Abstract
Objectives
The purpose of the present study was to systematically review literature on the effectiveness of surgical regenerative treatment for peri-implantitis.
Methods
Different databases were searched including the Cochrane Central Register of Controlled Trials, EMBASE and MEDLINE. Primary outcomes were changes in probing pocket depth (PPD), bleeding on probing (BOP), radiographic marginal bone level (RBL) and signs of infection. Secondary outcomes were facial marginal recession, aesthetic outcomes and cost of treatment. Only randomised controlled trials (RCTs) with a minimum of 12 months follow-up period after regenerative surgical treatment were selected according to PRISMA guidelines.
Main results
Five studies were selected. The highest mean reduction of PPD was 3.1 mm in a bovine-derived xenograft (BDX) group. The highest percentage reduction of BOP occurred in patients treated with implantoplasty and saline (a reduction of 85.2%). The highest mean defect fill of RBL was reported in the porous titanium granules group (3.6 mm). Mean reductions of PPD, RBL and facial marginal soft tissue recession were statistically insignificant (p-value > 0.05) in the studies included. However, the mean reduction in BOP was statistically significant (p-value < 0.05) in four studies as compared to the baseline (before treatment). A high heterogeneity among the studies included, regarding surgical protocols, defects morphology and selection of biomaterials, was found.
Conclusion
All studies included showed an improvement in clinical conditions after surgical regenerative treatment for peri-implantitis. However, no study has shown any statistical significance in its approach. There is a lack of scientific evidence in literature regarding which type of bone substitute has superiority in the treatment of peri-implantitis, as well as the role of barrier membranes, methods for detoxification of implant surfaces and antimicrobial prescriptions. For these reasons further well-designed RCTs are recommended.
Keywords: Peri-implantitis, Peri-implant diseases, Alveolar bone grafting, Alveolar bone loss, Bone regeneration
1. Introduction
Dental implants are increasingly becoming a viable and effective treatment for replacing missing teeth. Whilst the procedure has a high success rate, there is the potential for complications. Dental implants may fail due to local inflammation, such as peri-implant mucositis and peri-implantitis. If these conditions are left untreated, the recipient could end up losing the implant. As a direct result of the increasing prevalence of dental implants, a growing number of related complications are being discovered. Research indicates that one in four patients who receive dental implants are likely to suffer from a peri-implant disease at some point in their life (Wang et al., 2017). A peri-implant disease is defined as ‘an inflammatory process affecting the soft and/or hard tissues surrounding an implant in function’ (Chrcanovic et al., 2017, Romeo et al., 2005, Roos-Jansaker et al., 2003). Peri-implant diseases are often compared to periodontal diseases, and many studies have reported that the oral tissue that surrounds a dental implant has similar features to that which surround the natural teeth. These similarities might explain, for instance, how peri-implant mucositis can advance to peri-implantitis, in a similar way to the progression of gingivitis to periodontitis, which occurs around natural teeth (Renvert et al., 2013, Triplett et al., 2003, el Chaar and Jalbout, 2002). However, there are a number of key differences in the causes and treatment of each condition (Suarez et al., 2013, Kozlovsky et al., 2007). Peri-implant diseases are divided into two groups: peri-implant mucositis and peri-implantitis. Peri-implant mucositis is defined as ‘an inflammation of the peri-implant mucosa, but is not associated with bone resorption’ (Mombelli and Lang, 1998). The advancement of peri-implant mucositis is termed peri-implantitis, which is defined as ‘a peri-implant soft tissue inflammation that is associated with bone resorption with or without an increase in probing pocket depth (PPD) and suppuration’ (Thakkar et al., 2017, Ramanauskaite et al., 2016b, Lang and Berglundh, 2011).
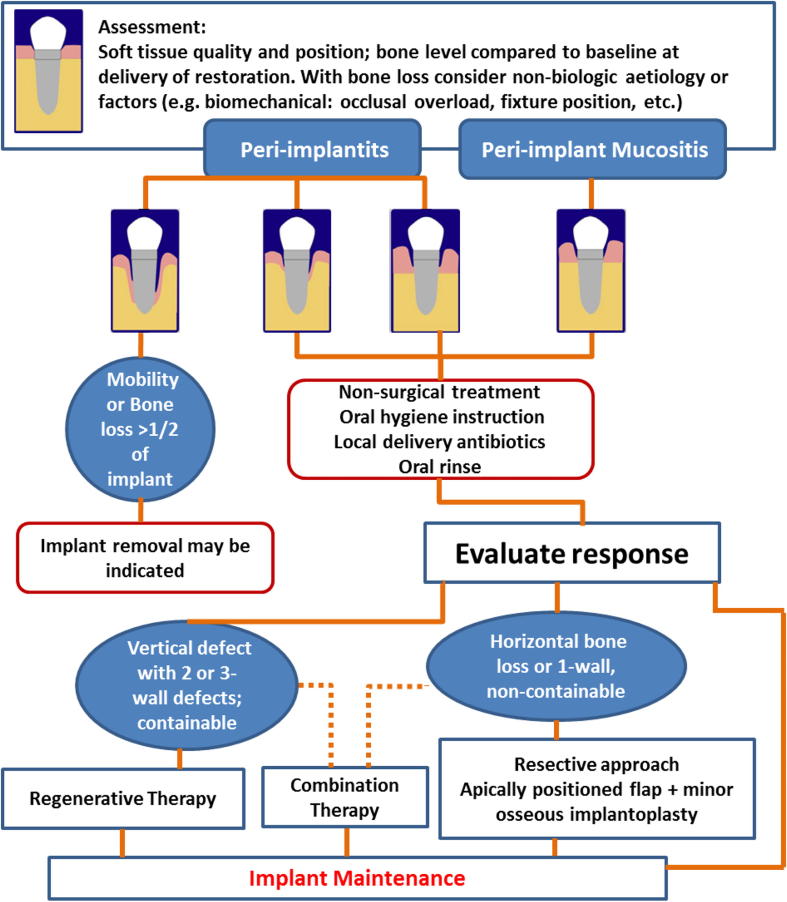
One of the treatment modalities for peri-implantitis is a regenerative technique that involves flap elevation, mechanical root debridement and the placement of bone graft material with or without a membrane (Ramanauskaite et al., 2016a, Nguyen-Hieu et al., 2012). Schwarz et al. (2006) studied the treatment of moderate peri-implantitis in 22 patients using either a nanocrystalline hydroxyapatite (NHA) or a natural bone mineral in combination with a collagen membrane. The researchers monitored the patients for two years following treatment and found that there was significant clinical progress in terms of reducing the peri-implant pocket depth and enhancing the clinical attachment level. Interestingly, however, the authors did not refer to the nature of the defects in bone quality in the study. This, of course, may have impacted on the effectiveness of the treatment. Mishler and Shiau (2014) developed an algorithm to help clinicians make decisions regarding the treatment of peri-implant conditions (Fig. 1). The algorithm supposes a standard-length diameter of 11.5 mm for a dental implant. Patients receiving shorter implants should be warned of the early risk of implant removal when the bone around the implant is resorbed. The authors suggested conservative treatment for peri-implant mucositis and mild peri-implantitis. While in moderate to advanced peri-implantitis, if the infrabony defect is vertical with two or three-wall defects that contain bone grafting material, then regenerative therapy is indicated. If, however, the defect is a horizontal or one-wall, and cannot contain bone grafting material, then non-regenerative approaches, such as an apically positioned flap or implantoplasty, are recommended.
