Abstract
Detection of early gastrointestinal tract malignancy can be challenging on white light endoscopy especially as lesions can be subtle and inconspicuous. With the advent of electronic chromoendoscopy technologies, lesions which have already been detected can be quickly and “conveniently” characterised. This review will discuss some of the indications and modern applications of chromoendoscopy in various conditions including Barrett’s oesophagus, oesophageal squamous cell carcinoma, early gastric cancer, inflammatory bowel disease and neoplastic colonic lesions. In carefully selected situations, chromoendoscopy could still be a useful adjunct to white light endoscopy in day-to-day clinical practice.
Keywords: Chromoendoscopy, endoscopy dyes, narrow band imaging (NBI), digestive system diagnostic techniques, endoscopy procedures
Introduction
Chromoendoscopy aids the endoscopist in highlighting and characterising lesions in the gastrointestinal tract (GIT). This is generally achieved by using a variety of dyes to distinguish normal from abnormal mucosa (1). The technique encourages endoscopists to study a particular area of interest with greater clarity and sharpness. Chromoendoscopy can complement imaging through enhancing features of mucosal surface topography and may assist in providing in-depth details that enables subsequent therapeutic decisions to be made with greater precision (2).
Spraying catheters allow the most controlled and precise application of the dye as a fine mist onto the GIT surface. The amount of staining solution required depends on the surface area to be stained. The endoscope should be slowly withdrawn, while the endoscope tip (with a 1–2 cm protruding spraying catheter) is directed in spiral movements onto the mucosa and simultaneously, the dye is sprayed continuously. In a stepwise manner, segments of approximately 20 cm should be stained each time and then carefully inspected. Special caution should be exercised whilst staining the upper oesophagus, due to the risk of aspiration. In addition, a foot pump can be useful in some cases, such as for pancolonic dye spraying in patients with inflammatory bowel disease (IBD).
In colonoscopy, good bowel preparation is critical; the use of spasmolytic agents such as scopolamine or glucagon can be used to avoid bowel peristalsis and an uneven distribution of the dye in some cases.
The use of chromoendoscopy has demonstrated good diagnostic yield in the evaluation of dysplastic lesions in Barrett’s oesophagus (BO) and oesophageal adenocarcinoma by facilitating targeted biopsies of suspicious areas (3-5). It also plays a key role in gastric metaplasia and adenocarcinoma, as well as in the colon particularly in patients with chronic colitis (6-8). Over the years, the introduction and adoption of ´virtual´ or electronic chromoendoscopy has rendered dye-based chromoendoscopy less relevant in day-to-day clinical practise. Virtual chromoendoscopy is technically less cumbersome, easier to apply and saves time (9,10). Nevertheless, chromoendoscopy still plays a major role in several conditions.
Classification of stains
Dyes used in chromoendoscopy have a transient effect. With the exclusion of known prior allergic reactions, these dyes are generally safe (11) Traditionally, they can be classified into three distinct types based on their staining properties.
Although chromoendoscopy may be replaced by virtual chromoendoscopy with similar results (12), for some specific cases the literature still shows advantages on using dyes. For each dye, scenarios where chromoendoscopy still has a role will be discussed in the following section:
The absorptive or vital stains are absorbed by specific cells and give rise to sharp detail of the cellular surface. Some examples of these stains include Lugol’s iodine (LI); which can be used in delineating squamous dysplasia and squamous cell cancer in the oesophagus. Methylene blue and crystal violet have been used in Barrett’s oesophagus, gastric intestinal metaplasia and colorectal dysplasia. Acetic acid (AA) used in detecting dysplastic Barrett’s lesions, are also categorized under these types of stains (13).
The non-absorptive or contrast stains include indigo carmine where on application they seep between grooves and crevices providing an overall detail of the contour of the mucosal surface. Indigo carmine is used throughout the GIT for various dysplastic lesions.
Reactive stains include Congo red and phenol red. The dye undergoes chemical reactions with specific cellular components and gives rise to a colour change. They are not commonly encountered in endoscopy (13).
