Abstract
Background:
Prostate cancer (PCa) remains the second leading cause of cancer-related death among men. Taxanes, such as docetaxel and cabazitaxel are utilized in standard treatment regimens for chemotherapy naïive castration-resistant PCa. However, tumors often develop resistance to taxane chemotherapeutics, highlighting a need to identify additional therapeutic targets. Fatty acid-binding protein 5 (FABP5) is an intracellular lipid carrier whose expression is upregulated in metastatic PCa and increases cell growth, invasion, and tumor formation. Here, we assessed whether FABP5 inhibitors synergize with semi-synthetic taxanes to induce cytotoxicity in vitro and attenuate tumor growth in vivo.
Methods:
PC3, DU-145, and 22Rv1 PCa cells were incubated with FABP5 inhibitors Stony Brook fatty acid-binding protein inhibitor 102 (SBFI-102) or SBFI-103 in the presence or absence of docetaxel or cabazitaxel, and cytotoxicity was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide assay. Cytotoxicity of SBFI-102 and SBFI-103 was also evaluated in noncancerous cells. For the in vivo studies, PC3 cells were subcutaneously implanted into BALB/c nude mice, which were subsequently treated with FABP5 inhibitors, docetaxel, or a combination of both.
Results:
SBFI-102 and SBFI-103 produced cytotoxicity in the PCa cells. Coincubation of the PCa cells with FABP5 inhibitors and docetaxel or cabazitaxel produced synergistic cytotoxic effects in vitro. Treatment of mice with FABP5 inhibitors reduced tumor growth and a combination of FABP5 inhibitors with a submaximal dose of docetaxel reduced tumor growth to a larger extent than treatment with each drug alone.
Conclusions:
FABP5 inhibitors increase the cytotoxic and tumor-suppressive effects of taxanes in PCa cells. The ability of these drugs to synergize could permit more efficacious antitumor activity while allowing for dosages of docetaxel or cabazitaxel to be lowered, potentially decreasing taxane-resistance.
Keywords: cabazitaxel, docetaxel, drug discovery, fatty acid-binding protein, Stony Brook fatty acid-binding protein inhibitor, taxanes
1 |. INTRODUCTION
Despite advances in anti-androgen and chemotherapeutic interventions, prostate cancer (PCa) remains the second leading cause of cancer-related death in men in the United States.1 After anti-androgen therapy, metastatic PCa often becomes castration-resistant and incurable, highlighting the need to develop next-generation therapeutics to treat aggressive metastatic PCa.2 It is well accepted that adenocarcinomas of the prostate utilize lipids to fuel their growth, and dysregulated lipid metabolism is observed in PCa.3,4 Recent work has demonstrated that the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ), which regulates the expression of proangiogenic genes, is overexpressed in metastatic prostate adenocarcinomas and is associated with reduced patient survival.5–7
Fatty acid-binding protein 5 (FABP5) is a member of a class of intracellular lipid chaperones that transports fatty acids to PPARγ, leading to increased expression of proangiogenic factors including vascular endothelial growth factor, which can result in a metastatic phenotype.5,8–12 The normal prostate lacks FABP5 expression but it becomes highly upregulated in PCa and the degree of its upregulation correlates with increasing Gleason scores, indicating that advanced metastatic prostate tumors express the highest levels of FABP5.5,8,12,13 Mirroring this expression pattern, PCa cell-lines with low metastatic potential lack FABP5 expression, whereas PCa cell-lines with high metastatic potential demonstrate elevated FABP5 expression levels.5,14 Introduction of FABP5 to PCa cell-lines with low metastatic potential enhances cell migration, invasion, and tumor formation, while its inhibition in PCa cell-lines with high metastatic potential attenuates these features.7,9,14 This positions FABP5 as a potential therapeutic target to treat PCa.
Our group has previously developed FABP5 inhibitors based upon the truxillic acid monoester scaffold, including the first-generation inhibitor Stony Brook fatty acid-binding protein inhibitor 26 (SBFI-26).15,16 Our recent efforts have led to the identification of second-generation FABP5 inhibitors that show enhanced potency or selectivity for FABP5.17 Recent work by the Ke group has shown that SBFI-26 suppresses PCa cell growth, migration, invasion, tumor formation, and metastasis in vitro and in vivo,18 suggesting that FABP5 inhibitors may constitute efficacious antitumor agents. Therefore, the first goal of this work was to determine whether our second-generation FABP5 inhibitors produce cytotoxicity in PCa cells in vitro and reduce tumor growth in vivo.
Docetaxel and cabazitaxel are used as standard chemotherapeutics for the treatment of naïive castration-resistant PCa.19–23 Despite the clinical availability of docetaxel, cabazitaxel, and newer-generation taxane chemotherapeutics, prostate tumors often develop resistance to these agents.20,24 Therefore, combination therapies consisting of docetaxel/cabazitaxel and other chemotherapeutic agents could lead to enhanced antitumor efficacy or permit the use of lower taxane doses in patients, thus reducing taxane-resistance as well as potentially decreasing the adverse effects associated with taxane chemotherapeutics.21,25–27 Thus, the second goal of this work was to determine whether FABP5 inhibitors potentiate the cytotoxic and tumor-suppressive effects of docetaxel/cabazitaxel.
2 |. MATERIALS AND METHODS
2.1 |. Cell-lines
PC3 cells were obtained from American Type Culture Collection (ATCC; CRL-1435; Manassas, VA) and were authenticated by the ATCC human short-tandem repeat profiling cell authentication service. DU-145 and 22Rv1 cells were also obtained from ATCC (HTB-81 and CRL-2505, respectively; ATCC). PC3, DU-145, and 22Rv1 cell-lines were each grown in Roswell Park Memorial Institute 1640 (RPMI 1640) (Gibco-Thermo Fisher Scientific, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS) (Gemini Bio-Products, West Sacramento, CA) and 100 units/mL of penicillin/streptomycin (Gibco-Thermo Fisher Scientific) in a humidified incubator containing 95% air and 5% CO2. WI-38 cells were obtained from ATCC (CCL-75). WI-38 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco-Thermo Fisher Scientific) supplemented with 10% FBS and 100 units/mL of penicillin/streptomycin in a humidified incubator containing 95% air and 5% CO2. RWPE-1 cells were purchased from ATCC (CRL-11609). RWPE-1 cells were grown in keratinocyte serum-free media (K-SFM) (Gibco-Thermo Fisher Scientific) supplemented with 25 mg of bovine pituitary extract (BPE), 1 mg of recombinant human epidermal growth factor (EGF), and 100 units/mL of penicillin/streptomycin in a humidified incubator containing 95% air and 5% CO2.
