Summary
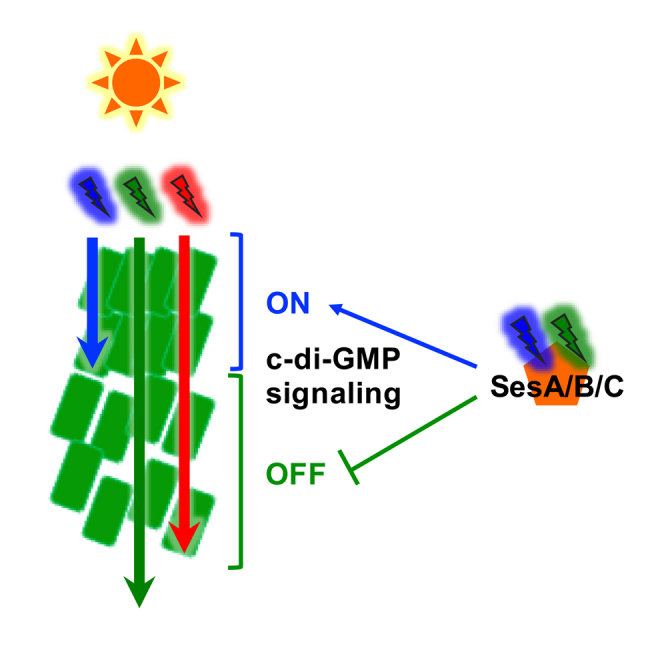
Cyanobacteriochrome (CBCRs) photoreceptors show various photochemical properties, but their ecophysiological functions remain elusive. Here, we report that the blue/green CBCRs SesA/B/C can serve as physiological sensors of cell density. Because cyanobacterial cells show lower transmittance of blue light than green light, higher cell density gives more green-light-enriched irradiance to cells. The cell-density-dependent suppression of cell aggregation under blue-/green-mixed light and white light conditions support this idea. Such a sensing mechanism may provide information about the cell position in cyanobacterial mats in hot springs, the natural habitat of Thermosynechococcus. This cell-position-dependent SesA/B/C-mediated regulation of cellular sessility (aggregation) might be ecophysiologically essential for the reorganization and growth of phototrophic mats. We also report that the green-light-induced dispersion of cell aggregates requires red-light-driven photosynthesis. Blue/green CBCRs might work as shade detectors in a different niche than red/far-red phytochromes, which may be why CBCRs have evolved in cyanobacteria.
Subject Areas: Sensor, Biological Sciences, Microbiology
Graphical Abstract
Highlights
-
•
Blue- and green-light-sensing cyanobacteriochromes can be sensors of cell density
-
•
They may provide information about the cell position in microbial mats
-
•
Green-light-induced dispersion of aggregates needs red-light-driven photosynthesis
-
•
Cyanobacteriochromes might work in a different niche than red/far-red phytochromes
Sensor; Biological Sciences; Microbiology
Introduction
Photoautotrophic cyanobacteria have developed sophisticated light response systems to capture and utilize the energy and information of incident light (Ho et al., 2017). Cyanobacteria-specific photoreceptors cyanobacteriochromes (CBCRs) are distantly related to more widespread phytochromes. CBCRs show the most diverse spectral properties among the naturally occurring photoreceptors, typified by a unique and prevailing blue-/green-light-absorbing variant (Anders and Essen, 2015, Cho et al., 2015, Enomoto et al., 2012, Ikeuchi and Ishizuka, 2008, Rockwell et al., 2012). However, where the CBCR-mediated “colorful” signaling systems function in nature has been elusive. We previously reported that the three CBCRs SesA/B/C synthesize/degrade a bacterial second messenger cyclic diguanylate (c-di-GMP) in response to blue/green light (Enomoto et al., 2012, Enomoto et al., 2014, Enomoto et al., 2015). The cooperative action of SesA/B/C enables blue light-ON and green light-OFF regulation of the c-di-GMP-dependent cell aggregation of the thermophilic cyanobacterium Thermosynechococcus vulcanus (Enomoto et al., 2015, Enomoto et al., 2018). The cooperation of multiple CBCRs for high spectral specificity was also reported for the process of complementary chromatic acclimation (Wiltbank and Kehoe, 2016), suggesting its physiological importance in nature. Here, we show that SesA/B/C can act as a physiological sensor of cell density in a different niche than red/far-red phytochromes. We discuss the ecophysiological functions of CBCRs and the possible driving force of CBCR evolution in cyanobacteria.
