Abstract
Background
Tributyltin (TBT) is known as an endocrine disruptor able to interfere with estrogen receptors (ERs) leading to toxic effects on the related endocrine pathways. TBT is an obesogen, reported to disrupt glucose homeostasis leading to diabetes. The aim of this study was to assess the influence of TBT and β-estradiol on the pancreatic islets of Langerhans in simultaneous exposures.
Experimental
Pancreatic islets of 15 male rat were isolated and exposed to TBT (10 μM), β-estradiol, and TBT plus β-estradiol for 24 h. Therewith, cellular viability, oxidative stress, apoptosis, and insulin secretion markers were investigated.
Results
TBT decreased the viability and increased the apoptosis, reactive oxygen species, and insulin secretion TBT led to increased amounts of apaptosis, reactive oxygen species (ROS), and insulin secretion in pancreatic islets; however, cellular viability was reduced. Co-exposure with β-estradiol ameliorated the entire mentioned variables near to the control level.
Conclusion
These results showed that β-estradiol protect pancreatic islets of Langerhans against TBT-induced toxicity by counteracting oxidative stress and apoptosis as well as insulin secretion. In this way, it is postulated that pancreatic ER pathways particularly in β-cells might be the determinant target of toxic effects of xenoestrogens like TBT. Hence, evaluation of xenoestrogens-induced ER dysfunction in the endocrine pancreas can be helpful in diabetic risk assessment of these contaminants. Pharmacological modifications of ER pathway in the β-cells seems promising for better management of diabetes.
Keywords: β-estradiol, Diabetes, Estrogen receptors, Islets of Langerhans, Tributyltin, Endocrinology, Health sciences, Pharmacology, Reproductive system, Toxicology
β-estradiol; Diabetes; Estrogen receptors; Islets of Langerhans; Tributyltin; Endocrinology; Health sciences; Pharmacology; Reproductive system; Toxicology
1. Introduction
Tributyltin (TBT) is a synthetic organic derivative of tin having three covalent bonds between a tin atom and carbon atoms. Its original and extensive use was to reduce the growth of marine organisms on the hulls of large ships, leading to an increased production and use in 1980s and 1990s. TBT along with the other organotins including monobutyltin and dibutyltin have been used as a stabilizer in manufacturing of plastic products, as a biocide in preservation of wood, textile, leather, paper, and industrial water systems. Due to widespread use as an antifouling paint, TBT was the most common pesticide contaminating marine and freshwater ecosystem during 2000s. In awareness of toxic effects of TBT on marine ecosystem, especially the phenomenon of imposex (superimpose of male sex characteristics on normal female gastropods), national and international bans were issued for application of TBT worldwide. In spite of regulatory bans, TBT is still detected in food chain including dairy products, meat, and fish and also in human body, due to its long persistence and bioaccumulation in the environment [1].
TBT has long been recognized as an endocrine disrupting chemical (EDC) interacting with nuclear receptors including retinoid-X receptor (RXR) and peroxisome proliferator-activated receptor γ (PPARγ) [2]. Due to the imposex, TBT has always been suspected to interact with estrogen receptor (ER) pathway. So, ER-dependent transcriptional expression and E-Screen assays were commonly used in both in vitro and in vivo studies to evaluate estrogenic potential of TBT along with the other heavy metals during the last two decades. The results of different studies indicated that TBT interacts with ERs more than the other metals. Hence, TBT can disrupt transcriptional activation of ER pathways and the related endocrine functions [3, 4, 5, 6, 7].
ERs are classified as nuclear ERs and G-protein coupled ERs mediating genomic effects and rapid cellular signaling effects, respectively. β-estradiol is the main endogenous estrogen in human, and is the main ligand for activating ERs and related endocrine effects. In addition to the sexual and reproductive positions, ERs have different metabolic functions in the body, and recently the presence of these receptors in the endocrine pancreas has been confirmed by numerous studies. These studies have found some important physiological roles for the pancreatic ERs such as integrity and maintenance of islets of Langerhans as well as biosynthesis and release of insulin [8, 9, 10, 11, 12].
Therefore, β-cells of the pancreas which produce and secrete insulin, the master regulator of glucose homeostasis in the body, have been suggested as a target for xenoestrogens. Xenoestrogens can overstimulate pancreatic ERs resulted in β-cell exhaustion and excessive insulin signaling. These events can lead to insulin resistance and diabetes in chronic exposures [13].
Diabetes, the leading metabolic disease, has an increasing prevalence worldwide and regardless to the quality of life (QOL) impairments, it burdens a huge cost to the health care system [14].
Considering the important role of pancreatic β-cells in the maintenance of glucose homeostasis, understanding their pathological mechanisms under the influence of environmental contaminants is valuable.
In this way, it can be postulated that TBT may disrupt glucose metabolic effects of the endocrine pancreas due to its interaction with ERs in the islets of Langerhans. There are some evidences on diabetogenic properties of TBT in vitro and in vivo. Chronic exposure to TBT has been shown to increase body weight, fasting blood glucose, fasting blood insulin, and glucose intolerance as well as a decrease in the expression of insulin signaling cascade in mice [15, 16]. In addition to the mentioned effects, both in vitro and in vivo studies have shown increased apoptosis, oxidative stress, protein kinase C and extracellular signal-regulated kinase (ERK)1/2 in the pancreatic islets of Langerhans and β-cells treated with TBT [17, 18].
