This randomized clinical trial examines the efficacy of gabapentin as pharmacotherapy for alchohol use disorder in adults with a history of alcohol withdrawal.
Key Points
Question
Is gabapentin efficacious in the treatment of alcohol use disorder in adults with a history of alcohol withdrawal symptoms?
Findings
In this randomized clinical trial, gabapentin compared with placebo significantly increased the number of people with total abstinence and reduced drinking. This effect was most significantly observed in those with greater pretreatment alcohol withdrawal symptoms—41% of participants with high alcohol withdrawal symptoms had total abstinence on gabapentin compared with 1% of participants in the placebo arm.
Meaning
This study showed that gabapentin is efficacious in promoting abstinence and reducing drinking in individuals with alcohol use disorder and especially so in those with more alcohol withdrawal symptoms.
Abstract
Importance
Although an estimated 30 million people meet criteria for alcohol use disorder (AUD), few receive appropriate pharmacotherapy. A more personalized, symptom-specific, approach might improve efficacy and acceptance.
Objective
To examine whether gabapentin would be useful in the treatment of AUD, especially in those with the most alcohol withdrawal symptoms.
Design, Setting, and Participants
This double-blind randomized clinical trial conducted between November 2014 and June 2018 evaluated gabapentin vs placebo in community-recruited participants screened and treated in an academic outpatient setting over a 16-week treatment period. A total of 145 treatment-seeking individuals who met Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) criteria for AUD and were not receiving other AUD intervention were screened, and 96 who also met recent alcohol withdrawal criteria were randomized to treatment after 3 abstinent days. Daily drinking was recorded, and percentage of disialo carbohydrate-deficient transferrin in the blood, a heavy drinking marker, was collected at baseline and monthly during treatment.
Interventions
Gabapentin up to 1200 mg/d, orally, vs placebo along with 9 medical management visits (20 minutes each).
Main Outcomes and Measures
The percentage of individuals with no heavy drinking days and those with total abstinence were compared between treatment groups and further evaluated based on prestudy alcohol withdrawal symptoms.
Results
Of 96 randomized individuals, 90 were evaluable (44 in the gabapentin arm and 46 in the placebo arm), with a mean (SD) age of 49.6 (10.1) years; 69 were men (77%) and 85 were white (94%). The evaluable participants had 83% baseline heavy drinking days (4 or more drinks/day for women, 5 or more for men) and met 4.5 alcohol withdrawal criteria from the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition). More gabapentin-treated individuals had no heavy drinking days (12 of 44 participants [27%]) compared with placebo (4 of 46 participants [9%]), a difference of 18.6% (95% CI, 3.1-34.1; P = .02; number needed to treat [NNT], 5.4), and more total abstinence (8 of 44 [18%]) compared with placebo (2 of 46 [4%]), a difference of 13.8% (95% CI, 1.0-26.7; P = .04; NNT, 6.2). The prestudy high–alcohol withdrawal group had positive gabapentin effects on no heavy drinking days (P < .02; NNT, 3.1) and total abstinence (P = .003; NNT, 2.7) compared with placebo, while within the low–alcohol withdrawal group, there were no significant differences. These findings were similar for other drinking variables, where gabapentin was more efficacious than placebo in the high–alcohol withdrawal group only. Gabapentin caused more dizziness, but this did not affect efficacy.
Conclusions and Relevance
These data, combined with others, suggest gabapentin might be most efficacious in people with AUD and a history of alcohol withdrawal symptoms. Future studies should evaluate sleep changes and mood during early recovery as mediators of gabapentin efficacy.
Trial Registration
ClinicalTrials.gov Identifier: NCT02349477
Introduction
Up to 30 million people in the United States meet criteria for alcohol use disorder (AUD), and the number is increasing over time,1 accounting for considerable morbidity and mortality.2 However, only 20% of those who might benefit from treatment receive it, and of those individuals, only 20% (or less than 1 million individuals) receive medication to help them with maintaining abstinence or reducing drinking. One reason is that available medications are not universally efficacious.3 However, there is reason to believe that some subgroups of patients might respond better to available treatments.3
One understudied phenotype or subtype of AUD are people who experience alcohol withdrawal syndrome (AWS).4 Although the quantity and frequency of drinking might predict who is at risk for AWS, there is considerable variation (some likely genetic) as to who will experience AWS on the cessation of drinking. More than half of inpatients with AUD5 and 35% of community individuals with AUD reported alcohol withdrawal symptoms, leading to a higher rate of alcohol problems at follow-up.6 Similarly, those who have undergone previous medicated detoxifications relapse quicker after cessation of alcohol use.7 While up to 30% of individuals with AUD presenting for clinical trials (who are similar to those seen in addiction outpatient clinics and in primary care) have acute AWS, it has been recognized that lower-grade AWS might be present, and last longer, in considerably more individuals. Many individuals also stop or reduce drinking prior to initiating treatment and therefore may experience mild to severe AWS without being formally diagnosed. In addition, a constellation of problems, such as irritability, anxiety, dysphoria, difficulties with concentration, and insomnia, might persist for a time after initial abstinence. Some have labeled these lingering symptoms as protracted withdrawal8 or protracted abstinence.9 In general, this constellation of symptoms and any desire to drink emanating from them are not likely to be influenced by antireinforcement or anticraving medications, such as naltrexone, which are likely to target more reward-based craving.10,11,12,13,14 Consistent with this notion, AWS is thought to be mediated primarily by γ-aminobutyric acid (GABA) and glutamate brain signaling15,16,17 in contrast to reward-based craving, in which opioid and dopamine brain signaling play a larger role.18,19 Therefore, medications that target brain GABA and glutamate brain signaling systems might be particularly useful in the treatment of AWS and, by extension, in those who have previously shown a biological propensity to experience AWS.16,20,21
Gabapentin has unique pharmacologic characteristics, binding to voltage-sensitive calcium channels at the α2δ-1 site,22 affecting their function23 as well as influencing receptor trafficking.24 Through these mechanisms, gabapentin is thought to secondarily influence GABA and glutamate tone/activity,25,26,27 which can be clinically measured.28
Gabapentin is efficacious for the treatment of acute alcohol withdrawal symptoms29,30 and also provides short-term relapse prevention after medicated alcohol detoxification,31 perhaps by an effect on sleep normalization.32,33 Post hoc analysis has shown effectiveness of treatment with gabapentin, in combination with flumazenil34 or naltrexone,35 in patients with current or historic alcohol withdrawal symptoms, consistent with basic science findings.36 Recent randomized clinical trials37,38 of gabapentin had mixed results but did not take alcohol withdrawal symptoms into account. Gabapentin is nevertheless worthy of further study given its earlier suggested effectiveness in those with alcohol withdrawal symptoms, its minimal cognitive effects,39,40 its lack of significant adverse interaction with alcohol,30,41,42 and its kidney excretion, which makes it potentially safer for individuals with liver disease.