Results and Discussion
The Ratio of Blue to Green Light Perceived by CBCRs SesA/B/C Can Be an Indicator of How Many Other Cells Shade Them
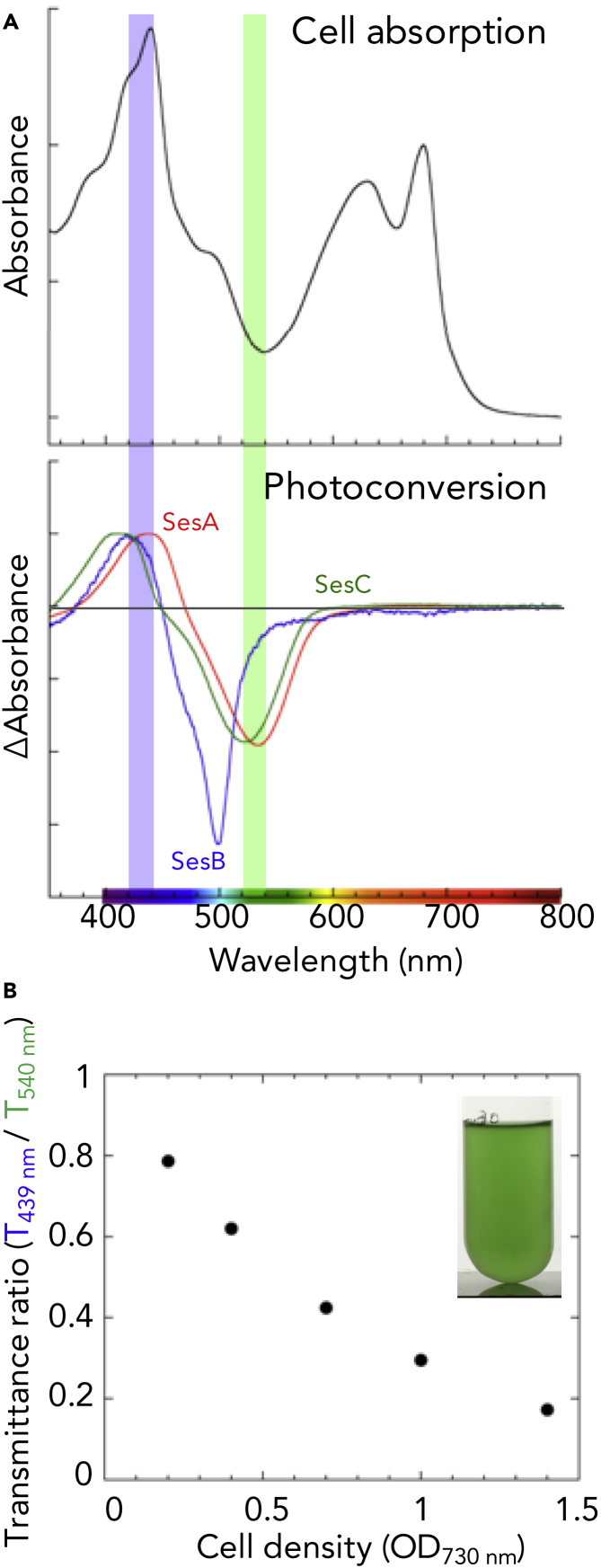
The absorption spectrum of Thermosynechococcus cells shows a peak at 435 nm and a trough at ∼530 nm, which roughly correspond to the blue absorption (∼430 nm) and the teal-green absorption (500–530 nm) of the three cyanobacteriochromes, SesA/B/C (Figure 1A) (Enomoto et al., 2015). Because white light can be differentially absorbed by cells containing chlorophyll, carotenoids, and phycocyanin/allophycocyanin, the light transmitted through a given cell layer is increasingly enriched in green light, depending on cell density (Figure 1B). Thus, we hypothesized that SesA/B/C-mediated cell aggregation may be governed by cell density under natural light.
Figure 1.
The Light Ratio of Blue to Green Light Perceived by SesA/B/C CBCRs Can be an Indicator of Self-Shading
(A) The cell absorption spectrum of Thermosynechococcusvulcanus (upper) and the difference spectra of the reversible photoconversion of SesA/B/C holoproteins (lower). The lower panel was created using the data reported in Ref. 7. The region of 420 nm–440 nm was highlighted in a blue shade and that of 520 nm–540 nm was highlighted in a green shade.
(B) The transmittance ratio of blue light (439 nm) to green light (540 nm) and the image of the liquid culture of T. vulcanus.
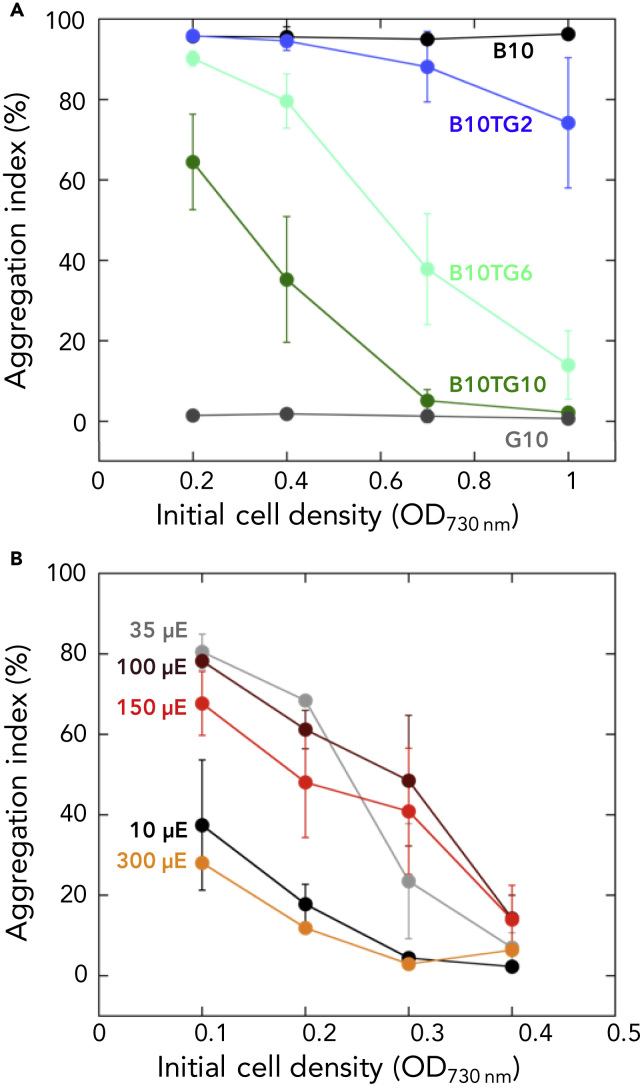
We assessed the effects of cell population density on cell aggregation under model light conditions. We used mixed light conditions of teal-green and blue (each 0–10 μmol photons m−2 s−1 as signaling light) in addition to red light for growth (30 μmol photons m−2 s−1). When ten μmol photons m−2 s−1 blue light and 2–10 μmol photons m−2 s−1 teal-green light were used as signaling light, we observed cell-density-dependent suppression of cell aggregation (Figure 2A). The more the green light was provided, the less the cell aggregation was induced. Under only blue or teal-green light (10 μmol photons m−2 s−1), cell aggregation did not depend on the cell density; cells showed secure cell aggregation irrespective of the density under only blue light, whereas they showed no cell aggregation under teal-green light (Figure 2A). This result suggests that the cell density dependency can be mediated by the blue/teal-green light-sensing system SesA/B/C but not by other light-independent regulatory systems, such as quorum sensing. This assay also confirmed that the ratio of blue light/teal-green light, but not the absolute intensity of blue light, is the signal for cell aggregation, as the more teal-green light that was added to 10 μmol photons m−2 s−1 blue light, the more cell aggregation was repressed (Figure 2A).
