Abstract
Objectives
Human cercarial dermatitis (HCD) is a water-borne zoonotic parasitic disease. Cercariae of the avian schistosomes of several genera are frequently recognized as the causative agent of HCD. Various studies have been performed regarding prevalence of bird schistosomes in different regions of the world. So far, no study has gathered and analyzed this data systematically. The aim of this systematic review and meta-analysis study was to determine the prevalence of avian schistosomes worldwide.
Methods
Data were extracted from six available databases for studies published from 1937 to 2017. Generally, 41 studies fulfilled the inclusion criteria and were used for data extraction in this systematic review. Most of studies have been conducted on the family Anatidae.
Results
The overall prevalence of avian schistosomes was estimated to be 34.0% (95%CI, 28%–41%) around the world. Furthermore, results displayed that, Allobilharzia visceralis and Trichobilharzia spp. had the highest frequency and their prevalence in the birds was 50.0% (95% CI, 3.0%–97.0%) and 32.0% (95% CI, 21.0%–0.36%), respectively. The results showed that the prevalence of avian schistosomes was 43.0% (95% CI, 29% - 56%) in the US and 38.0% (27.0% -50.0%) in Europe, which were higher than other continents, respectively.
Conclusions
The prevalence of 34% shows that the bird schistosomes are very common zoonotic worms among aquatic birds in the world. Also, this study shows the importance of avian schistosome research when facing animal and human health of the future.
Keywords: Human cercarial dermatitis, Avian schistosomes, Prevalence, Trichobilharzia, Allobilharzia
1. Introduction
The bird schistosomes are a group of blood flukes (Platyhelminthes, Digenea). They contain the largest clade of the family Schistosomatidae that includes ten genera: Dendritobilharzia, Gigantobilharzia, Allobilharzia, Austrobilharzia, Anserobilharzia, Trichobilharzia, Bilharziella, Macrobilharzia, Ornithobilharzia and Jilinobilharzia. In the two-host life cycle (fresh water snails as intermediate hosts and aquatic birds as definitive hosts), they develop as adults with in venous and arterial vessels or nasal tissue of their bird host. Bird schistosomes of the genus Trichobilharzia are categorized as nasal and visceral groups depending on their target tissue within the final hosts (Horák et al., 2002; Brant et al., 2006). Cercariae of the bird schistosomes released from intermediate hosts are recognized as the great causative agent of human cercarial dermatitis (HCD) or Swimmer's itch which is considered an emerging disease in various parts of the World (de Gentile et al., 1996). Experiments have demonstrated that avian schistosomes can migrate and partly develop in other bodies of their non-specific mammalian hosts, causing serious health risks. Larvae of different visceral avian schistosome have been detected in the lungs of experimentally infected monkeys and rodents (Horák et al., 2002), and foot paralysis has been seen in mice infected with the neurotropic species Trichobilharzia regenti (Horák et al., 1999; Hrádková and Horák, 1999). Therefore, as a result of these experiments, It is thus considered conceivable that avian schistosomes may be responsible for some nervous or pulmonary symptoms in humans. HCD occurs worldwide, for example Iceland, Austria, UK, The Netherlands, Iran, Chile, China and USA (Horák et al., 2002; Farahnak and Essalat, 2003; Brant, 2007; Valdovinos and Balboa, 2008; Hörweg et al., 2006; Skírnisson et al., 2009; Żbikowska, 2003). The incidence of bird schistosomes and dermatitis caused by them depends on the presence of appropriate intermediate and final hosts. Many of the migratory bird host species used by schistosomes, particularly Trichobilharzia, are in the family Anatidae. Furthermore, species in the snail families Lymnaeidae, Physidae, and Planorbidae are most often used as the intermediate hosts of bird schistosomes (Brant and Loker, 2009a; Brant and Loker, 2009b; Marszewska et al., 2018). While research papers document the global occurrence and burden of HCD, there are no studies that document a systematic review on the subject of the prevalence of bird schistosomes on a global scale. Therefore, it is the purpose of this study to estimate the world prevalence of avian schistosomes and thus HCD.
2. Methods
2.1. Search approach
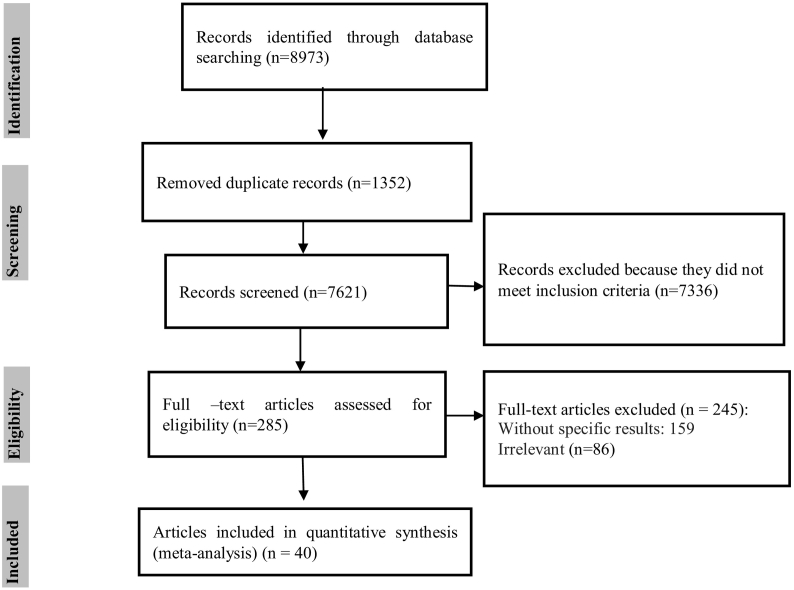
For studies published between 1937 (based on preliminary search) and 2017, six English language databases (PubMed, Web of Science, Ebsco, Science Direct, Google Scholar and Scopus) were searched. The search terms were “Human cercarial dermatitis”, “bird schistosomes”, “avian schistosomes”, “nasal schistosomes”, “visceral schistosomes” “Pseudobilharziella”, “Trichobilharzia”, “Allobilharzia”, “Dendritobilharzia”, “Gigantobilharzia”, “Austrobilharzia”, “Anserobilharzia”, “Bilharziella”, “Macrobilharzia”, “Ornithobilharzia ‘and’ Jilinobilharzia” alone or combined together using “OR” and/or “AND.”. The PRISMA diagram of the study plan is shown in Fig. 1.
