curves obtained on papillary muscle preparations over the entire range of lengths lie on the graphs as a dense bundle, and some divergence between them is only observed in the region of large preparation lengths. Note that cadmium and lead intoxications separately inhibit the contraction force while in combination they even enhance it compared with the control.
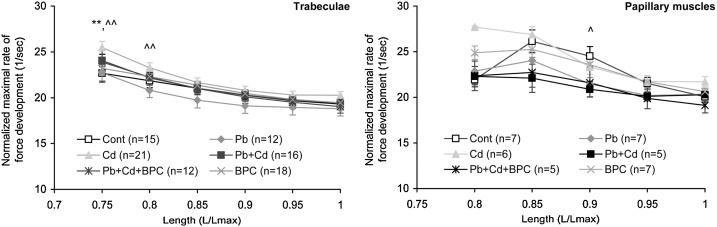
To obtain a more precise estimate for the dependence of the maximal isometric tension development rate [(dP/dt)]max on preparation length, we leveled out the gain in contraction amplitude by normalizing the rate to contraction amplitude. As is shown in Fig. 5, an increase in the length of trabecular and papillary muscle preparations in nearly all groups is accompanied by a drop in the maximal normalized rate of tension development, but it is virtually absent in the papillary muscle preparations from rats under combined exposure. Moreover, the trabecular preparations from the cadmium-exposed group are characterized by the greatest rate, while the preparations from the lead-exposed group display the lowest rate over the entire range of lengths compared with the trabeculae from the other exposed and control groups.
Fig. 5.
Length dependence of the normalized maximal rate of tension development in trabecular (left panel) and papillary muscle (right panel) preparations. The points with bars show mean values and standard errors of the mean. The differences are significant (p < 0.05) between the group: ** - Cd vs. Cont; ^ - Pb + Cd vs. Cont; ^^ - Pb vs. Cd.
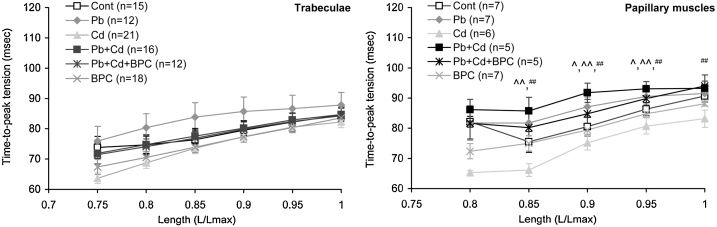
The value of time to peak (TTP) of isometric contractions in trabecular and papillary muscle preparations increases with preparation length in all groups (Fig. 6). TTP was the least for trabecular and papillary muscle preparations from the hearts of animals with cadmium intoxication and the greatest for trabecules under lead intoxication and for papillary muscle preparations under combined exposure for all preparation lengths.
Fig. 6.
Dependence of the time to peak (TTP) of isometric contraction amplitude on the extent of stretching in isolated trabecular (left panel) and papillary muscle (right panel) preparations. The points with bars show mean values and standard errors of the mean. The differences are significant (p < 0.05) between the groups: ^ - Pb + Cd vs. Cont; ^^ - Pb vs. Cd; ## - Pb + Cd vs. Cd.
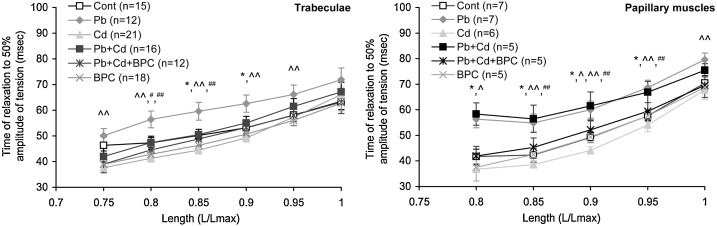
The course of isometric muscle relaxation is determined by the rate of detachment of myosin heads from actin, dissolution of calcium-troponin complexes, and outflux of calcium from sarcomeres into the sarcoplasmic reticulum (SR) and into the extracellular space. As an integral indicator of this process in isometric mode, an index is used which is determined by the time it takes for the myocardium to relax to 50 % level and is designated as t50 [52,53]. As follows from the curves shown in Fig. 7, the relaxation time increases with increasing the length of the trabecular and papillary muscle preparations in all exposed groups, being insignificantly different from t50 of the control group for most of the groups excluding the lead-exposed one. In the trabecular preparations from lead-exposed rats and in the papillary muscle preparations from the (Pb + Cd) group, the relaxation phase is slower and t50 is higher than in the corresponding preparations of the control group. On the contrary, the same preparations from the cadmium-exposed group display some increase in the relaxation rate and decrease in t50.
Fig. 7.
Dependence of the time of relaxation to 50 % isometric contraction amplitude on the extent of stretching in trabecular (left panel) and papillary muscle (right panel) preparations. The points with bars show mean values and standard errors of the mean. The differences are significant (p < 0.05) between the groups: * - Pb vs. Cont; ^ - Pb + Cd vs. Cont; ^^ - Pb vs. Cd; ## - Pb + Cd vs. Cd.
