Abstract
Background
Magnesium maintenance therapy is one of the types of tocolytic therapy used after an episode of threatened preterm labour (usually treated with an initial dose of tocolytic therapy) in an attempt to prevent the onset of further preterm contractions.
Objectives
To assess whether magnesium maintenance therapy is effective in preventing preterm birth after the initial threatened preterm labour is arrested.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 January 2013).
Selection criteria
Randomised controlled trials of magnesium therapy given to women after threatened preterm labour.
Data collection and analysis
The review authors independently assessed the studies for inclusion, assessed risk of bias and carried out data extraction. We checked data entry.
Main results
We included four trials involving 422 women. Three trials had high risk of bias and none included any long‐term follow‐up of infants. No differences in the incidence of preterm birth or perinatal mortality were seen when magnesium maintenance therapy was compared with placebo or no treatment; or alternative therapies (ritodrine or terbutaline). The risk ratio (RR) for preterm birth (less than 37 weeks) for magnesium compared with placebo or no treatment was 1.05, 95% confidence interval (CI) 0.80 to 1.40 (two trials, 99 women); and 0.99, 95% CI 0.57 to 1.72 (two trials, 100 women) for magnesium compared with alternative therapies. The RR for perinatal mortality for magnesium compared with placebo or no treatment was 5.00, 95% CI 0.25 to 99.16 (one trial, 50 infants); and 5.00, 95% CI 0.25 to 99.16 (one trial, 50 infants) for magnesium compared with alternative treatments.
Women taking magnesium preparations were less likely to report side effects (RR 0.67, 95% CI 0.47 to 0.96, three trials, 237 women), including palpitations or tachycardia (RR 0.26, 95% CI 0.13 to 0.52, three trials, 237 women) than women receiving alternative therapies. Women receiving magnesium were however, more likely to experience diarrhoea (RR 6.79, 95% CI 1.26 to 36.72, three trials, 237 women).
Authors' conclusions
There is not enough evidence to show any difference between magnesium maintenance therapy compared with either placebo or no treatment, or alternative therapies (ritodrine or terbutaline) in preventing preterm birth after an episode of threatened preterm labour.
Keywords: Female; Humans; Pregnancy; Magnesium Chloride; Magnesium Chloride/therapeutic use; Magnesium Compounds; Magnesium Compounds/therapeutic use; Magnesium Oxide; Magnesium Oxide/therapeutic use; Magnesium Sulfate; Magnesium Sulfate/therapeutic use; Obstetric Labor, Premature; Obstetric Labor, Premature/drug therapy; Premature Birth; Premature Birth/prevention & control; Randomized Controlled Trials as Topic; Ritodrine; Ritodrine/therapeutic use; Terbutaline; Terbutaline/therapeutic use; Tocolysis; Tocolysis/methods; Tocolytic Agents; Tocolytic Agents/therapeutic use
Plain language summary
Giving magnesium maintenance therapy to women to prevent preterm birth after stopping threatened preterm labour
Magnesium does not reduce preterm birth or improve the outcome for the infant when given to women after contractions of preterm labour have been stopped.
Babies born preterm, before 37 weeks of pregnancy, may not survive or they may have later physical health and developmental problems if they do survive. Women whose preterm labour is stopped with tocolytic therapy (medication to reduce uterine contractions) remain at high risk of preterm birth. A variety of agents (tocolytics) are used to halt the uterine contractions. These include betamimetics, calcium channel blockers, magnesium sulphate, and oxytocin receptor antagonists. Subsequent tocolytic maintenance medication has been advocated. Oral and intravenous magnesium has been used to prevent further early contractions.
We included four randomised controlled trials involving a total of 422 women in this review. The trials did not demonstrate any differences between magnesium maintenance therapy and placebo or other treatments (ritodrine or terbutaline) in the prevention of preterm birth or perinatal deaths. The trials were too small to exclude either important benefits or harms from magnesium maintenance therapy. Magnesium was less likely than the alternative tocolytics (betamimetics) to result in side effects, particularly palpitations or tachycardia, although diarrhoea was more likely. This finding is based on very few studies of low quality, and none of them looked at the infants' later development.
Background
Description of the condition
Preterm birth (before 37 weeks of gestation) is the principal cause of early neonatal mortality and morbidity and causes both significant immediate health problems and substantial long‐term problems in a proportion of surviving babies (AIHW 2006; Saigal 2008). Additionally, women who give birth to preterm babies are at increased risk of psychological distress (Eriksson 2002). Prevention of preterm birth remains an important priority.
Preterm labour, defined as birth occurring before 37 completed weeks' gestation, is a major cause of perinatal mortality and morbidity (Saigal 2008). A substantial proportion of women who have an episode of threatened preterm labour are actively treated with agents to stop the uterine contractions (acute tocolytic therapy). Women whose preterm labour is successfully arrested with acute tocolytic therapy still remain at risk of preterm birth until they reach 37 weeks of gestation, and they may therefore be treated with agents aimed at further prolonging gestation (maintenance tocolytic therapy).
Description of the intervention
A variety of therapeutic tocolytic agents that inhibit uterine contractions have been used to halt uterine activity in women in preterm labour and so prevent preterm birth. Separate Cochrane systematic reviews assessing betamimetics (Anotayanonth 2004), calcium channel blockers (King 2003), magnesium sulphate (Crowther 2002), and oxytocin receptor antagonists (Papatsonis 2005) cover these topics.
Women whose preterm labour is initially arrested with tocolytic therapy remain at high risk of preterm birth until they reach full term. For these women, the use of subsequent tocolytic maintenance medication has been advocated to reduce the risk of recurrence of preterm labour and to prevent preterm birth. Clinical practice guidelines vary as to when maintenance tocolysis is recommended to commence, but this usually begins once initial tocolytic medications have ceased. The maintenance tocolysis is usually given as oral medications and the duration of use is variable.
How the intervention might work
Magnesium was described as having an effect on uterine contractility, by increasing the duration of labour, in the late 1950s (Hall 1959). The exact mechanism of magnesium as a tocolytic agent (for initial use or for maintenance) is only partially understood. Magnesium decreases the frequency of depolarisation of smooth muscle, by modulating calcium uptake, binding and distribution in smooth muscle cells. The net result is inhibition of uterine contractions. However, the use of magnesium for tocolysis is not without risk of side effects, including diarrhoea, tachycardia and palpitations. In particular, uncoated magnesium salts are not used orally for maintenance therapy because of their potential to cause adverse gastrointestinal effects (Ricci 1991).
Why it is important to do this review
The effectiveness of maintenance tocolytic therapy to reduce the risk of recurrent preterm labour is unproven for women who have an episode of preterm labour that stops after acute tocolytics (Sanchez‐Ramos 1999).
This review assesses the efficacy of magnesium tocolytic maintenance therapy after preterm labour has been successfully arrested with initial tocolytic therapy. Another four Cochrane reviews have addressed other therapies for tocolytic maintenance therapy namely the use of terbutaline pumps (Nanda 2002); oral betamimetics (Dodd 2006); calcium channel blockers (Gaunekar 2004) and oxytocin antagonists (Papatsonis 2009).
Objectives
To assess whether magnesium maintenance therapy is effective in preventing preterm birth after the initial threatened preterm labour is arrested.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing randomised trials with reported data that compare outcomes in women and babies given magnesium therapy after threatened preterm labour with outcomes in controls given alternative therapy, placebo or no therapy. We planned to exclude quasi‐randomised trials, cluster‐randomised trials and cross‐over trials. We planned to include studies published as abstracts only, in addition to those published as full‐text papers.
Types of participants
Pregnant women who have had at least one episode of threatened preterm labour that settled without delivery.
Types of interventions
Magnesium maintenance therapy administered to the woman by any route prior to delivery, compared with either placebo, no treatment or alternative maintenance tocolytic therapy. We excluded trials where magnesium sulphate was used together with an alternative tocolytic as maintenance therapy.
Types of outcome measures
Primary outcomes
The primary outcomes considered were:
preterm birth;
perinatal mortality (defined as stillbirths and liveborn infants who died prior to hospital discharge);
any neurological disability at follow‐up.
Secondary outcomes
The secondary outcomes were:
maternal need for hospital readmission for threatened preterm labour;
low Apgar score (less than seven at five minutes);
neonatal respiratory disease;
use of mechanical ventilation;
air leak syndrome;
intraventricular haemorrhage;
periventricular haemorrhage;
periventricular leukomalacia;
necrotising enterocolitis;
retinopathy of prematurity;
