Abstract
Selective immunoglobulin A deficiency (SIgAD) is considered to be the most common human primary immune-deficiency disease in the world. However, the incidence in China is obviously lower than Caucasian races. The definition of SIgAD has changed over time with the progress of people's understanding. The scientific community did not reach a consensus on the definition until 1999. As a result, many previously reported cases need to be excluded under the current definition. SIgAD can lead to several spectra of diseases including infections and autoimmune diseases. We retrospectively summarized the SIgAD patients in Peking Union Medical College Hospital (PUMCH), and summarized the Chinese SIgAD reported in China and abroad in past 40 years. Fourty three SIgAD patients were confirmed in the study, in which 9 were healthy without clinical symptoms. Of the 34 patients with clinical symptoms, recurrent infections were found in 29 (85.3%) patients; 13 (38.2%) patients were with autoimmune diseases; 6 (17.6%)cases had allergic symptoms; 3 patients (8.8%) were with tumors, only one case (2.9%) had a family history. Compared with other countries, sIgAD patients in China showed similar symptoms, but the rate of recurrent infections and autoimmune diseases were higher than some other countries; most of the allergic symptoms are drug allergy, different with the allergic sequelae reported in other countries, such as asthma, rhinitis, food allergy and atopic dermatitis; and it is rare to have family history in Chinese patients. We also figured out that more female SIgAD patients tend to have more autoimmune diseases than men (P = 0.039).
Keywords: Autoimmune diseases, Chinese, Clinical manifestations, Recurrent infections, Selective Immunoglobulin A Deficiency (SigAD)
Introduction
Selective Immunoglobulin A Deficiency (SIgAD) is considered as one of the most common primary human immunodeficiency diseases in the world. The reported morbidity of SIgAD is between 1:143 and 1:18,500, varying due to regional and definition differences.1,2 The morbidity in China is between 1:420 and 1:17,812.3, 4, 5, 6, 7, 8, 9 The definition of SIgAD has changed over time with the progress of people's understanding. The scientific community did not reach a consensus on the definition of SIgAD until 1999.10 The current SIgAD diagnostic criteria is that immunoglobulinA (IgA) levels less than 0.07 g/L in a patient 4 years of age or older with normal immunoglobulin M (IgM) and immunoglobulin G (IgG), and no other identified causes of immunodeficiency.2 The mainly changes including IgA levels and age limitation. As a result, many previously reported cases need to be excluded under the current definition. According to the current definition, the incidence of Chinese SIgAD is between 1:2295 and 1:17,812, much lower than Caucasians. A vast majority of patients may have no symptoms expressed during lifespan,11 while some patients may present a spectrum of diseases. Main spectra of complications include infectious diseases, allergy, tumour, and autoimmune diseases. In this study, we retrospectively summarized the SIgAD patients in Peking Union Medical College Hospital (PUMCH), reviewed published Chinese SIgAD cases reported in the past 40 years, and compared with other countries to obtain more comprehensive knowledge of SIgAD in China.
Materials and methods
Diagnosis of SIgAD
In the present study, the diagnosis of SIgAD must simultaneously satisfy the three following criteria2,10:
-
1)
the patient is above 4 years old;
-
2)
serum IgA level is continuously below 0.07 g/L (at least 2 times), while IgG and IgM levels are normal or elevated;
-
3)
other reasons of IgA deficiency must be excluded.
Collection of clinical records
We searched all the inpatient medical records kept in PUMCH with discharge diagnosis including “selective IgA deficiency” from 1980 to July 2019, and select those cases who conforms to the current diagnosis criteria. We used PubMed, Chinese database, including Wanfang Data, CQVIP, CNKI and Chinese Medical Journal Net to search for literatures written in English and Chinese with open published time. The keywords including “selective IgA deficiency & China” and “selective IgA deficiency & Chinese”. Repeat cases or reported cases without individual clinical data were excluded. Collect demographic information, clinical manifestations and laboratory examination data and analysis. The study was approved by the Ethics Committee of Peking Union Medical College Hospital (ZJS-1248), but informed consent was't obtained for the retrospective study.
