Abstract
Background
Failure of implantation and conception may result from an inability of the blastocyst to escape from its outer coat, which is known as the zona pellucida. Artificial disruption of this coat is known as assisted hatching and has been proposed as a method for improving the success of assisted conception by facilitating embryo implantation.
Objectives
To determine the effect of assisted hatching (AH) of embryos from assisted conception on live birth and multiple pregnancy rates.
Search methods
We searched the Cochrane Menstrual Disorders and Subfertility Group Specialised Register (August 2012), the Cochrane Central Register of Controlled Trials (CENTRAL) (August 2012), MEDLINE (1966 to August 2012) and EMBASE (1980 to August 2012).
Selection criteria
Three authors identified and independently screened trials. We included randomised controlled trials (RCTs) of AH (mechanical, chemical or laser disruption of the zona pellucida prior to embryo replacement) versus no AH that reported live birth or clinical pregnancy.
Data collection and analysis
Three authors independently performed quality assessments and data extraction.
Main results
Thirty‐one trials reported clinical pregnancy data, including 1992 clinical pregnancies in 5728 women. There was no significant difference in the odds of live birth in the AH group compared with the control group (9 RCTs; odds ratio (OR) 1.03, 95% confidence interval (CI) 0.85 to 1.26, moderate quality evidence), with no evidence of significant heterogeneity (P = 0.38) or inconsistency (I2 = 6%). Analysis of the clinical pregnancy rates from the nine studies which reported live birth showed a non‐significant result (OR 1.03, 95% CI 0.85 to 1.25 ).
Analysis of all of the studies included in this update (31 RCTs) showed that the clinical pregnancy rate in women who underwent AH was slightly improved, but the level only just reached statistical significance (OR 1.13, 95% CI 1.01 to 1.27, moderate quality evidence). However, it is important to note that the heterogeneity for this combined analysis for clinical pregnancy rate was statistically significant (P = 0.001) and the I2 was 49%. Subgroup analysis of women who had had a previous failed attempt at IVF found improved clinical pregnancy rates in the women undergoing AH compared with the women in the control group (9 RCTs, n = 1365; OR 1.42, 95% CI 1.11 to 1.81) with I2 = 20%. Miscarriage rates per woman were similar in both groups (14 RCTs; OR 1.03, 95% CI 0.69 to 1.54, P = 0.90, moderate quality evidence). Multiple pregnancy rates per woman were significantly increased in women who were randomised to AH compared with women in the control groups (14 RCTs, 3447 women; OR 1.38, 95% CI 1.11 to 1.70, P = 0.004, low quality evidence).
Authors' conclusions
This update has demonstrated that whilst assisted hatching (AH) does appear to offer a significantly increased chance of achieving a clinical pregnancy, the extent to which it may do so only just reaches statistical significance. The 'take home' baby rate was still not proven to be increased by AH. The included trials provided insufficient data to investigate the impact of AH on several important outcomes. Most trials still failed to report on live birth rates.
Plain language summary
Assisted hatching of fertilised eggs to improve the chances of pregnancy in assisted conception (IVF and ICSI)
Assisted hatching is a technique sometimes used for IVF (in vitro fertilisation) and similar procedures. It involves thinning the coat surrounding the fertilised egg, or making a hole in it. It is suggested that this may improve the chance of the embryo attaching to the womb so that pregnancy can begin. In this review of randomised controlled trials there was no evidence of a benefit in the live birth rate with assisted hatching although there was an increase in multiple pregnancy rates. There was some evidence that assisted hatching improves the chances of pregnancy in women for whom IVF has been repeatedly unsuccessful, but more research is needed.
Summary of findings
Background
Description of the condition
The World Health Organization estimates that one in six couples experiences some delay in conception (WHO 1975), and an increasing number of couples require treatment by the assisted conception (AC) procedures of in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI). In the UK in 2008, 12,211 successful births giving rise to 15,082 babies were achieved from 50,687 assisted conception cycles (24.1%), increasing from one live birth in seven cycles in 1992 to one in four (HFEA 2000; HFEA 2010).
The implantation rate of embryos resulting from IVF cycles is generally less than 20% (Gardner 2000; Lopata 1996), culminating in a generally low 'take home baby rate' (Sengoku 2000). This may be the result of poor embryo quality, poor endometrial receptivity, or both (Denker 1993). The human embryo is surrounded by an outer glycoprotein coat (zona pellucida) that, during fertilisation, prevents penetration by multiple sperm or sperm from other species (Bleil 1980). After fertilisation, the zona maintains the three‐dimensional integrity of the uncompacted embryo, facilitates free passage of the compacted embryo through the fallopian tube into the uterus and protects the embryo from micro‐organisms and immune cells (Bronson 1970). The blastocyst‐stage embryo eventually hatches out of this protective coat prior to implantation (Cole 1967).
Human embryos resulting from superovulation develop more slowly in vitro compared to embryos in vivo, manifest a relatively high degree of cytogenetic abnormalities and undergo cellular fragmentation; and only a small proportion achieve blastocyst‐stage development (Hsu 1999). Cultured embryos also hatch and implant at lower rates than occurs naturally (Harlow 1982; Mercader 2001). It is unclear whether this is due to 'hardening' of the zona pellucida as a result of cross‐linking of its constituent glycoproteins (ZP1, ZP2, ZP3) in an in vitro environment (Cohen 1991). Zona thickness appears to be influenced by a woman's age, hormone profile (high early proliferative phase follicle‐stimulating hormone (FSH)), smoking and the cause of infertility, and correlates negatively with embryo implantation rates (Loret de Mola 1997). With IVF and ICSI treatment, the possible combination of delayed embryo hatching and advanced endometrial development may present an unfavourable environment for implantation (Check 1999; Hsu 1999).
Description of the intervention
Assisted hatching is a technique sometimes used for IVF and similar procedures. It involves thinning the coat surrounding a fertilised egg, or making a hole in it. This was thought to improve the chances of the embryo attaching to the womb so that pregnancy could begin. Artificially disrupting the zona pellucida is known as assisted hatching (AH) and was first suggested in the 1980s. It was subsequently observed in women undergoing embryo biopsy for pre‐implantation genetic diagnosis (Fehilly 1985).
A variety of techniques have since been employed to assist embryo hatching, including partial mechanical zona dissection, zona drilling and zona thinning, making use of acid tyrodes, proteinases, piezon vibrator manipulators and lasers (Al‐Nuaim 2002). In this update, one of the randomised controlled trials employs a new method of AH, namely that of mechanical expansion (Fang 2010). Regardless of the AH technique employed, it is also important to distinguish whether the zona has remained unbreached such as in thinning (chemically or lasered), been fully breached (when a hole is made chemically, with a laser or mechanically), or has been completely removed (chemically). This distinction may have implications for whether an embryo is able to undergo normal zona expansion and escape following AH (Blake 2001), and also subsequent monozygotic twinning (da Costa 2001; Menezo 2003; Schieve 2000).
How the intervention might work
There are a variety of mechanisms by which AH could improve embryo implantation. The most obvious is that AH overcomes the zona pellucida hardening caused by IVF and cell culture or cryopreservation. Additionally, there is some evidence that embryos that have undergone zona manipulation for AH tend to implant one day earlier than unhatched embryos (Rink 1995). Finally, as suggested by Cohen 1992, artificial opening could enhance hormonal and metabolite exchange in addition to messaging between the embryo and the endometrium.
Why it is important to do this review
For over a decade now, zona manipulation of some form has been offered to older women, those with high FSH levels, a high risk of zona hardening (as with in vitro oocyte maturation) and following repeated implantation failure (Al‐Nuaim 2002). However, there remains considerable uncertainty over whether AH significantly improves IVF and ICSI success rates or whether it is associated with negative consequences. The previous update showed that AH results in a significant increase in clinical pregnancy rate when compared with no AH. AH failed to result in a statistically significant increase in live birth rate. However, few trials reported on live birth rate. We hoped that by updating this review and incorporating more studies more conclusive evidence of AH's effects on both clinical pregnancy and live birth rate, as well as other outcomes such as miscarriage and multiple pregnancy rates, could be achieved.
Objectives
To determine the effect of assisted hatching (AH) of embryos from assisted conception on live birth and multiple pregnancy rates.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials (RCTs) were eligible for inclusion. We excluded non‐randomised studies (for example studies with evidence of inadequate sequence generation such as alternate days, patient numbers) as they are associated with a high risk of bias. Trials were only eligible for inclusion if data could be extracted per woman and not per cycle. We excluded trials which presented results as per cycle rather than per woman (unless it was clear in the text that per cycle and per woman were used interchangeably). Crossover trials were excluded as the design is not valid in this context.
Types of participants
The participants were women of all nationalities and reproductive ages undergoing assisted conception by IVF or ICSI using their own gametes and consenting to participation in a trial of AH after fertilisation.
In the subgroup analysis, poor prognosis referred to women with increased age, previous IVF failure, high FSH, use of frozen embryos, or where the primary study protocol referred to women with a poor prognosis.
Types of interventions
Trials were included that investigated any known method of AH after fertilisation. The techniques involved to disrupt the zona pellucida prior to embryo replacement were of the following forms:
mechanical (including a new technique of hydrostatic pressure injection after thawing);
chemical;
laser.
Assisted hatching took place to the following extents:
breaching the zona pellucida by a hole (by laser, chemical or mechanical means);
thinning the zona pellucida (but no actual hole created);
removing the whole of the zona pellucida.
In the trials, AH was performed on fresh embryos, cryopreserved embryos following thawing and prior to embryo transfer as well as vitrified‐warmed embryos which were transferred at the cleavage stage. The effects of these interventions were compared to a control group in which AH was not performed.
Trials directly comparing different AH methods (without a no hatching control group) were excluded because the objective of this review was to determine the overall effectiveness of the technique of AH.
Types of outcome measures
Primary outcomes
Live birth, defined as the birth of live offspring per woman
Multiple pregnancy rate per woman
Secondary outcomes
3. Clinical pregnancy, defined as the demonstration of fetal heart beats on ultrasound scan per woman
4. Miscarriage, loss of pregnancy up to 20 weeks gestation per woman
5. Monozygotic twinning
6. Ectopic pregnancy rate per woman
7. Congenital or chromosomal abnormalities
8. Failure to transfer any embryos per woman
9. Embryo damage
10. In vitro blastocyst development
Only trials which reported at least clinical pregnancy rate per woman were included. The first version of the review included trials with implantation as an outcome, however for this update we have removed implantation as a reason for inclusion. It is not possible to pool implantation as the data are reported per cycle. We recorded live births as an event per woman and not by the number of infants delivered because of the high number of multiple births.
Search methods for identification of studies
We searched for all published and unpublished RCTs of AH versus no AH, without language restrictions and in consultation with the Menstrual Disorders and Subfertility Group Trials Search Co‐ordinator.
Electronic searches
We searched the following electronic databases, trial registers and websites:
Menstrual Disorders and Subfertility Group (MDSG) Specialised Register of Controlled Trials;
Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library;
MEDLINE;
EMBASE.
See: Appendix 1; Appendix 2
Searching other resources
We handsearched reference lists of articles retrieved by the search.
Data collection and analysis
We conducted data collection and analysis in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two authors scanned titles and abstracts from the first searches, and the same methods were adopted by another author for the second searches. Trials that appeared relevant were selected and formally assessed for inclusion independently by three authors using an inclusion and exclusion form. Trials excluded at this stage are detailed in the table 'Characteristics of excluded studies'.
Data extraction and management
Data were extracted from eligible studies using a data extraction proforma. Three authors independently performed all assessments of trial quality and data extraction using forms designed for the review (Appendix 3; Figure 1 and Figure 2). Discrepancies in quality assessment or data extraction were resolved by consensus during discussions with another author (MWS).
1.
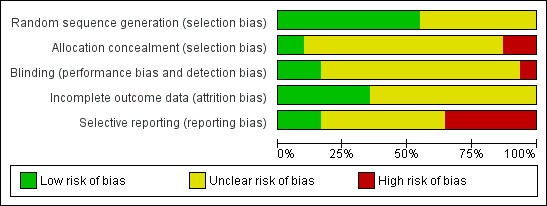
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
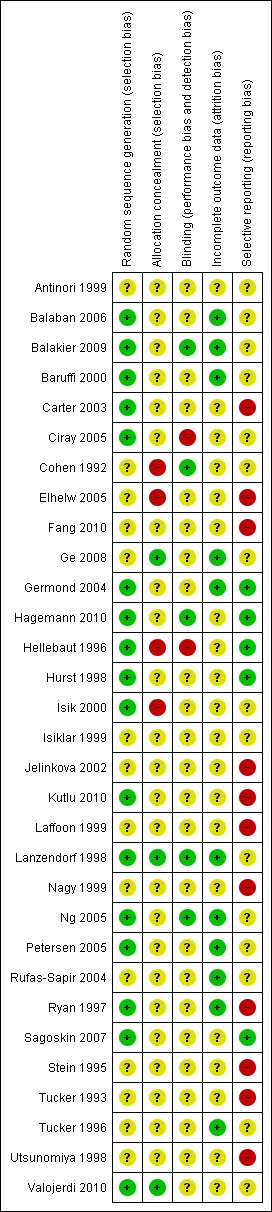
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies for risk of bias using the Cochrane risk of bias assessment tool (www.cochrane‐handbook.org) to assess: allocation (random sequence generation and allocation concealment); blinding of participants and personnel, blinding of outcome assessors; incomplete outcome data; selective reporting; and other bias. Disagreements were resolved by discussion or by a third review author.
For each trial it was determined whether adequate allocation concealment was described, and the trial was classed as being at low risk of bias if this was the case. If it was not, or it was unclear how allocation concealment was achieved, the trial was classed as being high risk or as having an unclear risk, respectively. For each trial we also determined whether an acceptable method of randomisation was described within the text, for example by stating that a computer‐generated randomisation list had been used. If this was the case, again the trial was classed as being at low risk in this respect. Similarly, if it was unclear, or the trial did not appear to be randomised, the trial was classed as having an unclear risk or being at high risk of bias, respectively. We determined who was blinded in each trial. If participants and medical staff in the trial were blinded to the allocation, the trial was at low risk. If it was not stated or was clear that this was not the case, the trial was again classed as having an unclear risk or as being at high risk of bias, respectively. Finally, selective reporting is an important issue in this review and is an important contributor to reporting bias with only a minority of trials reporting on the primary outcome of live birth. Each trial that reported live birth was classed as low risk whereas each trial which did not was classed as high risk.
Measures of treatment effect
All outcomes were dichotomous and the results were expressed for each trial as an odds ratio (OR) with 95% confidence interval (CI), and P values were calculated.
Unit of analysis issues
The primary analysis was per woman randomised. Data that did not allow valid analysis (for example 'per cycle' data) were not pooled. Multiple live births (for example twins or triplets) were counted as one live birth event.
Dealing with missing data
Attempts were made to obtain additional information on trial methodology, actual original trial data, or both, by contacting the principal authors of the trials. Reminders were sent (where necessary) to authors if there was no reply four weeks after the initial request. Only data that were available were analysed, and no imputation of data was undertaken.
Assessment of heterogeneity
Consideration of the clinical and methodological characteristics of included studies was undertaken to ascertain if they were sufficiently similar for meta‐analysis to provide a clinically meaningful result. Heterogeneity between the results of different trials was examined using the I2 statistic. Statistical heterogeneity was deemed significant if the P value was ≤ 0.1, that is an indication of more variation than would be expected by chance. I2 values were also examined and high values (> 40%) were taken to indicate substantial heterogeneity.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, the authors aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If there were 10 or more studies in an analysis, we planned to use a funnel plot to explore the possibility of small study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
Studies were combined via meta‐analysis using fixed‐effect models for AH versus no AH using RevMan 5.1 software (RevMan 2011). An increase in the odds of a particular outcome was displayed graphically in the meta‐analyses to the right of the centre‐line and a decrease in the odds of an outcome to the left of the centre‐line.
Subgroup analysis and investigation of heterogeneity
The following subgroup analyses were undertaken for the 2012 update.
Results based on number of attempts: first or repeat attempt at assisted conception.
Results based on mode of assisted conception: IVF or ICSI.
Results based on method of assisted hatching: chemical, laser or mechanical.
Results based on prognosis of woman: good or poor.
Results based on extent of AH: thinning, breaching, complete removal of zona pellucida.
Results based on type of embryo: fresh or frozen.
Sensitivity analysis
We performed sensitivity analysis to examine the stability of results in relation to:
adequacy of allocation concealment, by removing those trials with unclear or inadequate allocation concealment;
adequacy of the randomisation process, by removing those trials where the method of randomisation was unclear.
Results
Description of studies
Results of the search
A total of 31 randomised controlled trials met the inclusion criteria. Several publications reported two or more different comparisons in different populations (Antinori 1999; Cohen 1992; Ge 2008; Germond 2004; Kutlu 2010; Petersen 2005). All included trials were in published reports (full papers or abstracts) and available in English. They recruited a total of 5728 women undergoing IVF or ICSI, 2933 women in the assisted hatching and 2795 women in the control groups.
Included studies
Study design and setting
We included a total of 31 studies, including seven new studies in this update (Balakier 2009; Fang 2010; Ge 2008; Germond 2004; Hagemann 2010; Kutlu 2010; Valojerdi 2010).
The trials were carried out in 16 different countries: USA (Carter 2003; Cohen 1992; Hagemann 2010; Hurst 1998; Laffoon 1999; Lanzendorf 1998; Sagoskin 2007; Tucker 1993; Tucker 1996), Italy (Antinori 1999; Nagy 1999), Belgium (Hellebaut 1996), Turkey (Balaban 2006; Ciray 2005; Isik 2000; Isiklar 1999; Kutlu 2010), Brazil (Baruffi 2000; Petersen 2005), Australia (Ryan 1997), Germany (Jelinkova 2002), China (Fang 2010; Ge 2008; Ng 2005), Japan (Utsunomiya 1998), Israel (Rufas‐Sapir 2004; Stein 1995), Iran (Valojerdi 2010), Canada (Balakier 2009) and Egypt (Elhelw 2005). One study was a European multicentre study involving women at IVF centres in Switzerland, France, Germany and Spain (Germond 2004).
Participants
The age of participants ranged from 27 to 40 years (where reported). Some trials had subgroup data within them (for example Ge 2008; Germond 2004; Kutlu 2010; Rufas‐Sapir 2004; Stein 1995; and Tucker 1996 presented pregnancy for different age groups) whilst other studies only included women older than 35 years of age (for example Lanzendorf 1998) or less than 35 years old (Antinori 1999; Hurst 1998). Other studies included women of other specific age groups, for example 38 years old or younger (Balakier 2009; Hagemann 2010). Subgroup analysis based on the age of the women has not been achievable as studies did not categorise age groups in a universal way.
Twelve trials included women with a poor prognosis (Antinori 1999; Cohen 1992; Elhelw 2005; Ge 2008; Germond 2004; Hagemann 2010; Jelinkova 2002; Kutlu 2010; Petersen 2005; Rufas‐Sapir 2004; Stein 1995; Utsunomiya 1998), 12 trials included women with a good prognosis (Antinori 1999; Balakier 2009; Carter 2003; Ciray 2005; Ge 2008; Germond 2004; Hellebaut 1996; Hurst 1998; Kutlu 2010; Laffoon 1999; Sagoskin 2007; Tucker 1993), and the remainder did not provide information.
Interventions
Nine trials were repeat cycles and five included women undergoing their first assisted reproductive technology (ART) cycle; 17 trials did not report whether the treatment cycle was a first or repeat cycle or were mixed cycles. Eight trials included women undergoing ICSI alone, 14 were IVF only, and the rest were unstated or mixed ICSI and IVF cycles. Twenty‐three trials involved transfers of fresh embryos exclusively, six involved frozen or vitrified‐warmed embryos only, and the remaining trials used a combination of fresh or frozen embryos.
Eleven trials employed chemical means for assisted hatching, five employed mechanical means and 15 employed laser.
Fifteen trials utilised a breach of the zona pellucida with a hole (Antinori 1999; Cohen 1992; Germond 2004; Hagemann 2010; Hellebaut 1996; Hurst 1998; Isiklar 1999; Laffoon 1999; Lanzendorf 1998; Nagy 1999; Rufas‐Sapir 2004; Ryan 1997; Sagoskin 2007; Stein 1995; Tucker 1996) while 12 utilised a non‐breach thinning (Balaban 2006; Balakier 2009; Baruffi 2000; Ciray 2005; Elhelw 2005; Ge 2008; Kutlu 2010; Ng 2005; Petersen 2005; Tucker 1996; Utsunomiya 1998; Valojerdi 2010) and two performed a complete zona removal (Isik 2000; Jelinkova 2002). For one study this was unknown (Carter 2003), whilst another study used a new method of AH whereby the zona pellucida was expanding mechanically (Fang 2010). Three trials reported the thickness of the zona pellucida (in each case choosing a zona thickness of more than 12 µm as an inclusion criterion).
Twenty‐four trials reported the interval between AH and embryo transfer (19 trials reported less than four hours; three trials, four to eight hours; and two were greater than eight hours).
