Down syndrome (DS) is a neurodevelopmental disability most commonly caused by the complete triplication of chromosome 21 (Zigman et al., 2008), and is estimated to occur in 1 in 691 live births (Parker et al., 2010). Adults with DS have a heightened risk of early-onset Alzheimer’s disease (AD) (Wisniewski, Wisniewski, & Wen, 1985; Zigman, Schupf, Sersen, & Silverman, 1996). Virtually all adults with DS evidence AD neuropathology (i.e., β-amyloid plaques) in their 40s (Zigman & Lott, 2007) and 70–80% of adults with DS evidence clinical AD in their 60s and 70s (McCarron, McCallion, Reilly, & Mulryan, 2014). The heightened risk of AD in DS is thought to be due to the overproduction of brain β-amyloid, which is believed to occur as a result of the triplication of the gene coding for the amyloid precursor protein, located on chromosome 21.
Research on both non-DS (e.g., Aizenstein et al., 2008; Sperling, Mormino, & Johnson, 2014) and DS (e.g., Zigman & Lott, 2007) populations has shown that accumulation of brain β-amyloid begins years to decades before the onset of clinical AD. During this transitionary period, individuals progress from normative cognitive functioning to mild cognitive impairment (MCI), which involves subtle declines in cognitive functioning that do not yet significantly alter everyday functioning (Petersen, 2003; Thies & Bleiler, 2013). There is marked variability in both non-DS (e.g., Aizenstein et al., 2008; Palmqvist et al., 2017; Sperling et al., 2014) and DS (Lao et al., 2016) populations in the age of onset of AD neuropathology and in the rate of progression from normative cognitive functioning to MCI and then to clinical AD. In non-DS populations, lifestyle factors, such as time spent in cognitively stimulating (e.g., reading and doing puzzles), physical (e.g., running and biking), and social (e.g., playing cards with a friend) leisure activity have been posited to contribute to this variability (e.g., Amieva et al., 2010; Hertzog, Kramer, Wilson, & Lindenberger, 2008; Landau et al., 2012). The goal of the present study was to determine if engagement in leisure activity was associated with change in brain β-amyloid and decline in episodic memory across two time points (spanning 3 years) in 65 healthy (i.e., without clinical AD) adults with DS.
In non-DS populations, there is evidence that leisure activity is associated with later onset of AD neuropathology (e.g., Dougherty et al., 2016; Landau et al., 2012) and may buffer against the impact of early AD neuropathology on cognitive functioning (e.g., Bennett, Arnold, Valenzuela, Brayne, & Schneider, 2014; Foubert-Samier et al., 2014; Hertzog et al., 2008). The mechanisms driving this effect are hypothesized to vary by leisure domain. Cognitively stimulating leisure (i.e., activities requiring cognitive engagement and thinking) is thought to act through cognitive reserve, which posits that cognitively stimulating lifestyles may increase resistance to early AD neuropathology through compensatory brain mechanisms (Stern, 2009; Stern, 2012). In support of this idea, in non-DS populations, greater engagement in cognitively stimulating leisure has been found to be associated with a slower rate of decline in cognitive functioning in longitudinal studies (Bennett et al., 2014; Wilson, Scherr, Schneider, Tang, & Bennett, 2007). Moreover, in a sample of 86 adults without DS (65 without clinical AD, 10 with clinical AD, and 11 healthy young adults), greater engagement in cognitively stimulating leisure throughout the lifespan was associated with less brain β-amyloid (Landau et al., 2012). In this study, older adults without clinical AD who had engaged in the highest levels of cognitively stimulating leisure had levels of brain β-amyloid comparable to the healthy young adults, whereas those who had engaged in the lowest levels of cognitively stimulating leisure had levels of brain β-amyloid comparable to the older adults with clinical AD (Landau et al., 2012). Research has yet to examine the extent to which cognitively stimulating leisure activity is similarly associated with reduced AD neuropathology and/or protects against AD-related declines in cognitive functioning in adults with DS.
The benefit of physical leisure (i.e., activities involving physical energy expenditure) is posited to reduce early AD neuropathology and its impacts through increased cerebral blood flow, synaptic plasticity, and delay of loss of gray matter (e.g., Cotman, Berchtold, & Christie, 2007; Erickson, Leckie, & Weinstein, 2014). Indeed, physical leisure has been found to be associated with less brain atrophy and lower brain β-amyloid (e.g., Dougherty et al., 2016; Okonkwo et al., 2014). In a sample of 317 adults without clinical AD, Okonkwo and colleagues (2014) found that age was positively associated with brain β-amyloid in adults who did not engage in physical leisure, but not in those who did. Similarly, in a prospective longitudinal study, Lindsay and colleagues (2002) found that regular physical leisure was associated with reduced risk of clinical AD. To date, no study has examined the association between physical leisure activity and early AD neuropathology and/or decline in cognitive functioning in adults with DS. Yet, adults with DS have high rates of obesity and sedentary behavior (Factor, Heller, & Janicki, 2012; Yamaki, 2005). If physical leisure activity is indeed a way to stave off AD neuropathology and/or buffer against its effects, then efforts to increase physical leisure may be a critical target for social policy and intervention in DS.
The benefit of social leisure (i.e., activities involving social interaction) is posited to occur through three mechanisms – increased cognitive stimulation, increased physical activity, and improved affect (e.g., happiness from being around others) (Di Marco et al., 2014). Evidence from non-DS populations suggests that individuals who engage in high (as opposed to low) levels of social leisure experience less cognitive decline in the face of AD neuropathology (Amieva et al., 2010; Bennett, Schneider, Tang, Arnold, & Wilson, 2006; Foubert-Samier et al., 2014). In a longitudinal study of 89 older adults without clinical AD, Bennett and colleagues (2006) found that older adults with larger social networks exhibited higher cognitive functioning even when they had greater AD neuropathology, as measured at brain autopsy. Foubert-Samier and colleagues (2014) similarly found that risk of clinical AD was lower for older adults who remained engaged in or who became engaged in social leisure activity over time (30% risk) compared to older adults who remained disengaged in (52% risk) or who became disengaged in social leisure over time (42% risk). For adults with DS, a population at risk for social isolation and having few friends (e.g., Krauss, Seltzer, & Goodman, 1992), the protective effects of social leisure activity may be of particular importance in delaying AD neuropathology (i.e., brain β-amyloid) and/or buffering against its effects on cognitive functioning.
In the present study, we examined associations between three domains of leisure activity (cognitively stimulating, physical, and social) and overall leisure activity and brain β-amyloid and cognitive functioning indicative of MCI at baseline and at follow-up (approximately 3 years later) in 65 adults with DS aged 30–53 years. To assess MCI, we focused on episodic memory, as this domain was previously shown to be related to early brain β-amyloid in DS (Hartley et al., 2014; Hartley et al., 2017) and is similarly one of the first cognitive domains affected by MCI in non-DS populations (e.g., Backman, Small, & Fratiglioni; 2001; Irish, Lawlor, Coen, & O’Mara, 2011). The study aims were to: 1) determine if leisure activity at baseline was associated with brain β-amyloid and episodic memory at baseline; 2) evaluate whether leisure activity at baseline predicted increases in brain β-amyloid and/or declines in episodic memory from baseline to follow-up; 3) examine whether leisure activity moderated the relation between change in brain β-amyloid and change in episodic memory from baseline to follow-up.
