Abstract
Voltage-gated proton channels (HV1) are essential for various physiological tasks but are strongly inhibited by Zn2+ cations. Some determinants of Zn2+ binding have been elucidated experimentally and in computational studies. However, the results have always been interpreted under the assumption that Zn2+ binds to monomeric HV1 despite evidence that HV1 expresses as a dimer and that the dimer has a higher affinity for zinc than the monomer and experimental data that suggest coordination in the dimer interface. The results of former studies are also controversial, e.g., supporting either one single or two binding sites. Some structural determinants of the binding are still elusive. We performed a series of molecular dynamics simulations to address different structures of the human proton channel, the monomer and two plausible dimer conformations, to compare their respective potential to interact with and bind Zn2+ via the essential histidines. The series consisted of several copies of the system to generate independent trajectories and increase the significance compared to a single simulation. The amount of time simulated totals 29.9 μs for 126 simulations of systems comprising ∼59,000 to ∼187,000 atoms. Our approach confirms the existence of two binding sites in monomeric and dimeric human HV1. The dimer interface is more efficient for attracting and binding Zn2+ via the essential histidines than the monomer or a dimer with the histidines in the periphery. The higher affinity is due to the residues in the dimer interface that create an attractive electrostatic potential funneling the zinc cations toward the binding sites.
Significance
Our work addresses structural determinants of Zn2+ binding to the human voltage-gated proton channel hHV1 at the atomistic level with molecular dynamics simulations. Former computational studies considered monomeric HV1 despite evidence for the requirement of a dimer structure. Here, we used no constraint to get unbiased details about the binding and performed a comparison of different hHV1 structures with respect to the binding. Our approach provides new, to our knowledge, insights: it confirms that hHV1 binds zinc in two rather than one single site, identifies the residues participating in the binding, and suggests the dimeric structure with H140 and H193 in the interface is more efficient at attracting and binding Zn2+ via these essential histidines.
Introduction
Voltage-gated proton channels (HV1s) are quite unique. They mediate the highly selective voltage- and pH-dependent conductance of protons across the membrane into phagosomes (1,2) and out of many types of cells, including spermatozoa, electrically excitable cells, and respiratory epithelial cells (3). The channels play a fundamental role in many physiological tasks such as respiratory burst (4,5), pH homeostasis (6), innate immune response (7), sperm maturation and motility in humans (8,9), and many others. There are several reviews on the subject (see, for example, (10)). Structurally, they belong to the huge family of voltage-gated cation channels but only consist of one transmembrane domain. This domain is composed of four helices that constitute both the pore and the voltage-sensing domain (VSD), corresponding to the VSD of voltage-gated sodium and voltage-gated potassium channels (11). Native HV1s are dimers, which was supported by a variety of data first in human, mouse, and Ciona (the species discussed in this work (12, 13, 14)), but they possibly form larger quaternary assemblies (15, 16, 17). Dimerization was also shown to be essential for cooperativity and voltage dependence (17, 18, 19, 20, 21). The binding of zinc cations to HV1 strongly hampers the conductance of protons through the channel (22, 23, 24, 25, 26, 27, 28, 29). Zn2+ is commonly assumed to bind and thereby force the channel into a nonconductive conformation (16,21,23,25,30). The dimer especially is more sensitive to Zn2+, having a higher affinity for zinc than the monomer (21). Some determinants of Zn2+ binding have already been characterized in experimental and computational studies. However, antagonistic views of the binding have emerged during the last years because of contradicting experimental and computational results. We expose the different views in more detail hereafter.
One vs. two binding sites
The pH dependence of zinc inhibition in HV1 suggested that Zn2+ is coordinated by several residues with a pKa similar to histidine residues. Mutagenesis experiments of HV1 residues revealed two histidines, H140 and H193 in human HV1 (hHV1), that are essential for extracellular inhibition of HV1 by Zn2+ (25). Mutation to alanine of one histidine or the other considerably decreased Zn2+ potency. Mutation of both simultaneously practically abolished Zn2+ sensitivity. H140 is conserved in all three species—Ciona, mouse, and human—but H193 is less conserved in HV1 channels. In Ciona, for example, a homologous histidine in the S3-S4 extracellular loop is missing. The importance of the two histidines in humans was supported by experimental data (21,25). The first structural details were obtained later with the crystal structure of a chimeric Ciona-mouse proton channel HV1, mHV1cc (16). In the structure, one single zinc cation was identified per HV1 monomeric subunit. Histidine 136 (H140 in hHV1) is at a position enabling zinc coordination (∼5 Å between Zn2+ and the Cα atom of H136). But the second histidine, H189 (H193 in hHV1), was too disordered to be modeled in the density map, suggesting that the short segment containing this histidine (four residues of the S3-S4 loop not solved in the structure) was not stabilized by interaction with zinc. Two computational studies considered one single binding site in the molecular dynamics (MD) simulation of a structural model of hHV1 (31) and in a reduced quantum mechanical model of the binding site of mHV1 (32). In the first study, Zn2+ was placed in the expected binding site to simultaneously coordinate H140 and H193 but unbound quickly in the absence of constraint. In the subsequent simulation, the cation was constrained between the two histidines. An octahedral coordination around the metal was found. However, the most probable binding site has a tetrahedral geometry according to the quantum mechanical calculations. A recent NMR structure suggests two binding sites in the hHV1 monomer, one at H140 and the other at H193, because the two histidines are too far away in the structure to bind one zinc in between (33). The existence of two sites was suggested earlier by MD simulations (potential of mean force calculations) of zinc unbinding in mHV1 and Ci-HV1 (30). Histidine 188 (H140 in hHV1) was found to be part of one of the two sites in Ci-HV1 that lacks the second histidine in the S3-S4 loop. But in mHV1, no histidine was found to participate in the binding. The existence of only one binding site was also not verified in a preliminary MD simulation based on the crystal structure of mHV1 by the same authors. To date, one opinion is that HV1 has only one binding site. In humans, this site would involve the two essential histidine 140 and 193 residues. The other opinion involves two binding sites in human, one at H140 and one at H193. There are evident controversies in the literature regarding some details of the binding in monomeric HV1 at the atomistic level. However, the sole binding site is less supported by experimental data.
Monomer versus dimer
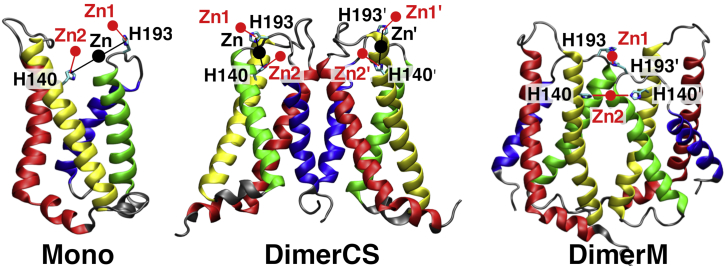
The binding is generally investigated under the assumption that it occurs within HV1 monomers. However, native HV1s are dimers under physiological conditions and more sensitive to zinc inhibition than the monomer. Furthermore, some electrophysiological data suggest that zinc binds in the dimer interface, involving residues from each monomeric subunit (21). The experimental structure of mHV1cc is a trimer with a crystallographic threefold axis on which the three H136 residues (H140 in hHV1) are located in the periphery (16). The subdivision of the trimer into potential dimeric configurations results in dimers in which the histidines are almost on opposite sides. The S3-S4 loop that contains the second essential histidine 189 (H193 in hHV1) was not solved. But again, inspection of the crystal structure indicates that these loops would also be far away from each other within the dimeric subunits. Former electrophysiological analysis of hHV1 wild-type (WT) and His mutants showed a stronger inhibition of the dimer compared to the monomer and suggested binding in the dimer interface (see Appendix S2 and (21)). The patch-clamp data propose binding to the two equivalent histidines from each hHV1 subunit in the interface. The different views are illustrated in Fig. 1 for the monomer (Mono) and two dimer conformations, DimerCS (from the crystal structure of mHV1cc) and DimerM (as suggested by (21)).
Figure 1.
Putative zinc binding site(s) in the hHV1 monomer and two plausible dimer structures. The transmembrane helices are colored from the N- to the C-termini (both intracellular) in red (S1), yellow (S2), green (S3), and blue (S4). The histidines essential for the binding in hHV1, H193 and H140, are depicted as sticks. The “one binding site” standpoint is depicted in black (Zn; between H140 and H193), and the “two binding sites” standpoint is depicted in red (Zn1 at H193 and Zn2 at H140, in Mono and DimerCS). In DimerM, the binding suggested in (21) also has two sites, one between the two histidine 193 residues (Zn1) and the other one between the two histidine 140 residues (Zn2).
