Abstract
Chronic granulomatous disease (CGD) is an inherited defect of phagocyte function due to defective NADPH oxidase. Patients with CGD are not able to effectively clear the infections because of the defect in the phagocyte production of oxygen free radicals and are prone to recurrent bacterial and fungal infections. Inflammatory complications are also noted in CGD such as colitis, non-infective granulomas causing gastrointestinal or urinary tract obstruction, hemophagocytic lymphohistiocytosis, and arthritis. Studies on toll-like receptor pathways and neutrophil extracellular traps in CGD have shed light on the role of NADPH oxidase in the innate immunity and pathogenesis of infections in CGD. Some reports also indicate a reduction of memory B cells and defective production of functional antibodies in CGD. Though the exact mechanisms for non-infective inflammatory complications in CGD are not yet clear, studies on efferocytosis and defective autophagy with inflammasome activation have made a substantial contribution to our understanding of the pathogenesis of inflammation in CGD. We also discuss the clinical and molecular features of p40phox defects and a newer genetic defect, EROS. Clinical phenotypes of X-linked carriers of CYBB are also discussed.
Keywords: Chronic granulomatous disease, Colitis, EROS, Genetics, Infections, Inflammation, p40phox
Introduction
Chronic granulomatous disease (CGD) is a primary immunodeficiency disorder that affects the phagocyte function. Initially, it was described as “fatal granulomatous disease of childhood”.1 Patients with CGD have impaired intracellular killing of the pathogen due to a defect in any one of the five subunits of NADPH oxidase complex, that are responsible for the production of reactive oxygen species.
Neutrophils are involved in immune regulation and pathogen clearance. Neutrophils and monocytes mediate oxidative burst by the production of reactive oxygen species (ROS) such as superoxide radicals via nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase complex. Defective NADPH oxidase machinery leads to impaired production of ROS and impaired pathogen clearance.
Pathogenesis
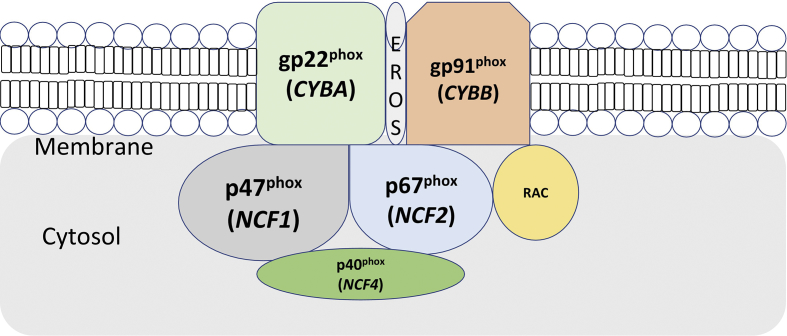
The functional NADPH oxidase complex is comprised of 5 subunits; two of which are localized in the cell membrane in the resting phase while three are in the cytoplasm. The two membrane-bound subunits are gp91phox (transmembrane glycoprotein with molecular mass of 91 kD) and p22phox (transmembrane non-glycoprotein with molecular mass 22 kD). The two proteins (gp91phox and p22phox) form a heterodimer named cytochrome b558 and hang on each other for stable expression and maturation (Fig. 1). Upon activation by the contact of cell membrane with a microorganism, the three cytoplasmic subunits (p47phox, p67phox, and p40phox) forms a heterotrimer and translocate to the cytochrome b558.
Figure 1.
A representative figure depicting the units of NADPH oxidase complex. Membrane bound units include gp91phox and p22phox. Cytosolic components include p47phox, p67phox, p40phox and Rac.
The genes encoding the five subunits of NADPH oxidase enzyme are; CYBB for gp91phox (located on the X chromosome), CYBA encoding p22phox, NCF1 encoding p47phox, NCF2 encoding p67phox, and NCF4 encoding p40phox. According to data from U.S. and European nations, almost ∼65% of patients with CGD have a molecular defect in CYBB (most of them are hemizygous males with a few heterozygous females due to unfavored lyonization). Autosomal recessive CGD constitutes for ∼30% of cases (∼25% cases occur due to a defect in p47phox, and rest 5% cases occur due to p67phox and p22phox deficiency).2, 3 Molecular defects in any one of these five genes can result in CGD. The clinical feature of the disease, severity of the disease, and survival of the patient greatly depends on the gene mutated, type of mutation, and position of mutation.4
Neutrophils are the phagocytes majorly involved in immune regulation and pathogen clearance. Neutrophils and phagocytic leukocytes mediate oxidative burst by the production of reactive oxygen species (ROS) such as superoxide radicals via nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase complex. Defective NADPH oxidase machinery leads to impaired production of ROS. Neutrophils from patients with chronic granulomatous disease (CGD) have impaired NADPH oxidase machinery leading to impaired ROS production and the inability to generate Neutrophil Extracellular Traps (NETs).5 Patients with CGD usually suffer from persistent bacterial or fungal infections due to poor phagocytic action of the phagocytes such as neutrophils.
Defective TLR signalling in CGD
Neutrophils provide defence by initiating production of proinflammatory cytokines and antimicrobial products at the site of infection. Neutrophils detect the presence of a pathogen through pattern recognition receptors (PRRs) that recognize pathogen-associated molecular patterns (PAMPs). One of the best characterized class of PRRs is toll-like receptors (TLRs). Expression of TLRs 1, 2, 4, 5, 6, 7, 8, 9, and 10 have been shown on human neutrophils.6 TLR agonists trigger the generation of reactive oxygen species (ROS), L-selectin shedding, the release of cytokines and inhibiting the chemotactic response to interleukin-8 (IL-8) stimulation in neutrophils. Defects in PRR signalling may contribute to the pathogenesis of chronic inflammation in various diseases. Release of damage associated molecular patterns (DAMPs) by tissue injury can act in an autocrine way resulting in amplification of inflammation and tissue damage.