In line with findings from non-DS populations (e.g., Okonkwo et al., 2014), we hypothesized that greater engagement in all domains of leisure activity and overall leisure activity at baseline would be associated with less brain β-amyloid at baseline and smaller increases in brain β-amyloid from baseline to follow up. Similar to findings from non-DS populations (e.g., Foubert-Samier et al., 2014), greater engagement in all domains of leisure activity and overall leisure activity at baseline were predicted to be associated with higher episodic memory at baseline and smaller declines in episodic memory from baseline to follow-up. Finally, leisure activity was hypothesized to moderate the association between change in brain β-amyloid and change in episodic memory. Specifically, for adults with DS with low (as opposed to high) engagement in cognitively stimulating, physical, social, and overall leisure activity, greater increase in brain β-amyloid was expected to be more strongly associated with greater decline in episodic memory.
Method
Sample
The present study involved 65 adults with DS drawn from a larger, ongoing longitudinal study, conducted between 2010 and 2017 at the University of Wisconsin-Madison and at the University of Pittsburgh Medical Center, examining early AD neuropathology in DS. Adults with DS, who were recruited through fliers and postings on DS listservs and in DS clinics. Inclusion criteria included being over the age of 30 years and having a mental age of ≥ 30 months, genetic testing confirming DS, no conditions contraindicative for brain imaging scans (e.g., pregnant or breastfeeding, metal in the body, or a history of claustrophobia or other behavioral concerns), and no evidence of mental health symptoms impacting cognitive functioning. Additionally, at both baseline and follow-up, caregivers were interviewed using the Dementia Scale for Down syndrome (DSDS; Gedye, 1995), a dementia screener with a specificity rate of 0.90 and a sensitivity rate of 0.85 for adults with DS with mild to moderate intellectual disability. Participants could not exhibit clinical AD symptoms, defined as having a DSDS cognitive cutoff score of > 3. In addition, a clinical case consensus review process involving review of all caregiver reported measures by a physician and two clinical psychologists with expertise in DS was conducted for all participants to ensure that no one exhibited signs of clinical AD at either time point.
A leisure activity questionnaire was added to the study protocol in 2012. From the larger study sample of 85 adults with DS, 65 adults with DS completed the leisure activity questionnaire at baseline (i.e., baseline visit was in 2012 or later). Independent samples t-tests and chi-square statistics indicated that the 65 participants included in the present analyses did not significantly differ from the larger study sample in chronological age, mental age, race/ethnicity, gender, or brain β-amyloid at baseline. Of these 65 participants, 11 did not have a follow-up visit (2 dropped out of the study and 9 will be tracked at a later time point in the larger ongoing study). Neuropsychological data was collected on the 65 participants at baseline and the remaining 54 participants at follow-up. However, only 41 of the 54 participants had usable brain imaging data at both time points (13 participants had excessive motion in magnetic resonance imagining [MRI] scans at either baseline or follow-up). Therefore, analyses examining change in brain β-amyloid only included these 41 participants. Independent samples t-tests and chi-square statistics indicated that there were no significant differences at baseline in chronological age, mental age, race/ethnicity, gender, or brain β-amyloid between the 65 participants at baseline and the 24 participants who did not complete a follow-up visit or who did not have useable brain imaging data at both time points.
Procedure
Along with their caregivers, the adults with DS participated in two cycles of data collection, approximately 3 years apart. At each cycle, the adults with DS completed a 2.5-hour neuropsychological battery, including assessments of episodic memory, and caregivers completed questionnaires regarding sociodemographic information and the leisure activity of the adults with DS. The adults with DS then underwent MRI and positron emission tomography (PET) scans to assess brain β-amyloid. The adults with DS received $250/cycle for their participation. Table 1 displays sociodemographic information for the 65 adults with DS who participated at baseline and the 54 adults with DS who returned at follow-up. At baseline, adults with DS were aged 30–53 years (M = 37.87, SD = 7.37), and had an average mental age of 5.52 years. Approximately half were male (52.31%) and the majority lived with family (66.15%).
Table 1.
Sociodemographic Characteristics of Adults with Down Syndrome at Baseline and Follow-Up
| Baseline (n=65) | Follow-Up (n=54) | |
|---|---|---|
| Chronological Age in years (M [SD]) | 37.87 (7.37) | 39.85 (6.96) |
| Range | 30.00 – 53.83 | 32.42 – 56.83 |
| Mental age in years (M [SD]) | 5.52 (1.51) | 5.58 (1.77) |
| Range | 2.00 – 10.00 | 2.50 – 10.00 |
| Gender (n [%]) | ||
| Female | 31 (47.69) | 26 (48.15) |
| Male | 34 (52.31) | 28 (51.85) |
| Ethnicity (n [%]) | ||
| White non-Hispanic | 65 (100.00) | 54 (100.00) |
| Residence (n [%]) | ||
| Family | 43 (66.15) | 33 (61.11) |
| Group home | 6 (9.23) | 6 (11.11) |
| Supported apartment | 9 (13.85) | 7 (12.96) |
| Independently | 7 (10.77) | 8 (14.81) |
| Employment (n [%]) | ||
| Full or part time | 21 (32.31) | 16 (29.63) |
| Full or part time with support | 13 (20.00) | 9 (16.67) |
| Supported workshop | 20 (30.78) | 11 (20.37) |
| Volunteer | 7 (10.77) | 6 (11.11) |
| Not employed | 4 (6.15) | 3 (5.56) |
| Missing | 0 (0.0) | 9 (16.67) |
| PiB Retention (M [SD]) | ||
| Global | 1.15 (0.16) | 1.23 (0.27) |
| PiB Status | ||
| PiB− | 52 (80.00) | 36 (66.67) |
| PiB+ | 5 (7.69) | 10 (18.52) |
| Missing | 8 (12.31) | 8 (14.81) |
Measures
Control variables.
Caregivers reported on the chronological age (in years) of the adults with DS. The Stanford-Binet, Fifth Edition Abbreviated Battery (SB5; Roid, 2003), which has previously been used with adults with DS (Couzens, Cuskelly, & Haynes, 2011), was administered to obtain an estimate of mental age (in years). Additionally, caregivers reported on the presence (coded 1) versus absence (coded 0) of 15 physical health problems (e.g., heart problems and diabetes). The total number of physical health problems was summed.
Episodic memory.
Two measures were used to assess episodic memory. The Cued Recall Test (Zimmerli & Devenny, 1995), previously shown to be sensitive to clinical AD symptoms in the DS population (Zimmerli & Devenny, 1995), was used to measure verbal episodic memory. The Free and Cued Recall (range 0 – 36) and Cued Recall Intrusions scores were used in analyses. Free and Cued Recall is the number of objects the adults with DS correctly named when asked to recall a list of objects without (free) and with (cued) object domain cues. Cued Recall Intrusions is the number of incorrect objects named during the cued recall trials. The Pictures Recognition subtest of the Rivermead Behavioral Memory Test for Children (RBMT; Wilson, Ivani-Chalian, & Aldrich, 1991) was used to assess visual episodic memory. During this task, the adults with DS were asked to identify the pictures they had learned 10 minutes prior, from a series of pictures (10 new, 10 previously learned). Pictures Recognition is the number of pictures correctly identified as having been previously learned minus the number of false positives (pictures incorrectly identified as having been previously learned).
Leisure activity.