Fig. 1.
A summary of the management for peri-implant diseases (Mishler and Shiau, 2014).
Various techniques have been advocated in literature for the surgical regenerative treatment of peri-implantitis, including the use of bone substitutes, methods for detoxification of implant surfaces, antimicrobial prescriptions and whether to use submerged or non-submerged techniques. This raises an important question: which approach is the best among the different regenerative techniques? In this review, an attempt has been made to answer this question using a structured search strategy and through the selection of the best available evidence, which was randomised clinical trials only.
2. Methods
2.1. Search strategy
Searches were completed in accordance with PRISMA guidelines and based on the concept of PICO, which is an abbreviation for Population (patients with peri-implantitis and classified according to the American Society of Anesthesiologists or ASA physical Status I or II), Intervention (surgical regenerative treatment of peri-implantitis), Comparison (different surgical approaches) and Outcome (primary and secondary outcomes) (Moher et al., 2009). The research questions were what were the overall outcomes of the surgical regenerative management of peri-implantitis, and which approach had better outcomes. Different databases were used to conduct a thorough search of the literature and to answer the focus questions. These databases were the Cochrane Central Register of Controlled Trials (CENTRAL), via the Cochrane Library, Ovid MEDLINE, EMBASE via OVID and PubMed. The final search was conducted on 13 July 2019. The following key words were combined: Peri-implantitis, OR Peri-implant Disease*, OR Peri-implant? Disease, OR Implantoplasy, OR Peri-implantitis Therapy AND Bone Graft, OR Bone Grafting, OR Osseous Defects, OR Collagen Membrane, OR Surgical Regenerative Therapy, OR Bone Substitute, OR Bone Regeneration, OR Chlorhexidine, OR Diode Laser OR Resective Surgery. Further attempts were made to find studies related to this systematic review. This was achieved through a comprehensive analysis of the reference lists of the studies found through the initial research process.
2.2. Study selection criteria
In this review only randomised controlled trials (RCTs) were included. Each RCT had to show a minimum follow-up period of 12 months after the surgical treatment of peri-implantitis.
2.3. Primary and secondary outcomes
The primary outcomes for this review were changes in probing pocket depth (PPD), bleeding on probing (BOP), radiographic marginal bone level (RBL) changes on dental radiographs or any signs or symptoms of infection. The secondary outcomes were facial marginal soft tissue recession, aesthetic outcomes evaluated by the patient or dentist and cost of the treatment.
2.4. Data extraction and management
After obtaining the full text of each of the included studies, data were extracted by three independent authors and any discrepancies were resolved by discussion. Inter-rater reliability was measured by Cohen's kappa coefficient and it was k = 0.8. Data collection and management followed the main principles of the checklist outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins and Green, 2011).
