Abstract
Background:
Measures of handgrip strength have not only emerged as a clinically viable screening tool for determining risk for morbidity, functional disability, and early mortality, but also for helping to identify cognitive deficits. However, the phenomena that links low handgrip strength with cognitive decline remains unclear. The role of the muscular and neural systems, and their adaptations to muscle strengthening activities over the life course, may provide important information for how age-related changes to muscle mass, strength, and neural capacity influence cognition. Moreover, disentangling how handgrip strength and cognitive function are associated may help to inform healthcare providers working with aging adults and guide targeted interventions aiming to preserve muscle and cognitive functioning.
Objective:
To 1) highlight and summarize evidence examining the associations of handgrip strength and cognitive functioning, and 2) provide directions for future research in this area.
Methods:
Articles from the PubMed database were searched from November 2018-May 2019. The search term algorithm, inclusion and exclusion criteria were pre-specified by investigators.
Results:
Several cross-sectional and longitudinal studies have revealed that measures of handgrip strength were associated with cognitive declines regardless of age demographics and the presence of comorbidities.
Conclusions:
Handgrip strength can be used in clinical and epidemiological settings for helping to determine the onset and progression of cognitive impairment. Future research should continue to examine how handgrip strength and cognitive function are linked.
Keywords: Alzheimer Disease, Aging, Cognition, Dementia, Muscle Weakness, Sarcopenia
Age-related reductions in muscle mass and strength begin at around 30 years of age and potentiate near the fifth or sixth decade of life [1, 2]. Sarcopenia (i.e., age-related loss of muscle mass) and dynapenia (i.e., age-related loss of muscle strength) help to characterize declines in muscle mass and strength during aging [3, 4], and influence health status during the aging process [5]. For example, sarcopenia and dynapenia are associated with chronic diseases, functional disabilities, and early mortality [6, 7]. Although a variety of measurements exist for examining age-related declines in muscle strength, measures of handgrip strength are the most common index of muscle strength due to ease of assessment, low cost, high feasibility, and validity of information provided [8, 9]. Lifespan changes in handgrip strength also imitate the changes in skeletal muscle mass and strength that occur over time [10, 11], and decreased handgrip strength is associated with several clinically-relevant health outcomes such as diabetes, frailty, and premature mortality [12]. Therefore, declines in handgrip strength are often used as a biomarker for identifying muscle weakness and are considered a “vital sign” of aging [13, 14].
While declines in handgrip strength have been thought to be primarily attributed to age-related changes in the muscular system, it has also been postulated that decreased handgrip strength is more a product of diminished neural and motor system capacity [15]. For example, during a strength capacity assessment, nervous system deficiencies may limit the amount of muscle force aging adults can generate to about half of what would be expected if the skeletal musculature were fully activated by the nervous system, primarily due to poor neuromuscular activation and motor unit recruitment [16, 17]. The dysfunction observed in the neural and motor systems during aging that influence handgrip strength declines may also be linked to the incipient and progressing cognitive impairment [18]. Hand dexterity is an important factor in handgrip strength performance that is partly mediated by nervous system functioning, and the cognitive demand that is necessary to complete motor tasks becomes more compromised at older age [15, 19]. Thus, the same age-related deficiencies to the nervous system that may limit cognitive function may also impact declines in muscle strength. As such, handgrip strength measurements may help to detect cognitive impairment.
Although some have suggested that decreased handgrip strength and cognitive function during aging are not necessarily mutual [20], others have indicated that maximal handgrip strength is a discriminating measure of neurological function and that handgrip strength may help to reveal onset changes in brain health [15]. Continuing to unravel how muscle strength, neural and muscle system integrity, and cognitive functioning are related may help to provide pathways for future research, inform practitioners working with aging adults, and guide interventions aiming to retain muscle strength and cognitive abilities. The purposes of this narrative review were to 1) highlight and summarize evidence examining the associations of handgrip strength and cognitive functioning, and 2) provide directions for future research in this area.
Methods and Materials
Articles from the PubMed database were searched. We selected the PubMed database for this review because articles indexed in PubMed contain a large amount of peer-reviewed studies related to handgrip strength and cognitive functioning. Database searches began in October 2018 and concluded in May 2019. The following search terms were executed: (grip OR handgrip) AND (strength OR weakness OR dynamometer) AND (middle-aged OR older OR aging) AND (adults OR individuals) AND (cognition OR cognitive function OR dementia OR cognitive impairment OR Alzheimer disease). The search terms included in the algorithm for our review were selected based on our study aims. To be considered for inclusion, articles must have been published in English language, included persons aged at least 45 years, assessed handgrip strength, evaluated a marker of cognitive function, and been published before the year 2000. Articles that were not original research and did not address the primary research question of interest were not considered.
Handgrip Strength and Cognitive Functioning for Studies with Middle-Aged and Older Adults
The National Institute of Aging has underscored the importance of supporting and conducting research in middle-aged adults (e.g., adults aged 45–64 years) for identifying and abating signs of neuronal aging [21]. Thus, research for the association between strength capacity and cognitive function should be thought as important for both middle-aged and older adults. Age-related declines in muscle strength begin at approximately 30 years of age, and onset cognitive deficits have been detected at around the same age [1, 2, 22]. Further, muscle strength and cognitive functioning can decline rapidly in middle-age [1, 2, 23]. Given that lifespan declines in handgrip strength correspond with the beginning of age-related reductions in muscle strength [1, 2, 10, 11], handgrip strength may also help to reveal age-related changes to cognitive functioning at middle-age.
There are several methods for assessing cognitive function [24]. The Mini-Mental State Examination is popular self-report assessment of cognitive function that examines orientation, memory, concentration, language, and praxis [25, 26]; whereas, other common assessments of cognitive function such as the animal naming test examine verbal fluency [27]. Malmstrom et al. [26] utilized the Mini-Mental State Exam and animal naming test for a cross-sectional investigation of over 900 African American adults aged 56.7±4.4 years and determined that handgrip strength was lower in those with scores in the lowest Mini-Mental State Examination tertile (handgrip strength: 30.13±17.01 kilograms) and animal naming test tertile (handgrip strength: 29.08±14.44 kilograms) relative to persons with scores in the highest tertiles for the Mini-Mental State Exam (handgrip strength: 33.32±13.07; p<0.001) and animal naming test (handgrip strength: 35.53±14.87; p=0.02). Another cross-sectional investigation of 2,565 adults aged at least 60 years determined that mean handgrip strength was 8.91-kilograms lower in persons with dementia compared to their non-dementia peers, and that lower handgrip strength was overall associated with dementia (β=−3.00; p<0.001) [28]. Similarly, in a cross-sectional study of 13,978 adults aged 61.1±10.2 years who completed several physical performance measures, every 1-kilogram increase in handgrip strength was associated with a 6% higher overall cognitive score [29].
Measures of handgrip strength may also relate to different determinants of cognitive function. For example, a cross-sectional study of 378 adults aged ≥60 years determined that handgrip strength was associated with poorer verbal memory (β=−0.13; p=0.039), slower processing speed (β=−0.20; p=0.001), and decreased working memory (β=−0.18; p=0.004) [30]. Similarly, Garcia-Cifunentes et al. [31] suggested that low handgrip strength was associated with 2.25 greater odds for cognitive impairment in a cross-sectional study of 1,654 adults aged at least 60 years, and future investigations should assess the temporal association of handgrip strength and cognitive function. Although several cross-sectional investigations that have included middle-aged adults found an association between handgrip strength and cognitive function, longitudinal study designs will help provide insights for this association over time.
A 6-year longitudinal investigation of 6,435 middle-aged and older adults revealed that those in the lowest handgrip strength quartile had a 36% higher hazard for new-onset cognitive dysfunction [32]. Another study of 13,828 adults aged at least 50 years who were followed for eight-years revealed that every five-kilogram lower handgrip strength was associated with 1.10 greater odds for any cognitive impairment, 1.18 greater odds for severe cognitive impairment, and 1.10 greater odds for poor cognitive functioning [33]. Likewise, a 5.9 year longitudinal investigation of 1,637 non-demented women aged at least 60 years determined that every 5-kilogram increase in handgrip strength was associated with a 16% decreased risk for all dementia and 21% decreased risk for probable Alzheimer’s disease [34]. Although some genetic factors such as Apolipoprotein E4 and Clusterin may increase the risk for Alzheimer’s disease, handgrip strength helped to predict longitudinal memory resilience in 642 middle-aged and older adults with such genetic risk factors [35]. Further, Boyle et al. [36] determined that low handgrip strength was associated with 28% greater risk for a first occurrence of mild cognitive impairment and 34% greater risk for persistent mild cognitive impairment in 761 adults aged at least 54 years over a 12-year annual follow-up.
