Abstract
Nonalcoholic steatohepatitis (NASH) is the fastest growing indication for liver transplant (LT)worldwide and is deemed to be the foremost indication in the near future. Recurrence of NASH can occur post LT and has been observed to be a common phenomenon. Baseline metabolic co-morbidities and worsening of metabolic profile post LT are the principal drivers of NASH recurrence. Liver biopsy remains the gold standard for establishing the diagnosis. However, noninvasive methods including transient elastography (TE) and magnetic resonance imaging (MRI) seem to be promising. The implications of recurrent NASH on post LT outcomes, graft steatosis, progression to fibrosis, overall survival, and cardiovascular associations warrant careful evaluation. Control of metabolic parameters and weight gain along with tailored immunosuppression remain the cornerstone of management. Extrapolation of the ever-increasing armamentarium of NASH pharmacotherapy specifically in this population of recurrent NAFLD remains a challenge for the future.
Keywords: Nonalcoholic steatohepatitis (NASH), recurrent NASH, nonalcoholic fatty liver disease (NAFLD), de novo NASH
Introduction
The worldwide prevalence of nonalcoholic fatty liver disease (NAFLD) has seen a meteoric rise to become one of the commonest causes of chronic liver disease worldwide. The current estimated global prevalence of NAFLD stands at 25.2%, and that of nonalcoholic steatohepatitis (NASH) ranges between 1.5% and 6.45% (1). As a consequence of the burgeoning prevalence of NAFLD, it has also established itself as the third most common indication for liver transplantation (LT) for chronic liver disease in the United States, with an ever-ascending curve (2). NAFLD can also occur in the post-transplant setting and can fall into either of the two categories, namely recurrent or de novo NAFLD. Recurrent NAFLD is re-occurrence of NAFLD in patients in whom the primary indication for transplant was NAFLD related cirrhosis (3). On the other hand recipients of LT can accrue multiple risk factors for NAFLD post-transplant and can develop post-transplant de novo NAFLD which is defined as the occurrence of liver steatosis or steatohepatitis in transplant recipients after at least six months of transplantation who were transplanted for indications other than NAFLD (4). Of these two entities, recurrent NAFLD is commoner and has been reported frequently in literature.
Epidemiology of recurrent NAFLD
Recurrent NAFLD presenting as recidivism of the parent disease has been universally reported in multiple studies. Studies have shown an alarmingly high prevalence of recurrent NAFLD after LT with one study showing that almost 90% of patients overall developed recurrent NAFLD, of which 25% had advanced fibrosis (5). In another study in patients with clinical histological phenotype of NASH-related cirrhosis which retrospectively analyzed the onset and progression of NAFLD in a time-dependent manner showed a post-transplant allograft steatosis of up to 100% in a 5-year time interval in comparison to only 25% in the control group consisting of patients with alcohol or cholestatic liver disease associated cirrhosis (6). In a 10-year single-center experience of 98 patients with NASH cirrhosis undergoing LT, it was shown that more than two-thirds developed recurrent NAFLD, one fourth had recurrent NASH, and 18% had stage II/IV or greater fibrosis (7). In another recent study of 226 patients undergoing LT for NASH with a mean follow-up of 7 years, 81 patients had biopsy-proven recurrent NASH, 15 had bridging fibrosis, and four patients developed recurrent NASH cirrhosis (8). A summary of recent studies showing the prevalence of recurrent NAFLD is shown in Table 1. At this point it is important to understand the fundamental differences of recurrent NAFLD from de novo NAFLD. A review from a recent meta-analysis of 12 studies involving 2,166 patients shows that de novo NAFLD has a variable prevalence of 14.7% to 52% post LT which is less commoner than recurrent NAFLD (4). Furthermore, the same meta-analysis also shows a variable prevalence of 0.96% to 32% of biopsy proven NASH involving eight studies in those having de novo NAFLD (4). Prevalence of de novo NAFLD is also dependent upon native disease etiology. Data suggests a pooled prevalence of de novo NAFLD of 37%, 35%, 22%, 19%, and 7% in alcoholic cirrhosis, cryptogenic cirrhosis, HBV cirrhosis, HCV cirrhosis and Cholestatic liver disease associated cirrhosis respectively (4).