Caregivers completed the Victoria Longitudinal Study activity questionnaire (VLS; Jopp & Hertzog, 2007) to assess the leisure activity of the adults with DS throughout the previous year. The VLS consists of 52 leisure activity items. Frequency of participation was rated using a 9-point scale: never (0), less than once a year (1), about once a year (2), 2 or 3 times a year (3), about once a month (4), 2 or 3 times a month (5), about once a week (6), 2 or 3 times a week (7), and daily (8). Items from these subscales were placed into the following domains: cognitively stimulating (game, experiential, and developmental subscales), physical (physical subscale), and social (social-private, social-public, and religious subscales). Total domain scores were summed across items (each rated on the 9-point scale) in each domain. An overall leisure activity total score was calculated by summing across the cognitively stimulating, physical, and social leisure domains. The VLS has been used with adults with DS and shown to have adequate internal consistency (Mihaila et al., 2017).
Brain β-amyloid.
Brain β-amyloid was assessed through level of retention of the radiotracer 11C-PiB (a continuous variable – standardized uptake value ratio [SUVR]) as indicated by PET scan. The measurement of 11C-PiB retention was obtained through the following procedure. First, structural T1-weighted 3 Tesla MRI scans were obtained using GE Medical Systems MRI scanners at the University of Wisconsin-Madison site and Siemens Magnetom Trio MRI scanners at the University of Pittsburgh Medical Center site. MRI data was used for PET-MRI registration to identify regions of interest and guide processing of PET scans. The adults with DS then received an intravenous injection of up to 15 mCi of the radiotracer 11C-PiB, while resting comfortably in the PET prep room. After a 35-minute uptake period, the adults with DS were positioned in a Siemens ECAT EXACT HR+ PET scanner (both sites) for a 30-minute time series acquisition scan initiated at 40 minutes post-injection. Following this scan, a 6 to 10-minute windowed transmission scan was obtained to correct for the attenuation of the annihilation radiation. PET data was then reconstructed using filtered back-projection and corrected for photon attenuation, deadtime, normalization, scatter, and radioactive decay (Lao et al., 2016).
Each T1-weighted MRI scan was processed and analyzed using the recon-all script of FreeSurfer version 5.3 (Fischl, 2012). Nine regions of interest were obtained from FreeSurfer’s white matter parcellation atlas (wmparc), including the orbito frontal cortex, superior frontal cortex, anterior cingulate gyrus, posterior cingulate gyrus, parietal cortex, lateral temporal cortex, insula, precuneus cortex, and the striatum. Using automated methods, PET-MRI registration was performed (Minoshima et al., 1993). Retention of 11C-PiB was measured using the SUVR determined over the 50 to 70-minute interval post-injection and using the cerebellar gray matter as the reference region. A Global PiB score was obtained by calculating the volume-weighted average 11C-PiB retention across the nine brain regions and was used to examine brain β-amyloid. The Global PiB score is heavily weighted for cortical PiB retention, however, it also includes striatal PiB retention, as it has been suggested that for adults with DS, brain β-amyloid may first accumulate in the striatum (Handen et al., 2012).
Data Analysis Plan
Descriptive statistics, including histograms of the residuals and quantile-comparison plots were conducted to examine the normality of data. Multiple linear regressions were conducted to examine associations between the leisure activity domains (cognitively stimulating, physical, and social) and overall leisure activity at baseline, and brain β-amyloid at baseline and change in brain β-amyloid from baseline to follow-up. Multiple linear regressions were also conducted to examine associations between leisure activity domains and overall leisure activity at baseline, and each measure of episodic memory (Free and Cued Recall Total, Cued Recall Intrusions, and Pictures Recognition) at baseline and change in each measure of episodic memory from baseline to follow-up. Control variables significantly associated with baseline leisure activity, episodic memory, and/or brain β-amyloid were included in models.
To address the final study aim, multiple linear regressions were used to examine the moderating effect of each domain of leisure activity and overall leisure activity at baseline on associations between change in brain β-amyloid from baseline to follow-up and change in episodic memory from baseline to follow-up. Models were run separately for each domain of leisure activity and overall leisure activity. The dependent variables were change in the measures of episodic memory. The independent variables were leisure activity at baseline and change in brain β-amyloid. Moderation interaction terms were created by calculating leisure activity at baseline X change in brain β-amyloid. Where significant moderating effects were found, the methods of Aiken and West (1991) were used to graph effects based on low (below the mean) and high (above the mean) leisure activity. In all analyses, leisure variables and Global PiB scores were z-scored to better capture sample variability and their interaction in moderation models. An alpha level of p ≤ .05 was used to assess significance. Multiple comparisons were not controlled for as only subtle changes are theorized to occur in the MCI stage. Thus, findings must be considered with caution.
Results
Distributions of study variables, histograms of residuals, and Shapiro-Wilk’s tests (p > .05) revealed that mental age and cognitively stimulating, physical, social, and overall leisure activity had data with normal distribution. However, the episodic memory variables had data with non-normal distribution. Free and Cued Recall had negative skewness of −3.72 (SE = .30) and kurtosis of 16.24 (SE = .59), Cued Recall Intrusions had positive skewness of 3.28 (SE = .30) and kurtosis of 12.70 (SE = .59), and Pictures Recognition had negative skewness of −.38 (SE = .30) and kurtosis of −1.26 (SE = .59). Log transformations, to correct for positive and negative skew, were performed on episodic memory variables. For the purpose of enhancing interpretability when graphing interactions, change in Cued Recall Intrusions and change in brain β-amyloid values were negated, such that greater negative change values are represented as positive, indicating greater decline.
Chronological age was included as a control variable in regressions given its significant association with brain β-amyloid at baseline (r = 0.63, p < .01). Mental age was also included as a control variable in regressions given its significant associations with episodic memory (Free and Cued Recall: r = 0.48, p < .01; Cued Recall Intrusions: r = −0.43, p < .01; and Pictures Recognition: r = 0.54, p < .01) and leisure activity (cognitively stimulating: r = 0.39, p < .01; physical: r = 0.12, p = .35; social: r = 0.29, p = .02 and overall: r = 0.39, p < .01) at baseline. Total number of physical health problems was not significantly associated with leisure activity (cognitively stimulating: r = 0.07, p = .58; physical: r = 0.05, p = .71; social: r = 0.11, p = .40 and overall: r = 0.10, p = .44), episodic memory (Free and Cued Recall: r = −0.19, p = .14; Cued Recall Intrusions: r = 0.07, p = .57; and Pictures Recognition: r = −0.12, p = .33), or brain β-amyloid at baseline (r = 0.04, p = .79), and thus not included in analyses.
Table 2 presents the results of analyses examining associations between the domains of leisure activity and overall leisure activity at baseline and brain β-amyloid at baseline and change in brain β-amyloid from baseline to follow-up. There were no significant associations. Table 3 presents the results of analyses between the domains of leisure activity and overall leisure activity at baseline and episodic memory at baseline. Greater cognitively stimulating and overall leisure were significantly associated with higher Free and Cued Recall at baseline. All remaining associations between leisure activity and episodic memory at baseline were not significant. Table 3 also shows associations between the domains of leisure activity and overall leisure activity at baseline and change in episodic memory from baseline to follow-up. Greater social leisure at baseline was significantly associated with smaller declines in Pictures Recognition from baseline to follow-up. All remaining associations were not significant.
Table 2.
