Abstract
Migration of polymorphonuclear leukocytes from bloodstream to the site of inflammation is an important event required for surveillance of foreign antigens. This trafficking of leukocytes from bloodstream to the tissue occurs in several distinct steps and involves several adhesion molecules. Defect in adhesion of leukocytes to vascular endothelium affecting their subsequent migration to extravascular space gives rise to a group of rare primary immunodeficiency diseases (PIDs) known as Leukocyte Adhesion Defects (LAD). Till date, four classes of LAD are discovered with LAD I being the most common form. LAD I is caused by loss of function of common chain, cluster of differentiation (CD)18 of β2 integrin family. These patients suffer from life-threatening bacterial infections and in its severe form death usually occurs in childhood without bone marrow transplantation. LAD II results from a general defect in fucose metabolism. These patients suffer from less severe bacterial infections and have growth and mental retardation. Bombay blood group phenotype is also observed in these patients. LAD III is caused by abnormal integrin activation. LAD III patients suffer from severe bacterial and fungal infections. Patients frequently show delayed detachment of umbilical cord, impaired wound healing and increased tendency to bleed. LAD IV is the most recently described class. It is caused by defects in β2 and α4β1 integrins which impairs lymphocyte adhesion. LAD IV patients have monogenic defect in cystic-fibrosis-transmembrane-conductance-regulator (CFTR) gene, resulting in cystic fibrosis. Pathophysiology and genetic etiology of all LAD syndromes are discussed in detail in this paper.
Keywords: Neutrophilic defect, Neutrophilic leukocytosis, Phagocyte rolling, Phagocytes, Primary immunodeficiency disorders
Introduction
Leukocyte adhesion defect (LAD) is a rare primary immunodeficiency disorder (PID) characterized by defect in adhesion of leukocytes to the endothelium of vessel wall and their subsequent migration to extravascular space. In patients with LAD, the neutrophils fail to migrate to the site of infection leading to a state of tissue neutropenia. Extreme neutrophilic leukocytosis in blood, absence of pus formation at the site of infection and non-healing ulcers are hallmarks of LAD. Apart from infections, the recent understanding about pathogenesis of LAD has expanded the clinical phenotype and patients have also been identified to have a variety of inflammatory complications. These inflammatory complications may occasionally be the predominant clinical presentation of disease and patients may need immuno-modulatory therapy rather than antimicrobials. In this review, we update on current understanding in the pathogenesis, clinical presentation and management of patients with various subtypes of LAD.
Leukocyte adhesion cascade
During an acute inflammatory response secondary to an antigenic stimulus in the body, polymorphonuclear leukocytes (PMN) are recruited from vasculature to the inflamed tissue within a span of 1–4 h. Normally, leukocytes rapidly move in bloodstream without adhering to vascular endothelium. However, in response to a local trauma or inflammation; endothelium gets activated resulting in leukocyte rolling along its wall. Migration of PMNs from bloodstream to the focus of inflammation involves 4 major steps: rolling, firm arrest, post-arrest modifications and transmigration into tissues. Leukocyte rolling on endothelium occurs via loose adhesions to the vessel wall. This step is mediated by selectins (P-selectin or E-selectin) present on activated endothelium, and their sialyated ligands (CD15s) which are constitutively expressed on leukocytes. Various chemo-attractants, such as the complement fragment C5a, IL-8, leukotriene B4 (LTB4), or bacterial product formyl-methionyl-leucyl-phenylalanine (fMLF), are produced by endothelium and displayed to rolling leukocytes in blood vessel lumen which in turn upregulate and activate endothelial integrins via intracellular signalling events. Chemokines bind to their G-Protein coupled receptors (GPCR) thereby triggering activation of Ca2+ and diacylglycerol regulated guanine nucleotide exchange factor I (CALDAG GEFI) and Phospholipase C (PLC). CALDAG GEFI in turn activates small GTPase Rap1 (Ras-related protein 1), which associates with effector proteins such as Rap1-interacting adaptor molecule (RIAM) and RAPL inducing the integrin activation. This results in rapid adhesion of leukocytes to endothelium by interaction between integrins on neutrophil and Intercellular Adhesion Molecule (ICAMs) on endothelium. This step is followed by transmigration of neutrophils between endothelial cells out to the site of inflammation.1
Genetics and pathogenesis of LAD
Leukocyte adhesion defect (LAD) is a group of rare autosomal recessively inherited immunodeficiency diseases caused by defects in leukocyte recruitment cascade. Till date, four different forms of leukocyte adhesion deficiencies have been reported which are named according to their chronological order of discoveries viz: LAD I, LAD II, LAD III and LAD IV (Table 1). LAD I affects third phase of adhesion cascade; impaired adhesion of leukocyte to endothelium. The function of β2 integrin, CD18 is lost in LAD I. LAD II is caused by defective rolling phase which results from defect in post-translational fucosylation. LAD III is caused by abnormal integrin activation, which results from loss of function of integrin adaptor molecule kindlin-3 and mutations in Fermitin family homolog 3 (FERMT3). Both β2 and α4β1 integrins are affected thus resulting in impaired monocyte adhesion but no defect in neutrophil adhesion occurs in Cystic fibrosis transmembrane conductance regulator (CFTR)-dependent leukocyte adhesion deficiency (LAD-IV).