The first report analyzing toll-like receptors (TLRs) in a patient with CGD showed that neutrophils, on stimulation with liposaccharide (LPS) (agonist of TLR4), secrete increased levels of TNF-α and IL-8. PBMCs of patients with CGD, when treated with TLR2 or TLR4 agonists, secreted very high levels TNF-α and IL-6 levels.7 Further, it was shown that ROS are needed for appropriate dampening of responses to these TLRs, in a positive feedback loop. The study inferred that the absence of ROS is related to increased expression of NF-κB subunits, contributing to higher levels of pro-inflammatory cytokines. Patients with CGD have also been shown to have reduced expression of TLR5 and TLR9 in the neutrophils. NADPH oxidase may be important for proper expression of TLR5 and TLR9 in neutrophils, while its absence results in impaired responses to stimulation by TLR ligands and less efficient phagocytosis.8 This regulation can be TLR specific, with NADPH oxidase complex promoting responses downstream of TLRs 5 and 9 signalling.6, 9 These results highlighted the importance of NADPH oxidase and ROS in regulating TLR signalling.
B cell dysfunction in CGD
There have been reports on defects in long term antibody memory responses in patients with CGD. Deficient ROS production in B cells has been reported to result in a reduction of memory B cells in patients with CGD. NADPH oxidase system was further shown to be important in the context of TLR9 stimulation in B cells.10 An age-dependent reduction in anti-measles antibody levels was also seen in patients with CGD. There was also reduced induction of measles-specific antibody secreting cells (ASCs) downstream of TLR9 activation. According to the study by Cotugno et al, reduced expression of TLR9 due to defect in NADPH oxidase might lead to a reduction in the production of long-term B cell memory.10 Recently, some studies have highlighted that B cell signalling and differentiation are impaired in patients with CGD.11 The process of conversion of naïve B cells to memory B cells as well as B cell differentiation has been shown to be impaired in patients with CGD. The altered phenotype of B lymphocytes might increase the susceptibility to opportunistic infections. A study conducted in patients with X-linked CGD has shown increased production of pro-inflammatory cytokines in PBMCs on stimulating with TLR agonists such as TLR9. This provides evidence that blood cells other than neutrophils are affected in patients with CGD.9, 11, 12 Studies have shown that total T cell numbers are low in CGD compared to controls after 3 years of age, however, the data on T cell proliferation and functions are limited.13 A recent study by Chiriaco et al14 demonstrated that human T cells do not express gp91phox and reactive oxygen species generation is gp91phox-NADPH oxidase-independent.
Neutrophil extracellular traps
Neutrophils die immediately after the phagocytosis of the antigen, but when treated by a mitogen phorbol 12-myristate 13-acetate (PMA), they undergo a rapid killing by a process completely different from apoptosis or necrosis.15 Work done by Zychlinsky and colleagues have shown that following the neutrophil suicide, large web-like structures are released which are composed of antimicrobial factors and decondensed chromatin. They named these structures as neutrophil extracellular traps (NETs).16 NETs are formed usually to protect against infection by large pathogens. The process of formation of NETs is known as neutrophil suicide or NETosis. Another study showed that the process of NETosis is NADPH oxidase-dependent.17 The process of NETosis involves disintegration of the nuclear envelope, mixing of nucleic acids and proteins with the intracellular vacuole, followed by releasing of NETs via plasma membrane perforation and cell lysis.18 Apart from PMA and IL-8, the NET formation is also triggered by innate immune receptors like cytokine receptors (tumour necrosis factor (TNF), interferon (IFN)) as well as bacteria, fungi, protozoa, antibody–antigen complexes, and autoantibodies. NETs consist of chromatin fibres containing DNA, histones (H1, H2A, H2B, H3, and H4) and several proteins like myeloperoxidase (MPO), neutrophil elastase (NE), proteinase 3 (PR3), cathepsin G, high mobility group protein B1 (HMGB1), and LL37, therefore, giving it characteristics of proinflammation.19
Treatment with hydrogen peroxide (H2O2) has been seen to rescue the production of NETs in neutrophils in patients with CGD. Compounds like N-acetylcysteine, or Trolox (ROS scavengers) have also been reported to inhibit NETosis.19 It has been seen that reconstitution of NET formation by gene therapy in a patient with CGD restored the neutrophil elimination of Aspergillus nidulans leading to cure of refractory invasive pulmonary aspergillosis. This report highlights the importance of functional NADPH oxidase in the NET formation and antifungal activity.20
Defects in autophagy
Both mice studies and human studies have shown that recruitment of light chain 3 (LC3) to phagosomes is reduced in CGD, thereby resulting in defective autophagy. The defect in autophagy has been shown to result in excess production of IL1-beta following stimulation with lipopolysaccharide.21 Studies in mice also revealed that treatment with anti-IL1 agents reduced the manifestation of experimental colitis and also restored the autophagy. Some human studies have also shown that anti-IL1 agents are effective in reducing the manifestation of colitis in CGD.22
Defects in efferocytosis
Efferocytosis is the mechanism by which the macrophages clear the dying cells. Impairment in macrophage efferocytosis has been documented in animal and human studies in CGD. Activated neutrophils usually express phosphatidyl-serine on its surface which in turn is sensed by the macrophages for phagocytosis and cell death (efferocytosis). Activated neutrophils in patients with CGD fail to externalize phosphatidyl-serine on its surface. Moreover, defective clearance of these activated neutrophils has been shown to result in heightened inflammatory response in CGD.23, 24 Therapy with peroxisome proliferator-activated receptor-gamma (PPR-gamma) agonists such as pioglitazone has been shown to enhance the function of efferocytosis in macrophages, improvement in colitis, and enhanced clearance of micro-organisms in patients with CGD.25
