Abstract
Background
Endocardial lead in the right ventricle is recognized as a cause for tricuspid regurgitation (TR), but the mechanism remains elusive. We sought to evaluate lead-specific features on the development of TR after endocardial lead implantation.
Methods
This was a prospective single-center study. The patients underwent 2-dimensional echocardiograms before endocardial lead implantation and at follow-up visits at 4 to 6 weeks, 6 months, and 12 months. We assessed the position of the endocardial lead at the tricuspid annulus by 3-dimensional echocardiography, the tricuspid leaflet interference by the endocardial lead by both 2- and 3-dimensional echocardiography, and the degree of lead slack radiologically. Patient characteristics and lead-related factors were evaluated in the prediction of new or worse TR by univariable and multivariable analyses.
Results
New or increased TR was detected in 38 of 128 patients at the 12-month follow-up. The postero-septal commissure was the most common lead position, and tricuspid leaflet interference detected in 21 patients was associated with a noncommissural lead position. The implantation of an implantable cardioverter defibrillator lead was not associated with new TR compared with the implantation of a pacemaker lead. Tricuspid leaflet interference (P < 0.0001), but not lead position or lead slack, was the only lead-specific factor associated with the development of TR.
Conclusion
After right ventricle endocardial lead implantation, leaflet interference determined by echocardiography, but not the nature of the lead, the lead position at the tricuspid annulus, and the radiological lead slack, predicted TR development at 1 year postimplantation.
Résumé
Contexte
Il est établi que la présence d’une sonde endocavitaire dans le ventricule droit est une cause de régurgitation tricuspide (RT), mais le mécanisme en cause n’est pas encore bien compris. Nous avons tenté d’évaluer la corrélation entre certaines caractéristiques des sondes et l’apparition d’une RT secondaire à l’implantation d’une sonde endocavitaire.
Méthodologie
Il s’agit d’une étude prospective menée dans un seul centre. Une échocardiographie bidimensionnelle a été réalisée avant la mise en place d’une sonde endocavitaire, ainsi qu’aux visites de suivi menées 4 à 6 semaines, 6 mois et 12 mois après l’intervention. Nous avons évalué la position de la sonde endocavitaire par rapport à l’anneau tricuspidien par échocardiographie tridimensionnelle, l’interférence de la sonde avec la valve tricuspide par échocardiographie bidimensionnelle et tridimensionnelle, et le degré de liberté de mouvement de la sonde par radiographie. Les caractéristiques des patients et les facteurs liés à la sonde ont été pris en compte dans la prédiction du risque de RT nouvelle ou d’aggravation d’une RT existante au moyen d’analyses univariées et multivariées.
Résultats
Une RT nouvelle ou aggravée a été détectée au suivi à 12 mois chez 38 des 128 patients. Dans la plupart des cas, la sonde se trouvait à la commissure postéroseptale; chez 21 patients, une interférence avec la valve tricuspide a été détectée alors que la sonde ne se trouvait pas à la commissure. La mise en place d'une sonde de défibrillateur implantable n’a pas été associée à l’apparition d’une RT, comparativement à l’implantation d'une sonde de stimulateur cardiaque. L’interférence avec la valve tricuspide (p < 0,0001) était le seul facteur lié à la sonde associé à l’apparition d’une RT; aucun lien n’a été établi avec la position et le degré de liberté de mouvement de la sonde.
Conclusion
Après la mise en place d’une sonde endocavitaire dans le ventricule droit, l’interférence avec la valve tricuspide établie par échocardiographie permettait de prédire l’apparition d’une RT dans l’année suivant la mise en place de la sonde sans égard au type de sonde, à sa position par rapport à l’anneau tricuspidien ou à la liberté de mouvement détectée par radiographie.
The development of tricuspid regurgitation (TR) after endocardial lead placement into the right ventricle (RV) was reported more than 4 decades ago, and in some patients, severe TR can ensue, necessitating surgical intervention.1, 2, 3, 4, 5 It is increasingly recognized that severe TR is associated with worse survival.6,7 With an aging population and the expanding indications for intracardiac devices, TR associated with the endocardial lead will likely become a more important health issue. A wide range of prevalence of TR after endocardial lead placement has been reported.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Many of the studies were retrospective in nature with small sample sizes and variable durations of follow-up. Recent studies have suggested that lead location can be assessed by 3-dimensional (3D) echocardiography and appears to be related to the worsening or the new development of TR.19, 20, 21 We have recently shown that new or worse TR occurred in 30% of patients after endocardial lead implantation.22 The objective of the study was to evaluate lead-specific risk factors for the development of TR after endocardial lead implantation including the nature of the lead (implantable cardioverter defibrillator [ICD] lead vs pacemaker lead), the location of the endocardial lead at the tricuspid annulus evaluated by 3D echocardiography, the tricuspid leaflet interference assessed by echocardiography, and the degree of slack of the lead on chest x-ray.
