Abstract
We report a case of a fibromyalgia (FM) patient with an history of brain-cancer presenting signs and symptoms of gadolinium toxicity following repeated administrations of a macrocyclic contrast agent, Gadovist. In the present report, we provide evidence supporting the hypothesis of a causal relationship linking gadolinium deposition to a clinical manifestation of disease, namely fibromyalgia. We unravel a role for gadolinium in the still unknown etiology of fibromyalgia as a metal toxicity disorder. Contrast agents are routinely administered in a clinical context. It is thus possible that the patients are mistakenly believed to show complaint of their primary disease, whereas, in some instances, their symptoms are associated with gadolinium deposition.
Keywords: Gadolinium deposition, Fibromyalgia syndrome, Chronic pain, Toxicity, Contrast agents, Magnetic resonance imaging
Introduction
Millions of doses of gadolinium-based contrast agents (GBCAs) are annually administered worldwide for diagnosis, staging evaluation, and follow-up of several diseases [1,2]. GBCAs are a staple in clinical radiology. Nevertheless, important unexpected pathological potential of these drugs was recognized after their approval in the clinical practice [3] and led to the first regulatory updates in 2007 [4]. More recently, new concern has emerged [5], [6], [7], [8]. Unquestionable retention of gadolinium species in human body has been demonstrated in pathological and healthy tissue [9], [10], [11], [12], [13], [14]. The long-standing hyperintense appearance of the dentate nucleus (DN) of the cerebellum in noncontrast-enhanced magnetic resonance T1-weighted images and associated gadolinium deposits in the brain parenchima [12] are a major theme of debate among the community of neuro-radiologists [5,[15], [16], [17], [18]]. In 2016, some initial data were published reporting symptoms, adverse reactions, and long-term consequences advocating incomplete excretion following GBCA administrations in patients with normal renal function [19], [20], [21], [22], [23]. Recognizing signs and symptoms of gadolinium toxicity in humans and understanding if deposition is causally associated with short- or long-term manifestations of disease is of enormous relevance from a global human health perspective and mandatory in the oath to first do no harm.
In this report, we present clinical and radiological findings of a fibromyalgia (FM) patient with an history of brain-cancer presenting signs and symptoms of gadolinium toxicity following repeated administrations of a macrocyclic contrast agent, Gadovist. We discuss the case that supports the hypothesis of a causal relationship linking gadolinium deposition to a clinical manifestation of disease, namely fibromyalgia.
Case report
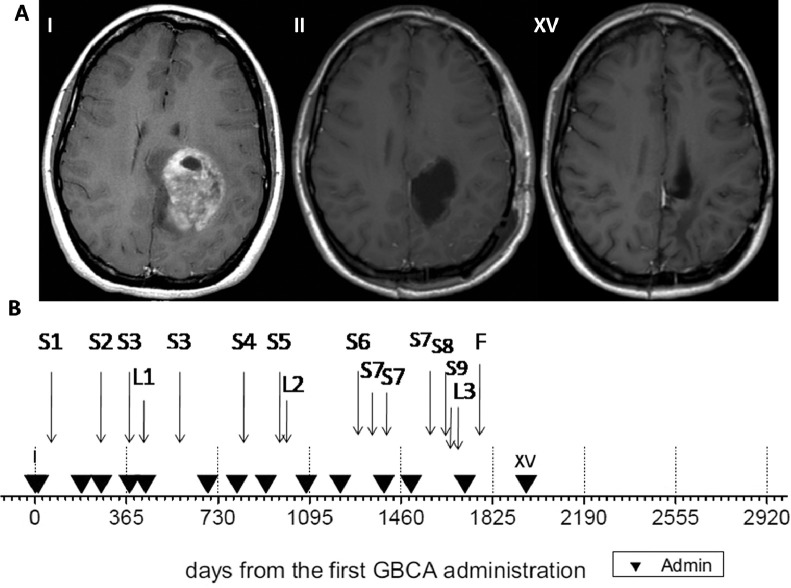
A 30-year-old woman presented to the neurological unit with a 4-month history of 5 episodes of migraine with aura and vomiting followed by days of severe fatigue. Computed tomography of the brain and soon after contrast-enhanced (CE) magnetic resonance imaging (MRI) revealed a cerebral mass consistent with a high-grade tumour (I in Fig. 1A, day 0 in Fig. 1B). She underwent surgery for resection, and histology revealed anaplastic astrocytoma III grade. The patient was treated with radiation therapy with a 2-cm margin for a total of 60 Gy (days 61-104) followed by 12 cycles of temozolamide. Both treatments were well tolerated. 3D conformed radiant procedure was performed by image-guided radiation therapy and positioning control with cone beam computed tomography. The patient did not receive any further therapy for her cancer. She continued to be followed closely with periodic scans (Fig. 1B and Table 1). Over the course of the following 5 years, she underwent 15 CE-MRI with no demonstrated tumour recurrence.