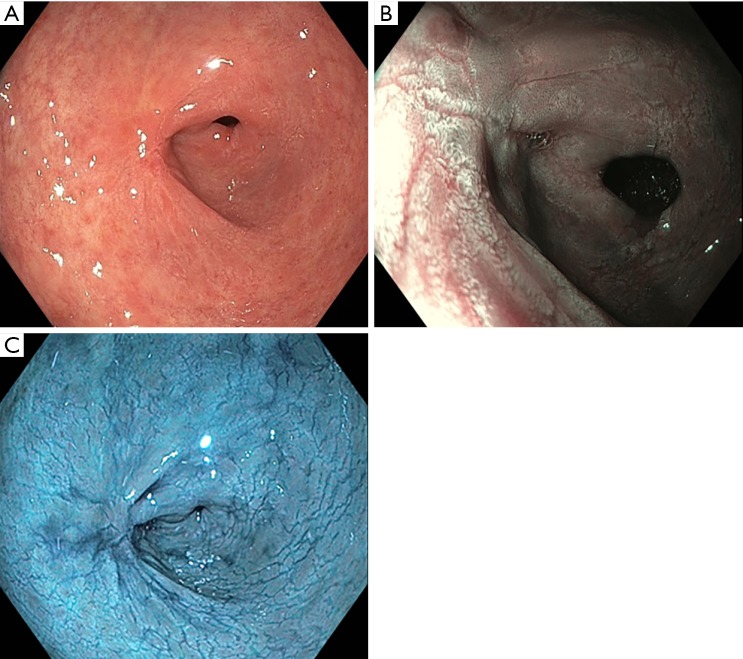
AA chromoendoscopy in Barrett’s neoplasia
This novel use of AA chromoendoscopy in BO was introduced in 2001 by Guelrud and colleagues (14). The principle is not dissimilar to AA employment in the field of gynaecology to screen for dysplastic uterine cervical lesions which was well established in the early 1990s (15). When sprayed onto the Barrett’s mucosa, a reversible acetylation of cellular proteins occurs leading to a so-called “aceto-whitening reaction” (Figure 1) (16). With neoplastic lesions which lack cellular proteins, the aceto-whitening reaction is not visible or is lost quickly compared to normal non-dysplastic BO epithelium. The duration of the aceto-whitening effect was further studied by Longcroft-Wheaton and colleagues who objectively measured and compared the effect of AA on dysplastic and non-dysplastic lesions. Not surprisingly, high-grade dysplastic lesions and intramucosal cancer took between 23–53 seconds to lose the aceto-whitening effect compared to non-dysplastic lesions which continued to hold on to this effect for up to a median duration of 311 seconds (P<0.05). In addition, using AA had a sensitivity and specificity of 98% and 84% respectively (17). This increases the yield of detecting dysplastic lesions and facilitates targeted biopsies. Coletta and colleagues also highlighted the role of AA in a recent meta-analysis where the pooled sensitivity and specificity was 92% and 96% for the diagnosis of high-grade dysplasia and oesophageal adenocarcinoma (18). Both these large studies were able to achieve the desired American Society for Gastrointestinal Endoscopy Preservation and Incorporation of Valuable Endoscopic Innovations (ASGE-PIVI) criteria (19). In terms of cost-efficacy, Bhandari and colleagues went on to compare protocol-guided random biopsies and AA guided biopsies in high-risk groups. They found that despite a 4% miss rate in the latter, substantial cost savings could be achieved (20). The gain in neoplasia detection was also tested by Tholoor and colleagues in a retrospective cohort study where a comparison was made between the yield of biopsies guided by AA chromoendoscopy and four quadrant random biopsies. They found a 14.7-fold increase in neoplasia detection on a per-biopsy analysis for AA guided biopsies. The number of biopsies required to detect neoplasia was also 15 times lower compared to the random biopsy group (4).
Figure 1.
Acetic acid reaction in Barrett’s oesophagus. (A) Barret’s oesophagus on white light endoscopy; (B) acetic acid on Barrett’s oesophagus: “aceto-whitening reaction” in intestinal metaplasia due to lack of normal stratified squamous epithelium.
Taken together, the studies show that cost-effectiveness for AA chromoendoscopy in detecting Barrett’s neoplasia may be noteworthy to implement considering the ease of preparation and wide availability (21).
LI chromoendoscopy in oesophageal squamous cell carcinoma
It is well known that oesophageal cancer has a very poor prognosis and a low 5-year survival rate (22). It is the eighth most common cancer in the world and is the sixth leading cause of cancer-related death (23,24). Early oesophageal squamous cell cancer (OSCC) are often subtle and difficult to identify on routine white light inspection (25).
Careful visualisation with the aid of dye-based chromoendoscopy in the form of LI may play a role in ensuring suspicious areas that can be detected allowing biopsies for confirmation (26,27). Appearing somewhat similar in principle with AA chromoendoscopy, the rationale behind LI is in its reaction with the glycogen, which is abundantly present in the normal stratified squamous epithelial tissue in the oesophagus. This gives rise to an intense brown stain visualised on normal squamous tissue. However, in neoplastic lesions, the glycogen-containing granules within the cell layers diminish. Consequently, LI does not properly “stain” the affected area giving rise to a pale “unstained” appearance (28). In 2008, Shimizu and colleagues introduced the “pink colour sign”, which has shown high sensitivity and specificity (91.9% and 94.0%) for diagnosing high-grade dysplasia and OSCC (Figure 2). This observation assists in the distinction between high and low-grade dysplasia/inflammation as the former gives a more distinct and pinkish hue, while the latter appears pale. An additional 2 to 3 minutes to observe this discoloration is necessary with the authors reporting that false positive LI stained areas (initially with yellow decolouration) could be ignored if they are not pink after this period of observation (29).