Associations between Leisure Activity at Baseline and Brain β-amyloid at Baseline and Change in Brain β-amyloid from Baseline to Follow-Up
| Baseline β-amyloid | Change in β-amyloid | |
|---|---|---|
| β (SE) | β (SE) | |
| Cognitively Stimulating Leisure Activity | ||
| Constant | (0.84)** | (0.96)** |
| Chronological Age | 0.66 (0.02)** | −0.75 (0.02)** |
| Mental Age | 0.15 (0.08) | −0.25 (0.09) |
| Cognitively Stimulating Leisure | −0.13 (0.12) | 0.16 (0.12) |
| Social Leisure Activity | ||
| Constant | (0.80)** | (0.94)** |
| Chronological Age | 0.66 (0.02)** | −0.75 (0.02)** |
| Mental Age | 0.12 (0.08) | −0.15 (0.08) |
| Social Leisure | −0.08 (0.12) | −0.06 (0.12) |
| Physical Leisure Activity | ||
| Constant | (0.81)** | (0.95)** |
| Chronological Age | 0.66 (0.02)** | −0.76 (0.02)** |
| Mental Age | 0.10 (0.07) | −0.16 (0.08) |
| Physical Leisure | −0.02 (0.11) | −0.00 (0.12) |
| Overall Leisure Activity | ||
| Constant | (0.82)** | (0.96)** |
| Chronological Age | 0.65 (0.02)** | −0.74 (0.02)** |
| Mental Age | 0.14 (0.08) | −0.22 (0.09) |
| Overall Leisure | −0.11 (0.12) | 0.09 (0.12) |
Note.
p<.10
p < .05,
p< .01.
Table 3.
Associations between Leisure Activity at Baseline and Episodic Memory at Baseline and Change in Episodic Memory from Baseline to Follow-Up
| Episodic Memory at Baseline | Change in Episodic Memory from Baseline to Follow-Up | |||||
|---|---|---|---|---|---|---|
| Free and Cued Recall | Cued Recall Intrusions | Pictures Recognition | Free and Cued Recall | Cued Recall Intrusions | Pictures Recognition | |
| β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | |
| Cognitively Stimulating Leisure Activity | ||||||
| Constant | (0.29)** | (0.09)** | (0.25)** | (0.19)** | (0.20)** | (0.26)† |
| Chronological Age | 0.24 (0.01)* | 0.12 (0.00) | 0.18 (0.01) | −0.24 (0.00) | −0.33 (0.00)* | 0.06 (0.01) |
| Mental Age | −0.36 (0.03)** | −0.36 (0.01)** | −0.41 (0.02)** | −0.13 (0.02) | −0.20 (0.02) | 0.03 (0.02) |
| Leisure | −.26 (0.04)* | −0.11 (0.01) | −0.11 (0.03) | −0.19 (0.03) | 0.14 (0.03) | 0.15 (0.03) |
| Social Leisure Activity | ||||||
| Constant | (0.29)† | (0.80)** | (0.25)** | (0.19)** | (0.20)** | (0.25)* |
| Chronological Age | 0.24 (0.01)* | 0.12 (0.00) | 0.18 (0.01) | −0.22 (0.00) | −0.30 (0.00)* | 0.04 (0. 01) |
| Mental Age | −0.41 (0.03)** | −0.36 (0.01)** | −0.47 (0.02)** | −0.15 (0.02) | −0.14 (0.02) | −0.00 (0.02) |
| Leisure | −0.19 (0.04)† | −0.19 (0.01) | 0.09 (0.03) | −0.18 (0.02) | −0.04 (0.03) | 0.31 (0.03) * |
| Physical Leisure Activity | ||||||
| Constant | (0.30)* | (0.09)** | (0.25)** | (0.19)** | (0.20)** | (0.25) |
| Chronological Age | 0.24 (0.01)* | 0.11 (0.00) | 0.19 (0.01) | −0.24 (0.00) | −0.31 (0.00)* | 0.09 (0.01) |
| Mental Age | −0.46 (0.03)** | −0.40 (0.01)** | −0.45 (0.02)** | −0.18 (0.02) | −0.15 (0.02) | 0.09 (0.02) |
| Leisure | 0.02 (0.04) | −0.02 (0.01) | 0.02 (0.03) | 0.15 (0.02) | 0.02 (0.03) | 0.15 (0.03) |
| Overall Leisure Activity | ||||||
| Constant | (0.29)† | (0.08)** | (0.25)** | (0.19)** | (0.20)** | (0.25)* |
| Chronological Age | 0.23 (0.01)* | 0.11 (0.00) | 0.18 (0.01) | −0.24 (0.00)† | −0.32 (0.00)* | 0.06 (0.01) |
| Mental Age | −0.38 (0.03)** | −0.35 (0.01)** | −0.44 (0.02)** | −0.15 (0.02) | −0.17 (0.02) | −0.00 (0.02) |
| Leisure | −0.23 (0.04)* | −0.15 (0.01) | −0.02 (0.03) | −0.14 (0.03) | 0.07 (0.03) | 0.26 (0.03)† |
Note.
p<.10,
p < .05,
p< .01.
Note. Due to log transformation of all episodic memory variables, greater values at baseline indicate worse performance at baseline. Greater change from baseline to follow-up values indicate less decline in episodic memory.
Table 4 displays analyses examining the moderating effect of the domains of leisure activity and overall leisure activity at baseline on the relation between change in brain β-amyloid and change in episodic memory from baseline to follow-up. Social leisure significantly altered the association between change in brain β-amyloid and change in Cued Recall Intrusions (β = − .41, p = .01). Overall leisure at baseline significantly altered the association between change in brain β-amyloid and change in Free and Cued Recall (β = −.42, p < .01) and the association between change in brain β-amyloid and change in Cued Recall Intrusions (β = −.46, p < .01). As shown in Figures 1 and 3, for adults with DS who engaged in high (above the mean) social (scores range: 50 – 74; 24 participants) and overall (scores range: 115 – 185; 20 participants) leisure activity at baseline, there was not an association between change in brain β-amyloid and change in Cued Recall Intrusions, across time points. However, for adults who engaged in low (below the mean) social (scores range: 19 – 49; 17 participants) and overall (scores range: 52 – 114; 21 participants) leisure at baseline, greater increase in brain β-amyloid from baseline to follow-up was associated with greater increase (i.e., decline in performance) in Cued Recall Intrusions from baseline to follow up. As shown in Figure 2, for adults with DS who engaged in high (above the mean) overall leisure activity at baseline, there was not an association between change in brain β-amyloid and change in Free and Cued Recall across time points. However, for adults who engaged in low (below the mean) overall leisure at baseline, greater increase in brain β-amyloid from baseline to follow-up was associated with greater decline in Free and Cued Recall from baseline to follow-up. The remaining moderation models were not significant.
Table 4.
