Abstract
Background
The prevalence of heterozygous familial hypercholesterolemia (FH) is 1 of 250 in the general population and approximately 1 of 125 in patients with atherosclerotic cardiovascular disease (ASCVD), yet only a minority are diagnosed. The diagnostic criteria for FH rely on a point system using low-density lipoprotein cholesterol (LDL-C), family history, cutaneous manifestations, and molecular diagnosis. The aim of the present study was to determine the prevalence of FH in the Relating Evidence to Achieve Cholesterol Targets (REACT) registry.
Methods
Patients were enrolled as ASCVD (n = 86) or FH (n = 109) and with an LDL-C level > 3.0 mmol/L despite maximally tolerated statin therapy. FH was diagnosed clinically using a validated clinical application integrating an imputation for baseline (untreated) LDL-C levels.
Results
There were 109 men and 86 women with a mean age of 63 ± 12 years. Diabetes (29.7%), hypertension (62.1%), smoking (37.9%), and family history of premature ASCVD (59.5%) were common. On-treatment LDL-C was 4.26 ± 0.94 mmol/L. On the basis of the dose and type of statin ± ezetimibe, imputed baseline LDL-C was 7.04 ± 2.90 mmol/L. A diagnosis of probable/definite FH was found in 54.7%, 49.5%, and 61.5% of patients according to the Simon Broome, Dutch Lipid Clinic Network criteria, and the new Canadian FH definition, respectively. Of note, 40% of patients in the ASCVD inclusion subgroup had probable or definite FH.
Conclusions
Our study reveals that a substantial proportion of patients with ASCVD whose LDL-C levels are > 3.0 mmol/L despite maximally tolerated statins have heterozygous FH. Clinicians should consider using the recently described algorithm to assess the possibility of FH in this high-risk population.
Résumé
Contexte
La prévalence de l’hypercholestérolémie familiale (HF) hétérozygote est de 1 cas sur 250 dans la population générale et d’environ 1 cas sur 125 chez les patients atteints d’une maladie cardiovasculaire athérosclérotique (MCVAS), pourtant on ne la diagnostique que dans une minorité de cas. Les critères diagnostiques de l’HF reposent sur un système de points utilisant comme paramètres le cholestérol à lipoprotéines de faible densité (C-LDL), les antécédents familiaux, les manifestations cutanées et le diagnostic moléculaire. La présente étude visait à déterminer la prévalence de l’HF parmi les patients répertoriés dans le registre REACT (Relating Evidence to Achieve Cholesterol Targets).
Méthodologie
Les patients admis à l’étude étaient considérés comme étant atteints d’une MCVAS (n = 86) ou d’une HF (n = 109) et présentaient un taux de C-LDL > 3,0 mmol/l malgré la prise d’un traitement par statine à la dose maximale tolérée. L’HF a été diagnostiquée sur le plan clinique à l’aide d’une application clinique validée incluant une imputation des taux de C-LDL initiaux (en l’absence de traitement).
Résultats
L’étude comptait 86 femmes et 109 hommes âgés en moyenne de 63 ± 12 ans. Le diabète (29,7 %), l’hypertension (62,1 %), le tabagisme (37,9 %) et les antécédents familiaux de MCVAS prématurée (59,5 %) étaient fréquents. Sous traitement, le taux de C-LDL était de 4,26 ± 0,94 mmol/l. D’après la dose et le type de statine ± ézétimibe administrés, le taux de C-LDL imputé au départ était de 7,04 ± 2,90 mmol/l. Un diagnostic d’HF probable ou certaine a été établi respectivement chez 54,7 %, 49,5 % et 61,5 % des patients selon les critères de Simon Broome et du Dutch Lipid Clinic Network, ainsi que la nouvelle définition canadienne de l’HF. Notons que 40 % des patients dans le sous-groupe d’inclusion de la MCVAS présentaient une HF probable ou certaine.
Conclusions
Notre étude révèle qu’une proportion importante de patients atteints de MCVAS dont les taux de C-LDL sont > 3,0 mmol/l malgré la prise de statines à la dose maximale tolérée présentent une HF hétérozygote. Les cliniciens devraient envisager d’utiliser l’algorithme récemment décrit pour évaluer la présence possible d’une HF dans cette population à haut risque.
