Abstract
Background
Urticarial vasculitis (UV) is a rare type of leukocytoclastic vasculitis characterized by long lasting urticarial skin lesions and poor response to treatment. As of yet, no clinical guidelines, diagnostic criteria, or treatment algorithms exist, and the approaches to the diagnostic workup and treatment of UV patients may differ globally. We conducted an online survey to examine how UV patients are diagnosed and treated by international specialists and to reveal the greatest challenges in managing UV patients worldwide.
Methods
Distribution of the questionnaire included an email to individuals in the World Allergy Organization (WAO) database, with no restrictions applied to the specialty, affiliation, or nationality of the participants (November 2018). The email contained a link (Internet address) to the online questionnaire. Responses were anonymous. The link to the questionnaire was further sent to the network of Urticaria Centers of Reference and Excellence (UCARE) in the Global Allergy and Asthma European Network (GA2LEN) as well as to the Turkish Dermatology Society and the Japanese Society of Allergology, who distributed the link to their members. In addition, the survey link was posted online in the group of the Russian Society of Allergologists and Immunologists.
Results
We received 883 completed surveys from physicians in 92 countries. UV was reported to be rare in clinical practice, with an average of 5 patients per physician per year. More than two-thirds of physicians reported wheals, burning of the skin, and residual hyperpigmentation in 60–100% of UV patients. The most frequently reported reason for receiving referrals of patients with UV was to establish the diagnosis. The most important features for establishing the diagnosis of UV were wheals of longer than 24 hours duration (72%), the results of skin biopsy (63%), and post-inflammatory hyperpigmentation (46%). The most common tests ordered in UV patients were complete blood count, erythrocyte sedimentation rate, C-reactive protein, complement components, antinuclear antibodies, and skin biopsy. Physicians considered UV to be of unknown cause in most patients, and drugs and systemic lupus erythematosus to be the most common identifiable causes. Two of 3 physicians reported that they use second-generation antihistamines in standard dose as the first-line therapy in patients with UV. The greatest perceived challenges in the management of UV were the limited efficacy of drugs and the absence of clinical guidelines and treatment algorithms.
Conclusions
UV is a challenging disease. Skin biopsy, a gold standard for UV diagnosis, is not performed by many physicians. This may lead to misdiagnosis of UV, for example, as chronic spontaneous urticaria, and to inadequate treatment. International consensus-based recommendations for the classification of UV and the diagnostic workup and treatment, as well as prospective studies evaluating potentially safe and effective drugs for the treatment of UV, are necessary.
Keywords: Urticarial vasculitis, Management, Worldwide, Treatment, Diagnosis
Abbreviations: ANA, antinuclear antibodies; CRP, C-reactive protein; CSU, Chronic spontaneous urticaria; ESR, erythrocyte sedimentation rate; GA2LEN, Global Allergy and Asthma European Network; HUV, Hypocomplementemic urticarial vasculitis; HUVS, Hypocomplementemic urticarial vasculitis syndrome; NUV, Normocomplementemic urticarial vasculitis; sgAHs, Second generation antihistamines; SLE, Systemic lupus erythematosus; UCARE, Urticaria Centers of Reference and Excellence; UV, Urticarial vasculitis; WAO, World Allergy Organization
Introduction
Urticarial vasculitis (UV) is a small vessel vasculitis characterized by long lasting urticarial skin lesions combined with the histopathological finding of leukocytoclastic vasculitis.1,2 The clinical picture of UV, or "aemorrhagic urticaria" as it used to be called, was first described in 2 male patients by Wills and Lond in 1890.3 In 1956, the histopathological finding of vasculitis was reported in 2 patients with urticaria.4 UV is a rare skin disorder.5 The worldwide prevalence of UV is unknown. As little as 2% and up to 27% of patients who initially present with urticaria have been reported to have UV.6, 7, 8, 9 UV tends to have a chronic course.2 The median age of disease onset and age at diagnosis is 35–51 years, with more female patients affected.10, 11, 12, 13, 14, 15
Although most UV cases are of unknown cause, UV can be associated with drugs, infections, autoimmune and autoinflammatory diseases, or malignancy.2,6,11 The clinical spectrum is wide and varies from a mild urticarial rash to severe disease with systemic manifestations including fever, fatigue, pulmonary, gastrointestinal, renal, eye, and joint involvement.12,13,16,17 UV skin lesions often resolve with hyperpigmentation, and they are frequently associated with burning sensations or pain.12,13,16 Besides recurrent wheals, which persist in most cases of more than 24 hours to up to several days, UV patients can present with intermittent angioedema.12
Based on blood complement levels (C3, C4), UV is divided into normocomplementemic (normocomplementemic urticarial vasculitis, NUV) and hypocomplementemic subsets including hypocomplementemic urticarial vasculitis (HUV) and hypocomplementemic urticarial vasculitis syndrome (HUVS, McDuffie syndrome18). Most UV patients present with NUV (approx. 80%), whereas HUV/HUVS affects 9–21% of UV patients.6,10,11 Systemic symptoms and underlying diseases are more frequent in patients with hypocomplementemic subsets of UV.6,11 In HUV/HUVS patients, 1-10-year survival rates are higher than 80%.14 Fatal cases can be associated with underlying disease such as malignancy.2
Pathophysiologically, UV is held to involve type III hypersensitivity with deposition of antigen-antibody complexes and complement factors in the vascular lumen.19 Activation of the complement system leads to cutaneous neutrophil influx, increase in vascular permeability, and mast cell degranulation with consecutive release of further chemokines and cytokines.19,20
The treatment of UV depends on the clinical phenotype, the presence of systemic symptoms and/or underlying diseases. It is often a difficult-to-treat condition. Systemic corticosteroids are effective for the treatment of cutaneous symptoms in more than 80% of UV patients, but their long-term use comes with considerable adverse effects.2 The addition of immunomodulatory/immunosuppressive drugs can enable corticosteroid tapering and improvement of treatment responses. The anti-Immunoglobulin (Ig) E antibody omalizumab, cyclophosphamide, dapsone, mycophenolate mofetil, plasmapheresis, colchicine, hydroxychloroquine, intravenous immunoglobulin, cyclosporine, and nonsteroidal anti-inflammatory drugs have been described to be effective in case reports and small case series, but controlled studies are missing.2 As of now, no drugs have been approved for the treatment of patients with UV.
