Abstract
Primary immunodeficiency diseases (PIDs) refer to a heterogenous group of disorders characterized clinically by increased susceptibility to infections, autoimmunity and increased risk of malignancies.
These group of disorders present with clinical manifestations similar to PIDs with known genetic defects but have either no genetic defect or have a somatic mutation and thus have been labelled as “Phenocopies of PIDs”. These diseases have been further subdivided into those associated with somatic mutations and those associated with presence of auto-antibodies against various cytokines.
In this review, we provide an update on clinical manifestations, diagnosis and management of these diseases.
Keywords: Anti-cytokine antibodies, Phenocopies, Primary immunodeficiency diseases, Somatic mutations
Introduction
Primary immunodeficiency diseases (PIDs) refer to a group of disorders distinguished clinically by increased susceptibility to infections, autoimmunity and increased risk of malignancies. More than 350 single gene defects have been identified to cause PIDs.1 A large majority of immune deficiency diseases are inherited and have an associated genetic defect. “Phenocopies of PIDs” is a special group of immune deficiencies that are caused by a somatic mutation (rather than genomic mutation) or autoantibodies against various cytokines thereby producing clinical manifestations similar to other PIDs. These diseases do not follow a Mendelian pattern of inheritance. Phenocopies of PIDs have been classified separately by the International Union of Immunological Societies (IUIS).2 Phenocopies of PIDs are rare and the majority of them have been identified in the last decade. In this review, we provide an update on clinical manifestations, diagnosis, and management of these diseases.
Understanding somatic mutations
When an individual has more than one cell populations that are distinct genetically, this is referred to as mosaicism.3 Both germ cells and somatic cells may demonstrate this phenomenon if mosaicism takes place during the developmental stages of cells. Contrary to this, if mosaicism occurs later in life then germ cells may get affected while leaving the somatic cells unaffected. Somatic mosaicism may arise due to somatic mutations; change in numbers or structure of chromosomes or epigenetic changes in DNA. Somatic mutations usually occur following the process of zygote formation.3 Somatic mosaicism may be detected using Sanger sequencing or next-generation sequencing. High throughput sequencing is required to detect somatic variants.4
Detection of somatic mutations through next-generation sequencing requires highly specialized filters and algorithms as these mutations occur with very low allele frequencies in the population. Additional filters (such as mapQ, baseQ, read depth, alternative allele fraction, read position, mismatches per read and strand bias) may be applied to remove artifacts. To identify changes in single nucleotide or large structural DNA, the reads are mapped to reference annotation. Single nucleotide variation (SNV) is the discrepancy between mapped sequence and reference annotation. The accuracy of sequencing increases by mapping a higher number of reads (i.e.by using higher read depth).
Isolating the exome gives a relatively cost-effective 100X coverage of the genome coding regions and may especially be useful for recognizing rare genomic variants from a population of cells.5 When a matched normal tissue is available, variant databases and “panel of normals” (PON) is applied to refine the level of specificity. Read-based phasing can analyze the relationship between putative somatic mutations and germline heterozygous variants and identifies true somatic mosaic mutations. Misaligned reads are a major cause of false positive variant calls. Variant allele fractions (VAFs) are the fraction of alleles in the sample that is mutated. VAF of a somatic mutation depends on the heterogeneity of the tissue or sample selected for sequencing as well as on the prevalence of the mutation. The VAF threshold can be used to differentiate between somatic and germline mutations. If a somatic mutation occurs earlier during development it usually acquires higher VAFs.6
Several recent technologies such as bulk sequencing, linked-read sequencing and single cell sequencing (sequencing of DNA from a single cell) enable refined phasing of variants and improve the accuracy of detection. Single cell sequencing may detect even the rare mosaic mutations.6 Hybrid experimental designs integrate both bulk and single cell sequencing for the detection of somatic mutations. Somatic mutations detected in bulk sequencing may be confirmed using single-cell sequencing.6
Limitations in the detection of somatic mutations
The availability of a matched normal tissue is a limitation in the process of detection of somatic mutations. DNA contamination, DNA damage, platform biases, read misalignment, obtaining coverage in regions of extreme GC/AT content, amplification errors and instrument errors are several other limitations. These factors are often observed at low allele frequencies and outnumber the mosaic mutations. Higher coverage and increasing the depth of sequencing may overcome several of these limitations.6
Phenocopies of PID caused by somatic mutations
Autoimmune Lymphoproliferative Syndrome (ALPS) caused by somatic mutation in TNFRSF6gene
ALPS, also known as Canale-Smith Syndrome is an uncommon PID characterized by Fas-mediated defective apoptosis thereby leading to abnormal lymphocyte homeostasis. Clinical manifestations include non-malignant reticuloendothelial cell proliferation (i.e. hepatosplenomegaly and lymphadenopathy) and autoimmunity. This disease also predisposes an individual to develop malignancies especially lymphomas.7
The protein Fas (Apo-1/CD95) is encoded by a tumor necrosis factor receptor superfamily member 6 (TNFRSF6) gene and is involved in maintaining lymphocyte homeostasis8 by causing apoptosis (Fig. 1). Mutation in this gene leads to dysfunction of CD95 protein and has been found to cause autoimmune diseases in mice8 and ALPS in humans.9,10
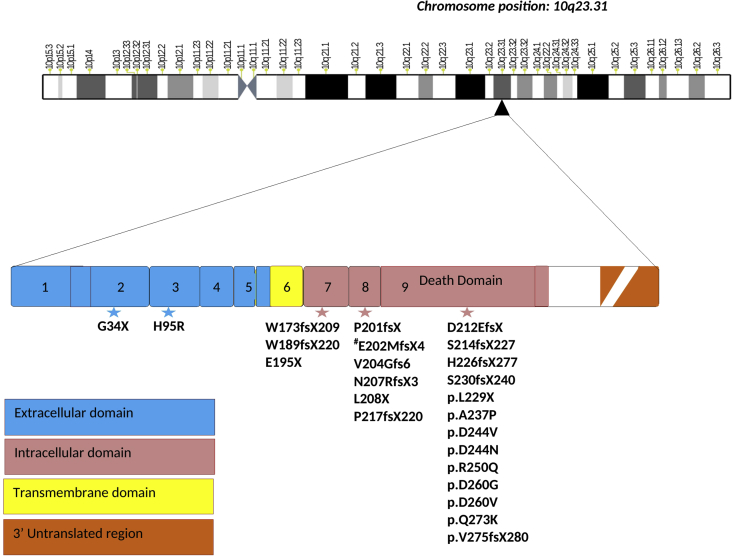
Figure 1.
Gene structure of TNFRSF6 (TNF-receptor superfamily 6) gene located at chromosome position10q23.31. The numbers 1 to 9 denote the number of exons. Exon 1–6 encode extracellular and trans membrane domain while intracellular domain is encoded from exon 7–9. The death domain is encoded within exon 9. Star denotes somatic mutations in the various domains of the gene, most of the mutations being frameshift. Intracellular domain (majorly death domain) is the hotspot for most mutations. a.a. denotes position of amino acids.
A large number of patients with ALPS have mutations in FAS (ALPS type Ia), FAS ligand (ALPS type Ib), caspase 8 or caspase 10 gene (ALPS type II). However, a proportion of patients with a clinical phenotype suggestive of ALPS with elevated double negative T (DNT) cells and defective apoptosis do not have these mutations. Somatic mutations in the FAS gene involving DNT cells were reported for the first time by Holzelova et al in 2004 in 6 patients.11 Although somatic mutations in the FAS gene have previously been identified in patients with lymphoma, Holzelova et al reported for the first time that a somatic mutation may also cause PID. Subsequently, Dowdell et al identified somatic FAS gene mutations in 12/31 patients who had manifestations similar to ALPS but were not found to have germline mutations in FAS or FAS ligand gene.12 Subsequently, other authors have also reported somatic mutations in these patients.13, 14, 15 Summary of all reported somatic mutations in the Fas gene known to cause ALPS are given in Table 1.
Table 1.
Somatic Mutations associated with TNFRSF6 gene.
| Author/Year | Gene | Exon/Intron | Mutation |
|---|---|---|---|
| Dowdell et al12/2010 | TNFRSF6 | Exon 7 Intron 7 Intron 7 Exon 9 Intron 8 Exon 8 Exon 8 Exon 8 Exon 8 Exon 3 Intron 7 Exon 9 |
p.E195X p.E202MfsX4 (splice mutation) p.E202MfsX4 (splice mutation) p.D244N p.E202MfsX4 (splice mutation) p.N207RfsX3 p.E202MfsX4 (splice mutation) p.V204Gfs6 p.L208X p.H95R p.E202MfsX4 (splice mutation) p.D212EfsX2 |
| Holzelova et al11/2004 | TNFRSF6 | Exon 8 Exon 8 Exon 9 Exon 9 Exon 7 Exon 8 |
p.P201fsX204 p.P201fsX204 p.D244V p.S214fsX227 p.W173fsX209 p.P201fsX204 |
| Martinez et al13/2016 | TNFRSF6 | Exon 9 | p.L229X p.D260G p.R250Q |
| Neven et al14/2011 | TNFRSF6 | Exon 7 Exon 8 Exon 9 Exon 9 Exon 9 Exon 9 Exon 9 |
p.W189fsX220 p.P217fsX220 p.H226fsX277 p.S230fsX240 p.D260V p.R250Q p.Q273K |
| Magerus-Chatinet et al15/2011 | TNFRSF6 | Exon 9 Exon 2 Exon 9 |
p.V275fsX280 p.G34X p.A237P |
TNFRSF6:Tumor necrosis factor receptor superfamily member 6.
