Summary
Many cancer vaccines are not successful in clinical trials, mainly due to the challenges associated with breaking immune tolerance. Herein, we report a new strategy using an adjuvant-protein-antigen (three-in-one protein conjugates with built-in adjuvant) as an anticancer vaccine, in which both the adjuvant (small-molecule TLR7 agonist) and tumor-associated antigen (mucin 1, MUC1) are covalently conjugated to the same carrier protein (BSA). It is shown that the protein conjugates with built-in adjuvant can increase adjuvant's stimulation, prevent adjuvant's systemic toxicities, facilitate the codelivery of adjuvants and antigens, and enhance humoral and cellular immune responses. The IgG antibody titers elicited by the self-adjuvanting three-in-one protein conjugates were significantly higher than those elicited by the vaccine mixed with TLR7 agonist (more than 15-fold) or other traditional adjuvants. Importantly, the potent immune responses against cancer cells suggest that this new vaccine construct is an effective strategy for the personalized antitumor immunotherapy.
Subject Areas: Conjugate, Biochemistry, Immunology
Graphical Abstract
Highlights
-
•
Adjuvant-protein-antigen protein conjugates act as new cancer vaccine strategy
-
•
Built-in adjuvant of TLR7 agonist can reduce toxicities and enhance immune stimulations
-
•
Three-in-one protein conjugates boost potent immune responses against cancer cells
Conjugate; Biochemistry; Immunology
Introduction
Aberrantly glycosylated mucin 1 (MUC1) is an important tumor-associated antigen of epithelial cells, mainly due to its overexpression on the tumor cell surface together with the formation of truncated glycans and exposed peptide epitopes (Lloyd et al., 1996). Therefore, the MUC1 glycoprotein constitutes a promising target (Acres and Limacher, 2005, Barratt-Boyes, 1996, Bhatia et al., 2019, Cheever et al., 2009, Singh and Bandyopadhyay, 2007) for tumor immunotherapies using a vaccination or chimeric antigen receptor T cell (CAR T) strategy. However, because most tumor-associated antigens act as autoantigens, which are tolerated by the immune system and unable to elicit potent immune responses, many antitumor vaccines have encountered difficulties in clinical trials (Tang et al., 2008, Tang et al., 2018). Therefore, it is highly necessary to develop a new strategy for antitumor vaccines to promote potent immunity to overcome the poor antigenicity of tumor-associated antigens and kill tumor cells (Gaidzik et al., 2013, Hossain and Wall, 2016, Martínez-Sáez et al., 2017, Rivalland et al., 2015).
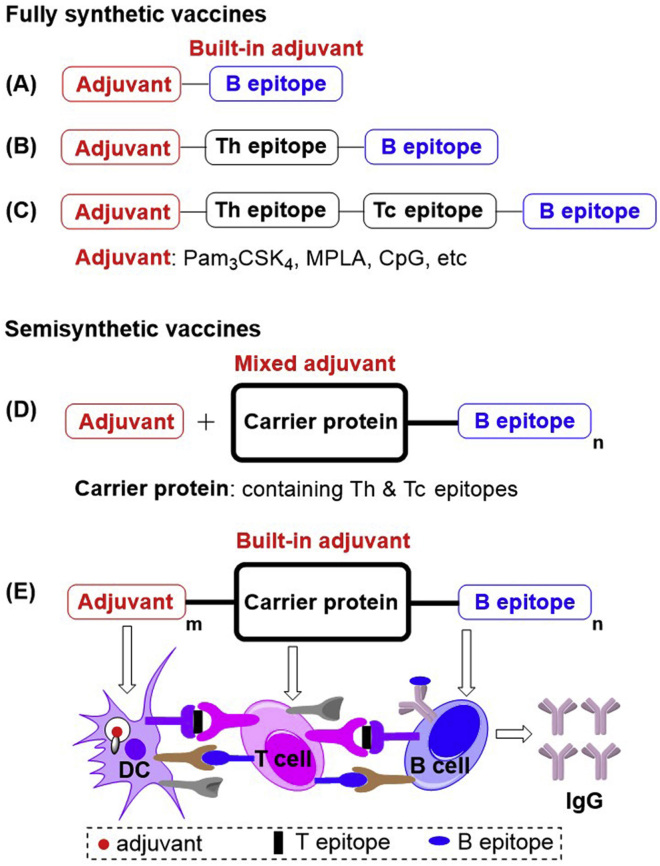
In cancer vaccine research, tremendous efforts have been made to improve the immunogenicity of tumor-associated antigens. In fully synthetic anticancer vaccines, such as two-component anticancer vaccines (Scheme 1A) (Cai et al., 2014, Hossain et al., 2018, Kaiser et al., 2010, Liu et al., 2016, Toyokuni et al., 1994, Wang et al., 2012, Wang et al., 2019, Wilkinson et al., 2010, Yin et al., 2017), three-component anticancer vaccines with Th epitopes (Scheme 1B) (Abdel-Aal et al., 2014, Cai et al., 2017, Ingale et al., 2007, Wilkinson et al., 2011, Wu et al., 2018a), and multicomponent anticancer vaccines with CD4+ T helper cell (Th) and CD8+ T cytotoxic/killer cell (Tc) epitopes (Scheme 1C) (Renaudet et al., 2008, Renaudet et al., 2010), built-in adjuvants have been proven to efficiently stimulate the immune system to recognize tumor-associated antigens and induce increased levels of antibodies against B epitopes. The generally utilized built-in adjuvants include Pam3CSK4 (TLR1/2 lipopeptide ligand) (Abdel-Aal et al., 2014, Cai et al., 2014, Cai et al., 2017, Hossain et al., 2018, Ingale et al., 2007, Kaiser et al., 2010, Toyokuni et al., 1994, Wilkinson et al., 2010, Wilkinson et al., 2011), monophosphoryl lipid A (MPLA; TLR4 agonist) (Wang et al., 2012), CpG-ODN 1826(TLR9 agonist) (Abdel-Aal et al., 2014), αGalCer (NKT cell agonist) (Yin et al., 2017), etc. Results have indicated that immune responses against tumor-associated antigens might increase gradually as the number of vaccine components increases, but the difficulty of synthesis is also raised. Previously, we reported that fully synthetic vaccines with a built-in or mixed NKT cell agonist acted as a potent adjuvant simplified vaccine construction and achieved antibody class switching from IgM to IgG (Chen et al., 2019, Du et al., 2019, Liu and Guo, 2017, Yin et al., 2017). Alternatively, in semisynthetic anticancer vaccines (Scheme 1D), tumor-associated antigens are usually conjugated to different carriers, including BSA (Cai et al., 2012, Dziadek et al., 2005, Hoffmann-Röder and Johannes, 2011), diphtheria toxoid cross-reactive material (CRM) 197 (DT) (Lee et al., 2014), tetanus toxoid (TTox) (Hoffmann-Röder et al., 2010, Oberbillig et al., 2012, Straßburger et al., 2018), keyhole limpet hemocyanin (KLH) (Xiao et al., 2016, Zhu et al., 2009), and virus-like particles (VLPs) (Wu et al., 2018b, Yin et al., 2018), then these conjugates are mixed with adjuvants. Because the multiple Th and Tc epitopes of carrier proteins can synergistically activate immunity, semisynthetic anticancer vaccines generally produce improved immune responses. However, it is still a major challenge to develop potent anticancer immunotherapies.
Scheme 1.
The Cancer Vaccines Comprising Built-in or Mixed Adjuvants.
(A) Two-component vaccines; (B) three-component vaccines with Th epitopes; (C) multicomponent vaccines with Th and Tc epitopes; (D and E) traditional semisynthetic vaccines with mixed adjuvants.