The current study was a double-blind, placebo-controlled randomized clinical trial of treatment with gabapentin in outpatient individuals with AUD who reported current or historical alcohol withdrawal symptoms. A secondary aim was to evaluate the relationship of recently experienced alcohol withdrawal symptoms to gabapentin response, with the hypothesis that gabapentin would be more efficacious in those with higher levels of self-reported alcohol withdrawal symptoms.
Methods
Trial Design and Participants
The study was a 16-week randomized clinical trial (ClinicalTrials.gov identifier: NCT02349477) of gabapentin vs placebo. The trial protocol was approved by the Medical University of South Carolina Institutional Review Board for Human Research and is included in Supplement 1. Participants had to be aged 18 to 70 years; meet Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) (DSM-5) criteria for AUD, including alcohol withdrawal, as determined by Structured Clinical Interview for DSM-543; have a minimum of 5 drinks per day in the 90 days prior to assessment; and be abstinent at least 3 days prior to randomization as measured by breath analysis and urinary ethyl glucuronide testing. Participants could be using cannabis or other drugs but not meet criteria for drug use disorder, except nicotine, and could have no other psychoactive drug detected in the urine. Participants could not be taking psychotropic medications other than antidepressants (with the dose stable for at least 1 month) or meet current criteria for any major depressive disorder, bipolar disorder, psychotic disorder, or eating disorder. A history of posttraumatic stress disorder with stable symptoms was allowed, given its comorbidity with AUD and the utility of gabapentin in some anxiety disorders.44 Participants had to be medically stable (including liver enzymes alanine aminotransferase and aspartate aminotransferase less than 3 times the upper limit of normal). Women could not be pregnant or breastfeeding, must be using a reliable form of contraception, or must be postmenopausal. A history of alcohol withdrawal seizure or a Clinical Institute Withdrawal Assessment for Alcohol–Revised (CIWA-Ar) scale45 score of 10 or more during assessment was exclusionary. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Recruitment
Participants were recruited from November 2014 through June 2018 primarily by community advertisement and were not engaged in other alcohol treatment. Participants provided written informed consent approved by the Medical University of South Carolina Institutional Review Board for Human Research before formal assessment. Individuals paid $50 for attending the end-of-study assessment to provide drinking and biological data.
Interventions
After at least 3 days of abstinence and assessment, participants were randomized (using prespecified computer random assignment by the investigational pharmacy) to receive gabapentin (day 1: 300 mg at bedtime; day 2: 300 mg in the morning and at bedtime; days 3 and 4: 300 mg in the morning, at noon, and at bedtime; and days 5 through 112: 300 mg in the morning and at noon and 600 mg at bedtime for a total of 1200 mg) or identical placebo capsules (given at the same quantity and times) in blister packs for 16 weeks. Study medications were identically overencapsulated with 25 mg of riboflavin (added to measure adherence) and distributed in labeled blister packs. Medical management, which consisted of a relatively brief (15-20 minutes) educational/supportive and adherence-enhancing clinical interaction,46,47 was conducted on weeks 1, 2, 3, 4, 6, 8, 10, 12, and 16 when adherence (pill count and urine riboflavin collection) and medication adverse effects, using the Systematic Assessment for Treatment of Emergent Events (SAFTEE) instrument,48 were assessed.
Assessment
Prior to randomization, multiple assessments were administered, including the Structured Clinical Interview for DSM-5, the Alcohol Withdrawal Symptom Checklist (AWSC),49 the Alcohol Dependence Scale (ADS),50 the Obsessive Compulsive Drinking Scale (OCDS),51 Form 90 (alcohol consumption, drug use, daily calendar method),52 the CIWA-Ar scale,45 and baseline physical complaints. Laboratory tests included a health screen, liver function tests, pregnancy test (in women), and alcohol use markers γ-glutamyltransferase (GGT), urine ethyl glucuronide, and percentage of disialo carbohydrate-deficient transferrin (%dCDT).53,54 White blood cell DNA samples were obtained for potential future analysis.
During each medical management session, the calendar-based timeline follow-back method55 was used to assess daily drinking since the last visit. In addition, OCDS and SAFTEE assessments were performed at each visit. To confirm verbal report of abstinence or heavy drinking, %dCDT was collected on weeks 3, 6, 10, and 16.
Outcome Measures
The primary a priori defined drinking outcome (efficacy) measure was the percentage of participants with no heavy drinking days (defined as 5 or more drinks per day for men and 4 or more drinks per day for women; standard drinks [defined as containing 14 g of ethanol, or translated into typical drinks: 1.5 oz of spirits, 5 oz of wine, or 12 oz of beer]) throughout the study.38,56,57 The main secondary outcome measure was the percentage of participants with no drinking days (ie, total abstinence) throughout the study. Verbal report was confirmed by %dCDT testing and drinking status corrected—that is, those who reported no drinking or no heavy drinking but had a %dCDT greater than 1.7% at any time during the study were considered to have heavy drinking days and not be abstinent. This led to 3 individuals being reclassified prior to unblinding of medication group allocation.
Additional analyses included the relationship of the level of reported recent alcohol withdrawal symptoms on the AWSC49 and medication response. Other drinking variables routinely reported in AUD randomized clinical trials that might be useful to clinicians were also evaluated. These included the percentage of heavy drinking days, percentage of days abstinent, number of drinks per day, and number of drinks per drinking day.