Heterozygous familial hypercholesterolemia (FH) is an autosomal dominant genetic disorder characterized by an elevated low-density lipoprotein cholesterol (LDL-C) (> 95th percentile for age and sex), clinical manifestations (tendinous xanthomas, xanthelasmas, and arcus cornealis), a family history of elevated LDL-C in a first-degree relative, or a personal or family history of premature (< 55 years for men and 65 years for women) atherosclerotic cardiovascular disease (ASCVD).1 The diagnosis is confirmed in the presence of a causal mutation in the low-density lipoprotein receptor, apolipoprotein B, or proprotein convertase subtilisin/kexin type 9 (PCSK9) genes.2 As much as 20% of patients with FH do not have a single mutation in these causal genes, but an accumulation of single nucleotide polymorphisms in genes that are associated with an increase in LDL-C: a “polygenic LDL score.”3 Prolonged exposure to high levels of LDL in patients with FH leads to the development of premature atherosclerosis. Compared with the general population, untreated patients with FH present with clinical ASCVD in the third decade for men and 10 years later in women. The prevalence of heterozygous FH is estimated at 1 of 250 in the general population and approximately 1 of 125 in patients with ASCVD.4, 5, 6
Despite FH being the most common monogenic disorder encountered in clinical practice, it is often not recognized clinically. The reasons for this are complex and may relate to the difficulty for clinicians in applying the current definitions. The accepted definitions for FH include the Dutch Lipid Clinic Network and Simon Broome Register criteria, as well as a new Canadian definition.1, 7 A diagnosis of FH relies on a baseline (ie, untreated) LDL-C level above 5 mmol/L in adults and clinical features with a point-based system that is often difficult to use in the clinical setting. Often, the baseline LDL-C is not available, because many patients are taking lipid-lowering drugs and the lack of electronic medical records makes it difficult to retrieve untreated LDL-C levels.
Therefore, a validated imputation was used to determine baseline LDL-C levels,8 and we examined the prevalence of FH in the Relating Evidence to Achieve Cholesterol Targets (REACT) registry, based on accepted international criteria and a new Canadian definition for FH.7 We used a computer algorithm, integrated into a practical clinical application, to determine the diagnosis of FH based on the criteria mentioned earlier (http://www.circl.ubc.ca/english/web_fh.html).9
Methods
The REACT registry was established to examine the need for further intensification of lipid-lowering therapies in patients with FH or ASCVD who did not reach the recommended LDL-C targets, despite maximally tolerated statins ± ezetimibe. The subjects gave written informed consent for the study after receiving a detailed explanation of the purposes, potential benefits, and risks associated with participation. The protocol was approved by the Ethics Review Board, University of Western Ontario. All data were de-identified, and the investigators were blinded to the patient’s physician or clinic of origin.
Inclusion criteria were as follows: (1) men and women aged > 18 years; (2) the presence of ASCVD defined as a documented acute coronary syndrome or myocardial infarction according to international criteria, prior arterial revascularization, or angiographically documented coronary artery disease (>50% cross-sectional stenosis of an epicardial coronary artery), the presence of documented cerebrovascular disease or peripheral arterial disease; or (3) the presence of FH defined by the treating physician according to accepted criteria (ie, documented physical signs of FH and presence of a first-degree relative with hypercholesterolemia or premature ASCVD as included in the Simon Broome Register10 or Dutch Lipid Clinic Network11 criteria); and (4) an LDL-C > 3.0 mmol/L when untreated (mostly due to perceived statin intolerance) or despite maximally tolerated lipid-lowering treatment.
A validated imputation for baseline LDL-C levels based on average response to statin and ezetimibe was used to determine baseline or untreated LDL-C levels according to lipid-lowering therapy when the baseline LDL-C was not available.8 Then, a clinical application was used to determine the presence of FH according to internationally accepted criteria (Simon Broome Register, Dutch Lipid Clinic Network) and the new simplified Canadian definition of FH (http://www.circl.ubc.ca/english/web_fh.html).9 Confirmation of FH with a molecular diagnosis was not available in this cohort.