2.2 |. Drugs
SBFI-102 and SBFI-103 were synthesized as described,17 and were annotated as compounds 4b and 4j, respectively.17 Docetaxel was obtained from Sigma-Aldrich (St. Louis, MO). Cabazitaxel was a gift from the Discovery Chemistry Laboratory (Institute of Chemical Biology and Drug Discovery, Stony Brook University, Stony Brook, NY).
2.3 |. Animals
Male BALB/c nude mice (BALB/cOlaHsd-Foxn1nu, 20-30 g, 7-8 weeks old) (Envigo RMS Inc, Indianapolis, IN) were used for all experiments. Animals were housed individually at room temperature and were kept on a 12:12-hour light:dark cycle with access to food and water ad libitum. Euthanasia was carried out utilizing CO2 asphyxiation. All of the experiments were approved by the Stony Brook University Animal Care and Use Committee (#850980).
2.4 |. Subcutaneous tumor implantation
Male BALB/c nude mice were subcutaneously inoculated with PC3 cells. Briefly, cells (1 × 106 per mouse) were resuspended in 100μL of a 1:1 mixture of phosphate-buffered saline (PBS):Matrigel (Corning Inc, Corning, NY) and implanted into a single dorsal lateral flank using a 21G needle. Tumor length (L) and tumor width (W) were measured twice weekly using digital calipers, and tumor volume (V) was calculated as (V = [LxW2]/2). When tumor volume reached approximately 150 to 200 mm3, animals were grouped and drug administration commenced. Humane endpoints for all animals were as follows: animals carrying a tumor burden greater than 35 days, body weight (which was recorded twice weekly) decreasing by greater than 15%, tumor ulceration, paralysis, failure to groom, bleeding, respiratory distress, and/or tumor volume reaching 1500 mm3.
2.5 |. Drug administration
SBFI-102, SBFI-103, and docetaxel were each reconstituted in a 1:1:8 vehicle consisting of dimethyl sulfoxide (DMSO) (Thermo Fisher Scientific, Hampton, NH):Cremaphor-EL (Sigma-Aldrich):saline. SBFI-102 and SBFI-103 were administered via intraperitoneal injection (ip) using a 27G needle at 20 mg/kg daily. Docetaxel was administered i.p. at 5 or 10 mg/kg weekly. All drugs were administered in a volume of 10 μL/g body weight.
2.6 |. Cytotoxicity assays
Cytotoxicity of SBFI-102, SBFI-103, docetaxel, and cabazitaxel (both individually, and in combination) were determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) colorimetric assay (Sigma-Aldrich). PC3 (2500 cells/well), DU-145, 22Rv1, WI-38 (5000 cells/well), and RWPE-1 (10 000 cells/well) cells were seeded into 96-well plates and incubated for 24 hours at 37°C in their respective media (PC3/DU-145/22Rv1 cells utilized RPMI 1640; WI-38 cells utilized DMEM; RWPE-1 cells utilized K-SFM). PC3, DU-145, and 22Rv1 cells were treated with RPMI 1640 supplemented with 1% FBS containing 0.1 μM to 100 μM SBFI-102 or SBFI-103, and/or 0.003nM to 300nM docetaxel or cabazitaxel (both individually, or in combination with SBFI-102 or SBFI-103). WI-38 cells were treated with DMEM supplemented with 1% FBS containing 0.1 μM to 100 μM SBFI-102 or SBFI-103. RWPE-1 cells were treated with K-SFM supplemented with 25 mg of BPE and 1 mg of recombinant human EGF containing 0.1 μM to 100μM SBFI-102 or SBFI-103. All drugs for in vitro experimentation were dissolved in a vehicle of DMSO at a final concentration of 0.1%. Additionally, the appropriate treatment media for each cell-line supplemented with 0.1% DMSO or 1% sodium dodecyl sulfate was used as either a positive or negative control, respectively. After a 72-hour incubation period, cells were washed with PBS and treated with MTT (0.5 mg/mL in serum-free RPMI 1640, serum-free DMEM, or K-SFM) for 4hours. The cells were subsequently solubilized using DMSO and the absorbance was read at 562 nm in an F5 Filtermax Multi-Mode Microplate Reader (Molecular Devices, Sunnyvale, CA).
2.7 |. Analysis of combined drug effects
Synergism between docetaxel/cabazitaxel and SBFI-102 or SBFI-103 was determined through the combination-index (CI) method using the median-effect principle of mass-action law, derived from Chou and Talalay28 using ComboSyn software. Briefly, individual drug concentrations that result in the desired fraction of cells affected (Fa) were measured (ie, the concentration of SBFI-102, SBFI-103, docetaxel, or cabazitaxel, which result in the same fraction of cells killed). The concentration resulting in the desired Fa (eg, Fa = 0.5 represents 50% of cells effected) for each drug was plotted on an XY-axis, and a straight line drawn to connect the data points. The coadministration of two drugs that achieves the same desired Fa was then plotted on the same axis. Data points that fall above the line (CI >1) represent antagonism, data points that fall on the line (CI = 1) represent an additive interaction, and data points that fall below the line (CI <1) represent synergism.
2.8 |. Quantification and statistical analysis
All data were obtained from at least three independent experiments and then values described in each figure legend depict each independent trial or animal. Data for all in vivo experiments were analyzed using a one-way analysis of variance with the Tukey post hoc test (GraphPad Prism, version 8.0.2). Data are represented as means ± SEM and P < .05 was considered statistically significant. The degree of significance is indicated in each figure legend.
3 |. RESULTS
We recently reported the development of second-generation FABP5 inhibitors, including compounds such as SBFI-102 and SBFI-103 (Figure 1A,B).17 These second-generation molecules were chosen as candidate inhibitors because they display greater potency than SBFI-26 (in the case of SBFI-102) or greater selectivity against related FABPs (in the case of SBFI-103).17 We first determined whether our second-generation FABP5 inhibitors produce cytotoxicity in PCa cells. The cytotoxic effects of SBFI-102 (Figure 2A) and SBFI-103 (Figure 2B) were assessed in human-derived PC3, DU-145, and 22Rv1 cells that express FABP5.14,18 SBFI-102 and SBFI-103 produced dose-dependent cytotoxicity in each cell-line tested: PC3 cells with IC50 values of 11.4 and 6.3 μM, respectively; DU-145 cells with half-maximal inhibitory concentration (IC50) values of 8.9 and 3.3 μM, respectively; and 22Rv1 cells with IC50 values of 10.1 and 3.1 μM, respectively. Next, we performed MTT assays using RWPE-1 cells (a normal prostate cell-line) to determine whether these compounds could produce comparable or lower cytotoxicity in a noncancerous cell-line. Both SBFI-102 and SBFI-103 showed less cytotoxicity in RWPE-1 cells, producing IC50 values of 26.0 and 20.6 μM, respectively (Figure 2A,B). We subsequently carried out MTT assays using WI-38 cells (a normal lung cell-line) to determine whether these compounds could produce comparable or lower cytotoxicity in a cell-line from a different tissue origin. As expected, both SBFI-102 and SBFI-103 showed less cytotoxicity in WI-38 cells, producing IC50 values of 29.4 and 29.6 μM, respectively (Figure 2A,B).
