This cohort study analyzes Swedish patient registries to identify the familial, lifestyle, cardiovascular, and other risk factors in the later development of neurodegenerative diseases among adults with stress-related disorders compared with their siblings and people without the condition.
Key Points
Question
Do severe psychiatric reactions induced by trauma or other life stressors increase the risk for neurodegenerative diseases?
Findings
In a nationwide cohort study of individuals with stress-related disorders and those without such disorders, the exposed individuals were at a considerably higher risk of developing neurodegenerative diseases compared with their matched unexposed counterparts. This risk elevation was more pronounced for vascular neurodegenerative diseases (risk increase of 80%) than for primary neurodegenerative diseases (risk increase of 31%).
Meaning
These findings suggest that stress-related disorders may be associated with the subsequent risk of neurodegenerative diseases, possibly through a cerebrovascular pathway.
Abstract
Importance
Posttraumatic stress disorder (PTSD) has been associated with increased risk for dementia. Less is known, however, about other stress-related disorders and their associations with neurodegenerative diseases.
Objective
To examine the association between stress-related disorders and risk for neurodegenerative diseases.
Design, Setting, and Participants
This population-matched and sibling cohort study was conducted in Sweden using data from nationwide health registers, including the Swedish National Patient Register. Individuals who received their first diagnosis of stress-related disorders between January 1, 1987, and December 31, 2008, were identified. Individuals who had a history of neurodegenerative diseases, had conflicting or missing information, had no data on family links, or were aged 40 years or younger at the end of the study were excluded. Individuals with stress-related disorders were compared with the general population in a matched cohort design; they were also compared with their siblings in a sibling cohort. Follow-up commenced from the age of 40 years or 5 years after the diagnosis of stress-related disorders, whichever came later, until the first diagnosis of a neurodegenerative disease, death, emigration, or the end of follow-up (December 31, 2013), whichever occurred first. Data analyses were performed from November 2018 to April 2019.
Exposures
Diagnosis of stress-related disorders (PTSD, acute stress reaction, adjustment disorder, and other stress reactions).
Main Outcomes and Measurements
Neurodegenerative diseases were identified through the National Patient Register and classified as primary or vascular. Alzheimer disease, Parkinson disease, and amyotrophic lateral sclerosis were evaluated separately. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) with 95% CIs after controlling for multiple confounders.
Results
The population-matched cohort included 61 748 exposed individuals and 595 335 matched unexposed individuals. A total of 44 839 exposed individuals and their 78 482 unaffected full siblings were included in the sibling cohort analysis. The median (interquartile range) age at the start of follow-up was 47 (41-56) years, and 24 323 (39.4%) of the exposed individuals were male. The median (interquartile range) follow-up was 4.7 (2.1-9.8) years. Compared with unexposed individuals, individuals with a stress-related disorder were at an increased risk of neurodegenerative diseases (HR, 1.57; 95% CI, 1.43-1.73). The risk increase was greater for vascular neurodegenerative diseases (HR, 1.80; 95% CI, 1.40-2.31) than for primary neurodegenerative diseases (HR, 1.31; 95% CI, 1.15-1.48). A statistically significant association was found for Alzheimer disease (HR, 1.36; 95% CI, 1.12-1.67) but not Parkinson disease (HR, 1.20; 95% CI, 0.98-1.47) or amyotrophic lateral sclerosis (HR, 1.20; 95% CI, 0.74-1.96). Results from the sibling cohort corroborated results from the population-matched cohort.
Conclusions and Relevance
This study showed an association between stress-related disorders and an increased risk of neurodegenerative diseases. The relative strength of this association for vascular neurodegenerative diseases suggests a potential cerebrovascular pathway.
Introduction
Chronic stress and a dysregulated stress response have been suggested in animal studies to influence the pathogenesis of neurodegenerative diseases such as Alzheimer disease (AD) and Parkinson disease (PD).1 Epidemiological studies support that stress exposure, including everyday life stress, work stress, life events, and trauma, is associated with dementia risk,2,3,4,5,6 whereas results for PD have been inconsistent.7,8,9,10 The potential association between stressful life events and amyotrophic lateral sclerosis (ALS) was not supported in 2 previous studies,11,12 but this association is underexplored to date.
Stress-related disorders are defined not only by their symptoms but also by the presence of at least 1 causative stressor. A stressful life event and its associated psychological distress might lead to a diagnosis of adjustment disorder, whereas a threatening traumatic event might lead to an immediate and transient acute stress reaction or chronic posttraumatic stress disorder (PTSD).13 These common psychiatric disorders14 have been associated with several long-term physiological health consequences, predominantly cardiovascular diseases.14,15,16 Regarding the association between stress-related disorders and neurodegenerative diseases, studies of male veterans17,18,19,20 and 2 cohort studies of the general population21,22 have demonstrated that PTSD is associated with increased dementia risk. A recent study with limited control for familial factors also provided supportive evidence for an association between all stress-related disorders and dementia.23 However, less is known about the association between stress-related disorders and other neurodegenerative diseases. Posttraumatic stress disorder24 and adjustment disorder25 were shown to be associated with an increased PD risk, whereas stress-related disorders were not found to be associated with ALS risk.26
Because adjustment disorder and acute stress reaction occur more frequently compared with PTSD, evaluating the associations of these disorders with neurodegenerative diseases is of importance. Furthermore, few studies have examined subtypes of dementia and other neurodegenerative diseases of different etiologies. Given that many previous studies on PTSD were based on male samples with war- or combat-related trauma who differed substantially from the general population, studies of the general population are especially useful. Therefore, the purpose of the present study was to examine the association between a range of stress-related disorders (PTSD, acute stress reaction, adjustment disorder, and other stress reactions) and subsequent risk for neurodegenerative diseases (including dementia, parkinsonian disorders, and ALS), using a nationwide population-matched and sibling-matched cohort design. Given the known associations between stress-related disorders and cardiovascular diseases14,15,16 and cerebrovascular impairments,27 we further aimed to test whether the associations differed between primary neurodegenerative diseases and neurodegenerative diseases with a primary vascular component.
Methods
Study Design
Using the nationwide Swedish population and health registers, we performed a population-matched cohort study to compare the risk of neurodegenerative diseases between individuals with stress-related disorders and individuals without such disorders after controlling for multiple potential confounders. To assess the role of unknown and unmeasured confounders, we also analyzed a sibling cohort to compare the risk of neurodegenerative diseases between individuals with stress-related disorders and their unaffected full siblings. This sibling comparison controlled for familial factors, both genetic and nongenetic, that are shared between full siblings and might be additional confounders for the association between stress-related disorders and neurodegenerative diseases. This cohort study was approved by the regional ethical review board in Stockholm, Sweden, which waived the informed consent requirement for register-based studies in Sweden.