3. Results
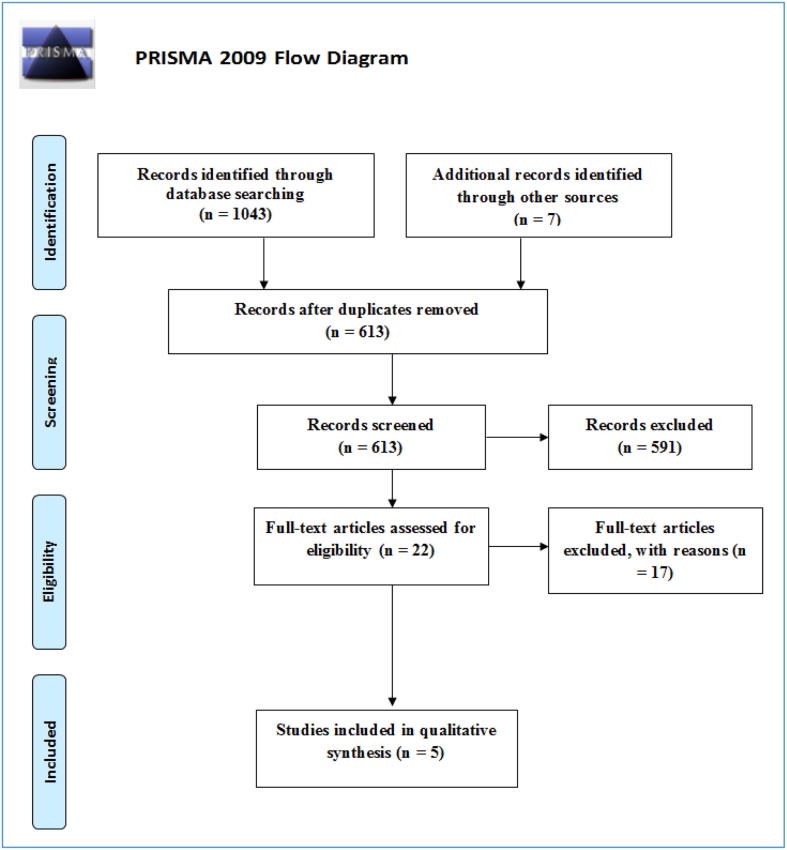
All of the collected studies were added into EndNote 8X software for research and reference management. The total number of included studies was 1050. Following the removal of duplicate studies, the total was 613. At the next stage, 591 studies were excluded as these were determined to be irrelevant to the research topic after viewing the title and abstract of each publication. Full reports on the remaining 22 studies were obtained for evaluation against the inclusion and exclusion criteria. A total of 17 studies were excluded, either because the studies were not a RCT (mainly cases series) or the follow-up period was less than 12 months. Therefore, five studies were included as part of this systematic review. Fig. 2 outlines a flow diagram of the search results according to PRISMA recommendations (Moher et al., 2009). The characteristics of the included studies are summarised in Table 1. In Table 2 a summary of the studies primary outcomes and main results are included.
Fig. 2.
The flow diagram following PRISMA recommendations (Moher et al., 2009).
Table 1.
Characteristics of studies included.
| Study ID | Aghazadeh et al. (2012) | Wohlfahrt et al. (2012) | Jepsen et al. (2016) | Schwarz et al. (2010) | Schwarz et al. (2013) |
|---|---|---|---|---|---|
| Study design | Single-blind randomised clinical trial. | Randomised, case-control, clinical trial. | Multicentre, multinational, randomised clinical trial. | Prospective, parallel-design clinical study. | Randomised controlled trial. |
| Follow-up duration | 12 months | 12 months | 12 months | 12 months | 48 months |
| Sample size (implant) | 22 patients in AB* group (34)/23 patients in BDX* group (37) | Case (PTG*): 16. Control: 17 |
Case (PTG): 33 Control group : 30 |
Class Ib group: 9; Class Ic group: 9; Class Ie group: 9. | CPS* group: 16 patients ERL* group: 16 patients |
| Age of participants (years) | AB: 70.1 (SD* = 6.2) BDX: 67 (SD = 7.5) |
Case: 65 (SD 10) Control: 57.2 (SD 12.3) |
Case: 57.7 (SD 12.6) Control: 59.1 (SD 12.2) |
Class Ib group: 48 (SD: 21); Class Ic group: 38 (SD: 12); Class Ie group: 42 (SD: 6). | Mean age 60.8 (SD: 10.9) |
| Gender | AB: 14 female, 8 male. BDX: 13 female, 10 male. |
Case: 7 male/9 female. Control: 7 male/10 female. |
Case: 16 male/17 female. Control: 11 male/19 female. |
3 male and 24 female. | 11 male and 21 female. |
| Inclusion criteria | A minimum of 1 osseointegrated implant with bone loss ≥ 2 mm; PPD ≥ 5 mm with BOP/suppuration; Selected implant should have an angular bone defect. | Age ≥ 18 years old; ASA* physical status class I or II; Ability to apply submerged implant treatment during 6 months of healing; Plaque scores < 20% preoperatively; Osseointegrated implants loaded for at least 12 months. | Osseointegrated implants were functionally loaded for at least 12 months; RBL ≥ 3 mm; PPD ≥ 5 mm; 3–4 walls defect with at least 270° (circumferential) + defect angle ≤ 35° from the implant’s axis. | Presence of at least one defect configuration classification: either class Ib, Ic, or Ie with PPD > 6 mm and an intra-bony component of >3 mm radiographically; Class II ≤ 1 mm; No implant mobility; No overhanging implant restorations; Absence of any evidence of occlusal overload; Presence of peri-implant keratinised mucosa; No systemic diseases ; Non-smoker. | Osseointegrated implant with class Ib, Ic, or Ie bone defect; PPD > 6 mm, and RBL > 3 mm; Class II ≥ 1 mm; No implant mobility; No overhanging implant restorations; Absence of any evidence of occlusal overload; Presence of ≥ 2 mm peri-implant keratinised mucosa; No systemic diseases; Non-smoker. |
| Detoxification method | Hydrogen peroxide 3% for 1 min. | 24% EDTA* | Rotary titanium brush + 3% hydrogen peroxide. | Er:YAG laser , Carbon curettes and saline. | CPS group = implantoplasty + saline. ERL group = implantoplasty + Er:YAG laser. |
| Bone substitute/membrane | Group A = AB + resorbable bovine collagen membrane. Group B = BDX + resorbable bovine collagen membrane. |
Porous Titanium Granules | Tigran titanium granules | Natural bone mineral with a collagen membrane. | Bovine-derived natural bone + native collagen membrane. |
| Submerge | Non-submerged | Submerged | Non-submerged | Non-submerged | Non-submerged |
| Antimicrobial | Azithromycin + 0.1% Chlorhexidine. | Amoxicillin 500 mg + metronidazole 400 mg | Amoxicillin 500 mg + metronidazole 400 mg | 0.2% Chlorhexidine digluconate. | 0.2% Chlorhexidine digluconate. |
| Analgesics | Ibuprofen 400 mg | Ibuprofen 600 mg | Ibuprofen 600 mg | Not reported. | Not reported. |
SD = standard deviation; AB = autogenous bone; BDX = bovine-derived xenograft; EDTA = ethylenediaminetetraacetic acid; ASA = American Society of Anaesthesiologists; PTG = Porous Titanium Granules; CPS = plastic curette + cotton pellets + sterile saline; ERL = Er:YAG laser.