Table 1. Summary of recent studies on post LT recurrent NAFLD.
| Authors (ref.) | Characteristics of study | Prevalence of NAFLD post LT | Important derivations |
|---|---|---|---|
| Sourianarayanane et al. 2017 (9) | Retrospective, n=77 | 54.6% recurrent NAFLD at 1 year | 16% had moderate or severe steatosis (>33%), 6.8% had NASH (with NAS ≥5), 2.3% had advanced fibrosis (stage ≥3) at 1 year |
| Bhati et al. 2017 (5) | Retrospective, n=103 | 90% recurrent NAFLD diagnosed histologically or with transient elastography | Liver biopsy: 20.6% had bridging fibrosis; TE: Advanced fibrosis (>F3) was seen in 26.8% |
| Kakar et al. 2019 (8) | Retrospective, n=226 | 49% had recurrent NASH at an average of 3 years | 15 bridging fibrosis (6 years); 4 NASH allograft cirrhosis (9 years) |
| Tokodai et al. 2019 (10) | Retrospective, n=95 | 41% recurrent NAFLD at 1-year | DM was only risk factor that was statistically associated with NASH recurrence |
NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
Risk factors for post-transplant NAFLD
The classical risk factors for traditional NAFLD, including obesity, weight gain, diabetes mellitus, hypertension, and hyperlipidemia holds true for the development of NAFLD in the allograft (11). Obesity or body mass index (BMI) at or after the point of transplant, post-transplant weight gain, hypertension and dyslipidemia have been found to be associated with both recurrent and de novo NAFLD although, diabetes mellitus was significantly more prevalent in the recurrent NAFLD group (P<0.01) (12). Other risk factors, although may be contributory, have not been shown to have a clear association with the development of post-transplant NAFLD.
Age in conjunction with components of metabolic syndrome increases the risk of metabolic co-morbidities, but its role as an independent risk factor for post-transplant NAFLD remains unclear (13). Similarly, the role of gender with women being at a higher risk for post LT NAFLD remains to be established (14). Genes may play an important role in the pathogenesis of post-transplant NAFLD with studies showing a co-relation between PNPLA3 and steatosis after LT (15). Post-transplant weight gain is a commonly described phenomenon with one study reporting a median weight gain of 5.1 and 9.5 kg at one year and three years respectively leading to 31% of the patients being obese at the 3-year mark post LT (16). Such post-transplant weight gain has been linked to the development of a metabolic syndrome phenotype with abnormal liver functions, possibly reflecting the development of NASH (16,17).
Post LT immunosuppression and its side effects need important considerations as risk factors for post-transplant NAFLD. The association between use of corticosteroids and development of metabolic risk factors and hepatic steatosis is mostly limited to the early post-transplant period and has been found to be dependent on the total daily dose (18). The commonly used calcineurin inhibitors (CNIs) tacrolimus and cyclosporine are known to have a negative effect on insulin resistance (19).
With regard to hepatic steatosis conflicting data is available comparing the two CNIs with one study reporting tacrolimus as a risk factor for de novo NAFLD whereas others finding no significant differences (20-22). Everolimus, on the other hand, has been associated with less weight gain, but no association has been made with NAFLD post LT (23). Similarly, the metabolic profile of everolimus may contribute to the development of de novo NAFLD, but no conclusive data has suggested the same (14).
