Abstract
2,3-Butanedione (BD) is a reactive diketone in artificial butter flavors that is thought to cause bronchiolitis obliterans in workers in microwave popcorn manufacturing. Bronchiolitis obliterans is generally not diagnosed until irreversible damage has occurred; therefore a biomarker of early exposure is needed. The potential systemic uptake of BD from inhalation exposure has not been evaluated. The objective here was to evaluate the systemic exposure of BD and bind to hemoglobin and albumin. [14C]BD was administered to male Harlan Sprague Dawley rats (100 mg/kg, intratracheal instillation) and B6C3F1 mice (157 mg/kg, oropharyngeal aspiration). Blood and plasma was collected 24 h after administration and analyzed for 14C content. At 24 h, 0.88 ± 0.07% of the administered dose was in rat blood, 0.66 ± 0.06% in rat plasma, 0.38 ± 0.13% in mouse blood and 0.17 ± 0.05% in mouse plasma. Albumin binding in rats was 269 ± 24.2 ng equiv./mg, which accounts for 38% of the radioactivity in plasma. In mice, binding was 85.0 ± 22.3 ng equiv./mg albumin, which accounts for 51% of the radioactivity in plasma. The binding to hemoglobin in rats was 38.2 ± 17.6 ng equiv./mg, and to globin was 29.1 ± 3.96 ng equiv./mg. In mice, the binding to hemoglobin was 16.2 ± 9.0 ng equiv./mg. The site(s) of adduction on hemoglobin and albumin was investigated by mass spectrometry. In rat globin, arginine adducts were detected at R-30 and R-104 of the beta chain in vitro and in vivo. In rat albumin, adducts were detected in vitro on R-219/221, R-360, and R-368, and in vivo on a variety of arginine residues. This study demonstrated that BD enters the systemic circulation and reacts with arginine on hemoglobin and albumin. These results indicate that hemoglobin and albumin adducts may be useful as biomarkers of BD exposure in humans.
Keywords: 2,3-butanedione; hemoglobin binding; albumin binding; systemic uptake
1. Introduction
Artificial butter flavors (ABFs) are proprietary mixtures consisting of more than 100 different volatile chemicals and have been used in microwave popcorn and other food industries. 2,3-Butanedione (BD, diacetyl) is a major component isolated from air samples at microwave popcorn production plants and is thought to play a major role in causing obliterative bronchiolitis (OB) in workers [1]. OB is an irreversible airway obstruction disease that is often fatal. Symptoms of OB include nonproductive cough, wheezing, and shortness of breath on exertion. Typically, these symptoms are gradual in onset and progression, and by the time respiratory symptoms become severe enough to seek medical attention, the disease has already caused irreversible lung damage. Although monitoring air can provide an estimate of a work place exposure to BD, a biomarker of exposure which provides a measure of internal dose is more suitable to monitor workers’ exposure. Therefore, development of a biomarker of BD exposure may allow early intervention, diagnosis, and treatment of exposed workers. Previous studies have indicated that the metabolism and elimination of BD occurs rapidly, with extensive exhalation as CO2 suggesting that BD itself is short lived, and would not make a good biomarker [2].
Hemoglobin and/or albumin adducts of electrophilic species have been widely used as a means for assessing exposure and internal dose for reactive chemicals [3, 4]. Since BD has been shown to bind arginine residues on proteins [5] it may be possible to use adducts of BD with blood proteins as a long-lived biomarker of exposure. Arginine adducts of methylglyoxal and glyoxal, a structurally similar compound to BD, have been characterized in vitro in human hemoglobin [6, 7]. A hotspot of modification with methylgyoxal at arginine-410 in human serum albumin has been reported [8]. Investigation of the reactivity of individual amino acids with BD, including N-alpha-acetylarginine, N-alpha-acetyllysine, and N-alpha-acetylcysteine, indicated that reaction occurred with N-alpha-acetylarginine. Adducts formed between BD and N-alpha-acetylarginine were characterized as stable cyclized and open-chain aminol addition products [9]. Therefore, it should be possible to extend this approach to detect BD-arginine adducts in hemoglobin and albumin.
Computational fluid dynamic and physiologically based pharmacokinetic modeling of BD fate in the airways of rats and humans indicated species differences in the extent of removal of inhaled BD in the upper respiratory tract and nose [10, 11]. The models described BD vapor interacting with the tissue compartments and equilibrating with the mucus, epithelial compartments and submucosal compartments, with loss by metabolism (by diacetyl reductase) and by equilibration, and removal by blood. In vitro metabolism of BD was determined in the olfactory and respiratory nasal mucosa and the tracheal mucosa of the rat. At an inspiratory flow rate of 400 mL/min, a 1-h exposure to BD indicated scrubbing by the upper respiratory tract of approximately 36%. Tracheal scrubbing was also considered and was estimated to be 7%, resulting in a concentration of BD exiting the trachea and reaching the bronchi of 61 ppm for a 100-ppm exposure. In nose breathing and mouth breathing humans, scaling of the model produced estimates of 79 and 96 ppm, respectively, for the concentration of BD exiting the trachea on exposure to 100 ppm BD.
The objectives of the current study were to evaluate the efficiency of [14C]BD uptake from the lung into the systemic circulation and the ability of [14C]BD to subsequently bind to cellular macromolecules. In this initial study, [14C]BD was administered to male Sprague Dawley rats by intratracheal instillation (ITI), and to male B6C3F1 mice by oropharyngeal aspiration (OPA). These nonphysiological modes of administration were chosen because they require significantly less radiolabeled BD than inhalation exposure, they avoid the significant uptake and metabolism of BD that occurs in the nose [10], and they allow direct deposit of a known amount of labeled BD in the lung. The results of these studies indicate that BD is absorbed into the systemic circulation and forms hemoglobin and albumin adducts. Mea: of hemoglobin and albumin adducts may be useful as biomarkers of BD exposure in humans.