To date, the following aspects have still not been answered conclusively: how many site(s) do HV1 proton channels present for zinc binding? Which residues participate in the binding? And which hHV1 conformation (monomer or dimer) is more efficient for binding zinc cations? To answer these questions and unravel contradictions, we analyzed the binding to different structures of the human proton channel. We performed a series of unconstrained MD simulations to generate independent trajectories and obtain results that are more significant than from a single simulation, get unbiased details about the binding at the atomistic level, and allow a valid comparison of the structures.
Materials and Methods
Systems
We considered three plausible “structures” of hHV1 to address zinc binding within a single monomeric unit (Mono), within the two HV1 subunits of a dimer with the essential histidines in the periphery (DimerCS, where CS stands for crystal structure), or between the two subunits of a dimer presenting the essential histidines in its interface (DimerM, where M stands for Musset et al. (21), who originally proposed this dimer structure).
We investigated the following situations: 1) with two Zn2+ ions, 2) with 100 mM “free” Zn2+ in the bulk solution, and 3) without Zn2+. When necessary, we will distinguish between them by appending, respectively, “⋅2Zn,” “⋅freeZn,” or “⋅noZn” to the structure name, e.g., DimerM⋅2Zn. For DimerM, we considered the WT and the mutants E192A and H140A + H193A. Mutations were always applied in both subunits. If necessary, we will append, respectively, “⋅WT,” “⋅E192A,” or “⋅H140A + H193A” to differentiate between them. If not specified, it will refer to WT. Because our results show that the two essential histidine 140 and 193 residues cannot participate in a unique binding site (see Results), we distinguish two, each involving one of the two histidines. We call “Zn1” the binding site involving H193 and “Zn2” the one involving H140 later on. In DimerCS, there are two separate Zn1 and two separate Zn2 sites, one in each hHV1 subunit. Here, Zn1 (respectively Zn2) refers indistinctly to the binding with H193 (resp. H140) in one and/or the other subunit. In DimerM, Zn1 refers to the site between the two H193 from each subunit and, respectively, for Zn2 with the two H140 from each subunit. The three structures are depicted with their putative binding sites in Fig. 1.
Zn2+ model
In former MD studies, details about the binding were obtained using artificial constraints (30,31). However, such constraints reflect prior assumptions of the designer of the simulation about features of the binding. To obtain unbiased details and to allow a valid comparison of the different hHV1 structures, it was necessary to eliminate the need for such constraints. Therefore, we first considered different molecular force field (FF) representations of Zn2+ cations. The representation that simulates stable zinc binding to the essential histidines without using artificial constraints was used in all the simulations discussed later on (Appendix S1 and Fig. S1; Table S2).
Homology modeling
A structural model of the transmembrane VSD of hHV1 (Mono), residues 84–218, was constructed via homology modeling. We used the amino acid sequence of hHV1 from the UniprotKB/Swiss-Prot database (34), Uniprot: Q96D96, and the crystal structure of the Ciona intestinalis VSD domain, Ci-VSP, as templates. Zn2+ binds to a nonconductive state of HV1. We thus considered the structure of Ci-VSP in the resting state in the Protein Databank (35), PDB: 4G80 (36). The amino acid sequences of hHV1 and Ci-VSP were aligned with T-COFFEE (37). The sequence similarity is 46% for the VSD domain, which is higher than for other channels that were used in earlier works (10,38, 39, 40). The choice of 4G80 as template structure was further motivated by the observation by Li et al. that Ci-VSP represents a better template for hHV1 than other channels (41). Structural models were generated with MODELLER9.18 (42,43). The Modeler objective function was considered to discriminate the solutions. The five best models were structurally refined using the 3D Refine online server (44). For each model submitted, five solutions were generated and ranked according to the 3Drefine score and the RWPlus scores. The best model was conserved for further works. Inspection with PROCHECK (45) revealed no structural problem. The same procedure was used for the generation of the dimer structure DimerCS based on the crystal structure of VSOP/HV1 (PDB: 3WKV (16)). This structure is a trimer, from which we extracted one dimer subunit to get the template for the homology modeling procedure.
Docking simulation
Putative structures of DimerM were generated via docking using the online server GRAMMX (46). An appropriate input structure was extracted from the MD trajectory of the monomer without zinc (Mono⋅noZn, MD 1) and used both as the receptor and the ligand. In the dimer proposed by Musset et al. (21), two binding sites are formed in the interface, one between the two His193 residues and one between the two His140 residues from each hHV1 subunit (21). We considered as an appropriate input structure a conformation of the monomer in which the orientations of the histidines side chains allow the formation of the two expected binding sites in the interface that would result in the assembly of two of this monomer conformation. All the solutions generated by the docking were inspected visually to find those that fulfill simultaneously the two following criteria: 1) the side chains of H140 and H193 are properly oriented to form the two expected binding sites in the dimer interface, and 2) the two subunits have orientations to each other that allow the resulting dimer to properly span a lipid bilayer membrane.
MD simulations
The hHV1 structures were oriented into the membrane with PPM, accessible online at the database for Orientation of Proteins in Membrane (47). The protonation state of the titratable residues was determined using PROPKA3.0 (48) at the PDB2PQR server (49). The systems (channel + lipid bilayer (POPC) + bulk solution with ions) were constructed with CHARMM-GUI (50). They were solvated in periodic boxes of ∼70 × 70 × 132 Å for the monomer and ∼120 × 120 × 138 Å for the dimers. 150 mM KCl was added to the bulk solution, but 100 mM ZnCl2 for the simulations with “free” Zn2+. We used MgCl2 during the construction because Zn2+ is not available in CHARMM-GUI. Mg2+ was exchanged for Zn2+ in the next step. The assembly files created by CHARMM-GUI were further processed for the simulations with the AMBER FF using the command “charmmlipid2amber” of AMBER16 (51) and manually for further input needs, e.g., exchanging Mg2+ for Zn2+.
All the MD simulations were done with AMBER16 (51) using the ff14SB FF for the protein (52) and the lipid14 FF for the lipid (53), except for those simulations that required CHARMM FF. The Joung and Cheatham parameters (54) were used for the ions other than Zn2+, and the TIP3P model was used for water (55). We used a similar protocol in all our MD simulations: minimization was done in 5000 steps of steepest descend followed by 5000 steps of conjugated gradients. The systems were then heated up very slowly because these are constructs and especially to allow them to adapt smoothly to the constraints applied between Zn2+ and the histidine 140 and 193 Nε atoms (2.1 Å; 10 kcal ⋅ mol−1 ⋅ Å−2) during the minimization and the heating (in 10 ns up to 310 K) of the DimerM⋅2Zn systems. The constraint was then decreased linearly during the relaxation step until annihilation after 8 ns. The systems were relaxed further for 2 ns. For consistency, all the other systems were also relaxed for 10 ns. At the end of this step, the time simulated totals 20 ns for all the systems. After relaxation, there was no constraint anymore in all the simulations. The previous steps were also not considered for analysis. Therefore, we assume that the results discussed later are for unconstrained MD simulations. For each system, we performed a series of five or 10 simulations, depending on the situation, starting at different temperatures to generate independent trajectories. For all the simulations, we used periodic boundary conditions, an NPT ensemble, a uniform integration step of 1 fs, and a cutoff of 12 Å with a switching function starting at 10 Å for van der Waals interactions. The particle mesh Ewald (PME) method was used to compute long-range electrostatic forces without cutoff (56,57). We used the CUDA version of the PME method to take advantage of the GPUs (58). Langevin dynamics was used to keep the temperature constant at 310 K, and a constant pressure of 1 bar was maintained using the Monte Carlo barostat. The trajectories were analyzed with the command cpptraj (59) of AmberTools16 and visualized with VMD (60). An overview of all the MD simulations is given in Table S1.
Electrostatic potential maps
For each hHV1 structure, we performed five independent MD simulations in the absence of ions. MD simulations without Zn2+ provide results that are independent of the model chosen (see Appendix S1) and eliminate the effects of the rapid binding that we observed in the simulations with free zinc on the dynamics of HV1. The trajectories of the five MDs were combined into one single trajectory to calculate the electrostatic potentials with the VMD plugin pmepot (61). The electrostatic potential is obtained by solving the Poisson equation
| (1) |
where the sum runs over all atoms, and ρi(r) is the charge distribution contributed by atom i at position r approximated by a spherical Gaussian
| (2) |
normalized to give the original charge upon integration. qi is the total charge of atom i.