On the basis of the described criteria, patients were classified as “definite FH,” “probable FH,” and hypercholesterolemia. The algorithm used to diagnose FH according to the new simplified Canadian FH criteria is shown in Figure 1. For the comparison of the Canadian definition with the 2 mostly used clinical definitions of FH, the proportion of probable/definite FH in subgroups of patients referred to as “probable FH” and “definite FH” as defined by the Dutch Lipid Clinic Network criteria and the “possible” and “definite FH” as defined by the Simon Broome Register criteria were used.
Figure 1.
Algorithm used to diagnose FH according to the simplified Canadian definition for the diagnosis of FH. *Secondary causes of high LDL-C should be ruled out (severe or untreated hypothyroidism, nephrotic syndrome, hepatic disease or biliary cirrhosis, and medication, especially antiretroviral agents). **Causal DNA mutation refers to the presence of a known FH-causing variant in the low-density lipoprotein receptor, apolipoprotein B, or PCSK9 gene based on presence of the variant in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/), HGMD (http://www.hgmd.cf.ac.uk/ac/index.php), or WDLV (https://www.ncbi.nlm.nih.gov/pubmed/23623477) databases in the proband or a first-degree relative. ASCVD, atherosclerotic cardiovascular disease; FH, familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol. Reproduced from Ruel et al.7 with permission from Elsevier.
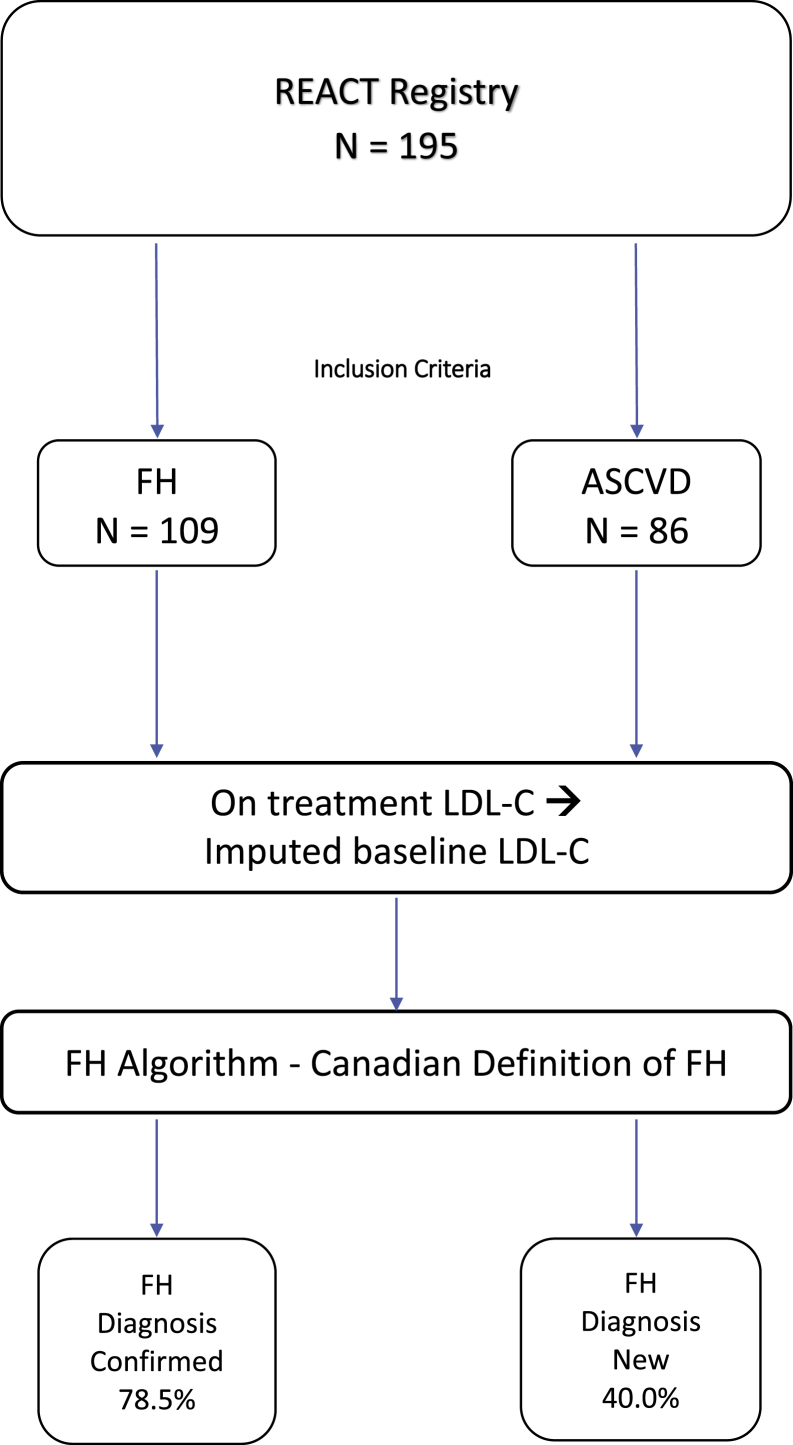
The REACT cohort was then separated into 2 subgroups: patients who were included on the basis of a clinical diagnosis of FH made by their treating physician and those enrolled because of the presence of ASCVD. The actual prevalence of FH was then determined in both groups. This allowed for validation of the clinician’s original diagnosis of FH, and for identification of “missed FH” patients in the ASCVD group. The study flow diagram is shown in Figure 2.
