This cohort study investigates the association of renin-angiotensin system blockade therapy discontinuation after estimated glomerular filtration rate decreases with the risk of mortality, major adverse cardiovascular events, and end-stage kidney disease.
Key Points
Question
Is there an association between discontinuing renin-angiotensin system blockade after estimated glomerular filtration rate (eGFR) decreases to less than 30 mL/min/1.73 m2 and the risk of all-cause mortality, major adverse cardiovascular events, and end-stage kidney disease in the subsequent 5 years?
Findings
In this cohort study of 3909 individuals who had initiated angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker therapy and experienced an eGFR decrease to below 30 mL/min/1.73 m2 during therapy, 35.1% of those who discontinued therapy within 6 months after the eGFR decrease died during the subsequent 5 years compared with 29.4% of those who did not discontinue therapy.
Meaning
These findings suggest that continued angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use is associated with cardiovascular health benefits in individuals with an eGFR below 30 mL/min/1.73 m2, without increased risk of progression to end-stage kidney disease.
Abstract
Importance
It is uncertain whether and when angiotensin-converting enzyme inhibitor (ACE-I) and angiotensin II receptor blocker (ARB) treatment should be discontinued in individuals with low estimated glomerular filtration rate (eGFR).
Objective
To investigate the association of ACE-I or ARB therapy discontinuation after eGFR decreases to below 30 mL/min/1.73 m2 with the risk of mortality, major adverse cardiovascular events (MACE), and end-stage kidney disease (ESKD).
Design, Setting, and Participants
This retrospective, propensity score–matched cohort study included 3909 patients from an integrated health care system that served rural areas of central and northeastern Pennsylvania. Patients who initiated ACE-I or ARB therapy from January 1, 2004, to December 31, 2018, and had an eGFR decrease to below 30 mL/min/1.73 m2 during therapy were enrolled, with follow-up until January 25, 2019.
Exposures
Individuals were classified based on whether they discontinued ACE-I or ARB therapy within 6 months after an eGFR decrease to below 30 mL/min/1.73 m2.
Main Outcomes and Measures
The association between ACE-I or ARB therapy discontinuation and mortality during the subsequent 5 years was assessed using multivariable Cox proportional hazards regression models, adjusting for patient characteristics at the time of the eGFR decrease in a propensity score–matched sample. Secondary outcomes included MACE and ESKD.
Results
Of the 3909 individuals receiving ACE-I or ARB treatment who experienced an eGFR decrease to below 30 mL/min/1.73 m2 (2406 [61.6%] female; mean [SD] age, 73.7 [12.6] years), 1235 discontinued ACE-I or ARB therapy within 6 months after the eGFR decrease and 2674 did not discontinue therapy. A total of 434 patients (35.1%) who discontinued ACE-I or ARB therapy and 786 (29.4%) who did not discontinue therapy died during a median follow-up of 2.9 years (interquartile range, 1.3-5.0 years). In the propensity score–matched sample of 2410 individuals, ACE-I or ARB therapy discontinuation was associated with a higher risk of mortality (hazard ratio [HR], 1.39; 95% CI, 1.20-1.60]) and MACE (HR, 1.37; 95% CI, 1.20-1.56), but no statistically significant difference in the risk of ESKD was found (HR, 1.19; 95% CI, 0.86-1.65).
Conclusions and Relevance
The findings suggest that continuing ACE-I or ARB therapy in patients with declining kidney function may be associated with cardiovascular benefit without excessive harm of ESKD.
Introduction
Angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin II receptor blockers (ARBs) are the mainstays of therapy for hypertension, albuminuric chronic kidney disease (CKD), heart failure with reduced ejection fraction, and coronary artery disease.1,2,3,4,5,6 However, the potential benefits of ACE-I and ARB therapy must be weighed against the potential risks, which include acute, largely hemodynamic reductions in estimated glomerular filtration rate (eGFR), hyperkalemia, and acute kidney injury (AKI).4,7,8 The risks of these adverse events are particularly relevant in individuals with lower eGFR. In real-world studies,9,10 more than half of the individuals initiating ACE-I or ARB therapy discontinued within 5 years after the initial prescription, with discontinuation increasingly common with more advanced CKD stage.
The Kidney Disease Improving Global Outcomes (KDIGO) guidelines suggest temporary discontinuation of ACE-I or ARB therapy in those with a GFR less than 60 mL/min/1.73 m2 (GFR categories G3a-G5) “who have serious intercurrent illness that increases the risk of AKI”11(p 12) but also emphasize to “not routinely discontinue [ACE-I/ARB] in people with GFR <30 mL/min/1.73 m2 as they remain nephroprotective.”11(p 103) The existing literature evaluating the risks and benefits of using ACE-Is or ARBs in individuals with advanced CKD is conflicting.12,13,14,15 The ongoing Multi-centre Randomised Controlled Trial of Angiotensin-Converting Enzyme Inhibitor/Angiotensin Receptor Blocker Withdrawal in Advanced Renal Disease (STOP-ACEi) aims to evaluate the difference in 3-year eGFR, randomizing 410 ACE-I or ARB users with advanced CKD to continue or discontinue ACE-I or ARB therapy.16 However, the benefits of ACE-I or ARB therapy may also extend to cardiovascular disease and mortality.