The mean electrostatic potential was determined by averaging the instantaneous electrostatic potentials φ(r) over the entire trajectory. An Ewald factor of 0.25 Å−1 for the inverse width of the Gaussian (β in Eq. 2) was chosen. Electrostatic potentials were calculated using a 72 × 72 × 128 grid for the monomer and a 120 × 120 × 144 grid for the dimers to ensure a density of grid points of at least 1/Å3 (the cell sizes in this situation were, respectively, 67 × 67 × 127 Å for the monomer and ∼117 × 117 × 135 Å for the dimers). All the atoms of the system were included in the calculation. The electrostatic potential thus represents the potential resulting from the entire system as it would be “sensed” by one Zn2+ introduced into the system and without the effect of other Zn2+ cations on the electrostatic potential or on the dynamics of the system.
Fluctuations
The mass weighted average of atomic fluctuations of the HV1 backbone (atoms C, Cα, and N) per residue was calculated using the fluctuation module of cpptraj according to Eq. 3:
| (3) |
Gene expression
We cloned the gene by restriction sites into a pQBI25-fC3 using 5′ BamH1 and 3′ EcoR1 restriction sites. Site-directed mutagenesis was performed using an overlapping PCR procedure. The clones were sequenced commercially to confirm mutations. tsA201 (human kidney cell line) cells were grown to 85% confluence in 35 mm culture dishes. The cells were transfected with 1.3 μg plasmid DNA using polyethylenimine (Sigma, St. Louis, MO). After 12 h at 37°C in 5% CO2, the cells were trypsinized and replated onto glass coverslips at low density for patch-clamp recording on the same and the next day. We selected green cells under fluorescence for recording. Whole-cell patch clamp showed no other voltage- or time-dependent conductance under our recording conditions. tsA201 cells showed no native proton conductance without transfection induced expression. The level of expression of all the mutants studied here was sufficiently high to neglect potential contamination by native HV1 currents.
Electrophysiology
Whole-cell patch-clamp or excised patch recordings were done as described in (62). Additional information is found in Appendix S3.
Results and Discussion
One vs. two binding sites in the monomer
To address the number of binding sites in the monomer, we constructed a structural model based on the crystal structure of Ci-VSP (36). In our construct, the distance between H140 and H193 is 14.3 Å (Cα atoms), which is too long to coordinate one zinc between the two histidines. We performed a 1 μs MD with the monomer in the absence of Zn2+ (Mono⋅noZn, MD 1 in Table S2). As shown in Fig. 2 A, the two histidines remain too distant to form one binding site during the entire time course of the simulation. Both the structural model and MD simulation suggest that hHV1 cannot bind one single zinc cation between the histidines, in agreement with the previous structural model (21), the experimental and computational data of (30) for Ciona and mouse HV1s, and the NMR structure of hHV1 (33). Consequently, each histidine might rather be involved in distinct binding sites. (This statement is supported by later simulations with two Zn2+ ions and with free Zn2+ (Mono⋅freeZn).)
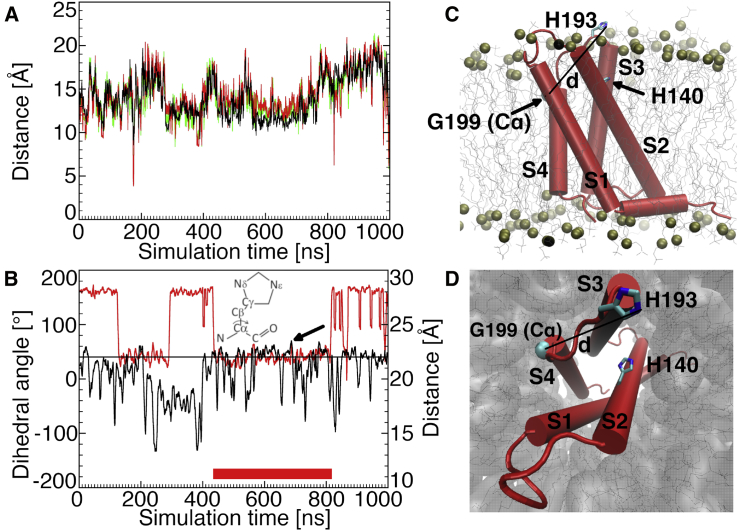
Figure 2.
MD simulation of the monomer without Zn2+ (Mono⋅noZn, MD 1). (A) Time evolution of distances between the H140 and H193 Cα (black), Nδ (green), and Nε atoms (red) is shown. The two histidines are too distant to form one binding site. (B) Time evolution of the distance between the H193 Nε and G199 Cα atoms (black) and of the H140 C-Cα-Cβ-Cγ dihedral angle (red) is shown. Values larger than 22 Å for the distance, above the black line, and lower than 90° for the dihedral angle correlate with the histidine side chains oriented toward the lipid bilayer, between S2 and S3. The thick red line shows the time window during which the side chains of both histidines are most often simultaneously oriented between S2 and S3 (approximately from 400 to 800 ns). The arrow shows the snapshot used for the right panels and for the generation of DimerM via docking. (C) The monomer inserted into the membrane is shown. (D) shows the same as (C), seen from the extracellular bulk solution.
Interestingly, in the simulation, we often observed orientations of the H140 and H193 residue side chains that were adequate to form the proposed binding sites in DimerM (Fig. 1, right panel). This was analyzed in closer detail by considering one dihedral angle for H140 and one distance for H193 (Fig. 2 B). H140 is located in a transmembrane helix (S2). Its backbone can thus undergo only small fluctuations. However, its side chain can be subject to larger fluctuations that we assessed by recording the C-Cα-Cβ-Cγ dihedral angle values. Small values around 40° correspond to the side chain being oriented toward the lipid bilayer, between helices S2 and S3, whereas large values around 160° correspond to orientations toward the interior of the channel (Fig. 2, B–D). H140’s side chain spends almost half of the time oriented toward the lipid bilayer (Fig. 2 B). H193 is located in the flexible S3-S4 extracellular loop. We found that its distance to G199 (rigid in S4) is a good indicator of its side chain’s orientation (measured: Nε side chain of H193 to Cα atom of G199). Values larger than 22 Å generally correlate with the side chain of H193 being oriented toward the lipid bilayer between helices S2 and S3, whereas the side chain is oriented toward the interior of the channel for smaller values. H193’s side chain spends a representative time oriented between helices S2 and S3 (Fig. 2, B–D). Altogether, for approximately half the time of the simulation (especially between 400 and 800 ns), the side chains of H140 and H193 are simultaneously oriented between helices S2 and S3 because it is expected to form the two binding sites in the interface of DimerM by substitution of the lipid bilayer in this region with a second HV1 monomer. One conformation was extracted from the trajectory for the generation of DimerM via docking.