The following groups were excluded from the study:
-
1)
children with diagnosed age younger than four;
-
2)
patients whose serum IgA level is not certainly or continuously below 0.07 g/L;
-
3)
patients diagnosed with other definite hypoimmunoglobulinemia;
-
4)
Repeat cases already discussed by PUMCH in published materials from 1980 to 2019 so as to prevent a case from being reported multiple times.
-
5)
published cases whose individual clinical data is unavailable.
Statistical approach
To describe demographic data, abnormal clinical manifestations and laboratory test results by case number, rate, median, mode, etc. Fisher's exact test was used to compare the difference of the rate of abnormal manifestations and abnormal library test ratio between male and female, or between children and adults. Statistical software is SPSS17.0, P < 0.05 is for statistically difference.
Clinical manifestations including: recurrent infections, autoimmune diseases, allergy, tumor, nerve involvement, articular bone involvement, kidney involvement, eye involvement, hematological system disease, lymphadenectasis, hepatomegaly or hepatosplenomegaly.
Abnormal laboratory findings including: increased WBC, decreased HGB and PLT; Proteinuria; increased ALT, AST, GGT, ALP, TBIL, DBIL, CH, TG, LDH, Cr and UA; increased IgM and IgG; positive Autoantibody; increased ESR and CRP.
Results
A total of 40 cases diagnosed as SIgAD were recorded in PUMCH from 1980 to July 2019, of which 14 satisfying the diagnostic criteria above were retrospectively studied, and 26 were excluded.
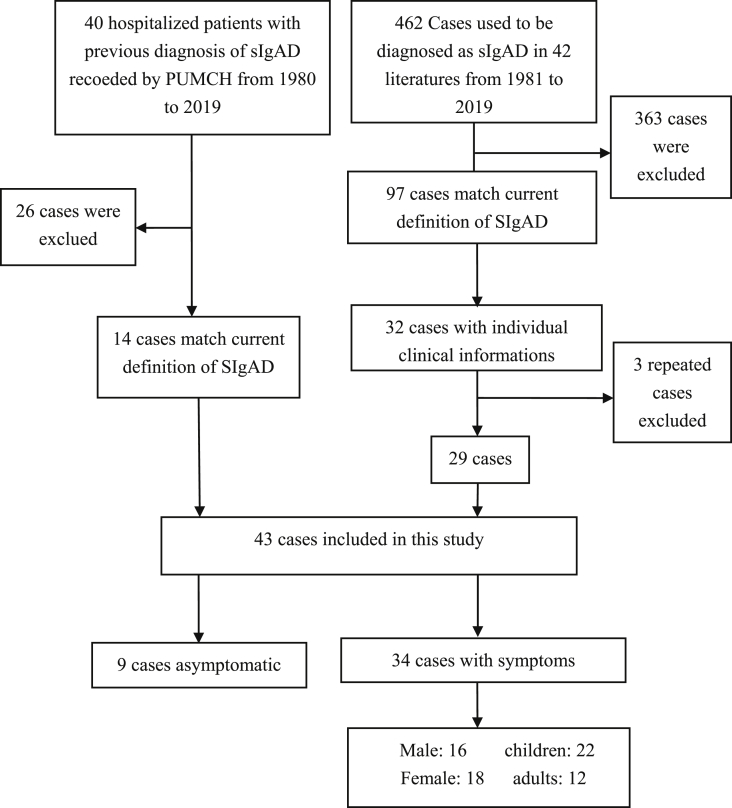
The literature types were case reports, clinical studies and epidemiological surveys. A total of 462 cases used to be diagnosed as SIgAD from 42 literatures published from 1981 to 2019 were included in this study. Ninety-seven cases were satisfying the current diagnostic criteria, of which 32 cases were with individual clinical data,5,12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 including 3 repetitive cases.13,18,19 As a result, a total of 43 cases, 14 cases from PUMCH and 29 cases reported in the literatures, were included in this study for analysis (Fig. 1).
Figure 1.
Cases screening process.