According to the evidence on the interaction of TBT with ERs from one side, and beneficial presence of ERs in the endocrine pancreas from the other side, uncovering the role of ER pathway in TBT-induced pancreatic toxicity is noteworthy. Since, β-estradiol is the endogenous full agonist of ERs in the body, this project evaluated its ability to confront with toxic effects of TBT in the pancreatic islets of Langerhans in rat.
2. Materials and methods
2.1. Chemicals
The insulin ELISA Kit and ApoFlowEx® FITC Kit were prepared from Mercodia (Sweden) and Exbio (Vestec, Czech Republic), respectively. All the other chemicals including TBT, β-estradiol, collagenase, bovine serum albumin (BSA), HEPES sodium salt, glucose, Pen-Strep, RPMI 1640 media, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) were prepared from the Sigma-Aldrich Company (GmbH Munich, Germany).
2.2. Animal treatment
This project was approved by the Research Ethics Committee at Ardabil University of Medical Sciences, Ardabil, Iran with an approval ID: IR. ARUMS.REC.1397.089 in 9/23/2019 and all the experiments dealing with laboratory animals matched the guidelines of the institute review board. 2–3 months old male Wistar rats weighting 200–250 g were acclimatized to laboratory conditions one week in advance of the experiments under the housing condition of a 12-hour dark/light cycle, 25 ± 1 °C and 50% humidity. Totally, 15 rats were used for the experiments of this project.
2.3. Islets isolation
A mixture at ratio of 10:1 ketamine and xylazine was injected to the animals intraperitoneally for induction of anesthesia. After complete anesthetization and laparotomy, the common bile duct was closed from the liver side and cannulated. Then fresh Krebs buffer was perfused to the pancreas so that the pancreas was swollen enough to ease cutting off step. Subsequent to isolating, the whole pancreas of each rat was chopped into small pieces in the cool Krebs buffer and was centrifuged for 1 min at 1200 g and 4 °C twice. Then collagenase enzyme was added to the sediment and after shaking for 3–5 min in 37 °C water bath, BSA was added to stop the digestion. The sediments were washed two times with Krebs buffer and the pancreatic islets of Langerhans with approximate same sizes were picked up using a stereo microscope. Eventually hale isolated islets were cultured in RPMI 1640 for 24 h at 37 °C to overcome negative side effects through stressful procedure of isolation [19].
2.4. Treatment of pancreatic islets of Langerhans
Pancreatic islets of Langerhans were grouped (10 islets in each group) and treated with different concentrations of TBT (10 nM, 100 nM, 1μM, 10 μM, 100 μM and 1 mM) in order to establish the median lethal concentration (LC50) of TBT in pancreatic islets of Langerhans. For determining the effective concentration of β-estradiol against TBT toxicity in this experimental set up, islets of Langerhans were treated with a combination of TBT LC50 and different concentrations of β-estradiol. Afterwards islets of Langerhans were grouped as control, β-estradiol, TBT and combination of β-estradiol and TBT and the experiment was set to assess the viability, ROS formation, insulin secretion and apoptosis of pancreatic beta cells in these groups.
2.5. Cell viability assessment
Viability of pancreatic islets were measured by MTT (tetrazolium bromide) which is a yellow substance and reduced by metabolically active cells due to the action of by NAD(P)H-dependent cellular oxidoreductase enzymes. Reduction of MTT results in generating purple formazan which can be quantified spectrophotometrically. After a 24-hour treatment, the islets of Langerhans were picked up from the medium and washed with Krebs buffer containing HEPES for two times. Fifty μl of MTT (0.5 mg/ml) was added to each group followed by a 4-hour incubation at 37 °C, adding 150 μL of DMSO and shaking the samples for 30 min at room temperature. At the end, the absorbance was read at 570 nm by an ELISA reader [19].
In fact, the dose-response tests using MTT assay were done in order to calculate the LC50 of TBT and subsequently the median effective concentration (EC50) of β-estradiol against TBT toxicity in the pancreatic islets of Langerhans, and the established concentrations were used in the following experiments.
2.6. Flowcytometry assessment of apoptosis versus necrosis
The rate of apoptosis and necrosis of the pancreatic cells were evaluated by analyzing plasma membrane changes by a flowcytometric assay. For this purpose, cells were dyed by Annexin-V, which binds to phosphatidylserine indicating apoptotic cells, and PI which bind to DNA indicating necrotic cells. After treatment period, islets of Langerhans were washed by PBS twice and transformed to single cells by using Trypsin/EDTA. Then the process of digestion was stopped by BSA and the cells were washed by PBS one more time. The exact protocol of manufacturer was done as followed: equal volume (5 μL) from both annexin V fluorescein isothiocyanate (FITC) and propidium iodide (PI) was added to the cell suspension at the approximate concentration of 3×105 cells/100 μL and incubated at room temperature. At the end, the samples were injected to flowcytometer (Mindray BriCyte E6) and the results were analyzed [20].
2.7. Measurement of cytosolic ROS
Presence of ROS was measured by a fluorometric assay based on the conversion of dichlorodifluorcein diacetate (DCFH-DA) a non-fluorescent compound to a fluorescent chemical named dichlorodifluorcein (DCF). The fluorescens of DCF is measured spectrometrically with maximum excitation of 495 nm and maximum emission of 529 nm in the spectra. When the treatment was completed, groups of pancreatic islets (10 islets in each group) were homogenized, supernatant was added to an assay buffer and DCFH-DA and the solution was incubated at 37 °C for 15 min. Then the absorbance was measured every 10 min up to 60 min by using a fluorimeter. The remaining pancreatic islets in this protocol was used to measure the concentration of protein by Bradford Protein Assay (BPA) in order to normalize the amount of the biochemical markers as described previously [20].
