Abstract
Premotor areas play a critical role in the control of repetitive movements. While research has shown that movement-related oscillations are abnormal during repetitive movements in persons with Parkinson’s disease (PD), there is limited research examining the contribution of premotor areas, such as the contralateral dorsal premotor area (PMd) and supplementary motor area (SMA), to this impairment. This study compared movement-related oscillations over premotor regions between participants with PD and control participants. Nine participants with PD off and on medication and nine matched control participants were studied. Participants performed cued index finger movements. Spectral power was derived from electroencephalographic recordings from electrodes FC3/FC4 and Cz over the regions of the contralateral PMd and SMA respectively. Movement-related alpha and beta band oscillations were suppressed over electrode FC3/FC4 (contralateral PMd) in participants with PD, particularly at higher movement rates, in both the off and on medication conditions compared to control subjects. The pattern of movement-related oscillations recorded from Cz (SMA) was similar between PD and control groups. This would suggest that the region of the contralateral PMd may be preferentially involved with the control of externally cued repetitive movements and that changes in this activity may contribute to the deterioration of repetitive finger movements at higher rates in persons with PD.
Keywords: Alpha band, Beta band, Desynchronization, Electroencephalography, Secondary motor regions
Repetitive movements in people with Parkinson’s disease (PD) are characterized by reductions in amplitude (hypokinesia), speed (bradykinesia) and rhythmicity and are frequently accompanied by involuntary hesitations and arrests. The incidence and severity of these impairments increases during small amplitude and/or higher rate movements [1–3]. Moreover, hypokinesia, hesitations and arrest of movement that occur at higher movement rates respond poorly to dopamine replacement therapy [2,3] and are not improved with external cueing [2,4]. Currently, the cortical mechanisms contributing to impaired repetitive movement in PD are poorly understood.
Motor cortical areas considered to be critically involved in the control of finger movements include the primary motor cortex (M1), dorsal premotor area (PMd), and supplementary motor area (SMA) [5–7]. The PMd is thought to be preferentially activated during externally cued movements whereas the SMA is considered to be important for the control of self-paced (internally generated) movements [8–10]. Functional neuroimaging studies of discrete movements have shown that internally generated movements in people with PD are associated with reduced activity in the SMA and increased activity in the PMd and M1 [11–14]. Motor and premotor cortical activity during externally paced movements is dependent upon movement rate with activity in the contralateral M1 and PMd increasing with increased rate [6,7]. Findings in the SMA have been equivocal, some studies showing no change in activity with increased rate of movement cueing [5,7] and others showing an increase [6]. Since externally paced movements often deteriorate more at higher movement rates in people with PD [2] and are associated with abnormally suppressed alpha and beta oscillations in M1 [15], this raises the possibility that impaired function of the PMd may contribute to this impairment.
The purpose of this study was to use scalp surface electroencephalography (EEG) to examine movement-related oscillations over the regions of the contralateral PMd and SMA in people with PD and matched control subjects across a range of externally paced movement rates (1–3 Hz). Given that movement was externally cued, we hypothesized that compared to control subjects(1) movement-related oscillations over the regions of the PMd of participants with PD would be suppressed and show impaired scaling with movement rate and (2) there would be no differences in movement-related oscillations over the SMA.
Methods for this study, including more detailed information about participants, have been described in detail in a previous publication [15] and are summarized below.
Participants with a diagnosis of idiopathic PD (n = 9; age = 65 ± 8 years) and age, gender and handedness matched control participants (n = 9; age = 65 ± 9 years) were tested on and off medication. The Institutional Review Board of Northwestern University approved the procedures. All participants gave their written informed consent according to the Declaration of Helsinki.
Each trial began with period of rest (REST). A series of acoustic tones was then presented at a pacing rate of 1 Hz and maintained for 15 intervals. The rate of the tone was then increased by 0.25 Hz until reaching 3.0 Hz [2,15,16]. All participants were asked to synchronize finger-flexion with the tones (MOVE). Participants with PD performed the task with their most affected hand, and control participants performed the task with the same side as their matched counterpart.