Table 1.
Salient features of Leukocyte Adhesion Defects type I-IV.
| LAD I | LADII | LAD III | LAD IV | |
|---|---|---|---|---|
| OMIM | 116920 | 266265 | 612840 | |
| Inheritance pattern | Autosomal recessive | Autosomal recessive | Autosomal recessive | Autosomal recessive |
| Integrins | Absent or decreased β2 integrins | Normal | Defective inside out signalling of β1,2,3 integrins | Decreased β2 and α4 β1 integrin |
| Selectin ligands | Normal | Defective Fucosylation | Normal | |
| Neutrophil functional defect | Tight adhesion, emigration | Rolling, tethering | Integrin activation, adhesion, chemotaxis, superoxide formation | – (Monocyte, Lymphocyte adhesion) |
| Delayed umbilical cord separation | Yes | No | Yes | No |
| Mutation | ITGB2 | SLC35C1 | FERMT3, CALDAG-GEF1 | CFTR |
| Leukocyte Defect | Neutrophils | Neutrophils | Neutrophils | Monocytes |
| Severity of infection | +++ | ++ | ++ | ++ |
Leukocyte adhesion defect I (LAD I)
Brief introduction
Initial cases of LAD were described in 1970s in 6 infants from 2 families who had delayed separation of cord along with severe, recurrent bacterial infections. Subsequently many patients have been described and it is the commonest form of leukocyte adhesion defects.
Pathogenesis
Patients with LAD I have defects in membrane expression of leukocyte adhesion glycoproteins of integrin superfamily.
Integrins are noncovalently associated, heterodimeric cell surface receptors, consisting of one α subunit (CD11a, CD11b, or CD11c) and a common β chain (CD18), which helps in surface expression of the CD11 chains. These proteins facilitate leukocyte adhesion to endothelium. Patients with LAD I have defective polymorphonuclear cell adherence, leading to defective chemotaxis and trafficking, as well as low natural killer (NK) and cytotoxic T-lymphocyte (CTL) activity.2 Absence of Complement Receptor 3 (CR3) leads to loss of complement-mediated phagocytosis and bacterial killing. Lymphoblasts from LAD I patients are shown to synthesize a normal α-subunit precursor that is never expressed on cell surface and is subsequently degraded in cytoplasm in absence of normal β2 subunit. Persistent neutrophil leukocytosis (usually >15,000 cells/μL) even in the absence of infection is common in all patients, resulting from both low-level ongoing infection and impaired exit of neutrophils from circulation.
Severity of symptoms, number of infections and survival directly depends upon the amount of β2 integrins (CD18) expressed on cell surface of leukocytes.3
Genetics
Leukocyte adhesion defect-I (LAD I) is an autosomal recessive genetic disorder that occurs due to mutations in ITGB2 gene located on chromosome 21q22.3 that encodes for CD18. Various mutations are known in this gene, including truncations, substitutions, frame shifts, deletions, and intronic mutations, that result in variable abnormalities of CD18 protein, ranging from complete absence of protein product or reduced protein size. Till date, around 86 different allelic mutations have been identified in the ITGB2 gene which comprises 35 missense mutations, 12 splice site mutations, 10 nonsense mutations, 23 deletions, 2 insertions and 4 small insertions and deletions (INDEL) variations.4 Many CD18 mutations are located in a region on exon 5 and 9, which encodes a highly conserved 241 amino acid sequence. This domain contains contact sites between α and β subunits and has an essential role in synthesis of subunit precursors.