Exposed Individuals With Stress-Related Disorders
Using the Swedish National Patient Register (NPR), we identified an exposed group of all Swedish-born individuals who received their first diagnosis of a stress-related disorder between January 1, 1987, and December 31, 2008 (n = 99 714; Figure 1). The NPR has had nationwide coverage of inpatient care since 1987 and has included more than 80% of outpatient specialist care since 2001. We defined stress-related disorders as any first outpatient or inpatient hospital visit with the main diagnosis of a stress-related disorder with the Swedish revisions of the International Classification of Diseases, Ninth Revision (ICD-9), codes 308 and 309, or Tenth Revision (ICD-10), code F43, as recorded in the NPR (eTable 1 in the Supplement). We then divided stress-related disorders into PTSD (ICD-9 code 309B; ICD-10 code F43.1), acute stress reaction (ICD-9 codes 308 and 309A; ICD-10 code F43.0), and adjustment disorder and other stress reactions (ICD-9 code 309X; ICD-10 codes F43.8 and F43.9). Because PTSD may initially be preceded by other stress-related disorders, we considered all exposed individuals who received a PTSD diagnosis within 1 year after their first stress-related disorder diagnosis as individuals with PTSD. We further classified the severity of stress-related disorders by the type of care received (ie, inpatient or outpatient), duration of inpatient stay, and number of outpatient visits due to diagnosis of a stress-related disorder within 1 year after the first diagnosis.
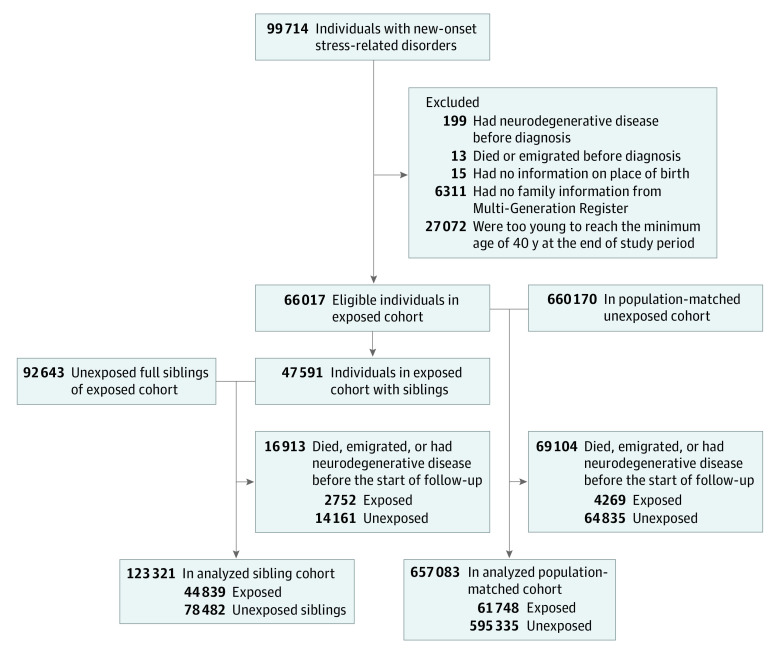
Figure 1. Flowchart of Study Cohorts.
We cross-linked the exposed group to other nationwide registers using the unique personal identity numbers assigned to all Swedish residents. Exposed persons with a history of neurodegenerative diseases (n = 199) or with conflicting (n = 13) or missing (n = 15) information were excluded from the analysis. To ensure a complete familial link from the Swedish Multi-Generation Register, which includes familial links for individuals born in or after 1932, we excluded individuals who were born before 1932 (n = 6311). Because the incidence rates of neurodegenerative diseases were low among individuals aged 40 years or younger, we also excluded individuals who were too young at index date to reach 40 years during the follow-up period (n = 27 072), leaving 66 017 eligible individuals in the exposed group (Figure 1).
Population-Matched Cohort
Individuals with stress-related disorders were compared with the general population in a matched cohort design. For each exposed person, 10 individuals free of stress-related disorders and neurodegenerative diseases at the diagnosis date of the index person were randomly selected from the Swedish Total Population Register using the method of incidence density sampling (n = 660 170) (Figure 1). Exposed and unexposed individuals were individually matched by birth year, sex, and county of birth.
Sibling Cohort
Individuals with stress-related disorders were also compared to their siblings in a sibling cohort. Through the Multi-Generation Register, we identified all clusters of full siblings that were discordant for stress-related disorders, including a total of 92 643 full siblings of the 47 591 individuals with stress-related disorders who were free of stress-related disorders and neurodegenerative diseases at the diagnosis date of the affected sibling (Figure 1).
Follow-up of the Cohorts
The date of stress-related disorder diagnosis was used as the index date for the exposed individuals and their matched unexposed counterparts and unaffected full siblings. Because the incidence rates of the neurodegenerative diseases were low among individuals aged 40 years or younger and diagnostic delays were common for these diseases, we started the follow-up of the study participants from age 40 years or 5 years after the index date, whichever came later, until the first diagnosis of a neurodegenerative disease, death, emigration, or the end of follow-up (December 31, 2013), whichever occurred first. This method also rendered a better control for potential surveillance bias, assuming a greater-than-expected surveillance of neurodegenerative diseases and other diseases compared with the diagnostic workup and treatment for stress-related disorders. For the matched unexposed individuals and unaffected full siblings, the follow-up was additionally censored at their first diagnosis of a stress-related disorder, if any, during the follow-up.
Identification of Neurodegenerative Diseases
We defined incident neurodegenerative diseases as the first outpatient or inpatient hospital visit with a diagnosis of a neurodegenerative disease with corresponding ICD-9 or ICD-10 codes, as recorded in the NPR (eTable 1 in the Supplement). We categorized neurodegenerative diseases according to their potential origin, including primary neurodegenerative diseases and neurodegenerative diseases with a primary vascular cause.28,29 We also separately studied AD, PD, and ALS. In addition, because the clinical diagnosis of AD or vascular dementia is not always optimal, we performed analyses for all dementias collectively (any dementia). The neurodegenerative disease diagnoses from the NPR have been validated against detailed clinical evaluations, showing a positive predictive value of 81% for dementia,30 71% for PD,31 and 91% for ALS.32 Individuals who received 2 neurodegenerative disease diagnoses during the follow-up contributed to the analyses of both outcomes.
Covariables
For the study participants, we obtained information on educational level, family income, and marital status from the Swedish Longitudinal Integration Database for Health Insurance and Labor Market. We defined family history of neurodegenerative diseases as a diagnosis of any neurodegenerative disease among the first-degree relatives (biological parents and full siblings) of the participants according to the NPR. We used the most updated information before the index date for each covariate in the analyses.
Other psychiatric disorders, such as depression, anxiety, and substance abuse, commonly co-occur with stress-related disorders. Therefore, we extracted from the NPR information on the first diagnosis of other psychiatric disorders. We described those who received a diagnosis more than 3 months before the diagnosis of stress-related disorders as having a history of other psychiatric disorders, and we described those who received a diagnosis within 3 months before and 1 year after the diagnosis of stress-related disorders as having a psychiatric comorbidity. Information on the occurrence of chronic pulmonary diseases and cardiovascular diseases before the end of follow-up was retrieved from the NPR as a proxy for smoking and vascular factors, respectively. Diagnosis codes for all aforementioned diseases are listed in eTable 1 in the Supplement.
Statistical Analysis
We used conditional Cox proportional hazards regression models to estimate hazard ratios (HRs) with 95% CIs of neurodegenerative diseases compared with stress-related disorders, using time after the start of follow-up as the underlying scale. In the population-matched cohort, we stratified all analyses by matching identifiers (birth year, sex, and county of birth) and partially or fully adjusted for multiple potential confounders, including educational level (<9 years, 9-12 years, >12 years, or unknown), family income level (top 20%, middle, lowest 20%, or unknown), marital status (single, married or cohabiting, or divorced or widowed), history of other psychiatric disorders (yes or no), and family history of neurodegenerative diseases (yes or no). The analyses were applied first for any stress-related disorder and then for different subtypes of stress-related disorders. In addition to examining any neurodegenerative disease, we separately examined the HRs for subtypes of neurodegenerative diseases (primary and vascular), specific neurodegenerative diseases (AD, PD, and ALS), and any dementia.