2. Materials and Methods
2.1. Chemicals
[1,4-14C]BD (label on the methyl carbon, specific activity of 58.2 mCi/mmol) was obtained from Midwest Research Institute (Kansas City, MO) and was purified by reversed phase HPLC prior to use in studies. The radiochemical purity of the material used in studies was between 95.6 and 97.0%. BD was obtained from Sigma-Aldrich (St. Louis, MO), and was redistilled prior to use (purity >99%). Identity was confirmed by 1H and 13C NMR (Varian Inova 500 MHz spectrometer), and GC-MS on an Agilent 7890 GC coupled to a 5975 Inert XL MSD in electron ionization mode, and purity was determined by chromatography on an Agilent 6890 GC-FID (Agilent Technologies, Santa Clara, CA).
2.2. Animals
All studies were conducted at RTI International (RTP, NC) and were approved by the Institutional Animal Care and Use Committee. Animals were housed in a facility that is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Animal procedures were in accordance with the “Guide for the Care and Use of Laboratory Animals’[12]. Male Harlan Sprague Dawley rats were purchased from Harlan Laboratories (Indianapolis, IN) and were dosed at body weights between 295–305 g. Male B6C3F1 mice were purchased from Charles River Laboratories, Inc., (Raleigh, NC) and were dosed at body weights between 27–31 g. Animals were provided certified NTP 2000 feed (Ziegler Brothers, Gardners, PA), and tap water (City of Durham) ad libitum. All animals were acclimated in the RTI animal facility for 7 days prior to use.
2.3. Administration of BD and Collection of Samples
Six male rats were administered a target dose of 100 mg/kg BD (30 μCi per animal, 78 μCi/mmol) in distilled deionized water by ITI under anesthesia (isoflurane) in a dose volume of 200 μL. Six male mice were administered a target dose of 200 mg/kg BD (10 μCi per animal, 193 μCi/mmol) in distilled deionized water by OPA under anesthesia (isoflurane) in a dose volume of 50 μL. Blood was collected by cardiac puncture under CO2 anesthesia at 24 h after dosing, with heparin as an anticoagulant. Aliquots of each blood sample were analyzed for radioactivity, and the remainder was centrifuged to prepare plasma and red blood cells. Red blood cells were washed three times with an equal volume of isotonic saline. All samples were stored at −20 °C.
2.4. Determination of Total Radioactivity in Blood and Plasma
Approximately 50 μL of blood or plasma was weighed and solubilized in 1 mL Soluene-350™, and then bleached by adding 125 μL of 70% perchloric acid, followed by 0.3 mL of 30% H2O2. Ultima Gold™ scintillation cocktail was added to samples and radioactivity was determined using a Packard 1900CA Liquid Scintillation Counter, using an external standard channels ratio quench correction method. Activities recovered in blood and plasma are expressed per g of sample. Samples were counted for 5 min. The concentration of BD equivalents in plasma and blood was calculated using radioactivity determined divided by the specific activity of the BD administered.
2.5. In vitro reaction of [14C]BD with Bovine Serum Albumin
To determine the [14C]BD bound to albumin in rats administered [14C]BD it was necessary to first evaluate the stability of albumin bound material during processing of plasma. Albumin bound [14C]BD was prepared by the incubation of bovine serum albumin with [14C]BD, followed by separation of the bound material from unreacted [14C]BD. A solution (1 mL) of bovine serum albumin (40 mg/mL) was incubated for 4 h at 37 °C with 200 μL of [14C]BD solution to give 0.142 mg/mL BD in final solution. The solution was diluted with 10 mL of 150 mM potassium phosphate buffer (pH 7) and concentrated using a Centriprep Ultracel YM-50 centrifugal filter unit (Millipore, Billerica, MA). The concentrated material yielded a [14C]BD-albumin purity of 98.1%, assessed by HPLC on a Discovery BIO GFC column, 30 cm x 4.6 mm, 5 μm (Supelco, Bellefonte, PA), eluted with 0.15 M potassium phosphate buffer, pH 7. The HPLC system used was a Waters 600E solvent delivery module with a Perkin Elmer (Waltham, MA) 785A UV absorbance detector and a β-Ram flow-through radioactivity detector equipped with a 250-μL glass solid scintillant cell (IN/US Systems Inc., Tampa, FL). The resulting adducted bovine serum albumin was then used to evaluate the stability and recovery of BD-albumin during the isolation of albumin from plasma from rats and mice by determining the % radioactivity recovered during albumin isolation.
2.6. Isolation and Analysis of Plasma Albumin
Rat and mouse albumin were isolated from plasma by precipitation with trichloroacetic acid (TCA) [13]. Plasma of rats (500 μL plasma) and mice (250 μL plasma + 250 μL water) administered [14C]BD was mixed with 500 μL of 10% aqueous TCA (VWR, West Chester, PA). The samples were mixed and placed on ice for 10 min before centrifuging for 20 min at 300g at 4 °C. The pellet was washed twice by resuspending in 500 μL of ethanol and centrifuged for 10 min at 900g at 4 °C. The combined ethanolic supernatants containing albumin were mixed with 5 mL of water and transferred to a Centriprep Ultracel YM-50 centrifugal filter unit. The filter units were centrifuged three times for 5 min at 1500g at 4 °C. After each spin, the filtrate was removed and combined for later determination of free BD, and an additional 5 mL of water was added to the centrifugal filter unit with the albumin concentrate. The final albumin concentrate was transferred to vials for later use. A second method of albumin isolation from rat plasma was investigated which involved addition of saturated ammonium sulfate to plasma to bring the final ammonium sulfate concentration in plasma to 50%. The plasma/ammonium sulfate solution was then kept on ice for 10 min and then centrifuged at 3000g for 30 min at 4 °C. The supernatant which contains albumin was transferred to Spectra/Por® dialysis tubing (MWCO 6000 – 8000, Spectrum Laboratories, Inc. Ranch Dominguez, CA), and then dialyzed against 1 L of water for 24 h at 4 °C. In each case, Aliquots of the albumin solutions were used for the determination of albumin concentration using a Pierce BCA (bicinchoninic acid) protein assay (Thermo Scientific, Waltham, MA). To a separate aliquot of albumin, Ultima Gold™ scintillation cocktail was added and radioactivity bound to albumin was determined using a Packard 1900CA Liquid Scintillation Counter. The concentration of BD equivalents bound to albumin was calculated using radioactivity determined divided by the specific activity of the BD administered.
