Abstract
Vinpocetine is being used worldwide by people of all ages, including pregnant women, for its purported multiple health benefits. However, limited data is available addressing the safety/toxicity of vinpocetine. The National Toxicology Program conducted studies to examine potential effects of vinpocetine on the developing rat. Disposition data is helpful to put the fetal findings into context and provide information on the potential risk for humans.
The current study reports the systemic exposure and toxicokinetic (TK) parameters of vinpocetine and metabolite, apovincaminic acid (AVA), in pregnant Harlan Sprague Dawley rats, fetuses and amniotic fluid following oral gavage exposure of dams to 5 and 20 mg/kg vinpocetine from gestational day 6 to 18. Vinpocetine was absorbed rapidly in dams with a maximum plasma concentration (Cmax) reaching ≤ 1.37h. Predicted Cmax and area under the concentration versus time curve (AUC) increased less than proportionally to the dose. Vinpocetine was rapidly distributed to the peripheral compartment. More importantly, significant transfer of vinpocetine from dam to fetuses was observed with fetal Cmax and AUC ≥ 55% of dams. Vinpocetine was cleared rapidly from dam plasma with an elimination half-life of ≤ 4.02h with no apparent dose-related effect. Vinpocetine was rapidly and highly metabolized to AVA with AVA plasma levels in dams ≥ 2.7-fold higher than vinpocetine, although in the fetuses, AVA levels were much lower than vinpocetine. Comparison of current rat data with literature human data demonstrates that systemic exposure to vinpocetine in rats following repeated exposure to 5mg/kg is similar to that following a single human relevant dose of 10mg suggesting that the findings from the toxicology study may be relevant to humans.
Introduction
Vinpocetine (apovincaminic acid ethyl ester) (Figure 1A) is a semi-synthetic derivative of vincamine, an alkaloid extract derived from the periwinkle plant (Vinca minor). Vinpocetine is available as a prescription pharmaceutical in Europe, Russia, China, and Japan, for treatment of cerebrovascular and cognitive disorders (Bereczki and Fekete, 2008). In the United States, although not considered an approved dietary ingredient by Food and Drug Administration, it is available as a dietary supplement with suggested use for claims of cognitive enhancement (Thal et al., 1989; Szatmari and Whitehouse, 2009; Peruzza and Dejacobis, 1986; Maconi et al., 1986; Feigin et al., 2001; Bereczki and Fekete, 2008). Recent marketing promotes the use of vinpocetine for additional purported benefits, including motion sickness, menopausal symptoms, tinnitus, visual impairment, and seizure disorders, among others; hence, there is potential use of vinpocetine by all age levels, including pregnant women and women of childbearing potential (Anonymous, 1984; Sitges et al., 2016; Taiji and Kanzanki, 1986; Thorne et al., 2002; Truss et al., 2000). A variety of brands and supplements labelled as vinpocetine are available on the United States market; there are currently over 300 products being tracked by the NIH Dietary Supplement Label Database (NIH, 2017). The recommended daily dose in humans can range from 5 to 30 mg according to current product labels. Due to its primary use as a dietary supplement, human exposure to vinpocetine typically occurs through oral consumption.
Figure 1.
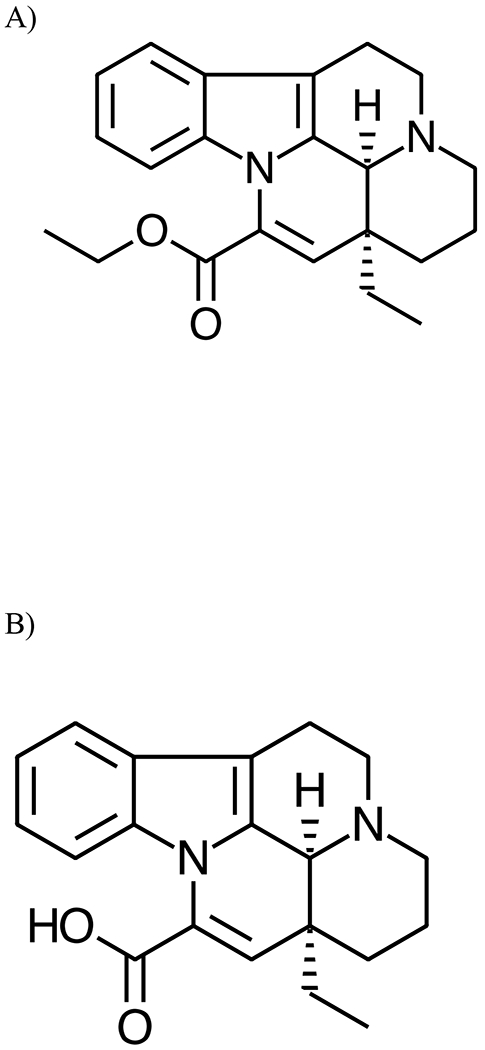
Structure of A) Vinpocetine (apovincaminic acid ethyl ester) and B) its Major Metabolite, Apovincaminic Acid (apovincaminic-22-oic acid).
There are limited toxicity data for vinpocetine in the literature. The oral median lethal dose (LD50) of vinpocetine in rats (strain not specified) is approximately 500 mg/kg (Cholnoky and Dömök, 1976; Palosi and Szporny, 1976). Toxicity of vinpocetine was investigated by Cholnoky and Dömök in a series of studies in rats (strain not specified) (Cholnoky and Dömök, 1976). Briefly, in a subchronic gavage study following exposure to vinpocetine at doses between 25 and 100 mg/kg increased salivation, liver, and thyroid weights were observed at the highest dose. Following intraperitoneal injection of 5 or 25 mg/kg for 3 months, mortality was reported. In chronic gavage study, at doses between 25 and 100 mg/kg, no adverse effects were reported (Cholnoky and Dömök, 1976). Although authors report that there are no reproductive or teratological effects following oral administration of 10 or 50 mg/kg vinpocetine in rats, the effects reported included uterine bleeding and fetal death (Cholnoky and Dömök, 1976).
Absorption, distribution, metabolism, and excretion (ADME) properties of vinpocetine has been characterized in animals and humans. Vinpocetine is rapidly absorbed, following a single oral administration in rats with peak plasma and tissue concentrations reaching ≤ 2h (summarized in Vereczkey, 1985; Sozaneski et al., 2012; Xia et al., 2010). In Wistar rats, following administration of [3H]vinpocetine, approximately 47% and 34%, respectively, of the dose was recovered in urine and feces at 48h following administration; < 5% was recovered in bile within 9h (Vereczkey et al., 1976; Vereczkey and Szporny, 1976). Highest radioactivity was recovered in the liver and small intestine, followed by the lung, stomach, kidney, and adrenals. With the exception of the liver and kidneys, residual radioactivity in tissues returned to minimal levels within 48h following administration. Urinary and fecal excretion of vinpocetine in rats was similar following a 5d repeat oral exposure. The plasma elimination half-life of vinpocetine in rats was ≤ 3h (Vereczkey et al., 1979a; Sozaneski et al., 2012; Xia et al., 2010). In rats, oral bioavailability of vinpocetine was 52% suggesting extensive first pass metabolism following oral administration (Vereczkey et al., 1979a). Absorption was also rapid in New Zealand White rabbits following oral administration, with a peak plasma concentration reaching ≤ 2 h (Xu et al., 2009; Nie et al., 2006; Ribiero et al., 2004); the plasma elimination half-life (approximately 6-14 h) was dependent on the type of formulation used (Ribiero 2004). In humans, similar to animals, absorption of vinpocetine following ingestion was fast with a maximum plasma concentration reached within ≤ 2h after ingestion with a plasma elimination half-life of ≤ 2 h (Elbary et al., 2002; Grandt et al., 1989; Miskolczi et al., 1990; Lohmann et al., 1992; Vereczkey et al., 1979b). The reported oral bioavailability in humans varies from 6.7 to 57% (Grandt et al., 1989; Vereczkey et al., 1979b).
The main metabolite of vinpocetine identified in the rat urine was apovincaminic acid (apovincaminic-22-oic acid) (AVA) (~ 75% of urinary excretion), arising from deesterification of vinpocetine (Figure 1B) (Vereczkey et al., 1979a; Vereczkey and Szporny, 1976). Formation of AVA following oral administration of vinpocetine in rats was rapid with a highest plasma concentration observed around 1h after administration with an elimination half-life between 3 and 10h (Vereczkey et al., 1979a; Xia et al., 2010).