Somatic mutations in the FAS gene causing ALPS type III is now the second most common type of ALPS (germline mutation in the Fas gene is the most common type).14 Clinical phenotype of patients with ALPS caused by somatic mutations in the FAS gene (ALPS-sFAS) is similar to other types of ALPS (i.e. splenomegaly, lymphadenopathy, hepatomegaly, autoimmunity, elevated DNT cells, high IL-10, high Vitamin B12, increased serum FAS ligand and histopathology findings). However, it has been reported that the onset of symptoms in patients with ALPS-sFAS is usually later and patients remain stable for a prolonged period,16 thereby leading to delay in the diagnosis. It has also been reported that absolute lymphocyte count of these patients is relatively low, total cholesterol and high-density lipoprotein (HDL) cholesterol is low and incidence of splenectomy is less in these patients when compared to patients with ALPS caused by germline mutations.17 In vitro apoptosis assay has shown that cells in these patients are also resistant to Fas-mediated apoptosis. However, the degree of apoptosis seen in these patients is higher than that seen in patients with ALPS type Ia. These clinical pointers may suggest a possibility of a somatic mutation in the FAS gene. However, it may be very difficult to predict this syndrome based on clinical phenotype alone. Therefore, all patients with a clinical suspicion of ALPS with elevated serum biomarkers and no germline mutations should be analyzed for somatic mutation in the FAS gene. Diagnosis is established by isolating DNT cells and doing the genetic analysis for the FAS gene in these cells. The mutation is absent in buccal mucosal epithelial cells.
Somatic mutations in the FAS gene have also been reported to be clinically useful in patients with haploinsufficient germline heterozygous FAS mutations.18 In these patients, somatic mutations act as second hit thereby leading to disease manifestation in a patient who would have otherwise been asymptomatic. Thus, symptomatic patients with haploinsufficient germline heterozygous mutations should also be evaluated for somatic FAS gene mutations.
Treatment is focused on disease manifestations and is similar to other types of ALPS. Autoimmune manifestations are treated with immunosuppressants (glucocorticoids, mycophenolate mofetil, rituximab, cyclosporine, and sirolimus).19 In refractory disease, intravenous immunoglobulin, plasmapheresis, and bortezomib has also been used.20 Lymphoma in these children can be treated with conventional therapy. Hematopoietic stem cell transplant has also been tried in a few patients.21
With the advancement in molecular technologies in the recent past, it is likely that more patients with this disease would be identified. This will enhance our understanding of the clinical phenotype of this disease and the risk of malignancy.
RALD: RAS-associated autoimmune leukoproliferative disease (ALPS like)
Germline mutations in the RAS gene have been found in patients with Costello syndrome, cardio-facio-cutaneous syndrome or Noonan syndrome. Somatic mutations in RAS gene have largely been reported to predispose to malignancies especially juvenile myelomonocytic leukemia and multiple myeloma. Ras-associated autoimmune leukoproliferative disorder (RALD) is a recently described PID characterized by non-malignant lymphoproliferation and autoimmune manifestations. This disease was initially considered to be a subset of ALPS.22 RALD is caused by activating somatic mutations affecting codons 12 or 13 in KRAS or NRAS gene involving myeloid and lymphoid lineages.23 The RAS gene encodes for GTPases that are important for differentiation, growth, and survival of cells (Figure 2, Figure 3). In patients with RAS gene mutations, there is activation of the signalling pathway and hyperactivation of RAS pathway.24 A revised classification and nomenclature for RALD were proposed in 2009 to distinguish it from ALPS.25
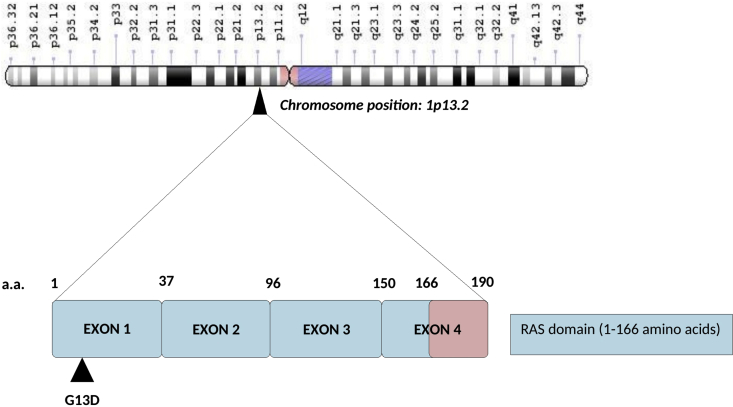
Figure 2.
Gene structure of NRAS (N-ras oncogene) gene located at chromosome position 1p13.2. The gene has 4 coding exons. Somatic mutations highlighted by a triangle are present in the GTP binding domain of the Ras binding domain present in Ras (rous sarcoma) proto-oncogene family (NRAS, KRAS, HRAS and RRAS).
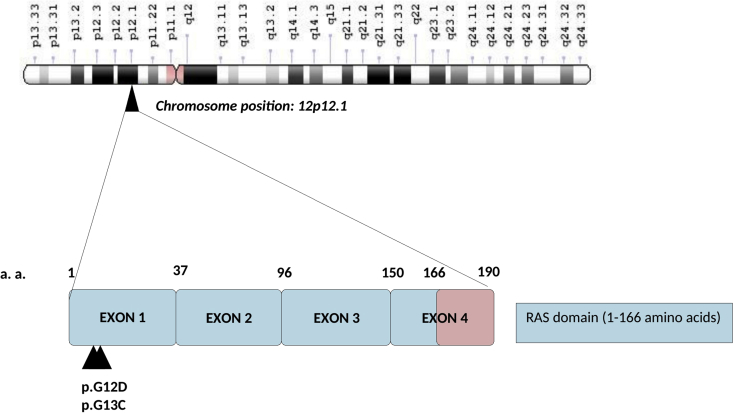
Figure 3.
Gene structure of KRAS (K-ras oncogene) gene consists of 4 coding exons and is located at chromosome position 12p12.1. Somatic mutations highlighted by a triangle are present in the GTP binding domain of the RAS binding domain.
Somatic mutation is known to cause RALD have been reported by Niemela et al23 and Shiota et al26 (Table 2).
Table 2.
Somatic Mutations associated with KRAS and NRAS gene.
This disease shares several clinical features with ALPS and JMML.27 The overlapping manifestations between patients with ALPS and RALD include splenomegaly, lymphadenopathy, hypergammaglobulinemia, increased circulating B cells, and autoimmunity.28 Patients with RALD may also have systemic lupus erythematosus (SLE) like autoimmune manifestations with low complements, elevated double-stranded DNA antibody (dsDNA) and antiphospholipid antibody (APLA) positivity. However, when compared with patients with ALPS, patients with RALD do not have increased serum vitamin B12 levels, DNT cells are normal and Fas-mediated apoptosis defect is not seen. However, RALD due to mutation in NRAS gene may have elevated DNT cells.29 The defect in apoptosis is because of resistance to interleukin-2 (IL-2) depletion-dependent apoptosis.30
There are few reports of cutaneous involvement in patients with RALD that present in with erythematous plaques with panniculitis like and histiocytoid sweet syndrome also known as RALD cutis.31,32 These manifestations are often benign and may develop because of monocytes infiltrating the skin.
Patients with RALD have persistent monocytosis and at times it is difficult to differentiate this entity from juvenile myelomonocytic leukemia (JMML) or chronic myelomonocytic leukemia (CMML). However, the presence of autoimmunity favors RALD and the presence of cytogenetic abnormality (e.g. Monosomy 7) favors JMML. Lymphoproliferation in patients with RALD is usually benign but malignant transformation27 and evolution into JMML33 is known and hence a close follow up is required.
Because of the limited literature on RALD, there are no well-defined treatment protocols. Corticosteroids and other immunomodulatory therapies are given for the management of autoimmunity in RALD. Rituximab has also been found to be effective in patients with refractory cytopenias.34
Cryopyrinopathy (Familial cold auto-inflammatory syndrome [FCAS]; Muckle-wells syndrome; chronic infantile neurologic, cutaneous, articular syndrome [CINCA]/neonatal-onset multisystem inflammatory disease [NOMID])
Cryopyrinopathies (Cryopyrin associated periodic fever syndrome; CAPS) are autoinflammatory disorders caused by gain-of-function mutations in the NLRP3 gene (Figure 4, Figure 5). This gene encodes cryopyrin protein that further leads to hyperactivation of IL-1β.35 These diseases follow an autosomal dominant pattern of inheritance. Recent studies on genetic etiology of patients with CAPS have shown that somatic mutations may account for a large majority of patients (5–69%) who have been found to be negative for germline mutation in the NLRP3 gene.36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 Vertical transmission of somatic NLRP3 gene mutation (similar to the Mendelian inheritance) has also been reported.47 A novel somatic mutation in the NLRC4 gene in a patient with clinical features consistent with NOMID has been identified.38
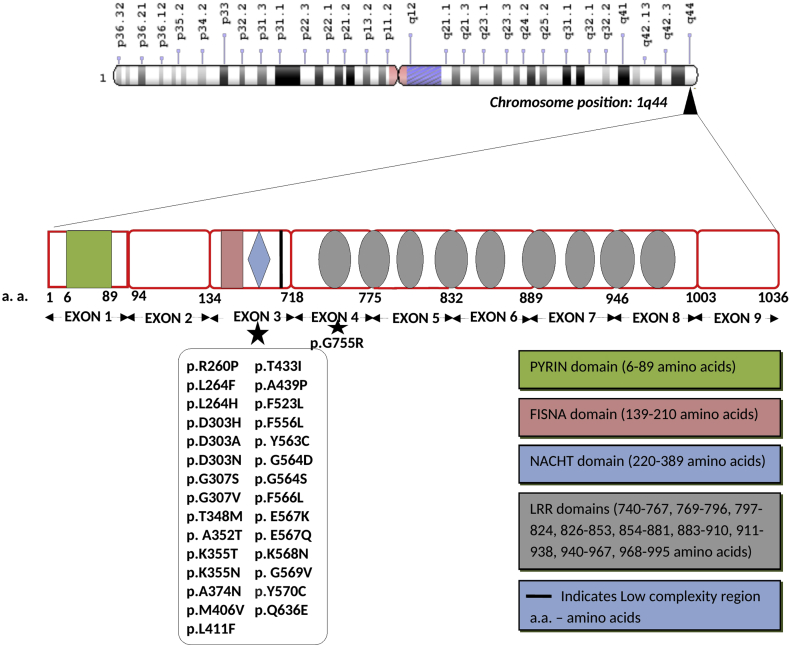
Figure 4.