Fig. 1.
(A). Axial contrast-enhanced T1-weighted magnetic resonance images of the brain: From left: first scan (day 0, I), second scan (ie, first after resection surgery) (day 12, II), fifteenth scan (day 1959, XV). Magnetic resonance imaging data have been obtained on 1.5 T, Philips Achieva, and after intravenous administration of the macrocyclic non-ionic contrast agent Gadovist (gadobutrol). (B). Timeline of patient's history since the first gadolinium-based contrast agent (GBCA) administration (day 0). Black triangles are contrast-enhanced administrations and associated brain scan examinations. L(i) reports radiological investigations of body compartments else than the brain. S(i) indicates the onset of symptoms as described in the text body.
Table 1.
Magnetic resonance imaging studies, symptoms and clinical signs, drug therapies and analysis in the patient’s history.
| CE-brain MRI | Day (*) | Symptoms and clinical signs | Radio-diagnostic investigations (other than brain MRI), drug therapies and analysis | ||
|---|---|---|---|---|---|
| I | 0 | ||||
| II | 12 | ||||
| S1 | Osteoarticular lumbar pain, “bone perceived as made of glass” | ||||
| III | 185 | ||||
| S2 | Restless legs, morning stiffness, severe leg and foot cramps, and “pins and needle” in foot | ||||
| IV | 264 | ||||
| S3 | Severe lower back aches and hip pain forced the patient to bed rest | ||||
| V | 377 | ||||
| L1 | MRI examination without contrast enhancement and X-ray scans revealed a lumbar disk-hernia | ||||
| VI | 441 | ||||
| VII | 689 | ||||
| VIII | 805 | ||||
| S4 | Anterior chest pain and tachycardia | ||||
| IX | 921 | ||||
| S5 | Recurrence of severe lumbar pain and progressive marked increase of morning stiffness | ||||
| L2 | A second lumbar MRI that revealed no disease progression | ||||
| X | 1082 | ||||
| XI | 1218 | ||||
| S6 | Severe exacerbation of lumbar aches and stiffness, forced bed rest several days | Anti-inflammatory pain killers were ineffective. Oral gabapentin administration had mild effect. | |||
| S7 | Flare-up of pain symptoms and lumbar stiffness | ||||
| XII | 1392 | ||||
| XIII | 1500 | ||||
| S8 | Onset of pain at the right shoulder and trapezium, stiffness presented at cervical site, pain at knees similar to the hips. Chest pain and tachycardia | ||||
| S9 | Unrefreshing sleep and severely fatigue unrelieved by rest. Exacerbating pain and severe stiffness impaired movements and forced the patient to bed. Depressive symptoms presented for the first time | Hypothesis of fibromyalgia. And referral to rheumatology unit | |||
| First rheumatology visit: physical examination, patient's history, and FM positive trigger point sensitivity. Requirement of blood analysis and pelvis MRI for excluding other rheumatic disorders and confirm FM diagnosis | Blood analysis (anti-nuclear antibodies, anti-nDNA antibodies research, anti-cyclic citrullinated peptide antibodies, anti-ENA antibodies, rheumatoid factor erythrocyte sedimentation rate, C-reactive protein, creatine kinase, and complete blood count) | ||||
| L3 | MRI scan of the pelvis without contrast enhancement | ||||
| Blood analyses negative. Other rheumatic pathologies such as spondilo-arthritis excluded based on MR images of the pelvis. | |||||
| XIV | 1715 | ||||
| F | Second rheumatology visit: FM diagnosis confirmed based on the presence of tender point sensitivity (14/18), widespread chronic pain for longer than 3 months, morning stiffness, non-restorative sleep, depression, anxiety, leg and foot cramps, chest pain, tachycardia, hypersensitivity to cold, “fibro-fog”, irritable bowel syndrome-constipation and hyperhidrosis. | ||||
| XV | 1959 |
CE-MRI, contrast-enhanced magnetic resonance imaging; S(i), symptoms.
L(i), radiological investigations of body compartments else than the brain.
(*) days from the first gadolinium-based administration.