Figure 2.
Lugol’s iodine chromoendoscopy. (A) OSCC on white light endoscopy; (B) Lugol’s iodine. Note the brownish colour in normal mucosa, not picked up by OSCC; (C) Lugol’s iodine after 2 to 3 minutes: “Pink colour sign”. OSCC, oesophageal squamous cell cancer.
Similar to AA, LI has significant diagnostic potential in early cancer detection where targeted biopsies can be facilitated. In addition, a clear demarcation between normal and abnormal margins can be easily delineated enabling planning for endoscopic resection at a later date (30,31). It is not usually recommended to use LI immediately prior to performing Endoscopic Submucosal Dissection (ESD) as the mucosa becomes “wrinkly” and appears to lose some of its “looseness” to the underlying submucosal tissue making it more difficult to perform an ESD. However, some centres continue to use LI as it provides clear delimitation of the resection margins. In this scenario, a lower concentration may be employed (e.g., 1.5%).
Some groups also advocate using sodium thiosulphate solution (STS) as an antidote after marking, prior to the ESD. Spraying of STS after oesophageal chromoendoscopy with LI has been reported to neutralize free iodine. STS is a water-soluble neutral chemical that is prepared by dissolving STS reagent in distilled water in a sterilized condition to a 5% concentration.
Once LI is sprayed through the endoscopic catheter and lesion assessment is completed, the site should be washed with water and then sprayed with STS. The time interval between the application of the iodine and STS sprays is important and should be performed as soon as possible (32).
Studies designed to analyse the utility of LI chromoendoscopy in OSCC includes detection of suspicious lesions and characterisation in the form of assessing the mucosal extension. In a prospective comparison study, LI was shown to be superior in demarcating areas of oesophageal cancer compared to white light (33). Dawsey and colleagues also found that staining with LI aided the diagnostic potential of dysplastic and cancer lesions (34). The sensitivity to detect OSCC on white light was 62%. This increased to 96% with LI chromoendoscopy. LI could also be used to screen patients with prior aerodigestive tract cancer. In a study undertaken by Tincani and colleagues, LI was able to further pick up another 20% of oesophageal cancers and high-grade dysplastic lesions following detailed white light endoscopic examination. All patients in this cohort had a history of recent head and neck or tracheobronchial squamous cell carcinoma. Similar outcomes were obtained in smaller cohorts by two other groups for high-risk individuals with prior head and neck cancers (35,36).
Similarly, with the increasing adoption of virtual chromoendoscopy such as narrow band imaging (NBI), the future role of LI chromoendoscopy in the detection and characterisation of OSCC has been questioned. Morita and colleagues, in a recent 2017 systematic review and meta-analyses, studied a large cohort of over 1,900 patients. They concluded that although for detection NBI and LI are similar, NBI was superior to LI in discerning high-grade dysplasia and cancer from other mucosal alterations (37). Despite the compelling evidence to move towards virtual chromoendoscopy, endoscopy practices throughout the world still varies. LI still has a role to play in centres that do not possess modern endoscopic systems or expertise to interpret findings on electronic chromoendoscopy platforms. It also appears to be more precise when determining margins for lesions deemed suitable to be resected endoscopically.
Detection of dysplastic lesions in IBD
In IBD, random biopsies were previously relied upon to detect dysplastic lesions that were flat and often difficult to pick up on white light endoscopy (38). Rutter and colleagues performed 2,904 random colonic biopsies for patients with longstanding extensive ulcerative colitis and reported that none of the biopsies were dysplastic. Following this, a study examining back-to-back colonoscopy with “pancolonic” indigo carmine examination by the same group of patients picked up mucosal abnormalities in 157 patients. There was an astounding 18.5-fold less biopsies performed using the chromoendoscopy-facilitated protocol (39). The sensitivity for random biopsies is so poor that endoscopists risks missing true flat dysplastic lesions in its early curative stage (40).
Data from various randomised and prospective trials favour the utility of dye-based chromoendoscopy given the proven overall sensitivity and accuracy in detecting suspicious lesions.