Gene structure of NLRP3 (NLR family pyrin domain containing 3)gene located at chromosome position1q44. Majority of the somatic mutations are present in the Exon 3 of the gene. LRR represents the leucine rich repeats. Various domains present in the gene are highlighted in the diagram.
Figure 5.
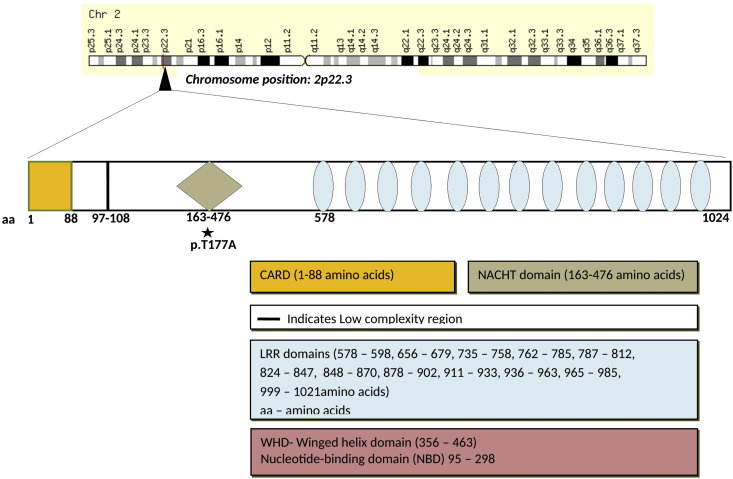
Gene structure of NLRC4 (NLR family CARD domain-containing protein 4)gene located at chromosome position 2p22.3. Various domains present in the gene are highlighted in the diagram. A somatic mutation p.T177A is present in the NACHT domain of the gene.
Although patients with CAPS usually present in childhood, patients who carry a somatic mutation may present even in adulthood.36 Clinical manifestations include fever, joint involvement, and pseudourticarial skin rash. Laboratory investigations often reveal neutrophilic leukocytosis and elevated acute-phase reactants (such as C-reactive protein and erythrocyte sedimentation rate). The spectrum of disease severity may vary from mild diseases as seen in patients with FCAS to moderate disease seen in MWS and severe disease seen in patients with CINCA (NOMID).48 The skin rash is the earliest manifestation and is often seen in infancy. Cryopyrinopathies caused by somatic mutations in the NLRP3 gene has a slightly different phenotype as compared to patients with germline mutations. Other than a delayed onset of diseases,36 patients have milder neurological symptoms.37
Therapy targeted against IL-1β (rilonacept and anakinra) is the treatment of choice.49 Summary of all reported somatic mutations in patients with Cryopyrinopathies is given in Table 3.
Table 3.
Summary of all reported somatic mutations in Cryopyrinopathies.
| Author/Year | Gene involved | Mutation |
|---|---|---|
| Saito et al43/2005 Zhou et al38/2015 |
NLRP3/CIAS1 | p.Y570C |
| Rowczenio et al34/2017 | NLRP3 | p. Y563C p. A352T p. G569V p. E567K p. E567Q p. G564D |
| Lasigliè et al35/2017 | NLRP3 | p.R260P p.G564S p.T433I |
| Aróstegui et al42/2002 | NLRP3 | p.D303H |
| Omoyinmi et al39/2014 | NLRP3 | p.F556L |
| Jiménez-Treviño et al45/2013 | NLRP3 | p.T348M |
| Mensa-Vilaro et al37/2016 | NLRP3 | p.Q636E |
| Nakagawa et al40/2015 | NLRP3 | p.D303A p.K355T p.L411F |
| Tanaka et al41/2011 | NLRP3 | p.G307S p.K355N p.M406V p.T433I p.F566L p.E567K p.K568N p.L264F p.D303H p.G307V p.A439P p.Y570C p.G755R |
| Kawasaki et al36/2017 | NLRC4 | p.T177A |
| Aksentijevich et al42/2002 | NLRP3 | p.L264H p.D303N p.A374N p.Y570C p.F523L |
Hypereosinophilic syndrome due to somatic mutations in STAT5b gene (STAT5b gain of function mutation)
Somatic mutations in the STAT5b gene have previously been reported to predispose to malignancies (Fig. 6). A novel somatic mutation in the STAT5b gene involving hematopoietic progenitor cells has recently been identified to cause eosinophilia, atopic dermatitis, urticarial rash, allergies, respiratory infections and episodic diarrhea in 2 patients. The striking feature in both patients was skin manifestations and eosinophilia. Family history suggestive of autoimmune diseases was present in 1 patient. Functional studies in T cells showed a marked increase in STAT5B responsiveness.50 Cross et al also reported a somatic mutation in STAT5b gene.51 Somatic Mutations associated with the STAT5b gene are given Table 4. Disease was relatively mild in one while another patient had severe disease and also had eosinophilic infiltrates in the gut biopsy. She was given umbilical cord stem cell transplant but died later.
Figure 6.
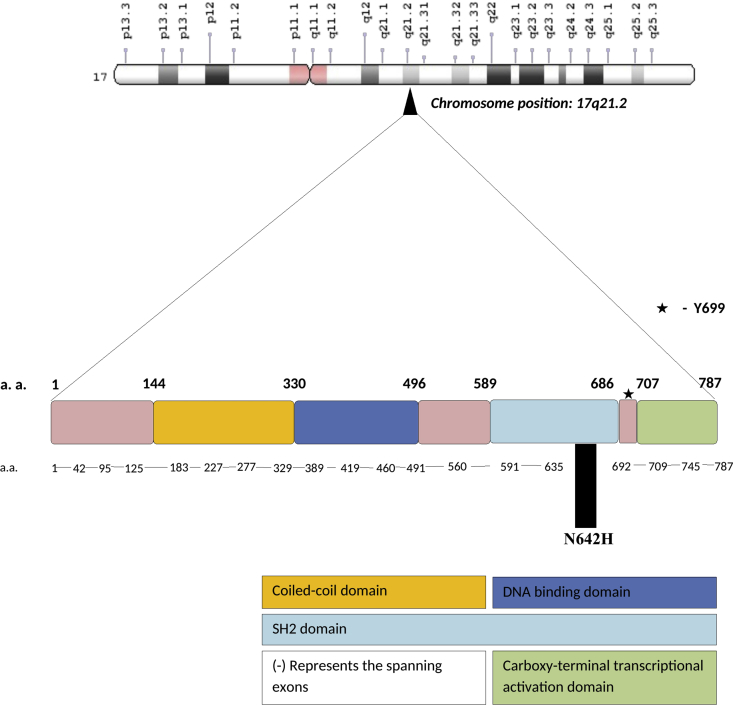
Gene structure of STAT 5b (Signal transducer and activator of transcription 5b) gene located at chromosome position17q21.2. It encodes 787 amino acid long protein and consists of 18 coding exons. A dash (−) represents the exons spanning the gene. N642H is the somatic gain of function mutation present in the Src homology 2(SH2) domain of the gene. Various domains present in the gene are highlighted in the diagram.
Table 4.
Somatic Mutations associated with STAT5b gene.
STAT5b: Signal transducer and activator of transcription.
Phenocopies of PIDs caused by autoantibodies against various cytokines
Chronic mucocutaneous candidiasis (isolated or associated with APECED syndrome) caused by autoantibodies to IL-17 and/or IL-22
Chronic mucocutaneous candidiasis (CMC) manifests as persistent or recurrent candida infections involving skin, nails, and mucous membranes without dissemination to internal organs and autosomal recessive mode of inheritance. Association of CMC and autoimmune hypoparathyroidism was first described in 1929 by Thorpe and Handley.52 It is also termed as autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED)53 and is caused by a loss-of-function mutation in autoimmune regulator (AIRE) gene. AIRE protein is essential for the process of central immune tolerance. Defect in AIRE allows autoreactive T cells to evade negative selection within the thymus, thereby leading to autoimmunity.54 CMC is often the first manifestation of APECED and appears before the age of 553.
IL-17 and IL-22 are pro-inflammatory cytokines produced predominantly by CD4+ T cells and T helper 17 (Th17) cells. Role of IL-17 and IL-22 immunity is well established in controlling the candida infections in humans.55,56 Several genetic defects leading to impaired IL-17 and IL-22 associated immunity predisposes to CMC. These include mutations in IL-17F, IL-17RA, IL-17RC, STAT3, STAT1, IL-12Rβ1, IL-12p40, CARD-9, and DECTIN-1 gene. In addition, the presence of antibodies against IL-17 cytokine has also been found to increase susceptibility to CMC. Studies have shown that a large majority of patients with APECED also have auto-antibodies against IL-17F, IL-17A, and IL-22. These antibodies are responsible for mucocutaneous candida infection in these patients.57 Paul et al evaluated 33 patients with APECED. All of them were identified to have antibodies againstIL-17F,IL-17A, and IL-22 while 29/33 patients developed CMC.58 Similar results have also been reported by Kisand et al59 in a multicentric and multinational study including 162 patients. These antibodies were detected in a large majority of patients especially in those patients who developed CMC. Surprisingly, these antibodies were also found in patients with thymoma who had CMC. It was also observed that titers of these antibodies declined with age in patients with APECED while titers of antibodies against type 1 interferon did not decrease. Serum levels of these antibodies were also found to correlate with the severity of candidiasis.57
Various techniques have been used to identify these antibodies. These include multiplex particle-based flow cytometry; western blotting and Enzyme Linked Immunosorbent Assay (ELISA).