Demographic of patients
Diagnosis of SIgAD was confirmed in 43 patients from PUMCH and literatures, 19 (44.2%) were males and 24 (55.8%) were females. The range of diagnostic age was from 4 to 61 years (median age was 13 years, mode age was 4 years). Thirty cases (60.76%) were under age 16 years, of which 14 were males and 16 were females. 13 cases were adults above age 18. There were 18 cases of Han nationality, 5 cases of Korean nationality, 2 cases of Mongolian nationality, and one case in each nationality of Hui, Bai, Tujia and Ozbek otherwise, 14 cases' nationality were non-available. Nine patients (20.9%) were healthy persons diagnosed as SIgAD in a few large epidemiological survey studies without clinical manifestations. Recurrent infections and autoimmune diseases were the most common manifestations in the 34 patients (79.1%) with clinical symptoms, and combined with many other manifestations. Among the 34 cases, 22 were children (64.7%) and 12 were adults (35.3%). 16 were male (47%) and 18 were female (53%) (Table 1). In addition, one child has a family history, her sister was also a patient with SIgAD.
Table 1.
Demographic informations of SIgAD Patients.
| symptomatic patients | asymptomatic patients | Total | |
|---|---|---|---|
| Cases n (%) | 34 (79.1%) | 9 (20.9%) | 43 (100%) |
| male/female n (%) | 16 (47.1%)/18 (52.9%) | 3 (33.3%)/6 (66.7%) | 19 (44.2%)/24 (55.8%) |
| Children/Adults n (%) | 22 (64.7%)/12 (35.3%) | 8 (88.9%)/1 (11.1%) | 30 (60.76%)/13 (29.24%) |
Clinical manifestations
The most common clinical manifestations of the 34 symptomatic patients were recurrent infections, autoimmune diseases. Drug allergies and tumors were also found in some patients. Multiple tissues, organs and systems were involved in SIgAD patients. Involved organs and systems included kidney, liver, neural, visual, articular skeleton, digestive and hematologic systems (Table 2).
Table 2.
Clinical manifestations of 34 SIgAD patients.
| Symptoms | Subtypes | Number of patients | Proportion (n/34) |
|---|---|---|---|
| Infection 29/34 (85.3%) | Respiratory tract infection | 25 | 73.5% |
| Mucocutaneous ulcerated | 4 | 11.8% | |
| Digestive tract infection | 9 | 26.47% | |
| Poliomyelitis | 2 | 5.8% | |
| Fever | 9 | 26.5% | |
| Concomitant autoimmune diseases 13/34 (38.2%) | SLE or SLE? | 8 | 23.5% |
| Hashimoto's thyroiditis | 2 | 5.9% | |
| Hypothyroidism | 1 | 2.9% | |
| Still | 1 | 2.9% | |
| CTD | 1 | 2.9% | |
| Allergy 6/34 (17.6%) | Photoallergy | 1 | 2.9% |
| Drug allergy | 5 | 14.7% | |
| Tumor 3/34 (8.8%) | Retinoblastoma | 1 | 2.9% |
| Pleuropulmonary blastoma | 1 | 2.9% | |
| Lymphoma? | 1 | 2.9% | |
| Involved systems | Nervous system | 7 | 20.6% |
| Vision | 5 | 14.7% | |
| Auditory sense | 1 | 2.9% | |
| Articulation | 9 | 26.5% | |
| Digestive tract | 9 | 26.5% | |
| Renal involvement | 12 | 35.3% | |
| Liver | 7 | 20.6% | |
| hematological system | 7 | 20.6% | |
| Other symptoms | lymphadenectasis | 6 | 17.6% |
| Abdominal mass | 1 | 2.9% |
Infections
Recurrent infections in different sites were found to be the most common symptom among patients, as they were found in 29 (85.29%) cases of the 34 symptomatic patients, of which 9 patients had recurrent fevers. Respiratory tract infections were found in 25/29 (86.2%) patients, with symptoms of susceptibility to catch colds, pneumonia, bronchitis, etc. Gastrointestinal tract infections were found in 9/29 (31.0%) patients, with symptoms of nausea, vomiting, recurrent abdominal pain, diarrhea, gastrointestinal bleeding and ulcer. 4 cases (13.8%) were with mucocutaneous ulcerated symptoms, such as ulceration, suppuration, furuncle or anal fistula. Two cases (6.9%) were with poliomyelitis.