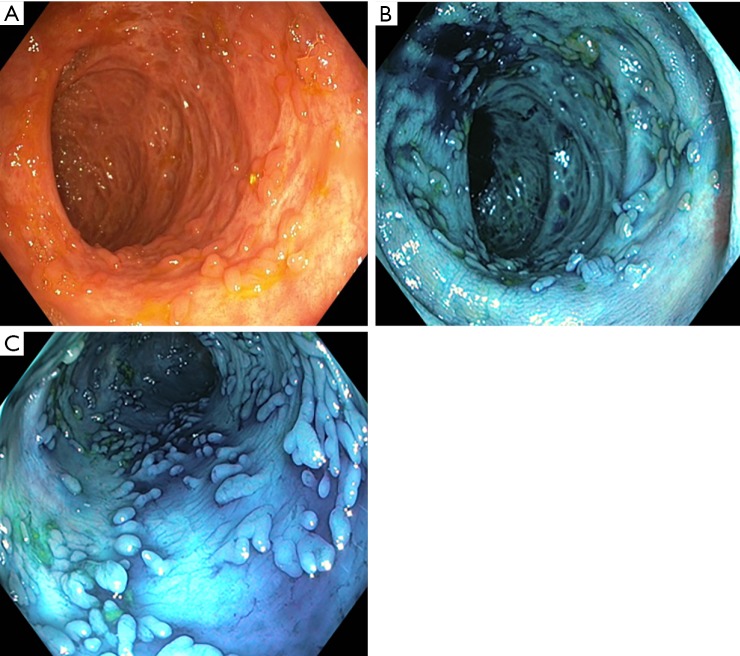
An international multidisciplinary group representing a wide spectrum of stakeholders regarding IBD surveillance developed the SCENIC International Consensus Statement on Surveillance and Management of Dysplasia in Inflammatory Bowel Disease. The authors recommended using dye-based chromoendoscopy given the proven overall sensitivity and accuracy in detecting suspicious lesions. Therefore, the panchromoendoscopy technique should be performed for detection of dysplastic lesions in IBD patients. This consists in spraying indigo carmine or methylene blue circumferentially throughout the colon either through the water jet channel by using a pump or through the biopsy channel by using a spray catheter (41-44) (Figure 3). A randomised controlled trial found compelling evidence of methylene blue chromoendoscopy outperforming targeted biopsies by 3 to 1 in detecting more neoplastic lesions with a sensitivity of 93% (45). The outcome was so convincing that the same group took their research further by combining chromoendoscopy with endomicroscopy. Circumscribed lesions identified with chromoendoscopy were assessed in detail with endomicroscopy to determine the need for targeted biopsies. The authors reported 4.75-fold increase in neoplastic lesion detection with half the amount of “usual random biopsies” required (46). However, these results warrant caution as this study combined another advance technique (endomicroscopy) which requires specialised facilities and a skill set that is not widely available in most centres.
Figure 3.
Colon chromoendoscopy with methylene blue in IBD patients. (A) Colonic mucosa in IBD patient; (B) colonic mucosa sprayed with methylene blue; (C) colonic mucosa sprayed with methylene blue. IBD, inflammatory bowel disease.
In a “colitic” bowel, one has to contend with inflammation and scarred segments. Should any doubt arise, one should fall back to random biopsies to avoid missing “invisible dysplasia” although this is becoming less of a need with the complement of high-resolution video endoscopy systems (47).
Detection of early gastric cancer (EGC) with indigo carmine or indigo carmine with AA
Indigo carmine can facilitate the evaluation of suspicious areas in the stomach in order to detect EGC (48). Combination of AA and indigo carmine has been used in the assessment of gastric adenomas (Figure 4). Kono and colleagues evaluated the sensitivity of the “reddish colour change” with this technique. This sign significantly increased the detection of EGC (multivariate analysis odds ratio =11) with superior sensitivity when compared to colour evaluation with white light endoscopy alone (87.0% versus 56.5%, P<0.05) (49).
Figure 4.
Chromoendoscopy in the stomach. (A) Gastric mucosa on white light; (B) gastric mucosa stained with acetic acid; (C) gastric mucosa stained with indigo carmine.
Dye-assisted chromoendoscopy in the assessment of colonic lesions
Crystal violet
Dye-assisted chromoendoscopy using crystal violet is useful to emphasise the pit pattern of the colonic mucosa and predict the histology. Crystal violet is an absorptive dye that stains epithelial cells in the colonic mucosal glands, making the pit look white after dyeing. It is generally used in a concentration of 0.05%. After spraying onto the colonic mucosa, staining takes 2 to 3 minutes.