Using data from a large, integrated health system, we assessed the association of ACE-I or ARB therapy discontinuation with the risk of all-cause mortality, major adverse cardiovascular events (MACE), and end-stage kidney disease (ESKD) among individuals receiving ACE-I or ARB therapy whose eGFR decreased to below 30 mL/min/1.73 m2. As a secondary objective, we evaluated the association of ACE-I or ARB therapy discontinuation with the same end points among users who experienced a decrease in eGFR by 40% or more within 1 year, a surrogate end point for kidney failure used by the US Food and Drug Administration.17
Methods
Study Setting and Study Population
This cohort study included individuals receiving ACE-I or ARB therapy through the Geisinger Health System, a fully integrated health care system that serves 45 counties in central and northeastern Pennsylvania. The local population has an estimated 1% annual outmigration rate.18 The electronic health records provide patient-level data on outpatient prescriptions, problem lists, laboratory test results, inpatient and outpatient encounters, and demographic characteristics. This study was approved by institutional review boards at the Geisinger Medical Center and Johns Hopkins Bloomberg School of Public Health. All data were deidentified, and consent was waived.
We first identified 162 654 individuals who initiated ACE-I or ARB therapy between January 1, 2004, and December 31, 2018. Next, we subset the population to the 10 810 individuals whose outpatient eGFR decreased to below 30 mL/min/1.73 m2 after therapy initiation. Individuals who discontinued ACE-I or ARB therapy before the initial decrease to below 30 mL/min/1.73 m2 were excluded (n = 5402). A window of 6 months after the eGFR decrease was constructed to assess ACE-I or ARB therapy discontinuation; thus, in the primary analysis, the baseline date of follow-up (T0) was 6 months after the day of the eGFR decrease to less than 30 mL/min/1.73 m2. Individuals who discontinued and restarted ACE-I or ARB therapy within the 6-month period before T0 (n = 233) and those who did not meet our formal criteria for discontinuation but were not receiving ACE-I or ARB therapy at T0 (n = 317) were excluded. Further exclusion criteria included T0 after the end of follow-up (n = 214), the absence of serum potassium level and systolic blood pressure measures in the year before the eGFR decrease (n = 303), age younger than 18 years at the time of the eGFR decrease (n = 3), and death before T0 (n = 324) or prevalent ESKD at T0 (n = 105) (eFigure 1 in the Supplement).
Treatment Strategies Under Comparison
We compared individuals who stopped ACE-I or ARB therapy within 6 months after a decrease in eGFR to below 30 mL/min/1.73 m2 with those who did not discontinue therapy. We defined discontinuation as a gap in therapy longer than 60 days that occurred before T0. We allowed for switching between ACE-I and ARB or to different medications within each class given evidence suggesting that ACE-Is and ARBs share similar mechanisms, benefits, and risks.4,5,11
End Points and Follow-up
The primary end point was mortality during the 5 years after T0. Secondary end points included MACE and ESKD. For all end points, follow-up data were available until January 25, 2019. MACE, defined as the first occurrence of death, myocardial infarction, percutaneous coronary intervention, or coronary artery bypass after T0, was ascertained using previously validated International Classification of Diseases (ICD) procedure and diagnosis codes.19 Data on ESKD were captured by linking to the US Renal Data System, which was available until July 31, 2018. For the remaining 6 months between this date and January 25, 2019, the ICD procedure and diagnosis codes, current procedural terminology, and health care common procedure coding system codes for kidney transplant and dialysis were used to ascertain ESKD cases (eTable 1 in the Supplement).
We investigated hyperkalemia and AKI as additional outcomes during follow-up. Hyperkalemia was defined as the first serum potassium level greater than 5.5 mEq/L (to convert to millimoles per liter, multiply by 1) that occurred as an outpatient or as the first measure of an inpatient episode. Diagnosis of AKI was determined using the ICD diagnosis codes (eTable 2 in the Supplement).
Baseline Covariates
We defined baseline serum potassium level, systolic blood pressure, and serum creatinine level as the most recent outpatient measures available within 1 year before the eGFR decrease to below 30 mL/min/1.73 m2. We used the CKD Epidemiology Collaboration equation to estimate GFR based on outpatient serum creatinine level.20 Baseline comorbidities, such as history of stroke, congestive heart failure, diabetes, and coronary artery disease, were defined based on the presence of the corresponding ICD codes. We also ascertained concurrent use of other medications, such as statin, antiplatelet agents, and β-blockers, at the time of the eGFR decrease and whether a patient was hospitalized, whether the patient had a nephrology visit, and the number of outpatient encounters during the 1-year period before the eGFR decrease. Other covariates included sex, race/ethnicity, age, and calendar year (categorized as 2004-2008, 2009-2013, 2014-2019) at the time of the eGFR decrease.