Material and Methods
This was a single-centre prospective study. The study was approved by the Research Ethics Board, and all subjects provided written informed consent. We excluded patients with preexisting RV dilatation or dysfunction, patients with more than mild TR, and patients scheduled for cardiac synchronization therapy because pre-existing RV dilatation or dysfunction is frequently present in these patients.
The subjects had preprocedural echocardiograms within 48 hours before lead implantation and follow-up echocardiograms at 4 to 6 weeks, 6 months, and 1 year after the procedure. Echocardiograms (both 2-dimensional and 3D) were obtained at each visit, in accordance with the American Society of Echocardiography guidelines.23,24 Tricuspid annular plane systolic excursion and RV annular systolic velocity were obtained in addition to left ventricle, right atrium, and RV dimensions.25
The grading of TR severity was based on existing guidelines.25 Mild or less TR was categorized into none/trace, mild, and mild-moderate based on the jet area (none/trace < 1.2 cm2, mild 1-3 cm2, mild to moderate 3-5 cm2) to provide a more refined grading so as to enhance our ability to detect evolutional changes of TR severity. The peak TR gradient was calculated using the modified Bernoulli equation, and the right atrium pressure was assessed on the basis of the American Society of Echocardiography guidelines.26
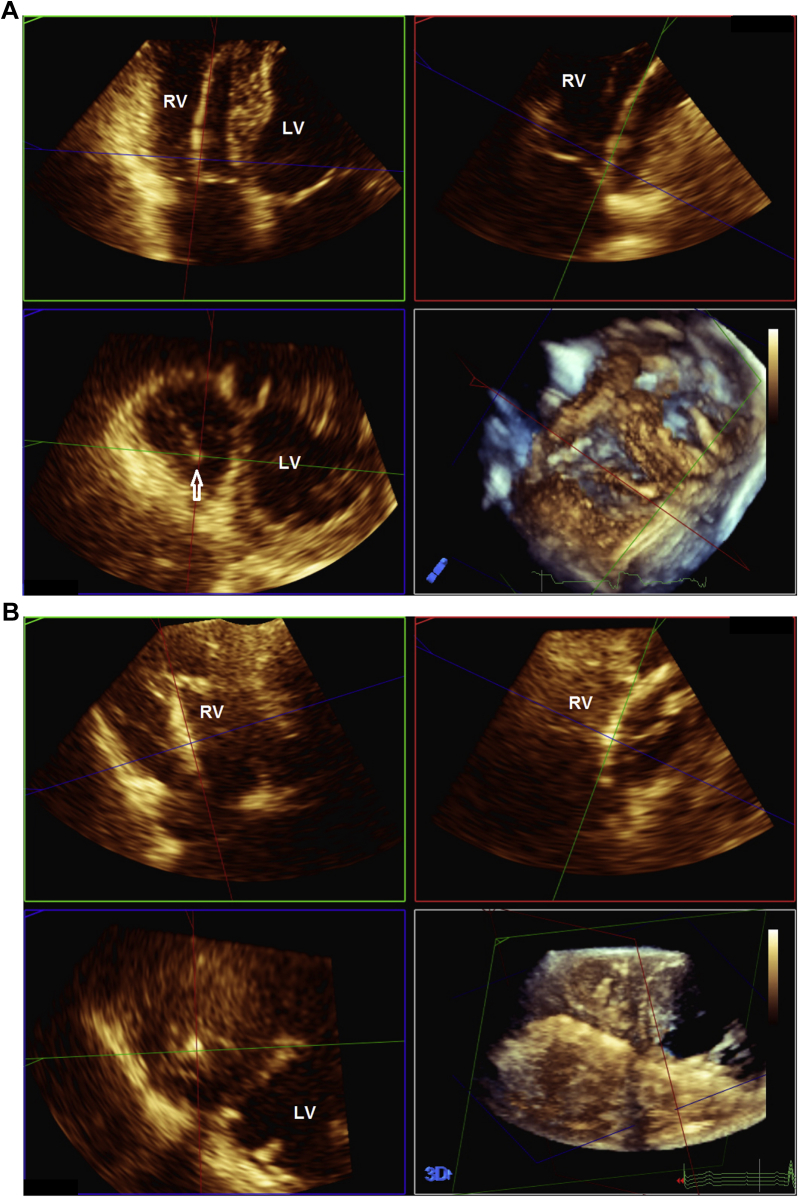
Three-dimensional images of the tricuspid valve were obtained at the first follow-up visit at 4 to 6 weeks and at 12 months after implantation according to the current guidelines.24 Both full volume and zoomed 3D volumes were obtained. 3D volumes were analyzed using a commercially available off-line software system (QLab version 10 Philips Healthcare, Andover, MA), and the position of the endocardial lead at the tricuspid annulus level was assessed using the multiplanar reconstruction (MPR) software (Fig. 1; Videos 1-3
 , view video online) We used the MPR approach rather than the 3D en face view of the tricuspid valve to determine the position, because we found that the latter generally provided suboptimal images of the tricuspid valve in the setting of nondilated RV. The position was described as commissural (in a commissure) or noncommissural (against a leaflet or centrally located) with reference to the specific commissure or leaflet involved.20,21 In addition, potential interference with the tricuspid leaflet motion by the endocardial lead was assessed using both 2-dimensional and 3D echocardiography to specifically assess for impingement, adherence, and entanglement.3,4,20,21 As previously described, leaflet interference was diagnosed when the systolic excursion of any of the tricuspid leaflets was impeded by the endocardial lead. Impingement was present when the leaflet was not tethered to the lead, whereas direct tethering of the lead to the leaflet was adherence and to the subvalvular chords indicated entanglement3 (Videos 4-6