Vinpocetine was nominated for testing to the National Toxicology Program because of consumer exposure through dietary supplement use, suspicion of toxicity, and lack of adequate toxicological data. As part of this assessment, the NTP conducted studies to examine potential effects of vinpocetine on developing rat following exposure of Hsd:Sprague Dawley® SD®) (HSD) rat dams beginning on gestational day (GD)6. Disposition data is helpful to put the presence (or absence) of fetal findings into context, and provide critical information on the potential risk for humans. Although there are some ADME data in the literature in rats as summarized above, no studies could be identified that examine ADME following repeated oral administration of vinpocetine in rats or in pregnant animals investigating the gestational transfer of vinpocetine. Therefore, the present study was designed to determine the systemic exposure and other TK parameters of vinpocetine and its major metabolite, AVA, in HSD rat dams and fetuses following exposure of dams from GD6 to 18, the same dosing paradigm used in NTP toxicology studies. Dose levels of 5 and 20mg/kg were selected to support the NTP toxicity study.
Materials and Methods
Chemicals and Reagents.
Vinpocetine (VIN, lot # VA201211001) was obtained from Maypro Industries LLC (Purchase, NY). The chemical identity of the lot was confirmed by infrared spectroscopy, proton and carbon-13 nuclear magnetic resonance spectroscopy and optical rotation analyses. The purity was determined to be > 99% based on both gas chromatography with flame ionization detection and high-performance liquid chromatography with ultraviolet detection (230nm). The lot was also analyzed by differential scanning calorimetry (approximate purity 99.9%, melting transition 149.9°C), for optical rotation (+131.6°) and by Karl-Fischer titrimetry for water content (< 0.07%). AVA and deuterated vinpocetine (d5-vinpocetine) with deuterium on the ethyl ester (chemical purity 99.7%; isotopic purity, 99.5%) was obtained from Toronto Research Chemicals Inc. (Ontario, Canada). Control HSD rat matrices for analytical method validation and matrix calibration curves were obtained from Bioreclamation IVT Inc. (Westbury, NY). Well plates for sample preparation using protein precipitation (96-well Impact™) were purchased from Phenomenex, (Torrance, CA). All other chemicals and reagents were procured from commercial sources.
Analytical Method Validation.
Methods using protein precipitation followed by liquid chromatography coupled with tandem mass spectrometry (LC/MS/MS) was used to quantitate vinpocetine and AVA in GD 18 plasma, amniotic fluid and fetal homogenate. Both Vinpocetine and AVA were quantitated in a single method. Fetal homogenate was prepared by homogenizing the pre-weighed frozen fetus using Covaris CryoPrep CP02 system (Woburn, MA). Methods were validated in GD 18 dam plasma and fetal homogenate. A partial validation was performed for amniotic fluid using the validated plasma method. The validation included an assessment of linearity, inter- and intra-day precision (estimated as relative standard deviation, RSD), inter- and intra-day accuracy (estimated as standard error, RE), absolute recovery, experimental limits of quantitation (LOQ) and detection (LOD).
Stock solutions of vinpocetine and AVA were prepared in methanol and further diluted in methanol to generate concentrations of standards in the working range. Stock solution of d5-vinpocetine (internal standard) was prepared in methanol and diluted in 0.2% formic acid in methanol to generate a working internal standard solution. A six-point combined vinpocetine and AVA solvent calibration curves, in the range of 0.5 to 100ng/mL per each analyte, were prepared using alternate stock solutions by diluting working standard solutions in 0.2% formic acid in methanol. Six-point matrix calibration curves were prepared by adding vinpocetine and AVA to respective blank matrix, using alternate stock solutions, to achieve final concentrations of each analyte in the range of 0.5 to 100ng/mL for plasma and amniotic fluid and 5 to 1000ng/g for fetal homogenate. Quality control (QC) samples to cover low, mid, and high end of the matrix calibration curves, were prepared similar to matrix standards but using an independent stock solution from above.
One hundred (100) μL of matrix (plasma, amniotic fluid or fetal homogenate) calibration standards, matrix blanks or QC samples were transferred into individual wells of a 96-well plate. To each well, 400μL of 25ng/mL d5-vinpocetine (in 0.2% formic acid in methanol) was added. The plate was covered, mixed by vortex for 2min, and allowed to stand for 10min at room temperature. The well plate was placed on a collection plate and 3–5psi of pressure was applied for approximately 2min. The collection plate was removed, a pierceable cover was placed and samples were analyzed by LC/MS/MS as described below.
Linearity, accuracy, precision (inter- and intraday), and absolute recovery were estimated for all matrices as appropriate. Dilution verification was conducted to demonstrate that concentrations outside the validated range could be accurately quantitated after dilution. Absolute recovery was calculated by comparing the response of matrix standards to the response of the solvent standard of similar concentration.
Stability of vinpocetine and AVA in extracted samples was evaluated in samples stored at ambient temperature and following three freeze-thaw cycles. Stability of the analytes in respective matrix was evaluated following three freeze-thaw cycles and in samples stored at −70°C for up to 61d to cover the study sample storage condition and duration.
LC-MS/MS Analysis.
All samples were analyzed by LC/MS/MS using a Shimadzu Prominence (Kyoto, Japan) LC coupled to a Sciex API 5000 (Ontario, Canada) mass spectrometer. A Phenomenex Kinetix column (C18, 2.6μm, 2.1mm x 100mm) was used for analyte separation. Mobile phases used were 0.2% aqueous formic acid (A) and 0.2% formic acid in acetonitrile (B) with a linear gradient from 10% B to 90% B in 5min at a flow rate of 0.35mL/min. The turboionspray ion source was operated in the positive ion mode with a source temperature of 250°C and an ion spray voltage of −3400V. Transitions monitored were m/z 351→280 for vinpocetine, 356→280 for d5-vinpocetine and 323→280 for AVA. The retention times were approximately 4.4, 4.4 and 3.6min for vinpocetine, d5-vinpocetine and AVA, respectively. Analytes were quantitated as described below.
Animals and Animal Maintenance.
Animal studies were conducted at Battelle Memorial Institute (Columbus, OH) and were approved by the Institutional Animal Care and Use Committee. Animals were housed in a facility (JM10, West Jefferson, Ohio) that is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Animal procedures were in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Research Council, 2011). Studies were conducted in compliance with the Food and Drug Administration Good Laboratory Practice Regulation (FDA, 1987).
Timed-mated female Hsd:Sprague Dawley® SD®)Rats (HSD) were obtained from Harlan laboratories (Indianapolis, IN). The day of mating was considered as GD0. Animals were quarantined immediately upon receipt at the study laboratory for up to 1 week. Animals were distributed randomly into groups based on body weights (range 188.4 to 252.9g) using Provantis software (Instem, Version 8.6.1.2) and individually housed in solid bottom polycarbonate cages suspended on stainless steel racks with Sani-Chips® hard wood bedding (P.J. Murphy Forest Products Corp., Montville, NJ). Food (irradiated and certified, NIH-07 wafer feed, Zeigler Brothers, Inc. Gardners, PA) and water (City of West Jefferson, Ohio) were provided ad libitum. Water was analyzed annually per Battelle’s standard operating protocol. No known contaminants that would interfere with the study were found in feed or water. During the quarantine and study periods, room temperature was maintained between 69 to 75°F and relative humidity was maintained within 35 to 65%. A 12-h light/dark cycle was maintained during the quarantine and study period.
Dose Formulation Preparation and Analysis.
Dose formulations of vinpocetine were prepared in 0.5% aqueous methylcellulose. A formulation method was validated (r ≥ 0.99; precision ≤ 5%; accuracy, ≤ ±10%) for the concentration range 0.1 to 200 mg/mL in 0.5% aqueous methylcellulose using a HPLC with ultraviolet detection. Prior to study start, stability of formulations was confirmed for up to 42 d, to be within 10% of day 0, when stored in sealed clear bottles in amber bags with Teflon-lined lids at room temperature or at 5°C. For the study, 1 and 4mg/mL concentrations were prepared and analyzed prior to dosing. All formulations were within 10% of the target concentration.
Study Design and Dose Administration.
Groups of dams were given a single daily oral dose of vinpocetine from GD6 to 18 at target doses of 5 or 20mg/kg. Doses were administered via intragastric gavage using a syringe equipped with a gavage needle (ball-tipped, 16-gauge) at a dosing volume of 5 mL/kg body weight. The volume administered was based on the body weights on the day of dosing. Following the last dose on GD18, animals were anesthetized using CO2/O2 (70:30) and approximately 3mL blood was collected via the vena cava or abdominal aorta from three dams at target times 0 (predose on GD18), 0.25, 0.5, 0.75, 1, 1.5, 2, 4, 8, 12 and 24h into tubes containing K3EDTA from 3 animals per timepoint. Immediately following collection, tubes were mixed gently and placed on wet ice. Plasma was isolated from blood within 30min of collection and kept on dry ice until transferred to an ultracold freezer (~ −70°C). Immediately following blood collection, each animal was exanguinated, the uterus was removed and amniotic fluid (pooled by the litter, approximately 1 mL) and fetuses (pooled by the litter) were collected. Samples were flash frozen in liquid nitrogen and stored on dry ice until transferred to an ultracold freezer (~ −70° C).