Structural details of the binding in the monomer
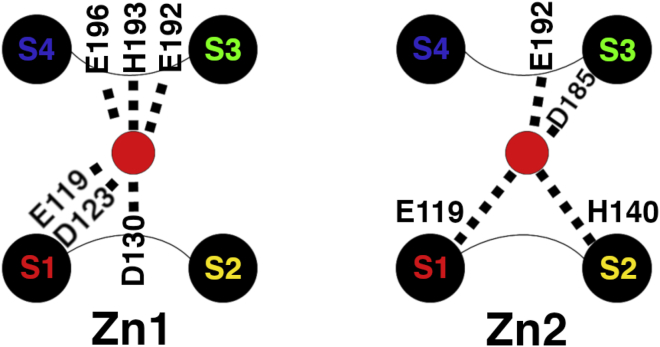
Here, we addressed the binding of two Zn2+ cations, one at H140 and one at H193, to the monomer. The results are summarized in Table S3 (Mono⋅2Zn) and in Fig. 3. The coordination spheres around Zn2+ discussed hereafter were stable during the simulations. The interaction with H140 is stable in all five simulations. With H193, it is lost in two simulations (MD simulations 2 and 3). This suggests that zinc binds more strongly with H140 (Zn2) than with H193 (Zn1). Although we could have expected stable binding in both sites on the timescale of the simulations, the fact that zinc can unbind from histidine shows that His-Zn interactions are not an artifact of the Zn2+ model used in our work. After unbinding from H193, Zn2+ binds E192 and D130 in MD 2 or E192, E119, and D123 in MD 3. Our simulations show a large contribution of E119 to Zn2, in agreement with the two experimental structures and the former MD studies (Table S3). They also reveal the contribution of glutamate 192 in the extracellular S3-S4 loop to either one of the two binding sites or both. In the latter case, E192 contacts each Zn2+ via one of the two oxygens of the side-chain carboxylate group (MD simulations 3 and 4). E192 was not identified in former studies for several reasons: first, the corresponding region was not solved in the crystal structure of mHV1cc. The structure consequently provides no information about the residues of the S3-S4 loop. Second, both mHV1 and Ci-HV1 do not have a glutamate at the equivalent position, but a serine (S188 in mHV1 and S242 in Ci-HV1). The importance of S242 was confirmed by experiment but slightly supported by the two-dimensional potential of mean force calculations (30). S242 is surrounded by two glutamates, E241 and E243, but none of them bound zinc in the previous MD simulation. In the simulation of hHV1 (31), the binding was constrained with the Nδ side-chain atoms of H193 and H140, which might have imposed structural and sterical restrictions disabling the side chain of E192 to contact the zinc cation. However, the NMR structure suggests its participation at the binding site including H193 (Zn1 in our work), as well as of glutamate 196 (33). We also identified this glutamate as possibly contributing to Zn1 in our simulations. Further residues that participate, but to a lesser extent, are the aspartate 123 and 130 residues in Zn1 and 185 in Zn2. D123 is equivalent to D119 in mHV1. In the MD simulations of mouse, Ciona, and hHV1, this aspartate apparently did not contribute to zinc binding (30,31). In the experimental structure of mHV1cc, it is found near Zn. However, it is too far away to interact directly with Zn, which might reflect the fact that it binds in only one of our simulations. However, the NMR structure of hHV1 also suggests that this residue may participate in Zn1. D185 can also participate in the binding in Zn2, as was suggested for the equivalent aspartate in mHV1 (D181) and Ci-HV1 (D233) by MD simulations (30). Finally, our simulations also identify D130 as a new potential residue for Zn1 (in two MD simulations).
Figure 3.
Zinc binding in the monomer from multiple MD simulations (Mono⋅2Zn). The same color scheme as in Fig. 1 is used for the transmembrane helices (S1–S4). The residues are shown with a number of square dots corresponding to the number of simulations in which they bind Zn2+ in Zn1 (left) and Zn2 (right) (see also Table S1).
Our simulations show that two binding sites are readily accommodated in the monomer, in perfect agreement with (30,33). Zn2 is essentially formed by H140 and E119. But E192 or, to a lesser extent, D185 can also participate. Zn1 is formed by H193, E192 and D130, or E196 but is less stable than Zn2. The coordination spheres around zinc are generally completed with water molecules. For Zn1, which is more exposed to the extracellular environment, interactions with the lipid can complete the coordination. We sometimes observe the rapid binding and unbinding of additional water molecules in Zn1 and Zn2 so that the cations were sometimes, transiently, penta- or hexacoordinated. However, the dominant coordination is tetrahedral, in agreement with (32).
DimerM has two binding sites in the dimer interface
Generation of the DimerM structure
Putative conformations of DimerM were generated via docking with GRAMM-X using one structure from the simulation of Mono⋅noZn (MD 1). Solutions of the docking were considered as “correct” for our problem if they simultaneously fulfilled the following two criteria: 1) two Zn2+ ions can be accommodated in the dimer interface, one between the side chains of the two H140 residues, one from each hHV1 subunit, and one between the side chains of the two H193 residues, one from each hHV1 subunit; and 2) the two HV1 subunits are adequately oriented with respect to each other to allow the resulting dimer to properly span a lipid membrane. Three docking solutions were identified by visual inspection of the results that fulfill the two previous criteria. The solutions were ranked by GRAMM-X at positions 166, 179, and 180, respectively. We will use the ranking numbers to distinguish them later on. The solutions were superimposed in Discovery Studio Visualizer (63) to assess their deviations (see also Fig. S2). Based on the Cα atoms, the calculated deviations are 3.06, 9.26, and 12.05 Å between solutions 166 and 179, 166 and 180, and 179 and 180, respectively. A major difference between solutions 166 and 179 and solution 180 is the angle between the inclination axes of the two subunits. They are much more parallel to each other and thus abler to correctly span a lipid membrane in 180 than in the other solutions (Fig. S2). The distances between the imidazole Nε atoms are less than 10 Å for H140-H140 and H193-H193 in the three solutions (3.9 and 9.7 Å, 3.7 and 7.5, and 8.2 and 8.7 in solutions 166, 179, and 180, respectively). Later on, we generally consider solution 180 only. However, we sometimes also used solutions 166 and 179 to verify that our results are not specific to solution 180.
Structural details of Zn2+ binding to DimerM
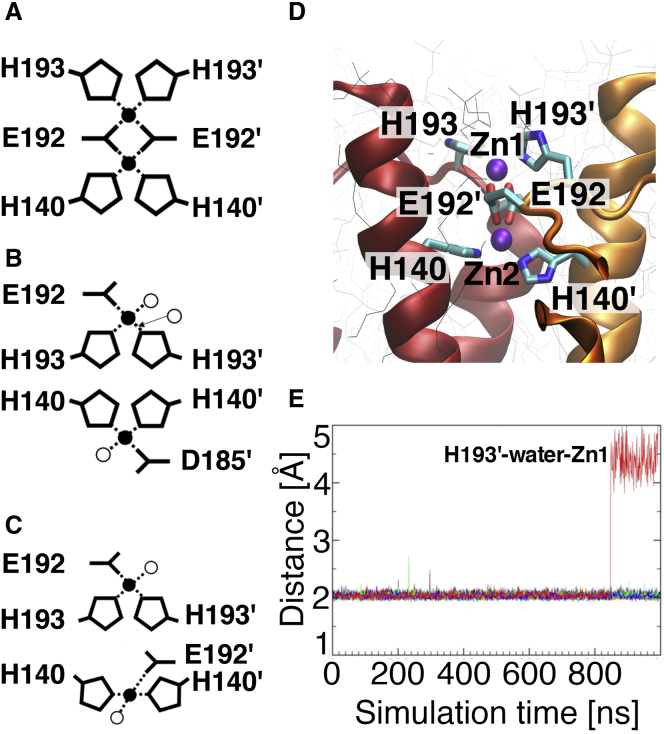
Here, we addressed the binding of two Zn2+ cations in the interface of DimerM, one between the two H140 residues from each HV1 subunit and one between the two H193 residues from each subunit. The results of these simulations are summarized in Table S2 (model of Pang) and represented in Fig. 4. The coordination spheres around Zn2+ discussed hereafter were stable during the simulations. DimerM readily accommodates two Zn2+ ions, one in each of the two proposed binding sites Zn1 and Zn2. We denote this configuration with one Zn between the two equivalent histidines from each HV1 subunit “His2-Zn.” Only one of the two H193-Zn interactions is lost in one of our five simulations, but it is spontaneously replaced by one water molecule bridging zinc with the side chain of that histidine (Fig. 4, B and E). As for the monomer, our simulations show a significant contribution of E192 to the binding. The two E192 residues most often coordinate the two zinc cations somehow connecting Zn1 and Zn2 (in three out of the five simulations; MD simulations 2–4 in Table S1; Fig. 4, A and D). Otherwise, the two sites are disconnected and either each E192 residue participates in one of them (MD 1, Fig. 4 C) or E192 is substituted with aspartate 185 in Zn2 (MD 5, Fig. 4 B).
Figure 4.
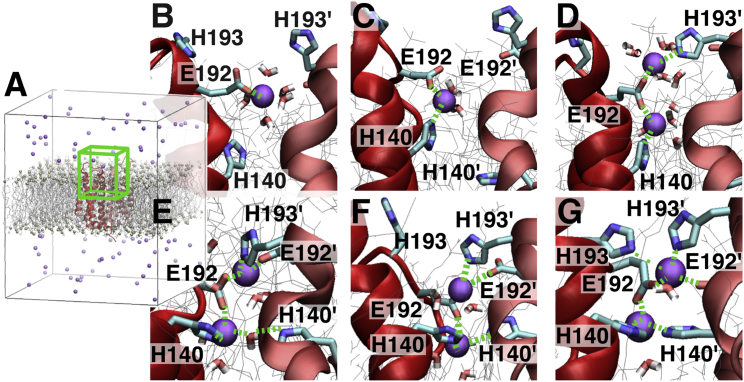
Binding in DimerM. (A–C) Schematic representations of the binding sites Zn1 (with H193) and Zn2 (with H140) obtained in the MD simulations are shown. The coordination pattern is found (A) in three (MD simulations 2, 3 and 4 in Table S2), (B) in one (MD 5 in Table S2), and (C) in one simulation (MD 1 in Table S2). The white circles represent water molecules. (D) A snapshot from one MD simulation showing the most frequent binding situation (A on the left) is given. (E) Time evolution of all the H140(Nε)-Zn and H193(Nε)-Zn distances in all five MD simulations is shown. 20 distances are plotted and are stable around 2.1 Å. Only one of the 10 H193-Zn interactions is lost but simultaneously replaced by a water-mediated interaction, shown in red in (E) and as a white circle with an arrow in (B).