Despite the current knowledge of UV, no clinical guidelines, diagnostic criteria or treatment algorithms have been published so far for this disorder.2 To help with the development of international consensus-based recommendations for the classification of UV and the diagnostic workup and treatment of patients with UV, we aimed at assessing the current real-life management of UV patients. It is currently unknown which specialists see UV patients and why they are referred to them. Furthermore, we do not know which criteria and diagnostic tests are used to diagnose UV. We also have very little information on which treatments are used and with which outcome. Until now, there are no studies on the worldwide management of UV, and the approaches to the diagnostic workup and treatment of UV patients may differ globally.
To address these unmet needs, we conducted an online survey and examined (a) how UV patients are diagnosed and treated by international specialists including differences in approaches and (b) the greatest challenges in managing UV patients worldwide. In addition, we aimed to identify further unmet needs in the management of patients with UV.
Methods
Study survey
A web-based questionnaire (Suppl. Fig. 1) was designed and circulated among the co-authors and the members of the WINiT: Skin Allergy Committee of the World Allergy Organization (WAO). We then tested the questionnaire in 7 physicians involved in the care of UV patients (n = 4 from Germany, n = 1 from Japan, n = 1 from Russia, n = 1 from Turkey) and revised and finalized the questionnaire based on their feedback. The questionnaire is comprised of 14 questions including 4 single-choice and 10 multiple-choice questions. We assessed demographic data (country of residence, specialty, clinical experience, and type of practice) and data on the management of UV patients (number of UV patients seen per year, establishment of the diagnosis by referring doctor/survey participant, reasons for referral of UV patients, occurrence of cutaneous symptoms, occurrence of extracutaneous symptoms, diagnostic criteria, diagnostic work-up, comorbidities/potential causes, treatment options, and greatest challenges in UV management).
Recruitment and dissemination
Distribution of the questionnaire included emails to individuals in the World Allergy Organization (WAO) database, with no restrictions applied to the specialty, affiliation, or nationality of the participants (November 2018). The email contained a link (Internet address) to the online questionnaire. Responses were anonymous. The link to the questionnaire was further sent to the Urticaria Centers of Reference and Excellence (UCARE, www.ga2len-ucare.org)21 in the Global Allergy and Asthma European Network (GA2LEN) as well as to the Turkish Dermatology Society and the Japanese Society of Allergology, who distributed the link to their members. In addition, the survey link was posted online in the group of the Russian Society of Allergologists and Immunologists. A reminder was sent in January 2019, and the survey was completed in February 2019.
Statistical analysis
SPSS version 25 (Armonk, NY: IBM Corp, USA), Microsoft Excel 2010 (Microsoft Corp, USA), and GraphPad Prism Version 6.0 (La Jolla, CA, USA) were used for statistical analysis. Categorical variables were reported as frequencies and percentages. Median score and interquartile range were calculated for continuous variables including non-normally distributed data. Comparisons between groups were performed by using the Pearson Chi-squared test and Mann–Whitney U test. A Kruskal-Wallis H test was conducted to determine differences in a number of UV patients seen per year between different groups of physicians. Pairwise comparisons were performed using Dunn's (1964) procedure with a Bonferroni correction for multiple comparisons. Adjusted p-values are presented, and p-values of ≤0.05 are considered to indicate statistically significant differences.
Results
Demographics of study population
Of the 883 specialist physicians from 92 countries who participated in this study, most resided in Europe (46%). The most prevalent countries of residence were the United States (18%), Turkey (15%), and Brazil (7%, Table 1, Suppl. Table 1, Table 2). Three of 4 participating physicians (77%) were allergists and clinical immunologists, 22% were dermatologists (Table 1, 16% had ≥ 1 specialty).
Table 1.
Demographic data.
| Parameter | Respondents (n = 883) | |
|---|---|---|
| Country of residence, % (n/total) | North America | 18.9 (167) |
| Latin America | 18.7 (165) | |
| Europe | 46.2 (408) | |
| Africa/Middle-East | 3.8 (34) | |
| Asia-Pacific | 12.4 (109) | |
| Specialty, % (n/total) | Allergy/Immunology | 76.8 (674/877) |
| Dermatology | 22.1 (194/877) | |
| Pediatrics | 10.7 (94/877) | |
| Rheumatology | 3.2 (28/877) | |
| General practice | 1.9 (17/877) | |
| Other | 3.9 (34/877) | |
| >1 specialty indicated by the same respondent | 16.3 (143/877) | |
| Place of work, % (n/total) | Private practice | 47.7 (420/879) |
| University clinic | 41.2 (362/879) | |
| Hospital | 36.8 (324/879) | |
| Specialized urticaria centre | 3.9 (35/879) | |
| Other | 2.9 (26/879) | |
| >1 place of work indicated by the same respondent | 24.8 (218/879) | |
| Years of practice, median (IQR) | 15 (7–26) | |
| Number of patients with urticarial vasculitis seen per year, median (IQR) | 5 (2–10) | |
IQR: interquartile range
Table 2.