Figure 2.
Flow diagram of the REACT registry. There were 195 subjects with ASCVD or a diagnosis of FH; 4 patients had both diagnoses and were included in the FH group (n = 109), and 86 patients were included in the ASCVD group. ASCVD, atherosclerotic cardiovascular disease; FH, familial hypercholesterolemia; low-density lipoprotein cholesterol; REACT, Relating Evidence to Achieve Cholesterol Targets.
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics (version 24.0, IBM Corp., Armonk, NY). Descriptive variables were calculated for the entire cohort and then according to the inclusion criterion subgroups (ASCVD or FH). Continuous variables with a normal distribution were presented as mean ± standard deviation. Student t tests were used to determine if variables differed significantly between the ASCVD and FH subjects. Differences in categorical variables were expressed as numbers and percentages, and a statistical difference between groups was examined by Pearson’s chi-square test. P ≤ 0.05 was statistically significant.
Results
As part of the REACT registry project, 195 patients with established ASCVD or FH (diagnosed based on traditional criteria) with an LDL-C level > 3.0 mmol/L despite maximally tolerated statin therapy ± ezetimibe were prospectively enrolled. A validated imputation was used to determine baseline (untreated) LDL-C levels, along with other clinical criteria to determine prevalence of FH, using accepted international criteria and the new Canadian definition for FH.7 The enrolling physicians identified ASCVD in 90 patients and FH in 109 patients, with 4 patients having both. For the present analysis, we assigned the 4 patients with FH and ASCVD to the FH group. Table 1 shows the demographic and clinical data of the REACT cohort and the FH and ASCVD subgroups.
Table 1.
Baseline demographics of patients
| Characteristic | REACT cohort | FH inclusion | ASCVD inclusion | P value FH vs ASCVD |
|---|---|---|---|---|
| N | 195 | 109 | 86 | |
| Men/women | 109/86 | 61/48 | 48/38 | 0.98 |
| Age, y (mean ± SD) [range] | 63 ± 12 [28-89] | 60 ± 12 [28-87] | 66 ± 10 [40-89] | < 0.001 |
| BMI, kg/m2 (mean ± SD) | 28.9 ± 5.1 | 29.0 ± 5.2 | 28.7 ± 4.85 | 0.64 |
| Cardiovascular disease reported for inclusion (%) | 46.2% | 3.7% | 100% | < 0.001 |
| Family history of ASCVD (%) | 59.5% | 74.3% | 40.7% | < 0.001 |
| Systemic hypertension (%) | 62.1% | 51.4% | 75.6% | < 0.001 |
| Type 2 diabetes (%) | 26.2% | 21.1% | 32.6% | 0.07 |
| Smoking (past and current) (%) | 37.9% | 34.9% | 41.9% | 0.32 |
| Total cholesterol, mmol/L (mean ± SD) | 6.34 ± 1.18 | 6.50 ± 1.27 | 6.14 ± 1.02 | 0.03 |
| LDL-C, mmol/L (mean ± SD) | 4.26 ± 0.94 | 4.46 ± 1.00 | 4.02 ± 0.81 | < 0.001 |
| HDL-C, mmol/L (mean ± SD) | 1.29 ± 0.49 | 1.30 ± 0.43 | 1.28 ± 0.57 | 0.79 |
| Triglycerides, mmol/L (mean ± SD) | 2.29 ± 2.00 | 2.26 ± 2.21 | 2.32 ± 1.72 | 0.86 |
| Imputed baseline LDL-C (mean ± SD) | 7.04 ± 2.90 | 7.53 ± 2.93 | 6.41 ± 2.75 | 0.007 |
| Lipid-lowering therapy∗ (%) | 76.9% | 78.0% | 75.6% | 0.69 |
| Reported goal-inhibiting statin intolerance (%) | 64.1% | 66.10% | 61.6% | 0.52 |
| Any statin (%) | 60.0% | 62.4% | 57.0% | 0.44 |
| Any statin + ezetimibe 10 mg/d (%) | 15.9% | 15.6% | 16.3% | 0.90 |
| High-intensity statin (%)† | 35.4% | 36.7% | 33.7% | 0.67 |
| Moderate-intensity statin (%)† | 19.0% | 21.1% | 16.3% | 0.39 |
| Low-intensity statin (%)† | 5.6% | 4.6% | 7.0% | 0.47 |
ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; FH, familial hypercholesterolemia; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density cholesterol lipoprotein; REACT, Relating Evidence to Achieve Cholesterol Targets; SD, standard deviation.