Hyperinflammation in CGD
Patients with CGD suffer from a variety of inflammatory conditions unrelated to infections that include granuloma formation, colitis, increased frequency of autoimmune diseases. Multiple reports suggest that absence of reactive oxygen species (ROS) generation by NOX2 leads to enhanced inflammation. The lower capacity to produce ROS is associated with an increased number of reduced thiol groups on T-cell membrane surfaces. Mice experiments have shown that reduced thiol group in T cells influences activation and proliferation of T cells and increased susceptibility to development of arthritis.26 Other mechanisms for hyperinflammation include decreased degradation of phagocytosed material with accumulated material causing persistent cellular activation, attenuated Ca 2+ signaling, redox-dependent termination of proinflammatory mediators and/or signaling, as well as redox-dependent cross-talk between phagocytes and lymphocytes (e.g. defective tryptophan catabolism).27
Clinical features
Patients with CGD present with recurrent episodes of cutaneous abscess, lymphadenitis, pneumonia, deep organ abscess and osteomyelitis. Common pathogens implicated in the infections are Staphylococcus aureus, Aspergillus sp., and Burkholderia cepacia. Onset of clinical manifestations usually occur by 2 years of age, however, some patients may have milder manifestations and present later in life due to a residual NADPH oxidase activity. The clinical feature of the disease, severity of the disease and survival of the patient greatly depends on the gene involved, kind of mutation, and position of mutation.2, 28, 29
Patients with CGD are previously thought to have predisposition only to infections with catalase positive microorganisms, however, with the advent of molecular techniques, infections with other microorganisms have also been documented.30 Weakly pathogenic organisms can result in severe infections in patients with CGD.31 These organisms are acquired from the environmental contact and cause the infections in the directly exposed organs like skin, respiratory tissues, lymph nodes, and gut.32 Infections in CGD are usually locally invasive, however, hematogenous spread of infections can also occur.33
Staphylococcus is usually implicated in liver abscess, cutaneous abscess, and suppurative adenitis. Pneumonia is typically caused by Aspergillus sp., Staphylococcus, enteric gram-negative bacteria, and Nocardia. Pulmonary infections are often insidious in onset, and generally chronic in the presentation. There may not be severe systemic dissemination, but infection in the lung parenchyma can expand in the vicinity like adjacent lung lobes, lymph nodes, and ribs. Invasive biopsy of lung tissue may be needed to identify the microorganism. Sometimes the lung lesions would involve the ribs that might require a rib resection.
Localized and disseminated BCG infections have also been noted in patients with CGD. In countries endemic to tuberculosis, infections due to Mycobacterium tuberculosis have also been documented in CGD.30, 34 Mulch pneumonitis is a severe inflammatory lung pathology seen in patients with CGD who are exposed to decayed living wastes. Aerosolized content causes severe inflammatory reactions in lung parenchyma and results in acute respiratory distress. Less common infections in CGD are osteomyelitis and visceral abscesses. Fungal pneumonia due to Aspergillus sp. in CGD often require prolonged courses of anti-fungal medications such as amphotericin B and voriconazole. Posaconazole, as salvage therapy in refractory filamentous fungal infections in CGD, has also been found to be beneficial.35 Apart from Aspergillus sp., Fusarium dimerum, Penicillium sp., Paecilomyces variotii, Scedosporium sp. have also been reported to cause pulmonary infections in CGD. Identification of fungus either by culture or molecular techniques is essential to guide the choice of therapy. For instance, Aspergillus nidulans have reduced susceptibility to amphotericin B and some species of Aspergillus are resistant to azole therapy.4
Liver abscesses due to Staphylococcus aureus in CGD are usually multi-locular. These lesions are mostly caseous, thick, and non-drainable by usual fine needle aspiration, requiring surgical intervention to clear.36 Recent reports have shown the beneficial effects of addition of glucocorticoids with intravenous antibiotics for the management of liver abscess in CGD in terms of reduction in the need of surgical intervention and enhanced resolution of abscess.37, 38, 39 Small bone osteomyelitis due to Serratia marcescens, septicemia due to Burkholderia cepecia and Chromobacter sp. are also characteristically seen in patients with CGD. Non-typhoidal Salmonellosis has also been reported in patients with CGD.
With the advent of molecular techniques like 16S rRNA sequencing, newer microorganisms have been identified to cause infections in CGD. These include methylotrophs such as Granulibacter bethesdensis, Methylobacterium lusitanum, and Acidomonas methanolica.40
Non-infectious inflammatory complications that are seen in CGD are colitis, granulomas causing intestinal or urogenital tract obstruction, hemophagocytic lymphohistiocytosis, and arthritis.33 Chronic inflammatory condition like retinitis, white matter disease, and lupus-like presentation are also rarely seen.
Diagnosis of CGD
Residual NADPH oxidase enzyme activity can be measured by oxygen consumption, superoxide generation or production of hydrogen peroxide in different assays. Superoxide production can be measured by Nitroblue Tetrazolium (NBT) reduction test, where a normal neutrophil on stimulation turns the pale yellow NBT to black solid formazan crystals. This reaction takes place inside the activated phagocyte. Patients with CGD show no or few formazan crystals inside the cells. The test can be performed on a microscopic slide and seen under a light microscope. This makes the NBT test an ideal assay for diagnosis of CGD even in resource-poor settings.