Assays and Biomarkers
Urine riboflavin was assayed in the Medical University of South Carolina Clinical Neurobiology Laboratory (directed by R.A.) using standard/calibration curves constructed from known amounts of riboflavin against which unknown amounts of urine riboflavin were calculated by fluorescence detection (448 nm excitation/510 nm emission). Values greater than 1300 ng/mL indicated adherence. The %dCDT was measured with a reference high-performance liquid chromatography assay.54 Using this international standardized assay, a value greater than 1.7% is close to 100% specific for sustained heavy drinking in the weeks prior to testing and can be used to corroborate or independently evaluate drinking in clinical trials.58 Urine ethyl glucuronide (Microgenics Diagnostics) and other blood chemistries, including GGT, were measured with an autoanalyzer.
Power Calculations and Statistical Analysis Plan
Data from individuals with an alcohol withdrawal history in 2 prior AUD trials that included gabapentin treatment34,35 indicated the percentage of individuals with no heavy drinking days (success rates) to be between 38% and 41% in the placebo-treated groups and between 71% and 79% in the gabapentin-treated group, estimating the power to detect a gabapentin effect to be between 0.83 and 0.98 at α = .05 with a sample size of 45 individuals per medication group.
For no heavy drinking (primary outcome) and no drinking/total abstinence (secondary outcome), the number of evaluable individuals who met that criteria by verbally reported drinking only or by %dCDT verification over the whole 16-week trial was analyzed using a 2-sample z test to produce confidence intervals and a χ2 P value; P values less than .05 were considered significant. In addition, the number needed to treat (NNT) or the number needed to harm (NNH)59 is reported. For analytic purposes, the 9 placebo-treated and 5 gabapentin-treated individuals that had missing drinking data were considered to be drinking or heavy drinking. A sensitivity analysis evaluating the same effects in only those who completed the study and were adherent with medication was also performed. All outcome data were collected by study staff and analyzed without knowledge of medication group assignment.
For evaluation of the level of alcohol withdrawal symptoms predicting gabapentin response, the prestudy AWSC (see eFigure in Supplement 2) was divided into low or high alcohol withdrawal based on median split. A χ2 test evaluating the medication within the different alcohol withdrawal groups on no heavy drinking and total abstinence was conducted for the total study period.
Evaluation of other drinking data was done using a linear mixed model with an unstructured variance/covariance matrix in which drinking parameters (percentage of heavy drinking days, percentage of drinking days, number of drinks per day, and number of drinks per drinking day) were evaluated over the 4 months of the study. These models measured the main effects of time (month), medication group, and alcohol withdrawal symptom group (low or high) and their potential interactions. In these models, missing data were assumed to be missing at random. Statistical analyses were performed using SPSS version 24 (IBM).
Results
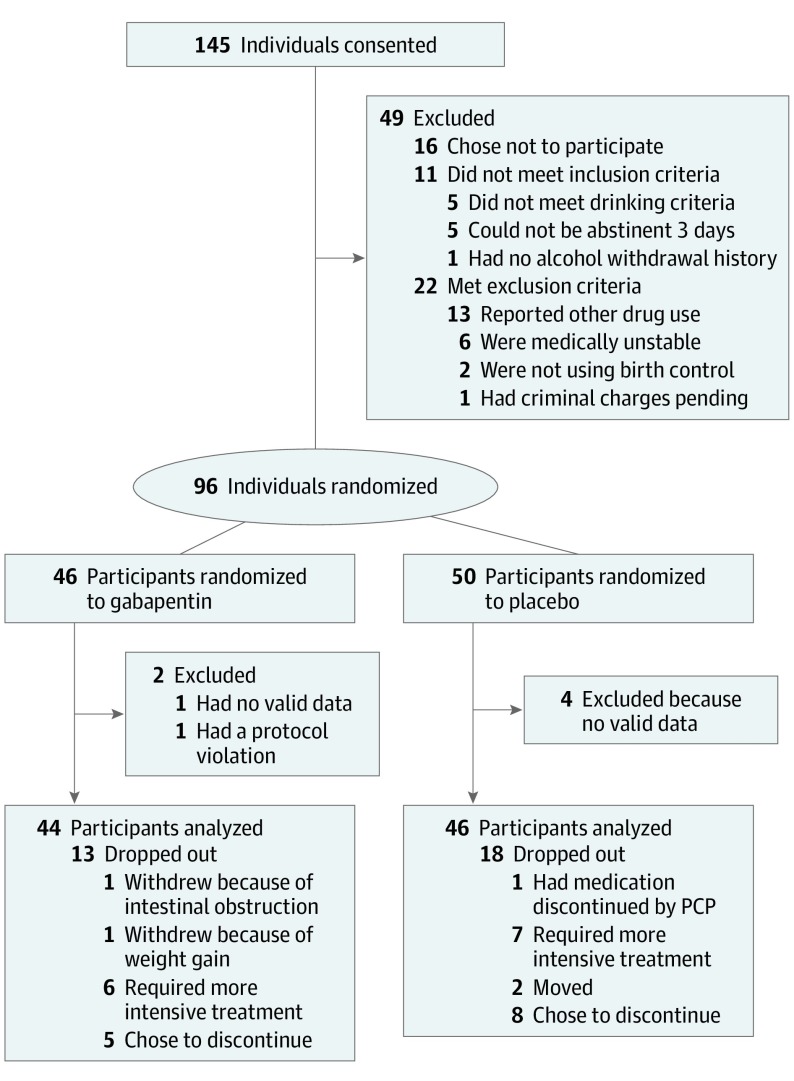
Participant recruitment and disposition are summarized in the CONSORT diagram (Figure 1). Essentially, 145 individuals meeting DSM-5 AUD criteria provided written informed consent. Of these, 96 individuals were randomized to placebo (n = 50) or gabapentin (n = 46). Four participants receiving placebo and 2 receiving gabapentin had no valid follow-up data or an early protocol violation (disclosure of an excluded medical disease that was withheld at screening). Eighteen of 46 participants (39%) receiving placebo and 13 of 44 participants (30%) receiving gabapentin did not complete treatment (reasons given in Figure 1). Participants in both groups attended an average of 7 of 9 medical management sessions. Table 1 summarizes the study entry demographic information and drinking variables. Of the 90 participants included in the final analysis, the mean (SD) age was 49.6 (10.1) years; 69 (77%) were men; 85 (94%) were white; 63 (70%) were employed; and 39 (43%) used nicotine products. They drank a mean of 86% of pretreatment days, with 83% being heavy drinking days, consuming 13 drinks per drinking day, and 25 participants (28%) had past treatments and 12 (13%) had undergone past medical detoxifications. They met a mean of 4.5 of 8 possible DSM-5 criteria for alcohol withdrawal. Sixty-four participants (71%) had elevated %dCDT and 68 (76%) had elevated GGT, both markers of heavy drinking. There were no important significant differences in any prestudy variables between the placebo and gabapentin groups.