Management involves treating endocrine and infectious manifestations.53 Ketoconazole is an effective and well-tolerated drug for the treatment of CMC.60
Autoantibodies against IL12p40
IL-12 cytokine is involved in protection against intracellular pathogens especially mycobacterium. Signaling via IL12p40 (a component of IL-12) leads to phosphorylation of Janus kinase 2 (JAK2) and Tyrosine kinase 2 (TYK2), that in turn causes phosphorylation and docking of signal transducer and activator of transcription 4 (STAT4) and subsequently causing transcription of genes related to interferon-γ (IFN-γ).61
Antibodies to IL12p40 may present with similar clinical manifestations as seen in patients with autoantibodies to IFN-γ. There has been only a single reported case of this immunodeficiency. A woman of Cambodian ethnicity who presented with recurrent lymphadenitis caused by Burkholderia gladioli was found to have a defect in the IL12-STAT4 signalling pathway. She was identified to have high titers of neutralizing auto-antibodies against IL12p40. High titers of this antibody have also been noted in patients with myasthenia gravis or thymoma. However, infections have not been reported in these patients so far.62
Autoantibodies to Interferon-α (IFN-α)
IFN-α works by phosphorylating STAT1/2 and aids in the transcription of type 1 interferon genes.63 Autoantibodies to type 1 IFNs have been reported in patients with autoimmunity (lupus, autoimmune thyroid diseases and type 1 diabetes),64 APECED,65 malignancies (metastatic colon cancer, breast cancer, and renal cell cancer)66 and sometimes even in healthy persons (200 donors).67 However, none of these patients have been reported to have infections except one patient who had varicella zoster reactivation that got disseminated and this individual was identified to have anti-IFN-α antibodies.68
Adult-onset immunodeficiency with susceptibility to mycobacterium (autoantibody to IFN-γ)
Interferon-γ (IFN-γ) is the predominant cytokine for defence against intracellular organisms. It is produced by T-cells and natural killer (NK) cells. IFN-γ activates macrophages to cause phagocytosis and killing of intracellular microorganisms such as Mycobacterium. The presence of autoantibodies against IFN-γ may impair the STAT1 phosphorylation and production of tumor necrosis factor-α (TNF-α) and IL-1256. The majority of patients with this rare immunodeficiency have been reported from Southeast Asia, Thailand and Taiwan.69 A gender predominance (female–>–male) for these diseases is seen in patients who are residing outside Asia while no gender bias has been reported in patients from Asian countries. Patients who have autoantibodies against IFN-γ have also been found to be positive for HLA- DQB1*05:01/05:02 and DRB1*15:02/16:0269. Moreover, it has also been revealed that anti-IFN-γ autoantibodies detect a major epitope (P121–131) at the C-terminus of IFN-γ.70 Functional activity of anti IFN- γ antibody was assessed using surface-enhanced laser desorption, affinity chromatography, ionization mass spectrometry, and N- terminal sequencing. The purified anti-IFN-γ antibody was found to inhibit the up-regulation of TNF-α production in response to endotoxin; to block the induction of IFN-γ inducible genes and to inhibit the up regulation of HLA class II expression on peripheral blood mononuclear cells (PBMCs).71
Clinical manifestations in patients with the presence of anti-IFN-γ antibody are similar to the manifestations seen in patients who have genetic defects in the IL-12/IFN-γ axis. These include infections with non-tubercular mycobacteria (NTM), coccidioidomycosis, listeriosis, salmonellosis, melioidosis, histoplasmosis, and penicilliosis.72 These patients, however, do not develop disseminated Bacillus Calmette-Guerin (BCG) disease. Browne et al reported that 88% of adult patients who presented with opportunistic infections had neutralizing anti-IFN-γ autoantibodies.73 While the lymph node is the predominant site of involvement,74 80% of the patients have been reported to have skin involvement in the form of neutrophilic dermatosis (Sweet syndrome).75 It has been suggested that patients with opportunistic infections and the presence of neutrophilic dermatoses should be evaluated for the presence of anti-IFN-γ antibodies.76
These autoantibodies can be measured using a particle-based technology77 or ELISA.78 Commonly available QuantiFERON-TB Gold In-tube (QFT-GIT) test can be used as a screening test for the detection of these autoantibodies. Undetectable or extremely low IFN-γ level may indicate the presence of neutralizing anti-IFN-γ antibodies.79
Management includes the use of appropriate antimicrobials as per the culture and drug sensitivity pattern. Immunomodulatory therapy to decrease the production of these autoantibodies may be needed in cases who fail to respond to antimicrobial agents alone. Cyclophosphamide has been found to reduce the production of autoantibody titres in cases who are refractory to antimycobacterial therapy alone.80 Other strategies include the use of intravenous immunoglobulin and plasmapheresis. There is recent evidence for the use of rituximab also.71,81
Autoantibodies against granulocyte macrophage colony stimulation factor (GM-CSF)
GM-CSF receptor is present on several cell lineages including neutrophils, dendritic cells, macrophage precursors, and megakaryocytes. GM-CSF is involved in immune activation, proliferation, and differentiation of these cells. GM-CSF is known to influence the terminal differentiation of monocytes to alveolar macrophages (by stimulating transcription factor PU) and thereby augments innate immunity.82,83
It has been shown that neutralizing anti-GM-CSF autoantibodies are present in healthy individuals and regulate excess GM-CSF activities in vivo.84 However, the overproduction of these autoantibodies is known to produce various diseases in humans. Neutralization of GM-CSF bioactivity by anti-GM-CSF autoantibody causes dysfunction of alveolar macrophages and reduced surfactant clearance resulting in pulmonary alveolar proteinosis (PAP). PAP is distinguished by the accumulation of surfactant components in alveoli with minimal interstitial inflammation or fibrosis and a varied clinical course ranging from spontaneous improvement to fatal respiratory failure. The basic pathology involves an abnormality of surfactant metabolism resulting in accumulation of acellular periodic acid-Schiff (PAS) positive proteinaceous material in pulmonary alveoli and the development of large foamy, monocyte-like alveolar macrophages.85 Primary PAP resulting from mutations in the GM-CSF receptor is the most severe form of the disease and leads to respiratory failure and death within the first few days of life.86 Secondary PAP is seen in patients who are on immunosuppressive therapies; who have hematological malignancies or because of the inhalation of toxins and is caused by a quantitative or qualitative deficiency of alveolar macrophages.87 Autoimmune PAP is a distinct clinical entity caused by IgG antibodies that block the effect of GM-CSF. Autoimmune PAP caused by anti-GM-CSF autoantibodies accounts for approximately 90% of all cases of PAP and commonly presents between 20 and 50 years of age. Clinical presentation varies from being asymptomatic to increasing dyspnea on exertion. Other clinical manifestations may include dry chronic cough, chest pain, weight loss, and fatigue. These autoantibodies may be detected in serum as well as in the bronchoalveolar lavage (BAL) fluid. In one study, anti-GM-CSF antibodies were detected in the serum of all patients with idiopathic PAP while none of the patients with secondary PAP or healthy individuals had these antibodies88,89. Levels of anti-GM-CSF antibodies in BAL fluid may correlate with disease severity and predict the need for subsequent therapeutic lung lavage.90,91
Autoantibodies against GM-CSF may also lead to neutrophil dysfunction.92 Defect in neutrophil function was found in patients with PAP who had anti-GM-CSF autoantibodies.93 Neutrophils had normal ultrastructure and differentiation markers; however, basal functions and antimicrobial properties after GM-CSF priming were impaired. This suggests that immune defect in these patients is not only restricted to the lungs. Anti-GM-CSF autoantibodies have also been reported in patients who have Nocardia infection involving lungs, central nervous system (CNS),94 septic arthritis and95 perinephric abscess.96 These autoantibodies have also been reported in patients with disseminated histoplasmosis97 and disseminated and CNS cryptococcus infection.98, 99, 100 Patients with these infections caused by anti-GM-CSF auto-antibodies usually do not have evidence for PAP. It has been postulated that infection may be an earlier presentation and PAP may develop later in patients who have anti-GM-CSF autoantibodies. Impaired phosphorylation of STAT5 and decreased production of MIP-1α in response to GM-CSF has been reported in these patients.101
In addition, neutralizing IgG anti-GM-CSF antibodies may be seen in up to 2–3% of patients treated with long term recombinant rhGM-CSF. Interestingly, non-neutralizing IgG anti-GM-CSF antibodies were also found in 70% of patients who were given vaccines where rhGM-CSF was used as an adjuvant. Autoantibodies to GM-CSF are also seen frequently in patients with myeloid leukemia and Crohn's disease. High titers antibodies may indicate an active disease.102, 103, 104, 105
Pulmonary function tests may reveal a restrictive pattern and may be used to assess disease activity. High-resolution computed tomography (HRCT) may show a characteristic “crazy paving” pattern (though the findings are not very specific). Bronchoalveolar lavage fluid analysis and estimation of auto-antibodies in BAL fluid and serum help establish the diagnosis.
Whole lung lavage remains the prime modality of the treatment of PAP. GM-CSF administration may be useful in patients who fail to respond to whole lung lavage.106 Prolonged antimicrobial therapy is given to patients with Cryptococcus and Nocardia infection. It is also believed that patients with very severe disease may have minimal residual GM-CSF activity and therefore may benefit from the removal of antibodies using either plasmapheresis or rituximab.107 There are no long term data on prolonged antimicrobial prophylaxis in these patients, however, it has been found to be useful in a few.108
Despite several advances in recent understanding about this disease, it still remains unanswered that why few patients develop only infections while others develop only PAP? What is the underlying mechanism for the production of these antibodies in pathogenic concentration? Is there a contribution of environmental or genetic factors? What is the most effective treatment strategy? Similarly, there are no data about whether a patient with an infection will eventually develop PAP later in life or not.