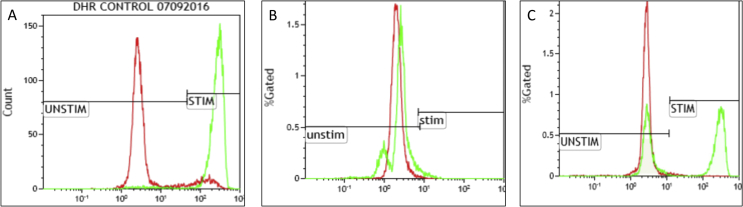
The activity of NADPH oxide enzyme can also be measured by its capacity to generate hydrogen peroxide after activation. Few well-known hydrogen peroxide-detecting agents are dihydrorhodamine-1,2,3 (DHR), resorufin, and luminol. DHR is a permeable dye which freely enters into the cell and oxidized into rhodamine-1,2,3 which emits a fluorescent signal in the green spectrum (525–575 nm) when excited with 488 nm light source. This assay measures the fluorescence signal from a single cell in a flow cytometer. The assay can also detect the carrier status of X-linked CGD. DHR by flow cytometry is the most sensitive and reliable assay for diagnosis of CGD (Fig. 2).
Figure 2.
Dihydrorhodamine test by flow cytometry showing oxidative capacity of the PMA-stimulated neutrophils (green). (A) Control sample depicting normal shift of the activated neutrophils with the median fluorescence intensity (MFI) of 277.95 compared to 2.75 in the unstimulated neutrophils (red). (B) DHR of a patient with a defect in CYBB showing absent shift of the stimulated neutrophils (green) with MFI of 2.57 compared to 2.02 in the control (red). (C) DHR of an X-linked carrier of CYBB defect showing a bimodal population of the stimulated neutrophils (green).
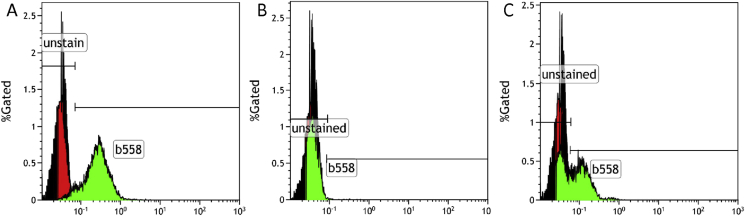
Flow cytometry can also be used to study the components of the NADPH oxidase complex-gp91phox, p47phox, p67phox, p22phox, and p40phox. Cytochrome b558 (gp91phox and p22phox) expression in the phagocytes can also be studied by flow cytometry (Fig. 3). Patients with a defect in CYBB or CYBA have an absent expression of b558, whereas X-linked carrier females show a bimodal distribution of population resembling a mosaic pattern as in DHR.
Figure 3.
Cytochrome b558 staining by flow cytometry. (A) Control sample showing normal staining and surface expression of b558. (B) Patient with a defect in CYBB showing absent surface staining for b558. (C) An X-linked carrier of CYBB defect showing mosaic pattern of expression of b558.
If the b558 assay is normal, intracellular staining of cytoplasmic components of the enzyme (p47phox and p67phox) can be performed. The results are interpreted in terms of median fluorescent intensity and percentage positivity. In such assays, the normal expression does not rule out disease, as there can be a mutation with intact protein expression but with diminished function.
Mutation analysis should be performed in patients with CGD to confirm the genetic defect and is needed for genetic counselling and prenatal screening. Disease carriers without disease phenotypes can reliably be screened by mutation analysis.
All the exons along with the exon-intron junctions of CYBB, CYBA, NCF2, and NCF4 genes can be amplified by polymerase chain reaction (PCR) from genomic DNA and run on 2% agarose gel. Amplified products, further, can be subjected to Sanger sequencing protocol for targeted sequencing. The data generated from Sanger sequencing could be analyzed by different bioinformatics pipelines such as codon code analyzer and clustalW. Next-generation sequencing platform can also be performed that can sequence many genes in a single run, and could potentially reduce the turnover time to arrive at the final genetic diagnosis as compared to the conventional Sanger sequencing.
The usual molecular defect in NCF1 involves deletion of GT at the start of exon 2 which induce a frame-shift and premature termination of the protein p47phox. However, analysis of the NCF1 sequencing results after Sanger or next-generation sequencing is difficult as NCF1 is accompanied by a pseudogene which shares >99% homology to NCF1 except for a GT sequence at the start of exon 2. It is therefore recommended to perform a gene scan to verify a GT deletion in the gene. The mutations other than GT deletion can be analyzed by NCF1 specific PCR from cDNA and confirmed by NCF1 specific amplification of genomic DNA. To perform gene scan, specific primers with fluorescent dye for GTGT site of exon 2 are to be designed and both NCF1 and pseudogene are amplified. The amplified product of both the NCF1 gene and pseudogene varies in 2 nucleotides in size. The mixture of both the amplified products is analyzed on GeneMapper software on applied biosystem analyzer. Peak height (correspond to the copy number) is calculated and a ration of NCF1 to pseudogene is determined.41
Newer genetic defects in CGD
p40phox defect
Defects in NCF4 result in an autosomal recessive form of CGD due to deficient p40phox. It is the third cytosolic NADPH oxidase component. NCF4 is located in gene locus 22q13.1 with a span of 18 kb and 10 exons.42 The pathogenic part of p40phox is less clear43 but current studies in mice highlighted that p40phox is involved in phagocytic activity via a phox homology (PX) domain.44, 45 The first case with NCF4 defect was reported in the year 2009 in a boy with granulomatous colitis46 who was non-responsive to glucocorticoids, sulfasalazine, and azathioprine. Investigations revealed deficient superoxide production in neutrophils and he was compound heterozygous for a frameshift mutation with a premature stop codon and a missense mutation predicting an R105Q substitution in the PX domain. Recently, a multi-centric study involving 8 different countries reported 24 patients with p40phox defects from 12 different families, of which 8 different in-frame or out-of-frame mutations were documented47 (Table 1). This study also highlighted the differences in clinical manifestations of the p40phox defect compared to classical CGD.