Leisure Activity at Baseline as a Moderator Between Change in Brain β-amyloid and Change in Episodic Memory from Baseline to Follow-Up
| Change Free and Cued Recall | Change Cued Intrusions | Change Pictures Recognition | |
|---|---|---|---|
| β (SE) | β (SE) | β (SE) | |
| Cognitively Stimulating Leisure Activity | |||
| Constant | (0.30)** | (0.22)** | (0.35) |
| Chronological Age | −0.11 (0.01) | −0.12 (0.00) | 0.08 (0.01) |
| Mental Age | −0.31 (0.02)† | −0.17 (0.02) | −0.07 (0.03) |
| Leisure | 0.24 (0.03) | 0.32 (0.02)* | 0.18 (0.04) |
| Change β-amyloid | 0.24 (0.04) | 0.19 (0.03) | −0.01 (0.05) |
| Leisure X Change β-amyloid | −0.25 (0.02) | −0.24 (0.02) | −0.12 (0.03) |
| Social Leisure Activity | |||
| Constant | (0.30)** | (0.21)** | (0.32) |
| Chronological Age | −0.06 (0.01) | −0.05 (0.00) | 0.11 (0.01) |
| Mental Age | −0.18 (0.02) | −0.01 (0.02) | −0.03 (0.02) |
| Leisure | 0.21 (0.03) | 0.28 (0.02)† | 0.42 (0.03)* |
| Change β-amyloid | 0.45 (0.04)* | 0.47 (0.03)* | 0.14 (0.05) |
| Leisure X Change β-amyloid | −0.29 (0.04)† | −0.41 (0.03)* | −0.13 (0.05) |
| Physical Leisure Activity | |||
| Constant | (0.31)* | (0.23)** | (0.34) |
| Chronological Age | −0.06 (0.01) | −0.07 (0.01) | 0.18 (0.01) |
| Mental Age | −0.13 (0.02) | 0.03 (0.02) | −0.04 (0.02) |
| Leisure | 0.15 (0.03) | 0.07 (0.02) | 0.01 (0.04) |
| Change β-amyloid | 0.31 (0.04) | 0.28 (0.03) | 0.10 (0.05) |
| Leisure X Change β-amyloid | −0.16 (0.05) | −0.16 (0.03) | 0.26 (0.05) |
| Overall Leisure Activity | |||
| Constant | (0.28)** | (0.20)** | (0.34)† |
| Chronological Age | −0.11 (0.01) | −0.11 (0.00) | 0.09 (0.01) |
| Mental Age | −0.29 (0.02)† | −0.14 (0.01) | −0.08 (0.02) |
| Leisure | 0.35 (0.03)* | 0.39 (0.02)* | 0.33 (0.04)† |
| Change β-amyloid | 0.27 (0.04) | 0.23 (0.03) | 0.01 (0.05) |
| Leisure X Change β-amyloid | −0.42 (0.04)** | −0.46 (0.03)** | −0.13 (0.04) |
Note.
p<.10,
p < .05,
p< .01.
Note. Due to log transformation of all episodic memory variables, greater change from baseline to follow-up values indicate less decline in episodic memory.
Figure 1.
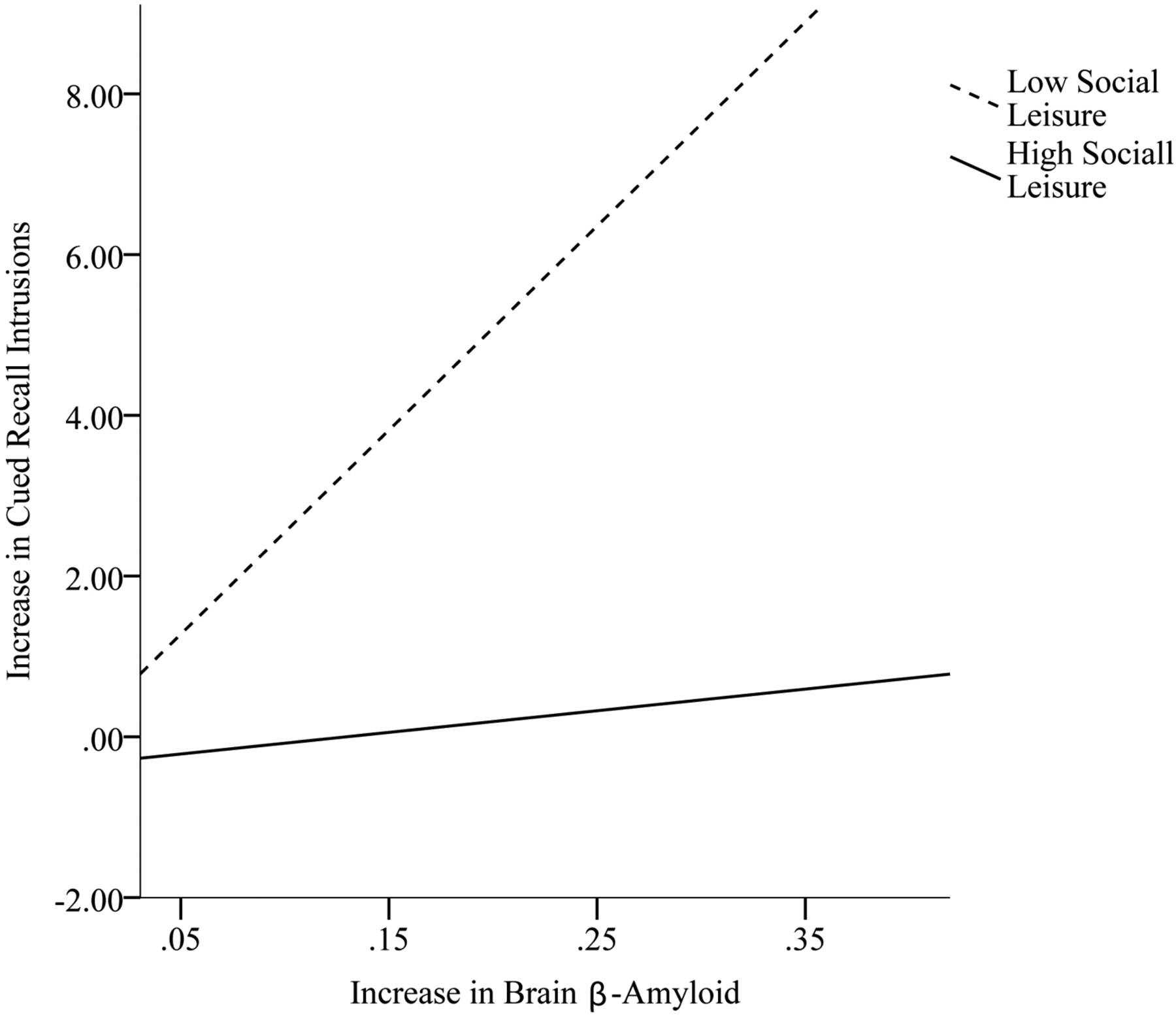
Social leisure activity at baseline as a moderator in the association between change in brain β-amyloid and change in Cued Recall Intrusions from baseline to follow-up.
Figure 3.