, view video online) We used the MPR approach rather than the 3D en face view of the tricuspid valve to determine the position, because we found that the latter generally provided suboptimal images of the tricuspid valve in the setting of nondilated RV. The position was described as commissural (in a commissure) or noncommissural (against a leaflet or centrally located) with reference to the specific commissure or leaflet involved.20,21 In addition, potential interference with the tricuspid leaflet motion by the endocardial lead was assessed using both 2-dimensional and 3D echocardiography to specifically assess for impingement, adherence, and entanglement.3,4,20,21 As previously described, leaflet interference was diagnosed when the systolic excursion of any of the tricuspid leaflets was impeded by the endocardial lead. Impingement was present when the leaflet was not tethered to the lead, whereas direct tethering of the lead to the leaflet was adherence and to the subvalvular chords indicated entanglement3 (Videos 4-6
 , view video online).
, view video online).
Figure 1.
Location of the endocardial lead at the tricuspid annulus by multiplanar reconstruction (MPR) analysis based on the 3-dimensional (3D) echocardiographic volume dataset showing the endocardial lead (arrow) at the postero-septal commissure (A) and at the centre position (B). LV, left ventricle; RV, right ventricle.
All patients had routine postero-anterior and lateral chest x-ray within 2 weeks after endocardial lead implantation. The contour of the lead on chest x-ray was assessed to determine the degree of lead slack and semiquantitated into grade 0 (no slack), grade 1 (minimal slack), grade 2 (normal slack), grade 3 (mildly excessive slack), and grade 4 (very excessive slack) as previously described.27 The percentage of the RV pacing was determined at the 12-month follow-up by interrogation of the device.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as absolute numbers and percentages. The Student t test was used to compare continuous data in normal distribution, and the Mann–Whitney U test was used for continuous data in skewed distribution. Categorical data were compared using the Fisher or chi-square test. Univariable and multivariable linear regression models were used to assess variables associated with new or worse TR after endocardial lead implantation. A 2-tailed P value < 0.05 was considered statistically significant.
Results
Between February 2013 and June 2015, 153 patients were recruited in the study, and 128 patients completed follow-up at 12 months (mean age of 66.8 ± 12.4 years and 94 women). Atrial fibrillation or flutter was present in 7 patients. A permanent pacemaker was implanted in 61 patients, and an ICD was implanted in 67 patients. New or worse TR developed in 38 patients. Increase in TR severity was by only 1 grade in 32 of 38 patients (84%).
