Abstract
Purpose
The efficacy of valproic acid (VPA) varies widely in clinical treatment of epileptic patients. Our study is aimed at exploring a potential association between polymorphisms of SCN1A, SCN2A, and UGT2B7 genetic factors and VPA responses.
Methods
In this observational study, a total of 114 epileptic patients only treated with VPA for at least 1 year were included to explore the genetic polymorphisms of drug responses (mean follow-up time: 3.68 ± 1.78 years). Thirty-one single-nucleotide polymorphisms (SNPs) in three candidate genes that related with drug-metabolizing enzymes and receptors were genotyped.
Results
Of the 31 SNPs, eight were significantly associated with VPA responses, including rs1381105, rs2162600, rs10197716, rs2119068, rs2119067, rs353116, rs353112 and rs6740895. The interaction between rs10197716 and rs2119068 was the most significantly correlated with VPA responses compared with other combinations (the highest VPA-responsive rate 0.92 versus the lowest VPA-responsive rate 0.33, p = 0.007).
Conclusion
The study indicated that eight SNPs and SNP-SNP interaction may be associated with VPA responses in Chinese Han epileptic patients. The SNPs were rs1381105 (SCN1A), rs2162600 (SCN1A), rs10197716 (SCN2A), rs2119068 (SCN2A), rs2119067 (SCN2A), rs353116 (SCN2A), rs353112 (SCN2A) and rs6740895 (SCN2A), respectively. The interaction between the three pairs of rs10197716-rs2119068, rs10197716-rs11889342 and rs7598931-rs12233719 was the most significant for VPA. This implied that these SNPs may play an important role in the pharmacogenomics mechanism of valproic acid.
1. Introduction
Epilepsy is a chronic neurological disease of brain dysfunction that has received widespread attention in society [1, 2]. Recent studies have shown that a total of 45.9 million people suffer from epilepsy in the world, which is one of the important causes of disability and death in the population [3].
Traditional antiepileptic drugs include valproic acid, carbamazepine and phenytoin. Valproic acid is a commonly used broad-spectrum antiepileptic drug, which is effective for various types of seizures, including tonic-clonic, myoclonic, and absence seizures [4]; carbamazepine is also commonly used as first-line antiepileptic drugs for focal onset seizures but may not be effective for absence or myoclonic seizures; phenytoin is mainly used to treat partial seizures, but no longer considered a first-line treatment due to concerns over adverse events [5]. After taking the above drugs, 20%-40% of patients with epilepsy still cannot effectively control seizures [6]. The mechanism of drug resistance has not been fully explained, but genetic factors have been recognized as an important cause of drug efficacy difference in the treatment of epilepsy [7, 8]. Drug-metabolizing enzymes, transporter, and receptor variants may be due to genetic polymorphisms that affect clinical outcomes in different AED therapy, and these clinical implications of gene polymorphisms associated with antiepileptic drugs have been reported [9]. In terms of drug metabolism enzyme genes, the CYP2C9 polymorphisms were associated with the dosage of phenytoin, and the widely studied CYP2C19 showed that different haplotypes had different toxicities, when epileptic patients took valproic acid [10, 11]. Polymorphisms of UGT2B7 affected the metabolism of AEDs such as VPA, CBZ and lamotrigine (LTG), and UGT2B7 was associated with oxcarbazepine (OXC) maintenance doses, which were useful for the personalization of OXC therapy [12, 13]. Some studies on ABCB1, a drug transporter gene, indicated that genetic polymorphisms were associated with resistance of AEDs in patients with epilepsy [14, 15]. SCN1A and SCN2A genes encoding the sodium channels were related to the efficacy, dose, and toxicity of multiple antiepileptic drugs [16]. These two genes were reported to be associated with efficacy of mono- or combination antiepileptic therapy, but the results were contradicted in different studies. For example, SCN1A (rs2298771) was correlated with responses to AEDs in epileptic patients [17], while some studies found that the SNP had no association with drug responses, similar in SCN2A [18]. As a key metabolic enzyme gene, UGT2B7 plays a crucial role in the metabolism of valproic acid; SCN1A and SCN2A are key genes encoding sodium channels. These gene mutations affect drug responsiveness in epilepsy. At present, the research results on the relationship between the above three genes and various antiepileptic drugs are not consistent. And current researches could not include all SNPs on these three genes. Therefore, based on the study of Han population taking valproic acid, new SNPs located in the SCN1A, SCN2A, and UGT2B7 were studied.
In summary, although the mechanism of AED responses has been still unclear, many studies have shown that the individual genetic variations were related to the responses of antiepileptic drugs. It is known that VPA is a broad-spectrum antiepileptic drug that is commonly used to control seizures [19]. And SCN1A, SCN2A, and UGT2B7 were reported that they are related to the efficacy of VPA by encoding sodium voltage-gated channels or influencing uridine 5′-diphospho-glucuronosyltransferases (UGTs) [20, 21]. In order to explore the associations between common SCN1A, SCN2A, and UGT2B7 gene polymorphisms, alone or in combination, with VPA responsiveness in epilepsy among 114 Chinese Han patients, we carried out a pharmacogenomics association analysis to identify whether genetic polymorphisms were correlated with VPA responses using a total of 31 SNPs in the three candidate genes.