FIGURE 1.
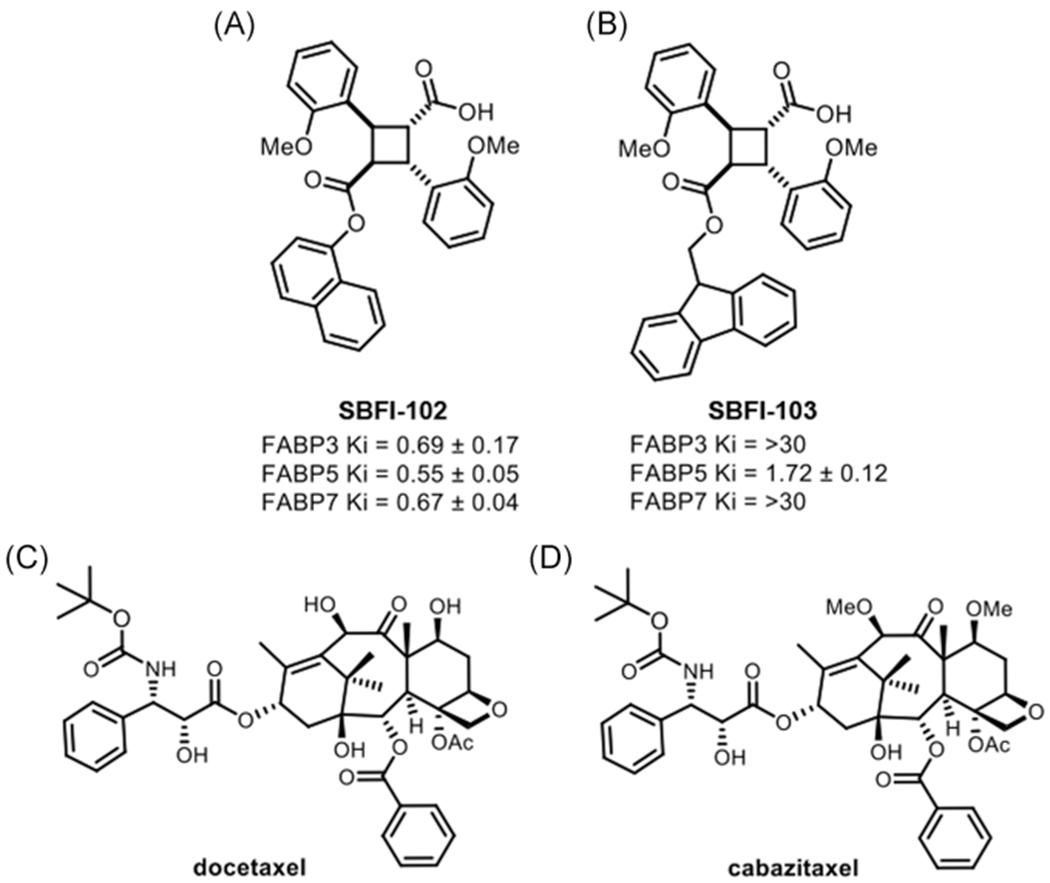
SBFI-102/SBFI-103 structures and in vitro affinities (Ki, μM), and docetaxel/cabazitaxel structures. The chemical structures and Ki values of (A) SBFI-102 and (B) SBFI-103 are shown and taken from Yan et al.17 The chemical structures for (C) docetaxel and (D) cabazitaxel. SBFI, Stony Brook fatty acid-binding protein inhibitor
FIGURE 2.
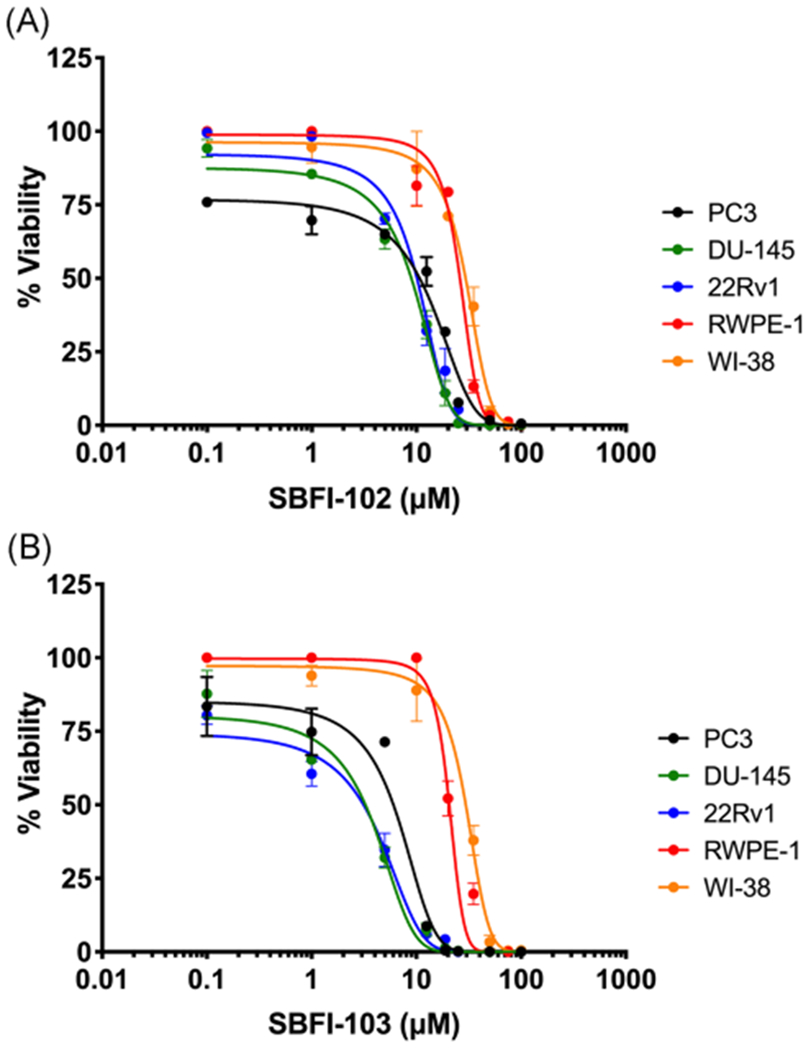
Cytotoxicity of SBFI-102 or SBFI-103 in PC3, DU-145, 22Rv1, RWPE-1, and WI-38 cells. A, SBFI-102 produced cytotoxicity in PC3, DU-145, 22Rv1, RWPE-1, and WI-38 cells with IC50 values of 11.4, 8.9, 10.1, 26.0, and 29.4 μM, respectively (n ≥3). B, SBFI-103 produced cytotoxicity in PC3, DU-145, 22Rv1, RWPE-1, and WI-38 cells with IC50 values of 6.3, 3.3, 3.1, 20.6, and 29.6 μM, respectively (n ≥3). IC50, half-maximal inhibitory concentration; SBFI, Stony Brook fatty acid-binding protein inhibitor [Color figure can be viewed at wileyonlinelibrary.com]
Docetaxel (Figure 1C) acts as a microtubule stabilizer and is the standard of care treatment for advanced metastatic PCa.26 We employed PC3, DU-145, and 22Rv1 cells to determine whether FABP5 inhibitors enhance the in vitro cytotoxic effects of docetaxel. As expected, docetaxel produced dose-dependent cytotoxicity in each cell-line tested: PC3 cells with an IC50 value of 1.9 nM (Figure 3A); DU-145 cells with an IC50 value of 0.8 nM (Figure 3B); and 22Rv1 cells with an IC50 value of 0.3 nM (Figure 3C); corroborating previously published literature.29–31 A combination of docetaxel with SBFI-102 or SBFI-103 resulted in greater cytotoxicity in all three cell-lines than each drug when administered independently (Figure 4). We employed the CI to assess the relationship between docetaxel and the FABP5 inhibitors as described.28 CI values were calculated across a range of docetaxel concentrations and a fixed concentration of SBFI-102 or SBFI-103 (7.5 