Propensity Score Matching
We performed nearest-neighbor propensity score matching without replacement to 1:1 match individuals who discontinued ACE-I or ARB therapy with those who did not discontinue therapy using a caliper of 0.25 SD of the propensity score.21 Covariates in the propensity score model included the aforementioned baseline variables. We chose these variables a priori to minimize indication bias that might confound the association between ACE-I or ARB therapy discontinuation and the outcome. Balance between the 2 comparison groups was evaluated using the standardized mean difference across covariates, with an absolute standardized mean difference below 0.1 indicating successful balance.22
Statistical Analysis
We described baseline characteristics of the study population by treatment group before and after propensity score matching using number (percentage) for categorical variables and mean (SD) for continuous variables. We used Kaplan-Meier curves to depict survival for 5 years after T0, stratified by treatment strategy. We then assessed the association of ACE-I or ARB therapy discontinuation with mortality using multivariable Cox proportional hazards regression analysis in the propensity score–matched sample, adjusting for all baseline covariates. We used linear spline forms of serum potassium level with knots at 4 and 5 mEq/L, systolic blood pressure with knots at 90 and 140 mm Hg, and age with knots at 45 and 65 years to allow for a nonlinear association. We applied similar methods to assess the associations of ACE-I or ARB therapy discontinuation with MACE, ESKD, hyperkalemia, and AKI. Using cumulative incidence curves for ESKD, we depicted the proportion of patients remaining free of ESKD, accounting for the competing risk of death.
We assessed whether the association of ACE-I or ARB therapy discontinuation with the end points differed by baseline diabetes, congestive heart failure, coronary artery disease, and history of stroke. We tested for potential effect modification by the presence of macroalbuminuria at baseline. Macroalbuminuria was defined as the latest measure within the 3-year window before the eGFR decreased to below 30 mL/min/1.73 m2 and a urine albumin to creatinine ratio greater than 300 mg/g; if not available, a urine protein to creatinine ratio greater than 700 mg/g or protein urine dipstick test result of ++ or greater were also accepted.
To evaluate the association of ACE-I or ARB therapy discontinuation after a 40% or greater decrease in eGFR within 1 year with the risks of mortality, MACE, and ESKD, we identified individuals who had a 40% or greater decrease in eGFR during treatment compared with the previous measure within a year. We repeated the primary analyses within this sample, with T0 defined as 6 months after the first time the eGFR decreased by 40% or more during ACE-I or ARB treatment.
Statistical significance was evaluated based on 2-sided testing at a significance level of P < .05. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc), R, version 3.6.0 (R Foundation for Statistical Computing), and Stata, version 15.1 (StataCorp LLC).
Negative Control
To assess whether the observed associations between ACE-I or ARB therapy discontinuation and the end points of interest were attributable to different health statuses between the 2 therapy groups, we compared the risk of bleeding, an outcome believed to be not affected by ACE-I or ARB use, between the propensity score–matched therapy groups, adjusting for antiplatelet agent use at the time of the eGFR decrease. Bleeding was ascertained from emergency department visits and hospitalization episodes using ICD diagnosis codes.
Sensitivity Analysis
Several sensitivity analyses were performed. First, we estimated the association between ACE-I or ARB therapy discontinuation and ESKD using the Fine-Gray competing risk regression models to account for the competing risk of death.23 Second, we used a target trial emulation technique that allowed for the inclusion of the individuals who died or developed ESKD during the 6 months after the eGFR decrease to below 30 mL/min/1.73 m2 with time-updated weighting (eMethods in the Supplement).24,25 Third, we excluded individuals with systolic blood pressure lower than 90 mm Hg or serum potassium level higher than 5.5 mEq/L at the time of the eGFR decrease to below 30 mL/min/1.73 m2. Fourth, we restricted the study population to individuals who had been receiving ACE-I or ARB therapy for at least 6 months at the time of the eGFR decrease. Fifth, we excluded individuals with AKI at the time of eGFR decrease, defined as an increase in serum creatinine level by more than 100% compared with the previous outpatient measure within 1 year. Sixth, we excluded patients with a history of cancer, determined by ICD diagnosis codes. Seventh, we evaluated whether therapy discontinuation after an eGFR decrease to 20 mL/min/1.73 m2 had similar associations with the end points of interest. Eighth, we used a previously described approach to explore the sensitivity of our findings to unmeasured confounding.26,27 All 95% CIs in the primary and sensitivity analyses were reported based on robust SEs.