Features of endocardial lead
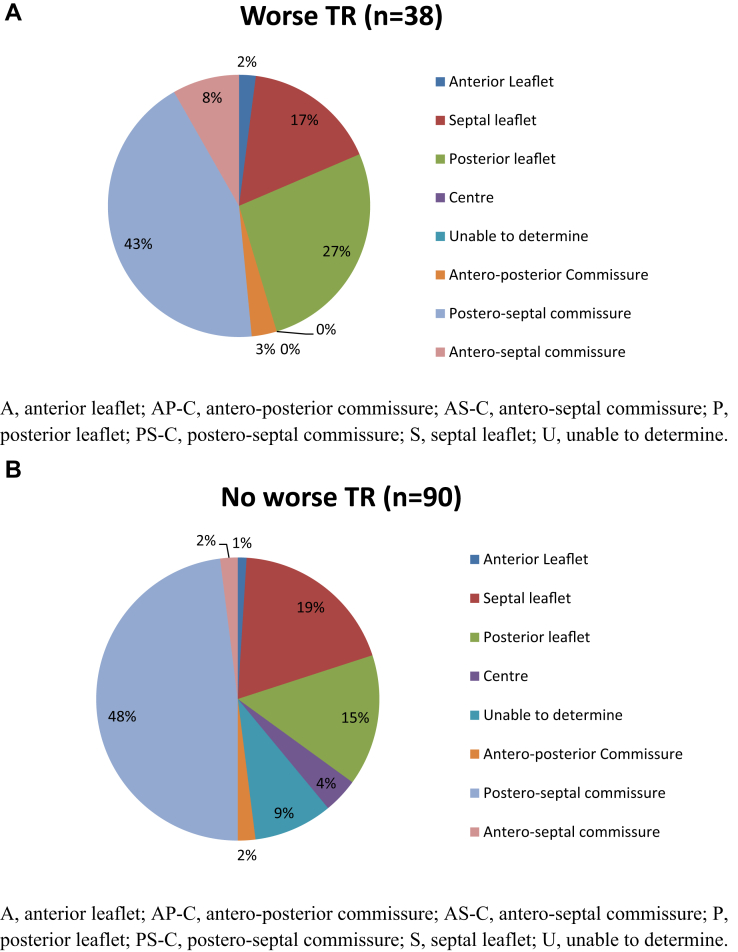
The 4 lead-specific features in patients with or without new or worse TR are presented in Table 1. Lead position at the commissure was common in both groups. Details of the lead positions at the tricuspid annulus for the 2 groups of patients are shown in Figure 2. Suboptimal 3D images prevented localization of the endocardial leads in 9 patients (1 patient with and 8 patients without new or worse TR). There were no significant differences in lead position at the tricuspid annulus between the 2 groups. Potential tricuspid leaflet interference was identified in 21 patients with impingement being the most common mechanism (Table 1). There were similar portions of ICD in both groups of patients. The slack scores for the 2 groups of patients were also similar. Leaflet interference was associated with the noncommissural lead position, with interference present in 18 of 53 patients with a noncommissural lead position and in 3 of 66 patients with a commissural lead position (P < 0.0001) (Table 2). Leaflet interference was not a specific feature for new TR, because 8 of these patients did not develop new or worse TR. No association was found between the leaflet interference and the slack score or the nature of the lead.
Table 1.
Leaflet-specific features in 128 patients
| New or worse TR, n = 38 (%) | No new or worse TR, n = 90 (%) | |
|---|---|---|
| Lead position | ||
| Commissural | 20 (52) | 46 (51) |
| Noncommissural | 17 (45) | 36 (40) |
| Unable to determine | 1 (3) | 8 (9) |
| Leaflet interference | ||
| Impingement | 10 (26) | 6 (7) |
| Adherence | 3 (8) | 1 (1) |
| Entanglement | 0 (0) | 1 (1) |
| Uncertain | 1 (3) | 8 (9) |
| No interference | 24 (63) | 74 (82) |
| Nature of lead | ||
| Pacemaker | 19 (50) | 42 (47) |
| ICD | 19 (50) | 48 (53) |
| Slack score | ||
| 0 | 0 (0) | 2 (2) |
| 1 | 8 (21) | 16 (18) |
| 2 | 23 (60) | 56 (62) |
| 3 | 6 (16) | 14 (16) |
| 4 | 1 (3) | 2 (2) |
ICD, implantable cardioverter defibrillator; TR, tricuspid regurgitation.
Figure 2.
Endocardial lead positions at the tricuspid annulus in patients with (A) and without (B) worse tricuspid regurgitation (TR) during follow-up.
Table 2.
Lead-specific features in 119 patients
| Lead interference, n = 21 (%) | No lead interference, n = 98 (%) | |
|---|---|---|
| Lead position | ||
| Commissural | 3 (14) | 63 (64) |
| Noncommissural | 18 (86) | 35 (36) |
| Nature of lead | ||
| Pacemaker | 16 (76) | 37 (38) |
| ICD | 5 (24) | 61 (62) |
| Slack score | ||
| 0 | 0 (0) | 2 (2) |
| 1 | 3 (14) | 19 (19) |
| 2 | 14 (67) | 60 (61) |
| 3 | 3 (14) | 15 (15) |
| 4 | 1 (5) | 2 (2) |
| TR | ||
| New or worse | 13 (62) | 24 (24) |
| Same | 8 (38) | 74 (76) |
ICD, implantable cardioverter defibrillator; TR, tricuspid regurgitation.