Study Sample Preparation and Analysis.
Study samples were prepared and analyzed similar to matrix standards as described above, using the validated analytical method, except that no standards of vinpocetine and AVA were added. Study samples that exceeded the matrix calibration range were diluted into the validated range using respective control matrix. Each sample set was bracketed by method blanks, matrix calibration standards and QC samples prepared at low and high ends of the calibration curve. Data from study samples were considered valid if: the matrix calibration curve was linear (r ≥ 0.99); at least 75% of matrix standards were within 15% of nominal (except at the LOQ where it was 20%); at least 67% of the QC samples were within 15% of nominal values.
Analyte Quantitation.
A linear regression with 1/X weighing was used to relate LC/MS/MS peak area response ratio of analyte to internal standard and concentration for plasma and amniotic fluid. A linear regression with 1/X2 weighing was used to relate LC/MS/MS peak area response ratio of analyte to internal standard and concentration for fetus homogenate. The concentration of each analyte was calculated using response ratio of analyte to internal standard, the regression equation, initial sample volume and dilution when applicable. All concentrations above LOD of the assay were reported. The concentration of analytes in plasma and amniotic fluid was expressed as ng/mL. The concentration of analytes in fetus homogenate was expressed as ng/g homogenate which is equivalent to ng/g fetus since no dilutions were made when fetal homogenate was prepared.
Study Sample Data Analysis.
Data sets were evaluated for aberrant concentrations and time points. Individual animal data were examined for acceptable agreement between target and actual collection times. All samples were collected within 6% of target for time points between 0 and 4 hours and within 15 min of target for time points between 4 and 24 h. Therefore, target time points were used for all analysis. Vinpocetine and AVA dam plasma, amniotic fluid and fetal homogenate concentrations that were above the LOD (Table 1) were used in TK analysis. All concentrations were evaluated to identify outliers by performing Q-tests. Based on these assessments, no values were eliminated from TK analysis.
Table 1.
Analytical Method Validationa and Stability Data for Vinpocetine and Apovincaminic Acid in Dam Plasma, Amniotic Fluid, and Fetal Homogenate
| Plasma | Amniotic Fluid | Fetal Homogenate | |
|---|---|---|---|
| Vinpocetine | |||
| Concentration Range (ng/mL or ng/g)b | 0.5 to 100 | 0.5 to 100 | 5-1000 |
| Linearity (r)c | ≥ 0.999 | 0.999 | 0.998 |
| LOQ (ng/mL or ng/g)d,e | 0.5 | 0.5 | 5 |
| LOD (ng/mL or ng/g)f | 0.05 | 0.05 | 0.7 |
| Accuracy (%RE) | |||
| Intra-day | −3.3 to 4.3 | −7.5 to 12.7 | −7.3 to 5.1 |
| Inter-day | −2.3 to 2.3 | NA | −2.0 to 2.7 |
| Precision (%RSD) | |||
| Intra-day | 0.7 to 9.6 | 0 to 4.7 | 0.5 to 8.9 |
| Inter-day | 1.7 to 6.1 | NA | 1.4 to 8.3 |
| Absolute Recovery (%)g | 89.6 to 124.4 | 81.2 to 102.0 | 103.8 to 150.3 |
| Dilution Verification, n=6 (% RE, %RSD)h | −7.9, 5.9 | 4.0, 2.3 | 6.9, 7.6 |
| Extracted Sample Stability, n=4 (%RE)i | |||
| Ambient temperature (7d)j | −7.5 to −12.9 | −0.2 to 12.4 | −8.8 to −0.4 |
| Freeze-thaw (3 cycles) | −16.5 to −6.0 | 0.4 to 8.6 | −8.8 to −2.9 |
| Matrix Stability (%RE)k | |||
| Freeze-Thaw (3 cycles) | −14.8 to −14.7 | −8.7 to −3.0 | 1.3 to 4.7 |
| Storage (61d) | −12.9 to −8.4 | −13.9 to −9.1 | 4.2 to 6.0 |
| Apovincaminic Acid | |||
| Concentration Range (ng/mL or ng/g)b | 0.5 to 100 | 0.5 to 100 | |
| Linearity (r)c | ≥ 0.995 | 0.997 | 0.998 |
| LOQ (ng/mL or ng/g)d,e | 0.5 | 0.5 | 5 |
| LOD (ng/mL or ng/g)f | 0.0567 | 0.140 | 0.777 |
| Accuracy (%RE) | |||
| Intra-day | −16.3 to 16.3 | −7.6 to 7.8 | −2.8 to 6.9 |
| Inter-day | −7.8 to 6.7 | NA | −1.6 to 2.0 |
| Precision (%RSD) | |||
| Intra-day | 0 to 23.7 | 0-10.7 | 0.8 to 15.2 |
| Inter-day | 3.2 to 14.4 | NA | 2.8 to 9.8 |
| Absolute Recovery (%)g | 160.2 to 249.7 | 131 to 172 | 119.3 to 159.3 |
| Dilution Verification, n=6 (% RE, %RSD)h | 3.9, 8.9 | −4.9, 8.5 | −2.9, 3.7 |
| Extracted Sample Stability, n=4 (%RE) i | |||
| Ambient temperature (7d)j | −2.4 to −5.4 | −7.3 to 14.7 | −14.1 to −2.4 |
| Freeze-thaw (3 cycles) | −13.7 to 3.6 | −10.5 to 5.7 | −9.8 to 2.7 |
| Matrix Stability (% RE)k | |||
| Freeze-Thaw (3 cycles) | −7.6 to 10.6 | 5.2 to 10.2 | −9.1 to 2.2 |
| Storage (61d) | −22.0 to −12.4 | −11.4 to −1.9 | −7.5 to −2.2 |
Full validations were performed in plasma and fetal homogenate. A partial validation was performed for amniotic fluid using plasma method.
Plasma and amniotic fluid concentrations are expressed as ng/mL and fetal homogenate as ng/g.
For both vinpocetine and AVA, plasma and amniotic fluid curves were fitted with linear 1/x weighted regression and fetal homogenate curves were fitted with linear 1/x2 weighted regression.
LOQ, limit of quantitation; LOD, limit of detection, RE, relative error; RSD, relative standard deviation; NA, not applicable.
Experimental LOQ, is the lowest concentration used in standard curve. Target values are given.
Estimated as the 3 times the standard deviation of the LOQ (n=6 replicates).
Values given are the range for the concentration range validated.
Highest concentration verified were: plasma and amniotic fluid, 1000 ng/mL; fetal homogenate, 10,000 ng/g
Values given are the range for 3 QC concentrations at 1, 30 and 75 ng/mL in plasma and amniotic fluid and 7, 320 and 750 ng/g in fetal homogenate.
Values given are mean percent recovered after refrigerator, autosampler or ambient temperature storage at least 7 days
Values given are for 2 target QC concentrations at 1 and 75 ng/mL in plasma and amniotic fluid and 7 and 750 ng/g in fetal homogenate.
WinNonlin (Version 6.4, Pharsight Corporation, Mountain View, CA) was used for TK analysis. The following mean concentration versus time data were data were analyzed using noncompartmental analysis (NCA): dam plasma AVA; amniotic fluid vinpocetine and AVA; fetal homogenate vinpocetine and AVA. Compartmental models were tested for dam plasma vinpocetine concentration versus time data sets. For each compartmental model, data sets were analyzed with and without weighting. The model and the weighing factor that resulted in the best goodness of fit was selected as the final model. Based on this, a one-compartmental model given below with first order input, first order output and 1/Ŷ2 weighting was used to calculate primary and secondary TK parameters.
Where: C(t) is the plasma concentration at time t; A, is the intercept of the distribution, elimination, and absorption phases with the concentration axis; k01 is the absorption rate constant; k10 is the elimination rate constant.
Half-lives for the absorption and elimination were calculated as 0.693/k01 and 0.693/k10, respectively. The area under the curve (AUC) was estimated to the last sampling time point (AUC0-t) using trapezoidal rule and further extrapolated to infinity (AUC∞) by dividing the last plasma concentration by k10. Volume of distribution to the central compartment (V1_F), and clearance (Cl1_F) from the central compartment, adjusted for bioavailability, were calculated using standard equation. Statistical comparisons for the dose-related effect of the TK parameters were performed using Satterwaithe approximation, using the means, standard errors, degrees of freedom, and number of samples in the model (statistical significance level set to p = 0.05). Cmax and AUC∞ were dose-normalized for the t-test procedures.