patent ductus arteriosus;
neonatal infection;
maternal side effects of therapy;
woman's assessment of the therapy;
the cost of therapy.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 January 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in a previous version of this review (2008 update), see Appendix 1.
For the 2010 update, we used the following methods when assessing the trial identified by the search. No new studies were identified for the 2013 update.
Selection of studies
At least two of the three review authors (CA Crowther, V Moore and S Han), independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, at least two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. We entered data into Review Manager software (RevMan 2011) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
In the previous version of this review, two review authors (CA Crowther and S Han) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreement by discussion or by involving the third review author.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determined whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies are at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook. We assessed methods as:
low risk of bias (e.g. where there are no missing data or where reasons for missing data are balanced across groups);
high risk of bias (e.g. where missing data are likely to be related to outcomes or are not balanced across groups);
unclear risk of bias (e.g. where there is insufficient reporting of attrition or exclusions to permit a judgement to be made).
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias. For example, was there a potential source of bias related to the specific study design? Was the trial stopped early due to some data‐dependent process? Was there extreme baseline imbalance? Has the study been claimed to be fraudulent?
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measure the same outcome, but used different methods.
Unit of analysis issues
We considered cluster‐randomised trials as inappropriate for inclusion in this review.
As infants from multiple pregnancies are not independent, we planned to use cluster trial methods in the analysis, where the data allowed, and where multiples made up a substantial proportion of the trial population, to account for non‐independence of variables (Gates 2004).
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If we detect asymmetry by a visual assessment, we will perform exploratory analyses to investigate it.
Where we suspected reporting bias (see 'Selective reporting bias' above), we attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. Where there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or where substantial statistical heterogeneity was detected, we have used random‐effects meta‐analysis to produce an overall summary where an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we have discussed the clinical implications of treatment effects differing between trials. If the average treatment effect had not been clinically meaningful, we would not have combined trials.
Where we have used random‐effects analyses, we have presented the results as the average treatment effect with its 95% confidence interval, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
We carried out separate comparisons for magnesium maintenance therapy compared with placebo or no treatment, and compared with alternative tocolytic maintenance therapy.
If we had identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. We planned to consider whether an overall summary was meaningful, and if it was, use random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses.
Route of administration of the magnesium (oral, intravenous, intramuscular).
Drug regimen of magnesium used (dose, timing after initial threatened preterm labour and duration).
We planned to use primary outcomes only in the subgroup analysis.
We planned to assess subgroup differences by interaction tests available within RevMan (RevMan 2011). We planned to report the results of subgroup analyses quoting the χ2 statistic and P value, and the interaction test I² value.
However, there were insufficient data to conduct the subgroup analyses based on the route of administration of the magnesium (oral, intravenous, intramuscular) and the drug regimen of magnesium used (dose, timing after initial threatened preterm labour and duration).
Sensitivity analysis
We carried out sensitivity analysis to explore the effect of trial quality for important outcomes in the review, by excluding those trials rated 'high risk of bias' or 'unclear risk of bias' for sequence generation and allocation concealment.
Results
Description of studies
Results of the search
No news trials were identified for inclusion or exclusion in this update of the review. In the previous update (Han 2010), we included four trials (Holcomb 1991; Ricci 1991; Ridgway 1990; Rust 1996), and excluded five trials (Facchinetti 1992; Lewis 1997; Martin 1990; Martin 1998; Terrone 2000) from this review.
Included studies
We included four trials that recruited 422 women. All four studies (Holcomb 1991; Ricci 1991; Ridgway 1990; Rust 1996) were from North America.
Ricci 1991 compared oral SLOW MAG (enteric‐coated magnesium chloride) with oral ritodrine or no treatment; Ridgway 1990 compared oral magnesium oxide with oral terbutaline sulphate; and Rust 1996 compared oral magnesium chloride with oral terbutaline sulphate and oral placebo. Holcomb 1991 compared gradual reduction in intravenous magnesium sulphate as maintenance therapy with no treatment (abrupt cessation of intravenous magnesium sulphate therapy that had been used for initial tocolysis of the preterm labour). None of the studies included any long‐term follow‐up of infants or children.
See the Characteristics of included studies table for more details.
Excluded studies
We excluded five trials (Facchinetti 1992; Lewis 1997; Martin 1990; Martin 1998; Terrone 2000). We have provided reasons for exclusion in the Characteristics of excluded studies tables.
Risk of bias in included studies
We have provided details of risk of bias for each of the included studies in the Characteristics of included studies. For a summary of the risk of bias across the included studies, see Figure 1 and Figure 2.
1.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All four studies (Rust 1996; Ridgway 1990; Ricci 1991; Holcomb 1991) included in the review were randomised trials. The randomisation process was adequately concealed in only one trial (Rust 1996), where randomisation and allocation was done centrally in the pharmacy. In the Ricci 1991 trial, randomisation was achieved by "sealed envelopes" with no further details given. The randomisation and allocation processes were unclear in the other trials (Holcomb 1991; Ridgway 1990).
Blinding
In Ricci 1991, it appears that the participants were aware of the treatment regimen to which they were assigned, as were the clinicians and outcome assessors involved (i.e. unblinded study) and no efforts made to standardise assessments of outcome were detailed. No information on blinding of outcome assessment procedures was provided in Holcomb 1991 and Ridgway 1990. In Rust 1996, both women and staff were blinded to the treatment allocation. However, how that blinding was achieved was not explained. None of the studies included any long‐term follow‐up of infants or children.
Incomplete outcome data
In Ridgway 1990, 17% of participants were lost to follow‐up. The Rust 1996 study lost 16% of participants after randomisation as they delivered before discharge; for the remaining participants who went on to receive maintenance therapy as outpatients, there was only a 2% loss to follow‐up. It appeared that in Ricci 1991 none of the randomised women were lost to follow‐up. Completeness of follow‐up was not mentioned in Holcomb 1991.
Selective reporting
In three of the four trials, there was insufficient information to judge the risk of selective reporting (Holcomb 1991; Ricci 1991; Ridgway 1990). Rust 1996 was judged to be at a low risk of reporting bias, with no evidence of selective reporting.
Other potential sources of bias
Ricci 1991 and Ridgway 1990 were judged to be at a high risk of other bias. In Ricci 1991 it was noted that the sample size was "not large enough to detect subtle differences", whilst in Ridgway 1990 there was no pre‐specified sample size. A post hoc power calculation indicated there was power of 0.5 to detect a difference of 0.2 in the proportion of women that delivered at less than 36 weeks. Holcomb 1991 was judged to be at an unclear risk of other bias, and only one trial (Rust 1996) was judged to be at low risk of other bias, with no obvious other sources of bias identified.
Three trials were judged to be at a high risk of bias overall (Holcomb 1991; Ricci 1991; Ridgway 1990), and one trial was judged to be at a low risk of bias (Rust 1996).
Effects of interventions
Magnesium versus placebo or no treatment
Three trials, involving 232 women were included in this comparison (Holcomb 1991; Ricci 1991; Rust 1996). However, most outcomes were only measured by one trial (Ricci 1991).
Primary outcomes
There was no significant difference in terms of the primary outcomes of preterm birth (risk ratio (RR) 1.05, 95% confidence interval (CI) 0.80 to 1.40, two trials, 99 women) (Analysis 1.1) or perinatal mortality (RR 5.00, 95% CI 0.25 to 99.16, one trial, 50 infants) (Analysis 1.2) between the magnesium and no magnesium groups. No data were available in relation to the outcomes of very preterm birth (less than 32 weeks of gestation) or extremely preterm birth (less than 28 weeks of gestation). None of the trials reported on any neurological follow‐up of the infants.
1.1. Analysis.