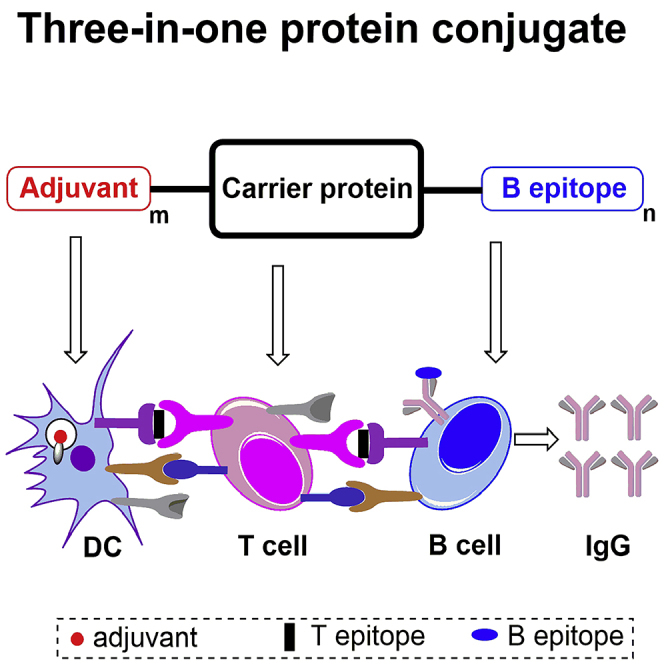
To address the challenge of antitumor vaccines, we present a novel strategy using three-in-one protein conjugates (Scheme 1E) with built-in adjuvants on carrier proteins that exploit the advantages of both fully synthetic vaccines and semisynthetic vaccines. In this strategy, the multiple built-in adjuvants have enhanced activity due to their cluster effect, prevent adjuvant's systemic toxicity, and the adjuvant's codelivery with antigens to the lymph nodes for immune stimulation is guaranteed. Additionally, the carrier proteins also contain various Tc and Th epitopes to elicit synergistic immune help for T-cell-dependent B epitopes.
Toll-like receptors (TLRs) are highly conserved cellular receptors that recognize unique molecular cell wall and nucleic acid components of invading pathogens and have the potential to regulate the activation of antigen-presenting cells, subsequently strengthening the signaling of costimulatory molecules and the secretion of many cytokines (Dowling, 2018). In particular, TLR7 mediate recognition of purine-rich ssRNA in the endosome to elicit immune responses to the recognized pathogens; numerous studies have demonstrated that conjugation of small molecular TLR7 agonists (TLR7a) to various polymers (Chan et al., 2009, Francica et al., 2016, Kim et al., 2016, Lynn et al., 2015, Lynn et al., 2019, Shinchi et al., 2015, Van Herck et al., 2018, Yoo et al., 2018) or proteins (Donadei et al., 2016, Feng et al., 2013, Gao et al., 2014, Gao et al., 2015, Gao et al., 2016, Oh and Kedl, 2010, Wu et al., 2007) can facilitate trafficking to the lymph nodes, enhance immunostimulatory activities, and decrease sideeffects due to the clustered arrangement of TLR7a adjuvants conjugated on carriers.
Therefore, we employed the strategy of a self-adjuvanting three-in-one protein conjugate for an antitumor vaccine for the first time and synthesized the vaccine conjugate TLR7a-BSA-MUC1 (adjuvant-protein-antigen), in which several small-molecule TLR7a (Donadei et al., 2016, Gao et al., 2014, Gao et al., 2015) were conjugated to the carrier protein BSA, the tumor-associated MUC1 antigens (Bermejo et al., 2018, Compañón et al., 2019) served as B epitopes, and their PDTRP motifs contained Tn antigens (Burchell et al., 1989, Du et al., 2019, Karsten et al., 1998). The immunological results revealed that the TLR7a-BSA-MUC1 vaccine triggered a robust response with production of antibodies targeting MUC1 antigens and exhibiting strong binding to MCF-7 cancer cells and B16-MUC1 cells expressing MUC1 antigens.
Results and Discussion
Preparation of Vaccine Components
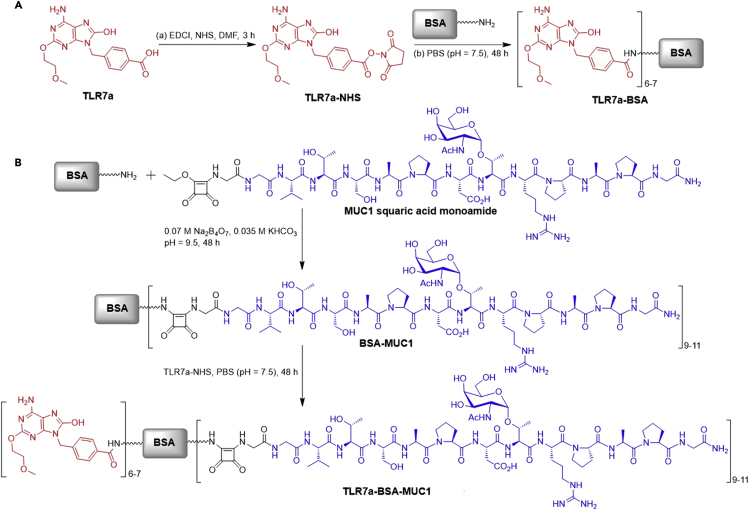
The synthesis of TLR7a-BSA conjugate and three-in-one protein conjugate TLR7a-BSA-MUC1 is shown in Scheme 2. The small-molecule TLR7a was converted to active ester TLR7a-NHS (Gao et al., 2016), which reacted with the protein to form conjugates. The MUC1 antigen (sequence, GVTSAPDTRPAPG) containing a Tn antigen in the PDTRP motif was synthesized and conjugated to BSA through the squaric acid diethyl ester method (Cai et al., 2012, Du et al., 2019, Dziadek et al., 2005). Next, a TLR7a was covalently attached to the carrier protein of the MUC1-BSA conjugate. MALDI-TOF MS indicated that there was an average of 6–7 TLR7a and 9–11 MUC1 glycopeptides covalently linked to BSA.
Scheme 2.
Synthesis of Vaccine Components
(A) Synthesis of TLR7a-BSA; (B) synthesis of TLR7a-BSA-MUC1 from MUC1 glycopeptide squaric acid monoamide and Fmoc-SPPS.
To evaluate immune responses induced by the TLR7a-BSA-MUC1 vaccine in vivo, a group of mice (n = 5) was intraperitoneally injected with TLR7a-BSA-MUC1 (dose of 21 μg of MUC1 peptide) on days 1, 15, and 29 (Scheme S8) (Chen et al., 2019, Du et al., 2019). To decipher the essentiality of the various components of the vaccine, control groups of mice were injected with BSA-MUC1, TLR7a-BSA and MUC1, TLR7a and BSA-MUC1, MPLA and BSA-MUC1, Pam3CSK4 and BSA-MUC1, alum (an adjuvant approved for human applications) and BSA-MUC1, with the same dose of MUC1 and optimized doses of the adjuvants (Table S1).
Evaluation of Cytokine Levels
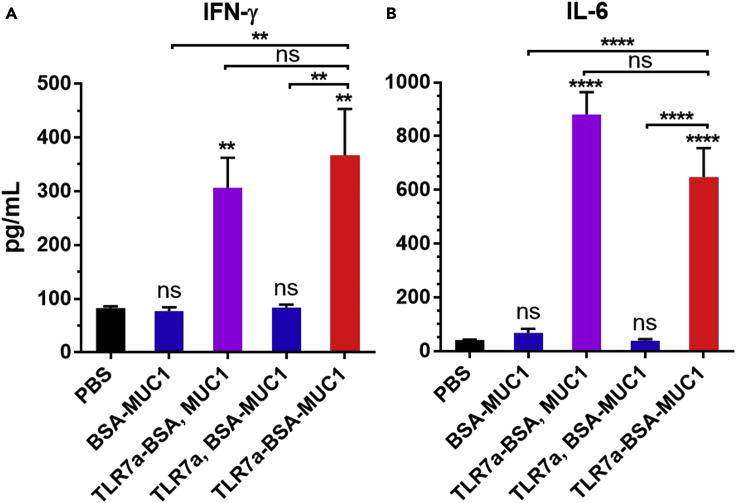
To investigate whether the covalent attachment of three components affected immune activation, the differences in the production of the Th1-type cytokine interferon-γ (IFN-γ) and Th2- and Th17-type pro-inflammatory cytokine interleukin 6 (IL-6) were evaluated (Figures 1 and S1) (Chen et al., 2019, Du et al., 2019). Mice immunized with the protein conjugates with built-in adjuvant (TLR7a-BSA and MUC1 or TLR7a-BSA-MUC1) showed significantly increased release of IFN-γ and IL-6, exhibiting four- to ten-fold higher cytokine levels than mice treated with a vaccine with mixed TLR7a adjuvants (TLR7a and BSA-MUC1) or without adjuvant.