Table 2.
A summary of the primary outcomes and main results of studies included.
| Study ID | Aghazadeh et al. (2012) | Wohlfahrt et al. (2012) | Jepsen et al. (2016) | Schwarz et al. (2010) | Schwarz et al. (2013) |
|---|---|---|---|---|---|
| PPD mean (SD) mm | AB group: Baseline 6 mm (1.3)/1 year after treatment 3.8 mm. BDX group: Baseline 6.2 mm (1.4)/1 year after treatment 3.3 mm. |
Case group: Baseline 6.5 mm (1.9)/1 year after treatment 4.9 mm (1.8). Control group: Baseline 6.5 mm (2.3)/1 year after treatment 4.4 mm (1.7). |
PTG group: Baseline 6.3 mm (1.3)/1 year after treatment 3.5 mm (1.5). Control group: Baseline 6.3 mm (1.6)/1 year after treatment 3.5 mm (1.1). |
Class Ib group: Baseline 6.7 (0.7), 1 year postoperatively 5.1 (0.6). Class Ic group: Baseline 7.1 (0.6), 1 year postoperatively 5.5 (0.5). Class Ie group: Baseline 7 mm (0.5), 1 year postoperatively 4.3 (0.5). |
CPS group: Baseline 5.5 (1.7)/48 months postoperatively 4.3 (1.2). ERL group: Baseline 5.1 (1.5)/48 months postoperatively 3.8 (1.1). |
| BOP mean (SD) % | AB group: Baseline 87.5% (20.1)/1 year after treatment 48.4%. BDX group: Baseline 79.4% (28.9)/1 year after treatment 26.7%. |
Case group: Baseline 5.5 (1.2)/reduction after 1 year 0.38 (2.1). Control group: Baseline 5 (1.8)/reduction after 1 year 0.56 (2.9). |
PTG group: Baseline 89.4% (20.7)/1 year after treatment 33.3% (31.7). Control group: Baseline 85.8% (23.9)/1 year after treatment 40.4% (37.1). |
Class Ib group: Baseline 81.5% (17.6), 1 year postoperatively 42.6% (14.6). Class Ic group: Baseline 83.3% (14.4), 1 year postoperatively 57.4% (8.7). Class Ie group: Baseline 85.2% (13), 1 year postoperatively 24.1% (8.8). |
CPS group: Baseline 100/48 months postoperatively 14.8% (16.4). ERL group: Baseline 95.2 (12.6)/48 months postoperatively 23.5% (23.4). |
| RBL, mean (SD) mm | AB group: Baseline 5.9 mm (1.8)/1 year after treatment 5.8 mm (0.3) BDX group: Baseline 5.2 mm (1.8)/1 year after treatment 4.2 mm (0.3). |
Case group: Baseline 6.8 mm (2.7)/defects change after 1 year 2 mm (1.7). Control group: Baseline 6.8 mm (2.30)/defects change after 1 year 0.1 mm (1.9). |
PTG group: Baseline 4.6 mm (2.1)/1 year after treatment 1.03 mm (1.4). Control group: Baseline 3.9 mm (2.1)/1 year after treatment 2.8 mm (1.8). |
No data | No data |
| Complications reported | Not reported | Uneventful | Uneventful | Uneventful | 4 patients (2 in each group) had pus formation and continuous bone loss around the implants. |
| Missing participants for follow-up | None | 1 control patient due to a psychological illness. | 4 patients in the control group were excluded at the time of analysis due to missing data. | Not reported | 4 from the CPS group and 7 from the ERL group due to missing recall sessions or severe signs of re-infection. |
| Main result | Xenograft (BDX) provides more evidence of radiographic bone fill than AB. The overall success of the treatment within both groups was limited. | PTG significantly increased radiographic bone defect identification compared to the control group. | PTG significantly increased radiographic bone defect identification in comparison to open flap debridement. | Class Ie bone defect produced a favourable result, while class Ib and class Ic were considered as unfavourable for this procedure. | Different methods of surface decontamination did not show significant differences in the treatment of peri-implantitis. |
From the five studies included in this paper, a total of 200 patients (226 implants) were treated. The mean age of the patients was 56 years (with a range between 26 and 76 years). A total of 63.5% of patients were women (127 patients), with the remainder (36.5%) being men (73 patients). The follow-up period of the studies ranged from 12 to 48 months. Three studies noted the smoking status and patients history of periodontal diseases (Aghazadeh et al., 2012, Jepsen et al., 2016, Wohlfahrt et al., 2012). These studies reported that between 40.9% and 69.6% of patients smoked, while between 50% and 95.2% had a history of periodontal treatment or tooth loss due to periodontal diseases. One study compared the use of autogenous bone grafts to bovine-derived xenografts with the use of a resorbable bovine collagen membrane in both groups (Aghazadeh et al., 2012). Two studies used porous titanium granules without a membrane (Jepsen et al., 2016, Wohlfahrt et al., 2012). Natural bone mineral with collagen membrane was employed in two studies (Schwarz et al., 2013, Schwarz et al., 2010). In terms of the detoxification methods applied in the treatment of the implant surfaces, two studies used 3% hydrogen peroxide and saline (Aghazadeh et al., 2012, Jepsen et al., 2016). One study used 24% EDTA gel and saline (Wohlfahrt et al., 2012), while Schwarz et al. (2013) used implantoplasty and Er:YAG laser. The fifth study included in this review used only sterile saline (Schwarz et al., 2010). Only one study employed the use of submerged implant treatment (Wohlfahrt et al., 2012), while the remainder used a non-submerged technique. In all of the studies, patients were prescribed both antibiotics (azithromycin, amoxicillin or metronidazole) and chlorhexidine mouthwash postoperatively, with the exception being Schwarz et al. (2010) who reported the use of chlorhexidine mouthwash but not prescription antibiotics. Only one study recorded and measured peri-implant keratinised mucosa (Wohlfahrt et al., 2012). This study established a weak positive correlation of r = 0.371 between the presence of peri-implant keratinised mucosa and increases in peri-implant bone levels. None of the studies reported any significant complications.