Physicians’ characteristics associated with seeing higher or lower numbers of UV patients per year.
| n, UV patients per year |
P-value | ||
|---|---|---|---|
| Median | IQR | ||
| Region of residence | |||
| NA (n = 163) | 2 | 1–4 | <0.001 |
| LA (n = 163) | 5 | 2–10 | |
| EU (n = 399) | 5 | 2–10 | |
| AME (n = 34) | 5 | 3–16 | |
| AP (n = 106) | 5 | 2–10 | |
| Specialtya | |||
| Dermatology (n = 153) | 5 | 3–13 | <0.001 |
| Allergy (n = 535) | 4 | 2–10 | |
| Pediatrics (n = 20) | 2 | 1–4 | |
| Place of worka | |||
| University clinic (n = 223) | 5 | 2–10 | <0.001 |
| Hospital (n = 159) | 4 | 2–7 | |
| Private practice (n = 245) | 3 | 1–6 | |
Respondents who fitted in two and more groups, e.g. had two specialties, were not included in the analysis; IQR: interquartile range; NA: North America; LA: Latin America; EU: Europe; AME: Africa/Middle-East; AP: Asia-Pacific
Almost 50% of participating physicians worked in private practice (Table 1). Participating physicians, on average, had 15 years of professional experience (range: 7–26 years). Eighty-four (84) percent % (716/854) of physicians responded that they are the ones who establish the diagnosis of UV in most of their UV patients, while only 16% primarily see patients with an already established diagnosis of UV.
Urticarial vasculitis is a rare disease
The median number of patients with UV seen per year was reported to be 5. Post hoc analyses revealed statistically significantly lower numbers of UV patients seen per year for physicians from North America (NA) as compared to Latin America (LA), Europe (EU), Africa/Middle-East (AME), or Asia-Pacific (AP, χ2(4) = 96.438, p < 0.001). The number of UV patients seen by dermatologists was significantly higher compared with allergists and pediatricians (χ2(5) = 26.910, p < 0.001). Pairwise comparisons showed that university physicians saw a higher number of UV patients compared with physicians working at non-university hospitals or in private practice (χ2(3) = 33.053, p < 0.001) (Table 2)
The most frequent reason for referring patients with UV to specialist physicians is to establish the diagnosis
"Establish a diagnosis" was the most frequent reason reported by respondents as to why patients with UV were referred to them. Other common reasons for referral of UV patients were treatment initiation and optimization, as well as requests for a second opinion and to perform basic and clinical research (Table 3).
Table 3.
The reasons why patients with UV are referred to the respondents’ place of work.
| Reasonsa | Respondents, % (n) |
|---|---|
| Establish a diagnosis | 77.2 (664) |
| Treatment initiation | 41.5 (357) |
| Second opinion | 34.2 (294) |
| Treatment optimization | 30.0 (258) |
| Clinical/basic research | 8.8 (76) |
| Other | 3.6 (31) |
Total number of respondents = 860;
Several responses were allowed from the same respondent
As compared to allergists, a higher number of dermatologists reported "treatment initiation" (50% vs 39%, χ2 = 6.374, p = 0.01) or "clinical/basic research" reasons (18% vs 6%, χ2 = 22.335, p < 0.001). The number of university physicians, who reported “establish a diagnosis”, “treatment initiation”, “second opinion”, and “clinical/basic research” as reasons for referral, was higher than the number of non-university or private practice physicians who did this (p < 0.05).
Physicians from North America or Europe more often reported “establish a diagnosis” as the reason for referral as compared to physicians from other regions (p < 0.05). A higher number of physicians from Europe compared with physicians from other regions reported that UV patients are referred to them for treatment initiation or to perform clinical/basic research (p ≤ 0.002).
Most physicians report that wheals, burning of the skin, and residual hyperpigmentation occur in 60–100% of UV patients
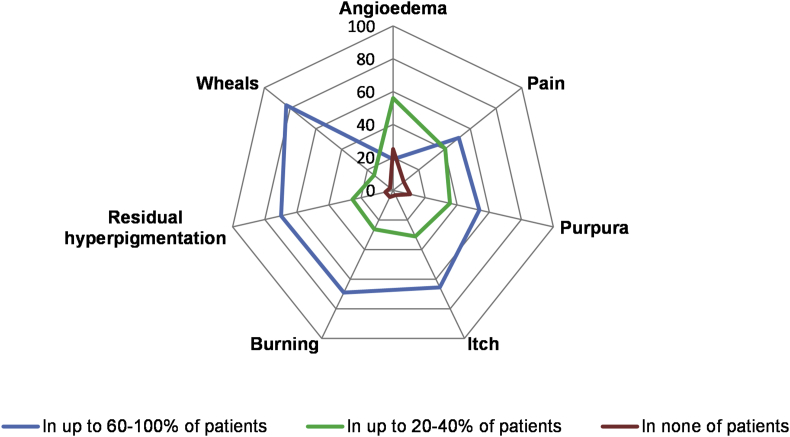
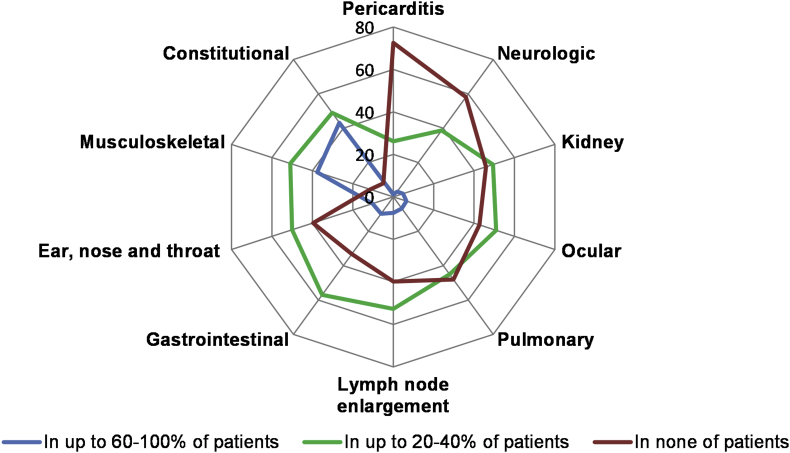
More than two-thirds of physicians observed wheals (83%), residual hyperpigmentation (70%), and burning of the skin (69%) in the majority, i.e. 60–100%, of UV patients (Fig. 1). With respect to systemic symptoms, 43% of physicians reported that most of their UV patients (i.e. 60–100%) have fatigue, asthenia, and/or fever. Thirty-eight (38) percent reported that their patients have musculoskeletal complaints, for example, arthralgia or arthritis. In contrast, only a few physicians, less than 10%, reported that more than 60% of their patients have ocular, pulmonary, kidney, or neurological symptoms (Fig. 2).