Fig. 1.
PRISMA flowchart describing the study design process.
2.2. Paper assortment
Included Studies were identified by two reviewers (EK and SD) independently and confirmed by a third reviewer (MF). Studies that met the inclusion criteria were included in this survey: (1) Full text articles available online (2) cross-sectional studies that estimated the prevalence of bird schistosomes with different diagnostic methods including morphological and molecular techniques (3) published papers in English. Duplicates, case reports, case series, experimental studies, mammalian schistosomes, published papers in other language and papers with unclear result section were excluded. In order to ensure that articles were not overlooked or not found in searches, the references of each paper were manually screened.
2.3. Quality assessment
The STROBE questionnaire was used for the determination of article quality. The questionnaire contains 22 questions with a maximum of 31 score. The score under 7.5 considered poor quality of study (Von Elm et al., 2007). For all eligible papers in this systematic review and meta-analysis, the obtained score was 18.
2.4. Data extraction
Data were extracted from papers including author (s), publication year, type of parasite, geographical area of the study, and number of examined samples, number of positive samples, prevalence rate, and laboratory technique used for the study and entered into an Excel sheet (Table 1).
Table 1.
The characteristics of selected studies for the meta-analysis.
| Author | Year | Country | Continent | Methods | No. examined | No. Infected | Ref. |
|---|---|---|---|---|---|---|---|
| McLeod J. A | 1937 | Canada | Americas | Morphology | 30 | 18 | (McLeod, 1937) |
| Cheatum E. L | 1941 | USA | Americas | Morphology | 72 | 41 | (Cheatum, 1941) |
| Brackett S | 1942 | USA | Americas | Morphology | 72 | 25 | (Brackett, 1942) |
| Guth BD | 1979 | USA | Americas | Morphology | 1244 | 169 | (Guth et al., 1979) |
| Blair D | 1979 | Australia | Oceania | Morphology | 548 | 310 | (Blair and Ottesen, 1979) |
| Strohm B. C | 1981 | USA | Americas | Morphology | 23 | 7 | (Blankespoor and Meier, 1981) |
| Pence D.B | 1982 | USA | Americas | Morphology | 5 | 5 | (Pence and Rhodes, 1982) |
| Appleton C.C | 1983 | Western Australia | Oceania | Morphology | 31 | 25 | (Appleton, 1983) |
| Palmer. D | 1984 | Switzerland | Europe | Morphology | 20 | 16 | (Palmer and Ossent, 1984) |
| Appleton C·C | 1986 | South Africa | Africa | Morphology | 1554 | 264 | (Appleton, 1986) |
| Athari A | 1990 | Iran | Asia | Morphology | 188 | 16 | (Athari et al., 1990) |
| Brent R. L | 1995 | USA | Americas | Morphology | 202 | 78 | (Loken et al., 1995) |
| Barber. E | 1995 | USA | Americas | Morphology | 96 | 68 | (Barber and Caira, 1995) |
| Kolarova. L | 1997 | Czech Republic | Europe | Morphology | 2051 | 239 | (Kolarova et al., 1997) |
| Martini F. S | 1999 | Spain | Europe | Morphology | 8 | 6 | (Simon-Martin and Simon-Vicente, 1999) |
| Rudolfova J | 2002 | Czech Republic | Europe | Morphology | 54 | 13 | (Rudolfova et al., 2002) |
| Bayssade-dufour C | 2006 | France | Europe | Morphological and molecular | 31 | 11 | (Bayssade-Dufour et al., 2006) |
| Davis NE | 2006 | New Zealand | Oceania | Morphology | 38 | 27 | (Davis, 2006) |
| Kolářová L | 2006 | Iceland | Europe | Morphological and molecular | 27 | 7 | (Kolářová et al., 2006) |
| Athari A | 2006 | Iran | Asia | Morphology | 138 | 25 | (Rostami-Jalilian, 2006) |
| Rudolfova J | 2007 | Czech Republic | Europe | Morphology | 102 | 23 | (Rudolfová et al., 2007) |
| Rudolfova J | 2007 | Poland | Europe | Morphology | 73 | 21 | (Rudolfová et al., 2007) |
| Brant SV | 2007 | USA | Americas | Morphological and molecular | 13 | 12 | (Brant, 2007) |
| Jouet D | 2008 | France | Europe | Morphology | 115 | 76 | (Jouet et al., 2008) |
| Jouet D | 2009 | France | Europe | Morphology | 399 | 174 | (Jouet et al., 2009) |