Cross-sectional and longitudinal investigations that have included middle-aged adults have helped to support the notion that the association between decreased handgrip strength and cognitive declines may occur before older adulthood. While identifying weakness and cognitive deficits at an earlier age may help in the efficacy and implementation of targeted interventions, the prevalence of age-related weakness and cognitive impairment remain overall higher in older adults [37, 38].
Handgrip Strength and Cognitive Functioning for Only Older Adults
Age is a hallmark risk factor for cognitive impairment and the older adult population is expected to increase globally. For example, projections indicate that 13.2 million Americans will be living with a cognitive impairment (about a 160% increase) by the year 2050 [39], which aligns with estimates suggesting that the older adult population in the United States will rapidly increase over the next few decades [40]. Reduced muscle strength is more prevalent in older adults than in other age groups [37], and remains a prominent risk factor for cognitive deficits [15]. The confluence of the health consequences related to aging, cognitive impairment, and weakness, along with the projected increase in each of these health factors, underscores the importance of addressing this emerging public health epidemic. Therefore, many have sought to understand how muscle strength, as measured by handgrip strength, factors into cognitive declines in older adults.
A cross-sectional study of 70 older adults aged 71.0±4.7 years uncovered that increased handgrip strength was significantly correlated with cognitive function tasks (r=0.42; p<0.01) [41]. Another cross-sectional investigation of adults aged 70 years and over found that those below the median split for handgrip strength had significantly lower scores on the Quality of Life-Alzheimer’s Disease Test compared to those at or above the median split (19.38±6.91 vs. 22.7±8.0; p<0.05) [42]. Additional evidence provided by Liu et al. [43] determined that increased handgrip strength was correlated with performance in completing instrumental activities of daily living (r=0.28; p<0.001) and Montreal Cognitive Assessment scores (r=0.28; p<0.001) in a cross-sectional study of 1,396 older adults aged 77.3±7.5 years. Moreover, the fully-adjusted linear regression models conducted by Yoon et al. [44] revealed that handgrip strength was significantly associated with processing speed (β=−0.32, p=0.017), working memory (β=0.24; p=0.026), and memory (β=0.25; p=0.042), but not cognitive flexibility (β=−0.30; p=0.833) in a cross-sectional investigation of 104 older adults aged 73.5±5.43 years. Although investigations utilizing a cross-sectional design have found a signal for the association between handgrip strength and cognitive function in older adults, utilizing longitudinal study designs may help to identify how time factors into this association.
A 4-year longitudinal investigation of 1,514 men aged 71.6±4.58 years and 1,223 women aged 71.5±4.85 years determined that every 6.14-kilogram increase in handgrip strength was associated with a 0.233 increase in Mini-Mental State Examination scores for men (p<0.01) and every 4.12-kilogram increase in handgrip strength was associated with a 0.197 increase in Mini-Mental State Examination scores for women (p<0.05) [45]. These observations were consistent with Alfaro-Acha et al. [46] who found that of the 2,160 older Mexican Americans aged 71.9±5.9 years in the lowest handgrip strength quartile at baseline had a 1.28 lower Mini Mental State Examination at 7-year follow-up (p<0.0001) and greater overall cognitive decline (β=−0.26; p<0.001) than those in the highest handgrip strength quartile. Given that Alzheimer’s disease is the most common type of dementia, Buchman et al. [47] analyzed data from 877 older adults without dementia and revealed that every 1-pound decrease in handgrip strength was associated with a 1.5% increased risk for Alzheimer’s disease and 9% increased risk for Alzheimer’s disease when examining annual rate of handgrip strength change during the 5-year study period. Given that age is a consistent risk factor for weakness and cognitive impairment, examining the association between handgrip strength and cognitive functioning in persons with advanced age may help to reveal new insights for this association.
The oldest old have a high prevalence of reduced muscle strength and dementia [37, 48], so it is possible that handgrip strength is strongly associated with cognitive functioning in this population. A cross-sectional study of 3,025 women aged at least 75 years indicated that low handgrip strength was associated with 1.81 greater odds for cognitive impairment [49]. Moreover, a prospective cohort study of 555 adults aged at least 85 years found that baseline handgrip strength difference (estimate=0.25; p<0.001), annual change (estimate=−0.75; p<0.001), and accelerated decline in handgrip strength (estimate=0.01; p<0.001) were each associated with Mini Mental State Examination scores [50]. Bullain et al. [51] utilized a cross-sectional study design for 629 participants aged at least 90 years and determined that those in the weakest handgrip strength category had 9.8 greater odds for dementia compared to those in the strongest handgrip strength category. Similarly, Bullain et al. [52] completed a population-based longitudinal study in 578 adults aged 93.3±2.6 years and found that those in the weakest handgrip strength quartile had a 2.01 greater risk for dementia relative to those in the strongest handgrip strength quartile over a 2.6 ±1.9 year follow-up.
While more investigations for the association of handgrip strength and cognitive function in persons with advanced age are warranted, the participant and investigator feasibility for conducting such investigations poses unique challenges. Such investigations and targeted interventions should also acknowledge that factors for cognitive impairment in younger populations such as the Apoliopoprotein E4 allele and healthy lifestyle behaviors are less relevant for oldest old populations [48].
Handgrip Strength and Cognitive Functioning in Aging Adults with Health Conditions
Aging adults living with certain health conditions may place themselves at greater risk for cognitive declines [53]. Measures of handgrip strength may be linked to such health conditions, which in turn, may help in identifying and preventing the onset and progression of cognitive impairment. Certain cardiovascular diseases such as congestive heart failure are strongly associated with cognitive deficits [54], and weakness is common in those with congestive heart failure hospital referrals [55]. Hypertension is a precursor for future cardiovascular diseases and cognitive impairment [56]; a study of 1,467 American adults from the National Health and Nutrition Examination Survey revealed that handgrip strength was lower in those with undiagnosed hypertension (β=−6.6; p<0.004) and diagnosed hypertension (β=−4.27; p=0.04) [57].
Likewise, chronic obstructive pulmonary disease increases the risk for cognitive impairment [58], and a prospective population based study of 502,293 adults aged 40–69 years found that every 5-kilogram lower handgrip strength was associated with 24% and 19% increased risk for chronic obstructive pulmonary disease in men and women, respectively [59]. Lower oxygen and higher carbon dioxide levels from chronic obstructive pulmonary disease may harm the brain and lead to snowballing multimorbidity [60]. Other chronic cardiometabolic diseases such as diabetes are also strongly associated with cognitive declines, in part, due to glycemic index and diabetes neuropathy [61]. Cross-sectional research from a nationally-representative sample of American adults in the National Health and Nutrition Examination Survey suggests that mean handgrip strength was lower for persons with undiagnosed (β=−10.02; p<0.0001) and diagnosed diabetes (β=−8.21; p<0.03) [57], while a 19-year longitudinal study of older Mexican Americans found that weak men had a 5% increased risk for incident diabetes and weak women had a 38% increased risk [62].
Weakness may factor into cerebrovascular diseases such as stroke, which are common in older adults and associated with cognitive decline [63]. Leong et al. [64] found that every 5-kilogram reduction in handgrip strength was associated with 9% increased risk for stroke in 142,861 adults aged 35–70 years over the 7-year study period. Depression is also a risk factor for future cognitive impairment [65]. Fukumori et al. [66] examined data from 4,314 participants aged 66.3±9.0 years and determined that lower handgrip strength (per 1 standard deviation decrease) was associated with higher 1.15 higher odds for depressive symptoms at baseline and 1.13 higher odds for the development of depressive symptoms at 1-year follow-up. Decreased handgrip strength has been shown to be associated tasks that require higher neuropsychological functioning such as instrumental activities of daily living [67], and the presence of an instrumental activity of daily living impairment is associated with future functional and cognitive declines [68].
Approximately 75% of older adults are living with multiple health conditions [69], and multimorbidity may also increase cognitive impairment risk [70]. Given that handgrip strength is associated with several health outcomes that are risk factors for future cognitive impairment, more attention should be given to preventing and treating these health outcomes so that cognitive impairment can be avoided later in life.