Results
Analytical method validation.
Analytical methods to quantitate vinpocetine and AVA in plasma, amniotic fluid, and fetus were developed and successfully validated. A summary of validation parameters investigated and corresponding results are shown in Table 1. Methods were linear (≥ 0.995), accurate (inter-day and intra-day %RE, −7.8 to 12.7) and precise (inter-day and intra-day %RSD 0 to 14.4) for both vinpocetine and AVA in all matrices examined. During the partial validation of the plasma method to amniotic fluid, interday precision and accuracy was not assessed for amniotic fluid. Experimental LOQ was 0.5ng/mL for both analytes in plasma and amniotic fluid. Experimental LOQ was set at 5 ng/mL for fetal homogenate in order to extend the upper end of the curve to 1000 ng/g. Standards as high as 1,000ng/mL in plasma or amniotic fluid and 10,000ng/g fetal homogenate could successfully be diluted into the validated concentration range with observed %RE in the range −7.9 to 8.5 and %RSD in the range 2.3 to 8.9. Stability of analytes in extracted samples were demonstrated when stored at ambient temperature or undergone 3 freeze-thaw cycles (% RE −16.5 to 14.7). Analyte stability was also confirmed in matrices of interest during 3 freeze-thaw cycles (%RE −14.8 to 10.6) or when stored ~ −70°C for at least 61d (% RE −22.0 to 10.6). These data confirm that analytical methods were suitable to quantitate vinpocetine and AVA in plasma, amniotic fluid, and fetal homogenate.
Vinpocetine and AVA in Dam Plasma:
Vinpocetine was detected at all timepoints in dam plasma. Plasma concentration versus time data were fitted using a one compartmental model with first order input and first order output; corresponding plots are shown in Figures 2A and 2B, for 5 and 20mg/kg, respectively, and TK parameters are given in Table 2. Vinpocetine was absorbed rapidly following gavage administration in dams with a maximum plasma concentration reached at ≤ 1.37h. Cmax_predicted increased less than proportionally to the dose with values increasing 2.8-fold with a 4-fold increase in dose (p=0.0375). Although not significant AUC∞ increased 3.3-fold with a 4-fold increase in dose (p>0.05). The volume of distribution exceeded the reported aqueous body water volume in rats (688mL/kg) (Davies and Morris, 1993) indicating distribution of vinpocetine into the peripheral compartment. Vinpocetine was cleared rapidly from dam plasma with a half-life of ≤ 4.02h with no apparent dose-related effect.
Figure 2.
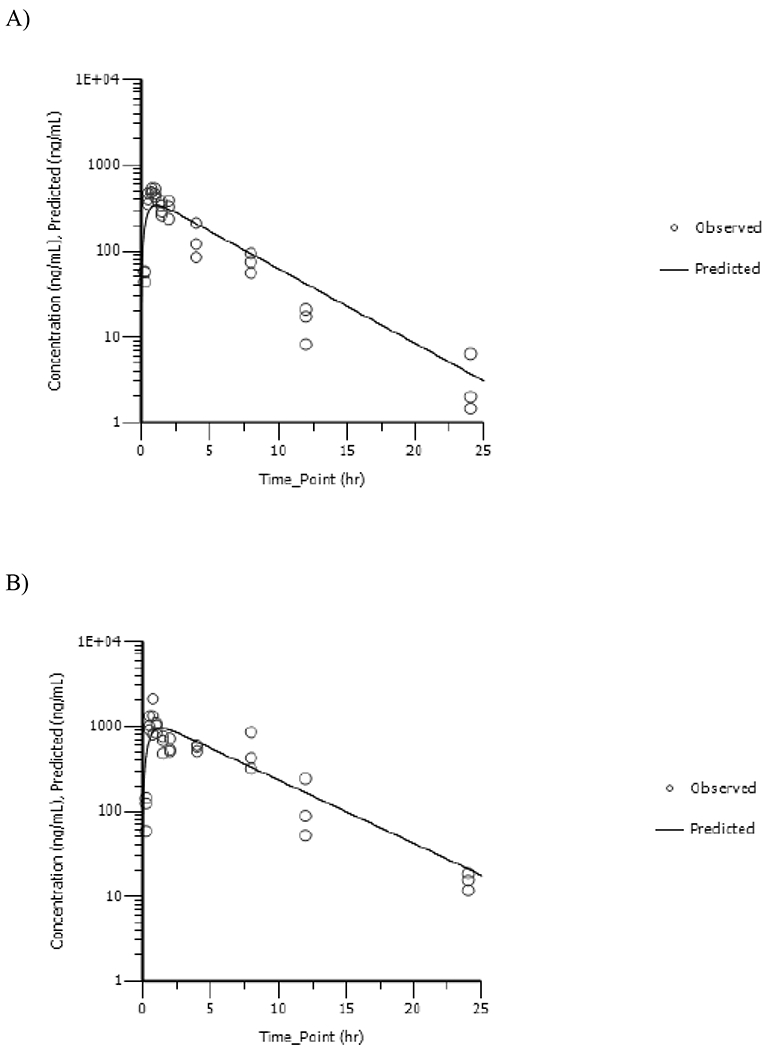
Dam Plasma Concentration versus Time Profiles of Vinpocetine Following a Single Daily Gavage Dose of A) 5mg/kg or B) 20mg/kg Vinpocetine in HSD rat Dams from GD6 to GD18. One-Compartmental Model with First Order Input, First Order Output and 1/Ŷ2 Weighting Was Used to Fit the Data.
Table 2.
Toxicokinetic Parameters a for Vinpocetine and Apovincaminic Acid in Dam Plasma Following a Daily Gavage Dose of Vinpocetine in HSD Rats from GD6 to GD18
| Parameter | Dose | |
|---|---|---|
| 5mg/kg | 20mg/kg | |
| Vinpocetineb | ||
| Cmax_Observed (ng/mL)c | 497 | 1420 |
| Cmax_Predicted (ng/mL) | 342 (36) | 948 (133) |
| Tmax Observed (h) | 0.750 | 0.750 |
| Tmax Predicted (h) | 1.11 (0.28) | 1.37 (0.40) |
| K01(h−1) | 2.44 (0.94) | 1.94 (0.88) |
| K01 Half-Life (h) | 0.284 (0.110) | 0.358 (0.162) |
| K10 (h−1) | 0.201 (0.015) | 0.173 (0.019) |
| K10 Half-Life (h) | 3.45 (0.25) | 4.02 (0.45) |
| AUC (0-T) (ng/mL.h) | 1830 | 7020 |
| AUC∞ (ng/mL.h) | 2120 (220) | 6960 (940) |
| Cl1_F (mL/h/kg) | 2350 (240) | 2870 (390) |
| V1_F (mL/kg) | 11700 (1700) | 16700 (3330) |
| Apovincaminic Acidd | ||
| Cmax_Observed (ng/mL) | 1070 | 2560 |
| Tmax Observed (h) | 1.50 | 1.00 |
| λz (h−1) | 0.170 | 0.157 |
| Half-life (h) | 4.07 | 4.41 |
| AUC (0-T) (ng/mL.h) | 6060 | 23500 |
| AUC∞ (ng/mL.h) | 6170 | 24200 |
| Cl1_F (mL/h/kg) | 811 | 826 |
| V1_F (mL/kg) | 4760 | 5250 |
Values given are mean (standard error, SE).
Based on one-compartmental model with first order input, first order output, and 1/Yhat2 weighting.
Observed values have no SE to report.
Based on non-compartmental analysis (NCA).
AVA was detected at all timepoints in dam plasma. AVA concentration versus time data were analyzed using NCA; corresponding plots are shown in Figures 3A and 3B, respectively, for 5 and 20mg/kg and TK parameters are given in Table 2. Maximum plasma AVA concentration was reached at ≤ 1.5h suggesting rapid metabolism of vinpocetine to AVA in rats. Cmax_observed increased less than proportionally to the dose with values increasing 2.4-fold with a 4-fold increase in dose. AUC∞ increased proportionally to the dose. AVA was cleared rapidly from the plasma with a half-life of ≤ 4.41h with no apparent dose-related effect.
Figure 3.