2. Methods
2.1. Participants
Participants of this study were derived from the demonstration platform of the epilepsy follow-up cohort of Linyi People's Hospital that constructed a long-term follow-up cohort. This platform was based on the clinical diagnosis and treatment records of patients with epilepsy. Baseline information, medical information, and frequency of epileptic seizures after the patient entered the cohort were recorded. We screened participants who had been followed up for more than one year and had been taking valproic acid. Finally, the study included 114 epileptic patients who received treatment with valproic acid from Linyi City People's Hospital. The research was approved by the ethics committee of School of Public Health, Shandong University in China. All participants or their legal guardian signed the written informed consent. All epileptic patients were diagnosed by clinicians according to the criteria of International League Against Epilepsy in combination with clinical manifestations and electroencephalograms [22]. Exclusion criteria included the presence of mental illness, such as autism, schizophrenia and affective disorder, presence of progressive or degenerative neurological disease, presence of any malignant tumor disease or severe organic heart disease or liver and kidney disease, history of alcohol or drug abuse and incomplete record of seizure frequency. For each participant, basic information was collected: age at first visit, gender, epilepsy etiology, seizure frequency and VPA dosage (mg). Patients were defined as VPA-responsive epilepsy if they have not experienced any type of seizure for at least 12 months after treatment with VPA. The remaining patients were included as VPA-resistant epilepsy [23].
2.2. Genotyping
Peripheral venous blood samples (5 ml) were collected from each subject in Eppendorf tubes containing ethylenediaminetetraacetic acid (EDTA). AssayDesigner3.1 software (Sequenom, Inc., CA, USA) was used for primer design and multiplex reactions according to actual conditions. The studied genetic polymorphisms were genotyped by using Sequenom MassARRAY System and MALDI-TOF mass spectrometry. Based on previous studies, we finally selected SCN1A, SCN2A and UGT2B7 [24–27]. The following criteria were used to select SNPs from the above genes [28]: selected SNPs has been reported to be related to nervous system diseases in previous studies; partially representative SNPs have been selected for too many SNP locus and high linkage disequilibrium (LD) r2 > 0.8 in block; for some genes with limited coding SNPs, some noncoding SNPs were also included according to LD.
2.3. Statistical Analysis
Minor allele frequency (MAF), Hardy–Weinberg equilibrium (HWE) test and linkage disequilibrium (LD) were conducted using Haploview 4.2 software (https://www.broadinstitute.org/haploview/haploview). We choose the default “confidence intervals (Gabriel et al.)” to define blocks [29]. And R software (version 3.5.1 for Windows, https://www.r-project.org/) was used to perform univariate analysis, logistic regression analysis and SNP-SNP interaction analysis. Basic characteristics including continuous and categorical variables were described using Student's t-test or chi-square test (Fisher's exact test). The association analysis between genotypes or alleles and drug response was tested using binary logistic regression with adjusting other covariates, including age, gender and VPA dosage. The criteria to select candidate SNPs were given as follows: MAF > 0.05, HWE > 0.05, and detection rate of each SNPs > 95%. p value < 0.05 is considered statistically significant.
3. Result
The sample population consisted of 114 patients with epilepsy: 90 VPA responders (mean follow-up time: 3.76 ± 1.77 years) and 24 VPA resisters (mean follow-up time: 3.40 ± 1.84 years). The characteristics including age, gender, VPA dosage and epilepsy etiology were not significant between the VPA-responsive and VPA-resistant groups. The basic characteristics of the study population are described in Table 1.
Table 1.
Basic characteristics of the epileptic patients.
| Characteristics | VPA responsive (n = 90) | VPA resistant (n = 24) | Total (n = 114) | p value |
|---|---|---|---|---|
| Age (years) | 29.17 ± 14.26 | 26.29 ± 13.16 | 28.56 ± 14.02 | 0.373 |
| Dose (mg) | 463.28 ± 288.00 | 569.58 ± 268.22 | 485.66 ± 286.12 | 0.103 |
| Gender | ||||
| Male | 65 (72.2) | 18 (75.0) | 83 (72.8) | 0.989 |
| Female | 25 (27.8) | 6 (25.0) | 31 (27.2) | |
| Etiology | ||||
| Idiopathic | 68 (75.6) | 13 (54.2) | 81 (71.1) | 0.122 |
| Symptomatic | 20 (22.2) | 10 (41.7) | 30 (26.3) | |
| Cryptogenic | 2 (2.2) | 1 (4.2) | 3 (2.6) |
Data were presented as mean ± standard deviation or n (%). VPA: valproic acid.