Table 1.
Molecular defects in NCF4 documented until date.
| Nucleotide change | Type of mutation | Amino acid or mRNA change | References |
|---|---|---|---|
| c.143_152dup10 | Insertion | p.Lys52ArgfsX79 | 46 |
| c.314G > A | Missense | p.Arg105Gln | 46 |
| c.118-1G > A | Essential splice | – | 47 |
| c.32+2T > G | Essential splice | – | 47 |
| c.314G > A | Missense | p.R105Q | 47 |
| c.172C > T | Missense | p.R58C | 47 |
| c.430C > A | Missense | p.P144T | 47 |
| c.716G > A | Nonsense | p.W239X | 47 |
| c.120_134del | In-frame deletion | – | 47 |
| c.118-1G > A | compound heterozygous splice-site variants | – | 47 |
| c.759-1G > C | compound heterozygous splice-site variants | – | 47 |
These patients usually develop superficial infections, mostly staphylococcal and mount high inflammation (less than classical CGD). Surprisingly, one of these reported cases developed invasive bacterial and fungal infections, however, had a better outcome with no mortality.
The laboratory investigations may be misleading as the widely used PMA-induced DHR oxidation test widely used for the diagnosis of CGD would give normal results. But the diagnosis can be made if serum-opsonized E. coli is used as a stimulant. In this category, it is also seen that the NADPH oxidase activity is normal in mononuclear phagocytes but diminished in EBV-transformed B cells. Surprisingly, in contradiction to the classical CGD, these patients do have the conserved capacity to kill fungi like Candida albicans and Aspergillus fumigatus, making them less prone for the same. The requirement of antifungal prophylaxis may not be high as they are less prone to fungal infections. In view of the milder phenotype, allogeneic HSCT needs to be considered on individual case scenarios. Prior to the relation of NCF4 with CGD, genome-wide association studies had identified polymorphism in NCF4 in patients with rheumatoid arthritis and Crohn's disease.48, 49
EROS defect
Recently, C17ORF62 (human orthologue of mouse Eros) protein, encoded by CYBC1 gene has been described to have a role in the regulation of expression of gp91phox and p22phox heterodimer leading to the generation of reactive oxygen species.50, 51
Eros, an acronym for ‘essential for reactive oxygen species’, is a newly described transmembrane protein in relation to the different subunits of NADPH oxidase enzyme. It is homologous to ycf4 protein in chloroplast photosystem I.52 Cytochrome b558 is a known heterodimer unit of NADPH oxidase complex. Eros is found to have an important role in the expression of oxidative units gp91phox and p22phox. It was observed that Eros deficient group of macrophages and neutrophils had a significantly low level of gp91phox, p22phox and Eros protein.50 It is also believed that this novel protein helps in stabilization of these phox protein subunits.
Since Eros is an essential component of innate immunity especially in neutrophils and macrophages, it was found to have a significant role in the functioning of oxidative burst. Since it has human cytochrome analogue, it is named as CYBC (in relation to hitherto named CYBC1 gene). Eros deficient macrophages were proven to be incapable of killing weakly pathogenic bacteria such as Salmonella typhimurium or Listeria monocytogenes.52 The respiratory burst is defective in Eros deficient cells. It is also found that Eros deficient cells lack the formation of neutrophilic traps. Thomas et al had published a description of a patient with Eros deficiency leading to infectious and inflammatory symptoms with absent DHR response consistent with classical CGD features.50
X-linked carriers of chronic granulomatous disease
The female carriers of X-linked CGD have been reported to have a varied spectrum of diseases. Generally, the superoxide burst is optimal in the carriers, and usually they do not develop significant infections. However, an X-CGD female carrier with X-linked inactivation can present with recurrent infections like CGD. When one X chromosome is silenced epigenetically, resulting in expression of only one X chromosome equivalent is called lyonization.53 This lyonization and random X chromosome inactivation thereby makes female X-chromosome gene dosage largely equivalent to that of males. Progressive skewing of X-chromosome inactivation occurs as age advances, and manifestations of CGD are reported to occur late in X-linked carriers.54, 55 Unusual late cases have also been reported in adults secondary to somatic mosaicism56 and denovo mutations.57
Autoimmune manifestations do occur in X-linked carriers with a wide spectrum ranging from Raynaud phenomenon, arthritis, oral ulcers to frank manifestations of lupus.58 There is also associated increased risk of antiphospholipid syndrome, pericardial effusion, juvenile idiopathic arthritis. The first case report of lupus in an X-linked CGD carrier dates back to 1957.59 In a prospective study of 19 X-linked carrier mothers, there was a history of arthritis, photosensitive rash, oral ulcers suggesting some kind of autoimmunity despite negative antinuclear ANA titers.60 There are multiple case reports suggesting the autoimmunity features in X-linked carriers.57, 61, 62, 63 The exact aetiology for autoimmune manifestations is unknown (Table 2).
Table 2.
Clinical manifestations of X-linked carriers of CGD.
| Cutaneous manifestations | Photosensitive malar rash Discoid lupus erythematosus Recurrent aphthous stomatitis |
| Gastrointestinal manifestations | Colitis/Diarrhea Inflammatory bowel disease |
| Autoimmune manifestations | Lupus Lupus-like syndrome Arthritis/JIA Oral ulcers Raynaud's phenomenon IgA nephropathy |
| Infections (in X-linked inactivation) Pneumonia, skin abscess, lymphadenitis, osteomyelitis, liver abscess |
Staphylococcal aureus, Aspergillus fumigatus, Nocardia, Burkholderia cepacia, Serratia marcescens |
| Ophthamic manifestations | Chorioretinitis |
X-linked carrier status can be reliably demonstrated by DHR test, as it is possible to demonstrate the two populations of neutrophils depending on lyonization and to quantify the percentage of functioning neutrophils. Interpretation of results can sometimes be difficult in flow cytometry-based DHR assay, as the two populations may not be distinct sometimes. A retrospective study involving 162 females with X-linked carriers of CYBB have shown that infection or autoimmune phenotypes depend upon the percentage DHR value. A %DHR+ value of less than 10% was strongly associated with infections.54
These carriers need symptomatic therapy targeting infections and autoimmunity when required. Cutaneous and discoid lupus typically responds to first-line treatment (hydroxychloroquine) and local medication, but systemic steroids are infrequently required for colitis, systemic lupus erythematosus (SLE) or arthritis. In cases with recurrent infections and low DHR, antimicrobial and antifungal prophylaxis would be required.64 Additionally, knowing the carrier status has implications in the antenatal diagnosis.
