SUMMARY
The vagus nerve conveys gastrointestinal cues to the brain to control eating behavior. In obesity, vagally mediated gut-brain signaling is disrupted. Here, we show that the cocaine- and amphetamine-regulated transcript (CART) is a neuropeptide synthesized proportional to the food consumed in vagal afferent neurons (VANs) of chow-fed rats. CART injection into the nucleus tractus solitarii (NTS), the site of vagal afferent central termination, reduces food intake. Conversely, blocking endogenous CART action in the NTS increases food intake in chow-fed rats, and this requires intact VANs. Viral-mediated Cartpt knockdown in VANs increases weight gain and daily food intake via larger meals and faster ingestion rate. In obese rats fed a high-fat, high-sugar diet, meal-induced CART synthesis in VANs is blunted and CART antibody fails to increase food intake. However, CART injection into the NTS retains its anorexigenic effect in obese rats. Restoring disrupted VAN CART signaling in obesity could be a promising therapeutic approach.
In Brief
Lee et al. report that consumption of an obesogenic diet inhibits calorie-induced synthesis and release of the neuropeptide CART from sensory vagal neurons. CART knockdown in these neurons mimics the hallmarks of obesity, weight gain, and overeating. Bypassing the vagus nerve with central CART administration effectively reduces feeding in obese rats.
Graphical Abstract
INTRODUCTION
The vagus nerve plays an important role in the control of food intake and energy homeostasis (de Lartigue, 2016). Vagal afferent terminals in the gut sense gastrointestinal signals, including hormones released from enteroendocrine cells (Lal et al., 2001; Williams et al., 2009), mechanical distension (Kentish and Page, 2014), and nutrients (Babic et al., 2012; Darling et al., 2014). This information is relayed centrally to neurons of the nucleus tractus solitarii (NTS) to control meal termination (Harding and Leek, 1973). In obesity, sensitivity of vagal afferent neurons (VANs) to satiation hormones (Covasa and Ritter, 2000; Daly et al., 2011; de Lartigue et al., 2012; Duca et al., 2013), distension (Daly et al., 2011; Kentish et al., 2012), and nutrients (Covasa et al., 2000, 2001; Duca et al., 2012) is reduced, thereby preventing gastrointestinal-mediated neuronal activation in the NTS (Covasa et al., 2000; Covasa and Ritter, 2000). Clinical studies using vagal neuromodulation are showing early signs of success for treating obesity (Ikramuddin et al., 2014), highlighting the vagus nerve as a viable peripheral therapeutic target.
The cocaine- and amphetamine-regulated transcript (CART), a neuropeptide transmitter that is expressed in a subpopulation of VANs (Broberger et al., 1999; de Lartigue et al., 2007; Kupari et al., 2019; Zheng et al., 2002) innervating the gut (Bai et al., 2019; Zheng et al., 2002), may be an important molecular signal for control of food intake. CART was originally discovered as a differentially expressed transcript in the striatum of rats in response to cocaine and amphetamine (Douglass et al., 1995) but was subsequently found to be distributed in regions of the brain associated with eating behavior (Koylu et al., 1997). Central administration of the active peptide CART55–102 inhibits eating in a dose- and time-dependent manner (Kristensen et al., 1998; Lambert et al., 1998), whereas neutralizing endogenous CART with CART antibody increases food intake (Kristensen et al., 1998; Lambert et al., 1998), suggesting CART has anorexigenic properties.
Extensive CART colocalization with the receptor for the gastrointestinal hormone cholecystokinin (CCK1R) in nodose ganglia (NG) led to the hypothesis that vagal CART mediates the satiating effects of CCK (Broberger et al., 1999). In support of this concept, peripheral administration of CART enhanced CCK-induced satiation (De Lartigue et al., 2010), and transient knockdown (KD) of NG CART prevented CCK-induced satiation (Heldsinger et al., 2012). Furthermore, CCK increases CART synthesis and release in cultured NG neurons (de Lartigue et al., 2007, 2010; Heldsinger et al., 2012). In vivo, postprandial CCK increases CART expression in NG neurons, a process that is potentiated by leptin and inhibited by ghrelin (de Lartigue et al., 2007, 2010, 2014). Nevertheless, the site of action of NG CART and its role in the control of food intake remain controversial. A recent study failed to observe eating-induced CART expression changes in the NG during the light phase (Yuan et al., 2016). Although 3rd and 4th ventricular injection of exogenous CART reduced food intake (Aja et al., 2002; Skibicka et al., 2009; Zheng et al., 2002), intraparenchymal NTS (intraNTS) injection failed to reduce short-term sucrose intake (Zheng et al., 2002), or 24 h intake of chow (Skibicka et al., 2009), raising the question of whether NG CART release into the NTS is capable of affecting eating behavior. Finally, although acute CART KD in NG neurons abolished satiation caused by exogenous administration of combined CCK and leptin (Heldsinger et al., 2012), it is unknown whether chronic disruption of NG CART expression has long-term effects on food intake and body weight.
The aim of this study was to determine whether CART signaling in the gut-brain axis could play a role in obesity. To address this question, we first investigated the mechanism that controls NG CART expression, the site of NG CART action, and its role in controlling food intake in lean animals. We next examined whether consuming a palatable energy-dense diet disrupts NG CART expression and whether this disruption is sufficient to promote hyperphagia and weight gain. Finally, we tested whether dietary intervention or restoring CART signaling in the NTS. acutely could reduce food intake in obese rats.