Blastocyst transfer occurred in three trials (Isik 2000; Isiklar 1999; Laffoon 1999), one of which involved complete zona removal (Isik 2000).
Outcomes
The outcome measures utilised for this review were reported by a varying number of trials:
nine trials reported live birth rate;
31 trials reported clinical pregnancy rate;
14 trials reported multiple pregnancy rate;
14 trials reported miscarriages;
six trials reported monozygotic twinning;
three trials reported ectopic pregnancy;
two trials reported congenital, chromosomal abnormalities or both;
four trials reported embryo damage;
and no trials reported in vitro blastocyst development post AH.
Further details about the included trials are provided in the table 'Characteristics of included studies'; Table 12 and Table 13.
1. Mean age of participants in assisted hatching and control groups.
| Study | AH n, mean age (SD) | Control n, mean age (SD) | OR for clinical pregnancy |
| Antinori 1999: First IVF | 73, 37.5 | 69, 36.0 | 1.27 (0.70, 2.32) |
| Antinori 1999: Repeat IVF | 96, 27.5 | 103, 27 | 1.86 (0.81, 4.25) |
| Balaban 2006 | 183, 32.4 (3.3) | 183, 32.7 (3.1) | 1.85 (1.19 to 2.86) |
| Balakier 2009 | 45, 32.5 (3.8) | 39, 33.8 (3.2) | 0.64 (0.27 to 1.55) |
| Ciray 2005 | 60, 33.1 (4.2) | 30, 34.0 (3.7) | 0.62 (0.26 to 1.49) |
| Baruffi 2000 | 51, 31.8 (3.6) | 52, 31.4 (3.6) | 0.74 (0.33 to 1.65) |
| Carter 2003 | 121, 34 (3.3) | 82, 34 (3.2) | 0.95 (0.54 to 1.67) |
| Cohen 1992 FSH <15 | 69, 36.50 (3.30) | 68, 36.70 (3.70) | 2.11 (1.18 to 3.77) |
| Cohen 1992 poor prognosis | 80, 36.7 (4.3) | 83, 35.3 (4.2) | 1.30 (0.66 to 2.55) |
| Cohen 1992 FSH > 15 | not stated | not stated | 1.30 (0.66 to 2.55) |
| Fang 2010 | 61, 32.3 (3.4) | 64, 32.1 (3.6) | 2.37 (1.07 to 5.28) |
| Ge 2008 fresh embryo | 387, 31.08 (4.68) | 373, 30.44 (4.15) | 0.99 (0.74 to 1.32) |
| Ge 2008 frozen embryo | 100, 31.84 (3.85) | 100, 30.66 (4.42) | 2.05 (0.99 to 4.22) |
| Germond 2004 first cycle of frozen‐thawed embryos | 62, 32.8 (4.2) | 53, 32.6 (3.8) | 0.09 (0.01 to 0.76) |
| Germond 2004 poor prognosis, first cycle of fresh embryos | 22, 39.3 (2.9) | 21, 38.3 (3.4) | 0.51 (0.10 to 2.45) |
| Hagemann 2010 | 59, 32.1 (3.0) | 62, 31.2 (3.5) | 0.81 (0.37 to 1.76) |
| Hellebaut 1996 | 60, 30.9 (4.3) | 60, 30.8 (3.9) | 1.15 (0.55 to 2.43) |
| Hurst 1998 | 13, 30.0 (0.9) | 7, 30.0 (0.8) | 0.40 (0.06 to 2.89) |
| Isik 2000 | 24, 30.5 (5.2) | 22, 29.1 (3.6) | 2.0 (0.62 to 6.49) |
| Jelinkova 2002 | 128, 32.3 (4.24) | 129, 32.1 (3.16) | 1.86 (1.12 to 3.10) |
| Kutlu 2010: Good prognosis | 73, 29.9 (2.9) | 66, 28.9 (3.4) | 1.06 (0.54, 2.08) |
| Kutlu 2010:Poor prognosis | 58, 38.0 (2.3) | 55, 37.4 (2.4) | 1.23 (0.58, 2.60) |
| Lanzendorf 1998 | 41, 38.30 (0.31) | 48, 38.50 (0.26) | 0.90 (0.38 to 2.10) |
| Mansour 2000 | 30, 37.30 (5.60) | 41, 36.30 (5.20) | 3.86 (0.91 to 16.41) |
| Nagy 1999 | 20, 32.0 (4.0) | 20, 31.4 (3.7) | 8.0 (1.44 to 44.3) |
| Ng 2005 | 80, 34.0 (range: 25 to 40) | 80, 34.0 (range: 26 to 40) | 0.81 (0.33 to 2.00) |
| Petersen 2005 one previous implantation failure | 35, 34.6 (4.6) | 35, 34.1 (5.3) | 1.15 (0.41 to 3.19) |
| Petersen 2005 several previous implanatation failures | 40, 35.7 (3.8) | 40, 35.3 (5.1) | 4.11 (1.04 to 16.29) |
| Sagoskin 2007 | 118, 34.0 (3.3) | 81, 34.0 (3.2) | 0.94 (0.53 to1.65) |
| Tucker 1993 | 110, 34.1 (4.8) | 108, 34.2 (4.1) | 1.37 (0.79 to 2.35) |
| Tucker 1996 | 50, 35.3 (4.2) | 50, 33.5 (4.3) | 0.74 (0.35 to 1.59 |
| Valojerdi 2010 | 200, 30.86 (5.82) | 200, 29.85 (5.14) | 0.53 (0.35 to 0.80) |
2. Prognostic factors in included trials.
| Study ID | Balanced age between groups | Balances no. of embryos transferred | Prognosis: poor/good | FSH levels | Blastocyst transfer | Complete/partial AH | Frozen cycles |
| Antinori 1999 | AH mean 1.5 years older | Yes | Good and Poor subgroups | No data | No | Complete hole | Not stated |
| Balaban 2006 | Yes | Yes | Unselected | < 10 | No | Thinning | Frozen |
| Balakier 2009 | AH mean 1.3 years older | Yes | Good | < 10 | No | Thinning | Fresh |
| Baruffi 2000 | Yes | Yes | Good | No data | No | Thinning | Fresh |
| Carter 2003 | Yes | Yes | Good | < 10 | No | Not stated | Fresh |
| Ciray 2005 | Yes | Yes | Good | < 15 | No | Thinning | Fresh |
| Cohen 1992 | Yes | Yes | Unstated | <= 15, and > 15 subgroups | No | Complete hole | Fresh |
| Elhelw 2005 | Yes | No data | Poor | No data | No | Thinning | Frozen |
| Fang 2010 | Yes | Yes | Not stated | No data | No | Mechanical expansion | Frozen thawed |
| Ge 2008 | Yes | Yes | Mixed | No data | No | Thinning | Fresh and Frozen Subgroups |
| Germond 2004 | Yes | Yes | Mixed, in subgroups | between 3 and 12 | No | Complete hole | Fresh and frozen, in subgroups |
| Hagemann 2010 | Mean age data only given for combined cycles 1 and 2 | Yes | under 38 years, >2 previous failed cycles, ZP thickness >13micrometers | No data | No | 20micrometer diameter opening | Fresh |
| Hellebaut 1996 | Yes | Yes | Good | No data | No | Complete hole | Fresh |
| Hurst 1998 | Yes | Yes | Good | < 10 | No | Complete hole | Fresh |
| Isik 2000 | AH mean 1.4 years older | Yes | Unstated | < 10 | Yes | Removal complete | Fresh |
| Isiklar 1999 | No data | Yes | Unstated | No data | Yes | Complete hole | Fresh |
| Jelinkova 2002 | Yes | Yes | Poor | No data | Yes | Removal complete | Fresh |
| Kutlu 2010 | Yes | Yes | Good and Poor Subgroups | No data | No | Complete hole | Fresh |
| Laffoon 1999 | No data | No data | Good | No data | No | Complete hole | Fresh |
| Lanzendorf 1998 | No | Yes | Poor | No data | No | Complete hole | Fresh |
| Nagy 1999 | Yes | Yes | Unstated | No data | No | Thinning | Frozen‐thaw cycles |
| Ng 2005 | Yes | Higher proportion of controls received 3 embryos | Unstated | < 11 | No | Thinning | Frozen‐thaw cycles |
| Petersen 2005 | Yes | Yes | Poor | No data | No | Thinning | Fresh |
| Rufas‐Sapir 2004 | No data | Yes | Poor | No data | No | Complete hole | Fresh |
| Ryan 1997 | No data | No data | Unstated | No data | No | Complete hole | Both |
| Sagoskin 2007 | Yes | Yes | Good | < 10 | No | Hole | Fresh |
| Stein 1995 | No data | No data | Poor | No data | No | Complete hole | Fresh |
| Tucker 1993 | Yes | Yes | Good | < 15 | No | Thinning | Fresh |
| Tucker 1996 | AH mean 1.8 years older | Yes | Not stated | No data | No | Complete hole | Fresh |
| Valojerdi 2010 | Yes | Yes | Not stated | No data | No | Partially thinned | Vitrified‐warmed embryo |
AH = assisted hatching ET = embryo transfer FSH = follicle‐stimulating hormone
Excluded studies
We excluded 58 studies from the review (see 'Characteristics of excluded studies'). Reasons for exclusion included: inadequate method of randomisation, no per woman data, inadequate reporting of clinical pregnancy and, in the remainder, the studies were not randomised. Three studies were found to be retrospective studies on close examination of the text.
Risk of bias in included studies
The overall methodological quality of the included trials was considered suboptimal, largely due to the lack of information on allocation and randomisation in many of the trials. Further details of the trials' risk of bias can be found in the table 'Characteristics of included studies'. Summaries of risk of bias for all the included studies are presented in Figure 1 and Figure 2.
Allocation
All 31 trials stated that randomised allocation had occurred. Ideally, studies should randomise women on the day of assessment of the embryos for suitability for embryo transfer. Regarding sequence generation, 17 studies were at low risk of selection bias, 14 studies had an unclear risk, and none of the studies was at high risk.
Four studies were at low risk of selection bias related to allocation concealment, 23 studies had an unclear risk, and four studies were felt to be at high risk. Ge 2008, Lanzendorf 1998, Ng 2005 and Valojerdi 2010 gave details of adequate concealment of allocation.
Blinding
Although blinding was unlikely to influence findings for the primary review outcome (live birth), only five trials (Balakier 2009; Cohen 1992; Hagemann 2010; Lanzendorf 1998; Ng 2005) employed double blinding with both the woman and the outcome assessor being unaware of the allocation. In 24 studies it was unclear if blinding was used or who was blinded (participant or assessor), and in the remaining two studies there was no blinding.
Incomplete outcome data
No trial reported losses to follow up. One trial reported a loss of participants in the early stages of the trial but gave reasons and numbers for the new number of women in the control and AH groups.
A total of 17 studies were at low risk of bias related to incomplete outcome data, and 14 studies had an unclear risk.
Selective reporting
All pre‐specified outcomes were reported within the outcomes of all of the studies. Studies which failed to report on live birth rate were rated as at high risk of reporting bias.
Other potential sources of bias
Age groups were matched in trials with similar means in the AH and control groups.
Twenty‐four trials were reported in full published papers (Balaban 2006; Balakier 2009; Baruffi 2000; Ciray 2005; Cohen 1992; Fang 2010; Ge 2008; Germond 2004; Hagemann 2010; Hellebaut 1996; Hurst 1998; Isik 2000; Isiklar 1999; Jelinkova 2002; Kutlu 2010; Lanzendorf 1998; Nagy 1999; Ng 2005; Petersen 2005; Sagoskin 2007; Stein 1995; Tucker 1993; Tucker 1996; Valojerdi 2010). Seven trials were published in conference abstract form only (Antinori 1999; Carter 2003; Elhelw 2005; Laffoon 1999; Rufas‐Sapir 2004; Ryan 1997; Utsunomiya 1998).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Live birth.
| Live birth | ||||||
| Patient or population: Women undergoing assisted conception Intervention: Assisted hatching compared with no assisted hatching | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Assisted hatching | |||||
| Live birth per woman randomised | 305 per 1000 | 311 per 1000 (271 to 356) | OR 1.03 (0.85 to 1.26) | 1921 (9 studies) | ⊕⊕⊕⊝ moderate1 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Many of the trials had some methodological limitations or missing information
Summary of findings 2. Multiple pregnancy.
| Multiple pregnancy | ||||||
| Patient or population: Women undergoing assisted reproduction Intervention: Assisted hatching compared with no assisted hatching | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Assisted hatching | |||||
| Multiple pregnancy rate per woman randomised | 102 per 1000 | 136 per 1000 (112 to 162) | OR 1.38 (1.11 to 1.7) | 3447 (14 studies) | ⊕⊕⊝⊝ low1,2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 There was methodological limitations or missing information in most trials 2 I square statistic was 57%
Summary of findings 3. Clinical pregnancy.
| Clinical pregnancy | ||||||
| Patient or population: Women undergoing assisted reproduction Intervention: Assisted hatching compared with no assisted hatching | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Assisted hatching | |||||
| Clinical pregnancy rate per woman randomised | 332 per 1000 | 360 per 1000 (334 to 387) | OR 1.13 (1.01 to 1.27) | 5728 (31 studies) | ⊕⊕⊕⊝ moderate1 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 There were methodological limitations or missing information in most of the trials
Summary of findings 4. Miscarriage.
| Miscarriage | ||||||
| Patient or population: Women undergoing assisted reproduction Intervention: Assisted hatching compared with no assisted hatching | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Miscarriage | |||||
| Miscarriage per woman randomised | 45 per 1000 | 46 per 1000 (32 to 68) | OR 1.03 (0.69 to 1.54) | 2131 (14 studies) | ⊕⊕⊕⊝ moderate1 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 There were methodological limitations or missing information in most of the trials
Primary outcomes
1. Live birth per woman
Few trials reported live birth data, with data available from only nine of the 31 trials. Overall, 595 live birth events were reported (that is not including individual births from multiple pregnancies), 313 in the AH group and 282 in the control group. Overall, there was no evidence of a significant difference between the odds of a live birth in women who underwent AH compared with those in the control group (9 RCTs, 1921 women; OR 1.03, 95% CI 0.85 to 1.26). There was no significant heterogeneity, with P = 0.38 and an I2 of 6% (Analysis 1.1) (Figure 3).
1.1. Analysis.
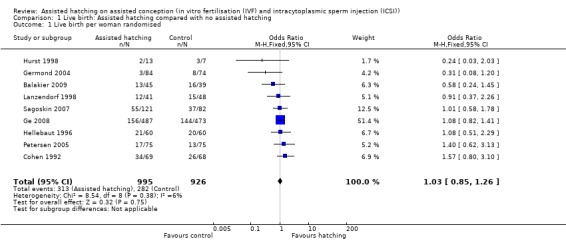
Comparison 1 Live birth: Assisted hatching compared with no assisted hatching, Outcome 1 Live birth per woman randomised.
3.
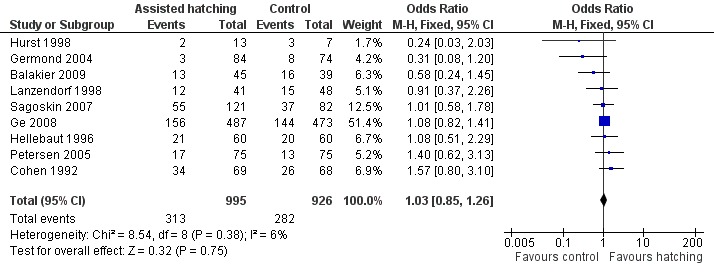
Forest plot of comparison: 1 Live birth rate, outcome: 1.1 Live birth per woman randomised.
Subgroup analysis
First or repeat attempt at ART: for women undergoing their first attempt at ART, one trial showed no significant difference in live births between the AH and control groups (1 RCT, 20 women; OR 0.24, 95% CI 0.03 to 2.03, P = 0.19). Similarly for women with previous failed attempts at ART, no significant difference in live birth outcome between the AH and control groups was found (1 RCT, 150 women; OR 1.40, 95% CI 0.62 to 3.13, P = 0.42) (Analysis 1.2).
Assisted conception procedure: for women undergoing ICSI, there was no significant difference in live birth outcome between the AH and control groups (1 RCT, 150 women; OR 1.40, 95% CI 0.62 to 3.13, P = 0.42). The same applied to women who underwent IVF, there was no significant difference in live birth outcome between the two groups (3 RCTs, 241 women; OR 1.00, 95% CI 0.60 to 1.68, P = 0.09, I2 of 58%) (Analysis 1.3).
Method of assisted hatching: for women undergoing a chemical method of assisted hatching, there was a no significant difference in live birth outcome between the AH and control groups (4 RCTs, 366 women; OR 1.13, 95% CI 0.74 to 1.74, P = 0.37, I2 of 5%). For women who underwent a laser method of AH, likewise there was no significant difference in live birth outcome between the groups (5 RCTs, 1555 women; OR 1.01, 95% CI 0.81 to 1.26, P = 0.27, I2 of 23%). None of the trials which employed mechanical forms of AH reported on live births (Analysis 1.4).
Prognosis: for women in poor prognosis groups, there was no significant difference in live birth outcome between the AH and control groups (4 RCTs, 576 women; OR 1.46, 95% CI 0.99 to 2.15, P = 0.65, I2 of 0%). The same was found for the women in good prognosis groups (5 RCTs, 1187 women; OR 0.94, 95% CI 0.74 to 1.19, P = 0.58, I2 of 0%) (Analysis 1.5).
1.2. Analysis.
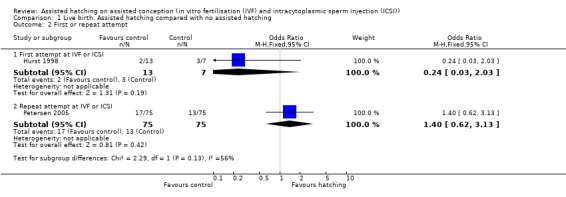
Comparison 1 Live birth: Assisted hatching compared with no assisted hatching, Outcome 2 First or repeat attempt.
1.3. Analysis.
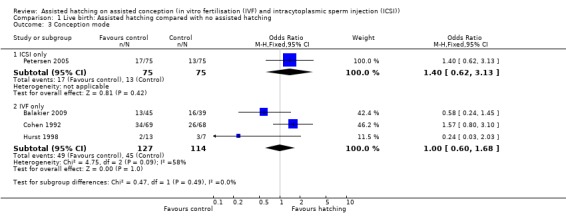
Comparison 1 Live birth: Assisted hatching compared with no assisted hatching, Outcome 3 Conception mode.
1.4. Analysis.
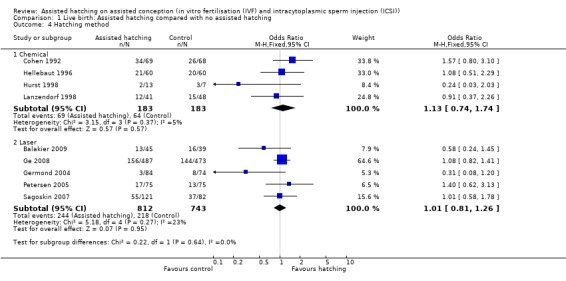
Comparison 1 Live birth: Assisted hatching compared with no assisted hatching, Outcome 4 Hatching method.
1.5. Analysis.
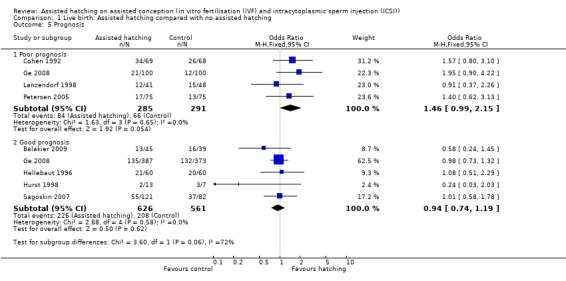
Comparison 1 Live birth: Assisted hatching compared with no assisted hatching, Outcome 5 Prognosis.
Sensitivity analysis
Allocation concealment: limiting the analysis to those trials that reported allocation concealment left only two trials (Ge 2008; Lanzendorf 1998). There was no significant difference in live birth rate between the AH group and the control group (OR 1.06, 95% CI 0.81 to 1.38, P = 0.25).
Method of randomisation: eight trials stated the method of randomisation (Balakier 2009; Ge 2008; Germond 2004; Hellebaut 1996; Hurst 1998; Lanzendorf 1998; Petersen 2005; Sagoskin 2007). Analysis of the data from these trials showed no statistically significant difference between the AH and control groups (OR 0.99, 95% CI 0.80 to 1.22, P = 0.19).
2. Multiple pregnancy per woman
Fourteen of the 31 trials reported on multiple pregnancy. Overall, 415 multiple pregnancies were reported in the 3447 women in the trials reporting on multiple pregnancies, with 244 multiple pregnancies occurring in the AH group and 171 in the control group. Overall, there was a significant increase in multiple pregnancy rates with AH compared to the controls (14 RCTs, 3447 women; OR 1.38, 95% CI 1.11 to 1.70, P = 0.004, I2 of 57 %) (Figure 4) (Analysis 2.1).