Figure 1.
Cytokine Quantification of Mouse IFN-γ and IL-6
The secretion of the cytokines IFN-γ (A) and IL-6 (B) was measured in serum samples from vaccinated mice at 2 h after the first immunization. Differences were determined by ANOVA using Tukey's HSD test. Asterisks represent statistically significant differences (∗∗∗∗p < 0.0001, ∗∗p < 0.01), and ns indicates no significant difference compared with the PBS group. Data are shown as the mean ± SEM of five mice and are representative of three separate experiments.
The ability to generate high levels of cytokines was mainly contributed by covalent conjugation of several copies of TLR7a on one carrier protein to achieve cluster effect, and facilitated draining of the adjuvant TLR7a together with the carrier protein to the lymph nodes, uptake and processing by the same one antigen-presenting cell (APC) (Gao et al., 2014, Gao et al., 2015, Gao et al., 2016, Wu et al., 2007). In addition, protein conjugates can prevent the small molecular TLR7a to rapidly enter the blood to cause systemic toxicity of waste inflammation (Dowling, 2018).
Evaluation of Antigen-Specific Antibodies
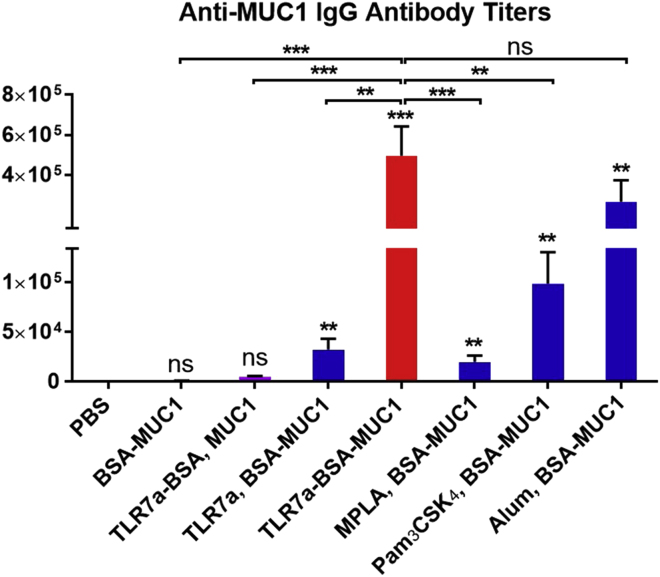
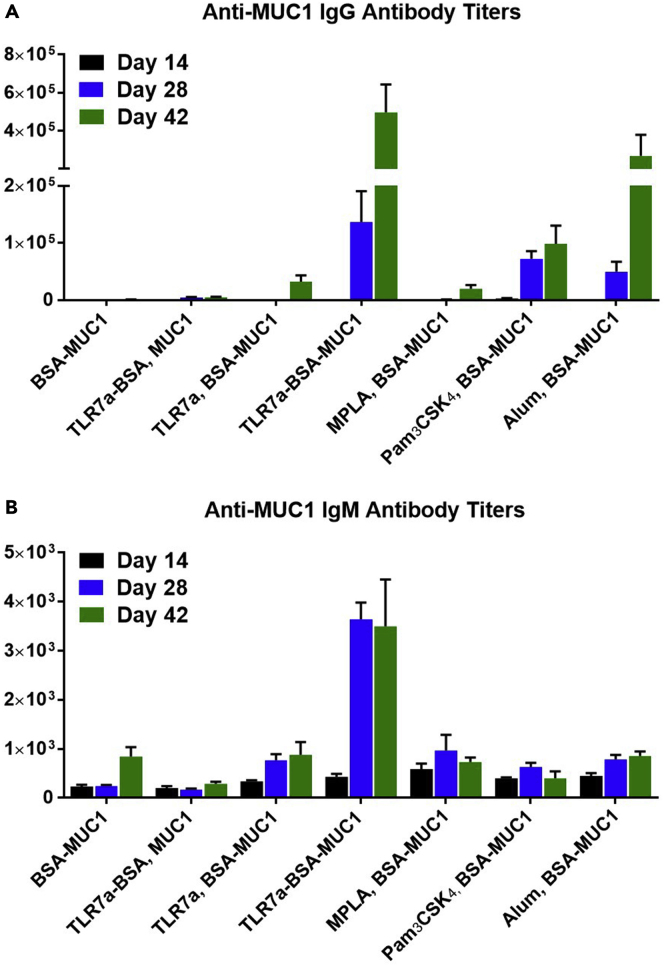
The anti-MUC1 antibody titers induced by vaccine candidates were measured by enzyme-linked immunosorbent assay (ELISA) (Du et al., 2019), in which a low concentration (0.125 μg/mL) of biotinylated MUC1 (Figures S39–S43) (Miermont et al., 2008) formed a complex with avidin to act as the coating antigen. As shown in Figure 2, the three-in-one protein conjugate vaccine (TLR7a-BSA-MUC1) elicited the highest IgG antibody titers against the MUC1 antigen: the titers were 500-fold higher than those of BSA-MUC1 without adjuvant, 100-fold higher than those of TLR7a-BSA and MUC1, and 15-fold higher than those of TLR7a and BSA-MUC1. Moreover, the anti-MUC1 IgG antibody titers of the three-in-one protein conjugate are also significantly higher than those of the mixed-adjuvant vaccines with the classic TLR4 agonist MPLA or TLR1/2 agonist Pam3CSK4 and slightly higher than those of the vaccine absorbed on the traditional alum adjuvant. This finding indicated that it is essential to covalently conjugate both the adjuvant TLR7a and the antigenic MUC1 glycopeptide to the carrier protein to produce the most potent vaccine, and this approach may provide the advantages of the cluster effect of the adjuvant and codelivery of the adjuvant and antigen on the carrier protein for immune cell uptake, processing, and immunomodulation (Donadei et al., 2016, Feng et al., 2013, Gao et al., 2014, Gao et al., 2015, Gao et al., 2016, Oh and Kedl, 2010, Wu et al., 2007).
Figure 2.
Quantification of Antigen-specific Antibodies Production via ELISA
Anti-MUC1 IgG antibody titers were detected in serum samples from vaccinated mice collected on day 42. Differences were determined by ANOVA and Tukey's HSD test. Asterisks represent statistically significant differences (∗∗∗p < 0.001, ∗∗p < 0.01), and ns indicates no significant difference compared with the PBS group. Data are shown as the mean ± SEM of five mice and are representative of three separate experiments.
The titers of the anti-MUC1 IgG antibodies elicited by the three-in-one protein conjugate increased gradually following immunizations (Figures 3A and S2–S4), whereas the IgM titers did not increase between days 28 and 42 (Figures 3B and S5–S7). Therefore, the three-in-one protein conjugates enhanced B cell stimulation and promoted differentiation into memory B cells and plasma cells for the induction of high-affinity IgG antibodies.
Figure 3.
Quantification of Antigen-Specific Antibodies Production via ELISA
Anti-MUC1 IgG (A) and IgM (B) antibody titers in serum samples from vaccinated mice were measured on days 14, 28, and 42. Data are shown as the mean ± SEM of five mice and are representative of three separate experiments.