Thus, under cadmium exposure the amplitude of isometric contractions of trabecules does not change substantially compared with the control group while decreasing in papillary muscles, and the rate of mechanical tension development and relaxation is observed to grow. Under exposure to lead, the amplitude of isometric contractions drops substantially in trabecules and practically does not change in papillary muscles, and the time to peak contraction amplitude and the relaxation time increase over the entire range of lengths.
3.3.4. Estimation of the effect of intoxication on post-rest potentiation
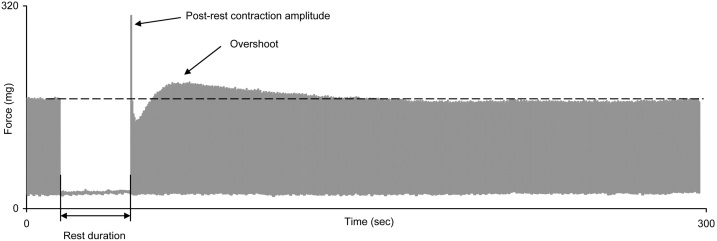
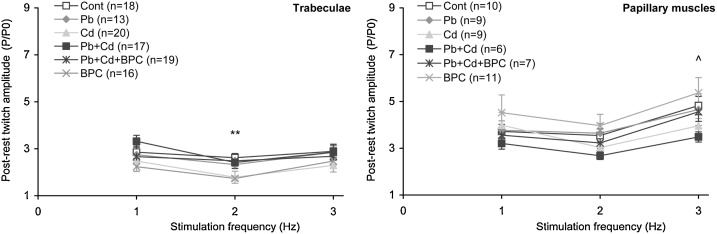
For estimating the effect of toxicants on the chronoinotropic regulation of myocardial contractility, we used the characteristics of post-rest potentiation (PRP) (Fig. 8) [54]. Post-rest contraction force is an indicator of calcium influx into the cardiomyocyte. There are grounds to believe that the main mechanism forming this potentiation is the accumulation of intracellular calcium in the SR during rest [33,55,56]. The length of the interval without stimulation was chosen to be equal to 60 s because it had been shown for the rat myocardium that the amplitude of the first contraction depends on the duration of the rest interval, achieving plateau after 0.5−1 min of rest [33,55,57].
Fig. 8.
The scheme of experimental data processing layout for recorded post-rest isometric contractions constructed by actual records of trabecular. There are isometric contractions after a 15 s pause at a frequency of 2 Hz.
In all the groups studied, the amplitude of the first contraction after a 60 s rest interval depended on stimulation frequency, and in most of the groups it was below the relative amplitude for the control one, particularly in the cadmium-exposed group (Fig. 9).
Fig. 9.
Dependence of the first isometric contraction amplitude after a 60 s pause reduced to the contraction amplitude before rest in steady state on the preparation stimulation frequency in trabecules (left panel) and papillary muscles (right panel) from the right ventricle of rats at constant preparation length 0.95 Lmax. The points with bars show mean values and standard errors of the mean. The differences are significant (p < 0.05) between the groups: ** - Cd vs. Cont; ^ - Pb + Cd vs. Cont.
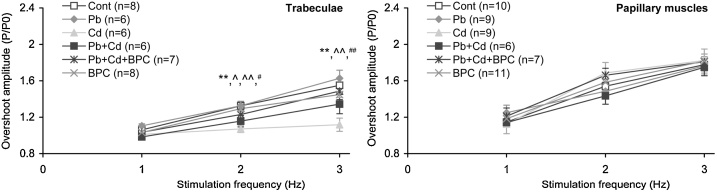
With increasing stimulation frequency for trabecular and papillary muscle preparations, the amplitude of isometric contractions during the transient overshoot after the 60 s rest interval grows monotonically in all groups of animals (Fig. 10). This frequency dependence of the transient pattern after potentiation decreases in trabecules in response to cadmium exposure and, to a lesser extent, under combined exposure to lead and cadmium. No significant inter-group differences were observed in papillary muscle preparations.
Fig. 10.
Dependence of the maximal isometric contraction during the transient overshoot after 60 s resting in steady state on the stimulation frequency applied to trabecular (left panel) and papillary muscle (right panel) preparations from the right ventricle of rats at constant preparation length 0.95 Lmax. The contraction amplitude during the transient process is reduced to that before resting. The points with bars show mean values and standard errors of the mean. The differences are significant (p < 0.05) between the groups: ** - Cd vs. Cont; ^ Pb + Cd - vs. Cont; ^^ - Pb vs. Cd; # - Pb + Cd vs. Pb; ## - Pb + Cd vs. Cd.