| Brant SV | 2009 | USA | Americas | Morphological and molecular | 378 | 92 | (Brant and Loker, 2009b) |
| Skírnisson K | 2009 | Iceland | Europe | Morphological | 110 | 39 | (Skírnisson and Kolářová, 2008) |
| Jouet D | 2010 | Iceland France |
Europe | Morphological and molecular | 373 | 150 | (Jouet et al., 2010) |
| Mahdavi SA | 2012 | Iran | Asia | Morphology | 110 | 15 | (Mahdavi et al., 2013) |
| Maleki SH | 2012 | Iran | Asia | Molecular techniques | 45 | 12 | (Maleki et al., 2012) |
| Gohardehi SH | 2013 | Iran | Asia | Morphology | 260 | 41 | (Gohardehi et al., 2013) |
| Birmani NA | 2013 | Pakistan | Asia | Morphology | 101 | 11 | (Birmani et al., 2013) |
| Kolářová L | 2013 | Iceland | Europe | molecular techniques | 19 | 14 | (Kolářová, 2013) |
| Aldhoun JA | 2014 | South African | Africa | Morphology | 555 | 45 | (Aldhoun and Horne, 2015) |
| Jouet D | 2015 | Iceland | Europe | Morphological and molecular | 46 | 3 | (Joue et al., 2015) |
| Jouet D | 2015 | Iceland | Europe | Morphological and molecular | 80 | 36 | (Joue et al., 2015) |
| Jouet D | 2015 | France | Europe | Morphological and molecular | 29 | 11 | (Joue et al., 2015) |
| Fakhar M | 2016 | Iran | Asia | Morphological and molecular | 508 | 45 | (Fakhar et al., 2016) |
| Prüter H | 2017 | Germany | Europe | molecular techniques | 106 | 35 | (Prüter et al., 2017) |
| Hayashi k | 2017 | Japan | Asia | molecular techniques | 13 | 4 | (Hayashi, 2017) |
| Brant S. V | 2017 | Argentina | Americas | molecular techniques | 40 | 1 | (Brant et al., 2017) |
2.5. Meta-analysis
For each study, the prevalence of bird schistosomes (the number of current positive of infection cases divided to total number of sample) and standard error (SE) were calculated.
We used forest plots for estimating pooled effect sizes and the effect of each study, with 95% confidence intervals (95% CI), to provide a visual summary of the data. To evaluate heterogeneity among studies that used common approaches we used the Cochran Q-test (p-value<.1) and the I-squared index, with I2 values between 25% and 50%, between 50% and 75% and above75% as thresholds for low, moderate, and high heterogeneity, respectively. When heterogeneity was present we used a random effects model (DerSimonian–Laird model); otherwise a fixed effects model (Mantel–Haenszel) used to compute overall effects. Sub-group analysis was used to evaluate the association between effect size and characteristics such as continent, parasite species and method. Potential publication bias was explored using funnel plot and Egger's test which evaluated whether precision of studies is related to the magnitude of their effect size. A trim-and-fill method was performed to detect the effect of missing studies on the overall effect of meta-analysis. All statistical analyses were done with the Stata software, v. 14 (Stata Corp LP, College Station, Texas, USA).
3. Results
Our initial electronic search identified 8973studies using our search strategy. Of those, 7336 studies were deemed ineligible after title screening. This narrowed the pool down to 285 potential studies that were then reviewed via the abstract and full text. The results of that screening excluded 245 studies because they did not meet inclusion criteria. Finally 40 articles (41 studies) were selected for inclusion in the meta-analysis (Fig. 1). From these 41 studies, the total number of birds included in meta-analysis was 9894 birds in which 2694 of them were infected. The most of the studies (17 studies) were performed in Europe.
Moreover, regarding detection method of bird schistosomes, morphological examination in 27 studies, both morphological and molecular in 9 and molecular method in 5 studies have been found (Table 1).
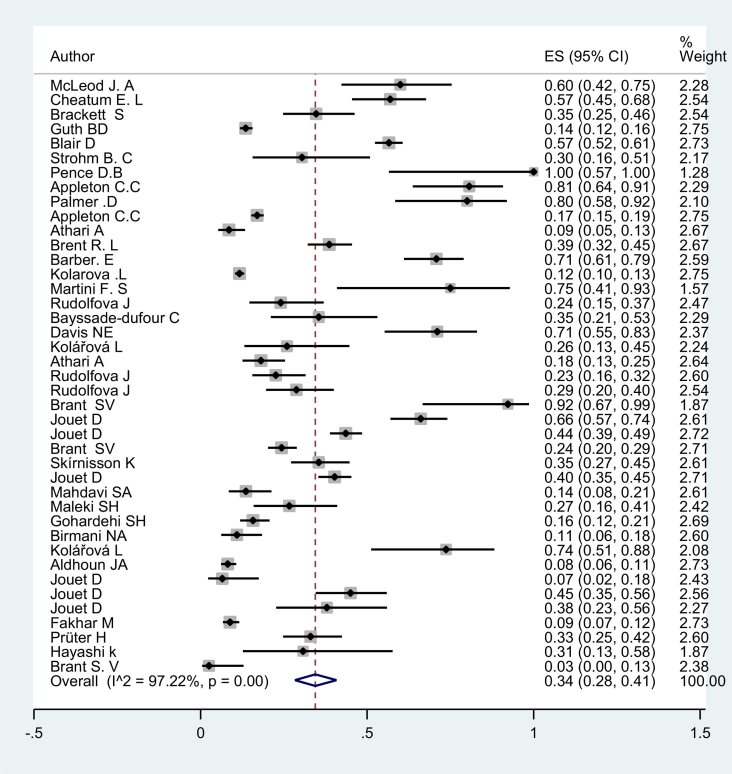
Based on a random effect meta-analysis (I2 = 97.20 % , P < 0.001) the pooled prevalence of bird schistosomes among examined ducks was estimated 34.0% (95%CI: 28–41%) which it was significantly difference from ES = 0 (z = 16.80, P < .001) (Fig. 2).