2.7. Preparation and Analysis Hemoglobin and Globin
The stability of hemoglobin bound BD to acid and other reagents used in protein isolation by precipitation was not known at the outset of this study. Therefore, we initially used the milder procedure of dialysis followed by size exclusion HPLC to determine the extent of hemoglobin binding. To 0.5 mL of red blood cells 0.5 mL of water was added and sample was centrifuged at 3000g for 30 min. The resulting hemoglobin solution was transferred to Spectra/Por® dialysis tubing (10 mm flat width, MWCO 6–8,000, Spectrum Laboratories, Inc. Rancho Dominguez, CA) and dialyzed against distilled water (1 L) at 4 °C for 24 h. The concentration of hemoglobin was determined using a Quantichrom™ Hemoglobin Assay Kit (BioAssay Systems, Hayward, CA). To determine the extent of binding, samples were analyzed by HPLC using a Discovery BIO GFC 150 column and BD-bound hemoglobin fractions were collected at 1-min intervals and radioactivity was determined by liquid scintillation counting as described above for albumin. The percentage of radioactivity associated with the hemoglobin peak was used to calculate the amount of radioactivity bound per mg hemoglobin. In addition to the analysis described above, for selected rat hemoglobin samples, globin was isolated from the dialyzed hemoglobin solution by adding 0.1% HCl in acetone followed by centrifugation at 3000g for 30 min at 4 °C. The globin pellet was washed three times with 0.5 mL ice cold acetone. The isolated globin was dried under vacuum, weighed, and analyzed for radioactivity as described above for albumin.
2.8. Investigation of Adduct formation in vitro and in vivo
To determine if radioactivity associated with hemoglobin and albumin in vitro and in vivo was a result of covalent adduct formation, BD was reacted with rat albumin and hemoglobin in vitro, and compared with albumin and hemoglobin from rats administered BD. An approach similar to that reported for hemoglobin by Gao and Wang (3) was used.
Male rat plasma was diluted 5x in PBS (pH 7.4) to give approximately 6.6 mg albumin/mL. Male rat RBCs were diluted 20x in PBS (pH 7.4) to give approximately 6.5 mg Hb/mL and centrifuged to remove cell membranes. BD was added to diluted plasma and hemoglobin for a final concentration of 4 mM. Corresponding controls were also prepared without the addition of BD. Samples were incubated for 24 h at 37 °C. After 24 h, the samples were split in half and one aliquot was filtered with YM-3 filters (Microcon) for 5 min, and the other for 60 min.
The modified hemoglobin and plasma were digested with either chymotrypsin (Promega, Madison, WI) or Lys-C/trypsin combination (enzyme:substrate 1:10 (w/w)) in 50 mM ammonium bicarbonate buffer at 37 °C. Chymotrypsin digestion was carried out for 24 h. Lys-C/trypsin digestions were performed in two stages: Lys-C digestion was performed overnight following which trypsin was added and the digestion was carried for an additional 24 h. Following digestion, all samples were dried on a Speed-Vac operating at 30 °C for 1 h. The dried peptides were reconstituted in 90:10 methanol:water for UPLC-MS/MS analysis.
Analyses were performed on an Orbitrap Velos mass spectrometer (Thermo, San Jose, CA) equipped with Waters Acquity UPLC system. Peptides were separated on an Acquity UPLC BEH300 C18 (1.7 μm × 2.1 × 100 mm) column operating at 45 °C. Mobile phases used were water with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B). Peptides were eluted using a 33-min gradient of 2–70% B at a flow rate of 0.4 mL/min. The mass spectrometer was operated in the positive ion mode. The FT mass analyzer was used to acquire the MS data at 7500 resolution and included settings of a mass range from m/z 200 to 2200, an ionization potential of 3.1 kV, an AGC target for 5 × 105 was set for FT full scan experiments. MS/MS data were acquired using a data-dependent acquisition using the ion trap as the mass detector and the AGC setting of 1 × 104 was used for the IT MSn experiments. The six most abundant ions from each MS survey scan further interrogated by MS/MS with dynamic exclusion for 19 sec following a repeat count of 3. A threshold of 3000 counts was set for the automated switching for MS/MS data acquisition.
All peptide and modified peptide identifications were performed using the Sequest HT algorithm of Proteome Discover 1.4 (Thermo, San Jose, CA) using the NCBI protein sequence for rat and mouse albumin and hemoglobin. The data extraction settings included limiting the data search to deconvoluted ions observed between 150 and 6000 Da. The MS/MS search function in Proteome Discover included an enzyme specificity with up to 4 missed cleavages allowed, a precursor ion mass tolerance of 0.8 Da, a product ion mass tolerance of 1.5 Da. Addition of butanedione (+BD, +86.0446 Da) and butanedione addition followed by water loss (+BD-H2O, +68.0262 Da) were custom built in Proteome Discover as chemical modifications of arginine residues and were used as variable modifications during peptide identification. MS/MS assignments for the identified peptides were manually validated.
2.9. Statistical Analysis
Comparisons of data obtained with globin and hemoglobin binding, and with albumin binding using two different isolation methods were conducted with a paired t-test, using Prism (version 5.00 for Windows, GraphPad Software, San Diego, CA).
3. Results
The dose of [14C]BD administered to male rats by instillation was close to the target dose of 100 mg/kg, with an actual dose ranging from 99.8–105 mg/kg (102 ± 1.86 mg/kg, mean ± SD). The radioactivity administered per rat was approximately 28–29 μCi (28.4 ± 0.250 μCi). The dose of [14C]BD administered to male mice by aspiration was approximately 157 mg/kg, and was subject to considerable variability because the animals weights varied and the dosing volume was kept constant at 50 μL. The actual dose ranged from 123–172 mg/kg (157 ± 19.3 mg/kg, mean ± SD). The radioactivity administered per mouse was approximately 8.61–12.0 μCi (10.7 ± 1.46 μCi).