RESULTS
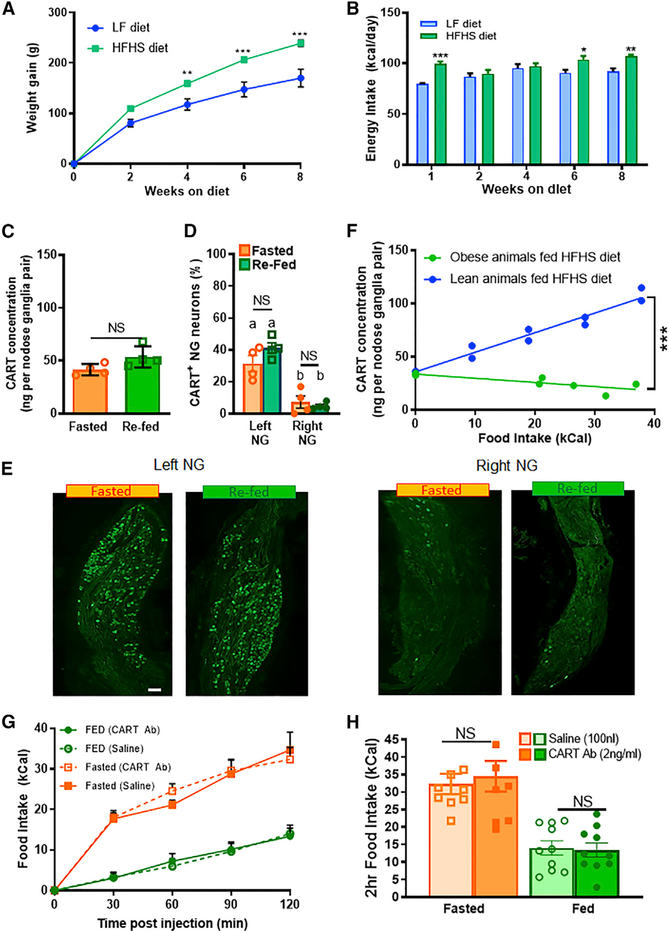
VAN CART Expression Increases In Proportion to Food Intake
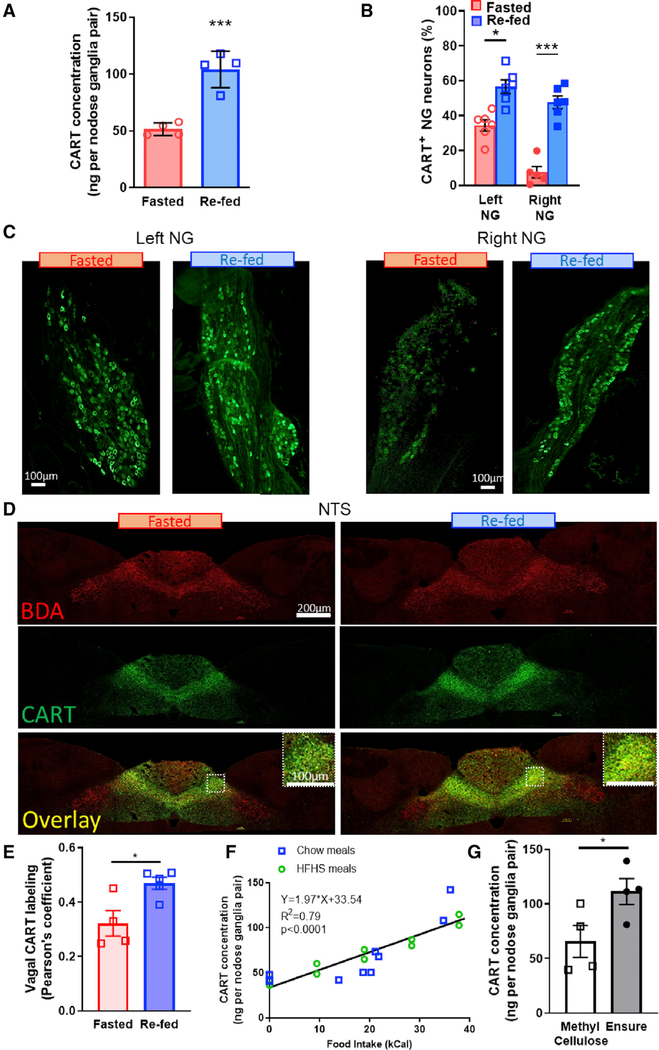
Lean chow-fed rats underwent a 48 h fast with or without 2 h of ad libitum access to food. Stomach contents were weighed to verify the absence or presence of food intake in both conditions (Figure S1A). 2 h refeeding increased both the CART protein concentration and the percentage of CART+ neurons in the NG compared with lean rats fasted 48 h (Figures 1A–1C; Figures S1B and S1C). Eating-induced CART expression in VANs was observed in both left and right NGs (Figures 1B and 1C): however, the effect was more pronounced in the right NG in lean rats (Figures 1B and 1C; Figure S1D) as a result of greater CART depression under fasting conditions in the right NG compared with the left NG.
Figure 1. VAN CART Expression Increases Proportional to Food Intake.
(A) EIA quantification of CART protein concentration from both left and right NG doubles with refeeding (n = 4; unpaired two-tailed t test, p = 0.0008).
(B) Percentage of CART-positive neurons increases in both left and right NG after refeeding (n = 6; two-way ANOVA, F(1,9) = 7.27; Sidak’s post hoc comparison, **p < 0.0025, ***p < 0.0001).
(C) Representative stitched images of CART expression in the left or right NG from lean rats fasted 48 h on wire floors or fasted 46 h and refed for 2 h. Scale bar, 100 μm.
(D) Representative images of vagal fibers (BDA, red), CART (green), or overlay in dorsal vagal complex (DVC) from rats fasted overnight for 16 h or fasted and refed for 2 h in the dark phase. Scale bar, 200 μm. Insert shows higher magnification of CART and BDA in the NTS. Scale bar, 100 μm.
(E) Quantification of CART and BDA overlap, using Pearson’s coefficient, increases with refeeding in the NTS (n = 4–5; unpaired two-tailed t test, p = 0.0176).
(F) EIA of NG CART concentration increases proportionally with caloric intake in lean animals fed chow or an HFHS diet (n = 20; linear regression, slope different from 0, F(1,18) = 69.36, p < 0.0001).
(G) EIA of NG CART from fasted rats 2 h after gavage of 3mL of methylcellulose (2% w/v, 0 kCal) or Intralipid (50%, 10 kCal) (n = 4; unpaired two-tailed t test, p = 0.05).
CART fibers are localized throughout the length of the NTS., and we confirmed previous findings (Zheng et al., 2002) that these are most densely found in the medial NTS (mNTS) and commissural NTS (Figure 1D). To determine the extent of CART expression in vagal fibers, we injected an anterograde tracer (biotinylated dextran amine [BDA], 10% w/v) into the NG. and colocalized CART and BDA in the NTS. We found extensive colabeling between CART and BDA in the mNTS and the commissural NTS in both fed and fasted conditions (Figures 1D and 1E). Refeeding increased CART expression (Figure S2A), with no effect on BDA staining, (Figure S2B) equally in both left and right mNTS (Figure S2C). Quantification of correlation using Pearson’s coefficient (Dunn et al., 2011) indicates that vagal CART expression increases with refeeding compared with fasted conditions in the NTS. (Figure 1E). These data indicate that CART synthesized in the NGs is transported to the NTS. within 2 h of a meal and stored in vagal fibers, presumably ready for release at a subsequent meal.