Autoimmune diseases
Thirteen (38.2%) patients were diagnosed with autoimmune diseases, including systemic lupus erythematosus (SLE) or suspected SLE in 8 (61.5%) patients; Hashimoto's thyroiditis in two (15.4%) patients, of which one with type I diabetes mellitus, another with Evans syndrome; hypothyroidism in one (7.7%) patient; systemic onset juvenile idiopathic arthritis (So-JIA) in one patient, and connective tissue disease (CTD) in one (12.5%) patient.
Allergy
Six (17.6%) patients had allergic symptoms, one case (17.7%)was photoallergic caused by SLE, and 5 (83.3%) were allergic to drugs. Among them, three were allergic to penicillin. None has allergic sequelae reported in other countries, such as asthma, rhinitis, food allergy and atopic dermatitis.
Tumours
Three children were with tumors, two were 4 years old, and the other was 10 years old one was with retinoblastoma, one was with pleuropulmonary blastoma (PPB), and the other one was NK/T-cell lymphoma.
Other manifestations of involved systems
Seven (20.6%) patients had neurological symptoms, including epilepsy, congenital hydrocephalus, cerebral infarction, viral encephalitis, acral numbness, facial paralysis, lost of consciousness, depression and so on. Five (14.7%) cases suffered from eyes involvement, such as iritis, cataract, visual impairment, eyes movement limited, and hemorrhage of the fundus. One case suffered from hearing loss. Nine(26.5%)cases had osteoarthrosis, including arthralgia, arthrocele, limited joint mobility and talalgia. Renal involvement were found in 12 (35.3%) cases, including 5 cases of lupus nephritis, 3 cases of nephrotic syndrome, 1 case of focal segmental glomerulosclerosis, 1 case of mild glomerular lesion, 1 case of renal calculi, and 1 case of mild hematuria and proteinuria. Seven (20.6%) cases had liver involvement, with hepatomegaly or hepatosplenomegaly, and liver function damage. Seven (20.6%) cases had hematological system involvement, with anemia or thrombocytopenia lymphadenectasis were found in 6 cases, located on neck, submandibular or inguinal region. In addition, recurrent abdominal mass was found in one patient, which showed plasma cell granuloma of pathology.
Laboratory examinations
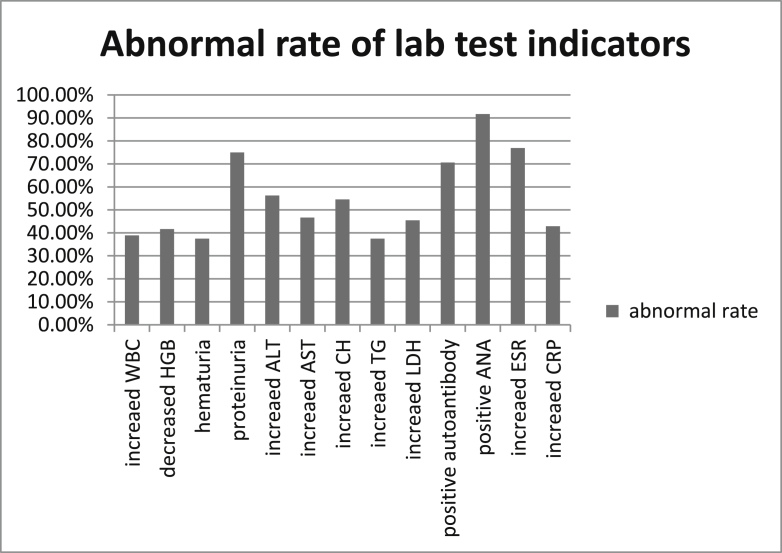
Immunoglobulin levels of IgG, IgM and IgA are available in each patient, but many of other lab tests were missing in many patients. In addition to the detailed laboratory results of 14 patients from PUMCH, only 4 cases reported in literatures had some simple lab test results, including blood and urine routine examinations, blood biochemical determinations. Other 4 patients with SLE only had positive autoantibodies results. IgG and IgM were normal or higher than normal in each patient, 8 cases had elevated IgM, while 9 cases were with elevated IgG. In the patients with available lab test results, abnormal laboratory tests including (Fig. 2): 7/18 WBC were increased, 1/18 WBC and 5/12 HGB were decreased, 4/16 PLT were increased and 3/16 PLT were decreased; 6/16 were hematuria, 12/16 were proteinuria, 4/12 were glycosuria; 7/9 had positive 24 h-urinary protein ranged from 0.07 to 0.7g/24 h (median 0.1g/24 h); 9/16 ALT, 7/15 AST, 2/15 GGT and 4/14 ALP were increased; 3/12 Tbil and Dbil were increased; 6/11 total cholesterol and 3/8 TG were increased; 5/11 LDH were increased; 4/14 blood Cr were decreased, 1/14 blood glucose was increased; 2/9 UA were decreased and 1/9 was increased. Autoantibodies were positive in 12/17 patients, among them, 11/12 ANA were increased. 10/13 ESR were increased and 6/14 CRP increased, 2/4 positive Coombs, and 5 cases had sinus tachycardia.
