Abstract
Background
Cerebral thromboembolism is a potentially devastating complication of atrial fibrillation (AF) and atrial flutter (AFl). The use of transesophageal echocardiogram (TEE) before electrophysiological procedures in anticoagulated patients is variable. Our objective was to determine the incidence and identify predictors of intracardiac left atrial appendage (LAA) thrombus on TEE in patients with AF/AFl before electrical cardioversion or ablation.
Methods
We reviewed TEEs of 401 patients undergoing an electrical cardioversion, AF, or AFl ablation from April 2013 to September 2015 at the McGill University Health Center. Clinical and echocardiographic variables were collected at the time of the TEE and follow-up visits. Multivariate logistic regression was used to determine predictors of LAA thrombus.
Results
Of 401 patients, 11.2% had LAA thrombus on TEE. The majority (87%) of patients were anticoagulated for at least 3 weeks before the TEE. The incidence of LAA thrombus was 21% (23/110) in patients taking warfarin vs 6.4% (15/236) in patients taking direct oral anticoagulants. Multivariate analysis identified prior stroke (odds ratio [OR], 2.7; 95% confidence interval [CI], 1.1-6.9) and heart failure (OR, 2.2; 95% CI, 1.0-4.7) as predictors of thrombus, whereas direct oral anticoagulant use (OR, 0.4; 95% CI, 0.2-0.8) was associated with reduced odds of thrombus.
Conclusions
LAA thrombus was identified in a significant proportion of patients undergoing TEE before cardioversion or ablation of AF/AFl despite preprocedural anticoagulation. Patients at increased risk of LAA thrombus (heart failure and prior stroke) may benefit from TEE before cardioversion, AF, or AFl ablation.
Résumé
Introduction
La thromboembolie cérébrale est une complication potentiellement dévastatrice de la fibrillation auriculaire (FA) et du flutter auriculaire. L’utilisation de l’échocardiographie transœsophagienne (ETO) avant les interventions en électrophysiologie chez les patients anticoagulés est variable. Notre objectif était de déterminer la fréquence et les prédicteurs des thrombi intracardiaques dans l’appendice auriculaire gauche (AAG) à l’ETO chez les patients atteints de FA ou de flutter auriculaire avant de procéder à une cardioversion électrique ou à une ablation.
Méthodes
Nous avons passé en revue les ETO de 401 patients qui avaient subi une cardioversion électrique, ou une ablation de la FA ou du flutter auriculaire entre avril 2013 et septembre 2015 au Centre universitaire de santé McGill. Nous avons recueilli les variables cliniques et échocardiographiques au moment de l’ETO et des visites de suivi. Nous avons utilisé la régression logistique multivariée pour déterminer les prédicteurs de thrombus dans l’AAG.
Résultats
Parmi les 401 patients, 11,2 % avaient un thrombus dans l’AAG à l’ETO. La majorité (87 %) des patients étaient anticoagulés au moins 3 semaines avant l’ETO. La fréquence des thrombus dans l’AAG était de 21 % (23/110) chez les patients qui prenaient de la warfarine vs 6,4 % (15/236) chez les patients qui prenaient des anticoagulants oraux directs. L’analyse multivariée a permis d’établir que l’accident vasculaire cérébral (AVC) antérieur (ratio d’incidence approché [RIA], 2,7; intervalle de confiance [IC] à 95 %, 1,1-6,9) et l’insuffisance cardiaque (RIA, 2,2; IC à 95 %, 1,0-4,7) étaient des prédicteurs de thrombus, alors que l’utilisation d’anticoagulants oraux directs (RIA, 0,4; IC à 95 %, 0,2-0,8) était associée une probabilité moindre de thrombus.
Conclusions
Une proportion importante de patients qui avaient subi l’ETO avant la cardioversion, ou l’ablation de la FA ou du flutter auriculaire avaient un thrombus dans l’AAG en dépit de l’anticoagulation avant l’intervention. Les patients exposés à un risque accru de thrombus dans l’AAG (insuffisance cardiaque et AVC antérieur) peuvent bénéficier de l’ETO avant la cardioversion, ou l’ablation de la FA ou du flutter auriculaire.