According to our simulations, the dimer with H193 and H140 in the interface allows the formation of two binding sites, one between the two H193 residues from each HV1 subunit and one between the two H140 residues from each subunit. The two binding sites are similarly stable. This result conforms to the experimental data of (21): extracellular zinc inhibition is comparable between the two mutants H193A and H140A. To exclude that the stable binding of Zn2+ between the histidines of DimerM is a result of docking solution 180 only, we also considered the two solutions 166 and 179 (five MD simulations for each, data not shown). The binding sites are consistent with those obtained previously. The His2-Zn coordination spheres especially are stable in Zn1 and Zn2 in all the simulations with the docking solutions 166 and 179. A coordination sphere consisting of two histidines and two glutamates (E192) is formed in two simulations with solution 166 (corresponding to Fig. 4 A). In the other simulations with this docking solution, the two binding sites are connected by only one of the two E192 residues. The tetrahedral coordination sphere is complete with one water molecule at each site. With docking solution 179, the two sites are disconnected in all the simulations. The coordination sphere in Zn1 is formed by the two H193 and the two E192 residues in three simulations (similar to Zn1 in Fig. 4 A) or by the two H193 resides, one E192 residue, and one water molecule in two simulations (corresponding to Fig. 4, B and C). In all five simulations, Zn2 is formed by the two H140 residues, a D185 residue, and one water molecule as in Fig. 4 C. Although some details of the binding sites differ, determining features are conserved among the three docking solutions. Essentially, our multiple simulations show that the dimer interface readily accommodates two zinc binding sites, one between the two H193 residues and one between the two H140 residues.
Effects of Zn2+ binding on DimerM dynamics
Zinc binding is generally assumed to lock the channel into a nonconductive conformation. This, in turn, should hinder motions necessary for the cooperative opening of the dimer and the conduction of protons through the channel (opening of the permeation pathway (20), disruption of the hydrophobic plug (39,64)). Here, we calculated the fluctuations of the protein backbone to evaluate the effects of zinc binding on HV1 motions. We considered the first 100 ns of the five simulations with DimerM⋅2Zn and with Dimer⋅noZn to make a comparison based on the same time interval. The observation of the fluctuations reveals two interesting features (Fig. S3). First, the protein backbone fluctuates less when zinc is bound. The zinc lock thus seems to dampen molecular fluctuations (motions) and might subsequently hinder protein activity, as has been noted in enzymes (65). This agrees with the assumption that Zn2+ binding hinders, or at least modulates, the gate opening and subsequently the proton conductance, most probably by restraining necessary fluctuations or motions; see, for example, (23,30,31). Second, the fluctuations are similar in the two subunits of the Zn-bound HV1, whereas they differ much between the two subunits of the Zn-unbound HV1. HV1 dimers function cooperatively, which means that the opening of one subunit affects (through positive or negative cooperation) the opening of the other (15,17, 18, 19, 20, 21). This implies that the two subunits should have different fluctuations, at least on the timescale of our simulations. We do not expect to observe the opening itself in our simulations (opening occurs on a timescale of seconds). However, the differences in the fluctuations of the two subunits of the Zn-unbound dimer might already reflect the independent motions that the two subunits must undergo during the cooperative opening.
Binding of Zn2+ from the bulk solution
The previous results show that both the monomer and the dimer have two binding sites. In the previous simulations, the zinc cations were placed manually in the putative binding sites during the preparation. Although zinc is free to unbind in our unconstrained simulations, the previous setup did not allow us to discriminate the structure that is more potent at attracting and binding zinc cations. Here, we considered the three structures Mono, DimerCS, and DimerM without zinc cations in the binding sites Zn1 and Zn2 but with 100 mM of “free” Zn2+ in the bulk solution. This led to the addition of 26, 78, and 80 Zn2+ for Mono, DimerCS, and DimerM, respectively. We used a high concentration of zinc cations to increase the probability of binding events during the simulations. For each system, 10 independent 50 ns MD simulations were done. For each simulation, we counted the number of Zn2+ ions that coordinated HV1 in general and the number of interactions formed with H140 and H193 in particular. The results are reported in Table S4.
The monomer attracts fewer Zn2+ cations than the dimers (approximately half), and DimerCS attracts almost as many Zn2+ ions as DimerM: 2.1 for Mono and 3.9 and 4.1 for DimerCS and DimerM, respectively, in average per simulation. The fact that Mono attracts fewer Zn2+ ions than the dimers can be explained by the fact that Mono has half as many potential residues to interact with Zn2+ than the dimers. Although DimerCS and DimerM attract overall almost as many Zn2+ ions, DimerM binds Zn2+ ions approximately six times more often via H140 and H193 than DimerCS (and Mono): 1.8, 0.3, and 0.3 in average per simulation, respectively. DimerCS has the same number of histidines as DimerM, but they are located in the periphery and cannot act in concert to interact with Zn2+. The fact that DimerM attracts more Zn2+ ions toward the essential histidines agrees with the higher zinc sensitivity of HV1 WT (dimer) in the patch-clamp experiments (21). We note here that we did not observe the binding of one Zn2+ ion concomitantly by H140 and H193 (H140-Zn-H193) in any simulation in monomeric or in dimeric HV1, supporting again the existence of two binding sites, rather than one single binding site, in HV1. The glutamate 192 and 196 and aspartate 130 residues are recurring ligands of Zn2+ in all the systems. This can be explained by the negative charge of their side chains and their exposure to the bulk solution: E192 and E196 are located in the extracellular loop S3-S4 and D130 in the extracellular loop S1-S2. Aspartate 130 binds zinc alone in our simulations, without additional HV1 residues. D185, which bound Zn2+ to a small extent in our previous simulations of DimerM⋅2Zn and Mono⋅2Zn, was not identified in these simulations. However, it is located deeper in HV1s, and it remains possible that this residue binds zinc in a later step. In several of the simulations with DimerM, the coordination spheres around the zinc cations binding H193 and/or H140 exhibit conformations similar to those formed in the previous simulations with two zinc cations (DimerM⋅2Zn). One Zn2+ ion is bound in Zn2 between the two H140 residues in four out of the 10 simulations. A second Zn2+ ion is bound to one of the two H193 residues in two of these simulations. In one of the two last MDs, the two Zn2+ ions are furthermore coordinated by one E192 residue, and the Zn2+ ion bound to one H193 residue in Zn1 is also coordinated by the other E192 residue. This simulation was extended to follow the structural reorganization over a longer time window of 3000 ns. The time evolution of the binding in this simulation is depicted in Fig. 5. We did not observe the stable second H193-Zn1 interaction, although it was formed repeatedly in the second part of the simulations, three times representing ∼12% of the last 1500 ns.
Figure 5.
Time evolution of the binding in DimerM (MD 7 of DimerM⋅180⋅WT⋅freeZn in Table S1). (A) The periodic box for the whole system is shown. (B–G) Snapshots during the time course of the simulation are shown. The region of the system corresponding to the green box in (A) is shown. The zinc cations are depicted as violet balls. Water molecules have been removed for clarity except for those within 3 Å of Zn2+, which are shown as dynamic bonds. The snapshots are taken at (B) 0.6 ns, (C) 0.8 ns, (D) 1.7 ns, (E) 5.9 ns, (F) 75 ns, and (G) 1420 ns of the trajectory. A first Zn2+ ion is coordinated by the glutamate 192 residue of one HV1 subunit (B), then by the histidine 140 residue (Zn2) of the same subunit (C). Then, a second Zn2+ is coordinated by E192 and H193′ residues (Zn1) of the other HV1 subunit (D). A reorganization of the binding sites then takes place: the first Zn2+ ion (Zn2) is further coordinated by the second histidine, H140′, first via a water-mediated interaction (E) and then directly (F), while the second (Zn1) is further coordinated by E192′ (E). The coordination spheres in (F) remain stable for the rest of the simulation, up to 3000 ns. The participation of H193 in the binding is transient but recurrent in the rest of the simulation (G). The binding sites in (G) are closely similar to the results of DimerM⋅2Zn (Fig. 4; Table S2).