Natural history of recurrent NAFLD
Few studies have addressed the time-dependent relationship of recurrent NAFLD in the post-transplant setting (5,6). A previous study analyzed graft steatosis and recurrence of NAFLD in patients who were originally transplanted for NAFLD cirrhosis with the aid of biopsy and transient elastography (TE) over an eighteen year follow up (5). In patients who underwent TE, 87.5% were found to have graft steatosis at a median time from LT of 75 months. Those undergoing liver biopsy showed the presence of NAFLD and NASH in 88.2% and 41.2% respectively at a median time from LT of 47 months. On TE, 26.8% of the patients had advanced fibrosis, and 5.4% had cirrhosis (without clinical evidence of decompensation), whereas, in the liver biopsy group, 20.6% had bridging fibrosis, but none had cirrhosis. Therefore, the recurrence of NAFLD post LT is extremely common and has a time-dependent graded pattern of progression. Further analysis in this cohort of illness also showed a higher prevalence of metabolic syndrome, T2DM, and fasting hyperglycemia in patients with recurrent NAFLD. Another study which analyzed the evolution of NASH recurrence in a time-dependent manner in 227 patients who were originally transplanted for NAFLD or cryptogenic cirrhosis and had at least one follow up biopsy showed an actuarial probability of development of NALFD of 8.2%, 13.6%, 24.9%, and 32.9%, respectively at 1, 2, 5 and 10 years (21). Furthermore, they also showed that patients with a primary diagnosis of NAFLD cirrhosis had a greater probability of developing post-transplant NAFLD than those having other etiologies (21). In another study Cantos et al. showed time-dependent probability of graft steatosis of up to 100% by five years in those transplanted for NAFLD cirrhosis in comparison to only 25% in those transplanted for other etiologies (6). Though these studies have demonstrated a relatively high incidence of post-transplant recurrent NAFLD, the incidence of steatohepatitis per se was shown to be 6% in one study and 11% in another (6,21). In another study involving 34 LT recipients graft steatosis was seen in 9, graft steatohepatitis in 25 recipients, whereas advanced fibrosis was seen in 3 recipients (22).
Establishing a diagnosis of recurrent NAFLD
Liver biopsy remains the gold standard for establishing a conclusive diagnosis of recurrent NAFLD as well as to rule out competing etiologies and has been advocated by the AASLD (American Association for Study of Liver Disease Practice) (24). Among other modalities for assessment of steatosis, MRI has been found to perform better than Ultrasound or Computed Tomography with sensitivity and specificity of 90% and 91% respectively, however still needs further validation (25). Similarly, the use of Transient Elastography for assessment of graft steatosis also needs further validation (19).
Data from a previous meta-analysis comparing noninvasive methods for assessment of post-liver transplant graft fibrosis shows that TE performs better than the serum-based biomarkers APRI and FIB 4 (TE odds ratio 21.17 (95% CI: 14.10–31.77, APRI 9.02, 95% CI: 5.79–14.07; and FIB-4 7.08, 95% CI: 4.00–12.55) (26). MRE has also been utilized for recurrent graft fibrosis assessment in a very select population of HCV patients and showed 87.5%sensitivity and specificity of 79.2% using a cut-off of 4.2 (27). Hence, to conclude liver biopsy remains the gold standard for assessment of post-transplant recurrence of graft steatosis and fibrosis while other modalities like TE, MRE need further validation.
The implication of recurrent NAFLD on post-transplant survival
Limited data is available on the impact of recurrent NAFLD and post-transplant survival. In one study involving 588 liver transplant recipients, of which 9.4% were transplanted for NASH, post-transplant allograft steatosis was not associated with post-transplant survival (28). Although studies have shown worse outcomes with patients being transplanted NASH related HCC as well as patients being re-transplanted for NASH, no such data exists with NASH recurrence post-transplant (28-30). Hence, till further prospective data is generated it would be prudent to assume that survival is not influenced by recurrence of NASH.
Tailored immunosuppression—its implication in recurrent NAFLD
Metabolic effects of immunosuppression therapy, including corticosteroids and CNIs, has been a matter of concern in the post-transplant setting. Both corticosteroids and CNIs have been linked with adverse impact on the metabolic profile post-liver transplant and become profound with background NASH (5). However, the studies linking the effects of immunosuppressants, specifically on allograft steatosis, need further validation. Both tacrolimus, as well as cumulative steroid dose, have been implicated in development in recurrent allograft NAFLD in one study (6). However, another study found no association between the use of tacrolimus and post-transplant steatosis (21). The use of cyclosporine was found to be a predictor of post-transplant steatosis on univariate analysis in one study; however on multivariate assessment did not attain statistical significance (21). Therefore, although the risks of worsening metabolic profiles are common with the use of immunosuppressive regimens in the post-transplant period, their impact on graft steatosis needs further substantiation.