Atrial fibrillation (AF) is the most common arrhythmia in clinical practice, with an overall prevalence of 0.4% in the general population and more than 9% in patients aged more than 80 years.1, 2, 3 AF and atrial flutter (AFl) are associated with significant morbidity with a 2 to 5 times increased risk of stroke, transient ischemic attack (TIA), or systemic embolism, as well as a 1.5 times increased risk of mortality.4, 5, 6 In patients with nonvalvular AF, 89% of thrombi arise from the left atrial appendage (LAA), which is the main source of stroke and systemic embolism.7, 8, 9
Transesophageal echocardiography (TEE) is the gold standard for identifying preprocedural LAA thrombus and is routinely used before cardioversion and catheter ablation (CA) to minimize the risk of periprocedural stroke in patients who are not on therapeutic anticoagulation for at least 3 weeks before the procedure, as per international guidelines.10, 11, 12
TEE also may be performed in therapeutically anticoagulated patients at higher risk for LAA thrombi (eg, patients with prior LAA thrombus or prior stroke) or in those undergoing a left-sided electrophysiology (EP) procedure, such as AF ablation, in which catheters are introduced in close proximity to the LAA. The use of TEE in patients who are therapeutically anticoagulated before electrical cardioversion and CA for AF and AFl vary among centers. In the 2018 Heart Rhythm Society consensus statement on catheter and surgical ablation of AF, approximately 51% of the Task Force members would perform TEE in all patients undergoing AF ablation, regardless of the presenting rhythm and anticoagulation use.12
Various risk factors have been identified as predictors for preablation LAA thrombus detected by TEE in patients with AF/AFl, including the presence of structural heart disease, left atrial (LA) enlargement, and increased Congestive Heart Failure, Hypertension, Age, Diabetes, Stroke/Transient Ischemic Attack (CHADS2) scores.9, 13, 14, 15, 16 Randomized studies of direct oral anticoagulants (DOACs) have shown significant reductions in stroke and systemic embolism compared with warfarin, prompting further investigation of DOACs as a potentially protective agents for LAA thrombus in patients with AF/AFl.17, 18, 19
The purpose of this study was to determine the incidence and predictors of LAA thrombus identified in patients by TEE performed before cardioversion or AF and AFl ablation.
Methods
Study population
At McGill University Heath Centre (MUHC), all patients cared for by the EP service with AF or persistent AFl underwent TEE before cardioversion or AF/AFL ablation, regardless of duration of AF/AFl and anticoagulation. We conducted a retrospective review of 513 consecutive TEEs performed before emergent or elective CA or cardioversion of AF or AFl by the cardiac EP service at the MUHC from April 2013 to September 2015. We retained only the initial TEE for each patient and excluded 112 subsequent TEEs from patients who had more than 1 TEE. The final study cohort included 401 patients. The study was approved by the MUHC Research Ethics Board.
Outcome and comorbidities
We extracted demographic and clinical information at the time of TEE before the EP procedure (baseline) and at all follow-up visits. Information on age, sex, arrhythmia type (AFl, paroxysmal AF, persistent AF), traditional cardiac risk factors, and type and duration of anticoagulation therapy was collected for each patient. In addition, CHADS2 and Congestive Heart Failure, Hypertension, Age (≥ 75 years), Diabetes, Stroke/Transient Ischemic Attack, Vascular Disease, Age (65-74 years), Sex (Female) (CHA2DS2-VASc) scores were calculated.20
For patients on warfarin therapy, time in therapeutic range (TTR) was calculated as the fraction of international normalized ratios (INRs) in therapeutic range during the month before the TEE (for patients with at least 2 INR results available within this 30-day period). All patients underwent TEE whether or not their INR was therapeutic. Patients with a negative TEE had their procedure performed and anticoagulation postprocedure adjusted if required.
Echocardiographic data collected included left ventricular ejection fraction (LVEF), LA diameter and volume, presence of valvular heart disease (defined as moderate to severe mitral or aortic stenosis or regurgitation, or mechanical prosthetic valve), and presence of LAA thrombus. Spontaneous echo contrast or sludge without thrombus was not classified as LAA thrombus. TEEs were analyzed by 2 level 3 echocardiography cardiologists to ascertain the presence of LAA thrombus. Complications related to TEEs were also recorded.
Statistical analysis
Continuous variables were presented as median values (interquartile range [IQR]) and compared using the Mann–Whitney–Wilcoxon test. Age, sex, hypertension, diabetes, heart failure, prior stroke, vascular disease (defined as prior myocardial infarction, peripheral arterial disease, or aortic plaque), anticoagulation therapy (DOAC or warfarin), and type of arrhythmia (paroxysmal AF, persistent AF, or AFl) were potential predictors in the multivariate logistic regression. An additional multivariate logistic regression was performed with the inclusion of valvular disease as a predictor in a subset of the patients with available information on the presence of valvular disease. LA diameter, LVEF, and laboratory measures were not included in the model because of missing data. Predictors of LAA thrombus were determined using multivariate logistic regression modeling with stepwise selection (P ≤ 0.2). A P value of ≤ 0.05 was considered statistically significant.
Results
Study population and anticoagulation therapy
Baseline characteristics of the 401 patients are displayed in Table 1. The median age of the cohort was 65 years (IQR, 56-76 years), and 25.9% were female. Most patients had paroxysmal AF (43%) followed by AFl (36%) and persistent AF (8%). The majority of patients (87%) were anticoagulated: 27.4% on warfarin, 22.9% on rivaroxaban, 21.4% on apixaban, and 15.2% on dabigatran. Compared with patients on DOACs, patients on warfarin had more congestive heart failure, hypertension, diabetes, coronary artery disease, valvular disease, and poor renal function (P < 0.05 for all of the stated comorbidities). The mean CHADS2 score was (1.3 ± 1.3). The distribution of CHA2DS2-VASc scores was 15.9% with a score of 0, 21.9% with a score of 1, 18.9% with a score of 2, 18.9% with a score of 3, and 24.4% with a score of 4 and more. Baseline characteristics were also presented by type of procedure (cardioversion, AF ablation, and AFl ablation) in Supplemental Table S1.
