Abstract
Background.
Preeclampsia (PE) is a disorder prevalent in 3–8% of pregnancies, characterized by hypertension, endothelial dysfunction and cardiac dysfunction, including hypertrophy and impaired global longitudinal strain (GLS), which indicates reduced contractility and tissue injury. Despite several clinical studies highlighting impaired cardiac function in these women, the underlying mechanisms have not been studied, in part, due to lack of an appropriate animal model. The Reduced Uterine Perfusion Pressure (RUPP) rat model produces a PE-like phenotype, including adverse cardiac remodeling. However, whether this translates to impaired cardiac function is not known. The aim of this study was to test the hypothesis that placental ischemia in the RUPP rat leads to impaired left ventricular (LV) systolic function and GLS.
Study Design.
RUPP (n=10) rats underwent surgery to induce placental ischemia on gestational day (GD) 14. Sham (n=10) and RUPP rats had indwelling carotid catheters placed on GD 18, and blood pressure and echocardiography measurements were made on GD 19.
Results.
The RUPP group exhibited increased mean arterial pressure compared to the Sham group (123±3 vs. 97±2 mmHg, P<0.01). RUPP hearts exhibited impaired LV ejection fraction (60±2 vs. 78±2 %, P<0.01) and GLS (−17.89±0.5 vs. −26.31±2.7 %, P=0.02), in addition to cardiac hypertrophy (0.97±0.04 vs. 0.91±0.02 g, P=0.02).
Conclusions.
Cardiac dysfunction and impaired strain are present in RUPP rats during pregnancy. These findings represent an animal model of PE that could be used to understand the mechanisms of cardiac dysfunction in this disease and ultimately, improve or prevent cardiac abnormalities in these patients.
Keywords: placental ischemia, cardiac dysfunction, echocardiography, speckle tracking, placental ischemia, preeclampsia
Introduction
Preeclampsia (PE) is a hypertensive disorder prevalent in 3–8% of pregnancies worldwide [1–4], caused by impaired spiral artery remodeling, leading to poor perfusion of the placenta and placental ischemia. The consequent release of anti-angiogenic, such as sFlt-1 and pro-inflammatory (eg. Tumor necrosis factor- α, TNF-α) factors by the placenta are thought to play a major role in the manifestation of this disease. Despite being the leading cause of maternal death and major contributor to maternal and perinatal morbidity worldwide [5], there is no effective drug treatment for PE. Currently, the only cure is delivery of the fetus and placenta, which exposes obvious short-term risks for both mother and baby, in addition to increased long-term risk of developing cardiovascular disease. In fact, a meta-analysis showed mothers who were diagnosed with PE during pregnancy are at a 4-fold and 2-fold higher risk of developing hypertension and ischemic heart disease, respectively [6].
In normal pregnancy, blood volume increases by approximately 40–50%, accompanied by 45% increase in cardiac output to support growth of the uteroplacental unit. Cardiac hypertrophy accompanied by increases in vascular density is also normal during pregnancy [7], to maintain cardiac efficiency, and typically reverses within 7 days postpartum. In addition, pregnancy is associated with significant decreases in mean arterial pressure (MAP) and peripheral resistance. In contrast, PE is associated with increased blood pressure, decreased cardiac output and impaired systolic and diastolic function, accompanied by adverse cardiac remodeling [8,9]. Myocardial Performance Index, which typically increases with age or disease, is also increased in preeclamptic women [10]. Furthermore, from 28–32 weeks gestation, preeclamptic women exhibit impaired circumferential, radial and global longitudinal strain (GLS), which are indicators of impaired contractility and tissue injury [11–13]. These data are consistent with clinical data, which shows hypertensive pregnant women have higher circulating cardiac troponin I (cTnI) than normotensive pregnant women, and that levels increase further in the presence of proteinuria, as seen in PE [14]. The long-term risk of cardiac related morbidity and mortality has also been shown in PE, with studies showing both diastolic and systolic dysfunction are persistent 12 months following parturition, in the absence of hypertension. Furthermore, the risk of developing essential hypertension within 2 years postpartum is increased in these patients [9]. These data suggest irreversible damage to the heart during pregnancy, despite clearance of circulating placental factors postpartum.