and 1.0 μM, respectively, a submaximal concentration below the IC50 for both FABP5 inhibitors in each cell-line) (Table 1). We observed synergistic relationships between docetaxel and the FABP5 inhibitors in each cell-line (CI <1), especially at higher concentrations which is important given that CI values for anticancer agents calculated at high concentrations are more relevant to the therapy than CI values calculated at low concentrations.28
FIGURE 3.
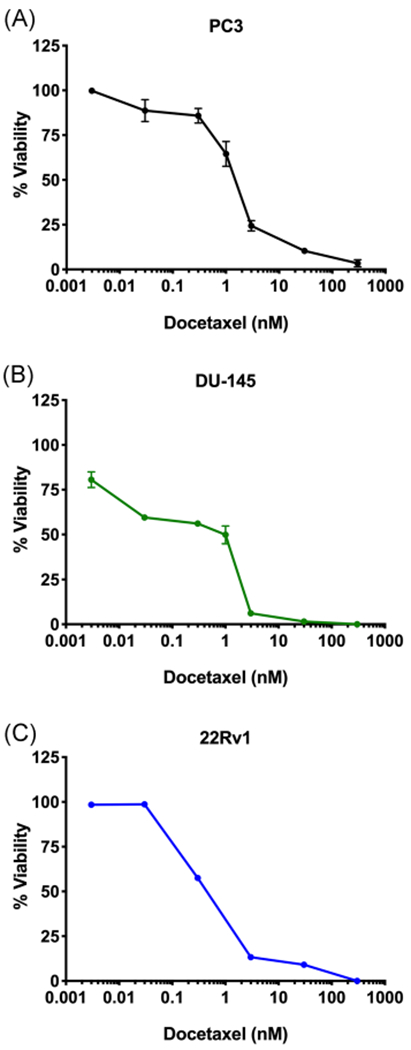
Cytotoxicity of docetaxel in PC3, DU-145, and 22Rv1 cells. Docetaxel produced cytotoxicity in (A) PC3, (B) DU-145, and (C) 22Rv1 cells with IC50 values of 1.9, 0.8, and 0.3 nM, respectively (n ≥3). IC50, half-maximal inhibitory concentration [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.
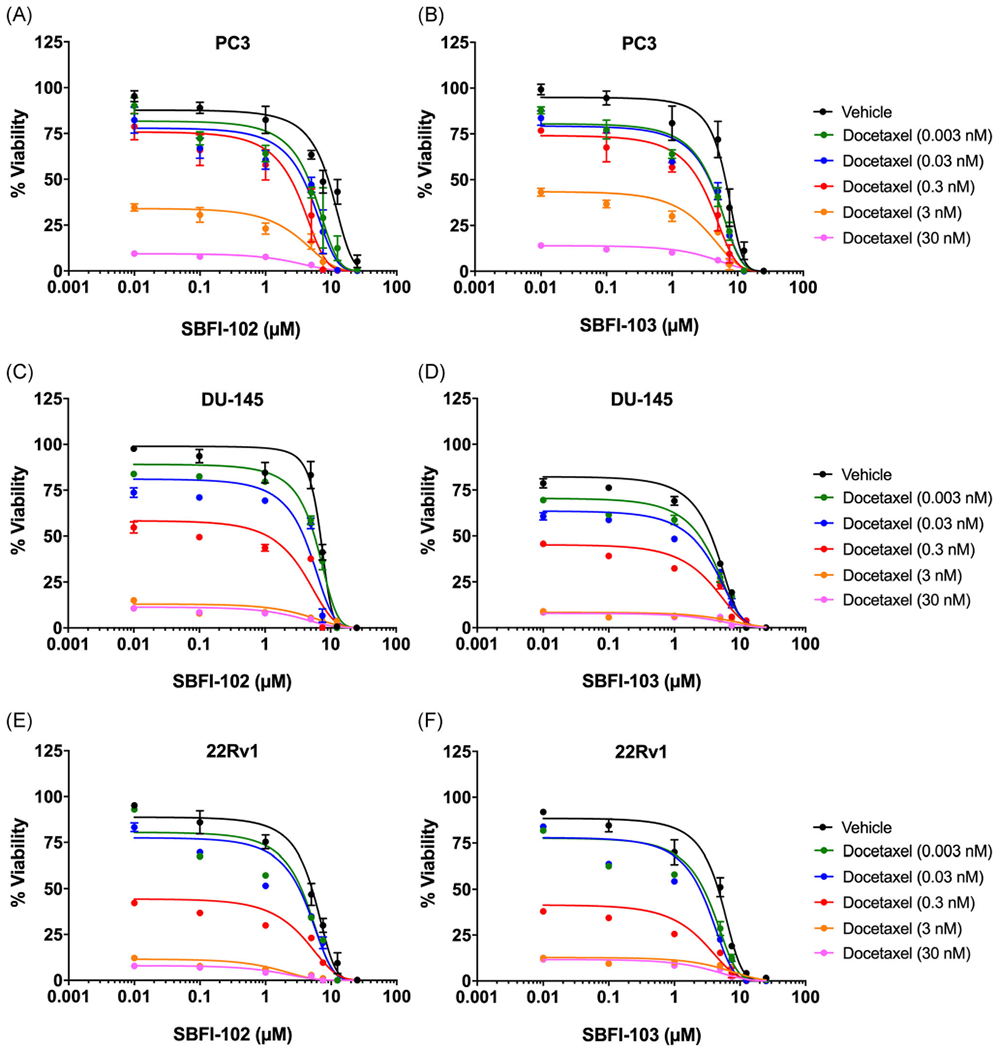
Cytotoxicity of PC3, DU-145, and 22Rv1 cells following combinatorial treatment with docetaxel and SBFI-102 or SBFI-103. Cytotoxicity of PC3 cells incubated with docetaxel in the presence of (A) SBFI-102 or (B) SBFI-103 (n ≥3). Cytotoxicity of DU-145 cells incubated with docetaxel in the presence of (C) SBFI-102 or (D) SBFI-103 (n ≥3). Cytotoxicity of 22Rv1 cells incubated with docetaxel in the presence of (E) SBFI-102 or (F) SBFI-103 (n ≥3). SBFI, Stony Brook fatty acid-binding protein inhibitor [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Synergy analysis for SBFI-102 or SBFI-103 and docetaxel combinations in PC3, DU-145, and 22Rv1 cell-lines
| Cell-line | SBFI-102, μM | SBFI-103, μM | Docetaxel, nM | Fa value | CI value | Relationship |
|---|---|---|---|---|---|---|
| PC3 | 7.5 | – | 0.03 | 0.786 | 0.897 | Synergistic |
| PC3 | 7.5 | – | 0.3 | 0.950 | 0.009 | Synergistic |
| PC3 | 7.5 | – | 3.0 | 0.992 | 0.160 | Synergistic |
| PC3 | – | 1.0 | 0.03 | 0.402 | 0.710 | Synergistic |
| PC3 | – | 1.0 | 0.3 | 0.432 | 0.889 | Synergistic |
| PC3 | – | 1.0 | 3.0 | 0.700 | 0.394 | Synergistic |