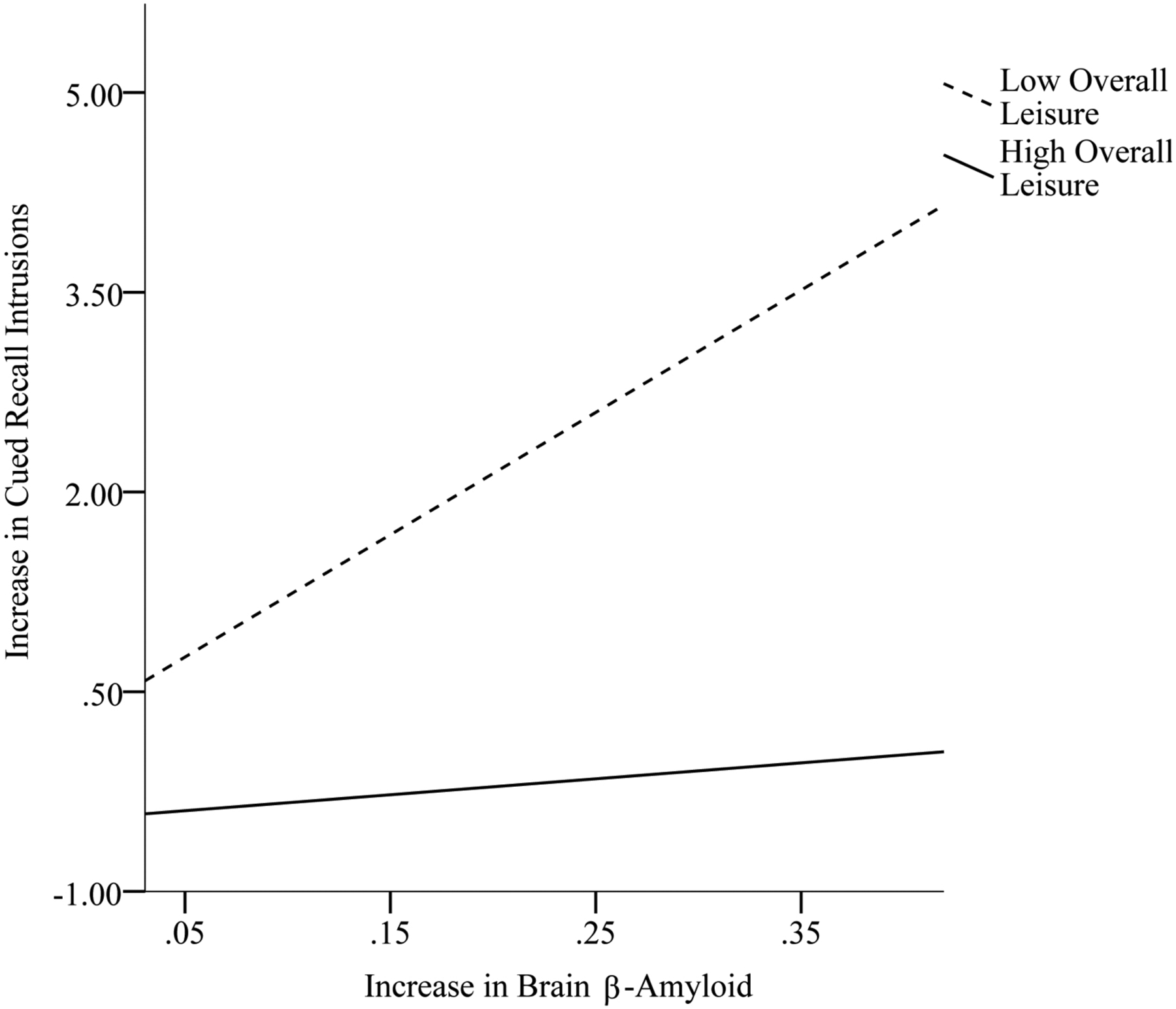
Overall leisure activity at baseline as a moderator in the association between change in brain β-amyloid and change in Cued Recall Intrusions from baseline to follow-up.
Figure 2.
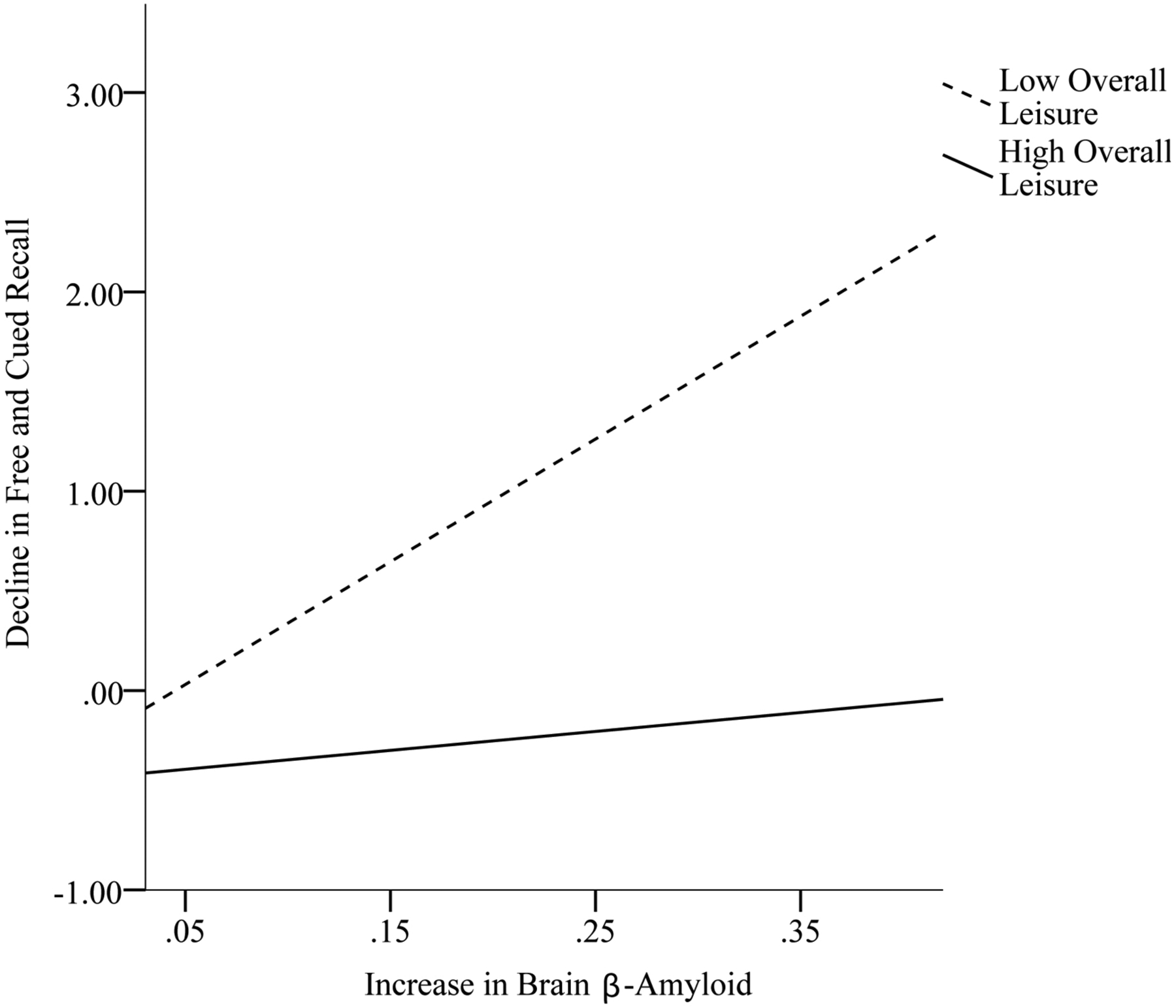
Overall leisure activity at baseline as a moderator in the association between change in brain β-amyloid and change in Free and Cued Recall from baseline to follow-up.
Discussion
Adults with DS have a heightened rate and early onset of AD relative to non-DS populations, purportedly due to having a third copy of chromosome 21 (Zigman et al., 2008). Yet, there is marked variability in the onset and rate of early AD neuropathology across adults with DS who share this genotype (Lao et al., 2016). In non-DS populations, leisure activity has been theorized to be a lifestyle factor that may, in part, explain variability in the progression from normative cognitive functioning to MCI and then finally to clinical AD (e.g., Amieva et al., 2010; Landau et al., 2012). As a group, adults with DS have been found to engage in limited leisure activity relative to non-DS populations (e.g., Krauss et al., 1992; Mihaila et al., 2017; Yamaki, 2005). The present study provides the first examination of whether leisure activity is associated with early AD neuropathology (i.e., brain β-amyloid) and declines in episodic memory in healthy (i.e., without clinical AD) adults with DS.