An accelerometer (Measurement Specialties EGAXT3–15-/L2M) placed on the index finger was used to capture finger kinematics. Bipolar surface electromyography (EMG) signals were recorded from the first dorsal interosseous (FDI) muscle (Grass P511, Grass Technologies). EEG signals were recorded from a montage of 74 scalp-surface electrodes conformed to the international 10–20 system with increased density of electrodes over the right and left sensorimotor areas (Neuroscan Syamps System/Neuroscan 4.1)[15].
Analysis of event-related power was focused on signals obtained from electrodes FC3 or FC4 (overlying the region of the PMd) contralateral to the moving hand, and electrode Cz (overlying the region of the SMA). A 5-point Laplacian spatial filter was applied to minimize signals common to adjacent electrodes (e.g. C3 and FC3). EEG data were epoched according to movement onset which was manually marked using the EMG and acceleration signals. Epoched data was filtered and inspected for noise accordingly [15]. Movement onset times were superimposed on REST data and MOVE data was then normalized to the REST [17]. Epochs were then averaged across each pacing rate (1–3 Hz) and task condition (REST, MOVE).
A short-time Fourier transform method was used to obtain within-subject time-frequency power spectra profiles [18]. Analysis was focused on the alpha (9–14 Hz) and beta (20–25 Hz) bands as previous research has shown that synchronization – desynchronization transition is greatest within these bands [17]. To obtain time-power plots, normalized power in each frequency band was averaged for each group. The amplitude of movement-related oscillations was derived from measures of the peak-to-peak oscillations observed in the grand average waveform. Area under the curve was calculated at each pacing rate and averaged across groups to capture the overall magnitude of synchronization and desynchronization of movement related oscillations (MROs) across a movement cycle relative to rest.
Analysis was completed to determine if differences in power at REST contributed to power during the MOVE condition. The REST condition was epoched into 1-s segments, and a fast Fourier transform was applied to each segment. The power spectrum was normalized to 1 and summed resulting in a chi-square distribution. The mean spectrum of one group was divided by the mean spectrum of the second group. From the resulting F distribution, statistical comparison of spectrum between groups was completed by obtaining the 95th percentile confidence limits from an F table. Any value below or above these limits was designated a significant difference between spectrums [19].
To test the main hypotheses, a non-parametric Mann-Whitney-U test was used due to the non-Gaussian distribution of the MRO measures, to examine differences in peak-to-peak amplitude and area under the curve between the PD and control groups. The Wilcoxon test was used to test for differences between the PDOFF and PDON groups and across tone rates. Statistical analysis was completed using SPSS and the level of significance for all tests was set at α < 0.05.
Results of analysis of the kinematic data have been published previously [15]. Participants with PD showed an increase in hypokinesia and hastening at pacing rates ranging from 1.75 to 3 Hz both OFF and ON medication compared to the control participants.