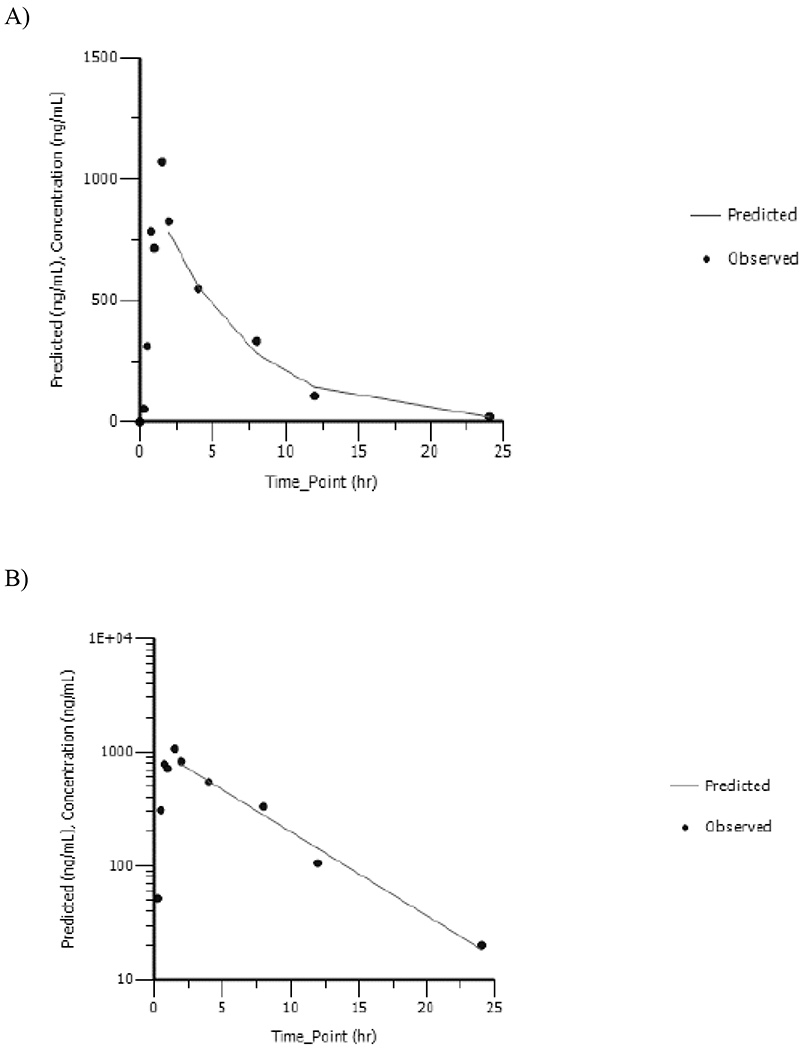
Dam Plasma Concentration versus Time Profiles of Apovincaminic Acid Following a Single Daily Gavage Dose of A) 5mg/kg or B) 20mg/kg Vinpocetine in HSD rat Dams from GD6 to GD18. Data Were Analyzed Using Noncompartmental Analysis.
Vinpocetine and AVA in Fetuses:
Fetuses were pooled by litter and homogenized for analyses. Vinpocetine was detected at all timepoints following exposure in GD18 fetuses. Fetus concentration versus time data were analyzed using NCA: corresponding plots are shown in Figures 4A and 4B, respectively, for 5 and 20mg/kg and TK parameters are given in Table 3. Maximum vinpocetine concentration in fetuses was reached at ≤ 1h suggesting rapid gestational transfer from dam to fetuses. Cmax_observed increased less than proportionally to the dose with values increasing 2.4-fold with a 4-fold increase in dose. AUC∞ in fetuses increased proportional to the dose with values increasing 3.7-fold as the dose increased 4-fold. Vinpocetine was cleared rapidly from the fetuses with a half-life of ≤ 3.23h with no apparent dose-related effect.
Figure 4.
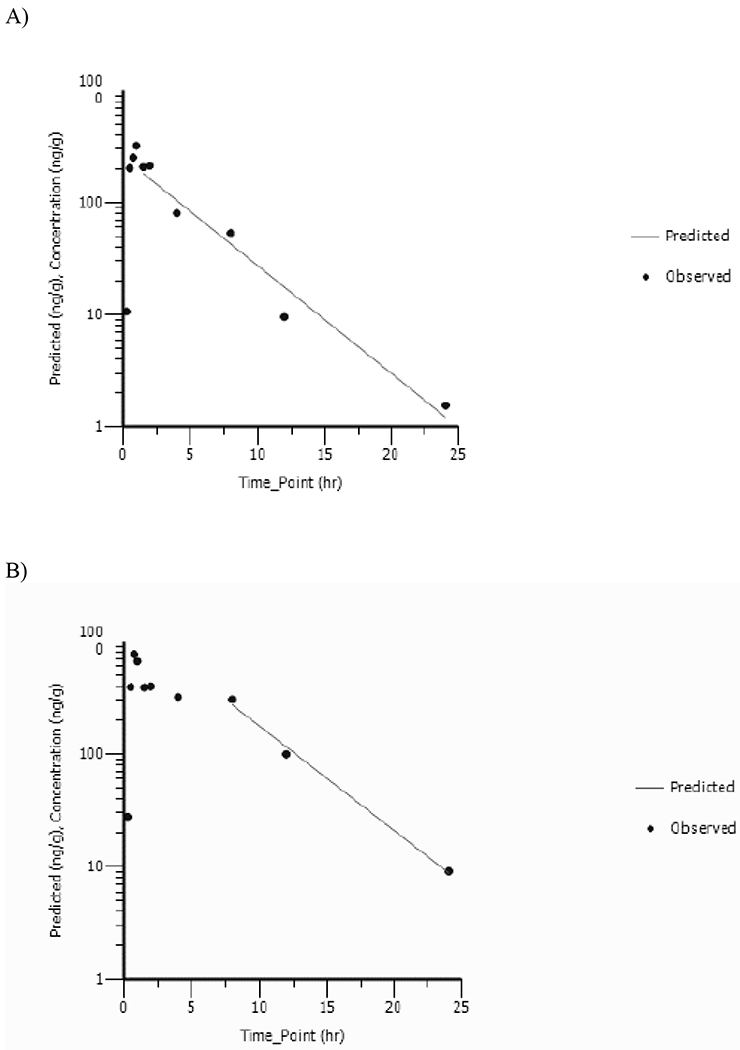
Fetus Concentration versus Time Profiles of Vinpocetine Following a Single Daily Gavage Dose of A) 5mg/kg or B) 20mg/kg Vinpocetine in HSD rat dams from GD6 to GD18. Data Were Analyzed Using Noncompartmental Analysis.
Table 3.
Toxicokinetic Parameters a for Vinpocetine and Apovincaminic Acid in Fetuses Following a Daily Gavage Dose of Vinpocetine in HSD Rats from GD6 to GD18.
| Parameter | Dose | |
|---|---|---|
| 5 mg/kg | 20 mg/kg | |
| Vinpocetine | ||
| Cmax_Observed (ng/g) | 321 | 768 |
| Tmax Observed (h) | 1.00 | 0.750 |
| λz (h−1) | 0.222 | 0.214 |
| Half-life (h) | 3.12 | 3.23 |
| AUC (0-T) (ng/g.h) | 1150 | 4260 |
| AUC∞ (ng/g.h) | 1160 | 4300 |
| Cl1_F (g/h/kg) | 4330 | 4650 |
| V1_F (g/kg) | 19500 | 21700 |
| Apovincaminic Acid | ||
| Cmax_Observed (ng/g) | 25.0 | 83.7 |
| Tmax Observed (h) | 8.00 | 4.00 |
| λz (h−1) | 0.136 | 0.108 |
| Half-life (h) | 5.09 | 6.42 |
| AUC (0-T) (ng/g.h) | 334 | 1160 |
| AUC∞ (ng/g.h) | 353 | 1290 |
| Cl1_F (g/h/kg) | 14100 | 15600 |
| V1_F (g/kg) | 104000 | 144000 |
Based on non-compartmental analysis.
AVA was detected at all timepoints in the fetuses. AVA concentration versus time data were analyzed using NCA: corresponding plots are shown in Figures 5A and 5B and TK parameters are given in Table 3. Maximum fetal AVA concentration was reached at ≤ 8h. Cmax_observed and AUC∞ increased 3.3- and 3.7-fold with a 4-fold increase in dose suggesting dose-proportional increase in systemic exposure parameters of AVA in the fetuses. AVA was cleared rapidly from the fetuses with a half-life of ≤ 6.42h with no apparent dose-related effect.
Figure 5.