Magnifying chromoendoscopy with crystal violet can aid in identifying invasive cancers. The combination of virtual and dye-based chromoendoscopy methods is highly effective in differentiating intramucosal or superficial submucosal cancers from deep submucosal cancers (50). Classifications based on NBI, such as the Japan NBI Expert Team (JNET) classification, can also predict the depth of invasion. However, JNET has limited performance for the differentiation of superficial and invasive subtypes (i.e., JNET 2B and 3). Therefore, a three-step strategy for management of these lesions has been suggested: the first step is to identify the lesion; the second step is to observe capillary and surface patterns of the identified lesion by NBI followed by examining the pit pattern of the lesion by dye-assisted chromoendoscopy (if doubt whether the superficial or deep submucosa is involved) (51,52).
Indigo carmine
Indigo carmine chromoendoscopy (IC) is a blue dye spraying technique used to improve the detection of neoplastic lesions by enhancing the contrast of raised and deepened areas such as pit patterns. It has been shown to significantly increase the adenoma detection rate (ADR) (53).
Kudo’s “pit patterns” classification distinguishes neoplastic and non-neoplastic polyps via magnifying endoscopy. According to their appearance, structure and staining patterns, Type I pits appear as roundish pits; Type II pits appear as stellar or papillary pits; Type III-s pits are small roundish, tubular pits (smaller than Type I) and Type III-L are roundish and tubular pits (larger than Type I); Type IV pits appear as branch-like or gyrus-like pits and Type V pits appear as non-structured pits. Type I and II are considered benign changes (e.g., normal, hyperplastic, inflammatory polyps), whereas pit pattern classes III–V are considered to show neoplastic and malignant changes (54).
Tischendorf and colleagues compared the value of magnifying chromoendoscopy and NBI in classifying colorectal polyps. Using the Kudo classification of mucosal patterns, NBI with magnification resulted in a sensitivity of 90.5% and a specificity of 89.2% for the differentiation of neoplastic versus non-neoplastic lesions. This performance was comparable to magnifying chromoendoscopy with a sensitivity of 91.7% and a specificity of 90%, respectively. Using vascular patterns for differentiation, NBI with magnification correctly identified 93.7% of neoplastic polyps and 89.2% of non-neoplastic colorectal lesions, whereas magnifying chromoendoscopy had a specificity of 95% but a sensitivity of only 66.7% (55).
The future in dye-based chromoendoscopy
Dye-based chromoendoscopy historically has aided endoscopists to more accurately detect and characterise lesions throughout the GIT and still has a relevant role. The most challenging feature regarding dye-based chromoendoscopy when compared to the current virtual chromoendoscopy techniques is the longer procedural time. Novel trials are taking place trying to mitigate this issue. For instance, the use of orally ingested tablets of methylene blue combined with the bowel preparation for screening colonoscopy has showed promising results in increasing adenoma detection (56).
Conclusions
Random sampling of mucosa has been a primary method used for surveillance. This approach has been ineffective, time-consuming, expensive and has low diagnostic yield. This has led to a more focused approach, taking targeted biopsies of any mucosal abnormalities using dye-based chromoendoscopy, virtual chromoendoscopy or a combination of dye-based chromoendoscopy with high magnification imaging (48).
Chromoendoscopy has important implications for the detection, demarcation and characterisation of dysplasia as exemplified by the scenarios above. The procedure is safe and provides important clinical information which eventually leads to better treatment options (56).