Figure 2.
Abnormal rate of some lab test indicators.
Symptoms and laboratory test differences between children and adults, or between males and females
There was no significant difference in the incidence of any abnormal clinical manifestation between adults and children; The incidence of autoimmune diseases associated with women (10/18,55.6%) was higher than that of men (3/16,18.8%) (P = 0.039), and there was no difference in remaining clinical symptoms between males and females. By comparing the ratios of abnormal laboratory tests between adults and children, or between males and females, there was no statistically significant difference found.
Discussion
Two points of the diagnostic criteria need to be explained. We have excluded children under age four from our study because children at this age level do not have developed mature immune systems yet, and thus may have transient IgA deficiencies resembling SIgAD. As for the diagnostic level of serum IgA, studies from literature search used different criteria due to differences in measurement techniques, including below 0.05 g/L23 and below 0.07 g/L.1,2 We have applied the limit 0.07 g/L in our present study because this is the minimum value that most laboratories can detect. Transient IgA deficiency is sometimes misdiagnosed as SIgAD, especially in younger children.24 Therefore, all the patients from PUMCH included in the study must have IgA levels under 0.07 g/L for at least 2 times.
Fourty cases recorded by PUMCH used to be diagnosed as SIgAD, but only 14 cases (35%) met the current diagnostic criteria. Similarly, 462 cases used to be reported as SIgAD, in which 97 cases (21%) matched the current definition. 65%–79% of the cases were excluded because of the decreased IgG and/or IgM levels, diagnosis age under 4 years, or IgA levels above 0.07 g/L. About 2/3 of cases are excluded following further understandings of SIgAD, so the informations summarized from previous literatures need to be further updated. Several large-scale epidemiological surveys3, 4, 5, 6, 7, 8, 9 of China showed the incidence of sIgAD in China was between 1:420 to 1:17,812, which need to be corrected to 1:2295–1:17,812 according to the current definition. The incidence of China is much lower than Caucasian race (1:142)2 but similar to Japan (1:18,550),2 geographical or ethnical reasons need to be considered.
Although a majority of the cases occur sporadically, SIgAD can be transmitted autosomally. MSH5 (6p21)25 and TNFRSF13B (i.e., TACI, 17p11)26 have been reported to correlate with this disorder. The genetic basis is also supported by the different rate of occurrence in different races, as SIgAD is more common in Caucasians than in Asians.1 The study is a retrospective study over 40 years, genetic analysis has not been popularized in past years. None of the patients had done gene analysis. Only one patient in the study, who visited PUMCH in 2010, had a family history, her sister was also with SIgAD. We speculated her family may have genetic abnormalities. Family history is extremely rare (2.9%) in this study, much lower than that reported in other countries (20%–25%2), which may be related to the heterogeneity of the pathogenic genes of SIgAD and races difference.