Null Results for the Association of Handgrip Strength and Cognitive Functioning in Aging Adults
While there have been several investigations that have shown positive results for the association between handgrip strength and cognitive functioning, other studies have reported null results. For example, Sattler et al. [71] found that handgrip strength did not predict mild cognitive impairment or Alzheimer’s disease in 300 older adults. Weakness, as measured by handgrip strength, is an important part of frailty assessments, but Gary et al. [72] concluded that although an association between frailty and non-Alzheimer’s dementia existed, handgrip strength alone was not associated with dementia, possible Alzheimer’s disease, and non-Alzheimer’s dementia in 2,619 older adults. Another investigation of 1,249 adults aged 72.2±5.8 years revealed that several physical performance tests at baseline, including handgrip strength, were not associated with cognitive decline during a 4.4 year follow-up after [73].
In a 3-year longitudinal investigation aiming to determine the association between physical and cognitive function in 169 community-dwelling adults aged 72.4±4.8 years, both handgrip strength at baseline and changes in handgrip strength over the study period were not associated with cognitive function [74]. Although handgrip strength was associated with functional declines and mortality in a large sample of adults aged 70–90 years, handgrip strength did not predict subsequent cognitive decline [75]. Measures of handgrip strength also did not predict rate of change on memory tasks, and changes in handgrip strength moderately correlated with memory changes in 425 community-dwelling older adults, suggesting that changes in handgrip strength and cognitive functioning may move together over time [76].
Interventions for Muscle Strength and Cognitive Function
Intervention strategies for improving either muscle strength, cognitive functioning, or both may improve the health aspects of these outcomes. A review article by Okura et al. [77] suggests that cardiorespiratory fitness interventions, especially in combination with other healthy behaviors, may help to reduce the risk of cognitive impairment. Martin et al. [78] completed a systematic review of cognitive-based interventions for healthy older persons and persons with cognitive impairment, and suggested that cognitive interventions may lead to performance gains but such effects may not be attributed to the cognitive training directly. A systematic review and meta-analysis of 33 investigations examining the efficacy of cognitive interventions on improving general cognition in dementia completed by Huntley et al. [79] revealed that cognitive stimulation may improve Mini-Mental State Examinations scores (g=0.51; p<0.001) compared to non-active (g=0.35; p=0.019) and active controls. Moreover, social engagement has demonstrated efficacy for improving cognitive functioning, as a meta-analysis of 51 articles examining the association between social isolation and cognitive function determined that higher social engagement and social networks were associated with better cognitive function (r=0.052; p<0.05) [80].
Healthy behaviors may also help to improve cognitive functioning. For example, a systematic review of studies examining the effect of diet (which may have implications on muscle strength) on cognitive outcomes suggested that improvements in cognitive performance (specifically memory) were consistent in studies that included B vitamins, folic acid, eicosapentaenoic acid, docosahexaenoic acid, and flavonol supplementation [81]. Dietary supplementation with vitamin D, resveratrol, lipoic acid, and green tea have demonstrated neuroprotective actions with aging [82–86]. A systematic review and meta-analysis of investigations evaluating the effects of physical activity (aerobic and resistance training) and cognitive activity on cognitive outcomes showed that combined physical and cognitive activity interventions improved cognition relative to the control group (g=0.316; p<0.001) [87]. Interestingly, the same systematic review and meta-analysis indicated that studies which compared physical and cognitive activity with physical activity alone showed small but significant improvements in cognitive outcomes in favor of combined physical and cognitive activity interventions; whereas, no significant differences were found between combined physical and cognitive activity, and cognitive activity only interventions [87]. These findings demonstrate that interventions including cognitive stimuli (i.e., cognitive tasks, social engagement), healthy behaviors, or both may show promise for preventing or slowing cognitive impairment.
While engaging in healthy lifestyle behaviors may help to preserve muscle strength and cognitive function, concurrent improvements to handgrip strength may not occur. For example, Rhodes et al. [88] randomly assigned 44 healthy, sedentary women aged 65–75 years to either a one year progressive resistance training exercise program or control group, and determined that although muscle strength gains occurred in those who engaged in progressive resistance exercise, there were no gains in handgrip strength. Yaginuma et al. [89] similarly had participants self-select to be in a 12-week body mass-based home exercise intervention (n=160; age: 69.0±6.0 years) or control group (n=37; age: 69.0±7.0 years) and found that increases in lower body strength and muscle size failed to translate to improvements in handgrip strength. Conversely, a recent meta-analytic review by Labott et al. [90] concluded meaningful but small improvements in handgrip strength occurred in healthy community-dwelling older adults who participated in a variety of exercise training approaches, but that task specific and multimodal exercise training may provide a proper stimulus to particularly improve handgrip strength. Central nervous system functioning is also involved in handgrip strength and cognitive function, and engaging in physical activity may lead to positive adaptations in central nervous system functioning (e.g., brain stem, hypothalamus, basal ganglia) [91]. Although engaging in healthy lifestyle behaviors may preserve muscle strength, cognitive function, and central nervous system functioning, it is relatively uncertain if such improvements would be observed in handgrip strength.
Future Directions
Measures of maximal handgrip strength remain a simple-to-use, inexpensive, and viable screening tool for determining muscle weakness during aging. There is an accumulating amount of evidence that suggests handgrip strength is associated with cognitive functioning in middle-aged and older adults, and with health conditions that may increase cognitive impairment risk. Targeted interventions for preventing declines in cognitive function through healthy behaviors, including muscle strengthening activities, have shown potential for preventing and treating cognitive impairment [87]. Interventions should also be multidisciplinary, as single-component interventions may not be helpful for preserving cognitive function [92]. Beginning such healthy behaviors early in life will help to improve the implementation and efficacy of any intervention. Similarly, the use of technology, artificial intelligence, and the internet may serve as a unique platform for expanding reach for aging adults with transportation barriers and those who live in rural areas. Incorporating psychological framework, cognitive-based tasks, and social supports (e.g., community-based participatory research) may also help in improving health behavior adherence and the association between weakness and cognitive function.
Although cross-sectional and longitudinal evidence suggests an association between handgrip strength and cognitive function in aging adults, improvements in handgrip strength alone may especially be challenging to detect for older frail adults who are in a health behavior intervention [93]. The concept of anabolic resistance has been mostly attributed to the inability of muscle protein signaling to respond to an anabolic stimulus in aging adults relative to those who are younger [94]. Future research should help to provide an understanding for responders and non-responders as it pertains to the trainability of handgrip measurement with exercise training and any accompanying changes in cognitive function. Essentially, attempting to determine if there is “neural resistance” to cognitive stimuli with aging. Older frail adults may especially benefit from such research.
Mechanisms for sarcopenia and dynapenia have been primarily attributed to age-related reductions in function to the muscular and nervous systems [6, 95]. Engaging in activities that help to preserve the capacity of both these body systems (e.g., neuroprotection) may be especially important for cognitive function [96]. For example, persons who consistently engage in muscle strengthening activities throughout the life course often have greater levels of muscle mass and strength through adaptations to the muscular and neural systems compared to those who do not engage in these activities [97]. When declines to muscle mass and strength, and neural functioning occurs during aging, those who engaged in muscle strengthening activities may not experience cognitive deficits [96]. Although interventions aiming to improve strength capacity may have shown promise, implementing intervention programs later in life may have challenges related to adherence, and efficacy may have been greater if interventions were practiced earlier in life. Therefore, the neural mechanisms that influence muscle mass and strength may also influence cognitive functioning. It should be noted, however, that measures of handgrip strength may have limitations for determining efficacy in strength capacity before and after an intervention.
A recent systematic review of studies examining the associations between changes of handgrip strength and cognitive functioning provided interesting perspectives [20]. Zammit et al. [20] recommended evaluating the bidirectional association between handgrip strength and cognitive functioning, and possible third factors that propel these associations. Research that has found a bidirectional association between handgrip strength and cognitive functioning suggests that strength capacity and cognitive function may parallel, such that losses of functioning in one factor may predict losses of functioning in the other [98]. An investigation by Kim et al. [99] also helped to determine the bidirectional association between handgrip strength and cognitive impairment in older Koreans. Some possible explanations for these findings were provided by Kim et al. [99] including cognitive impairment may lead to more sedentary behavior, which factors into reduced muscle strength; while physical activity participation preserves muscle strength and improves cognitive function through enhanced brain plasticity. Although these discussion points are plausible, it might be important to consider how to improve some of the modifiable risk factors related to each outcome.