Results
Study Population
A total of 3909 (mean [SD] age, 73.7 [12.6] years; 2406 [61.6%] female) individuals from the Geisinger Health System met the inclusion criteria. A total of 1235 individuals discontinued ACE-I or ARB therapy within the 6 months after the eGFR decreased to below 30 mL/min/1.73 m2, and 2674 individuals did not discontinue therapy. Compared with their counterparts, individuals who discontinued ACE-I or ARB therapy were more often male, had lower eGFR, had higher serum potassium levels, had a higher prevalence of congestive heart failure, and were more likely to be receiving antiplatelet agents at the time of the eGFR decrease (Table). These individuals were less likely to have diabetes and less likely to be taking statins and β-blockers. A total of 1205 individuals (98%) in the discontinuation group were successfully matched to controls, resulting in a total of 2410 individuals in the propensity score–matched sample. The 2 treatment groups were closely balanced on all baseline covariates after propensity score matching, with the absolute standardized mean difference below 0.1 for all covariates.
Table. Baseline Characteristics by ACE-I or ARB Therapy Discontinuation Status Before and After Propensity Score Matchinga.
| Baseline Characteristic | Before Matching (n = 3909) | After Matching (n = 2410) | ||||
|---|---|---|---|---|---|---|
| Discontinued Therapy (n = 1235) | Control (n = 2674) | Standardized Mean Difference | Discontinued Therapy (n = 1205) | Control (n = 1205) | Standardized Mean Difference | |
| Age, mean (SD), y | 73.0 (12.9) | 74.0 (12.4) | 0.083 | 73.1 (12.8) | 73.3 (13.2) | 0.011 |
| eGFR, mean (SD), mL/min/1.73 m2b | 23.2 (5.7) | 25.5 (4.2) | 0.462 | 23.6 (5.3) | 23.9 (5.3) | 0.073 |
| Potassium, mean (SD), mEq/Lb | 4.8 (0.8) | 4.6 (0.6) | 0.266 | 4.8 (0.7) | 4.8 (0.7) | 0.036 |
| Systolic blood pressure, mean (SD), mm Hgb | 125.0 (22.5) | 127.1 (20.2) | 0.095 | 125.3 (22.3) | 125.4 (20.1) | 0.004 |
| No. of outpatient visits, mean (SD)c | 6.7 (5.5) | 5.9 (4.5) | 0.158 | 6.6 (5.4) | 6.4 (5.0) | 0.032 |
| Female sex | 711 (57.6) | 1695 (63.4) | 0.119 | 696 (57.8) | 706 (58.6) | 0.017 |
| Black race | 22 (1.8) | 52 (1.9) | 0.012 | 22 (1.8) | 25 (2.1) | 0.018 |
| Coronary artery diseased | 547 (44.3) | 1180 (44.1) | 0.003 | 533 (44.2) | 528 (43.8) | 0.008 |
| Congestive heart failured | 404 (32.7) | 807 (30.2) | 0.055 | 395 (32.8) | 398 (33.0) | 0.005 |
| Diabetesd | 582 (47.1) | 1332 (49.8) | 0.054 | 572 (47.5) | 589 (48.9) | 0.028 |
| History of stroked | 239 (19.4) | 560 (20.9) | 0.040 | 236 (19.6) | 245 (20.3) | 0.019 |
| Statin use | 694 (56.2) | 1721 (64.4) | 0.167 | 681 (56.5) | 692 (57.4) | 0.018 |
| β-Blocker use | 709 (57.4) | 1682 (62.9) | 0.112 | 689 (57.2) | 676 (56.1) | 0.022 |
| Antiplatelet agent use | 524 (42.4) | 1113 (41.6) | 0.016 | 505 (41.9) | 501 (41.6) | 0.007 |
| Hospitalizationc | 20 (1.6) | 44 (1.7) | 0.002 | 20 (1.7) | 19 (1.6) | 0.007 |
| Nephrology visitc | 206 (16.7) | 512 (19.2) | 0.064 | 204 (16.9) | 188 (15.6) | 0.036 |
| Calendar years | ||||||
| 2004-2008 | 175 (14.2) | 463 (17.3) | 0.086 | 173 (14.4) | 176 (14.6) | 0.007 |
| 2009-2013 | 449 (36.4) | 1022 (38.2) | 0.039 | 437 (36.3) | 429 (35.6) | 0.014 |
| 2014-2019 | 611 (49.5) | 1189 (44.5) | 0.100 | 595 (49.4) | 600 (49.8) | 0.008 |
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; eGFR, estimated glomerular filtration rate.
Data are presented as number (percentage) of patients unless otherwise indicated.
Most recent measure within the year before eGFR decreased to below 30 mL/min/1.73 m2.
Assessed during the 1-year window before baseline.
Any time before baseline.