While there is an obvious association between cardiac dysfunction and PE, there have not been any studies to address the underlying mechanisms, due at least in part, to lack of an appropriate animal model. The Reduced Uterine Perfusion Pressure (RUPP) rat model, where silver clips are placed on branches of the ovarian arteries and abdominal aorta above the uterine arteries on GD 14, recapitulates many of the characteristics of PE, including hypertension, endothelial dysfunction, impaired renal function, increased circulating sFlt-1 and TNF-α, and reduced placental growth factor (PlGF) and vascular endothelial growth factor (VEGF). In addition, following only 5 days of placental ischemia, RUPP hearts exhibit, fibrotic deposits, increased collagen mRNA expression and apoptosis. RUPP hearts also express increases in brain natriuretic peptide (BNP), myosin heavy chain α/β (MHC α/β), and atrial natriuretic peptide (ANP) compared to normal pregnant controls, indicating pathological cardiac remodeling [15]. However, whether this adverse remodeling effects cardiac function is not known. Thus, this study aims to determine whether RUPP rats exhibit impaired cardiac function. We tested the hypothesis that placental ischemia in the RUPP rat causes cardiac dysfunction and decreased GLS during pregnancy, accompanied by increases in circulating cTnI.
Methods
Animals.
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center, and conducted in accordance with National Institute of Health Guide for the Care and Use of Laboratory Animals. Female Sprague-Dawley rats were received from Charles River Laboratories (Wilmington, MA, USA) on GD 10 and housed in the Center for Comparative Research facility at the University of Mississippi Medical Center. On GD 14, animals were assigned to Sham (n=10) or RUPP (n=10) groups. In the RUPP group, rats were anesthetized (~3% isoflurane in 2 L/min O2), the uterus was exteriorized and silver clips were placed on the abdominal aorta (above the kidneys) and branches of the ovarian arteries, as previously described [16]. In Sham animals, the uterus was exteriorized and replaced without placement of the clips. All animals were maintained on standard chow (Teklad, Indianapolis, IN, USA) and water, ad libitum. On GD 18, indwelling carotid catheters were placed to measure blood pressure via pressure transducer on GD 19. Following blood pressure measurement, all animals underwent comprehensive echocardiographic assessment. Finally, blood was collected, and the heart was immediately excised, arrested in a cardioplegic solution and weighed.
Speckle-tracking echocardiography.
Echocardiograms were performed using a Vevo 3100 high-resolution in vivo imaging platform and the MX250 scan head for small animals. For each animal, four views were collected to measure a range of cardiac structural and functional parameters, including GLS: parasternal long-axis, parasternal short-axis, four-chamber apical, suprasternal view. Rats were anesthetized, and temperature and heart rate were monitored continuously. Body weight was recorded and used for some calculations in the results section, where indicated.
Biochemical analyses.
On GD 19, blood was collected from the abdominal aorta into an EDTA-coated vacutainer (BD, Franklin Lakes, NJ, USA), spun at 2500 RPM for 12 min at 4°C and stored at −20°C. Circulating cTnI, sFlt-1 and TNF-α were measured in serum using a Cardiac Troponin I ELISA kit (MyBioSource, San Diego, CA), VEGFR1 ELISA kit and TNF-α ELISA kit (R&D Systems, Minneapolis, MN), respectively, as per the manufacturers’ instructions.
Statistical analysis.
Groups were compared using the students’ t-test, where P<0.05 was considered statistically significant. All graphs and statistical analysis were performed using Graphpad Prism 7 (GraphPad Software, San Diego, CA). All data are presented as mean+SEM.