Fig. 2.
Forest plot of prevalence of bird schistosomes. The middle-point in each line indicates the prevalence and the length of each line indicates the 95% confidence interval of each study. Diamonds indicate the 95% confidence interval for pooled prevalence.
The results of subgroup analysis showed that bird schistosomes in Americas (43%) and Europe (34%) were higher than those in other regions of the world. Prevalence of bird schistosomes by morphological examination was 36.0% (95%CI: 28.0–44.0%), molecular and morphological 32.0% (95%CI: 19.0–46.0%) and molecular was 30.0% (95%CI: 11.0–53.0%).
Allobilharzia visceralis (50%) and Trichobilharzia spp. (35%) had the higher prevalence than other schistosome species (Table 2).
Table 2.
Subgroup meta-analysis to compare prevalence of global bird schistosomes.
| Subgroup | n | Prevalence (%) (95%CI) |
I-square (%) |
p | |
|---|---|---|---|---|---|
| Continent | Americas | 11 | 43.0(29.0–56.0) | 96.2 | <0.001 |
| Oceania | 3 | 18.0(10.0–17.0) | 81.3 | ||
| Asia | 8 | 13.0(10.0–18.0) | 73.1 | ||
| Europe | 17 | 38.0(27.0–50.0) | 87.3 | ||
| Africa | 2 | 14.0(13.0–16.0) | 70.8 | ||
| Method | Morphological | 27 | 36.0 (28.0–44.0) | 97.6 | 0.79 |
| Morphological and molecular | 9 | 32.0 (19.0–46.0) | 95.4 | ||
| Molecular | 5 | 30.0 (11.0–53.0) | 90.2 | ||
| aParasite species | Bird schistosomes | 6 | 31.0 (18.0–46.0) | 96.50 | 0.58 |
| Trichobilharzia regenti | 6 | 21.0 (6.0–37.0) | 96.7 | ||
| Allobilharzia visceralis | 4 | 50.0(0.30–0.97) | 95.3 | ||
| Trichobilharzia sp | 12 | 32.0(0.21–0.36) | 97.9 |
For some subgroups there was only one study.
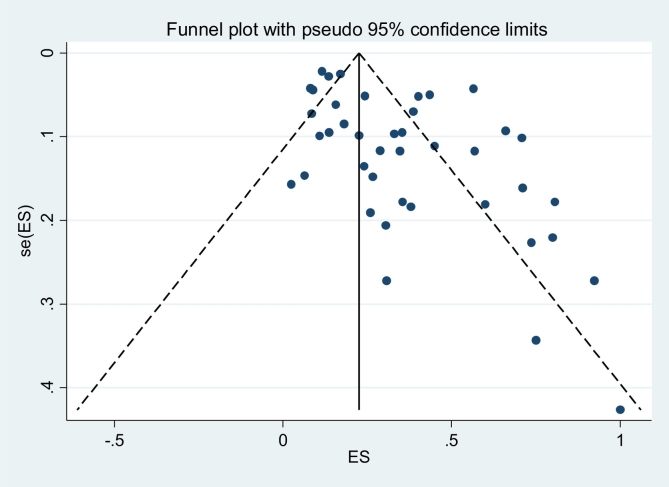
Both Funnel plot and the results of Egger's test (t = 2.57, P = .001) showed that there is an evidence of substantial publication bias (Figure 3).
Fig. 3.
Funnel plot for global prevalence of bird schistosomes.
After applying fill-and-trim method, the pooled estimate of prevalence found 34.0% (95%CI: 28–41%) which there is no difference from the pooled estimate in Fig. 2.
4. Discussion
HCD is a skin condition that affects people in water bodies all over the world for both recreation and occupation. HCD can temporarily disable the inflicted person by secondary infections as a result of the constant scratching. HCD reduces the recreational use of water bodies and can lead to economic losses for those areas [57, 58]. Clearly, the avian schistosomes are responsible for the outbreaks of reported HCD around different parts of the world such as Americas, Western Europe, Asia (Kolárová, 2007; Horák et al., 2015; Marcogliese, 2001). We have conducted a comprehensive systematic review of studies that examined the prevalence of schistosomes in bird hosts worldwide. Our findings show that the total sum prevalence of avian schistosomes worldwide is 34.0% (95%CI, 28%–41%). This global rate is high, and likely higher if more countries had a history of bird examinations for parasites. Thus, it is not a local problem, but a global one with high prevalence and thus likely neglected.
Prevalence of bird schistosomes in different countries of the world is most likely related to several issues such as climate changes and land alterations, changes in the behavioral and physiological patterns and species compositions of avian hosts, changes in phenology of aquatic migratory birds to sedentary. Climate changes affect behavior of migratory birds having a key role in spread of avian schistosomes, as well, lead to variation in seasonal / temperature dependent activities in the snail and schistosomes. These changes affect the frequency of transmission of parasites and severity of infection (Skírnisson et al., 2009; Marcogliese, 2001; Cotton, 2003; Suter, 1994).
The highest and the lowest prevalence of bird schistosomes were recorded in Americas, 41% and Asia, 13%. High prevalence of birds schistosomes in Americas may attribute to the fact that high local concentration of and the close relationship between birds and snails may facilitate the transmission of parasites. The occurrence of this parasite may be influenced by the seasonal migration of their bird hosts and the susceptibility of local snail populations (Brant, 2007; Brant and Loker, 2009b). The fact that most studies have been carried out in Canada, USA, and Western Europe, may be another reason may be another reason for the high prevalence of avian schistosomes in Americas. The northern latitudes also have the highest summer concentration of wild migratory Anatidae, so it is expected that HCD will be high there.