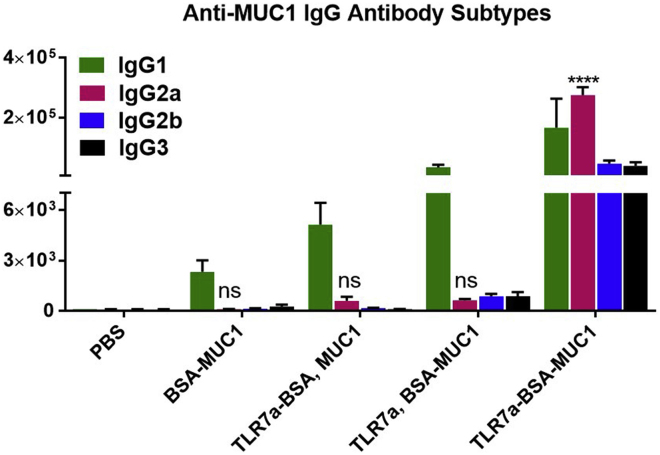
Evaluation of Antibody Subtypes
To evaluate the immunological properties of these vaccines, an analysis of IgG subtypes was performed. As depicted in Figures 4 and S8–S15, the IgG1 subclasses of the MUC1-specific antibodies were dominant for most vaccines, such as BSA-MUC1 alone, BSA-TLR7a and MUC1, or TLR7a and BSA-MUC1. In contrast, when the three-in-one protein conjugate with covalently ligated TLR7a adjuvants was used, it induced Th1-skewed immune responses and produced approximately similar titers of IgG1, IgG2a, IgG2b, and IgG3 antibodies, and the titers of the Th1-type IgG2a antibodies were the highest. In addition, the vaccines containing the mixed adjuvants (MPLA, Pam3CSK4, or alum) and BSA-MUC1 displayed skewed Th2-type responses with high levels of IgG1 antibodies and much lower levels of IgG2a antibodies than those induced by the TLR7a-BSA-MUC1 vaccine (Figure S9).
Figure 4.
Quantification ofAntibody Subtypes Production via ELISA
Anti-MUC1 IgG antibody subtypes were measured in serum samples from vaccinated mice collected on day 42. Differences were determined by ANOVA and Tukey's HSD test. Asterisks represent statistically significant differences (∗∗∗∗p < 0.0001), and ns indicates no significant difference compared with the PBS group. Data are shown as the mean ± SEM of five mice and are representative of three separate experiments.
Previous studies indicate that this small molecule TLR7a is an effective adjuvant to induce cytokines optimal for Th1 cell immunity and antibody production (Dowling, 2018). Herein, the results demonstrated that the built-in adjuvant with several TLR7a molecules in the TLR7a-BSA-MUC1 conjugates greatly enhanced the efficacy of TLR7a, preferentially promoted Th1-type adaptive immunity, and implemented antibody subclass and isotype switching, which is preferred in antitumor immunotherapy.
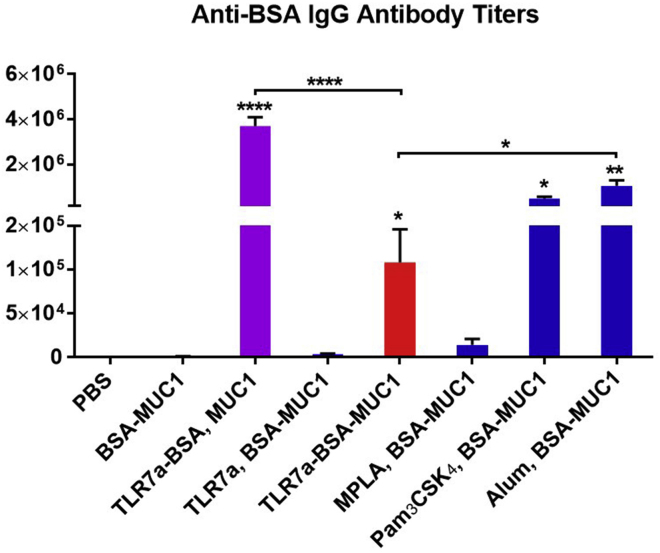
Evaluation of Carrier-Protein-Specific Antibodies
On the one hand, the vaccine needs the Th and Tc epitopes from the carrier protein to promote the immune responses against the tumor-associated antigens; on the other hand, eliciting high antibody titers against the carrier protein will increase the burden of immune system. The titers of the IgG antibodies against the carrier protein BSA were also analyzed (Figures 5 and S16). For the anti-BSA IgG antibody titers, the three-in-one protein conjugate vaccine (TLR7a-BSA-MUC1) were higher than BSA-MUC1, TLR7a and BSA-MUC1, or MPLA and BSA-MUC1, but lower than TLR7a-BSA and MUC1, Pam3CSK4 and BSA-MUC1, or alum and BSA-MUC1.
Figure 5.
Anti-BSA IgG Antibody Titers Were Detected in Serum Samples from Vaccinated Mice Collected on Day 42
Differences were determined by ANOVA using Tukey's HSD test. Asterisks represent statistically significant differences (∗∗∗∗p < 0.0001, ∗∗p < 0.01, ∗p < 0.05), and ns indicates no significant difference compared with the PBS group. Data are shown as the mean ± SEM of five mice and are representative of three separate experiments.
Compared with TLR7a-BSA and MUC1, the reduced carrier-specific antibody titers induced by the three-in-one protein conjugates were presumably attributed to the shielding effect of conjugating MUC1 and TLR7a adjuvant to the carrier BSA (Clough et al., 1985, Miermont et al., 2008).
Immunological Studies with Cancer Cells
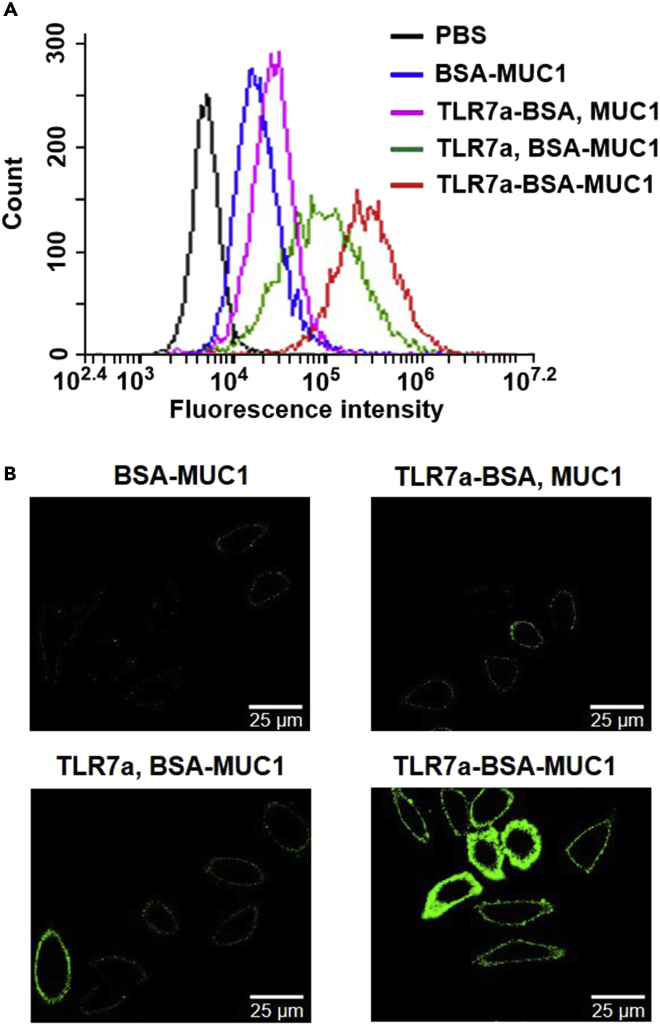
To determine whether the serum antibodies could bind to MCF-7 cells and B16-MUC1 cells (Wang et al., 2013), fluorescence-activated cell sorting (FACS) analysis (Figures 6A, S17, and S19), confocal fluorescence microscopy (Figures 6B, S20, and S21), and fluorescence microscopy (Figures S23 and S24) were conducted (Du et al., 2019). The results showed that the antibodies induced by the TLR7a-BSA-MUC1 vaccine displayed the highest binding affinity for MCF-7 cells and B16-MUC1 cells, as shown by measuring the mean fluorescence intensity of the cancer cells, whereas relatively lower binding affinity was observed with the antibodies elicited by the BSA-MUC1, BSA-TLR7a and MUC1, or TLR7a and BSA-MUC1 vaccine. Additionally, the antibodies induced by the vaccines composed of a mixed adjuvant, such as MPLA, Pam3CSK4, or alum, with the BSA-MUC1 conjugate exhibited lower binding affinity than those elicited by the TLR7a-BSA-MUC1 vaccine. To further investigate the antibody's binding ability to cell lines that do not express tumor-associated MUC1 antigens on the surface, B16-F10 cells (Hirabayashi et al., 1985) were used as a negative control in both FACS analysis and confocal fluorescence microscopy assays (Figures S18 and S22). We found that all serum antibodies induced by these vaccines (BSA-MUC1, TLR7a-BSA and MUC1, TLR7a and BSA-MUC1, TLR7a-BSA-MUC1) displayed very weak binding affinity for B16-F10 cells, overall there was no difference compared with the PBS group.