The radioactivity determined in blood and in plasma at 24 h after dosing is shown in Table 1. A single time point at 24 h was chosen to provide blood samples where the majority of the unbound BD and metabolites would have been removed by metabolism. Since adducts in blood are proportional to the area under the curve for the reactive chemical, free BD in blood would have had the maximum opportunity to react with albumin and hemoglobin by 24 h. The concentration of 14C in blood was higher for male rats (17343 ± 905 ng equiv./g) compared with male mice (7118 ± 1850 ng equiv./g). Similarly, the concentration of 14C in plasma was higher for male rats (22466 ± 1882 ng equiv./g) compared with male mice (5468 ± 1248 ng equiv./g). The percentage of administered dose in blood 24 h following administration was calculated based on the estimated total blood volume of 5.4 and 8.5% of body weight in rat and mouse, respectively [14], and was 0.88% in rats and 0.38% in mice (Table 1). The percentage of the administered dose in plasma was approximately half that in blood for mice, and approximately three quarters in rats (Table 1).
Table 1.
Radioactive BD-equivalentsa in bloodb and plasmac 24 h following administration of [14C]BD via intratracheal instillation in male Harlan Sprague Dawley rats and oropharyngeal aspiration in B6C3F1 mice
| Blood (ng BD equiv./g) | % Dose in Blood | Plasma (ng BD equiv./g) | % Dose in Plasma | |
| Male Rat | 17343 ± 905 | 0.88 ± 0.07 | 22466 ± 1882 | 0.66 ± 0.06 |
| Male Mouse | 7118 ±1850 | 0.38 ± 0.13 | 5468 ±1248 | 0.17 ± 0.05 |
Values represent mean ± SD, n=6. Values determined from radioactivity in blood (dpm/g) divided by specific activity of BD (dpm/ng BD)
Percentage of the administered dose in rat and mouse blood was calculated assuming blood represents 5.4 and 8.5% of body weight in rat and mouse, respectively [14].
Percentage of the administered dose in rat and mouse plasma was calculated assuming a plasma volume of 3.12 and 5% of body weight, in rat and mouse, respectively [14].
To determine the extent of binding of 14C to albumin in rats and mice, albumin was isolated from plasma by TCA precipitation using a method reported by Jacobs et al. [13]. Prior to use of this method on samples from animals treated in vivo with BD, the ability of adducts to withstand the albumin isolation procedure was established using bovine serum albumin reacted in vitro with [14C]BD and purified using Centriprep Ultracel YM-50 centrifugal filter unit. Size exclusion HPLC of the purified [14C]BD-bovine serum albumin indicated that approximately 98% of the radioactivity was associated with albumin. Using the purified [14C]BD-bovine serum albumin, the recovery of [14C]BD-albumin through the albumin isolation and purification procedure was 79%, and when mixed with rat plasma was 62%. Size exclusion HPLC confirmed that the radioactivity remained associated with albumin. Because the TCA method use strong acid, a second method was tested for rat albumin isolation using ammonium sulfate precipitation. The albumin-bound radioactivity estimated using these methods in rats and mice administered [14C]BD is shown in Table 2. For rat albumin, there was good agreement between the TCA precipitation and ammonium sulfate precipitation method ensuring the stabililty of adducts . A paired t-test indicated no significant difference between albumin binding assessed by each method (p-value = 0.086).
Table 2.
RRadioactive BD-equivalentsa bound to albuminb 24 h following administration of [14C]BD via intratracheal instillation in male Harlan Sprague Dawley rats and oropharyngeal aspiration in B6C3F1 mice
| Radioactivity Bound (ng equiv./mg Albumin) | % of Dose Boundb | ||
| TCA Precipitation | Ammonium Sulfate Precipitation | ||
| Male Rats | 269 ± 24.2 | 261 ± 24.2 | 0.26 ± 0.02c |
| Male Mice | 85.0 ± 22.3 | ND | 0.090 ± 0.024 |
Values represent mean ± SD, n=6. Values determined from radioactivity bound to albumin (dpm/mg) divided by specific activity of BD (dpm/ng BD).
Calculated assuming, in rat and mouse, respectively, a plasma volume of 3.12 and 5% of body weight and an albumin concentration of 31.6 and 32.7 mg/mL plasma [14].
For rats, the data from TCA precipitation was used. ND = not determined.
The amount of BD bound to rat albumin was approximately 3-fold higher than that in mouse. Albumin binding in rats was 269 ± 24.2 ng equiv./mg, which accounts for 38% of the radioactivity in plasma. In mice, binding was lower than observed in rats and was 85.0 ± 22.3 ng equiv./mg albumin, which accounts for 51% of the radioactivity in plasma. Hence, in rats and mice, 0.26 and 0.09%, respectively, of the dose administered was associated bound to albumin.
Red blood cells incubated in vitro with [14C]BD contained a radioactive peak that chromatographed with a single major UV-absorbing peak (hemoglobin) on size exclusion chromatography, and could be readily separated from unbound [14C]BD. Based on retention time, this indicated the binding of [14C]BD to hemoglobin. This method was used to investigate the binding of [14C]BD in hemoglobin from animals administered [14C]BD following dialysis to remove small molecules (Table 3). Similar to that observed with albumin, the amount of BD bound to rat hemoglobin was approximately 3-fold higher than that in mouse. The binding to hemoglobin in the rat was 38.2 ± 17.6 ng equiv./mg hemoglobin and in mouse was 16.2 ± 9.0 ng equiv./mg. Therefore, in rats and mice, 0.30 and 0.14%, respectively, of the dose administered was bound to hemoglobin. Radioactivity bound to globin in rats administered [14C]BD was also determined following isolation of globin using precipitation with acidic acetone (Table 3). The mean value estimated for globin was 29.1 ± 3.96 ng equiv./mg globin and for hemoglobin was 38.2 ± 17.6 ng equiv./mg hemoglobin. A paired t-test indicated no significant difference between hemoglobin and globin binding (p-value = 0.297).
Table 3.