Placing credence in the individual components for the bidirectional association of handgrip strength and cognitive functioning may help to improve our understanding of the association. For example, if priority were to be given to preserving muscle strength for preventing cognitive deficits, several mediating factors such as physical activity participation, healthy diet, and practicing such behaviors earlier in life have shown promise for improving brain plasticity and reducing cognitive declines during aging (Figure 1A) [100]. Conversely, if priority were given to preserving cognitive functioning for maintaining muscle strength, completing cognitively driven tasks or social engagement may not help muscle strength (Figure 1B). Thus, muscle strengthening activities alone may help cognitive functioning; whereas, cognitive activities alone may not help improve muscle strength. It should be noted, however, that addressing either muscle strength or cognitive function individually may not be as effective as using multidimensional approaches. Nevertheless, the health aspects of muscle strength may help to protect against adverse health outcomes from cognitive impairment such as chronic disease, falls, and functional limitations, thereby helping to preserve quality of life, autonomous living, and longevity. More research is needed for unraveling the direction of causation for the association of muscle strength and cognitive function and how potential mediators (e.g., age) may influence this association. Further, investigating potential common causes of age-related declines in strength and cognitive function are also warranted [101–103].
Figure 1.
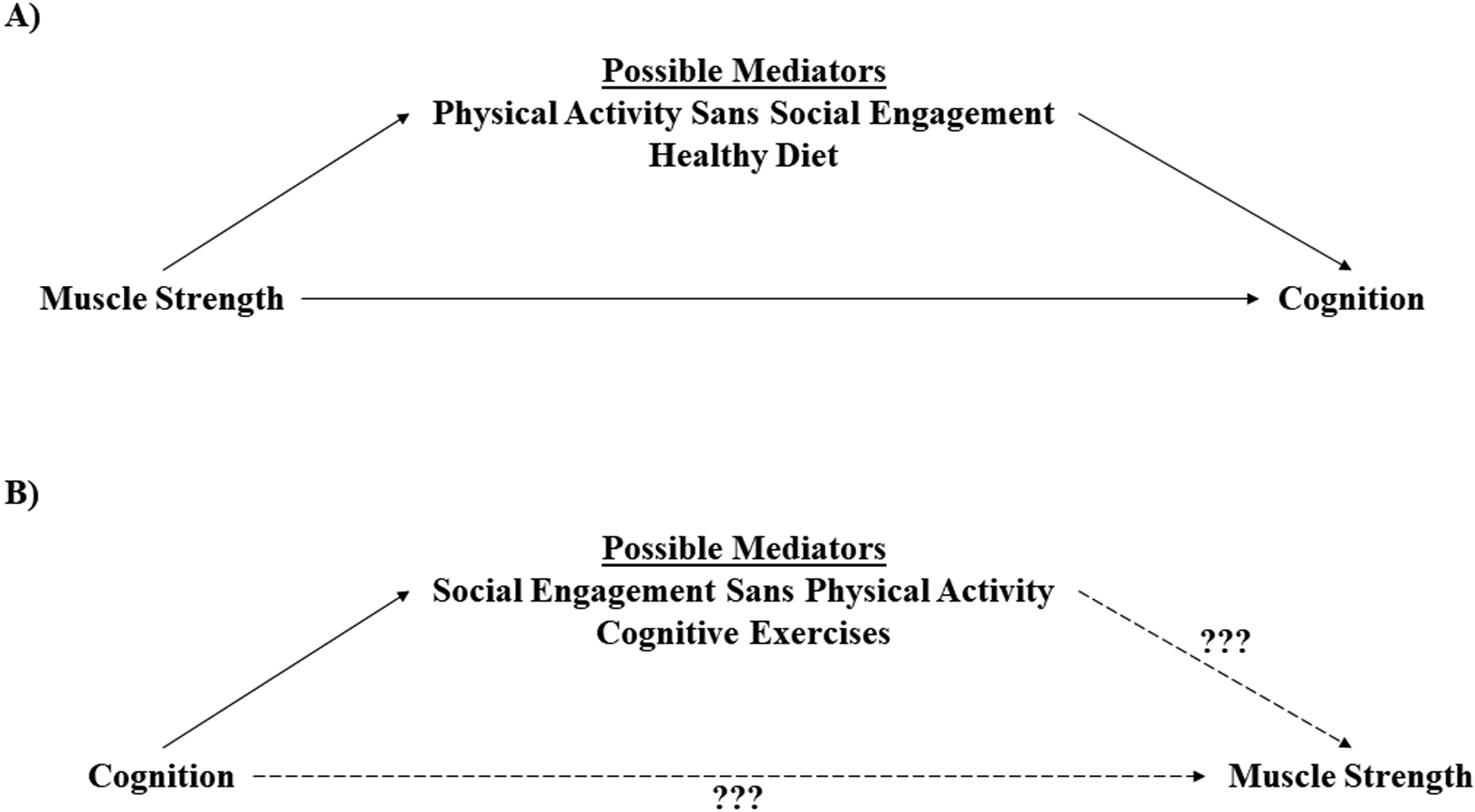
A theoretical model for improving individual components for the bidirectional association of muscle strength and cognition.
A=placing credence in muscle strengthening activities alone, B=placing credence in cognitive functioning activities alone.
Over the past decade, there has been growing interest in the central neural control of mobility in aging [104–106], and evidence for a predementia syndrome characterized by slow gait speed and cognitive complaints (e.g., motoric cognitive risk syndrome) [107–109]. This work, along with the conceptual consistency within the field of geroscience [110, 111], we postulate that certain common causes may underline associations between handgrip strength and cognitive function. These may include factors such as inflammation, poor cerebrovascular health, degeneration of functional and structural connectivity in selected brain regions, and/or changes in neurotransmitter function (e.g., dopamine), all of which theoretically could contribute to systematic neurodegeneration of both motor and cognitive circuits [112]. Developing a better understanding for these common causes of reduced motor and cognitive function will not only help to explain the observed association between handgrip strength and cognitive function, but it will also provide perspectives into potential neurotherapeutic targets for enhancing both physical and cognitive health in aging adults. Moreover, determining how measures of muscle strength, including handgrip strength, are connected to domains of the brain and processing systems may provide specificity for examining how handgrip strength and cognitive function are related.
Physiological brain aging is characterized by a loss of synaptic contacts and neuronal apoptosis that provokes age-dependent decline of sensory processing, motor performance, and cognitive function [113]. In the central nervous system, the size of neurons, number of synapses, integrity of white matter, volume of grey matter, and neurotransmitter levels have all been reported to decrease with age [114–118]. These neurodegenerative and neurochemical changes are thought to underlie a decline in both cognitive and motor function [118–121].
The “common cause hypothesis” suggests that common factors are responsible for age-related deterioration in cognitive and non-cognitive process, thereby positing reasons why handgrip strength and cognitive function are linked [103]. Physiological brain aging is characterized by a loss of synaptic contacts and neuronal apoptosis that provokes age-dependent decline of sensory processing, motor performance, and cognitive function [113]. In the central nervous system, neuronal size, synaptic numbers, white matter integrity, grey matter volume, and neurotransmitter levels are among some of the neurochemical and neuroanatomical structures that are reported to decrease with age [114–118]. This myriad of neurodegenerative and neurochemical changes are thought to result in reduced cortical plasticity with advancing age [122], and collectively underlie a decline in both cognitive and motor function [119–121, 123]. Simply stated, it is reasonable to believe that systemic changes throughout the brain occur with advancing age results in central neurodegeneration throughout virtually all brain regions. Because handgrip strength requires hand dexterity and vigorous contractions of the hand flexors [15], persons with diminished central nervous system functioning may have difficulty completing tasks that require high levels of coordinated motor function and physical exertion [112]. Other physical measures such as knee extension strength and gait speed have also been shown to be associated with cognitive function [124, 125], but may lack in feasibility relative to handgrip strength. As such, reevaluating handgrip strength measurements, methodologies, and technologies may help to provide novel insights into central nervous system functioning and cognitive status, while also improving handgrip strength as a diagnostic tool for cognitive impairment and preserving the practicality of handgrip strength assessments.