Fig. 1.
Most physicians report that wheals, residual hyperpigmentation, and burning of the skin occur in most of their UV patients∗. The figure depicts the % of physicians who reported the skin symptoms in up to 60–100% of UV patients (the blue line), in up to 20–40% of UV patients (the green line) and in none of the patients (the red line). ∗Several responses were allowed from the same respondent
Fig. 2.
The spectrum and rate of systemic symptoms in UV patients is variable∗. The figure depicts the % of physicians who reported the systemic symptoms in up to 60–100% of UV patients (the blue line), in up to 20–40% of UV patients (the green line) and in none of the patients (the red line). ∗Several responses were allowed from the same respondent
Wheals of longer than 24 hours duration, the results of histological analysis, and post-inflammatory hyperpigmentation are the most important features for establishing the diagnosis of UV
When asked to select the 3 most important features for establishing the diagnosis of UV, the most frequent ones were wheals of longer than 24 hours duration (72%), the results of histological analysis (63%), and post-inflammatory hyperpigmentation (46%) (Table 4).
Table 4.
The criteria for UV diagnosis.
| Answer Choicesa | Respondents, % (n) |
|---|---|
| Wheals predominantly >24 h | 72.4 (492) |
| Histological analysis | 63.2 (429) |
| Presence of post-inflammatory hyperpigmentation | 46.1 (313) |
| Poor response to antihistamines | 27.8 (189) |
| Systemic symptoms (e.g. fever, arthralgia, abdominal pain) | 24.9 (169) |
| High levels of inflammation markers, e.g. ESR, CRP | 22.2 (151) |
| Low complement levels | 17.2 (117) |
| Presence of underlying disease (e.g. malignancy, SLE) | 5.8 (40) |
| Other | 2.9 (20) |
Total number of respondents = 679;
Each respondent was asked to choose only the 3 most important signs and/or symptoms; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; SLE: systemic lupus erythematosus
Physicians residing in North America (74%), dermatologists (79%), and university physicians (75%) more frequently indicated that the results of histological analyses are the most important to establish the diagnosis of UV (p ≤ 0.01). Physicians who did not choose histological analysis chose “wheals predominantly >24 h” (80%), “presence of post-inflammatory hyperpigmentation” (55%), and/or “poor response to antihistamines” (42%) as important features for establishing the diagnosis of UV. Many of them were from Africa/Middle-East, non-dermatologists, and worked in a non-university hospital (Table 5).
Table 5.
Characteristics of physicians who chose or did not choose histological analysis as an important feature for establishing the diagnosis of UV
| Parameter | Chose histological analysis | Did not choose histological analysis but other features | |
|---|---|---|---|
| Region of residency | North America (n = 113) | 73.5% (83) | 26.5% (30) |
| Europe (n = 317) | 68.8% (218) | 31.2% (99) | |
| Latin America (n = 132) | 53.8% (71) | 46.2% (61) | |
| Asia-Pacific (n = 92) | 48.9% (45) | 51.1% (47) | |
| Africa/Middle-East (n = 25) | 48% (12) | 52% (13) | |
| Specialty | Dermatology (n = 118) | 79.7% (94) | 20.3% (24) |
| Allergology (n = 420) | 63.1% (265) | 36.9% (155) | |
| Other (n = 29) | 31.0% (9) | 69.0% (20) | |
| Place of work | University clinic (n = 183) | 75.4% (138) | 24.6% (45) |
| Private practice (n = 188) | 62.8% (118) | 37.2% (70) | |
| Hospital (n = 118) | 55.1% (65) | 44.9% (53) | |
The most common tests ordered in UV patients are complete blood count, erythrocyte sedimentation rate, C-reactive protein, complement components, antinuclear antibodies and skin biopsy
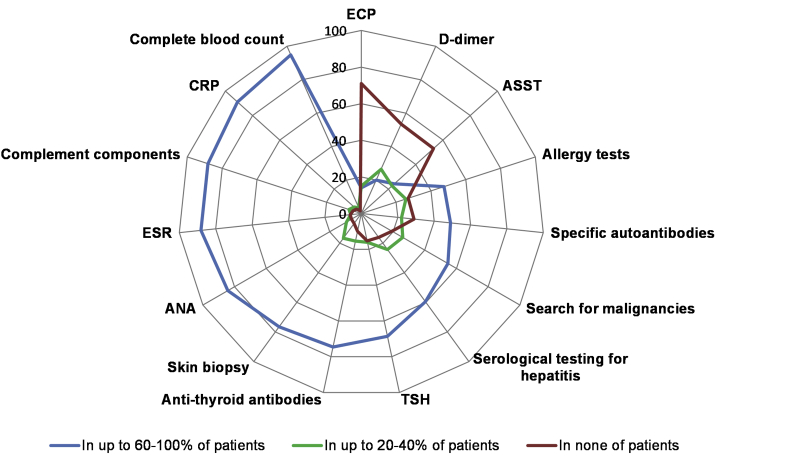
Most physicians reported that they order a complete blood count (95%), C-reactive protein (CRP) (91%), complement components (88%), ESR (88%), antinuclear antibodies (ANA) (84%), a skin biopsy (77%), antithyroid antibodies (75%), and thyroid stimulating hormone (67%) in most of their UV patients, i.e. in 60% or more. Some physicians responded that they order eosinophil cationic peptide (29%), autologous serum skin test (47%), and D-dimer (47%) in some of their UV patients. Two-thirds (73%) of physicians ordered allergy tests in UV patients (Fig. 3). More dermatologists than allergists performed skin biopsy in the majority, i.e. 60–100%, of UV patients (87% vs 77%, χ2 = 5.081, p = 0.024).
Fig. 3.