To test the hypothesis that VAN CART expression encodes food intake in a calorie-dependent manner, we quantified CART protein concentration by enzyme immunoassay (EIA) in the NG in response to defined meal sizes in lean rats. After an overnight fast, consumption of a pre-weighed chow or a high-fat, high-sugar (HFHS) meal increased NG CART protein concentration in a calorie-dependent manner (Figure 1F). Using analysis of covariance (ANCOVA), we found no difference in CART expression between chow and HFHS meals when calorie intake was used as a covariate (Figure S1E). However, when the weights of meals were used as a covariate, CART expression showed significantly different slopes between chow and HFHS (Figure S1F). Importantly, in the light phase when animals have access to food but opt not to eat, NG CART expression levels were similar to those of fasted animals (Figure S3). To dissociate the role of calories and volume on CART expression, we gavaged trained rats with either Ensure (3 mL, 10 kCal) or 2% methylcellulose (3 mL, 0 kCal), and found that Ensure, but not methylcellulose, increased EIA quantification of NG CART abundance (Figure 1G). Altogether, these findings are consistent with the idea that CART expression does not depend on weight or volume of food, nor the absence of hunger, but rather encodes information about calorie intake.
Exogenous CART Administration into the NTS Reduces Food Intake
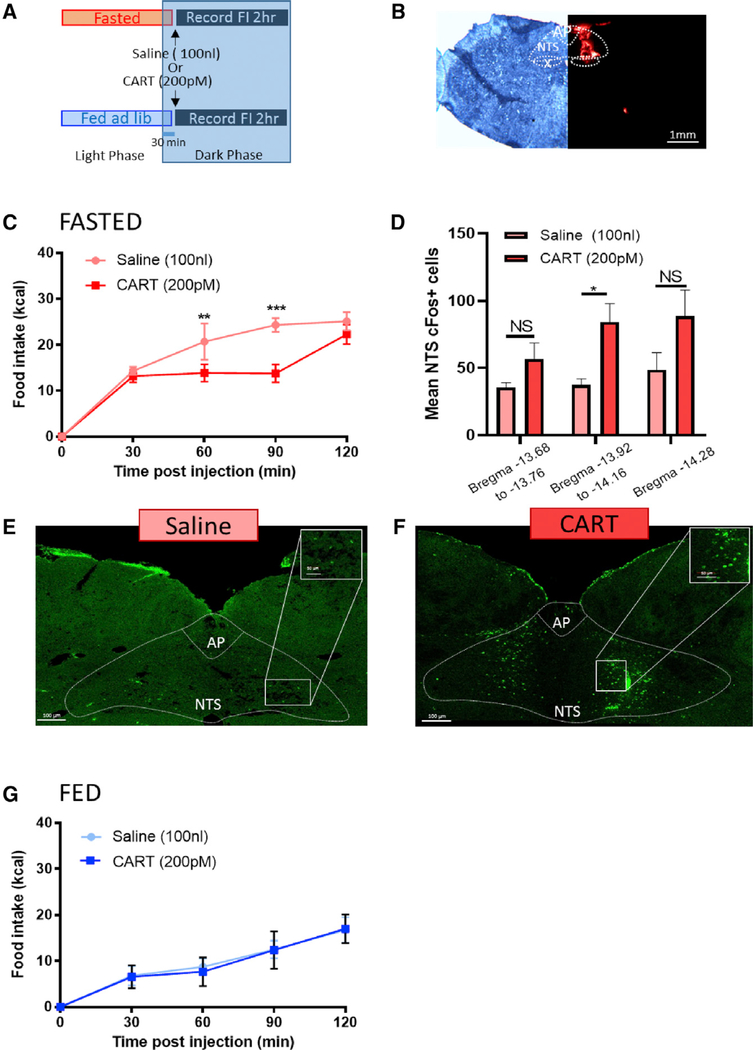
Because CART is synthesized in the NG (Broberger et al., 1999), transported to the NTS (Broberger et al., 1999; Zheng et al., 2002), and at least in culture released from VANs in response to postprandial gastrointestinal humoral signals (De Lartigue et al., 2010), we hypothesized that NG CART inhibits food intake at the levels of the NTS. Therefore, we first sought to determine whether injection of exogenous CART peptide into the NTS is anorexigenic and activates NTS neurons. Thirty min into the dark phase, we injected either CART (200 pM) or saline (100 nL) into the right mNTS in rats fasted on wire-bottom floors overnight. Following infusion, rats were immediately returned to their home cage and food intake was measured for 2 h (Figure 2A). The site of injection was validated by retrobeads (Figure 2B). CART transiently reduced food intake in lean fasted rats 60 and 90 min postinjection (Figure 2C). In separate fasted rats, c-Fos expression was increased in mNTS and area postrema neurons 90 min following NTS infusion of CART compared with saline (Figures 2D–2F; Figure S4). This demonstrates that CART in the NTS is capable of reducing food intake and activates NTS and area postrema (AP) neurons at the site of vagal afferent terminal fields. In fed lean rats, exogenous CART failed to reduce food intake (Figure 2G), suggesting that the anorexigenic effect of CART depends on the feeding state and on low endogenous NG CART levels.
Figure 2. Exogenous CART Administration into the NTS Reduces Food Intake in Lean Rats.
(A) Experimental paradigm.
(B) Unilateral cannula placement into the right NTS. Scale bar, 1 mm.
(C) In overnight fasted lean animals, intraNTS CART injection reduced food intake at 60 and 90 min (n = 5; two-way ANOVA, interaction F(4,12) = 10.08, p < 0.0008; Sidak’s multiple comparison test, **p = 0.0017, ***p < 0.0001).
(D) intraNTS injection of CART increases c-Fos expression in neurons of the mNTS (n = 6; two-way ANOVA, interaction F(2,19) = 0.552, p = 0.58; Sidak’s multiple comparison test, *p = 0.034).
(E and F) Representative images of c-Fos expression in the dorsal vagal complex 90 min after (E) saline or (F) CART. Scale bar, 100 μm.
(G) In fed lean animals, intraNTS CART injection has no effect on food intake compared with saline treatment (n = 5; two-way ANOVA, interaction F(8,32) = 0.788, p = 0.617).