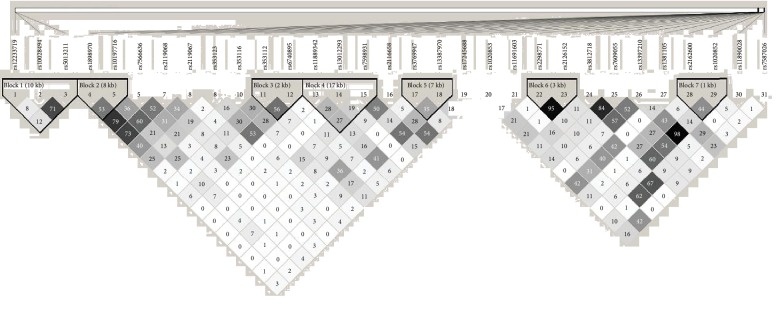
All studied SNPs conformed to HWE > 0.05 and MAF > 0.05. Linkage disequilibrium blocks are displayed in Figure 1. The figure showed that SNP-SNP LD information was located in SCN1A, SCN2A and UGT2B7 genes. According to confidence intervals algorithm, seven haplotype blocks were identified. The frequency of a haplotype block did not differ significantly among VPA-responsive and VPA-resistant group.
Figure 1.
Linkage disequilibrium (LD) of SCN1A, SCN2A, and UGT2B7 for 31 single-nucleotide polymorphisms (SNPs) in 114 patients. Numbers 1-3 were in UGT2B7; 4-19 were in SCN2A; 20-31 were in SCN1A. Haploview analysis of LD (r2) between pairwise comparisons of SNPs <500 kb apart is shown in the figure, with a darker square for larger r2. Seven haplotype blocks were identified using confidence intervals algorithm.
Among the studied SNPs, there were eight SNPs associated with VPA efficacy in the SCN1A, SCN2A, and UGT2B7, summarized in Table 2. The analysis of SCN1A showed that GT genotype of rs1381105 (0.36 (95% CI: 0.14-0.94)), TC genotype, “TC+CC” combination genotype and allele C of rs2162600 (0.34 (95% CI: 0.13-0.92), 0.36 (95% CI: 0.14-0.92), and 0.46 (95% CI: 0.22-1.02), respectively) were significantly associated with VPA resistance. The rest of the SNPs associated with VPA responses belonged to SCN2A, which were TC genotype of rs2119067 (0.37 (95% CI: 0.13-0.96)), CT, CC genotype, “CT+CC” combination genotype and allele C of rs353116 (2.81 (95% CI: 1.01-8.43), 5.83 (95% CI: 1.24-45.05), 2.34 (95% CI: 1.27-9.37) and 2.62 (95% CI: 1.27-5.80), respectively), GG genotype and allele G of rs10197716 (0.19 (95% CI: 0.04-0.78) and 0.46 (95% CI: 0.23-0.90), respectively), GG genotype, “CG+GG” combination genotype and allele G of rs2119068 (9.09 (95% CI: 1.51-176.64), 3.01 (95% CI: 1.15-8.03), and 2.48 (95% CI: 1.22-5.33), respectively), “GC+CC” combination genotype and allele C of rs353112 (95% CI: 3.16 (1.21-8.91) and 3.07 (95% CI: 1.39-7.60), respectively), CT genotype, “CT+CC” combination genotype and allele C of rs6740895 (5.80 (95% CI: 1.65-28.94), 7.02 (95% CI: 2.03-34.46) and 5.91 (95% CI: 1.96-25.84), respectively). Therefore, genetic variation might be considered a potential predict factor for drug resistance.
Table 2.
Genotypes/alleles frequencies of SCN1A (2A) and UGT2B7 in VPA responsive and VPA resistant groups (p < 0.05).
| Genes | SNPs | Genotypes/alleles | VPA responsive | VPA resistant | OR (95% CI) | p value |
|---|---|---|---|---|---|---|
| SCN1A | rs1381105 | GG | 57 (63.3) | 11 (45.8) | Reference | |
| GT | 27 (30.0) | 13 (54.2) | 0.36 (0.14-0.94) | 0.039 | ||
| TT | 6 (6.7) | 0 (0.0) | — | |||
| GT+TT | 33 (36.7) | 13 (54.2) | 0.49 (0.19-1.229) | 0.129 | ||
| G | 141 (78.3) | 35 (72.9) | Reference | |||
| T | 39 (21.7) | 13 (27.1) | 0.78 (0.38-1.69) | 0.516 | ||
|
| ||||||
| SCN1A | rs2162600 | TT | 65 (72.2) | 12 (50.0) | Reference | |
| TC | 22 (24.4) | 11 (45.8) | 0.34 (0.13-0.92) | 0.033 | ||
| CC | 3 (3.3) | 1 (4.2) | 0.46 (0.05-9.96) | 0.524 | ||
| TC+CC | 25 (27.8) | 12 (50.0) | 0.36 (0.14-0.92) | 0.033 | ||
| T | 152 (84.4) | 35 (72.9) | Reference | |||
| C | 28 (15.6) | 13 (27.1) | 0.46 (0.22-1.02) | 0.049 | ||
|
| ||||||
| SCN2A | rs10197716 | AA | 27 (30.0) | 3 (12.5) | Reference | |
| AG | 45 (50.0) | 12 (50.0) | 0.35 (0.07-1.31) | 0.148 | ||
| GG | 18 (20.0) | 9 (37.5) | 0.19 (0.04-0.78) | 0.030 | ||
| AG+GG | 63 (70.0) | 21 (87.5) | 2.50 (0.89-6.67) | 0.075 | ||
| A | 99 (55.5) | 18 (37.5) | Reference | |||
| G | 81 (45.5) | 30 (62.5) | 0.46 (0.23-0.90) | 0.024 | ||
|
| ||||||
| SCN2A | rs2119068 | CC | 24 (26.7) | 12 (52.2) | Reference | |
| CG | 49 (54.4) | 10 (43.5) | 2.37 (0.88-6.61) | 0.091 | ||