3.1. Effects of interventions on probing pocket depth (PPD)
All of the interventions significantly reduced PPD in all groups when compared to baseline (before the intervention). However, the reduction was broadly insignificant (p-value > 0.05) in all studies. The highest mean reduction was 3.1 mm, which was reported in Aghazadeh et al. (2012). This study applied a bovine-derived xenograft (BDX) with a collagen membrane. The lowest PPD reduction mean was 1.2 mm in patients who were treated with implantoplasty and saline (Schwarz et al., 2013).
3.2. Effects of interventions on bleeding on probing (BOP)
BOP was significantly reduced in four of the five studies when compared to baseline (Aghazadeh et al., 2012, Jepsen et al., 2016, Schwarz et al., 2013, Schwarz et al., 2010). However, no difference was reported between the groups or compared to the baseline in Wohlfahrt et al. (2012). None of the studies evidenced a statistical reduction between the tested groups, with the exception of Schwarz et al. (2010) that presented a higher Class Ie defect as compared to other groups from the same study. The highest percentage reduction occurred in patients treated only with implantoplasty and saline (a reduction of 85.2%) (Schwarz et al., 2013). In contrast, the lowest reduction was 25.9% in the Class Ic defect group (Schwarz et al., 2010).
3.3. Effects of interventions on radiographic bone level (RBL)
Unfortunately, the impact of treatment on RBLs were not reported in two of the studies (Schwarz et al., 2013, Schwarz et al., 2010). In the remaining studies, there was evidence of increased bone level as compared to baseline in all of the intervention groups. However, this increase was insignificant in all of the groups. Two studies found that the use of porous titanium granules led to a significant increase in radiographic defect fill when compared to the control groups (Jepsen et al., 2016, Wohlfahrt et al., 2012). Aghazadeh et al. (2012) showed that the use of a bovine-derived xenograft led to an increase in defect fill as compared to the autogenous bone group. However, this increase was limited and relatively insignificant. The highest mean defect fill was reported in the porous titanium granules group (3.6 mm) (Jepsen et al., 2016), while the lowest mean defect fill was 0.1 mm in the control group reported by Wohlfahrt et al. (2012).
3.4. Effects of interventions on secondary outcomes
Facial marginal soft tissue recession was measured and recorded in two studies (Schwarz et al., 2013, Schwarz et al., 2010). Schwarz et al. (2010) reported a marginal decrease in facial mucosal gain between the baseline (mean of mucosal recession = 0.4 mm) and at 12 months (mean of mucosal recession = 0.8 mm). However, this was not statistically significant. In a later study, Schwarz et al. (2013) found almost no changes in the mucosal recession in the Er:YAG laser group between baseline and the 48-month follow-up (mean of mucosal recession = 2.2 mm). In contrast, there was a slight improvement in the CPS group (group was treated with plastic curettes + cotton pellets + sterile saline). This improvement included a 1.2 mm mucosal recession at baseline, improving to 0.9 mm at the 48-month follow-up visit (0.3 mm mean gain of mucosal height). In both groups, there was no statistically significant difference.
Additional secondary outcomes, such as aesthetic changes evaluated by the patient or dentist and the cost of the treatment, were not reported on in any of the studies.
4. Discussion
All of the included studies showed improvement of the clinical conditions when compared to their baseline parameters, and they also reported a clinical relevance in their findings. However, none of the five included studies proved any statistical significance in their approach as compared to the other studies, regarding the clinical parameters (PPD, BOP, RBL or facial marginal recession). The highest mean reduction in PPD was 3.1 mm which was reported in Aghazadeh et al. (2012) and the second highest was 2.8 mm reported in Jepsen et al. (2016), both studies had used bone substitutes (bovine-derived xenograft and porous titanium granules, respectively). This could suggest that the use of bone substitutes may result in a larger PPD reduction as compared to other non-regenerative surgical treatments.
For BOP, four of the studies showed a significant improvement when compared to their baseline. However, none of them proved that their technique was reliable or better than the control groups or other techniques. Furthermore, the improvement that was achieved as compared to their baseline may be a normal outcome of patient care after surgical treatment.
For the RBL clinical parameter, the included studies showed that the use of bone substitutes led to an increase in RBL as compared to baseline. The highest mean defect fill was reported in the porous titanium granules group (3.6 mm) (Jepsen et al., 2016), followed by the porous titanium granules group (2 mm) reported in Wohlfahrt et al. (2012), then the bovine-derived xenograft group (1.1 mm) reported in Aghazadeh et al. (2012) and the least defect fill was reported in the autogenous bone group (0.2 mm) in Aghazadeh et al. (2012). This suggests that the order of the best material to fill a bone defect to the least is: porous titanium granules, bovine-derived xenograft and autogenous bone, respectively. However, radiographic examination did not prove that there was complete re-osseointegration between the implant and bone graft and this needed histological examination, which may be an impractical study on human patients. Another limitation of RBL is that autogenous bone grafts are not as radiopaque as porous titanium granules and might be resorbed at a faster rate than the latter (Jepsen et al., 2016).