The percentages of physicians who performed or ordered different tests for the diagnostic workup in UV patients∗. The figure depicts the % of physicians who performed or ordered tests in up to 60–100% of UV patients (the blue line), in up to 20–40% of UV patients (the green line) and in none of the patients (the red line). ∗Several responses were allowed from the same respondent; TSH: thyroid stimulating hormone; ESR: erythrocyte sedimentation rate; ANA: antinuclear antibodies; ECP: eosinophil cationic protein; ASST: autologous serum skin test; CRP: C-reactive protein
UV is considered to be of unknown cause in most patients, and drugs and systemic lupus erythematosus are the most common identifiable causes
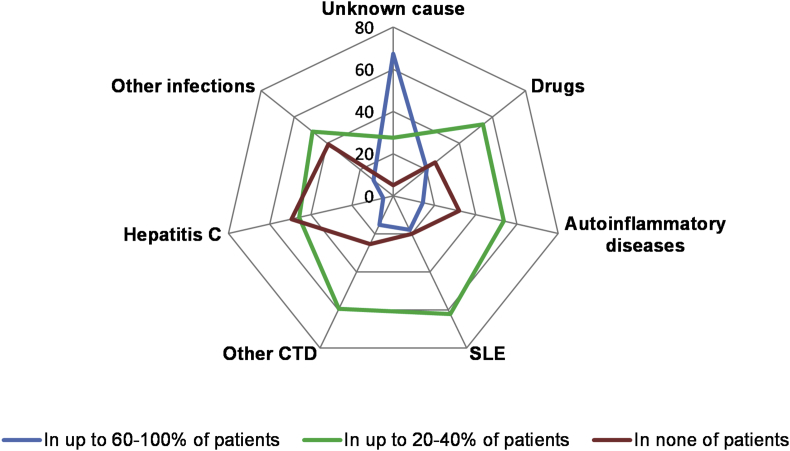
Nine of 10 physicians (89%; 540/609) reported that they look for underlying causes in their patients with UV. Two of three physicians (67%; 395/586) responded that they consider UV to be of unknown cause in most patients. With respect to revealed UV causes, 20% and 18% physicians identified drugs and systemic lupus erythematosus (SLE), respectively, in most patients (Fig. 4). Professionals who reported that they look for underlying causes of UV had more years of practice as compared to respondents who did not (median: 17 vs 10; p = 0.002).
Fig. 4.
The percentage of physicians who reported different comorbidities/potential causes of UV. The figure depicts the % of physicians who reported different comorbidities/potential causes of UV in up to 60–100% of UV patients (the blue line), in up to 20–40% of UV patients (the green line) and in none of the patients (the red line). CTD: connective tissue diseases; SLE: systemic lupus erythematosus
Many respondents prefer second generation antihistamines and/or corticosteroids as the first-line therapy of UV
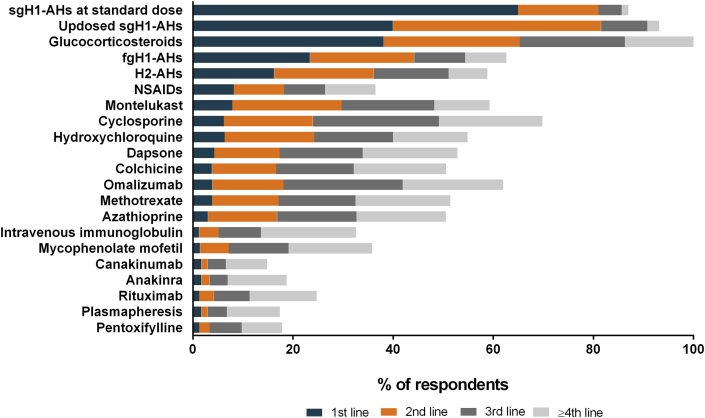
Two of three (65%) physicians reported that they use a second generation antihistamine (sgAH) in standard dose as the first-line therapy in patients with UV. High doses of an antihistamine and corticosteroids were the first-line therapy of 40% and 38% of physicians, respectively. Only 13% of physicians from North America reported that they use corticosteroids as the first-line therapy. Updosing of sgAHs and/or corticosteroids were the most common second-line therapy, cyclosporine and omalizumab the most common third- and fourth-line therapy (Fig. 5).
Fig. 5.
Medications used in UV. sgH1-AHs: second generation H1-antihistamines; fgH1-AHs: first generation H1-antihistamines; H2-AHs: H2-antihistamines; NSAIDs: nonsteroidal anti-inflammatory drugs
The greatest perceived challenges in the management of UV are limited efficacy of drugs and absence of clinical guidelines and treatment algorithms
Most physicians indicated that there are many challenges in managing patients with UV. The most commonly reported ones were that the treatments used are of limited efficacy (60%) and have adverse effects (48%) and that there are no clinical guidelines and treatment algorithms (59%) (Table 6).
Table 6.
The greatest challenges in managing UV patients.
| Answer Choicesa | Respondents, % (n) |
|---|---|
| Many drugs have limited efficacy | 59.9 (362) |
| No clinical guidelines and treatment algorithms exist | 58.8 (355) |
| Many drugs have potentially serious adverse effects | 47.7 (288) |
| Clinical diagnostic criteria are not clear | 43.0 (260) |
| Often severe and difficult-to-treat disease | 39.7 (240) |
| It is difficult to find an underlying disease (a cause of UV is usually unknown) | 38.9 (235) |
| Need for help from other specialists, especially in the case of underlying disease | 34.3 (207) |
| Novel treatment is not available or costs too high in my country of residence | 30.8 (186) |
| Histological diagnostic criteria are not clear | 25.7 (155) |
| I don't have enough clinical experience in the management of UV | 22.5 (136) |
Total number of respondents = 604;
Several responses were allowed from the same respondent
Discussion
This is the first worldwide research project that assesses personal physician preferences in managing UV patients.
As reported by the almost 900 physicians who participated in this project, UV is a rare disease with only 5 patients seen per physician per year on average. In the literature, UV has been described in 2.7% (n = 21) of 766 patients with cutaneous vasculitis.15 Although worldwide prevalence and incidence of UV is unknown, a point prevalence and the mean annual incidence rate of HUV/HUVS per one million Swedish inhabitants was estimated to be 9.5 and 0.7, respectively.14 After age and sex adjusted to the 2000 US caucasian population, the population-based incidence rate of UV was 0.5 per 100,000 person-years.22
In UV, as in other inflammatory skin diseases, the management should be aimed at (a) excluding differential diagnoses, primarily chronic spontaneous urticaria (CSU), (b) identifying relevant triggers and/or underlying causes, (c) assessing disease activity, impact, and control, and (d) appropriate treatment.