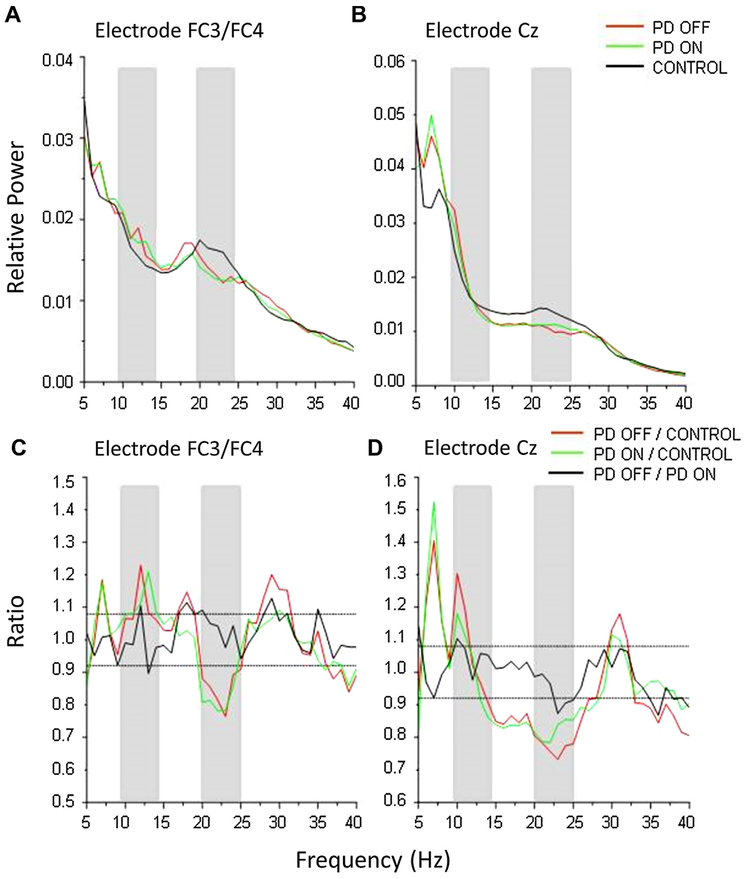
Fig. 1 shows the difference in relative power in electrodes FC3/FC4 and Cz recorded over the regions of the contralateral PMd and SMA respectively at rest between the PD and control groups. Fig. 1C and D shows the ratio of the mean spectra between groups in which a peak above or below the 95th lower confidence limit (dashed lines) indicates significance. The participants with PD showed a significant increase in relative power in the alpha band (~9–14 Hz) and suppression in the beta band (~13–30 Hz) in both medication states compared to controls for both electrodes FC3/FC4 (Fig. 1C) and Cz (Fig. 1D).
Fig. 1.
Relative power spectra from electrodes FC3/FC4 (A) and Cz (B) and the ratio of power spectra recorded from electrodes FC3/FC4 (C) and Cz (D). Gray bars indicate frequency band of interest in the analysis of MROs.
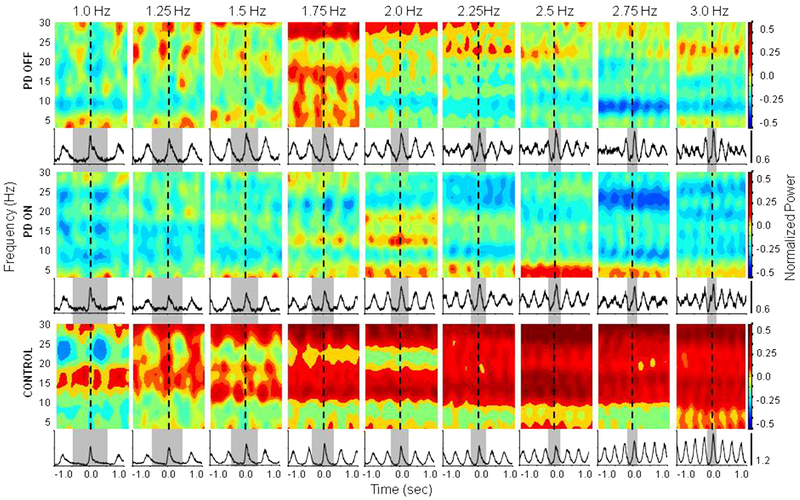
A visual depiction of the average time-frequency power spectra across all tone rates from electrode FC3/FC4 is shown in Fig. 2. The control group showed a progressive increase in power (more red) in both the alpha and beta bands with increasing movement rate. In contrast, both PD groups showed an attenuation of power in both bands across all tone rates.
Fig. 2.