| GG | 17 (18.9) | 1 (4.3) | 9.09 (1.51-176.64) | 0.045 | ||
| CG+GG | 66 (73.3) | 11 (47.8) | 3.01 (1.15-8.03) | 0.025 | ||
| C | 97 (53.9) | 34 (73.9) | Reference | |||
| G | 83 (46.1) | 12 (26.1) | 2.48 (1.22-5.33) | 0.015 | ||
|
| ||||||
| SCN2A | rs2119067 | TT | 52 (57.8) | 10 (41.7) | Reference | |
| TC | 34 (37.8) | 14 (58.3) | 0.37 (0.13-0.96) | 0.045 | ||
| CC | 4 (4.4) | 0 (0.0) | — | |||
| TC+CC | 38 (42.2) | 14 (58.3) | 0.40 (0.15-1.05) | 0.067 | ||
| T | 138 (76.7) | 34 (70.8) | Reference | |||
| C | 42 (23.3) | 14 (29.2) | 0.62 (0.30-1.33) | 0.209 | ||
|
| ||||||
| SCN2A | rs353116 | TT | 30 (34.5) | 14 (58.3) | Reference | |
| CT | 41 (47.1) | 8 (33.3) | 2.81 (1.01-8.43) | 0.053 | ||
| CC | 16 (18.4) | 2 (8.3) | 5.83 (1.24-45.05) | 0.046 | ||
| CT+CC | 57 (65.5) | 10 (41.7) | 2.34 (1.27-9.37) | 0.017 | ||
| T | 101 (58.0) | 36 (75.0) | Reference | |||
| C | 73 (42.0) | 12 (25.0) | 2.62 (1.27-5.80) | 0.012 | ||
|
| ||||||
| SCN2A | rs353112 | GG | 38 (42.2) | 16 (66.7) | Reference | |
| GC | 40 (44.4) | 8 (33.3) | 2.40 (0.91-6.83) | 0.084 | ||
| CC | 12 (13.3) | 0 (0.0) | — | |||
| GC+CC | 52 (57.8) | 8 (33.3) | 3.16 (1.21-8.91) | 0.022 | ||
| G | 116 (64.4) | 40 (83.3) | Reference | |||
| C | 64 (35.6) | 8 (16.7) | 3.07 (1.39-7.60) | 0.009 | ||
|
| ||||||
| SCN2A | rs6740895 | TT | 53 (58.9) | 21 (87.5) | Reference | |
| CT | 30 (33.3) | 3 (12.5) | 5.80 (1.65-28.94) | 0.014 | ||
| CC | 7 (7.8) | 0 (0.0) | — | |||
| CT+CC | 37 (41.1) | 3 (12.5) | 7.02 (2.03-34.46) | 0.006 | ||
| T | 136 (75.6) | 45 (93.8) | Reference | |||
| C | 44 (24.4) | 3 (6.2) | 5.91 (1.96-25.84) | 0.005 | ||
OR: odds ratio; CI: confidence interval; SNPs: single-nucleotide polymorphisms; VPA: valproic acid. Due to no significant SNP in the recessive model, it is not listed in the table.
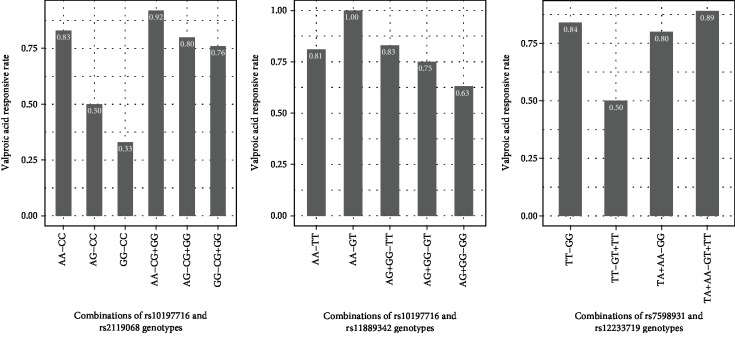
The significant association between SNP interactions and VPA responses is summarized in Table 3. A total of 19 pairs of SNP-SNP interactions were related to the drug efficacy based on “codominant (epistasis)” model. VPA-responsive rates with different genotype combinations of three pairs (p < 0.01) are descripted in Figure 2. They were rs10197716 (SCN2A)-rs2119068 (SCN2A), rs10197716 (SCN2A)-rs11889342 (SCN2A), and rs7598931 (SCN2A)-rs12233719 (UGT2B7), respectively. Since GG carriers were few in rs2119068, genotype CG and GG were merged into one group, namely, the CG+GG group. Similar processes were also conducted in rs10197716, rs7598931, and rs12233719 for the same reason. The VPA-responsive rate of GG-CC carriers was only 0.33, while the carriers of AA-CG+GG (combination of AA in rs10197716 and CG+GG in rs2119068) and AG-CG+GG (combination of AG and CG+GG) were higher than that of the GG-CC group (0.92 vs. 0.33 and 0.80 vs. 0.33, p < 0.05), which was about 2.5 times that of the GG-CC group in Figure 2(a). The AG+GG-GT (combination of AG+GG in rs10197716 and GT in rs11889342) group and AG+GG-GG (combination of AG+GG and GG) group, 0.75 and 0.63, respectively, were lower than that the AA-GT group (p < 0.05), which was nearly 1.00 with VPA-responsive rate in the combinations of rs10197716 and rs11889342 genotypes shown in Figure 2(b). The TT-GT+TT (combination of TT and GT+TT) group was significantly lower than the other three combinations (p < 0.05) from rs7598931 and rs12233719 showed in Figure 2(c). It is worth noting that the interaction of rs7598931 and rs12233719 (p < 0.001), which were in SCN2A and UGT2B7 respectively, were associated with the VPA responsiveness, but these two SNPs were not associated with VPA responses in the univariate logistic analysis. Responsive rates of other combinations (SNP-SNP p value > 0.01 and p < 0.05) need to be explored further.