ACE-I or ARB Therapy Discontinuation and Mortality, MACE, and ESKD
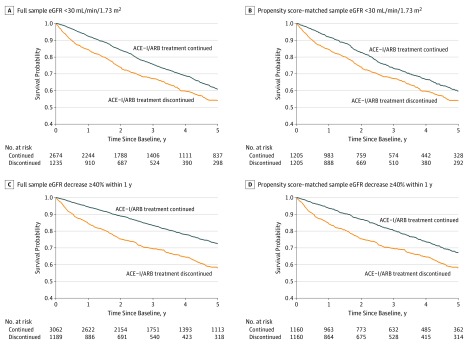
During a median follow-up of 2.9 years (interquartile range [IQR], 1.3-5.0 years), 434 (35.1%) of the 1235 individuals who discontinued ACE-I or ARB therapy within 6 months after their eGFR decreased to below 30 mL/min/1.73 m2 and 786 (29.4%) of the 2674 individuals who did not discontinue therapy died within 5 years after T0 (Figure 1A and B). Of note, 347 (28%) of the 1235 patients who discontinued ACE-I or ARB therapy within 6 months after the eGFR decrease restarted therapy during the follow-up period. The association between ACE-I or ARB therapy discontinuation and higher risk of mortality remained after adjusting for baseline covariates in the propensity score–matched sample (hazard ratio [HR], 1.39; 95% CI, 1.20-1.60).
Figure 1. Cumulative Incidence of All-Cause Mortality by Angiotensin-Converting Enzyme Inhibitor (ACE-I) and Angiotensin II Receptor Blocker (ARB) Discontinuation Status.
eGFR indicates estimated glomerular filtration rate.
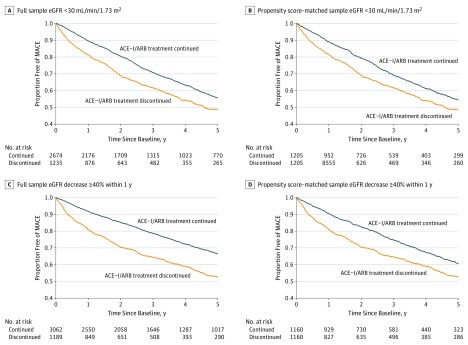
During a median follow-up of 2.7 years (IQR, 1.2-5.0 years), the risk of MACE was higher during the 5-year period among those who discontinued ACE-I or ARB therapy (494 [40.0%]) compared with those who did not discontinue therapy (910 [34.0%]) (Figure 2A and B). The association between ACE-I or ARB therapy discontinuation and MACE remained in the propensity score–matched sample (HR, 1.37; 95% CI, 1.20-1.56).
Figure 2. Cumulative Incidence of Major Adverse Cardiovascular Events (MACE) by Angiotensin-Converting Enzyme Inhibitor (ACE-I) and Angiotensin II Receptor Blocker (ARB) Discontinuation Status.
eGFR indicates glomerular filtration rate.
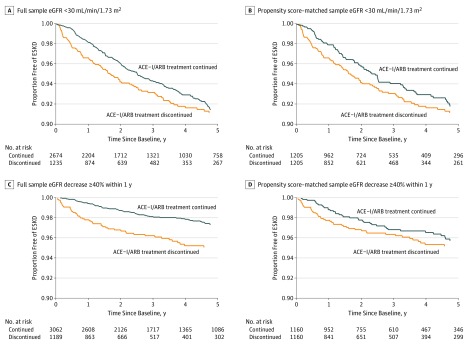
During a median follow-up of 2.7 years (IQR, 1.2-5.0 years), 87 individuals (7.0%) developed ESKD within 5 years among those who discontinued ACE-I or ARB therapy within 6 months after their eGFR decreased below 30 mL/min/1.73m2 compared with the 176 (6.6%) who did not discontinue (Figure 3A and B). In adjusted analysis within the propensity score–matched sample, ACE-I or ARB discontinuation was not significantly associated with the risk of ESKD (HR, 1.19; 95% CI, 0.86-1.65).
Figure 3. Cumulative Incidence of End-stage Kidney Disease (ESKD) Accounting for the Competing Risk of Death by Angiotensin-Converting Enzyme Inhibitor (ACE-I) and Angiotensin II Receptor Blocker (ARB) Discontinuation Status.
eGFR indicates estimated glomerular filtration rate.
There was no effect modification of the associations of ACE-I or ARB therapy discontinuation with mortality and MACE by baseline history of stroke, diabetes, congestive heart failure, or coronary artery disease. However, the presence of baseline diabetes modified the association between ACE-I or ARB therapy discontinuation and ESKD (HR was estimated to be 1.56 for those with baseline diabetes and 0.61 for those without baseline diabetes; P = .01). Of the 915 individuals from the discontinuation group and the 1982 from the nondiscontinuation group who had a baseline measure of urine albumin to creatinine ratio, urine protein to creatinine ratio, or protein urine dipstick test, 1794 were included in the propensity score–matched sample. No significant effect modification was found of the association of ACE-I or ARB therapy discontinuation with mortality, MACE, and ESKD by the presence of macroalbuminuria.