In subgroup analyses, we calculated the HRs by sex, age group (≤35 years, 36-51 years, or ≥52 years at index date according to tertile distribution), attained age (<70 or ≥70 years), calendar year at index date (1987-1999 or 2000-2008), history of other psychiatric disorders (yes or no), family history of neurodegenerative diseases (yes or no), and time since the start of follow-up (0-4 years, 5-9 years, or ≥10 years). Furthermore, to assess the implication of the severity of stress-related disorders, we subgrouped the exposed individuals by the status of psychiatric comorbidity as well as by the type and intensity of psychiatric care received within 1 year after the diagnosis.
Standardized cumulative incidence of neurodegenerative diseases was estimated and plotted for exposed individuals and their matched unexposed counterparts by using regression standardization over covariate distributions after fitting the multivariable-adjusted flexible parametric model.33,34
We repeated the main analyses in the sibling cohort, using Cox proportional hazards regression models stratified by family identifiers and adjusted for birth year, sex, county of birth, and all aforementioned covariables. Wald test was used to test the difference in subgroups, and we compared HRs between the population-matched and sibling cohorts using a Z test.35 We tested the potential roles of smoking and vascular factors in the associations by also adjusting for chronic pulmonary diseases and cardiovascular diseases diagnosed before the end of follow-up. A 2-sided P < .05 was considered statistically significant.
All analyses were conducted in SAS, version 9.4 (SAS Institute Inc), and Stata 15 (StataCorp LP). Data analyses were performed from November 2018 to April 2019.
Results
In total, 61 748 exposed individuals and 595 335 matched unexposed individuals were included in the analysis of the population-matched cohort, whereas 44 839 exposed individuals and their 78 482 unaffected full siblings were included in the analysis of the sibling cohort (Figure 1). The median (interquartile range) age at the start of follow-up was 47 (41-56) years, and 24 323 (39.4%) of the exposed individuals were male.
The population-matched cohort accrued a total of 4 314 225 person-years at risk, with the median (interquartile range) follow-up of 4.7 (2.1-9.8) years. Having a history of other psychiatric disorders was more prevalent among individuals with stress-related disorders compared with their matched unexposed counterparts (19 178 [31.1%] vs 35 167 [5.9%]) (Table 1). In addition, individuals with stress-related disorders tended to have lower socioeconomic status (ie, lower educational level [>12 years of school: 15 155 (24.5%) vs 183 326 (30.8%)] and lower family income [lowest 20%: 10 059 (16.3%) vs 76 939 (12.9%)]) and were more likely to be divorced or widowed (11 845 [19.2%] vs 63 630 [10.7%]).
Table 1. Characteristics of Study Cohorts.
| Variable | No. (%) | |||
|---|---|---|---|---|
| Population-Matched Cohort | Sibling Cohort | |||
| Exposed | Matched Unexposed | Exposed | Unexposed Siblings | |
| No. of participants | 61 748 | 595 335 | 44 839 | 78 482 |
| Follow-up, median (IQR), y | ||||
| Age at the start | 47 (41-56) | 48 (41-56) | 47 (41-55) | 49 (42-57) |
| Time | 4.6 (2.1-9.2) | 4.7 (2.1-9.8) | 4.6 (2.1-9.2) | 5.4 (2.5-11.2) |
| Age at the end | 54 (47-63) | 54 (47-63) | 54 (47-62) | 57 (49-65) |
| Male, % | 39.4 | 39.4 | 39.4 | 50.2 |
| Educational level, y | ||||
| <9 | 4437 (7.2) | 40 496 (6.8) | 2966 (6.6) | 8662 (11.0) |
| 9-12 | 42 070 (68.1) | 369 894 (62.1) | 30 702 (68.5) | 51 532 (65.7) |
| >12 | 15 155 (24.5) | 183 326 (30.8) | 11 120 (24.8) | 18 110 (23.1) |
| Unknown | 86 (0.2) | 1619 (0.3) | 51 (0.1) | 178 (0.2) |
| Yearly family income level | ||||
| Lowest 20% | 10 059 (16.3) | 76 939 (12.9) | 7391 (16.5) | 10 552 (13.5) |
| Middle | 34 595 (56.0) | 322 064 (54.1) | 25 246 (56.3) | 41 957 (53.5) |
| Top 20% | 9574 (15.5) | 123 336 (20.7) | 6855 (15.3) | 15 103 (19.2) |
| Unknown | 7520 (12.2) | 72 996 (12.3) | 5347 (11.9) | 10 870 (13.9) |
| Marital status | ||||
| Single | 23 905 (38.7) | 226 803 (38.1) | 17 511 (39.1) | 27 700 (35.3) |
| Married or cohabiting | 25 998 (42.1) | 304 902 (51.2) | 19 024 (42.4) | 40 405 (51.5) |
| Divorced or widowed | 11 845 (19.2) | 63 630 (10.7) | 8304 (18.5) | 10 377 (13.2) |
| History of other psychiatric disordersa | ||||
| Yes | 19 178 (31.1) | 35 167 (5.9) | 13 602 (30.3) | 7711 (9.8) |
| No | 42 570 (68.9) | 560 168 (94.1) | 31 237 (69.7) | 70 771 (90.2) |
| Family history of neurodegenerative diseases | ||||
| Yes | 4379 (7.1) | 39 379 (6.6) | 3282 (7.3) | 6338 (8.1) |
| No | 57 369 (92.9) | 555 956 (93.4) | 41 557 (92.7) | 72 144 (91.9) |
| Type of stress-related disorders | ||||
| Diagnosis type | ||||
| PTSD | 3743 (6.0) | NA | 2709 (6.0) | NA |
| Acute stress reaction | 27 938 (45.3) | NA | 20 121 (44.9) | NA |
| Adjustment disorder and other stress reaction | 30 067 (48.7) | NA | 22 009 (49.1) | NA |
| Psychiatric comorbidityb | ||||
| Yes | 12 385 (20.1) | NA | 9101 (20.3) | NA |
| No | 49 363 (79.9) | NA | 35 738 (79.7) | NA |
Abbreviations: IQR, interquartile range; NA, not applicable; PTSD, posttraumatic stress disorder.
The first diagnosis of a psychiatric disorder, other than stress-related disorders, that occurred more than 3 months before the index date (ie, the diagnosis date of exposed patients, or the diagnosis date of the index patient for matched unexposed individuals and siblings).
A new-onset psychiatric disorder, other than stress-related disorders, that was diagnosed from 3 months before to 1 year after the diagnosis of a stress-related disorder.