Figure 2.
SesA/B/C-Mediated Blue/Green Light Signaling Senses Cell Density to Regulate Cell Aggregation under Both Blue Ligh and Green Light
(A) Effects of cell population density on cell aggregation under LED irradiation. The horizontal axis shows the initial cell density, and the vertical axis shows the aggregation index (percentage of aggregated cells) after 48 h of cultivation at low temperatures of 31°C in the presence of red light (634 nm, 30 μE m−2 s−1). The intensity of signaling light is indicated as labels; B10TG2: blue light (448 nm, 10 μE m−2 s−1) + teal-green light (507 nm, 2 μE m−2 s−1). Data are represented as the mean ± SEM. See also Figure S2.
(B) Cell aggregation under the four different intensities of the white light fluorescent lamp. Data are represented as the mean ± SEM. See also Figure S1.
To extend our model light system to naturally occurring conditions, we assessed the cell density dependency of cell aggregation under white light irradiation. Under white light ranging from 10 to 300 μmol photons m−2 s−1, cell aggregation decreased as the cell density increased (Figure 2B). The levels of aggregation were different under the tested light intensities although the cellular growth showed no difference (Figure S1). Under low light of 10 μmol photons m−2 s−1, aggregation was weak even at the lowest cell density, probably because the light was insufficient for activation of the SesA/B/C photoreceptors. The aggregation at the lowest cell density under high light of 300 μmol photons m−2 s−1 tended to be weaker than that under 35 or 100 μmol photons m−2 s−1. This lowered cell aggregation suggested that other regulatory systems rather than light-quality-sensing SesABC may dominantly control cell aggregation under high irradiance. These results suggest that cell-density-dependent control of cell aggregation can also be mediated by the blue-/teal-green-light-sensing proteins SesA/B/C under white light.
Blue-/Green-Light-Regulated Reversible Formation and Dispersion of Cell Aggregates Are Driven by Red-Light-Driven Photosynthesis
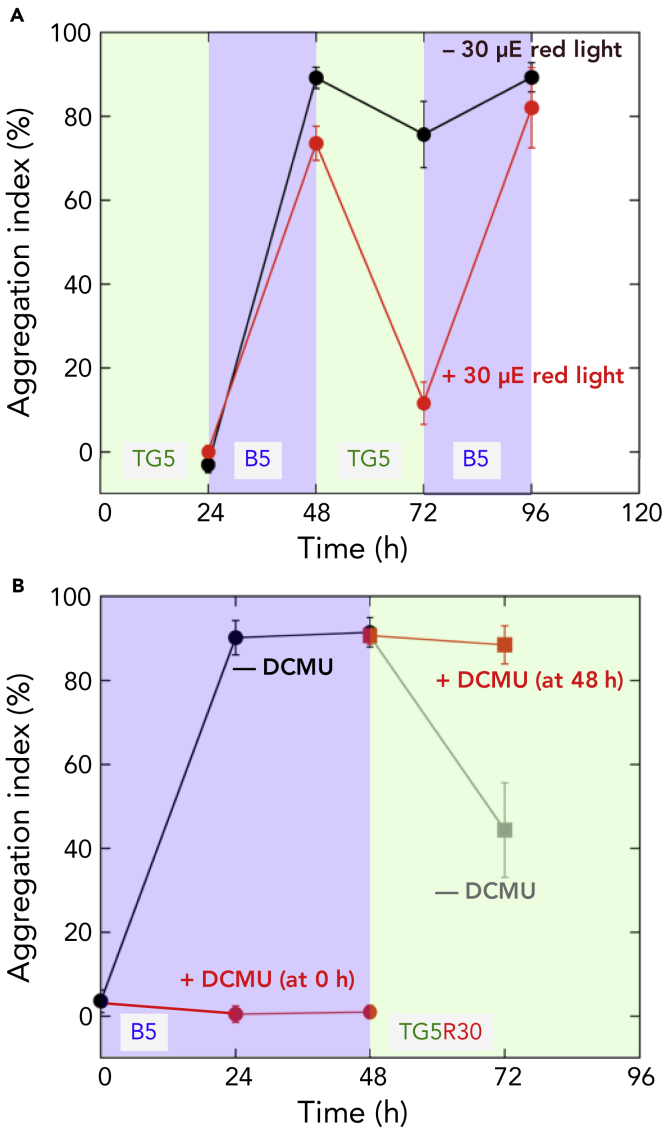
To dissect the contribution of each wavelength of light, we assessed the role of red light in blue-/green-light-regulated cell aggregation. When cell aggregates were transferred from blue light conditions to teal-green light conditions at 31°C with red background light, they dispersed in 24 h (Figure 3A). This result indicates that cell aggregation is reversible even under cell-aggregation-enhancing low-temperature conditions. The omission of the background red light impaired the dispersion of cell aggregates (Figure 3A). These results suggested that the background red light may support dispersion via photosynthetic activity, given that photosynthetic pigments poorly absorb teal-green light. Consistently, the addition of the photosynthesis inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) impaired both the formation and dispersion of cell aggregates (Figure 3B). Thus, growth light is critical for the decision and active responses to induce a sessile lifestyle (cell aggregation) or planktonic lifestyle (dispersion). Many cyanobacteria, including Thermosynechococcus, possess chlorophyll a and phycocyanin pigments for photosynthesis but not far-red light-absorbing chlorophyll d/f or green-light-absorbing phycoerythrin. This pigment composition means that blue, orange, and