To summarize, our multiple simulations with “free” zinc in the bulk solution show that DimerM is more efficient than the monomer and DimerCS at attracting and binding zinc cations via the essential histidine 140 and 193 residues.
Attractive potential of the different structures
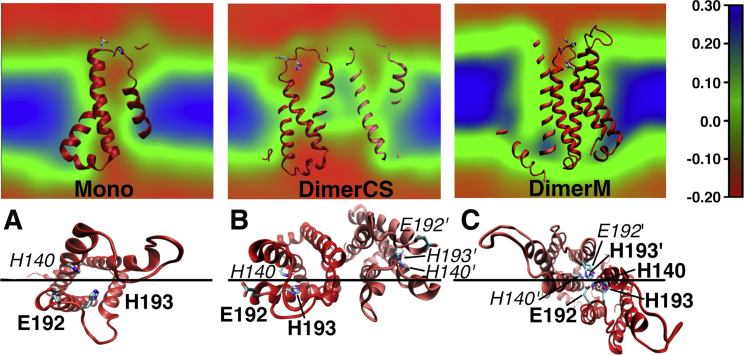
The previous results might depend on the FF model used for Zn2+ (see Appendix S1 and Table S2). Here, we performed simulations in the complete absence of ions. The results obtained here are thus independent of the Zn2+ model and of the effects of the binding on the dynamics of HV1s. For each structure (Mono, DimerCS, and DimerM), five independent 100 ns MD simulations were performed. The five trajectories were combined to calculate the averaged electrostatic potential (EP). The EP calculated in this way thus represents the potential originating from the unperturbed system formed by the channel, lipid, and bulk solution as it would be sensed by one Zn2+. The calculated EP maps (EPMs) are shown in Fig. 6. The slides for the EPMs in the upper panel were chosen to show the potential between the histidines (the region passing the binding sites), as depicted in the lower panel of Fig. 6. An attractive potential (red in upper panel of Fig. 6) is found in the interior of the channels of Mono and DimerCS, and between the two HV1 subunits of DimerM. However, it is disrupted between the interior of the channel and the bulk solution in Mono and DimerCS, but not in DimerM. Consequently, the Zn2+ cations must overcome a less attractive region (green cap above the channels) to penetrate into the channels and reach the histidine residues in Mono and DimerCS. In DimerM, the attractive potential between the two HV1 subunits reaches the bulk solution and consequently funnels the Zn2+ ion toward H193 and H140. This nicely correlates with the higher number of binding events with the two histidines in DimerM compared to Mono and DimerCS in the simulations with free zinc.
Figure 6.
Electrostatic potential maps (EPMs) of (A) the monomer (Mono) and dimers (B) DimerCS, and (C) DimerM. The potential is shown in a plane through the membrane that goes between H140 and H193 side chains as depicted by the black line in the lower panel. In the lower panel, the channels are seen from the extracellular (foreground) to the intracellular (background) sides of the membrane. The residues visible in front of the EPMs in the upper panel are labeled in bold in the lower panel, whereas those located behind the EPMs are labeled in italics. Color coding in the EPMs is shown on the right (in volts). EPMs are averaged over the five MD simulations for each HV1 structure. The attractive potential (red) in the interior of the channels is disrupted from the bulk solution in Mono and in DimerCS but continuous between the bulk solution and the dimer interface of DimerM, funneling the zinc cations toward the binding sites.
DimerM mutants
Our simulations indicate E192 as a substantial residue for the binding. Here, we used the same approach as previously to investigate in more detail the role of this glutamate and of the two histidine residues 193 and 140 in DimerM. For each hHV1 mutant, E192A and H140A + H193A, a series of five or 10 MD simulations was performed with two Zn2+ ions placed manually in the binding sites Zn1 and Zn2, with 100 mM free Zn2+ in the bulk solution, and without zinc. The mutations were considered in the two HV1 subunits simultaneously.
DimerM⋅E192A
Mutation of E192 to alanine has almost no effect on the binding in Zn1 and Zn2 in the simulations with two Zn2+ ions (DimerM⋅E192A⋅2Zn). Despite the missing negative charges of the glutamate side chains, the two Zn2+ ions are tightly bound between the histidines (Table S5). The missing interactions with E192 are generally replaced by interactions with water molecules to complete the tetrahedral coordination sphere around Zn2+. Thus, our simulations rather emphasize a minor role of this residue in the binding. This is not completely surprising because this or an equivalent residue is often missing in other HV1 channels. Actually, the role played by E192 in hHV1 is more evident from the simulations with free Zn2+ (DimerM⋅E192A⋅freeZn). In these simulations, the mutant coordinates fewer Zn2+ ions than WT: 2.9 and 4.1, respectively, on average per simulation (Table S4). This result further correlates with the smaller (approximately half) number of interactions formed with H140 and H193: 1.1 and 2.1 in average per simulation for the mutant and WT, respectively (but still more than three times that for Mono and DimerCS). Here, Zn2+ binding between the two H140 residues (H1402-Zn2) happens in only one simulation (four for WT) and no coordination between the two H193 residues (H1932-Zn1) is observed. We propose that the presence of E192 contributes to increase the attraction of the cations toward the binding sites but is not essential to tightly stabilize Zn2+ in the binding sites between the histidines. This proposition is also supported by the calculation of the EPM that indicates that the attractive funnel between the two subunits is considerably decreased in the mutant in comparison to WT (results not shown). We tested the role of E192 in vivo in patch-clamp experiments. The experiments show no significant difference of zinc inhibition between WT and the mutant (Fig. S4). This result can be correlated to the minor role of E192 in the binding. Nevertheless, it is surprising because our simulations emphasize a substantial role of this residue in the attraction. This must, however, not be contradictory. Patch-clamp experiments last much longer (seconds) than MD simulations (nanoseconds). One explanation for this apparent contradiction might be that the experiments capture effects that happen on longer timescales than that of the simulations. Indeed, our simulations with two Zn2+ show that the binding between the histidines in Zn1 and Zn2 is stable also without E192. Furthermore, the attractive potential in the dimer interface is diminished in the absence of E192 but does not completely vanish. It might remain sufficient to attract Zn2+ toward the histidines, but on a longer timescale than that of our simulations.
DimerM⋅H140A + H193A
Contrary to the E192 results, the mutation of H140 and H193 to alanine leads to a significant destabilization of the binding sites (Table S4). In Zn1, Zn2+ is bound between the two E192 residues in three out of five simulations, but in Zn2, it is never bound by the two E192 residues in common. In the simulations with free Zn, E192 residues interact with Zn2+ almost as often in the mutant as in WT—19 and 21 times, respectively (Table S4). However, we observed almost no simultaneous participation of the two E192 residues from each subunit to the binding of the same cation. These results confirm the previous observation that H193 and H140 are essential to the formation of stable binding sites between the two HV1 subunits of DimerM and that E192 plays a minor role to the stability of these binding sites. Interestingly, if the interactions with H140 are no longer available, Zn2 always binds to D185 in one of the two subunits, a situation that was observed in (30) in which no participation of histidine but participation of aspartate 181 or 233 (D185 in hHV1) to the deepest binding site was found, respectively, in mHV1 or Ci-HV1 (Table S3). The attractive potential between the two HV1 subunits is diminished, as for the E192A mutant previously, but to a lower extent. The results of both the E192A and H140A + H193A mutants together suggest that the additive contributions of all the glutamate 192 residues and histidine 140 and 193 residues result in the efficient attraction of Zn2+ cations toward the binding sites in the dimer interface.