The cerebellum could be viewed as part of the brain that is a “messenger” of cognitive declines which can be measured by physically-driven tasks such as handgrip strength. For example, the cerebellum is linked to areas of the brain that support cognitive functioning such as the cerebral cortex and hippocampus [126, 127]. This may help to explain why the cerebellum contributes to executive functions [128]. Moreover, cerebellar involvement could be viewed as secondary relative to cerebral involvement in certain dementias, and that cerebellar atrophy mostly occurs at the latter stages of Alzheimer’s disease [129]. Such cerebrocerebellar interactions influencing brain connectivity may also explain why handgrip strength is associated with white matter volume [130]. These findings indicate that the declines in cerebral functioning that are linked to certain dementias could impair cerebellum functioning, which in turn limits the motor and neural pathways that are responsible for performing a maximal grip force task.
Similarly, corticostriatal functional connectivity studies have demonstrated connections between the dorsal striatum and motor related cortical areas [131–133]. Interestingly, cortical circuits related to cognition and executive function have also been shown to be from other distinct cortico-striatal networks that are involved in action selection, planning, and decision-making [134, 135]. For instance, it has long been suggested that there are numerous basal ganglia-thalamocortical circuits, one of which is a dorsolateral prefrontal cortex circuit [134]. A variety of inflammatory stimuli have been found to preferentially target basal ganglia function and lead to impaired motivation and motor activity [136]. As such, it is possible that a decline in dopaminergic output from the basal ganglia may lead not only to less goal-directed motor output, but also reduced ability for integrating automatic motor programs, leading to motor cognitive resources for a movement task [137]. However, as cognitive ability also declines with age, this may limit older adults’ ability to “compensate” using this cognitive strategy for movement.
Limitations
While this narrative review underscores evidence about the association of handgrip strength and cognitive function, it is not intended to be an all-encompassing systematic review of the literature. Therefore, our narrative review shares common limitations of narrative reviews in general [138]. Articles that did not meet our criteria for the narrative portion of this review could have been used to support text within the narrative review and our implications for future research. Nevertheless, narrative reviews allow for a detailed description of published articles on a topic, provides rational for future research, and speculates on new types of interventions that may arise [139].
Conclusions
As an isometric grip force task, measures of handgrip strength are intricately linked to cognitive functioning. Several cross-sectional and longitudinal investigations have identified that handgrip strength and cognitive function are associated. Unraveling the direction of causation and potential mediators for this association may help healthcare providers and interventionists prevent weakness and cognitive impairment. Measures of handgrip strength should become more commonplace for helping to identify not only muscle weakness, but also cognitive impairment in clinical and epidemiological settings.
ACKNOWLEDGEMENTS
BCC effort was supported in part by a grant for the NIA (R01AG044424). RRB was funded by a grant from the NIHG (5T32HL130357). RM was funded in part by the College of Human Sciences and Education at North Dakota State University.
Footnotes
CONFLICTS OF INTEREST
In the past 5-years, BCC has received research funding from the NIH, Regeneron Pharmaceuticals, Astellas Pharma Global Development, Inc., RTI Health Solutions, Biophytis, and the Osteopathic Heritage Foundations. BCC has also received consulting fees from Regeneron Pharmaceuticals, Abbott Laboratories, and the Gerson Lehrman Group. Additionally, BCC is co-founder with equity, and serves as the Chief of Aging Research, of AEIOU Scientific, LLC. WJK was a paid consultant for handgrip strength in the National Health and Nutrition Examination Survey. KAS, KJH, DJT, RRB, and RPM report no conflicts of interest.
REFERENCES
- [1].Keller K, Engelhardt M (2013) Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J. 3, 346–50. [PMC free article] [PubMed] [Google Scholar]
- [2].Landi F, Calvani R, Tosato M, Martone AM, Fusco D, Sisto A, Ortolani E, Savera G, Salini S, Marzetti E (2017) Age-related variations of muscle mass, strength, and physical performance in community-dwellers: Results from the Milan EXPO Survey. J Am Med Dir Assoc. 18, 88. e17–88. e24. [DOI] [PubMed] [Google Scholar]
- [3].Clark BC, Manini TM (2008) Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 63, 829–34. [DOI] [PubMed] [Google Scholar]
- [4].Clark BC, Manini TM (2010) Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care. 13, 271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hunter GR, Singh H, Carter SJ, Bryan DR, Fisher GJ (2019) Sarcopenia and Its Implications for Metabolic Health. J Obes. 8031705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V (2008) Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 18, 388–95. [DOI] [PubMed] [Google Scholar]
- [7].Li R, Xia J, Zhang X, Gathirua-Mwangi WG, Guo J, Li Y, McKenzie S, Song Y (2018) Associations of muscle mass and strength with all-cause mortality among US older adults. Med Sci Sports Exerc. 50, 458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McGrath RP (2019) Understanding the Feasibility and Validity of Muscle Strength Measurements in Aging Adults. J Am Med Dir Assoc. 20, 99–100. [DOI] [PubMed] [Google Scholar]
- [9].Clark BC (2018) Neuromuscular Changes with Aging and Sarcopenia. J Frailty Aging. 8, 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, Der G, Gale CR, Inskip HM, Jagger C, Kirkwood TB, Lawlor DA, Robinson SM, Starr JM, Steptoe A, Tilling K, Kuh D, Cooper C, Sayer AA (2014) Grip strength across the life course: normative data from twelve British studies. PLoS One. 9, e113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dodds RM, Syddall HE, Cooper R, Kuh D, Cooper C, Sayer AA (2016) Global variation in grip strength: a systematic review and meta-analysis of normative data. Age Ageing. 45, 209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McGrath RP, Kraemer WJ, Al Snih S, Peterson MD (2018) Handgrip Strength and Health in Aging Adults. Sports Med. 48, 1993–2000. [DOI] [PubMed] [Google Scholar]
- [13].Bohannon RW (2015) Muscle strength: clinical and prognostic value of hand-grip dynamometry. Curr Opin Clin Nutr Metabl Care. 18, 465–70. [DOI] [PubMed] [Google Scholar]
- [14].Bohannon RW (2008) Hand‐grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 31, 3–10. [DOI] [PubMed] [Google Scholar]
- [15].Carson RG (2018) Get a grip: Individual variations in grip strength are a marker of brain health. Neurobiol Aging. 71, 189–222. [DOI] [PubMed] [Google Scholar]
- [16].Shinohara M, Li S, Kang N, Zatsiorsky VM, Latash ML (2003) Effects of age and gender on finger coordination in MVC and submaximal force-matching tasks. J Appl Physiol. 94, 259–70. [DOI] [PubMed] [Google Scholar]
- [17].Clark BC, Manini TM, Wages NP, Simon JE, Clark LA (2019) Voluntary vs Electrically Stimulated Activation in Age-Related Muscle Weakness. JAMA Netw Open. 2, e1912052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bishop NA, Lu T, Yankner BA (2010) Neural mechanisms of ageing and cognitive decline. Nature. 464, 529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB (2010) Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 34, 721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zammit AR, Robitaille A, Piccinin AM, Muniz-Terrera G, Hofer SM (2018) Associations between aging-related changes in grip strength and cognitive function in older adults: A systematic review. J Gerontol A Biol Sci Med Sci. 74, 519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].National Institute on Aging. Aging Well in the 21st Century: Strategic Directions for Research on Aging. https://www.nia.nih.gov/sites/default/files/2017-07/nia-strategic-directions-2016.pdf. Accessed 19 August 2019.