Starting a few weeks after the second GBCA administration, the patient presented with symptoms of bone pain in the lumbar region (initially advocated bed rest during recovery after surgery) (S1 in Fig. 1B and Table 1), followed by restless legs within few months, morning stiffness, severe leg and foot cramps, and “pins and needle” in foot (S2). Over the course of the subsequent year, severe lower backaches and hip pain forced the patient to bed rest (S3). Non-CE MRI and X-ray scans revealed a lumbar disk-hernia, but no other abnormalities or lesion have been found (L1). Some months later, anterior chest pain and tachycardia led to emergency unit access without relevant cardiac abnormalities (S4). The patient presented the recurrence of severe lumbar pain and a marked progressive increase of morning stiffness (S5). A second lumbar MRI revealed no disease progression able to explain the symptoms (L2). Some months later, exacerbation of lumbar aches and stiffness once more forced the patient to bed up to experiencing insomnia for the excruciating bone pain (S6). Anti-inflammatory pain killers were ineffective. Oral gabapentin administration had a mild effect. A few months later, the patient presented a flare-up of pain symptoms and lumbar stiffness (S7). Afterwards, the onset of pain at the right shoulder and trapezium occurred: pain was described similar to that persisting in the lower back and hips. Cold and humidity have been reported to markedly worse the symptoms. A few days after, stiffness presented at the cervical site, and aches occurred at knees similar to the hips. Chest pain and tachycardia (S8), non-restorative sleep and fatigue unrelieved by rest closely onset. Precipitation of patient's conditions occurred when pain and stiffness forced the patient to bed suffering exacerbating pain described as originating deep in the bone and muscles and triggered by any attempt of locomotor action for several hours (S9). Intriguingly, passive movements guided by another person have been reported to provoke much less pain than voluntary movements. A few days after, for the first time in her history, the patient had manifest depressive symptoms. The clinical symptomatology was considered suggestive of fibromyalgia (FM). She was referred to the rheumatology unit. The clinical history was deemed consistent with FM by a first rheumatology specialist, and, on physical examination, trigger point sensitivity was assessed positively. To finally confirm the FM diagnosis and exclude other rheumatic pathologies, laboratory tests and MRI of the pelvis were required. The laboratory results came back all negative, namely, anti-nuclear antibodies, anti-nDNA antibodies research, anti-cyclic citrullinated peptide antibodies, anti- ENA antibodies, and rheumatoid factor were all in the normal range as well as erythrocyte sedimentation rate, C-reactive protein, creatine kinase, and complete blood count. The MRI of the pelvis (L3) excluded spondilo-arthritis. In the meanwhile, scheduled brain CE-MRI lacked to present tumour recurrence and evidence able to explain the worsening of symptoms. On physical examination by a second rheumatology specialist, trigger point sensitivity was once more assessed reporting positivity 14/18 and main symptoms of widespread chronic pain for longer than three months, morning stiffness, unrefreshing sleep, depression, hypersensitivity to cold and ``fibro-fog''. The FM diagnosis was thus confirmed based on the 2010/2011 criteria [24,25] that emphasized the importance of associated symptoms along with widespread chronic pain and crystallized FM as a multi-symptom and systemic disorder. The patient also satisfied the American College of Rheumatology 1990 classification criteria for FM [26] that persist in the clinical practice to support the diagnosis and require tender point sensitivity (≥11/18).
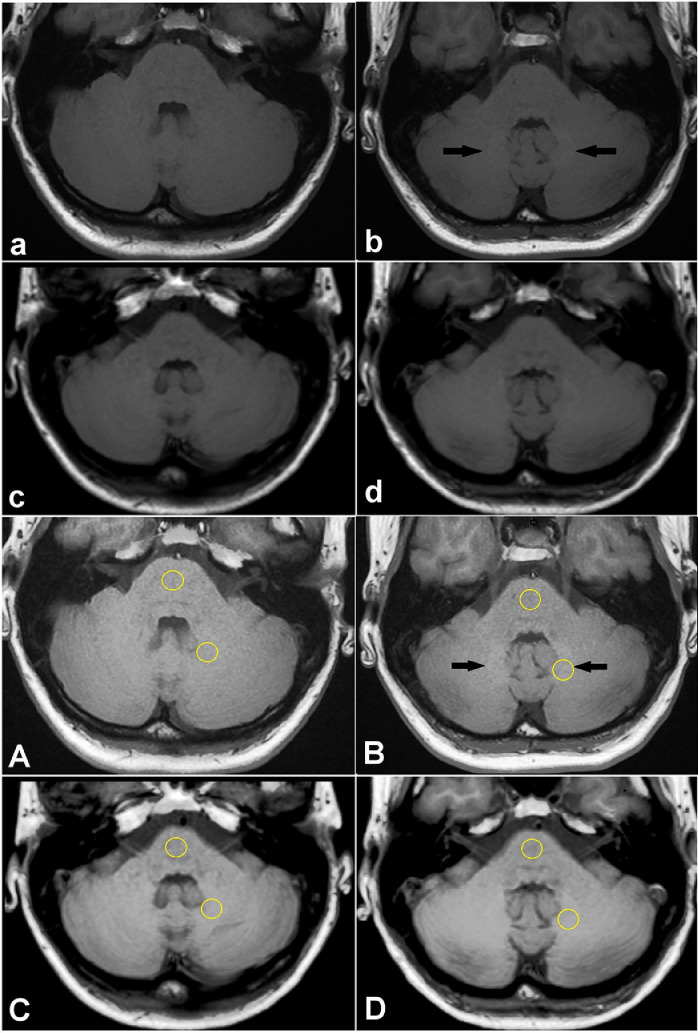