Bold values denote statistical significance at the P < 0.05 level.
Any lipid-lowering therapy includes statins, ezetimibe, bile acid sequestrants, niacin, fibrates, and omega-3.
High-intensity statin includes atorvastatin 40-80 mg and rosuvastatin 20-40 mg; moderate-intensity statin includes atorvastatin 10-20 mg, rosuvastatin 5-10 mg, simvastatin 20-40 mg, pravastatin 40-80 mg, and fluvastatin 40 mg; low-intensity statin includes simvastatin 10 mg, pravastatin 5-20 mg, and fluvastatin 20 mg.
In the total group of patients, there were 109 men and 86 women (76% white) with a mean age of 63 ± 12 years; diabetes (29.7%), hypertension (62.1%), past/current smoking (37.9%), and family history of premature ASCVD (59.5%) were common. The on-treatment LDL-C was 4.26 ± 0.94 mmol/L; 76.9% of patients were taking lipid-lowering therapies with 35.4% receiving high-intensity statin (rosuvastatin 20 or 40 mg; atorvastatin 40 or 80 mg/d). An important proportion (64.1%) of all patients reported goal-inhibiting statin intolerance.
A validated clinical application was used to determine a diagnosis of FH incorporating clinical criteria. We have previously established the 95th percentile for LDL-C in a population of > 3 million subjects. By using a cut-point of LDL-C > 5.0 mmol/L as the baseline (untreated) value, 140 of 195 patients (72%) had severe hypercholesterolemia, the threshold for considering a diagnosis of FH in adults. The imputed baseline LDL-C was determined to be 7.04 ± 2.90 mmol/L, above the threshold of 5.0 mmol/L for the 95th percentile in adults aged > 40 years.8
A diagnosis of probable/definite FH was found in 54.7%, 49.5%, and 61.5% of patients according to the Simon Broome Register, Dutch Lipid Clinic Network criteria, and the new Canadian FH definition (Fig. 1), respectively (Table 2). Overall, approximately 55% of our patients fitted a definition for FH.
Table 2.
Proportion of probable/definite FH in subgroups of patients included in the registry because of FH versus ASCVD, according to known standardized clinical definitions of FH
| Dutch Lipid Clinic Network | Simon Broome Register | Canadian definition for FH | |
|---|---|---|---|
| REACT cohort (n = 195) | 49.5% | 54.7% | 61.5% |
| Subgroups: FH (n = 109) | 61.7% | 79.4% | 78.5% |
| ASCVD (n = 86) | 34.1% | 23.5% | 40.0% |
ASCVD, atherosclerotic cardiovascular disease; FH, familial hypercholesterolemia; REACT, Relating Evidence to Achieve Cholesterol Targets.