Endogenous CART Release from VANs into the NTS Is Required for Satiation
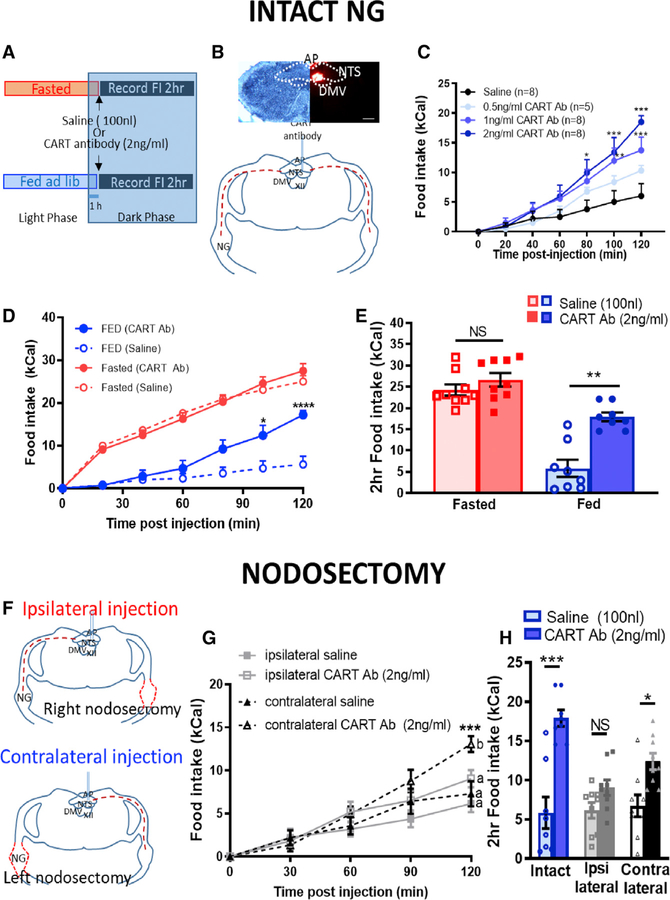
We then sought to test the physiological role of endogenous CART in the NTS by infusing CART antibody into the mNTS in ad libitum-fed rats or after an overnight fast during the early dark phase (Figure 3A). In light of the lateral asymmetry in NG CART expression (Figures 1B and 1C; Figure S1D), cannulas were implanted into the right mNTS and the placement was verified by retrobead infusion (Figure 3B). In ad libitum chow-fed lean rats, CART antibody injection into the NTS 1 h into the dark phase dose-dependently increased food intake (Figure 3C). The highest dose of CART antibody (2 ng/mL) resulted in an increase of up to 3-fold in food intake 2 h postinjection (Figures 3C–3E). To validate the use of CART antibody to inhibit endogenous CART, we neutralized CART antibody by 24 h incubation with CART peptide. This prevented the stimulatory effect of the antibody on eating (Figure S5A), indicating that overeating occurs when endogenous CART signaling is blocked.
Figure 3. Endogenous CART Release from VANs into the NTS Is Required for Satiation.
(A) Experimental paradigm.
(B) Unilateral cannula placement into the right mNTS.
(C) CART antibody injected into the NTS of ad libitum-fed rats 1 h into the dark phase dose-dependently stimulates food intake over 2 h compared with saline (n = 5–8; two-way ANOVA, interaction F(12,150) = 4.295, p < 0.0001; Tukey’s post hoc comparison, *p < 0.05, **p < 0.01, ***p < 0.001).
(D) In intact animals, CART antibody increases food intake compared with saline in fed conditions, but not in fasted conditions (n = 8/9; three-way ANOVA, interaction F(6,6) = 1.57, p < 0.016; Sidak’s multiple comparison test, *p < 0.05, ****p < 0.0001).
(E) 2 h food intake post-CART antibody injection in fasted versus fed lean rats (n = 9/8; two-way repeated measure (RM) ANOVA, interaction F(1,7) = 21.83, p = 0.002; Sidak’s multiple comparison test, **p = 0.002).
(F) Unilateral nodosectomy of the right NG with ipsilateral or the left NG with contralateral NTS injection of saline (100nL) or CART antibody (2ng/mL).
(G) In fed conditions, nodosectomy prevented hyperphagia of ipsilateral (n = 9; two-way RM ANOVA, interaction (4,32) = 2.233, p = 0.088; Bonferroni’s multiple comparison test, *p < 0.05), but not contralateral (n =8; two-way RM ANOVA, interaction F(4,28) = 7.533, p = 0.0003; Bonferroni’s multiple comparison test, *p < 0.05, ***p < 0.001), NTS injection of CART antibody.
(H)2 h food intake following intraNTSCART antibody injection in intact, ipsilateral nodosectomy, and contralateral nodosectomy rats (n = 8/9/9; two-way ANOVA, interaction F(2,23) = 5.885, p = 0.009; Sidak’s multiple comparison test, *p < 0.05, ***p < 0.001).
In fasted lean rats, CART antibody had no effect on 2 h food intake (Figures 3D and 3E). Thus, CART antibody-mediated hyperphagia occurs in the fed state, when VAN CART expression is high, but not in the fasted state, when CART expression is depressed (Figures 1A–1C).
The NTS receives numerous CART+ afferent inputs from central sites (Zheng et al., 2002). Therefore, to test whether afferent terminals originating from a vagal source are necessary for endogenous CART-induced satiation in the NTS, we removed vagal inputs by unilateral nodosectomy with ipsilateral or contralateral CART antibody infusion into the right mNTS (Figure 3F). Right nodosectomy severely blunted ipsilateral CART antibody-induced hyperphagia (Figures 3G and 3H), while left nodosectomy with contralateral CART antibody infusion into the right mNTS did not prevent increased food intake (Figures 3G and 3H). Comparing 2 h intake of intact, left-nodosectomy, or right-nodosectomy rats confirms that vagal inputs into the NTS are required to block the stimulatory effects of intraNTS CART antibody infusions on eating (Figure 3H). Altogether, these data support the idea that intact vagal afferent inputs into the NTS are required for the anorexigenic effect of endogenous CART.