Acquired angioedema: AutoAb to C1 inhibitor
Angioedema secondary to deficiency of C1 esterase inhibitor (C1–INH) due to acquired causes is known as acquired angioedema (AAE) and is associated with the presence of anti C1–INH inactivating autoantibodies.109 Diseases associated with AAE include malignancy (especially lymphoma) monoclonal gammopathy of uncertain significance, autoimmune disorders like systemic lupus erythematosus (SLE) and dermatomyositis and human immunodeficiency virus (HIV) infection.110, 111, 112, 113, 114, 115, 116 In one study, monoclonal gammopathy of undetermined significance was the most common cause of AAE followed by non-Hodgkin lymphoma.117
Clinically, it is difficult to differentiate between C1–INH deficiency either hereditary or acquired as both are characterized by recurrent, non-pitting, self-resolving and non-pruritic edema. Some episodes can be associated with life-threatening airway edema.
Laboratory investigations would show low C4 and C1q with normal C3. C1–INH function and antigen are decreased. Reduced C1q helps differentiate AAE from HAE as C1q is normal in patients with HAE.
Plasma-derived human C1–INH concentrate is the first-line of therapy for managing severe attacks of AAE. However, its availability is limited in developing countries. In such situations, plasma concentrate may be given to replenish C1–INH. Tranexamic acid and stanazolol have also been used as maintenance therapy.118,119 Patients with AAE may develop resistance to recombinant C1–INH on follow-up due to its cleavage by autoantibodies. These patients may need a higher dose of C1–INH concentrate as compared to patients with HAE.118 Icatibant, a synthetic selective bradykinin B2 receptor antagonist may also be used in patients with AAE and is particularly helpful in patients who develop resistance to C1–INH therapy.120 AAE when occurs in association with malignancies often resolves after treating the underlying disease.121 Plasmapheresis and cytotoxic therapy has been used in resistant cases.122
Thymoma with hypogammaglobulinemia(Good syndrome)
Good syndrome (GS), was first described by Robert Alan Good and is characterized by a triad of thymoma, hypogammaglobulinemia, and immunodeficiency causing recurrent respiratory and systemic infections and autoimmunity.123 The thymus plays a prime role in balancing the immune system. Immunological manifestations are variable and range from asymptomatic state to severe disease manifestations such as immunodeficiency, autoimmunity, or both.62,124
Hypogammaglobulinemia is the hallmark of Good syndrome that predisposes to recurrent sinopulmonary infections with encapsulated bacterial organisms (Streptococcus pneumonia and Haemophilus influenza).125 Patients with thymoma may occasionally develop clinical manifestations that suggest cellular immunodeficiencies such as non-tubercular mycobacterial infections, Pneumocystis jirovecii pneumonia, mucocutaneous candidiasis, giardiasis, cryptococcosis, varicella zoster virus and cytomegalovirus infection, Kaposi's sarcoma and progressive multifocal leukoencephalopathy.126,127 Occasionally, B cell lymphopenia may be noted at presentation.128 Anti-cytokine autoantibodies may also be seen in these patients and are a potential cause of immunodeficiency.129,130
Management includes organism-specific antimicrobial therapy and intravenous immunoglobulin (IVIg).126 Secondary prophylaxis for Pneumocystis jirovecii pneumonia should be given.
Autoantibodies to interleukin-6
IL-6 is involved in both acute and chronic inflammation and is synthesized by many cell types including lymphocytes, macrophages, and hepatocytes. Autoantibodies against IL-6 can predispose an individual to develop serious bacterial infections especially with Staphylococcus sp.131, 132, 133 Low C-reactive protein in the serum despite serious bacterial infections is a ‘soft’ clue towards a diagnosis of this rare immunodeficiency.
Conclusion
‘Phenocopies of PIDs’ may be caused by a somatic mutation or anti-cytokine antibodies. Somatic mutations that were initially considered to predispose to malignancies have now been implicated in causing PIDs. Several patients who have clinical and laboratory manifestations suggestive of a PID but have not been found to have a genetic defect may have either a somatic mutation or an antibody against cytokines. Better understanding and advancement in the techniques for the detection of somatic mutations may lead to the identification of several novel defects.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Yu J.E., Orange J.S., Demirdag Y.Y. New primary immunodeficiency diseases: context and future. Curr Opin Pediatr. 2018;30(6):806–820. doi: 10.1097/MOP.0000000000000699. [DOI] [PubMed] [Google Scholar]
- 2.Bousfiha A., Jeddane L., Picard C. The 2017 IUIS phenotypic classification for primary immunodeficiencies. J Clin Immunol. 2018;38(1):129–143. doi: 10.1007/s10875-017-0465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Youssoufian H., Pyeritz R.E. Mechanisms and consequences of somatic mosaicism in humans. Nat Rev Genet. 2002;3(10):748–758. doi: 10.1038/nrg906. [DOI] [PubMed] [Google Scholar]
- 4.Wang S.A., Tam W., Tsai A.G. Targeted next-generation sequencing identifies a subset of idiopathic hypereosinophilic syndrome with features similar to chronic eosinophilic leukemia, not otherwise specified. Mod Pathol. 2016;29(8):854–864. doi: 10.1038/modpathol.2016.75. [DOI] [PubMed] [Google Scholar]
- 5.Churko J.M., Mantalas G.L., Snyder M.P., Wu J.C. Overview of high throughput sequencing technologies to elucidate molecular pathways in cardiovascular diseases. Circ Res. 2013;112(12):1613–1623. doi: 10.1161/CIRCRESAHA.113.300939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dou Y., Gold H.D., Luquette L.J., Park P.J. Detecting somatic mutations in normal cells. Trends Genet TIG. 2018;34(7):545–557. doi: 10.1016/j.tig.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George L.A., Teachey D.T. Optimal management of autoimmune lymphoproliferative syndrome in children. Paediatr Drugs. 2016;18(4):261–272. doi: 10.1007/s40272-016-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagata S., Golstein P. The Fas death factor. Science. 1995;267(5203):1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 9.Fisher G.H., Rosenberg F.J., Straus S.E. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81(6):935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 10.Jackson C.E., Fischer R.E., Hsu A.P. Autoimmune lymphoproliferative syndrome with defective Fas: genotype influences penetrance. Am J Hum Genet. 1999;64(4):1002–1014. doi: 10.1086/302333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holzelova E., Vonarbourg C., Stolzenberg M.-C. Autoimmune lymphoproliferative syndrome with somatic Fas mutations. N Engl J Med. 2004;351(14):1409–1418. doi: 10.1056/NEJMoa040036. [DOI] [PubMed] [Google Scholar]
- 12.Dowdell K.C., Niemela J.E., Price S. Somatic FAS mutations are common in patients with genetically undefined autoimmune lymphoproliferative syndrome. Blood. 2010;115(25):5164–5169. doi: 10.1182/blood-2010-01-263145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez-Feito A., Melero J., Mora-Díaz S. Autoimmune lymphoproliferative syndrome due to somatic FAS mutation (ALPS-sFAS) combined with a germline caspase-10 (CASP10) variation. Immunobiology. 2016;221(1):40–47. doi: 10.1016/j.imbio.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Neven B., Magerus-Chatinet A., Florkin B. A survey of 90 patients with autoimmune lymphoproliferative syndrome related to TNFRSF6 mutation. Blood. 2011;118(18):4798–4807. doi: 10.1182/blood-2011-04-347641. [DOI] [PubMed] [Google Scholar]
- 15.Magerus-Chatinet A., Neven B., Stolzenberg M.-C. Onset of autoimmune lymphoproliferative syndrome (ALPS) in humans as a consequence of genetic defect accumulation. J Clin Investig. 2011;121(1):106–112. doi: 10.1172/JCI43752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García García G.M., Bureo Dacal J.C., Suárez-Varela Pineda S., Elduayen Izaguirre R. Adult onset autoimmune lymphoproliferative syndrome due to somatic FAS mutation. Intern Med J. 2015;45(4):462–464. doi: 10.1111/imj.12714. [DOI] [PubMed] [Google Scholar]
- 17.Bleesing J.J., Nagaraj C.B., Zhang K. Autoimmune lymphoproliferative syndrome. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. Gene Reviews®. University of Washington, Seattle; Seattle (WA): 1993-2019. [PubMed] [Google Scholar]