Clinical manifestations
Infections in LAD I
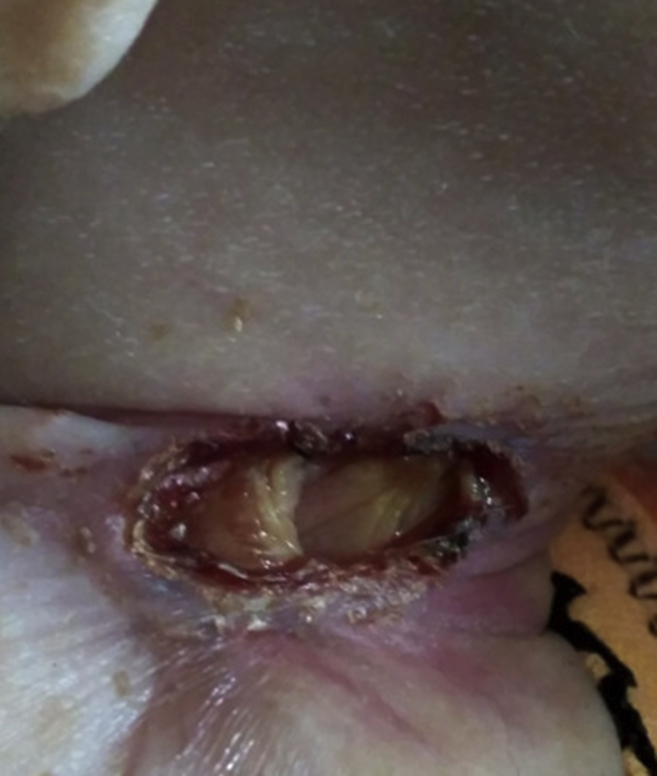
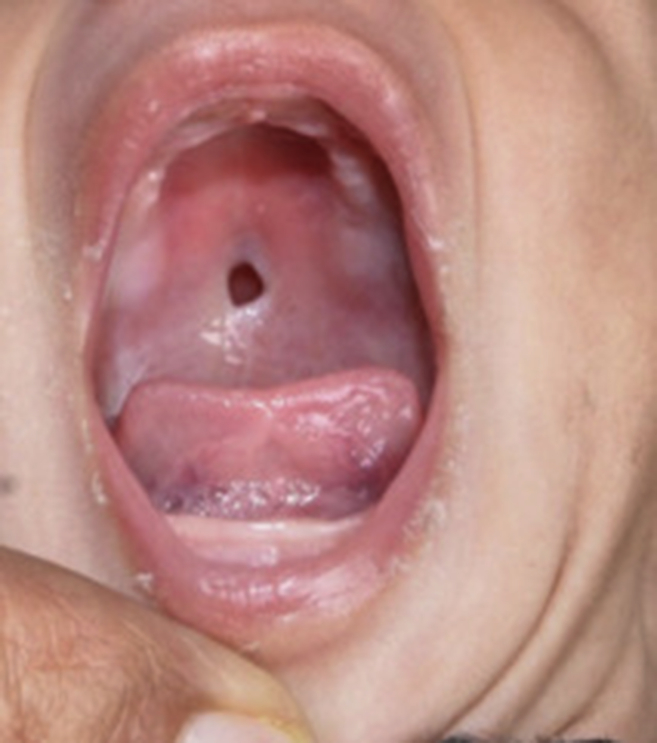
Recurrent infections that are primarily localised to the skin, gingiva and lungs are predominant clinical manifestation in children with LAD. These infections are usually apparent from early stages of life. Delayed separation of umbilical stump with severe omphalitis is often the earliest clinical presentation. Recurrent infections are caused by Staphylococcus aureus, Pseudomonas aeruginosa, and Klebsiella sp. Fungal infections are also seen.5 Absence of pus at the site of infection is characteristic. Leukocytes fail to migrate to sites of infection, thus resulting in inability to form pus and erythema at site of infection. However, there are occasional reports of pus formation at the site of infection.6 Impaired wound healing either traumatic or surgical, is also observed. Non-healing ulcers are another important manifestation of this disorder (Fig. 1). These non-healing ulcers are commonly seen in peri-anal and peri-umbilical regions but have also been reported at unusual sites such as palate7(Fig. 2). Apart from infections, inflammation at site of ulcers is also seen. Recent studies show that there is an excessive IL-17 and IL-23 response at site of infection in patients with LAD I in the absence of neutrophils.8, 9 Recurrent skin infections, inflammation and non-healing ulcers lead to occurrence of lesions that appear morphologically like pyoderma gangrenosum. Other inflammatory conditions like colitis have also been described in patients with LAD-I. Unusual presentations like that with Budd-Chiari syndrome have been reported.10
Figure 1.
Showing a non-healing ulcer without pus over neck of a patient.
Figure 2.
Palatal ulcer.