Comparison 1 Magnesium versus placebo or no treatment, Outcome 1 Preterm birth.
1.2. Analysis.

Comparison 1 Magnesium versus placebo or no treatment, Outcome 2 Perinatal mortality.
Secondary outcomes
There was no significant difference in maternal readmission for threatened preterm labour (RR 0.79, 95% CI 0.45 to 1.38, one trial, 50 women) (Analysis 1.3), respiratory distress syndrome (RR 3.00, 95% CI 0.13 to 70.30, one trial, 40 infants) (Analysis 1.4), or periventricular haemorrhage (RR 3.00, 95% CI 0.13 to 70.30, one trial, 50 infants) (Analysis 1.5) between the magnesium versus no magnesium groups.
1.3. Analysis.

Comparison 1 Magnesium versus placebo or no treatment, Outcome 3 Maternal readmission for threatened preterm labour.
1.4. Analysis.

Comparison 1 Magnesium versus placebo or no treatment, Outcome 4 Respiratory distress syndrome.
1.5. Analysis.

Comparison 1 Magnesium versus placebo or no treatment, Outcome 5 Periventricular haemorrhage.
No data were reported in any of the trials on other indicators for neonatal morbidity (low Apgar score, use of mechanical ventilation, air leak syndrome, intraventricular haemorrhage, periventricular leukomalacia, necrotising enterocolitis, retinopathy of prematurity, patent ductus arteriosus and neonatal infection).
No difference was found for neonatal intensive care unit admissions (RR 1.57, 95% CI 0.76 to 3.24, one trial, 133 infants) (Analysis 1.6), neonatal length of stay (mean difference (MD) 1.18 days, 95% CI ‐0.46 to 2.82, two trials, 180 infants) (Analysis 1.7) or gestational age at birth (MD ‐0.55 weeks, 95% CI ‐1.34 to 0.25, two trials, 183 infants) (Analysis 1.8). While these three outcomes were not pre‐specified in the protocol, we considered these to be important.
1.6. Analysis.

Comparison 1 Magnesium versus placebo or no treatment, Outcome 6 Neonatal intensive care unit admissions.
1.7. Analysis.

Comparison 1 Magnesium versus placebo or no treatment, Outcome 7 Neonatal length of stay (days).
1.8. Analysis.

Comparison 1 Magnesium versus placebo or no treatment, Outcome 8 Gestational age at delivery (weeks).
Women receiving magnesium were more likely to report side effects (RR 1.88, 95% CI 1.11 to 3.20, one trial, 133 women) (Analysis 1.9), particularly diarrhoea (RR 7.67, 95% CI 2.41 to 24.41, one trial, 133 women) (Analysis 1.9). There were no data on women's assessment of therapy, or on costs.
1.9. Analysis.