Table 1.
Baseline characteristics
| Baseline characteristics | All patients (N = 401) N (%) | LAA thrombus (N = 45) (%) | No LAA thrombus (N = 356) (%) | P value |
|---|---|---|---|---|
| Age, y [median, IQR] | 65 (56-72) | 69 (60-76) | 65 (56-72) | 0.02 |
| < 65 | 185 (46.0) | 18 (40.0) | 167 (46.8) | 0.02 |
| 65-75 | 141 (35.1) | 13 (28.9) | 128 (35.9) | 0.39 |
| ≥ 75 | 76 (18.9) | 14 (31.1) | 62 (17.4) | 0.026 |
| Female | 104 (25.9) | 13 (28.9) | 91 (25.5) | 0.62 |
| Type of arrhythmia (N = 375): | ||||
| Paroxysmal AF | 149 (39.7) | 10 (25.0) | 139 (41.6) | 0.04 |
| Persistent AF | 71 (18.9) | 13 (32.5) | 57 (17.1) | 0.018 |
| AFl | 155 (41.3) | 17 (42.5) | 138 (41.3) | 0.89 |
| Hypertension (N = 382) | 173 (43.4) | 20 (47.6) | 153 (45.1) | 0.76 |
| Diabetes (N = 381) | 85 (21) | 13 (31.0) | 73 (21.6) | 0.172 |
| Cerebrovascular accident/TIA (N = 380) | 34 (9) | 9 (21.4) | 25 (7.4) | 0.003 |
| Heart failure (N = 381) | 135 (35.4) | 27 (62.8) | 108 (32.0) | < 0.001 |
| Vascular disease (n = 380) | 89 (23.4) | 14 (33.3) | 75 (22.2) | 0.108 |
| Valvular disease (n = 294) | 60 (20.3) | 20 (54.1) | 40 (15.5) | < 0.001 |
| CHADS2 score [median, IQR] | 1 (0-2) | 2 (1-3) | 1 (0-2) | 0.008 |
| CHA2DS2-VASc score [median, IQR] | 2 (1-3) | 3 (2-4) | 2 (1-3) | 0.002 |
| 0 | 64 (15.9) | 3 (6.7) | 61 (17.1) | 0.072 |
| 1 | 88 (21.9) | 6 (13.3) | 82 (23.0) | 0.141 |
| 2 | 76 (18.9) | 8 (17.8) | 68 (19.1) | 0.84 |
| 3 | 76 (18.9) | 12 (26.7) | 64 (17.9) | 0.16 |
| ≥ 4 | 98 (24.4) | 16 (35.6) | 82 (23.0) | 0.06 |
| Left atrial diameter by TTE (N = 94) | 43 (39-46) | 48 (42-54) | 43 (38-46) | 0.018 |
| Left ventricular ejection fraction (N = 295) | 58 (43-60) | 50 (20-60) | 58 (45-60) | 0.013 |
| Labs [median, IQR] | ||||
| Hemoglobin (N = 320) | 136 (125-147) | 136 (125-147) | 135 (123-144) | 0.41 |
| Creatinine (N = 328) | 86 (73-100) | 86 (73-99) | 93.5 (77.5-120.5) | 0.082 |
| INR (N = 105)∗ | 2.4 (1.9-2.8) | 2.5 (1.9-2.8) | 2.4 (1.9-2.7) | 0.55 |
| Type of procedure | ||||
| Cardioversion | 78 (19.5) | 11 (24.4) | 65 (18.3) | 0.30 |
| AF ablation | 175 (43.6) | 16 (37.2) | 159 (44.7) | 0.25 |
| AFl ablation | 148 (36.9) | 16 (35.5) | 132 (37.1) | 0.84 |
| Anticoagulation/antiplatelets | ||||
| Warfarin | 110 (27.4) | 23 (51.1) | 81 (24.4) | < 0.001 |
| Direct oral anticoagulants | 236 (61.9) | 15 (35.7) | 221 (65.2) | < 0.001 |
| Apixaban | 86 (21.4) | 8 (17.8) | 78 (21.9) | 0.53 |
| Low dose | 2 (0.5) | 0 (0) | 2 (0.6) | 0.62 |
| High dose | 84 (20.9) | 8 (17.8) | 76 (21.3) | 0.59 |
| Dabigatran | 58 (15.2) | 3 (7.1) | 55 (16.2) | 0.12 |
| Low dose | 8 (2.0) | 0 (0.0) | 8 (2.3) | 0.31 |
| High dose | 50 (13.1) | 3 (7.1) | 47 (13.8) | 0.23 |
| Rivaroxaban | 92 (22.9) | 4 (8.9) | 88 (24.7) | 0.018 |
| Low dose | 4 (1) | 0 (0) | 4 (1.1) | 0.48 |
| High dose | 88 (2.9) | 4 (8.9) | 84 (23.5) | 0.03 |
| Antiplatelet agents | 35 (9.2) | 3 (7.2) | 32 (9.4) | 0.63 |
| None | 21 (5.2) | 4 (8.9) | 17 (4.8) | 0.24 |
AF, atrial fibrillation; AFl, atrial flutter; CHADS2, Congestive Heart Failure, Hypertension, Age, Diabetes, Stroke/Transient Ischemic Attack; CHA2DS2-VASc, Congestive Heart Failure, Hypertension, Age (≥ 75 years), Diabetes, Stroke/Transient Ischemic Attack, Vascular Disease, Age (65-74 years), Sex (Female); INR, international normalized ratio; IQR, interquartile range; LAA, left atrial appendage; TIA, transient ischemic attack; TTE, transthoracic echocardiogram.