During the follow-up, 3822 individuals with incident neurodegenerative diseases were identified, leading to a crude incidence rate of 1.50 for exposed individuals and 0.82 for unexposed individuals per 1000 person-years. We observed a statistically significant association between stress-related disorders and any neurodegenerative disease in the partially adjusted models; the HR decreased but remained statistically significant in the fully adjusted models (Table 2). Compared with unexposed individuals, individuals with a stress-related disorder were at an increased risk of neurodegenerative diseases (HR, 1.57; 95% CI, 1.43-1.73), after controlling for all confounders. The observed association was more pronounced for vascular neurodegenerative diseases compared with primary neurodegenerative diseases (HR, 1.80 [95% CI, 1.40-2.31] vs HR, 1.31 [95% CI, 1.15-1.48]; P for difference = .03). For the specific neurodegenerative diseases, we obtained a statistically significant association for AD only (HR, 1.36; 95% CI, 1.12-1.67), although comparable HRs were also observed for PD (HR, 1.20; 95% CI, 0.98-1.47) and ALS (HR, 1.20; 95% CI, 0.74-1.96). We found a similar association between stress-related disorders and any dementia (HR, 1.80; 95% CI, 1.60-2.03).
Table 2. Crude Incidence Rates and Hazard Ratios With 95% CIs for Neurodegenerative Diseases Among Individuals With Stress-Related Disorders vs Matched Unexposed Counterparts or Unaffected Full Siblings.
| Model Information | Analyses of Population-Matched Cohort | Analyses of Sibling Cohort | ||||
|---|---|---|---|---|---|---|
| No. (IR)a | HR (95% CI)b | No. (IR)a | HR (95% CI)b | |||
| Exposed | Unexposed | Exposed | Siblings | |||
| Any neurodegenerative disease | ||||||
| Controlled for sex, birth year, and county of birth | 588 (1.50) | 3234 (0.82) | 1.90 (1.73-2.07) | 363 (1.27) | 580 (1.02) | 1.63 (1.39-1.92) |
| As above + educational level, family income, and marital status | 1.87 (1.71-2.05) | 1.63 (1.39-1.91) | ||||
| As above + history of other psychiatric disorders | 1.57 (1.42-1.73) | 1.41 (1.19-1.67) | ||||
| As above + family history of neurodegenerative diseases | 1.57 (1.43-1.73) | NA | ||||
| Specific neurodegenerative diseases (full models)c | ||||||
| Primary neurodegenerative diseases | 319 (0.81) | 2344 (0.60) | 1.31 (1.15-1.48) | 203 (0.71) | 381 (0.67) | 1.29 (1.04-1.61) |
| Amyotrophic lateral sclerosis | 20 (0.05) | 171 (0.04) | 1.20 (0.74-1.96) | 14 (0.05) | 27 (0.05) | 1.15 (0.46-2.87) |
| Alzheimer disease | 125 (0.32) | 933 (0.24) | 1.36 (1.12-1.67) | 77 (0.27) | 159 (0.28) | 1.33 (0.92-1.93) |
| Parkinson disease | 124 (0.32) | 968 (0.25) | 1.20 (0.98-1.47) | 79 (0.28) | 157 (0.28) | 1.24 (0.87-1.78) |
| Vascular neurodegenerative diseases | 100 (0.25) | 419 (0.11) | 1.80 (1.40-2.31) | 65 (0.23) | 79 (0.14) | 1.97 (1.27-3.04) |
Abbreviations: HR, hazard ratio; IR, incidence rate; NA, not applicable.
Incidence rate per 1000 person-years.
HRs and 95% CIs were derived from Cox proportional hazards regression models, stratified by matching identifier (birth year, sex, and county of birth for population-matched cohort) and family identifier (for sibling cohort) and adjusted for covariables (model information column). Time since the start of follow-up was used as the underlying time scale.
The calculation of HRs and 95% CIs for specific neurodegenerative diseases was based on fully adjusted Cox proportional hazards regression models.
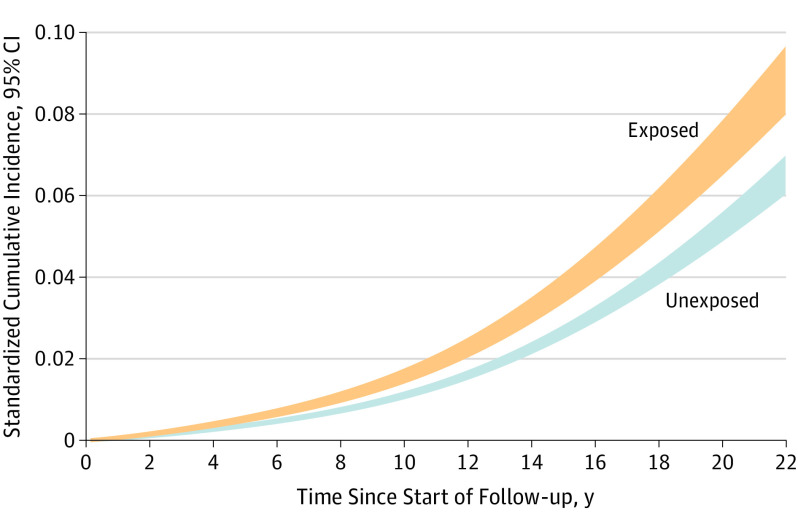
The cumulative incidence plot shows the temporal pattern of neurodegenerative diseases among exposed individuals and their matched unexposed counterparts (Figure 2). Time 0 in the figure represents at least 5 years after the diagnosis of stress-related disorders. These curves indicate that the increased incidence of neurodegenerative disease was observed right after the beginning of follow-up and appeared to be constant throughout the entire follow-up period.
Figure 2. Standardized Cumulative Incidence of Neurodegenerative Diseases Among Individuals With Stress-Related Disorders (Exposed) and Matched Unexposed Individuals (Unexposed).
Standardized cumulative incidence was estimated for the exposed and unexposed individuals using regression standardization over covariates’ distributions, after fitting flexible parametric models and adjustment for birth year, sex, county of birth, educational level, family income, marital status, history of psychiatric disorders, and family history of neurodegenerative diseases. Time 0 represents at least 5 years after the diagnosis of stress-related disorders.
The association between stress-related disorders and neurodegenerative diseases was not greatly modified by sex, history of other psychiatric disorders, family history of neurodegenerative diseases, or time since the start of follow-up (Table 3). The association was, however, considerably stronger among individuals with younger age at index date compared with those with older age at index date (≤35 years: HR, 2.45 [95% CI, 1.61-3.74]; 36-51 years: HR, 1.71 [95% CI, 1.46-2.00]; ≥52 years: HR, 1.45 [95% CI, 1.27-1.65]; P for trend = .04) and during the earlier compared with later calendar period (1987-1999 HR, 1.70 [95% CI, 1.51-1.91] vs 2000-2008 HR, 1.37 [95% CI, 1.15-1.62]; P for difference = .04). Separate analyses for primary and vascular neurodegenerative diseases revealed largely similar risk result patterns.