NG CART KD Is Sufficient to Increase Food Intake and Body Weight
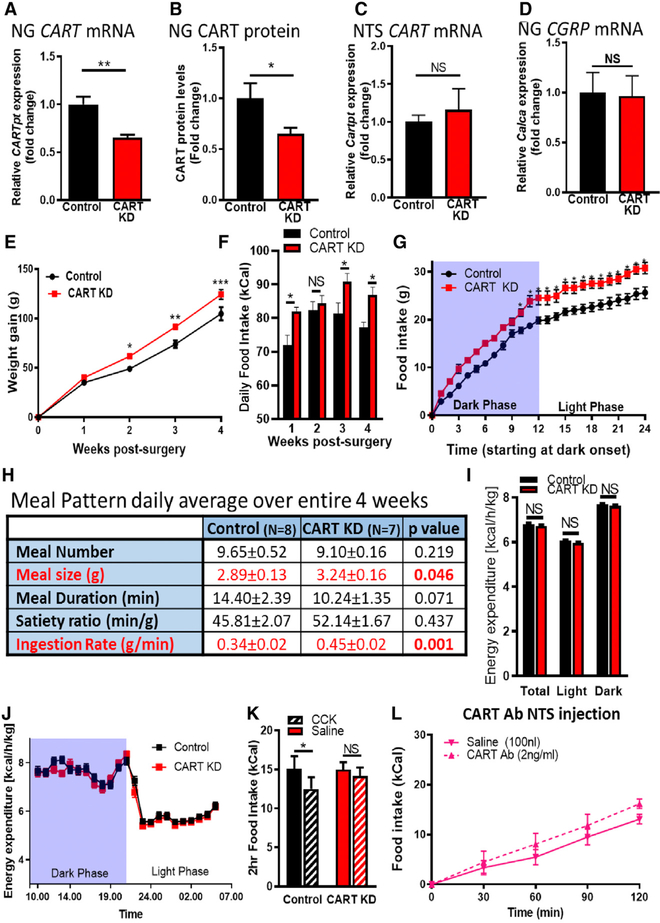
To determine whether reduced vagal CART expression would affect long-term energy homeostasis, we used a lentiviral (LV)-mediated short hairpin RNA (shRNA) approach for selective KD of CART expression in VANs. In vivo, injection of LV-short hairpin CART (shCART) bilaterally into the NG significantly reduced CART mRNA (33.0% ± 0.3%) and protein expression (34.8% ± 0.6%) compared with rats injected with the same lentiviral vector expressing control non-specific target shRNA (LV-shCTR; Figures 4A and 4B). Conversely, CART expression in the NTS was not altered (Figure 4C), and calcitoningene-related protein mRNA (Calca) was identical in the NG of control and CART KD rats (Figure 4D), indicating that NG injection of LV-shCART is tissue specific and selectively targets CART.
Figure 4. NG CART KD Is Sufficient to Increase Food Intake and Body Weight.
(A and B) LV-mediated KD reduced (A) CART mRNA expression (n = 7; unpaired two-tailed t test, p = 0.0022) and (B) CART protein concentration (n = 7; unpaired one-tailed t test, p = 0.025).
(C and D) NTS CART mRNA expression (n = 7; unpaired one-tailed t test, p = 0.293) (C) and CGRP mRNA in the NG (n = 7; unpaired two-tailed t test, p = 0.452) (D) are unaffected by NG CART KD.
(E and F) Body weight gain per week (n = 10/8; two-way ANOVA, interaction F(3, 48) = 1.907, p = 0.141; Sidak’s multiple comparison test, *p = 0.02, ***p < 0.0043) (E) and average daily chow intake per week (n = 8 control, n = 7 KD; two-way ANOVA, interaction F(3,39) = 1.233, p = 0.31; Sidak’s multiple comparison test, *p < 0.05) (F) are increased in KD.
(G) Cumulative food intake over a 24 h period highlighting hyperphagia occurs primarily in the dark phase (n = 8/7; two-way ANOVA, interaction F(24,312) = 1.9, p = 0.008; Sidak’s post hoc multiple comparison test, *p < 0.05).
(H) Table attributing increased food intake to an uncompensated increase in ingestion rate and meal size (n = 8/7; unpaired Student’s t test).
(I) Average daily energy expenditure (days 14–19 postoperation) is unchanged between groups (n = 7/ 9; two-way ANOVA, F(22,322) = 0.914, p = 0.577).
(J) Average hourly energy expenditure over 24 h is unaffected by NG CART KD (n = 7/9; two-way ANOVA, F(22,322) = 0.914, p = 0.577).
(K) CCK-induced satiation is blunted in CART KD rats (n = 7/9; two-way ANOVA, F(1,13) = 1.86, p = 0.20; Sidak’s multiple comparison test, *p = 0.021).
(L) In CART KD rats, intraNTS CART antibody failed to increase food intake (n = 8/7; two-way ANOVA, F(4,24) = 1.04, p = 0.407).
NG CART KD significantly increased body weight up to 20% more than controls by four weeks postinfection (Figure 4E). Concurrently, NG CART KD increased daily chow intake 17% compared with controls (Figures 4F and 4G). Overconsumption of food was driven by increased ingestion rate and meal size with no change in meal duration or meal number (Figure 4H). Hyperphagia and meal pattern differences were only observed during the dark phase, when rats consume most of their food (Figures S6A–S6C). No change in energy expenditure was observed (Figures 4I and S4J; Figure S6D). Reduced NG CART expression also abolished satiation induced by exogenous CCK (intraperitoneal [IP], 4 μg/kg) in 4 h fasted rats (Figure 4K), confirming previous work suggesting that CART is required to mediate CCK-induced satiety (Heldsinger et al., 2012). Therefore, we conclude that even partial loss of CART in the NG is sufficient to cause hyperphagia and weight gain by chronically increasing ingestion rate and meal size.
To fully evaluate whether endogenous CART released from the NG into the NTS is required to inhibit eating in response to a meal, we tested whether the hyperphagic effects of NTS CART antibody injection would be abolished by CART KD in the NGs. Viral-mediated CART KD in VANs abolished the stimulatory effect of CART antibody injected into the NTS under fed conditions (Figure 4L; Figure S5B). These data confirm that CART antibody injections specifically inhibit endogenous CART and, crucially, that the inhibitory effects of endogenous CART released into the NTS come from a vagal source.