Autoimmunity in LAD I
Primary immunodeficiency can directly predispose to autoimmunity through mechanisms that normally regulate immune responses such as defect of regulatory T cells 11. Autoimmune manifestations were observed in a group of patients in long-term. Interestingly patients who had underwent a hematopoietic stem cell transplant also had autoimmune manifestations like immune cytopenias over long-term.12 In a recent study, autoantibodies like anti-nuclear antibodies (ANA), perinuclear anti-neutrophil cytoplasmic antibodies (pANCA), anti-beta 2 glycoprotein antibodies (β2GPI) and anti-cardiolipin antibodies were detected in three out of four LAD I patients who underwent HSCT. In few cases, development of type-1 Diabetes mellitus has been observed in patients receiving HSCT.12 LAD-1 syndrome have been associated with chronic enterocolitis13 and Crohn-like colitis.2
Inflammatory periodontitis in LAD 1
Oral ulcers, severe periodontitis and eventual loss of permanent teeth are frequently observed in LAD I. Role of neutrophils has been highlighted in oral health and causation of periodontitis has been documented.10 A recent study shows that the LAD subgingival microbiome is different from the Localized Aggressive Periodontitits. Parvimonas micra, Porphyromonas endodontalis, Eubacterium brachy and Treponema species are detected in LAD I periodontitis. Pseudomonas aeruginosa, a bacterium not typically found in subgingival plaque is detected in LAD-I. Thus suggesting a role of microbial products from LAD-associated communities in stimulating the local inflammatory response. The bacterial lipo-polysaccharide (LPS) translocates into the lesions of LAD-periodontitis and potentially triggers immunopathology.14
Diagnosis
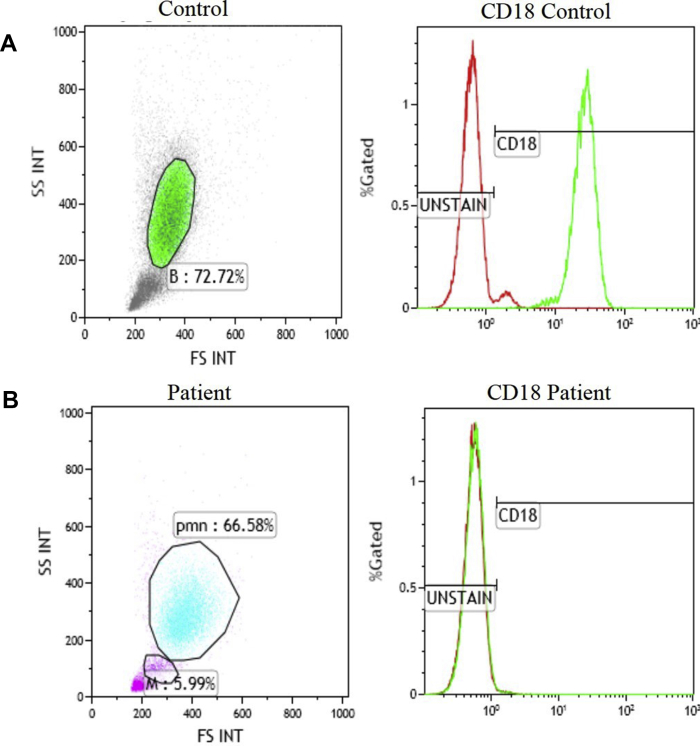
Diagnosis of LAD I is made on the basis of clinical symptoms and marked leukocytosis. Flow cytometry for expression of surface CD11b/CD18 in stimulated and unstimulated neutrophils helps in diagnosis.15 Results of Flow-cytometry evaluation for CD18 however, are inconsistent. Expression of CD18 does not always correlate with the genetic mutation and may even sometimes be normal in patients with LAD I. Combined evaluation for CD18 and CD11a has been proposed to overcome this issue.3 Finding a mutation in ITGB2 gene is confirmatory and is helpful for genetic counselling and prenatal diagnosis. Flow cytometry on fetal blood is reported to have been used for pre-natal diagnosis16 (Fig. 3). This may be of utility in centres where genetic diagnosis is not readily available.
Figure 3.
Flow-CytometricImmunophenotyping for CD18 Leukocytes on peripheral blood: neutrophil gated using SSC/FSC and CD18 expression is measured in (A) healthy control against (B) LAD patient. CD18 expression is normal in control while its absent in the patient.
Management
Bone marrow transplantation is required for the most severe cases with no or very low expression of CD18 protein (<1% of normal expression). In mild to moderate cases, antimicrobial treatment and prophylaxis is helpful. Intravenous immunoglobulins have been used in some patients which resulted in reduced frequency of infections and healing of pyoderma gangrenosum like lesions in a patient.16 Subcutaneous immunoglobulins have also been successfully used in non-healing ulcers in patients with LAD I.17 Granulocyte infusions along with Granulocyte Colony Stimulating Factor (G CSF) have been used to treat ecthyma gangrenosum in a patient with LAD I.18 Recently inflammatory lesions in a patient with LAD I were effectively treated with Interleukin (IL)-12 and IL-23 blockade with ustekinumab.9 Gene therapy for LAD I has been studied and results in mouse models are promising.19, 20, 21, 22
Leukocyte adhesion defect II (LAD II)
Brief introduction
LAD II is not as common as LAD I, it was first described in 1992 in two unrelated Arab boys, then 3 and 5 years old, both offsprings of consanguineous mating. Both showed severe psychomotor and growth retardation. They also had recurrent infections with marked neutrophilia and periodontitis. Their red cells expressed a non-fucosylated variant of the H-antigen and were found to have the rare Bombay blood phenotype. Another set of fucose-containing surface molecules, the sialyl Lewis X, an important ligand for selectins on leukocyte surfaces were also absent in these boys.23 Because these features were different from those of a previously described LADI, this new syndrome was designated LAD II.