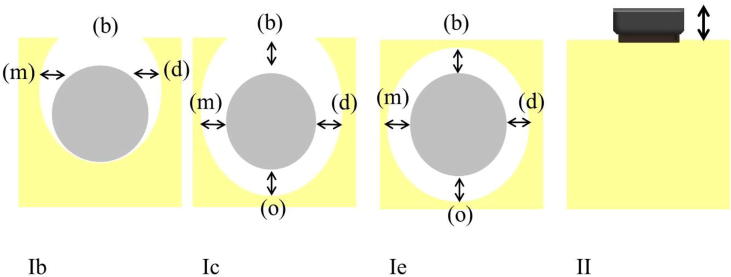
Schwarz et al. (2010) investigated the impact of regenerative techniques on defect configuration. The researchers divided Class I defects into three subcategories: Class Ib, Class Ic and Class Ie (Fig. 3). They found that Class Ie had better clinical outcomes (PPD and BOP) than the other two subcategories. They also concluded that Class Ie had favourable treatment outcomes when treated with a natural bone mineral and a collagen membrane, while Class Ic had unfavourable outcomes. This classification might be similar to the infrabony defects classification (three osseous walls), and the findings may support the algorithm (Fig. 1) that was developed by Mishler and Shiau (2014), which showed the importance of the bony walls in defects to support and well contain the bone graft materials during the healing process.
Fig. 3.
Intraoperative assessment of defect configuration. Class Ib represents buccal dehiscence + semicircular bone resorption. Class Ic presents buccal dehiscence + circular bone resorption. Class Ie demonstrates circular bone resorption under maintenance of the peri-implant bony walls. Class II demonstrates a vertical and horizontal bone resorption. (b) buccal aspect; (o) oral aspect; (m) mesial aspect; (d) distal aspect.
Source: Schwarz et al. (2010).
Schwarz et al. (2013) investigated the impact of two different surface debridement and decontamination methods as a surgical treatment for peri-implantitis. They divided the patients into two groups, and the treatment procedure for both groups included access flap surgery, granulation tissue removal and implantoplasty. Then, the first group was treated with Er:YAG laser (ERL group) and the second group was treated with plastic curettes + cotton pellets + sterile saline (CPS group). In addition, in both groups, the bony defects were filled with a natural bone mineral and covered by a collagen membrane. The researchers followed the patients up to 48 months postoperatively. They concluded that there was no statistically significant difference between the groups and the treatment outcomes were not influenced by the different methods of surface decontamination. Thereafter, the researchers continued to follow the patients and examined them 84 months after the surgical intervention. They published their results in 2017 (Schwarz et al., 2017), and their conclusion was the same as in Schwarz et al. (2013) that treatment outcomes were not influenced by the different methods of surface decontamination.
4.1. Autogenous bone graft vs. other types of bone substitutes
In the studies included various types of bone grafting materials were used in the treatment of peri-implantitis. In the literature, autogenous bone is considered the ‘gold standard’ of bone grafting materials (Warreth et al., 2015). However, in the included studies, the least defect fill was in the autogenous bone group. This finding is in line with other studies, which have shown that autogenous bone grafts have more volume loss (40%) during the healing process as compared to synthetic bone substitutes, which may retain their volume for years (Iezzi et al., 2007). On the other hand, porous titanium granules, which were recently confirmed to have osteoconductive properties (Mijiritsky et al., 2013), had a higher defect fill in the presented studies. This will lead us to rethink whether autogenous bone graft is still the gold standard for treatment of peri-implantitis or not, and it is difficult from this review to answer this question as it needs more clinical and histological studies.
4.2. The role of barrier membranes in regenerative surgical techniques
In the literature there is disagreement on the benefits of barrier membranes. Some studies showed that higher reductions of PPD and BOP were found when barrier membranes were used to cover bone grafts as compared to grafts alone (Chan et al., 2014), while others demonstrated no additional benefits to using barrier membranes and claimed that the membranes added more costs (Roos-Jansaker et al., 2014). In this review, three out of the five studies used resorbable collagen membranes (Aghazadeh et al., 2012, Schwarz et al., 2013, Schwarz et al., 2010), while the other two studies did not use any membrane (Jepsen et al., 2016, Wohlfahrt et al., 2012). It cannot be concluded from the results of these studies if barrier membranes had any additional effect.
4.3. Quality of the evidence
An assessment of the risk posed by bias in the included RCT studies was undertaken using an assessment tool from the Cochrane handbook for systematic reviews of interventions (Higgins and Green, 2011). Table 3 shows the risk of bias assessment for each of the included studies. The overall risk of bias was low for the selected studies except for Schwarz et al., 2013, Schwarz et al., 2010, which were assessed as unclear risk. Schwarz et al. (2010) did not give any clear information on their random sequence generation and allocation concealment, and the study was judged to have a high risk of bias due to incomplete outcome data as they did not evaluate the bony defects preoperatively and bone graft treatments postoperatively by radiographs. Instead, they used the clinical attachment level, which might not be as an accurate tool to evaluate peri-implantitis treatment as compared to dental radiographs. In addition, Schwarz et al. (2013) did not give clear information on allocation concealment, and the incomplete data section was assessed as a high risk as they did not use radiographs to evaluate the peri-implantitis treatment. Furthermore, although 11 patients missed the recall sessions, and the majority of these patients (seven) were in one group (ERL group), the authors did not disclose whether the missing participants affected the results of the study. Aghazadeh et al. (2012) failed to mention if the researchers knew the patients’ allocation sequence before the surgical treatment.
Table 3.
Risk of bias assessment.
| Study ID | Random sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective reporting | Other biases | Overall risk of bias |
|---|---|---|---|---|---|---|---|
| Aghazadeh et al. (2012) | + | ? | + | + | + | + | + |
| Wohlfahrt et al. (2012) | + | + | + | + | + | + | + |
| Jepsen et al. (2016) | + | + | + | + | + | + | + |
| Schwarz et al. (2010) | ? | ? | + | − | + | + | ? |
| Schwarz et al. (2013) | + | ? | + | − | ? | + | ? |
+ = low risk − = high risk ? = unclear risk.