Figure 6.
Binding of the Mouse Serum to MCF-7 Cells was Determined by FACS and Confocal Microscopy Analysis
(A) FACS analysis of the binding of vaccinated mouse serum samples to MCF-7 cancer cells. Incubation with PBS group samples (black) served as a control.
(B) Confocal fluorescence microscopy images of MCF-7 cells incubated with serum samples from vaccinated mice (magnification: 63×). The images are representative of five independent experiments. Scale bar = 25 μm.
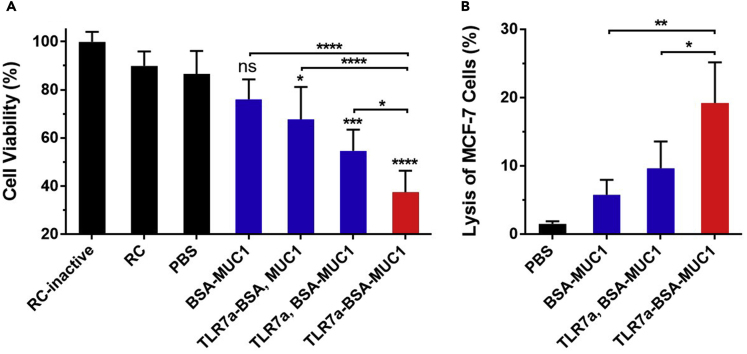
The elicited antibodies were able to bind to MCF-7 cancer cells and may initiate lysis of the recognized cancer cells via activation of the complement-dependent cytotoxicity (CDC) of rabbit sera (RC) (Cai et al., 2014, Du et al., 2019). Sera or complement was diluted 50-fold to determine the cell viability of MCF-7 cells by applying a tetrazolium bromide (MTT) assay. Control groups in these trials included MCF-7 cells incubated with an inactive form of rabbit sera (RC-inactive), rabbit sera, or PBS. As shown in Figure 7A, the MUC1-specific antibodies elicited by the TLR7a-BSA-MUC1 vaccine led to lower than 40% cell viability, and these antibodies were predicted to kill the MCF-7 cells by mediating CDC activation distinctly stronger than that induced by the antibodies elicited by the vaccines composed of the noncovalently linked adjuvants or antigens.
Figure 7.
Cell viability and Cytotoxicity Assay
(A) Complement-dependent cytotoxicity of antisera from each group: the cell viability of MCF-7 cells was determined by the MTT assay.
(B) Assay of cytotoxic T-lymphocyte-mediated immune responses: in vitro cytotoxicity of splenocytes collected from each group on day 42 to MCF-7 cells. Differences were determined by one-way ANOVA and Tukey's HSD test.
Asterisks indicate statistically significant differences (∗∗∗∗p < 0.0001, ∗∗p < 0.01, ∗p < 0.05). Data are the mean ± SD of five mice and are representative of five independent experiments.
Evaluation of the Mouse T-Cell-Mediated Response to Vaccination
We further studied whether the vaccine candidates could evoke a cytotoxic T lymphocyte (CTL) response. Splenocytes were obtained from immunized mice and incubated with MCF-7 cancer cells (Figure 7B) (Song et al., 2017). The splenocytes isolated from mice immunized with TLR7a and BSA-MUC1 or TLR7a-BSA-MUC1 exhibited significantly higher cytotoxicity to MCF-7 cells than those isolated from BSA-MUC1-vaccinated mice. CTLs activated by TLR7a-BSA-MUC1 displayed more efficient cytotoxicity than those activated by TLR7a and BSA-MUC1, which further suggested that the TLR7a-BSA-MUC1 conjugate could provoke stronger T-cell-mediated immunity than TLR7a and BSA-MUC1. Thus, the three-in-one protein conjugate is an applicable vaccine strategy to trigger a strong CTL immune response and simultaneously enhance immunogenicity.
Concluding Remarks
We investigated the structure-activity relationships of different constructs of anticancer vaccines with conjugated and mixed adjuvants. In this respect, this study indicated that the most potent antitumor vaccine was the three-in-one protein conjugate construct with a small-molecule TLR7 agonist as the adjuvant and tumor-associated antigen MUC1 as the B epitope covalently attached to a carrier protein containing multiple Tc and Th epitopes; this construct could not only stimulate exceptionally high IgG antibody titers but also affect the distribution of IgG subclasses toward Th1-polarized immune responses. It is essential that the TLR7 agonist be covalently coupled to the MUC1-BSA conjugate, probably due to the agonist providing a multivalent effect and subsequently facilitating codelivery to the lymph nodes to enhance the stimulation of immunity. As a result, the antibodies induced by the three-in-one vaccine bound to MCF-7 cancer cells strongly and killed the bound cancer cells through CDC activation; splenocytes from mice immunized with the three-in-one protein conjugate also lysed MCF-7 cancer cells relatively efficiently.
This three-in-one protein conjugate represents a novel anticancer vaccine strategy with a relatively simple formulation that improves immune responses, thus providing potential applications for personalized anticancer immunotherapy against tumor-associated antigens and tumor-specific neoantigens (Hilf et al., 2019, Keskin et al., 2019, Ott et al., 2017, Sahin et al., 2017).
Limitations of the Study
Three-in-one protein conjugates with built-in adjuvant can facilitate the codelivery of adjuvants and antigens. However, in order to induce potent immune responses, only when the modification with linker does not notably reduce the adjuvant's activity, the adjuvants are applicable for this strategy. Currently we are investigating different kinds of molecular adjuvants to validate this vaccine strategy.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank the National Key Research and Development Program of China (No. 2017YFA0505200), the National Natural Science Foundation of China (No.21772056), the self-determined research funds of CCNU from the colleges' basic research and operation of MOE (No. CCNU18TS011), Program of Introducing Talents of Discipline to Universities of China (111 program, B17019), and the Research Fund of East China University of Technology (No.DHBK2017114).
Author Contributions
J.G. and G.-F.Y. conceived the project. J.-J.D. and C.-W.W. designed and carried out the synthesis. X.-F.G. and Y.-K.T. contributed to synthesis. W.-B.X. and L.Z. carried out the immunizations in mice. J.-J.D., C.-W.W., and S.-H.Z. performed the immunological evaluation. J.-J.D. and C.-W.W. wrote the manuscript, and all the authors contributed to the discussion.
Declaration of Interests
J.G., G.-F.Y., J.-J.D., and C.-W.W. have filed a patent application. The authors declare no competing interests.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100935.
Contributor Information
Guang-Fu Yang, Email: gfyang@mail.ccnu.edu.cn.
Jun Guo, Email: jguo@mail.ccnu.edu.cn.