Pathogenesis
Functionally, Golgi GDP-fucose transporter (GFTP) transfers GDP-fucose from endoplasmic reticulum into Golgi-apparatus where GDP-fucose is used for post-translational fucosylation of glycol-conjugates. Post-translational α1, 3 fucosylation of terminal N-acetylglucosamines of O- and N-glycans is required for synthesis of functional selectin ligands. Thus, in absence of Golgi-localised GFTP, GDP-fucose is not available for synthesis of functional selectin ligands in the Golgi apparatus, subsequently affecting leukocyte rolling. Adhesion and transmigration via β2 integrin is intact in LAD II, possibly permitting some neutrophil mobilization to sites of inflammation, thereby allowing some level of neutrophil defence. Presence of Bombay blood group also results from defective fucose metabolism.
Genetics
LAD II is alternatively called congenital deficiency of glycosylation-IIc (CDG-IIc) and is caused by loss of function mutations in solute carrier family 35 member C1 (SLC35C1) or FUCT1 gene, which encodes for Golgi-localised GDP-fucose transporter.24, 25 The human gene encoding for specific fucose transporter that is mutated in LAD II is located on chromosome 11.26 Rolling step of neutrophil adhesion is impaired by hypofucosylation of protein CD15s (sialyl Lewis X, SLeX) on neutrophils.27 Mutations in GFTP can be divided into two groups: in one, mutated protein is normally located in Golgi apparatus but its function is impaired; in second group transporter is improperly located in endoplasmic reticulum and its function is impaired. Till now 5 different mutations are identified in the SLC35C1 gene which includes 3 missense mutaions (c.1010A > G, c.923C > G and c.439C > T), one deletion (c.588delG) and one nonsense mutation (c.969G > A).4 Additionally two polymorphisms are also identified in the SLC35C1 gene: c.718A/G and c.772T/C.4
Clinical manifestations
Susceptibility to bacterial infections is not as severe with mild cases of pneumonia and superinfections of varicella lesions (Table 1). This variant is characterized by growth and mental retardation, hypotonia, seizures, strabismus, and persistent periodontitis. Microcephaly, coarse facies, cerebral atrophy, craniosynostosis and autistic features are seen in some patients.27, 28 Dysmorphic features like simian crease, depressed nasal bridge, toe abnormalities and long eye-lashes have been reported.29 Wound healing is not impaired in LAD II patients. A characteristic feature of LAD II is rare Bombay blood group phenotype, which occurs due to the absence of H-antigen.
Diagnosis
Diagnosis of LAD II is done by flow-cytometric measurement of sialyl Lewis X (CD15) on neutrophils. Detection of mutation in SLC35C1 gene by genetic testing confirms the diagnosis4.
Management
Infections in LAD II are not as severe as in LAD I and thus treatment of infections, as they occur, is the usual practise. Antimicrobial prophylaxis is also recommended by some workers (Ref). Due to small number of patients described till date, treatment for LAD II is not standardized. Fucose supplementation has been used successfully as a treatment in some patients with LAD II.30, 31
Leukocyte adhesion defect III (LAD III)
Brief introduction
Leukocyte adhesion deficiency 3 (LAD-III), initially named LAD I/variant (LAD-Iv) is caused by a general defect in integrin activation32.
Pathogenesis
Leukocyte integrin expression and genetic sequence of CD18 are normal in LAD III, and rolling of LAD III leukocytes is also intact in these patients. Activation of all major leukocyte integrins (β 1, 2, and 3) by chemokines and chemoattractants is severely impaired, and lymphocytes fail to arrest on endothelial integrin ligands. LAD III platelets have decreased binding to soluble fibrinogen, do not respond properly to thrombin via thrombin receptors, and therefore have poor platelet granule secretion through integrin activation.32
Genetics
This syndrome is caused by mutations in FERMT3, which encodes KINDLIN3, an adaptor protein expressed in hematopoietic cells that regulates integrin activation.33 So far 9 different mutations in Fermit in family homolog 3(FERMT3) are known to cause LAD III, which include one missense mutation (c.922G > A), 4 nonsense mutations (c.48G > A, c.687G > A, c.1525C > T, c.S > T), two splice site variants (c.161-2A > C, c.1671-2A > G), one insertion (c.238_244dup7) and one deletion (c.1275delT).4 The KINDLIN3 protein binds β subunit of integrins by interacting with C-terminal NXXY/F site which stabilizes active conformations of integrin subunits. KINDLIN3 deficiency causes abnormal activation in all β integrins. Defective function of β 2 integrin causes an immune deficiency similar to LAD I and of β3 integrin causes a bleeding defect, as observed in Glanzmann thrombasthenia.34 A mutation is also observed in Ca2+ and diacylglycerol regulated guanine nucleotide exchange factor I (CALDAG-GEF1), an important element in integrin activation that leads to impaired Ras-related protein 1 (RAP-1) signalling. Kindlin-3 is encoded in the same locus as gene encoding CALDAG-GEF1 (RASGRP2) (11q13), although two genes are 5,03,029 base pairs apart.35 Based on location and severity of mutation, LAD III leukocytes may also display loss of adhesion to vascular cell adhesion molecule-1 (VCAM-1).