Our data on the insignificance of changes in potentiated contractions in both types of preparations under lead intoxication are in good agreement with the indirect estimate of the effect of chronic lead exposure on SR activity by the extent of papillary muscle post-rest contractions [58]. However, under cadmium intoxication we observed a drop in the amplitude of the first post-rest contractions in the preparations of both types at all applied frequencies (Fig. 10). This can be explained by a shift in the intracellular calcium balance towards its decrease in the intracellular calcium depot. Despite the inhibition of cAMP-dependent phosphorylation of phospholamban [27,59], which under rhythmic stimulation facilitates SR filling through the calcium pump of the reticulum (SERCA), the result of post-rest potentiation provides evidence that the balance of calcium release and uptake in the SR in general reduces its filling with calcium under cadmium exposure compared with the control. Cadmium is known as the inhibitor of Na/Ca exchanger and l-type and T-type calcium channels [60,61], which may explain the discharge of calcium from cardiomyocytes under cadmium intoxication.
The rate of contraction development and the value of time to peak amplitude are integral characteristics of myocardium contractility. They are determined by the processes of calcium influx into the cytosol from outside and from the intracellular sources (SR in the first place), the kinetics of calcium binding to troponin C, and the kinetics of attachment of myosin heads to actin (formation of cross bridges). The rate of force decay (relaxation) is determined by the kinetics of detachment of actin-myosin cross-bridges, the kinetics of dissolution of calcium-troponin complexes, and the transport of calcium from the cytosol into the SR and into the extracellular space (primarily through the Na/Ca exchange mechanism).
The rate of active tension build-up was increased under cadmium exposure compared with the control, which a priori could be associated both with an increase in the rate of cross-bridge cycling, i.e. a shift towards the fast V1 myosin isoform, and with an increase in the amount of calcium in the SR. But since we have shown that under post-rest potentiation the amplitude of the first contraction under cadmium exposure decreased in comparison with the control (which points to reduced calcium filling of the SR rather than anything else), the increased rate of active tension build-up is likely to be explained by a shift in the isomyosin ratio. The reduced relaxation time that we observed may also correspond to a shift towards the faster myosin isoform under cadmium intoxication.
The increase in the time to peak amplitude of contraction and the time of relaxation under lead intoxication (with insignificant changes in the magnitude of potentiated contractions, i.e. in SR activity) is associated with a shift in the ratio of myosin isoforms towards the slower V3. Such effects of lead intoxication had been discovered by us previously [1].
What leaps to the eye is paradoxical differences in the extent and, for some effects, character of the effect of intoxication with the same metals on myocardial trabecular and papillary muscle preparations. We noted this phenomenon already in a subchronic lead intoxication experiment [1,2]. The fact that now this effect has been discovered again not only for lead but also for cadmium and for combined lead-cadmium intoxication allows it to be regarded as being of a non-random character. However, to be able to propose at least a hypothetical mechanism of these differences, special experimental studies should be conceived and carried out.
At the same time, we believe that in estimating the cardiotoxicity of metals (lead and cadmium in any case) there are grounds to attach key importance to analyzing the contractile dysfunctions of the ventricle wall myocardium (in a reciprocal relationship with changes in arterial pressure) and, thus, trabecular preparations rather than papillary muscles. We proceed from the assumption that an induced decrease of any extent in the active contraction force of the ventricular wall myocardium and its dynamics over time would manifest itself in explicit or latent disturbances of the systemic hemodynamics, which are indeed reported for the effects of the metals under consideration. At the same time, such hemodynamics would be affected only by a reduction in the force of contraction of papillary muscles such that would not allow them to hold the leaflets of the mitral or tricuspid valve reliably closed throughout the systole. Meanwhile, whereas the problem of arterial hypertension under chronic exposure to lead and cadmium is actual indeed even if different researchers may have differing opinions about it (see the Introduction), we have failed to find any direct statements of a relationship between such toxic exposure and the development of valvular heart disease.
It is true though that Yang et al. [18] assessed in a prospective study involving a small sample of Danish residents the left-ventricle function by using Doppler imaging of the transmitral blood flow and the mitral annular movement and speckle tracking, and they revealed a significant decrease in the systolic (but not diastolic) function with an increase in the body burden of lead and cadmium (although the multivariate analyses did not allow them to discriminate which of these metals was predominant in this association). Therefore, these researchers suggested that “environmental exposure to lead, cadmium, or both might be a risk factor for systolic left-ventricle dysfunction, a condition often proceeding to heart failure”.
If valvular heart disease associated with the action of lead and/or cadmium is possible indeed, then there are no grounds to ignore the intoxication-induced changes in the contractile function of papillary muscles that we have found. For the time being, we just assume that within the framework of the general cardio-toxicological characterization of these metals priority belongs to the patterns that are revealed in trabecular preparations.
Under combined lead-cadmium intoxication, some characteristics changed in the direction typical of the effect of lead (for instance, active tension in trabecules), while others in the direction typical of the effect of cadmium (passive tension in papillary muscles). In some cases, the opposite action of lead and cadmium when administered alone changed under combined exposure into no effect (time to peak and relaxation time in trabecular preparations). In general, since departures from the norm caused by subchronic isolated effects of lead and cadmium on cardiomyocytes are oppositely directed, their combined action should be expected to produce a complex picture depending on both the dose of the toxicants and the individual susceptibility of the organism.