4.
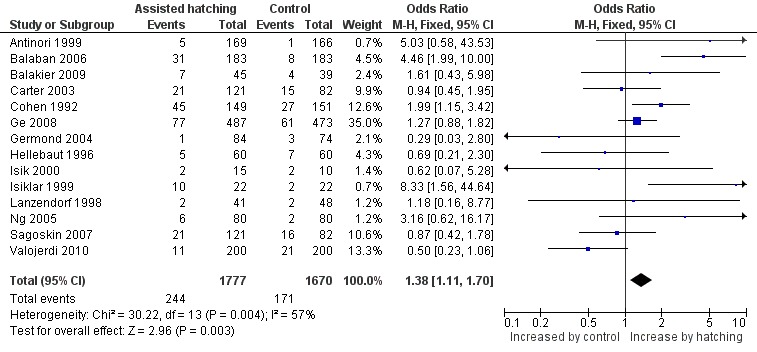
Forest plot of comparison: 4 Multiple pregnancy rate, outcome: 4.1 Multiple pregnancy rate per woman randomised.
2.1. Analysis.
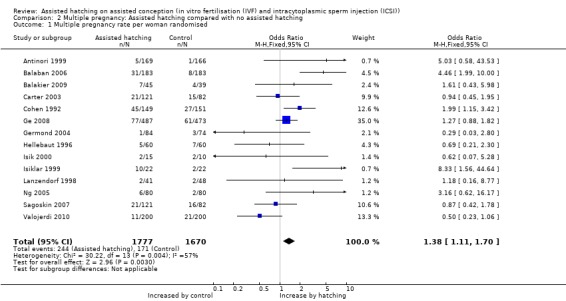
Comparison 2 Multiple pregnancy: Assisted hatching compared with no assisted hatching, Outcome 1 Multiple pregnancy rate per woman randomised.
Subgroup analysis
First attempt or repeat attempt at ART: for women undergoing their first attempt at ART, there were two trials which reported on multiple pregnancy rate, and these found no statistically significant difference between the AH and control groups (2 RCTs, 294 women; OR 0.62, 95% CI 0.12 to 3.19, P = 0.27). There were four trials where women had previous failed attempts at ART which reported multiple pregnancy rate. These also showed no statistically significance between AH and control groups with no significant heterogeneity (4 RCTs, 765 women; OR 1.12, 95% CI 0.70 to 1.80, P = 0.29, I2 of 20%) (Analysis 2.2).
Assisted conception procedure: for women undergoing ICSI, there was evidence of a statistically significant difference in multiple pregnancy rate between the AH and control groups (2 RCTs, 391 women; OR 3.54, 95% CI 1.70 to 7.39, P = 0.09, I2 of 65%). Similar results were found for women undergoing IVF (6 RCTs, 1126 women; OR 1.87, 95% CI 1.28 to 2.72, P = 0.17, I2 of 36%) (Analysis 2.3).
Method of assisted hatching: there was an increase in multiple pregnancy rates, which bordered on statistical significance, for women in the trials undergoing the laser form of AH (9 RCTs, 2869 women; OR 1.27, 95% CI 1.00 to 1.61, P = 0.006, I2 of 63%) and a significant increase in multiple pregnancies among women in the one trial undergoing a mechanical method of assisted hatching. For the laser trials, however, there was significant heterogeneity, and for the mechanical method only one trial reported on multiple pregnancy, so there was a wide CI (44 women; OR 8.33, 95% CI 1.56 to 44.64). No increase in multiple pregnancy rate was seen with the chemical method (4 RCTs, 534 women; OR 1.55, 95% CI 0.98 to 2.47, P = 0.35, I2 = 10) (Analysis 2.4).
Prognosis: there was no evidence of significant differences between the AH and control groups in the rate of multiple pregnancy amongst women with good prognosis (6 RCTs, 1569 women; OR 1.08, 95% CI 0.81 to 1.44, P = 0.69, I2 of 0%). However, there was a significant difference in the AH group in women with a poor prognosis (5 RCTs, 883 women; OR 1.88, 95% CI 1.19 to 2.96, P = 0.48, I2 of 0%), with no heterogeneity (Analysis 2.5).
Degree of zona manipulation: for the one trial in which women underwent complete removal of the zona pellucida, there was no statistically significant increase in multiple pregnancy rate amongst women in the AH group compared to those in the control group (1 RCT, 25 women; OR 0.62, 95% CI 0.07 to 5.28, P = 0.66). The same applied to trials employing breaching (7 RCTs, 1249 women; OR 1.51, 95% CI 1.05 to 2.17, P = 0.06, I2 of 51%). For trials employing thinning (5 RCTs, 1970 women; OR 1.39, 95% CI 1.05 to 1.84, P = 0.003, I2 of 76%), there was a statistically significant increase in multiple pregnancy rates in the AH group compared to controls (Analysis 2.6).
Multiple pregnancy per pregnancy: overall the multiple pregnancy rate per clinical pregnancy achieved was statistically significant for women in the AH group compared to the control group (14 trials, OR 1.39, 95% CI 1.09 to 1.77, P = 0.07) (Analysis 2.7).
2.2. Analysis.
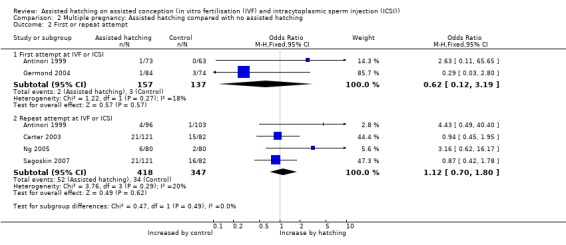
Comparison 2 Multiple pregnancy: Assisted hatching compared with no assisted hatching, Outcome 2 First or repeat attempt.
2.3. Analysis.
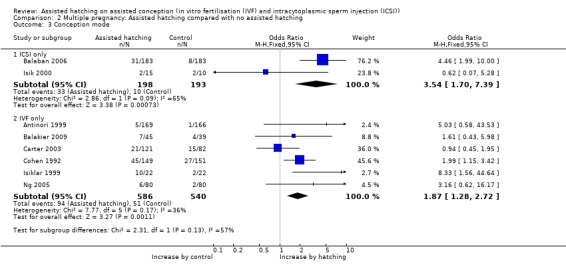
Comparison 2 Multiple pregnancy: Assisted hatching compared with no assisted hatching, Outcome 3 Conception mode.
2.4. Analysis.
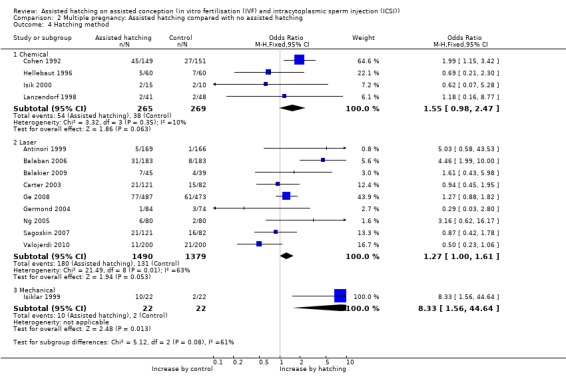
Comparison 2 Multiple pregnancy: Assisted hatching compared with no assisted hatching, Outcome 4 Hatching method.
2.5. Analysis.
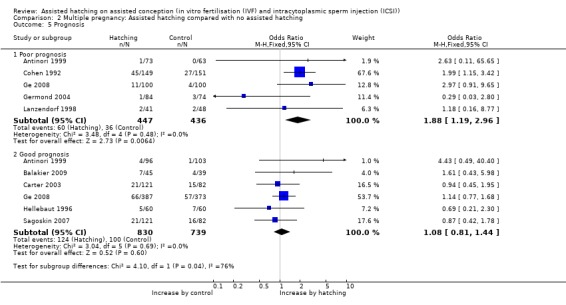
Comparison 2 Multiple pregnancy: Assisted hatching compared with no assisted hatching, Outcome 5 Prognosis.
2.6. Analysis.
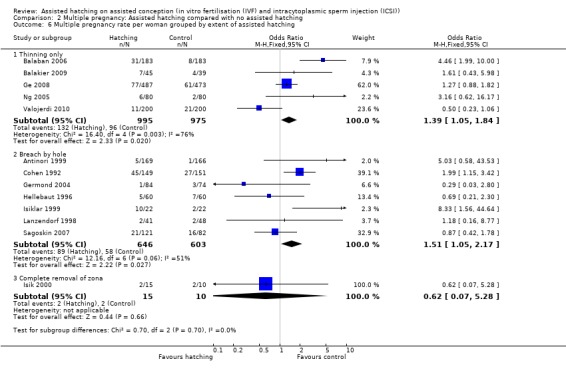
Comparison 2 Multiple pregnancy: Assisted hatching compared with no assisted hatching, Outcome 6 Multiple pregnancy rate per woman grouped by extent of assisted hatching.
2.7. Analysis.
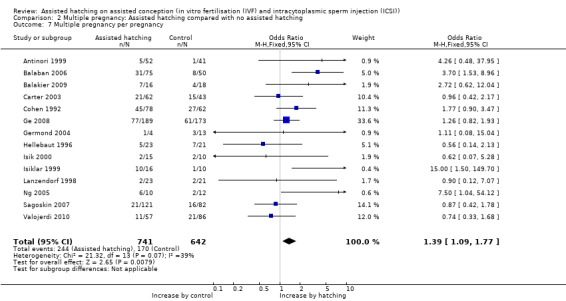
Comparison 2 Multiple pregnancy: Assisted hatching compared with no assisted hatching, Outcome 7 Multiple pregnancy per pregnancy.
Secondary outcomes
3. Clinical pregnancy rate per woman
Thirty‐one trials reported clinical pregnancy data, including 1992 clinical pregnancies in 5728 women. There were 1064 clinical pregnancies in the AH group and 928 in the control group. Overall, the OR for clinical pregnancy per woman randomised was 1.13 (95% CI 1.01 to 1.27) (Analysis 3.1), showing a borderline statistically significant difference overall favouring the AH group compared to controls. There was, however, evidence of heterogeneity in this analysis (P = 0.001, I2 of 49%) indicating that, due to wide variation between trials, it may be inappropriate to perform a combined analysis (Figure 5).
3.1. Analysis.
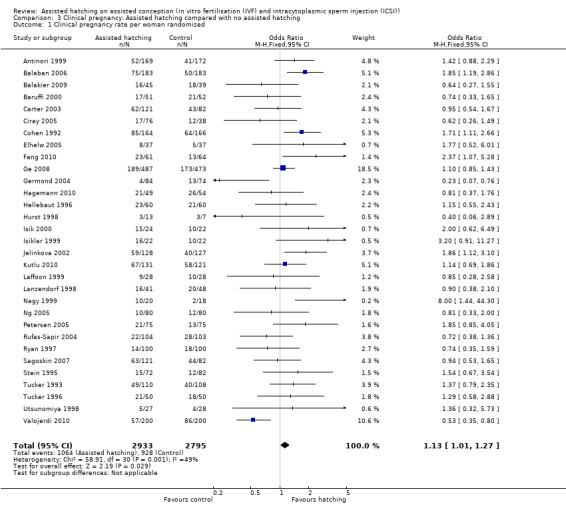
Comparison 3 Clinical pregnancy: Assisted hatching compared with no assisted hatching, Outcome 1 Clinical pregnancy rate per woman randomised.
5.
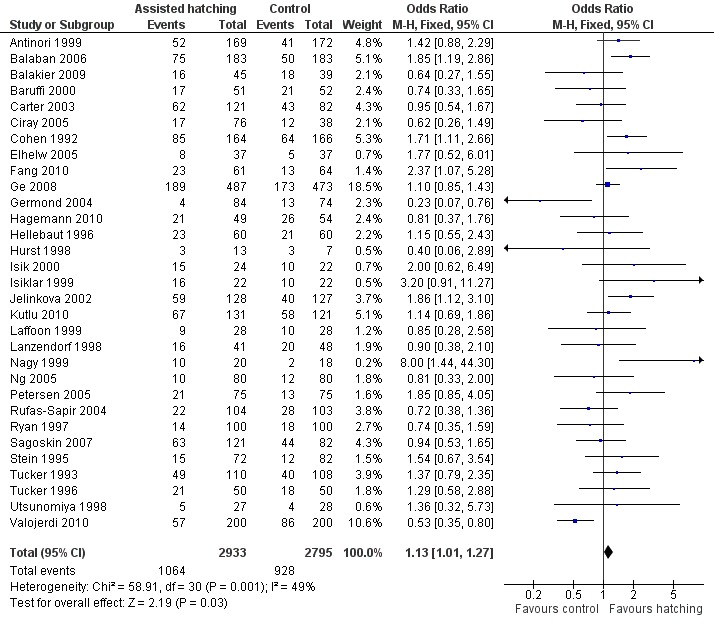
Forest plot of comparison: 2 Clinical pregnancy, outcome: 2.1 Clinical pregnancy rate per woman randomised.
Among the nine trials that reported on live births there was no significant increase in clinical pregnancy for women in the AH group compared with the control group (OR 1.03, 95% CI 0.85 to 1.25, P = 0.18, I2 of 29%) (Analysis 4.1).
4.1. Analysis.
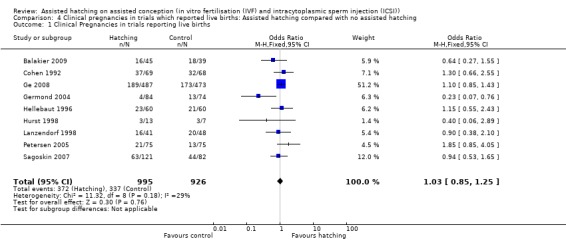
Comparison 4 Clinical pregnancies in trials which reported live births: Assisted hatching compared with no assisted hatching, Outcome 1 Clinical Pregnancies in trials reporting live births.
Subgroup analysis
First or repeat attempt at ART: in the six trials with women experiencing their first cycle of IVF or ICSI there was no evidence of an improved clinical pregnancy rate between women in the AH group and women in the control group (6 RCTs, 650 women; OR 0.77, 95% CI 0.54 to 1.10, P = 0.19, I2 of 32%). Amongst women, who had previously failed attempts at IVF or ICSI, there was evidence of an improved clinical pregnancy rate (9 trials, 1365 women; OR 1.42, 95% CI 1.11 to 1.81, P = 0.27, I2 of 20%) (Analysis 3.2).
Assisted conception procedure: in the subgroup of women undergoing IVF, there was evidence of a statistically significantly improved clinical pregnancy rate in the AH group compared to the control group (14 RCTs, 2300 women; OR 1.29, 95% CI 1.08 to 1.54, P = 0.12, I2 of 32%). The same applied to women undergoing ICSI cycles (8 RCTs, 1205 women; OR 1.34, 95% CI 1.05 to 1.71, P = 0.26, I2 of 21%) (Analysis 3.3).
Method of assisted hatching: for women undergoing a chemical method of assisted hatching, there was evidence of an improved clinical pregnancy rate, which was statistically significant, amongst women in the AH group compared with those in the control group (11 RCTs, 1536 women; OR 1.33, 95% CI 1.08 to 1.64, P = 0.47, I2 of 0%). In contrast, for women undergoing laser forms of assisted hatching, there was no evidence of a statistically significant improvement amongst women in the test group compared with those in the control group (15 RCTs, 3606 women; OR 1.04, 95% CI 0.90 to 1.19, P = 0.0008, I2 of 62%). The same applied to women undergoing mechanical forms of AH (5 RCTs, 586 women; OR 1.30, 95% CI 0.89 to 1.88, P = 0.09, I2 of 51) (Analysis 3.4).
Prognosis: for women in the poor prognosis group, a statistically significant better outcome in clinical pregnancy rate was found amongst those in the AH group compared to the control group (12 RCTs, 1675 women; OR 1.49, 95% 1.19 to 1.85, P = 0.37, I2 of 8%), but there was no evidence of a statistically significant improvement amongst women with good prognosis (12 trials, 2253 women; OR 1.02, 95% CI 0.86 to 1.21, P = 0.89) (Analysis 3.5).
Degree of zona manipulation: for women undergoing complete removal of the zona pellucida, there was a statistically significant increase in clinical pregnancy rate amongst those in the AH group compared to the control group (2 RCTs, 301 women; OR 1.93, 95% CI 1.21 to 3.09, P = 0.70, I2 of 0%). Although only two trials reported on this, the CI was not excessively wide and the OR may be of clinical relevance. Similarly, examining the effects of mechanical expansion of the zona pellucida, there was an improvement which was statistically significant in the AH group (1 RCT, 125 women; OR 2.37, 95% CI 1.07 to 5.28, P = 0.003), but only one trial examined this technique of AH. There was no significant difference between AH and control groups in trials which reported on zona pellucida thinning as a means of zona manipulation (12 RCTs, 2936 women; OR 1.05, 95% CI 0.90 to 1.23, P = 0.01, I2 of 55%). Likewise, there was no significant difference between AH and control groups in trials which reported on zona pellucida piercing (breaching with a hole) as a means of zona manipulation (15 RCTs, 2163 women; OR 1.14, 95% CI 0.94 to 1.37, P = 0.03, I2 of 45%). The heterogeneity of the latter two groups suggested too much variation amongst trials examining thinning and piercing, however (Analysis 3.6).
Fresh or frozen embryo transfer: in fresh embryo groups, there was a statistically significant increase amongst women in the AH group when compared with the control group (24 RCTs, 4050 women; OR 1.14, 95% CI 1.01 to 1.30, P = 0.33, I2 of 10%). This was not the case for frozen embryo transfers (eight RCTs, 1478 women; OR 1.14, CI 0.90 to 1.44, P < 0.0001, I2 of 81%) (Analysis 3.7).
3.2. Analysis.
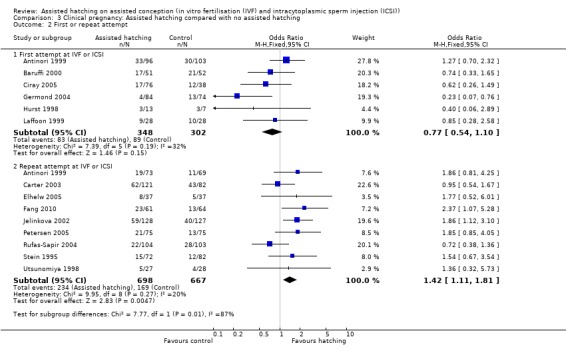
Comparison 3 Clinical pregnancy: Assisted hatching compared with no assisted hatching, Outcome 2 First or repeat attempt.
3.3. Analysis.
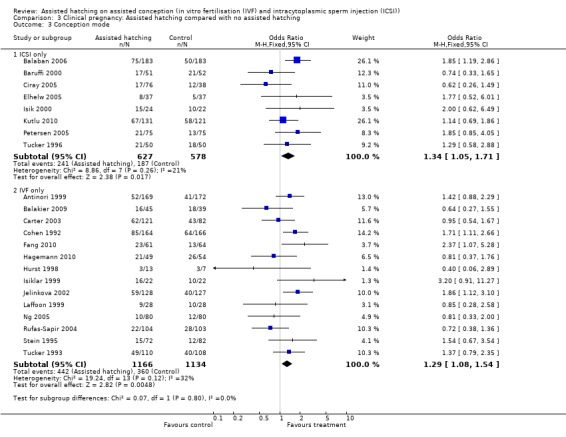
Comparison 3 Clinical pregnancy: Assisted hatching compared with no assisted hatching, Outcome 3 Conception mode.
3.4. Analysis.
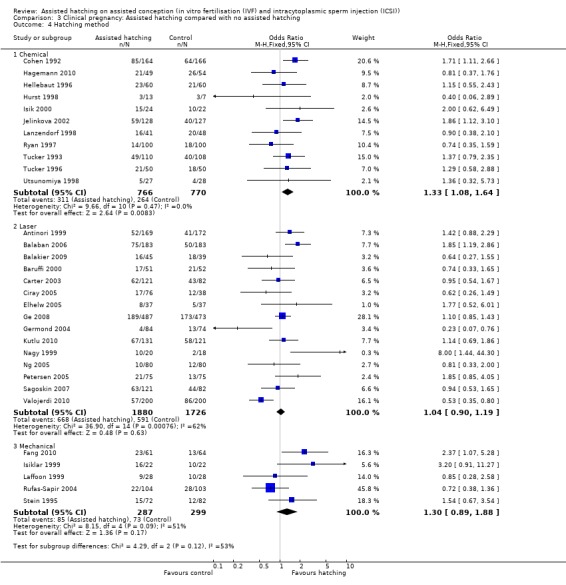
Comparison 3 Clinical pregnancy: Assisted hatching compared with no assisted hatching, Outcome 4 Hatching method.