Some months after the FM diagnosis, a scheduled CE-MRI for tumour follow-up was performed (XV, day 1959) without evidence of disease recurrence. A few days later, the patient presented worsening of symptoms in particular related to osteoarticular pain, tiredness, depressed mood, mental confusion, "pins and needle'', calf and feet cramps, prickling sensation in the bottom of the eyes, increasing unexplained fatigue unrelieved by rest and increased parestesia in the right feet and the right lower leg. Since then, she denied the consent for GBCA administration. Since non-CE scans have been considered insufficient for a proper evaluation of a III grade astrocytoma follow-up, no other MRI brain scans have been performed. The temporal correlation among symptoms worsening and the last MRI led to conjecture a role for MRI diagnostic. The debate and emerging concern on the safety of GBCAs and their restrictions and suspensions in Europe [7] led to hypothesize a causal role for the agents. The case was reviewed in light of new knowledge and awareness. Non-CE T1-weighted MR images of the posterior fossa a the level of dentate nucleus of the cerebellum are reported in Figure 2. Image contrast enhancement by the ImageJ process tool † (A,B,C,D in Fig. 2) allows easier visual detection of the structures abnormally hyperintense respect to the adjacent tissue. Mild eye-visually appreciable signal enhancement can be observed in the dentate nucleus, comparing images among the first (a and A in Fig. 2) and the sixth examinations (b and B in Fig. 2). Instead, the fourteenth scan (c and C in Fig. 2) shows no visually appreciable signal enhancement and fifteenth scan (d and D in Fig. 2) shows only minimal almost non eye-visually appreciable signal enhancement in the DN comparing to the adjacent regions of the cerebellum, despite a higher signal in the central and upper region than in the pons and lower lateral cerebellar hemispheres can be noted (d in Fig. 2). However, a quantitative analysis reveals subtle intensity differences and a linear relationship among DN-to-pons ratio signal enhancement versus the number of administrations (R2 = 0.956, by linear fit of Microsoft Excel software, numerical data reported on caption of Fig. 2, plot not shown). Thus, reduced visually appreciable hyperintense signal in the DN among the sixth and the subsequent scans in our data is a sign of more homogeneous deposition throughout the posterior fossa reasonably involving the surrounding cerebellar cortex. The patient received macrocyclic non-ionic contrast agent Gadovist (ie, gadobutrol, 1.0 mmol/ml; Bayer Healthcare, Berlin, Germany) at dose of 0.125 mmol/Kg. The exact amount of total administered agent cannot be accessed because nor the weight nor the administered dose are routinely reported. A rough estimation for the range of patient's bodyweight of 56-64 Kg is a dose of 7 to 8 ml of Gadovist 1.0 mmol/ml at single administration, for a total of 105 to 120 ml.
Fig. 2.
Unenhanced axial T1-weighted MR images (MRI) of the posterior fossa at the level of the dentate nucleus of our patient (first and second line) and same views taking advantage of contrast enhancement by ImageJ (third and fouth line): (a, A, I MRI) before any gadolinium administration (dentate nucleus-to-pons signal intensity ratio = 1.0259), (b, B, VI MRI) sixth exam (dentate nucleus-to-pons signal intensity ratio = 1,0739), (c, XIV MRI) fourteenth exam (dentate nucleus-to-pons signal intensity ratio = 1,1212), (d, XV MRI) fifteenth exam (dentate nucleus-to-pons signal intensity ratio = 1,1581). Else than stronger quantitavite analysis as reported, a qualitative difference can be appreciated among the first (a, A) and the last (d, D) image: a wide higher signal in the central and upper region of the cerebellum than in the pons and lower lateral cerebellar hemispheres can be observed, thus masking visually appreciable dentate nucleus hyperintensity, that is conversely revealed by quantitative analysis. Regions of interest (ROI) used for quantitative analysis on dentate nucleus and pons are shown. MRI, magnetic resonance imaging.
Discussion
Different topics are entangled in this case and involve specific knowledge (ie; fibromyalgia, biochemistry of lanthanides, gadolinium toxicity, gadolinium deposition) often not co-occurrent in the growing specialization and compartmentalization of the medical knowledge. This patient presents a typical case of fibromyalgia syndrome [27,28]. At the same time, this case represents an example of the mimetic nature of signs and symptoms associated with gadolinium deposition and the difficulties of their recognition in the complexity of a clinical case. Multiple factors may confound, if unaware of the uncountable pathologic interferences of the gadolinium ion in human physiology.