Factors associated with TR
In Table 3, we compared the clinical and echocardiographic variables previously reported to be associated with new or worse TR after endocardial lead implantation in the 2 groups of patients.2,3,7,15,28 Clinical variables including age, sex, and atrial fibrillation were not associated with new TR. The percentages of RV pacing were 19.3% ± 34.0% in patients with and 20.5% ± 37.5% in patients without new or worse TR (P = 0.86). The nature of the lead (pacemaker vs ICD), the lead slack score, the lead position at the annulus by 3D echocardiography, and the leaflet interference were included as variables. Leaflet interference was the only predictor for new TR progression by univariable and multivariable analysis.
Table 3.
Univariable and multivariable factors associated with the progression of tricuspid regurgitation during follow-up
| Variable | Univariable |
Multivariable |
||
|---|---|---|---|---|
| Coefficient | P | Coefficient | P | |
| Age | 0.0133 | 0.41 | ||
| Sex | 0.086 | 0.35 | ||
| AF | 1.22 | 0.12 | 0.1693 | 0.3707 |
| % RV pacing at follow-up | 0.0788 | 0.63 | ||
| RVFAC | 0.0039 | 0.83 | ||
| RVSP | −0.0026 | 0.86 | ||
| LVEF | 0.0035 | 0.78 | ||
| ICD lead | 0.1335 | 0.73 | ||
| Lead slack score | 0.0444 | 0.87 | ||
| Lead position | −0.0370 | 0.64 | ||
| Lead interference | −0.2556 | 0.0019 | −0.0840 | < 0.0001 |
AF, atrial fibrillation; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; RV, right ventricle; RVFAC, right ventricular fractional area change; RVSP, right ventricular systolic pressure; TR, tricuspid regurgitation.
Discussion
We have shown that TR occurred in 30% of patients at 1 year after endocardial lead implantation. In the present study, we prospectively evaluated 4 lead-specific features and found that tricuspid leaflet interference by the endocardial lead was a predictor for new or worse TR, whereas lead position at the tricuspid annulus, nature of lead, and radiological lead slack were not. This finding may be an important factor to consider in developing strategies to present this lead-related complication.
Contrary to studies showing development of significant TR within 6 months after device implantation,14,15,29,30 we have recently shown that development of new or increased TR was common after RV endocardial lead placement at the 12-month follow-up, but the majority of new TR was mild in severity, although it was associated with increases in the dimensions of the right heart chambers.21 It may be argued that mild TR has little clinical significance, but the progression of TR is a dynamic process, and in the long term there is the likelihood that TR begets TR such that severe TR leading to overt RV failure may ensue in some of these patients who developed new or increased TR.31 Thus, it is important to identify risk factors and the potential mechanisms for the development of TR in this clinical setting.
Mechanism of TR
The mechanisms of lead-associated TR remain speculative. Patient factors such as atrial fibrillation and left ventricular ejection fraction, and non–lead-related causes such as contraction dyssynchrony due to RV pacing have been reported to predict the development of TR.21,28,32 In the present study, patient factors including age, sex, atrial fibrillation, and RV dyssynchrony measured by the percentage of RV pacing were not associated with TR development. Atrial fibrillation is increasingly recognized as a cause for TR,18,33 but our study contained few patients with atrial fibrillation such that the role of atrial fibrillation could not be properly addressed. Furthermore, left ventricle and RV systolic function as evidenced by left ventricular ejection fraction and RV fractional area change did not predict TR progression.
Lead-related effects on tricuspid leaflets are likely important and supported by pathologic and intraoperative findings.4,5 This is the first prospective study to assess lead-specific factors in the development of TR, and we examined 4 features of the endocardial lead, namely, nature of the lead, lead slack, lead location at the annulus, and echocardiographic evidence of leaflet interference. The last 3 parameters would be potentially amenable for corrective action if found to predict TR.
Lead slack assessed the course of the lead within the RV. The degree of lead slack was based on the radiological appearance, which was a measure of the lead distortion or redundancy. Excessive slack may interfere with proper function of the tricuspid annulus or leaflets, but we did not find an association between slack and leaflet interference or TR. This may be explained by the floppy nature of the tricuspid leaflet, which is able to envelop the lead regardless the degree of lead slack. This may also be the reason why the thicker and less flexible ICD leads were not associated with new or worse TR, contrary to the findings of Kim et al.29 Lead interference appeared more common with pacemaker lead (Table 2), but this finding needs to be interpreted with caution because 8 of 9 patients excluded because of uncertain lead positions were patients with pacemaker leads, and the nature of lead (pacemaker vs ICD) was not a predictor of new TR in the univariable and multivariable analyses (Table 3).