Additional Outcomes
During a median follow-up of 2.3 years (IQR, 0.9-4.6 years), a lower proportion of individuals who discontinued ACE-I or ARB therapy within 6 months after eGFR decreased to below 30 mL/min/1.73 m2 experienced hyperkalemia (n = 193 [15.6%]) compared with those who did not discontinue therapy (n = 593, [22.2%]) (eFigure 2 in the Supplement). In the propensity score–matched sample, discontinuation of ACE-I or ARB therapy was associated with a lower risk of hyperkalemia (HR, 0.65; 95% CI, 0.54-0.79). Although the proportion of individuals developing AKI was slightly lower among individuals who discontinued ACE-I or ARB therapy (343 [27.8%]) than among those who did not discontinue therapy (806 ([30.1%]) in the overall sample, the risk of AKI was not significantly different after accounting for baseline covariates in the propensity score–matched sample (HR, 0.92; 95% CI, 0.79-1.07).
ACE-I or ARB Therapy Discontinuation After eGFR Decrease by 40% or More
Among the 4251 individuals with an eGFR decrease by 40% or more during 1 year while receiving ACE-I or ARB therapy, 1189 (28.0%) discontinued ACE-I or ARB therapy within 6 months after the eGFR decreased and the remaining 3062 (72.0%) did not discontinue therapy (eTable 3 in the Supplement). During a median follow-up of 3.3 years (IQR, 1.5-5.0 years), 388 individuals (32.6%) who discontinued ACE-I or ARB therapy died compared with the 627 (20.5%) of those who did not discontinue therapy. A similar trend was found for 5-year MACE, with 448 individuals (37.7%) who discontinued ACE-I or ARB therapy and 777 (25.4%) who did not discontinue therapy experiencing MACE. A total of 48 individuals (4.0%) who discontinued ACE-I or ARB therapy developed ESKD compared with 63 (2.1%) of those who did not discontinue therapy (Figure 1, Figure 2, and Figure 3C and D). In adjusted analyses within the propensity score–matched sample, ACE-I or ARB therapy discontinuation was associated with a higher risk of mortality (HR, 1.53; 95% CI, 1.31-1.79) and MACE (HR, 1.40; 95% CI, 1.22-1.62) but not ESKD (HR, 1.50; 95% CI, 0.91-2.47).
Negative Control
Individuals who discontinued ACE-I or ARB therapy within 6 months after their eGFR decreased to below 30 mL/min/1.73 m2 had similar risks of bleeding compared with their propensity score–matched peers who did not discontinue therapy (HR, 0.88; 95% CI, 0.66-1.16) (eFigure 3 in the Supplement).
Sensitivity Analysis
All sensitivity analyses yielded similar associations of ACE-I or ARB therapy discontinuation with mortality, MACE, and ESKD (eResults in the Supplement). Our results were moderately sensitive to an unobserved confounder. To attribute the observed associations to an unobserved covariate that increased the odds of mortality and MACE by 50%, the covariate would need to more than double the odds of ACE-I or ARB therapy discontinuation.
Discussion
In this large, real-world study of individuals who experienced an eGFR decrease while receiving ACE-I or ARB therapy, discontinuation of therapy after the eGFR decrease was associated with a higher risk of death and MACE but no statistically significant difference in the risk of ESKD. The findings were robust to a number of sensitivity analyses, including using a target trial emulation technique and excluding individuals with hypotension, hyperkalemia, AKI, or a history of cancer at the time of the eGFR decrease. ACE-I or ARB therapy discontinuation was associated with a lower risk of hyperkalemia, consistent with existing evidence7; however, this risk did not appear to outweigh the potential cardiovascular and survival benefits of continuing ACE-I or ARB therapy.
Although our study is one of the first to evaluate the long-term risks of ACE-I or ARB therapy discontinuation among individuals who experience CKD progression, other researchers have evaluated the association of ACE-I or ARB use with long-term outcomes or discontinuation with short-term changes in eGFR. A meta-analysis28 of clinical trials showed that ACE-I or ARB therapy use was associated with a reduced risk of kidney failure and cardiovascular events in patents with CKD. Similarly, an observational cohort study29 of US veterans with CKD found survival benefits associated with the administration of ACE-I or ARB across all eGFR levels, including eGFR less than 30 mL/min/1.73 m2. A trial13 that randomized 224 patients with serum creatinine levels of 3.1 to 5.0 mg/dL (to convert to micromoles per liter, multiply by 88.4) to benazepril or placebo showed a 43% lower risk of the composite of doubling of serum creatinine level, ESKD, or death associated with benazepril. Post hoc secondary analyses of the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial and the Ramipril Efficacy in Nephropathy (REIN) trial suggested benefits of ACE-I or ARB therapy in individuals with low GFRs.14,15 However, an observational study of 52 individuals with stage G4 or G5 CKD reported an eGFR increase after ACE-I or ARB treatment discontinuation, and the authors concluded that “discontinuation of ACEi/ARB has undoubtedly delayed the onset of RRT [renal replacement therapy] in the majority of those studied.”12(p 3981) An ongoing clinical trial16 aims to detect the difference in 3-year eGFR among individuals with stages G4 to G5 CKD. Our results add a longer observation period, a larger sample size of individuals with eGFR below 30 mL/min/1.73 m2, and ascertainment of outcomes with less concern for acute GFR events associated with therapy.