red light but not green or far-red light is effective for the active photosynthesis-driven responses. In other words, irradiation with only blue light can induce cell aggregation with active photosynthesis (Figure 3B). In contrast, green light should be accompanied by orange light or red light for the induction of cell dispersion. Both the formation and dispersion of cellulose-dependent cell aggregates (Kawano et al., 2011) may be energy-demanding responses because cells will have to produce a massive amount of extracellular cellulose to stabilize the cell aggregates during formation and degrade the cellulose when they escape from the aggregates.
Figure 3.
Red-Light-Driven Photosynthesis is Necessary to Support the Reversible Formation and Dispersion of Cell Aggregates
(A) Reversible formation and dispersion of cell aggregation induced by switching blue light and teal-green light every 24 h in the presence or absence of the background red light irradiation. Data are represented as the mean ± SD.
(B) Effects of the photosynthesis inhibitor DCMU on the formation and dispersion of cell aggregates. DCMU (final concentration 10 μM) was added at the indicated time. Data are represented as the mean ± SD.
The Physiological and Ecological Relevance of Blue-/Green-Type CBCR Signaling
Many bacteria often establish multicellular, matrix-embedded communities, such as microbial mats or biofilms, which are crucial for their physiology, ecology, and infections (Nadell et al., 2016, Flemming et al., 2016). Likewise, thermophilic cyanobacteria usually form a microbial mat in hot springs (Ward et al., 1998, Bolhuis et al., 2014). Analysis of the transmittance of light in typical thermophilic cyanobacterial mats revealed that green and far-red light are penetrating, whereas blue and red light are mostly absorbed by photosynthetic pigments (Ohkubo and Miyashita, 2017, Pierson et al., 1990). This tendency fits well with the light transmittance properties of the cell suspension of Thermosynechococcus (Figure 1A). Thus, reversible regulation of sessility by blue/green CBCR signaling could account for the behavioral responses of Thermosynechococcus in the mats.
Physiologically, low-temperature-enhanced and blue-light-induced cell aggregation would protect photosynthesis from photoinhibition due to self-shading effects, as suggested previously (Kawano et al., 2011). Blue light irradiation easily damages the oxygen-evolving complex of photosystem II (Ohnishi et al., 2005). Usually, the rapid repair cycle of photosystem II supports the replacement of the damaged reaction center polypeptides with newly synthesized ones (Nishiyama and Murata, 2014). At low temperatures, on the other hand, the protein turnover is decelerated, leading to impaired repair of the photosystem. Under these photoinhibition-inducing conditions, the formation of cell aggregates may be beneficial for the protection of the photosynthetic apparatus by self-shading. However, low temperature is not necessary to induce sessile lifestyles because blue light signals can induce cell aggregation alone, even at 45°C (Figure S2) (Maeda et al., 2018). Because the formation of cell aggregates is enhanced at relatively low temperatures, it will be beneficial for cells to remain in the hot spring environment and to avoid being displaced by water currents to lethally low-temperature environments such as rivers and oceans.