In our study, infections (85.3%) were the most common complication observed, which was higher than findings in Israeli (39.7%)24 and Brazilian populations.27 Infections are so common that, we speculate, few people would connect recurrent infections to SIgAD and thus have the immunoglobulin levels tested. IgA are mostly found in mucous secretions, including the mucus in respiratory tract and digestive tract. In normal populations, secretory immunoglobulins in the mucus cover the inner surface of those tracts, preventing pathogenic infections. People with SIgAD have diminished abilities to resist incoming infectious pathogens because of the absence of secretory immune proteins. Similar to the findings in this study, infection in the respiratory tract has been observed in 40% (6 out of 15) and 60% (21 out of 35) subjects in two other Chinese samples, from Xi'an and Yanbian respectively.6,8 IgG2 deficiency is speculated to lead to more severe infections and complications,1 and this matched with our observation of a patient with IgG2 deficiency in our study. The patient had ulcers on fingers and thigh, and the infection caused excision of his left middle finger. He was also diagnosed with NK/T-cell lymphoma.
Autoimmune diseases (38.2%) were also an important category of complications in our study, much higher than the reported proportions in Brazil (19%), Spain (11.5%), Turkey (17.3%) and Israel (20.6%).2,23,24,27 Besides the proportional difference, we have also observed the difference in disease types. In our study, 23.5% of the patients were diagnosed or suspected with SLE, while few cases were reported in the Brazilian, Spanish, and Israeli papers. We speculate that this is due to race difference, as SLE is more common in people of non-Caucasian ancestry.28 It has been reported that the incidence of SLE in Chinese SIgAD is only 0.8%.2 Our result (23.5%) is much higher than that, we speculate a large number of previous patients diagnosed with sIgAD have been excluded for the changes of diagnostic criteria of sIgAD, so our result is higher than previous article.
Such a high ratio of autoimmune patients can be explained by the positive ANA levels among the patients. Since ANA levels (usually if higher than 1:160) are used as diagnostic methods of SLE, autoimmune hepatitis, and other diseases, patients of such diseases usually express the elevated positive ANA levels. In the population, women are more likely to have autoimmune diseases than men, because of the influence of sex hormones and chromosomes on the function of the innate and adaptive immune systems.29 In this study, the prevalence of autoimmune diseases in female (55.6%) patients was higher than that in male (18.8%) patients (P = 0.039), which was consistent with population.
Six allergic cases (17.6%) have been observed in our study. We have only noted one patient with SLE was allergic to light, other 5 were all had allergy against drug, especially penicillin. The occurrence rate of allergic diseases gives us a similar result between Chinese and Caucasian. Among Caucasians, the occurrence of allergies ranged from 17.8% to 48.4%.23,27 How ever allergic sequelae such as asthma, rhinitis, atopic dermatitis, and food allergy were more often in those countries.2 Even though rashes on the face have been found in another patient of our study, who administrated with Nabumetone 3 days later, we could not rule out the potentiality of SLE because of the similarity between this disease and the dermatitis defined as allergy.
We have also observed three cases (8.8%)of tumor in our study. One 4-year-old girl was with retinoblastoma, one 4-year-old girl was with pleural pulmonary blastoma (PPB), and the other 10-year-old boy had lymphoma. All the 3 patients were children, but there was no significant difference between children and adults due to the small number of cases. Lymphoid and gastrointestinal cancers are the most prevalent malignancies in SIgAD patients,1,30 Hodgkin lymphoma, ALL, Wilm's, Burkitt's, and ganglioneuroma were all reported in abroad. Tumors combined with SIgAD in China need futher observations.
We used Fisher's exact test to compare the difference in rates of abnormal clinical features and abnormal laboratory tests between men and women, children and adults. No significant difference was found (P > 0.05), mainly due to the lack of detection values in most patients. Only 14 patients from PUMCH had more detailed informations, and 4 cases from literatures had some simple results. The difference is not obvious because of the insufficient sample size and the insufficient amount of valid data. However, we noticed that elevated ALT found in 6/8 males and in 3/8 females, elevated CH found in 3/8 males and in 3/3 females, while elevated LDH found in 4/7 males and 1/4 females. These indicators may be worthy to pay more attention in follow-up studies.