Radioactive BD-equivalentsaa bound to hemoglobin and globin (rats only) 24 h following administration of [14C]BD via intratracheal instillation in male Harlan Sprague Dawley rats and oropharyngeal aspiration in B6C3F1 mice
| Radioactivity Bound (ng equiv./mg Hemoglobin) | Radioactivity Bound (ng equiv./mg Globin) | % of Dose Boundb | |
| Male Rats | 38.2 ± 17.6 | 29.1 ± 3.96 | 0.30 ± 0.14 |
| Male Mice | 16.2 ± 9.0 | ND | 0.14 ± 0.08 |
Values represent mean ± SD, n=6. Values determined from radioactivity bound to hemoglobin or globin (dpm/mg) divided by specific activity of BD (dpm/ng BD)
Calculated using a blood volume of 5.4% (rats) and 8.5% (mice) of body weight and a hemoglobin concentration of 150 mg/mL (Davies and Morris, 1993). ND = not determined.
To determine whether BD was covalently bound to globin and albumin, following reaction with BD in vitro and in rats administered BD in vivo, analysis of globin and albumin peptides was conducted using UPLC-MS/MS analysis. The expected modifications are at arginine residues, which is one of the target sites for cleavage for trypsin, the most commonly used enzyme for proteolysis. Two enzyme systems were used: chymotrypsin, which cleaves peptides adjacent to large hydrophobic amino acids; and Lys C which cleaves adjacent to lysine, followed by trypsin which cleaves adjacent to lysine and arginine. After peptide hydrolysis and chromatography, peptides were analyzed by UPLC-MS/MS with high resolution. Unmodified proteins were compared with BD reacted proteins. The data acquired were analyzed by MS/MS searching for peptides modified at arginine residues by addition of butanedione (+BD, +86.0446 Da) and butanedione addition followed water loss (+BD-H2O, +68.0262 Da). These modifications were consistent with adducts described previously for the reaction of arginine with BD [8].
Hemoglobin has 6 arginine residues, with three each in the alpha and beta chains. Peptides corresponding to modifications on alpha chain at R-92, and on the beta chain at R-30 and R-104 were detected in vitro (Table 4). With peptides from hemoglobin isolated from in vivo treatment, modifications at the R-31 on the alpha chain and at R-30 and R-104 on the beta chain were detected (Table 5). An example analysis of a peptide from hemoglobin modified with BD is shown in Figure 1, with the spectrum of the unmodified peptide (top), the in vitro modified peptide (middle) and the peptide from a rat administered BD (bottom). The peptide corresponded to RLLGNMIVIVLGHHL, β104–115, with modification at arginine-104. The structure of the proposed adduct of BD with arginine is given in Figure 2.
Table 4.
LC-MS/MS Analysis of arginine modifications in hemoglobin in vitro
| Rat Hemoglobin in vitro | Unmodified Peptide | Modified Peptide | |||||||
| Arginine Position | Digestion | Peptide | Calculated m/z [M+H+] | Charge State | Measured m/z | Calculated m/z [M+H+] | Charge State | Measured m/z | Adduct |
| R-92, αa | Cb | RVDPVNF | 846.448 27 |
+1 | 846.448 27 |
933.482 23 |
+2 | 467.244 75 |
86.044 60 |
| R-92, α | C | RVDPVNFKFLSHCL | 1762.82 792 |
+3 | 588.280 82 |
86.044 60 |
|||
| R-92, α | C | STLSDLHAHKLRVDPVNF | 2135.98 647 |
+4 | 534.752 08 |
86.044 60 |
|||
| R-30, β | C | WGKVNADNVGAEALGRL | 1856.99 503 |
+3 | 619.669 86 |
86.044 60 |
|||
| R-104, β | Lys C/Tc | LHVDPENFR | 1126.56 092 |
+3 | 376.191 82 |
1212.59 788 |
+3 | 404.870 81 |
86.044 60 |
| R-104, β | C | HVDPENFRLL | 1240.78 891 |
+2 | 620.898 09 |
1307.72 798 |
+2 | 654.367 63 |
68.026 20 |
| R-104, β | C | RLLGNMIVIVLGHHL | 1685.00 953 |
+3 | 562.341 36 |
1753.93 742 |
+2 | 877.472 35 |
68.026 20 |
Table 5.
LC-MS/MS Analysis of arginine modifications in hemoglobin in vivo
| Rat Hemoglobin in vivo | Unmodified Peptide | Modified Peptide | |||||||
| Arginine Position | Digestion | Peptide | Calculated m/z [M+H+] | Charge State | Measured m/z | Calculated m/z [M+H+] | Charge State | Measured m/z | Adduc |
| R-31, αa | Lys C/Tb | NCWGKIGGHGGEYGEEALQR | 2231.19 267 |
+2 | 1116.09 998 |
68.026 20 |
|||
| R-30, β | Cc | VNADNVGAEALGRL | 1856.99 503 |
+2 | 677.851 93 |
68.026 20 |
|||
| R-104, β | Lys C/T | GTFAHLSELHCDKLHVDPENFR | 2652.19 831 |
+5 | 531.245 48 |
86.044 60 |
|||
| R-104, β | C | RLLGNMIVIVLGHHL | 1685.00 953 |
+3 | 562.34 136 |
1753.93 742 |
+2 | 877.472 35 |
68.026 20 |
Figure 1.