Results
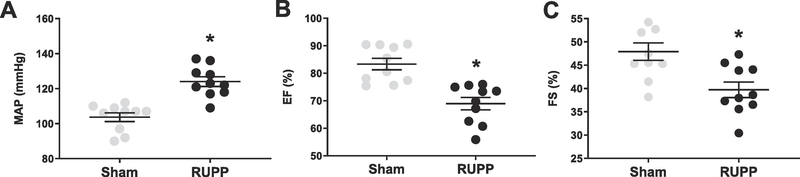
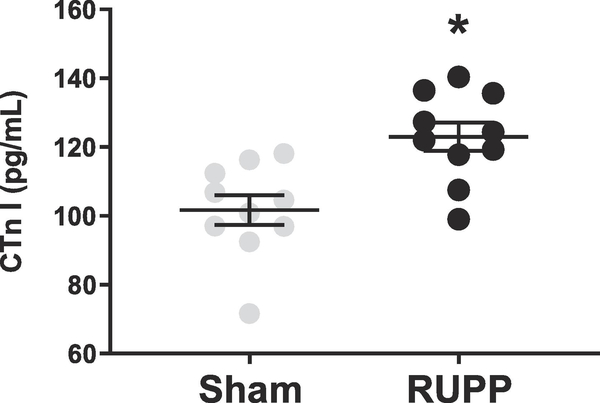
Animals in the RUPP group exhibited increased blood pressure, accompanied by increased circulating placental factors, including sFlt-1 and TNF-α on GD19 (Table 1). Left ventricular (LV) ejection fraction and fractional shortening were significantly reduced in the RUPP group compared to Sham animals (Figure 1). Global longitudinal strain, which is a marker of cardiac remodeling and injury was also impaired in the RUPP group (Figure 2). This data was accompanied by increases in circulating cTnI (Figure 3).
Table 1.
Characteristics of RUPP-operated compared to Sham-operated animals on GD 19
| Sham | RUPP | P value | |
|---|---|---|---|
| n | 10 | 10 | |
| Body weight, g | 305 ± 3.6 | 282 ± 4.3 | <0.01 |
| Circulating sFlt-1, pg/ml | 49 ± 9.0 | 469 ± 20 | <0.01 |
| Circulating TNF-α, pg/ml | 21 ± 2.9 | 49 ± 5.7 | <0.01 |
| Heart rate, bpm | 388 ± 15 | 373 ± 9 | 0.57 |
| Cardiac output, ml/min | 93 ± 9 | 89 ± 4 | 0.67 |
| Stroke volume, μl | 284 ± 27 | 255 ± 46 | 0.49 |
| MV E, mm/s | 750 ± 48 | 730 ± 41 | 0.73 |
| MV A, mm/s | 512 ± 70 | 532 ± 40 | 0.70 |
| MV E/A | 1.45 ± 0.1 | 1.38 ± 0.7 | 0.71 |
| Isovolumetric relaxation time, ms | 20 ± 2.8 | 25 ± 2.1 | 0.30 |
| Isovolumetric contraction time, ms | 17 ± 2.4 | 18 ± 1.9 | 0.68 |
| LVID during diastole, mm | 6.1 ± 0.3 | 7.2 ± 0.3 | 0.03 |
| LVID during systole, mm | 2.7 ± 0.4 | 3.7 ± 0.2 | 0.04 |
| LVPW during diastole, mm | 1.9 ± 0.1 | 1.7 ± 0.7 | 0.07 |
| LVPW during systole, mm | 3.2 ± 0.1 | 2.4 ± 0.1 | <0.01 |
| LV mass, mg | 799 ± 38 | 957 ± 0.1 | <0.01 |
| Aortic diameter at diastole, mm | 2.4 ± 0.11 | 2.6 ± 0.1 | 0.24 |
TNF-α, tumor necrosis factor-α; MV E, peak mitral valve (MV) early filling, MV A, peak MV active filling; LVID, left ventricular (LV) internal diameter; LVPW, LV posterior wall thickness
Figure 1.
Mean arterial pressure (A; MAP), ejection fraction (B; EF) and fractional shortening (C; FS) in Sham- and RUPP- operated animals on GD19 (n=10/group). Mean+SEM. *P<0.05
Figure 2.
Global longitudinal strain (GLS) in Sham- and RUPP- operated animals on GD19 (n=10/group). Mean+SEM. *P<0.05
Figure 3.
Circulating levels of cardiac troponin I (cTnI) in Sham- and RUPP-operated rats on GD19 (n=10/group). Mean+SEM. *P<0.05
Discussion
Little is known about the mechanisms of maternal cardiac dysfunction in PE. While several clinical studies have shown a strong correlation in women diagnosed with PE and reduced cardiac performance, lack of an appropriate animal model has made investigations into underlying mechanisms of this phenotype difficult. Thus, highlighting the importance of the current study. We utilized the RUPP rat model of PE to show increased heart mass (Table 1), decreased LV ejection fraction and fractional shortening (Figure 1B and 1C) and impaired GLS, giving rise to the only reported animal model of PE that also mimics cardiac dysfunction seen in PE patients.