| DU-145 | 7.5 | – | 0.03 | 0.934 | 1.302 | – |
| DU-145 | 7.5 | – | 0.3 | 0.963 | 0.968 | Synergistic |
| DU-145 | 7.5 | – | 3.0 | 0.998 | 0.123 | Synergistic |
| DU-145 | – | 1.0 | 0.03 | 0.516 | 4.473 | – |
| DU-145 | – | 1.0 | 0.3 | 0.676 | 2.697 | – |
| DU-145 | – | 1.0 | 3.0 | 0.935 | 0.445 | Synergistic |
| 22Rv1 | 7.5 | – | 0.03 | 0.795 | 3.846 | – |
| 22Rv1 | 7.5 | – | 0.3 | 0.904 | 0.003 | Synergistic |
| 22Rv1 | 7.5 | – | 3.0 | 0.999 | 0.005 | Synergistic |
| 22Rv1 | – | 1.0 | 0.03 | 0.457 | 1.673 | – |
| 22Rv1 | – | 1.0 | 0.3 | 0.744 | 0.373 | Synergistic |
| 22Rv1 | – | 1.0 | 3.0 | 0.905 | 0.267 | Synergistic |
Abbreviations: Fa, fraction of cells affected; CI, combination-index; SBFI, Stony Brook fatty acid binding protein inhibitor.
Cabazitaxel (Figure 1D) is a second-line chemotherapeutic for advanced metastatic PCa, and is often utilized after tumors begin to develop docetaxel-resistance.21,22 Similar to docetaxel, cabazitaxel produced dose-dependent cytotoxicity in each cell-line tested: PC3 cells with an IC50 value of 1.6 nM (Figure 5A); DU-145 cells with an IC50 value of 0.2 nM (Figure 5B); and 22Rv1 cells with an IC50 value of 0.3 nM (Figure 5C); similar to previously published literature.32,33 Combination of cabazitaxel with SBFI-102 or SBFI-103 resulted in greater cytotoxicity in all three cell-lines than each drug when administered alone (Figure 6). CI values were calculated across a range of cabazitaxel concentrations, and synergistic relationships between cabazitaxel and the FABP5 inhibitors were observed (Table 2).
FIGURE 5.
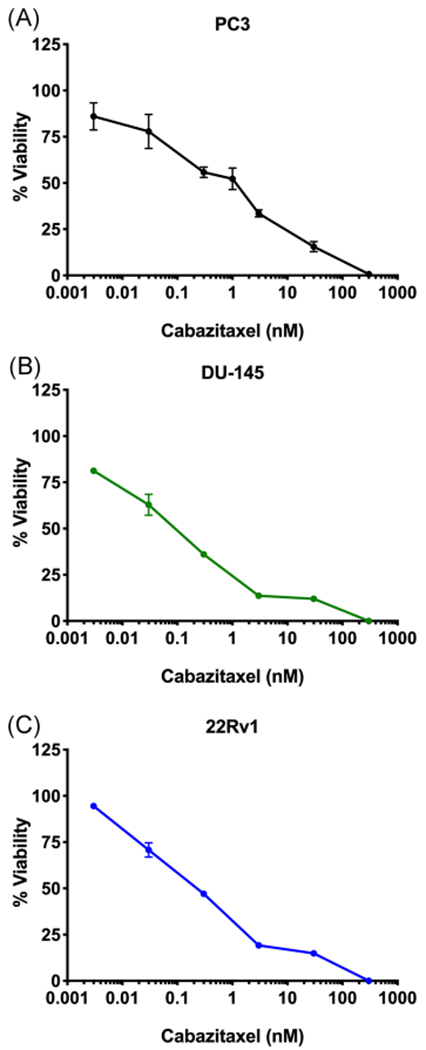
Cytotoxicity of cabazitaxel in PC3, DU-145, and 22Rv1 cells. Cabazitaxel produced cytotoxicity in (A) PC3, (B) DU-145, and (C) 22Rv1 cells with IC50 values of 1.6, 0.2, and 0.3 nM, respectively (n ≥3). IC50, half-maximal inhibitory concentration [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 6.