Table 3.
SNP-SNP interaction analysis among 31 studied single-nucleotide polymorphisms.
| Chromosome | Gene | SNPs | Chromosome | Gene | SNPs | p value |
|---|---|---|---|---|---|---|
| 2:166122240 | SCN1A | rs7587026 | 2:165270773 | SCN2A | rs2119067 | 0.046 |
| 2:166035836 | SCN1A | rs11691603 | 2:165270773 | SCN2A | rs2119067 | 0.025 |
| 2:166069891 | SCN1A | rs2162600 | 2:165307150 | SCN2A | rs7598931 | 0.033 |
| 2:166035836 | SCN1A | rs11691603 | 2:165283510 | SCN2A | rs6740895 | 0.028 |
| 2:166122240 | SCN1A | rs7587026 | 2:165283510 | SCN2A | rs6740895 | 0.026 |
| 2:165310162 | SCN2A | rs2116658 | 2:165268464 | SCN2A | rs10197716 | 0.029 |
| 2:165290104 | SCN2A | rs11889342 | 2:165283510 | SCN2A | rs6740895 | 0.048 |
| 2:165337455 | SCN2A | rs13387970 | 2:165304831 | SCN2A | rs13012293 | 0.028 |
| 2:165268464 | SCN2A | rs10197716 | 2:165270664 | SCN2A | rs2119068 | 0.009 |
| 2:165268464 | SCN2A | rs10197716 | 2:165290104 | SCN2A | rs11889342 | 0.001 |
| 2:165290104 | SCN2A | rs11889342 | 2:165330368 | SCN2A | rs3769947 | 0.013 |
| 2:166053034 | SCN1A | rs3812718 | 4:69096731 | UGT2B7 | rs12233719 | 0.017 |
| 2:166065518 | SCN1A | rs13397210 | 4:69096731 | UGT2B7 | rs12233719 | 0.010 |
| 2:166071149 | SCN1A | rs1020852 | 4:69096731 | UGT2B7 | rs12233719 | 0.013 |
| 2:165310162 | SCN2A | rs2116658 | 4:69105219 | UGT2B7 | rs10028494 | 0.015 |
| 2:165337455 | SCN2A | rs13387970 | 4:69107059 | UGT2B7 | rs5013211 | 0.026 |
| 2:165307150 | SCN2A | rs7598931 | 4:69107059 | UGT2B7 | rs5013211 | 0.012 |
| 2:165307150 | SCN2A | rs7598931 | 4:69096731 | UGT2B7 | rs12233719 | 0.001 |
| 2:165310162 | SCN2A | rs2116658 | 4:69096731 | UGT2B7 | rs12233719 | 0.024 |
SNPs: single-nucleotide polymorphisms.
Figure 2.
VPA-responsive rate among different combinations of genotypes. The responsive rate of AA-CG+GG group or AG-CG+GG group was significantly higher than that of the GG-CC group (p values were 0.007 and 0.028, respectively) in (a). The responsive rate of AG+GG-GT group or AG+GG-GG group was significantly lower than that of the AA-GT group (p values were 0.047 and 0.013, respectively) in (b). The responsive rate of TA+AA-GG group, TT-GG group, or TA+AA-GT+TT was significantly higher than that of the TT-GT+TT group (p values were 0.046, 0.016, and 0.023, respectively) in (c). VPA: valproic acid.
4. Discussion
In our study, we found the relationship between the SNPs screened from SCN1A, SCN2A and UGT2B7, respectively, and VPA responses in Chinese Han people with epilepsy. Our results indicated that two SNPs (rs1381105 and rs2162600) located in SCN1A and six SNPs including rs10197716, rs2119068, rs2119067, rs353116, rs353112 and rs6740895 located in SCN2A had different distributions in the VPA resistance group and the responsive group, which were possible for altering sensitivity for VPA. Through SNP-SNP interaction analysis, a total of 19 pairs of SNPs were associated with VPA responsiveness. The three most obvious pairs were rs10197716 (SCN2A)-rs2119068 (SCN2A), rs10197716 (SCN2A)-rs11889342 (SCN2A) and rs7598931 (SCN2A)-rs12233719 (UGT2B7). In comparison with previous studies, we found that some of our findings were controversial or novel.