INR values presented only for patients on warfarin.
Incidence of preprocedural LAA thrombus on TEE
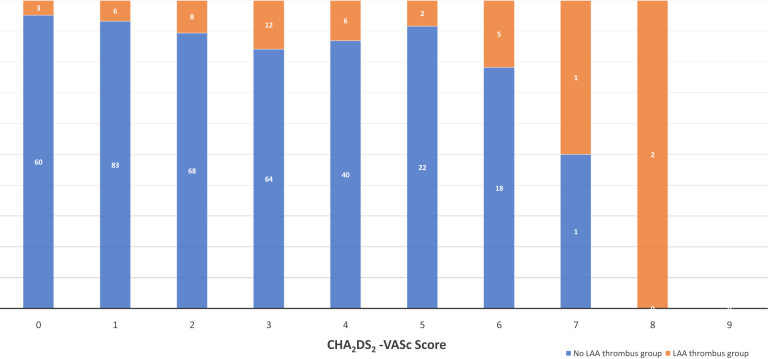
Of 401 patients who underwent preprocedural TEE, 45 (11.2%) had LAA thrombus documented by TEE. Patients with documented LAA thrombus were more likely to be older (69 years [IQR, 60-76] vs 65 years [IQR, 56-72]), to have a prior cardiovascular accident (CVA)/TIA (21.4% vs 7.4%), to have heart failure (62.8% vs 32.0%), to have persistent AF (33% vs 17%), to have valvular disease (54.1% vs 15.5%), to have increased LA diameter (48 cm [IQR, 42-54] vs 43 cm [IQR 38-46]), and to have reduced LVEF (50% [IQR, 20-60] vs 58% [IQR, 45-60]) (patients with LAA thrombus vs no LAA thrombus, P < 0.05 for all) (Table 1). Increased age and comorbidities translated to significantly higher CHADS2 and CHA2DS2-VASc scores among patients with LAA thrombus (P < 0.05 for both). Nevertheless, the incidence of LAA thrombus on TEE among patients with low CHA2DS2-VASc score was not negligible: LAA thrombus was detected in 3 patients (4.7%), 6 patients (6.7%), and 8 patients (10.5%) with CHA2DS2-VASc scores of 0, 1, and 2, respectively (Fig. 1).
Figure 1.
Prevalence of left atrial appendage (LAA) thrombus on transesophageal echocardiography (TEE) before cardiac electrophysiology (EP) procedures based on Congestive Heart Failure, Hypertension, Age (≥ 75 years), Diabetes, Stroke/Transient Ischemic Attack, Vascular Disease, Age (65-74 years), Sex (Female) (CHA2DS2-VASc) score.
Among anticoagulated patients, there was a higher prevalence of LAA thrombus in patients taking warfarin (21%) compared with patient taking DOACs (6.4%) (P < 0.001). For patients taking warfarin with at least 2 INR measurements within 30 days before TEE (n = 70), the mean TTR was 50% (mean TTR of 36.8% for patients with LAA thrombus compared with 52.7% for patients without LAA thrombus, P = 0.19). Of these patients, 15 (21.4%) had a TTR of 100% (including 3 patients referred for cardioversion, 6 patients referred for AF ablation, and 6 patients referred for AFL ablation). The prevalence of LAA thrombus among patients with a TTR of 100% was 6.7% (0.0%, 16.7%, and 0.0% of patients referred for cardioversion, AF ablation, and AFL ablation, respectively). All patients with LAA thrombus had their EP procedure delayed and anticoagulation therapy reevaluated.