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Kaltenbach T, Sano Y, Friedland S, et al. American Gastroenterological Association. American Gastroenterological Association (AGA) Institute technology assessment on image-enhanced endoscopy. Gastroenterology 2008;134:327-40. 10.1053/j.gastro.2007.10.062 [DOI] [PubMed] [Google Scholar]
- 2.Subramanian V, Ragunath K. Advanced endoscopic imaging: a review of commercially available technologies. Clin Gastroenterol Hepatol 2014;12:368-76.e1. 10.1016/j.cgh.2013.06.015 [DOI] [PubMed] [Google Scholar]
- 3.Qumseya BJ, Wang H, Badie N, et al. Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett’s esophagus: a meta-analysis and systematic review. Clin Gastroenterol Hepatol 2013;11:1562-70.e1-2. [DOI] [PMC free article] [PubMed]
- 4.Tholoor S, Bhattacharyya R, Tsagkournis O, et al. Acetic acid chromoendoscopy in Barrett’s esophagus surveillance is superior to the standardized random biopsy protocol: results from a large cohort study (with video). Gastrointest Endosc 2014;80:417-24. 10.1016/j.gie.2014.01.041 [DOI] [PubMed] [Google Scholar]
- 5.Seewald S, Ang TL, Groth S, et al. Detection and endoscopic therapy of early esophageal adenocarcinoma. Curr Opin Gastroenterol 2008;24:521-9. 10.1097/MOG.0b013e3282ff8b1f [DOI] [PubMed] [Google Scholar]
- 6.Ohnita K, Isomoto H, Shikuwa S, et al. Magnifying chromoendoscopic findings of early gastric cancer and gastric adenoma. Dig Dis Sci 2011;56:2715-22. 10.1007/s10620-011-1638-6 [DOI] [PubMed] [Google Scholar]
- 7.Pohl J, Schneider A, Vogell H, et al. Pancolonic chromoendoscopy with indigo carmine versus standard colonoscopy for detection of neoplastic lesions: a randomised two-centre trial. Gut 2011;60:485-90. 10.1136/gut.2010.229534 [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto T, Saito Y, Nakajima T, et al. Comparison of magnifying chromoendoscopy and narrow-band imaging in estimation of early colorectal cancer invasion depth: a pilot study. Dig Endosc 2011;23:118-23. 10.1111/j.1443-1661.2010.01049.x [DOI] [PubMed] [Google Scholar]
- 9.dos Santos CE, Lim JC, Lopes CV, et al. Computerized virtual chromoendoscopy versus indigo carmine chromoendoscopy combined with magnification for diagnosis of small colorectal lesions: a randomized and prospective study. Eur J Gastroenterol Hepatol 2010;22:1364-71. 10.1097/MEG.0b013e32833a5d63 [DOI] [PubMed] [Google Scholar]
- 10.Pohl J, Lotterer E, Balzer C, et al. Computed virtual chromoendoscopy versus standard colonoscopy with targeted indigocarmine chromoscopy: a randomised multicentre trial. Gut 2009;58:73-8. 10.1136/gut.2008.153601 [DOI] [PubMed] [Google Scholar]
- 11.Repici A, Ciscato C, Wallace M, et al. Evaluation of genotoxicity related to oral methylene blue chromoendoscopy. Endoscopy 2018;50:1027-32. 10.1055/a-0630-1004 [DOI] [PubMed] [Google Scholar]
- 12.Feuerstein JD, Rakowsky S, Sattler L, et al. Meta-analysis of dye-based chromoendoscopy compared with standard- and high-definition white-light endoscopy in patients with inflammatory bowel disease at increased risk of colon cancer. Gastrointest Endosc 2019;90:186-195.e1. 10.1016/j.gie.2019.04.219 [DOI] [PubMed] [Google Scholar]
- 13.Kheir AO, Soetikno R, Kaltenbach T. Chromoendoscopy. In: Konda VJA, Waxman I. editors. Endoscopic Imaging Techniques and Tools. Switzerland: Springer International Publishing, 2016:29-49. [Google Scholar]
- 14.Guelrud M, Herrera I, Essenfeld H, et al. Enhanced magnification endoscopy: a new technique to identify specialized intestinal metaplasia in Barrett’s esophagus. Gastrointest Endosc 2001;53:559-65. 10.1067/mge.2001.114059 [DOI] [PubMed] [Google Scholar]
- 15.Van Le L, Broekhuizen FF, Janzer-Steele R, et al. Acetic acid visualization of the cervix to detect cervical dysplasia. Obstet Gynecol 1993;81:293-5. [PubMed] [Google Scholar]
- 16.Lambert R, Rey JF, Sankaranarayanan R. Magnification and chromoscopy with the acetic acid test. Endoscopy 2003;35:437-45. 10.1055/s-2003-38766 [DOI] [PubMed] [Google Scholar]
- 17.Longcroft-Wheaton G, Brown J, Basford P, et al. Duration of acetowhitening as a novel objective tool for diagnosing high risk neoplasia in Barrett’s esophagus: a prospective cohort trial. Endoscopy 2013;45:426-32. 10.1055/s-0032-1326630 [DOI] [PubMed] [Google Scholar]