The most frequent reason for referring patients with UV to specialist physicians is to establish the diagnosis. Wheals of longer than 24 hours duration and residual hyperpigmentation were the most popular clinical features for UV diagnosis reported. Most physicians observed long-lasting wheals, residual hyperpigmentation and burning of the skin in 60–100% of UV patients (Fig. 6a, b). Regarding systemic symptoms, many physicians reported constitutional and musculoskeletal complaints in most UV patients. This is in line with the results of previous studies where the most frequent skin and systemic manifestations of UV were urticarial lesions and arthritis/arthralgia, respectively.14,15
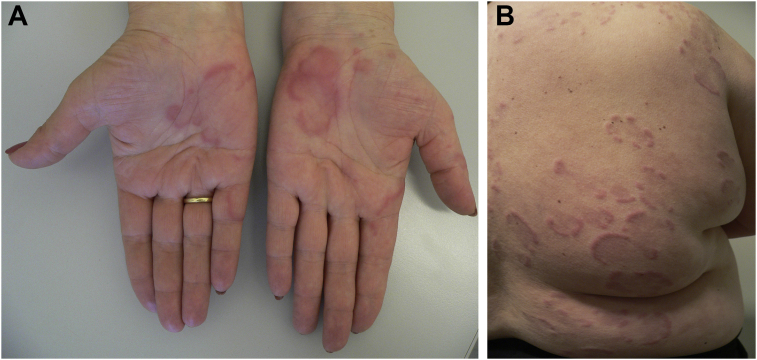
Fig. 6.
Long-lasting urticarial lesions on the hands (A) and the trunk (B) in two female adult patients with urticarial vasculitis
The histopathological evaluation of a lesional skin biopsy is considered the gold standard approach for diagnosing cutaneous vasculitis including UV.23 However, only 63% of physicians indicated that they perform skin biopsies for UV diagnosis. The international urticaria guideline recommends to perform histologic analyses of a skin biopsy as part of the extended diagnostic programme for the identification of underlying causes or eliciting factors and for ruling out possible differential diagnoses including UV.24 Importantly, some patients with clinical symptoms of UV, for example, poor response to antihistamines or wheals >24 hours, can have CSU. It is currently unclear why more than one-third of physicians including 37% of allergologists do not do skin biopsies for the diagnostic work-up of UV. Our results show that these physicians, instead of basing their diagnosis of UV on a skin biopsy, use persistent wheals, residual hyperpigmentation, and poor response to antihistamines as criteria for diagnosing UV. This approach can be expected to carry the risk of misdiagnosis. Our results also show that these physicians were mostly from Africa/Middle-East, non-dermatologists, and/or work in non-university hospitals (half of all participating physicians worked in private practice), and this information can help to increase the awareness and knowledge on UV in this physician population.
Most physicians ordered complete blood count, C-reactive protein, complement components, ESR, ANA, antithyroid antibodies, and thyroid-stimulating hormone in most patients. Inflammation markers, such as CRP or ESR, can be useful for differential diagnosis between UV and CSU.24 Analysis of serum levels of complement components allows distinguishing between NUV and HUV/HUVS. The relevance of antithyroid antibodies, and thyroid-stimulating hormone in UV, is unclear. Reportedly, a higher number of UV patients had elevated antithyroid antibodies as compared with patients with CSU.25 In a few cases, levothyroxine treatment resulted in improvement or remission of UV in patients with hypothyroidism due to autoimmune thyroid disease.2,26
Although 89% physicians look for underlying causes of UV, many of them considered UV to be of unknown cause in most patients. The literature supports this notion. In a small proportion of patients, UV appears to be due to drug intake or different systemic diseases, such as SLE, hepatitis C, or malignancy. In a systematic review, we could see comorbid SLE, malignancy, and infection in 11%, 7%, and 6% of UV patients, respectively.2 Withdrawal of relevant drugs, antiviral treatment of chronic hepatitis C, surgical therapy, or chemotherapy and/or radiation therapy of malignant neoplasms are usually associated with improvement or cure of UV.2,27
The severity of UV and the occurrence of relapses determine the treatment approach. Most respondents prefer second generation antihistamines and/or corticosteroids as the first-line therapy of UV. We previously reported that antihistamines are not effective in most UV cases.2 However, H1-antihistamines are safe and can be considered for the treatment of itch in UV patients, in children and/or patients without systemic symptoms. Corticosteroids are the mainstay of UV therapy and are also commonly used as initial therapy in this study and previous reports.2,14 In this study, we did not analyze which corticosteroids are used for the treatment of patients with UV. Also, it remains unclear how corticosteroids were dosed and for how long they were used. Corticosteroid dosing differed between previous studies, and long-term administration of corticosteroids can induce dose-dependent adverse effects.
Various alternative treatment options have been tried including immunosuppressive therapy and biologicals. For example, more than 10 cases of successful use of omalizumab, a monoclonal anti-IgE antibody, in UV patients with none or minimal response to treatment with antihistamines have been reported.2,28, 29, 30 Complete remission was achieved in 5 of 6 patients with UV within 7 days after treatment with IL-1β inhibitors. In all but 1 patient, treatment with IL-1β inhibitors allowed discontinuation of corticosteroids. UV relapsed after an IL-1 blocker discontinuation.31 In another study, canakinumab, a long-acting fully humanized monoclonal anti–IL-1β antibody, was effective in 10 patients with active UV.32 Other frequently reported treatment modalities include cyclosporine, colchicine, hydroxychloroquine, azathioprine, methotrexate, and dapsone.