Clinical manifestations
It presents with a distinct infantile bleeding diathesis similar to Glanzmann-type thrombasthenia along with defective leukocyte adhesion. LAD-III patients suffer from mild LAD-I-like immunodeficiency with recurrent bacterial and fungal infections, serious skin and soft-tissue infections without pus formation regardless of a marked leukocytosis. Patients frequently show delayed detachment of umbilical cord and impaired wound healing. Osteopetrosis-like bone defect is observed in some patients.
Diagnosis
Diagnosis of LAD III is confirmed by finding mutations in Ca2+ and diacylglycerol regulated guanine nucleotide exchange factor I (CALDAG GEFI) and Fermitin family homolog 3(FERMT3).36
Management
Bone marrow transplantation is necessary and curative.32
Leukocyte adhesion defect IV (LAD IV)
Brief introduction
In 2015, recent variant of Leukocyte Adhesion Defects viz, LAD IV was characterized in patients with monogenic defect in cystic fibrosis transmembraneconductance regulator (CFTR) gene, resulting in cystic fibrosis.37 Cystic fibrosis (CF) is a life-threatening genetic disease caused by imbalance in ionic transport across secretory epithelia in several organs, such as lungs, gastrointestinal tract, pancreas and liver resulting in abnormal mucociliary clearance, and dehydration of periciliary liquid. A salient feature of CF is severe lung infections characterized by exaggerated neutrophilic inflammation that often go along with chronic bacterial airway infection and progressive bronchiectasis, aggravating chronic pulmonary disease and respiratory failure.
Pathogenesis
The function of CFTR appears to be involved in optimal phagocytosis and killing of Pseudomonas aeruginosa by monocytes. Both β1 and β2 integrin–dependent activation by chemo attractants, as well as rapid adhesion and chemotaxis, is severely compromised in CF monocytes but not in polymorphonuclear leukocytes. This defect especially impairs monocyte trafficking into lungs, causing entrapment of monocytes in lung parenchyma. Monocytes from patients with CF have defect in adhesion to intercellular adhesion molecule-1 (β2 integrins ligand: lymphocyte function-associated antigen-1 [LFA-1] and macrophage-1 antigen [Mac-1]), fibrinogen (Mac-1 ligand), and vascular cell adhesion molecule-1 (α4β1 integrin ligand).
Genetics
Mutations in CFTR cause reduced Ras homolog gene family member A (RhoA) and cell division control protein 42 (Cdc42) activation, resulting in impaired α4 and β2 integrin activation and monocyte function.38 The F508del mutation in CFTR is most common, which is found in 90% of CF patients. Several other mutations like N1303K, G85E, and G91R lead to a misfolded CFTR protein that is prematurely degraded. About 5–10% of CFTR mutations like G542X results in premature truncation or nonsense alleles while some CFTR mutations such as G551D and A455E encode properly processed, full-length CFTR protein that lacks normal ion channel activity.38 CFTR-correcting drugs VRT325 and VX809, have the ability to recover CFTR expression on monocytes and restore functionality of β1 and β2 integrins in CF patients.