Visually appreciable T1-shortening is just the handier clue to unravel gadolinium deposited in the brain parenchima in a number of cases: the presence of homogeneous deposition leads to even quantitatively undetectable signal enhancement among adjacent regions. Roberts and colleagues [29] presented a significative example supporting this point. They report a case of heavy deposition of gadolinium within the DN and throughout the cerebellar cortex demonstrated by laser ablation-inductively coupled plasma mass spectrometry (ICP-MS), despite the lack of T1-weighted hyperintensity within the DN on MRI. It is worth to stress that appreciable changes in signal intensity are subjected to signal intensity of the surrounding tissue: therefore, widespread retention in the brain or the presence of species that lack relaxivity properties might lead to undetectable visual changes, even quantitatively. Furthermore, the visual limit of detection of gadolinium species in the brain is unknown, as much as, thus, the correlation among signal intensity and concentration. The lack of T1-shortening and associated signal enhancement is insufficient to exclude the presence of residual gadolinium. This might be a crucial point for clinical outputs and implications. Visibility of gadolinium is intrinsically dependent not just on its speciation, but also on the compartment where it is stored [30]. GBCAs are paramagnetic metal complexes as water proton relaxation agents for nuclear magnetic resonance imaging: gadolinium is ‘MR invisible’ and becomes indirectly ‘MR visible’ only under specific physicochemical conditions where its relaxation-enhancement properties are sufficiently preserved [30,31]. This makes the challenge for its in vivo detection in human brain even more tricky, and pushes us to better consider the indirect signs of its presence, disclosed by symptoms, and a careful analysis of patient's history. At time of diagnosis, FM was considered completely independent from the brain cancer, but new awareness of the issue of gadolinium deposition built a new indirect link through the diagnostic procedures and a clear role, at least for this case, for gadolinium deposition following GBCA administrations. The interference of the Gd(III) ion and its pharmaceutical chelates into several biochemical pathways might be able to explain FM symptoms as a consequence of impairment and alterations at the cellular, intracellular, and systemic levels [32]. It affects calcium currents and the functionality of calcium channels [33], [34], [35], [36], so altering the numerous stimulus-coupled cellular responses that rely on calcium influx [37]; calcium- and magnesium-requiring enzymes, such as Ca2+, Mg2+ - adenosine triphosphatase (ATPase) of sarcoplasmic reticulum [38]; neurotransmitters such as serotonin, noradrenaline, and dopamine by the inhibition of their transporter (respectively SERT, NAT, and DAT) [39]; endogenous metal cations following transmetallation, particularly zinc in blood [40], being this metal a strong competitor of gadolinium chelators; and mitochondria [41], [42], [43]. Recently, a toxic effect of GBCAs on neurons in vitro affecting mitochondrial respiratory function has been further demonstrated [44]. The impact of gadolinium on mitochondria might be of particular relevance to explain persistent fatigue, weak resistance, and reduced physical performances.
Starting from this case, our thesis is that fibromyalgia might be one of the clinical manifestations of gadolinium toxicity in the humans. Nevertheless, the relationship is not bijective: gadolinium deposition can cause fibromyalgia, but fibromyalgia could be the clinical manifestation of toxicity of other metals. Indeed: it is a matter of fact that there are FM patients that never met GBCAs in their clinical history; and it is already known from past clinical studies that fibromyalgia can be caused by exogenous metals other than gadolinium [45]. Among others, Stejskal and colleagues [46,47] measured high levels of several toxic metals and toxic levels of physiological ones in a cohort of fibromyalgia patients. They advocated metal-induced inflammation the primary origin of the symptoms. They reported symptoms resolution in a number of (but not all) patients after the removal of the hypothesized sources of metal-induced toxicity (not lanthanides). Nevertheless, despite this evidence, up to date the relevance of metal toxicity in the fibromyalgia disease is neglected among rheumatology experts [27].