Comparison 1 Magnesium versus placebo or no treatment, Outcome 9 Maternal side effects.
For all outcomes in this comparison (where there was more than one study contributing data to a meta‐analysis), there was no observed heterogeneity (I² = 0).
Magnesium versus alternative treatment
Three trials, involving 237 women were included in this comparison (Ridgway 1990; Ricci 1991; Rust 1996).
Primary outcomes
No differences were found between magnesium maintenance therapy and alternative treatment in terms of the primary outcomes of preterm birth (RR 0.99, 95% CI 0.57 to 1.72, two trials, 100 women) (Analysis 2.1) or perinatal mortality (RR 5.00, 95% CI 0.25 to 99.16, one trial, 50 infants) (Analysis 2.2). No data were available in relation to the outcomes of very preterm birth (less than 32 weeks of gestation) or extremely preterm birth (less than 28 weeks of gestation). None of the trials reported on any neurological follow‐up of the infants.
2.1. Analysis.

Comparison 2 Magnesium versus alternative treatment, Outcome 1 Preterm birth.
2.2. Analysis.

Comparison 2 Magnesium versus alternative treatment, Outcome 2 Perinatal mortality.
Secondary outcomes
No differences were seen for the secondary outcomes of maternal need for hospital readmission for threatened preterm labour (RR 1.00, 95% CI 0.62 to 1.62, two trials, 100 women) (Analysis 2.3) and periventricular haemorrhage (RR 1.00, 95% CI 0.07 to 15.12, one trial, 50 infants) (Analysis 2.4).
2.3. Analysis.

Comparison 2 Magnesium versus alternative treatment, Outcome 3 Maternal readmission for threatened preterm labour.
2.4. Analysis.

Comparison 2 Magnesium versus alternative treatment, Outcome 4 Periventricular haemorrhage.
Similarly no differences were shown between groups for neonatal intensive care admissions (RR 0.98, 95% CI 0.53 to 1.80, one trial, 137 infants) (Analysis 2.5), neonatal length of stay (MD ‐2.63 days, 95% CI ‐5.70 to 0.43, two trials, 180 infants; I² = 25%) (Analysis 2.6), and gestational age at delivery (MD 0.56 weeks, 95% CI ‐0.17 to 1.30, three trials, 237 infants) (Analysis 2.7) (these three outcomes were not pre‐specified in the review, though they were considered to be important).
2.5. Analysis.

Comparison 2 Magnesium versus alternative treatment, Outcome 5 Neonatal intensive care unit admissions.
2.6. Analysis.

Comparison 2 Magnesium versus alternative treatment, Outcome 6 Neonatal length of stay (days).
2.7. Analysis.
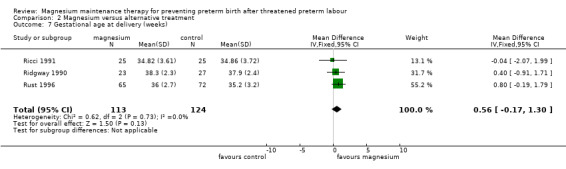
Comparison 2 Magnesium versus alternative treatment, Outcome 7 Gestational age at delivery (weeks).
For all above mentioned outcomes in this comparison there was no observed heterogeneity (I² = 0) (excluding neonatal length of stay, where the I² has been reported).
There was no difference found in the need to cease therapy because of side effects (RR 1.08, 95% CI 0.27 to 4.28, two trials, 100 women; I² = 0%) (Analysis 2.8). Fewer women taking magnesium preparations reported any side effects however, compared with women receiving alternative treatments, (betamimetics, ritodrine or terbutaline) (RR 0.67, 95% CI 0.47 to 0.96, three trials, 237 women). As we identified moderate statistical heterogeneity for this outcome (T² = 0.03; I² = 33%), a random‐effects model was used (Analysis 2.8). It is possible that the differing comparison treatments across the three trials (Ricci 1991: oral ritodrine, Ridgway 1990 and Rust 1996: oral terbutaline sulphate) contributed to this moderate level of heterogeneity.
2.8. Analysis.