Overall, we found that leisure activity at baseline was not related to brain β-amyloid at baseline or change in brain β-amyloid from baseline to follow-up in adults with DS. Thus, in the present sample, engaging in leisure activity was not related to lower brain β-amyloid accumulation in adults with DS. However, there was evidence that leisure activity may play a role in the healthy aging of adults with DS by delaying decline in episodic memory. Specifically, cognitively stimulating and overall leisure activity were related to better episodic memory at baseline, and social leisure was related to smaller declines in episodic memory from baseline to follow-up. These associations were found in models controlling for both chronological and mental age. Together, these findings suggest that engagement in leisure activity, and in particular, cognitively stimulating (e.g., puzzles and computer games) and social (e.g., outing with a friend) leisure activity, may reduce the rate of decline in episodic memory in adults with DS.
We also found evidence that leisure activity moderates the association between early brain β-amyloid accumulation and episodic memory declines in adults with DS. In line with our hypothesis, social and overall leisure activity moderated the association between change in brain β-amyloid and decline in episodic memory across the 3-year study period. For adults with DS who engaged in high (as opposed to low) levels of social and overall leisure activity, increased brain β-amyloid across the 3 years was not associated with decline in episodic memory. In contrast, for adults with DS who engaged in low levels of social and overall leisure activity, increased brain β-amyloid across the 3 years was associated with decline in episodic memory over the same period. Thus, while higher engagement in social and overall leisure activity was not directly related to level of brain β-amyloid, these activities may mitigate the deleterious effects of brain β-amyloid on episodic memory.
Overall, these findings build on evidence from non-DS populations that leisure activity may be an important lifestyle factor in understanding why certain individuals maintain normative cognitive functioning over time, despite AD neuropathology, whereas others transition more rapidly to MCI (e.g., Bennett et al., 2014; Stern, 2009; Wilson et al., 2007). In adults with DS, cognitively stimulating and social leisure activity may be particularly salient domains of leisure that contribute to healthy aging and should be targeted in social policies and interventions. It is unclear why physical leisure activity was not related to episodic memory in our sample of adults with DS, particularly given findings of its importance in non-DS populations (e.g., Okonkwo et al., 2014). It is possible that our global, caregiver-reported measure of physical leisure activity did not effectively capture differences in physical leisure activity and/or adults with DS did not engage in much physical activity. Future studies in adults with DS should use accelerometers to capture actual levels of physical activity. Lastly, it is unclear why leisure activity had more robust associations with the Cued Recall test than with the Picture Recognition test. It is possible that verbal episodic memory (Cued Recall) is more strongly related to leisure activity than visual episodic memory (Picture Recognition). Alternatively, the Picture Recognition test may be a less sensitive measure of episodic memory, as it had a more restricted range than the Cued Recall test.
The present study has several strengths. Due to its longitudinal design and the use of a battery of direct measures of episodic memory, it was possible to capture subtle changes over time. Analyses were conducted controlling for chronological and mental age. There were also a number of limitations. At baseline, the sample was restricted to adults with DS over the age of 30 years who had a mental age ≥ 30 months and who exhibited no clinical signs of AD. Findings may not generalize outside of this population. It is possible that leisure activity plays a different role both earlier and/or later on in the trajectory of AD neuropathology. Additionally, the adults with DS in our sample did not have mental health symptoms deemed to be severe enough to impair cognitive functioning. However, it is possible that even mild mental health symptoms (e.g., depressive symptoms) alter leisure activity participation; this should be explored in future studies. Next, while the present study incorporated two cycles of data collection of brain β-amyloid and episodic memory, engagement in leisure activity was only captured at baseline; thus, it was not possible to disentangle how change in leisure activity across the 3-year period may have played a role in associations between brain β-amyloid and episodic memory. Moreover, the present data do not allow us to make statements about causality; it is possible that having a lower baseline leisure activity level may have been the result of earlier declines in episodic memory and/or increased β-amyloid that then persisted across time. Finally, the present study did not control for multiple comparisons as only subtle changes may occur in the transitionary period from normative cognitive functioning to MCI. Findings should be considered with caution until replicated.
In summary, the present study is the first to examine the relation between leisure activity and brain β-amyloid and MCI in adults with DS, a population at genetic risk for both AD (Zigman et al., 2008) and low leisure activity (e.g., Mihaila et al., 2017). Findings suggest that cognitively stimulating and social leisure activity may alter the association between early brain β-amyloid on declines in episodic memory in the DS population. These findings suggest that the DS population provides a unique scientific opportunity to model the impact of lifestyle factors on AD and may afford insights relevant for other populations. Leisure activity can be thought of as a proxy for underlying structural and functional brain mechanisms which are thought to drive associated benefits in delaying AD (Bennett et al., 2014; Okonkwo et al., 2014). Future longitudinal research should be aimed at examining the association between these underlying mechanisms and biomarkers of AD neuropathology and episodic memory in the DS population.
References
- Aiken LS, & West SG (1991). Multiple Regression: Testing and Interpreting Interactions. Newbury Park, CA: Sage Publications. [Google Scholar]
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas Nicholas D., … Klunk WE (2008). Frequent amyloid deposition without significant cognitive impairment among the elderly. Archives of Neurology, 65, 1509–1517. doi: 10.1001/archneur.65.11.1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amieva H, Stoykova R, Matharan F, Helmer C, Antonucci TC, & Dartigues J-F (2010). What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosomatic Medicine, 72(9), 905–911. doi: 10.1097/PSY.0b013e3181f5e121 [DOI] [PubMed] [Google Scholar]
- Bäckman L, Small BJ, & Fratiglioni L (2001). Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain: A Journal of Neurology, 124, 96–102. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Arnold SE, Valenzuela MJ, Brayne C, & Schneider JA (2014). Cognitive and social lifestyle: links with neuropathology and cognition in late life. Acta Neuropathologica, 127(1), 137–150. doi: 10.1007/s00401-013-1226-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Tang Y, Arnold SE, & Wilson RS (2006). The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. The Lancet Neurology, 5, 406–412. doi: 10.1016/S1474-4422(06)70417-3 [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, & Christie LA (2007). Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in Neurosciences, 30, 464–472. doi: 10.1016/j.tins.2007.06.0111 [DOI] [PubMed] [Google Scholar]
- Couzens D, Cuskelly M, & Haynes M (2011). Cognitive development and Down syndrome: Age-related change on the Stanford-Binet Test (Fourth edition). American Journal on Intellectual and Developmental Disabilities, 116, 181–204. doi: 10.1352/1944-7558-116.3.181 [DOI] [PubMed] [Google Scholar]
- Di Marco LY, Marzo A, Muñoz-Ruiz M, Ikram MA, Kivipelto M, Ruefenacht D, … Frangi AF (2014). Modifiable Lifestyle Factors in Dementia: A Systematic Review of Longitudinal Observational Cohort Studies. Journal of Alzheimer’s Disease, 42(1), 119–135. doi: 10.3233/JAD-132225 [DOI] [PubMed] [Google Scholar]