The pathogenesis of epilepsy is complex and is thought to be due to excitatory and inhibitory imbalances in the central nervous system currently, which is closely related to neurotransmitter imbalance and ion channels [30]. It has been well documented that changes in expression and affinity of binding site in the sodium channels (SCNs) may be resistant to AEDs [31]. And VPA is a kind of histone deacetylase inhibitor that inhibit the voltage-sensitive sodium channels of neuronal firing to exert neurosuppressive effects [32]. Therefore, SCN gene mutation encoding sodium channels may lead to abnormal receptor function and affect the efficacy of VPA. rs6740895 is an intron variant located in SCN2A that encodes sodium voltage-gated channel alpha subunit 2. In the association analysis, we found that the distribution of genotypes with rs6740895 (SCN2A) was significantly different in VPA resistance group and responsive group, and allele C was associated with VPA responsiveness. Until now, the correlation between this site mutation and the efficacy of antiepileptic drugs has not been reported, and the detailed function of this site has not been explained clearly. The possible mechanism that the SNP alters the VPA response may be to alter the structure or function of the sodium channel receptors [33]. rs2298771 is a kind of missense variant in SCN1A, which causes the change of alanine to threonine [34]. However, we did not find a correlation between the SNP and VPA response. In fact, the result of rs2298771 (SCN1A) associated with response of AEDs so far is controversial. A few studies found that mutant genotype or allele did not improve or decrease efficacy with taking mono- or combination antiepileptic drugs, but some studies indicated that genetic polymorphism had a significant correlation with responsiveness of AEDs [24, 35]. The SNP rs3812718 an intron variant located in SCN1A had also different results in a variety of studies. 5′-diphosphate glucuronosyltransferase is essential for drug metabolism. As an important gene of this enzyme, UGT2B7 plays an important role in VPA metabolism. Wang et al. did not identify the relationship between rs12233719 (UGT2B7) and VPA metabolism in a meta-analysis [13]. Our study found that there was no correlation between this locus and the efficacy of VPA. There are several reasons for inconsistent results in different studies. Firstly, sample size is a key factor in the association analysis, which may cause false positive and low power. Secondly, genetic differences in different races may be also an important factor. Thirdly, different treatments, mono- or combination AEDs, may also affect treatment results of AEDs [17, 36, 37]. A SNP-SNP interaction, rs7598931 and rs12233719 located in SCN2A and UGT2B7 separately, associated with VPA responses was first found. It is amazing that these SNPs were not associated with VPA responses alone in our association analysis. The interaction analysis implied that a potential association between combinations of SNP-SNP genotypes and VPA responses in the association study could be ignored possibly. More interaction analysis may help discover new association between SNP-SNP interaction and drug responsiveness. At present, it is not clear whether the interactions of different SNP pairs are truly associated with VPA responses, and mechanisms remain to be studied. Therefore, the molecular mechanism is required to further research.
We studied the association between genetic polymorphisms and mono antiepileptic therapy (VPA), avoiding confounder for multiple drug interactions or competing for common targets [38]. Meanwhile, multiple SNPs that have never been reported in the past were chosen in Chinese Han epilepsy patients. This not only reveals new SNP locus information but also avoids racial differences. Moreover, we have also discovered the interaction among different SNPs for the first time, while the mechanism will be needed for further exploration. It is worth noting that some limitations existed in our study. Firstly, the sample size was relatively small compared with previous studies, which may reduce the statistical power. Secondly, our research sample lacked some recorded factors that had been reported in previous studies, such as age of onset and seizure type. Thirdly, the studied SNPs do not consist of all genetic information, so other mutations that were not covered in this study may also be associated with VPA responses. Further research based on enough sample size is not only to explore more potential gene mutations but also to clarify mechanisms and pathways with VPA responses.
5. Conclusion
Our study indicated that multiple SNPs, including rs1381105 (SCN1A), rs2162600 (SCN1A), rs10197716 (SCN2A), rs2119068 (SCN2A), rs2119067 (SCN2A), rs353116 (SCN2A), rs353112 (SCN2A) and rs6740895 (SCN2A), especially the SNP-SNP interaction (rs10197716-rs2119068, rs10197716-rs11889342 and rs7598931-rs12233719) were associated with VPA responsiveness in Chinese Han patients with epilepsy. Such gene mutations were found to potentially affect drug responses, facilitating personalized treatment for VPA in epileptic patients.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number 81773547).
Contributor Information
Fengyuan Che, Email: che1971@126.com.
Fuzhong Xue, Email: xuefzh@sdu.edu.cn.
Data Availability
The data used to support the findings of this study are available from the corresponding authors upon request.
Conflicts of Interest
The authors declare that they have no competing interests regarding the publication of this paper.
Authors' Contributions
Yuan Lu and Quanping Su contributed equally to this study. Yuan Lu and Quanping Su should be considered co-first authors.