Comparison 2 Magnesium versus alternative treatment, Outcome 8 Maternal side effects.
Women taking magnesium preparations were less likely to report palpitations or tachycardia than women receiving alternative treatments (RR 0.26, 95% CI 0.13 to 0.52, three trials, 237 women; I² = 0%) (Analysis 2.8) but were much more likely to experience diarrhoea (RR 6.79, 95% CI 1.26 to 36.72, three trials, 237 women) (Analysis 2.8). Considering the outcome diarrhoea, we identified moderate statistical heterogeneity in the meta‐analysis (T² = 0.95; I² = 43%) and thus a random‐effects model was used (Analysis 2.8). As above, it is possible that the differing comparison treatments across the three trials contributed to this heterogeneity.
No differences between groups were shown for the side effects nausea (RR 0.91, 95% CI 0.49 to 1.70, three trials, 237 women; I² = 0%) or vomiting (RR 0.89, 95% CI 0.19 to 4.06, three trials, 237 women; T² = 0.73; I² = 60%).
No data were available from the included trials for other indicators of neonatal morbidity (a low Apgar score at five minutes, use of mechanical ventilation, or pulmonary air leak, intraventricular haemorrhage, periventricular leukomalacia, necrotising enterocolitis, retinopathy of prematurity, patent ductus arteriosus and neonatal infection). There were no data on the mother's assessment of therapy, or on costs.
Magnesium versus alternative treatment, placebo or no treatment (high‐quality trials only)
When the pre‐specified sensitivity analyses were undertaken to examine the influence of study quality, Holcomb 1991, Ricci 1991 and Ridgway 1990 were excluded because the randomisation and allocation concealment processes were unclear in these trials.
The Rust 1996 study was at low risk of bias on randomisation, allocation concealment, blinding of outcomes assessment and selective reporting, but 18% of women were lost to follow‐up in this trial and it was unclear whether the incomplete outcome data were adequately addressed. The Rust 1996 trial found no significant difference between magnesium and terbutaline or magnesium and placebo on gestational age at birth, although it found magnesium to be more favourable than terbutaline for reducing length of stay for the infant (MD ‐4.4 days, 95% CI ‐8.69 to ‐0.11). The authors note that this benefit may be due to the confounding effect of the larger number of twins in the terbutaline group, and report that when twins were controlled for, neither magnesium or terbutaline showed beneficial or detrimental effects on maternal or neonatal outcomes. In this trial, women taking magnesium were more likely than those in the placebo group to experience side effects (RR 1.88, 95% CI 1.11 to 3.20), although there was no difference seen between magnesium and terbutaline (RR 0.85, 95% CI 0.59 to 1.24), the latter being consistent with the two other studies (Ricci 1991; Ridgway 1990).
Discussion
Given that magnesium has been used widely for many years as tocolytic maintenance medication in women who have had an episode of threatened preterm labour, it was surprising that evidence to support its use was so sparse. Of the four trials identified, only one was rated of reasonable quality for randomisation, allocation concealment, blinding of outcomes assessment and free of selective reporting. The trials available for inclusion in this review do not demonstrate any differences between magnesium maintenance therapy and placebo or other treatments in the prevention of preterm birth or perinatal mortality. However, the trials were too small to exclude either important benefits or harms from magnesium maintenance therapy. Magnesium was less likely than the alternative tocolytics (betamimetics) to result in side effects, particularly palpitations or tachycardia, although diarrhoea was more likely.
Authors' conclusions
Implications for practice.
The role of magnesium maintenance therapy in preventing preterm birth is unproven.
Implications for research.
Researchers who consider that magnesium might be an effective maintenance therapy should conduct further well‐designed randomised controlled trials. Any future trials should follow up infants past the neonatal period to assess neurological outcomes at childhood follow‐up.
What's new
| Date | Event | Description |
|---|---|---|
| 14 February 2013 | New search has been performed | Review updated. |
| 31 January 2013 | New citation required but conclusions have not changed | Search updated; no new studies identified. Methods have been updated. |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 1, 1998
| Date | Event | Description |
|---|---|---|
| 31 May 2010 | New citation required but conclusions have not changed | New author involved in the review. All authors made contributions to this update. |
| 31 May 2010 | New search has been performed | Search updated. One trial previously awaiting classification has been included (Holcomb 1991). Three trials (4 reports) previously awaiting classification have now been excluded (Lewis 1997; Martin 1998; Terrone 2000). Risk of bias tables have been incorporated. |
| 10 November 2008 | Amended | Contact details updated. |
| 11 September 2008 | Amended | Converted to new review format. |
| 22 May 2003 | Amended | The title of this Review has been changed, from Issue 3, 2003 of The Cochrane Library, to make the scope of the Review clearer. The previous title was 'Magnesium for preventing preterm birth after threatened preterm labour'. |
Acknowledgements
For this update of the review, we thank Emily Bain, who provided review support, assisted by a grant from the Australian National Health and Medical Research Council.
Thanks to Philippa Middleton who provided review support for the previous versions of this review, assisted by a grant from the Commonwealth Department of Health and Ageing, Australia.
Special thanks to Lynn Hampson, Sonja Henderson, Frances Kellie and Leanne Jones, Cochrane Pregnancy and Childbirth Group, Liverpool for conducting the updated search and providing review author support.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Methods used to assess trials included in previous versions of this review
The following methods were used to assess Holcomb 1991, Ricci 1991, Ridgway 1990, Rust 1996.
Trials under consideration were evaluated for inclusion and methodological quality, without consideration of their results. These assessments were performed separately by each reviewer with discrepancies resolved by discussion. There was no blinding of authorship.
Quality scores for concealment of allocation were assigned to each trial using the criteria described in the Cochrane Handbook (Clarke 2002). A = adequate, B = unclear, C = inadequate, D = not used.
In addition, quality scores were assigned to each trial for completeness of follow‐up and blinding as described below.
Completeness of follow‐up: (A) less than 3% of participants lost to follow‐up; (B) 3% to less than 10% of participants lost to follow‐up; (C) 10% to less than 20% of participants lost to follow‐up; (D) 20% or more lost to follow‐up; (E) unclear.
For blinding: (A) neither investigator, outcome assessor, nor participant knew or were likely to guess the allocated treatment; (B) either the investigator, outcome assessor, or the participant knew the allocation, or it is likely that for a significant proportion (at least 20%) of participants the allocation could be correctly identified; (C) no blinding, investigator, outcome assessor and participant knew or were likely to guess the allocated treatment; (D) unclear.