2.8. Measurement of insulin secretion
After a 24-hour treatment, 1 ml of Krebs medium was added to each group of pancreatic islets in the vials and were centrifuged at 3000 g for 1 min. Then supernatant was removed and the pancreatic islets were incubated in a medium containing 2.8 mM glucose for 30 min. Afterwards the vials containing pancreatic islets were divided into two groups; one receiving 2.8 mM and the other 16.7 mM glucose in order to assess the basal and stimulated insulin secretion, respectively. Eventually followed by an hour incubation, the supernatants were taken to read the insulin concentration by an ELISA kit (Cat number: 10-1250-01) prepared from the Mercodia Co. according to the protocol of the manufacturer.
2.9. Measurement of total protein concentration
This measurement was standardized by BSA solutions concentrating between 0 and 10 μg/ml in a buffer. Five μl of remaining samples of ROS and insulin assay were also diluted with 795 μl distilled water. Total protein concentration was assessed by adding 200 μl Bradford reagent to the standard and sample solutions and after 5 min the absorbance was read at 595 nm by the spectrophotometer [21].
2.10. Statistical analysis of data
The results were expressed as the value of the mean ± standard error of four experiments in each group. One Way Analysis of Variance (ANOVA) was used for comparison between groups. Then the post hoc Tukey was used to calculate p value. For each experiment, significant differences between groups was presented at three statics of p values (<0.05, <0.01, and <0.001).
3. Results
3.1. Cellular viability
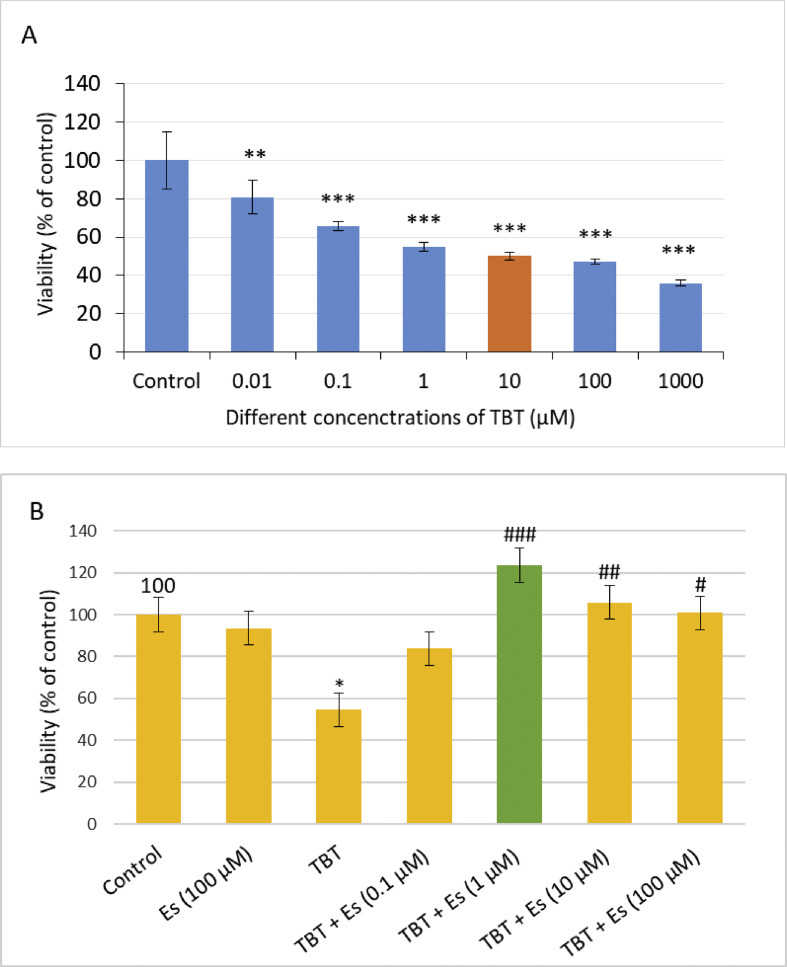
Exposure of pancreatic islets of Langerhans to 6 logarithmic concentrations of TBT ranging from 10 nM to 1000 μM for 24 h caused a concentration-dependent decrease in the viability of pancreatic islets of Langerhans. Ten μM TBT was established as LC50; the concentration at which TBT decreased the viability of pancreatic islets by 50%. To obtain the EC50 of β-estradiol, a range of concentrations from 100 nM to 100 μM was assessed against TBT-induced toxicity at LC50 and as indicated in Figure 1 and 1μM was the concentration of β-estradiol that elevated the viability of the TBT treated islets of Langerhans by 50%.
Figure 1.
Cellular viability of pancreatic islets of Langerhans from the rat after exposure to different concentrations of TBT (A) and TBT (10 μM) plus different concentration of Es (B). Values are expressed as mean ± SEM with a replication number, n = 4. ∗ significantly different from control at P-value < 0.05, ∗∗significantly different from control at P-value < 0.01, ∗∗∗significantly different from control at P-value < 0.001. # significantly different from TBT at P-value < 0.05, ## significantly different from TBT at P-value < 0.01, ### significantly different from TBT at P-value < 0.001. TBT: tributyltin, ES: β-estradiol.