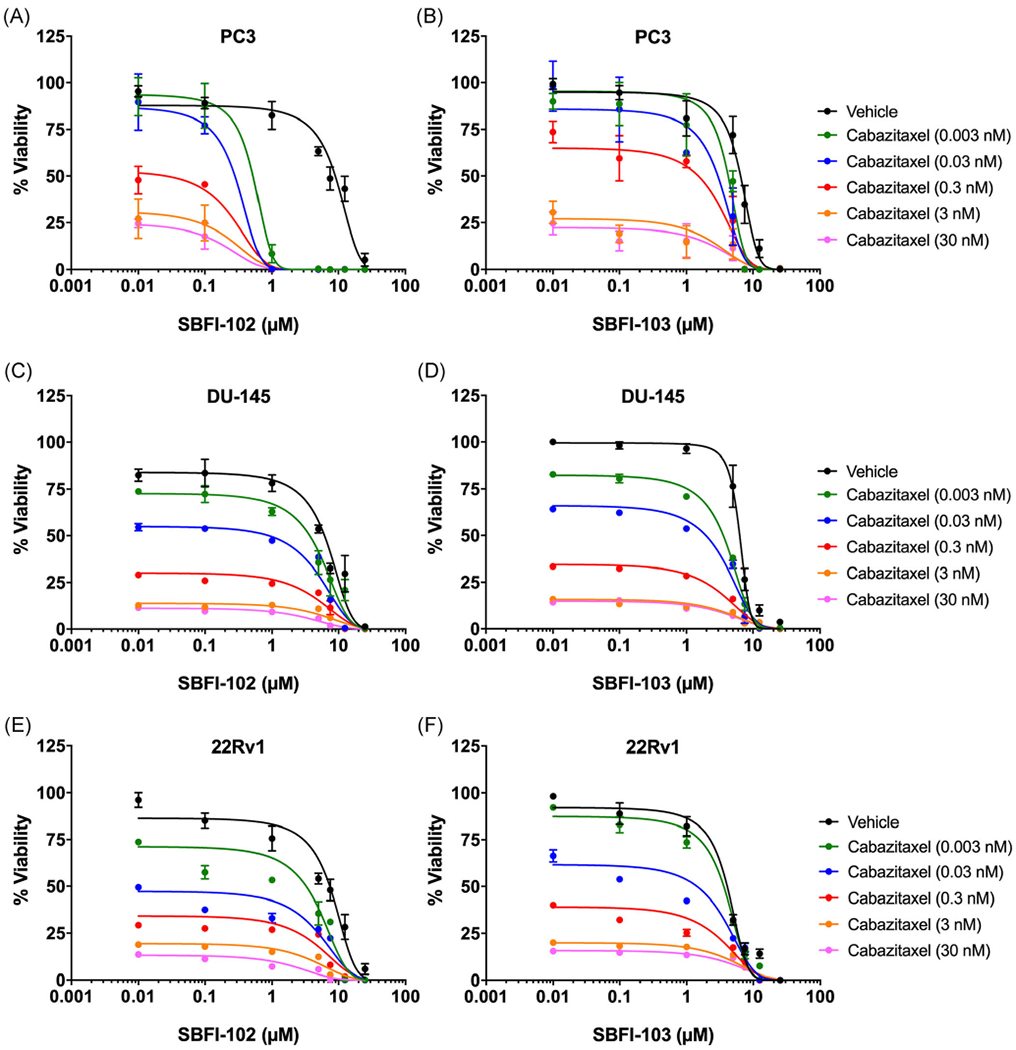
Cytotoxicity of PC3, DU-145, and 22Rv1 cells following combinatorial treatment with cabazitaxel and SBFI-102 or SBFI-103. Cytotoxicity of PC3 cells incubated with cabazitaxel in the presence of (A) SBFI-102 or (B) SBFI-103 (n ≥3). Cytotoxicity of DU-145 cells incubated with cabazitaxel in the presence of (C) SBFI-102 or (D) SBFI-103 (n ≥3). Cytotoxicity of 22Rv1 cells incubated with cabazitaxel in the presence of (E) SBFI-102 or (F) SBFI-103 (n ≥3). SBFI, Stony Brook fatty acid-binding protein inhibitor [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 2.
Synergy analysis for SBFI-102 or SBFI-103 and cabazitaxel combinations in PC3, DU-145, and 22Rv1 cell-lines
| Cell-line | SBFI-102, μM | SBFI-103, μM | Docetaxel, nM | Fa value | CI value | Relationship |
|---|---|---|---|---|---|---|
| PC3 | 7.5 | – | 0.03 | 0.998 | 0.0001 | Synergistic |
| PC3 | 7.5 | – | 0.3 | 0.999 | 0.0001 | Synergistic |
| PC3 | 7.5 | – | 3.0 | 0.999 | 0.0001 | Synergistic |
| PC3 | – | 1.0 | 0.03 | 0.379 | 0.899 | Synergistic |
| PC3 | – | 1.0 | 0.3 | 0.420 | 0.942 | Synergistic |
| PC3 | – | 1.0 | 3.0 | 0.853 | 0.373 | Synergistic |
| DU-145 | 7.5 | – | 0.03 | 0.829 | 0.709 | Synergistic |
| DU-145 | 7.5 | – | 0.3 | 0.885 | 0.322 | Synergistic |
| DU-145 | 7.5 | – | 3.0 | 0.939 | 0.136 | Synergistic |
| DU-145 | – | 1.0 | 0.03 | 0.464 | 0.449 | Synergistic |
| DU-145 | – | 1.0 | 0.3 | 0.716 | 0.300 | Synergistic |
| DU-145 | – | 1.0 | 3.0 | 0.882 | 0.194 | Synergistic |
| 22Rv1 | 7.5 | – | 0.03 | 0.777 | 0.707 | Synergistic |
| 22Rv1 | 7.5 | – | 0.3 | 0.899 | 0.396 | Synergistic |
| 22Rv1 | 7.5 | – | 3.0 | 0.998 | 0.003 | Synergistic |
| 22Rv1 | – | 1.0 | 0.03 | 0.577 | 0.731 | Synergistic |
| 22Rv1 | – | 1.0 | 0.3 | 0.728 | 0.549 | Synergistic |
| 22Rv1 | – | 1.0 | 3.0 | 0.823 | 0.659 | Synergistic |
Abbreviations: Fa, fraction of cells affected; CI, combination-index; SBFI, Stony Brook fatty acid binding protein inhibitor.