Data were extracted independently by the two reviewers and double entered. Discrepancies were resolved by discussion. There was no blinding of authorship. Whenever possible, unpublished data were sought from investigators.
A priori it was decided that all eligible trials would be included in the initial analyses and sensitivity analyses would be carried out to evaluate the effects of trial quality. It was planned to do this through excluding trials given less than an A rating for quality for concealment of allocation, then D or E for completeness of follow‐up, then C or D for blinding.
Dichotomous outcomes are presented in the form of relative risks with 95% confidence intervals (fixed‐effect model). Continuous outcomes are presented as weighted mean differences with 95% confidence intervals (fixed‐effect model).
Data and analyses
Comparison 1. Magnesium versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth | 2 | 99 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.80, 1.40] |
| 1.1 < 37 weeks | 2 | 99 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.80, 1.40] |
| 1.2 < 32 weeks | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 < 28 weeks | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Perinatal mortality | 1 | 50 | Risk Ratio (IV, Fixed, 95% CI) | 5.0 [0.25, 99.16] |
| 2.1 Stillbirth | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Death before discharge among live‐born infants | 1 | 50 | Risk Ratio (IV, Fixed, 95% CI) | 5.0 [0.25, 99.16] |
| 3 Maternal readmission for threatened preterm labour | 1 | 50 | Risk Ratio (IV, Fixed, 95% CI) | 0.79 [0.45, 1.38] |
| 4 Respiratory distress syndrome | 1 | 50 | Risk Ratio (IV, Fixed, 95% CI) | 3.00 [0.13, 70.30] |
| 5 Periventricular haemorrhage | 1 | 50 | Risk Ratio (IV, Fixed, 95% CI) | 3.00 [0.13, 70.30] |
| 6 Neonatal intensive care unit admissions | 1 | 133 | Risk Ratio (IV, Fixed, 95% CI) | 1.57 [0.76, 3.24] |
| 7 Neonatal length of stay (days) | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | 1.18 [‐0.46, 2.82] |
| 8 Gestational age at delivery (weeks) | 2 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐1.34, 0.25] |
| 9 Maternal side effects | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Experienced any side effects | 1 | 133 | Risk Ratio (IV, Fixed, 95% CI) | 1.88 [1.11, 3.20] |
| 9.2 Nausea | 1 | 133 | Risk Ratio (IV, Fixed, 95% CI) | 0.73 [0.30, 1.81] |
| 9.3 Vomiting | 1 | 133 | Risk Ratio (IV, Fixed, 95% CI) | 0.42 [0.08, 2.08] |
| 9.4 Diarrhoea | 1 | 133 | Risk Ratio (IV, Fixed, 95% CI) | 7.67 [2.41, 24.41] |
| 9.5 Abdominal pain | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.6 Palpitations/tachycardia | 1 | 133 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.15, 7.21] |
| 9.7 Faintness/hypotension | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.8 Chest pain | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.9 Discontinued therapy | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Magnesium versus alternative treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth | 2 | 100 | Risk Ratio (IV, Fixed, 95% CI) | 0.99 [0.57, 1.72] |
| 1.1 < 37 weeks | 2 | 100 | Risk Ratio (IV, Fixed, 95% CI) | 0.99 [0.57, 1.72] |
| 1.2 < 32 weeks | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 < 28 weeks | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Perinatal mortality | 1 | 50 | Risk Ratio (IV, Fixed, 95% CI) | 5.0 [0.25, 99.16] |
| 2.1 Stillbirth | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Death before discharge among live‐born infants | 1 | 50 | Risk Ratio (IV, Fixed, 95% CI) | 5.0 [0.25, 99.16] |
| 3 Maternal readmission for threatened preterm labour | 2 | 100 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.62, 1.62] |
| 4 Periventricular haemorrhage | 1 | 50 | Risk Ratio (IV, Fixed, 95% CI) | 1.0 [0.07, 15.12] |
| 5 Neonatal intensive care unit admissions | 1 | 137 | Risk Ratio (IV, Fixed, 95% CI) | 0.98 [0.53, 1.80] |
| 6 Neonatal length of stay (days) | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐2.63 [‐5.70, 0.43] |
| 7 Gestational age at delivery (weeks) | 3 | 237 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [‐0.17, 1.30] |
| 8 Maternal side effects | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Experienced any side effects | 3 | 237 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.47, 0.96] |
| 8.2 Nausea | 3 | 237 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.49, 1.70] |
| 8.3 Vomiting | 3 | 237 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.19, 4.06] |
| 8.4 Diarrhoea | 3 | 237 | Risk Ratio (IV, Random, 95% CI) | 6.79 [1.26, 36.72] |
| 8.5 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.6 Palpitations/tachycardia | 3 | 237 | Risk Ratio (IV, Random, 95% CI) | 0.26 [0.13, 0.52] |
| 8.7 Faintness/hypotension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.8 Chest pain | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.9 Discontinued therapy | 2 | 100 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.27, 4.28] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Holcomb 1991.
| Methods | Randomised controlled trial. | |
| Participants | Women (n = 49; 25 in the magnesium group, 24 in the comparison group) between 26 to 35 weeks' gestation with preterm labour and intact amnion, and contractions have been suppressed for at least 12 hours with intravenous magnesium sulphate. Study conducted in Washington University Medical Center, USA. | |
| Interventions | (1) After the initial intravenous magnesium treatment, the dosage of magnesium sulphate was decreased at a rate of 0.5 g/hour for 4 hours. (2) The intravenous magnesium treatment was discontinued abruptly after the initial dose. All women received intravenous magnesium to suppress the initial contractions. |
|
| Outcomes | Preterm delivery, length of pregnancy prolongation, gestational age at delivery, delivery within 3 days and 7 days of treatment. | |
| Notes | No detail of whether the pregnancies were single/multiple. No losses to follow‐up and post‐randomisation exclusion mentioned. No pre‐specified sample size. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as: "women were randomly assigned to one of the two groups"; no other information available. |
| Allocation concealment (selection bias) | Unclear risk | No information was given on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details on blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficent information to make the judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to make the judgement. |
| Other bias | Unclear risk | Insufficent information to make the judgement. |
Ricci 1991.
| Methods | Randomised controlled trial. | |
| Participants | Women (n = 75; 25 in each group) of low socio‐economic status, presenting to hospital in Miami, USA, at 24 to 34 weeks' gestation of a singleton pregnancy, who had been 12 hours without contractions following threatened preterm labour, which was treated with intravenous magnesium sulphate. However, cervical change was not required as part of the definition of preterm labour and "it is probable that a large proportion of the women were never actually in preterm labour". | |
| Interventions | (1) 10 mg oral ritodrine every 2 hours for 24 hours, then 20 mg every 4 hours. (2) 535 mg SLOW MAG (enteric‐coated magnesium chloride) every 4 hours. (3) No treatment. Duration of treatment unclear. | |