Strengths and Limitations
Our study has several strengths. First, we used a doubly robust method to minimize confounding by indication through propensity score matching and further adjustment for baseline demographic and clinical variables. Second, we conducted a number of sensitivity analyses with robust findings. Third, our study sample contained approximately 4000 individuals in routine clinical practice, which is a considerable sample size of individuals receiving ACE-I or ARB therapy with an eGFR decrease to below 30 mL/min/1.73 m2. Fourth, we assessed a negative control and observed similar risks of bleeding between the 2 treatment groups. This result helped mitigate concerns that the observed higher risks of death and MACE associated with ACE-I or ARB therapy discontinuation might reflect poorer health status among those who discontinued ACE-I or ARB therapy. Fifth, we studied individuals who experienced a 40% or greater decrease in eGFR within 1 year to address potential misclassification of advanced CKD and to provide additional insights for clinical practice.
Our study also has several limitations. First, ACE-I or ARB use was obtained through prescription records, and we could not verify actual medication dispensation or intake. Second, our study was observational and susceptible to unmeasured confounding. However, the 2 comparison groups were matched closely not only on measured clinical and demographic factors but also on variables that included the number of outpatient visits, hospitalization status, and the presence of a nephrology visit during the year before eGFR decrease. Third, we performed an intention-to-treat analysis that provided an effect estimate based on the initial treatment decision; however, many patients who discontinued therapy then restarted therapy within the follow-up period. Thus, our effect estimate was likely conservative because of dilution caused by nonadherence to discontinuation status during follow-up.30 Fourth, we treated ACE-I or ARB therapy discontinuation as binary without dose-response assessment. Fifth, ESKD is itself a treatment decision and likely varies by practitioner. Sixth, most of the study population was white.
Conclusions
We found a higher risk of mortality and MACE associated with ACE-I or ARB therapy discontinuation after an eGFR decrease to below 30 mL/min/1.73 m2 but no significant difference in the risk of ESKD. Similar patterns held for individuals with a 40% or greater decrease in eGFR. Our findings suggest that continuing ACE-I or ARB therapy in patients with declining kidney function may provide cardiovascular and survival benefits without excess risks of ESKD.
eFigure 1. Derivation of Study Population
eFigure 2. Cumulative Incidence of Hyperkalemia, by ACE-I/ARB Discontinuation Status
eFigure 3. Cumulative Incidence of Bleeding, by ACE-I/ARB Discontinuation Status in the Propensity-Score Matched Sample
eTable 1. Diagnosis and Procedure Codes Used to Define End-Stage Kidney Disease (ESKD)
eTable 2. International Classification of Disease, 9th and 10th Editions, Clinical Modification (ICD-9-CM, ICD-10-CM) Used to Define Disease Conditions
eTable 3. Baseline Characteristics of Patients With ≥40% eGFR Decline
eMethods. Target Trial Emulation
eResults. Sensitivity Analyses
References
- 1.Agodoa LY, Appel L, Bakris GL, et al. ; African American Study of Kidney Disease and Hypertension (AASK) Study Group . Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285(21):2719-2728. doi: 10.1001/jama.285.21.2719 [DOI] [PubMed] [Google Scholar]
- 2.Lewis EJ, Hunsicker LG, Clarke WR, et al. ; Collaborative Study Group . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851-860. doi: 10.1056/NEJMoa011303 [DOI] [PubMed] [Google Scholar]
- 3.Marin R, Ruilope LM, Aljama P, Aranda P, Segura J, Diez J; Investigators of the ESPIRAL Study; Efecto del tratamiento antihipertensivo Sobre la Progresión de la Insuficiencia RenAL en pacientes no diabéticos . A random comparison of fosinopril and nifedipine GITS in patients with primary renal disease. J Hypertens. 2001;19(10):1871-1876. doi: 10.1097/00004872-200110000-00023 [DOI] [PubMed] [Google Scholar]
- 4.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed). 2016;69(12):1167. doi: 10.1016/j.rec.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 6.Ferrari R, Guardigli G, Ceconi C. Secondary prevention of CAD with ACE inhibitors: a struggle between life and death of the endothelium. Cardiovasc Drugs Ther. 2010;24(4):331-339. doi: 10.1007/s10557-010-6244-x [DOI] [PubMed] [Google Scholar]
- 7.Ahuja TS, Freeman D Jr, Mahnken JD, Agraharkar M, Siddiqui M, Memon A. Predictors of the development of hyperkalemia in patients using angiotensin-converting enzyme inhibitors. Am J Nephrol. 2000;20(4):268-272. doi: 10.1159/000013599 [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson LA, Abel GA, Chaudhry AN, et al. ACE inhibitor and angiotensin receptor-II antagonist prescribing and hospital admissions with acute kidney injury: a longitudinal ecological study. PLoS One. 2013;8(11):e78465. doi: 10.1371/journal.pone.0078465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiao Y, Shin J-I, Sang Y, et al. Discontinuation of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in chronic kidney disease. Mayo Clin Proc. 2019;94(11):2220-2229. doi: 10.1016/j.mayocp.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahmoudpour SH, Asselbergs FW, Souverein PC, de Boer A, Maitland-van der Zee AH. Prescription patterns of angiotensin-converting enzyme inhibitors for various indications: a UK population-based study. Br J Clin Pharmacol. 2018;84(10):2365-2372. doi: 10.1111/bcp.13692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Kidney Foundation KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. 2013https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf. Accessed July 26, 2018.