3.5. Analysis.
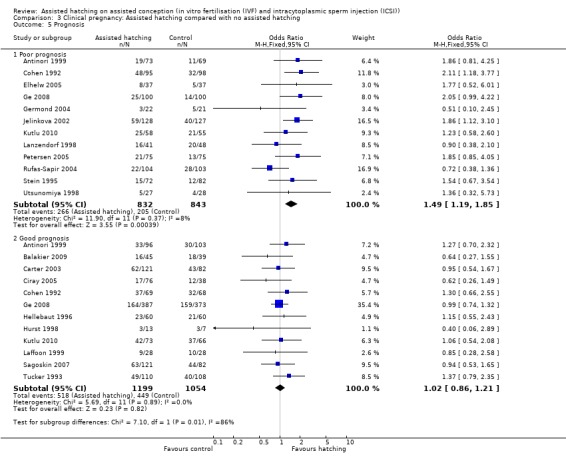
Comparison 3 Clinical pregnancy: Assisted hatching compared with no assisted hatching, Outcome 5 Prognosis.
3.6. Analysis.
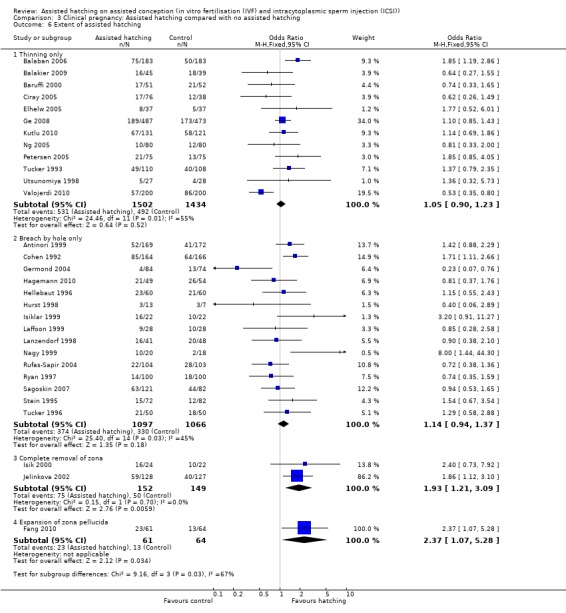
Comparison 3 Clinical pregnancy: Assisted hatching compared with no assisted hatching, Outcome 6 Extent of assisted hatching.
3.7. Analysis.
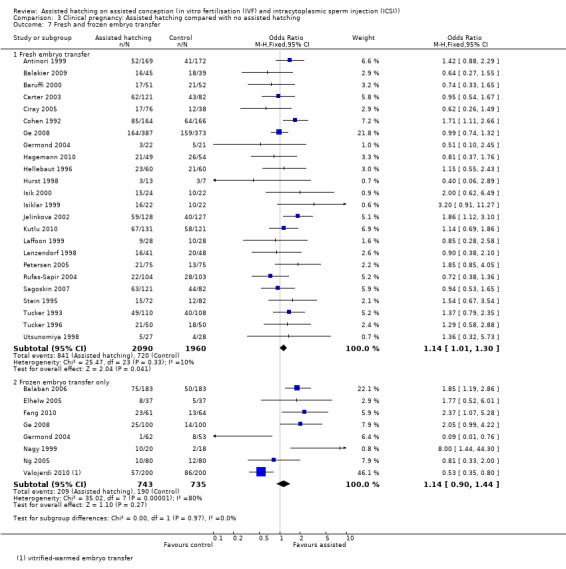
Comparison 3 Clinical pregnancy: Assisted hatching compared with no assisted hatching, Outcome 7 Fresh and frozen embryo transfer.
Sensitivity analysis
Allocation concealment: limiting the analysis to trials which reported allocation concealment left only three trials (Ge 2008; Lanzendorf 1998; Ng 2005). There was no significant difference in clinical pregnancy rate in the AH group when compared to the control group (OR 1.05, 95% CI 0.83 to 1.35, P = 0.27).
Method of randomisation: 16 trials stated an acceptable method of randomisation (Balaban 2006; Balakier 2009; Baruffi 2000; Carter 2003; Ciray 2005; Ge 2008; Germond 2004; Hagemann 2010; Hellebaut 1996; Hurst 1998; Isik 2000; Kutlu 2010; Petersen 2005; Ryan 1997; Sagoskin 2007; Valojerdi 2010). Analysis of the data from these trials showed no statistically significant difference between the AH and control groups (OR 0.96, 95% CI 0.83 to 1.11, P = 0.03).
4. Miscarriage per woman
Fourteen trials reported miscarriage rates, accounting for 2131 women. There were 99 miscarriages in total, 52 miscarriages occurring in the AH group and 47 in the control group. Overall, there was no significant difference in miscarriage rates between AH and control (14 RCTs, 2131 women; OR 1.03, 95% CI 0.69 to 1.54, P = 0.90, I2 of 0%) (Figure 6) (Analysis 5.1).
6.
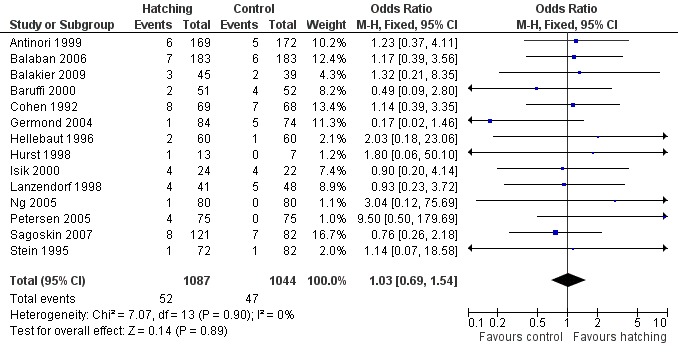
Forest plot of comparison: 3 Miscarriage rate, outcome: 3.1 Miscarriage per woman randomised.
5.1. Analysis.
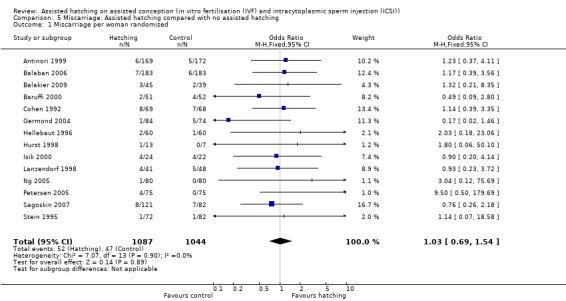
Comparison 5 Miscarriage: Assisted hatching compared with no assisted hatching, Outcome 1 Miscarriage per woman randomised.
Subgroup analysis
First or repeat attempt at ART: for women undergoing their first attempt at ART, there was no evidence of a statistically significant difference in miscarriage rate between AH and control groups (3 RCTs, 264 women; OR 0.91, 95% CI 0.29 to 2.80, P = 0.64, I2 of 0%). There was no evidence of heterogeneity in this group of trials. The same applied to women who had previously failed attempts at ART (4 RCTs, 663 women; OR 2.14, 95% CI 0.72 to 6.35, P = 0.59, I2 of 0%) (Analysis 5.2).
Assisted conception procedure: for women undergoing ICSI, there was no evidence of a statistically significant difference in miscarriage rate between the AH and control groups (4 RCTs, 665 women; OR 1.20, 95% CI 0.58 to 2.43, P = 0.38, I2 of 2%). There was no significant heterogeneity between studies. The same results were found for women undergoing IVF (6 RCTs; OR 1.28, 95% CI 0.65 to 2.52, P = 1.00, I2 of 0%) (Analysis 5.3).
Method of assisted hatching: there was no statistically significant evidence of a difference in miscarriage rate between women who underwent a chemical means of AH and those in the control group, with no significant heterogeneity (5 RCTs, 412 women; OR 1.11, 95% CI 0.56 to 2.21, P = 0.98, I2 of 0%). The same applied to women who underwent a laser means of AH when compared with the control group (8 RCTs, 1565 women; OR 0.98, 95% CI 0.59 to 1.63, P = 0.48, I2 of 0%), and for women who underwent a mechanical means of AH when compared with the control group (one trial only) (Analysis 5.4).
Prognosis: for AH and control groups in the poor prognosis group there was no statistically significant difference in miscarriage rate, with no significant heterogeneity (6 RCTs, 830 women; OR 1.06, 95% CI 0.57 to 1.99, P = 0.40, I2 of 2%). Likewise, for AH and control groups in the good prognosis group there was no statistically significant difference in miscarriage rate with no significant heterogeneity (5 RCTs, 626 women; OR 1.03, 95% CI 0.50 to 2.14, P = 0.94, I2 of 0%).
5.2. Analysis.
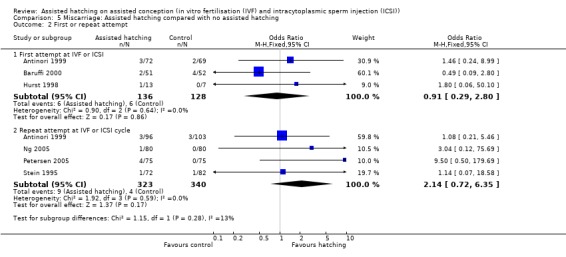
Comparison 5 Miscarriage: Assisted hatching compared with no assisted hatching, Outcome 2 First or repeat attempt.
5.3. Analysis.
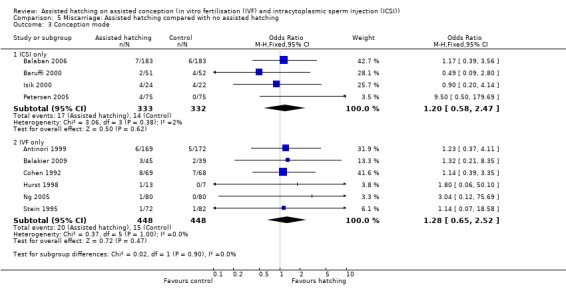
Comparison 5 Miscarriage: Assisted hatching compared with no assisted hatching, Outcome 3 Conception mode.
5.4. Analysis.
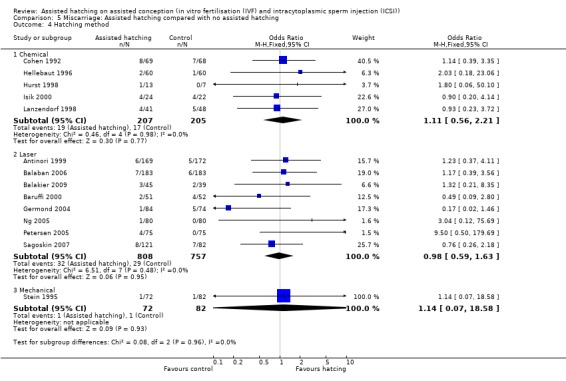
Comparison 5 Miscarriage: Assisted hatching compared with no assisted hatching, Outcome 4 Hatching method.
5. Ectopic pregnancy
Four trials reported ectopic pregnancy data: Lanzendorf 1998 reported one ectopic pregnancy in the control group and none in the AH group. Hagemann 2010, Hellebaut 1996 and Hurst 1998 reported an absence of ectopic pregnancies.
6. Monozygotic twinning
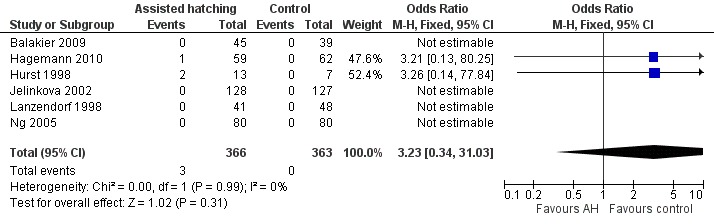
Six trials reported data on monozygotic twinning (Figure 7): Hurst 1998 reported two monozygotic twins from the three pregnancies in the AH group and none in the control group (0 from three pregnancies). Hagemann 2010 reported one case of monozygotic twins in the AH group also. Balakier 2009, Isik 2000, Jelinkova 2002, Lanzendorf 1998 and Ng 2005 reported an absence of monozygotic twins in either group. There was an overall rate of 0.8% for the AH group and 0% for the control group (Analysis 6.1).
7.
Forest plot of comparison: 5 Monozygotic twinning rate, outcome: 5.1 Monozygotic twinning per woman randomised.
6.1. Analysis.
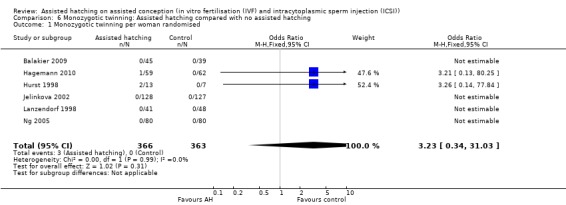
Comparison 6 Monozygotic twinning: Assisted hatching compared with no assisted hatching, Outcome 1 Monozygotic twinning per woman randomised.
7. Congenital or chromosomal abnormalities
Two trials (Hurst 1998; Lanzendorf 1998) reported an absence of congenital or chromosomal abnormalities, and one trial (Hagemann 2010) reported fetal abnormalities in both the AH and the control groups.
8. Failure to transfer any embryos per woman
No trials reported data on this outcome.
9. Embryo damage
Three trials reported an absence of embryo damage (Hurst 1998; Lanzendorf 1998; Stein 1995).
10. In vitro blastocyst development
No trials reported data on in vitro blastocyst development.
No further analyses were performed because of the paucity of data on these secondary outcomes.
Discussion
Summary of main results
Live birth
In this update, the primary outcome remained live birth rate. Yet only nine of the 31 studies reported this outcome, representing only 34% of all women randomised in the studies. Although the live birth rate may not be representative of all the studies in this review, these studies are representative of those with robust randomisation methods and were considered to be of good quality.
There is no evidence as yet that assisted hatching (AH) impacts on live birth rate, and subgroup analysis does not provide evidence of any effects. It was disappointing that the conclusions of the review were still limited by the paucity of available data in probably the most important and sought after statistic on the impact of AH on assisted conception, namely the 'take home baby rate'. This reflects the gap that currently exists between the practice of assisted conception and clinical obstetrics and the absence of a central database of patient records that would facilitate follow up of these women by authorised agencies, like the Human Fertilisation and Embryology Authority (HFEA) in the UK. That only nine of the included trials from nine authors reported live birth data suggests haste on the part of the other trialists to disseminate data limited to short‐term outcomes, and to all intents and purposes these data are incomplete.
Multiple pregnancy
Overall, there was a statistically significant increase in multiple pregnancies per clinical pregnancy (38% increase in OR), indicating that AH does seem to increase the chances of multiple pregnancies. Given this significance in combination with the lack of concrete evidence of an increase in success at achieving live birth, it may bring us to consider the overall risks versus benefits of this technique.
The lack of reporting of live birth data in this group of studies is unfortunate as it limits the interpretation of the results, given this high multiple pregnancy rate, because as many as 5% of multiple pregnancies are lost between 20 and 40 weeks gestation. In addition, most studies were transferring two to four embryos although the numbers transferred were balanced between groups. The reason for the increase in multiple pregnancies can be attributed to an increase in implantation rates resulting in higher pregnancy rates or monozygotic twinning, or both, with AH. This must be taken into consideration in the planning of this procedure.
It is likely that reducing the number of embryos transferred to one will not completely eliminate monozygotic twinning. Implantation rate was not considered as an outcome in this update for two reasons. The pooling of embryo implantation data for meta‐analysis is statistically problematic. Implantation is traditionally expressed 'per embryo transferred', without regard to the number of women. However, more than one embryo is normally transferred per woman, resulting in an embryo clustering effect and necessitating more advanced analysis to render the results meaningful. A statistically valid and easier approach is to express implantation 'per woman randomised'. This also has the advantage of being more useful in aiding understanding of the resulting live births. This approach requires, however, that the number of women with at least one gestation sac is reported, which is not the case in practice.
Clinical pregnancy
All 31 included trials reported on clinical pregnancy. There were 1992 pregnancies amongst the 5728 participants, 1064 in the AH group and 928 in the control group. Similar to the previous update in 2007, this update has shown that, overall, AH does seem to increase the chance of achieving a clinical pregnancy, however the level to which it does so only just reaches statistical significance (OR 1.13, 95% CI 1.01 to 1.27).
Restricting analysis of clinical pregnancy rate to those trials that went on to report live birth, the clinical pregnancy result showed statistically insignificant differences between the AH and the control groups (OR 1.03, 95% CI 0.85 to 1.25, P = 0.18). Analysis of clinical pregnancy rate of the robust studies, which described allocation concealment and their method of randomisation as well as reporting on the live birth rate, gave a clinical pregnancy rate of 1.08 (95% CI 0.82 to 1.41, P = 0.60), again suggesting that AH may not give statistically significant increased chances of achieving clinical pregnancy.
Despite this, similar to 2007, further subgroup analysis of all 31 studies suggests that women undergoing IVF or ICSI cycles who have previously been unsuccessful may benefit from AH as well as those women with a poor prognosis. AH involving complete removal of the zona pellucida shows statistically significant differences in clinical pregnancy rates. The same applies for AH involving expansion of the zona pellucida; however, in this update, there was only one trial which employed this method. In contrast to the previous update, this update showed AH only had statistically significant effects among participants receiving fresh embryos for embryo transfer rather than AH using either fresh or frozen embryos for embryo transfer.
Miscarriage
This review did not find sufficient evidence to draw conclusions on the impact of AH on miscarriage rates overall or for any of the subgroups considered.
Other outcomes
The impact of AH on ectopic pregnancy, congenital and chromosomal abnormalities, blastocyst formation and embryo damage could unfortunately not be answered by this review because of the paucity of available data. This was disappointing as it leaves many unanswered questions about the perceived risks of the procedure, from embryo damage to chromosomal and congenital abnormalities.
Overall completeness and applicability of evidence
A large number of trials were incorporated into this review, with a large sample size being investigated. The results of 5698 women in 31 trials are included in this review, leading to a generally acceptable level of evidence. However, failure of many trials to report on primary outcomes (live birth, multiple pregnancy) and variable levels of reporting on other outcomes will inevitably allow potential bias to be introduced into the analysis. This calls for standardised outcome reporting for future assisted conception trials.
Quality of the evidence
The overall quality of the evidence was low to moderate: please see Table 1; Table 2; Table 3; Table 4.
Potential biases in the review process
Three authors with varying levels of expertise undertook the search process several times in order to minimise the risk of authors introducing bias, and there was no conflict of interest.
Agreements and disagreements with other studies or reviews
Overall, the addition of the nine new trials in this update has not changed the findings regarding live birth that have been shown in previous reviews, namely that AH does not significantly increase the chances of a live birth. Clinical pregnancy rate again was shown to be slightly increased in women undergoing AH and this just reached a level of statistical significance.
Authors' conclusions
Implications for practice.
Live birth is the primary outcome yet only nine trials reported on this. Therefore, there could be under reporting of live birth outcomes leading to this result (22 of the 31 trials did not report live birth rates). The addition of the new trials resulted in a further 2082 participants in this review update (36% of the 5728 participants). Subgroups including women who had previously had failed attempts at assisted reproduction and poor prognosis women did have increased clinical pregnancy rates in the assisted hatching (AH) groups, which reached significance.
There was a significant increase in multiple pregnancy rates. This significant increase in the rate of twinning raises concerns regarding the number of embryos transferred and AH. The statistically significant chance of a multiple pregnancy if a clinical pregnancy is achieved may bring the clinician to consider the overall safety of offering this procedure to women in the future, or offering the procedure only to specific subgroups for which AH may be favourable.
Implications for research.
This review once again highlighted a wide range of currently unresolved issues that provide potential avenues for future research, including the need for high quality trials which report live births, clinical pregnancies and adverse events (including multiple pregnancies, miscarriages and long‐term adverse outcomes), and which are powered to investigate effects in clinical subgroups.
The potential of assisted hatching in assisted conception makes it imperative that studies of high methodological quality (preferably multicentre trials with appropriate design, adequate power and appropriate duration of follow up) are undertaken to provide these urgently needed answers.
What's new
| Date | Event | Description |
|---|---|---|
| 28 February 2013 | Amended | Minor correction to review title (format only) |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 8 August 2012 | New search has been performed | Review updated August 2012. Seven new studies in this update (Balakier 2009; Fang 2010; Ge 2008; Germond 2004; Hagemann 2010; Kutlu 2010; and Valojerdi 2010). |
| 8 August 2012 | New citation required but conclusions have not changed | Seven new studies added; no change to conclusions. |
| 17 June 2008 | New search has been performed | New search identified four new randomised controlled trials, which have been added. Conclusions have not changed. |
| 15 May 2008 | Amended | Converted to new review format. |
| 18 September 2007 | New citation required and conclusions have changed | Substantive amendment |
Notes
Carter 2003 was included after information regarding the number of couples was provided by the authors.
Acknowledgements
We wish to thank Sue Furness, Sarah Hetrick and Michelle Proctor of the Cochrane Menstrual Disorders and Subfertility Group for help with literature searches, and Deborah Thornton (Librarian at St. Mary's Hospital, Manchester) for assistance in retrieving journal articles. Andy Vail performed the meta‐regression in previous updates, for which we are grateful. Catherine Fullwood reviewed the update and provided statistical overview. We gratefully acknowledge those authors who provided extra information about their studies: Professors S Hellebaut, University of Ghent; BS Hurst, Charlotte, North Carolina; SE Lanzendorf, Eastern Virginia Medical School; F Olivennes, Hospital Antoine‐Beclere, Clamart; and MC Magli (SISMER, Reproductive Medicine Unit, Bologna).