Three-dimensional echocardiography is useful in the assessment of the location of the endocardial lead at the level of the tricuspid annulus. Similar to the observation of Seo et al.,19 we showed that the postero-septal commissure was a common location for the endocardial lead in patients with and in those without new or increased TR, in contrast to the finding by Mediratta et al.20 and Addetia et al.,21 who found no lead-associated TR when the lead was at the central position or postero-septal commissure. In our patients, the postero-septal commissure was the most common location for the endocardial lead and not necessarily a “safe lead position” to avoid the development of TR, although there was an association of lead interference with the noncommissure position. The studies by Mediratta et al.20 and Addetia et al.21 were retrospective studies with patient characteristics different from those in our study, and 3D echocardiograms were obtained at 3.9 ± 3.9 years and 3.8 ± 3.0 years, respectively, postimplantation of endocardial lead such that considerable RV remodeling and TV annulus dilatation may have occurred.
In the studies by Mediratta et al.20 and Addetia et al.,21 the presence of leaflet interference was inferred if moderate or greater TR was detected in the presence of an endocardial lead, even though non–lead-related causes might be present. The majority of patients in the study by Mediratta et al.20 did not have preimplantation echocardiograms, and significant TR was present in many patients who had preimplantation studies. The studies by Mediratta et al.20 and Addetia et al.21 included many of the same patients. We agree with Addetia et al.21 that “a word of caution about the implications of our results for the general population of patients who have pacemakers inserted: this study included a highly selected population.” In our experience, the MPR mode was superior to the 3D en face view of the tricuspid valve in evaluating the position of and leaflet interaction with the endocardial lead. We believe our findings are more representative of the incidence and severity of TR after RV endocardial lead implementation. We found that endocardial lead in the noncommissural position and abutting on the tricuspid leaflet during ventricular systole was not uncommon and did not differentiate patients with TR from those without new or worse TR. We specifically examined for echocardiographic findings of lead interference with leaflet coaptation, and our results would support that these findings provided more reliable evidence of interference with leaflet coaptation than lead position. Nonetheless, 8 of 21 patients with leaflet interference did not develop new TR, likely explained by the redundant nature of the tricuspid leaflets, which have the ability to envelop the endocardial lead and to maintain adequate coaptation. Long-term studies are needed because it is plausible that these patients may develop TR in a longer follow-up.
Study limitations
Although studies have suggested that by 6 months the impact of lead implantation on TR and RV would have been apparent,14,15,29,30 the time course of TR development and progression after endocardial lead placement is not well defined. A follow-up longer than 1 year will be needed to provide a better appreciation of the long-term effects of TR. Our grading of TR severity to allow a more refined assessment of milder degrees of TR was based largely on the jet area. We believe this was appropriate because other measures of TR, such as jet width and proximal iso-velocity surface area, were not applicable in these cases of mild TR. The validity of the grading method was supported by the observation that a 1-grade increase in TR was associated with unfavorable functional consequences.22 The differences in grading TR severity need to be considered in comparing our results with results from other studies. We did not find that the position of endocardial lead in relation to the commissures or leaflets to be associated with new or worse TR. It is plausible that the position may have an effect on the development of TR in a longer follow-up because the patients reported by Seo et al.19 and Addetia et al.20 were largely studied several years after implantation. Echocardiographic features of tricuspid leaflet interference by the endocardial lead such as those used in the present study have not been well defined or validated.3 More studies are needed to evaluate these echocardiographic features on short-term and long-term effects on tricuspid valve function.
Conclusions
New or increased TR occurred in 30% of patients after RV endocardial lead implantation at a follow-up of 1 year. The implantation of an ICD lead was not associated with TR compared with the implantation of a pacemaker lead. Echocardiographic evidence of leaflet interference, not the lead position at the tricuspid annulus or lead slack, was a predictor of new or worse TR at follow-up of 1 year. Leaflet interference was associated with a noncommissural lead position. A longer-term follow-up is needed to assess the progression and clinical impact of endocardial lead-associated TR. Studies are also needed to evaluate the effect on TR development by modification of the lead implantation technique to minimize tricuspid leaflet interference.
Funding Sources
Supported in part by the Ontario Ministry of Health Innovation Fund.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The study was approved by and conducted according to the guidelines of the Research Ethics Board.
See page 322 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca and at https://doi.org/10.1016/j.cjco.2019.10.002.
Supplementary Material
Multiplanar reconstruction (MPR) showing the endocardial lead was located at the postero-septal commissure.
MPR showing the endocardial lead against the posterior tricuspid leaflet.
MPR showing the endocardial lead at the center position.