References
- 1.Fiest K. M., Sauro K. M., Wiebe S., et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. 2017;88(3):296–303. doi: 10.1212/WNL.0000000000003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher R. S., Acevedo C., Arzimanoglou A., et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 3.Beghi E., Giussani G., Nichols E., et al. Global, regional, and national burden of epilepsy, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology. 2019;18(4):357–375. doi: 10.1016/S1474-4422(18)30454-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perucca E. Pharmacological and therapeutic properties of Valproate. CNS Drugs. 2002;16(10):695–714. doi: 10.2165/00023210-200216100-00004. [DOI] [PubMed] [Google Scholar]
- 5.Nevitt S. J., Marson A. G., Weston J., Smith C. T., Cochrane Epilepsy Group Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review. Cochrane Database of Systematic Reviews. 2017;2(2, article CD001911) doi: 10.1002/14651858.cd001911.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briggs D. E., French J. A. What makes epilepsy drug refractory. Expert Review of Neurotherapeutics. 2003;3(1):127–131. doi: 10.1586/14737175.3.1.127. [DOI] [PubMed] [Google Scholar]
- 7.Dalic L., Cook M. J. Managing drug-resistant epilepsy: challenges and solutions. Neuropsychiatric Disease and Treatment. 2016;12:2605–2616. doi: 10.2147/NDT.S84852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji J., Li G., Ma Y., Pan S., Yuan R. Expression of multidrug resistance genes in peripheral blood of patients with refractory epilepsy and the reverse effect of oxcarbazepine on its expression. Iranian Journal of Public Health. 2018;47(1):40–48. [PMC free article] [PubMed] [Google Scholar]
- 9.Fricke-Galindo I., Ortega-Vázquez A., Monroy-Jaramillo N., et al. Allele and genotype frequencies of genes relevant to anti-epileptic drug therapy in Mexican-Mestizo healthy volunteers. Pharmacogenomics. 2016;17(17):1913–1930. doi: 10.2217/pgs-2016-0078. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhry A. S., Urban T. J., Lamba J. K., et al. CYP2C9∗1BPromoter polymorphisms, in linkage withCYP2C19∗2, affect phenytoin autoinduction of clearance and maintenance dose. The Journal of Pharmacology and Experimental Therapeutics. 2010;332(2):599–611. doi: 10.1124/jpet.109.161026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noai M., Soraoka H., Kajiwara A., et al. Cytochrome P 450 2C19 polymorphisms and valproic acid-induced weight gain. Acta Neurologica Scandinavica. 2016;133(3):216–223. doi: 10.1111/ane.12473. [DOI] [PubMed] [Google Scholar]
- 12.Ma C. L., Wu X. Y., Jiao Z., Hong Z., Wu Z. Y., Zhong M. K. SCN1A, ABCC2 and UGT2B7 gene polymorphisms in association with individualized oxcarbazepine therapy. Pharmacogenomics. 2015;16(4):347–360. doi: 10.2217/pgs.14.186. [DOI] [PubMed] [Google Scholar]
- 13.Wang P., Lin X. Q., Cai W. K., et al. Effect of UGT2B7 genotypes on plasma concentration of valproic acid: a meta-analysis. European Journal of Clinical Pharmacology. 2018;74(4):433–442. doi: 10.1007/s00228-017-2395-z. [DOI] [PubMed] [Google Scholar]
- 14.Li S. X., Liu Y. Y., Wang Q. B. ABCB1 gene C3435T polymorphism and drug resistance in epilepsy: evidence based on 8, 604 subjects. Medical Science Monitor. 2015;21:861–868. doi: 10.12659/MSM.894023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y., Wang X., Li H., et al. Polymorphisms of ABCG2, ABCB1 and HNF4α are associated with Lamotrigine trough concentrations in epilepsy patients. Drug Metabolism and Pharmacokinetics. 2015;30(4):282–287. doi: 10.1016/j.dmpk.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Ma C. L., Wu X. Y., Zheng J., Wu Z. Y., Hong Z., Zhong M. K. Association of SCN1A, SCN2A and ABCC2 gene polymorphisms with the response to antiepileptic drugs in Chinese Han patients with epilepsy. Pharmacogenomics. 2014;15(10):1323–1336. doi: 10.2217/pgs.14.89. [DOI] [PubMed] [Google Scholar]
- 17.Zhou B. T., Zhou Q. H., Yin J. Y., et al. Effects of SCN1A and GABA receptor genetic polymorphisms on carbamazepine tolerability and efficacy in Chinese patients with partial seizures: 2-year longitudinal clinical follow-up. CNS Neuroscience & Therapeutics. 2012;18(7):566–572. doi: 10.1111/j.1755-5949.2012.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haerian B. S., Baum L., Kwan P., Tan H. J., Raymond A. A., Mohamed Z. SCN1A, SCN2A and SCN3A gene polymorphisms and responsiveness to antiepileptic drugs: a multicenter cohort study and meta-analysis. Pharmacogenomics. 2013;14(10):1153–1166. doi: 10.2217/pgs.13.104. [DOI] [PubMed] [Google Scholar]
- 19.Tomson T., Battino D., Perucca E. Valproic acid after five decades of use in epilepsy: time to reconsider the indications of a time-honoured drug. The Lancet Neurology. 2016;15(2):210–218. doi: 10.1016/S1474-4422(15)00314-2. [DOI] [PubMed] [Google Scholar]