Edmond Edi‐Osagie contributed to designing the original review, publishing the protocol, data collection, developing a search strategy, undertaking searches, screening search results, organising retrieval of papers, screening retrieved papers against inclusion criteria, appraising quality of papers, abstracting data from papers, writing to authors of papers for additional information, data management for the review, interpretation of data, providing a methodological perspective, providing a clinical perspective, providing a policy perspective, writing the review, providing general advice on the review, and performing previous work that was the foundation of the review.
Lee Hooper developed the second search strategy, undertook the February 2002 searches, screened these search results, assessed inclusion of all potential studies, appraised the quality of and abstracted data from all included studies, analysed the data (meta‐analysis in RevMan (RevMan 2008), subgrouping, sensitivity analyses, meta‐regression in STATA), interpreted the data, provided a methodological perspective, provided a consumer perspective, wrote the methodology and results sections of the review, and edited the original review.
Phil McGinlay contributed to designing the original review, data collection for the review, developing a search strategy, undertaking searches, screening search results, organising retrieval of papers, screening retrieved papers against inclusion criteria, and appraising quality of papers. Mr McGinlay unfortunately passed away before completion of this review and although he was acknowledged as an author in the two initial versions of this review, in the 2007 version he was removed from the title list.
Appendices
Appendix 1. MEDLINE
MEDLINE (1966 to April 2012)
1 randomised controlled trial.pt. (201065) 2 controlled clinical trial.pt. (68353) 3 Randomised controlled trials/ (37180) 4 random allocation/ (53076) 5 double‐blind method/ (81524) 6 single‐blind method/ (8937) 7 or/1‐6 (341829) 8 clinical trial.pt. (405523) 9 exp clinical trials/ (164946) 10 (clin$ adj25 trial$).ti,ab,sh. (109338) 11 ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).ti,ab,sh. (80778) 12 placebos/ (23682) 13 placebo$.ti,ab,sh. (100239) 14 random$.ti,ab,sh. (355156) 15 Research design/ (40564) 16 or/8‐15 (742407) 17 animal/ not (human/ and animal/) (2870499) 18 7 or 16 (746072) 19 18 not 17 (684366) 20 (assist$ adj5 hatch$).ti,ab,sh. (160) 21 (zona$ adj5 (pellucid$ or manipulat$ or disrupt$ or thin$ or drill$)).ti,ab,sh. (3190) 22 (zona$ adj5 (dissect$ or tyrode$ or proteinase$ or piezon$ or krypton$ or yag$)).ti,ab,sh. (166) 23 pzd.tw. (57) 24 or/20‐23 (3346) 25 7 and 16 and 19 and 24 (75)
Appendix 2. EMBASE
EMBASE (1980 to April 2012)
1 Controlled study/ or randomised controlled trial/ (1972522) 2 double blind procedure/ (55961) 3 single blind procedure/ (5342) 4 crossover procedure/ (16269) 5 drug comparison/ (81243) 6 placebo/ (77846) 7 random$.ti,ab,hw,tn,mf. (303696) 8 latin square.ti,ab,hw,tn,mf. (976) 9 crossover.ti,ab,hw,tn,mf. (28767) 10 cross‐over.ti,ab,hw,tn,mf. (10308) 11 placebo$.ti,ab,hw,tn,mf. (122744) 12 ((doubl$ or singl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).ti,ab,hw,tn,mf. (94676) 13 (comparative adj5 trial$).ti,ab,hw,tn,mf. (5072) 14 (clinical adj5 trial$).ti,ab,hw,tn,mf. (392129) 15 or/1‐14 (2384221) 16 nonhuman/ (2564036) 17 animal/ not (human/ and animal/) (12787) 18 or/16‐17 (2567625) 19 15 not 18 (1389301) 20 (assist$ adj5 hatch$).ti,ab,hw. (168) 21 (zona$ adj5 (pellucid$ or manipulat$ or disrupt$ or thin$ or drill$)).ti,ab,hw. (3135) 22 (zona$ adj5 (dissect$ or tyrode$ or proteinase$ or piezon$ or krypton$ or yag$)).ti,ab,hw. (143) 23 pzd.tw. (53) 24 or/20‐23 (3236) 25 15 and 19 and 24 (420)
Appendix 3. Data Extraction Proforma
Trial quality characteristics
Refer to Figure 1 and Figure 2.
(1) Method of randomisation:
(a) randomised allocation ‐ method of randomisation clearly stated and correct;
(b) randomised allocation ‐ method of randomisation not stated or unclear.
(2) Allocation concealment:
(a) randomisation sequence adequately concealed;
(b) allocation concealment unclear;
(c) allocation concealment inadequate.
(3) Blinding:
(a) presence or absence of blinding of participants;
(b) presence or absence of blinding of outcome assessors.
(4) Power calculation reported.
(5) Intention‐to‐treat analysis stated or implied.
(6) Publication as full paper or abstract only.
Trial design and flow
(7) Trial flow:
(a) numbers of women recruited;
(b) numbers of women randomised;
(c) numbers of women excluded;
(d) numbers of women analysed;
(e) numbers of women lost to follow up.
(8) Study setting:
(a) single‐ or multi‐centre;
(b) location;
(c) timing.
(9) Indication for zona manipulation:
(a) both diagnostic and therapeutic;
(b) therapeutic.
Study participants
(10) Baseline characteristics:
(a) age (mean and standard deviation in each study arm);
(b) primary or secondary infertility;
(c) cause and duration of infertility;
(d) previous treatment.
(11) Other subgroup criteria:
(a) women undergoing IVF only;
(b) women undergoing ICSI only;
(c) women over the age of 35 undergoing IVF, ICSI or both;
(d) women with high early proliferative phase FSH levels undergoing IVF, ICSI or both;
(e) women with repeated implantation failure undergoing IVF, ICSI or both;
(f) women with a higher risk of zona hardening undergoing IVF, ICSI or both.
Interventions
(12) Embryo manipulation:
(a) mechanical zona disruption ‐ zona dissection, piezon vibrator, micro‐manipulator;
(b) chemical zona disruption ‐ acid tyrodes, proteinases;
(c) laser zona manipulation ‐ krypton, ND‐Yag, carbon dioxide.
(13) Complete (holes), partial (thinning) zona breach, complete removal.
Outcomes
(14) Primary:
(a) live birth (per woman randomised).
(15) Secondary:
(a) clinical pregnancy (per woman randomised);
(b) miscarriage (per woman randomised);
(c) multiple pregnancy (per woman randomised);
(d) ectopic pregnancy;
(e) monozygotic twinning;
(f) congenital and chromosomal abnormalities;
(g) embryo damage (per embryo generated).
Data and analyses
Comparison 1. Live birth: Assisted hatching compared with no assisted hatching.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth per woman randomised | 9 | 1921 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.85, 1.26] |
| 2 First or repeat attempt | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 First attempt at IVF or ICSI | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.03] |
| 2.2 Repeat attempt at IVF or ICSI | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.62, 3.13] |
| 3 Conception mode | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 ICSI only | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.62, 3.13] |
| 3.2 IVF only | 3 | 241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.60, 1.68] |
| 4 Hatching method | 9 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Chemical | 4 | 366 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.74, 1.74] |
| 4.2 Laser | 5 | 1555 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.81, 1.26] |
| 5 Prognosis | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Poor prognosis | 4 | 576 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.99, 2.15] |
| 5.2 Good prognosis | 5 | 1187 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.74, 1.19] |
Comparison 2. Multiple pregnancy: Assisted hatching compared with no assisted hatching.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Multiple pregnancy rate per woman randomised | 14 | 3447 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.11, 1.70] |
| 2 First or repeat attempt | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 First attempt at IVF or ICSI | 2 | 294 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.12, 3.19] |
| 2.2 Repeat attempt at IVF or ICSI | 4 | 765 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.70, 1.80] |
| 3 Conception mode | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 ICSI only | 2 | 391 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.54 [1.70, 7.39] |
| 3.2 IVF only | 6 | 1126 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.28, 2.72] |
| 4 Hatching method | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Chemical | 4 | 534 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.98, 2.47] |
| 4.2 Laser | 9 | 2869 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.00, 1.61] |
| 4.3 Mechanical | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.33 [1.56, 44.64] |
| 5 Prognosis | 9 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Poor prognosis | 5 | 883 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.19, 2.96] |
| 5.2 Good prognosis | 6 | 1569 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.81, 1.44] |
| 6 Multiple pregnancy rate per woman grouped by extent of assisted hatching | 13 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Thinning only | 5 | 1970 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.05, 1.84] |
| 6.2 Breach by hole | 7 | 1249 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.05, 2.17] |
| 6.3 Complete removal of zona | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.07, 5.28] |
| 7 Multiple pregnancy per pregnancy | 14 | 1383 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.09, 1.77] |
Comparison 3. Clinical pregnancy: Assisted hatching compared with no assisted hatching.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical pregnancy rate per woman randomised | 31 | 5728 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [1.01, 1.27] |
| 2 First or repeat attempt | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 First attempt at IVF or ICSI | 6 | 650 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.54, 1.10] |
| 2.2 Repeat attempt at IVF or ICSI | 9 | 1365 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.11, 1.81] |
| 3 Conception mode | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 ICSI only | 8 | 1205 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.05, 1.71] |
| 3.2 IVF only | 14 | 2300 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.08, 1.54] |
| 4 Hatching method | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Chemical | 11 | 1536 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.08, 1.64] |
| 4.2 Laser | 15 | 3606 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.90, 1.19] |
| 4.3 Mechanical | 5 | 586 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.89, 1.88] |
| 5 Prognosis | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Poor prognosis | 12 | 1675 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.19, 1.85] |
| 5.2 Good prognosis | 12 | 2253 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.86, 1.21] |
| 6 Extent of assisted hatching | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Thinning only | 12 | 2936 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.90, 1.23] |
| 6.2 Breach by hole only | 15 | 2163 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.94, 1.37] |
| 6.3 Complete removal of zona | 2 | 301 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.21, 3.09] |
| 6.4 Expansion of zona pellucida | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.07, 5.28] |
| 7 Fresh and frozen embryo transfer | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Fresh embryo transfer | 24 | 4050 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.01, 1.30] |
| 7.2 Frozen embryo transfer only | 8 | 1478 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.90, 1.44] |
Comparison 4. Clinical pregnancies in trials which reported live births: Assisted hatching compared with no assisted hatching.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical Pregnancies in trials reporting live births | 9 | 1921 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.85, 1.25] |
Comparison 5. Miscarriage: Assisted hatching compared with no assisted hatching.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Miscarriage per woman randomised | 14 | 2131 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.69, 1.54] |
| 2 First or repeat attempt | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 First attempt at IVF or ICSI | 3 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.29, 2.80] |
| 2.2 Repeat attempt at IVF or ICSI cycle | 4 | 663 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.72, 6.35] |
| 3 Conception mode | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 ICSI only | 4 | 665 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.58, 2.47] |
| 3.2 IVF only | 6 | 896 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.65, 2.52] |
| 4 Hatching method | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Chemical | 5 | 412 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.56, 2.21] |
| 4.2 Laser | 8 | 1565 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.59, 1.63] |
| 4.3 Mechanical | 1 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.07, 18.58] |
| 5 Prognosis | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Poor prognosis | 6 | 830 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.57, 1.99] |
| 5.2 Good prognosis | 5 | 626 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.50, 2.14] |
| 6 Miscarriage per clinical pregnancy | 14 | 687 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.62, 1.50] |
5.5. Analysis.
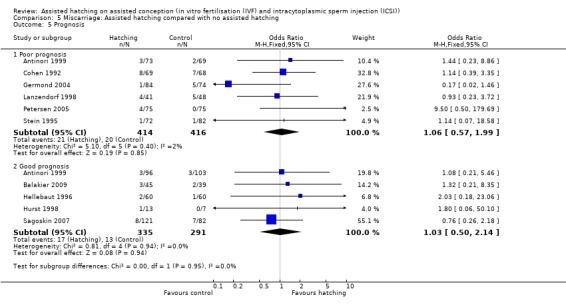
Comparison 5 Miscarriage: Assisted hatching compared with no assisted hatching, Outcome 5 Prognosis.
5.6. Analysis.
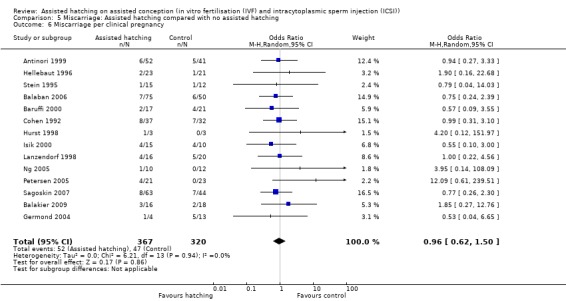
Comparison 5 Miscarriage: Assisted hatching compared with no assisted hatching, Outcome 6 Miscarriage per clinical pregnancy.
Comparison 6. Monozygotic twinning: Assisted hatching compared with no assisted hatching.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Monozygotic twinning per woman randomised | 6 | 729 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.23 [0.34, 31.03] |
Comparison 7. Robust studies (randomisation method and allocation concealment stated & live birth reported): Assisted hatching compared with no assisted hatching.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live Births | 1 | 960 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.41] |
| 2 Clinical Pregnancies | 1 | 960 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.85, 1.43] |
7.1. Analysis.
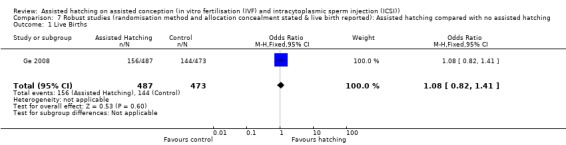
Comparison 7 Robust studies (randomisation method and allocation concealment stated & live birth reported): Assisted hatching compared with no assisted hatching, Outcome 1 Live Births.
7.2. Analysis.
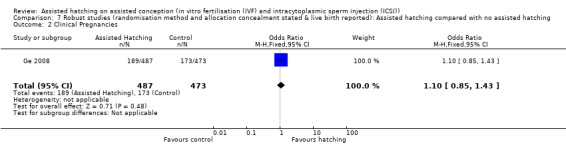
Comparison 7 Robust studies (randomisation method and allocation concealment stated & live birth reported): Assisted hatching compared with no assisted hatching, Outcome 2 Clinical Pregnancies.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Antinori 1999.
| Methods | Randomisation stated, but method unclear or incorrect Allocation concealment unclear Unclear if single/multicentre Participants not blinded or unclear Assessor not blinded or unclear Unclear if power calculation performed ITT analysis unclear Published as abstract | |
| Participants | 341 Women from Italy undergoing IVF. Subgrouped by previous IVF experience: a) without previous IVF experience (n=199) or b) with more than 6 previous IVF failures (n=142) Mean age: control group 27.0; AH group 27.5 years | |
| Interventions | AH (laser; complete zona breach; unclear how long from egg retrieval to AH; unclear how long from AH to transfer) ‐ 169 women randomised, 221 embryos transferred (estimated) versus Control ‐ no AH ‐172 women randomised, 247 embryos transferred (estimated) |
|
| Outcomes | Clinical pregnancy, miscarriage, multiple pregnancy | |
| Notes | No reply No of embryos transferred: AH 2.3, Control 2.4 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stated, but method unclear or incorrect. Day not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear. Participants not blinded or unclear. Assessor not blinded or unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis unclear and no evidence of any losses |
| Selective reporting (reporting bias) | Unclear risk | This is a conference abstract. There is no evidence of a full paper, live birth was not reported |
Balaban 2006.
| Methods | Randomisation by computer‐generated numbers | |
| Participants | 366 women from Turkey undergoing ICSI treatment only Exclusion: women undergoing IVF |
|
| Interventions | AH (laser thinning) n = 183 versus No AH (laser thinning) n = 183 Unclear on how long before transfer, frozen‐thawed embryos only |
|
| Outcomes | Primary: implantation rate, Secondary: clinical pregnancy, miscarriage and multiple pregnancy rate |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated numbers |
| Allocation concealment (selection bias) | Unclear risk | No details in text |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses to follow up and all women were analysed |
| Selective reporting (reporting bias) | Unclear risk | The original protocol was not viewed but all outcomes listed in the methods section were reported. Live birth was not reported |
Balakier 2009.
| Methods | Single centre Unclear if power calculation performed ITT analysis unclear Published as full paper | |
| Participants | 84 women from Canada with no more than one unsuccessful previous IVF attempt, aged ≤ 37 years of age, and with a day 3 FSH ≤ 10mIU/mL
Mean age: Control: 33.8 ± 3.2; AH: 32.5 ± 3.8 54 women underwent their first IVF cycle, the other 30 (13 AH) their second cycle. |
|
| Interventions | Laser assisted thinning n = 45: the total length of laser cut was approximately 30‐40μm, and about 60‐80% of the outer layer of the zona pellucid was thinned without complete breaching, applying 2ms laser beams. versus control n = 39 |
|
| Outcomes | Clinical pregnancy;Multiple pregnancies; Spontaneous miscarriages; Live births | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | No details in text |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study was double blinded to patients and medical personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses to follow up and all women were analysed |
| Selective reporting (reporting bias) | Unclear risk | Original protocol not viewed. Live birth was reported |
Baruffi 2000.
| Methods | Single centre Unclear if power calculation performed ITT analysis unclear Published as full paper | |
| Participants | 103 women from Brazil aged 37 years or less, undergoing ICSI for the first time. Mean zona thickness: control group 17.1 μm (SD 1.7); AH 16.6 μm (SD 2.2). Mean age: control group 31.4 (3.6); AH group 31.8 (3.6) | |
| Interventions | AH (laser; thinning partial; 48 hours egg retrieval to AH; 0 hours AH to transfer), 51 women randomised, 141 embryos transferred versus No AH, 52 women randomised, 149 embryos transferred |
|
| Outcomes | Implantation, clinical pregnancy, miscarriage | |
| Notes | No reply No of embryos transferred AH 2.76; Control 2.87 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were selected at random, using a randomisation table |
| Allocation concealment (selection bias) | Unclear risk | No information in the text |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information in the text |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses to follow up and all women were analysed |
| Selective reporting (reporting bias) | Unclear risk | Original protocol not viewed. Live birth not reported |
Carter 2003.
| Methods | Single centre Unclear if power calculation performed Published as abstract and authors provided additional information | |
| Participants | 203 women from fertility clinic in US Age < 40 years FSH < 10, ovulatory menstrual cycles, day 3 ET with good embryo quality Women with more than one failed IVF cycle were excluded | |
| Interventions | Laser hatching (n=121) versus No hatching (n=82) |
|
| Outcomes | Clinical pregnancy rate, multiple pregnancy rate | |
| Notes | Additional information provided by authors
Drop‐outs were included for the denominator in this review No. of embryos AH 2.2; Control 2.1 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, method by computer generation on day three |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants not blinded or unclear Assessor not blinded or unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated but included drop‐outs |
| Selective reporting (reporting bias) | High risk | This was a conference abstract only and was not published as a full paper although the authors did provide some additional information. Live birth was not reported |
Ciray 2005.
| Methods | Single centre Power calculation not reported ITT analysis not stated Published as full paper | |
| Participants | 114 women from Turkey undergoing ART for ASRM grade 3 to 4 endometrosis only (poor prognosis) Age < 40 years: AH group 33.1 (4.2); Control group 34.0 (3.7) Basal FSH: AH group 7.4 (3.5); Control group 9.0 (5.1) | |
| Interventions | Laser hatching (thinning to a quarter), 76 women randomised, 146 embryos transferred (16 cancelled) versus No hatching, only fresh embryo transfer cycles, 38 women randomised, 72 embryos transferred (8 cancelled) |
|
| Outcomes | Clinical pregnancy rate, implantation rate | |
| Notes | No. of embryos: AH 2.4; Control 2.4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, method stated 2:1 date, with the aid of computer programme |
| Allocation concealment (selection bias) | Unclear risk | Unclear no details |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding of participants or assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All women appear to have been analysed |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported but original protocol not viewed, live birth was not reported |
Cohen 1992.
| Methods | Single centre Unclear if power calculation performed ITT analysis unclear Published as full paper | |
| Participants | 330 women from North America undergoing IVF Mean age: control group 36.7 (3.7); AH group 36.5 (3.3) | |
| Interventions | AH by acid tyrodes (chemical; complete zona breach hole; 68 to 72 hours egg retrieval to AH; 4 to 8 hours AH to transfer), 69 women with FSH <15 (trial 1), 80 women with poor prognosis (trial 2, thick zonae pellucida, low developmental rate, excessive fragmentation), 15 women with FSH >15 (trial 3) No AH, 68 women with FSH <15 (trial 1), 83 women with poor prognosis (trial 2, thick zonae pellucida, low developmental rate, excessive fragmentation), 15 women with FSH >15 (trial 3) |
|
| Outcomes | Implantation, clinical pregnancy (rates given for trials 1, 2 and 3), live births (rates given for women in trial 1 only), multiple pregnancy (rates given form women in trials 1 and 2 only) | |
| Notes | Attempted to contact author about this study. Reply received, but no additional information was offered No. of embryos AH 3.5; Control 3.4 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Pre‐printed randomisation list day not stated |
| Allocation concealment (selection bias) | High risk | Allocation concealment inadequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants blinded Assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis unclear |
| Selective reporting (reporting bias) | Unclear risk | Original protocol not viewed |
Elhelw 2005.