Figure 1. CONSORT Diagram.
CONSORT indicates Consolidated Standards of Reporting Trials; PCP, primary care physician.
Table 1. Study Population Demographic Information and Drinking Variables.
| Characteristic | No. (%) | P Valuea | ||
|---|---|---|---|---|
| Total (n = 90) | Gabapentin (n = 44) | Placebo (n = 46) | ||
| Age, mean (SD), y | 49.6 (10.1) | 50.3 (10.4) | 48.9 (9.9) | .53 |
| PTSD (current or past) | 23 (26) | 12 (27) | 11 (24) | .72 |
| Nicotine use | 39 (43) | 18 (41) | 21 (46) | .65 |
| Antidepressant use | 22 (24) | 9 (21) | 13 (28) | .39 |
| Sex (male) | 69 (77) | 34 (77) | 35 (76) | .89 |
| Married/cohabitating | 40 (44) | 21 (48) | 19 (41) | .54 |
| Education (≤12 y) | 10 (11) | 5 (11) | 5 (11) | .94 |
| Employed | 63 (70) | 29 (66) | 34 (74) | .41 |
| Race (white) | 85 (94) | 41 (93) | 44 (96) | .61 |
| Alcohol use and severity indicators, mean (SD) | ||||
| Drinks per dayb | 11.0 (4.4) | 10.9 (4.4) | 11.0 (4.6) | .93 |
| Drinks per drinking dayb | 12.9 (4.5) | 13.2 (4.7) | 12.5 (4.4) | .46 |
| Days abstinent, %b | 13.9 (20.2) | 15.1 (22.2) | 12.8 (18.1) | .59 |
| Heavy drinking days, %b | 82.7 (22.2) | 82.9 (23.0) | 82.5 (21.6) | .93 |
| Days since last drinkc | 4.4 (2.1) | 4.4 (2.2) | 4.4 (2.0) | .93 |
| ADSd | 17.6 (7.3) | 19.5 (7.6) | 15.8 (6.8) | .02 |
| OCDSe score | 26.3 (9.4) | 27.5 (9.3) | 25.1 (9.6) | .22 |
| Alcohol Withdrawal Symptom Checklistf | 9.6 (6.6) | 10.5 (7.1) | 8.8 (6.0) | .22 |
| DSM-5 AW items positive, mean (SD) | 4.5 (1.3) | 4.6 (1.2) | 4.3 (1.3) | .38 |
| Had past alcohol treatments | ||||
| Treatment | 25 (28) | 16 (36) | 9 (20) | .09 |
| Detoxification | 12 (13) | 7 (16) | 5 (11) | .51 |
| Alcohol blood tests (biomarkers) | ||||
| %dCDT ≥ 1.7 | 64 (71) | 31 (72) | 33 (72) | .97 |
| GGT > 36 U/L | 68 (76) | 34 (77) | 34 (74) | .71 |
Abbreviations: ADS, Alcohol Dependence Scale; AW, alcohol withdrawal; DSM-5, Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition); GGT, γ-glutamyltransferase; OCDS, Obsessive Compulsive Drinking Scale; %dCDT, percentage of disialo carbohydrate-deficient transferrin.
The χ2 statistic was used for all categorical variables, and the ANOVA statistic was used for all continuous variables.
Calculated using the 90 days prior to screening.
Days abstinent prior to randomization.
Range, 0-47.
Range, 0-56.
Range, 0-44.
Drinking Outcomes
Data for the primary outcome variable, the percentage of individuals with no heavy drinking days, and the secondary outcome variable, the percentage of individuals with no drinking days, are summarized in Table 2. For no heavy drinking, using verbal report only, gabapentin (12 of 44 participants [27%]) was superior to placebo (6 of 46 participants [13%]) at the trend level (14.2% difference; 95% CI, −2.1 to 30.6; P = .09; NNT, 7.0), but when verbal report was confirmed by the highly specific %dCDT heavy drinking marker,60,61 the difference was statistically significant (18.6% difference; 95% CI, 3.1-34.1; P = .02; NNT, 5.4). For the secondary variable (no drinking days/total abstinence), when using verbal report only, gabapentin treatment (9 of 44 participants [21%]) was superior to placebo (2 of 46 participants [4%]) (16.1% difference; 95% CI, 2.8-29.4; P = .02; NNT, 6.2) and in the same direction after %dCDT confirmation (13.8% difference; 95% CI, 1.0-26.7; P = .04; NNT, 7.2).
Table 2. Number of Individuals With No Heavy Drinking Days or No Drinking Days by Medication Group.
| Outcome Variable | No. (%) | Difference, % (95% CI) | P Value (χ2) | |
|---|---|---|---|---|
| Gabapentin (n = 44) | Placebo (n = 46) | |||
| No heavy drinking days | ||||
| Verbal report only | 12 (27) | 6 (13) | 14.2 (−2.1 to 30.6) | .09 |
| %dCDT corrected | 12 (27) | 4 (9) | 18.6 (3.1 to 34.1) | .02 |
| No drinking days (abstinent) | ||||
| Verbal report only | 9 (21) | 2 (4) | 16.1 (2.8 to 29.4) | .02 |
| %dCDT corrected | 8 (18) | 2 (4) | 13.8 (1.0 to 26.7) | .04 |
Abbreviation: %dCDT, percentage of disialo carbohydrate-deficient transferrin.
Performing a sensitivity analysis on the 21 placebo-treated and 26 gabapentin-treated participants who completed the study (had all 16-week drinking data) and who adhered to pill taking (at least 75% riboflavin-positive urine samples), there were 12 gabapentin-treated individuals compared with 1 placebo-treated individual with no heavy drinking days (χ21 = 5.08; P = .02; NNT, 2.4) and 8 gabapentin-treated individuals compared with 1 placebo-treated individual who remained abstinent throughout the study (χ21 = 9.95; P = .002; NNT, 3.9). These results were similar or stronger when drinking status was adjusted based on within-treatment %dCDT levels.