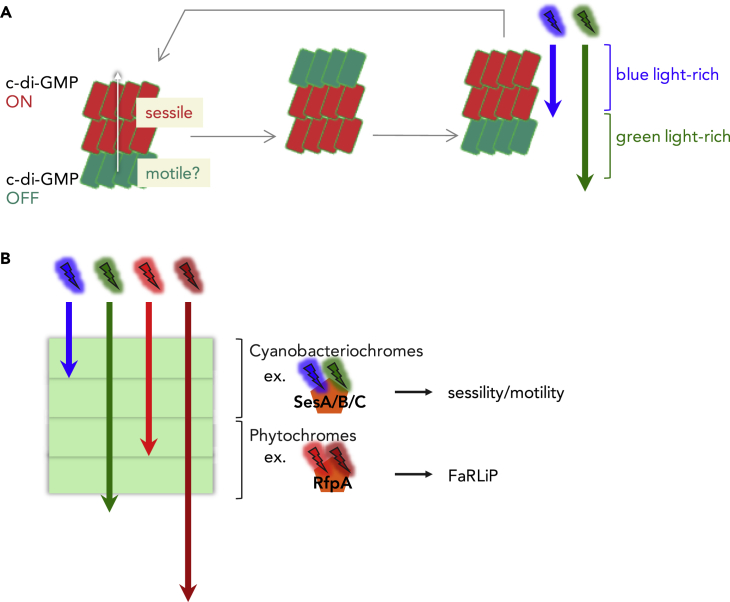
Ecologically, light-induced regulation of sessility and motility would be necessary for the remodeling (or reorganizing) of phototrophic mats. The phototactic motility of Thermosynechococcus is regulated intricately by light quality and intensity (Kondou et al., 2001). c-di-GMP is often involved in the regulation of bacterial motility (Savakis et al., 2012, Jenal et al., 2017). As cell aggregates develop, the internal cells in a floc will sense a green-light-rich environment and turn off c-di-GMP signaling, favoring the motile-planktonic lifestyle. Given that the Thermosynechococcus vulcanus strain used in this study shows positive phototaxis, the internal cells might move through the crowd of cells toward the sun. When these cells reach the upper region, they will sense the relatively blue-light-rich environment and turn on c-di-GMP signaling, leading to a sessile mode of life. Then, the next generation of internal cells will sense the green-light-rich environment and turn off c-di-GMP signaling. Repeating this process may lead to dynamic cell movements inside a static microbial mat (Figure 4A). This dynamism might facilitate nutrient uptake and gas exchange, which are necessary for efficient photosynthetic growth, and might also be helpful for the expansion of a microbial mat and the colonization of another niche. The other potential advantage is for every cell of the community to obtain access to light for photosynthetic viability and growth. Further in situ and ex situ studies are needed to reveal the role and significance of c-di-GMP heterogeneity and cellular behaviors in a cyanobacterial community. Several blue/green CBCR variants are involved in the regulation of community-based phototactic motility (Narikawa et al., 2011, Savakis et al., 2012, Song et al., 2011, Wilde and Mullineaux, 2017, Yoshihara et al., 2004) and floc formation (Conradi et al., 2019), supporting the idea that blue/green CBCRs work as cell-shade-sensing systems to orchestrate motile cell communities under natural light conditions.
Figure 4.
SesA/B/C Invoke Heterogeneity of C-di-GMP Signaling in a Cyanobacterial Community and Act in Different Layers than Red/Far-Red Light-Responsive Phytochromes
(A) A hypothesis of dynamic cell movements inside a microbial mat. Under natural light conditions, the internal cells in a floc will sense a green light-rich environment and turn off c-di-GMP signaling, favoring the motile-planktonic lifestyle. If the internal c-di-GMP-OFF cells retain positive phototactic motility, they may move and reach the upper region, resulting in the reorganization of the heterogeneous c-di-GMP levels in the floc. This would invoke sequential cellular movements in the cyanobacterial community.
(B) A hypothesis of niche differentiation of photoreceptors. Blue light rapidly attenuates in the top layer of photosynthetic microbial mats (Jorgensen et al., 1987, Ohkubo and Miyashita, 2017). Blue/green cyanobacteriochromes may be a shade detector in an upper layer of microbial mats, where sufficient red light is still available. On the other hand, phytochromes may be effective in a darker deep area, where red light diminishes, and therefore cyanobacteria cannot out-compete other bacterial species.
Two Strategies of Light Acclimation in Mats
The phototrophic microbial mats in the Nakabusa hot spring in Japan contain unicellular Thermosynechococcus and chlorophyll f-producing filamentous cyanobacteria (Ohkubo and Miyashita, 2017, Stolyar et al., 2014). The production of chlorophyll f as well as specific subunits of PSI, PSII, and phycobiliproteins for absorbing long-wavelength light is induced by far-red light via the knotless phytochrome RfpA (Gan et al., 2014, Zhao et al., 2015, Ho et al., 2017). This far-red-light-induced acclimation enables active photosynthesis under the red light-depleted niche when cells are embedded in other chlorophyll a-containing cyanobacteria. However, even these far-red-light-responsive organisms, such as Leptolyngbya sp. JSC-1, possess proteins harboring both a blue-/teal-green-type CBCR domain and domain(s) of c-di-GMP production or degradation, like SesA/B/C. This fact suggests that blue/teal-green regulation of cell aggregation (sessility) may be more widely distributed than the far-red light induction of chlorophyll f and long-wavelength photosynthesis. Notably, blue light penetrates mats less than red light due to the higher absorption and scattering of blue light (Jorgensen et al., 1987, Ohkubo and Miyashita, 2017). The green light may be a useful reference beam to the blue monitoring beam. The uppermost cyanobacterial layer of the microbial mats in the Nakabusa hot spring does not contain phycoerythrin or phycoerythrocyanin, which absorb green light for photosynthesis. This fact may support the idea that green light can be a reference for