We then examined the FH and ASCVD groups separately (Fig. 2). Fewer patients had hypertension in the FH group than in the ASCVD group (51.4% vs. 75.6%, P < 0.001, Table 1). Surprisingly, body mass index and smoking were not significantly different between the FH and ASCVD groups (P = 0.64 and 0.32, respectively), whereas the presence of type 2 diabetes was likely more common in the ASCVD group (P = 0.07). Figure 3 shows individual patient data according to the LDL-C at entry into the study and the imputed baseline LDL-C according to the type and dose of medication. Figure 3 shows the FH subgroup of patients (left panel) and those with a diagnosis of ASCVD (right panel) at entry. The on-treatment and imputed baseline LDL-C for the patients with FH were 4.46 ± 1.00 mmol/L and 7.53 ± 2.93 mmol/L, and 4.02 ± 0.81 mmol/L and 6.41 ± 2.75 mmol/L in those with ASCVD, respectively. Although the diagnosis of FH was a requirement, some patients labeled as “FH” by their treating physician lacked the LDL-C criterion, as determined by the accepted FH definitions.
Figure 3.
Current or on-treatment LDL-C and imputed baseline LDL-C based on dose and type of lipid-lowering treatment in the FH (left) and ASCVD (right) inclusion group separately. The mean ± standard deviation is shown in red. ASCVD, atherosclerotic cardiovascular disease; FH, familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol.
Discussion
FH has a prevalence of approximately 0.004 (1/250) and accounts for approximately 0.008 (1/125) patients presenting in an acute coronary care setting.4, 5, 6 Untreated, FH leads to an inaugural ASCVD presentation in the fourth decade of life in men and 10 years later in women.3, 12 Registry data from The Netherlands show that early detection of FH through cascade screening and prompt initiation of statin use have led to an improvement in disease-free survival, approaching that of a normal population.13 Unfortunately, a lack of awareness on the part of physicians, the relative complexity of diagnosis on clinical grounds, and the lack of availability of genetic screening delay the diagnosis of FH until an ASCVD event occurs in many subjects.
In the current study, we focused on subjects with ASCVD and high residual cardiovascular risk because of an elevated LDL-C (>3.0 mmol/L despite maximally tolerated lipid-lowering therapies). It should be noted that goal-inhibiting statin intolerance was reported in 64% of this highly selected group of patients and that 51% of patients with ASCVD were not taking statins. This analysis of 195 patients from community and specialized clinics reveals that in patients with ASCVD and on-treatment LDL-C > 3 mmol/L, the prevalence of probable or definite FH is high, which is unrecognized. By using the imputed LDL-C as initial criterion for a diagnosis of FH, many patients have a baseline LDL-C > 5.0 mmol/L, the threshold for FH in adults aged > 40 years.6 In some patients, the imputed LDL-C was > 13 mmol/L, a threshold considered to reflect severe hypercholesterolemia, possibly homozygous FH. However, our imputed LDL-C estimation has the inherent limitation of presenting an average response to a specific dose of statin and ezetimibe and relies on the assumption that the patient is fully compliant. Therefore, a patient with an on-treatment LDL-C of 7 mmol/L but not taking the prescribed high-dose statin will have an imputed LDL-C > 13 mmol/L. Excluding all patients with an imputed LDL-C > 13 mmol/L did not significantly change our conclusions. The mean imputed baseline LDL-C after removal of patients with an imputed LDL-C > 13 mmol/L was 7.04 ± 2.31 mmol/L for the patients included with a diagnosis of FH and 6.14 ± 2.39 mmol/L for the patients with ASCVD as inclusion criterion (P = 0.01).
A diagnosis of FH guides the intensity of therapy, affects prognosis,5, 14 and has important implications for cascade screening and prevention in first-degree relatives.15, 16 Early diagnosis and treatment of FH can decrease the risk of ASCVD development. PCSK9 inhibitors have been found to markedly decrease LDL-C in patients with FH with or without a background of statin therapy.17 In high-risk patients with ASCVD with an elevated residual risk because of elevated LDL-C despite maximally tolerated statin therapy ± ezetimibe, evolocumab and alirocumab reduce cardiovascular outcomes in large, randomized clinical trials.18, 19 PCSK9 inhibitors are now recommended in such patients by national guidelines in Canada and the United States.20, 21, 22
In the present study, we used a practical clinical application to determine the presence of FH in a highly selected population of patients with a prior diagnosis of FH (according to the treating physician) or ASCVD with an elevated residual LDL-C despite maximally tolerated therapy. In the ASCVD high-risk population, 75.6% of patients were on a lipid-lowering therapy and only 33.7% were on high-intensity statins. In fact, approximately 2 of 3 of subjects in both groups reported partial or complete goal-inhibiting statin intolerance, which did not allow the patient to reach the recommended LDL-C target according to the Canadian Cardiovascular Society guidelines.20 In the patients with ASCVD, 34% to 40% of patients had probable/definite FH according to the Dutch Lipid Clinic Network or the new Canadian definition for FH. The Simon Broome Register criteria fared less well because, in many cases, the total cholesterol criterion was lacking.