Table 3. Hazard Ratios With 95% CIs for Neurodegenerative Diseases Among Individuals With Stress-Related Disorders vs Matched Unexposed Counterparts, by Different Characteristics.
| Variable | Any Neurodegenerative Diseases | Primary Neurodegenerative Diseases | Vascular Neurodegenerative Diseases | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. (IR)a | HR (95% CI)b | No. (IR)a | HR (95% CI)b | No. (IR)a | HR (95% CI)b | ||||
| Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | ||||
| By sex | |||||||||
| Male | 237 (1.48) | 1448 (0.90) | 1.47 (1.26-1.70) | 133 (0.83) | 1027 (0.64) | 1.26 (1.04-1.53) | 38 (0.24) | 232 (0.14) | 1.30 (0.89-1.89) |
| Female | 351 (1.51) | 1786 (0.77) | 1.65 (1.46-1.88) | 186 (0.80) | 1317 (0.57) | 1.33 (1.13-1.58) | 62 (0.27) | 187 (0.08) | 2.43 (1.73-3.43) |
| By age at the index date (tertiles), y | |||||||||
| ≤35 | 35 (0.31) | 138 (0.12) | 2.45 (1.61-3.74) | 16 (0.14) | 123 (0.11) | 1.37 (0.78-2.39) | 4 (0.04) | 5 (0) | 5.72 (0.89-36.9) |
| 36-51 | 226 (1.08) | 1166 (0.55) | 1.71 (1.46-2.00) | 134 (0.64) | 910 (0.43) | 1.45 (1.19-1.76) | 32 (0.15) | 114 (0.05) | 2.26 (1.43-3.57) |
| ≥52 | 327 (4.67) | 1930 (2.73) | 1.45 (1.27-1.65) | 169 (2.40) | 1311 (1.85) | 1.21 (1.02-1.44) | 64 (0.91) | 300 (0.42) | 1.63 (1.19-2.21) |
| By attained age, y | |||||||||
| <70 | 377 (1.01) | 1927 (0.52) | 1.64 (1.45-1.85) | 219 (0.59) | 1501 (0.40) | 1.35 (1.16-1.58) | 59 (0.16) | 198 (0.05) | 2.03 (1.45-2.84) |
| ≥70 | 211 (10.9) | 1153 (6.42) | 1.49 (1.27-1.75) | 97 (5.01) | 721 (4.02) | 1.24 (0.99-1.56) | 39 (2.02) | 185 (1.03) | 1.48 (1.00-2.20) |
| By calendar year at index date | |||||||||
| 1987-1999 | 409 (1.57) | 2211 (0.84) | 1.70 (1.51-1.91) | 211 (0.81) | 1617 (0.61) | 1.29 (1.11-1.51) | 79 (0.30) | 295 (0.11) | 2.20 (1.65-2.95) |
| 2000-2008 | 179 (1.36) | 1023 (0.80) | 1.37 (1.15-1.62) | 108 (0.82) | 727 (0.57) | 1.33 (1.07-1.65) | 21 (0.16) | 124 (0.10) | 1.14 (0.68-1.91) |
| By history of other psychiatric disordersc | |||||||||
| Yes | 247 (2.15) | 96 (1.64) | 1.44 (1.06-1.96) | 122 (1.06) | 60 (1.02) | 1.30 (0.86-1.97) | 44 (0.38) | 14 (0.24) | 1.59 (0.74-3.43) |
| No | 341 (1.23) | 1975 (0.76) | 1.67 (1.49-1.88) | 197 (0.71) | 1482 (0.57) | 1.29 (1.11-1.50) | 56 (0.20) | 231 (0.09) | 2.48 (1.83-3.35) |
| By family history of neurodegenerative diseases | |||||||||
| Yes | 87 (3.84) | 68 (3.14) | 1.52 (1.36-1.69) | 48 (2.11) | 56 (2.58) | 1.25 (1.09-1.44) | 11 (0.48) | 5 (0.23) | 1.79 (1.37-2.34) |
| No | 501 (1.36) | 2546 (0.73) | 1.54 (1.02-2.33) | 271 (0.73) | 1841 (0.53) | 1.11 (0.66-1.87) | 89 (0.24) | 335 (0.10) | 7.45 (0.47-118) |
| By time since the start of follow-up, y | |||||||||
| 0-4 | 217 (0.99) | 1146 (0.53) | 1.44 (1.23-1.68) | 133 (0.60) | 840 (0.39) | 1.41 (1.16-1.72) | 29 (0.13) | 130 (0.06) | 1.19 (0.74-1.91) |
| 5-9 | 144 (1.45) | 777 (0.77) | 1.60 (1.32-1.95) | 75 (0.75) | 579 (0.58) | 1.22 (0.94-1.59) | 33 (0.33) | 102 (0.10) | 2.79 (1.79-4.36) |
| ≥10 | 227 (3.13) | 1311 (1.69) | 1.65 (1.41-1.94) | 111 (1.52) | 925 (1.19) | 1.25 (1.01-1.55) | 38 (0.52) | 187 (0.24) | 1.71 (1.13-2.59) |
Abbreviations: HR, hazard ratio; IR, incidence rate.
Incidence rate per 1000 person-years.
Cox proportional hazards regression models were stratified by matching identifiers (birth year, sex, and county of birth) and adjusted for educational level, family income, marital status, history of psychiatric disorders, and family history of neurodegenerative diseases, when applicable.
The first diagnosis of a psychiatric disorder, other than stress-related disorders, occurred more than 3 months before the index date (ie, the diagnosis date of exposed patients, or the diagnosis date of the index patient for matched unexposed individuals).
The analyses on subtypes of stress-related disorders demonstrated comparable estimates for PTSD and other types of stress-related disorders (eTable 2 and eTable 3 in the Supplement). In addition, although not statistically significant, the results suggested that exposed individuals with more severe or persistent stress-related disorders, indicated by the presence of psychiatric comorbidity, longer inpatient care, or more frequent outpatient visits, had an even higher risk of neurodegenerative diseases compared with patients with less severe stress-related disorders (eTable 4 in the Supplement).
The results from the sibling cohort largely corroborated the results from the population-matched cohort (HR for any neurodegenerative diseases, 1.41; 95% CI, 1.19-1.67; P for difference between cohorts = .33) (Table 2; eTable 3 and eTable 5 in the Supplement). Moreover, although we observed slightly lower HRs for vascular neurodegenerative diseases after also adjusting for the diagnosis of cardiovascular diseases occurring before the end of follow-up, additional adjustment for chronic pulmonary diseases did not modify the estimates (eTable 6 in the Supplement).
Discussion
In this nationwide population-matched and sibling-matched cohort study, we found that stress-related disorders were associated with an increased subsequent risk of neurodegenerative diseases. The association was robust and remained after adjustment for potential confounders, including familial factors. The stronger association observed for neurodegenerative diseases with a vascular component, compared with primary neurodegenerative diseases, suggested a considerable role of a possible cerebrovascular pathway. For specific neurodegenerative diseases, we found a statistically significant association for AD but not for PD or ALS.
The finding that individuals with a stress-related disorder were at an increased risk of developing a neurodegenerative disease has support from previous studies.17,18,19,20,21,22,23,24,25 However, the main body of existing evidence has focused solely on PTSD17,18,19,20,21,22,24 and dementia18,19,20,21,22,23 and is often derived from studies of male veterans, who differ substantially from the general population and in terms of trauma exposure.17,18,19,20 Furthermore, the evidence base for the association between stress-related disorders and neurodegenerative diseases other than dementia has thus far been limited. To our knowledge, the present study is the first to date to consider all stress-related disorders and risk of the most common neurodegenerative diseases, including dementia, parkinsonian disorders, and ALS, in the same study. Given the comparable results between the population-matched and sibling cohort analyses, this study suggests that the association between stress-related disorders and neurodegenerative diseases cannot be explained by familial factors shared by siblings.