A number of rat myocardium mechanical characteristics which change under lead-cadmium intoxication were found to be normalized fully or partially if such intoxication developed against the background of BPC administration (Table 1). Thus, growth in passive tension in papillary muscle preparations under combined exposure was leveled out if this exposure took place along with BPC administration. The background administration of the BPC is associated with a reduced combined toxic effect on the active tension amplitude in trabecules, time to peak contraction, and relaxation time in papillary muscles. Moreover, no increase in the proportion of α-MHC took place if the exposure to these metals was done along with BPC administration.
The combined toxic action of lead and cadmium was found to be attenuated in cases of exposure with the BPC judging by some other effects displayed by various organs and systems [32]. We may therefore assume that in relation to cardiotoxicity as well this attenuation could be associated not only with a direct action on the myocardium but also with an indirect one through complex inter-systemic relations. An overview of our investigations on the problem of antitoxic biological prophylaxis the reader may find in [62]. Recently Rana et al. [63] mentioned similar mechanisms of bioprotective action of plants and plants derived compounds against toxicity of lead, cadmium, mercury and arsenic.
4. Conclusions
-
1
We have found that under subchronic intoxication of rats with lead and cadmium (administered separately or in combination) resulting in a moderately pronounced toxic action of these metals on different organs and systems, including the cardio-vascular one, the mechanisms of heterometric and chronoinotropic regulation of myocardium contractility are maintained but modified quantitatively to this or that extent.
-
2
Under cadmium intoxication, compared with the myocardium of control animals:
-
3
the cross-sectional area of isolated ventricular preparations (both trabecules and papillary muscles) is reduced;
-
4
no reliable changes in the amplitude of isometric contractions were found in both types of muscle;
-
5
the rate of mechanical tension development and relaxation in a single twitch increases, which corresponds to a shift in the isoform composition of the myosin towards V1 rapidly cycling isomyosins;
-
6
myocardial stiffness increases;
-
7
the amplitude of the first post-rest contraction decreases.
-
8
Under lead intoxication, compared with the myocardium of the same control group of animals:
-
9
the cross-sectional area of isolated ventricular preparations (both trabecules and papillary muscles) is increased;
-
10
the amplitude of isometric contractions of trabecules drops and that of papillary muscles practically does not change over the entire range of preparation lengths;
-
11
the time to peak contraction amplitude and the relaxation time increase over the entire range of lengths, which could be associated with a shift in the isoform composition of the myosin towards slower V3 isomyosins;
-
12
the stiffness of the trabecular and papillary muscle preparations does not exceed that of the preparations from control animals;
-
13
the amplitude of the first post-rest contraction changes insignificantly.
-
14
Under combined lead-cadmium intoxication, some contractile characteristics changed in the direction typical of the effect of lead while others – of cadmium.
-
15
A number of rat myocardium characteristics which change under the effect of lead-cadmium intoxication normalize fully or partially if the latter developed against the background administration of a tested bioprotective complex (BPC). This is in agreement with the fact that there was no increase in the proportion of α-MHC caused by intoxication if the toxic exposure was administered along with the BPC.
-
16
An increase in arterial pressure observed in rats with lead intoxication could be a precondition to the development of concentric heart hypertrophy, the realism of which is conveyed by the above-presented results (increased cross-sectional area of papillary muscle and particularly of trabecular preparations, a shift towards slower V3 isomyosin and increased time to peak contraction amplitude and relaxation time), as well as by cardiomyocyte morphometry by optical microscopy of histological myocardial preparations.
CRediT authorship contribution statement
Yuri L. Protsenko: Conceptualization, Writing - original draft. Svetlana V. Klinova: Investigation, Writing - original draft. Oksana P. Gerzen: Investigation. Larisa I. Privalova: Methodology, Writing - review & editing. Ilzira A. Minigalieva: Supervision, Writing - review & editing. Alexander A. Balakin: Investigation. Oleg N. Lookin: Investigation. Ruslan V. Lisin: Investigation. Ksenya A. Butova: Investigation. Salavat R. Nabiev: Investigation. Leonid B. Katsnelson: Writing - review & editing. Larisa V. Nikitina: Investigation, Writing - review & editing. Boris A. Katsnelson: Conceptualization, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work was carried out partly within the framework of the IIF UrB RAS theme No AAAA-A18-118020590031-8 and AAAA-A18-118020590031.
This work was performed using the equipment of the Shared Research Center of Scientific Equipment SRC IIP UrB RAS.
Footnotes
This is a summary of the “Results and Discussion” Section of the special paper in which they are presented in details [32].