Extrahepatic implications of recurrent NAFLD
To date, literature regarding the relationship between allograft steatosis and extrahepatic manifestations, especially cardiovascular outcomes, are limited. In one study involving two hundred and fifty-four patients, post-transplant allograft steatosis when analyzed in a time-dependent sequence was found not to be predictive of cardiovascular outcomes. (HR, 1.08; 95% CI: 0.73–1.59; P=0.70) (28). However, the study also noted that the cardiovascular event rates were higher (40% at five years) irrespective of the status of allograft steatosis in patients transplanted for NASH in comparison to those for other etiologies (5–10% at five years) (28). This possibly reflects the impact of metabolic profile alteration and occult underlying cardiovascular abnormalities in these subsets of the population. Dureja et al. also reported similar findings demonstrating increased higher rates of cardiovascular and infection-related mortality in the cohort with NAFLD recurrence, although the overall survival is similar to other etiologies (22). This, in essence, takes home the point that although allograft steatosis may not be associated with worse post-transplant outcomes, the associated metabolic profile and underlying cardiovascular co-morbidities in patients who are transplanted for NAFLD may have a synergistic effect towards worse post-transplant outcomes.
NAFLD recurrence: implications in management
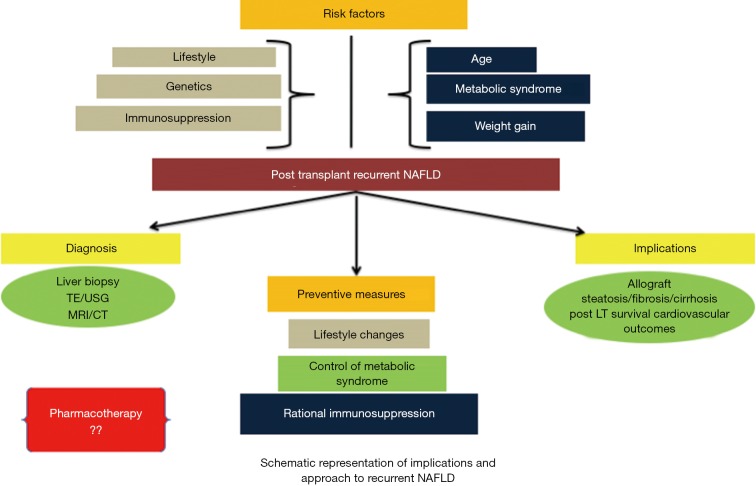
As with the non-transplanted population with NAFLD, lifestyle modification, prevention of weight gain, dietary restriction and achieving weight loss also remain the cornerstones in the management of post LT recurrent NAFLD although controlled trials in this specific population are lacking (19). In one study, Krasnoff et al. evaluated 151 OLT patients for the role of exercise and dietary counseling and found a greater increase in exercise capacity and overall general health in the intervention group (31). An approach to implications and approach to recurrent NAFLD is shown in Figure 1. However, there remains a need for further long-term studies specifically addressing this population in this regard. Similarly, the impact of pharmacotherapy for the treatment of established recurrent NAFLD has not been substantiated by validated studies. Whether the therapeutic armamentarium against NASH in the non-transplanted population can be extrapolated to the population of recurrent NAFLD remains to be explored and warrants well-constructed studies (19).
Figure 1.
Approach to implications and management in recurrent NAFLD. NAFLD, nonalcoholic fatty liver disease.