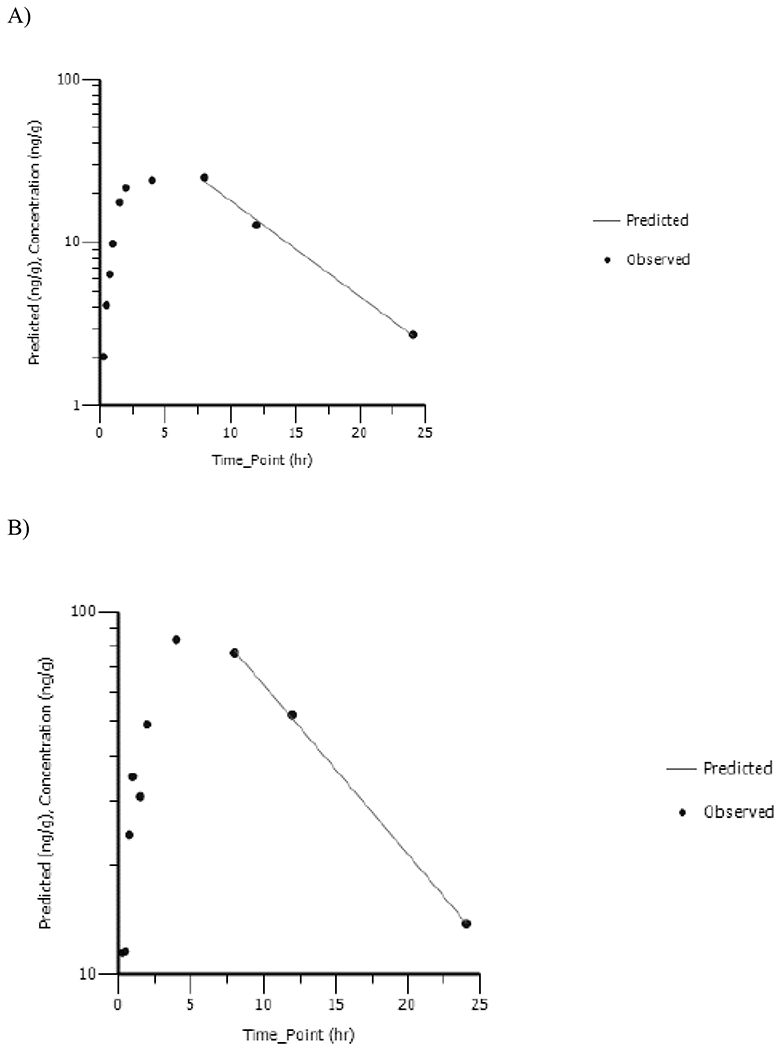
Fetus Homogenate Concentration versus Time Profiles of Apovincaminic Acid Following a Single Daily Gavage Dose of A) 5 mg/kg or B) 20 mg/kg Vinpocetine in HSD Rat Dams from GD6 to GD18. Data Were Analyzed Using Noncompartmental Analysis.
Vinpocetine and AVA in Amniotic Fluid:
Amniotic fluid was pooled by litter for analyses. Vinpocetine was detected in GD18 amniotic fluid although the levels were lower than in dam plasma or fetuses. Amniotic fluid concentration versus time data were analyzed using NCA and corresponding TK parameters are given in Table 4. Cmax_observed was reached at ≤ 1h and increased less than proportionally to the dose with values increasing 2.1-fold with 4-fold increase in dose. AUC∞ increased 3.2-fold as dose increased 4-fold. Vinpocetine was cleared rapidly from amniotic fluid with a half-life of ≤ 3.90h with no apparent dose-related effect.
Table 4.
Toxicokinetic Parametersa for Vinpocetine and Apovincaminic Acid in Amniotic Fluid Following a Daily Gavage Dose of Vinpocetine in HSD Rats from GD6 to GD18.
| Parameter | Dose | |
|---|---|---|
| 5mg/kg | 20mg/kg | |
| Vinpocetine | ||
| Cmax_Observed (ng/mL) | 21.4 | 45.0 |
| Tmax Observed (h) | 1.00 | 0.750 |
| λz (h−1) | 0.178 | 0.184 |
| Half-life (h) | 3.90 | 3.77 |
| AUC (0-T) (ng/g·h) | 97.3 | 310 |
| AUC∞ (ng/mL.h) | 98.7 | 316 |
| Cl1_F (mL/h/kg) | 50600 | 63300 |
| V1_F (mL/kg) | 285000 | 345000 |
| Apovincaminic Acid | ||
| Cmax_Observed (ng/mL) | 4.93 | 23.0 |
| Tmax Observed (h) | 24.0 | 24.0 |
| λz (h−1) | NA | NA |
| Half-life (h) | NA | NA |
| AUC (0-T) (ng/mL/h) | 99.1 | 440 |
| AUC∞ (ng/mL.h) | NA | NA |
| Cl1_F (mL/h/kg) | NA | NA |
| V1_F (mL/kg) | NA | NA |
Based on non-compartmental analysis.
AVA was detected in amniotic fluid, although the levels were lower than in dam plasma and fetuses. Amniotic fluid concentration versus time data were analyzed using NCA and corresponding TK parameters are given in Table 4. A linear terminal phase could not be defined for the AVA concentration versus time profiles in amniotic fluid since levels were still increasing at the final sample collection timepoint of 24 h. Cmax_observed and AUC(0-T) increased 2.1 and 3.2-fold, respectively, with a 4-fold increase in dose. Due to lack of linear terminal phase, half-life of elimination, clearance and AUC∞ could not be estimated.
Discussion
Vinpocetine is being used worldwide as a pharmaceutical or dietary supplement. Due to its purported claims of multiple benefits, there is potential use of vinpocetine by all age ranges, including pregnant women. Although a daily dose between 5 and 30 mg is recommended for humans, there is no restriction for multiple daily use of vinpocetine-containing products leading to potential exposure exceeding 30 mg per day. However, there are limited data addressing the safety/toxicity of vinpocetine in the current literature.
NTP undertook studies investigating the developmental toxicity of vinpocetine following in utero exposure. Although there are some TK data for vinpocetine and its major metabolite, AVA, in adult rodents in the literature following a single administration, to the best of our knowledge, there are no data following repeated exposure and/or exposure in utero. Therefore, we also designed a study to address this data gap and subsequently to help interpret data from NTP toxicology studies of vinpocetine.
Following exposure of pregnant rats to 5 or 20 mg/kg vinpocetine from GD6 through GD18, vinpocetine was rapidly absorbed and distributed in the body. Cmax_predicted and AUC∞ increased less than proportionally to the dose, as evidenced by the dose-normalized data, although the effect was significant only for Cmax_predicted (Table 2, Figures 6A and 6B). Following exposure of rat dams, unchanged parent was detected in fetuses and amniotic fluid confirming gestational transfer of vinpocetine. Similar to dams, Cmax_predicted and AUC∞ increased less-than proportionally to the dose in fetuses (Figure 6A). More importantly, fetal vinpocetine levels were, based on the two doses examined, 81 to 94% of dams for Cmax, and 55 to 62% of dams for AUC∞, demonstrating that the transfer of vinpocetine from dam to fetus in utero was significant. To the best of our knowledge, this is the first study demonstrating gestational transfer of vinpocetine. Vinpocetine is cleared rapidly in dams and fetuses following oral exposure of dams with an estimated plasma half-life of ≤ 4.02h.
Figure 6.
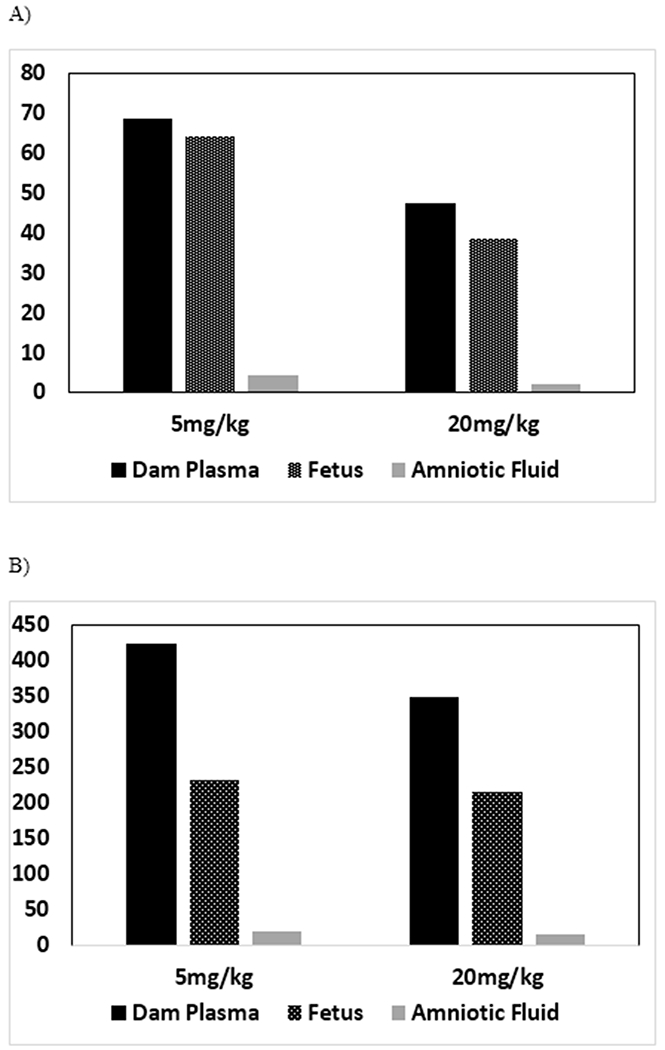
Comparison of Dose-Normalized A) Cmax and B) AUC∞ of Vinpocetine in Dam Plasma, Amniotic Fluid and Fetus Following a Single Daily Gavage Dose of Vinpocetine in HSD rat Dams from GD6 to GD18
Vinpocetine was rapidly and highly metabolized to AVA in the rat dam as evidenced by the Tmax for AVA of ≤ 1.50h. Based on the Cmax and AUC∞, respectively, the levels of AVA in dams were 2.7- to 3.1-fold and 2.9 to 3.5-fold higher than vinpocetine at the two doses examined. Additionally, similar to vinpocetine, AVA also increased less than proportional to the dose (Figures 7A and 7B). This suggests that the less than dose proportional increase of vinpocetine is likely due to decreased absorption with increasing dose rather than increased metabolism. AVA was also detected in fetuses, although the levels were much lower than vinpocetine; levels in the fetus were ≤ 3.3% and ≤ 5.7% of dams based on Cmax (observed or predicted, depending on the matrix) and AUC (AUC∞ or AUC0-T), respectively, suggesting either little transfer of AVA from the dam to the fetus and/or little metabolism of vinpocetine to AVA in the fetus.