To summary, the main clinical complications of SIgAD in China are recurrent infections and autoimmune diseases, while malignancy and allergy are infrequent. Allergy symptoms are always drug allergy. Most patients are without family histories. Women are more likely to have autoimmune diseases than men. For patients above age four, the diagnosis needs to be differentiated with transient IgA deficiency by multiple evaluations of immunoglobulin levels.
Funding
1. CAMS Innovation Fund for Medical Sciences(CIFMS) (2016-I2M-1-008).
2. The Capital Health Research and Development of Special (2016-2-40114).
3. Public Welfare Scientific Research Project of China (201402012).
4. Golden Brige Engineering Seed Found of Beijing Association for Science and Technology(JQ17032).
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30(1):10–16. doi: 10.1007/s10875-009-9357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odineal D.D., Gershwin M.E. The epidemiology and clinical manifestations of autoimmunity in selective IgA deficiency. Clin Rev Allergy Immunol. 2019 Jul 2 doi: 10.1007/s12016-019-08756-7. [DOI] [PubMed] [Google Scholar]
- 3.Guo L.Z., Shen G.Q., Su Y.X. Investigation of selective IgA deficiency in some populations in Nanjing and Shanghai. Zhonghua Yixue Zazhi. 1985;65(6):365–366. [Chinese] [Google Scholar]
- 4.Wang N., Lu P., Ling B., Zhu Z., Hammarström L. Caucasian origin of disease associated HLA haplotypes in Chinese blood donors with IgA deficiency. J Clin Immunol. 2014;34(2):157–162. doi: 10.1007/s10875-013-9983-1. [DOI] [PubMed] [Google Scholar]
- 5.Feng L., Yu S.J., Song S.Y. Epidemiological investigation of selective IgA deficiency in six ethnic groups in different regions of China. Zhonghua Yixue Zazhi. 1992;72(2):88–90. [Chinese] [Google Scholar]
- 6.Wang H., Wang R.Y., Liu H. Clinical analysis of 35 cases of selective IgA deficiency. Shan Xi Yi Xue Za Zhi. 2000;29(7):440. [Chinese] [Google Scholar]
- 7.Zhang J., Wang H., Zhou S.H., Zhan X.M. Epidemiological investigation of selective IgA deficiency in children. Yi Xue Xin Xi. 1995;8(10):479–480. [Chinese] [Google Scholar]
- 8.Zhao H.W. Epidemiological investigation of selective IgA deficiency in Yanbian area. Zhongguo Xi Bu Ke Ji. 2013;12(05):58–71. [Chinese] [Google Scholar]
- 9.Ou F.X., Baunogel Feng H. Epidemiological investigation of selective IgA deficiency in Mongolian children and adolescents. Neimenggu Yi Xue Za Zhi. 1994;14(4):241–242. [Chinese] [Google Scholar]
- 10.Conley M.E., Notarangelo L.D., Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (pan-American group for immunodeficiency) and ESID (European Society for Immunodeficiencies) Clin Immunol. 1999;93(3):190–197. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 11.Hammarström L., Vorechovsky I., Webster D. Selective IgA deficiency (SIgAD) and common variable immunodeficiency (CVID) Clin Exp Immunol. 2000;120(2):225–231. doi: 10.1046/j.1365-2249.2000.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z.L., Tao Y. Four cases of systemic lupus erythematosus complicating with Selective Immunoglobulin A Deficiency and review of literature. Chin J Allergy ClinImmunol. 2015;9(1):36–39. [Chinese] [Google Scholar]
- 13.Zhang F., Tu P., Zhang Y., Zhou W., Tang F.L., Kuang J. Selective IgA deficiency. J Clin Dermatol. 2012;41(4):211–213. [Chinese] [Google Scholar]
