Abstract
Left ventricular assist devices provide hemodynamic support to improve quality of life and long-term survival in patients with end-stage heart failure. The HeartMate 3 (Abbott, Abbott Park, IL) left ventricular assist device uses magnetically levitated impeller technology, improving durability and reducing pump thrombosis. Sternum-sparing implantation may reduce perioperative bleeding and infection, improve mobility, decrease hospitalization duration, and reduce right ventricular dysfunction. We describe the first Canadian HeartMate 3 implantation via bilateral minithoracotomy. Our case supports the compatibility of the HeartMate 3 device with sternum-sparing approaches and highlights the feasibility of intrapericardial tunnelling of the outflow graft.
Résumé
Les dispositifs d’assistance ventriculaire gauche procurent un soutien hémodynamique permettant d’améliorer la qualité de vie et la survie à long terme chez les patients présentant une insuffisance cardiaque terminale. Le dispositif d’assistance ventriculaire gauche HeartMate 3 (Abbott, Abbott Park, IL) fait appel à une pompe à flux centrifuge à lévitation magnétique, qui rehausse la durabilité de l’appareil et réduit le risque de thrombose de la pompe. L’implantation sans sternotomie peut diminuer le risque d’hémorragie périopératoire et d’infection, favoriser la mobilité, réduire la durée de l’hospitalisation et atténuer la dysfonction ventriculaire droite. Nous décrivons la première implantation d’un dispositif HeartMate 3 réalisée au Canada par minithoracotomie bilatérale. Le cas présenté montre la possibilité d’implanter le dispositif HeartMate 3 sans sternotomie et met en lumière la faisabilité d’une tunnellisation intrapéricardique de la prothèse d’éjection.
Novel Teaching Points.
-
•
Our initial Canadian experience supports that the HeartMate 3 LVAD is compatible with a bilateral minithoracotomy approach, providing less-invasive options for patients requiring LVAD implantation.
-
•
Intrapericardial tunnelling of the HeartMate 3 outflow graft is feasible and does not hinder flow. This technique can be combined with a bilateral minithoracotomy approach to possibly mitigate adhesion-related technical difficulty during re-entry for cardiac transplantation.
In patients with end-stage heart failure, durable left ventricular assist devices (LVADs) support hemodynamics, improving quality of life and survival.1 The HeartMate 3 (Abbott, Abbott Park, IL), approved by Health Canada in January 2018, is a new-generation LVAD with magnetically levitated impeller technology designed to reduce pump thrombosis.2 LVAD implantation via sternum-sparing minithoracotomy demonstrates potential to reduce transfusion, postoperative right ventricular dysfunction, sternal wound complications, and mechanical ventilation time.3, 4, 5 We describe the first Canadian HeartMate 3 LVAD implantation through bilateral minithoracotomy.
Case Presentation
A 57-year-old man with New York Heart Association IIIB symptoms and ischemic cardiomyopathy was admitted in rapid atrial fibrillation. After attempted cardioversion, he became milrinone dependent (0.375 μg/kg/min). Echocardiography revealed moderate to severe left ventricular (LV) dysfunction with a laminated apical thrombus. He was classified as Interagency Registry for Mechanically Assisted Circulatory Support Class 3 and consented to HeartMate 3 LVAD implantation as a bridge to transplantation.
Cannulation was through the right-sided femoral artery and vein through a 2-cm cut-down using the Seldinger technique and transesophageal echocardiographic guidance. Venous and arterial cannulation were performed using a 25F multi-sideport venous cannula (Medtronic, Dublin, Ireland) and a 19F arterial cannula (Medtronic), respectively.
In the second intercostal space, a 5-cm right anterior minithoracotomy incision was made, and in the left sixth intercostal space, an 8-cm left anterolateral minithoracotomy was performed. Soft tissue protectors and minithoracotomy retractors (Geister, Tuttlingen, Germany) were placed. On the right, pericardiotomy was performed, and the pericardium was retracted to improve exposure by displacing the ascending aorta rightward. On the left, pericardial retraction sutures were placed to optimize exposure of the LV apex.
The sewing cuff was secured to the apex with 4 pledgeted Ethibond (Ethicon, Somerville, NJ) mattress sutures and a running Prolene hemostatic suture line (Fig. 1A). Cardiopulmonary bypass was initiated, followed by apical coring. The LV cavity was examined, and the laminated clot was removed. The device inflow cannula was fixed to the apical cuff (Fig. 1B). The outflow graft was tunneled within the pericardium to be accessed through the right thoracotomy using a Stearns grasping instrument, and care was taken to maintain proper graft orientation. It was then cut to length and anastomosed end to side to the proximal ascending aorta using a side-biting clamp (Fig. 1C). Graft orientation was reconfirmed after de-airing maneuvers and side-biting clamp removal. The driveline was then tunnelled to its exit site in the right upper quadrant. The HeartMate 3 pump was started, and cardiopulmonary bypass was weaned after 89 minutes. The patient’s hemodynamics were stabilized at 5200 rpm.