Figure 7.
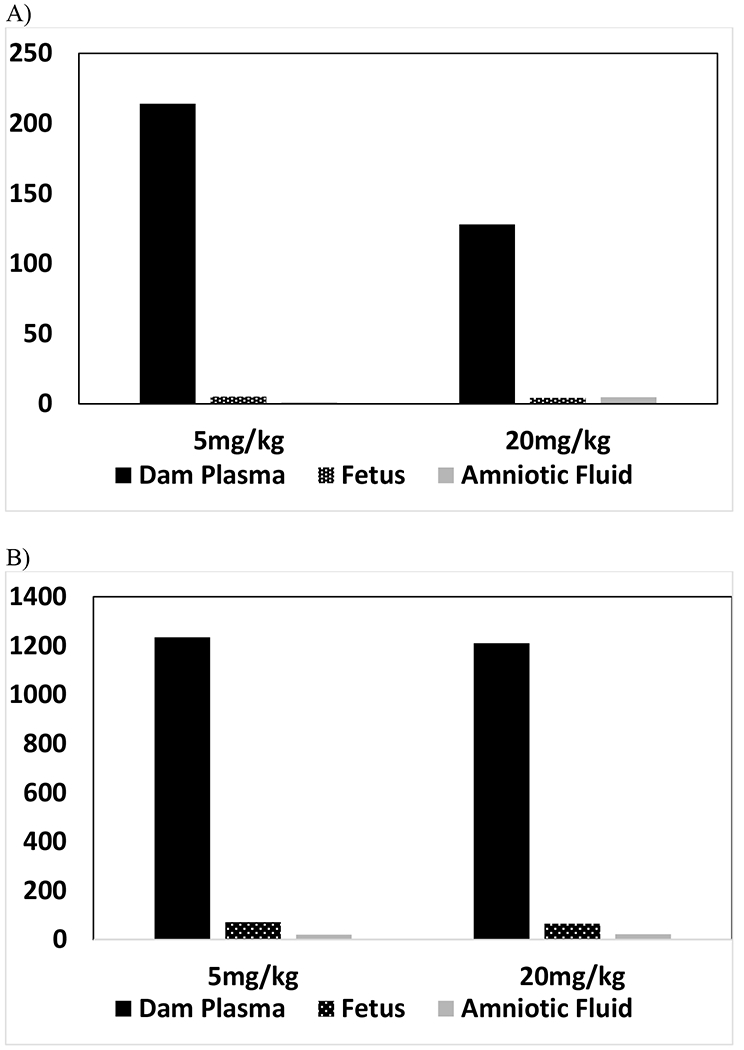
Comparison of Dose-Normalized A) Cmax and B) AUC∞ of Apovincaminic acid in Dam Plasma, Amniotic Fluid and Fetus Following a Single Daily Gavage Dose of Vinpocetine in HSD rat Dams from GD6 to GD18
Vinpocetine and AVA TK in adult rats has been published by other investigators following a single oral dose (summarized in Vereczkey, 1985; Sozaneski et al., 2012; Xia et al., 2010). Data reported in the literature are similar to those observed in the current study with reported Tmax ≤ 1.5h and plasma half-life of elimination ≤ 3h. A comparison of dose-normalized systemic exposure parameters between studies (Table 5), including the current investigation, shows that Cmax and AUC values, respectively, for vinpocetine are within 3- and 7-fold and for AVA are within 2- and 7-fold, despite differences between the doses (1 to 20 mg/kg), exposure paradigm (single versus repeat exposure), pregnancy status and strain of rats used between studies (Table 5). These data along with the short half-life and high clearance values observed for vinpocetine suggests that accumulation of vinpocetine following repeated dosing is low.
Table 5.
Comparison of Dose-Normalized AUC and Cmax of Vinpocetine and Apovincaminic Acid in Rodents
| Rat Strain | Dose | Vinpocetine | Apovincaminic Acid | Reference | ||
|---|---|---|---|---|---|---|
| (mg/kg) | Cmax/Dose (ng/mL)/(mg/kg) | AUC/Dose (ng/ml).h/(mg/kg) | Cmax/Dose (ng/mL)/(mg/kg) | AUC/Dose (ng/ml).h/(mg/kg) | ||
| Hsd:Sprague Dawley® SD® Rats | 5 | 68.4 | 424 | 214 | 1234 | Current study |
| Hsd:Sprague Dawley® SD® Rats | 20 | 47.4 | 348 | 128 | 1210 | Current study |
| Wistar | 2.5 | NA | 261.6 | NA | 3089 | Vereczkey et al., 1979b |
| Sparague Dawley | 1 | 23.8 | 57.4 | 86.6 | 472 | Xie et al., 2010 |
| Wistar | 2 | 67.6 | 262 | NA | NA |
Sozanski et al., 2011 |
Disposition of vinpocetine following a single or repeat oral exposure using different vinpocetine formulations in humans has been reported by several investigators. The pattern of disposition is similar to rodents. Briefly, it is rapidly absorbed with a maximum plasma concentration, Cmax, reaching ≤ 3h (summarized in NTP 2013; Elbary et al., 2002; Grandt et al., 1989; Miskolczi et al., 1990; Vereczkey at al., 1979; Lohmann et al., 1992). Reported plasma elimination half-lives range between 1.22 to 2.20h (Elbary et al., 2002; Grandt et al., 1989; Miskolczi et al., 1990).
We compared our rat data to published human data using both external dose (mg/kg and mg/m2) and systemic exposure parameters (Cmax and AUC) of vinpocetine and these data are summarized in Table 6. A human dose of 10mg translates to 0.14mg/kg, assuming a 70kg person and to 5.4 mg/m2 using a human Km value of 37kg/m2 (Reagen-Shaw et al., 2008). Similarly, rat doses of 5 and 20 mg/kg translate to 30 and 120mg/m2 using a rat Km value of 6kg/m2 (Table 6). Based on mg/kg and mg/m2 dose, respectively, rat dose of 5 mg/kg is 35- and 5.5-fold higher than the recommended human dose of 10mg. Using systemic exposure parameters, which takes into account species differences in disposition, the rat to human exposure multiple was ≤ 13.6 and ≤ 8.5 for Cmax and AUC, respectively, suggesting that systemic exposure to vinpocetine in rats following repeated 5mg/kg dose is similar to that following a single 10mg human dose.
Table 6.