The finding of an increased risk of neurodegenerative diseases compared with all individual diagnoses of stress-related disorders is in line with a recent Danish study that reported associations of different stress-related disorders with dementia,23 suggesting the role of a general stress psychopathological condition rather than disorder-specific neurodegeneration. Moreover, we found stronger associations for vascular neurodegenerative diseases than for primary neurodegenerative diseases, which may indicate the importance of cardiovascular factors in mediating the observed associations. Despite limited information on vascular factors, owing to the register-based nature of the present study, our sensitivity analysis indicated that the association was somewhat attenuated after additionally adjusting for cardiovascular diseases diagnosed before the end of follow-up. No study has addressed this specific question to date, but previous studies on stress-related disorders and dementia found equal magnitudes of association for both vascular dementia and AD,17 whereas work-related stress was associated with only vascular dementia.3 Regarding the specific neurodegenerative diagnoses, we found a statistically significant association only between stress-related disorders and risk of AD. For PD and ALS, although the estimates also implied an increased risk of PD compared with stress-related disorders, the associations were not statistically significant. This finding might be partly attributable to the relatively young cohort and the low incidence of PD and ALS at a younger age.
Biological mechanisms have been suggested to explain the association between stress and neurodegeneration. Chronic, repeated, or intense stress may lead to impairment in the hypothalamic-pituitary-adrenal axis and to altered stress hormone levels.36 It has been suggested that, through increased cortisol level, stress might induce the activation of microglia and astrocytes as well as the overproduction of proinflammatory cytokines and oxidative stress. This process might lead to chronic neuroinflammation and subsequently increase the risk of neurodegenerative diseases.1,37,38,39 Psychological stress has also been associated with cerebrovascular impairments, possibly through similar mechanisms.27 Furthermore, cardiovascular diseases are among the most well-established long-term physiological health consequences of stress-related disorders.14,15,16 Therefore, vascular factors could underlie the association of stress-related disorders with neurodegenerative diseases and may explain the relatively stronger association for vascular neurodegenerative diseases compared with primary neurodegenerative diseases.28 Vascular factors could also be implicated in the pathophysiological process of AD by worsening symptoms and accelerating the disease progression.40 Furthermore, AD and vascular dementia often occur simultaneously, and these diagnoses are often hard to distinguish clinically. Thus, vascular factors may also mediate the observed associations with primary neurodegenerative diseases, although to a lesser extent. The diminished magnitude of the association between stress-related disorders and vascular neurodegenerative diseases after additional adjustment for cardiovascular diseases supports such a hypothesis. In addition to biological pathways, the observed associations could also be attributable to lifestyle changes after the diagnosis of stress-related disorders, such as increased smoking and alcohol use, substance abuse, or sleep disorders,41,42 which might lead to an altered risk for neurodegenerative diseases.
Strengths and Limitations
This study has strengths and limitations. The main strengths are its population-based cohort design and the novel use of sibling comparison, which enabled us to address unmeasured confounding by familial factors. We also considered a wide range of other important confounders and performed subgroup and sensitivity analyses to assess the robustness of the findings. The complete follow-up and the fact that all Swedish citizens have free access to uniform, publicly funded health care reduced selection bias and contributed to the internal validity of the study. Another important strength is the use of prospectively and independently collected data on stress-related disorders and neurodegenerative diseases, which minimizes information bias.
Reverse causation and surveillance bias are potential limitations of the study. Symptoms of stress-related disorders, such as cognitive impairment and depressive symptoms,43,44,45 might be early signs of dementia and PD. Alternatively, stress-induced decline in cognitive function may lead to an earlier onset of dementia among patients with stress-related disorders. To address these issues, we used a 5-year lag time in all analyses to alleviate concerns that stress-related disorders might be secondary to preclinical neurodegenerative diseases or that neurodegenerative diseases might be detected in the workup of stress-related disorders. The study period started in 1987 because the ICD codes for stress-related disorders were only available in ICD-9 onward. As a result, individuals with severe stress reactions before 1987 did not receive a diagnosis and might have been misclassified as unexposed. The observed association might therefore be an underestimation of the real association between stress-related disorders and neurodegenerative diseases.
Other limitations are the lack of validation studies for the diagnoses of stress-related disorder in the NPR and the lack of outpatient specialist care data in the NPR until 2001. In addition, although validation studies have shown satisfactory to excellent positive predictive values and specificity for neurodegenerative diseases identified through the NPR, the sensitivity of these diagnoses, especially dementia (ie, 50% sensitivity for dementia diagnosis based on inpatient care records30), is suboptimal. This misclassification would, however, most likely bias the studied associations toward the null.46 Furthermore, the register-based definitions of stress-related disorders and neurodegenerative diseases might have included patients with more severe stress-related disorders or neurodegenerative diseases, compared with all patients with these diseases. This might be especially relevant for the period of 1987 to 2000, when these diseases were only ascertained through inpatient hospital visits. Therefore, the association of stress-related disorders with milder forms of neurodegenerative diseases, as well as the generalizability of these findings to individuals with milder stress-related disorders, needs further investigation. Moreover, because the study cohort was relatively young and neurodegenerative diseases were commonly diagnosed at older age, we did not have enough statistical power for analysis of rarer outcomes, such as ALS and PD.
Because the study lacked detailed information on potential risk factors for stress-related disorders and neurodegenerative diseases, residual confounding is another limitation. For example, smoking may be a risk factor for dementia47 and ALS.48 We therefore performed sensitivity analyses, adjusting for chronic pulmonary diseases as a proxy for smoking, which had only minimal implication for the results. The association might also be confounded by factors such as lower cognitive function,49,50 certain personality traits, and lower stress resilience,51 which may predispose both individuals with stress-related disorders and those with neurodegenerative diseases.52,53 These factors are presumably shared within families; therefore, the similar results obtained from the population-matched and sibling analyses do not support important confounding of these factors.
Conclusions
The findings of this cohort study appear to support the hypothesis that individuals with a stress-related disorder diagnosis are at an increased risk of developing a neurodegenerative disease later in life, independent of multiple confounders such as familial factors. The underlying mechanisms behind this association, primarily the role of cerebrovascular factors, warrant further studies.