Supplemental Information
References
- Abdel-Aal A.-B.M., Lakshminarayanan V., Thompson P., Supekar N., Bradley J.M., Wolfert M.A., Cohen P.A., Gendler S.J., Boons G.-J. Immune and anticancer responses elicited by fully synthetic aberrantly glycosylated MUC1 tripartite vaccines modified by a TLR2 or TLR9 agonist. ChemBioChem. 2014;15:1508–1513. doi: 10.1002/cbic.201402077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acres B., Limacher J.-M. MUC1 as a target antigen for cancer immunotherapy. Expert Rev. Vaccin. 2005;4:493–502. doi: 10.1586/14760584.4.4.493. [DOI] [PubMed] [Google Scholar]
- Barratt-Boyes S.M. Making the most of mucin: a novel target for tumor immunotherapy. Cancer Immunol. Immunother. 1996;43:142–151. doi: 10.1007/s002620050315. [DOI] [PubMed] [Google Scholar]
- Bermejo I.A., Usabiaga I., Compañón I., Castro-López J., Insausti A., Fernández J.A., Avenoza A., Busto J.H., Jiménez-Barbero J., Asensio J.L. Water sculpts the distinctive shapes and dynamics of the tumor-associated carbohydrate Tn antigens: implications for their molecular recognition. J. Am. Chem. Soc. 2018;140:9952–9960. doi: 10.1021/jacs.8b04801. [DOI] [PubMed] [Google Scholar]
- Bhatia R., Gautam S.K., Cannon A., Thompson C., Hall B.R., Aithal A., Banerjee K., Jain M., Solheim J.C., Kumar S. Cancer-associated mucins: role in immune modulation and metastasis. Cancer Metastasis Rev. 2019;38:223–236. doi: 10.1007/s10555-018-09775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell J., Taylor-Papadimitriou J., Boshell M., Gendler S., Duhig T. A short sequence, within the amino acid tandem repeat of a cancer-associated mucin, contains immunodominant epitopes. Int. J. Cancer. 1989;44:691–696. doi: 10.1002/ijc.2910440423. [DOI] [PubMed] [Google Scholar]
- Cai H., Huang Z.-H., Shi L., Sun Z.-Y., Zhao Y.-F., Kunz H., Li Y.-M. Variation of the glycosylation pattern in MUC1 glycopeptide BSA vaccines and its influence on the immune response. Angew. Chem. Int. Ed. 2012;51:1719–1723. doi: 10.1002/anie.201106396. [DOI] [PubMed] [Google Scholar]
- Cai H., Orwenyo J., Giddens J.P., Yang Q., Zhang R., LaBranche C.C., Montefiori D.C., Wang L.-X. Synthetic three-component HIV-1 V3 glycopeptideimmunogens induce glycan-dependent antibody responses. Cell Chem. Biol. 2017;24:1513–1522.e4. doi: 10.1016/j.chembiol.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H., Sun Z.-Y., Chen M.-S., Zhao Y.-F., Kunz H., Li Y.-M. Synthetic multivalent glycopeptide-lipopeptide antitumor vaccines: impact of the cluster effect on the killing of tumor cells. Angew. Chem. Int. Ed. 2014;53:1699–1703. doi: 10.1002/anie.201308875. [DOI] [PubMed] [Google Scholar]
- Chan M., Hayashi T., Kuy C.S., Gray C.S., Wu C.C.N., Corr M., Wrasidlo W., Cottam H.B., Carson D.A. Synthesis and immunological characterization of toll-like receptor 7 agonistic conjugates. Bioconjug. Chem. 2009;20:1194–1200. doi: 10.1021/bc900054q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever M.A., Allison J.P., Ferris A.S., Finn O.J., Hastings B.M., Hecht T.T., Mellman I., Prindiville S.A., Viner J.L., Weiner L.M. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.-Z., Zhang R.-Y., Wang X.-F., Yin X.-G., Wang J., Wang Y.-C., Liu X., Du J.-J., Liu Z., Guo J. Peptide-free synthetic nicotine vaccine candidates with α-galactosylceramide as adjuvant. Mol. Pharm. 2019;16:1467–1476. doi: 10.1021/acs.molpharmaceut.8b01095. [DOI] [PubMed] [Google Scholar]
- Clough E.R., Jolivet M., Audibert F., Barrwell J.W., Schlesinger D.H., Chedid L. Production of anti-sporozoite antibodies in absence of response to carrier by coupling an MDP derivative to a malaria peptide-tetanus toxoid conjugate. Biochem. Biophys. Res. Commun. 1985;131:70–76. doi: 10.1016/0006-291x(85)91771-1. [DOI] [PubMed] [Google Scholar]
- Compañón I., Guerreiro A., Mangini V., Castro-López J., Escudero-Casao M., Avenoza A., Busto J.H., Castillón S., Jiménez-Barbero J., Asensio J.L. Structure-Based design of potent tumor-associated antigens: modulation of peptide presentation by single-atom O/S or O/Se substitutions at the glycosidic linkage. J. Am. Chem. Soc. 2019;141:4063–4072. doi: 10.1021/jacs.8b13503. [DOI] [PubMed] [Google Scholar]
- Donadei A., Balocchi C., Mancini F., Proietti D., Gallorini S., O’Hagan D.T., D’Oro U., Berti F., Baudner B.C., Adamo R. The adjuvant effect of TLR7 agonist conjugated to a meningococcal serogroup C glycoconjugate vaccine. Eur. J. Pharm. Biopharm. 2016;107:110–119. doi: 10.1016/j.ejpb.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Dowling D.J. Recent advances in the discovery and delivery of TLR7/8 agonists as vaccine adjuvants. Immunohorizons. 2018;2:185–197. doi: 10.4049/immunohorizons.1700063. [DOI] [PubMed] [Google Scholar]
- Du J.-J., Zou S.-Y., Chen X.-Z., Xu W.-B., Wang C.-W., Zhang L., Tang Y.-K., Zhou S.-H., Wang J., Yin X.-G. Liposomal antitumor vaccines targeting mucin 1 elicit a lipid-dependent immunodominant response. Chem. Asian J. 2019;14:2116–2121. doi: 10.1002/asia.201900448. [DOI] [PubMed] [Google Scholar]
- Dziadek S., Kowalczyk D., Kunz H. Synthetic vaccines consisting of tumor-associated MUC1 glycopeptide antigens and bovine serum albumin. Angew. Chem. Int. Ed. 2005;44:7624–7630. doi: 10.1002/anie.200501593. [DOI] [PubMed] [Google Scholar]
- Feng Y., Forsell M.N.E., Flynn B., Adams W., Loré K., Seder R., Wyatt R.T., KarlssonHedestam G.B. Chemical cross-linking of HIV-1 Env for direct TLR7/8 ligand conjugation compromises recognition of conserved antigenic determinants. Virology. 2013;446:56–65. doi: 10.1016/j.virol.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francica J.R., Lynn G.M., Laga R., Joyce M.G., Ruckwardt T.J., Morabito K.M., Chen M., Chaudhuri R., Zhang B., Sastry M. Thermoresponsive polymer nanoparticles Co-deliver RSV F trimers with a TLR-7/8 adjuvant. Bioconjug. Chem. 2016;27:2372–2385. doi: 10.1021/acs.bioconjchem.6b00370. [DOI] [PubMed] [Google Scholar]
- Gaidzik N., Westerlind U., Kunz H. The development of synthetic antitumour vaccines from mucinglycopeptide antigens. Chem. Soc. Rev. 2013;42:4421–4442. doi: 10.1039/c3cs35470a. [DOI] [PubMed] [Google Scholar]
- Gao D., Liu Y., Diao Y., Gao N., Wang Z., Jiang W., Jin G. Synthesis and evaluation of conjugates of novel TLR7 inert ligands as self-adjuvantingimmunopotentiators. ACS Med. Chem. Lett. 2015;6:249–253. doi: 10.1021/ml5003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D., Liu Y., Li W., Zhong F., Zhang X., Diao Y., Gao N., Wang X., Jiang W., Jin G. Synthesis and immunoregulatory activities of conjugates of a Toll-like receptor 7 inert ligand. Bioorg. Med. Chem. Lett. 2014;24:5792–5795. doi: 10.1016/j.bmcl.2014.10.034. [DOI] [PubMed] [Google Scholar]