3.2. Apoptosis and necrosis
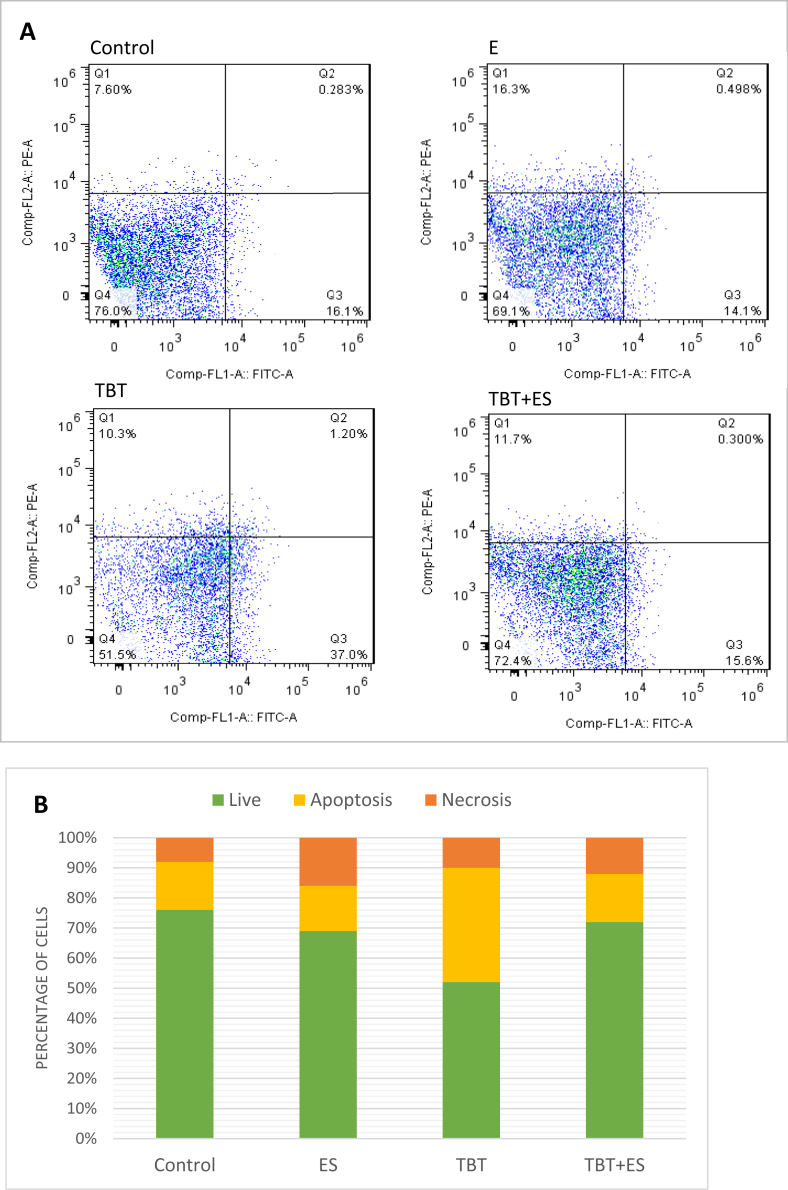
As shown in Figure 2, the percentage of live cells in the control group was 76% while this percent decreased up to 52% in the pancreatic islets exposed to TBT. Co-treatment of pancreatic islets with β-estradiol and TBT resulted in viability in 72.4% of the cells. TBT increased the percentage of apoptotic cells by almost 38%, whereas there is a reduction of apoptosis in co-treated group. Unlike apoptosis, the percent of necrotic cells was increased.
Figure 2.
Flowcytometry assessment of live, early apoptotic, late apoptotic, and necrotic cells of pancreatic islets of Langerhans exposed to TBT and Es. Lower left field (FITC− and PI−): live cells, lower right field (FITC+ and PI−): early apoptotic cells, upper right field (FITC+ and PI+): late apoptotic cells, and upper left field (FITC− and PI+): necrotic cells (A). Percentage of cells in the stages of live, apoptosis, and necrosis (B). TBT: tributyltin, Es: β-estradiol.
3.3. ROS formation
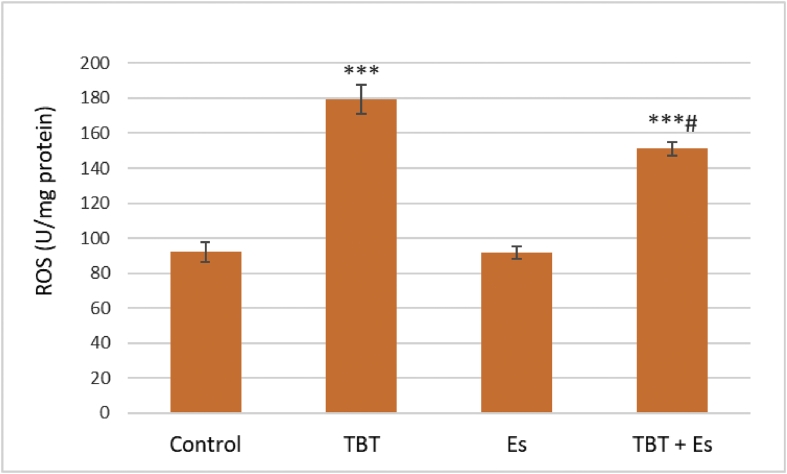
TBT increased ROS formation in the pancreatic islets of Langerhans, while β-estradiol decreased the level of raised ROS in co-treatment group (Figure 3).