Conflict of interest
The authors declare no conflict of interest.
Authorship statement
Gummadi Anjani; concept and design, manuscript preparation, manuscript editing.
Pandiarajan Vignesh; concept and design, literature search, manuscript preparation, manuscript editing and manuscript review.
Vibhu Joshi; concept and design, literature search.
Jitendra Kumar Shandilya; concept and design, literature search.
Dharmagat Bhattarai; concept and design, literature search, clinical studies and manuscript review.
Jyoti Sharma; manuscript editing and manuscript review.
Amit Rawat; concept and design, manuscript editing and manuscript review.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Mouy R., Fischer A., Vilmer E., Seger R., Griscelli C. Incidence, severity, and prevention of infections in chronic granulomatous disease. J Pediatr. 1989;114(4 Pt 1):555–560. doi: 10.1016/s0022-3476(89)80693-6. [DOI] [PubMed] [Google Scholar]
- 2.van den Berg J.M., van Koppen E., Ahlin A. Chronic granulomatous disease: the European experience. PLoS One. 2009;4(4):e5234. doi: 10.1371/journal.pone.0005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winkelstein J.A., Marino M.C., Johnston R.B. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltim) 2000;79(3):155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Holland S.M. Chronic granulomatous disease. Hematol Oncol Clin N Am. 2013;27(1):89–99. doi: 10.1016/j.hoc.2012.11.002. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberg B.E., Grinstein S. Unconventional roles of the NADPH oxidase: signaling, ion homeostasis, and cell death. Sci STKE. 2007;2007(379):pe11. doi: 10.1126/stke.3792007pe11. [DOI] [PubMed] [Google Scholar]
- 6.Prince L.R., Whyte M.K., Sabroe I., Parker L.C. The role of TLRs in neutrophil activation. Curr Opin Pharmacol. 2011;11(4):397–403. doi: 10.1016/j.coph.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Bylund J., MacDonald K.L., Brown K.L. Enhanced inflammatory responses of chronic granulomatous disease leukocytes involve ROS-independent activation of NF-kappa B. Eur J Immunol. 2007;37(4):1087–1096. doi: 10.1002/eji.200636651. [DOI] [PubMed] [Google Scholar]
- 8.Rieber N., Hector A., Kuijpers T., Roos D., Hartl D. Current concepts of hyperinflammation in chronic granulomatous disease. Clin Dev Immunol. 2012;2012:252460. doi: 10.1155/2012/252460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi F., Means T.K., Luster A.D. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102(7):2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 10.Cotugno N., Finocchi A., Cagigi A. Defective B-cell proliferation and maintenance of long-term memory in patients with chronic granulomatous disease. J Allergy Clin Immunol. 2015;135(3):753–761. doi: 10.1016/j.jaci.2014.07.012. e2. [DOI] [PubMed] [Google Scholar]
- 11.Mohsenzadegan M., Fattahi F., Fattahi F. Altered pattern of Naïve and memory B cells and B1 cells in patients with chronic granulomatous disease. Iran J Allergy, Asthma Immunol. 2014;13(3):157–165. [PubMed] [Google Scholar]
- 12.Bleesing J.J., Souto-Carneiro M.M., Savage W.J. Patients with chronic granulomatous disease have a reduced peripheral blood memory B cell compartment. J Immunol. 2006;176(11):7096–7103. doi: 10.4049/jimmunol.176.11.7096. [DOI] [PubMed] [Google Scholar]
- 13.Heltzer M., Jawad A.F., Rae J., Curnutte J.T., Sullivan K.E. Diminished T cell numbers in patients with chronic granulomatous disease. Clin Immunol. 2002;105(3):273–278. doi: 10.1006/clim.2002.5291. [DOI] [PubMed] [Google Scholar]
- 14.Chiriaco M., Casciano F., Di Matteo G. Impaired X-CGD T cell compartment is gp91phox-NADPH oxidase independent. Clin Immunol. 2018;193:52–59. doi: 10.1016/j.clim.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Takei H., Araki A., Watanabe H., Ichinose A., Sendo F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J Leukoc Biol. 1996;59(2):229–240. doi: 10.1002/jlb.59.2.229. [DOI] [PubMed] [Google Scholar]
- 16.Brinkmann V., Reichard U., Goosmann C. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 17.Röhm M., Grimm M.J., D'Auria A.C., Almyroudis N.G., Segal B.H., Urban C.F. NADPH oxidase promotes neutrophil extracellular trap formation in pulmonary aspergillosis. Infect Immun. 2014;82(5):1766–1777. doi: 10.1128/IAI.00096-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuchs T.A., Abed U., Goosmann C. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang H., Biermann M.H., Brauner J.M., Liu Y., Zhao Y., Herrmann M. New insights into neutrophil extracellular traps: mechanisms of formation and role in inflammation. Front Immunol. 2016;7:302. doi: 10.3389/fimmu.2016.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bianchi M., Hakkim A., Brinkmann V. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood. 2009;114(13):2619–2622. doi: 10.1182/blood-2009-05-221606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Veerdonk F.L., Dinarello C.A. Deficient autophagy unravels the ROS paradox in chronic granulomatous disease. Autophagy. 2014;10(6):1141–1142. doi: 10.4161/auto.28638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Luca A., Smeekens S.P., Casagrande A. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc Natl Acad Sci USA. 2014;111(9):3526–3531. doi: 10.1073/pnas.1322831111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown J.R., Goldblatt D., Buddle J., Morton L., Thrasher A.J. Diminished production of anti-inflammatory mediators during neutrophil apoptosis and macrophage phagocytosis in chronic granulomatous disease (CGD) J Leukoc Biol. 2003;73(5):591–599. doi: 10.1189/jlb.1202599. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Boyanapalli R., Frasch S.C., Riches D.W.H., Vandivier R.W., Henson P.M., Bratton D.L. PPARγ activation normalizes resolution of acute sterile inflammation in murine chronic granulomatous disease. Blood. 2010;116(22):4512–4522. doi: 10.1182/blood-2010-02-272005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Boyanapalli R.F., Falcone E.L., Zerbe C.S. Impaired efferocytosis in human chronic granulomatous disease is reversed by pioglitazone treatment. J Allergy Clin Immunol. 2015;136(5):1399–1401. doi: 10.1016/j.jaci.2015.07.034. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelderman K.A., Hultqvist M., Holmberg J., Olofsson P., Holmdahl R. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc Natl Acad Sci USA. 2006;103(34):12831–12836. doi: 10.1073/pnas.0604571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schäppi M.G., Jaquet V., Belli D.C., Krause K.-H. Hyperinflammation in chronic granulomatous disease and anti-inflammatory role of the phagocyte NADPH oxidase. Semin Immunopathol. 2008;30(3):255–271. doi: 10.1007/s00281-008-0119-2. [DOI] [PubMed] [Google Scholar]
- 28.Martire B., Rondelli R., Soresina A. Clinical features, long-term follow-up and outcome of a large cohort of patients with Chronic Granulomatous Disease: an Italian multicenter study. Clin Immunol. 2008;126(2):155–164. doi: 10.1016/j.clim.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Segal B.H., Leto T.L., Gallin J.I., Malech H.L., Holland S.M. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltim) 2000;79(3):170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Arnold D.E., Heimall J.R. A review of chronic granulomatous disease. Adv Ther. 2017;34(12):2543–2557. doi: 10.1007/s12325-017-0636-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanmun D., Witasp E., Jitkaew S. Involvement of a functional NADPH oxidase in neutrophils and macrophages during programmed cell clearance: implications for chronic granulomatous disease. Am J Physiol Cell Physiol. 2009;297(3):C621–C631. doi: 10.1152/ajpcell.00651.2008. [DOI] [PubMed] [Google Scholar]
- 32.Roos D. Chronic granulomatous disease. Br Med Bull. 2016;118(1):50–63. doi: 10.1093/bmb/ldw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henrickson S.E., Jongco A.M., Thomsen K.F., Garabedian E.K., Thomsen I.P. Noninfectious manifestations and complications of chronic granulomatous disease. J Pediatric Infect Dis Soc. 2018;7(suppl 1):S18–S24. doi: 10.1093/jpids/piy014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gennery A.R., Holland S.M. Primary immunodeficiencies: not just paediatric diseases. Eur Respir J. 2015;45(6):1521–1523. doi: 10.1183/09031936.00020215. [DOI] [PubMed] [Google Scholar]
- 35.Segal B.H., Barnhart L.A., Anderson V.L., Walsh T.J., Malech H.L., Holland S.M. Posaconazole as salvage therapy in patients with chronic granulomatous disease and invasive filamentous fungal infection. Clin Infect Dis. 2005;40(11):1684–1688. doi: 10.1086/430068. [DOI] [PubMed] [Google Scholar]
- 36.Wu J., Wang W.-F., Zhang Y.-D., Chen T.-X. Clinical features and genetic analysis of 48 patients with chronic granulomatous disease in a single center study from Shanghai, China (2005-2015): new studies and a literature review. J Immunol Res. 2017;2017:8745254. doi: 10.1155/2017/8745254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leiding J.W., Freeman A.F., Marciano B.E. Corticosteroid therapy for liver abscess in chronic granulomatous disease. Clin Infect Dis. 2012;54(5):694–700. doi: 10.1093/cid/cir896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin K.-S., Lee M.S. Concomitant use of corticosteroid and antimicrobials for liver abscesses in patients with chronic granulomatous disease. Korean J Pediatr. 2016;59(4):196–201. doi: 10.3345/kjp.2016.59.4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Straughan D.M., McLoughlin K.C., Mullinax J.E. The changing paradigm of management of liver abscesses in chronic granulomatous disease. Clin Infect Dis. 2018 17;66(9):1427–1434. doi: 10.1093/cid/cix1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falcone E.L., Petts J.R., Fasano M.B. Methylotroph infections and chronic granulomatous disease. Emerg Infect Dis. 2016;22(3):404–409. doi: 10.3201/eid2203.151265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kulkarni M., Hule G., de Boer M. Approach to molecular diagnosis of chronic granulomatous disease (CGD): an experience from a large cohort of 90 Indian patients. J Clin Immunol. 2018;38(8):898–916. doi: 10.1007/s10875-018-0567-y. [DOI] [PubMed] [Google Scholar]
- 42.Zhan S., Vazquez N., Zhan S. Genomic structure, chromosomal localization, start of transcription, and tissue expression of the human p40-phox, a new component of the nicotinamide adenine dinucleotide phosphate-oxidase complex. Blood. 1996;88(7):2714–2721. [PubMed] [Google Scholar]