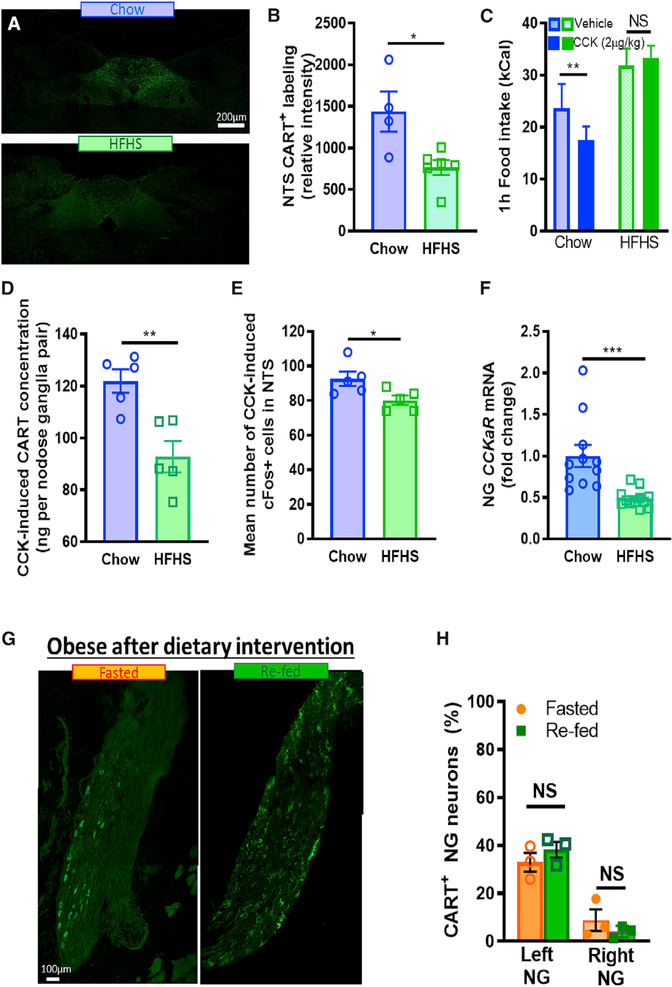
Calorie-Dependent Increase in VAN CART Expression Is Blunted in Diet-Induced Obesity
The NG have reduced sensitivity to peripheral postprandial signals in diet-induced obesity (DIO) (de Lartigue, 2016). Therefore, we hypothesized that NG CART expression would be blunted after chronic exposure to HFHS diet. Rats fed an HFHS diet for 8 weeks had 40% increased weight gain and were hyperphagic compared with lean chow-fed rats (Figures 5A and 5B). Unlike lean rats, refeeding failed to increase the percentage of CART+ neurons or CART protein concentration in the NG of obese rats (Figures 5C–5E). Furthermore, CART protein concentration was not increased in a calorie-dependent manner in DIO rats (Figure 5F). Thus, chronic exposure to energy-dense palatable foods impairs vagal sensitivity to peripheral food cues and/or intracellular signaling mechanisms, resulting in constitutively blunted postprandial NG CART synthesis in obesity. Interestingly, refeeding failed to significantly increase the percentage of CART+ neurons of both left and right NG in DIO rats; however, meal-induced CART expression was significantly lower in the right NG (Figures 5D and 5E). In obese rats, CART antibody injection into the NTS did not alter food intake in either fed or fasted conditions (Figures 5G and 5H), suggesting that there are low levels of endogenous CART released in the NTS in obesity. In support of this, we found that CART immunoreactivity was reduced in HFHS-fed rats compared with lean rats selectively in the commissural NTS (Figures 6A and 6B).
Figure 5. Calorie-Dependent Increase in VAN CART Expression Is Blunted in DIO.
(A) Body weight gain (n = 6; Two-way ANOVA, interaction F(4,40) p<0.0001; Sidak’s multiple comparison **p<0.0079, ***p<0.0001) and (B) energy intake (n = 6; Two-way ANOVA, Interaction F(4,40) p = 0.0003; Sidak’s multiple-comparison test, *p = 0.0178, **p = 0.0054, ***p = 0.0002) of rats fed either HFHS or chow diet for 8 weeks.
(C) EIA quantification of CART protein concentration from both left and right NG from obese rats fasted 48 h or fasted and refed 2 h (n = 4; unpaired two-tailed t-test p = 0.078).
(D) The percent of CART+ neurons remains unchanged in response to 2 h refeeding in left or right NG (n = 4, two-way ANOVA, interaction F(1,12) = 0.9906, p = 0.34, Sidak’s post-hoc comparison).
(E) Representative stitched images of left or right NG CART expression from DIO rats either fasted 48 h or refed 2 h. Scale bar, 100 μm.
(F) NG CART concentration increased linearly with caloric intake in lean rats fed a HFHS meal (n = 10), but not in DIO rats (n = 7; differences in linear regression slopes F(1,13) = 119, p<0.0001).
(G) In DIO rats, intraNTS injection of CART antibody (2ng/ml) had no effect on caloric intake compared with saline (100 nl) in fasted or refed conditions (n=10/9; three-way ANOVA, F(4,4) = 0.12, p = 0.98). (H) 2 h food intake post CART antibody injection in fasted vs. fed DIO rats (n = 9/10; two-Way ANOVA, interaction F(1,8) = 0.27, p = 0.62).
Figure 6. Reduced Vagal Sensitivity to CCK in Obesity Is Associated with Impaired CCK Signaling and CART Expression in NG.
(A) Representative images of CART in DVC from rats fed chow or HFHS diet for 8 weeks. Scale bar, 100 μm.
(B) Quantification of NTS CART is lower in the NTS of HFHS-fed compared with chow-fed rats (n = 4–6; unpaired two-tailed t-test, p = 0.0166).
C) CCK (IP, 2ug/kg) reduces 1 h food intake in chow, but not HFHS, fed rats (n = 5; Two-Way ANOVA, interaction F(1,8) = 5.834, p = 0.04; Sidak’s multiple comparison **p < 0.0085).
(D) EIA quantification of CART protein concentration from pooled left and right NG increases more in response to CCK (IP, 2ug/kg) in chow than HFHS fed rats (n = 5; unpaired two-tailed t-test, p = 0.0048).
(E) c-Fos in the NTS increases more in response to CCK (IP, 2ug/kg) in chow fed compared to HFHS fed rats (n=5; unpaired two-tailed t-test, p = 0.04).
(F) CCKa receptor mRNA expression is lower in the NG of HFHS-fed, compared to chow-fed, rats (n = 11–12; unpaired two-tailed t-test, p = 0.0008).
(G) Representative stitched images of right NG from rats obese rats fed a high fat diet for 8 weeks and switched to chow for 5 months. Dietary intervention failed to rescue low CART expression in NG in DIO rats in both fasted and refed conditions. Scale bar, 100 μm.