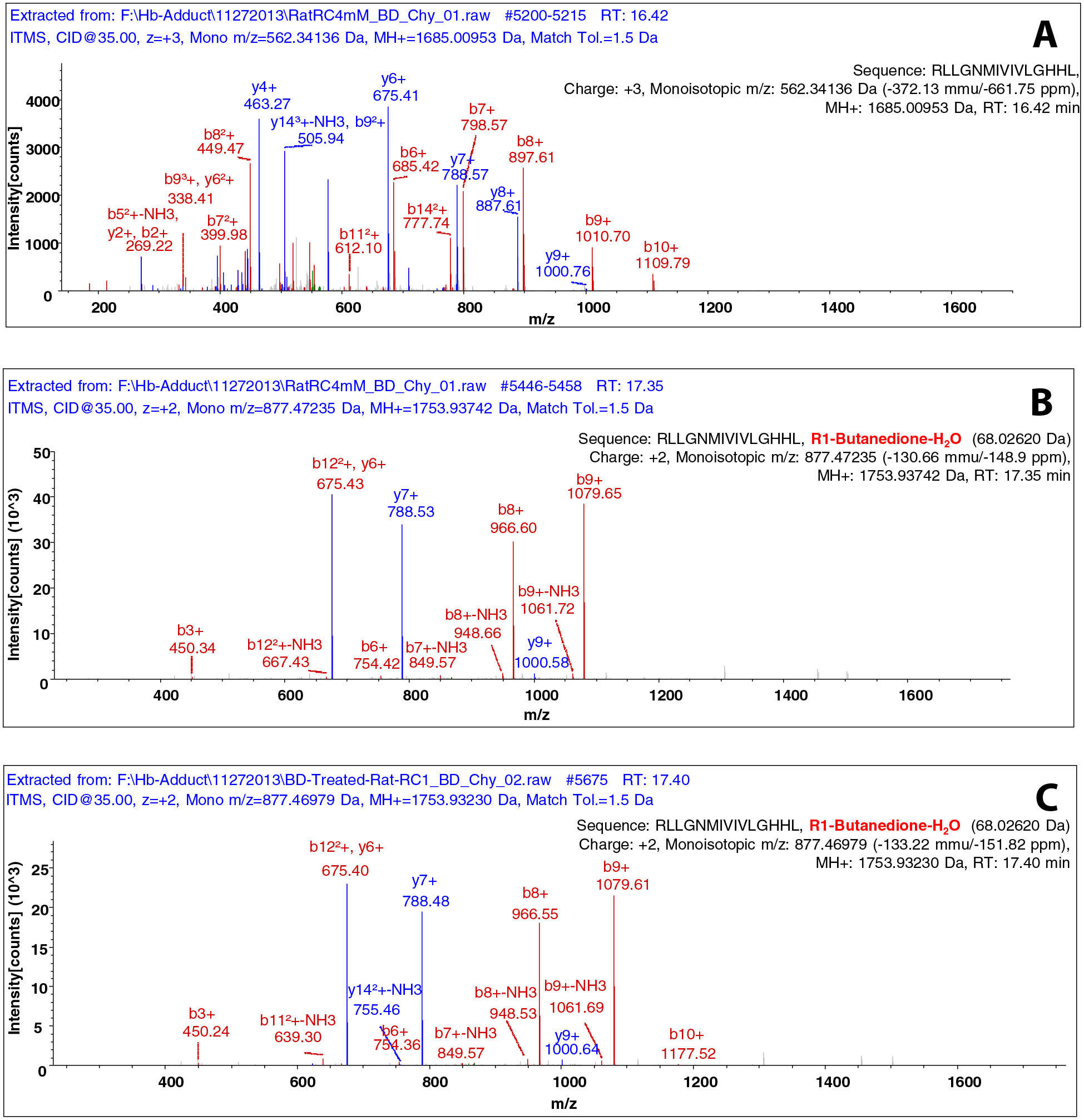
LC-MS/MS analysis of peptides modified in vitro and in vivo by BD. Mass spectrum of the globin peptide RLLGNMIVIVLGHHL, β104–115 following chymotrypsin digestion: unmodified (A), from reaction in vitro with BD (B) and from a rat administered BD in vivo (C).
Figure 2.
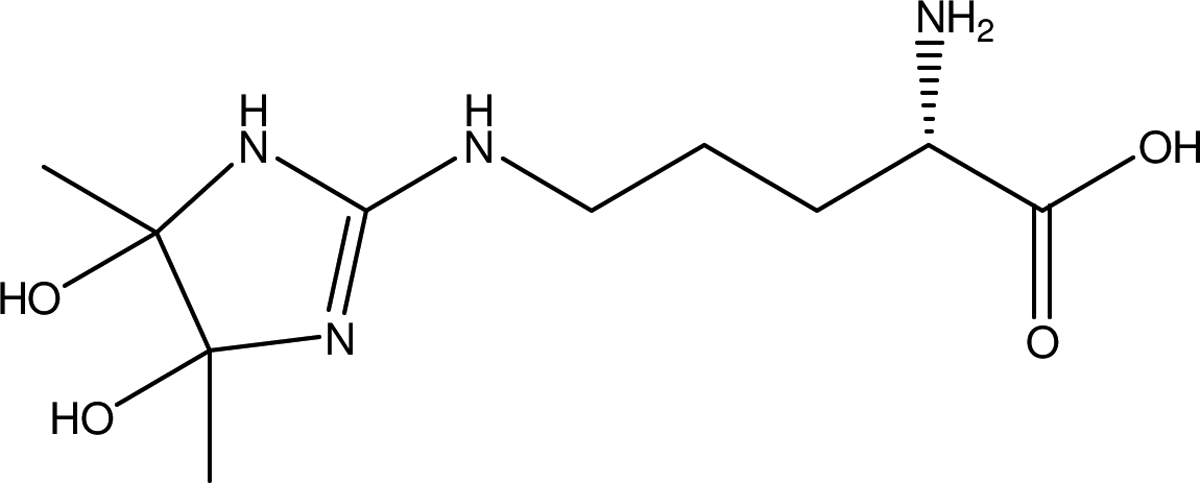
Proposed structure for the adduct of BD with Arginine, resulting in an increase of 86 Da
Albumin contains substantially more arginine residues than hemoglobin. Investigations with in vitro modified albumin indicated reaction with R-219 or 221, R-360, and R-368 (Table 6). With in-vivo modified albumin, a considerable number of modified peptides were detected, with modifications at R-34, R-168 or R-169, R-360, R-368, R-434, R-453, R-454, R-493, and R-498 (Table 7).
Table 6.