We employed subcutaneous tumor growth assays to determine whether the interactions between SBFI-102/SBFI-103 and docetaxel extend to the in vivo setting. Docetaxel was chosen as it is the standard of care first-line treatment for chemotherapy naïve castration-resistant PCa, and PC3 cells were chosen due to their heightened tumorigenicity and expression of FABP5 relative to DU-145 and 22Rv1 cells.14 BALB/c nude mice were implanted with PC3 cells and inhibitor treatments were initiated when tumors reached a volume of approximately 150 to 200 mm3. Administration of SBFI-102 and SBFI-103 (20 mg/kg, ip, once daily) significantly reduced tumor growth (Figure 7A). Similarly, administration of docetaxel (5 or 10 mg/kg, ip, once weekly) reduced tumor growth, with the 5 mg/kg dose producing similar inhibition of tumor growth as observed with the FABP5 inhibitors, while the 10 mg/kg dose produced near complete inhibition of growth (Figure 7A–D). To determine whether SBFI-102 and SBFI-103 enhance the tumor suppressive effects of docetaxel, we administered the FABP5 inhibitors in combination with the submaximal dose of docetaxel (5 mg/kg). Consistent with the in vitro efficacy data, coadministration of docetaxel with SBFI-102 or SBFI-103 produced greater inhibition of tumor growth than treatment with each compound alone, with effects that were comparable in magnitude to the 10 mg/kg docetaxel dose (Figure 8A–D).
FIGURE 7.

Inhibition of subcutaneous tumor growth by docetaxel or FABP5 inhibitors. PC3 cells (1 × 106) were implanted subcutaneously into male BALB/c nude mice. From day 15 onwards, mice were treated with vehicle, SBFI-102 (20 mg/kg, daily), SBFI-103 (20 mg/kg, daily), or docetaxel (5 mg/kg or 10 mg/kg, weekly). A, Tumor growth over the time course of treatments. B-D, Tumor volumes at days 25, 30, and 35, respectively. *P < .05 versus vehicle treatment; **P < .01 versus vehicle treatment; ***P < .001 versus vehicle treatment; #P < .05 versus 10 mg/kg docetaxel treatment; ##P < .01 versus 10 mg/kg docetaxel treatment; (n = 5). FABP5, fatty acid-binding protein; SBFI, Stony Brook fatty acid-binding protein inhibitor [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 8.

Inhibition of subcutaneous tumor growth by docetaxel and FABP5 inhibitors. PC3 cells (1 × 106) were implanted subcutaneously into male BALB/c nude mice. From day 15 onwards, mice were treated with vehicle, SBFI-102 (20 mg/kg, daily) in combination with docetaxel (5 mg/kg, weekly), SBFI-103 (20 mg/kg, daily) in combination with docetaxel (5 mg/kg, weekly), or docetaxel (5 mg/kg or 10 mg/kg, weekly). A, Tumor growth over the time course of treatments. B-D, Tumor volumes at days 25, 30, and 35, respectively. **P < .01 versus vehicle treatment; ***P < .001 versus vehicle treatment; #P < .05 versus 10 mg/kg docetaxel treatment; NS versus 10 mg/kg docetaxel treatment; (n = 5). FABP5, fatty acid-binding protein; SBFI, Stony Brook fatty acid-binding protein inhibitor; NS, no significance [Color figure can be viewed at wileyonlinelibrary.com]
4 |. DISCUSSION
Dysregulated lipid metabolism and signaling are one of the hallmarks of PCa. Upregulation of FABP5 enhances PCa cell migration, invasion, and tumor formation and effects principally attributed to the activation of PPARγ.3–5,8–12 Recent work by the Ke group has established that pharmacological inhibition of FABP5 using small molecule inhibitors (eg, our first-generation inhibitor SBFI-26) suppresses the metastatic potential of PCa.18 Therefore, lipid signaling and metabolic pathways may serve as new targets for the development of therapeutics to treat PCa. Current treatment modalities for PCa consist of anti-androgen and chemotherapeutic interventions, yet metastatic prostate tumors often develop resistance to taxane chemotherapeutics.19,20,24 Combination therapies consisting of taxanes and novel agents could present an opportunity to enhance antitumor efficacy while simultaneously lowering taxane doses and potentially reducing taxane-resistance in metastatic PCa.25,26
Our study demonstrates that FABP5 inhibitors, when combined with docetaxel or cabazitaxel, produce synergistic cytotoxicity in PCa cell-lines in vitro, and also potentiate the antitumor effects of docetaxel in vivo. Indeed, combinations of docetaxel or cabazitaxel with SBFI-102 or SBFI-103 showed CI values less than 1 (indicative of synergistic interactions). In further support of these findings, docetaxel, or cabazitaxel alone did not completely eradicate PC3, DU-145, or 22Rv1 cells even at concentrations greater than 10-fold above their respective IC50 values, while combinations of taxanes with FABP5 inhibitors resulted in complete cell death at lower concentrations. Consistent with the in vitro results, coadministration of FABP5 inhibitors with a submaximal dose of docetaxel produced a greater reduction in tumor growth than treatment with either agent alone. It is also noteworthy that SBFI-102 and SBFI-103 displayed greater toxicity in PCa cells compared with noncancerous cells and may, therefore, have more limited side-effects compared with taxanes. For example, concentrations of SBFI-102 and SBFI-103 that produced limited or no cytotoxicity in noncancerous cells resulted in near-complete cell death in PC3, DU-145, and 22Rv1 cells. Consequently, our study demonstrates that FABP5 inhibition represents an attractive avenue to treat PCa, highlighting the necessity to develop next-generation potent and selective FABP5 inhibitors.
5 |. CONCLUSIONS
Docetaxel and cabazitaxel are efficacious chemotherapeutic agents used to treat castration-resistant metastatic PCa. However, prostate tumors often develop taxane-resistance, and taxane-treatment can lead to numerous adverse effects that can lead to the termination of therapy. Our study is the first to demonstrate that FABP5 inhibitors, such as SBFI-102 and SBFI-103 increase the cytotoxic effects of two clinically used chemotherapeutics in PCa cells. The ability of these drugs to synergize could lead to new combination therapies with enhanced tumor-suppressive efficacy.
ACKNOWLEDGMENT
We would like to thank Dr. Dale Deutsch for the use of his facilities and equipment.
Funding information
Translational Research Opportunities Grant from the Stony Brook University School of Medicine and the Department of Anesthesiology
Abbreviations:
- BPE
bovine pituitary extract
- CI
combination-index
- EGF
epidermal growth factor
- Fa
fraction of cells affected
- FABP
fatty acid-binding protein
- FBS
fetal bovine serum
- i.p.
intraperitoneal injection
- K-SFM
keratinocyte serum-free media
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide
- PCa
prostate cancer
- PPARγ
peroxisome proliferator-activated receptor gamma
- SBFI
Stony Brook fatty acid-binding protein inhibitor
Footnotes
CONFLICT OF INTERESTS
IO and MK hold patents covering the FABP inhibitors used in this study, which have been licensed to Artelo Biosciences.