- [22].Salthouse TA (2009) When does age-related cognitive decline begin? Neurobiol Aging. 30, 507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Singh-Manoux A, Kivimaki M, Glymour MM, Elbaz A, Berr C, Ebmeier KP, Ferrie JE, Dugravot A (2012) Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ. 344, d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Young J, Meagher D, MacLullich A (2011) Cognitive assessment of older people. BMJ. 343, d5042. [DOI] [PubMed] [Google Scholar]
- [25].Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [26].Malmstrom TK, Wolinsky FD, Andresen EM, Philip Miller J, Miller DK (2005) Cognitive ability and physical performance in middle‐aged African Americans. J Am Geriatr Soc. 53, 997–1001. [DOI] [PubMed] [Google Scholar]
- [27].Mueller KD, Koscik RL, LaRue A, Clark LR, Hermann B, Johnson SC, Sager MA (2015) Verbal fluency and early memory decline: results from the Wisconsin registry for Alzheimer’s prevention. Arch Clin Neuropsychol. 30, 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ong HL, Chang S, Abdin E, Vaingankar J, Jeyagurunathan A, Shafie S, Magadi H, Chong SA, Subramaniam M (2016) Association of grip strength, upper arm circumference, and waist circumference with dementia in older adults of the wise study: A cross-sectional analysis. J Nutr Health Aging. 20, 996–1001. [DOI] [PubMed] [Google Scholar]
- [29].Zuo M, Gan C, Liu T, Tang J, Dai J, Hu X (2019) Physical predictors of cognitive function in individuals with hypertension: Evidence from the CHARLS basline survey. West J Nurs Res. 41, 592–614. [DOI] [PubMed] [Google Scholar]
- [30].Arts MH, Collard RM, Comijs HC, Zuidersma M, de Rooij SE, Naarding P, Oude Voshaar RC (2016) Physical frailty and cognitive functioning in depressed older adults: findings from the NESDO study. J Am Med Dir Assoc. 17, 36–43. [DOI] [PubMed] [Google Scholar]
- [31].Garcia-Cifuentes E, David-Pardo D, Borda M, Perez-Zepeda M, Cano C (2017) Two-way bridge between muscular dysfunction and cognitive impairment: secondary analyses of SABE-Bogota Study. J Frailty Aging. 6, 141–143. [DOI] [PubMed] [Google Scholar]
- [32].Jeong S, Kim J (2018) Prospective Association of Handgrip Strength with Risk of New-Onset Cognitive Dysfunction in Korean Adults: A 6-Year National Cohort Study. Tohoku J Exp Med. 244, 83–91. [DOI] [PubMed] [Google Scholar]
- [33].McGrath R, Robinson-Lane SG, Cook S, Clark BC, Herrmann S, O’Connor ML, Hackney KJ (2019) Handgrip Strength Is Associated with Poorer Cognitive Functioning in Aging Americans. J Alsheimers Dis. 70, 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yamada M, Mimori Y, Kasagi F, Miyachi T, Ohshita T, Sasaki HJ (2009) Incidence and risks of dementia in Japanese women: Radiation Effects Research Foundation Adult Health Study. J Neurol Sci. 283, 57–61. [DOI] [PubMed] [Google Scholar]
- [35].McDermott KL, McFall GP, Andrews SJ, Anstey KJ, Dixon RA (2016) Memory resilience to alzheimer’s genetic risk: Sex effects in predictor profiles. J Gerontol B Psychol Sci Soc Sci. 72, 937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA (2010) Physical frailty is associated with incident mild cognitive impairment in community‐based older persons. J Am Geriatr Soc. 58, 248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Looker AC, Wang C-Y (2015) Prevalence of reduced muscle strength in older US adults: United States, 2011–2012. NCHS Data Brief. 179, 1–8. [PubMed] [Google Scholar]
- [38].Geda YE (2012) Mild cognitive impairment in older adults. Curr Psychiatry Rep. 14, 320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hebert LE, Weuve J, Scherr PA, Evans DA (2013) Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 80, 1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Colby SL, Ortman JM (2014) Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau. [Google Scholar]
- [41].Ramnath U, Rauch L, Lambert E, Kolbe-Alexander TJ (2018) The relationship between functional status, physical fitness and cognitive performance in physically active older adults: A pilot study. PLoS One. 13, e0194918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Arrieta H, Rezola-Pardo C, Echeverria I, Iturburu M, Gil SM, Yanguas JJ, Irazusta J, Rodriguez-Larrad A (2018) Physical activity and fitness are associated with verbal memory, quality of life and depression among nursing home residents: preliminary data of a randomized controlled trial. BMC Geriatr. 18, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liu T, Wong GH, Luo H, Tang JY, Xu J, Choy JC, Lum TY (2018) Everyday cognitive functioning and global cognitive performance are differentially associated with physical frailty and chronological age in older Chinese men and women. Aging Mental Health. 22, 936–41. [DOI] [PubMed] [Google Scholar]
- [44].Yoon D, Hwang S, Lee D, Lee C, Song WJ (2018) Physical frailty and cognitive functioning in Korea rural community-dwelling older adults. J Clin Med. 7, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Auyeung TW, Lee J, Kwok T, Woo J (2011) Physical frailty predicts future cognitive decline—a four-year prospective study in 2737 cognitively normal older adults. J Nutr Health Aging. 15, 690–4. [DOI] [PubMed] [Google Scholar]
- [46].Alfaro-Acha A, Al Snih S, Raji MA, Kuo Y-F, Markides KS, Ottenbacher KJ (2006) Handgrip strength and cognitive decline in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 61, 859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA (2007) Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology. 29, 66–73. [DOI] [PubMed] [Google Scholar]
- [48].Kravitz E, Schmeidler J, Beeri MS (2012) Cognitive decline and dementia in the oldest-old. Rambam Maimonides Med J. 3, e0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Abellan van Kan G, Cesari M, Gillette-Guyonnet S, Dupuy C, Nourhashémi F, Schott AM, Beauchet O, Annweiler C, Vellas B, Rolland Y (2012) Sarcopenia and cognitive impairment in elderly women: results from the EPIDOS cohort. Age Ageing. 42, 196–202. [DOI] [PubMed] [Google Scholar]
- [50].Taekema DG, Gussekloo J, Maier AB, Westendorp RG, de Craen AJ (2010) Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing. 39, 331–7. [DOI] [PubMed] [Google Scholar]
- [51].Bullain SS, Corrada MM, Shah BA, Mozaffar FH, Panzenboeck M, Kawas CH (2013) Poor physical performance and dementia in the oldest old: the 90+ study. JAMA Neurol. 70, 107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bullain SS, Corrada MM, Perry SM, Kawas CH (2016) Sound Body Sound Mind? Physical Performance and the Risk of Dementia in the Oldest‐Old: The 90+ Study. J Am Geriatr Soc. 64, 1408–15. [DOI] [PubMed] [Google Scholar]
- [53].Morley JE (2017) Cognition and chronic disease. J Am Med Dir Assoc. 18, 369–71. [DOI] [PubMed] [Google Scholar]
- [54].Čelutkienė J, Vaitkevičius A, Jakštienė S, Jatužis DJ (2016) Expert opinion-cognitive decline in heart failure: more attention is needed. Card Fail Rev. 2, 106–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Joyce E, Gopal DM, Luk A, Groarke JD, Shah SP, Stewart GC, Givertz MM, Mehra MR (2015) Grip strength assessment and early outcomes in hospitalized acute decompensated heart failure: a prospective study. J Card Fail. 21, S117. [Google Scholar]
- [56].Aronow WS (2017) Hypertension and cognitive impairment. Ann Transl Med. 5, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mainous AG, Tanner RJ, Anton SD, Jo A (2015) Grip Strength as a Marker of Hypertension and Diabetes in Healthy Weight Adults. Am J Prev Med. 49, 850–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hung WW, Wisnivesky JP, Siu AL, Ross JS (2009) Cognitive decline among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 180, 134–7. [DOI] [PubMed] [Google Scholar]
- [59].Celis-Morales CA, Welsh P, Lyall DM, Steell L, Petermann F, Anderson J, IIiodromiti S, Sillars A, Graham N, Mackay DF, Pell JP, Gill JMR, Sattar N, Gray SR (2018) Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ. 361, k1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Tondo G, De Marchi F, Terazzi E, Prandi P, Sacchetti M, Comi C, Cantello R (2018) Chronic obstructive pulmonary disease may complicate Alzheimer’s disease: a comorbidity problem. Neurol Sci. 39, 1585–9. [DOI] [PubMed] [Google Scholar]
- [61].Zilliox LA, Chadrasekaran K, Kwan JY, Russell JW (2016) Diabetes and cognitive impairment. Curr Diab Rep. 16, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].McGrath R, Vincent BM, Al Snih S, Markides KS, Peterson MD (2017) The association between muscle weakness and incident diabetes in older Mexican Americans. J Am Med Dir Assoc. 18, 452.e7–452.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gorelick PB, Nyenhuis D (2015) Stroke and cognitive decline. JAMA. 314, 29–30. [DOI] [PubMed] [Google Scholar]
- [64].Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A, Orlandini A, Seron P, Ahmed SH, Rosengren A, Kelishadi R, Rahman O, Swaminathan S, Iqbal R, Gupta R, Lear SA, Oguz A, Yusoff K, Zatonska K, Chifamba J, Igumbor E, Mohan V, Anjana RM, Gu H, Li W, Yusuf S, Prospective Urban Rural Epidemiology (PURE) Study Investigators (2015) Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 386, 266–73. [DOI] [PubMed] [Google Scholar]
- [65].Steenland K, Karnes C, Seals R, Carnevale C, Hermida A, Levey AJ (2012) Late-life depression as a risk factor for mild cognitive impairment or Alzheimer’s disease in 30 US Alzheimer’s disease centers. J Alzheimers Dis. 31, 265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Fukumori N, Yamamoto Y, Takegami M, Yamazaki S, Onishi Y, Sekiguchi M, Otani K, Konno S, Kikuchi S, Fukuhara S (2015) Association between hand-grip strength and depressive symptoms: Locomotive Syndrome and Health Outcomes in Aizu Cohort Study (LOHAS). Age Ageing. 44, 592–8. [DOI] [PubMed] [Google Scholar]
- [67].McGrath R, Erlandson K, Vincent B, Hackney K, Herrmann S, Clark BJ (2018) Decreased Handgrip Strength is Associated with Impairments in Each Autonomous Living Task for Aging Adults in the United States. J Frailty Aging. 1–5. [DOI] [PubMed] [Google Scholar]
- [68].Mlinac ME, Feng MC (2016) Assessment of activities of daily living, self-care, and independence. Arch Clin Neuropsychol. 31, 506–16. [DOI] [PubMed] [Google Scholar]
- [69].United Sates Department of Health and Human Services. Multiple Chronic Conditions Chartbook. https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/prevention-chronic-care/decision/mcc/mccchartbook.pdf. Accessed 19 August 2019.