References
- 1.Protsenko Y.L., Katsnelson B.A., Klinova S.V., Lookin O.N., Balakin A.A., Nikitina L.V., Gerzen O.P., Minigalieva I.A., Privalova L.I., Gurvich V.B., Sutunkova M.P., Katsnelson L.B. Effects of subchronic lead intoxication of rats on the myocardium contractility. Food Chem. Toxicol. 2018;120:378–389. doi: 10.1016/j.fct.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 2.Protsenko Y.L., Katsnelson B.A., Klinova S.V., Lookin O.N., Balakin A.A., Nikitina L.V., Gerzen O.P., Nabiev S.R., Minigalieva I.A., Privalova L.I., Gurvich V.B., Sutunkova M.P., Katsnelson L.B. Further analysis of rat myocardium contractility changes associated with a subchronic lead intoxication. Food Chem. Toxicol. 2019;129:233–241. doi: 10.1016/j.fct.2018.12.054. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y.D., Eom S.Y., Yim D.H., Kim I.S., Won H.K., Park C.H., Kim G.B., Yu S.D., Choi B.S., Park J.D., Kim H. Environmental exposure to arsenic, lead, and cadmium in people living near Janghang copper smelter in Korea. J. Korean Med. Sci. 2016;31(4):489–496. doi: 10.3346/jkms.2016.31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Hassanin A.S., Samak M.R., Abdel-Rahman G.N., Abu-Sree Ya.H., Saleh E.M. Risk assessment of human exposure to lead and cadmium in maize grains cultivated in soils irrigated either with low-quality water or freshwater. Toxicol. Rep. 2020;7:10–15. doi: 10.1016/j.toxrep.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez A.F., Buha A., Constantin C., Wallace D.R., Sarigiannis D., Neagu M., Antonijevic B., Hayes A.W., Wilks M.F., Tsatsakis A. Critical assessment and integration of separate lines of evidence for risk assessment of chemical mixtures. Arch. Toxicol. 2019;93:2741–2757. doi: 10.1007/s00204-019-02547-x. [DOI] [PubMed] [Google Scholar]
- 6.Minigalieva I.A., Katsnelson B.A., Panov V.G., Varaksin A.N., Gurvich V.B., Privalova L.I., Sutunkova M.P., Klinova S.V. Experimental study and mathematical modeling of toxic metals combined action as a scientific foundation for occupational and environmental health risk assessment (a synthesis of results obtained by the Ekaterinburg research team, Russia) Toxicol. Rep. 2017;4C:194–201. doi: 10.1016/j.toxrep.2017.04.002. https://dx.doi.org/10.1016%2Fj.toxrep.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eum K.D., Lee M.S., Paek D. Cadmium in blood and hypertension. Sci. Total Environ. 2008;407(1):147–153. doi: 10.1016/j.scitotenv.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 8.Peters J.L., Perlstein T.S., Perry M.J., McNeely E., Weuve J. Cadmium exposure in association with history of stroke and heart failure. Environ. Res. 2010;110(2):199–206. doi: 10.1016/j.envres.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee M.S., Park S.K., Hu H., Lee S. Cadmium exposure and cardiovascular disease in the 2005 Korea National Health and Nutrition Examination Survey. Environ. Res. 2011;111(1):171–176. doi: 10.1016/j.envres.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caciari T., Sancini A., Fioravanti M., Capozzella A., Casale T., Montuori L., Fiaschetti M., Schifano M.P., Andreozzi G., Nardone N., Tomei G., Ciarrocca M., Rosati M.V., Tomei F. Cadmium and hypertension in exposed workers: a meta-analysis. Int. J. Occup. Med. Environ. Health. 2013;26(3):440–456. doi: 10.2478/s13382-013-0111-5. [DOI] [PubMed] [Google Scholar]
- 11.Julin B., Bergkvist C., Wolk A., Åkesson A. Cadmium in diet and risk of cardiovascular disease in women. Epidemiology. 2013;24(6):880–885. doi: 10.1097/EDE.0b013e3182a777c9. [DOI] [PubMed] [Google Scholar]
- 12.Tellez-Plaza M., Jones M.R., Dominguez-Lucas A., Guallar E., Navas-Acien A. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr. Atheroscler. Rep. 2013;15(10):356. doi: 10.1007/s11883-013-0356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tellez-Plaza M., Guallar E., Howard B.V., Umans J.G., Francesconi K.A., Goessler W., Silbergeld E.K., Devereux R.B., Navas-Acien A. Cadmium exposure and incident cardiovascular disease. Epidemiol. 2013;24(3):421–429. doi: 10.1097/EDE.0b013e31828b0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myong J.P., Kim H.R., Jang T.W., Lee H.E., Koo J.W. Association between blood cadmium levels and 10-year coronary heart disease risk in the general Korean population: the Korean National Health and Nutrition Examination Survey 2008-2010. PLoS One. 2014;9(11):e111909. doi: 10.1371/journal.pone.0111909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borné Y., Barregard L., Persson M., Hedblad B., Fagerberg B., Engström G. Cadmium exposure and incidence of heart failure and atrial fibrillation: a population-based prospective cohort study. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsson S.C., Wolk A. Urinary cadmium and mortality from all causes, cancer and cardiovascular disease in the general population: systematic review and meta-analysis of cohort studies. Int. J. Epidemiol. 2016;45(3):782–791. doi: 10.1093/ije/dyv086. [DOI] [PubMed] [Google Scholar]