Conclusions
NASH remains the fastest growing indication for LT. Following LT NAFLD can be of two forms: recurrent or de novo. Recurrent NAFLD is common and can be present in greater than three-fourths of patients in this cohort. Baseline metabolic profile, post-transplant rapid weight gain, poor metabolic control and adverse profile of immunosuppressants use remains the primary risk factors associated with recurrent NAFLD. Liver biopsy remains the gold standard to establish the diagnosis of recurrent NAFLD, and noninvasive methods need further evaluation. Although overall graft and patient survival are not associated with recurrence of NAFLD per se, cardiovascular outcomes are worse on account of poor metabolic profile. Control of metabolic risk factors and tailored immunosuppression remain the cardinal strategies for prevention of progression to recurrent NAFLD. Extrapolation of the therapeutic armamentarium in non-transplant settings this population warrants further evaluation and quality studies.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84. 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 2.Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011;141:1249-53. 10.1053/j.gastro.2011.06.061 [DOI] [PubMed] [Google Scholar]
- 3.Patil DT, Yerian LM. Evolution of nonalcoholic fatty liver disease recurrence after liver transplantation. Liver Transpl 2012;18:1147-53. 10.1002/lt.23499 [DOI] [PubMed] [Google Scholar]
- 4.Losurdo G, Castellaneta A, Rendina M, et al. Systematic review with meta-analysis: de novo non-alcoholic fatty liver disease in liver transplanted patients. Aliment Pharmacol Ther 2018;47:704-14. 10.1111/apt.14521 [DOI] [PubMed] [Google Scholar]
- 5.Bhati C, Idowu MO, Sanyal AJ, et al. Long-term outcomes in patients undergoing liver transplantation for nonalcoholic steatohepatitis-related cirrhosis. Transplantation 2017;101:1867-74. 10.1097/TP.0000000000001709 [DOI] [PubMed] [Google Scholar]
- 6.Contos MJ, Cales W, Sterling RK, et al. Development of nonalcoholic fatty liver disease after orthotopic liver transplantation for cryptogenic cirrhosis. Liver Transpl 2001;7:363-73. 10.1053/jlts.2001.23011 [DOI] [PubMed] [Google Scholar]
- 7.Malik SM, deVera ME, Fontes P, et al. Recurrent disease following liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Transpl 2009;15:1843-51. 10.1002/lt.21943 [DOI] [PubMed] [Google Scholar]
- 8.Kakar S, Dugum M, Cabello R, et al. Incidence of Recurrent NASH-Related Allograft Cirrhosis. Dig Dis Sci 2019;64:1356-63. 10.1007/s10620-018-5413-9 [DOI] [PubMed] [Google Scholar]
- 9.Sourianarayanane A, Arikapudi S, McCullough AJ, et al. Nonalcoholic steatohepatitis recurrence and rate of fibrosis progression following liver transplantation. Eur J Gastroenterol Hepatol 2017;29:481-7. 10.1097/MEG.0000000000000820 [DOI] [PubMed] [Google Scholar]
- 10.Tokodai K, Karadagi A, Kjaernet F, et al. Characteristics and risk factors for recurrence of nonalcoholic steatohepatitis following liver transplantation. Scand J Gastroenterol 2019;54:233-9. 10.1080/00365521.2019.1577484 [DOI] [PubMed] [Google Scholar]
- 11.Burke A, Lucey MR. Non-alcoholic fatty liver disease, non-alcoholic steatohepatitis and orthotopic liver transplantation. Am J Transplant 2004;4:686-93. 10.1111/j.1600-6143.2004.00432.x [DOI] [PubMed] [Google Scholar]
- 12.Vallin M, Guillaud O, Boillot O, et al. Recurrent or de novo nonalcoholic fatty liver disease after liver transplantation: natural history based on liver biopsy analysis. Liver Transpl 2014;20:1064-71. 10.1002/lt.23936 [DOI] [PubMed] [Google Scholar]
- 13.Collins BH, Pirsch JD, Becker YT, et al. Long-term results of liver transplantation in older patients 60 years of age and older. Transplantation 2000;70:780-3. 10.1097/00007890-200009150-00012 [DOI] [PubMed] [Google Scholar]