Gadolinium deposition associated to linear agents is to date widely recognized and the suspension of marketing authorization in Europe for most of the agents belonging to this category [7] confirms the legitimacy of concerns for the clinical relevance of the deposition. It is now clear that the stability of the gadolinium-ligand complex plays a crucial role, and that gadolinium deposition is not a peculiarity of linear agents [16]. Our case might be relevant for its involvement of a macrocyclic agent. Millions of magnetic resonance studies are annually performed, and a relevant percent of them are contrast-enhanced [48]; moreover, GBCAs have been used as alternative contrast agents for other diagnostic examinations [1]. The issue of gadolinium deposition might be more relevant and widespread than expected. Also, it might be unrecognised in the clinical setting when lacking specific investigations that allow lanthanide detection and when co-occurrence of primary diseases might mask the role of gadolinium in triggering or contributing to the symptoms. The patients might be often mistakenly believed to show complaint of their major disease and symptoms associated with gadolinium retention are unrecognized. In 2018, Fitzgerald and colleagues [49] published the results of a study querying radiologists’ practices regarding gadolinium deposition (data collected from November-December 2015). Among a total of 94 responder radiologists from 30 countries, more than 60% had observed brain gadolinium deposition on brain studies, but more than half of them did not include this finding in the radiological report. This choice has been mainly explained by the “fear of provoking patient/clinician anxiety and incomplete understanding of the implications of gadolinium deposition”. Unfortunately, this might have led to underestimate the incidence of gadolinium deposition in the brain parenchima.
Addressing the causes of a disease is the most promising avenue to its cure and prevention. Gadolinium deposition is clearly linked to GBCA administration. Thus, we have the duty to prevent its occurrence and strongly balance the risk-benefits ratio of GBCA administration, particularly when repeated. Actions should be implemented to make aware of this issue not only radiologists, but any physician prescribing diagnostic exams involving the administration of GBCAs, and patients with proper informed consent. Last but not least, when benefit for diagnostic purpose appears much more valuable than the risk for deposition, pre-administration of compounds possibly useful to prevent deposits appears a more promising approach than attempting a subsequent gadolinium removal (particularly concerning the bone), as initially shown in a preclinical study on mice by Rees and colleagues [50].
Conclusion
That gadolinium is highly toxic for mammals is not a novelty and that gadolinium deposition can occur in vivo is a reality. Biochemistry of gadolinium can explain the heterogeneity and systemic nature of the symptoms associated with its deposition in humans. GBCAs are routinely administered in a clinical context, where pathophysiological ongoing processes exist (and often co-occurrent drug intake). It is thus possible that the patients are mistakenly believed to show complaint of their major disease. Conversely, some of their symptoms are unrecognized symptoms associated with gadolinium deposition. At the same time, these symptoms match with FM symptoms and dysfunctions. The etiology of FM is still unknown: gadolinium might be one of the mysterious sources of the symptoms in a number of FM patients. Gadolinium deposition in humans might have its primary long-term clinical manifestation as FM. This point may have implications regarding patient outcomes and therapies.
Software references
† Schneider CA., Rasband WS, Eliceiri KW. "NIH Image to ImageJ: 25 years of image analysis". Nat. Methods 9, 671-675, 2012.
Footnotes
Acknowledgments: Thanks to Laura Bianchi, Giuseppe Zanotti and Nazareno Paolocci for their support and encouragement in the making of this contribution and for their helpful suggestions.
References
- 1.Essig M., Giesel E., Le-Huu M. Perfusion MRI in CNS disease: current concepts. Neuroradiology. 2004;46:S201–S207. doi: 10.1007/s00234-004-1331-y. [DOI] [PubMed] [Google Scholar]
- 2.Lohrke J., Frenzel T., Endrikat J. 25 years of contrast-enhanced MRI: developments, current challenges and future perspectives. Adv Ther. 2016;33:1–28. doi: 10.1007/s12325-015-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein E.J., Schmidt-Lauber C., Kay J. Nephrogenic systemic fibrosis: a systemic fibrosing disease resulting from gadolinium exposure. Best Pract Res Clin Rheumatol. 2012;26:489–503. doi: 10.1016/j.berh.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Food & Drug Administration . 2007. FDA requests boxed warning for contrast agents used to improve MRI images [press release]http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108919.html Available from: Accessed Nov 2018. [Google Scholar]