Figure 1.
(A) Anastomosis of the sewing cuff to left ventricular (LV) apex. (B) HeartMate 3 (Abbott, Abbott Park, IL) left ventricular assist device (LVAD) positioned intrapericardially through left minithoracotomy incision. (C) LVAD outflow graft anastomosed to proximal ascending aorta through right second interspace minithoracotomy.
The patient was extubated after 14.28 hours and discharged from the cardiovascular intensive care unit in 3 days. All vasoactive agents were discontinued 5 days postoperatively. Figure 2 depicts the incisions on postoperative day 9. No transfusions were required during the hospital stay. Discharge was delayed for social reasons, and the patient left the hospital on postoperative day 32.
Figure 2.
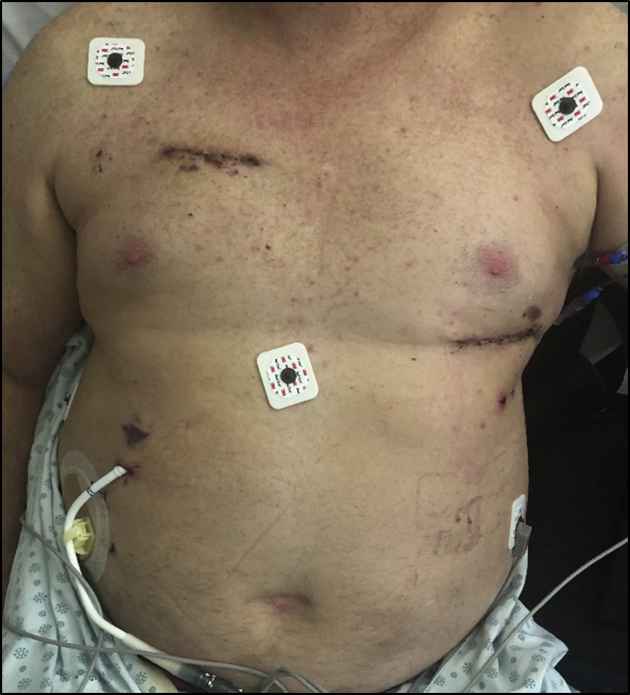
Healing incisions on postoperative day 9 after HeartMate 3 LVAD insertion via bilateral minithoracotomy approach.
Discussion
We outline the first Canadian HeartMate 3 LVAD implantation through a minimally invasive bilateral minithoracotomy approach. Potential benefits have been suggested, including less blood transfusion, less postoperative right ventricular failure, decreased intubation time, and fewer wound complications.3, 4, 5 In our experience, exposure of the LV apex through thoracotomy is favourable for suturing the sewing ring and confirming hemostasis. Because the sternum and anterior pericardium remain intact over the outflow graft and right ventricle, substernal adhesions are minimized and the risks associated with redo midline sternotomy for future heart transplant are mitigated.4, 5 Although this procedure can be performed off-pump, we believe bypass offers an increased degree of safety, and peripheral cannulation improves access for aortic outflow graft anastomosis.
The HeartMate 3 device is designed for intrapericardial implantation, which facilitates minimally invasive implantation. Although larger than other intrapericardial LVADs (largest diameter ∼75 mm), the device can be easily implanted with 2 minimally invasive thoracotomy incisions. We believe this sternal-sparing approach has advantages and, as experience grows, could become a preferred implantation technique with the potential to improve operative outcomes and optimize patients for subsequent transplant.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The authors are in agreement that this case report has adhered to all relevant ethical guidelines.
See page 262 for disclosure information.
References
- 1.Pagani F.D., Miller L.W., Russell S.D. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312–321. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 2.Krabatsch T., Netuka I., Schmitto J.D. Heartmate 3 fully magnetically levitated left ventricular assist device for the treatment of advanced heart failure -1 year results from the Ce mark trial. J Cardiothorac Surg. 2017;12:23. doi: 10.1186/s13019-017-0587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitto J.D., Molitoris U., Haverich A., Strueber M. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: upper hemisternotomy combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg. 2012;143:511–513. doi: 10.1016/j.jtcvs.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 4.Potapov E.V., Kukucka M., Falk V., Krabatsch T. Off-pump implantation of the HeartMate 3 left ventricular assist device through a bilateral thoracotomy approach. J Thorac Cardiovasc Surg. 2017;153:104–105. doi: 10.1016/j.jtcvs.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Sileshi B., O’Hara B.K., Davis M.E. Outcomes of patients implanted using a left thoracotomy technique for a miniaturized centrifugal continuous-flow pump. ASAIO J. 2016;62:539–544. doi: 10.1097/MAT.0000000000000407. [DOI] [PubMed] [Google Scholar]