- Dougherty RJ, Ellingson LD, Schultz SA, Boots EA, Meyer JD, Lindheimer JB, … Cook DB (2016). Meeting physical activity recommendations may be protective against temporal lobe atrophy in older adults at risk for Alzheimer’s disease. Alzheimer’s & Dementia (Amsterdam, Netherlands), 4, 14–17. doi: 10.1016/j.dadm.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Leckie RL, & Weinstein AM (2014). Physical activity, fitness, and gray matter volume. Neurobiology of Aging, 35, S20–S28. doi: 10.1016/j.neurobioloaging.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor A, Heller T, & Janicki M (2012). Bridging the aging and developmental disabilities service networks: Challenges and best practices. Chicago: Institute on Disability and Human Development, University of Illinois at Chicago. [Google Scholar]
- Fischl B (2012). FreeSurfer. NeuroImage, 62(2), 774–781. doi: 10.10.16/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foubert-Samier A, Le Goff M, Helmer C, Pérès K, Orgogozo JM, Barberger-Gateau P, … Dartigues JF (2014). Change in leisure and social activities and risk of dementia in elderly cohort. The Journal of Nutrition, Health and Aging, 18, 876–882. doi: 10.1007/s12603-014-0475-7 [DOI] [PubMed] [Google Scholar]
- Gedye A (1995). Dementia Scale for Down’s Syndrome: Manual. Vancouver, BC: Gedye Research and Counseling. [Google Scholar]
- Handen BL, Cohen AD, Channamalappa U, Bulova P, Cannon SA, Cohen WI, … Klunk WE (2012). Imaging brain amyloid in nondemented young adults with Down syndrome using Pittsburgh compound B. Alzheimer’s and Dementia, 8, 496–501. doi: 10.1016/j.jalz.2011.09.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SL, Handen BL, Devenny DA, Hardison R, Mihaila I, Price JC, … Christian BT (2014). Cognitive functioning in relation to brain amyloid-β in healthy adults with Down syndrome. Brain: A Journal of Neurology, 137, 2556–2563. doi: 10.1093/brain/awu173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SL, Handen BL, Devenny D, Mihaila I, Hardison R, Lao PJ, … Christian BT (2017). Cognitive decline and brain amyloid-β accumulation across 3 years in adults with Down syndrome. Neurobiology of Aging, 58, 68–76. doi: 10.1016/j.neurobiolaging.2017.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, & Lindenberger U (2008). Enrichment Effects on Adult Cognitive Development: Can the Functional Capacity of Older Adults Be Preserved and Enhanced? Psychological Science in the Public Interest, 9, 1–65. doi: 10.1111/j.1539-6053.2009.01034.x [DOI] [PubMed] [Google Scholar]
- Irish M, Lawlor BA, Coen RF, & O’Mara SM (2011). Everyday episodic memory in amnestic mild cognitive impairment: a preliminary investigation. BMC Neuroscience, 12, 80. doi: 10.1186/1471-2202-12-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jopp DS, & Hertzog C (2007). Activities, self-referent memory beliefs, and cognitive performance: Evidence for direct and mediated relations. Psychology and Aging, 22, 811–825. doi: 10.1037/0882-7974.22.4.811 [DOI] [PubMed] [Google Scholar]
- Krauss MW, Seltzer MM, & Goodman SJ (1992). Social support networks of adults with mental retardation who live at home. American Journal of Mental Retardation, 96, 432–441. [PubMed] [Google Scholar]
- Landau SM, Marks SM, Mormino EC, Rabinovici GD, Oh H, O’Neil JP, … Jagust WJ (2012). Association of lifetime cognitive engagement and low β-amyloid deposition. Archives of Neurology, 69, 623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao PJ, Betthauser TJ, Hillmer AT, Price JC, Klunk WE, Mihaila I, … Christian BT (2016). The effects of normal aging on amyloid-β deposition in nondemented adults with Down syndrome as imaged by carbon 11-labeled Pittsburgh compound B. Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association, 12, 380–390. doi: 10.1016/j.jalz.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay J, Laurin D, Verreault R, Hébert R, Helliwell B, Hill GB, & McDowell I (2002). Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. American Journal of Epidemiology, 156, 445–453. [DOI] [PubMed] [Google Scholar]
- McCarron M, McCallion P, Reilly E, & Mulryan N (2014). A prospective 14-year longitudinal follow-up of dementia in persons with Down syndrome. Journal of Intellectual Disability Research, 58, 61–70. doi: 10.1111/jir.12074 [DOI] [PubMed] [Google Scholar]
- Mihaila I, Hartley SL, Handen BL, Bulova PD, Tumuluru RV, Devenny DA, … Christian BT (2017). Leisure Activity in Middle-Aged and Older Adults with Down Syndrome. Intellectual and Developmental Disabilities, 55, 97–109. doi: 10.1352/1934-9556-55.2.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Koeppe RA, Mintun MA, Berger KL, Taylor SS, Frey KA, & Kuhl DE (1993). Automated detection of the intercommissural (AC-PC) line for stereotactic localization of functional brain images. Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine, 34, 322–329. [PubMed] [Google Scholar]
- Okonkwo OC, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, … Sager MA (2014). Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology, 83, 1753–1760. doi: 10.1101/cshperspect.a006239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S, Scholl M, Strandberg O, Mattsson N, Stomrud E, Zetterberg H, … Hansson O (2017). Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nature Communications, 8, 1–13. doi: 10.1038/s41467-017-01150-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SE, Mai CT, Canfield MA Rickard R, Wang Y, Meyer RE, … Correa A (2010). Updated national birth prevalence estimates for selected bird defects in the United States, 2004–2005. Birth Defects Research Part A: Clinical and Molecular Teratology, 88, 1008–1016. doi: 10.1002/bdra.20735 [DOI] [PubMed] [Google Scholar]
- Petersen RC (2003). Mild cognitive impairment: Aging to Alzheimer’s disease. New York, NY US: Oxford University Press. [Google Scholar]
- Roid GH (2003). Stanford-Binet Intelligence Scales, 5th Edition Itasca, IL: Riverside. [Google Scholar]
- Sperling R, Mormino E, & Johnson K (2014). The evolution of preclinical Alzheimer’s disease: Implications for prevention trials. Neuron, 84, 608–622. doi: 10.1016/j.neuron.2014.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y (2009). Cognitive reserve. Neuropsychologia, 47(10), 2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y (2012). Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurology, 11, 1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies W & Bleiler L (2013). 2013 Alzheimer’s Disease Facts and Figures. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 9, 208–45. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Wilson B, Ivani-Chalian CF, & Aldrich F (1991). Rivermead behavioral memory test for children. Bury St Edmunds, U.K.: Thames Valley Test Co. [Google Scholar]
- Wilson RS, Scherr PA, Schneider JA, Tang Y, & Bennet DA (2007). Relation of cognitive activity to risk of developing Alzheimer disease. Neurology, 69, 1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb [DOI] [PubMed] [Google Scholar]
- Wisniewski KE, Wisniewski HM, & Wen GY (1985). Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Annals of Neurology, 17, 278–282. doi: 10.1002/ana.410170310 [DOI] [PubMed] [Google Scholar]
- Yamaki K (2005). Body Weight Status among Adults with Intellectual Disability in the Community. Mental Retardation, 43, 1–10. doi: [DOI] [PubMed] [Google Scholar]
- Zigman WB, Devenny DA, Krinsky-McHale SJ, Jenkins EC, Urv Tiina K., Wegiel J, … Silverman W (2008). Alzheimer’s Disease in Adults with Down Syndrome. International Review of Research In Mental Retardation, 36, 103–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman WB, & Lott IT (2007). Alzheimer’s disease in Down syndrome: Neurobiology and risk. Mental Retardation and Developmental Disabilities Research Reviews, 13, 237–246. doi: 10.1002/mrdd.20163 [DOI] [PubMed] [Google Scholar]
- Zigman WB, Schupf N, Sersen E, & Silverman W (1996). Prevalence of dementia in adults with and without Down syndrome. American Journal on Mental Retardation, 100, 403–412. [PubMed] [Google Scholar]
- Zimmerli E, & Devenny DA (1995, March). Cued recall as a screen for dementia in the MR population. Paper presented at the Gatlinburg conference on research and theory in mental retardation and developmental disabilities, Gatlinburg, TN. [Google Scholar]