Effect of Alcohol Withdrawal Severity on Medication Effects
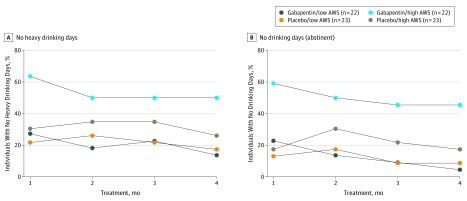
Because the main hypothesis of this study was that gabapentin would be more efficacious in individuals with AUD with more alcohol withdrawal, as suggested in past work,29,30 and because ethical and feasibility issues limited the ability to enroll people in acute withdrawal (CIWA-Ar scores greater than 10), we undertook an additional analysis of the level of self-reported alcohol withdrawal symptoms when participants reduced or stopped drinking in the 2 weeks before randomization using the modified AWSC (eFigure in Supplement 2). Figure 2 shows the percentage of individuals with heavy drinking days and any drinking days in the medication groups predicated by prestudy alcohol withdrawal scores (median split greater or less than 8.5) over the study period. Those with high alcohol withdrawal scores had less relapse to heavy drinking (χ21 = 5.75; P = .02; NNT, 3.1) and more total abstinence (χ21 = 8.69; P = .003; NNT, 2.7) when treated with gabapentin (10 of 22 [46%] and 9 of 22 [41%], respectively) compared with placebo (3 of 23 [13%] and 1 of 23 [4%]), while those with low alcohol withdrawal scores had similar relapse to heavy drinking (χ21 = 0.18; P = .67; NNH, 25.3) and similar total abstinence (χ21 = 0.98; P = .32; NNH, 23.0) when treated with gabapentin (2 of 22 [9%] and 0 of 22 [0%], respectively) compared with placebo (3 of 23 [13%] and 1 of 23 [4%]). The same pattern was found when the data were corrected for %dCDT.
Figure 2. Percentage of Individuals With Heavy Drinking or No Drinking Days During the Study.
Percentage of individuals with either (A) no heavy drinking days or (B) no drinking days (complete abstinence) over the course of the study by medication and alcohol withdrawal level (AWS low or high). At baseline (data not shown), all study participants had drinking days and heavy drinking days, so in this depiction they would start at 0%. AWS indicates alcohol withdrawal symptoms.
To evaluate gabapentin efficacy across a range of other drinking variables, we conducted sensitivity analyses taking AWSC score groups (as above) into account in a linear mixed model (Table 3). Although there was a main effect of time, it did not significantly interact with medication group or AWSC scores; thus, time interaction terms were dropped from the model. In this analysis, there was no main effect of gabapentin over placebo on any drinking variable, but there were significant interactions with AWSC scores such that in the high AWSC group, gabapentin compared with placebo reduced the percentage of heavy drinking days (F1,86.802 = 4.53; P = .04) and promoted more abstinence days (F1,86.419 = 5.68; P = .02) but did not decrease drinks per day (F1,85.252 = 3.40; P = .07) or drinks per drinking day (F1,71.659 = 0.14; P = .71).
Table 3. Gabapentin vs Placebo Treatment on Various Drinking Variables.
| Variable | Mean (SD) | P Valuea | |||
|---|---|---|---|---|---|
| Gabapentin (n = 44) | Placebo (n = 46) | Medication | AWS | AWS × Medication | |
| Heavy drinking days, % | |||||
| Low AWS | 47.6 (6.2) | 31.1 (6.2) | .51 | .01 | .04 |
| High AWS | 19.5 (6.3) | 28.2 (6.1) | |||
| Days abstinent, % | |||||
| Low AWS | 44.9 (6.7) | 63.9 (6.6) | .57 | .02 | .02 |
| High AWS | 75.5 (6.7) | 63.9 (6.6) | |||
| Number of drinks per day | |||||
| Low AWS | 4.6 (0.7) | 3.6 (0.7) | .87 | .05 | .07 |
| High AWS | 2.2 (0.7) | 3.5 (0.7) | |||
| Number of drinks per drinking day | |||||
| Low AWS | 8.4 (0.8) | 7.8 (0.8) | .79 | .78 | .71 |
| High AWS | 7.8 (1.1) | 7.9 (0.8) | |||
Abbreviation: AWS, alcohol withdrawal symptoms.
All P values were derived from a linear model with main effects of medication and AWS and their interaction terms.
Adverse Medication Effects
Adverse effects as recorded with the SAFTEE interview at each study visit showed significantly more gabapentin-treated (n = 25) vs placebo-treated (n = 15) individuals reported mild to moderate dizziness (χ21 = 5.34; P = .02), but in a separate analysis, the presence or absence of dizziness did not significantly account for gabapentin’s effectiveness. While there was no other significant between-group differences in unique individuals reporting other symptoms, there were more within-individual reports of nervousness (χ21 = 10.62; P = .001) and headache (χ21 = 9.70; P = .002) in the gabapentin-treated group (none severe), and more reports of insomnia (χ21 = 10.81; P = .001) in the placebo-treated group (1 severe).
Discussion
These results add to the growing literature on the use of gabapentin for the treatment of AUD. While some previous studies37 have shown efficacy, others have not,38 and a recent meta-analysis62 suggested weak support for its efficacy in treatment of AUD. Although multiple reasons might contribute to the variability of results, an important issue might be the individual’s alcohol withdrawal status/history.34,35 In the current study, we chose to explicitly study the hypothesis that a history of alcohol withdrawal symptoms would influence gabapentin efficacy by evaluating gabapentin only in those who met current or historical criteria for DSM-5 alcohol withdrawal. We further used a previously validated alcohol withdrawal self-rating instrument49 to gauge the relationship between the level of alcohol withdrawal symptoms in the weeks prior to randomization and gabapentin response.