Figure 3.
ROS formation in pancreatic islets of Langerhans from the rat after exposure to TBT (10 μM), ES (1 μM), and TBT (10 μM) plus Es (1 μM). Values are expressed as mean ± SEM with a replication number, n = 4. ∗∗∗ significantly different from control at P-value < 0.001, # significantly different from TBT at P-value < 0.05, TBT: tributyltin, Es: β-estradiol, ROS: reactive oxygen species.
3.4. Insulin secretion
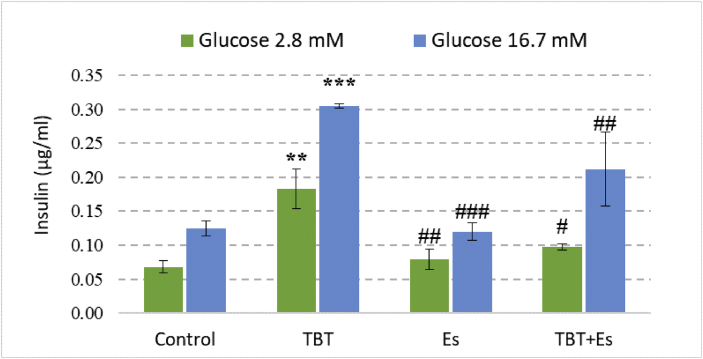
The secretion of insulin at basal and stimulated phases was evaluated under the influence of TBT and β-estradiol. In comparison with the control group, both chemicals increased the insulin secretion at both basal and stimulatory phase but β-estradiol could ameliorate the increment in co-treated group (Figure 4) (see Figure 5).
Figure 4.
Insulin secretion from the pancreatic islets of Langerhans from the rat after 24 h of exposure to TBT (10 μM), Es (1 μM), and TBT (10 μM) plus Es (1 μM). The pancreatic islets were divided into two groups for 1 h incubation with respective concentrations of glucose (basal; 2.8 mM) and (stimulant; 16.7 mM). Data are expressed as mean ± SEM with a replication number, n = 4. ∗∗ Significantly different from control at P-value < 0.01, ∗∗∗ Significantly different from control at P-value < 0.001. # Significantly different from TBT at P-value < 0.05, ## Significantly different from TBT at P-value < 0.05, ### Significantly different from TBT at P-value < 0.001. TBT: tributyltin, Es: β-estradiol.
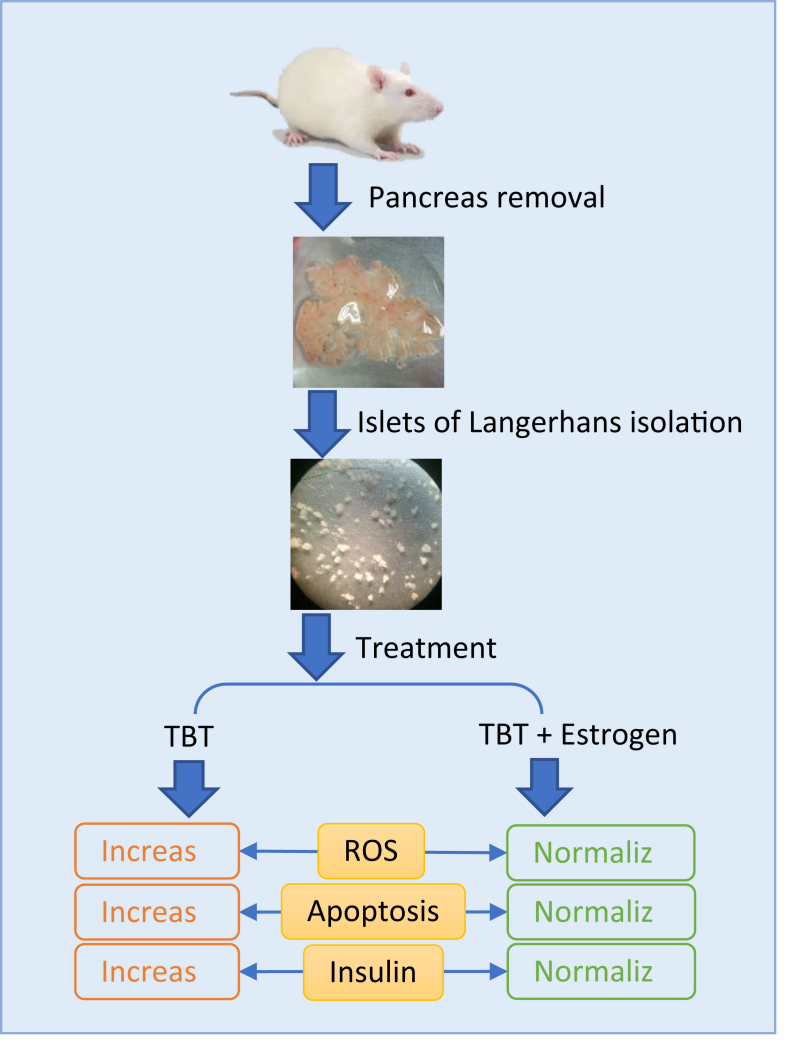
Figure 5.