- 14.Gao J.J. One case of retinoblastoma complicated with selective IgA deficiency. Zhonghua Yan Ke Za Zhi. 1989;25(6):375–376. [Chinese] [Google Scholar]
- 15.Chen Y., Jin M., Zhao W. DICER1-Negative Pleuropulmonary Blastoma in a patient with selective IgA deficiency. Pediatr Blood Cancer. 2016;63(4):757–758. doi: 10.1002/pbc.25856. [DOI] [PubMed] [Google Scholar]
- 16.Hui C.K. Recurrent intestinal obstruction in a patient with selective IgA deficiency. Malays J Med Sci. 2016;23(6):123–127. doi: 10.21315/mjms2016.23.6.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yi Z.W. Three cases of nephrotic syndrome complicated with selective IgA deficiency. Hunan Yi Xue. 1990;7(2):99. [Chinese] [Google Scholar]
- 18.Zong S.J., Dong Y. A case report of selective IgA deficiency. Zhongguo Yi XueKeXue Yuan XueBao. 1981;3(3):201–203. [Chinese] [PubMed] [Google Scholar]
- 19.Ai M.X., Li X.H., Zeng X.F. One case of systemic lupus erythematosus complicated with selective IgA deficiency. Chin J Intern Med. 2001;40(6):416. [Chinese] [Google Scholar]
- 20.Department of Pediatrics, Peking University People's Hospital One case of selective IgA deficiency. Zhonghua Er Ke Za Zhi. 1981;(2):128. [Chinese] [Google Scholar]
- 21.Song S.Y. A case report of Selective IgA deficiency with plasmacytic granuloma. Zhonghua Yixue Zazhi. 1995;75(8):469. [Chinese] [Google Scholar]
- 22.Jia Y.P., Sun J.Y. A case of Selective IgA deficiency complicated with Evans syndrome and Hashimoto thyroiditis. Zhonghua Er Ke Za Zhi. 2001;39(11):661. [Chinese] [Google Scholar]
- 23.Dominguez O., Giner M.T., Alsina L., Martín M.A., Lozano J., Plaza A.M. Clinical Phenotypes associated with SIgAD - a review of 330 cases and proposed followup protocol. An Pediatr. 2012;76(5):216–217. doi: 10.1016/j.anpedi.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Shkalim V., Monselize Y., Segal N., Zan-Bar I., Hoffer V., Garty B.Z. Selective IgA deficiency in children in Israel. J Clin Immunol. 2010 Sep;30(5):761–765. doi: 10.1007/s10875-010-9438-x. [DOI] [PubMed] [Google Scholar]
- 25.Sekine H., Ferreira R.C., Pan-Hammarström Q. Role for Msh5 in the regulation of Ig class switch recombination. Proc Natl Acad Sci U S A. 2007;104(17):7193–7198. doi: 10.1073/pnas.0700815104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castigli E., Ceha R.S. TACI, isotyping, CVID and IgAD. Immunol Res. 2007;38(1–3):102–111. doi: 10.1007/s12026-007-8000-2. [DOI] [PubMed] [Google Scholar]
- 27.Jacob C.M., Pastorino A.C., Fahl K., Carneiro-Sampaio M., Monteiro R.C. Autoimmunity in IgA Deficiency: revisiting the role of IgA as a silent housekeeper. J Clin Immunol. 2008;28(Suppl 1):S56–S61. doi: 10.1007/s10875-007-9163-2. [DOI] [PubMed] [Google Scholar]
- 28.Rahman A., Isenberg D.A. Systemic lupus erythematosus. N Engl J Med. 2008;358(9):929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 29.Selmi C., Gershwin M.E. Sex and autoimmunity: proposed mechanisms of disease onset and severity. Expert Rev Clin Immunol. 2019;15(6):607–615. doi: 10.1080/1744666X.2019.1606714. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham-Rundles C., Pudifin D.J., Armstrong D., Good R.A. Selective IgA deficiency and neoplasia. Vox Sang. 1980;38(2):61–67. doi: 10.1111/j.1423-0410.1980.tb02332.x. [DOI] [PubMed] [Google Scholar]