| Outcomes | Preterm birth, gestational age at delivery, birthweight, time gained in utero, recurrent preterm labour, Apgar score, side effects, discontinuation of therapy. | |
| Notes | Singleton pregnancies only. No losses to follow‐up mentioned. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as: "women were randomised to 1 of 3 groups using sealed envelopes", no other information available. |
| Allocation concealment (selection bias) | Unclear risk | No information was given on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding of women, clinicians or outcome assessors in relation to the treatment. Although some aspects of weekly assessments were blinded, only women who received magnesium therapy had blood tests, and side effects were assessed unblinded although "neither of the physicians who actually questioned the women with regard to side effects had a vested interest". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | As above. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to make a judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a judgement. |
| Other bias | High risk | Sample size "not large enough to detect subtle differences". |
Ridgway 1990.
| Methods | Randomised controlled trial. | |
| Participants | Women (n = 50) who had threatened preterm labour at 25 to 35 weeks of pregnancy but had uterine quiescence for 12 to 24 hours after parenteral tocolysis. Study conducted in San Antonio, Texas, USA. | |
| Interventions | (1) 200 mg magnesium oxide, orally, every 3 to 4 hours until 36 week's gestation (n = 23). (2) 2.5 to 5 mg terbutaline sulphate, orally, every 3 to 4 hours until 36 week's gestation (n = 27). Unclear how the dose of terbutaline was assigned. | |
| Outcomes | Dubowitz at < 36 weeks, gestational age at delivery, birthweight, days on therapy, side effects (palpitations, vomiting, diarrhoea), discontinuation of therapy. | |
| Notes | 1 set of twins in each group (4% of all pregnancies were multiple pregnancies). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomised to maintenance therapy. No information on where women were recruited from, what randomisation procedure was used. |
| Allocation concealment (selection bias) | Unclear risk | No information was given on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information was given on blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 17% of participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make the judgement. |
| Other bias | High risk | No pre‐specified sample size. Post hoc power calculation indicated there was power of 0.5 to detect a difference of 0.2 in the proportion of women that delivered at less than 36 weeks. |
Rust 1996.
| Methods | Randomised controlled trial. | |
| Participants | Women (n = 248) of 24 to 34 weeks' gestation admitted to university hospital in Mississippi, USA, for idiopathic preterm labour who had preterm labour arrested with parenteral tocolysis and demonstrated uterine quiescence documented by tocodynamometry, absence of further cervical dilatation and intact membranes. Randomised after "stabilisation". Treated as outpatients in a "comprehensive system of preterm birth prevention" including home uterine contraction assessment, preterm labour education, weekly appointments at preterm birth prevention clinic. | |
| Interventions | (1) Magnesium chloride containing 128 mg elemental magnesium, orally, every 4 hours (data reported on 65 women). (2) 5 mg terbutaline, orally, every 4 hours (data reported on 72 women). (3) Inert substance, every 4 hours (data reported on 68 women). Also, corticosteroid therapy for women at risk of delivering within 7 days. No mention of duration of treatment. | |
| Outcomes | Preterm birth < 37 weeks, uterine activity calculation, pregnancy prolongation, gestational age at delivery, latency period, birthweight, neonatal intensive care unit admissions, neonatal length of stay, side effects. | |
| Notes | A priori power calculation. Adequate sample sizes.
43 (18%) participants were lost to follow‐up (after randomisation, 39 women delivered prior to discharge, and 4 women were lost to follow‐up). A total of 17 of the 205 pregnancies followed up were twin pregnancies (8%) (10 in the terbutaline group, 3 in the placebo group and 4 in the magnesium group). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out by a computerised method in the pharmacy. |
| Allocation concealment (selection bias) | Low risk | Described as "women were randomised via a computerised method in the pharmacy to 1 of 3 groups". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients and care providers blinded to treatment group for care and outcomes assessment. Placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 18% of participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No obvious risk of selective reporting. |
| Other bias | Low risk | No obvious risk of other bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Facchinetti 1992 | Combined therapies ‐ magnesium plus ritodrine was compared with ritodrine plus placebo. |
| Lewis 1997 | Combined therapies ‐ 30 minutes before discontinuation of magnesium sulphate, all women were given 2.5 mg of terbutaline orally, which was continued every 6 hours until 36 weeks' gestation. |
| Martin 1990 | 2 treatments were compared but for only 48 hours after threatened preterm labour. After 48 hours, all women received oral magnesium gluconate for long‐term tocolysis. Thus this study did not provide data in relation to long‐term maintenance therapy. |
| Martin 1998 | Comparing the effects of 2 preparations of magnesium for tocolysis. |
| Terrone 2000 | Acute tocolysis, no preterm settling. |
Differences between protocol and review
For the previous version of the review, the objective was changed from "to assess the effects of magnesium maintenance therapy on preventing preterm birth after threatened preterm labour" in the protocol, to be more specific: "to assess whether magnesium maintenance therapy is effective in preventing preterm birth after the initial threatened preterm labour is arrested".
For the previous version of the review, the secondary neonatal outcomes were changed from a broad description of "other neonatal morbidity" in the protocol to detailed specifications.
Contributions of authors
CA Crowther and V Moore contributed to the development of the protocol, identification and selection of studies for inclusion, data extraction and preparation of the text for the original version of this review (Crowther 1998).
For the previous update of this review, S Han and CA Crowther assessed identified studies for eligibility and risk of bias, contributed to data extraction and data entry, S Han prepared the initial draft of the risk of bias tables, and review text.
For this update of the review, all authors have contributed to the final version.
Sources of support
Internal sources
Australian Research Centre for Health of Women and Babies, Robinson Institute, Discipline of Obstetrics and Gynaecology, The University of Adelaide, Australia.
Discipline of Public Health, The University of Adelaide, Australia.
External sources
Commonwealth Department of Health and Ageing, Australia.
National Health and Medical Research Council, Australia.
Declarations of interest