The limitations of our study include a possible bias due to selection of participants, the use of an online non-validated questionnaire, and the heterogeneous approach to the UV diagnosis. Moreover, our results on the impact of physician specialty should be interpreted with caution, since allergology, the most common specialty in our study, is a subspecialty, and dermatology is the main specialty in some countries.
Unmet needs
The greatest challenges reported by physicians are the absence of clinical guidelines and treatment algorithms as well as the limited efficacy of available drugs and their adverse effects.
First, there is a need for the proper definition and classification of UV. The recently published update of the nomenclature for cutaneous vasculitis based on the 2012 Revised Chapel Hill Consensus Conference distinguishes HUV/HUVS from NUV, “a skin-limited vasculitis, not accompanied by systemic involvement” with the absence of anti-C1q antibodies.1 However, this classification does not take into account that some NUV patients have systemic symptoms, and it does not differentiate HUV from HUVS. In addition, clinical and histological criteria of UV should be clarified and developed. For this, further specialists such as dermatohistopathologists should be involved.
Second, there is a need for easy-to-perform, cost-effective, and noninvasive tests for differentiating UV from urticaria.33 For example, Suh et al. showed that dermoscopic red or purpuric dots/globules and a purple-brown background may be useful distinguishing dermoscopic features of UV and can help in differentiating early UV from early common urticaria lesions.34 Further prospective studies should assess whether these dermoscopic signs correlate with clinical symptoms and skin biopsy results. The delay in diagnosis and the rate of misdiagnosis in patients with UV are currently unknown but must be expected to be long and high, respectively. The development and availability of better diagnostic tests can help to change this.
Third, no tools for assessing disease activity, impact, and control of UV exist so far. Although not yet validated, the UV activity score (UVAS) for patient daily self-assessment may be promising tool for assessing UV activity/severity.32 It includes 5 subscales corresponding to the 5 key symptoms of UV: wheals, burning/pruritus, residual skin pigmentation, joint pain, and general symptoms. UVAS scores range from 0 to 10 per day (0 = none; 10 = very severe).
Fourth, prognostic factors of UV duration and severity should be evaluated. Serum, histologic, and immunopathologic markers should be assessed as predictors of response to treatment.
Finally, no drugs have been approved for the treatment of patients with UV. Prospective studies that investigate new, effective, and safe drugs for the treatment of UV are warranted. Together with the development of a stepwise algorithm of treatment of UV, this will improve the management of UV in everyday clinical practice.
Conclusion
In summary, UV is a rare but challenging disease worldwide. Many physicians do not perform skin biopsy, a gold standard for UV diagnosis. This may lead to delays in diagnosis and misdiagnoses, for example, as chronic spontaneous urticaria, and to inadequate treatment. Other pitfalls and challenges in the management of UV include the limited efficacy and safety of treatments and the absence of clinical guidelines and treatment algorithms. These unmet needs should be addressed by further research and with future initiatives.
Funding
Not applicable.
Declaration of Competing Interest
The authors declare that they have no relevant conflicts of interest in relation to this work.
Acknowledgements
The authors thank the members of the GA2LEN network of Urticaria Centers of Reference and Excellence (UCARE, www.ga2len-ucare.com) for their support of this study. The study was also supported by the ‘‘Russian Academic Excellence Project 5–100”. P.K. was supported by a GA2LEN stipend. The authors also thank the Board of Directors of the World Allergy Organization for support of the project on which this report is based.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2020.100107.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Caproni M., Verdelli A. An update on the nomenclature for cutaneous vasculitis. Curr Opin Rheumatol. 2019;31:46–52. doi: 10.1097/BOR.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 2.Kolkhir P., Grakhova M., Bonnekoh H., Krause K., Maurer M. Treatment of urticarial vasculitis: a systematic review. J Allergy Clin Immunol. 2019;143:458–466. doi: 10.1016/j.jaci.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Wills W., Lond M. Two cases of haemorrhagic urticaria. Lancet. 1890;135:1296–1297. [Google Scholar]
- 4.McCommbs R.P., Patterson J.F., Macmahon H.E. Syndromes associated with allergic vasculitis. N Engl J Med. 1956;255:251–261. doi: 10.1056/NEJM195608092550601. [DOI] [PubMed] [Google Scholar]
- 5.Zuberbier T., Maurer M. Urticarial vasculitis and Schnitzler syndrome. Immunol Allergy Clin. 2014;34:141–147. doi: 10.1016/j.iac.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Dincy C.V., George R., Jacob M., Mathai E., Pulimood S., Eapen E.P. Clinicopathologic profile of normocomplementemic and hypocomplementemic urticarial vasculitis: a study from South India. J Eur Acad Dermatol Venereol. 2008;22:789–794. doi: 10.1111/j.1468-3083.2007.02641.x. [DOI] [PubMed] [Google Scholar]
- 7.O'Donnell B., Black A.K. Urticarial vasculitis. Int Angiol. 1995;14:166–174. [PubMed] [Google Scholar]
- 8.Cardoso P.A., de Oliveira Z.P., Alves V.A., Candelori I., Croce J., Rivitti E.A. Urticarial vasculitis. Allergol Immunopathol. 1990;18:191–195. [PubMed] [Google Scholar]