Graphical abstract showing briefely the main procedure and finding of the study. TBT: tributyltin, ROS: reactive oxygen species.
4. Discussion
This study was done to evaluate the effects of TBT on pancreatic islets of Langerhans and the ability of β-estradiol as an endogenous agonist of ERs to counteract the toxicity. Viability of pancreatic islets was measured using MTT assay and the dose-response curves were plotted in order to establish LC50 of TBT and EC50 of β-estradiol. The present results showed that TBT decreased viability of pancreatic islets in a dose-dependent manner while the viability of pancreatic islets receiving 10 μM decreased by 50%. Co-administration of β-estradiol prevented TBT-induced cellular damage and increased the viability of pancreatic islets but not dose-dependently. Dose-dependent effect of β-estradiol on cellular viability and apoptosis was previously reported. The concentrations between 10−10-10−6 have been known to physiological concentrations of β-estradiol while the concentrations above 10−6 are considered as pharmacological concentrations [22]. As expected, β-estradiol at physiological concentrations was able to increase the viability of pancreatic islets but not at pharmacological concentrations. When the concentration of β-estradiol increased above its physiological concentration, its ability to counteract with TBT-induced cell death was decreased. The EC50 of β-estradiol against TBT toxicity was established 1 μM in the pancreatic islets of Langerhans isolated in this experimental set up.
In the next step, we performed a flowcytometric assay in order to evaluate cell death pathways. As expected, the ratio of live cells in the pancreatic islets treated with TBT was decreased (by 24%), while the number of dead cells was approximately doubled when compared with control. TBT caused cellular death mainly through apoptotic pathway than necrosis in the pancreatic islets so that the number of apoptotic cells increased 2.5 times while the number of necrotic cells increased 1.5 times. This observation confirms the previous studies reporting apoptotic cell death due to TBT toxicity in the pancreas. An in vivo study found that chronic exposure to TBT in mice increased the number of apoptotic cells in the total pancreas, dose-dependently [18]. In another study conducted on the pancreatic β-cells, it was indicated that TBT increased apoptosis associated with cleavage of poly (ADP-ribose) polymerase and phosphorylation of mitogen-activated protein kinases including JNK and ERK1/2 [23]. In our experiment, adding β-estradiol to the pancreatic islets treated with TBT increased viability of the cells near to control. Inhibitory effect of β-estradiol on cell death is shown to be mediated mainly through a decrease in apoptosis. Confronting effect of β-estradiol against TBT toxicity in relation with apoptosis indicates that opposing effects of these two chemicals on the cell death pathways may have a same operator such as ERs in the islets of Langerhans. ER-α was found responsible for antiapoptotic effects of β-estradiol in the pancreatic β-cells and maintenance of insulin release, and thus antidiabetic actions of estrogens may be related to these events [11]. However, apoptosis is the main mode of cell death in pancreatic β-cells and thus chemicals whose therapeutic or toxic effects are mediated through apoptotic pathway may have a determinant role in the fate of these cells. Considering the functions of pancreatic ERs in favor of the cellular viability and inhibition of apoptosis, identification and evaluation of compounds which are capable of interfering with ERs would be of great importance in pharmacology and toxicology of pancreatic islets for understanding and proposing new mechanisms involved in development and control of diabetes.
The results of our study regarding ROS formation confirmed findings of MTT assay and indicated pro-oxidative and antioxidant properties of TBT and β-estradiol, respectively. Oxidative stress plays an important role in the pathophysiology of pancreatic beta cells and activation of stress-activated protein kinases including JNK and p38 have been reported to be involved in ROS induced pancreatic β-cell death [24, 25]. Role of oxidative stress in the cellular toxicity of TBT has been previously confirmed both in vitro and in vivo [26, 27, 28]. On the other hand, β-estradiol via ERs can cope with ROS through transcriptional upregulation of cellular antioxidant elements [29]. However, our results regarding inhibitory effect of β-estradiol on TBT-induced ROS formation support previous studies, which proved the role of ER pathway in antioxidant properties of β-estradiol.
In this work, co-administration of β-estradiol also prevented TBT-induced rise in the release of insulin via pancreatic islets. There has been a controversy regarding the effects of TBT on insulin secretion. There is evidence that chronic and oral treatment of mice with TBT decreases insulin secretion at doses, which cause cellular toxicity and apoptosis [18]. On the other side, two recent studies conducted both in vitro and in vivo, reported that TBT resulted in an increase in insulin secretion from the pancreatic β-cells along with an increase in the body weight, fasting blood glucose, glucose intolerance, and insulin resistance condition characterized by a decrease in the expression of molecules involved insulin receptor cascade [15, 17]. Findings regarding TBT-induced insulin secretion are in accordance with the two latter reports. This observation can be explained by IP3/ryanodine-induced ROS production leading to increased concentration of intracellular Ca2+ [17]. Furthermore, secretion of insulin as a growth factor in order to relieve TBT toxicity can be another explanation for this result. In this way, decreasing effect of β-estradiol on insulin secretion may be related to its anti-oxidant and anti-apoptotic effects. However, activity of ERs in the pancreatic islets influences both viability and functions of β-cells whose integrity is important in the homeostasis of glucose metabolism in the body.