- 17.Franceschini N., Fry R.C., Balakrishnan P., Navas-Acien A., Oliver-Williams C., Howard A.G., Cole S.A., Haack K., Lange E.M., Howard B.V., Best L.G., Francesconi K.A., Goessler W., Umans J.G., Tellez-Plaza M. Cadmium body burden and increased blood pressure in middle-aged American Indians: the Strong Heart Study. J. Hum. Hypertens. 2017;3:225–230. doi: 10.1038/jhh.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang W.Y., Zhang Z.Y., Thijs L., Cauwenberghs N., Wei F.F., Jacobs L., Luttun A., Verhamme P., Kuznetsova T., Nawrot T.S., Staessen J.A. Left ventricular structure and function in relation to environmental exposure to lead and cadmium. J. Am. Heart Assoc. 2017;6(2) doi: 10.1161/JAHA.116.004692. pii: e004692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozturk I.M., Buyukakilli B., Balli E., Cimen B., Gunes S., Erdogan S. Determination of acute and chronic effects of cadmium on the cardiovascular system of rats. Toxicol. Mech. Methods. 2009;19(4):308–317. doi: 10.1080/15376510802662751. [DOI] [PubMed] [Google Scholar]
- 20.Ferramola M.L., Pérez Díaz M.F.F., Honoré S.M., Sánchez S.S., Antón R.I., Anzulovich A.C., Giménez M.S. Cadmium-induced oxidative stress and histological damage in the myocardium. Effects of a soy-based diet. Toxicol. Appl. Pharmacol. 2012;265:380–389. doi: 10.1016/j.taap.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Almenara C.C., Broseghini-Filho G.B., Vescovi M.V., Angeli J.K., de O. Faria T., Stefanon I., Vassallo D.V., Padilha A.S. Chronic cadmium treatment promotes oxidative stress and endothelial damage in isolated rat aorta. PLoS One. 2013;8(7):e68418. doi: 10.1371/journal.pone.0068418. https://dx.doi.org/10.1371%2Fjournal.pone.0068418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittziyev K.G., Brin V.B., Mittziyev A.K., Kabisov O.T. Impact of changes calcium homeostasis on the cardiovascular effects of chronic cadmium intoxication, Bulletin of new medical technologies. Electronic edition. 2013;1:137. URL: https://cyberleninka.ru/article/n/vliyanie-izmenennogo-kaltsievogo-gomeostazisa-na-serdechno-sosudistye-effekty-hronicheskoy-kadmievoy-intoksikatsii (Accessed: 16.07.2019) (in Russian) [Google Scholar]
- 23.Mittziyev K.G., Brin V.B., Mittziyev A.K. Hemodynamic effects of chronic cadmium intoxication under a modified calcium homeostasis. Kuban Sci. Med. Bull. 2013;5:142–145. (in Russian) [Google Scholar]
- 24.Turdi S., Sun W., Tan Y., Yang X., Cai L., Ren J. Inhibition of DNA methylation attenuates low-dose cadmium-induced cardiac contractile and intracellular Ca(2+) anomalies. Clin. Exp. Pharmacol. Physiol. 2013;40(10):706–712. doi: 10.1111/1440-1681.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C., Zhang S., Liu Z., Tian Y., Sun Q. Cadmium toxicity induces ER stress and apoptosis via impairing energy homoeostasis in cardiomyocytes. Biosci. Rep. 2015;35(3):e00214. doi: 10.1042/BSR20140170. https://dx.doi.org/10.1042%2FBSR20140170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallagher C.M., Meliker J.R. Blood and urine cadmium, blood pressure, and hypertension: a systematic review and meta-analysis. Environ. Health Perspect. 2010;118(12):1676–1684. doi: 10.1289/ehp.1002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopp S.J., Barany M., Erlanger M., Perry E.F., Perry H.M. The influence of chronic low-level cadmium and/or lead feeding on myocardial contractility related to phosphorylation of cardiac myofibrillar proteins. Toxicol. Appl. Pharmacol. 1980;54:48–56. doi: 10.1016/0041-008x(80)90007-1. [DOI] [PubMed] [Google Scholar]
- 28.Kopp S.J., Perry H.M., Jr, Glonek T., Erlanger M., Perry E.F., Barany M., D’Agrosa L.S. Cardiac physiologic-metabolic changes after chronic low-level heavy metal feeding. Am. J. Physiol. 1980;239:H22–H30. doi: 10.1152/ajpheart.1980.239.1.H22. [DOI] [PubMed] [Google Scholar]
- 29.Chao S.H., Suzuki Y., Zysk J.R., Cheung W. Activation of calmodulin by various metal cations as a function of ionic radius. Mol. Pharmacol. 1984;26(1):75–82. [PubMed] [Google Scholar]