(H) Percent CART+ NG neurons remains blunted with re-feeding. (n = 3; unpaired two-tailed t-test p = 0.703)
Next, we assessed the mechanism responsible for blunted NG CART expression in obesity. Previous work has demonstrated that CCK is necessary and sufficient for postprandial CART NG expression in lean animals (de Lartigue et al., 2007). In obese rats, CCK induced satiation and c-Fos expression in the NTS have both been demonstrated to be reduced (Covasa and Ritter, 1998). Therefore, we hypothesized that reduced sensitivity to CCK in the NG of obese rats was responsible for blunted NG CART expression. To test this, we fed rats either chow or HFHS diet for 8 weeks. Exogenous administration of CCK (IP, 2 μg/kg) reduced food intake in chow-fed rats, but not HFHS-fed rats (Figure 6C), and this coincided with reduced CART concentration in the NG measured by EIA (Figure 6D), and c-Fos in the NTS measured by immunocytochemistry (Figure 6E), in HFHS-fed compared with chow-fed rats. In a separate batch of age-matched rats, we found 51% reduction in CCKa receptor mRNA expression in HFHS-fed compared with chow-fed rats (Figure 6F), suggesting reduced CCKa receptor expression may be at least partially responsible for blunted NG CART expression in DIO.
Blunted Vagal CART Expression in DIO Is Not Restored by a Dietary Intervention
Next, we addressed whether the downregulation of CART expression seen in DIO rats is reversible by a dietary intervention. Rats fed an HFHS diet for 8 weeks, when they weighed significantly more than chow-fed rats, were switched back to chow for more than 5 months (Figure S7A). After an initial weight loss in the first 2 weeks of dietary intervention, body weight plateaued before ending at a higher level compared with animals exclusively maintained on chow (Figure S7A). Daily food intake was greater while on an HFHS diet and was still higher more than 5 months after dietary switch compared with chow controls (Figure S7B). At this later time point, CART expression remained blunted in NG neurons after refeeding (Figure 6G). The percentage of CART+ neurons in both left and right NG (Figures 6G and 6H) was similar to that of obese rats maintained on an HFHS diet for 8 weeks (Figures 5D and 5E), with no quantifiable increase in postprandial CART expression. Importantly, age-matched chow-fed controls retained calorie-induced CART expression (Figures S7C and S7D). Therefore, preventing consumption of an obesogenic diet alone is not sufficient to restore NGCART expression following the onset of DIO.
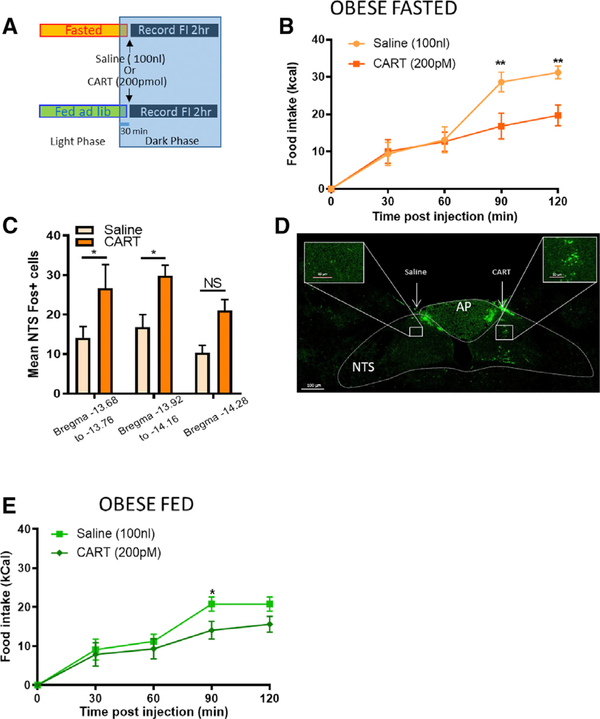
The Anorexigenic Effect of CART NTS Injection Is Preserved in DIO Rats
Finally, we tested whether NTS neurons remain sensitive to CART and whether CART could reduce food intake in DIO rats. Administration of exogenous CART into the NTS of DIO rats reduced food intake in fasted rats, though with slower kinetics (Figures 7A and 7B). Unilateral intraNTS administration of CART increased c-Fos expression at the site of injection compared with saline in the NTS of DIO rats, irrespective of injection side in a counterbalanced design (Figures 7C and 7D). Unlike fed lean rats, which were unresponsive to exogenous CART (Figure 2G), fed DIO rats reduced 90 min food intake in response to CART administration into the NTS (Figure 7E). These data further indicate that the anorexigenic effect of exogenous CART depends on low endogenous NG CART levels. Altogether, these data provide important evidence that NTS neurons remain sensitive to CART in DIO.
Figure 7. The Anorexigenic Effect of CART NTS Injection Is Preserved in DIO Rats.
(A) Experimental paradigm in obese rats.
(B) In fasted obese rats, right intraNTS injection of CART reduced caloric intake at 90 and 120 min compared with saline (n = 5; two-way ANOVA, interaction F(4,20) = 5.518, p = 0.004; Sidak’s multiple comparison test, **p < 0.003).
(C) CART was injected into the right or left NTS, and the same animal received contralateral injection of saline. After counterbalancing, intraNTS injection of CART increased c-Fos expression in neurons of the NTS compared with saline in the same obese fasted rats (n = 6; two-way ANOVA, interaction F(2,30) = 0.062, p = 0.94; Sidak’s multiple comparison test, *p < 0.05).
(D) Representative image of c-Fos expression in the dorsal vagal complex 90 min after intraNTS infusion of saline (left) and CART (right).
(E) In fed DIO rats, counterbalanced injection of CART intraNTS reduced caloric intake compared with saline (n = 6; two-way ANOVA, F(4,20) = 1.48, p = 0.245; Sidak’s multiple comparison test, *p < 0.05).
Scale bar, 100 mm. Inserts: NTS is shown in higher magnification. Scale bar, 50 mm.