Cardiac dysfunction is emerging as an important phenotype of PE, with long term consequences such as increased risk of myocardial infarction and heart failure [6,17]. During pregnancy, clinical studies have shown reduced ejection fraction, fractional shortening and hypertrophy [9,17,18], with more severe cardiac dysfunction being correlated to severity of PE. In fact, one recent study showed that cardiac function can be predictive of the severity of PE that may develop during pregnancy. The investigators showed that women with both early and late onset PE exhibited increased systemic vascular resistance, however only the early onset group had reduced cardiac output, and this was associated with severity of PE later in gestation [19]. A limitation of the current standard of care is that ejection fraction and cardiac output are crude measures of cardiac performance that may remain unchanged in PE despite cardiac injury.
Speckle tracking echocardiography (STE) is a clinically relevant and sensitive technology, which has been used to show decreased GLS and early onset subclinical heart failure in PE in a number of studies, despite normal ejection fraction [13,20–22]. One of the major benefits of STE is that it can differentiate between active and passive contraction, and also allows assessment of subendocardial longitudinal fibers, which may be particularly disposed to ischemia and myocardial dysfunction when afterload is reduced, as in pregnancy [23]. While increased blood pressure can be causative of cardiac injury and dysfunction, GLS is shown to be more severely impaired in PE women compared to non-proteinuric hypertensive pregnancies, suggesting that factors beyond blood pressure may be responsible. Importantly, GLS itself has also been shown to be associated with fibrosis in other cardiovascular diseases [24,25].
The RUPP model has been used extensively by our lab and others to understand the pathogenesis of PE [26–32]. Our group has previously showed that following only 5 days of placental ischemia, RUPP hearts exhibit fibrotic deposits and increased collagen mRNA expression, as well as markers of pathological cardiac hypertrophy and apoptosis [15]. The findings in this study are an extension of this work and also recapitulate what has been shown in the human PE population. Here, we have reported significantly increased heart mass (Table 1), decreased LV ejection fraction and decreased fractional shortening (Figure 1B and 1C), with decreases in GLS in the RUPP group, compared to the Sham (Figure 2). Furthermore, cTnI, a marker of cardiomyocyte injury that is shown to be increased in women with PE, is increased in blood samples of RUPP rats on GD19 (Figure 3). Indeed, it is important to note that most animal models of hypertension require approximately two weeks of intervention for the development of cardiac dysfunction, suggesting that placental factors play an important and rapid role in the development of cardiac dysfunction in PE.
Previous studies from our lab and others, coupled with the findings in the current study, provide an excellent rationale to investigate the possible role of placental factors in cardiac dysfunction in the RUPP rat in the future. One factor that could contribute is the anti-angiogenic placental factor, sFlt-1. sFlt-1 has been implicated in the development of peripartum cardiomyopathy and heart failure, but its role in PE has not been explored. In fact, clinical studies show that the impaired angiogenesis correlates with the extent of elevation in circulating sFlt-1, and our group has previously shown reduced vascular density in RUPP hearts [15]. Thus, the anti-angiogenic environment that exists in PE could play a role in impairing cardiac adaptations to pregnancy.
Many clinical studies have shown the relationship between PE and cardiac dysfunction postpartum, with 60% of women developing Stage B heart failure within 12 months and 20% developing new-onset subclinical heart failure within four years postpartum [11–13,18]. Small for gestational age births, a hallmark of PE pregnancy, has also been linked to long term heart failure, ischemic heart disease and atrial and ventricular dysrhythmia in a number of studies [33,34]. In the RUPP rat, we have previously shown that animals exhibit impaired ejection fraction and fractional shortening compared to normal pregnant controls, 8 weeks postpartum [35]. However, understanding cardiac dysfunction during pregnancy is equally important as it represents an opportunity to investigate therapeutic intervention and improve long term outcomes.