Right ventricular inflow view showing impingement of the anterior tricuspid leaflet by the pacemaker lead associated with incomplete coaptation and tricuspid regurgitation.
MPR showing adherence of the septal tricuspid leaflet to the pacemaker lead. Adherence of the septal leaflet to the lead was clearly imaged in the short-axis view in the lower left panel.
Modified apical 4-chamber view showing entanglement of the subvalvular chords by the pacemaker lead. The subvalvular chords, but not the leaflets, were tethered to the lead.
References
- 1.Nachnani G.H., Gooch A.S., Hsu I. Systolic murmurs induced by pacemaker catheters. Arch Intern Med. 1969;124:202–205. [PubMed] [Google Scholar]
- 2.Chang J.D., Manning W.J., Ebrille E., Zimetbaum P.J. Tricuspid valve dysfunction following pacemaker or cardioverter-defibrillator implantation. J Am Coll Cardiol. 2017;69:2331–2341. doi: 10.1016/j.jacc.2017.02.055. [DOI] [PubMed] [Google Scholar]
- 3.Al-Mohaissen M.A., Chan K.L. Prevalence and mechanism of tricuspid regurgitation following implantation of endocardial leads for pacemaker or cardioverter-defibrillator. J Am Soc Echocardiogr. 2012;25:245–252. doi: 10.1016/j.echo.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Lin G., Nishimura R.A., Connolly H.M. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter-defibrillator leads. J Am Coll Cardiol. 2005;45:1672–1675. doi: 10.1016/j.jacc.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 5.Pfannmueller B., Hirnie G., Seegurger J. Tricuspid valve repair in the presence of a permanent ventricular pacemaker lead. Eur J Cardiothorac Surg. 2011;39:657–661. doi: 10.1016/j.ejcts.2010.08.051. [DOI] [PubMed] [Google Scholar]
- 6.Nath J., Foster E., Heidenreich P.A. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43:405–409. doi: 10.1016/j.jacc.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 7.Topilsky Y., Nkomo V.T., Vatury O. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging. 2014;7:1185–1194. doi: 10.1016/j.jcmg.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Hoke U., Auger D., Thijssen J. Significant lead-induced tricuspid regurgitation is associated with poor prognosis at long-term. Heart. 2014;100:960–968. doi: 10.1136/heartjnl-2013-304673. [DOI] [PubMed] [Google Scholar]
- 9.Lee R., Friedman S., Kono A., Greenberg M.L., Palac R.T. Tricuspid regurgitation following implantation of endocardial leads: incidence and predictors. Pacing Clin Electrophysiol. 2015;38:1267–1274. doi: 10.1111/pace.12701. [DOI] [PubMed] [Google Scholar]
- 10.Fanari Z., Hammami S., Hammami M., Hammami Shurijh M. The effects of right ventricular apical pacing with intravenous pacemaker and implantable cardioverter defibrillator on mitral and tricuspid regurgitation. J Electrocardiol. 2015;48:791–797. doi: 10.1016/j.jelectrocard.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Al-Bawardy R., Krishnaswamy A., Rajeswaran J. Tricuspid regurgitation and implantable devices. Pacing Clin Electrophysiol. 2015;38:259–266. doi: 10.1111/pace.12530. [DOI] [PubMed] [Google Scholar]
- 12.Delling F., Hassan Z., Piatkowski G. Tricuspid regurgitation and mortality in patients with transvenous permanent pacemaker leads. Am J Cardiol. 2016;117:988–992. doi: 10.1016/j.amjcard.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadreddini M., Haroun M.J., Buikema L. Tricuspid valve regurgitation following temporary or permanent endocardial lead insertion, and the impact of cardiac resynchronization therapy. Open Cardiovasc Med J. 2014;8:113–120. doi: 10.2174/1874192401408010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leibowitz D., Rosenheck S., Pollak A., Geist M., Gilon D. Transvenous pacemaker leads do not worsen tricuspid regurgitation: a prospective echocardiographic study. Cardiology. 2000;93:74–77. doi: 10.1159/000007005. [DOI] [PubMed] [Google Scholar]
- 15.Kucukarslan N., Kirilman A., Ulusoy E. Tricuspid insufficiency does not increase early after permanent implantation of pacemaker leads. J Card Surg. 2006;21:391–394. doi: 10.1111/j.1540-8191.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- 16.Arabi P., Ozer N., Ates A.H. Effects of pacemaker and implantable cardioverter defibrillator electrodes on tricuspid regurgitation and right sided heart functions. Cardiol J. 2015;22:637–644. doi: 10.5603/CJ.a2015.0060. [DOI] [PubMed] [Google Scholar]
- 17.Najib M., Vitalla S., Challa S. Predictors of severe tricuspid regurgitation in patients with permanent pacemaker of automatic implantable cardioverter-defibrillator leads. Tex Heart Inst J. 2013;40:529–533. [PMC free article] [PubMed] [Google Scholar]