eTable 1. Swedish Revisions of the International Classification of Diseases (ICD) Codes for Exposure, Outcome, and Covariable Identification
eTable 2. Crude Incidence Rates (IRs) and Hazard Ratios (HRs) With 95% Confidence Intervals (CIs) for Neurodegenerative Diseases Among Patients With Different Types of Stress-Related Disorders, Compared to Their Matched Unexposed Individuals
eTable 3. Crude Incidence Rates (IRs) and Hazard Ratios (HRs) With 95% Confidence Intervals (CIs) for Any Dementia (Including Alzheimer Disease, Vascular Dementia, and Other Dementia) Among Individuals With Different Types of Stress-Related Disorders, Compared to Their Matched Unexposed Individuals or Full Siblings
eTable 4. Crude Incidence Rates (IRs) and Hazard Ratios (HRs) With 95% Confidence Intervals (CIs) for Neurodegenerative Diseases Among Patients With Any Stress-Related Disorder by Psychiatric Care Indicators, Compared to Their Matched Unexposed Individuals
eTable 5. Crude Incidence Rates (IRs) and Hazard Ratios (HRs) With 95% Confidence Intervals (CIs) for Any Neurodegenerative Diseases Among Patients With Any Stress-Related Disorder, Compared to Their Full Sibling, by Different Characteristics
eTable 6. Hazard Ratios (HRs) With 95% Confidence Intervals (CIs) for Any or Specific Neurodegenerative Diseases Among Patients With Any Stress-Related Disorder, Compared to Their Matched Unexposed Individuals or Full Siblings, Additionally Adjusted for the Occurrence of Cardiovascular Diseases or Chronic Pulmonary Diseases Before the End of Follow-up
References
- 1.Vyas S, Rodrigues AJ, Silva JM, et al. . Chronic stress and glucocorticoids: from neuronal plasticity to neurodegeneration. Neural Plast. 2016;2016:6391686. doi: 10.1155/2016/6391686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansson L, Guo X, Waern M, et al. . Midlife psychological stress and risk of dementia: a 35-year longitudinal population study. Brain. 2010;133(Pt 8):2217-2224. doi: 10.1093/brain/awq116 [DOI] [PubMed] [Google Scholar]
- 3.Andel R, Crowe M, Hahn EA, et al. . Work-related stress may increase the risk of vascular dementia. J Am Geriatr Soc. 2012;60(1):60-67. doi: 10.1111/j.1532-5415.2011.03777.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radford K, Delbaere K, Draper B, et al. . Childhood stress and adversity is associated with late-life dementia in aboriginal Australians. Am J Geriatr Psychiatry. 2017;25(10):1097-1106. doi: 10.1016/j.jagp.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 5.Hikichi H, Aida J, Kondo K, et al. . Increased risk of dementia in the aftermath of the 2011 Great East Japan earthquake and tsunami. Proc Natl Acad Sci U S A. 2016;113(45):E6911-E6918. doi: 10.1073/pnas.1607793113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang HX, Wahlberg M, Karp A, Winblad B, Fratiglioni L. Psychosocial stress at work is associated with increased dementia risk in late life. Alzheimers Dement. 2012;8(2):114-120. doi: 10.1016/j.jalz.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 7.Rod NH, Hansen J, Schernhammer E, Ritz B. Major life events and risk of Parkinson’s disease. Mov Disord. 2010;25(11):1639-1645. doi: 10.1002/mds.22850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlajinac H, Sipetic S, Marinkovic J, et al. . The stressful life events and Parkinson’s disease: a case-control study. Stress Health. 2013;29(1):50-55. doi: 10.1002/smi.2424 [DOI] [PubMed] [Google Scholar]
- 9.Clark AJ, Ritz B, Prescott E, Rod NH. Psychosocial risk factors, pre-motor symptoms and first-time hospitalization with Parkinson's disease: a prospective cohort study. Eur J Neurol. 2013;20(8):1113-1120. doi: 10.1111/ene.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sieurin J, Andel R, Tillander A, Valdes EG, Pedersen NL, Wirdefeldt K. Occupational stress and risk for Parkinson’s disease: a nationwide cohort study. Mov Disord. 2018;33(9):1456-1464. doi: 10.1002/mds.27439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang F, Ye W, Fall K, et al. . Loss of a child and the risk of amyotrophic lateral sclerosis. Am J Epidemiol. 2008;167(2):203-210. doi: 10.1093/aje/kwm289 [DOI] [PubMed] [Google Scholar]
- 12.Parkin Kullmann JA, Hayes S, Pamphlett R. Is psychological stress a predisposing factor for amyotrophic lateral sclerosis (ALS)? An online international case-control study of premorbid life events, occupational stress, resilience and anxiety. PLoS One. 2018;13(9):e0204424. doi: 10.1371/journal.pone.0204424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 14.Gradus JL. Prevalence and prognosis of stress disorders: a review of the epidemiologic literature. Clin Epidemiol. 2017;9:251-260. doi: 10.2147/CLEP.S106250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song H, Fang F, Arnberg FK, et al. . Stress related disorders and risk of cardiovascular disease: population based, sibling controlled cohort study. BMJ. 2019;365:1255. doi: 10.1136/bmj.l1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gradus JL, Farkas DK, Svensson E, et al. . Associations between stress disorders and cardiovascular disease events in the Danish population. BMJ Open. 2015;5(12):e009334. doi: 10.1136/bmjopen-2015-009334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaffe K, Vittinghoff E, Lindquist K, et al. . Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613. doi: 10.1001/archgenpsychiatry.2010.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qureshi SU, Kimbrell T, Pyne JM, et al. . Greater prevalence and incidence of dementia in older veterans with posttraumatic stress disorder. J Am Geriatr Soc. 2010;58(9):1627-1633. doi: 10.1111/j.1532-5415.2010.02977.x [DOI] [PubMed] [Google Scholar]
- 19.Meziab O, Kirby KA, Williams B, Yaffe K, Byers AL, Barnes DE. Prisoner of war status, posttraumatic stress disorder, and dementia in older veterans. Alzheimers Dement. 2014;10(3)(suppl):S236-S241. doi: 10.1016/j.jalz.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 20.Mawanda F, Wallace RB, McCoy K, Abrams TE. PTSD, psychotropic medication use, and the risk of dementia among US veterans: a retrospective cohort study. J Am Geriatr Soc. 2017;65(5):1043-1050. doi: 10.1111/jgs.14756 [DOI] [PubMed] [Google Scholar]
- 21.Flatt JD, Gilsanz P, Quesenberry CP Jr, Albers KB, Whitmer RA. Post-traumatic stress disorder and risk of dementia among members of a health care delivery system. Alzheimers Dement. 2018;14(1):28-34. doi: 10.1016/j.jalz.2017.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang TY, Wei HT, Liou YJ, et al. . Risk for developing dementia among patients with posttraumatic stress disorder: a nationwide longitudinal study. J Affect Disord. 2016;205:306-310. doi: 10.1016/j.jad.2016.08.013 [DOI] [PubMed] [Google Scholar]
- 23.Gradus JL, Horváth-Puhó E, Lash TL, et al. . Stress disorders and dementia in the Danish population. Am J Epidemiol. 2019;188(3):493-499. doi: 10.1093/aje/kwy269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan YE, Bai YM, Hsu JW, et al. . Post-traumatic stress disorder and risk of Parkinson disease: a nationwide longitudinal study. Am J Geriatr Psychiatry. 2017;25(8):917-923. doi: 10.1016/j.jagp.2017.03.012 [DOI] [PubMed] [Google Scholar]