In essence, we found gabapentin to be efficacious in preventing relapse to heavy drinking (NNT, 5.4) and, perhaps more importantly, in promoting abstinence (NNT, 7.2). These are conservative outcomes for AUD randomized clinical trials,56 but both are accepted by the US Food and Drug Administration as indicators of medication efficacy in AUD randomized clinical trials.57 However, when taking the amount of alcohol withdrawal symptoms into account, significant gabapentin effects were seen only in those with the higher levels of self-reported alcohol withdrawal symptoms. There was less relapse to heavy drinking (NNT, 3.1) and more total abstinence (NNT, 2.7) in those with more intense alcohol withdrawal symptoms treated with gabapentin, and we also found an interaction between the level of those symptoms on other continuous drinking outcomes, including percentage of heavy drinking days, percentage of days abstinent, and to some extent the number of drinks per day—all favoring gabapentin treatment over placebo. In fact, in our previous studies, we found more abstinence when gabapentin was combined with flumazenil34 compared with placebo and less relapse to heavy drinking when gabapentin was combined with naltrexone compared with naltrexone alone.35 In both studies, individuals with a greater history of alcohol withdrawal showed the most efficacy on those drinking outcomes. It is also of interest that Mason et al37 reported that gabapentin decreased relapse to heavy drinking and increased total abstinence in a less severe population, based on alcohol consumption levels, and without significant current alcohol withdrawal symptoms. However, in a meta-analysis62 in which the alcohol withdrawal status of the populations was not considered, the percentage of heavy drinking days was the only variable that showed superior gabapentin efficacy. In a larger clinical context, the NNT for gabapentin efficacy was equal to or better than reported for naltrexone in the COMBINE Study (NNT, 7),47 and with alcohol withdrawal status taken into account, gabapentin benefited even more people (NNT, 3).
Many studies have supported the efficacy of gabapentin in alcohol withdrawal treatment.44,63 Given gabapentin’s direct and indirect pharmacologic effects on brain GABA and glutamate systems15,17 and the role of these systems in alcohol withdrawal and relapse drinking,16,64 its efficacy makes sense. Of additional biological interest, the gene regulating the voltage-sensitive calcium channel α2δ-1 protein is upregulated by chronic alcohol exposure and withdrawal.65 Because this is the putative site of gabapentin action,23 this site might be a fruitful target for potential future AUD pharmacologic treatment development as well.
Limitations
This study had several limitations. Although the noncompletion rate was similar to that for other AUD gabapentin treatment trials37,38 and a National Institutes of Health National Institute on Alcohol Abuse and Alcoholism–sponsored large multisite study,47 it should be noted that 13 of 44 (30%) of individuals on gabapentin and 18 of 46 (39%) on placebo did not complete the trial. Perhaps, adding other supportive counseling or Alcoholics Anonymous attendance could increase retention in treatment. Also, self-reported alcohol withdrawal symptoms prior to study entry might not fully capture the extent of withdrawal severity. In addition, those with complex psychiatric and medical conditions, including history of alcohol withdrawal seizures, were excluded. Furthermore, given its kidney excretion, gabapentin should be studied in patients with AUD with more severe liver disease, a condition in which medications are greatly needed.66
Conclusions
The weight of the evidence now suggests that gabapentin might be most efficacious after the initiation of abstinence to sustain it and that it might work best in those with a history of more severe alcohol withdrawal symptoms. To further confirm this, future studies should specifically evaluate symptoms related to protracted alcohol withdrawal during gabapentin treatment. Armed with this knowledge, clinicians may have another alternative when choosing a medication to treat AUD and thereby encourage more patient participation in treatment with enhanced expectation of success.
Trial Protocol
eFigure. A) Modified Alcohol Withdrawal Symptom Checklist (Pittman et al. 2007) given to participants prior to randomization and B) number of subjects in each group reporting that symptom.
Data Sharing Statement
References
- 1.Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74(9):911-923. doi: 10.1001/jamapsychiatry.2017.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rehm J, Dawson D, Frick U, et al. Burden of disease associated with alcohol use disorders in the United States. Alcohol Clin Exp Res. 2014;38(4):1068-1077. doi: 10.1111/acer.12331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litten RZ, Wilford BB, Falk DE, Ryan ML, Fertig JB. Potential medications for the treatment of alcohol use disorder: an evaluation of clinical efficacy and safety. Subst Abus. 2016;37(2):286-298. doi: 10.1080/08897077.2015.1133472 [DOI] [PubMed] [Google Scholar]
- 4.Schuckit MA, Smith TL, Daeppen JB, et al. Clinical relevance of the distinction between alcohol dependence with and without a physiological component. Am J Psychiatry. 1998;155(6):733-740. [DOI] [PubMed] [Google Scholar]
- 5.Caetano R, Clark CL, Greenfield TK. Prevalence, trends, and incidence of alcohol withdrawal symptoms: analysis of general population and clinical samples. Alcohol Health Res World. 1998;22(1):73-79. [PMC free article] [PubMed] [Google Scholar]
- 6.Schuckit MA, Danko GP, Smith TL, Hesselbrock V, Kramer J, Bucholz K. A 5-year prospective evaluation of DSM-IV alcohol dependence with and without a physiological component. Alcohol Clin Exp Res. 2003;27(5):818-825. doi: 10.1097/01.ALC.0000067980.18461.33 [DOI] [PubMed] [Google Scholar]
- 7.Wright TM, Myrick H, Malcolm R, et al. Impact of lifetime alcohol quit attempts and medicated detoxifications on time to relapse during an index alcohol detoxification. J Addict Med. 2007;1(1):15-20. doi: 10.1097/ADM.0b013e318044ce4f [DOI] [PubMed] [Google Scholar]