Conclusions
MD simulations are an ideal tool to investigate structural details of biological processes at the molecular level. However, the results might strongly depend on the setup and parameters used for the simulations. We identified one Zn2+ FF model that binds zinc to hHV1 without requiring artificial constraints during the simulations. We used this model to address and obtain the first, to our knowledge, unbiased atomistic details of the binding in different structures of hHV1. Furthermore, we performed multiple simulations to provide results that are more significant than from a single simulation. Our results support the binding of two Zn2+ ions in two binding sites in monomeric as well as in dimeric hHV1. In hHV1, one of the two sites involves histidine 193 and the other histidine 140. Our simulations confirm the participation of glutamate 119 and possibly of aspartates 123 and 185 in the monomer. They also identify new residues: E192 and, to a lesser extent, E196 in the monomer. These residues are also suggested to participate in the binding by a recent NMR structure of hHV1. Our simulations support the dimer structure suggested by earlier patch-clamp data (DimerM in this work). In this dimer, the two Zn2+ ions bind in the interface: one between the two histidine 193 residues and one between the two histidine 140 residues from each monomeric subunit. Glutamate 192 residues also participate in the binding in this interface, but not E196 residues. We compared the efficiency of the different hHV1 structures to attract and bind Zn2+ cations via H140 and H193 in more detail. The simulations with free zinc in the bulk solution revealed that DimerM attracts and binds Zn2+ via the essential histidines more efficiently. We analyzed the EP of the structures and explained the higher efficiency of DimerM by the additive contributions of the histidine and glutamate 192 residue side chains in the interface. Together, these residues create an attractive potential reaching into the bulk solution and funneling Zn2+ toward the binding sites. Our results show that the binding restrains the flexibility of all transmembrane helices. This might in turn restrain fluctuations in HV1 that are necessary for the opening of the gate and the subsequent conduction of protons through the channel. Further simulations and analyses will be necessary to mechanistically elucidate how zinc binding restricts the channel into a nonactive conformation. Finally, our results suggest that investigations of HV1 proton channels properties in the presence of inhibitors should consider the oligomeric nature of these channels. Conducting experiments solely with the monomer or interpreting the results in view of the monomer might not represent the reality of zinc binding to native (oligomeric) HV1 and its effects of the binding on the biophysical properties.
Author Contributions
B.M. conceived the project. C.J. designed the research and carried out simulations and analysis. G.C. recorded patch clamp. All the authors wrote the manuscript.
Acknowledgments
We thank Yuan-Ping Pang for technical support and interpretation of the results of the simulations using the cationic dummy atom approach.
This work was supported by grants from the Paulmanns-Wunschkinder Stiftung to C.J., the W. Lutz Stiftung to G.C., and the Deutsche Forschungsgemeinschaft (MU 3574/4-1) to B.M.
Editor: Philip Biggin.
Footnotes
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2019.12.035.
Supporting Citations
References (66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81) appear in the Supporting Material.
Supporting Material
References
- 1.Murphy R., DeCoursey T.E. Charge compensation during the phagocyte respiratory burst. Biochim. Biophys. Acta. 2006;1757:996–1011. doi: 10.1016/j.bbabio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Capasso M., Bhamrah M.K., Dyer M.J. HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat. Immunol. 2010;11:265–272. doi: 10.1038/ni.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capasso M., DeCoursey T.E., Dyer M.J. pH regulation and beyond: unanticipated functions for the voltage-gated proton channel, HVCN1. Trends Cell Biol. 2011;21:20–28. doi: 10.1016/j.tcb.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson L.M., Chappell J.B., Jones O.T. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem. J. 1987;246:325–329. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeCoursey T.E., Morgan D., Cherny V.V. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422:531–534. doi: 10.1038/nature01523. [DOI] [PubMed] [Google Scholar]
- 6.Taylor A.R., Chrachri A., Brownlee C. A voltage-gated H+ channel underlying pH homeostasis in calcifying coccolithophores. PLoS Biol. 2011;9:e1001085. doi: 10.1371/journal.pbio.1001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki M., Tojo A., Okamura Y. Autoimmune disorder phenotypes in Hvcn1-deficient mice. Biochem. J. 2013;450:295–301. doi: 10.1042/BJ20121188. [DOI] [PubMed] [Google Scholar]
- 8.Lishko P.V., Botchkina I.L., Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 2010;140:327–337. doi: 10.1016/j.cell.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 9.Lishko P.V., Kirichok Y. The role of Hv1 and CatSper channels in sperm activation. J. Physiol. 2010;588:4667–4672. doi: 10.1113/jphysiol.2010.194142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeCoursey T.E. Voltage-gated proton channels: molecular biology, physiology, and pathophysiology of the H(V) family. Physiol. Rev. 2013;93:599–652. doi: 10.1152/physrev.00011.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S.Y., Letts J.A., MacKinnon R. Functional reconstitution of purified human Hv1 H+ channels. J. Mol. Biol. 2009;387:1055–1060. doi: 10.1016/j.jmb.2009.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S.Y., Letts J.A., Mackinnon R. Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc. Natl. Acad. Sci. USA. 2008;105:7692–7695. doi: 10.1073/pnas.0803277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch H.P., Kurokawa T., Larsson H.P. Multimeric nature of voltage-gated proton channels. Proc. Natl. Acad. Sci. USA. 2008;105:9111–9116. doi: 10.1073/pnas.0801553105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tombola F., Ulbrich M.H., Isacoff E.Y. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron. 2008;58:546–556. doi: 10.1016/j.neuron.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujiwara Y., Kurokawa T., Okamura Y. Gating of the designed trimeric/tetrameric voltage-gated H+ channel. J. Physiol. 2013;591:627–640. doi: 10.1113/jphysiol.2012.243006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeshita K., Sakata S., Nakagawa A. X-ray crystal structure of voltage-gated proton channel. Nat. Struct. Mol. Biol. 2014;21:352–357. doi: 10.1038/nsmb.2783. [DOI] [PubMed] [Google Scholar]
- 17.Smith S.M., DeCoursey T.E. Consequences of dimerization of the voltage-gated proton channel. Prog. Mol. Biol. Transl. Sci. 2013;117:335–360. doi: 10.1016/B978-0-12-386931-9.00012-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez C., Koch H.P., Larsson H.P. Strong cooperativity between subunits in voltage-gated proton channels. Nat. Struct. Mol. Biol. 2010;17:51–56. doi: 10.1038/nsmb.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tombola F., Ulbrich M.H., Isacoff E.Y. The opening of the two pores of the Hv1 voltage-gated proton channel is tuned by cooperativity. Nat. Struct. Mol. Biol. 2010;17:44–50. doi: 10.1038/nsmb.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu F., Rebolledo S., Larsson H.P. Subunit interactions during cooperative opening of voltage-gated proton channels. Neuron. 2013;77:288–298. doi: 10.1016/j.neuron.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musset B., Smith S.M., DeCoursey T.E. Zinc inhibition of monomeric and dimeric proton channels suggests cooperative gating. J. Physiol. 2010;588:1435–1449. doi: 10.1113/jphysiol.2010.188318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahaut-Smith M.P. The effect of zinc on calcium and hydrogen ion currents in intact snail neurones. J. Exp. Biol. 1989;145:455–464. doi: 10.1242/jeb.145.1.455. [DOI] [PubMed] [Google Scholar]
- 23.Cherny V.V., DeCoursey T.E. pH-dependent inhibition of voltage-gated H(+) currents in rat alveolar epithelial cells by Zn(2+) and other divalent cations. J. Gen. Physiol. 1999;114:819–838. doi: 10.1085/jgp.114.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratanayotha A., Kawai T., Okamura Y. Molecular and functional characterization of the voltage-gated proton channel in zebrafish neutrophils. Physiol. Rep. 2017;5:E13345. doi: 10.14814/phy2.13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramsey I.S., Moran M.M., Clapham D.E. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki M., Takagi M., Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- 27.Sakata S., Kurokawa T., Okamura Y. Functionality of the voltage-gated proton channel truncated in S4. Proc. Natl. Acad. Sci. USA. 2010;107:2313–2318. doi: 10.1073/pnas.0911868107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakata S., Miyawaki N., Okamura Y. Comparison between mouse and sea urchin orthologs of voltage-gated proton channel suggests role of S3 segment in activation gating. Biochim. Biophys. Acta. 2016;1858:2972–2983. doi: 10.1016/j.bbamem.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Humez S., Fournier F., Guilbault P. A voltage-dependent and pH-sensitive proton current in Rana esculenta oocytes. J. Membr. Biol. 1995;147:207–215. doi: 10.1007/BF00233548. [DOI] [PubMed] [Google Scholar]