- 18.Van De Heyning C., Elbarasi E., Masiero S. Prospective study of tricuspid regurgitation associated with permanent leads after cardiac rhythm device implantation. Can J Cardiol. 2019;35:389–395. doi: 10.1016/j.cjca.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Seo Y., Ishizu T., Nakajima H. Clinical utility of 3-dimensional echocardiography in the evaluation of tricuspid regurgitation caused by pacemaker leads. Circ J. 2008;72:1465–1470. doi: 10.1253/circj.cj-08-0227. [DOI] [PubMed] [Google Scholar]
- 20.Mediratta A., Addetia K., Yamat M. 3D echocardiographic location of implantable device leads and mechanism of associated tricuspid regurgitation. JACC Cardiovasc Imaging. 2014;7:337–347. doi: 10.1016/j.jcmg.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Addetia K., Maffessanti F., Mediratta A. Impact of implantable transvenous device lead location on severity of tricuspid regurgitation. J Am Soc Echocardiogr. 2014;27:1164–1175. doi: 10.1016/j.echo.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anvardeen K., Rao R., Hazra S. Prevalence and significance of tricuspid regurgitation post-endocardial lead placement. JACC Cardiovasc Imaging. 2019;12:562–564. doi: 10.1016/j.jcmg.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Lang R.M., Badano L.P., Tsang W. EAE/ASE recommendations of image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr. 2012;25:3–46. doi: 10.1016/j.echo.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Rudski L.G., Lai W.W., Afilalo J. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Zoghbi W.A., Adams D., Bonow R.O. Recommendations for noninvasive evaluation of native valvular regurgitation. A report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Ha A.C.T., Vezi B., Keren A. Predictors of fracture risk of a small caliber implantable cardioverter defibrillator lead. PACE. 2010;33:437–443. doi: 10.1111/j.1540-8159.2009.02626.x. [DOI] [PubMed] [Google Scholar]
- 28.Vaturi M., Kusniec J., Shapira Y. Right ventricular pacing increases tricuspid regurgitation grade regardless of the mechanical interference to the valve by the electrode. Eur J Echocardiogr. 2010;11:550–553. doi: 10.1093/ejechocard/jeq018. [DOI] [PubMed] [Google Scholar]
- 29.Kim J.B., Spevack D.M., Tunick P.A. The effect of transvenous pacemaker and implantable cardioverter defibrillator lead placement on tricuspid valve function: an observational study. J Am Soc Echocardiogr. 2008;21:284–287. doi: 10.1016/j.echo.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Klustein M., Balkin J., Butnaru A. Tricuspid incompetence following permanent pacemaker implantation. Pacing Clin Eletrophysiol. 2009;32:S135–S137. doi: 10.1111/j.1540-8159.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- 31.Fender E.A., Zack C.J., Nishimura R.A. Isolated tricuspid regurgitation: outcomes and therapeutic interventions. Heart. 2018;104:798–806. doi: 10.1136/heartjnl-2017-311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito M., Iannaccone A., Kaye G., Negishi K., Marwick T.H., PROTECT-PACE Investigators Effect of right ventricular pacing on right ventricular mechanics and tricuspid regurgitation in patients with high-grade atrioventricular block and sinus rhythm (from the Protection of Left Ventricular Function During Right Ventricular Pacing Study) Am Heart J. 2015;116:1875–1882. doi: 10.1016/j.amjcard.2015.09.041. [DOI] [PubMed] [Google Scholar]
- 33.Utsunomiya H., Itabashi Y., Mihara H. Functional tricuspid regurgitation caused by chronic atrial fibrillation: a real-time 3-dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.116.004897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiplanar reconstruction (MPR) showing the endocardial lead was located at the postero-septal commissure.
MPR showing the endocardial lead against the posterior tricuspid leaflet.
MPR showing the endocardial lead at the center position.
Right ventricular inflow view showing impingement of the anterior tricuspid leaflet by the pacemaker lead associated with incomplete coaptation and tricuspid regurgitation.
MPR showing adherence of the septal tricuspid leaflet to the pacemaker lead. Adherence of the septal leaflet to the lead was clearly imaged in the short-axis view in the lower left panel.
Modified apical 4-chamber view showing entanglement of the subvalvular chords by the pacemaker lead. The subvalvular chords, but not the leaflets, were tethered to the lead.