- 8.Bonnet U, Banger M, Leweke FM, Maschke M, Kowalski T, Gastpar M. Treatment of alcohol withdrawal syndrome with gabapentin. Pharmacopsychiatry. 1999;32(3):107-109. doi: 10.1055/s-2007-979203 [DOI] [PubMed] [Google Scholar]
- 9.Mason BJ, Light JM, Williams LD, Drobes DJ. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: effects of gabapentin. Addict Biol. 2009;14(1):73-83. doi: 10.1111/j.1369-1600.2008.00133.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl). 2002;160(1):19-29. doi: 10.1007/s002130100919 [DOI] [PubMed] [Google Scholar]
- 11.Drobes DJ, Anton RF, Thomas SE, Voronin K. Effects of naltrexone and nalmefene on subjective response to alcohol among non-treatment-seeking alcoholics and social drinkers. Alcohol Clin Exp Res. 2004;28(9):1362-1370. doi: 10.1097/01.ALC.0000139704.88862.01 [DOI] [PubMed] [Google Scholar]
- 12.Anton RF. Naltrexone for the management of alcohol dependence. N Engl J Med. 2008;359(7):715-721. doi: 10.1056/NEJMct0801733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64(9):1069-1077. doi: 10.1001/archpsyc.64.9.1069 [DOI] [PubMed] [Google Scholar]
- 14.Schacht JP, Randall PK, Latham PK, et al. Predictors of naltrexone response in a randomized trial: reward-related brain activation, OPRM1 genotype, and smoking status. Neuropsychopharmacology. 2017;42(13):2640-2653. doi: 10.1038/npp.2017.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75(1):218-265. doi: 10.1016/j.bcp.2007.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koob GF, Kenneth Lloyd G, Mason BJ. Development of pharmacotherapies for drug addiction: a Rosetta stone approach. Nat Rev Drug Discov. 2009;8(6):500-515. doi: 10.1038/nrd2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Littleton J. Neurochemical mechanisms underlying alcohol withdrawal. Alcohol Health Res World. 1998;22(1):13-24. [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18(24):10663-10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schacht JP, Anton RF, McNamara PJ, Im Y, King AC. The dopamine transporter VNTR polymorphism moderates the relationship between acute response to alcohol and future alcohol use disorder symptoms. Addict Biol. 2019;24(5):1109-1118. doi: 10.1111/adb.12676 [DOI] [PubMed] [Google Scholar]
- 20.Addolorato G, Leggio L, Hopf FW, Diana M, Bonci A. Novel therapeutic strategies for alcohol and drug addiction: focus on GABA, ion channels and transcranial magnetic stimulation. Neuropsychopharmacology. 2012;37(1):163-177. doi: 10.1038/npp.2011.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krystal JH, Staley J, Mason G, et al. Gamma-aminobutyric acid type A receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Arch Gen Psychiatry. 2006;63(9):957-968. doi: 10.1001/archpsyc.63.9.957 [DOI] [PubMed] [Google Scholar]
- 22.Maneuf YP, Luo ZD, Lee K. Alpha2delta and the mechanism of action of gabapentin in the treatment of pain. Semin Cell Dev Biol. 2006;17(5):565-570. doi: 10.1016/j.semcdb.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 23.Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev. 2015;67(4):821-870. doi: 10.1124/pr.114.009654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendrich J, Van Minh AT, Heblich F, et al. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci U S A. 2008;105(9):3628-3633. doi: 10.1073/pnas.0708930105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol. 2006;6(1):108-113. doi: 10.1016/j.coph.2005.11.003 [DOI] [PubMed] [Google Scholar]
- 26.Yoshizumi M, Parker RA, Eisenach JC, Hayashida K. Gabapentin inhibits γ-amino butyric acid release in the locus coeruleus but not in the spinal dorsal horn after peripheral nerve injury in rats. Anesthesiology. 2012;116(6):1347-1353. doi: 10.1097/ALN.0b013e318254e6fd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suto T, Severino AL, Eisenach JC, Hayashida K. Gabapentin increases extracellular glutamatergic level in the locus coeruleus via astroglial glutamate transporter-dependent mechanisms. Neuropharmacology. 2014;81:95-100. doi: 10.1016/j.neuropharm.2014.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai K, Nanga RP, Lamprou L, et al. The impact of gabapentin administration on brain GABA and glutamate concentrations: a 7T 1H-MRS study. Neuropsychopharmacology. 2012;37(13):2764-2771. doi: 10.1038/npp.2012.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mariani JJ, Rosenthal RN, Tross S, Singh P, Anand OP. A randomized, open-label, controlled trial of gabapentin and phenobarbital in the treatment of alcohol withdrawal. Am J Addict. 2006;15(1):76-84. doi: 10.1080/10550490500419110 [DOI] [PubMed] [Google Scholar]
- 30.Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588. doi: 10.1111/j.1530-0277.2009.00986.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700. doi: 10.4088/JCP.v68n1108 [DOI] [PubMed] [Google Scholar]
- 32.Karam-Hage M, Brower KJ. Gabapentin treatment for insomnia associated with alcohol dependence. Am J Psychiatry. 2000;157(1):151. doi: 10.1176/ajp.157.1.151 [DOI] [PubMed] [Google Scholar]
- 33.Karam-Hage M, Brower KJ. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry Clin Neurosci. 2003;57(5):542-544. doi: 10.1046/j.1440-1819.2003.01161.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anton RF, Myrick H, Baros AM, et al. Efficacy of a combination of flumazenil and gabapentin in the treatment of alcohol dependence: relationship to alcohol withdrawal symptoms. J Clin Psychopharmacol. 2009;29(4):334-342. doi: 10.1097/JCP.0b013e3181aba6a4 [DOI] [PubMed] [Google Scholar]
- 35.Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717. doi: 10.1176/appi.ajp.2011.10101436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberto M, Gilpin NW, O’Dell LE, et al. Cellular and behavioral interactions of gabapentin with alcohol dependence. J Neurosci. 2008;28(22):5762-5771. doi: 10.1523/JNEUROSCI.0575-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi: 10.1001/jamainternmed.2013.11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falk DE, Ryan ML, Fertig JB, et al. ; National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group (NCIG) Study Group . Gabapentin enacarbil extended-release for alcohol use disorder: a randomized, double-blind, placebo-controlled, multisite trial assessing efficacy and safety. Alcohol Clin Exp Res. 2019;43(1):158-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldberg JF, Burdick KE. Cognitive side effects of anticonvulsants. J Clin Psychiatry. 2001;62(suppl 14):27-33. [PubMed] [Google Scholar]