- Gao D., Zeng J., Wang X., Liu Y., Li W., Hu Y., Gao N., Diao Y., Wang Z., Jiang W. Conjugation of weak ligands with weak antigens to activate TLR-7: a step toward better vaccine adjuvants. Eur. J. Med. Chem. 2016;120:111–120. doi: 10.1016/j.ejmech.2016.04.070. [DOI] [PubMed] [Google Scholar]
- Hilf N., Kuttruff-Coqui S., Frenzel K., Bukur V., Stevanović S., Gouttefangeas C., Platten M., Tabatabai G., Dutoit V., van der Burg S.H. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565:240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y., Hamaoka A., Matsumoto M., Matsubara T., Tagawa M., Wakabayashi S., Taniguchi M. Syngeneic monoclonal antibody against melanoma antigen with interspecies cross-reactivity recognizes GM3, a prominent ganglioside of B16 melanoma. J. Biol. Chem. 1985;260:13328–13333. [PubMed] [Google Scholar]
- Hoffmann-Röder A., Johannes M. Synthesis of a MUC1-glycopeptide–BSA conjugate vaccine bearing the 3′-deoxy-3′-fluoro-Thomsen–Friedenreich antigen. Chem. Commun. (Camb.) 2011;47:9903–9905. doi: 10.1039/c1cc13184b. [DOI] [PubMed] [Google Scholar]
- Hoffmann-Röder A., Kaiser A., Wagner S., Gaidzik N., Kowalczyk D., Westerlind U., Gerlitzki B., Schmitt E., Kunz H. Synthetic antitumor vaccines from tetanus toxoid conjugates of MUC1 glycopeptides with the thomsen–Friedenreich antigen and a fluorine-substituted analogue. Angew. Chem. Int. Ed. 2010;49:8498–8503. doi: 10.1002/anie.201003810. [DOI] [PubMed] [Google Scholar]
- Hossain M.K., Wall K.A. Immunological evaluation of recent MUC1 glycopeptide cancer vaccines. Vaccines. 2016;4:25. doi: 10.3390/vaccines4030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M.K., Vartak A., Karmakar P., Sucheck S.J., Wall K.A. Augmenting vaccine immunogenicity through the use of natural human anti-rhamnose antibodies. ACS Chem. Biol. 2018;13:2130–2142. doi: 10.1021/acschembio.8b00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingale S., Wolfert M.A., Gaekwad J., Buskas T., Boons G.-J. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat. Chem. Biol. 2007;3:663–667. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser A., Gaidzik N., Becker T., Menge C., Groh K., Cai H., Li Y.-M., Gerlitzki B., Schmitt E., Kunz H. Fully synthetic vaccines consisting of tumor-associated MUC1 glycopeptides and a lipopeptide ligand of the toll-like Receptor 2. Angew. Chem. Int. Ed. 2010;49:3688–3692. doi: 10.1002/anie.201000462. [DOI] [PubMed] [Google Scholar]
- Karsten U., Diotel C., Klich G., Paulsen H., Goletz S., Müller S., Hanisch F.-G. Enhanced binding of antibodies to the DTR motif of MUC1 tandem repeat peptide is mediated by site-specific glycosylation. Cancer Res. 1998;58:2541–2549. [PubMed] [Google Scholar]
- Keskin D.B., Anandappa A.J., Sun J., Tirosh I., Mathewson N.D., Li S., Oliveira G., Giobbie-Hurder A., Felt K., Gjini E. Neoantigen vaccine generates intratumoral T cell responses in phase Ibglioblastoma trial. Nature. 2019;565:234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.G., Choi B., Yang H.-J., Han J.-A., Jung H., Cho H., Kang S., Hong S.Y. Covalent conjugation of small-molecule adjuvants to nanoparticles induces robust cytotoxic T cell responses via DC activation. Bioconjug. Chem. 2016;27:2007–2013. doi: 10.1021/acs.bioconjchem.6b00277. [DOI] [PubMed] [Google Scholar]
- Lee H.-Y., Chen C.-Y., Tsai T.-I., Li S.-T., Lin K.-H., Cheng Y.-Y., Ren C.-T., Cheng T.-J.R., Wu C.-Y., Wong C.-H. Immunogenicity study of globo H analogues with modification at the reducing or nonreducingend of the tumor antigen. J. Am. Chem. Soc. 2014;136:16844–16853. doi: 10.1021/ja508040d. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang W., He Q., Yu F., Song T., Liu T., Zhang Z., Zhou J., Wang P.G., Zhao W. Fully synthetic self-adjuvanting MUC1-fibroblast stimulating lipopeptide 1 conjugates as potential cancer vaccines. Chem. Commun.(Camb.) 2016;52:10886–10889. doi: 10.1039/c6cc04623a. [DOI] [PubMed] [Google Scholar]
- Liu Z., Guo J. NKT-cell glycolipid agonist as adjuvant in synthetic vaccine. Carbohydr. Res. 2017;452:78–90. doi: 10.1016/j.carres.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Lloyd K.O., Burchell J., Kudryashov V., Yin B.W.T., Taylor-Papadimitriou J. Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines: DEMONSTRATION OF SIMPLER and FEWERGLYCANCHAINS IN tumor cells. J. Biol. Chem. 1996;271:33325–33334. doi: 10.1074/jbc.271.52.33325. [DOI] [PubMed] [Google Scholar]
- Lynn G.M., Chytil P., Francica J.R., Lagová A., Kueberuwa G., Ishizuka A.S., Zaidi N., Ramirez-Valdez R.A., Blobel N.J., Baharom F. Impact of polymer-TLR-7/8 agonist (adjuvant) morphology on the potency and mechanism of CD8 T cell induction. Biomacromolecules. 2019;20:854–870. doi: 10.1021/acs.biomac.8b01473. [DOI] [PubMed] [Google Scholar]
- Lynn G.M., Laga R., Darrah P.A., Ishizuka A.S., Balaci A.J., Dulcey A.E., Pechar M., Pola R., Gerner M.Y., Yamamoto A. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat. Biotechnol. 2015;33:1201–1210. doi: 10.1038/nbt.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Sáez N., Peregrina J.M., Corzana F. Principles of mucin structure: implications for the rational design of cancer vaccines derived from MUC1-glycopeptides. Chem. Soc. Rev. 2017;46:7154–7175. doi: 10.1039/c6cs00858e. [DOI] [PubMed] [Google Scholar]
- Miermont A., Barnhill H., Strable E., Lu X., Wall K.A., Wang Q., Finn M.G., Huang X. Cowpea mosaic virus capsid: a promising carrier for the development of carbohydrate based antitumor. Vaccin. Chem. Eur. J. 2008;14:4939–4947. doi: 10.1002/chem.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberbillig T., Mersch C., Wagner S., Hoffmann-Röder A. Antibody recognition of fluorinated MUC1 glycopeptide antigens. Chem. Commun.(Camb.) 2012;48:1487–1489. doi: 10.1039/c1cc15139h. [DOI] [PubMed] [Google Scholar]
- Oh J.Z., Kedl R.M. The capacity to induce cross-presentation dictates the success of a TLR7 agonist-conjugate vaccine for eliciting cellular immunity. J. Immunol. 2010;185:4602–4608. doi: 10.4049/jimmunol.1001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott P.A., Hu Z., Keskin D.B., Shukla S.A., Sun J., Bozym D.J., Zhang W., Luoma A., Giobbie-Hurder A., Peter L. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudet O., BenMohamed L., Dasgupta G., Bettahi I., Dumy P. Towards a self-adjuvanting multivalent B and T cell epitope containing synthetic glycolipopeptide cancer vaccine. ChemMedChem. 2008;3:737–741. doi: 10.1002/cmdc.200700315. [DOI] [PubMed] [Google Scholar]