Predictors of LAA thrombus
Univariate regressions demonstrate that most potential predictors were associated with the presence of LAA thombus except for DOAC therapy (Supplemental Table S2). In a multivariate regression analysis, prior CVA/TIA (odds ratio [OR], 2.71; 95% confidence interval [CI], 1.07-6.85) and heart failure (OR, 2.21; 95% CI, 1.04-4.71) were independently associated with increased odds of LAA thrombus (Table 2). DOAC use (OR, 0.40; 95% CI, 0.19-0.82) was associated with reduced odds of preprocedure LAA thrombus (Table 2). An additional multivariable analysis limited to patients with information regarding valvular heart disease found that prior CVA/TIA (OR, 3.24; 95% CI, 1.07-9.78) and valvular heart disease (OR, 3.93; 95% CI, 1.58-9.79) were independently associated with increased odds of LAA thrombus.
Table 2.
Multivariable analysis of potential predictors of LAA thrombus on TEE
| Covariates | OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.02 | 0.99-1.06 | 0.13 |
| Heart failure | 2.21 | 1.04-4.71 | 0.04 |
| Prior stroke | 2.71 | 1.07-6.85 | 0.04 |
| Direct oral anticoagulants | 0.40 | 0.19-0.82 | 0.01 |
CI, confidence interval; LAA, left atrial appendage; OR, odds ratio; TEE, transesophageal echocardiogram.
Periprocedural stroke and systemic embolic events
We observed 1 periprocedural thromboembolic event. The patient was a 49-year-old man with paroxysmal AF and a CHA2DS2VASc score 2 (for prior CVA) who underwent electrical cardioversion. There was spontaneous echo contrast without thrombus noted on his preprocedure TEE. He was taking warfarin with an INR of 1.88 the day he was cardioverted and was being bridged with heparin (with continued warfarin until INR was > 2). He developed right middle cerebral artery ischemia the following day with resolution of symptoms with conservative management.
Discussion
Our study demonstrates that among patients with AF and AFl undergoing a preprocedural TEE (1) 11.2% of patients had an LAA thrombus; (2) had prior CVA/TIA event, heart failure, and valvular heart disease were associated with increased odds of preprocedural LAA thrombus; and (3) DOAC use was associated with a reduced odds of LAA thrombus.
Prevalence of LAA thrombus
The prevalence of LAA thrombus in our retrospective review was high compared with current literature.9, 13, 14, 21, 22 Among patients who were therapeutically anticoagulated before TEE, the reported prevalence of thrombus ranged from 0.3% and 7.7% compared with 11.2% in our study.9, 13, 14, 15, 21, 22, 23, 24 The high prevalence of LAA thrombus in our patients may be due to a sicker population at our center, including a higher proportion of patients with valvular disease (20%), heart failure (35%), and mean TTR of 50% among warfarin-treated patients. Prior studies excluded patients with valvular disease or sampled a small portion of the population with valvular heart disease and AF (6%-11%).12, 13, 15, 21, 22, 23 A higher proportion of patients with valvular heart disease and heart failure included in our cohort may partially explain the increased prevalence of LAA thrombus.22, 25, 26 In addition, although the proportion of patients with persistent AF is similar in our cohort as in other studies, the duration of time in persistent AF before the procedure may be longer in our center because our practice included cardioversion or ablation of patients with long-standing persistent AF and heart failure. A recently published Canadian study22 reported a significantly lower prevalence of LAA thrombus (0.3%) compared with our study and other studies already cited.9, 13, 14, 15, 21, 22, 23, 24 In addition to reporting on a lower-risk population limited to patients undergoing AF ablation, the low rate of thrombus may have been the result of a strict protocol whereby all patients were anticoagulated with DOAC or warfarin for at least 4 weeks and patients on warfarin were seen in a dedicated clinic to ensure the INR was therapeutic during the 4 weeks before AF ablation.22 However, the present study may more closely reflect real-world anticoagulation practice because preprocedural INRs were not managed using a strict protocol and all patients underwent TEE regardless of anticoagulation status or preprocedural INR value.
Clinical risk factors for LAA thrombus
CHADS2 and CHA2DS2-VASc score have consistently been identified as predictors of LAA thrombus; however, the individual comorbidities of heart failure and prior CVA/TIA have shown varied results.13, 23, 27, 28, 29, 30, 31, 32, 33 Our study results were similar to those of Wysokinski et al.,32 who reported an increased odds of thrombus associated with congestive heart failure (OR, 5.12; 95% CI, 2.91-9.03) and prior stroke (OR, 2.56; 95% CI, 1.37-4.76). Fukuda et al.,23 Willens et al.,30 and Yamashita et al.33 also showed heart failure as a risk factor in multivariate analyses, although prior CVA/TIA was not shown to be predictive of LAA thrombus. Our study did not show an association between diabetes and LAA thrombus risk. We found a higher rate of thrombi in patients with persistent AF, but the association was not significant in multivariate analysis.30, 32, 33 Wysokinski et al.32 found that permanent AF (vs nonpermanent AF) was associated with increased risk of LAA thrombus in multivariate analysis (OR, 2.49; 95% CI, 1.26-4.93).