- 40.Schacht JP, Randall PK, Waid LR, et al. Neurocognitive performance, alcohol withdrawal, and effects of a combination of flumazenil and gabapentin in alcohol dependence. Alcohol Clin Exp Res. 2011;35(11):2030-2038. doi: 10.1111/j.1530-0277.2011.01554.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend. 2006;83(1):25-32. doi: 10.1016/j.drugalcdep.2005.10.008 [DOI] [PubMed] [Google Scholar]
- 42.Myrick H, Anton R, Voronin K, Wang W, Henderson S. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227. doi: 10.1111/j.1530-0277.2006.00299.x [DOI] [PubMed] [Google Scholar]
- 43.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition. Version. 2.0 New York, NY: New York State Psychiatric Institute; 1997. [Google Scholar]
- 44.Ahmed S, Bachu R, Kotapati P, et al. Use of gabapentin in the treatment of substance use and psychiatric disorders: a systematic review. Front Psychiatry. 2019;10(228):228. doi: 10.3389/fpsyt.2019.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357. doi: 10.1111/j.1360-0443.1989.tb00737.x [DOI] [PubMed] [Google Scholar]
- 46.Pettinati HM, Weiss RD, Miller WR, Donovan D, Ernst DB, Rounsaville BJ. Medical Management Treatment Manual: A Clinical Research Guide for Medically Trained Clinicians Providing Pharmacotherapy as Part of the Treatment for Alcohol Dependence. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2004. [Google Scholar]
- 47.Anton RF, O’Malley SS, Ciraulo DA, et al. ; COMBINE Study Research Group . Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017. doi: 10.1001/jama.295.17.2003 [DOI] [PubMed] [Google Scholar]
- 48.Johnson BA, Ait-Daoud N, Roache JD. The COMBINE SAFTEE: a structured instrument for collecting adverse events adapted for clinical studies in the alcoholism field. J Stud Alcohol Suppl. 2005;(15):157-167. doi: 10.15288/jsas.2005.s15.157 [DOI] [PubMed] [Google Scholar]
- 49.Pittman B, Gueorguieva R, Krupitsky E, Rudenko AA, Flannery BA, Krystal JH. Multidimensionality of the Alcohol Withdrawal Symptom Checklist: a factor analysis of the Alcohol Withdrawal Symptom Checklist and CIWA-Ar. Alcohol Clin Exp Res. 2007;31(4):612-618. doi: 10.1111/j.1530-0277.2007.00345.x [DOI] [PubMed] [Google Scholar]
- 50.Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91(3):199-209. doi: 10.1037/0021-843X.91.3.199 [DOI] [PubMed] [Google Scholar]
- 51.Anton RF, Moak DH, Latham PK. The obsessive compulsive drinking scale: a new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry. 1996;53(3):225-231. doi: 10.1001/archpsyc.1996.01830030047008 [DOI] [PubMed] [Google Scholar]
- 52.Tonigan JS, Miller WR, Brown JM. The reliability of Form 90: an instrument for assessing alcohol treatment outcome. J Stud Alcohol. 1997;58(4):358-364. doi: 10.15288/jsa.1997.58.358 [DOI] [PubMed] [Google Scholar]
- 53.Litten RZ, Bradley AM, Moss HB. Alcohol biomarkers in applied settings: recent advances and future research opportunities. Alcohol Clin Exp Res. 2010;34(6):955-967. doi: 10.1111/j.1530-0277.2010.01170.x [DOI] [PubMed] [Google Scholar]
- 54.Helander A, Wielders JP, Jeppsson JO, et al. ; IFCC Working Group on Standardization of Carbohydrate-Deficient Transferrin (WG-CDT) . Toward standardization of carbohydrate-deficient transferrin (CDT) measurements: II. performance of a laboratory network running the HPLC candidate reference measurement procedure and evaluation of a candidate reference material. Clin Chem Lab Med. 2010;48(11):1585-1592. doi: 10.1515/CCLM.2010.322 [DOI] [PubMed] [Google Scholar]
- 55.American Psychiatric Association Handbook of Psychiatric Measures. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 56.Falk D, Wang XQ, Liu L, et al. Percentage of subjects with no heavy drinking days: evaluation as an efficacy endpoint for alcohol clinical trials. Alcohol Clin Exp Res. 2010;34(12):2022-2034. doi: 10.1111/j.1530-0277.2010.01290.x [DOI] [PubMed] [Google Scholar]
- 57.Center for Drug Evaluation and Research Alcoholism: Developing Drugs for Treatment. Guidance for Industry. Silver Spring, MD: Center for Drug Evaluation and Research; 2015. [Google Scholar]
- 58.Anton RF, Latham PK, Voronin KE, et al. Nicotine-use/smoking is associated with the efficacy of naltrexone in the treatment of alcohol dependence. Alcohol Clin Exp Res. 2018;42(4):751-760. doi: 10.1111/acer.13601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med. 1988;318(26):1728-1733. doi: 10.1056/NEJM198806303182605 [DOI] [PubMed] [Google Scholar]
- 60.Anton RF, Lieber C, Tabakoff B; CDTect Study Group . Carbohydrate-deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multisite study. Alcohol Clin Exp Res. 2002;26(8):1215-1222. doi: 10.1111/j.1530-0277.2002.tb02658.x [DOI] [PubMed] [Google Scholar]
- 61.Helander A, Husa A, Jeppsson JO. Improved HPLC method for carbohydrate-deficient transferrin in serum. Clin Chem. 2003;49(11):1881-1890. doi: 10.1373/clinchem.2003.023341 [DOI] [PubMed] [Google Scholar]
- 62.Kranzler HR, Feinn R, Morris P, Hartwell EE. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction. 2019;114(9):1547-1555. doi: 10.1111/add.14655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leggio L, Kenna GA, Swift RM. New developments for the pharmacological treatment of alcohol withdrawal syndrome. a focus on non-benzodiazepine GABAergic medications. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1106-1117. doi: 10.1016/j.pnpbp.2007.09.021 [DOI] [PubMed] [Google Scholar]
- 64.Griffin WC III, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology. 2014;39(3):707-717. doi: 10.1038/npp.2013.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melendez RI, McGinty JF, Kalivas PW, Becker HC. Brain region-specific gene expression changes after chronic intermittent ethanol exposure and early withdrawal in C57BL/6J mice. Addict Biol. 2012;17(2):351-364. doi: 10.1111/j.1369-1600.2011.00357.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134. doi: 10.1016/j.amjmed.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure. A) Modified Alcohol Withdrawal Symptom Checklist (Pittman et al. 2007) given to participants prior to randomization and B) number of subjects in each group reporting that symptom.
Data Sharing Statement