Clinical manifestations
LAD IV causes persistent lung infections in patients, progressively limiting the ability to breathe. Patients develop disseminated bronchiectasis, congenital bilateral absence of vas deferens (CBAVD), chronic rhinosinusitis and chronic pancreatitis.39
Diagnosis
Diagnosis of LAD IV can be made through Cystic fibrosis transmembrane conductance regulator (CFTR) gene mutation screening.37, 38
Management
Antimicrobial prophylaxis is used to treat infections and chest physiotherapy is helpful in patients. Pancreatic enzyme replacement in patients with chronic pancreatitis and lung transplant serve as curative measures.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 2.Castriconi R., Dondero A., Cantoni C. Functional characterization of natural killer cells in type I leukocyte adhesion deficiency. Blood. 2007;109(11):4873–4881. doi: 10.1182/blood-2006-08-038760. [DOI] [PubMed] [Google Scholar]
- 3.Rechavi E., Abuzaitoun O., Vernitsky H. Highlighting the problematic reliance on CD18 for diagnosing leukocyte adhesion deficiency type 1. Immunol Res. 2015;64(2):476–482. doi: 10.1007/s12026-015-8706-5. [DOI] [PubMed] [Google Scholar]
- 4.van de Vijver E., Maddalena A., Sanal Ö. Hematologically important mutations: leukocyte adhesion deficiency.Blood Cells. Mol Dis. 2012;48(1):53–61. doi: 10.1016/j.bcmd.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolach B., Gavrieli R., Wolach O. Leucocyte adhesion deficiency-A multicentre national experience. Eur J Clin Investig. 2019;49(2):e13047. doi: 10.1111/eci.13047. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A., Gupta A., Rawat A., Ahuja C., Suri D., Singh S. Brain abscess in a child with leukocyte adhesion defect: an unusual association. J Clin Immunol. 2016;36(7):624–626. doi: 10.1007/s10875-016-0315-0. [DOI] [PubMed] [Google Scholar]
- 7.Sharma A., Jindal A.K., Pilania R.K., Gautam P., Daroch S. Palatal ulcer in leukocyte adhesion deficiency: an unusual occurrence. J Clin Immunol. 2018;38(7) doi: 10.1007/s10875-018-0545-4. 736–736. [DOI] [PubMed] [Google Scholar]
- 8.Stark M.A., Huo Y., Burcin T.L., Morris M.A., Olson T.S., Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22(3):285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Moutsopoulos N.M., Uzel G., Notarangelo L.D. Interleukin-12 and interleukin-23 blockade in leukocyte adhesion deficiency type 1. N Engl J Med. 2017;376(12):1141–1146. doi: 10.1056/NEJMoa1612197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva L.M., Brenchley L., Moutsopoulos N.M. Primary immunodeficiencies reveal the essential role of tissue neutrophils in periodontitis. Immunol Rev. 2019;287(1):226–235. doi: 10.1111/imr.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arkwright P.D., Abinun M., Cant A.J. vol. 99. 2002. Autoimmunity in Human Primary Immunodeficiency Diseases Blood; pp. 2694–2702. [DOI] [PubMed] [Google Scholar]
- 12.Notarangelo L.D., Martire B., Moratto D. Long term outcome of eight patients with type 1 Leukocyte Adhesion Deficiency (LAD-1): not only infections, but high risk of autoimmune complications. Clin Immunol. 2018;191:75–80. doi: 10.1016/j.clim.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 13.D'Agata I.D., Paradis K., Chad Z., Bonny Y., Seidman E. Leucocyte adhesion deficiency presenting as a chronic ileocolitis. Gut. 1996;39(4):605–608. doi: 10.1136/gut.39.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moutsopoulos N.M., Chalmers N.I., Barb J.J. Subgingival microbial communities in leukocyte adhesion deficiency and their relationship with local immunopathology. PLoS Pathog. 2015;11(3):1–19. doi: 10.1371/journal.ppat.1004698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanegane H., Hoshino A., Okano T. Flow cytometry-based diagnosis of primary immunodeficiency diseases. Allergol Int. 2018;67(1):43–54. doi: 10.1016/j.alit.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Nord K.M., Pappert A.S., Grossman M.E. Pyoderma gangrenosum-like lesions in leukocyte adhesion deficiency I treated with intravenous immunoglobulin. Pediatr Dermatol. 2011;28(2):156–161. doi: 10.1111/j.1525-1470.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki-Nakashimada M., Maravillas-Montero J.L., Berrón-Ruiz L. Successful adjunctive immunoglobulin treatment in patients affected by leukocyte adhesion deficiency type 1 (LAD-1) Immunol Res. 2015;61(3):260–268. doi: 10.1007/s12026-014-8619-8. [DOI] [PubMed] [Google Scholar]