Conclusion
Mounting evidence suggests that cardiac dysfunction and injury occurs in women with PE. However, little to no studies have investigated the underlying mechanisms due, in part at least, to lack of an animal model. In the current study we have showed reduced LV ejection fraction, fractional shortening and GLS, which not only recapitulate what has been shown in the human PE population, but also provide the first evidence of cardiac dysfunction in an animal model of PE. These findings also provide rationale in using the RUPP rat to investigate mediators of cardiac abnormalities in PE; an important but understudied area of research.
Acknowledgments
Funding sources
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health (P01HL051971, P20GM104357) and the American Heart Association (18POST33990293).
References
- [1].Ananth CV, Keyes KM, Wapner RJ Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ, 347 (2013), p. f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hutcheon JA, Lisonkova S, Joseph K. Epidemiology of pre-eclapmsia and other hypertensive disorders of pregnancy. Best Pr Res Clin Obs Gynaecol, 25 (2011), p. 391–403. [DOI] [PubMed] [Google Scholar]
- [3].Duley L. Pre-eclampsia and the hypertensive disorders of pregnancy. Br Med Bull, 67 (2003), p. 161–176. [DOI] [PubMed] [Google Scholar]
- [4].Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi J-M. Pre-eclampsia: Pathophysiology, diagnosis, and management. Vasc Health Risk Manag, 7 (2011), p. 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sibai B, Dekker G, Kupferminc M Pre-eclampsia. Lancet, 365 (2005), p. 785–799. [DOI] [PubMed] [Google Scholar]
- [6].Bellamy L, Casas J-P, Hingorani AD, Williams DJ Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ, 335 (2007), p. 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Umar S, Nadadur R, Iorga A, Amjedi M, Matori H, Eghbali M. Cardiac structural and hemodynamic changes associated with physiological heart hypertrophy of pregnancy are reversed postpartum. J Appl Physiol, 113 (2012), p. 1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Melchiorre K, Sharma R, Thilaganathan B. Cardiovascular implications in preeclampsia: an overview. Circulation, 130 (2014), p. 703–714. [DOI] [PubMed] [Google Scholar]
- [9].Melchiorre K, Thilaganathan B. Maternal cardiac function in preeclampsia. Curr Opin Obstet Gynecol, 23 (2011), p. 440–447. [DOI] [PubMed] [Google Scholar]
- [10].Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature, 485 (2012), p. 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cong J, Fan T, Yang X, Shen J, Cheng G, Zhang Z. Maternal cardiac remodeling and dysfunction in preeclampsia: a three-dimensional speckle-tracking echocardiography study. Int J Cardiovasc Imaging, 31 (2015), p. 1361–1368. [DOI] [PubMed] [Google Scholar]
- [12].Davis EF, Lewandowski AJ, Leeson P. Cardiac dysfunction and preeclampsia can imaging give clues to mechanism? Circ Cardiovasc Imaging, 5 (2012), p. 691–692. [DOI] [PubMed] [Google Scholar]
- [13].Shahul S, Rhee J, Hacker MR, Gulati G, Mitchell JD, Hess P, et al. Subclinical left ventricular dysfunction in preeclamptic women with preserved left ventricular ejection fraction: A 2D speckle-tracking imaging study. Circ Cardiovasc Imaging, 5 (2012), p. 734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fleming SM, O’Gorman T, Finn J, Grimes H, Daly K, Morrison JJ Cardiac troponin I in pre-eclampsia and gestational hypertension. Br J Obstet Gynaecol, 107 (2000), p. 1417–1420. [DOI] [PubMed] [Google Scholar]
- [15].Gutkowska J, Granger JP, Lamarca BB, Danalache BA, Wang D, Jankowski M. Changes in cardiac structure in hypertension produced by placental ischemia in pregnant rats: effect of tumor necrosis factor blockade. J Hypertens, 29 (2011), p. 1203–1212. [DOI] [PubMed] [Google Scholar]
- [16].LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-α. Hypertension, 46 (2005), p. 1022–1025. [DOI] [PubMed] [Google Scholar]
- [17].Melchiorre K, R Sutherland G, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension, 58 (2011), p. 709–715. [DOI] [PubMed] [Google Scholar]