the recognition of differential light quality. In other words, blue/teal-green CBCRs may be superior to red/far-red phytochromes for the shade detection in the upper region of the cyanobacterial layer of microbial mats (Figure 4B) because blue and teal-green light are less penetrating than red and far-red light, respectively, in thermophilic cyanobacterial mats (Jorgensen et al., 1987, Ohkubo and Miyashita, 2017). Red/far-red phytochromes, which are well known as a shade detector for shade avoidance of land plants, are distributed in various organisms, such as plants, algae, fungi, and bacteria (Fiorucci and Fankhauser, 2017, Anders and Essen, 2015), suggesting that phytochromes may be the ancestor of the related but cyanobacteria-specific photoreceptors CBCRs (Rockwell and Lagarias, 2019). The blue/green variant is distinguished by a conserved Asp-Xaa-Cys-Phe (DXCF) motif in the GAF domain. DXCF-type CBCRs are one of the most prevailing variants in cyanobacteria, including early diverging and mat-forming strains (Cho et al., 2015, Ponce-Toledo et al., 2017, Rockwell et al., 2012, Rockwell et al., 2015). Notably, the blue/green photochemistry is missing in any other type of photoreceptor. Thus, the new demand for blue/green sensors in a unique niche might be a driving force for CBCR evolution in cyanobacteria. Although the basal clade of the phylogenetic tree of cyanobacteriochrome GAF domains include the green/red sensors (Rockwell et al., 2015), the clade also includes DXCF-based blue-/green light-responsive proteins (Rockwell et al., 2012, Ma et al., 2012), suggesting that blue/green sensors can have ancient origin. Furthermore, the only CBCR identified in the basal cyanobacterial genus Gloeobacter (Glr3432 in Gloeobacterviolaceus PCC 7421) is a DXCF variant (Moore et al., 2019, Shih et al., 2013). It is of note that the green/red sensors for complementary chromatic acclimation may also be regarded as early developed CBCRs given their uniqueness of photochemistry and ancientness in the phylogenetic tree (Hirose et al., 2019). In complementary chromatic acclimation, cells express more green-light-absorbing phycoerythrin under green-light-rich irradiance, whereas they halt phycoerythrin production under red-light-rich irradiance (Sanfilippo et al., 2019). The green reference beam may be better than the far-red beam to evaluate whether the cells are shaded by phycoerythrin-containing cyanobacteria (green-less, red-less, far-red-rich) or by phycoerythrin-less cyanobacteria (green-rich, red-less, far-red-rich). For a clearer picture of the CBCR's evolution, more thorough coverage of cyanobacteriochrome sequences with the confirmed photochemical properties is awaited.
Limitations of the Study
The hypotheses that we constructed in this study need to be examined experimentally. Specifically, the distribution and heterogeneity of c-di-GMP levels in cell aggregates and physiological functions thereof should be addressed. However, the establishment of a c-di-GMP reporter system in cyanobacteria has been hindered because the reported systems such as FRET-based biosensors are only amenable in a limited number of organisms so far. The transcriptional fusion with a c-di-GMP responsive promoter and a versatile reporter protein such as GFP and luciferase may be a more accessible option. However, such a promoter has not been identified in cyanobacteria, and also the transcriptional change would be too slow to monitor the c-di-GMP fluctuations if the cells move inside a microbial mat. Furthermore, cyanobacteria harbor a high amount of photopigments, whose autofluorescence can mask the fluorescent signal. In nature, various factors other than light, for example, oxygen concentration, and any signals from other bacterial species could affect the c-di-GMP signaling. Experimental validation will lead to a better understanding of how signaling molecules orchestrate the dynamics and structure of bacterial communities.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by a grant-in-aid for Young Scientists (B) (JSPS KAKENHI Grant No. 17K15244) from Japan Society for the Promotion of Science (GE) and by Core Research for Evolutional Science and Technology, Japan Science and Technology Agency (MI). GE was supported by EMBO Long-Term Fellowship (ALTF 274–2017). We thank Dr. Chihiro Azai and Prof. Dr. Satoshi Hanada for insightful discussions and suggestions. We are also grateful to Prof. Dr. Annegret Wilde and Ms. Annik Jakob for their critical reading of the manuscript.
Author Contributions
Conceptualization, G.E. and M.I.; Methodology, G.E.; Investigation, G.E.; Writing—Original Draft, G.E.; Writing—Review & Editing, G.E. and M.I.; Funding Acquisition, G.E. and M.I.; Resources, G.E. and M.I.
Declaration of Interests
The authors declare no competing interests.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100936.