- 25.Svensson E, Farkas DK, Gradus JL, Lash TL, Sorensen HT. Adjustment disorder and risk of Parkinson's disease. Eur J Neurol. 2016;23(4):751-756. doi: 10.1111/ene.12933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longinetti E, Mariosa D, Larsson H, et al. . Neurodegenerative and psychiatric diseases among families with amyotrophic lateral sclerosis. Neurology. 2017;89(6):578-585. doi: 10.1212/WNL.0000000000004179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burrage E, Marshall KL, Santanam N, Chantler PD. Cerebrovascular dysfunction with stress and depression. Brain Circ. 2018;4(2):43-53. doi: 10.4103/bc.bc_6_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalaria RN. Cerebrovascular disease and mechanisms of cognitive impairment: evidence from clinicopathological studies in humans. Stroke. 2012;43(9):2526-2534. doi: 10.1161/STROKEAHA.112.655803 [DOI] [PubMed] [Google Scholar]
- 29.Korczyn AD. Vascular parkinsonism—characteristics, pathogenesis and treatment. Nat Rev Neurol. 2015;11(6):319-326. doi: 10.1038/nrneurol.2015.61 [DOI] [PubMed] [Google Scholar]
- 30.Rizzuto D, Feldman AL, Karlsson IK, Dahl Aslan AK, Gatz M, Pedersen NL. Detection of dementia cases in two Swedish health registers: a validation study. J Alzheimers Dis. 2018;61(4):1301-1310. doi: 10.3233/JAD-170572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldman AL, Johansson AL, Gatz M, et al. . Accuracy and sensitivity of Parkinsonian disorder diagnoses in two Swedish national health registers. Neuroepidemiology. 2012;38(3):186-193. doi: 10.1159/000336356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mariosa D, Hammar N, Malmström H, et al. . Blood biomarkers of carbohydrate, lipid, and apolipoprotein metabolisms and risk of amyotrophic lateral sclerosis: a more than 20-year follow-up of the Swedish AMORIS cohort. Ann Neurol. 2017;81(5):718-728. doi: 10.1002/ana.24936 [DOI] [PubMed] [Google Scholar]
- 33.Hinchliffe SR, Lambert PC. Flexible parametric modelling of cause-specific hazards to estimate cumulative incidence functions. BMC Med Res Methodol. 2013;13:13. doi: 10.1186/1471-2288-13-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9(2):265-290. doi: 10.1177/1536867X0900900206 [DOI] [Google Scholar]
- 35.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219-219. doi: 10.1136/bmj.326.7382.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873-904. doi: 10.1152/physrev.00041.2006 [DOI] [PubMed] [Google Scholar]
- 37.Greenberg MS, Tanev K, Marin MF, Pitman RK. Stress, PTSD, and dementia. Alzheimers Dement. 2014;10(3)(suppl):S155-S165. doi: 10.1016/j.jalz.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 38.Jauregui-Huerta F, Ruvalcaba-Delgadillo Y, Gonzalez-Castañeda R, Garcia-Estrada J, Gonzalez-Perez O, Luquin S. Responses of glial cells to stress and glucocorticoids. Curr Immunol Rev. 2010;6(3):195-204. doi: 10.2174/157339510791823790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Austin KW, Ameringer SW, Cloud LJ. An integrated review of psychological stress in Parkinson’s disease: biological mechanisms and symptom and health outcomes. Parkinsons Dis. 2016;2016:9869712. doi: 10.1155/2016/9869712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120(3):287-296. doi: 10.1007/s00401-010-0718-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohlenhoff BS, O’Donovan A, Weiner MW, Neylan TC. Dementia risk in posttraumatic stress disorder: the relevance of sleep-related abnormalities in brain structure, amyloid, and inflammation. Curr Psychiatry Rep. 2017;19(11):89. doi: 10.1007/s11920-017-0835-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl). 2001;158(4):343-359. doi: 10.1007/s002130100917 [DOI] [PubMed] [Google Scholar]
- 43.Cohen BE, Neylan TC, Yaffe K, Samuelson KW, Li Y, Barnes DE. Posttraumatic stress disorder and cognitive function: findings from the mind your heart study. J Clin Psychiatry. 2013;74(11):1063-1070. doi: 10.4088/JCP.12m08291 [DOI] [PubMed] [Google Scholar]
- 44.Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12(1):125-133. doi: 10.1037/0894-4105.12.1.125 [DOI] [PubMed] [Google Scholar]
- 45.Johnsen GE, Asbjørnsen AE. Consistent impaired verbal memory in PTSD: a meta-analysis. J Affect Disord. 2008;111(1):74-82. doi: 10.1016/j.jad.2008.02.007 [DOI] [PubMed] [Google Scholar]
- 46.Rothman KJ, Greenland S. Precision and validity in epidemiologic studies In: Modern Epidemiology. 2nd ed. Philadelphia, PA: Lippincott-Raven; 1998. [Google Scholar]
- 47.Zhong G, Wang Y, Zhang Y, Guo JJ, Zhao Y. Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One. 2015;10(3):e0118333. doi: 10.1371/journal.pone.0118333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ingre C, Roos PM, Piehl F, Kamel F, Fang F. Risk factors for amyotrophic lateral sclerosis. Clin Epidemiol. 2015;7:181-193. doi: 10.2147/CLEP.S37505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilbertson MW, Paulus LA, Williston SK, et al. . Neurocognitive function in monozygotic twins discordant for combat exposure: relationship to posttraumatic stress disorder. J Abnorm Psychol. 2006;115(3):484-495. doi: 10.1037/0021-843X.115.3.484 [DOI] [PubMed] [Google Scholar]
- 50.Kremen WS, Koenen KC, Boake C, et al. . Pretrauma cognitive ability and risk for posttraumatic stress disorder: a twin study. Arch Gen Psychiatry. 2007;64(3):361-368. doi: 10.1001/archpsyc.64.3.361 [DOI] [PubMed] [Google Scholar]
- 51.Breslau N, Schultz L. Neuroticism and post-traumatic stress disorder: a prospective investigation. Psychol Med. 2013;43(8):1697-1702. doi: 10.1017/S0033291712002632 [DOI] [PubMed] [Google Scholar]
- 52.Sieurin J, Gustavsson P, Weibull CE, et al. . Personality traits and the risk for Parkinson disease: a prospective study. Eur J Epidemiol. 2016;31(2):169-175. doi: 10.1007/s10654-015-0062-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johansson L, Guo X, Duberstein PR, et al. . Midlife personality and risk of Alzheimer disease and distress: a 38-year follow-up. Neurology. 2014;83(17):1538-1544. doi: 10.1212/WNL.0000000000000907 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Swedish Revisions of the International Classification of Diseases (ICD) Codes for Exposure, Outcome, and Covariable Identification
eTable 2. Crude Incidence Rates (IRs) and Hazard Ratios (HRs) With 95% Confidence Intervals (CIs) for Neurodegenerative Diseases Among Patients With Different Types of Stress-Related Disorders, Compared to Their Matched Unexposed Individuals
eTable 3. Crude Incidence Rates (IRs) and Hazard Ratios (HRs) With 95% Confidence Intervals (CIs) for Any Dementia (Including Alzheimer Disease, Vascular Dementia, and Other Dementia) Among Individuals With Different Types of Stress-Related Disorders, Compared to Their Matched Unexposed Individuals or Full Siblings
eTable 4. Crude Incidence Rates (IRs) and Hazard Ratios (HRs) With 95% Confidence Intervals (CIs) for Neurodegenerative Diseases Among Patients With Any Stress-Related Disorder by Psychiatric Care Indicators, Compared to Their Matched Unexposed Individuals
eTable 5. Crude Incidence Rates (IRs) and Hazard Ratios (HRs) With 95% Confidence Intervals (CIs) for Any Neurodegenerative Diseases Among Patients With Any Stress-Related Disorder, Compared to Their Full Sibling, by Different Characteristics
eTable 6. Hazard Ratios (HRs) With 95% Confidence Intervals (CIs) for Any or Specific Neurodegenerative Diseases Among Patients With Any Stress-Related Disorder, Compared to Their Matched Unexposed Individuals or Full Siblings, Additionally Adjusted for the Occurrence of Cardiovascular Diseases or Chronic Pulmonary Diseases Before the End of Follow-up