DATA AVAILABILITY STATEMENT
The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable request.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Frieling JS, Basanta D, Lynch CC. Current and emerging therapies for bone metastatic castration-resistant prostate cancer. Cancer Control. 2015;22:109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deep G, Schlaepfer I. Aberrant lipid metabolism promotes prostate cancer: role in cell survival under hypoxia and extracellular vesicles biogenesis. Int J Mol Sci. 2016;17:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zadra G, Photopoulos C, Loda M. The fat side of prostate cancer. Biochim Biophys Acta. 2013;1831:1518–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forootan FS, Forootan SS, Malki MI, et al. The expression of C-FABP and PPARγ and their prognostic significance in prostate cancer. Int J Oncol. 2014;44:265–275. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad I, Mui E, Galbraith L, et al. Sleeping beauty screen reveals Pparg activation in metastatic prostate cancer. Proc Natl Acad Sci USA. 2016;113:8290–8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao Z, Malki MI, Forootan SS, et al. A novel cutaneous fatty acid-binding protein-related signaling pathway leading to malignant progression in prostate cancer cells. Genes Cancer. 2013;4: 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan EA, Forootan SS, Adamson J, et al. Expression of cutaneous fatty acid-binding protein (C-FABP) in prostate cancer: potential prognostic marker and target for tumourigenicity-suppression. Int J Oncol. 2008;32:767–775. [PubMed] [Google Scholar]
- 9.Forootan FS, Forootan SS, Gou X, et al. Fatty acid activated PPARgamma promotes tumorigenicity of prostate cancer cells by up regulating VEGF via PPAR responsive elements of the promoter. Oncotarget. 2016;7:9322–9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adamson J, Morgan EA, Beesley C, et al. High-level expression of cutaneous fatty acid-binding protein in prostatic carcinomas and its effect on tumorigenicity. Oncogene. 2003;22:2739–2749. [DOI] [PubMed] [Google Scholar]
- 11.Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7:489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jing C, Beesley C, Foster CS, et al. Identification of the messenger RNA for human cutaneous fatty acid-binding protein as a metastasis inducer. Cancer Res. 2000;60:2390–2398. [PubMed] [Google Scholar]
- 13.Fujita K, Kume H, Matsuzaki K, et al. Proteomic analysis of urinary extracellular vesicles from high Gleason score prostate cancer. Sci Rep. 2017;7:42961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaguchi K, Kinameri A, Suzuki S, Senga S, Ke Y, Fujii H. The cancer-promoting gene fatty acid-binding protein 5 (FABP5) is epigenetically regulated during human prostate carcinogenesis. Biochem J. 2016;473:449–461. [DOI] [PubMed] [Google Scholar]
- 15.Berger WT, Ralph BP, Kaczocha M, et al. Targeting fatty acid binding protein (FABP) anandamide transporters–a novel strategy for development of anti-inflammatory and anti-nociceptive drugs. PLoS One. 2012;7:e50968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaczocha M, Rebecchi MJ, Ralph BP, et al. Inhibition of fatty acid binding proteins elevates brain anandamide levels and produces analgesia. PLoS One. 2014;9:e94200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan S, Elmes MW, Tong S, et al. SAR studies on truxillic acid mono esters as a new class of antinociceptive agents targeting fatty acid binding proteins. Eur J Med Chem. 2018;154:233–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Jameel W, Gou X, Forootan SS, et al. Inhibitor SBFI26 suppresses the malignant progression of castration-resistant PC3-M cells by competitively binding to oncogenic FABP5. Oncotarget. 2017;8:31041–31056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. [DOI] [PubMed] [Google Scholar]
- 20.Galletti G, Leach BI, Lam L, Tagawa ST. Mechanisms of resistance to systemic therapy in metastatic castration-resistant prostate cancer. Cancer Treat Rev. 2017;57:16–27. [DOI] [PubMed] [Google Scholar]
- 21.Antonarakis E, Paller ES. Cabazitaxel: a novel second-line treatment for metastatic castration-resistant prostate cancer. Drug Des Devel Ther. 2011;5:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. The Lancet. 2010;376:1147–1154. [DOI] [PubMed] [Google Scholar]
- 23.Higano CS, Crawford ED. New and emerging agents for the treatment of castration-resistant prostate cancer. Urol Oncol. 2011;29:1–8. [DOI] [PubMed] [Google Scholar]
- 24.Hongo H, Kosaka T, Oya M. Analysis of cabazitaxel-resistant mechanism in human castration-resistant prostate cancer. Cancer Sci. 2018;109:2937–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cella D, Peterman A, Hudgens S, Webster K, Socinski MA. Measuring the side effects of taxane therapy in oncology: The Functional Assessment of Cancer Therapy-taxane (FACT-taxane). Cancer. 2003;98:822–831. [DOI] [PubMed] [Google Scholar]
- 26.Baker J, Ajani J, Scotté F, et al. Docetaxel-related side effects and their management. Eur J Oncol Nurs. 2009;13:49–59. [DOI] [PubMed] [Google Scholar]
- 27.Sperlich C, Saad F. Optimal management of patients receiving cabazitaxel-based chemotherapy. Can Urol Assoc J. 2013;7:S18–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. [DOI] [PubMed] [Google Scholar]
- 29.Attia RT, Tolba MF, Trivedi R, Tadros MG, Arafa HMM, Abdel-Naim AB. The chemomodulatory effects of glufosfamide on docetaxel cytotoxicity in prostate cancer cells. PeerJ. 2016;4:e2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsakalozou E, Eckman AM, Bae Y. Combination effects of docetaxel and doxorubicin in hormone-refractory prostate cancer cells. Biochem Res Int. 2012;2012:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corcoran C, Rani S, O’Brien K, et al. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One. 2012;7:e50999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oprea-Lager DE, Bijnsdorp IV, van Moorselaar RJ, van den Eertwegh AJ, Hoekstra OS, Geldof AA. ABCC4 decreases docetaxel and not cabazitaxel efficacy in prostate cancer cells in vitro. Anticancer Res. 2013;33:387–391. [PubMed] [Google Scholar]
- 33.Shimizu Y, Tamada S, Kato M, et al. Androgen receptor splice variant 7 drives the growth of castration resistant prostate cancer without being involved in the efficacy of taxane chemotherapy. J Clin Med. 2018;7:444. [DOI] [PMC free article] [PubMed] [Google Scholar]