In addition, low prevalence rate in Asia despite the large numbers and diversity of migratory anatid birds, especially in Iran may be mostly due to a low priority among researchers. Due to the wildlife conservation by the environmental organization, access to study the parasites of these birds is very difficult and unlike other countries such as USA and Canada, there is not a widespread hunting season to provide birds to examine. Likewise, the natural habitat of these migratory birds is limited, mostly located in Mazandaran Province, northern Iran.
Moreover, in Europe, France and Iceland report a high prevalence of bird schistosomes in areas with suitable natural conditions for bird schistosomes (populations of suitable snails and bird hosts). In France, there are reported cases of HCD in various freshwater areas (de Gentile et al., 1996; Gay et al., 1999; Marszewska et al., 2016; Caumes et al., 2003) and researchers there are concerned with the distribution and diversity of bird schistosomes due to the position of this country along the migratory flyways of the bird hosts. Bird schistosomes in relationship with the increasing populations of protected water birds could clarify the increased hazard of HCD in these recreational regions (Jouet et al., 2009).
A review of studies shows that different genera of bird schistosomes play a role in the outbreaks of HCD. Kolářová et al. (2013) referred to the known causes of HCD around the world such as Trichobilharzia, Gigantobilharzia and Austrobilharzia. Trichobilharzia is the largest genus of Schistosomatidae family that covers >40 species of bird schistosomes (Horák et al., 2002). Usually, bird schistosomes were reported from geese, ducks and swans (Kolářová et al., 2013; Fain, 1955; Fain, 1956; ITO, 1960a).
Iran is located on the migration airways of water birds. Accordingly, Iran's wetlands are a temporary or permanent refuge for many migratory birds and due to specific climatic conditions and abundant food resources, Mazandaran province in northern Iran is considered a unique region for wintering of these birds. This species has been isolated from Anas clypeata and Anas platyrhynchos in northern Iran. In a study conducted in the Mazandaran Province, Fakhar et al. (2016) reported that isolated eggs from the nasals of A. clypeata and Anas platyrhynchos grouped in a sister clade to the European Trichobilharzia regenti.
Pulmonate snails such as those in the families Planorbidae, Physidae or Lymnaeidae are commonly used as intermediate hosts. It is interesting that host of avian and mammalian schistosomes causing HCD are Indoplanorbis and Biomphalaria in the Planorbidae family and Stagnicola in the Lymnaeidae family (Fain, 1955; Fain, 1956; ITO, 1960a; ITO, 1960b).
Regulations on the collection of bird hosts had led many to search for bird schistosomes in their feces. However, this method will detect visceral schistosomes (although if only one species is found, it cannot be ruled out that there is a second species), but not the presence of nasal schistosomes where eggs are discharged in the mucosa or hatch when the bird has its bill in the water to feed. However, birds can be surveyed throughout the year and important outcomes can be achieved by necropsy of the birds in the hunting season. It is suggested that mostly the blood system and the surrounding tissue of the preferred organs should be examined, since visceral schistosomes and their eggs are frequently found in the mesentery veins, intestinal wall, liver, kidney, heart, and lung. Nasal worms can be recovered from the nasal tissues and different parts of the CNS (Kolářová et al., 2010). It is difficult to identify cercariae to species since diverse species or genera can be morphologically similar and thus difficult to determine under the microscope (Joue et al., 2015). Future studies on the morphology of cercariae in addition to genetic work would aid in identifying these worms in the field or lab, at least to a species group. In addition to morphological information, other supporting data, for example, host species, location of the worms and their eggs, can help to determine the species (Kolářová et al., 2010). The researchers confirmed that combination of molecular methods with morphological examination of flukes facilitates taxonomical determination, life-cycle description and their geographic distribution (Rudolfova et al., 2002). Also, molecular methods may be used to identify precisely and discover phylogenetic relationships as well as make connections between adults and larvae (Brant and Loker, 2009b; Joue et al., 2015; Aldhoun et al., 2009).
Evidence shows that the emergence of HCD caused by bird schistosomes results from dispersal of schistosomes to new areas and an increased access of the snail hosts. Long term research of hosts and schistosomes in established and new endemic regions are essential to comprehend the host range and patterns of utilization of these hosts by schistosomes over time and space. Considering the infection of birds and outbreaks of HCD, it is essential for the environmental and agricultural organization and authorities of health, to collaborate in order to control this disease. Also, hygiene education should be given to those who are at high risk of HCD because of their occupation, such as rice farmers. Although these schistosomes do not establish themselves in mammals, therefore, do not cause a transmissible infection, we believe it is serious not to underestimate mammalian infections by bird schistosomes. These parasites, with their unrecognized life cycles and adaptations to different vertebrates, display an unseen region of parasitology.
In conclusion, to the best of our understanding, this is the first systematic review and meta-analysis that provides a comprehensive view of the global status of avian schistosomes in their final hosts. As a whole according to the presented data it seems that avian schistosomes are the most neglected of the neglected zoonotic parasitic worm among aquatic birds in the world. So, spreading of avian schistosomes to new areas, local abundance of the snail hosts and the use of water reservoirs for recreational and agricultural purposes, may contribute to a higher number of outbreaks of cercarial dermatitis in the world. Thus, it is suggested that the distribution and abundance of this illness should be considered in detail and appropriate trending must be performed to evaluate the outbreaks of cercarial dermatitis globally.
Acknowledgments
Acknowledgements
The authors would like to thank of financially supported by Vice Chancellors for Research and Technology of Mazandaran University of Medical Sciences (grant number: 1692).