- 9.Peteiro C., Toribio J. Incidence of leukocytoclastic vasculitis in chronic idiopathic urticaria. Study of 100 cases. Am J Dermatopathol. 1989;11:528–533. doi: 10.1097/00000372-198912000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Davis M.D., Daoud M.S., Kirby B., Gibson L.E., Rogers R.S. Clinicopathologic correlation of hypocomplementemic and normocomplementemic urticarial vasculitis. J Am Acad Dermatol. 1998;38:899–905. [PubMed] [Google Scholar]
- 11.Kulthanan K., Cheepsomsong M., Jiamton S. Urticarial vasculitis: etiologies and clinical course. Asian Pac J Allergy Immunol. 2009;27:95–102. [PubMed] [Google Scholar]
- 12.Jachiet M., Flageul B., Deroux A. The clinical spectrum and therapeutic management of hypocomplementemic urticarial vasculitis: data from a French nationwide study of fifty-seven patients. Arthritis Rheum. 2015;67:527–534. doi: 10.1002/art.38956. [DOI] [PubMed] [Google Scholar]
- 13.Tosoni C., Lodi-Rizzini F., Cinquini M. A reassessment of diagnostic criteria and treatment of idiopathic urticarial vasculitis: a retrospective study of 47 patients. Clin Exp Dermatol. 2009;34:166–170. doi: 10.1111/j.1365-2230.2008.02891.x. [DOI] [PubMed] [Google Scholar]
- 14.Sjowall C., Mandl T., Skattum L., Olsson M., Mohammad A.J. Epidemiology of hypocomplementaemic urticarial vasculitis (anti-C1q vasculitis) Rheumatology. 2018;57:1400–1407. doi: 10.1093/rheumatology/key110. [DOI] [PubMed] [Google Scholar]
- 15.Loricera J., Calvo-Río V., Mata C. Urticarial vasculitis in northern Spain: clinical study of 21 cases. Medicine. 2014;93:53–60. doi: 10.1097/MD.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis M.D., Brewer J.D. Urticarial vasculitis and hypocomplementemic urticarial vasculitis syndrome. Immunol Allergy Clin. 2004;24:183–213. doi: 10.1016/j.iac.2004.01.007. vi. [DOI] [PubMed] [Google Scholar]
- 17.Grotz W., Baba H.A., Becker J.U., Baumgartel M.W. Hypocomplementemic urticarial vasculitis syndrome: an interdisciplinary challenge. Dtsch Arztebl Int. 2009;106:756–763. doi: 10.3238/arztebl.2009.0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDuffie F.C., Sams W.M., Maldonado J.E., Andreini P.H., Conn D.L., Samayoa E.A. Hypocomplementemia with cutaneous vasculitis and arthritis. Possible immune complex syndrome. Mayo Clin Proc. 1973;48:340–348. [PubMed] [Google Scholar]
- 19.Mehregan D.R., Gibson L.E. Pathophysiology of urticarial vasculitis. Arch Dermatol. 1998;134:88–89. doi: 10.1001/archderm.134.1.88. [DOI] [PubMed] [Google Scholar]
- 20.Venzor J., Lee W.L., Huston D.P. Urticarial vasculitis. Clin Rev Allergy Immunol. 2002;23:201–216. doi: 10.1385/CRIAI:23:2:201. [DOI] [PubMed] [Google Scholar]
- 21.Maurer M., Metz M., Bindslev-Jensen C. Definition, aims, and implementation of GA2LEN urticaria centers of reference and excellence. Allergy. 2016;71:1210–1218. doi: 10.1111/all.12901. [DOI] [PubMed] [Google Scholar]
- 22.Arora A., Wetter D.A., Gonzalez-Santiago T.M., Davis M.D.P., Lohse C.M. Incidence of leukocytoclastic vasculitis, 1996 to 2010: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:1515–1524. doi: 10.1016/j.mayocp.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlson J.A. The histological assessment of cutaneous vasculitis. Histopathology. 2010;56:3–23. doi: 10.1111/j.1365-2559.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- 24.Zuberbier T., Aberer W., Asero R. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393–1414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 25.Cherrez-Ojeda I., Vanegas E., Mata V.L. Autoimmune thyroid disease and urticarial vasculitis: is there a significant association? Allergy Asthma Clin Immunol. 2019;15:25. doi: 10.1186/s13223-019-0339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granier F., Marchand C., Navarranne A., Hermier C., Perrot H. Urticaire systémique associée à une thyroïdite auto-immune. Rev Med Interne. 1987;8:169–172. doi: 10.1016/s0248-8663(87)80166-2. [DOI] [PubMed] [Google Scholar]
- 27.Younis A.A. Urticarial vasculitis as an initial manifestation of colonic carcinoma: a case report and review of the literature. Reumatismo. 2018;70:259–263. doi: 10.4081/reumatismo.2018.1052. [DOI] [PubMed] [Google Scholar]
- 28.Rattananukrom T., Svetvilas P., Chanprapaph K. Successful treatment of normocomplementemic urticarial vasculitis with omalizumab: a report of three cases and literature review. Asian Pac J Allergy Immunol. 2019;2 doi: 10.12932/AP-050918-0402. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.de Brito M., Huebner G., Murrell D., Bullpitt P., Hartmann K. Normocomplementaemic urticarial vasculitis: effective treatment with omalizumab. Clin Transl Allergy. 2018;8:37. doi: 10.1186/s13601-018-0222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degirmentepe E.N., Kızıltac K., Etikan P., Singer R., Memet B., Kocaturk E. Omalizumab as a Succesfull therapy in normocomplementemic urticarial vasculitis: a series of four patients and review of the literature. Ann Dermatol. 2019;31:335–338. doi: 10.5021/ad.2019.31.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bettuzzi T., Deroux A., Jachiet M. Dramatic but suspensive effect of interleukin-1 inhibitors for persistent urticarial vasculitis: a French multicentre retrospective study. Ann Rheum Dis. 2019:1446–1448. doi: 10.1136/annrheumdis-2019-215605. [DOI] [PubMed] [Google Scholar]
- 32.Krause K., Mahamed A., Weller K., Metz M., Zuberbier T., Maurer M. Efficacy and safety of canakinumab in urticarial vasculitis: an open-label study. J Allergy Clin Immunol. 2013;132 doi: 10.1016/j.jaci.2013.04.008. 751-4.e5. [DOI] [PubMed] [Google Scholar]
- 33.Maurer M., Magerl M., Metz M., Siebenhaar F., Weller K., Krause K. Practical algorithm for diagnosing patients with recurrent wheals or angioedema. Allergy. 2013;68:816–819. doi: 10.1111/all.12153. [DOI] [PubMed] [Google Scholar]
- 34.Suh K.S., Kang D.Y., Lee K.H. Evolution of urticarial vasculitis: a clinical, dermoscopic and histopathological study. J Eur Acad Dermatol Venereol. 2014;28:674–675. doi: 10.1111/jdv.12263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.