Study limitations
There are important limitations to this study. The relatively small number of subjects included in the registry tends to suggest that this is, fortunately, not a frequently encountered problem and that for a majority of patients, current treatment with statins ± ezetimibe is adequate. The present group of subjects might not effectively represent the Canadian population with ASCVD but nevertheless raise awareness that among Canadians at high risk of cardiovascular disease and elevated LDL-C despite lipid-lowering therapy, a diagnosis of FH should be considered in a significant percentage. Second, the use of an imputed LDL-C may overestimate baseline LDL-C in some patients who are not compliant with their medication and lead to an inaccurate diagnosis of FH. Nevertheless, clinicians should be aware that an elevated LDL-C (> 3.0 mmol/L) in statin-treated subjects should raise the possibility of underlying FH, and more aggressive LDL-C lowering is warranted, as is cascade screening.
Conclusions
This REACT analysis reveals that a substantial proportion of patients with ASCVD whose LDL-C levels remain well above target have probable or definite FH. Use of an imputed LDL-C in statin-treated patients and simplified tools for FH diagnosis significantly help identify FH in patients. Clinicians should consider using the recently described algorithm to assess the possibility of FH in patients with ASCVD with an LDL > 3.0 mmol/L despite maximally tolerated statins. Appropriate diagnosis of FH in these patients not only guides aggressive therapy but also has important implications for cascade screening and prevention in first-degree relatives.
Acknowledgements
The authors thank the Canadian Collaborative Research Network team members for providing services in the management of the present clinical research project.
Funding Sources
This work was supported an unrestricted research grant provided by Sanofi-Canada for the REACT program to Canadian Collaborative Research Network.
Disclosures
Statements and opinions expressed in the articles and communications herein are those of the authors and not necessarily those of the Editors, Society, or publisher, and the Editors, Society, and publisher disclaim any responsibility or liability for such material. All authors have substantially contributed to the conception and design of the study, or acquisition of data, or analysis and interpretation of data. Briefly, M.Z., M.K., D.B., D.G., R.A.H., E.L., D.N., N.S., M.T., M.G., and J.G. contributed to the conception and design of the work, and to the acquisition of data. L.A., M.Z., S.A., I.R., M.T., M.G., and J.G. reviewed and analyzed the data. L.A. and J.G. drafted the manuscript. All co-authors critically revised the manuscript for important intellectual content, and all gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy. This manuscript is original, has not been published before, and is not currently being considered for publication elsewhere. No important aspects of the study have been omitted.
L.A., M.Z., S.A., M.K., D.B., D.N., I.R., and M.T. declared no potential conflicts of interest with respect to the research, authorship, or publication of this article. D.G. obtained research grants and honoraria from Akcea, Aegerion, Amgen, Gemphire, HDL Therapeutics, Ionis Pharmaceuticals, Novartis, Regeneron, and Sanofi; and received research grants from AstraZeneca, Esperion, Ironwood, Pfizer, and honoraria from Nestlé. R.A.H. reports consulting fees from Acasti, Aegerion, Akcea/Ionis, Amgen, and Sanofi. E.L. has obtained research support from Amgen, Sanofi, and Astra Zeneca and received consulting and lecture honoraria from Amgen and Sanofi. N.S. has obtained research grants and Speakers Bureau for Sanofi/Regeneron and Amgen. J.G. Co-Chairs FHCanada, a registry of FH in Canada, and has obtained research grants from Amgen, Sanofi, and Pfizer and honoraria from Amgen and Sanofi. M.G. held the grant provided by Sanofi Canada for the REACT program.
Footnotes
Ethics Statement: The research reported in this article has adhered to the relevant ethical guidelines.
See page 196 for disclosure information.