Declaration of competing interest
Authors declare that have no competing interests.
References
- Aldhoun J., Kolářová L., Horák P., Skírnisson K. Bird schistosome diversity in Iceland: molecular evidence. J. Helminthol. 2009;83:173–180. doi: 10.1017/S0022149X09289371. [DOI] [PubMed] [Google Scholar]
- Aldhoun J.A., Horne E.C. Schistosomes in South African penguins. Parasitol. Res. 2015;114:237–246. doi: 10.1007/s00436-014-4185-1. [DOI] [PubMed] [Google Scholar]
- Appleton C. Studies on Austrobilharzia terrigalensis (Trematoda: Schistosomatidae) in the Swan Estuary, Western Australia: frequency of infection in the intermediate host population. Int. J. Parasitol. 1983;13:51–60. doi: 10.1016/s0020-7519(83)80065-4. [DOI] [PubMed] [Google Scholar]
- Appleton C. Occurrence of avian Schistosomatidae (Trematoda) in South African birds as determined by a faecal survey. Afr. Zool. 1986;21:60–67. [Google Scholar]
- Athari A., Sahba G., Amini H., Jafarian S. 1990. Investigation of Cercaraial Dermatitis in Iran. Proceeding of 7th International Congress of Parasitology. [Google Scholar]
- Barber K.E., Caira J. Investigation of the life cycle and adult morphology of the avian blood fluke Austrobilharzia variglandis (Trematoda: Schistosomatidae) from Connecticut. J. Parasitol. 1995;81:584–592. [PubMed] [Google Scholar]
- Bayssade-Dufour C., Jouet D., Rudolfova J., Horák P., Ferté H. Seasonal morphological variations in bird schistosomes. Parasite. 2006;13:205–214. doi: 10.1051/parasite/2006133205. [DOI] [PubMed] [Google Scholar]
- Birmani N., Dharejo A., Khan M., Shaikh A. New record of Dendritobilharzia pulverulenta (Trematoda: Schistosomatidae) from Pakistan. J Anim Plant Sci. 2013;23:1215–1218. [Google Scholar]
- Blair D., Ottesen P. Nasal schistosomiasis in Australian anatids. J. Parasitol. 1979;65:982–984. [Google Scholar]
- Blankespoor B.C.S.H.D., Meier P.G. Natural infections of the dermatitis-producing Schistosome Gigantobilharzia huronemis Najim, 1950 in passerines in southeastern Michigan1. Proc. Helminthol. Soc. Wash. 1981;48:80–82. [Google Scholar]
- Brackett S. Five new species of avian schistosomes from Wisconsin and Michigan with the life cycle of Gigantobilharzia gyrauli (Brackett, 1940) J. Parasitol. 1942;28:25–42. [Google Scholar]
- Brant S.V. The occurrence of the avian schistosome Allobilharzia visceralis Kolárová, Rudolfová, Hampl et Skírnisson, 2006 (Schistosomatidae) in the tundra swan, Cygnus columbianus (Anatidae), from North America. Folia Parasitol. 2007;54:99–104. doi: 10.14411/fp.2007.013. [DOI] [PubMed] [Google Scholar]
- Brant S.V., Loker E.S. Schistosomes in the Southwest United States and their potential for causing cercarial dermatitis or ‘swimmer's itch. J. Helminthol. 2009;83:191–198. doi: 10.1017/S0022149X09308020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant S.V., Loker E.S. Molecular systematics of the avian schistosome genus Trichobilharzia (Trematoda: Schistosomatidae) in North America. J. Parasitol. 2009;95:941–963. doi: 10.1645/GE-1870.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant S.V., Morgan J.A., Mkoji G.M., Snyder S.D., Rajapakse R.P.V.J., Loker E.S. An approach to revealing blood fluke life cycles, taxonomy, and diversity: provision of key reference data including DNA sequence from single life cycle stages. J. Parasitol. 2006;92:77–88. doi: 10.1645/GE-3515.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant S.V., Loker E.S., Casalins L., Flores V. Phylogenetic placement of a schistosome from an unusual marine snail host, the false limpet (Siphonaria lessoni) and gulls (Larus dominicanus) from Argentina with a brief review of marine schistosomes from snails. J. Parasitol. 2017;103:75–83. doi: 10.1645/16-43. [DOI] [PubMed] [Google Scholar]
- Caumes E., Felder-Moinet S., Couzigou C., Darras-Joly C., Latour P., Léger N. Failure of an ointment based on IR3535 (ethyl butylacetylaminopropionate) to prevent an outbreak of cercarial dermatitis during swimming races across Lake Annecy, France. Ann. Trop. Med. Parasitol. 2003;97:157–163. doi: 10.1179/000349803235001633. [DOI] [PubMed] [Google Scholar]
- Cheatum E. Dendritobilharzia anatinarum n. sp., a blood fluke from the mallard. J. Parasitol. 1941;27:165–170. [Google Scholar]
- Cotton P.A. Avian migration phenology and global climate change. Proc Natl Acad Sci India Sect. 2003;100:12219–12222. doi: 10.1073/pnas.1930548100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. A survey of waterfowl for echinostomes and schistosomes from Lake Wanaka and the Waitaki River watershed. New Zealand, J Helminthol. 2006;80:33–40. doi: 10.1079/joh2005310. [DOI] [PubMed] [Google Scholar]
- Fain A. Recherches sur les schistosomes d'oiseaux au Ruanda-Urundi (Congo Belge) Rev. Zool. Bot. Afr. 1955;51:373–387. [Google Scholar]