Mean normalized power from 8 to 30 Hz recorded from electrode FC3/FC4 over the region of the contralateral PMd and mean normalized FDI EMG for each group across each pacing rate. Dashed lines indicate movement onset. Gray bars indicated one movement cycle
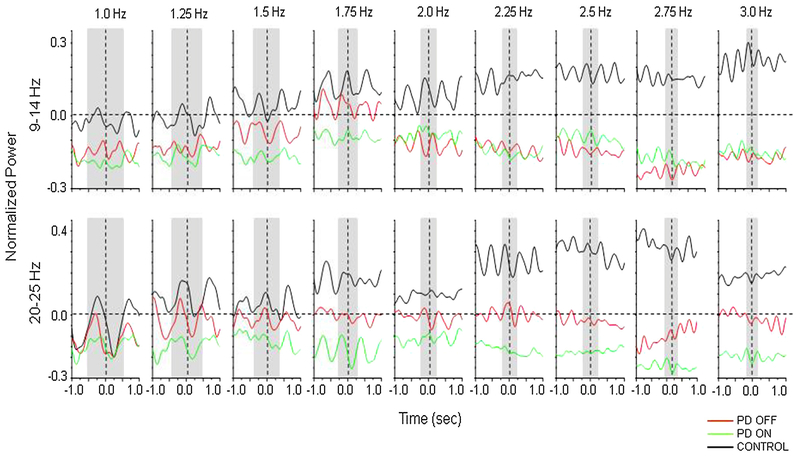
Time-frequency plots for the frequencies of interest (9–14 Hz and 20–25 Hz) are shown in Fig. 3. Statistical analysis of peak-to-peak amplitude (peak within the grey shaded region) revealed significant differences between groups. The PD group and controls significantly differed in the alpha and beta bands in both the off and on medication states (PDOFF vs. Control: Z(1) < 2.427, p < 0.015; PDON vs. Control: Z(1) < −3.804, p < 0.001). A significant medication effect (PDOFF vs PDON) was also revealed for both bands (Z(1) < −2.312, p < 0.021). For area under the curve, significant main effects of group were observed for alpha band oscillations in both the off and on medication states (PDOFF vs. Control: Z(1) = − 3.706, p < 0.001; PDON vs. Control: Z(1) = −2.281, p = 0.023), but not in the beta band. In contrast, there was a significant effect of medication in the beta band (Z(1) = −2.957, p = 0.003), but not the alpha band.
Fig. 3.
Mean normalized power averaged across 9–14 Hz (alpha band) and 20–25 Hz (beta band) recorded from electrode FC3/FC4 over the region of the contralateral PMd. Vertical dashed lines indicate movement onset and gray bars indicate one movement cycle.
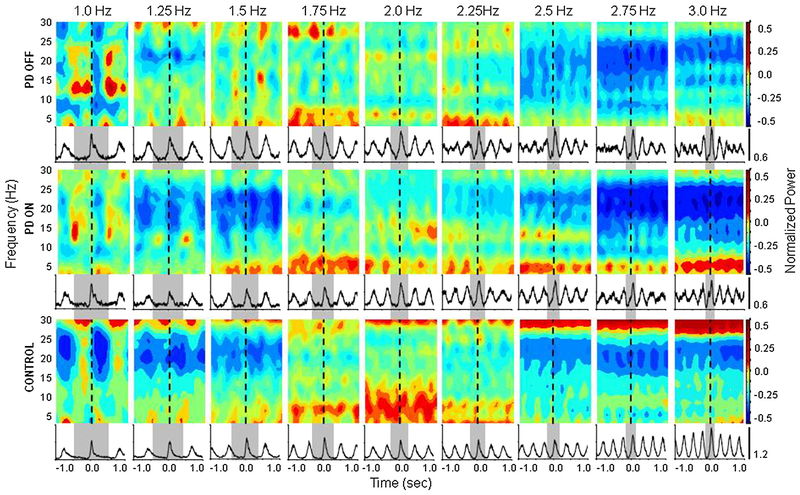
A visual depiction of the average time-frequency power spectra across all tone rates from electrode Cz is shown in Fig. 4. In both control subjects and participants with PD in the on medication state, MROs at lower (1.0–1.5 Hz) and higher (2.5–3 Hz) tone rates were associated with a distinct suppression of power in the beta range of 20–25 Hz. The pattern was different at tone rates of 1.75–2.25 Hz, with an increase in power within the alpha and beta bands. Statistical analysis of peak amplitude revealed a group effect between the PDOFF and control groups in the alpha band only (Z(1) = −2.186, p = 0.029). There was no significant difference between the PDON and control groups, and there was no medication effect. Statistical analysis of area under the curve revealed that both the PDOFF and PDON groups significantly differed from the control group in the alpha band only (PDOFF vs. Control: Z(1) = −2.718, p = 0.007; PDON vs. Control: Z(1) = −2.345, p = 0.019).