| Methods | Power calculation not reported ITT not stated Published as abstract only | |
| Participants | 74 women from Egypt undergoing ICSI only Poor prognosis Previous 2 implantation failures Cryo‐thaw cycles only | |
| Interventions | Laser hatching (thinning to quarter) versus no hatching. AH done 1 hour before embryo transfer AH: 37 women randomised, 121 embryos transferred Control: 37 women randomised, 130 embryos transferred | |
| Outcomes | Implantation rate, clinical pregnancy rate | |
| Notes | No author contact as all details in article No data re no. of embryo transfer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated, no details |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participant blinding unclear Assessor blinding unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details |
| Selective reporting (reporting bias) | High risk | Conference abstract only. No evidence of a full paper. Live birth not reported |
Fang 2010.
| Methods | Single centre randomised controlled trial | |
| Participants | 125 women in China who had their first IVF/ICSI cycles between 2006 and 2008 with fresh IVF‐ET failures or without fresh embryo transfers Mean age: 32.3 in AH group, 32.1 in control group Setting: Fertility centre, China (2006 to 2008) |
|
| Interventions | Mechanical assisted hatching: expanding/stretching the zona pellucida via injected hydrostatic pressure AH: 61 women, 178 embryos Control: 64 women, 190 embryos |
|
| Outcomes | Clinical pregnancy and intrauterine implantation rates | |
| Notes | Unclear if power calculation performed ITT analysis unclear Published as full paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Embryologists blinded to the group assignment, unclear if participants were too |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | High risk | Original protocol not viewed. Live birth not reported |
Ge 2008.
| Methods | Randomised controlled trial | |
| Participants | 760 women from China having IVF with fewer than five failed cycles of ART with normal baseline FSH concentration. Those participants with uterine abnormality or low fertilisation capacity (rate of fertilisation less than 20% and late ICSI following fertilisation failure of IVF) were excluded Mean age: Fresh, 31.08 in AH, 30.44 control; Frozen, 31.84 in AH, 30.66 control |
|
| Interventions | Laser thinning to about 50% of the initial ZP thickness AH: 387 women with fresh embryos, 100 women with frozen‐thawed embryos Control: 373 women with fresh embryos, 100 women with frozen‐thawed embryos |
|
| Outcomes | Implantation rate, pregnancy rate and live birth | |
| Notes | Unclear if power calculation performed ITT not stated Published as full paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomised according to a randomisation list based on sequential numbers in sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Both patients and the clinician were blinded to group allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated in the text |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fresh embryo transfer cycles: a total of 831 IVF/ICSI cycles were performed during the study period. Of these, 772 met the inclusion criteria but 12 participants abandoned embryo transfer for various reasons such as avoiding potential risks of ovarian hyperstimulation syndrome Frozen‐thawed embryo transfer: a total of 245 frozen‐thawed cycles were also performed, of which 45 were excluded either because they didn't meet the criteria of the study or embryo transfer was abandoned |
| Selective reporting (reporting bias) | Unclear risk | Original protocol not viewed |
Germond 2004.
| Methods | Multicentre RCT | |
| Participants | 153 women in four European IVF centres aged between 20 and 45 years old, having at least one functional ovary, having normal FSH and prolactin levels, having no clinically significant findings within six months before starting treatment, and having a normal uterine cavity | |
| Interventions | Laser assisted hatching using diode laser AH: 56 women undergoing their first cycle of frozen‐thawed embryos, 23 women who had a poor prognosis using fresh embryos Control: 53 women undergoing their first cycle of frozen‐thawed embryos, 21 women who had a poor prognosis using fresh embryos |
|
| Outcomes | Clinical pregnancies, live births, miscarriages and multiple pregnancies | |
| Notes | Power calculation performed
ITT not stated Published as full paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised according to a randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not stated in the text |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated in the text |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Low risk | Live birth reported |
Hagemann 2010.
| Methods | Randomised, single centre, crossover trial | |
| Participants | 103 women in the United States under 38 years of age with any embryo with a zona pellucida thickness > 13μm and more than 2 previous failed IVF cycles Mean age: 32.1 years in the hatched group, 31.2 in the unhatched group | |
| Interventions | AH performed by acidic Tyrode's solution AH: 49 women Control: 54 women |
|
| Outcomes | Clinical intrauterine pregnancy rate, implantation rate, spontaneous pregnancy loss and live birth rate | |
| Notes | Power calculation: study states it has inadequate power. The study as ultimately performed only had sufficient statistical power to identify a 30% absolute effect size with alpha = 0.05 and beta = 0.80
ITT analysis unclear Published as full paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by the IVF lab staff by drawing 1 of 200 opaque envelopes from a box |
| Allocation concealment (selection bias) | Unclear risk | Opaque envelopes drawn, but not numbered |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study arm to which participants belonged was blinded to care givers, with the exception of the IVF embryologyists, as well as to participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Low risk | Live birth reported (but results not included in this study as results were only given for both cycles combined and not for just the first cycle, which is the data we are using). No other evidence of reporting bias |
Hellebaut 1996.
| Methods | Randomised, single centre trial | |
| Participants | 120 women from Belgium undergoing IVF or ICSI Mean age: control group 30.8 (3.9); AH group 30.9 (4.3) years | |
| Interventions | AH (mechanical; complete zona breach hole; 48 hours egg retrieval to AH; 0.2 hours AH to transfer) versus no AH AH: 60 women randomised, 168 embryos transferred Control: 60 women randomised, 162 embryos transferred | |
| Outcomes | Implantation, clinical pregnancy, live birth, miscarriage, ectopic pregnancy | |
| Notes | Attempted to contact author about this study. A reply including much useful additional information was received
No. of embryos transferred: AH 2.8 (0.6); Control 2.7 (0.6) Unclear if power calculation performed ITT analysis unclear Published as full paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer on day of transfer |
| Allocation concealment (selection bias) | High risk | Allocation concealment inadequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants not blinded Assessor not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis unclear |
| Selective reporting (reporting bias) | Low risk | Live birth reported. Authors responded to requests for details. No other evidence of bias, all outcomes stated were reported |
Hurst 1998.
| Methods | Single centre randomised trial | |
| Participants | 20 women from North America undergoing IVF, either with no prior IVF (30 years or less, FSH < 10 IU/L, normal endometrium and sperm) or prior IVF (35 years or less, 6 embryos, 50% fertilisation, normal endometrium) Mean age: control group 30 (0.8); AH group 30 (0.9) | |
| Interventions | AH by acid tyrodes (chemical; complete zona breach hole; ? hours egg retrieval to AH; ? hours AH to transfer) versus no AH AH: 13 women randomised, 52 embryos transferred Control: 7 women randomised, 28 embryos transferred | |
| Outcomes | Implantation, clinical pregnancy, live births | |
| Notes | Attempted to contact author about this study. A reply including much useful additional information was received. No of embryos transferred: AH 4.0; Control 4.0 Unclear if power calculation performed ITT analysis unclear Published as full paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomised |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants not blinded or unclear Assessor not blinded or unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis unclear |
| Selective reporting (reporting bias) | Low risk | Protocol not viewed but outcomes were reported and included live birth |
Isik 2000.
| Methods | Single centre, randomised trial | |
| Participants | 46 women from Turkey with > 5 day 3 cleavage stage embryos (FSH at day 3: control 6.1 (3.0); AH 5.5 (1.4) IU/L)undergoing ICSI Mean duration of infertility: 6.7 years Mean age: control group 29.1 (3.6); AH group 30.5 (5.2) years | |
| Interventions | AH enzymatic (chemical; complete and total zona breach; 120 to 144 hours egg retrieval to AH; 0.5 to 1 hours AH to transfer) versus no AH AH: 24 women randomised, 71 embryos transferred Control: 22 women randomised, 63 embryos transferred | |
| Outcomes | Implantation | |
| Notes | Author response No. of embryos transferred, blastocyst transfer: AH 2.95 (0.9); Control 2.86 (0.8) Unclear if power calculation performed ITT analysis unclear Published as full paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a random number table on day three |
| Allocation concealment (selection bias) | High risk | Allocation not concealed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants not blinded or unclear Assessor not blinded or unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Protocol not viewed, however live birth was not reported |
Isiklar 1999.
| Methods | Single centre randomised trial | |
| Participants | 44 women from Turkey undergoing IVF Mean age not stated | |
| Interventions | AH (mechanical; complete zona breach; ? hours egg retrieval to AH; ? hours AH to transfer) versus no AH AH: 22 women randomised, 83 embryos transferred Control: 22 women randomised, 78 embryos transferred | |
| Outcomes | Implantation, clinical pregnancy, multiple pregnancy | |
| Notes | Attempted to contact author about this study No of embryos transferred AH 3.7 Control 3.5 Unclear if power calculation performed ITT analysis unclear Published as abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised on day three |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants not blinded or unclear Assessor not blinded or unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis unclear |
| Selective reporting (reporting bias) | Unclear risk | This publication was in abstract form only, no full paper publication was identified. The authors do not report on live birth |
Jelinkova 2002.
| Methods | Single centre, randomised trial | |
| Participants | 255 IVF participants only; at least 2 previous failures
Age AH: 32.3 (4.24), control: 32.1 (3.16) Germany |
|
| Interventions | AH (chemical removal by acid, complete zona breach) AH: 128 women Control: 127 women |
|
| Outcomes | Clinical pregnancy rate, implantation rate | |
| Notes | Attempted to contact author about this study No. of embryos transferred: AH 2.2; Control 2.2 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stated, but method and timing unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants not blinded or unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | High risk | Protocol not viewed, outcomes were reported on but do not include live birth |
Kutlu 2010.
| Methods | Single centre, randomised trial | |
| Participants | 252 infertile couples having ART treatments at Medicana Camlica Hospital, Istanbul, Turkey. Subgrouped by prognosis: poor (n=113) or good (n=139) | |
| Interventions | AH was performed by laser method AH: 73 women aged under 35 years, 58 women aged 35 or over Control: 66 women aged under 35, 55 women aged 35 or over |
|
| Outcomes | Clinical pregnancy rate, implantation rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed in a computerised manner |
| Allocation concealment (selection bias) | Unclear risk | Not stated within the text |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated within the text |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis unclear |
| Selective reporting (reporting bias) | High risk | Original protocol not viewed. Live birth not reported |
Laffoon 1999.
| Methods | Single centre, randomised trial | |
| Participants | 56 women from North America aged less than 40 years undergoing IVF. Mean age not stated | |
| Interventions | AH (mechanical; complete zona breach; ? hours egg retrieval to AH; ? hours AH to transfer) versus no AH AH: 28 women randomised, embryos transferred not stated Control: 28 women randomised, embryos transferred not stated | |
| Outcomes | Clinical pregnancy | |
| Notes | Attempted to contact author about this study No. of embryos transferred not stated Unclear if power calculation performed ITT analysis unclear Published as abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Timing and method not stated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants not blinded or unclear Assessor not blinded or unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis unclear |
| Selective reporting (reporting bias) | High risk | Published as a conference abstract. Unable to find a full paper publication. Live birth was not reported |
Lanzendorf 1998.
| Methods | Single centre, randomised trial | |
| Participants | 94 women from North America aged at least 36 years (mean basal FSH control 7.6 IU/L (SD 2.0); AH 7.9 IU/L (SD 2.5)), undergoing IVF (some with ICSI), half had been previously treated with IVF Mean age: control group 38.5 (0.26); AH group 38.3 (0.31) | |
| Interventions | AH by acid tyrodes (chemical; complete zona breach; 55 hours egg retrieval to AH; ? hours AH to transfer) versus no AH AH: 42 women randomised, 180 embryos transferred Control: 52 women randomised, 212 embryos transferred | |
| Outcomes | Implantation, clinical pregnancy, multiple pregnancy, live births | |
| Notes | Attempted to contact author about this study. A reply including much useful additional information was received No. of embryos stated: AH 4.4; Control 4.4 Unclear if power calculation performed ITT analysis performed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, method stated |
| Allocation concealment (selection bias) | Low risk | Allocation concealment using sealed envelopes on day of aspiration |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants blinded Assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting, although original protocol not viewed. Authors did report on live birth |
Nagy 1999.
| Methods | Single centre, randomised trial | |
| Participants | 38 women from Italy with cryopreserved embryos undergoing IVF and ICSI Mean age: control group 31.4 (3.7); AH group 32.0 (4.0) | |
| Interventions | AH (laser; complete zona breach; ? hours egg retrieval to AH; ? hours AH to transfer) with concomitant removal of damaged blastomeres versus no AH and no damaged blastomere removal AH: 20 women randomised, 65 embryos transferred Control: 18 women randomised, 52 embryos transferred | |
| Outcomes | Clinical pregnancy | |
| Notes | Attempted to contact author about this study. Reply received No. of embryos: AH: 2.9, control: 3.2 Unclear if power calculation performed ITT analysis unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stated, but method unclear or incorrect |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants not blinded or unclear Assessor not blinded or unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis unclear |
| Selective reporting (reporting bias) | High risk | Published as a conference abstract only. No evidence of a full paper publication. The authors did not report on live birth |
Ng 2005.
| Methods | Randomised trial | |
| Participants | 160 women from Hong Kong with frozen embryo transfer Mean age 34.0 years | |
| Interventions | Laser assisted thinning 1/4 with frozen embryos compared to frozen embryos AH: 80 women Control: 80 women |
|
| Outcomes | Clinical pregnancy, miscarriage and multiple pregnancy rates | |
| Notes | No author contact as all details clearly stated in article No. of embryos stated: AH, transferred 2 in 52.5% and 3 in 41.3%; Control, transferred 2 in 36.2% and 3 in 61.3% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation pm day of frozen embryo transfer, computergenerated randomisation in sealed envelopes on day of ET |
| Allocation concealment (selection bias) | Unclear risk | 'sealed envelopes' used but unclear if these were opaque and how they were numbered |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double blinding until completion of the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Original protocol not viewed. The authors did not report on live birth |
Petersen 2005.
| Methods | Randomised trial | |
| Participants | 150 Women from Brazil undergoing ART cycles All participants had one failed treatment cycle Mean age 34 years | |
| Interventions | ICSI cycles only
AH quarter‐laser thinning versus control AH: 35 women with one previous implantation failure, 40 women with repeated implantation failures Control: 35 women with one previous implantation failure, 40 women with repeated implantation failures |
|
| Outcomes | Live birth, clinical pregnancy, miscarriage and multiple pregnancy | |
| Notes | Author response No. of ET: mean 2.7 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Unclear ‐ a code ID to mask identity of the participant but not clear how or who generated this |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised appear to have been analysed |
| Selective reporting (reporting bias) | Unclear risk | Original protocol not viewed but authors did report on live birth |
Rufas‐Sapir 2004.
| Methods | Unknown randomisation method and allocation concealment. occurred on day of embryo transfer | |
| Participants | 207 women 3 consecutive failed IVF cycles All ages Undergoing IVF only |
|
| Interventions | Mechanical partial zonal dissection: complete breach technique versus control AH ‐ 104 women Control ‐ 103 women |
|
| Outcomes | Clinical pregnancy, miscarriage | |
| Notes | Author response AH 3.4; ET Control 3.7 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unknown randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Unknown allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Ryan 1997.
| Methods | Single centre, randomised trial | |
| Participants | 200 women from Sydney Australia undergoing ART cycles | |
| Interventions | AH: tyrodes complete breach ‐ hole chemical means on both fresh and frozen‐thawed embryos: 100 women Control: 100 women |
|
| Outcomes | Clinical pregnancy | |
| Notes | Additional information was received from the 1st author regarding the definition of pregnancy. No further publication is planned Mean ET 2.17 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Unclear if allocation concealed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear if blinding took place |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised appear to have been analysed |
| Selective reporting (reporting bias) | High risk | Original protocol not viewed. Authors did not report on live birth |
Sagoskin 2007.
| Methods | Randomised trial | |
| Participants | 199 women from USA undergoing IVF or ICSI Good prognosis group with only one previous implantation failure Fresh embryo transfer cycles only | |
| Interventions | Laser hatching (breach with hole) AH: 121 randomised, 118 analysed, 254 embryos; Control: 82 randomised, 81 analysed, 170 embryos | |
| Outcomes | Live birth, clinical pregnancy, miscarriage and multiple pregnancy rate | |
| Notes | No author contact as all details clearly stated in article ET: AH 2.2 (0.4); Control 2.1 (0.3) Power calculation not reported Published as a full paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment assignments were determined by a computer‐generated randomised series in a 2:1 ratio of treatments to controls |
| Allocation concealment (selection bias) | Unclear risk | Not stated within the text |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated within the text |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT unclear |
| Selective reporting (reporting bias) | Low risk | Live birth reported |
Stein 1995.
| Methods | Single centre, randomised trial | |
| Participants | 154 women from Israel with repeated implantation failure (> 3 attempts) undergoing IVF Mean age not stated | |
| Interventions | AH (mechanical; complete zona breach; ? hours egg retrieval to AH; 1.5 hours AH to transfer) versus no AH AH: 72 women randomised, 230 embryos transferred Control: 82 women randomised, 295 embryos transferred | |
| Outcomes | Clinical pregnancy, miscarriage | |
| Notes | Attempted to contact author about this study, no reply received Unclear if power calculation performed ITT analysis unclear Published as full paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stated, but method unclear or incorrect |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants not blinded or unclear Assessor not blinded or unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis unclear |
| Selective reporting (reporting bias) | High risk | Original protocol not viewed but authors did not report on live birth |
Tucker 1993.
| Methods | Single centre, randomised trial | |
| Participants | 218 women from North America undergoing IVF (mean basal FSH: control group 9.0 (5.3); AH group 8.8 (3.7) IU/L) Mean age: control group 34.2 (4.1); AH group 34.1 (4.8) | |
| Interventions | AH with acid tyrodes thinning to 1/4; 72 hours egg retrieval to AH; 1 to 3 hours AH to transfer) versus no AH AH: 110 women randomised, 333 embryos transferred Control: 108 women randomised, 312 embryos transferred | |
| Outcomes | Implantation, clinical pregnancy | |
| Notes | Attempted to contact author about this study, no reply received ET: AH 2.9, Control 3.0 Unclear if power calculation performed ITT analysis unclear Published as full paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stated, but method unclear or incorrect |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants not blinded or unclear Assessor not blinded or unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis unclear |
| Selective reporting (reporting bias) | High risk | Original protocol not viewed but authors did not report on live birth |
Tucker 1996.
| Methods | Single centre, randomised trial | |
| Participants | 100 women from North America undergoing ICSI Mean age: control group 33.5 (4.3); AH group 35.3 (4.2) | |
| Interventions | AH with acid tyrodes (chemical; complete zona breach; 72 hours egg retrieval to AH; 4 hours AH to transfer) versus no AH AH: 50 women randomised, 189 embryos transferred Control: 50 women randomised, 184 embryos transferred | |
| Outcomes | Implantation, clinical pregnancy | |
| Notes | Attempted to contact author about this study, no reply received ET: AH 3.7, control 3.8 Unclear if power calculation performed ITT analysis unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stated, but method unclear or incorrect |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants not blinded or unclear Assessor not blinded or unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Women randomised appear to be analysed |
| Selective reporting (reporting bias) | Unclear risk | Original protocol not viewed but authors did not report on live birth |
Utsunomiya 1998.
| Methods | Single centre, randomised trial | |
| Participants | 55 women from Japan, undergoing either ICSI or IVF No data provided on age | |
| Interventions | AH with acid (chemical): 27 women
No other details about the day of treatment provided Control: 28 women |
|
| Outcomes | Clinical pregnancy rate only (gestation sac on ultrasound) | |
| Notes | No attempt to contact author No. of ET not stated Unclear if power calculation performed ITT analysis unclear Published as abstract only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stated, but method unclear or incorrect |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants not blinded or unclear Assessor not blinded or unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis is unclear |
| Selective reporting (reporting bias) | High risk | Published as a conference abstract only and did not report on live births |
Valojerdi 2010.
| Methods | Single centre, randomised trial | |
| Participants | 400 women in Iran undergoing first treatment cycle and women with previous failed cycles Mean age: control group 29.85 (5.14); AH group 30.86 (5.82); 82 |
|
| Interventions | Partially thinned by laser AH: 200 women randomised Control: 200 women randomised |
|
| Outcomes | Clinical pregnancy, implantation rates | |
| Notes | Power calculation not reported ITT analysis unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list participants |
| Allocation concealment (selection bias) | Low risk | Sequential numbers in sealed envelopes (200 participants in each group) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Original protocol was not viewed but authors did not report on live birth |
AH = assisted hatching IVF = in vitro fertilisation ICSI = intracytoplasmic sperm injection ITT = intention‐to‐treat Mean age given in years (standard deviation). Note: only arms where all or no embryos transferred were treated with AH were accepted for data extraction.