References
- 1.Genest J., Hegele R.A., Bergeron J. Canadian Cardiovascular Society position statement on familial hypercholesterolemia. Can J Cardiol. 2014;30:1471–1481. doi: 10.1016/j.cjca.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 2.Nordestgaard B.G., Chapman M.J., Humphries S.E. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34 doi: 10.1093/eurheartj/eht273. 3478-90a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Futema M., Shah S., Cooper J.A. Refinement of variant selection for the LDL cholesterol genetic risk score in the diagnosis of the polygenic form of clinical familial hypercholesterolemia and replication in samples from 6 countries. Clin Chem. 2015;61:231–238. doi: 10.1373/clinchem.2014.231365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akioyamen L.E., Genest J., Shan S.D. Estimating the prevalence of heterozygous familial hypercholesterolaemia: a systematic review and meta-analysis. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abul-Husn N.S., Manickam K., Jones L.K. Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science. 2016;354 doi: 10.1126/science.aaf7000. [DOI] [PubMed] [Google Scholar]
- 6.Nanchen D., Gencer B., Auer R. Prevalence and management of familial hypercholesterolaemia in patients with acute coronary syndromes. Eur Heart J. 2015;36:2438–2445. doi: 10.1093/eurheartj/ehv289. [DOI] [PubMed] [Google Scholar]
- 7.Ruel I., Brisson D., Aljenedil S. Simplified Canadian definition for familial hypercholesterolemia. Can J Cardiol. 2018;34:1210–1214. doi: 10.1016/j.cjca.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Ruel I., Aljenedil S., Sadri I. Imputation of baseline LDL cholesterol concentration in patients with familial hypercholesterolemia on statins or ezetimibe. Clin Chem. 2018;64:355–362. doi: 10.1373/clinchem.2017.279422. [DOI] [PubMed] [Google Scholar]
- 9.CardioRisk Calculator: simplifies cardiovascular risk stratification and a Canadian dyslipidemia guidelines application. http://www.circl.ubc.ca/cardiorisk-calculator.html Available at:
- 10.Risk of fatal coronary heart disease in familial hypercholesterolaemia. Scientific Steering Committee on behalf of the Simon Broome Register Group. BMJ. 1991;303:893–896. doi: 10.1136/bmj.303.6807.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . WHO; Geneva, Switzerland: 1999. Familial Hypercholesterolemia - Report of a Second WHO Consultation. [Google Scholar]
- 12.Perak A.M., Ning H., de Ferranti S.D. Long-term risk of atherosclerotic cardiovascular disease in us adults with the familial hypercholesterolemia phenotype. Circulation. 2016;134:9–19. doi: 10.1161/CIRCULATIONAHA.116.022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Versmissen J., Oosterveer D.M., Yazdanpanah M. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ. 2008;337:a2423. doi: 10.1136/bmj.a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khera A.V., Won H.H., Peloso G.M. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol. 2016;67:2578–2589. doi: 10.1016/j.jacc.2016.03.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gidding S.S., Champagne M.A., de Ferranti S.D. The Agenda for Familial Hypercholesterolemia: A Scientific Statement From the American Heart Association. Circulation. 2015;132:2167–2192. doi: 10.1161/CIR.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 16.Knowles J.W., Rader D.J., Khoury M.J. Cascade screening for familial hypercholesterolemia and the use of genetic testing. JAMA. 2017;318:381–382. doi: 10.1001/jama.2017.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qian L.J., Gao Y., Zhang Y.M. Therapeutic efficacy and safety of PCSK9-monoclonal antibodies on familial hypercholesterolemia and statin-intolerant patients: a meta-analysis of 15 randomized controlled trials. Sci Rep. 2017;7:238. doi: 10.1038/s41598-017-00316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabatine M.S., Giugliano R.P., Keech A.C. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz G.G., Steg P.G., Szarek M. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;369:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 20.Anderson T.J., Gregoire J., Pearson G.J. 2016 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can J Cardiol. 2016;32:1263–1282. doi: 10.1016/j.cjca.2016.07.510. [DOI] [PubMed] [Google Scholar]
- 21.Brunham L.R., Ruel I., Aljenedil S. Canadian Cardiovascular Society Position Statement on Familial Hypercholesterolemia: Update 2018. Can J Cardiol. 2018;34:1553–1563. doi: 10.1016/j.cjca.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Grundy S.M., Stone N.J., Bailey A.L. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]