- 30.Chao S.H., Bu C.-H., Cheung W.Y. Activation of troponin C by Cd2+ and Pb2+ Arch. Toxicol. 1990;64:490–496. doi: 10.1007/BF01977632. [DOI] [PubMed] [Google Scholar]
- 31.Shen J., Wan X., Zhou D., Li T., Tang L., Gong T., Su J., Liang P. Modelling cadmium-induced cardiotoxicity using human pluripotent stem cell-derived cardiomyocytes. J. Cell. Mol. Med. 2018;22(9):4221–4235. doi: 10.1111/jcmm.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klinova S.V., Minigalieva I.A., Privalova L.I., Valamina I.E., Makeyev O.H., Shuman E.A., Korotkov A.A., Panov V.G., Sutunkova M.P., Ryabova J.V., Bushueva T.V., Shtin T.N., Gurvich V.B., Katsnelson B.A. Further verification of some postulates of the combined toxicity theory: new animal experimental data on separate and joint adverse effects of lead and cadmium. Food Chem. Toxicol. 2020;136 doi: 10.1016/j.fct.2019.110971. [DOI] [PubMed] [Google Scholar]
- 33.Milani-Nejad N., Brunello L., Gyorke S., Janssen P.M. Decrease in sarcoplasmic reticulum calcium content, not myofilament function, contributes to muscle twitch force decline in isolated cardiac trabecules. J. Muscle Res. Cell. Motil. 2014;35(3-4):225–234. doi: 10.1007/s10974-014-9386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiser P.J., Kline W.O. Electrophoretic separation and quantitation of cardiac myosin heavy chain isoforms in eight mammalian species. Am. J. Physiol. 1998;284:H1048–H1053. doi: 10.1152/ajpheart.1998.274.3.H1048. [DOI] [PubMed] [Google Scholar]
- 35.Minigalieva I.A., Katsnelson B.A., Panov V.G., Varaksin A.N., Gurvich V.B., Privalova L.I., Sutunkova M.P., Klinova S.V. Experimental study and mathematical modeling of toxic metals combined action as a scientific foundation for occupational and environmental health risk assessment (a synthesis of results obtained by the Ekaterinburg research team, Russia) Toxicol. Rep. 2017;4C:194–201. doi: 10.1016/j.toxrep.2017.04.002. https://dx.doi.org/10.1016%2Fj.toxrep.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puri V.N. Cadmium induced hypertension. Clin. Exp. Hypertens. 1999;21(1-2):79–84. doi: 10.3109/10641969909068651. [DOI] [PubMed] [Google Scholar]
- 37.Walker H.L., Moses H.A. Cadmium: Hypertension induction and lead mobilization. J. Med. Assoc. 1979;71(12):1187–1189. [PMC free article] [PubMed] [Google Scholar]
- 38.Boscolo P., Carmignani M. Mechanisms of cardiovascular regulation in male rabbits chronically exposed to cadmium. Occup. Environ. Med. 1986;43(9):605–610. doi: 10.1136/oem.43.9.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorza L., Mercadier J.J., Schwartz K., Thornell L.E., Sartore S., Schiaffino S. Myosin types in the human heart. An immunofluorescence study of normal and hypertrophied atrial and ventricular myocardium. Circ. Res. 1984;54:694–702. doi: 10.1161/01.res.54.6.694. [DOI] [PubMed] [Google Scholar]
- 40.Hirzel H.O., Tuchschmid C.R., Schneider J., Krayenbuehl H.P., Schaub M.C. Relationship between myosin isoenzyme composition, hemodynamics, and myocardial structure in various forms of human cardiac hypertrophy. Circ. Res. 1985;57:729–740. doi: 10.1161/01.res.57.5.729. [DOI] [PubMed] [Google Scholar]
- 41.Sugiura S., Yamashita H. Functional characterization of cardiac myosin isoforms. Jpn. J. Physiol. 1998;48(3):173–179. doi: 10.2170/jjphysiol.48.173. [DOI] [PubMed] [Google Scholar]
- 42.Chen A., Kim S.S., Chung E., Dietrich K.N. Thyroid hormones in relation to lead, mercury, and cadmium exposure in the National Health and Nutrition Examination Survey, 2007–2008. Environ. Health Perspect. 2013;121(2):181–187. doi: 10.1289/ehp.1205239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Granzier H.L., Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ. Res. 2004;94:284–295. doi: 10.1161/01.RES.0000117769.88862.F8. [DOI] [PubMed] [Google Scholar]
- 44.Linke W.A. Titin gene and protein functions in passive and active muscle. Annu. Rev. Physiol. 2018;80:11. doi: 10.1146/annurev-physiol-021317-121234. 1–11.23. [DOI] [PubMed] [Google Scholar]