- 18.Mellouli F., Ksouri H., Barbouche R. Successful treatment of fusarium solani ecthyma gangrenosum in a patient affected by leukocyte adhesion deficiency type 1 with granulocytes transfusions. BMC Dermatol. 2010;10(1):10. doi: 10.1186/1471-5945-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer T.R., Allen J.M., Hai M. Successful treatment of canine leukocyte adhesion deficiency by foamy virus vectors. Nat Med. 2008;14(1):93–97. doi: 10.1038/nm1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter M.J., Zhao H., Tuschong L.M. Gene therapy for canine leukocyte adhesion deficiency with lentiviral vectors using the murine stem cell virus and human phosphoglycerate kinase promoters. Hum Gene Ther. 2011;22(6):689–696. doi: 10.1089/hum.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leon-Rico D., Aldea M., Sanchez-Baltasar R. Lentiviral vector-mediated correction of a mouse model of leukocyte adhesion deficiency type I. Hum Gene Ther. 2016;27(9):668–678. doi: 10.1089/hum.2016.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Marzio D., Baronio M., Lucantoni M. Successful anti-TNF-α treatment in a girl with LAD-1 disease and autoimmune manifestations. J Clin Immunol. 2014;34(7):788–791. doi: 10.1007/s10875-014-0086-4. [DOI] [PubMed] [Google Scholar]
- 23.Frydman M., Etzioni A., Eidlitz-Markus T. Rambam-Hasharon syndrome of psychomotor retardation, short stature, defective neutrophil motility, and Bombay phenotype. Am J Med Genet. 1992;44(3):297–302. doi: 10.1002/ajmg.1320440307. [DOI] [PubMed] [Google Scholar]
- 24.Lühn K., Wild M.K., Eckhardt M., Gerardy-Schahn R., Vestweber D. The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat Genet. 2001;28(1):69–72. doi: 10.1038/ng0501-69. [DOI] [PubMed] [Google Scholar]
- 25.Lübke T., Marquardt T., Etzioni A., Hartmann E., von Figura K., Körner C. Complementation cloning identifies CDG-IIc, a new type of congenital disorders of glycosylation, as a GDP-fucose transporter deficiency. Nat Genet. 2001;28(1):73–76. doi: 10.1038/ng0501-73. [DOI] [PubMed] [Google Scholar]
- 26.Etzioni A., Tonetti M. Leukocyte adhesion deficiency II - from A to almost Z. Immunol Rev. 2000;178:138–147. doi: 10.1034/j.1600-065x.2000.17805.x. [DOI] [PubMed] [Google Scholar]
- 27.Yakubenia S., Wild M.K. Leukocyte adhesion deficiency II. FEBS J. 2006;273(19):4390–4398. doi: 10.1111/j.1742-4658.2006.05438.x. [DOI] [PubMed] [Google Scholar]
- 28.Gazit Y., Mory A., Etzioni A. Leukocyte adhesion deficiency type II: long-term follow-up and review of the literature. J Clin Immunol. 2010;30(2):308–313. doi: 10.1007/s10875-009-9354-0. [DOI] [PubMed] [Google Scholar]
- 29.Marquardt T., Brune T., Lühn K. Leukocyte adhesion deficiency II syndrome, a generalized defect in fucose metabolism. J Pediatr. 1999;134(6):681–688. doi: 10.1016/S0022-3476(99)70281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marquardt T., Lühn K., Srikrishna G., Freeze H.H., Harms E., Vestweber D. Correction of leukocyte adhesion deficiency type II with oral fucose. Blood. 1999;94(12):3976–3985. [PubMed] [Google Scholar]
- 31.Etzioni A., Tonetti M. Fucose supplementation in leukocyte adhesion deficiency type II. Blood. 2000;95(11):3641–3643. [PubMed] [Google Scholar]
- 32.Stepensky P.Y., Wolach B., Gavrieli R. Leukocyte adhesion deficiency type III: clinical features and treatment with stem cell transplantation. J Pediatr Hematol Oncol. 2015;37(4):264–268. doi: 10.1097/MPH.0000000000000228. PMID: 25072369. [DOI] [PubMed] [Google Scholar]
- 33.Mory A., Feigelson S.W., Yarali N. Kindlin-3: a new gene involved in the pathogenesis of LAD-III. Blood. 2008;112(6) doi: 10.1182/blood-2008-06-163162. 2591–2591. [DOI] [PubMed] [Google Scholar]
- 34.Moser M., Nieswandt B., Ussar S., Pozgajova M., Fässler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14(3):325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 35.Pasvolsky R., Feigelson S.W., Kilic S.S. A LAD-III syndrome is associated with defective expression of the Rap-1 activator CalDAG-GEFI in lymphocytes, neutrophils, and platelets. J Exp Med. 2007;204(7):1571–1582. doi: 10.1084/jem.20070058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etzioni A. Genetic etiologies of leukocyte adhesion defects. Curr Opin Immunol. 2009;21(5):481–486. doi: 10.1016/j.coi.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Fan Z., Ley K. Leukocyte adhesion deficiency IV. Monocyte integrin activation deficiency in cystic fibrosis. Am J Respir Crit Care Med. 2016;193(10):1075–1077. doi: 10.1164/rccm.201512-2454ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorio C., Montresor A., Bolomini-Vittori M. Mutations of cystic fibrosis transmembrane conductance regulator gene cause a monocyte-selective adhesion deficiency. Am J Respir Crit Care Med. 2016;193(10):1123–1133. doi: 10.1164/rccm.201510-1922OC. [DOI] [PubMed] [Google Scholar]
- 39.Best practice guidelines for molecular genetic diagnosis of cystic fibrosis and CFTR- related disorders--updated European recommendations. Eur J Hum Genet. 2009;17(1):51–65. doi: 10.1038/ejhg.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]