ET= embryo transfer
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelmassih 2002 | Pooled oocytes and then randomised; no per woman data provided |
| Antinori 1996a | Not a randomised controlled trial Mentions randomly selected not randomly allocated |
| Antinori 1996b | No randomised comparison between control and assisted hatching groups |
| Balaban 2002 | Not randomised No appropriate controls |
| Bider 1997 | Not randomised |
| Blake 2001 | Not randomised No embryo transfer occurred, so no review outcomes could be measured |
| Carter 2003a | No per woman data |
| Chao 1997 | Assessment of pregnancy was by HCG only, 14 days after embryo transfer |
| Check 1996 | Not randomised Benefits of AH confounded by concurrent assessment of 2 different culture media |
| Chen 1999 | Not randomised Benefits of assisted hatching confounded by concurrent assessment of two different culture media |
| Cieslak 1999 | Comparison of two types of assisted hatching; no 'no assisted hatching' control group was used More than one cycle per woman |
| Cohen 1990 | Not randomised |
| Debrock 2011 | Primary outcome was implantation, results per embryo transfer and not per woman |
| Demirol 2003 | No pregnancy data provided |
| Dirnfeld 2003 | No hatching |
| Dokras 1994 | No appropriate outcome measure |
| Domitrz 2000 | Benefits of assisted hatching confounded by concurrent assessment of two different culture media |
| Ebner 2002 | No per woman data |
| Edirisinghe 1999 | Not randomised |
| Feng 2009 | Not a prospective study. A retrospective study |
| Figueira 2012 | eggs were from egg donors, not the womens' own eggs |
| Frydman 2006 | No per woman data |
| Gabrielsen 2004 | Pseudo‐randomised (alternate days) |
| Grace 2007 | No control. Comparing assisted hatching in good embryos with assisted hatching in poor embryos. |
| Hershlag 1999 | Not randomised The control group were from the period 1990 to 1993, while the assisted hatching group were from 1994 to 1996 (historical controls) |
| Hiraoka 2009 | No control. Comparing a half thinning versus a quarter thinning. |
| Hur 2011 | Not clear if randomised, results appear to be per embryo transfer rather than per woman |
| Huttelova 1999 | Not randomised Benefits of AH confounded by concurrent assessment of 2 different culture media |
| Komarovsky 2002 | No per women data |
| Komarovsky 2003 | No per women data |
| Lee 1999 | Not randomised |
| Levron 2003 | No per women data |
| Ma 2007 | No per women data |
| Magli 1998 | No per women data |
| Mahadevan 1998 | Not randomised No concurrent controls |
| Mansour 2000 | Randomisation by alternate days |
| Meldrum 1998 | Not randomised No concurrent controls |
| Montag 1999 | Not randomised No concurrent controls |
| Nagy 2003 | No per woman data |
| Nakayama 1998 | No appropriate outcome measure |
| Nakayama 1999 | No per woman data |
| Ng 2008 | No control. Compared 2 methods of laser |
| Obruca 1994 | Not randomised No concurrent controls |
| Olivennes 1997 | No per woman data |
| Peterson 2006 | results per embryo transfer only no per woman data |
| Rienzi 2002 | Assisted hatching was part of the ICSI method |
| Ringler 1999 | It was not clear how many women were included in the study, or for how many cycles (only cycles were mentioned), and a mixture of participants and donated eggs were used for the study |
| Schoolcraft 1994 | Not randomised Control and intervention groups recruited at different times |
| Shahin 2003 | No per women data |
| Sifer 2005 | Per cycle data only No per woman data |
| Szell 1998 | Not randomised Benefits of assisted hatching confounded by concurrent assessment of two different culture media |
| Tao 1997 | Not randomised Some of the women in the assisted hatching group were randomised, but most were allocated assisted hatching routinely, with no control option |
| Tucker 1991 | Not randomised |
| Urman 2002 | Alternate randomisation |
| Valojerdi 2008 | Inadequate method of randomisation |
| Yano 2007 | No per woman data, only per cycle data |
| Zech 1998 | Numbers in tables do not add up correctly and the text and tables are contradictory on the age groups used in the prospective part of the study |
| Zhang 2009 | Not a prospective study. A retrospective study. |
HCG = human chorionic gonadotropin
Differences between protocol and review
1. In the 2005 update the following subgroups were investigated:
age (where reported in the studies) ≥ 35 years;
first cycle versus previous failed cycles of IVF, ICSI, or both;
ICSI only cycles;
chemical versus laser versus mechanical;
thinning versus breach with hole versus complete removal.
In the 2007 update, the subgroup of poor prognosis women (age ≥ 35, poor ovulation induction, previous failed cycles, or where protocol refers to poor prognosis women) and new subgroups of fresh and frozen embryo transfer cycles were added.
No new subgroups were added in the 2012 update
2. For the 2012 update the review was reformatted in line with current recommended Cochrane methods. Current Cochrane methodological standards require one identification name per paper. Papers that had one method but separated results into subgroups had these results pooled for the overall individual outcomes (i.e. live birth, clinical pregnancy, etc.), however the subgroups results were separated accordingly in the subgroup analysis (i.e. fresh versus frozen, first versus repeat attempt, etc.).
Contributions of authors
Mourad Seif contributed to conceiving the review, designing the review, publishing the protocol, co‐ordinating the review, data collection for the review, developing a search strategy, undertaking searches, screening search results, organising retrieval of papers, screening retrieved papers against inclusion criteria, arbitration on quality and data extraction of papers, interpretation of data, providing a methodological perspective, providing a clinical perspective, providing a policy perspective, editing the review, providing general advice on the review, and performing previous work that was the foundation of the review.
Cindy Farquhar updated the review in July 2005 by completing the new searches, selecting the studies, restructuring the table of comparisons, extracting the data, and rewriting parts of the review. She assisted with the updating of the review in 2007 by identifying the studies and assisting with data extraction and writing.
Debbie Blake was involved in the 2005 update by cross‐checking newly extracted data, assessing inclusion criteria, subgroup analysis, editing, and providing a scientific perspective to the updated text.
Sangeeta Das was involved in the 2007 update by completing the new searches, retrieval of papers, screening retrieved papers against inclusion criteria, extracting data, and rewriting the review.
Sarah‐Kate Carney was involved in the 2012 update by completing the new searches, retrieval of papers, screening retrieved papers against inclusion criteria, extracting data, and rewriting the review.
Linsey Nelson was involved in the 2012 updated by completing the new searches, retrieval of papers, screening retrieved papers against inclusion criteria, extracting data, and editing the review.
Andy Vail assisted the authors by performing the meta‐regression for maternal age and pregnancy outcomes. Catherine Fullwood provided a statistical overview.
Sources of support
Internal sources
Central Manchester and Manchester Children's University Trust, UK.
University of Manchester, UK.
University of Auckland, New Zealand.
External sources
Ministry of Health, New Zealand.
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Antinori 1999 {published data only}
- Antinori S, Versaci C, Dani L, Barbaro E, Antinori M, Cerusico C, et al. Laser assisted hatching at the extremes of the IVF spectrum: first cycle and after 6 cycles. A randomized prospective trial [abstract]. Fertility and Sterility 1999;72(3 Suppl 1):S111. [Google Scholar]
Balaban 2006 {published data only}
- Balaban B, Urman B, Yakin K, Isiklar A. Laser assisted hatching increases pregnancy and implantation rates in cryopreserved embryos that were allowed to cleave in‐vitro after thawing: a prospective randomised study. Human Reproduction; 2006;21(8):2136‐40. [DOI] [PubMed] [Google Scholar]
Balakier 2009 {published data only}
- Balakier H, Mandel R, Sojecki A, Motamedi G, Zaver S, Librach C. Laser zona thinning in women aged < or = 37 years: a randomized study. Fertility and Sterility 2009;91(4 Suppl):1479‐82. [PUBMED: 18793768] [DOI] [PubMed] [Google Scholar]
Baruffi 2000 {published data only}
- Baruffi RL, Mauri AL, Petersen CG, Ferreira RC, Coelho J, Franco JG. Zona thinning with noncontact diode laser in patients aged < or = 37 years with no previous failure of implantation: a prospective randomized study. Journal of Assisted Reproduction and Genetics 2000;17(10):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carter 2003 {published and unpublished data}
- Carter J, Graham J, Han T, Davis A, Richter K, Widra E. Preliminary results of a prospective randomized study to assess the value of laser assisted hatching before cleavage stage embryo transfer among good‐prognosis in vitro fertilization (IVF) patients. Fertility and Sterility 2003;80 Suppl 3:S94. [Google Scholar]
Ciray 2005 {published data only}
- Ciray HN, Bener F, Karagenc L, Ulug U, Bahceci M. Impact of assisted hatching on ART outcome in women with endometriosis. Human Reproduction 2005;20(9):2546‐9. [DOI] [PubMed] [Google Scholar]
Cohen 1992 {published data only}
- Cohen J, Alikani M, Trowbridge J, Rosenwaks Z. Implantation enhancement by selective assisted hatching using zona drilling of human embryos with poor prognosis. Human Reproduction 1992;7(5):685‐91. [DOI] [PubMed] [Google Scholar]
- Liu H‐C, Alikani M, Cohen J, Rosenwaks Z. Assisted hatching facilitates earlier implantation after IVF‐ET. Fertility and Sterility Abstracts. 1992; Vol. 58:61 (O‐136). [PubMed]
- Liu H‐C, Cohen J, Alikani M, Noyes N, Rosenwaks Z. Assisted hatching facilitates earlier implantation. Fertility and Sterility 1993;60(5):871‐5. [PubMed] [Google Scholar]
Elhelw 2005 {published data only}
- Elhelw B, Sadek MM, Al Nomrosy KM. Laser assisted hatching may enhance implantation and pregnancy rates on cryopreserved‐thawed embryos in patients with repeated implantation failures. A prospective randomised study. ESHRE Copenhagen ‐ poster abstract. 2005.
Fang 2010 {published data only}
- Fang C, Li T, Miao BY, Zhuang GL, Zhou C. Mechanically expanding the zona pellucida of human frozen thawed embryos: a new method of assisted hatching. Fertility and Sterility 2010;94(4):1302‐7. [PUBMED: 19782973] [DOI] [PubMed] [Google Scholar]
Ge 2008 {published data only}
- Ge HS, Zhou W, Zhang W, Lin JJ. Impact of assisted hatching on fresh and frozen‐thawed embryo transfer cycles: a prospective, randomized study. Reproductive Biomedicine Online 2008;16(4):589‐96. [PUBMED: 18413070] [DOI] [PubMed] [Google Scholar]
Germond 2004 {published data only}
- Primi M‐P, Senn A, Montag M, Ven H, Mandelbaum J, Veiga A, et al. A European multicentre prospective randomized study to assess the use of assisted hatching with a diode laser and the benefit of immunosuppressive/antibiotic treatment in different patient populations. Human Reproduction 2004;19(10):2325‐33. [DOI] [PubMed] [Google Scholar]
Hagemann 2010 {published data only}
- Hagemann AR, Lanzendorf SE, Jungheim ES, Chang AS, Ratts VS, Odem RR. A prospective, randomized, double‐blinded study of assisted hatching in women younger than 38 years undergoing in vitro fertilization. Fertility and Sterility 2010;93(2):586‐91. [PUBMED: 19268926] [DOI] [PubMed] [Google Scholar]
Hellebaut 1996 {published and unpublished data}
- Hellebaut S, Sutter P, Dozortsev D, Onghena A, Qian C, Dhont M. Does assisted hatching improve implantation rates after in vitro fertilization or intracytoplasmic sperm injection in all patients? A prospective randomized study. Journal of Assisted Reproduction and Genetics 1996;13(1):19‐22. [DOI] [PubMed] [Google Scholar]
Hurst 1998 {published and unpublished data}
- Hurst BS, Tucker KE, Awoniyi CA, Schlaff WD. Assisted hatching does not enhance IVF success in good‐prognosis patients. Journal of Assisted Reproduction and Genetics 1998;15(2):62‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Isik 2000 {published data only}
- Isik AZ, Vicdan K, Kaba A, Dagli G. Comparison of zona manipulated and zona intact blastocyst transfers: a prospective randomized trial. Journal of Assisted Reproduction and Genetics 2000;17(3):135‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Isiklar 1999 {published data only}
- Isiklar A, Balaban B, Aksoy S, Alatas C, Mercan R, Nuhoglu A, et al. The effect of mechanical assisted hatching on progression of cleavage stage embryos to the blastocyst stage [abstract]. Fertility and Sterility 1999;72(3 Suppl 1):S162. [Google Scholar]
Jelinkova 2002 {published data only}
- Jelinkova L, Pavelkova J, Reeka N, Paulus W, Zivny J, Sterzik K. Chemical removal of the zona pellucida improves implantation. Human Reproduction 2002;17:131. [DOI] [PubMed] [Google Scholar]
- Jelinkova L, Pavelkova J, Strehler E, Paulus W, Zivny J, Sterzik K. Improved implantation rate after chemical removal of the zona pellucida. Fertility and Sterility 2003;79 Suppl 6:1299‐303. [DOI] [PubMed] [Google Scholar]
Kutlu 2010 {published data only}
- Pelin Kutlu, Ozhan Atwar, Omer Faruk Vanlioglu. Laser assisted zona thinning technique has no beneficial effect on the ART outcomes of two different maternal age groups. Journal of Assisted Reproduction and Genetics 2010;27(8):457‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laffoon 1999 {published data only}
- Laffoon IS, Sokoloski JE, Volk EA, Hughes L, Krivinko DM, Sanfilippo JS, et al. The effect of assisted hatching on the outcome of assisted reproductive technology cycles in women under 39 years of age [abstract]. Fertility and Sterility 1999;72(3 Suppl 1):S243. [Google Scholar]
Lanzendorf 1998 {published and unpublished data}
- Lanzendorf SE, Nehchiri F, Mayer JF, Oehninger S, Muasher SJ. A prospective, randomized, double‐blind study for the evaluation of assisted hatching in patients with advanced maternal age. Human Reproduction 1998;13(2):409‐13. [DOI] [PubMed] [Google Scholar]
Nagy 1999 {published data only}
- Nagy ZP, Rienzi L, Iacobelli M, Morgia F, Ubaldi F, Schimberni M, et al. Laser‐assisted hatching and removal of degenerated blastomere(s) of frozen‐thawed embryos improves pregnancy rate [abstract]. Fertility and Sterility 1999;72(3 Suppl 1):S4. [Google Scholar]
Ng 2005 {published data only}
- Ng EHY, Naveed F, Lau EYL, Yeung WSB, Chan CCWC, Tang OS, et al. A randomized double‐blind controlled study of the efficacy of laser‐assisted hatching on implantation and pregnancy rates of frozen‐thawed embryo transfer at the cleavage stage. Human Reproduction 2005;20:979‐85. [DOI] [PubMed] [Google Scholar]
Petersen 2005 {published data only}
- Petersen CG, Mauri AL, Baruffi RL, Oliveira JBA, Massaro FC, Elder K, et al. Implantation failures: success of assisted hatching with quarter‐laser zona thinning. Reproductive Biomedicine Online 2005;10 Suppl 2:224‐9. [DOI] [PubMed] [Google Scholar]
Rufas‐Sapir 2004 {published data only}
- Rufas‐Sapir O, Stein A, Orvieto R, Avrech OM, Kotler N, Pinkas H, et al. Is assisted hatching beneficial in patients with recurrent implantation failures. Clinical & Experimental Obstetrics & Gynecology 2004;31 Suppl 2:110‐2. [PubMed] [Google Scholar]
Ryan 1997 {published data only}
- Ryan JP, Pike IL, Catt JW, Porter RN, Saunders DM. Failure of assisted hatching to increase pregnancy rates following the transfer of fresh or frozen‐thawed day 2 human embryos. Human Reproduction Abstracts of 13th Annual Meeting of the ESHRE. 1997:188.
Sagoskin 2007 {published data only}
- Sagoskin AW, Levy MJ, Tucker MJ, Richter KS, Widra EA. Laser assisted hatching in good prognosis patients undergoing in vitro fertilisation embryo transfer: a randomised control trial. Fertility and Sterility 2007;87(2):283‐7. [DOI] [PubMed] [Google Scholar]
Stein 1995 {published data only}
- Stein A, Rufas O, Amit S, Avrech O, Pinkas H, Ovadia J, et al. Assisted hatching by partial zona dissection of human pre‐embryos in patients with recurrent implantation failure after in vitro fertilization. Fertility and Sterility 1995;63(4):838‐41. [DOI] [PubMed] [Google Scholar]
Tucker 1993 {published data only}
- Tucker MJ, Luecke NM, Wiker SR, Wright G. Chemical removal of the outside of the zona pellucida of day 3 human embryos has no impact on implantation rate. Journal of Assisted Reproduction and Genetics 1993;10(3):187‐91. [DOI] [PubMed] [Google Scholar]
Tucker 1996 {published data only}
- Tucker MJ, Morton PC, Wright G, Ingargiola PE, Sweitzer CL, Elsner CW, et al. Enhancement of outcome from intracytoplasmic sperm injection: does co‐culture or assisted hatching improve implantation rates?. Human Reproduction 1996;11(11):2434‐7. [DOI] [PubMed] [Google Scholar]
Utsunomiya 1998 {published data only}
- Utsunomiya T, Sato M, Hirotsuru K. Assisted hatching by zona thinning to multiple‐failure in vitro fertilization patients [abstract]. Fertility and Sterility 1998;70(3 Suppl 1):S328. [Google Scholar]
Valojerdi 2010 {published data only}
- Valojerdi MR, Eftekhari‐Yazdi P, Karimian L, Hassani F, Movaghar B. Effect of laser zona thinning on vitrified‐warmed embryo transfer at the cleavage stage: a prospective, randomized study. Reproductive Biomedicine Online 2010;20(2):234‐42. [PUBMED: 20113961] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdelmassih 2002 {published data only}
- Abdelmassih S, Cardoso J, Abdelmassih V, Dias JA, Abdelmassih R, Nagy ZP. Laser‐assisted ICSI: a novel approach to obtain higher oocyte survival and embryo quality rates. Human Reproduction 2002;17 Suppl 10:2694‐7. [DOI] [PubMed] [Google Scholar]
Antinori 1996a {published data only}
- Antinori S, Selman HA, Caffa B, Panci C, Dani G, Versaci C. Zona opening of human embryos using a non‐contact UV laser for assisted hatching in patients with poor prognosis of pregnancy. Human Reproduction 1996;11:2488‐92. [DOI] [PubMed] [Google Scholar]
Antinori 1996b {published data only}
- Antinori S, Panci C, Selman HA, Caffa B, Dani G, Versaci C. Zona thinning with the use of laser: a new approach to assisted hatching in humans. Human Reproduction 1996;11:590‐4. [DOI] [PubMed] [Google Scholar]
Balaban 2002 {published data only}
- Balaban B, Urman B, Alatas C, Mercan R, Mumcu A, Isiklar A. A comparison of four different techniques of assisted hatching. Human Reproduction 2002;17:1239‐43. [DOI] [PubMed] [Google Scholar]
Bider 1997 {published data only}
- Bider D, Livshits A, Yonish M, Yemini Z, Mashiach S, Dor J. Assisted hatching by zona drilling of human embryos in women of advanced age. Human Reproduction 1997;12:317‐20. [DOI] [PubMed] [Google Scholar]
Blake 2001 {published data only}
- Blake CA, Forsberg AS, Johansson BR, Wikland M. Laser zona pellucida thinning ‐ an alternative approach to assisted hatching. Human Reproduction 2001;16(9):1959‐64. [DOI] [PubMed] [Google Scholar]
Carter 2003a {published data only}
- Carter J, Graham J, Han T, Davis A, Richter K, Widra E. Preliminary results of a prospective randomized study to assess the value of laser assisted hatching before cleavage stage embryo transfer among good‐prognosis In Vitro Fertilization (IVF) patients. Fertility and Sterility 2003;80 Suppl 3:S94. [Google Scholar]
Chao 1997 {published data only}
- Chao KH, Chen SU, Chen HF, Wu MY, Yang YS, Ho HN. Assisted hatching increases the implantation and pregnancy rate of in vitro fertilization (IVF)‐embryo transfer (ET), but not that of IVF‐tubal ET in patients with repeated IVF failures. Fertility and Sterility 1997;67:904‐8. [DOI] [PubMed] [Google Scholar]
Check 1996 {published data only}
- Check JH, Hoover L, Nazari A, O'Shaughnessy A, Summers D. The effect of assisted hatching on pregnancy rates after frozen embryo transfer. Fertility and Sterility 1996;65:254‐7. [DOI] [PubMed] [Google Scholar]
Chen 1999 {published data only}
- Chen C, Kattera S, Lim MN. Improved pregnancy rates in assisted reproduction using assisted hatching and delayed embryo transfer. Proceedings of the 11th World Congress on In Vitro Fertilization and Human Reproductive Genetics, Bologna, Italy. Monduzzi, 1999:169‐72.