- Renaudet O., Dasgupta G., Bettahi I., Shi A., Nesburn A.B., Dumy P., BenMohamed L. Linear and branched glyco-lipopeptide vaccines Follow distinct cross-presentation pathways and generate different magnitudes of antitumor immunity. PLoS One. 2010;5:e11216. doi: 10.1371/journal.pone.0011216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivalland G., Loveland B., Mitchell P. Update on Mucin-1 immunotherapy in cancer: a clinical perspective. Expert Opin. Biol. Ther. 2015;15:1773–1787. doi: 10.1517/14712598.2015.1088519. [DOI] [PubMed] [Google Scholar]
- Sahin U., Derhovanessian E., Miller M., Kloke B.-P., Simon P., Löwer M., Bukur V., Tadmor A.D., Luxemburger U., Schrörs B. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- Shinchi H., Crain B., Yao S., Chan M., Zhang S.S., Ahmadiiveli A., Suda Y., Hayashi T., Cottam H.B., Carson D.A. Enhancement of the immunostimulatory activity of a TLR7 ligand by conjugation to polysaccharides. Bioconjug. Chem. 2015;26:1713–1723. doi: 10.1021/acs.bioconjchem.5b00285. [DOI] [PubMed] [Google Scholar]
- Singh R., Bandyopadhyay D. MUC1: a target molecule for cancer therapy. Cancer Biol. Ther. 2007;6:481–486. doi: 10.4161/cbt.6.4.4201. [DOI] [PubMed] [Google Scholar]
- Song C., Zheng X.-J., Liu C.-C., Zhou Y., Ye X.-S. A cancer vaccine based on fluorine-modified sialyl-Tn induces robust immune responses in a murine model. Oncotarget. 2017;8:47330–47343. doi: 10.18632/oncotarget.17646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straßburger D., Glaffig M., Stergiou N., Bialas S., Besenius P., Schmitt E., Kunz H. Synthetic MUC1 antitumor vaccine with incorporated 2,3-sialyl-T carbohydrate antigen inducing strong immune responses with isotypespecificity. ChemBioChem. 2018;19:1142–1146. doi: 10.1002/cbic.201800148. [DOI] [PubMed] [Google Scholar]
- Tang C.-K., Katsara M., Apostolopoulos V. Strategies used for MUC1 immunotherapy: human clinical studies. Expert Rev. Vaccin. 2008;7:963–975. doi: 10.1586/14760584.7.7.963. [DOI] [PubMed] [Google Scholar]
- Tang J., Pearce L., O'Donnell-Tormey J., Hubbard-Lucey V.M. Trends in the global immuno-oncology landscape. Nat. Rev. Drug Discov. 2018;17:783. doi: 10.1038/nrd.2018.167. [DOI] [PubMed] [Google Scholar]
- Toyokuni T., Hakomori S.-i., Singhal A.K. Synthetic carbohydrate vaccines: synthesis and immunogenicity of Tn antigen conjugates. Bioorg. Med. Chem. 1994;2:1119–1132. doi: 10.1016/s0968-0896(00)82064-7. [DOI] [PubMed] [Google Scholar]
- Van Herck S., Deswarte K., Nuhn L., Zhong Z., Portela Catani J.P., Li Y., Sanders N.N., Lienenklaus S., De Koker S., Lambrecht B.N. Lymph-node-targeted immune activation by engineered block copolymer amphiphiles–TLR7/8 agonist conjugates. J. Am. Chem. Soc. 2018;140:14300–14307. doi: 10.1021/jacs.8b08595. [DOI] [PubMed] [Google Scholar]
- Wang F., Li Q., Ni W., Fang F., Sun X., Xie F., Wang J., Wang F., Gao S., Tai G. Expression of human full-length MUC1 inhibits the proliferation and migration of a B16 mouse melanoma cell line. Oncol. Rep. 2013;30:260–268. doi: 10.3892/or.2013.2440. [DOI] [PubMed] [Google Scholar]
- Wang H., Yang B., Wang Y., Liu F., Fernández-Tejada A., Dong S. β-Glucan as an immune activator and a carrier in the construction of a synthetic MUC1 vaccine. Chem. Commun.(Camb.) 2019;55:253–256. doi: 10.1039/c8cc07691j. [DOI] [PubMed] [Google Scholar]
- Wang Q., Zhou Z., Tang S., Guo Z. Carbohydrate-monophosphoryl lipid A conjugates are fully synthetic self-adjuvanting cancer vaccines eliciting robust immune responses in the mouse. ACS Chem. Biol. 2012;7:235–240. doi: 10.1021/cb200358r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B.L., Day S., Malins L.R., Apostolopoulos V., Payne R.J. Self-adjuvanting multicomponent cancer vaccine candidates combining per-glycosylated MUC1 glycopeptides and the toll-like receptor 2 agonist Pam3CysSer. Angew. Chem. Int. Ed. 2011;50:1635–1639. doi: 10.1002/anie.201006115. [DOI] [PubMed] [Google Scholar]
- Wilkinson B.L., Malins L.R., Chun C.K.Y., Payne R.J. Synthesis of MUC1–lipopeptide chimeras. Chem. Commun.(Camb.) 2010;46:6249–6251. doi: 10.1039/c0cc01360a. [DOI] [PubMed] [Google Scholar]
- Wu C.C.N., Hayashi T., Takabayashi K., Sabet M., Smee D.F., Guiney D.D., Cottam H.B., Carson D.A. Immunotherapeutic activity of a conjugate of a Toll-like receptor 7 ligand. Proc. Natl. Acad. Sci. U S A. 2007;104:3990–3995. doi: 10.1073/pnas.0611624104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.-J., Li W.-H., Chen P.-G., Zhang B.-D., Hu H.-G., Li Q.-Q., Zhao L., Chen Y.-X., Zhao Y.-F., Li Y.-M. Targeting STING with cyclic di-GMP greatly augmented immune responses of glycopeptide cancer vaccines. Chem. Commun.(Camb.) 2018;54:9655–9658. doi: 10.1039/c8cc04860f. [DOI] [PubMed] [Google Scholar]
- Wu X., Yin Z., McKay C., Pett C., Yu J., Schorlemer M., Gohl T., Sungsuwan S., Ramadan S., Baniel C. Protective epitope discovery and design of MUC1-based vaccine for effective tumor protections in immunotolerant mice. J. Am. Chem. Soc. 2018;140:16596–16609. doi: 10.1021/jacs.8b08473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao A., Zheng X.-J., Song C., Gui Y., Huo C.-X., Ye X.-S. Synthesis and immunological evaluation of MUC1 glycopeptide conjugates bearing N-acetyl modified STn derivatives as anticancer vaccines. Org. Biomol. Chem. 2016;14:7226–7237. doi: 10.1039/c6ob01092j. [DOI] [PubMed] [Google Scholar]
- Yin X.-G., Chen X.-Z., Sun W.-M., Geng X.-S., Zhang X.-K., Wang J., Ji P.-P., Zhou Z.-Y., Baek D.J., Yang G.-F. IgG antibody response elicited by a fully synthetic two-component carbohydrate-based cancer vaccine candidate with α-galactosylceramide as built-in adjuvant. Org. Lett. 2017;19:456–459. doi: 10.1021/acs.orglett.6b03591. [DOI] [PubMed] [Google Scholar]
- Yin Z., Wu X., Kaczanowska K., Sungsuwan S., ComellasAragones M., Pett C., Yu J., Baniel C., Westerlind U., Finn M.G. Antitumor humoral and T cell responses by mucin-1 conjugates of bacteriophage Qβ in wild-type mice. ACS Chem. Biol. 2018;13:1668–1676. doi: 10.1021/acschembio.8b00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo E., Salyer A.C.D., Brush M.J.H., Li Y., Trautman K.L., Shukla N.M., De Beuckelaer A., Lienenklaus S., Deswarte K., Lambrecht B.N. Hyaluronic acid conjugates of TLR7/8 agonists for targeted delivery to secondary lymphoid tissue. Bioconjug.Chem. 2018;29:2741–2754. doi: 10.1021/acs.bioconjchem.8b00386. [DOI] [PubMed] [Google Scholar]
- Zhu J., Wan Q., Lee D., Yang G., Spassova M.K., Ouerfelli O., Ragupathi G., Damani P., Livingston P.O., Danishefsky S.J. From synthesis to biologics: preclinical data on a chemistry derived anticancer vaccine. J. Am. Chem. Soc. 2009;131:9298–9303. doi: 10.1021/ja901415s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.