- 18.Coletta M, Sami SS, Nachiappan A, et al. Acetic acid chromoendoscopy for the diagnosis of early neoplasia and specialized intestinal metaplasia in Barrett’s esophagus: a meta-analysis. Gastrointest Endosc 2016;83:57-67.e1. 10.1016/j.gie.2015.07.023 [DOI] [PubMed] [Google Scholar]
- 19.Sharma P, Savides TJ, Canto MI, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on imaging in Barrett’ s Esophagus. Gastrointest Endosc 2012;76:252-4. 10.1016/j.gie.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 20.Bhandari P, Kandaswamy P, Cowlishaw D, et al. Acetic acid-enhanced chromoendoscopy is more cost-effective than protocol-guided biopsies in a high-risk Barrett’s population. Dis Esophagus 2012;25:386-92. 10.1111/j.1442-2050.2011.01267.x [DOI] [PubMed] [Google Scholar]
- 21.Hoffman A, Korczynski O, Tresch A, et al. Acetic acid compared with i-scan imaging for detecting Barrett's esophagus: a randomized, comparative trial. Gastrointest Endosc 2014;79:46-54. 10.1016/j.gie.2013.07.013 [DOI] [PubMed] [Google Scholar]
- 22.Shiozaki H, Tahara H, Kobayashi K, et al. Endoscopic screening of early esophageal cancer with the Lugol dye method in patients with head and neck cancers. Cancer 1990;66:2068-71. [DOI] [PubMed] [Google Scholar]
- 23.GLOBOCAN. Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. International Agency for Research on Cancer-World Heatlh Organization. 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer/aspx
- 24.Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013;19:5598-606. 10.3748/wjg.v19.i34.5598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansour NM, Anandasabapathy S. Lugol’s Chromoendoscopy in the Screening of Esophageal Squamous Cell Carcinoma: Time to Take a Closer Look? Clin Gastroenterol Hepatol 2018;16:1562-63. 10.1016/j.cgh.2018.05.034 [DOI] [PubMed] [Google Scholar]
- 26.Li J, Xu R, Liu M, et al. Lugol Chromoendoscopy Detects Esophageal Dysplasia With Low Levels of Sensitivity in a High-Risk Region of China. Clin Gastroenterol Hepatol 2018;16:1585-92. 10.1016/j.cgh.2017.11.031 [DOI] [PubMed] [Google Scholar]
- 27.Ina H, Shibuya J, Ohashi I, et al. The frequency of a concomitant early esophageal cancer in male patients with oral and oropharyngeal cancer. Screening results using Lugol dye endoscopy. Cancer 1994;73:2038-41. [DOI] [PubMed] [Google Scholar]
- 28.Inoue H, Rey JF, Lightdale C. Lugol chromoendoscopy for esophageal squamous cell cancer. Endoscopy 2001;33:75-9. [PubMed] [Google Scholar]
- 29.Shimizu Y, Omori T, Yokoyama A, et al. Endoscopic diagnosis of early squamous neoplasia of the esophagus with iodine staining: high-grade intra-epithelial neoplasia turns pink within a few minutes. J Gastroenterol Hepatol 2008;23:546-50. 10.1111/j.1440-1746.2007.04990.x [DOI] [PubMed] [Google Scholar]
- 30.Mori M, Adachi Y, Matsushima T, et al. Lugol staining pattern and histology of esophageal lesions. Am J Gastroenterol 1993;88:701-5. [PubMed] [Google Scholar]
- 31.Toriie S, Akasaka Y, Yamaguchi K, et al. New trial for endoscopical observation of esophagus by dye spraying method. G E N 1976;30:159-65. [PubMed] [Google Scholar]
- 32.Kondo H, Fukuda H, Ono H, et al. Sodium thiosulfate solution spray for relief of irritation caused by Lugol's stain in chromoendoscopy. Gastrointest Endosc 2001;53:199-202. 10.1067/mge.2001.110730 [DOI] [PubMed] [Google Scholar]
- 33.Meyer V, Burtin P, Bour B, et al. Endoscopic detection of early esophageal cancer in a high-risk population: does Lugol staining improve videoendoscopy? Gastrointest Endosc 1997;45:480-4. 10.1016/S0016-5107(97)70177-9 [DOI] [PubMed] [Google Scholar]
- 34.Dawsey SM, Fleischer DE, Wang GQ, et al. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer 1998;83:220-31. [DOI] [PubMed] [Google Scholar]
- 35.Tincani AJ, Brandalise N, Altemani A, et al. Diagnosis of superficial esophageal cancer and dysplasia using endoscopic screening with a 2% lugol dye solution in patients with head and neck cancer. Head Neck 2000;22:170-4. [DOI] [PubMed] [Google Scholar]
- 36.Boller D, Spieler P, Schoenegg R, et al. Lugol chromoendoscopy combined with brush cytology in patients at risk for esophageal squamous cell carcinoma. Surg Endosc 2009;23:2748-54. 10.1007/s00464-009-0489-0 [DOI] [PubMed] [Google Scholar]