Fig. 4.
Mean normalized power from 8 to 30 Hz recorded from electrode Cz over the region of the SMA and mean normalized FDI EMG for each group across each pacing rate. Dashed lines indicate movement onset. Gray bars indicated one movement cycle.
The principal finding of this experiment was that participants with PD showed a significant suppression of movement-related oscillations in both the alpha and beta bands recorded from electrode FC3/FC4 (over the region of the contralateral PMd), particularly at higher movement rates, compared to control subjects. However, the pattern of movement-related oscillations recorded from electrode Cz (over the regions of the SMA) was similar between groups and medication states. Kinematic results from this study [15] revealed that movement impairment in repetitive finger movements emerges around 2.0 Hz and higher in persons with PD. Thus, these findings suggest that changes in premotor cortical activity may contribute to externally cued repetitive movement impairment at high rates in PD.
MROs recorded from electrode FC3/FC4 over the region of the contralateral PMd in control subjects showed a progressive increase in power/synchronization with increasing movement rate. This is quite different from the pattern observed over the sensori-motor region which is associated with increased and prolonged desynchronization with increasing movement rate [15,20]. These findings suggest there is a change in the role of the PMd with increasing movement rate, possibly reflecting a transition from discrete (sensorimotor feedback driven) to more continuous or automatic control of movement at higher rates [20,21]. Control of discrete movements is considered to be preferentially mediated by cerebellar-premotor pathways, while the control of continu ous movement is mediated by the basal ganglia-thalamocortical pathways [22,23]. A progressive increase in PMd synchronization with movement rate was absent in the PD group, suggesting that sustained activity in this region is required for the performance of higher rate movements. This is in keeping with imaging studies that have shown increased movement-related activity in the PMd of patients with PD [11–14]. Wu and Hallett [21] showed that the performance of automatic movements in people with PD was associated with increased activity in the cerebellum and premotor cortex. This would suggest that maintained desynchronization of PMd at higher movement rates may reflect a reduced capacity to transition from sensory feedback driven discrete movements to more automatic movements that are more basal ganglia dependent. Consistent with this idea, parkinsonian medications significantly improved both the magnitude and area under curve of oscillations over the region of the PMd in the PD group.
The results of this study also revealed differences in the distribution of activity recorded from electrode Cz over the region of the SMA between groups but in the alpha band only, and minimal differences across tone rates. Previous research has demonstrated equivocal results regarding the involvement of the SMA with increasing movement rate [5–7]. Perhaps the differences in results lie in the movement rates tested. Indeed, results of this study revealed that power over the region of the SMA did increase up to 2.0 Hz, but decreased again at higher rates in this study. Interestingly, this pattern of activity was observed in both PD groups and the control group. Thus, the change in activity over the region of the SMA around this rate may also represent a transition between motor control strategies (discrete to continuously) as discussed previously. However, there were minimal differences between groups. Given that the task used in this study is externally cued, the minimal differences between groups in SMA activity may suggest that this region may not be playing an active role in the control and/or dysregulation of externally cued repetitive movements, in particular at high movement rates, in persons with PD.