Cieslak 1999 {published data only}
- Cieslak J, Ivakhnenko V, Wolf G, Sheleg S, Verlinsky Y. Three‐dimensional partial zona dissection for preimplantation genetic diagnosis and assisted hatching. Fertility and Sterility 1999;71(2):308‐13. [DOI] [PubMed] [Google Scholar]
Cohen 1990 {published data only}
- Cohen J, Elsner C, Kort H, Malter H, Massey J, Mayer MP, et al. Impairment of the hatching process following IVF in the human and improvement of implantation by assisting hatching using micromanipulation. Human Reproduction 1990;5(1):7‐13. [DOI] [PubMed] [Google Scholar]
Debrock 2011 {published data only}
- Debrock D, Peeraer K, Spiessens C, Willeman D, Loecker P, D'Hooghe TM. The effect of modified quarter laser‐assisted zona hardening on the implantation rate per embryo in frozen/vitrified‐thawed/warmed embryo transfer cycles: a prospective randomized controlled trial. Human Reproduction June 2011;26:1997‐2007. [DOI] [PubMed] [Google Scholar]
Demirol 2003 {published data only}
- Demirol A, Sari T, Gurgan T. Comparison of the laser‐assisted ICSI and conventional ICSI results in recurrent ICSI failure patients with few oocytes. Human Reproduction 2003;18 Suppl 1:61. [Google Scholar]
Dirnfeld 2003 {published data only}
- Dirnfeld M, Shiloh H, Bider D, Harari E, Koifman M, Lahav Baratz S, et al. A prospective randomized controlled study of the effect of short coincubation of gametes during insemination on zona pellucida thickness. Gynecological Endocrinology 2003;17:397‐403. [DOI] [PubMed] [Google Scholar]
Dokras 1994 {published data only}
- Dokras A, Ross C, Gosden B, Sargent IL, Barlow DH. Micromanipulation of human embryos to assist hatching. Fertility and Sterility 1994;61:514‐20. [DOI] [PubMed] [Google Scholar]
Domitrz 2000 {published data only}
- Domitrz J, Wolczynski S, Syrewicz M, Kuczynski W, Szamatowicz J, Grochowski D, et al. Enzymatic assisted hatching in the infertile couple after failed attempts IVF ET. Ginekologia Polska 2000;71:1047‐52. [PubMed] [Google Scholar]
Ebner 2002 {published data only}
- Ebner T, Moser M, Yaman C, Sommergruber M, Hartl J, Jesacher K, et al. Prospective hatching of embryos developed from oocytes exhibiting difficult oolemma penetration during ICSI. Human Reproduction 2002;17:1317‐20. [DOI] [PubMed] [Google Scholar]
Edirisinghe 1999 {published data only}
- Edirisinghe WR, Ahnonkitpanit V, Promviengchai S, Suwajanakorn S, Pruksananonda K, Chinpilas V, et al. A study failing to determine significant benefits from assisted hatching: patients selected for advanced age, zonal thickness of embryos, and previous failed attempts. Journal of Assisted Reproduction and Genetics 1999;16:294‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feng 2009 {published data only}
- Feng HL, Hershlag A, Scholl GM, Cohen MA. A retroprospective study comparing three different assisted hatching techniques. Fertility and sterility 2009;91(4 Suppl):1323‐5. [PUBMED: 18394610] [DOI] [PubMed] [Google Scholar]
Figueira 2012 {published data only}
- Figueira R, Paes D, Setti A, Iaconelli A, Borges E. Relevance of assisted hatching in an oocyte donation programme using egg cryobanking: a prospective randomised study. European Journal of Obstetrics, Gynecology, and Reproductive Biology May 2012;164:48‐51. [DOI] [PubMed] [Google Scholar]
Frydman 2006 {published data only}
- Frydman N, Madoux S, Hesters L, Duvernoy C, Feyereisen E, Du A, et al. A randomised double‐blind controlled study on the efficacy of laser zona pellucida thinning on live birth rates in cases of advanced female age. Human Reproduction 2006;21(8):2131‐5. [DOI] [PubMed] [Google Scholar]
Gabrielsen 2004 {published data only}
- Gabrielsen A, Agerholm I, Toft B, Hald F, Petersen K, Aagaard J, et al. Assisted hatching improves implantation rates on cryopreserved‐thawed embryos: a randomized prospective study. Human Reproduction 2004;19:2258‐62. [DOI] [PubMed] [Google Scholar]
Grace 2007 {published data only}
- Grace J, Bolton V, Braude P, Khalaf Y. Assisted hatching is more effective when embryo quality was optimal in previous failed IVF/ICSI cycles. Journal of Obstetrics and Gynaecology 2007;27(1):56‐60. [PUBMED: 17365461] [DOI] [PubMed] [Google Scholar]
Hershlag 1999 {published data only}
- Hershlag A, Paine T, Cooper GW, Scholl GM, Rawlinson K, Kvapil G. Monozygotic twinning associated with mechanical assisted hatching. Fertility and Sterility 1999;71(1):144‐6. [DOI] [PubMed] [Google Scholar]
Hiraoka 2009 {published data only}
- Hiraoka K, Hiraoka K, Horiuchi T, Kusuda T, Okano S, Kinutani M, et al. Impact of the size of zona pellucida thinning area on vitrified‐warmed cleavage‐stage embryo transfers: a prospective, randomized study. Journal of Assisted Reproduction and Genetics 2009;26(9‐10):515‐21. [PUBMED: 19830543] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hur 2011 {published data only}
- Hur YS, Park JH, Ryu EY, Yoon HJ, Yoon SH, Hur CY, et al. Effect of artificial shrinkage on outcome in fresh blastocyst transfer cycles. Clinical and Experimental Reproductive Medicine 2011;38(2):87‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huttelova 1999 {published data only}
- Huttelova R, Becvarova V, Mardesic T, Muller P, Hulvert J, Voboril J. Assisted hatching combined with long‐term culture. Proceedings of the 11th World Congress on In Vitro Fertilization & Human Reproductive Genetics, Bologna, Italy. Monduzzi, 1999:169‐72.
Komarovsky 2002 {published data only}
- Komarovsky D, Strassburger D, Raziel A, Kasterstein E, Schachter M, Friedler S, et al. A prospective randomized trial of assisted hatching in cryopreserved embryo transfer. Human Reproduction 2002;17:76. [Google Scholar]
Komarovsky 2003 {published data only}
- Komarovsky D, Bern O, Kasterstein E, Strassburger D, Raziel A, Friedler S, et al. A prospective randomized comparison between laser and chemically‐assisted hatching in thawed embryo transfers. Human Reproduction 2003;18 Suppl 1:77. [Google Scholar]
Lee 1999 {published data only}
- Lee JE, Lee DR, Paik HR, Shim HN, Cho JH, Roh SI, et al. Biochemical assisted hatching (BAH) increased the implantation and pregnancy rate in human cryopreserved embryo transfer [abstract]. Fertility and Sterility 1999;72(3 Suppl 1):S4‐S5. [Google Scholar]
Levron 2003 {published data only}
- Levron J, Ferber Meiri B, Bider D, Shulman A, Levin T, Shporn E. A prospective randomized study comparing laser and tyrode's medicated methods of assisted hatching. Fertility and Sterility 2003;80 Suppl 3:S202. [Google Scholar]
Ma 2007 {published data only}
- Ma S, Rowe T, Ho Yuen B. Impact of assisted hatching on the outcome of intracytoplasmic sperm injection: a prospective, randomized clinical trial and pregnancy follow‐up. Fertility and Sterility 2006;85(4):895‐900. [DOI] [PubMed] [Google Scholar]
Magli 1998 {published and unpublished data}
- Magli MC, Gianaroli L, Ferraretti AP, Fortini D, Aicardi G, Montanaro N. Rescue of implantation potential in embryos with poor prognosis by assisted zona hatching. Human Reproduction 1998;13(5):1331‐5. [DOI] [PubMed] [Google Scholar]
Mahadevan 1998 {published data only}
- Mahadevan MM, Miller MM, Maris MO, Moutos D. Assisted hatching of embryos by micromanipulation for human in vitro fertilization: UAMS experience. Journal of the Arkansas Medical Society 1998;94:529‐31. [PubMed] [Google Scholar]
Mansour 2000 {published data only}
- Mansour RT, Rhodes CA, Aboulghar MA, Serour GI, Kamal A. Transfer of zona‐free embryos improves outcomes in poor prognosis patients: a prospective randomised controlled study. Human Reproduction 2000;15:1061‐4. [DOI] [PubMed] [Google Scholar]
Meldrum 1998 {published data only}
- Meldrum DR, Wisot A, Yee B, Garzo G, Yeo L, Hamilton F. Assisted hatching reduces the age‐related decline in IVF outcome in women younger than age 43 without increasing miscarriage or monozygotic twinning. Journal of Assisted Reproduction and Genetics 1998;15:418‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montag 1999 {published data only}
- Montag M, Ven H. Laser‐assisted hatching in assisted reproduction. Croatian Medical Journal 1999;40:398‐403. [PubMed] [Google Scholar]
Nagy 2003 {published data only}
- Nagy ZP, Toledo A, Mitchell Leef D, Elsner C, Massey J, Kort H. A prospective randomized study to evaluate the effect of laser micro‐hole drilling of zona pellucida prior to ICSI on implantation and pregnancy results. Human Reproduction 2003;18 Suppl 1:60‐1. [Google Scholar]
Nakayama 1998 {published data only}
- Nakayama T, Fujiwara H, Tastumi K, Fujita K, Higuchi T, Mori T. A new assisted hatching technique using a piezo‐micromanipulator. Fertility and Sterility 1998;69(4):784‐8. [DOI] [PubMed] [Google Scholar]
Nakayama 1999 {published data only}
- Nakayama T, Fujiwara H, Yamada S, Tastumi K, Honda T, Fujii S. Clinical application of a new assisted hatching method using piezo‐micromanipulator for morphologically low‐quality embryos in poor‐prognosis infertile patients. Fertility and Sterility 1999;71(6):1014‐8. [DOI] [PubMed] [Google Scholar]
Ng 2008 {published data only}
- Ng EH, Lau EY, Yeung WS, Cheung TM, Tang OS, Ho PC. Randomized double‐blind comparison of laser zona pellucida thinning and breaching in frozen‐thawed embryo transfer at the cleavage stage. Fertility and Sterility 2008;89(5):1147‐53. [PUBMED: 17662284] [DOI] [PubMed] [Google Scholar]
Obruca 1994 {published data only}
- Obruca A, Strohmer H, Sakkas D, Menezo Y, Kogosowski A, Barak Y. Use of lasers in assisted fertilization and hatching. Human Reproduction 1994;9:1723‐6. [DOI] [PubMed] [Google Scholar]
Olivennes 1997 {published data only}
- Olivennes F, Hazout AD. A prospective randomized study of the use of assisted hatching in IVF‐ET patients with high day‐3 FSH. Increased clinical pregnancy rate with assisted hatching but high rate of miscarriages. Fertility and Sterility. Abstracts of the Meeting of the American Society for Reproductive Medicine. 1997:S227.
Peterson 2006 {published data only}
- Peterson C, Mauri A, Baruffi R, Oliveira J, Felipe V, Massaro F, Franco J. Laser‐assisted hatching of cryopreserved‐thawed embryos by thinning one quarter of the zona. Reproductive BioMedicine Online September 2006;13(5):668‐75. [DOI] [PubMed] [Google Scholar]
Rienzi 2002 {published data only}
- Rienzi L, Ubaldi F, Iacobelli M, Martinez F, Ferrero S, Greco E. Controlled comparison of ICSI and laser‐assisted ICSI in low responder patients. Human Reproduction 2002;17:36. [Google Scholar]
Ringler 1999 {published data only}
- Ringler GE, Marrs RP, Stein AL, Varygas JM, Schiewe MC. Improved pregnancy rates using assisted hatching on day 3 frozen‐thawed embryos [abstract]. Fertility and Sterility 1999;72(3 Suppl 1):S86. [Google Scholar]
Schoolcraft 1994 {published data only}
- Schoolcraft WB, Schlenker T, Gee M, Jones GS, Jones HW. Assisted hatching in the treatment of poor prognosis in vitro fertilization candidates. Fertility and Sterility 1994;62(3):551‐4. [DOI] [PubMed] [Google Scholar]
Shahin 2003 {published data only}
- Shahin A, Krussel JS, Sayed EH, Ahmed AG, Al Hussaini TK, Hirchenhain J. A prospective randomized study on laser assisted hatching in good prognosis patients. Human Reproduction 2003;18 Suppl 1:53. [Google Scholar]
Sifer 2005 {unpublished data only}
- Sifer C, Sellami A, Martin‐Pont B, Bottero J, Porcher R, Poncelet C, et al. A prospective randomised study to assess the benefit of zona pellucida partial digestion prior to frozen‐thawed embryo transfers. ESHRE Copenhagen ‐ oral abstract. 2005. [DOI] [PubMed]
- Sifer C, Sellami A, Poncelot C, Kulski P, Martin‐Pont B, Bottero J, et al. A prospective randomised study to assess the benefit of zona pellucida partial digestion prior to frozen‐thawed embryo transfers. In: ESHRE Copenhagen ‐ oral abstract, 2005. Human Reproduction 2006;21(9):2384‐9. [DOI] [PubMed] [Google Scholar]
Szell 1998 {published data only}
- Szell AZ, Antaran JM, Chetkowski RJ. Pregnancy and implantation rates from the transfer of human embryos cultured in P1 or human tubal fluid medium and transferred with or without assisted hatching. Fertility and Sterility 1998;70:S495. [Google Scholar]
Tao 1997 {published data only}
- Tao J, Tamis R. Application of assisted hatching for 2‐day‐old, frozen‐thawed embryo transfer in a poor prognosis population. Journal of Assisted Reproduction and Genetics 1997;14(2):128‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tucker 1991 {published data only}
- Tucker MJ, Cohen J, Massey JB, Mayer MP, Wiker SR, Wright G. Partial dissection of the zona pellucida of frozen‐thawed human embryos may enhance blastocyst hatching, implantation and pregnancy rates. American Journal of Obstetrics and Gynecology 1991;165(2):342‐5. [DOI] [PubMed] [Google Scholar]
Urman 2002 {published data only}
- Urman B, Balaban B, Alatas C, Aksoy S, Mumcu A, Isiklas A. Zona‐intact versus zona‐free blastocyst transfer: a prospective randomised study. Fertility and Sterility 2002;70(3 Suppl 1):S238. [DOI] [PubMed] [Google Scholar]
Valojerdi 2008 {published data only}
- Valojerdi MR, Eftekhari‐Yazdi P, Karimian L, Ashtiani SK. Effect on laser zona pellucida opening on clinical outcome of assisted reproduction technology in patients with advanced female age, recurrent implantation failure, or frozen‐thawed embryos. Fertility and Sterility 2008;90(1):84‐91. [DOI] [PubMed] [Google Scholar]
Yano 2007 {published data only}
- Yano K, Yano C, Kubo T, Ohashi I, Maeda N, Fukaya T. Chemical zona pellucida thinning with acidified Tyrode's solution: comparison between partial and circumferential techniques. Journal of Assisted Reproduction and Genetics 2007;24(10):471‐5. [PUBMED: 17701000] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zech 1998 {published data only}
- Zech H, Stecher A, Vanderzwalmen P, Murach KF. Investigation of the usefulness of laser‐assisted hatching for women of ages above and below forty years [abstract]. Fertility and Sterility 1998;70(3 Suppl 1):S428. [Google Scholar]
Zhang 2009 {published data only}
- Zhang XJ, Yang YZ, Lv Q, Min LH, Li XL, Bai P. Effect of the size of zona pellucida thinning by laser assisted hatching on clinical outcome of human frozen‐thawed embryo transfers. Cryo Letters 2009;30(6):455‐61. [PUBMED: 20309502] [PubMed] [Google Scholar]
Additional references
Al‐Nuaim 2002
- Al‐Nuaim LA, Jenkins JM. Assisted hatching in assisted reproduction. British Journal of Obstetrics and Gynaecology August 2002;109:856‐62. [DOI] [PubMed] [Google Scholar]
Bleil 1980
- Bleil JD, Wasserman PM. Structure and function of the zona pellucida: identification and characterisation of the proteins of the mouse oocyte zona pellucida. Developmental Biology 1980;76:185‐202. [DOI] [PubMed] [Google Scholar]
Bronson 1970
- Bronson RA, McLaren A. Transfer to mouse oviduct of eggs with and without the zona pellucida. Journal of Reproductive Fertility 1970;22:129‐36. [DOI] [PubMed] [Google Scholar]
Check 1999
- Check JH, Choe JK, Katsoff D, Summers‐Chase D, Wilson C. Controlled ovarian hyperstimulation adversely affects implantation following in vitro fertilization‐embryo transfer. Journal of Assisted Reproduction and Genetics 1999;16(8):416‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1991
- Cohen J. Assisted hatching of human embryos. Journal of In Vitro Fertilization and Embryo Transfer 1991;8(4):179‐90. [DOI] [PubMed] [Google Scholar]
Cole 1967
- Cole RJ. Cinematographic observation on the trophoblast and zona pellucida of the mouse blastocyst. Journal of Embryology and Experimental Morphology 1967;17:481‐90. [PubMed] [Google Scholar]
da Costa 2001
- Costa ALE, Abdelmassih S, Oliveira FG, Abdelmassih V, Abdelmassih R, Nagy ZP, et al. Monozygotic twins and transfer at the blastocyst stage after ICSI. Human Reproduction 2001;16(2):333‐6. [DOI] [PubMed] [Google Scholar]
Denker 1993
- Denker HW. Implantation: a cell biological paradox. Journal of Experimental Zoology 1993;266:541‐58. [DOI] [PubMed] [Google Scholar]
Fehilly 1985
- Fehilly CB, Cohen J, Simons RF, Fishel SB, Edwards RG. Cryopreservation of cleaving embryos and expanded blastocysts in the human: a comparative study. Fertility and Sterility 1985;44:638‐44. [DOI] [PubMed] [Google Scholar]
Gardner 2000
- Gardner DK, Lane M, Schoolcraft WB. Culture and transfer of viable blastocysts: a feasible proposition for human IVF. Human Reproduction 2000;15 Suppl 6:9‐23. [PubMed] [Google Scholar]
Harlow 1982
- Harlow GM, Quinn P. Development of pre‐implantation mouse embryos in vitro and in vivo. Australian Journal of Biology and Science 1982;35:187‐93. [DOI] [PubMed] [Google Scholar]
HFEA 2000
- Human Fertilisation, Embryology Authority (UK). Patient's Guide to IVF Clinics 2000. London: The HFEA, 2000. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Hsu 1999
- Hsu MI, Mayer J, Aronshon M, Lazendorf S, Muasher S, Kolm P, et al. Embryo implantation in in vitro fertilization and intracytoplasmic sperm injection: impact of cleavage status, morphology grade, and number of embryos transferred. Fertility and Sterility 1999;72(4):679‐85. [DOI] [PubMed] [Google Scholar]
Lopata 1996
- Lopata A. Implantation of the human embryo. Human Reproduction 1996;11 Suppl 1:175‐84. [DOI] [PubMed] [Google Scholar]
Loret de Mola 1997
- Loret De Mola JR, Garside WT, Bucci J, Tureck RW, Heyner S. Analysis of the human zona pellucida during culture: correlation with diagnosis and the preovulatory hormonal environment. Journal of Assisted Reproduction and Genetics 1997;14:332‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menezo 2003
- Menezo Y, Cassuto G, Chavrier M. Culture conditions and not prolonged culture time are responsible for monozygotic twinning in human in vitro fertilization. Fertility and Sterility 2003;80(2):462‐3. [DOI] [PubMed] [Google Scholar]
Mercader 2001
- Mercader A, Simon C, Galan A, Herrer R, Albert C, Remohi J, et al. An analysis of spontaneous hatching in a human endometrial epithelial coculture system: is assisted hatching justified?. Journal of Assisted Reproduction and Genetics 2001;18(6):315‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagan: Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rink 1995
- Rink K, Descloux L, Delacretaz G, Senn A, Nocera D, Germond M. Zona pellucida drilling by a 1.48um laser: influence on the biomechanics of the hatching process [abstract]. SPIE ‐ The International Society for Optical Engineering Proceedings, Barcelona. 1995:2624.
Schieve 2000
- Schieve LA, Meikle SF, Peterson HB, Jen G, Burnett NM, Wilcox LS. Does assisted hatching pose a risk for monozygotic twinning in pregnancies conceived through in vitro fertilization. Fertility and Sterility 2000;74:288‐94. [DOI] [PubMed] [Google Scholar]
Sengoku 2000
- Sengoku K. Present state and future in reproductive medicine. Hokkaido Igaku Zasshi 2000;75(4):237‐42. [PubMed] [Google Scholar]
WHO 1975
- World Health Organization. The epidemiology of infertility. Vol. 582, World Health Organization Technical Report Service, 1975:1‐37. [PubMed] [Google Scholar]
References to other published versions of this review
Edi‐Osagie 2003
- Edi Osagie E, Hooper L, Seif MW. The impact of assisted hatching on live birth rates and outcomes of assisted conception: a systematic review. Human Reproduction 2003;18(9):1828‐35. [DOI] [PubMed] [Google Scholar]