- Fain A. Les schistosomes d'oiseaux du genre Trichobilharzia Skrjabin et Zakharov, 1920, 1920 au Ruanda Urundi. Rev. Zool. Bot. Afr. 1956;54:147–178. [Google Scholar]
- Fakhar M., Ghobaditara M., Brant S.V., Karamian M., Gohardehi S., Bastani R. Phylogenetic analysis of nasal avian schistosomes (Trichobilharzia) from aquatic birds in Mazandaran Province. northern Iran, Parasitol. Int. 2016;65:151–158. doi: 10.1016/j.parint.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Farahnak A., Essalat M. A study on cercarial dermatitis in Khuzestan province. south western Iran, BMC Public Health. 2003;3:35. doi: 10.1186/1471-2458-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay P., Bayssade-Dufour C., Grenouillet F., Bourezane Y., Dubois J. Etude experimentale de dermatites cercariennes provoquees par Trichobilharzia en France. Med. Mal. Infect. 1999;29:629–637. [Google Scholar]
- de Gentile L., Picot H., Bourdeau P., Bardet R., Kerjan A., Piriou M. Cercarial dermatitis in Europe: a new public health problem? Health Organ Bull. 1996;74:159–163. [PMC free article] [PubMed] [Google Scholar]
- Gohardehi S., Fakhar M., Madjidae M. Avian schistosomes and human cercarial dermatitis in a wildlife refuge in Mazandaran province. northern Iran, Zoonoses Public Health. 2013;60:442–447. doi: 10.1111/zph.12020. [DOI] [PubMed] [Google Scholar]
- Guth B.D., Blankespoor H.D., Reimink R.L., Johnson W.C. Prevalence of dermatitis-producing Schistosomes in natural bird populations of lower Michigan1. Proc. Helminthol. Soc. Wash. 1979;46:58–63. [Google Scholar]
- Hayashi K. First detection of Allobilharzia visceralis (Schistosomatidae, Trematoda) from Cygnus cygnus in Japan. Parasitol. Int. 2017;66:925–929. doi: 10.1016/j.parint.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Horák P., Dvořák J., Kolářová L., Trefil L. Trichobilharzia regenti, a pathogen of the avian and mammalian central nervous systems. Parasitology. 1999;119:577–581. doi: 10.1017/s0031182099005132. [DOI] [PubMed] [Google Scholar]
- Horák P., Kolářová L., Adema C. 2002. Biology of the Schistosome Genus Trichobilharzia. [DOI] [PubMed] [Google Scholar]
- Horák P., Mikeš L., Lichtenbergová L., Skála V., Soldánová M., Brant S.V. Avian schistosomes and outbreaks of cercarial dermatitis. Clin. Microbiol. Rev. 2015;28:165–190. doi: 10.1128/CMR.00043-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörweg C., Sattmann H., Auer H. Cercarial dermatitis in Austria: questionnaires as useful tools to estimate risk factors? Wien. Klin. Wochenschr. 2006;118:77–80. doi: 10.1007/s00508-006-0674-2. [DOI] [PubMed] [Google Scholar]
- Hrádková K., Horák P. Neurotropic behaviour of Trichobilharzia regenti in ducks and mice. J. Helminthol. 1999;76:137–141. doi: 10.1079/JOH2002113. [DOI] [PubMed] [Google Scholar]
- ITO J. Contributions to the morphology of cercariae obtained from a snail host, Semisulcospira libertina in Japan. Jpn. J. Med. Sci. Biol. 1960;13:59–72. doi: 10.7883/yoken1952.13.59. [DOI] [PubMed] [Google Scholar]
- ITO J. Studies on the morphology and life cycle of Pseudobilharziella corvi Yamaguti, 1941 (Trematoda: Schistosomatidae) Jpn. J. Med. Sci. Biol. 1960;13:53–58. doi: 10.7883/yoken1952.13.53. [DOI] [PubMed] [Google Scholar]
- Joue D., Kolářová L., Patrelle C., Ferté H., Skírnisson K. Trichobilharzia anseri n. sp. (Schistosomatidae: Digenea), a new visceral species of avian schistosomes isolated from greylag goose (Anser anser L.) in Iceland and France. Infect. Genet. Evol. 2015;34:298–306. doi: 10.1016/j.meegid.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Jouet D., Ferté H., Depaquit J., Rudolfová J., Latour P., Zanella D. Trichobilharzia spp. in natural conditions in Annecy Lake, France. Parasitol. Res. 2008;103:51–58. doi: 10.1007/s00436-008-0926-3. [DOI] [PubMed] [Google Scholar]
- Jouet D., Ferté H., Hologne C., Kaltenbach M., Depaquit J. Avian schistosomes in French aquatic birds: a molecular approach. J. Helminthol. 2009;83:181–189. doi: 10.1017/S0022149X09311712. [DOI] [PubMed] [Google Scholar]
- Jouet D., Skírnisson K., Kolářová L., Ferté H. Final hosts and variability of Trichobilharzia regenti under natural conditions. Parasitol. Res. 2010;107:923–930. doi: 10.1007/s00436-010-1953-4. [DOI] [PubMed] [Google Scholar]
- Kolárová L. Schistosomes causing cercarial dermatitis: a mini-review of current trends in systematics and of host specificity and pathogenicity. Folia Parasitol. 2007;54:81–87. [PubMed] [Google Scholar]
- Kolářová L. Trichobilharzia mergi sp. nov.(Trematoda: Digenea: Schistosomatidae), a visceral schistosome of Mergus serrator (L.)(Aves: Anatidae) Parasitol. Int. 2013;62:300–308. doi: 10.1016/j.parint.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Kolarova L., Horák P., Sitko J. Cercarial dermatitis in focus: schistosomes in the Czech Republic. Helminthologia. 1997;34:127–139. [Google Scholar]