Supplemental Information
References
- Anders K., Essen L.O. The family of phytochrome-like photoreceptors: diverse, complex and multi-colored, but very useful. Curr.Opin.Struct. Biol. 2015;35:7–16. doi: 10.1016/j.sbi.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Bolhuis H., Cretoiu M.S., Stal L.J. Molecular ecology of microbial mats. FEMS Microbiol. Ecol. 2014;90:335–350. doi: 10.1111/1574-6941.12408. [DOI] [PubMed] [Google Scholar]
- Cho S.M., Jeoung S.C., Song J.Y., Kupriyanova E.V., Pronina N.A., Lee B.W., Jo S.W., Park B.S., Choi S.B., Song J.J., Park Y.I. Genomic survey and biochemical analysis of recombinant candidate cyanobacteriochromes reveals enrichment for near UV/violet sensors in the halotolerant and alkaliphilic cyanobacterium Microcoleus IPPAS B353. J. Biol. Chem. 2015;290:28502–28514. doi: 10.1074/jbc.M115.669150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradi F.D., Zhou R.Q., Oeser S., Schuergers N., Wilde A., Mullineaux C.W. Factors controlling floc formation and structure in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 2019;201 doi: 10.1128/JB.00344-19. e00344–00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto G., Hirose Y., Narikawa R., Ikeuchi M. Thiol-based photocycle of the blue and teal light-sensing cyanobacteriochrome Tlr1999. Biochemistry. 2012;51:3050–3058. doi: 10.1021/bi300020u. [DOI] [PubMed] [Google Scholar]
- Enomoto G., Ni Ni W., Narikawa R., Ikeuchi M. Three cyanobacteriochromes work together to form a light color-sensitive input system for c-di-GMP signaling of cell aggregation. Proc. Natl. Acad. Sci. U S A. 2015;112:8082–8087. doi: 10.1073/pnas.1504228112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto G., Nomura R., Shimada T., Ni Ni W., Narikawa R., Ikeuchi M. Cyanobacteriochrome SesA is a diguanylatecyclase that induces cell aggregation in Thermosynechococcus. J. Biol. Chem. 2014;289:24801–24809. doi: 10.1074/jbc.M114.583674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto G., Okuda Y., Ikeuchi M. Tlr1612 is the major repressor of cell aggregation in the light-color-dependent c-di-GMP signaling network of Thermosynechococcusvulcanus. Sci. Rep. 2018;8:5338. doi: 10.1038/s41598-018-23628-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorucci A.S., Fankhauser C. Plant strategies for enhancing access to sunlight. Curr. Biol. 2017;27:R931–R940. doi: 10.1016/j.cub.2017.05.085. [DOI] [PubMed] [Google Scholar]
- Flemming H.C., Wingender J., Szewzyk U., Steinberg P., Rice S.A., Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 2016;14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- Gan F., Zhang S., Rockwell N.C., Martin S.S., Lagarias J.C., Bryant D.A. Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light. Science. 2014;345:1312–1317. doi: 10.1126/science.1256963. [DOI] [PubMed] [Google Scholar]
- Hirose Y., Chihong S., Watanabe M., Yonekawa C., Murata K., Ikeuchi M., Eki T. Diverse chromatic acclimation processes regulating phycoerythrocyanin and rod-shaped phycobilisome in cyanobacteria. Mol. Plant. 2019;12:1167–1169. doi: 10.1016/j.molp.2019.07.002. [DOI] [PubMed] [Google Scholar]
- Ho M.Y., Soulier N.T., Canniffe D.P., Shen G., Bryant D.A. Light regulation of pigment and photosystem biosynthesis in cyanobacteria. Curr.Opin. Plant Biol. 2017;37:24–33. doi: 10.1016/j.pbi.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Ikeuchi M., Ishizuka T. Cyanobacteriochromes: a new superfamily of tetrapyrrole-binding photoreceptors in cyanobacteria. Photochem.Photobiol.Sci. 2008;7:1159–1167. doi: 10.1039/b802660m. [DOI] [PubMed] [Google Scholar]
- Jenal U., Reinders A., Lori C. Cyclic di-GMP: second messenger extraordinaire. Nat. Rev. Microbiol. 2017;15:271–284. doi: 10.1038/nrmicro.2016.190. [DOI] [PubMed] [Google Scholar]
- Jorgensen B.B., Cohen Y., Des Marais D.J. Photosynthetic action spectra and adaptation to spectral light distribution in a benthic cyanobacterial mat. Appl. Environ. Microbiol. 1987;53:879–886. doi: 10.1128/aem.53.4.879-886.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y., Saotome T., Ochiai Y., Katayama M., Narikawa R., Ikeuchi M. Cellulose accumulation and a cellulose synthase gene are responsible for cell aggregation in the cyanobacterium Thermosynechococcus vulcanus RKN. Plant Cell Physiol. 2011;52:957–966. doi: 10.1093/pcp/pcr047. [DOI] [PubMed] [Google Scholar]
- Kondou Y., Nakazawa M., Higashi S., Watanabe M., Manabe K. Equal-quantum action spectra indicate fluence-rate-selective action of multiple photoreceptors for photomovement of the thermophilic cyanobacterium Synechococcus elongatus. Photochem. Photobiol. 2001;73:90–95. doi: 10.1562/0031-8655(2001)073<0090:eqasif>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ma Q., Hua H.H., Chen Y., Liu B.B., Kramer A.L., Scheer H., Zhao K.H., Zhou M. A rising tide of blue-absorbing biliprotein photoreceptors: characterization of seven such bilin-binding GAF domains in Nostoc sp. PCC7120. FEBS J. 2012;279:4095–4108. doi: 10.1111/febs.12003. [DOI] [PubMed] [Google Scholar]
- Maeda K., Tamura J., Okuda Y., Narikawa R., Midorikawa T., Ikeuchi M. Genetic identification of factors for extracellular cellulose accumulation in the thermophilic cyanobacterium Thermosynechococcus vulcanus: proposal of a novel tripartite secretion system. Mol. Microbiol. 2018;109:121–134. doi: 10.1111/mmi.13977. [DOI] [PubMed] [Google Scholar]