- 45.Linke W.A., Popov V.I., Pollack G.H. Passive and active tension in single cardiac myofibrils. Biophys. J. 1994;67(2):782–792. doi: 10.1016/S0006-3495(94)80538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koser F., Loescher C., Linke W.A. Posttranslational modifications of titin from cardiac muscle: how, where and what for? FEBS J. 2019;286:2240–2260. doi: 10.1111/febs.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamasaki R., Wu Y., McNabb M., Greaser M., Labeit S., Granzier H. Protein kinase A phosphorylates titin’s cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ. Res. 2002;90(11):1181–1188. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- 48.Fukuda N., Wu Y., Nair P., Granzier H.L. Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner. J. Gen. Physiol. 2005;125(3):257–271. doi: 10.1085/jgp.200409177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linke W.A., Hamdani N. Gigantic business titin properties and function through thick and thin. Circ. Res. 2014;114:1052–1068. doi: 10.1161/CIRCRESAHA.114.301286. [DOI] [PubMed] [Google Scholar]
- 50.Allen D.G., Kentish J.C. The cellular basis of the length-tension relation in cardiac muscle. J. Mol. Cell. Cardiol. 1985;17:821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- 51.DeTombe P.P., Mateja R.D., Tachampa K., AitMou Y., Farman G.P., Irving T.C. Myofilament length dependent activation. J.Mol.Cell. Cardiol. 2010;48:851–858. doi: 10.1016/j.yjmcc.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Biesiadecki B.J., Davis J.P., Ziolo M.T., Janssen P.M. Tri-modal regulation of cardiac muscle relaxation; intracellular calcium decline, thin filament deactivation, and cross-bridge cycling kinetics. Biophys. Rev. 2014;6(3-4):273–289. doi: 10.1007/s12551-014-0143-5. https://dx.doi.org/10.1007%2Fs12551-014-0143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janssen P.M. Kinetics of cardiac muscle contraction and relaxation are linked and determined by properties of the cardiac sarcomere. Am. J. Physiol. 2010;299(4):H1092–H1099. doi: 10.1152/ajpheart.00417.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schouten V., van Deen J.K., De Tombe P., Verveen A. Force-interval relationship in heart muscle of mammals. A calcium compartment model. Biophys. J. 1987;51(1):13–26. doi: 10.1016/S0006-3495(87)83307-6. https://dx.doi.org/10.1016%2FS0006-3495(87)83307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maier L.S., Bers D.M., Pieske B. Differences in Ca2+-handling and sarcoplasmic reticulum Ca2+-content in isolated rat and rabbit myocardium. J. Mol. Cell. Cardiol. 2000;32(12):2249–2258. doi: 10.1006/jmcc.2000.1252. [DOI] [PubMed] [Google Scholar]
- 56.Sabourin J., Boet A., Rucker-Martin C., Lambert M., Gomez A.M., Benitah J.P., Perros F., Humbert M., Antigny F. Ca2+ handling remodeling and STIM1L/Orai1/TRPC1/TRPC4 upregulation in monocrotaline-induced right ventricular hypertrophy. J. Mol. Cell. Cardiol. 2018;118:208–224. doi: 10.1016/j.yjmcc.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Kogler H., Hartmann O., Leineweber K., van Nguyen P., Schott P., Brodde O.-E., Hasenfuss G. Mechanical load-dependent regulation of gene expression in monocrotaline-induced right ventricular hypertrophy in the rat. Circ. Res. 2003;93(3):230–237. doi: 10.1161/01.RES.0000085042.89656.C7. [DOI] [PubMed] [Google Scholar]
- 58.Fioresi M., Simões M.R., Furieri L.B., Broseghini-Filho G.B., Vescovi M.V.A., Stefanon I., Vassallo D.V. Chronic lead exposure increases blood pressure and myocardial contractility in rats. PLoS One. 2014;9(5):e96900. doi: 10.1371/journal.pone.0096900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Presti C.F., Jones L.R., Lindemann J.P. Isoproterenol-induced phosphorylation of a 15-kilodalton sarcolemmal protein in intact myocardium. J. Biol. Chem. 1985;260:3860–3867. [PubMed] [Google Scholar]
- 60.Uehara A., Yasukochi M., Imanaga I., Nishi M., Takeshima H. Store-operated Ca2+ entry uncoupled with ryanodine receptor and junctional membrane complex in heart muscle cells. Cell Calcium. 2002;31(2):89–96. doi: 10.1054/ceca.2001.0257. [DOI] [PubMed] [Google Scholar]
- 61.Wasserstrom J.A., Vites A.-M. Activation of contraction in cat ventricular myocytes: effects of low Cd2+ concentration and temperature. Am. J. Physiol. 1999;277(2):H488–H498. doi: 10.1152/ajpheart.1999.277.2.H488. [DOI] [PubMed] [Google Scholar]
- 62.Privalova L.I., Katsnelson B.A., Sutunkova M.P., Minigalieva I.A., Gurvich V.B., Makeyev O.H., Shur V.Ya., Valamina I.E., Klinova S.V., Shishkina E.V., Zubarev I.V. Looking for biological protectors against adverse health effects of some nanoparticles that can pollute workplace and ambient air (a summary of authors’ experimental results) J. Environ. Protect. 2017;8:844–866. [Google Scholar]
- 63.Rana M.N., Tangpong J., Rahman Md.M. Toxicodynamics of lead, cadmium, mercury and arsenic- induced kidney toxicity and treatment strategy: a mini review. Toxicol. Rep. 2018;5:704–713. doi: 10.1016/j.toxrep.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]