Caroline Crowther was the principal investigator of the Australasian Collaborative Trial of Magnesium Sulphate for the Prevention of Mortality and Cerebral Palsy in Infants born very preterm. This multi‐centre randomised controlled trial assessed the effects of magnesium sulphate given to women expected to deliver a very preterm infant(s) (less than 30 weeks) within 24 hours. She is also principal investigator for the Magenta Trial assessing the use of antenatal magnesium sulphate for fetal neuroprotection immediately prior to birth in women between 30 and 34 weeks' gestation.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Holcomb 1991 {published data only}
- Holcomb WL, Daftery A, Petrie RH. Magnesium tocolysis: is "weaning" important?. American Journal of Obstetrics and Gynecology 1991;164:375. [Google Scholar]
Ricci 1991 {published data only}
- Ricci JM, Hariharan S, Helfgott A, Reed K, O'Sullivan MJ. Oral tocolysis with magnesium chloride: a randomized controlled prospective clinical trial. American Journal of Obstetrics and Gynecology 1991;1654:603‐10. [DOI] [PubMed] [Google Scholar]
- Ricci JM, Hariharan S, Helfgott A, Reed K, O'Sullivan MJ. Oral tocolysis with magnesium chloride: a randomized controlled prospective clinical trial. Proceedings of the 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 January 23‐27; Houston, Texas, USA. Houston, 1990:156.
Ridgway 1990 {published data only}
- Ridgway LE, Muise K, Patterson RM, Wright JW, Newton E, Gibbs RS. A prospective randomized comparison of oral terbutaline and magnesium oxide for the maintenance of tocolysis. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23‐27; Houston, Texas, USA. 1990:170. [DOI] [PubMed]
- Ridgway LE, Muise K, Wright JW, Patterson RM, Newton ER. A prospective randomized comparison of oral terbutaline and magnesium oxide for the maintenance of tocolysis. American Journal of Obstetrics and Gynecology 1990;163:879‐82. [DOI] [PubMed] [Google Scholar]
Rust 1996 {published data only}
- Rust O, Bofill J, Arriola R, Andrew M, Morrison J. The clinical efficacy of oral tocolytic therapy. American Journal of Obstetrics and Gynecology 1996;175:838‐42. [DOI] [PubMed] [Google Scholar]
- Rust OA, Bofil JA, Andrew M, Arriola R, Morrison JC. The clinical efficacy of oral tocolytic therapy. American Journal of Obstetrics and Gynecology 1996;174:316. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Facchinetti 1992 {published data only}
- Facchinetti F, Battaglia C, Benatti R, Borella P, Genazzani A. Oral magnesium supplementation improves fetal circulation. Magnesium Research 1992;5(3):179‐81. [PubMed] [Google Scholar]
Lewis 1997 {published data only}
- Lewis D, Bergstedt S, Adair C, Edwards M, Burlison S, Gallaspy J, et al. Successful magnesium sulfate tocolysis: is '"weaning" the drug necessary?. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S7. [DOI] [PubMed] [Google Scholar]
- Lewis DF, Bergstedt S, Edwards MS, Burlison S, Gallaspy JW, Brooks GG, et al. Successful magnesium sulfate tocolysis: is "weaning" the drug necessary?. American Journal of Obstetrics and Gynecology 1997;177(4):742‐5. [DOI] [PubMed] [Google Scholar]
Martin 1990 {published data only}
- Martin RW, Perry KG, Martin JN, Hess LW, Morrison JC. Oral magnesium for tocolysis: a comparison of magnesium gluconate and enteric‐coated magnesium chloride. Proceedings of 37th Annual Meeting of the Society for Gynecologic Investigation; 1990 March 21‐24; St Louis, USA. 1990:167.
Martin 1998 {published data only}
- Martin RW, Perry KG Jr, Martin JN Jr, Seago DP, Roberts WE, Morrison JC. Oral magnesium for tocolysis: a comparison of magnesium gluconate and enteric‐coated magnesium chloride. Journal of the Mississippi State Medical Association 1998;39(5):180‐2. [PubMed] [Google Scholar]
Terrone 2000 {published data only}
- Terrone DA, Rinehart BK, Kimmel ES, May WL, Larmon JE, Morrison JC. A prospective, randomized, controlled trial of high and low maintenance doses of magnesium sulfate for acute tocolysis. American Journal of Obstetrics and Gynecology 2000;182(6):1477‐82. [DOI] [PubMed] [Google Scholar]
Additional references
AIHW 2006
- Australian Institute of Health and Welfare. Australia's Health 2006. Canberra: AIHW, 2006. [Google Scholar]
Anotayanonth 2004
- Anotayanonth S, Subhedar NV, Neilson JP, Harigopal S. Betamimetics for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004352.pub2] [DOI] [PubMed] [Google Scholar]
Clarke 2002
- Clarke M, Oxman AD, editors. Cochrane Reviewer's Handbook 4.1.5. The Cochrane Library 2002, Issue 3..
Crowther 2002
- Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD001060] [DOI] [PubMed] [Google Scholar]
Dodd 2006
- Dodd JM, Crowther CA, Dare MR, Middleton P. Oral betamimetics for maintenance therapy after threatened preterm labour. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD003927.pub2] [DOI] [PubMed] [Google Scholar]
Eriksson 2002
- Eriksson BS, Pehrsson G. Evaluation of psycho‐social support to parents with an infant born preterm. Journal of Child Health Care 2002;6:19‐33. [DOI] [PubMed] [Google Scholar]
Gates 2004
- Gates S, Brocklehurst P. How should trials recruiting women with multiple pregnancies be analysed?. British Journal of Obstetrics and Gynaecology 2004;111:213‐9. [DOI] [PubMed] [Google Scholar]
Gaunekar 2004
- Gaunekar NN, Crowther CA. Maintenance therapy with calcium channel blockers for preventing preterm birth after threatened preterm labour. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004071.pub2] [DOI] [PubMed] [Google Scholar]
Hall 1959
- Hall D, McGaughery H Jr, Corey E, Thornton W. The effects of magnesium therapy on the duration of labour. American Journal of Obstetrics and Gynecology 1959;78:27‐32. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
King 2003
- King JF, Flenady V, Papatsonis D, Dekker G, Carbonne B. Calcium channel blockers for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002255] [DOI] [PubMed] [Google Scholar]
Nanda 2002
- Nanda K, Cook LA, Gallo ME, Grimes DA. Terbutaline pump maintenance therapy after threatened preterm labor for preventing preterm birth. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD003933] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papatsonis 2005
- Papatsonis D, Flenady V, Cole S, Liley H. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004452.pub2] [DOI] [PubMed] [Google Scholar]
Papatsonis 2009
- Papatsonis D, Flenady V, Liley H. Maintenance therapy with oxytocin antagonists for inhibiting preterm birth after threatened preterm labour. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005938.pub2] [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Saigal 2008
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371(9608):261‐9. [DOI] [PubMed] [Google Scholar]
Sanchez‐Ramos 1999
- Sanchez‐Ramos L, Kaunitz AM, Gaudier FL, Delke I. Efficacy of maintenance therapy after acute tocolysis: a meta‐analysis. American Journal of Obstetrics and Gynecology 1999;181(2):484‐90. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Crowther 1998
- Crowther CA, Moore V. Magnesium maintenance therapy for preventing preterm birth after threatened preterm labour. Cochrane Database of Systematic Reviews 1998, Issue 1. [DOI: 10.1002/14651858.CD000940] [DOI] [Google Scholar]
Han 2010
- Han S, Crowther CA, Moore V. Magnesium maintenance therapy for preventing preterm birth after threatened preterm labour. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD000940.pub2] [DOI] [PubMed] [Google Scholar]
Keirse 1993
- Keirse MJNC. Magnesium sulphate vs betamimetics for maintenance after preterm labour [revised 22 April 1993]. In: Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software: 1995.