- 18.Rieux-Laucat F. What's up in the ALPS. Curr Opin Immunol. 2017;49:79–86. doi: 10.1016/j.coi.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Teachey D.T., Lambert M.P. Diagnosis and management of autoimmune cytopenias in childhood. Pediatr Clin N Am. 2013;60(6):1489–1511. doi: 10.1016/j.pcl.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khandelwal P., Davies S.M., Grimley M.S. Bortezomib for refractory autoimmunity in pediatrics. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2014;20(10):1654–1659. doi: 10.1016/j.bbmt.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 21.Sleight B.J., Prasad V.S., DeLaat C. Correction of autoimmune lymphoproliferative syndrome by bone marrow transplantation. Bone Marrow Transplant. 1998;22(4):375–380. doi: 10.1038/sj.bmt.1701306. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira J.B., Bidère N., Niemela J.E. NRAS mutation causes a human autoimmune lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 2007;104(21):8953–8958. doi: 10.1073/pnas.0702975104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niemela J.E., Lu L., Fleisher T.A. Somatic KRAS mutations associated with a human nonmalignant syndrome of autoimmunity and abnormal leukocyte homeostasis. Blood. 2011;117(10):2883–2886. doi: 10.1182/blood-2010-07-295501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meynier S., Rieux-Laucat F. FAS and RAS related Apoptosis defects: from autoimmunity to leukemia. Immunol Rev. 2019;287(1):50–61. doi: 10.1111/imr.12720. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira J.B., Bleesing J.J., Dianzani U. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010;116(14):e35–e40. doi: 10.1182/blood-2010-04-280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiota M., Yang X., Kubokawa M. Somatic mosaicism for a NRAS mutation associates with disparate clinical features in RAS-associated leukoproliferative disease: a report of two cases. J Clin Immunol. 2015;35(5):454–458. doi: 10.1007/s10875-015-0163-3. [DOI] [PubMed] [Google Scholar]
- 27.Calvo K.R., Price S., Braylan R.C. JMML and RALD (Ras-associated autoimmune leukoproliferative disorder): common genetic etiology yet clinically distinct entities. Blood. 2015;125(18):2753–2758. doi: 10.1182/blood-2014-11-567917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takagi M., Shinoda K., Piao J. Autoimmune lymphoproliferative syndrome-like disease with somatic KRAS mutation. Blood. 2011;117(10):2887–2890. doi: 10.1182/blood-2010-08-301515. [DOI] [PubMed] [Google Scholar]
- 29.Picard C., Bobby Gaspar H., Al-Herz W. International union of immunological Societies: 2017 primary immunodeficiency diseases committee report on inborn errors of immunity. J Clin Immunol. 2018;38(1):96–128. doi: 10.1007/s10875-017-0464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy-Mendelovich S., Lev A., Rechavi E. T and B cell clonal expansion in Ras-associated lymphoproliferative disease (RALD) as revealed by next-generation sequencing. Clin Exp Immunol. 2017;189(3):310–317. doi: 10.1111/cei.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran T.A.N., Grow W.B., Chang C.-C. Superficial and deep cutaneous involvement by RAS-associated autoimmunne leukoproliferative disease (RALD cutis): a histologic mimicker of histiocytoid sweet syndrome. Am J Dermatopathol. December 2018;41(8):606–610. doi: 10.1097/DAD.0000000000001332. [DOI] [PubMed] [Google Scholar]
- 32.Giacaman A., Bauzá Alonso A., Salinas Sanz J.A. Cutaneous involvement in an 8-year-old boy with Ras-associated autoimmune leucoproliferative disorder (RALD) Clin Exp Dermatol. 2018;43(8):913–916. doi: 10.1111/ced.13668. [DOI] [PubMed] [Google Scholar]
- 33.Lanzarotti N., Bruneau J., Trinquand A. RAS-associated lymphoproliferative disease evolves into severe juvenile myelo-monocytic leukemia. Blood. 2014;123(12):1960–1963. doi: 10.1182/blood-2014-01-548958. [DOI] [PubMed] [Google Scholar]
- 34.Toyoda H., Deguchi T., Iwamoto S. Weekly rituximab followed by monthly rituximab treatment for autoimmune disease associated with RAS-associated autoimmune leukoproliferative disease. J Pediatr Hematol Oncol. 2018;40(8):e516–e518. doi: 10.1097/MPH.0000000000001276. [DOI] [PubMed] [Google Scholar]
- 35.Stojanov S., Kastner D.L. Familial autoinflammatory diseases: genetics, pathogenesis and treatment. Curr Opin Rheumatol. 2005;17(5):586–599. doi: 10.1097/bor.0000174210.78449.6b. [DOI] [PubMed] [Google Scholar]
- 36.Rowczenio D.M., Gomes S.M., Aróstegui J.I. Late-onset cryopyrin-associated periodic syndromes caused by somatic NLRP3 mosaicism-UK single center experience. Front Immunol. 2017;8:1410. doi: 10.3389/fimmu.2017.01410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lasigliè D., Mensa-Vilaro A., Ferrera D. Cryopyrin-associated periodic syndromes in Italian patients: evaluation of the rate of somatic NLRP3 mosaicism and phenotypic characterization. J Rheumatol. 2017;44(11):1667–1673. doi: 10.3899/jrheum.170041. [DOI] [PubMed] [Google Scholar]
- 38.Kawasaki Y., Oda H., Ito J. Identification of a high-frequency somatic NLRC4 mutation as a cause of autoinflammation by pluripotent cell-based phenotype dissection. Arthritis Rheum. 2017;69(2):447–459. doi: 10.1002/art.39960. [DOI] [PubMed] [Google Scholar]
- 39.Mensa-Vilaro A., Teresa Bosque M., Magri G. Brief report: late-onset cryopyrin-associated periodic syndrome due to myeloid-restricted somatic NLRP3 mosaicism. Arthritis Rheum. 2016;68(12):3035–3041. doi: 10.1002/art.39770. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Q., Aksentijevich I., Wood G.M. Brief report: cryopyrin-associated periodic syndrome caused by a myeloid-restricted somatic NLRP3 mutation. Arthritis Rheum. 2015;67(9):2482–2486. doi: 10.1002/art.39190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omoyinmi E., Melo Gomes S., Standing A. Brief Report: whole-exome sequencing revealing somatic NLRP3 mosaicism in a patient with chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum. 2014;66(1):197–202. doi: 10.1002/art.38217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakagawa K., Gonzalez-Roca E., Souto A. Somatic NLRP3 mosaicism in Muckle-Wells syndrome. A genetic mechanism shared by different phenotypes of cryopyrin-associated periodic syndromes. Ann Rheum Dis. 2015;74(3):603–610. doi: 10.1136/annrheumdis-2013-204361. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka N., Izawa K., Saito M.K. High incidence of NLRP3 somatic mosaicism in patients with chronic infantile neurologic, cutaneous, articular syndrome: results of an International Multicenter Collaborative Study. Arthritis Rheum. 2011;63(11):3625–3632. doi: 10.1002/art.30512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aksentijevich I., Nowak M., Mallah M. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46(12):3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saito M., Fujisawa A., Nishikomori R. Somatic mosaicism of CIAS1 in a patient with chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum. 2005;52(11):3579–3585. doi: 10.1002/art.21404. [DOI] [PubMed] [Google Scholar]
- 46.Aróstegui J.I., Lopez Saldaña M.D., Pascal M. A somatic NLRP3 mutation as a cause of a sporadic case of chronic infantile neurologic, cutaneous, articular syndrome/neonatal-onset multisystem inflammatory disease: novel evidence of the role of low-level mosaicism as the pathophysiologic mechanism underlying mendelian inherited diseases. Arthritis Rheum. 2010;62(4):1158–1166. doi: 10.1002/art.27342. [DOI] [PubMed] [Google Scholar]
- 47.Jiménez-Treviño S., González-Roca E., Ruiz-Ortiz E., Yagüe J., Ramos E., Aróstegui J.I. First report of vertical transmission of a somatic NLRP3 mutation in cryopyrin-associated periodic syndromes. Ann Rheum Dis. 2013;72(6):1109–1110. doi: 10.1136/annrheumdis-2012-202913. [DOI] [PubMed] [Google Scholar]
- 48.Neven B., Prieur A.-M., Quartier dit Maire P. Cryopyrinopathies: update on pathogenesis and treatment. Nat Clin Pract Rheumatol. 2008;4(9):481–489. doi: 10.1038/ncprheum0874. [DOI] [PubMed] [Google Scholar]
- 49.Terreri M.T.R.A., Bernardo W.M., Len C.A. Guidelines for the management and treatment of periodic fever syndromes: cryopyrin-associated periodic syndromes (cryopyrinopathies - CAPS) Rev Bras Reumatol. 2016;56(1):44–51. doi: 10.1016/j.rbre.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 50.Ma C.A., Xi L., Cauff B. Somatic STAT5b gain-of-function mutations in early onset nonclonal eosinophilia, urticaria, dermatitis, and diarrhea. Blood. 2017;129(5):650–653. doi: 10.1182/blood-2016-09-737817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cross N.C.P., Hoade Y., Tapper W.J. Recurrent activating STAT5B N642H mutation in myeloid neoplasms with eosinophilia. Leukemia. 2019;33(2):415–425. doi: 10.1038/s41375-018-0342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thorpe E.S., Handley H.E. Chronic tetany and chronic mycelial stomatitis in a child aged four and one-half years. Am J Dis Child. 1929;38(2):328–338. [Google Scholar]
- 53.Husebye E.S., Perheentupa J., Rautemaa R., Kämpe O. Clinical manifestations and management of patients with autoimmune polyendocrine syndrome type I. J Intern Med. 2009;265(5):514–529. doi: 10.1111/j.1365-2796.2009.02090.x. [DOI] [PubMed] [Google Scholar]
- 54.Peterson P., Pitkänen J., Sillanpää N., Krohn K. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED): a model disease to study molecular aspects of endocrine autoimmunity. Clin Exp Immunol. 2004;135(3):348–357. doi: 10.1111/j.1365-2249.2004.02384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mengesha B.G., Conti H.R. The role of IL-17 in protection against mucosal Candida infections. J Fungi Basel Switz. 2017;3(4) doi: 10.3390/jof3040052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Browne S.K., Holland S.M. Anticytokine autoantibodies in infectious diseases: pathogenesis and mechanisms. Lancet Infect Dis. 2010;10(12):875–885. doi: 10.1016/S1473-3099(10)70196-1. [DOI] [PubMed] [Google Scholar]