LC-MS/MS Analysis of arginine modifications in albumin in vitro
| Rat Albumin | Unmodified Peptide | Modified Peptide | |||||||
| Arginine Position | Peptide | Calculated m/z [M+H+] | Charge State | Measured m/z | Calculated m/z [M+H+] | Charge State | Measured m/z | Adduct | |
| R-219, 221 | Lys C/Tb | EKALVAAVRQRMK | 1672.87041 | +2 | 836.93884 | 86.04460 | |||
| R-360 | Lys C/T | DVFLGTFLYEYSR | 1609.78569 | +2 | 805.39648 | 1696.83586 | +2 | 848.92157 | 86.04460 |
| R-368 | Lys C/T | RHPDYSVSLLLRLAK | 1855.93998 | +3 | 619.31818 | 86.04460 | |||
Uniprot accession number P02770.
Lys C/T, Lys/C digestion followed lowed by trypsin digestion.
Table 7.
LC-MS/MS Analysis of arginine modifications in albumin in vivo
| Rat Albumin | Unmodified Peptide | Modified Peptide | |||||||
| Arginine Position | Peptide | Calculated m/z [M+H+] | Charge State | Measured m/z | Calculated m/z [M+H+] | Charge State | Measured m/z | Adduct | |
| R-34 | Lys C/Tb | EAHKSEIAHR | 1263.78020 | +2 | 632.3937 | 86.04460 | |||
| R-168, 169 | Cc | HEVARRHPYFY | 1611.79130 | +2 | 806.39929 | 68.02620 | |||
| R-360 | C | YEYSRRHPDY | 1471.74955 | +3 | 491.25470 | 86.04460 | |||
| R-368 | C | LLRLAKKYEATL | 1505.76543 | +2 | 753.38635 | 86.04460 | |||
| R-434 | C | GFQNAVLVRY | 1252.71697 | +2 | 626.862 | 86.04460 | |||
| R-452 | Lys C/T | APQVSTPTLVEAAR | 1439.78162 | +3 | 480.59872 | 1526.74333 | +2 | 763.8753 | 86.04460 |
| R-454 | C | GRVGTKCCTLPEAQRL | 1818.84767 | +2 | 909.92747 | 86.04460 | |||
| R-483 | C | LSAILNRLCVL | 1283.73 | +2 | 642.37183 | 68.02620 | |||
| R-508 | Lys C/T | TPVSEKVTKCCSGSLVER | 2009.92966 | +3 | 670.64807 | 86.04460 | |||
Uniprot accession number P02770.
Lys C/T, Lys/C digestion followed by trypsin digestion;
C, chymotrypsin digestion.
4. Discussion
In these studies we investigated the feasibility of developing a biomarker for exposure to BD using either albumin or hemoglobin adducts. These results demonstrate that BD can bind in vitro with albumin and hemoglobin, and suggest that BD administered to rats and mice is absorbed from the lungs, enters the systemic circulation and covalently binds with albumin and hemoglobin. The isolation of albumin using strong acidic conditions such as TCA precipitation method did not change the level of bound radioactivity compared to the ammonium sulfate method (p-value = 0.086). This suggests that BD-derived radioactivity is covalently bound to albumin and hemoglobin in a manner that is sufficiently stable to enable isolation of proteins. Incubation of albumin and hemoglobin with BD in vitro also supports the covalent binding hypothesis. This was further supported by companion studies conducted on the reaction of BD with N-α-acetylarginine indicating that multiple adducts are formed by reaction with the guanidino group resulting in predominantly a cyclized and an open chained product supporting that the binding observed in vivo may have been due to formation of adducts [9]. The demonstration of peptides modified at arginine in both hemoglobin and albumin both in vitro and in vivo in this study supports the covalent binding hypothesis, confirming that adducted arginine residues can be observed on the proteins that contain radioactivity derived from BD.
Methylglyoxal, which is structurally similar to BD, has been reported to react with arginine residues in albumin [8] and in hemoglobin [6]. Methylglyoxal is involved in the generation of advanced glycation endproducts that are implicated in complications of diabetes, cataracts, and atherosclerosis [15]. The main adduct formed by reaction of methylglyoxal with arginine in albumin is a hydroimidazolone: Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine (MG-H1) [8], and the same adduct is formed in hemoglobin [6]. MG-H1 is produced as a protein degradation product that can be quantitated in urine and plasma by LC-MS, and is elevated in diabetics [16]. The MG-H1 adduct is formed in collagen, and has been described in treatment of human microvascular endothelial cells in culture with methylglyoxal [17]. This adduct is formed at arginine hotspots in collagen, resulting in impaired integrin binding, and causing impaired extracellular matrix attachment, viability and angiogenic activity. Glyoxal has also been investigated for its reaction with hemoglobin, and modifications at arginine residues have been described recently in human hemoglobin at Arg 31α, Arg-40β, and Arg-104β [7]. Dicarbonyl compounds including methylglyoxal and glyoxal have been implicated in modifications of proteome, lipidome, and genome [18]. It has been suggested that dicarbonyl induced glycation, oxidative stress, and inflammatory responses may contribute to the etiology of human neurodegenerative diseases [19]. A recent publication has described the covalent interaction of BD with β-amyloid, accompanied by an exacerbation of toxicity in SH-SY5Y cells. Covalent binding of BD to the Arg5 of Aβ1−42 resulted in a conformation change thought to enhance its cytotoxicity. In addition, it was demonstrated that BD could cross an MDR1-MDCK cell monolayer, a model for the blood-brain barrier [20]. BD has been detected in plasma (0.25–0.75 nmol/mL) of volunteers consuming alcohol [21]. It has been proposed that reaction of acetaldehyde, a metabolite of ethanol, with pyruvate in the presence of thiamine forms acetoin which can undergo reduction to 2,3-butanediol, or oxidation to BD [22].
The studies reported here suggest that it may be feasible to develop a biomarker for exposure to BD using either albumin or hemoglobin adducts. We have shown that about 38 to 45% of the radioactivity in plasma is bound to albumin. In addition, the expected long lifespan of adducts in hemoglobin and albumin provide enhanced accumulation and persistence, enabling sensitive measurement of exposure and internal dose days to weeks following exposure [3, 23]. Additional studies are needed to determine if BD adducts can be detected in the blood following inhalation exposure.
2,3-Butanedione plays a major role in obliterative bronchiolitis in workers in microwave popcorn manufacturing.