- [18].Melchiorre K, Sutherland GR, Baltabaeva A, Liberati M, Thilaganathan B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension, 57 (2011), p. 85–93. [DOI] [PubMed] [Google Scholar]
- [19].di Pasquo E, Ghi T, Dall’asta A, Angeli L, Fieni S, Pedrazzi G, et al. Maternal cardiac parameters can help in differentiating the clinical profile of preeclampsia and in predicting progression from mild to severe forms. Am J Obstet Gynecol (2019), in press. [DOI] [PubMed] [Google Scholar]
- [20].Shahul S, Ramadan H, Nizamuddin J, Mueller A, Patel V, Dreixler J, et al. Activin A and late postpartum cardiac dysfunction among women with hypertensive disorders of pregnancy novelty and significance. Hypertension,72 (2018), p. 188–193. [DOI] [PubMed] [Google Scholar]
- [21].Shahul S, Ramadan H, Mueller A, Nizamuddin J, Nasim R, Perdigao JL, et al. Abnormal mid-trimester cardiac strain in women with chronic hypertension predates superimposed preeclampsia. Pregnancy Hypertens, 10 (2017), p. 251–255. [DOI] [PubMed] [Google Scholar]
- [22].Shahul S, Medvedofsky D, Wenger JB, Nizamuddin J, Brown SM, Bajracharya S, et al. Circulating antiangiogenic factors and myocardial dysfunction in hypertensive disorders of pregnancy. Hypertension, 67 (2016), p. 1273–1280. [DOI] [PubMed] [Google Scholar]
- [23].Sengupta SP, Bansal M, Hofstra L, Sengupta PP, Narula J. Gestational changes in left ventricular myocardial contractile function: new insights from two-dimensional speckle tracking echocardiography. Int J Cardiovasc Imaging, 33 (2017), p. 69–82. [DOI] [PubMed] [Google Scholar]
- [24].Haland TF, Almaas VM, Hasselberg NE, Saberniak J, Leren IS, Hopp E, et al. Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging, 17 (2016), p. 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Leader CJ, Moharram M, Coffey S, Sammut IA, Wilkins GW, Walker RJ Myocardial global longitudinal strain : An early indicator of cardiac interstitial fibrosis modified by spironolactone , in a unique hypertensive rat model. PLoS ONE, (2019), p. e0220837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol, 294 (2008), p. H541–H550. [DOI] [PubMed] [Google Scholar]
- [27].George EM, Granger JP Linking placental ischemia and hypertension in preeclampsia: Role of endothelin 1. Hypertension, 60 (2012), p. 507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bakrania BA, Duncan J, Warrington JP, Granger JP . The Endothelin Type A Receptor as a Potential Therapeutic Target in Preeclampsia. Int J Mol Sci, 18 (2017), p. 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li J, LaMarca BB, Reckelhoff JF A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Hear Circ Physiol, 303 (2012), p. H1–H8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].LaMarca BB, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, et al. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats effect of tumor necrosis factor-α blockade. Hypertension, 52 (2008), p. 1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].LaMarca BB, Ryan MJ, Gilbert JS, Murphy SR, Granger JP Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep, 9 (2007), p.480–485. [DOI] [PubMed] [Google Scholar]
- [32].Gilbert JS, Dukes M, LaMarca BB, Cockrell K, Babcock SA, Granger JP Effects of reduced uterine perfusion pressure on blood pressure and metabolic factors in pregnant rats. Am J Hypertens, 20 (2007), p. 686–691. [DOI] [PubMed] [Google Scholar]
- [33].Silverberg O, Park AL, Cohen E, Fell DB, Ray JG Premature cardiac disease and death in women whose infant was preterm and small for gestational age: A retrospective cohort study, JAMA Cardiol, 3 (2019), p. 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease : a retrospective cohort study of 129 290 births. Lancet, 357 (2001), p. 2002–2006. [DOI] [PubMed] [Google Scholar]
- [35].Paauw ND, Joles JA, Spradley FT, Bakrania BA, Zellenger ZK, Franx A, et al. Exposure to placental ischemia impairs postpartum maternal renal and cardiac function in rats. Am J Physiol Regul Integr Comp Physiol, 312 (2017), p. R664–R670. [DOI] [PMC free article] [PubMed] [Google Scholar]