- 57.Sarkadi A.K., Taskó S., Csorba G., Tóth B., Erdős M., Maródi L. Autoantibodies to IL-17A may be correlated with the severity of mucocutaneous candidiasis in APECED patients. J Clin Immunol. 2014;34(2):181–193. doi: 10.1007/s10875-014-9987-5. [DOI] [PubMed] [Google Scholar]
- 58.Puel A., Döffinger R., Natividad A. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207(2):291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kisand K., Bøe Wolff A.S., Podkrajšek K.T. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207(2):299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Long-term therapy of chronic mucocutaneous candidiasis with ketoconazole: experience with twenty-one patients. Am J Med. 1983;74(1):23–29. doi: 10.1016/0002-9343(83)90511-9. [DOI] [PubMed] [Google Scholar]
- 61.Presky D.H., Yang H., Minetti L.J. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc Natl Acad Sci U S A. 1996;93(24):14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burbelo P.D., Browne S.K., Sampaio E.P. Anti-cytokine autoantibodies are associated with opportunistic infection in patients with thymic neoplasia. Blood. 2010;116(23):4848–4858. doi: 10.1182/blood-2010-05-286161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Szabo G., Dolganiuc A. The role of plasmacytoid dendritic cell-derived IFN alpha in antiviral immunity. Crit Rev Immunol. 2008;28(1):61–94. doi: 10.1615/critrevimmunol.v28.i1.40. [DOI] [PubMed] [Google Scholar]
- 64.Prümmer O., Seyfarth C., Scherbaum W.A., Drees N., Porzsolt F. Interferon-alpha antibodies in autoimmune diseases. J Interferon Res. 1989;9(Suppl 1):S67–S74. [PubMed] [Google Scholar]
- 65.Meager A., Visvalingam K., Peterson P. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006;3(7):e289. doi: 10.1371/journal.pmed.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trown P.W., Kramer M.J., Dennin R.A. Antibodies to human leucocyte interferons in cancer patients. Lancet Lond Engl. 1983;1(8316):81–84. doi: 10.1016/s0140-6736(83)91737-3. [DOI] [PubMed] [Google Scholar]
- 67.Ross C., Hansen M.B., Schyberg T., Berg K. Autoantibodies to crude human leucocyte interferon (IFN), native human IFN, recombinant human IFN-alpha 2b and human IFN-gamma in healthy blood donors. Clin Exp Immunol. 1990;82(1):57–62. doi: 10.1111/j.1365-2249.1990.tb05403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pozzetto B., Mogensen K.E., Tovey M.G., Gresser I. Characteristics of autoantibodies to human interferon in a patient with varicella-zoster disease. J Infect Dis. 1984;150(5):707–713. doi: 10.1093/infdis/150.5.707. [DOI] [PubMed] [Google Scholar]
- 69.Ku C.-L., Lin C.-H., Chang S.-W. Anti-IFN-γ autoantibodies are strongly associated with HLA-DR*15:02/16:02 and HLA-DQ*05:01/05:02 across Southeast Asia. J Allergy Clin Immunol. 2016;137(3):945–948. doi: 10.1016/j.jaci.2015.09.018. e8. [DOI] [PubMed] [Google Scholar]
- 70.Chang P.H., Chuang Y.C. Anti-interferon-γ autoantibody-associated disseminated Mycobacterium abscessus infection mimicking parotid cancer with multiple metastases: a case report. Medicine (Baltim) 2017;96(39):e8118. doi: 10.1097/MD.0000000000008118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kampmann B., Hemingway C., Stephens A. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J Clin Investig. 2005;115(9):2480–2488. doi: 10.1172/JCI19316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dorman S.E., Holland S.M. Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev. 2000;11(4):321–333. doi: 10.1016/s1359-6101(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 73.Browne S.K., Burbelo P.D., Chetchotisakd P. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367(8):725–734. doi: 10.1056/NEJMoa1111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wongkulab P., Wipasa J., Chaiwarith R., Supparatpinyo K. Autoantibody to interferon-gamma associated with adult-onset immunodeficiency in non-HIV individuals in Northern Thailand. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0076371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chan J.F.-W., Trendell-Smith N.J., Chan J.C.-Y. Reactive and infective dermatoses associated with adult-onset immunodeficiency due to anti-interferon-gamma autoantibody: sweet's syndrome and beyond. Dermatol Basel Switz. 2013;226(2):157–166. doi: 10.1159/000347112. [DOI] [PubMed] [Google Scholar]
- 76.Jutivorakool K., Sittiwattanawong P., Kantikosum K. Skin manifestations in patients with adult-onset immunodeficiency due to anti-interferon-gamma autoantibody: a relationship with systemic infections. Acta Derm Venereol. 2018;98(8):742–747. doi: 10.2340/00015555-2959. [DOI] [PubMed] [Google Scholar]
- 77.Ding L., Mo A., Jutivorakool K., Pancholi M., Holland S.M., Browne S.K. Determination of human anticytokine autoantibody profiles using a particle-based approach. J Clin Immunol. 2012;32(2):238–245. doi: 10.1007/s10875-011-9621-8. [DOI] [PubMed] [Google Scholar]
- 78.Browne S.K. Anticytokine autoantibody-associated immunodeficiency. Annu Rev Immunol. 2014;32:635–657. doi: 10.1146/annurev-immunol-032713-120222. [DOI] [PubMed] [Google Scholar]
- 79.Wu U.-I., Chuang Y.-C., Sheng W.-H. Use of QuantiFERON-TB Gold In-tube assay in screening for neutralizing anti-interferon-γ autoantibodies in patients with disseminated nontuberculous mycobacterial infection. Clin Microbiol Infect. 2018;24(2):159–165. doi: 10.1016/j.cmi.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 80.Chetchotisakd P., Anunnatsiri S., Nanagara R., Nithichanon A., Lertmemongkolchai G. Intravenous cyclophosphamide therapy for anti-IFN-gamma autoantibody-associated Mycobacterium abscessus infection. J Immunol Res. 2018;2018:6473629. doi: 10.1155/2018/6473629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Browne S.K., Zaman R., Sampaio E.P. Anti-CD20 (rituximab) therapy for anti-IFN-γ autoantibody-associated nontuberculous mycobacterial infection. Blood. 2012;119(17):3933–3939. doi: 10.1182/blood-2011-12-395707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bonfield T.L., Raychaudhuri B., Malur A. 1 regulation of human alveolar macrophage differentiation requires granulocyte-macrophage colony-stimulating factor. Am J Physiol Lung Cell Mol Physiol. 2003;285(5):L1132–L1136. doi: 10.1152/ajplung.00216.2003. [DOI] [PubMed] [Google Scholar]
- 83.Chen B.D., Mueller M., Chou T.H. Role of granulocyte/macrophage colony-stimulating factor in the regulation of murine alveolar macrophage proliferation and differentiation. J Immunol Baltim Md. 1950;141(1):139–144. 1988. [PubMed] [Google Scholar]
- 84.Uchida K., Nakata K., Suzuki T. Granulocyte/macrophage-colony-stimulating factor autoantibodies and myeloid cell immune functions in healthy subjects. Blood. 2009;113(11):2547–2556. doi: 10.1182/blood-2009-05-155689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trapnell B.C., Whitsett J.A., Nakata K. Pulmonary alveolar proteinosis. N Engl J Med. 2003;349(26):2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 86.Carey B., Trapnell B.C. The molecular basis of pulmonary alveolar proteinosis. Clin Immunol Orlando Fla. 2010;135(2):223–235. doi: 10.1016/j.clim.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ladeb S., Fleury-Feith J., Escudier E., Tran Van Nhieu J., Bernaudin J.F., Cordonnier C. Secondary alveolar proteinosis in cancer patients. Support Care Cancer. 1996;4(6):420–426. doi: 10.1007/BF01880639. [DOI] [PubMed] [Google Scholar]
- 88.Kitamura T., Tanaka N., Watanabe J. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190(6):875–880. doi: 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kitamura T., Uchida K., Tanaka N. Serological diagnosis of idiopathic pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2000;162(2 Pt 1):658–662. doi: 10.1164/ajrccm.162.2.9910032. [DOI] [PubMed] [Google Scholar]
- 90.Lin F.-C., Chang G.-D., Chern M.-S., Chen Y.-C., Chang S.-C. Clinical significance of anti-GM-CSF antibodies in idiopathic pulmonary alveolar proteinosis. Thorax. 2006;61(6):528–534. doi: 10.1136/thx.2005.054171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Piccoli L., Campo I., Fregni C.S. Neutralization and clearance of GM-CSF by autoantibodies in pulmonary alveolar proteinosis. Nat Commun. 2015;6 doi: 10.1038/ncomms8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Golde D.W., Territo M., Finley T.N., Cline M.J. Defective lung macrophages in pulmonary alveolar proteinosis. Ann Intern Med. 1976;85(3):304–309. doi: 10.7326/0003-4819-85-3-304. [DOI] [PubMed] [Google Scholar]
- 93.Uchida K., Beck D.C., Yamamoto T. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med. 2007;356(6):567–579. doi: 10.1056/NEJMoa062505. [DOI] [PubMed] [Google Scholar]
- 94.Oerlemans W.G., Jansen E.N., Prevo R.L., Eijsvogel M.M. Primary cerebellar nocardiosis and alveolar proteinosis. Acta Neurol Scand. 1998;97(2):138–141. doi: 10.1111/j.1600-0404.1998.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 95.Clague H.W., Harth M., Hellyer D., Morgan W.K. Septic arthritis due to Nocardia asteroides in association with pulmonary alveolar proteinosis. J Rheumatol. 1982;9(3):469–472. [PubMed] [Google Scholar]
- 96.Andersen B.R., Ecklund R.E., Kellow W.F. Pulmonary alveolar proteinosis with systemic nocardiosis. A case report. JAMA. 1960;174:28–31. doi: 10.1001/jama.1960.03030010030008. [DOI] [PubMed] [Google Scholar]