- 5.US Food and Drug Administration . 2017. FDA Briefing Document: Gadolinium retention after gadolinium based contrast magnetic resonance imaging in patients with normal renal function.https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM577014.pdf September 8 Accessed August 2018. [Google Scholar]
- 6.FDA . 2015. Drug safety communication. FDA evaluating the risk of brain deposits with repeated use of Gadolinium-based contrast agents for magnetic resonance imaging (MRI)https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-evaluating-risk-brain-deposits-repeated-use-gadolinium-based Accessed July 2017. [Google Scholar]
- 7.EMA’s final opinion confirms restrictions on use of linear gadolinium agents in body scans . 2017. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/gadolinium_contrast_agents_31/European_Commission_final_decision/WC500240575.pdf EMA/625317/2017 November 23 Available from: Accessed July 2019. [Google Scholar]
- 8.US Food and Drug Administration . 2018. FDA drug safety communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings.https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-gadolinium-based-contrast-agents-gbcas-are-retained-body [Google Scholar]
- 9.Gibby W.A., Gibby K.A., Gibby W.A. Comparison of Gd-DTPA-BMA (Omniscan) versus Gd-HPDO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Invest Radiol. 2004;39:138–142. doi: 10.1097/01.rli.0000112789.57341.01. [DOI] [PubMed] [Google Scholar]
- 10.White G.W., Gibby W.A., Tweedle M.F. Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HPDO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled mass spectroscopy. Invest Radiol. 2006;41:272–278. doi: 10.1097/01.rli.0000186569.32408.95. [DOI] [PubMed] [Google Scholar]
- 11.Murata N., Gonzalez-Cuyar L.F., Murata K. Macrocyclic and other non–group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: Preliminary results from 9 patients with normal renal function. Invest Radiol. 2016;51:447–453. doi: 10.1097/RLI.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 12.McDonald R.J., McDonald J.S., Kallmes D.F. Intracranial gadolinium deposition after contrast enhanced MR imaging. Radiology. 2015;275:772–782. doi: 10.1148/radiol.15150025. [DOI] [PubMed] [Google Scholar]
- 13.Darrah T.H., Prutsman-Pfeiffer J.J., Poreda R.J. Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics. 2009;1:479–488. doi: 10.1039/b905145g. [DOI] [PubMed] [Google Scholar]
- 14.Kiviniemi A., Gardberg M., Ek P. Gadolinium retention in gliomas and adjacent normal brain tissue: association with tumor contrast enhancement and linear/macrocyclic agents. Neuroradiology. 2019;61:535–544. doi: 10.1007/s00234-019-02172-6. [DOI] [PubMed] [Google Scholar]
- 15.Olchowy C., Cebulski K., Lasecki M. The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity - A systematic review. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nehra A.K., McDonald R.J., Bluhm A.M. Accumulation of gadolinium in human cerebrospinal fluid after gadobutrol-enhanced MR imaging: A observational cohort study. Radiology. 2018;288:416–423. doi: 10.1148/radiol.2018171105. [DOI] [PubMed] [Google Scholar]
- 17.Pullicino R., Radon M., Biswas S. A review of the current evidence on gadolinium deposition in the brain. Clin Neuroradiol. 2018;28:159–169. doi: 10.1007/s00062-018-0678-0. [DOI] [PubMed] [Google Scholar]
- 18.Adin M.E., Kleinberg L., Vaidya D. Hyperintense dentate nuclei on T1-weighted MRI: relation to repeat gadolinium administration. AJNR Am J Neuroradiol. 2015;36:1859–1865. doi: 10.3174/ajnr.A4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts D.R., Lindhorst S.M., Welsh C.T. High levels of gadolinium deposition in the skin of a patient with normal renal function. Invest Radiol. 2016;51:280–289. doi: 10.1097/RLI.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 20.Semelka R.C., Commander C.W., Jay M. Presumed gadolinium toxicity in subjects with normal renal function. A report of 4 cases. Invest Radiol. 2016;51:661–665. doi: 10.1097/RLI.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 21.Semelka R.C., Ramalho J., Vakharia A. Gadolinium deposition disease: inital description of a disease that has been around for a while: A family of disorders. Magn Res Imaging. 2016;34:1383–1390. doi: 10.1016/j.mri.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Burke L.M.B., Ramalho M., AlObaidy M. Self-reported gadolinium toxicity: a survey of patients with chronic symptoms. Magn Res Imaging. 2016;34:1078–1080. doi: 10.1016/j.mri.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Swaminathan S. Gadolinium toxicity: iron and ferroportin as central targets. Magn Reson Imaging. 2016;34:1373–1376. doi: 10.1016/j.mri.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe F., Clauw D.J., Fitzcharles M.A., Goldenberg D.L., Katz R.S., Mease P. The American college of rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe F., Clauw D.J., Fitzcharles M.A., Goldenberg D.L., Hauser W., Katz R.S. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR preliminary diagnostic criteria for fibromyalgia. J Rheumatol. 2011;38:1113–1122. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe F., Smythe H.A., Yunus M.B. The american college of rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 27.Arnold L.M., Choy E., Clauw D.J. Fibromyalgia and chronic pain syndromes. A white paper detailing current challenges in the field. Clin J Pain. 2016;32:737–746. doi: 10.1097/AJP.0000000000000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassisi G., Sarzi-Puttini P., Alciati A. Symptoms and signs in fibromyalgia syndrome. Reumatismo. 2008;60:15–24. [PubMed] [Google Scholar]