- Kolářová L., Rudolfová J., Hampl V., Skírnisson K. Allobilharzia visceralis gen. nov., sp. Nov.(Schistosomatidae-Trematoda) from Cygnus cygnus (L.) (Anatidae), Parasitol. Int. 2006;55:179–186. doi: 10.1016/j.parint.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Kolářová L., Horák P., Skírnisson K. Methodical approaches in the identification of areas with a potential risk of infection by bird schistosomes causing cercarial dermatitis. J. Helminthol. 2010;84:327–335. doi: 10.1017/S0022149X09990721. [DOI] [PubMed] [Google Scholar]
- Kolářová L., Horák P., Skírnisson K., Marečková H., Doenhoff M. Cercarial dermatitis. a neglected allergic disease, Clin Rev Allergy Immunol. 2013;45:63–74. doi: 10.1007/s12016-012-8334-y. [DOI] [PubMed] [Google Scholar]
- Loken B.R., Spencer C.N., Granath W.O. Prevalence and transmission of cercariae causing schistosome dermatitis in Flathead Lake. Montana, J Parasitol. 1995;81:646–649. [PubMed] [Google Scholar]
- Mahdavi S., Farahnak A., Mobedi I., Rad M.M., Azadeh H. Survey of migratory birds (Anatidae: Anas platyrhynchos) for schistosome parasites from Mazandaran Province. Northern Iran, Iran J Parasitol. 2013;8:333. [PMC free article] [PubMed] [Google Scholar]
- Maleki S., Athari A., Haghighi A., Taghipour N., Gohardehi S., Tabaei S.S. Species identification of birds nasal Trichobilharzia in sari. North of Iran, Iran J Parasitol. 2012;7:82. [PMC free article] [PubMed] [Google Scholar]
- Marcogliese D.J. Implications of climate change for parasitism of animals in the aquatic environment. Can. J. Zool. 2001;79:1331–1352. [Google Scholar]
- Marszewska A., Cichy A., Heese T., Żbikowska E. The real threat of swimmers' itch in anthropogenic recreational water body of the Polish Lowland. Parasitol. Res. 2016;115:3049–3056. doi: 10.1007/s00436-016-5060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszewska A., Strzała T., Cichy A., Dąbrowska G.B., Żbikowska E. Agents of swimmer's itch—dangerous minority in the Digenea invasion of Lymnaeidae in water bodies and the first report of Trichobilharzia regenti in Poland. Parasitol. Res. 2018;117:3695–3704. doi: 10.1007/s00436-018-6068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod J. Two new schistosomid trematodes from water-birds. J. Parasitol. 1937;23:456–466. [Google Scholar]
- Palmer D., Ossent P. Nasal schistosomiasis in mute swans in Switzerland. Rev. Suisse Zool. 1984;91:709–715. [Google Scholar]
- Pence D.B., Rhodes M.J. Trichobilharzia physellae (Digenea: Schistosomatidae) from endemic waterfowl on the high plains of Texas. J. Wildl. Dis. 1982;18:69–74. doi: 10.7589/0090-3558-18.1.69. [DOI] [PubMed] [Google Scholar]
- Prüter H., Sitko J., Krone O. Having bird schistosomes in mind—the first detection of Bilharziella polonica (Kowalewski 1895) in the bird neural system. Parasitol. Res. 2017;116:865–870. doi: 10.1007/s00436-016-5359-9. [DOI] [PubMed] [Google Scholar]
- Rostami-Jalilian M. Determination of definitive and intermediate hosts of cercarial dermatitis-producing agents in northern Iran. Arch Iran Med. 2006;9:11–15. [PubMed] [Google Scholar]
- Rudolfova J., Sitko J., Horak P. Nasal schistosomes of wildfowl in the Czech Republic. Parasitol. Res. 2002;88:1093–1095. doi: 10.1007/s00436-002-0634-3. [DOI] [PubMed] [Google Scholar]
- Rudolfová J., Littlewood D., Sitko J., Horák P. Bird schistosomes of wildfowl in the Czech Republic and Poland. Folia Parasitol. 2007;54:88–93. [PubMed] [Google Scholar]
- Simon-Martin F., Simon-Vicente F. The life cycle of Trichobilharzia salmanticensis n. sp.(Digenea: Schistosomatidae), related to cases of human dermatitis. Res. Rev. Parasitol. 1999;59:13–18. [Google Scholar]
- Skírnisson K., Kolářová L. Diversity of bird schistosomes in anseriform birds in Iceland based on egg measurements and egg morphology. Parasitol. Res. 2008;103:43–50. doi: 10.1007/s00436-008-0925-4. [DOI] [PubMed] [Google Scholar]
- Skírnisson K., Aldhoun J., Kolářová L. A review on swimmer's itch and the occurrence of bird schistosomes in Iceland. J. Helminthol. 2009;83:165–171. doi: 10.1017/S0022149X09336408. [DOI] [PubMed] [Google Scholar]
- Suter W. Overwintering waterfowl on Swiss lakes: how are abundance and species richness influenced by trophic status and lake morphology? Hydrobiologia. 1994;279:1–14. [Google Scholar]
- Valdovinos C., Balboa C. Cercarial dermatitis and Lake Eutrophication in south-central Chile. Epidemiol. Infect. 2008;136:391–394. doi: 10.1017/S0950268807008734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Żbikowska E. Is there a potential danger of “swimmer's itch” in Poland? Parasitol. Res. 2003;89:59–62. doi: 10.1007/s00436-002-0684-6. [DOI] [PubMed] [Google Scholar]