- [70].Vassilaki M, Aakre JA, Cha RH, Kremers WK, St. Sauver JL, Mielke MM, Geda YE, Machulda MM, Knopman DS, Petersen RC, Roberts RO (2015) Multimorbidity and risk of mild cognitive impairment. J Am Geriatr Soc. 63, 1783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sattler C, Erickson KI, Toro P, Schröder J (2011) Physical fitness as a protective factor for cognitive impairment in a prospective population-based study in Germany. J Alzheimers Dis. 26, 709–18. [DOI] [PubMed] [Google Scholar]
- [72].Gray SL, Anderson ML, Hubbard RA, LaCroix A, Crane PK, McCormick W, Bowen JD, McCurry SM, Larson EB (2013) Frailty and incident dementia. J Gerontol A Biol Sci Med Sci. 68, 1083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Veronese N (2016) What physical performance measures predict incident cognitive decline among intact older adults? A 4.4year follow up study. Exp Gerontol. 81, 110–8. [DOI] [PubMed] [Google Scholar]
- [74].Abe T, Soma Y, Kitano N, Jindo T, Sato A, Tsunoda K, Tsuji T, Okura T (2017) Change in hand dexterity and habitual gait speed reflects cognitive decline over time in healthy older adults: a longitudinal study. J Phys Ther Sci. 29, 1737–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Stessman J, Rottenberg Y, Fischer M, Hammerman‐Rozenberg A, Jacobs JM (2017) Handgrip strength in old and very old adults: mood, cognition, function, and mortality. J Am Geriatr Soc. 65, 526–32. [DOI] [PubMed] [Google Scholar]
- [76].Christensen H, Korten A, Mackinnon A, Jorm AF (2000) Are changes in sensory disability, reaction time, and grip strength associated with changes in memory and crystallized Intelligence? A longitudinal analysis in an elderly community sample. Gerontology. 46, 276–92. [DOI] [PubMed] [Google Scholar]
- [77].Okura T, Saghazadeh M, Soma Y, Tsunoda K (2013) Physical fitness, physical activity, exercise training and cognitive function in older adults. J Phys Fitness Sports Med. 2, 275–86. [Google Scholar]
- [78].Martin M, Clare L, Altgassen AM, Cameron MH, Zehnder FJ (2011) Cognition‐based interventions for healthy older people and people with mild cognitive impairment. Cochrane Database Syst Rev. 1, CD006220. [DOI] [PubMed] [Google Scholar]
- [79].Huntley J, Gould R, Liu K, Smith M, Howard RJ (2015) Do cognitive interventions improve general cognition in dementia? A meta-analysis and meta-regression. BMJ Open. 5, e005247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Evans IE, Martyr A, Collins R, Brayne C, Clare LJ (2018) Social isolation and cognitive function in later life: A systematic review and meta-analysis. J Alzheimers Dis. (Epub). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].McGrattan AM, McEvoy CT, McGuinness B, McKinley MC, Woodside JV (2018) Effect of dietary interventions in mild cognitive impairment: a systematic review. Br J Nutr. 120, 1388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Li F, Gong Q, Dong H, Shi JJ (2012) Resveratrol, a neuroprotective supplement for Alzheimer’s disease. Curr Pharm Des. 18, 27–33. [DOI] [PubMed] [Google Scholar]
- [83].Annweiler C, Karras SN, Anagnostis P, Beauchet OJ (2014) Vitamin D supplements: a novel therapeutic approach for Alzheimer patients. Front Pharmacol. 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gutierrez-Merino C, Lopez-Sanchez C, Lagoa R, Samhan-Arias AK, Bueno C, Garcia-Martinez VJ (2011) Neuroprotective actions of flavonoids. Curr Med Chem. 18, 1195–212. [DOI] [PubMed] [Google Scholar]
- [85].Rawson ES, Venezia AC (2011) Use of creatine in the elderly and evidence for effects on cognitive function in young and old. Amino Acids. 40, 1349–62. [DOI] [PubMed] [Google Scholar]
- [86].Maczurek A, Hager K, Kenklies M, Sharman M, Martins R, Engel J, Carlson DA, Munch G (2008) Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Adv Drug Deliv Rev. 60, 1463–70. [DOI] [PubMed] [Google Scholar]
- [87].Gheysen F, Poppe L, DeSmet A, Swinnen S, Cardon G, De Bourdeaudhuij I, Chastin S, Fias W (2018) Physical activity to improve cognition in older adults: can physical activity programs enriched with cognitive challenges enhance the effects? A systematic review and meta-analysis. Int J Behav Nutr Phys Act. 15, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rhodes EC, Martin AD, Taunton JE, Donnelly M, Warren J, Elliot J (2000) Effects of one year of resistance training on the relation between muscular strength and bone density in elderly women. Br J Sports Med. 34, 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yaginuma Y, Abe T, Thiebaud RS, Kitamura T, Kawanishi M, Fukunaga TJ (2017) Can handgrip strength improve following body mass-based lower body exercise? Biores Open Access. 6, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Labott BK, Bucht H, Morat M, Morat T, Donath LJG (2019) Effects of Exercise Training on Handgrip Strength in Older Adults: A Meta-Analytical Review. Gerontology. 1–13. [DOI] [PubMed] [Google Scholar]
- [91].Morgan JA, Corrigan F, Baune BT (2015) Effects of physical exercise on central nervous system functions: a review of brain region specific adaptations. J Mol Psychiatry. 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Brasure M, Desai P, Davila H, Nelson VA, Calvert C, Jutkowitz E, Butler M, Fink HA, Ratner E, Hemmy LS, McCarten JR, Barclay TR, Kane RL (2018) Physical Activity Interventions in Preventing Cognitive Decline and Alzheimer-Type Dementia: A Systematic Review. Ann Intern Med. 168, 30–8. [DOI] [PubMed] [Google Scholar]
- [93].Tieland M, Verdijk LB, de Groot LC, van Loon LJ (2015) Handgrip strength does not represent an appropriate measure to evaluate changes in muscle strength during an exercise intervention program in frail older people. Int J Sport Nutr Exerc Metab. 25, 27–36. [DOI] [PubMed] [Google Scholar]
- [94].Morton RW, Traylor DA, Weijs PJ, Phillips SM (2018) Defining anabolic resistance: implications for delivery of clinical care nutrition. Curr Opin Crit Care. 24, 124–30. [DOI] [PubMed] [Google Scholar]
- [95].Vandervoort AA (2002) Aging of the human neuromuscular system. Muscle Nerve. 25, 17–25. [DOI] [PubMed] [Google Scholar]
- [96].Vecchio LM, Meng Y, Xhima K, Lipsman N, Hamani C, Aubert IJ (2018) The neuroprotective effects of exercise: Maintaining a healthy brain throughout aging. Brain Plast. 4, 17–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gabriel DA, Kamen G, Frost GJSM (2006) Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med. 36, 133–49. [DOI] [PubMed] [Google Scholar]
- [98].McGrath R, Vincent BM, Hackney KJ, Robinson-Lane SG, Downer B, Clark BC (2019) The Longitudinal Associations of Handgrip Strength and Cognitive Function in Aging Americans. J Am Med Dir Assoc. ppi: S1525–8610(19)30649–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kim GR, Sun J, Han M, Nam CM, Park S (2019) Evaluation of the directional relationship between handgrip strength and cognitive function: the Korean Longitudinal Study of Ageing. Age Ageing. 1, 426–32. [DOI] [PubMed] [Google Scholar]
- [100].Williams KN, Kemper S (2010) Interventions to reduce cognitive decline in aging. Journal of psychosocial nursing and mental health services. J Psychosc Nurs Ment Health Serv. 48, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Baltes PB, Lindenberger U (1997) Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging. 12, 12–21. [DOI] [PubMed] [Google Scholar]
- [102].Christensen H, Mackinnon AJ, Korten A, Jorm AFJP, aging (2001) The “common cause hypothesis” of cognitive aging: evidence for not only a common factor but also specific associations of age with vision and grip strength in a cross-sectional analysis. Psychol Aging. 16, 588. [DOI] [PubMed] [Google Scholar]