- 29.Roberts D.R., Welsh C.A., LeBel D.P., II Distribution map of gadolinium deposition within the cerebellum following GBCA administration. Neurology. 2017;88:1206–1208. doi: 10.1212/WNL.0000000000003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tweedle M.F. Gadolinium deposition: is it chelated of dissociated gadolinium? how can we tell? Magn Res Imaging. 2016;34:1377–1382. doi: 10.1016/j.mri.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Lauffer R.B. Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: theory and design. Chem Rev. 1987;87:901–927. 46. [Google Scholar]
- 32.Lattanzio S.M. The gadolinium hypothesis for fibromyalgia and unexplained widespread chronic pain. Med Hyp. 2019;129 doi: 10.1016/j.mehy.2019.109240. [DOI] [PubMed] [Google Scholar]
- 33.Lansman J.B. Blockade of current through single calcium channels by trivalent lanthanide cations. Effect of ionic radius on the rates of ion entry and exit. J Gen Physiol. 1990;95:679–696. doi: 10.1085/jgp.95.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mlinar B., Enyeart J.J. Block of current through T-type calcium channels by trivalent metal cations and nickel in neural rat and human cells. J Physiol. 1993;469:639–652. doi: 10.1113/jphysiol.1993.sp019835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourne G., Trifaro J. The gadolinium ion: a potent blocker of calcium channels and catecholamine release from cultured chromaffin cells. Neuroscience. 1982;7:1615–1622. doi: 10.1016/0306-4522(82)90019-7. [DOI] [PubMed] [Google Scholar]
- 36.Biagi B.A., Enyeart J.J. Gadolinium blocks low-threshold and high-threshold calcium currents in pituitary-cells. Am J Physiol. 1990;259:C515–C520. doi: 10.1152/ajpcell.1990.259.3.C515. [DOI] [PubMed] [Google Scholar]
- 37.Evans C.H. Plenum Press; New York: 1990. Biochmistry of the Lanthanides. [Google Scholar]
- 38.Itoh N., Kawakita M. Characterization of Gd3+ and Tb3+ binding sites on Ca2+, Mg2+ -adenosine triphosphatase of sarcoplasmic reticulum. J Biochem (Tokyo) 1984;95:661–669. doi: 10.1093/oxfordjournals.jbchem.a134655. [DOI] [PubMed] [Google Scholar]
- 39.Bryan-Lluka L., Bonish H. Lanthanides inhibit human noradrenaline, 5-hydroxytryptamine and dopamine transporters. Naunyn. Schmiedebergs. Arch. Pharmacol. 1997;355:699–706. doi: 10.1007/pl00005002. [DOI] [PubMed] [Google Scholar]
- 40.Sherry A.D., Caravan P., Lenkinski R.E. Primer on gadolinium chemistry. J Magn Reson Imaging. 2009;30:1240–1248. doi: 10.1002/jmri.21966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu H., Yuan L., Yang X. La3+, Gd3+ and Yb3+ induced changes in mitochondrial structure, membrane permeability, cytochrome c release and intracellular ROS level. Chem Biol Interact. 2003;146:27–37. doi: 10.1016/s0009-2797(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 42.Feng X., Xia Q., Yuan L. Impaired mitochondrial function and oxidative stress in rat cortical neurons: Implications for gadolinium-induced neurotoxicity. Neurotoxicology. 2010;31:391–398. doi: 10.1016/j.neuro.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Zhao J., Zhou Z., Jin J.C. Mitochondrial dysfunction induced by different concentrations of gadolinium ion. Chemosphere. 2014;100:194–199. doi: 10.1016/j.chemosphere.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 44.Bower D.V., Richter J.K., von Tengg-Kobligk H. Gadolinium-based MRI contrast agents induce mitochondrial toxicity and cell death in human neurons, and toxicity increases with reduced kinetic stability of the agent. Invest Radiol. 2019;54:453–463. doi: 10.1097/RLI.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 45.Bjørklund G., Dadarb M., Aasethc J. Delayed-type hypersensitivity to metals in connective tissue diseases and fibromyalgia. Environ Res. 2018;161:573–579. doi: 10.1016/j.envres.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Stejskal V., Ockert K., Bjørklund G. Metal-induced inflammation triggers fibromyalgia in metal-allergic patients. Neuroendocrinol Lett. 2013;34:559–565. [PubMed] [Google Scholar]
- 47.Stejskal V. Metals as a common trigger of inflammation resulting in non-specific symptoms: Diagnosis and treatment. IMAJ. 2014;16:753–758. [PubMed] [Google Scholar]
- 48.Leyba K., Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28:154–162. doi: 10.1097/MNH.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fitzgerald R.T., Agarwal V., Hoang J.K. The impact of gadolinium deposition on radiology practice: An international survey of radiologists. Curr Probl Diagn Radiol. 2019;48:220–223. doi: 10.1067/j.cpradiol.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Rees J.A., Deblonde G.J.P., An D.D. Evaluating the potential of chelation therapy to prevent and treat gadolinium deposition from MRI contrast agents. Sci Rep. 2018;8:4419. doi: 10.1038/s41598-018-22511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]