- 97.Hartung M., Salfelder K. Pulmonary alveolar proteinosis and histoplasmosis: report of three cases. Virchows Arch A Pathol Anat Histol. 1975;368(4):281–287. doi: 10.1007/BF00432306. [DOI] [PubMed] [Google Scholar]
- 98.Crum-Cianflone N.F., Lam P.V., Ross-Walker S., Rosen L.B., Holland S.M. Autoantibodies to granulocyte-macrophage colony-stimulating factor Associated with severe and unusual manifestations of cryptococcus gattii infections. Open Forum Infect Dis. 2017;4(4) doi: 10.1093/ofid/ofx211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sunderland W.A., Campbell R.A., Edwards M.J. Pulmonary alveolar proteinosis and pulmonary cryptococcosis in an adolescent boy. J Pediatr. 1972;80(3):450–456. doi: 10.1016/s0022-3476(72)80503-1. [DOI] [PubMed] [Google Scholar]
- 100.Rosen L.B., Freeman A.F., Yang L.M. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol Baltim Md. 1950;190(8):3959–3966. doi: 10.4049/jimmunol.1202526. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kuo C.-Y., Wang S.-Y., Shih H.-P. Disseminated cryptococcosis due to anti-granulocyte-macrophage colony-stimulating factor Autoantibodies in the absence of pulmonary alveolar proteinosis. J Clin Immunol. 2017;37(2):143–152. doi: 10.1007/s10875-016-0364-4. [DOI] [PubMed] [Google Scholar]
- 102.Sergeeva A., Ono Y., Rios R., Molldrem J. High titer autoantibodies to GM-CSF in patients with AML, CML and MDS are associated with active disease. Leukemia. 2008;22(4):783–790. doi: 10.1038/sj.leu.2405104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sergeeva A., Ono Y., Rios R., Molldrem J.J. IgG, IgA and IgM autoantibodies to GM-CSF are present in AML, CML and MDS patients and IgG titer is associated with disease progression. Blood. 2004;104(11) 1071-1071. [Google Scholar]
- 104.Gathungu G., Kim M.-O., Ferguson J.P. Granulocyte-macrophage colony-stimulating factor autoantibodies: a marker of aggressive Crohn's disease. Inflamm Bowel Dis. 2013;19(8):1671–1680. doi: 10.1097/MIB.0b013e318281f506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gathungu G., Zhang Y., Tian X. Impaired granulocyte-macrophage colony-stimulating factor bioactivity accelerates surgical recurrence in ileal Crohn's disease. World J Gastroenterol. 2018;24(5):623–630. doi: 10.3748/wjg.v24.i5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hadda V., Tiwari P., Madan K. Pulmonary alveolar proteinosis: experience from a tertiary care center and systematic review of Indian literature. Lung India Off Organ Indian Chest Soc. 2016;33(6):626–634. doi: 10.4103/0970-2113.192876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Griese M. Pulmonary alveolar proteinosis: a comprehensive clinical perspective. Pediatrics. 2017;140(2) doi: 10.1542/peds.2017-0610. [DOI] [PubMed] [Google Scholar]
- 108.Rosen L.B., Rocha Pereira N., Figueiredo C. Nocardia-induced granulocyte macrophage colony-stimulating factor is neutralized by autoantibodies in disseminated/extrapulmonary nocardiosis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2015;60(7):1017–1025. doi: 10.1093/cid/ciu968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alsenz J., Bork K., Loos M. Autoantibody-mediated acquired deficiency of C1 inhibitor. N Engl J Med. 1987;316(22):1360–1366. doi: 10.1056/NEJM198705283162202. [DOI] [PubMed] [Google Scholar]
- 110.Sinclair D., Smith A., Cranfield T., Lock R.J. Acquired C1 esterase inhibitor deficiency or serendipity? The chance finding of a paraprotein after an apparently low C1 esterase inhibitor concentration. J Clin Pathol. 2004;57(4):445–447. doi: 10.1136/jcp.2003.013524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mészáros T., Füst G., Farkas H. C1-inhibitor autoantibodies in SLE. Lupus. 2010;19(5):634–638. doi: 10.1177/0961203309357059. [DOI] [PubMed] [Google Scholar]
- 112.Cacoub P., Frémeaux-Bacchi V., De Lacroix I. A new type of acquired C1 inhibitor deficiency associated with systemic lupus erythematosus. Arthritis Rheum. 2001;44(8):1836–1840. doi: 10.1002/1529-0131(200108)44:8<1836::AID-ART321>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 113.Beretta A., Zanichelli A., Agostoni A., Cicardi M., Gringeri A. C1 inhibitor function and anti-C1 inhibitor autoantibodies in patients with HIV type 1 infection. AIDS Res Hum Retrovir. 1999;15(1):95–96. doi: 10.1089/088922299311781. [DOI] [PubMed] [Google Scholar]
- 114.Sugisaki K., Itoh K., Tamaru J. Acquired C1-esterase inhibitor deficiency and positive lupus anticoagulant accompanied by splenic marginal zone B-cell lymphoma. Clin Exp Rheumatol. 2007;25(4):627–629. [PubMed] [Google Scholar]
- 115.Jacquin-Porretaz C., Castelain F., Daguindau E. [Acquired C1 esterase inhibitor deficiency via bradykinin-mediated angioedema: four cases] Ann Dermatol Venereol. 2018;145(10):598–602. doi: 10.1016/j.annder.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 116.Sbattella M., Zanichelli A., Ghia P. Splenic marginal zone lymphomas in acquired C1-inhibitor deficiency: clinical and molecular characterization. Med Oncol Northwood Lond Engl. 2018;35(9):118. doi: 10.1007/s12032-018-1183-7. [DOI] [PubMed] [Google Scholar]
- 117.Bork K., Staubach-Renz P., Hardt J. Angioedema due to acquired C1-inhibitor deficiency: spectrum and treatment with C1-inhibitor concentrate. Orphanet J Rare Dis. 2019;14(1):65. doi: 10.1186/s13023-019-1043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cicardi M., Zingale L.C., Pappalardo E., Folcioni A., Agostoni A. Autoantibodies and lymphoproliferative diseases in acquired C1-inhibitor deficiencies. Medicine (Baltim) 2003;82(4):274–281. doi: 10.1097/01.md.0000085055.63483.09. [DOI] [PubMed] [Google Scholar]
- 119.Cugno M., Cicardi M., Agostoni A. Activation of the contact system and fibrinolysis in autoimmune acquired angioedema: a rationale for prophylactic use of tranexamic acid. J Allergy Clin Immunol. 1994;93(5):870–876. doi: 10.1016/0091-6749(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 120.Zanichelli A., Bova M., Coerezza A., Petraroli A., Triggiani M., Cicardi M. Icatibant treatment for acquired C1-inhibitor deficiency: a real-world observational study. Allergy. 2012;67(8):1074–1077. doi: 10.1111/j.1398-9995.2012.02853.x. [DOI] [PubMed] [Google Scholar]
- 121.Branellec A., Bouillet L., Javaud N. Acquired C1-inhibitor deficiency: 7 patients treated with rituximab. J Clin Immunol. 2012;32(5):936–941. doi: 10.1007/s10875-012-9691-2. [DOI] [PubMed] [Google Scholar]
- 122.Donaldson V.H., Bernstein D.I., Wagner C.J., Mitchell B.H., Scinto J., Bernstein I.L. Angioneurotic edema with acquired C1- inhibitor deficiency and autoantibody to C1- inhibitor: response to plasmapheresis and cytotoxic therapy. J Lab Clin Med. 1992;119(4):397–406. [PubMed] [Google Scholar]
- 123.Good R.A., Varco R.L. A clinical and experimental study of agammaglobulinemia. J Lancet. 1955;75(6):245–271. [PubMed] [Google Scholar]
- 124.Khawaja M.R., Nelson R.P., Miller N. Immune-mediated diseases and immunodeficiencies associated with thymic epithelial neoplasms. J Clin Immunol. 2012;32(3):430–437. doi: 10.1007/s10875-011-9644-1. [DOI] [PubMed] [Google Scholar]
- 125.Tormoehlen L.M., Pascuzzi R.M. Thymoma, myasthenia gravis, and other paraneoplastic syndromes. Hematol Oncol Clin N Am. 2008;22(3):509–526. doi: 10.1016/j.hoc.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 126.Tarr P.E., Sneller M.C., Mechanic L.J. Infections in patients with immunodeficiency with thymoma (Good syndrome). Report of 5 cases and review of the literature. Medicine (Baltim) 2001;80(2):123–133. doi: 10.1097/00005792-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 127.Rawat A., Dhir V., Gupta A. Good's syndrome presenting with recurrent giardiasis”. J Clin Immunol. 2014;34(7):751–752. doi: 10.1007/s10875-014-0080-x. [DOI] [PubMed] [Google Scholar]
- 128.Kennedy J.L., Schroeder N., Palacios T. Fifty-five-year-old man with chronic yeast infections. Allergy Asthma Proc. 2014;35(5):415–422. doi: 10.2500/aap.2014.35.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Meager A., Vincent A., Newsom-Davis J., Willcox N. Spontaneous neutralising antibodies to interferon--alpha and interleukin-12 in thymoma-associated autoimmune disease. Lancet Lond Engl. 1997;350(9091):1596–1597. doi: 10.1016/s0140-6736(05)64012-3. [DOI] [PubMed] [Google Scholar]
- 130.Jansen A., van Deuren M., Miller J. Prognosis of Good syndrome: mortality and morbidity of thymoma associated immunodeficiency in perspective. Clin Immunol. 2016;171:12–17. doi: 10.1016/j.clim.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 131.de Beaucoudrey L., Puel A., Filipe-Santos O. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205(7):1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Homann C., Hansen M.B., Graudal N. Anti-interleukin-6 autoantibodies in plasma are associated with an increased frequency of infections and increased mortality of patients with alcoholic cirrhosis. Scand J Immunol. 1996;44(6):623–629. doi: 10.1046/j.1365-3083.1996.d01-344.x. [DOI] [PubMed] [Google Scholar]
- 133.Nanki T., Onoue I., Nagasaka K. Suppression of elevations in serum C reactive protein levels by anti-IL-6 autoantibodies in two patients with severe bacterial infections. Ann Rheum Dis. 2013;72(6):1100–1102. doi: 10.1136/annrheumdis-2012-202768. [DOI] [PubMed] [Google Scholar]