Comparison of Exposure Between Rats1 and Humans
| Rat Dose | Human Dose | Exposure Multiple (Rat Dose/Human Dose)2 | |||||
|---|---|---|---|---|---|---|---|
| mg/kg(mg/m2)3 | mg/kg(mg/m2)4 | mg/kg | mg/m2 | Cmax | AUC | ||
| Ref 15 | Ref 2 | Ref 1 | Ref 2 | ||||
| 5 (30) | 0.143 (5.4) | 35 | 5.5 | 5.3 | 13.6 | 4.1 | 8.5 |
| 20 (120) | - | 140 | 120 | 14.7 | 37.6 | 13.4 | 27.9 |
Data used was from the current study. In dams for 5mg/kg and for 20 mg/kg, respectively, Cmax (ng/mL) was 342 and 948 and AUC (ng.h/mL) was 2120 and 6960 (Table 2)
Exposure multiples given are the ratio of rat dose/human dose estimated using mg/kg, mg/m2, Cmax and AUC
mg/m2 values were estimated using Km value of 6kg/m2 for rats (Reagen-Shaw et al., 2008)
Human doses mg/kg and mg/m2 were calculated assuming 10mg dose taken by a 70kg person (0.143mg/kg) and using a Km value of 37kg/m2 for humans (Reagen-Shaw et al., 2008)
Two human studies were used for comparison. The following Cmax (ng/mL) and AUC (ng.h/mL) values, respectively, reported following use of 10mg of vinpocetine product were used: 64.3 and 519.7 (Ref 1, Elbary et al., (2002); 25.2 and 249.43 (Ref 2, Kharshoum (2013))
Conclusion
We have successfully validated analytical methods to quantitate vinpocetine and AVA simultaneously in plasma, amniotic fluid and fetal tissue. Validated methods were used to determine vinpocetine and AVA in these matrices from a TK study following perinatal exposure of rat dams to 5 and 20 mg/kg vinpocetine beginning on GD 6. Our data demonstrate that vinpocetine is rapidly absorbed in rats and distributed to the peripheral compartment. More importantly, significant transfer of vinpocetine from dam to fetus was observed with fetus Cmax and AUC values ≥ 55% of dams. Comparison of rat and human exposure using Cmax and AUC demonstrates that systemic exposure to vinpocetine in rats following repeated 5 mg/kg dose is similar to that following a single human relevant dose of 10 mg.
Acknowledgements
The authors are grateful to Drs. Anika Dzierlenga and Kristen Ryan for their review of this manuscript. This work was performed for the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health , U.S. Department of Health and Human Services, under contract No. HHSN273201400027C).
Footnotes
DECLARATION OF INTEREST
The authors report no declarations of interest.
References
- Anonymous. (1984). Cavinton, injection, tablet. Geor. Med 14, 363–364. [PubMed] [Google Scholar]
- Bereczki D and Fekete I (2008). Vinpocetine for acute ischemic stroke. Cochrane Database Syst Rev. 1:CD000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholnoky E, and Dömök LI (1976). Summary of safety tests of ethyl apovincaminate. Arzneimittelforschung, 26, 1938–1944. [PubMed] [Google Scholar]
- Elbary AA, Foda N, El-Gazayerly O and El Khatib M (2002). Reversed phase liquid chromatographic determination of vinpocetine in human plasma and its pharmacokinetic application. Anal Lett, 35:1041–1054. [Google Scholar]
- Feigin VL, Doronin BM, Popova TF, Gribatcheva EV and Tchervov DV (2001). Vinpocetine treatment in acute ischaemic stroke: a pilot single-blind randomized clinical trial. Eur. J. Neurol 8, 81–85. [DOI] [PubMed] [Google Scholar]
- Grandt R, Beitinger H, Schaltenbrand R and Braun W (1989). Vinpocetine pharmacokinetics in elderly subjects. Arzneimittelforschung, 39, 1599–1602. [PubMed] [Google Scholar]
- Lohmann A, Dingler E, Sommer W, Schaffler K, Wober W and Schmidt W (1992). Bioavailability of vinpocetine and interference of the time of application with food intake. Arzneimittelforschung, 42, 914–917. [PubMed] [Google Scholar]
- Maconi E, Binaghi F, and Pitzus F (1986). A double-blind clinical trial of vinpocetine in the treatment of cerebral insufficiency of vascular and degenerative origin. Current Therapeutic Research 40, 702–709. [Google Scholar]
- Miskolczi P, Kozma K, Polgar M and Vereczkey L (1990). Pharmacokinetics of vinpocetine and its main metabolite apovincaminic acid before and after the chronic oral administration of vinpocetine to humans. Eur J Drug Metab Pharmacokinet, 15, 1–5 [DOI] [PubMed] [Google Scholar]
- National Research Council (2011). Guide for the care and use of laboratory animals: Eighth edition. Washington DC: The National Academic Press [Google Scholar]
- Nie S, Fan X, Peng Y, Yang X, Wang C and Pan W (2006). In vitro and in vivo studies on the complexes of vinpocetine with hydroxypropyl-β-cyclodextrin. Arch. Pharm. Res 30, 991–1001. [DOI] [PubMed] [Google Scholar]
- National Institute of Health Dietary supplement database (last accessed August 2017). https://dsld.nlm.nih.gov/dsld/rptQSearch.jsp?item=vinpocetine&db=adsld
- National Toxicology Program (2013) Chemical Information Review Document for vinpocetine [CAS No. 42971-09-5]. [Google Scholar]
- Peruzza M and DeJacobis M (1986). A double-blind placebo controlled evaluation of the efficacy and safety of vinpocetine in the treatment of patients with chronic vascular or degenerative senile cerebral dysfunction. Avd. Therapy 3, 201–209. [Google Scholar]
- Ribeiro LSS, Falcão AC, Patrício JAB, Ferreira DC, and Veiga FJB (2004). Cyclodextrin multicomponent complexation and controlled release delivery strategies to optimize the oral bioavailability of vinpocetine. J. Pharm. Sci 96, 2018–2028. [DOI] [PubMed] [Google Scholar]
- Sitges M, Aldana BI and Reed RC (2016). Effect of the anti-depressant sertraline, the novel anti-seizure drug vinpocetine and several conventional antiepileptic drugs on the epileptiform EEG activity induced by 4-aminopyridine. Neurochem. Res 41, 1365–1374. [DOI] [PubMed] [Google Scholar]
- Sozanski T, Magdalan J, Trocha M, Szumny A, Merwid-Lad A, Slupski W, Karazniewicz-Lada M, Kielbowicz G, Ksiadzyna D and Szelag A 2011. Omeprazole does not change the oral bioavailability or pharmacokinetics of vinpocetine in rats. Pharmacol Rep, 63, 1258–1263. [DOI] [PubMed] [Google Scholar]
- Szatmári S and Whitehouse P (2003). Vinpocetine for cognitive impairment and dementia. Cochrane Database of Systematic Reviews, Issue 1 Art. No.: CD003119 DOI: 10.1002/14651858.CD003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiji H, and Kanzaki J (1986). Clinical study of vinpocetine in the treatment of vertigo. Jpn. Pharmacol Ther 14, 577–589. (abstract in English) [Google Scholar]
- Thal LJ, Salmon DP, Lasker B, Bower D and Klauber MR (1989). The safety and lack of efficacy of vinpocetine in Alzheimer’s disease. J. Am. Geriatr. Soc 37, 515–320. [DOI] [PubMed] [Google Scholar]
- Thorne Research, Inc. (2002). Monograph: vinpocetine. Altern. Med. Rev 7, 240–243. [PubMed] [Google Scholar]
- Truss MC, Stief CG, Ückert S, Becker AJ, Schultheiss D, Machtens S and Jonas U (2000). Initial clinical experience with the selective phosphodiesterase-I isoenzyme inhibitor vinpocetine in the treatment of urge incontinence and low compliance bladder. World J. Urol 18, 439–443. [DOI] [PubMed] [Google Scholar]
- Vereczkey L, Zólyomi G, and Szporny L (1976). Pharmacokinetic data on tritium labeled ethyl apovincaminate. Arzneim.-Forsch. (Drug Res.) 26, 1929–1933. [PubMed] [Google Scholar]
- Vereczkey L, and Szporny L (1976). Metabolism of ethyl apovincaminate in the rat. Arzneimittelforschung, 26(10a):1933–1938. [PubMed] [Google Scholar]
- Vereczkey L, Szentirmay Z and Szporny L (1979a). Kinetic metabolism of vinpocetine in the rat. Arzneim.-Forsch. (Drug Res.) 29, 953–956. [PubMed] [Google Scholar]
- Vereczkey L, Czira G, Tamas J, Szentirmay Z, Botar Z, and Szporny L (1979b). Pharmacokinetics of vinpocetine in humans. Arzneimittelforschung, 29(6):957–960. [PubMed] [Google Scholar]
- Vereczkey L (1985). Pharmacokinetics and metabolism of vincamine and related compounds. Eur. J. Drug Metab. Pharmacokinet 10, 89–103. [DOI] [PubMed] [Google Scholar]
- Xia HM, Su LN, Guo JW, Liu GM, Pang ZQ, Jiang XG, and Chen J (2010). Determination of vinpocetine and its primary metabolite, apovincaminic acid, in rat plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci, 878:1959–1966. [DOI] [PubMed] [Google Scholar]
- Xu H, He L, Nie S, Guan J, Zhang X, Yang X and Pan W (2009). Optimized preparation of vinpocetine proliposomes by a novel method and in vivo evaluation of its pharmacokinetics in New Zealand rabbits. J. Control. Release 140, 61–68. [DOI] [PubMed] [Google Scholar]