- 14.Kappus M, Abdelmalek M. De novo and recurrence of nonalcoholic steatohepatitis after liver transplantation. Clin liver Dis 2017;21:321-35. 10.1016/j.cld.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 15.Finkenstedt A, Auer C, Glodny B, et al. Patatin-like phospholipase domain-containing protein 3 rs738409-G in recipients of liver transplants is a risk factor for graft steatosis. Clin Gastroenterol Hepatol 2013;11:1667-72. 10.1016/j.cgh.2013.06.025 [DOI] [PubMed] [Google Scholar]
- 16.Richards J, Gunson B, Johnson J, et al. Weight gain and obesity after liver transplantation. Transpl Int 2005;18:461-6. 10.1111/j.1432-2277.2004.00067.x [DOI] [PubMed] [Google Scholar]
- 17.Palmer M, Schaffner F, Thung SN. Excessive weight gain after liver transplantation. Transplantation 1991;51:797-800. 10.1097/00007890-199104000-00012 [DOI] [PubMed] [Google Scholar]
- 18.Sprinzl MF, Weinmann A, Lohse N, et al. Metabolic syndrome and its association with fatty liver disease after orthotopic liver transplantation. Transpl Int 2013;26:67-74. 10.1111/j.1432-2277.2012.01576.x [DOI] [PubMed] [Google Scholar]
- 19.Germani G, Laryea M, Rubbia-Brandt L, et al. Management of recurrent and de novo NAFLD/NASH after liver transplantation. Transplantation 2019;103:57-67. 10.1097/TP.0000000000002485 [DOI] [PubMed] [Google Scholar]
- 20.Dumortier J, Giostra E, Belbouab S, et al. Non-alcoholic fatty liver disease in liver transplant recipients: another story of “seed and soil”. Am J Gastroenterol 2010;105:613-20. 10.1038/ajg.2009.717 [DOI] [PubMed] [Google Scholar]
- 21.Yalamanchili K, Saadeh S, Klintmalm GB, et al. Nonalcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or nonalcoholic fatty liver disease. Liver Transpl 2010;16:431-9. [DOI] [PubMed] [Google Scholar]
- 22.Dureja P, Mellinger J, Agni R, et al. NAFLD recurrence in liver transplant recipients. Transplantation 2011;91:684-9. 10.1097/TP.0b013e31820b6b84 [DOI] [PubMed] [Google Scholar]
- 23.Charlton M, Rinella M, Patel D, et al. Everolimus is associated with less weight gain than tacrolimus 2 years after liver transplantation: results of a randomized multicenter study. Transplantation 2017;101:2873-82. 10.1097/TP.0000000000001913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328-57. 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 25.van Werven JR, Marsman HA, Nederveen AJ, et al. Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology 2010;256:159-68. 10.1148/radiol.10091790 [DOI] [PubMed] [Google Scholar]
- 26.Bhat M, Tazari M, Sebastiani G. Performance of transient elastography and serum fibrosis biomarkers for non-invasive evaluation of recurrent fibrosis after liver transplantation: a meta-analysis. PLoS One 2017;12:e0185192. 10.1371/journal.pone.0185192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee VS, Miller FH, Omary RA, et al. Magnetic resonance elastography and biomarkers to assess fibrosis from recurrent hepatitis C in liver transplant recipients. Transplantation 2011;92:581-6. 10.1097/TP.0b013e31822805fa [DOI] [PubMed] [Google Scholar]
- 28.Narayanan P, Mara K, Izzy M, et al. Recurrent or de novo allograft steatosis and long-term outcomes after liver transplantation. Transplantation 2019;103:e14-21. 10.1097/TP.0000000000002317 [DOI] [PubMed] [Google Scholar]
- 29.Kern B, Feurstein B, Fritz J, et al. High incidence of hepatocellular carcinoma and postoperative complications in patients with nonalcoholic steatohepatitis as a primary indication for deceased liver transplantation. Eur J Gastroenterol Hepatol 2019;31:205-10. 10.1097/MEG.0000000000001270 [DOI] [PubMed] [Google Scholar]
- 30.Thuluvath AJ, Chen PH, Thuluvath PJ, et al. Poor survival after retransplantation in NASH cirrhosis. Transplantation 2019;103:101-8. 10.1097/TP.0000000000002135 [DOI] [PubMed] [Google Scholar]
- 31.Krasnoff JB, Vintro AQ, Ascher NL, et al. A randomized trial of exercise and dietary counseling after liver transplantation. Am J Transplant 2006;6:1896-905. 10.1111/j.1600-6143.2006.01391.x [DOI] [PubMed] [Google Scholar]