BD enters systemic circulation following intratracheal instillation in rats and oropharengeal aspiration mice.
Significant fraction of systemic dose was bound to hemoglobin and albumin to form adducts.
One of the major sites of adduction was on arginine residues.
5. Acknowledgements
The authors are grateful to Drs. Helen Cunny and Aris Martone for their review of this manuscript, and Ms. Kathy Ancheta for her assistance in preparation of the manuscript. This work was performed for the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health, U.S. Department of Health and Human Services, under contract No. N01-ES-75563 (HHSN29120077563).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL, Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant, N Engl J Med, 347 (2002) 330–338. [DOI] [PubMed] [Google Scholar]
- [2].NIEHS, Disposition and Excretion of [14C]2,3-Butanedione in Male Rats following Oral Administration. Final Study Report (RTl/64U-6855/06P). issue Date: September 8, 1997, (1997).
- [3].Osterman-Golkar S, Ehrenberg L, Segerback D, Hallstrom I, Evaluation of genetic risks of alkylating agents. II. Haemoglobin as a dose monitor, Mutat. Res, 34 (1976) 1–10. [DOI] [PubMed] [Google Scholar]
- [4].Farmer PB, Monitoring of human exposure to carcinogens through DNA and protein adduct determination, Toxicol Lett, 82-83 (1995) 757–762. [DOI] [PubMed] [Google Scholar]
- [5].Yankeelov JA, Modification of Arginine in Proteins by Oligomers of 2,3-Butanedione, Biochemistry, 9 (1970) 2433–2439. [DOI] [PubMed] [Google Scholar]
- [6].Gao Y, Wang Y, Site-selective modifications of arginine residues in human hemoglobin induced by methylglyoxal, Biochemistry, 45 (2006) 15654–15660. [DOI] [PubMed] [Google Scholar]
- [7].Banerjee S, Chakraborti AS, Structural alterations of hemoglobin and myoglobin by glyoxal: a comparative study, Int J Biol Macromol, 66 (2014) 311–318. [DOI] [PubMed] [Google Scholar]
- [8].Ahmed N, Dobler D, Dean M, Thornalley PJ, Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity, J Biol Chem, 280 (2005) 5724–5732. [DOI] [PubMed] [Google Scholar]
- [9].Mathews JM, Watson SL, Snyder RW, Burgess JP, Morgan DL, Reaction of the butter flavorant diacetyl (2,3-butanedione) with N-alpha-acetylarginine: a model for epitope formation with pulmonary proteins in the etiology of obliterative bronchiolitis, J Agric Food Chem, 58 (2010) 12761–12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Morris JB, Hubbs AF, Inhalation dosimetry of diacetyl and butyric acid, two components of butter flavoring vapors, Toxicol Sci, 108 (2009) 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gloede E, Cichocki JA, Baldino JB, Morris JB, A validated hybrid computational fluid dynamics-physiologically based pharmacokinetic model for respiratory tract vapor absorption in the human and rat and its application to inhalation dosimetry of diacetyl, Toxicol Sci, 123 (2011) 231–246. [DOI] [PubMed] [Google Scholar]
- [12].Institute for Laboratory Animal Research, Guide for the Care and Use of Laboratory Animals, The National Academies Press, Washington, DC, 1996. [Google Scholar]
- [13].Jacobs R, Demmelmair H, Rittler P, Kellermann J, Koletzko B, Krick M, Jauch KW, Hartl WH, Isolation of plasma albumin by ethanol extraction is inappropriate for isotope ratio measurements during the acute phase response, J Chromatogr B Analyt Technol Biomed Life Sci, 817 (2005) 145–151. [DOI] [PubMed] [Google Scholar]
- [14].Davies B, Morris T, Physiological parameters in laboratory animals and humans, Pharm Res, 10 (1993) 1093–1095. [DOI] [PubMed] [Google Scholar]
- [15].Degenhardt TP, Thorpe SR, Baynes JW, Chemical modification of proteins by methylglyoxal, Cell Mol Biol (Noisy-le-grand), 44 (1998) 1139–1145. [PubMed] [Google Scholar]
- [16].Ahmed N, Babaei-Jadidi R, Howell SK, Thornalley PJ, Beisswenger PJ, Glycated and oxidized protein degradation products are indicators of fasting and postprandial hyperglycemia in diabetes, Diabetes Care, 28 (2005) 2465–2471. [DOI] [PubMed] [Google Scholar]
- [17].Dobler D, Ahmed N, Song L, Eboigbodin KE, Thornalley PJ, Increased dicarbonyl metabolism in endothelial cells in hyperglycemia induces anoikis and impairs angiogenesis by RGD and GFOGER motif modification, Diabetes, 55 (2006) 1961–1969. [DOI] [PubMed] [Google Scholar]
- [18].Thornalley PJ, Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems--role in ageing and disease, Drug Metabol Drug Interact, 23 (2008) 125–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Williams WM, Weinberg A, Smith MA, Protein modification by dicarbonyl molecular species in neurodegenerative diseases, J Amino Acids, 2011 (2011) 461216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].More SS, Vartak AP, Vince R, The butter flavorant, diacetyl, exacerbates beta-amyloid Chem Res Toxicol, 25 (2012) 2083–2091. [DOI] [PubMed] [Google Scholar]
- [21].Otsuka M, Harada N, Itabashi T, Ohmori S, Blood and urinary levels of ethanol, acetaldehyde, and C4 compounds such as diacetyl, acetoin, and 2,3-butanediol in normal male students after ethanol ingestion, Alcohol, 17 (1999) 119–124. [DOI] [PubMed] [Google Scholar]
- [22].Otsuka M, Mine T, Ohuchi K, Ohmori S, A detoxication route for acetaldehyde: metabolism of diacetyl, acetoin, and 2,3-butanediol in liver homogenate and perfused liver of rats, Journal of Biochemistry, 119 (1996) 246–251. [DOI] [PubMed] [Google Scholar]
- [23].Fennell TR, Sumner SC, Walker VE, A model for the formation and removal of hemoglobin adducts, Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 1 (1992) 213–219. [PubMed] [Google Scholar]