- 43.Matute J.D., Arias A.A., Dinauer M.C., Patiño P.J. p40phox: the last NADPH oxidase subunit. Blood Cells Mol Dis. 2005;35(2):291–302. doi: 10.1016/j.bcmd.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Ellson C., Davidson K., Anderson K., Stephens L.R., Hawkins P.T. PtdIns3P binding to the PX domain of p40phox is a physiological signal in NADPH oxidase activation. EMBO J. 2006;25(19):4468–4478. doi: 10.1038/sj.emboj.7601346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suh C.I., Stull N.D., Li X.J. The phosphoinositide-binding protein p40phox activates the NADPH oxidase during FcγIIA receptor–induced phagocytosis. J Exp Med. 2006;203(8):1915–1925. doi: 10.1084/jem.20052085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matute J.D., Arias A.A., Wright N.A.M. A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40 phox and selective defects in neutrophil NADPH oxidase activity. Blood. 2009;114(15):3309–3315. doi: 10.1182/blood-2009-07-231498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van de Geer A., Nieto-Patlán A., Kuhns D.B. Inherited p40phox deficiency differs from classic chronic granulomatous disease. J Clin Investig. 2018;128(9):3957–3975. doi: 10.1172/JCI97116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rioux J.D., Xavier R.J., Taylor K.D. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39(5):596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olsson L.M., Lindqvist A.K., Källberg H. A case-control study of rheumatoid arthritis identifies an associated single nucleotide polymorphism in the NCF4 gene, supporting a role for the NADPH-oxidase complex in autoimmunity. Arthritis Res Ther. 2007;9(5):R98. doi: 10.1186/ar2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas D.C., Charbonnier L.-M., Schejtman A. EROS/CYBC1 mutations: decreased NADPH oxidase function and chronic granulomatous disease. J Allergy Clin Immunol. 2019;143(2):782–785. doi: 10.1016/j.jaci.2018.09.019. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnadottir G.A., Norddahl G.L., Gudmundsdottir S. A homozygous loss-of-function mutation leading to CYBC1 deficiency causes chronic granulomatous disease. Nat Commun. 2018 25;9(1):4447. doi: 10.1038/s41467-018-06964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas D.C., Clare S., Sowerby J.M. Eros is a novel transmembrane protein that controls the phagocyte respiratory burst and is essential for innate immunity. J Exp Med. 2017 03;214(4):1111–1128. doi: 10.1084/jem.20161382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lyon M.F. Gene action in the X-chromosome of the mouse (Mus musculus L.) Nature. 1961;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- 54.Marciano B.E., Zerbe C.S., Falcone E.L. X-linked carriers of chronic granulomatous disease: illness, lyonization, and stability. J Allergy Clin Immunol. 2018;141(1):365–371. doi: 10.1016/j.jaci.2017.04.035. [DOI] [PubMed] [Google Scholar]
- 55.Rösen-Wolff A., Soldan W., Heyne K., Bickhardt J., Gahr M., Roesler J. Increased susceptibility of a carrier of X-linked chronic granulomatous disease (CGD) to Aspergillus fumigatus infection associated with age-related skewing of lyonization. Ann Hematol. 2001;80(2):113–115. doi: 10.1007/s002770000230. [DOI] [PubMed] [Google Scholar]
- 56.Wolach B., Scharf Y., Gavrieli R., de Boer M., Roos D. Unusual late presentation of X-linked chronic granulomatous disease in an adult female with a somatic mosaic for a novel mutation in CYBB. Blood. 2005;105(1):61–66. doi: 10.1182/blood-2004-02-0675. [DOI] [PubMed] [Google Scholar]
- 57.Severe phenotype of chronic granulomatous disease presenting in a female with a de novo mutation in gp91-phox and a non familial, extremely skewed X chromosome inactivation. Clin Immunol. 2003;109(3):308–317. doi: 10.1016/j.clim.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Roesler J. Carriers of X-linked chronic granulomatous disease at risk. Clin Immunol. 2009;130(2):233. doi: 10.1016/j.clim.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 59.Landing B.H., Shirkey H.S. A syndrome of recurrent infection and infiltration of viscera by pigmented lipid histiocytes. Pediatrics. 1957;20(3):431–438. [PubMed] [Google Scholar]
- 60.Cale C.M., Morton L., Goldblatt D. Cutaneous and other lupus-like symptoms in carriers of X-linked chronic granulomatous disease: incidence and autoimmune serology. Clin Exp Immunol. 2007;148(1):79–84. doi: 10.1111/j.1365-2249.2007.03321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barton L.L., Johnson C.R. Discoid lupus erythematosus and X-linked chronic granulomatous disease. Pediatr Dermatol. 1986;3(5):376–379. doi: 10.1111/j.1525-1470.1986.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 62.Brandrup F., Koch C., Petri M., Schiødt M., Johansen K.S. Discoid lupus erythematosus-like lesions and stomatitis in female carriers of X-linked chronic granulomatous disease. Br J Dermatol. 1981;104(5):495–505. doi: 10.1111/j.1365-2133.1981.tb08163.x. [DOI] [PubMed] [Google Scholar]
- 63.Smitt J.H.S., Weening R.S., Krieg S.R., Bos J.D. Discoid lupus erythematosus-like lesions in carriers of X-linked chronic granulomatous disease. Br J Dermatol. 1990;122(5):643–650. doi: 10.1111/j.1365-2133.1990.tb07286.x. [DOI] [PubMed] [Google Scholar]
- 64.Battersby A.C., Cale A.M., Goldblatt D., Gennery A.R. Clinical manifestations of disease in X-linked carriers of chronic granulomatous disease. J Clin Immunol. 2013;33(8):1276–1284. doi: 10.1007/s10875-013-9939-5. [DOI] [PubMed] [Google Scholar]