The results of this study may be driven by differences in activity at rest. If power at rest was high in the PD group, then normalizing to rest would result in reduced power during movement. Results revealed that power at rest in both PD groups was significantly higher compared to controls within the alpha band, but significantly lower in the beta band across both electrodes of interest. Thus, the significant differences revealed for the alpha band may be partially affected by the normalization process and may represent a more global change in activity at rest rather than movement only. However, the differences revealed for the beta band activity may reliably account for differences in activity related to movement. Results of this study revealed significant differences in both the alpha and beta bands over the region of the PMd, but only differences in the alpha band over the region of the SMA. Taken together, these results support the notion that differences in MROs over the region of the PMd have a larger influence than the SMA on impairments in externally cued repetitive finger movements at high rates in persons with PD.
Limitations include the small sample size which may have limited the ability to discern differences between groups and conditions. Additionally, the inclusion/exclusion criteria for this study restricted the cohort to those with a predominantly akinetic-rigid syndrome to avoid confounding kinematic and EEG signals associated with large amplitude tremor. Interpretation to the broader population of people with PD should be limited. Source localization was not completed and it is possible that volume conduction of signals from other regions, including M1, may have contributed to the movement-related oscillations recorded over the regions of interest in this study.
In conclusion, these results demonstrate a lack of progressive increase in power of movement-related oscillations over the region of the contralateral PMd in people with PD compared to controls. Activity over the region of the SMA was similar between groups. This would suggest that the region of the contralateral PMd may be preferentially involved with the control of externally cued repetitive movements and that changes in this activity may contribute to the deterioration of repetitive finger movements at higher rates.
HIGHLIGHTS.
Motor cortical oscillations recorded over the PMd are suppressed in persons with PD
Motor cortical oscillations recorded over the SMA were similar between groups
Changes in premotor cortical oscillations may impact repetitive movement
Acknowledgement
This work was supported by National Institutes of Health grant number RO1 NS054199–01A1
Footnotes
Conflict of interest
None of the authors have potential conflicts of interest to be disclosed
Contributor Information
Elizabeth L. Stegemöller, Email: esteg@iastate.edu.
David P. Allen, Email: dp.allen62@gmail.com.
Tanya Simuni, Email: t-simuni@northwestern.edu.
Colum D. MacKinnon, Email: cmakinn@umn.edu.
References
- [1].Vercruysse S, Spildooren J, Heremans E, Vandernbossche J, Levin O, Wenderoth N, et al. , Freezing in Parkinson’s disease: a spatiotemporal motor disorder beyond gait, Mov. Disord 27 (2012) 254–263. [DOI] [PubMed] [Google Scholar]
- [2].Stegemöller EL, Simuni T, MacKinnon CD, Timing and frequency barriers during repetitive finger movements in patients with Parkinson’s disease, Mov. Disord 24 (2009) 1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Espay AJ, Giuffrida JP, Chen R, Payne M, Mazzella F, Dunn E, et al. , Differential response of speed amplitude, and rhythm to dopaminergic medications in Parkinson’s disease, Mov. Disord 26 (2011) 2504–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pastor MA, Jahanshahi M, Artieda J, Obeso JA, Performance of repetitive wrist movements in Parkinson’s disease, Brain 115 (1992) 875–891. [DOI] [PubMed] [Google Scholar]