- 12.Ahmed AK, Kamath NS, El Kossi M, El Nahas AM. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant. 2010;25(12):3977-3982. doi: 10.1093/ndt/gfp511 [DOI] [PubMed] [Google Scholar]
- 13.Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354(2):131-140. doi: 10.1056/NEJMoa053107 [DOI] [PubMed] [Google Scholar]
- 14.Remuzzi G, Ruggenenti P, Perna A, et al. ; RENAAL Study Group . Continuum of renoprotection with losartan at all stages of type 2 diabetic nephropathy: a post hoc analysis of the RENAAL trial results. J Am Soc Nephrol. 2004;15(12):3117-3125. doi: 10.1097/01.ASN.0000146423.71226.0C [DOI] [PubMed] [Google Scholar]
- 15.Ruggenenti P, Perna A, Remuzzi G; Gruppo Italiano di Studi Epidemiologici in Nefrologia . ACE inhibitors to prevent end-stage renal disease: when to start and why possibly never to stop: a post hoc analysis of the REIN trial results. J Am Soc Nephrol. 2001;12(12):2832-2837. [DOI] [PubMed] [Google Scholar]
- 16.Bhandari S, Ives N, Brettell EA, et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant. 2016;31(2):255-261. doi: 10.1093/ndt/gfv346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Gansevoort RT, Coresh J, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020;75(1):84-104. doi: 10.1053/j.ajkd.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 18.Shin J-I, Secora A, Alexander GC, et al. Risks and benefits of direct oral anticoagulants across the spectrum of GFR among incident and prevalent patients with atrial fibrillation. Clin J Am Soc Nephrol. 2018;13(8):1144-1152. doi: 10.2215/CJN.13811217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel P, Hu Y, Kolinovsky A, et al. Hidden burden of electronic health record-identified familial hypercholesterolemia: clinical outcomes and cost of medical care. J Am Heart Assoc. 2019;8(13):e011822. doi: 10.1161/JAHA.118.011822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics. 1996;52(1):249-264. doi: 10.2307/2533160 [DOI] [PubMed] [Google Scholar]
- 22.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 24.Emilsson L, García-Albéniz X, Logan RW, Caniglia EC, Kalager M, Hernán MA. Examining bias in studies of statin treatment and survival in patients with cancer. JAMA Oncol. 2018;4(1):63-70. doi: 10.1001/jamaoncol.2017.2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cain LE, Saag MS, Petersen M, et al. ; Antiretroviral Therapy Cohort Collaboration, the Centers for AIDS Research Network of Integrated Clinical Systems, and the HIV-CAUSAL Collaboration . Using observational data to emulate a randomized trial of dynamic treatment-switching strategies: an application to antiretroviral therapy. Int J Epidemiol. 2016;45(6):2038-2049. doi: 10.1093/ije/dyv295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gastwirth JL, Krieger AM, Rosenbaum PR. Dual and simultaneous sensitivity analysis for matched pairs. Biometrika. 1998;85(4):907-920. doi: 10.1093/biomet/85.4.907 [DOI] [Google Scholar]
- 27.Liu W, Kuramoto SJ, Stuart EA. An introduction to sensitivity analysis for unobserved confounding in nonexperimental prevention research. Prev Sci. 2013;14(6):570-580. doi: 10.1007/s11121-012-0339-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie X, Liu Y, Perkovic V, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67(5):728-741. doi: 10.1053/j.ajkd.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 29.Molnar MZ, Kalantar-Zadeh K, Lott EH, et al. Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker use, and mortality in patients with chronic kidney disease. J Am Coll Cardiol. 2014;63(7):650-658. doi: 10.1016/j.jacc.2013.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2(3):109-112. doi: 10.4103/2229-3485.83221 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Derivation of Study Population
eFigure 2. Cumulative Incidence of Hyperkalemia, by ACE-I/ARB Discontinuation Status
eFigure 3. Cumulative Incidence of Bleeding, by ACE-I/ARB Discontinuation Status in the Propensity-Score Matched Sample
eTable 1. Diagnosis and Procedure Codes Used to Define End-Stage Kidney Disease (ESKD)
eTable 2. International Classification of Disease, 9th and 10th Editions, Clinical Modification (ICD-9-CM, ICD-10-CM) Used to Define Disease Conditions
eTable 3. Baseline Characteristics of Patients With ≥40% eGFR Decline
eMethods. Target Trial Emulation
eResults. Sensitivity Analyses