- 20.Escayg A., Goldin A. L. Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia. 2010;51(9):1650–1658. doi: 10.1111/j.1528-1167.2010.02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y. X., Zhuo W. Y., Lin H., et al. The influence of UGT2B7 genotype on valproic acid pharmacokinetics in Chinese epilepsy patients. Epilepsy Research. 2015;114:78–80. doi: 10.1016/j.eplepsyres.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Dreifuss F. E., Martinez-Lage M., Roger J. W. M. Proposal for classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1985;26(3):268–278. [PubMed] [Google Scholar]
- 23.Kwan P., Arzimanoglou A., Berg A. T., et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 24.Mittal B., Kumari R., Lakhan R., Garg R. K., Kalita J., Misra U. K. Pharmacogenomic association study on the role of drug metabolizing, drug transporters and drug target gene polymorphisms in drug-resistant epilepsy in a north Indian population. Indian Journal of Human Genetics. 2011;17(4, article 80357) Supplement 1:32–S40. doi: 10.4103/0971-6866.80357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yip T. S. C., O'Doherty C., Tan N. C. K., Dibbens L. M., Suppiah V. SCN1A variations and response to multiple antiepileptic drugs. The Pharmacogenomics Journal. 2014;14(4):385–389. doi: 10.1038/tpj.2013.43. [DOI] [PubMed] [Google Scholar]
- 26.Van den Berg R. J., Kok P., Voskuyl R. A. Valproate and sodium currents in cultured hippocampal neurons. Experimental Brain Research. 1993;93(2):279–287. doi: 10.1007/bf00228395. [DOI] [PubMed] [Google Scholar]
- 27.Chung J. Y., Cho J. Y., Yu K. S., et al. Pharmacokinetic and pharmacodynamic interaction of lorazepam and valproic acid in relation to UGT2B7 genetic polymorphism in healthy subjects. Clinical Pharmacology & Therapeutics. 2007;83(4):595–600. doi: 10.1038/sj.clpt.6100324. [DOI] [PubMed] [Google Scholar]
- 28.Ferraro T. N., Buono R. J. The relationship between the pharmacology of antiepileptic drugs and human gene variation: an overview. Epilepsy & Behavior. 2005;7(1):18–36. doi: 10.1016/j.yebeh.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Gabriel S. B. The structure of haplotype blocks in the human genome. Science. 2002;296(5576):2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 30.Hui Y. Y., Ahmad N., Makmor-Bakry M. Pathogenesis of epilepsy: challenges in animal models. Iranian Journal of Basic Medical Sciences. 2013;16(11):1119–1132. [PMC free article] [PubMed] [Google Scholar]
- 31.Grover S., Gourie-Devi M., Baghel R., et al. Genetic profile of patients with epilepsy on first-line antiepileptic drugs and potential directions for personalized treatment. Pharmacogenomics. 2010;11(7):927–941. doi: 10.2217/pgs.10.62. [DOI] [PubMed] [Google Scholar]
- 32.Göttlicher M. Valproic acid: an old drug newly discovered as inhibitor of histone deacetylases. Annals of Hematology. 2004;83(Supplement 1):S91–S92. doi: 10.1007/s00277-004-0850-2. [DOI] [PubMed] [Google Scholar]
- 33.Catterall W. A. Forty years of sodium channels: structure, function, pharmacology, and epilepsy. Neurochemical Research. 2017;42(9):2495–2504. doi: 10.1007/s11064-017-2314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Domitrz I., Kosiorek M., Żekanowski C., Kamińska A. Genetic studies of Polish migraine patients: screening for causative mutations in four migraine-associated genes. Human Genomics. 2016;10(1):p. 3. doi: 10.1186/s40246-015-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertok S., Dolžan V., Goričar K., Podkrajšek K. T., Battelino T., Rener-Primec Z. The association of _SCN1A_ p.Thr1067Ala polymorphism with epilepsy risk and the response to antiepileptic drugs in Slovenian children and adolescents with epilepsy. Seizure. 2017;51:9–13. doi: 10.1016/j.seizure.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Daci A., Beretta G., Vllasaliu D., et al. Polymorphic variants of SCN1A and EPHX1 influence plasma carbamazepine concentration, metabolism and pharmacoresistance in a population of Kosovar Albanian epileptic patients. PLoS One. 2015;10(11, article e0142408) doi: 10.1371/journal.pone.0142408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manna I., Gambardella A., Bianchi A., et al. A functional polymorphism in the SCN1A gene does not influence antiepileptic drug responsiveness in Italian patients with focal epilepsy. Epilepsia. 2011;52(5):e40–e44. doi: 10.1111/j.1528-1167.2011.03097.x. [DOI] [PubMed] [Google Scholar]
- 38.Marson A. G., Williamson P. R., Clough H., Hutton J. L., Chadwick D. W., On Behalf Of The Epilepsy Monothera Carbamazepine versus valproate monotherapy for epilepsy: a meta-analysis. Epilepsia. 2002;43(5):505–513. doi: 10.1046/j.1528-1157.2002.20801.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding authors upon request.