- [5].Blinkenberg M, Bonde C, Holm S, Svarer C, Anderson J, Paulson O, et al. , Rate dependence of regional cerebral activation during performance of a repetitive motor task: a PET study, J. Cereb. Blood Flow Matab 16 (1996) 794–803. [DOI] [PubMed] [Google Scholar]
- [6].Jenkins IH, Passingham RE, Brooks DJ, The effect of movement frequency on cerebral activation: a positron emission tomography study, J. Neurol. Sci 151 (1997) 195–205. [DOI] [PubMed] [Google Scholar]
- [7].Sadato N, Ibanez V, Deiber MP, Campbell G, Leonardo M, Hallett M, Frequency-dependent changes of regional cerebral blood flow during finger movements, J. Cereb. Blood Flow Matab 16 (1996) 23–33. [DOI] [PubMed] [Google Scholar]
- [8].Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ, Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects, Brain 118 (1995) 1216–1228. [DOI] [PubMed] [Google Scholar]
- [9].Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ, Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow, Brain 123 (2000) 1216–1228. [DOI] [PubMed] [Google Scholar]
- [10].Tanji J, Mushiake H, Comparison of neuronal activity in the supplementary motor area and primary motorcortex, Cogn. Brain Res 3 (1996) 143–150. [DOI] [PubMed] [Google Scholar]
- [11].Buhmann C, Gorsler A, Baumer T, Hidding U, Demiralay C, Hinkelmann K, et al. , Abnormal excitability of premotor-motor connections in de novo Parkinson’s disease, Brain 127 (2004) 2732–2746. [DOI] [PubMed] [Google Scholar]
- [12].Catalan MJ, Ishii K, Honda M, Samii A, Hallett M, A PET study of sequential finger movements of varying length in patients with Parkinson’s disease, Brain 122 (1999) 483–495. [DOI] [PubMed] [Google Scholar]
- [13].Rascol O, Sabatini U, Chollet F, Celsis P, Montastruc JL, Marcvergnes JP, et al. , Supplementary and primary sensory motor area in Parkinson’s disease – regional cerebral blood-flow changes during finger movements and effects of apomorphine, Arch. Neurol 49 (1992) 144–148. [DOI] [PubMed] [Google Scholar]
- [14].Samuel M, Ceballos-Baumann AO, Blin J, Uema T, Boecker H, Passingham RE, et al. , Evidence for lateral premotor and parietal overactivity in Parkinson’s disease during sequential and bimanual movements – a PET study, Brain 120 (1997) 963–976. [DOI] [PubMed] [Google Scholar]
- [15].Stegemöller EL, Allen DP, Simuni T, MacKinnon CD, Motor cortical oscillations are abnormally suppressed during repetitive movement in patients with Parkinson’s disease, Clin. Neurophysiol 127 (2016) 664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stegemöller EL, Zadikoff C, Rosenow JM, MacKinnon CD, Deep brain stimulation improves movement amplitude but not hastening of repetitive finger movements, Neurosci. Lett 27 (2013) 552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pfurtscheller G, da Silva FHL, Event-related EEG/MEG synchronization and desynchronization: basic principles, Clin. Neurophysiol 110 (1999) 1842–1857. [DOI] [PubMed] [Google Scholar]
- [18].Allen DP, MacKinnon CD, Time-frequency analysis of movement-related spectral power in EEG during repetitive movements: a comparison of methods, J. Neurosci. Methods 186 (2010) 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Diggle PJ, A point process modelling approach to raised incidence of a rare phenomenon in the vicinity of a pre-specified point, J. R. Stat. Soc 160 (1990) 491–505. [Google Scholar]
- [20].Toma K, Mima T, Matsuoka T, Gerloff C, Ohnishi T, Koshy B, et al. , Movement rate effect on activation and functional coupling of motor cortical areas, J. Neurophysiol 88 (2002) 3377–3385. [DOI] [PubMed] [Google Scholar]
- [21].Wu T, Hallett M, A functional MRI study of automatic movements in patients with Parkinson’s disease, Brain 128 (2005) 2250–2259. [DOI] [PubMed] [Google Scholar]
- [22].Ivry R, The representation of temporal information in perception and motor control, Curr. Opin. Neurobiol 6 (1996) 851–857. [DOI] [PubMed] [Google Scholar]
- [23].Ivry R, Spencer RMC, The neural representation of time, Curr. Opin. Neurobiol 14 (2004) 225–232. [DOI] [PubMed] [Google Scholar]