- [103].Salthouse TA, Hambrick DZ, McGuthry KE (1998) Shared age-related influences on cognitive and noncognitive variables. Psychol Aging. 13, 486–500. [DOI] [PubMed] [Google Scholar]
- [104].Rosso AL, Studenski SA, Chen WG, Aizenstein HJ, Alexander NB, Bennett DA, Black SE, Camicioli R, Carlson MC, Ferrucci L, Guralnik JM, Hausdorff JM, Kaye J, Launer LJ, Lipsiz LA, Verghese J, Rosano C (2013) Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci. 68, 1379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Sorond FA, Cruz-Almeida Y, Clark DJ, Viswanathan A, Scherzer CR, De Jager P, Csiszar A, Laurineti PJ, Hausdorff JM, Chen WG, Ferrucci L, Rosano C, Studenski SA, Black SE, Lipsiz LA (2015) Aging, the Central Nervous System, and Mobility in Older Adults: Neural Mechanisms of Mobility Impairment. J Gerontol A Biol Sci Med Sci. 70, 1526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Varma VR, Hausdorff JM, Studenski SA, Rosano C, Camicioli R, Alexander NB, Chen WG, Lipsitz LA, Carlson MC (2016) Aging, the central nervous system, and mobility in older adults: interventions. J Gerontol A Biol Sci Med Sci. 71, 1451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Verghese J, Ayers E, Barzilai N, Bennett DA, Buchman AS, Holtzer R, Katz MJ, Lipton RB, Wang C (2014) Motoric cognitive risk syndrome: multicenter incidence study. Neurology. 83, 2278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Verghese J, Wang C, Lipton RB, Holtzer R (2012) Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 68, 412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Allali G, Ayers EI, Verghese J (2015) Motoric cognitive risk syndrome subtypes and cognitive profiles. J Gerontol A Biol Sci Med Sci. 71, 378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F (2014) Geroscience: linking aging to chronic disease. Cell. 159, 709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Seals DR, Melov S (2014) Translational geroscience: emphasizing function to achieve optimal longevity. Aging. 6, 718–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Clark BC, Woods AJ, Clark LA, Criss CR, Shadmehr R, Grooms DR (2019) The Aging Brain & the Dorsal Basal Ganglia: Implications for Age-Related Limitations of Mobility. Adv Geriatr Med Res. 1, pii:e190008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Rossini PM, Rossi S, Babiloni C, Polich J (2007) Clinical neurophysiology of aging brain: from normal aging to neurodegeneration. Prog Neurobiol 83, 375–400. [DOI] [PubMed] [Google Scholar]
- [114].Haug H, Eggers R (1991) Morphometry of the human cortex cerebri and corpus striatum during aging. Neurobiol Aging 12, 336–8. [DOI] [PubMed] [Google Scholar]
- [115].Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM (2004) Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage 21, 1174–81. [DOI] [PubMed] [Google Scholar]
- [116].Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS (2001) A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14, 21–36. [DOI] [PubMed] [Google Scholar]
- [117].Volkow ND, Gur RC, Wang G-J, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J (1998) Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 155, 344–9. [DOI] [PubMed] [Google Scholar]
- [118].Wenk GL, Pierce DJ, Struble RG, Price DL, Cork LC (1989) Age-related changes in multiple neurotransmitter systems in the monkey brain. Neurobiol Aging 10, 11–9. [DOI] [PubMed] [Google Scholar]
- [119].Jebsen RH, Taylor N, Trieschmann R, Trotter MJ, Howard LA (1969) An objective and standardized test of hand function. Arch Phys Med Rehabil 50, 311–9. [PubMed] [Google Scholar]
- [120].Kolb B, Forgie M, Gibb R, Gorny G, Rowntree S (1998) Age, experience and the changing brain. Neurosci Biobehav Rev 22, 143–59. [DOI] [PubMed] [Google Scholar]
- [121].Potvin A, Syndulko K, Tourtellotte W, Lemmon J, Potvin J (1980) Human neurologic function and the aging process. J Am Geriatr Soc 28, 1–9. [DOI] [PubMed] [Google Scholar]
- [122].Freitas C, Perez J, Knobel M, Tormos JM, Oberman LM, Eldaief M, Bashir S, Vernet M, Pena-Gomez C, Pascual-Leone A (2011) Changes in cortical plasticity across the lifespan. Front Aging Neurosci 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Hackel ME, Wolfe GA, Bang SM, Canfield JS (1992) Changes in hand function in the aging adult as determined by the Jebsen Test of Hand Function. Phys Ther 72, 373–7. [DOI] [PubMed] [Google Scholar]
- [124].Nakamoto H, Yoshitake Y, Takai Y, Kanehisa H, Kitamura T, Kawanishi M, Mori S (2012) Knee extensor strength is associated with Mini-Mental State Examination scores in elderly men. Eur J Appl Physiol 112, 1945–53. [DOI] [PubMed] [Google Scholar]
- [125].Peel NM, Alapatt LJ, Jones LV, Hubbard RE (2018) The association between gait speed and cognitive status in community-dwelling older people: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci 74, 943–8. [DOI] [PubMed] [Google Scholar]
- [126].Jacobs HI, Hopkins DA, Mayrhofer HC, Bruner E, van Leeuwen FW, Raaijmakers W, Schmahmann JD (2017) The cerebellum in Alzheimer’s disease: evaluating its role in cognitive decline. Brain 141, 37–47. [DOI] [PubMed] [Google Scholar]
- [127].Yu W, Krook-Magnuson E (2015) Cognitive collaborations: bidirectional functional connectivity between the cerebellum and the hippocampus. Front Syst Neurosci 9, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Bellebaum C, Daum I (2007) Cerebellar involvement in executive control. Cerebellum 6, 184–92. [DOI] [PubMed] [Google Scholar]
- [129].Tabatabaei‐Jafari H, Walsh E, Shaw ME, Cherbuin N, Alzheimer’s Disease Neuroimiging Initiative (ADNI) (2017) The cerebellum shrinks faster than normal ageing in A lzheimer’s disease but not in mild cognitive impairment. Hum Brain Mapp 38, 3141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Kilgour AH, Todd OM, Starr JM (2014) A systematic review of the evidence that brain structure is related to muscle structure and their relationship to brain and muscle function in humans over the lifecourse. BMC Geriatr 14, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Jung WH, Jang JH, Park JW, Kim E, Goo EH, Im OS, Kwon JS (2014) Unravelling the intrinsic functional organization of the human striatum: a parcellation and connectivity study based on resting-state fMRI. PLoS One 9, e106768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Choi EY, Yeo BT, Buckner RL (2012) The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol 108, 2242–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Di Martino A, Scheres A, Margulies DS, Kelly A, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP (2008) Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex 18, 2735–47. [DOI] [PubMed] [Google Scholar]
- [134].Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9, 357–81. [DOI] [PubMed] [Google Scholar]
- [135].Balleine BW, Liljeholm M, Ostlund SB (2009) The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res 199, 43–52. [DOI] [PubMed] [Google Scholar]
- [136].Felger JC, Treadway MT (2017) Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology 42, 216–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Wu T, Hallett M, Chan P (2015) Motor automaticity in Parkinson’s disease. Neurobiol Dis 82, 226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Delfini. Primer: Problems with Narrative Reviews. http://www.delfini.org/Delfini_Primer_NarrativeReviewProbs.pdf. Accessed 2 December 2019.
- [139].Ferrari RJ (2015) Writing narrative style literature reviews. Medical Writing. 24, 230–235. [Google Scholar]