- 30.Qiu F., Chamberlin A., Larsson H.P. Molecular mechanism of Zn2+ inhibition of a voltage-gated proton channel. Proc. Natl. Acad. Sci. USA. 2016;113:E5962–E5971. doi: 10.1073/pnas.1604082113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De La Rosa V., Bennett A.L., Ramsey I.S. Coupling between an electrostatic network and the Zn2+ binding site modulates Hv1 activation. J. Gen. Physiol. 2018;150:863–881. doi: 10.1085/jgp.201711822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwaki M., Takeshita K., Kandori H. Zn2+-binding to the voltage-gated proton channel Hv1/VSOP. J. Phys. Chem. B. 2018;122:9076–9080. doi: 10.1021/acs.jpcb.8b04890. [DOI] [PubMed] [Google Scholar]
- 33.Bayrhuber M., Maslennikov I., Riek R. Nuclear magnetic resonance solution structure and functional behavior of the human proton channel. Biochemistry. 2019;58:4017–4027. doi: 10.1021/acs.biochem.9b00471. [DOI] [PubMed] [Google Scholar]
- 34.UniProt Consortium UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berman H.M., Westbrook J., Bourne P.E. The protein data bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q., Wanderling S., Perozo E. Structural mechanism of voltage-dependent gating in an isolated voltage-sensing domain. Nat. Struct. Mol. Biol. 2014;21:244–252. doi: 10.1038/nsmb.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Notredame C., Higgins D.G., Heringa J. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- 38.Ramsey I.S., Ruchti E., Clapham D.E. Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc. Natl. Acad. Sci. USA. 2009;106:7642–7647. doi: 10.1073/pnas.0902761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chamberlin A., Qiu F., Larsson H.P. Hydrophobic plug functions as a gate in voltage-gated proton channels. Proc. Natl. Acad. Sci. USA. 2014;111:E273–E282. doi: 10.1073/pnas.1318018111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kulleperuma K., Smith S.M., Pomès R. Construction and validation of a homology model of the human voltage-gated proton channel hHV1. J. Gen. Physiol. 2013;141:445–465. doi: 10.1085/jgp.201210856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Q., Shen R., Perozo E. Resting state of the human proton channel dimer in a lipid bilayer. Proc. Natl. Acad. Sci. USA. 2015;112:E5926–E5935. doi: 10.1073/pnas.1515043112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webb B., Sali A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinformatics. 2016;54:5.6.1–5.6.37. doi: 10.1002/cpbi.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sali A., Blundell T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 44.Bhattacharya D., Nowotny J., Cheng J. 3Drefine: an interactive web server for efficient protein structure refinement. Nucleic Acids Res. 2016;44:W406–W409. doi: 10.1093/nar/gkw336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laskowski R.A., MacArthur M.W., Thornton J.M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Cryst. 1993;26:283–291. [Google Scholar]
- 46.Tovchigrechko A., Vakser I.A. GRAMM-X public web server for protein-protein docking. Nucleic Acids Res. 2006;34:W310–W314. doi: 10.1093/nar/gkl206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lomize M.A., Pogozheva I.D., Lomize A.L. OPM database and PPM web server: resources for positioning of proteins in membranes. Nucleic Acids Res. 2012;40:D370–D376. doi: 10.1093/nar/gkr703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsson M.H., Søndergaard C.R., Jensen J.H. PROPKA3: consistent treatment of internal and surface residues in empirical pKa predictions. J. Chem. Theory Comput. 2011;7:525–537. doi: 10.1021/ct100578z. [DOI] [PubMed] [Google Scholar]
- 49.Dolinsky T.J., Nielsen J.E., Baker N.A. PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004;32:W665–W667. doi: 10.1093/nar/gkh381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jo S., Lim J.B., Im W. CHARMM-GUI membrane builder for mixed bilayers and its application to yeast membranes. Biophys. J. 2009;97:50–58. doi: 10.1016/j.bpj.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Case D.A., Betz R.M., Kollman P.A. University of California; San Francisco, CA: 2016. AMBER 2016. [Google Scholar]
- 52.Maier J.A., Martinez C., Simmerling C. ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015;11:3696–3713. doi: 10.1021/acs.jctc.5b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dickson C.J., Madej B.D., Walker R.C. Lipid14: the Amber lipid force field. J. Chem. Theory Comput. 2014;10:865–879. doi: 10.1021/ct4010307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joung I.S., Cheatham T.E., III Determination of alkali and halide monovalent ion parameters for use in explicitly solvated biomolecular simulations. J. Phys. Chem. B. 2008;112:9020–9041. doi: 10.1021/jp8001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jorgensen W.L., Chandrasekhar J., Klein M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983;79:926–935. [Google Scholar]
- 56.Darden T., York D., Pedersen L. Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993;98:10089–10092. [Google Scholar]
- 57.Essmann U., Perera L., Pedersen L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995;103:8577–8593. [Google Scholar]
- 58.Salomon-Ferrer R., Götz A.W., Walker R.C. Routine microsecond molecular dynamics simulations with AMBER on GPUs. 2. Explicit solvent particle mesh Ewald. J. Chem. Theory Comput. 2013;9:3878–3888. doi: 10.1021/ct400314y. [DOI] [PubMed] [Google Scholar]
- 59.Roe D.R., Cheatham T.E., III PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013;9:3084–3095. doi: 10.1021/ct400341p. [DOI] [PubMed] [Google Scholar]
- 60.Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–38, 27–28.. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 61.Aksimentiev A., Schulten K. Imaging alpha-hemolysin with molecular dynamics: ionic conductance, osmotic permeability, and the electrostatic potential map. Biophys. J. 2005;88:3745–3761. doi: 10.1529/biophysj.104.058727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Musset B., Cherny V.V., DeCoursey T.E. Detailed comparison of expressed and native voltage-gated proton channel currents. J. Physiol. 2008;586:2477–2486. doi: 10.1113/jphysiol.2007.149427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dassault Systèmes BIOVIA . Dassault Systèmes; San Diego, CA: 2016. Discovery studio visualizer v.17.2. [Google Scholar]
- 64.Banh R., Cherny V.V., DeCoursey T.E. Hydrophobic gasket mutation produces gating pore currents in closed human voltage-gated proton channels. Proc. Natl. Acad. Sci. USA. 2019;116:18951–18961. doi: 10.1073/pnas.1905462116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hammes-Schiffer S. Impact of enzyme motion on activity. Biochemistry. 2002;41:13335–13343. doi: 10.1021/bi0267137. [DOI] [PubMed] [Google Scholar]
- 66.Li P., Merz K.M., Jr. Metal ion modeling using classical mechanics. Chem. Rev. 2017;117:1564–1686. doi: 10.1021/acs.chemrev.6b00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vallee B.L., Auld D.S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990;29:5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- 68.Christianson D.W. Structural biology of zinc. Adv. Protein Chem. 1991;42:281–355. doi: 10.1016/s0065-3233(08)60538-0. [DOI] [PubMed] [Google Scholar]
- 69.Glusker J.P. Structural aspects of metal liganding to functional groups in proteins. Adv. Protein Chem. 1991;42:1–76. doi: 10.1016/s0065-3233(08)60534-3. [DOI] [PubMed] [Google Scholar]
- 70.Alberts I.L., Nadassy K., Wodak S.J. Analysis of zinc binding sites in protein crystal structures. Protein Sci. 1998;7:1700–1716. doi: 10.1002/pro.5560070805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patel K., Kumar A., Durani S. Analysis of the structural consensus of the zinc coordination centers of metalloprotein structures. Biochim. Biophys. Acta. 2007;1774:1247–1253. doi: 10.1016/j.bbapap.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 72.Andreini C., Bertini I., Cavallaro G. Minimal functional sites allow a classification of zinc sites in proteins. PLoS One. 2011;6:e26325. doi: 10.1371/journal.pone.0026325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laitaoja M., Valjakka J., Jänis J. Zinc coordination spheres in protein structures. Inorg. Chem. 2013;52:10983–10991. doi: 10.1021/ic401072d. [DOI] [PubMed] [Google Scholar]
- 74.Lemkul J.A., Huang J., MacKerell A.D., Jr. An empirical polarizable force field based on the classical Drude oscillator model: development history and recent applications. Chem. Rev. 2016;116:4983–5013. doi: 10.1021/acs.chemrev.5b00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.MacKerell A.D., Bashford D., Karplus M. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 76.Huang J., Rauscher S., MacKerell A.D., Jr. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods. 2017;14:71–73. doi: 10.1038/nmeth.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stote R.H., Karplus M. Zinc binding in proteins and solution: a simple but accurate nonbonded representation. Proteins. 1995;23:12–31. doi: 10.1002/prot.340230104. [DOI] [PubMed] [Google Scholar]
- 78.Yu H., Whitfield T.W., Roux B. Simulating monovalent and divalent ions in aqueous solution using a Drude polarizable force field. J. Chem. Theory Comput. 2010;6:774–786. doi: 10.1021/ct900576a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duarte F., Bauer P., Kamerlin S.C. Force field independent metal parameters using a nonbonded dummy model. J. Phys. Chem. B. 2014;118:4351–4362. doi: 10.1021/jp501737x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pang Y.-P. Novel zinc protein molecular dynamics simulations: steps toward antiangiogenesis for cancer treatment. J. Mol. Model. 1999;5:196–202. [Google Scholar]
- 81.Hodgkin A.L., Huxley A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.