The present study was not able to investigate the association with LA enlargement in multivariable analyses; however, like other studies, our univariate analysis indicated a significant difference between LA diameter in patients with and without thrombus.30, 32, 33 Scherr et al.13 found that LA enlargement was an independent predictor of LAA thrombus in patients with AF before AF ablation (OR, 1.6; 95% CI, 1.1-2.3);13 however, Tang et al.27 found that LA enlargement did not predict LAA thrombus (OR, 1.04; 95% CI, 0.95-1.13).
DOAC associated with reduced LAA thrombus
The presence of thrombus was greater in those taking warfarin at 21% compared with those taking a DOAC (6.3%). The differential distribution of comorbidities among those taking warfarin or DOAC may partly explain the increased incidence of LAA thrombus in warfarin users. Specifically, patients taking warfarin had more congestive heart failure, hypertension, diabetes, coronary artery disease, impaired renal function, and valvular heart disease, and higher CHA2DS2-VASc scores as opposed to patients on DOAC; however, the significance of all these variables could not be tested because of missing data.
The TTR in patients with LAA thrombus on warfarin in our study was 36.8% vs 52.7% for the subgroup with no LAA thrombus on warfarin. Poor TTR is associated with higher rates of stroke and thromboembolism.34 The mean TTRs in the warfarin groups in the pivotal anticoagulation trials Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY), Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonist for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF), and Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) were 64%, 58%, and 62%, respectively,35, 36, 37, 38 which is higher than in our study (50.0%).
The incidence of LAA thrombus in the DOAC subgroup in our study was not negligible. The rate of LAA thrombus in the dabigatran, rivaroxaban, and apixaban groups were 5%, 4%, and 9%, respectively; however, information on compliance with DOAC therapy was not available. These findings are consistent with Frenkel et al.’s19 retrospective analysis of 388 initial TEEs of patients on 4 weeks of continuous DOAC or warfarin therapy before CA of AF and AFl. LAA thrombus was found in 4.4% of the DOAC group vs 2.9% of the warfarin group (P = 0.45).19
The Randomized Evaluation of Dabigatran Etexilate Compared to Warfarin in Pulmonary Vein Ablation: Assessment of an Uninterrupted Peri-Procedural Anticoagulation Strategy (RE-CIRCUIT) study randomized patients (mean CHA2DS2-VASc = 2.1, 68% paroxysmal AF) to warfarin (318/635) or uninterrupted dabigatran (317/635) for 4 to 8 weeks before AF ablation, and all patients underwent TEE imagining before the procedure. LAA thrombus was reported in 1 patient. On follow-up, 1 patient in the warfarin group had a periprocedural TIA during follow-up.39 Another prospective study comparing DOAC therapy with warfarin before AF ablation was the Active-Controlled Multi-Center Study With Blind-Adjudication Designed to Evaluate the Safety of Uninterrupted Rivaroxaban and Uninterrupted Vitamin K Antagonists in Subjects Undergoing Catheter Ablation for Non-Valvular Atrial Fibrillation (VENTURE-AF) trial, in which 248 patients were randomized to periprocedural anticoagulation with rivaroxaban 20 mg once daily vs uninterrupted warfarin therapy (target INR 2-3). Paroxysmal AF was present in 75% of patients, and both groups had a low mean CHA2DS2VASc score of 1.5 to 1.7. At 30 days, there were 2 thromboembolic events in the warfarin arm compared with 0 in the rivaroxaban arm.40
The incidence of LAA thrombus in patients on DOAC therapy in the major DOAC studies was as follows. In a subgroup analysis of the Re-Ly trial, the rates of LA thrombus detection among patients undergoing TEE before cardioversion was 1.5% for patients on dabigatran.41 In the ARISTOTLE trial, TEE records were available in 86 patients on apixaban and none of these patients had LA thrombus.42 In the ROCKET-AF trial, there were no TEE data collected to assess prevalence of LA thrombus in patients on rivaroxaban undergoing cardioversions or CA of AF.43
Study limitations
The main limitation of our study is that it presents a single-center experience that may differ from other centers because of possible treatment differences and distribution of risk factors. Potentially important predictors of LAA thrombus, including LA diameter, LVEF, and valvular heart disease, were excluded from multivariate analysis because of missing data. Transthoracic echocardiograms performed at other centers were not available for review or had missing measurements. Moreover, we could not verify compliance to DOAC therapy, and the TTR with warfarin therapy was only 50%.
Conclusion
The incidence of LAA thrombus in patients who are anticoagulated before cardioversion or ablation procedure for AF or AFl is not insignificant, even in low-risk patients. Although the CHADS2 and CHA2DS2VASc scores are predictive of the presence of LAA thrombus, identification of specific risk factors, including prior CVA/TIA, congestive heart failure, and valvular heart disease, further stratify the specific patients at risk for thrombus before an electrophysiological procedure. The role of other potential risk factors, including LA enlargement and valvular disease, warrants additional evaluation. The use of DOACs preprocedure may reduce but not entirely eliminate the possibility of LAA thrombus, particularly in high-risk patients. The value of routine TEE in high-risk patients despite therapeutic anticoagulation before EP procedures requires further investigation.