4.4. Limitations of the included studies and the review
The included studies have many limitations that may affect the overall validity of this systematic review. First, all five studies selected for the review contained a relatively small number of patients and a short follow-up period. Second, the definition of peri-implantitis and the clinical parameters varied in the inclusion criteria of the included studies. Some of them defined RBL as ≥2 mm (Aghazadeh et al., 2012) and some defined it as ≥3 mm (Jepsen et al., 2016), while others defined it as ≥4 mm (Wohlfahrt et al., 2012). Also, PPD was not the same in the inclusion criteria among the studies. It was defined as ≥5 mm in three of the studies (Aghazadeh et al., 2012, Jepsen et al., 2016, Wohlfahrt et al., 2012), while it was >6 mm in the other two (Schwarz et al., 2013, Schwarz et al., 2010). Third, there was heterogeneity in the study design among the studies; the variation in bone substitutes and detoxification methods could make the comparison between the techniques difficult. Moreover, the heterogeneity may make the meta-analysis of the included studies impractical. Another limitation of the studies is the fact that evaluation of re-osseointegration between the implants and bone grafts could not be assessed by radiographs only, a histological examination would also be needed. Furthermore, many of the included studies reported a high percentage of smokers and a history of periodontal diseases among the patients, which are potential risk factors for peri-implantitis and could have compromised the treatment outcomes. In addition, most of the studies did not report the amount of peri-implant keratinised mucosa, which might also have affected the treatment outcomes. Also, the cost of treatment, which might be important to evaluate the cost-effectiveness of each technique, and aesthetic outcome evaluation, which were part of the secondary outcomes for this review, were not reported in any of the studies. Another limitation of this systematic review is that the studies included were only in English. Thus, any relevant studies in other languages might have been missed. Finally, the number of included studies was only five, which may not be enough to draw any solid conclusions.
5. Conclusion
Within the limits of this systematic review, regenerative surgical treatment showed clinical improvements in the included studies as compared to their baseline conditions (before surgical treatment). However, none of the five selected studies proved any statistical significance in their approaches. Porous titanium granules had the highest radiographic bone defect fill for the RBL parameter as compared to the other bone substitutes, and there were better PPD outcomes in the bovine-derived xenograft group and before the autogenous bone graft group. The shape of the bony defect may play an important role in the success of regenerative treatment, as shown in the review Class Ie had favourable PPD and BOP outcomes when compared to Class Ib and Class Ic. In addition, Class Ic had unfavourable results. There was no clear evidence to support any additional advantages for the use of Er:YAG laser, submerged techniques or barrier membranes. The results of this systematic review should be used with caution when it comes to clinical practice, as this review has many limitations. There is a need for well-designed, long-term, randomised clinical trials with sufficiently large sample sizes and a long follow-up period.
Funding
No funding was received for this research.
Declaration of Competing Interest
The authors have no conflict of interest to declare.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aghazadeh A., Rutger G., Persson, Renvert S. A single-centre randomized controlled clinical trial on the adjunct treatment of intra-bony defects with autogenous bone or a xenograft: results after 12 months. J. Clin. Periodontol. 2012;39:666–673. doi: 10.1111/j.1600-051X.2012.01880.x. [DOI] [PubMed] [Google Scholar]
- Chan H.L., Lin G.H., Suarez F., Maceachern M., Wang H.L. Surgical management of peri-implantitis: a systematic review and meta-analysis of treatment outcomes. J. Periodontol. 2014;85:1027–1041. doi: 10.1902/jop.2013.130563. [DOI] [PubMed] [Google Scholar]
- Chrcanovic B.R., Albrektsson T., Wennerberg A. Bone quality and quantity and dental implant failure: a systematic review and meta-analysis. Int. J. Prosthodont. 2017;30:219–237. doi: 10.11607/ijp.5142. [DOI] [PubMed] [Google Scholar]
- el Chaar E.S., Jalbout Z.N. Regeneration of an osseous peri-implantitis lesion. Periodontal. Clin. Investig. 2002;24:5–10. [PubMed] [Google Scholar]
- Higgins, J., Green, S., 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) [Online]. The cochrane collaboration. Available: http://handbook-5-1.cochrane.org/ [Accessed 25 December 2017].