- Moore K.R., Magnabosco C., Momper L., Gold D.A., Bosak T., Fournier G.P. An expanded ribosomal phylogeny of cyanobacteria supports a deep placement of plastids. Front. Microbiol. 2019;10:1612. doi: 10.3389/fmicb.2019.01612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadell C.D., Drescher K., Foster K.R. Spatial structure, cooperation and competition in biofilms. Nat. Rev. Microbiol. 2016;14:589–600. doi: 10.1038/nrmicro.2016.84. [DOI] [PubMed] [Google Scholar]
- Narikawa R., Suzuki F., Yoshihara S., Higashi S., Watanabe M., Ikeuchi M. Novel photosensory two-component system (PixA-NixB-NixC) involved in the regulation of positive and negative phototaxis of cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2011;52:2214–2224. doi: 10.1093/pcp/pcr155. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Murata N. Revised scheme for the mechanism of photoinhibition and its application to enhance the abiotic stress tolerance of the photosynthetic machinery. Appl. Microbiol. Biotechnol. 2014;98:8777–8796. doi: 10.1007/s00253-014-6020-0. [DOI] [PubMed] [Google Scholar]
- Ohkubo S., Miyashita H. A niche for cyanobacteria producing chlorophyll f within a microbial mat. ISME J. 2017;11:2368–2378. doi: 10.1038/ismej.2017.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi N., Allakhverdiev S.I., Takahashi S., Higashi S., Watanabe M., Nishiyama Y., Murata N. Two-step mechanism of photodamage to photosystem II: step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center. Biochemistry. 2005;44:8494–8499. doi: 10.1021/bi047518q. [DOI] [PubMed] [Google Scholar]
- Pierson B.K., Sands V.M., Frederick J.L. Spectral irradiance and distribution of pigments in a highly layered marine microbial mat. Appl. Environ. Microbiol. 1990;56:2327. doi: 10.1128/aem.56.8.2327-2340.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce-Toledo R.I., Deschamps P., López-García P., Zivanovic Y., Benzerara K., Moreira D. An early-branching freshwater cyanobacterium at the origin of plastids. Curr. Biol. 2017;27:386–391. doi: 10.1016/j.cub.2016.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell N.C., Lagarias J.C. Phytochrome evolution in 3D: deletion, duplication, and diversification. New Phytol. 2019;225:2283–2300. doi: 10.1111/nph.16240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell N.C., Martin S.S., Gulevich A.G., Lagarias J.C. Phycoviolobilin formation and spectral tuning in the DXCF cyanobacteriochrome subfamily. Biochemistry. 2012;51:1449–1463. doi: 10.1021/bi201783j. [DOI] [PubMed] [Google Scholar]
- Rockwell N.C., Martin S.S., Lagarias J.C. Identification of DXCF cyanobacteriochrome lineages with predictable photocycles. Photochem. Photobiol. Sci. 2015;14:929–941. doi: 10.1039/c4pp00486h. [DOI] [PubMed] [Google Scholar]
- Sanfilippo J.E., Garczarek L., Partensky F., Kehoe D.M. Chromatic acclimation in cyanobacteria: a diverse and widespread process for optimizing photosynthesis. Annu. Rev. Microbiol. 2019;73:407–433. doi: 10.1146/annurev-micro-020518-115738. [DOI] [PubMed] [Google Scholar]
- Savakis P., De Causmaecker S., Angerer V., Ruppert U., Anders K., Essen L.O., Wilde A. Light-induced alteration of c-di-GMP level controls motility of Synechocystis sp. PCC 6803. Mol. Microbiol. 2012;85:239–251. doi: 10.1111/j.1365-2958.2012.08106.x. [DOI] [PubMed] [Google Scholar]
- Shih P.M., Wu D., Latifi A., Axen S.D., Fewer D.P., Talla E., Calteau A., Cai F., Tandeau de Marsac N., Rippka R. Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc. Natl. Acad. Sci. U S A. 2013;110:1053–1058. doi: 10.1073/pnas.1217107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.Y., Cho H.S., Cho J.I., Jeon J.S., Lagarias J.C., Park Y.I. Near-UV cyanobacteriochrome signaling system elicits negative phototaxis in the cyanobacterium Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. U S A. 2011;108:10780–10785. doi: 10.1073/pnas.1104242108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolyar S., Liu Z., Thiel V., Tomsho L.P., Pinel N., Nelson W.C., Lindemann S.R., Romine M.F., Haruta S., Schuster S.C. Genome sequence of the thermophilic cyanobacterium Thermosynechococcus sp. strain NK55a. Genome Announc. 2014;2 doi: 10.1128/genomeA.01060-13. e01060–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D.M., Ferris M.J., Nold S.C., Bateson M.M. A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Microbiol. Mol. Biol. Rev. 1998;62:1353–1370. doi: 10.1128/mmbr.62.4.1353-1370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde A., Mullineaux C.W. Light-controlled motility in prokaryotes and the problem of directional light perception. FEMS Microbiol. Rev. 2017;41:900–922. doi: 10.1093/femsre/fux045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltbank L.B., Kehoe D.M. Two cyanobacterial photoreceptors regulate photosynthetic light harvesting by sensing teal, green, yellow, and red light. mBio. 2016;7 doi: 10.1128/mBio.02130-15. e02130–02115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara S., Katayama M., Geng X., Ikeuchi M. Cyanobacterial phytochrome-like PixJ1 holoprotein shows novel reversible photoconversion between blue- and green-absorbing forms. Plant Cell Physiol. 2004;45:1729–1737. doi: 10.1093/pcp/pch214. [DOI] [PubMed] [Google Scholar]
- Zhao C., Gan F., Shen G., Bryant D.A. RfpA, RfpB, and RfpC are the master control elements of far-red light photoacclimation (FaRLiP) Front. Microbiol. 2015;6:1303. doi: 10.3389/fmicb.2015.01303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.