Funding Sources
This work was supported by a Clinical Research Scholar Award to Vidal Essebag from Fonds de Recherche du Quebec - Santé and funding from Bayer Inc.
Disclosures
V.E. has received honoraria from Bayer, Boehringer Ingelheim, Pfizer, and Servier. T.H. has received research grants and honoraria from Bayer, Boehringer Ingelheim, Pfizer, and Servier. G.T. is a consultant for Ionis Pharmaceuticals and has received honoraria from Amgen, Sanofi, Servier Canada, and Boehringer Ingelheim. The remaining authors have no disclosures.
Footnotes
Ethics Statement: The research reported in this paper has adhered to research data guidelines.
See page 236 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca and at https://doi.org/10.1016/j.cjco.2019.06.004.
Supplementary Material
References
- 1.Kannel W.B., Wolf P.A., Benjamin E.J., Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 2.Feinberg W.M., Blackshear J.L., Laupacis A., Kronmal R., Hart R.G. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 3.Go A.S., Hylek E.M., Phillips K.A. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin E.J., Wolf P.A., D’Agostino R.B. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 5.Wolf P.A., Abbott R.D., Kannel W.B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 6.Miyasaka Y., Barnes M.E., Bailey K.R. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol. 2007;49:986–992. doi: 10.1016/j.jacc.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 7.Blackshear J.L., Odell J.A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 8.Stoddard M.F., Singh P., Dawn B., Longaker R.A. Left atrial thrombus predicts transient ischemic attack in patients with atrial fibrillation. Am Heart J. 2003;145:676–682. doi: 10.1067/mhj.2003.91. [DOI] [PubMed] [Google Scholar]
- 9.Zhan Y., Joza J., Al Rawahi M. Assessment and management of the left atrial appendage thrombus in patients with nonvalvular atrial fibrillation. Can J Cardiol. 2018;34:252–261. doi: 10.1016/j.cjca.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Andrade J.G., Verma A., Mitchell L.B. 2018 Focused Update of the Canadian Cardiovascular Society Guidelines for the Management of Atrial Fibrillation. Can J Cardiol. 2018;34:1371–1392. doi: 10.1016/j.cjca.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Kirchhof P., Benussi S., Kotecha D. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 12.Calkins H., Hindricks G., Cappato R. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1–e160. doi: 10.1093/europace/eux274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scherr D., Dalal D., Chilukuri K. Incidence and predictors of left atrial thrombus prior to catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:379–384. doi: 10.1111/j.1540-8167.2008.01336.x. [DOI] [PubMed] [Google Scholar]
- 14.Wallace T.W., Atwater B.D., Daubert J.P. Prevalence and clinical characteristics associated with left atrial appendage thrombus in fully anticoagulated patients undergoing catheter-directed atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2010;21:849–852. doi: 10.1111/j.1540-8167.2010.01729.x. [DOI] [PubMed] [Google Scholar]
- 15.Puwanant S., Varr B.C., Shrestha K. Role of the CHADS2 score in the evaluation of thromboembolic risk in patients with atrial fibrillation undergoing transesophageal echocardiography before pulmonary vein isolation. J Am Coll Cardiol. 2009;54:2032–2039. doi: 10.1016/j.jacc.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 16.Khan M.N., Usmani A., Noor S. Low incidence of left atrial or left atrial appendage thrombus in patients with paroxysmal atrial fibrillation and normal EF who present for pulmonary vein antrum isolation procedure. J Cardiovasc Electrophysiol. 2008;19:356–358. doi: 10.1111/j.1540-8167.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 17.Di Biase L., Briceno D.F., Trivedi C. Is transesophageal echocardiogram mandatory in patients undergoing ablation of atrial fibrillation with uninterrupted novel oral anticoagulants? Results from a prospective multicenter registry. Heart Rhythm. 2016;13:1197–1202. doi: 10.1016/j.hrthm.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Ruff C.T., Giugliano R.P., Braunwald E. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 19.Frenkel D., D’Amato S.A., Al-Kazaz M. Prevalence of left atrial thrombus detection by transesophageal echocardiography: a comparison of continuous non-vitamin k antagonist oral anticoagulant versus warfarin therapy in patients undergoing catheter ablation for atrial fibrillation. JACC Clin Electrophysiol. 2016;2:295–303. doi: 10.1016/j.jacep.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Lip G.Y., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 21.Zoppo F., Brandolino G., Berton A. Predictors of left atrium appendage clot detection despite on-target warfarin prevention for atrial fibrillation. J Interv Card Electrophysiol. 2012;35:151–158. doi: 10.1007/s10840-012-9707-0. [DOI] [PubMed] [Google Scholar]