- Iezzi G., Degidi M., Scarano A., Petrone G., Piattelli A. Anorganic bone matrix retrieved 14 years after a sinus augmentation procedure: a histologic and histomorphometric evaluation. J. Periodontol. 2007;78:2057–2061. doi: 10.1902/jop.2007.070062. [DOI] [PubMed] [Google Scholar]
- Jepsen K., Jepsen S., Laine M.L., Anssari D., Moin, Pilloni A., Zeza B., Sanz M., Ortiz-Vigon A., Roos-Jansaker A.M., Renvert S. Reconstruction of peri-implant osseous defects: a multicenter randomized trial. J. Dent. Res. 2016;95:58–66. doi: 10.1177/0022034515610056. [DOI] [PubMed] [Google Scholar]
- Kozlovsky A., Tal H., Laufer B.Z., Leshem R., Rohrer M.D., Weinreb M., Artzi Z. Impact of implant overloading on the peri-implant bone in inflamed and non-inflamed peri-implant mucosa. Clin. Oral. Implants Res. 2007;18:601–610. doi: 10.1111/j.1600-0501.2007.01374.x. [DOI] [PubMed] [Google Scholar]
- Lang N.P., Berglundh T. Periimplant diseases: where are we now?–Consensus of the seventh european workshop on periodontology. J Clin Periodontol. 2011;38(Suppl 11):178–181. doi: 10.1111/j.1600-051X.2010.01674.x. [DOI] [PubMed] [Google Scholar]
- Mijiritsky E., Yatzkaier G., Mazor Z., Lorean A., Levin L. The use of porous titanium granules for treatment of peri-implantitis lesions: preliminary clinical and radiographic results in humans. Br. Dent. J. 2013;214:E13. doi: 10.1038/sj.bdj.2013.222. [DOI] [PubMed] [Google Scholar]
- Mishler O.P., Shiau H.J. Management of peri-implant disease: a current appraisal. J. Evidence Based Dent. Pract. 2014;14:53–59. doi: 10.1016/j.jebdp.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., Altman, D., 2009. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement [Online]. The PRISMA Group Available: www.prisma-statement.org [Accessed 28 December 2017]. [PMC free article] [PubMed]
- Mombelli A., Lang N.P. The diagnosis and treatment of peri-implantitis. Periodontol. 1998;2000(17):63–76. doi: 10.1111/j.1600-0757.1998.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Nguyen-Hieu T., Borghetti A., Aboudharam G. Peri-implantitis: from diagnosis to therapeutics. J. Investig. Clin. Dent. 2012;3:79–94. doi: 10.1111/j.2041-1626.2012.00116.x. [DOI] [PubMed] [Google Scholar]
- Ramanauskaite A., Daugela P., FARIA DE R., ALMEIDA, SAULACIC N. Surgical non-regenerative treatments for peri-implantitis: a systematic review. J. Oral. Maxillofac. Res. 2016;7:e14. doi: 10.5037/jomr.2016.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanauskaite A., Daugela P., Juodzbalys G. Treatment of peri-implantitis: meta-analysis of findings in a systematic literature review and novel protocol proposal. Quintessence Int. 2016;47:379–393. doi: 10.3290/j.qi.a35131. [DOI] [PubMed] [Google Scholar]
- Renvert S., Polyzois I., Persson G.R. Treatment modalities for peri-implant mucositis and peri-implantitis. Am. J. Dent. 2013;26:313–318. [PubMed] [Google Scholar]
- Romeo E., Ghisolfi M., Murgolo N., Chiapasco M., Lops D., Vogel G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part I: clinical outcome. Clin. Oral Implants Res. 2005;16:9–18. doi: 10.1111/j.1600-0501.2004.01084.x. [DOI] [PubMed] [Google Scholar]
- Roos-Jansaker A.M., Persson G.R., Lindahl C., Renvert S. Surgical treatment of peri-implantitis using a bone substitute with or without a resorbable membrane: a 5-year follow-up. J. Clin. Periodontol. 2014;41:1108–1114. doi: 10.1111/jcpe.12308. [DOI] [PubMed] [Google Scholar]
- Roos-Jansaker A.M., Renvert S., Egelberg J. Treatment of peri-implant infections: a literature review. J. Clin. Periodontol. 2003;30:467–485. doi: 10.1034/j.1600-051x.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- Schwarz F., Bieling K., Latz T., Nuesry E., Becker J. Healing of intrabony peri-implantitis defects following application of a nanocrystalline hydroxyapatite (Ostim) or a bovine-derived xenograft (Bio-Oss) in combination with a collagen membrane (Bio-Gide). A case series. J. Clin. Periodontol. 2006;33:491–499. doi: 10.1111/j.1600-051X.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- Schwarz F., Hegewald A., John G., Sahm N., Becker J. Four-year follow-up of combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination. J. Clin. Periodontol. 2013;40:962–967. doi: 10.1111/jcpe.12143. [DOI] [PubMed] [Google Scholar]
- Schwarz F., John G., Schmucker A., Sahm N., Becker J. Combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination: a 7-year follow-up observation. J. Clin. Periodontol. 2017;44:337–342. doi: 10.1111/jcpe.12648. [DOI] [PubMed] [Google Scholar]
- Schwarz F., Sahm N., Schwarz K., Becker J. Impact of defect configuration on the clinical outcome following surgical regenerative therapy of peri-implantitis. J. Clin. Periodontol. 2010;37:449–455. doi: 10.1111/j.1600-051X.2010.01540.x. [DOI] [PubMed] [Google Scholar]
- Suarez F., Monje A., Galindo-Moreno P., Wang H.L. Implant surface detoxification: a comprehensive review. Implant Dent. 2013;22:465–473. doi: 10.1097/ID.0b013e3182a2b8f4. [DOI] [PubMed] [Google Scholar]
- Thakkar, J., Oh, J., Inglehart, M., Aronovich, S., 2017. Etiology, diagnosis and treatment of peri-implantitis-a national survey of AAOMS members. J. Oral Maxillofacial Surg. 75 (10 Supplement 1), e355–e356.
- Triplett R.G., Andrews J.A., Hallmon W.W. Management of peri-implantitis. Oral Maxillofacial Surgery Clinics of North America. 2003;15:129–138. doi: 10.1016/s1042-3699(02)00078-x. [DOI] [PubMed] [Google Scholar]
- Wang W.C.W., Lagoudis M., Yeh C.-W., Paranhos K.S. Management of peri-implantitis – a contemporary synopsis. Singapore Dent. J. 2017;38:8–16. doi: 10.1016/j.sdj.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Warreth A., Boggs S., Ibieyou N., El-Helali R., Hwang S. Peri-implant diseases: an overview. Dent Update. 2015;42 doi: 10.12968/denu.2015.42.2.166. 166–8, 171–4, 177–80 passim. [DOI] [PubMed] [Google Scholar]
- Wohlfahrt J.C., Lyngstadaas S.P., Ronold H.J., Saxegaard E., Ellingsen J.E., Karlsson S., Aass A.M. Porous titanium granules in the surgical treatment of peri-implant osseous defects: a randomized clinical trial. Int. J. Oral Maxillofac Implants. 2012;27:401–410. [PubMed] [Google Scholar]