The present study aimed to evaluate the effects of TBT and β-estradiol on rat pancreatic islets. The results indicated that β-estradiol could protect pancreatic islets of Langerhans through increasing cell viability and also decreasing apoptosis, and ROS formation, as well as modifying insulin secretion against TBT toxicity. Considering the previous background regarding the interaction of TBT with ERs, it can be postulated that ER pathway may be involved in TBT-induced damage to the pancreatic islets of Langerhans. Accordingly, evaluating the integrity of endocrine pancreas as a unique organ involved in the homeostasis of glucose metabolism seems to be helpful in the risk assessment of TBT and the other xenoestrogens-induced diabetes.
Declarations
Author contribution statement
F. Ghaemmaleki: Performed the experiments; Wrote the paper.
P. Mohammadi and M. Abdollahi: Contributed reagents, materials, analysis tools or data.
M. Baeeri: Analyzed and interpreted the data.
M. Navaei-Nigjeh: Performed the experiments.
S. Mostafalou: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Council of Research at Ardabil University of Medical Sciences, Ardabil, Iran (grant number: 9705).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to thank the Institute of Pharmaceutical Sciences (TIPS) at Tehran University of Medical Sciences, Tehran, Iran for technical support. Authors wish to thank INSF for their facilitations to access the required full articles and Ms Marzieh Daniali for her English language editing.
References
- 1.Antizar-Ladislao B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. a review. Environ. Int. 2008;34:292–308. doi: 10.1016/j.envint.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Lagadic L., Katsiadaki I., Biever R., Guiney P.D., Karouna-Renier N., Schwarz T., Meador J.P. Tributyltin: advancing the science on assessing endocrine disruption with an unconventional endocrine-disrupting compound. Rev. Environ. Contam. Toxicol. 2018;245:65–127. doi: 10.1007/398_2017_8. [DOI] [PubMed] [Google Scholar]
- 3.Cho E.M., Lee H.S., Moon J.S., Kim I.S., Sim S., Ohta A. Organotin compounds act as inhibitor of transcriptional activation with human estrogen receptor. J. Microbiol. Biotechnol. 2012;22:378–384. doi: 10.4014/jmb.1105.05033. [DOI] [PubMed] [Google Scholar]
- 4.Choe S.Y., Kim S.J., Kim H.G., Lee J.H., Choi Y., Lee H., Kim Y. Evaluation of estrogenicity of major heavy metals. Sci. Total Environ. 2003;312:15–21. doi: 10.1016/S0048-9697(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 5.Iguchi T., Watanabe H., Ohta Y., Blumberg B. Developmental effects: oestrogen-induced vaginal changes and organotin-induced adipogenesis. Int. J. Androl. 2008;31:263–268. doi: 10.1111/j.1365-2605.2008.00863.x. [DOI] [PubMed] [Google Scholar]
- 6.Santos M.M., Micael J., Carvalho A.P., Morabito R., Booy P., Massanisso P., Lamoree M., Reis-Henriques M.A. Estrogens counteract the masculinizing effect of tributyltin in zebrafish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006;142:151–155. doi: 10.1016/j.cbpc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Sharan S., Nikhil K., Roy P. Effects of low dose treatment of tributyltin on the regulation of estrogen receptor functions in MCF-7 cells. Toxicol. Appl. Pharmacol. 2013;269:176–186. doi: 10.1016/j.taap.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Alonso-Magdalena P., Ropero A.B., Carrera M.P., Cederroth C.R., Baquie M., Gauthier B.R., Nef S., Stefani E., Nadal A. Pancreatic insulin content regulation by the estrogen receptor ERα. PloS One. 2008;3 doi: 10.1371/journal.pone.0002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balhuizen A., Kumar R., Amisten S., Lundquist I., Salehi A. Activation of G protein-coupled receptor 30 modulates hormone secretion and counteracts cytokine-induced apoptosis in pancreatic islets of female mice. Mol. Cell. Endocrinol. 2010;320:16–24. doi: 10.1016/j.mce.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Contreras J.L., Smyth C.A., Bilbao G., Young C.J., Thompson J.A., Eckhoff D.E. 17beta-Estradiol protects isolated human pancreatic islets against proinflammatory cytokine-induced cell death: molecular mechanisms and islet functionality. Transplantation. 2002;74:1252–1259. doi: 10.1097/00007890-200211150-00010. [DOI] [PubMed] [Google Scholar]
- 11.Le May C., Chu K., Hu M., Ortega C.S., Simpson E.R., Korach K.S., Tsai M.-J., Mauvais-Jarvis F. Estrogens protect pancreatic β-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc. Natl. Acad. Sci. Unit. States Am. 2006;103:9232–9237. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S., Le May C., Wong W.P., Ward R.D., Clegg D.J., Marcelli M., Korach K.S., Mauvais-Jarvis F. Importance of extranuclear estrogen receptor-α and membrane G protein–coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009;58:2292–2302. doi: 10.2337/db09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nadal A., Alonso-Magdalena P., Soriano S., Quesada I., Ropero A.B. The pancreatic beta-cell as a target of estrogens and xenoestrogens: implications for blood glucose homeostasis and diabetes. Mol. Cell. Endocrinol. 2009;304:63–68. doi: 10.1016/j.mce.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Baena-Diez J.M., Penafiel J., Subirana I., Ramos R., Elosua R., Marin-Ibanez A., Guembe M.J., Rigo F., Tormo-Diaz M.J., Moreno-Iribas C., Cabre J.J., Segura A., Garcia-Lareo M., Gomez de la Camara A., Lapetra J., Quesada M., Marrugat J., Medrano M.J., Berjon J., Frontera G., Gavrila D., Barricarte A., Basora J., Garcia J.M., Pavone N.C., Lora-Pablos D., Mayoral E., Franch J., Mata M., Castell C., Frances A., Grau M., Investigators F. Risk of cause-specific death in individuals with diabetes: a competing risks analysis. Diabetes Care. 2016;39:1987–1995. doi: 10.2337/dc16-0614. [DOI] [PubMed] [Google Scholar]