- 22.Alqarawi W., Birnie D.H., Spence S. Prevalence of left atrial appendage thrombus detected by transoesophageal echocardiography before catheter ablation of atrial fibrillation in patients anticoagulated with non-vitamin K antagonist oral anticoagulants. Europace. 2019;21:48–53. doi: 10.1093/europace/euy129. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda S., Watanabe H., Shimada K. Left atrial thrombus and prognosis after anticoagulation therapy in patients with atrial fibrillation. J Cardiol. 2011;58:266–277. doi: 10.1016/j.jjcc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Wu X., Wang C., Zhang C. Computed tomography for detecting left atrial thrombus: a meta-analysis. Arch Med Sci. 2012;8:943–951. doi: 10.5114/aoms.2012.32400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petty G.W., Khandheria B.K., Whisnant J.P. Predictors of cerebrovascular events and death among patients with valvular heart disease: a population-based study. Stroke. 2000;31:2628–2635. doi: 10.1161/01.str.31.11.2628. [DOI] [PubMed] [Google Scholar]
- 26.De Caterina R., Camm A.J. What is ‘valvular’ atrial fibrillation? A reappraisal. Eur Heart J. 2014;35:3328–3335. doi: 10.1093/eurheartj/ehu352. [DOI] [PubMed] [Google Scholar]
- 27.Tang R.B., Dong J.Z., Liu X.P. Is CHA2DS2-VASc score a predictor of left atrial thrombus in patients with paroxysmal atrial fibrillation? Thromb Haemost. 2011;105:1107–1109. doi: 10.1160/TH10-12-0800. [DOI] [PubMed] [Google Scholar]
- 28.Tang R.B., Liu X.H., Kalifa J. Body mass index and risk of left atrial thrombus in patients with atrial fibrillation. Am J Cardiol. 2009;104:1699–1703. doi: 10.1016/j.amjcard.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akoum N., Fernandez G., Wilson B. Association of atrial fibrosis quantified using LGE-MRI with atrial appendage thrombus and spontaneous contrast on transesophageal echocardiography in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24:1104–1109. doi: 10.1111/jce.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willens H.J., Gomez-Marin O., Nelson K., DeNicco A., Moscucci M. Correlation of CHADS2 and CHA2DS2-VASc scores with transesophageal echocardiography risk factors for thromboembolism in a multiethnic United States population with nonvalvular atrial fibrillation. J Am Soc Echocardiogr. 2013;26:175–184. doi: 10.1016/j.echo.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Dorenkamp M., Sohns C., Vollmann D. Detection of left atrial thrombus during routine diagnostic work-up prior to pulmonary vein isolation for atrial fibrillation: role of transesophageal echocardiography and multidetector computed tomography. Int J Cardiol. 2013;163:26–33. doi: 10.1016/j.ijcard.2011.06.124. [DOI] [PubMed] [Google Scholar]
- 32.Wysokinski W.E., Ammash N., Sobande F. Predicting left atrial thrombi in atrial fibrillation. Am Heart J. 2010;159:665–671. doi: 10.1016/j.ahj.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita E., Takamatsu H., Tada H. Transesophageal echocardiography for thrombus screening prior to left atrial catheter ablation. Circ J. 2010;74:1081–1086. doi: 10.1253/circj.cj-09-1002. [DOI] [PubMed] [Google Scholar]
- 34.White H.D., Gruber M., Feyzi J. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med. 2007;167:239–245. doi: 10.1001/archinte.167.3.239. [DOI] [PubMed] [Google Scholar]
- 35.Granger C.B., Alexander J.H., McMurray J.J. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 36.Pokorney S.D., Simon D.N., Thomas L. Patients’ time in therapeutic range on warfarin among US patients with atrial fibrillation: results from ORBIT-AF registry. Am Heart J. 2015;170(141-8):8.e1. doi: 10.1016/j.ahj.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Connolly S.J., Ezekowitz M.D., Yusuf S. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 38.Patel M.R., Mahaffey K.W., Garg J. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 39.Calkins H., Willems S., Gerstenfeld E.P. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med. 2017;376:1627–1636. doi: 10.1056/NEJMoa1701005. [DOI] [PubMed] [Google Scholar]
- 40.Cappato R., Marchlinski F.E., Hohnloser S.H. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J. 2015;36:1805–1811. doi: 10.1093/eurheartj/ehv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagarakanti R., Ezekowitz M.D., Oldgren J. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation. 2011;123:131–136. doi: 10.1161/CIRCULATIONAHA.110.977546. [DOI] [PubMed] [Google Scholar]
- 42.Flaker G., Lopes R.D., Al-Khatib S.M. Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) J Am Coll Cardiol. 2014;63:1082–1087. doi: 10.1016/j.jacc.2013.09.062. [DOI] [PubMed] [Google Scholar]
- 43.Piccini J.P., Stevens S.R., Lokhnygina Y. Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. J Am Coll Cardiol. 2013;61:1998–2006. doi: 10.1016/j.jacc.2013.02.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.