- 37.Morita FH, Bernardo WM, Ide E, et al. Narrow band imaging versus lugol chromoendoscopy to diagnose squamous cell carcinoma of the esophagus: a systematic review and meta-analysis. BMC Cancer 2017;17:54. 10.1186/s12885-016-3011-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farraye FA, Odze RD, Eaden J, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology 2010;138:738-45. 10.1053/j.gastro.2009.12.037 [DOI] [PubMed] [Google Scholar]
- 39.Rutter MD, Saunders BP, Schofield G, et al. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut 2004;53:256-60. 10.1136/gut.2003.016386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Günther U, Kusch D, Heller F, et al. Surveillance colonoscopy in patients with inflammatory bowel disease: comparison of random biopsy vs. targeted biopsy protocols. Int J Colorectal Dis 2011;26:667-72. 10.1007/s00384-011-1130-y [DOI] [PubMed] [Google Scholar]
- 41.Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology 2015;148:639-651.e28. 10.1053/j.gastro.2015.01.031 [DOI] [PubMed] [Google Scholar]
- 42.Picco MF, Pasha S, Leighton JA, et al. Procedure time and the determination of polypoid abnormalities with experience: implementation of a chromoendoscopy program for surveillance colonoscopy for ulcerative colitis. Inflamm Bowel Dis 2013;19:1913-20. [DOI] [PubMed] [Google Scholar]
- 43.Mohammed N, Kant P, Abid F, et al. High definition white light endoscopy (Hdwle) versus high definition with chromoendoscopy (Hdce) in the detection of dysplasia in long standing ulcerative colitis: a randomized controlled trial. Gastrointest Endosc 2015;81:AB148 10.1016/j.gie.2015.03.1237 [DOI] [Google Scholar]
- 44.Marion JF, Waye JD, Present DH, et al. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol 2008;103:2342-9. 10.1111/j.1572-0241.2008.01934.x [DOI] [PubMed] [Google Scholar]
- 45.Kiesslich R, Fritsch J, Holtmann M, et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology 2003;124:880-8. 10.1053/gast.2003.50146 [DOI] [PubMed] [Google Scholar]
- 46.Kiesslich R, Goetz M, Lammersdorf K, et al. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology 2007;132:874-82 10.1053/j.gastro.2007.01.048 [DOI] [PubMed] [Google Scholar]
- 47.Sridhar S, Wu GY. editors. Diagnostic and Therapeutic Procedures in Gastroenterology. Clinical Gastroenterology. Humana Press, Springer Nature, 2019. [Google Scholar]
- 48.Konda VJA, Waxman I. Endoscopic imaging, Techniques and tools. ISBN 978-3-319-30053-5. DOI 10.1007/978-3-319-30053-5 [DOI]
- 49.Kono Y, Takenaka R, Kawahara Y, et al. Chromoendoscopy of gastric adenoma using an acetic acid indigocarmine mixture. World J Gastroenterol 2014;20:5092-7. 10.3748/wjg.v20.i17.5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee HH, Lee BI. Image-Enhanced Endoscopy in Lower Gastrointestinal Diseases: Present and Future. Clin Endosc 2018;51:534-40. 10.5946/ce.2018.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uraoka T, Oka S, Ichihara S, et al. Endoscopic management of colorectal tumors less than 10 mm in size: Current status and future perspectives in Japan from a questionnaire survey. Dig Endosc 2018;30 Suppl 1:36-40. 10.1111/den.13060 [DOI] [PubMed] [Google Scholar]
- 52.Di Stefano AFD, Radicioni MM, Vaccani A, et al. Methylene blue MMX® tablets for chromoendoscopy. Bioavailability, colon staining and safety in healthy volunteers undergoing a full colonoscopy. Contemp Clin Trials 2018;71:96-102. 10.1016/j.cct.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 53.Cellier C, Perrod G, Colas C, et al. Back-to-Back Comparison of Colonoscopy With Virtual Chromoendoscopy Using a Third-Generation Narrow-Band Imaging System to Chromoendoscopy With Indigo Carmine in Patients With Lynch Syndrome. Am J Gastroenterol 2019;114:1665-70. 10.14309/ajg.0000000000000386 [DOI] [PubMed] [Google Scholar]
- 54.Li M, Ali SM, Umm-a-OmarahGilani S, et al. Kudo’s pit pattern classification for colorectal neoplasms: A meta-analysis. World J Gastroenterol 2014;20:12649-56. 10.3748/wjg.v20.i35.12649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tischendorf JJ, Wasmuth HE, Koch A, et al. Value of magnifying chromoendoscopy and narrow band imaging (NBI) in classifying colorectal polyps: a prospective controlled study. Endoscopy 2007;39:1092-6. 10.1055/s-2007-966781 [DOI] [PubMed] [Google Scholar]
- 56.Akarsu M, Akarsu C. Evaluation of New Technologies in Gastrointestinal Endoscopy. JSLS 2018. doi: . 10.4293/JSLS.2017.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]