- 15.Li B., Guo J., Xi Z., Xu J., Zuo Z., Wang C. Tributyltin in male mice disrupts glucose homeostasis as well as recovery after exposure: mechanism analysis. Arch. Toxicol. 2017;91:3261–3269. doi: 10.1007/s00204-017-1961-6. [DOI] [PubMed] [Google Scholar]
- 16.Xu J., Ou K., Chen C., Li B., Guo J., Zuo Z., Wang C. Tributyltin exposure disturbs hepatic glucose metabolism in male mice. Toxicology. 2019;425:152242. doi: 10.1016/j.tox.2019.152242. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y.W., Lan K.C., Tsai J.R., Weng T.I., Yang C.Y., Liu S.H. Tributyltin exposure at noncytotoxic doses dysregulates pancreatic beta-cell function in vitro and in vivo. Arch. Toxicol. 2017;91:3135–3144. doi: 10.1007/s00204-017-1940-y. [DOI] [PubMed] [Google Scholar]
- 18.Zuo Z., Wu T., Lin M., Zhang S., Yan F., Yang Z., Wang Y., Wang C. Chronic exposure to tributyltin chloride induces pancreatic islet cell apoptosis and disrupts glucose homeostasis in male mice. Environ. Sci. Technol. 2014;48:5179–5186. doi: 10.1021/es404729p. [DOI] [PubMed] [Google Scholar]
- 19.Mostafalou S., Baeeri M., Bahadar H., Soltany-Rezaee-Rad M., Gholami M., Abdollahi M. Molecular mechanisms involved in lead induced disruption of hepatic and pancreatic glucose metabolism. Environ. Toxicol. Pharmacol. 2015;39:16–26. doi: 10.1016/j.etap.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Bahadar H., Maqbool F., Mostafalou S., Baeeri M., Rahimifard M., Navaei-Nigjeh M., Abdollahi M. Assessment of benzene induced oxidative impairment in rat isolated pancreatic islets and effect on insulin secretion. Environ. Toxicol. Pharmacol. 2015;39:1161–1169. doi: 10.1016/j.etap.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Mohammadi P., Rahimifard M., Baeeri M., Abdollahi M., Mostafalou S. Mechanistic assessment of cadmium toxicity in association with the functions of estrogen receptors in the Langerhans islets. Iran J Basic Med Sci. 2019;22:445–451. doi: 10.22038/ijbms.2019.33939.8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Celojevic D., Petersen A., Karlsson J.O., Behndig A., Zetterberg M. Effects of 17β-estradiol on proliferation, cell viability and intracellular redox status in native human lens epithelial cells. Mol. Vis. 2011;17:1987–1996. [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C.F., Yang C.Y., Tsai J.R., Wu C.T., Liu S.H., Lan K.C. Low-dose tributyltin exposure induces an oxidative stress-triggered JNK-related pancreatic beta-cell apoptosis and a reversible hypoinsulinemic hyperglycemia in mice. Sci. Rep. 2018;8:5734. doi: 10.1038/s41598-018-24076-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou N., Torii S., Saito N., Hosaka M., Takeuchi T. Reactive oxygen species-mediated pancreatic β-cell death is regulated by interactions between stress-activated protein kinases, p38 and c-Jun N-terminal kinase, and mitogen-activated protein kinase phosphatases. Endocrinology. 2008;149:1654–1665. doi: 10.1210/en.2007-0988. [DOI] [PubMed] [Google Scholar]
- 25.Newsholme P., Haber E., Hirabara S., Rebelato E., Procópio J., Morgan D., Oliveira-Emilio H., Carpinelli A.R., Curi R. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J. Physiol. 2007;583:9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitra S., Gera R., Siddiqui W.A., Khandelwal S. Tributyltin induces oxidative damage, inflammation and apoptosis via disturbance in blood–brain barrier and metal homeostasis in cerebral cortex of rat brain: an in vivo and in vitro study. Toxicology. 2013;310:39–52. doi: 10.1016/j.tox.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Nakatsu Y., Kotake Y., Ohta S. Concentration dependence of the mechanisms of tributyltin-induced apoptosis. Toxicol. Sci. 2007;97:438–447. doi: 10.1093/toxsci/kfm039. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Chen Y., Sun L., Liang J., Guo Z., Xu L. Protein phosphatases 2A as well as reactive oxygen species involved in tributyltin-induced apoptosis in mouse livers. Environ. Toxicol. 2014;29:234–242. doi: 10.1002/tox.21751. [DOI] [PubMed] [Google Scholar]
- 29.Panza S., Santoro M., De Amicis F., Morelli C., Passarelli V., D'Aquila P., Giordano F., Cione E., Passarino G., Bellizzi D., Aquila S. Estradiol via estrogen receptor beta influences ROS levels through the transcriptional regulation of SIRT3 in human seminoma TCam-2 cells. Tumour Biol. 2017;39 doi: 10.1177/1010428317701642. 1010428317701642. [DOI] [PubMed] [Google Scholar]