DISCUSSION
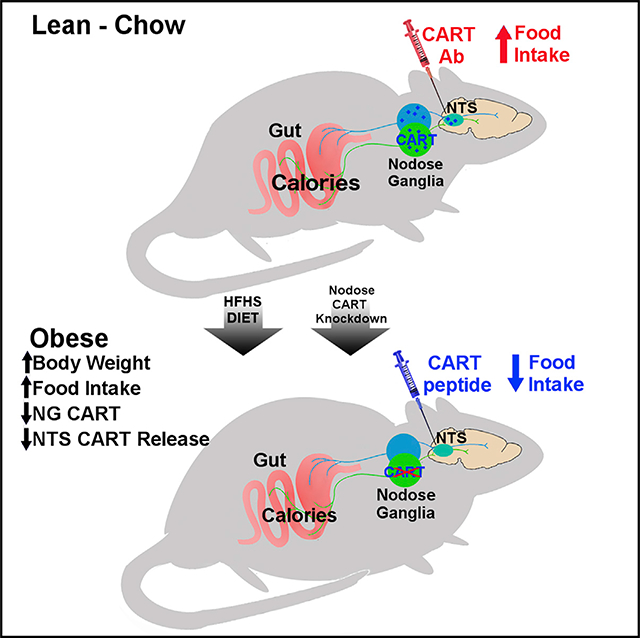
CART expression in the NG neurons has been implicated in gut hormone-mediated inhibition of food intake (de Lartigue et al., 2007, 2010; Heldsinger et al., 2012). Here we address many outstanding questions about NG CART signaling and its role in the control of eating behavior under physiological conditions. Specifically, we show that (1) CART expression in the NG encodes caloric information to the brain, (2) CART activation of NTS neurons is sufficient to reduce food intake, and (3) release of endogenous CART from the NG into the NTS is necessary to inhibit eating. Furthermore, we identify that in DIO, CART expression in the NG is constitutively low irrespective of the feeding state, possibly via a mechanism involving impaired CCK sensitivity by sensory vagal neurons. Partial loss of CART expression in the NG, as mimicked by viral-mediated KD, increased food intake and body weight in chow-fed rats, providing supporting evidence that blunted NG CART expression is sufficient for hyperphagia and weight gain. Although dietary switch from an HFHS diet to chow failed to restore postprandial NG CART expression in DIO, intraNTS CART injection retained its anorexigenic effect in DIO rats. Altogether, the data identify CART as an important anorexigenic signal in VANs capable of modulating energy intake; disruption of CART vagal signaling causes weight gain, the hallmark of obesity.
We confirm previous reports (de La Serre et al., 2015; de Lartigue et al., 2007, 2010, 2014; Lee et al., 2011) that in lean animals, CART expression is low after a fast and is upregulated after a meal in the dark phase. We find that both 48 h fasting in the immunohistochemistry experiments and overnight fasts in the EIA resulted in low CART levels, which were increased with re-feeding. Thus, CART expression changes occur consistently across a spectrum of physiological conditions. Furthermore, we replicate a contradictory report (Yuan et al., 2016) demonstrating that food availability had no impact on CART expression in the left NG during the light phase. We hypothesized that food intake may account for the apparent paradox and therefore quantified the amount of food consumed in either of these experimental feeding paradigms. Notably, gastric content was present in similar levels in both ad libitum-fed and fasted rats 3–5 h into the light phase. Gastric content in both of these groups was significantly higher than in animals fasted 48 h on wire floors and trended lower than in animals fasted and refed for 2 h in the dark phase. Presumably, the low levels of food in the ad libitum-fed rodents is because rats consume few to no calories in the early hours of the light phase, whereas the elevated levels of gastric content in the fasted animals are a result of coprophagy. Based on the differences in gastric content between the experimental paradigms, we conclude that nutrient-induced postprandial signals, rather than food availability or the absence of hunger alone, are required to increase CART expression. In support of this, gavage of calorie-rich Ensure increased NG CART expression compared with the same volume of non-absorbable methylcellulose. Future work will be necessary to determine the role of individual macronutrients on NG CART synthesis; however, extensive literature supports the view that food ingestion results in release of gastrointestinal hormones, and we confirm previous studies demonstrating a causal relationship between the gastrointestinal satiety hormone CCK and the postprandial rise in CART expression in VANs (de Lartigue et al., 2007). We extend previous findings with three additional lines of evidence to support a role for CCK in CART expression. First, ingestion of fewer grams of HFHS diet was required compared with low-fat chow for equivalent increases in NG CART concentration, consistent with fat as a potent stimulator of CCK release (Liddle et al., 1985). Second, CART expression was constitutively low in DIO animals, when vagal sensitivity to CCK (Daly et al., 2011) and CCK-induced satiation are blunted (Covasa and Ritter, 1998). Finally, reduced sensitivity to CCK in obesity, as characterized by reduced CCK-induced c-Fos labeling in the NTS and reduced satiation, results in blunted CCK-induced CART expression in the NG compared with those of lean controls. Thus, one key reason for the discrepancy in the literature is that experimental paradigms in which animals do not have a surge in CCK release are unlikely to result in CART upregulation. Additional factors that may account for the discrepancy in the literature include that circadian cycle influences CART expression (Turek et al., 2005) and that CART expression changes are more pronounced in the right NG. Thus, the signal-to-noise ratio may be too small to observe changes in CART expression in the left NG of partially fasted animals in the light phase, especially given that basal fasting levels of CART in the left NG are high. Altogether, our findings emphasize the importance of controlling for the time of last meal and the calories consumed when studying the neurochemical phenotype of metabolic neurons.
We provide direct evidence that CART is an anorexigenic neuropeptide that controls food intake at the level of the NTS. CART peptide infused into the NTS is sufficient to reduce food intake when endogenous NG CART is low. Conversely, blocking endogenous CART signaling within the NTS using CART antibody resulted in a dose-dependent increase in food intake compared with saline. Although there is solid evidence concerning the identity of the CART receptor (Yosten et al., 2020), the absence of a viable antagonist necessitates the use of CART antibody. This approach has previously been used to immunoneutralize CART-induced anorexia centrally (Kristensen et al., 1998), and we validated this approach by demonstrating that pre-incubating CART antibody with CART peptide abolished the stimulatory effect of intraNTS CART antibody on food intake. Although antibodies are too large to penetrate synapses (Cartwright et al., 2014), neuropeptides are extensively released extrasynaptically (Trueta and De-Miguel, 2012; Zhu et al., 1986). In our experiments, we find that CART antibody alone fails to increase food intake in fasted conditions when endogenous vagal CART is low, suggesting that baseline levels of CART in the brain (Bakhtazad et al., 2016) are insufficient to influence food intake. The effects of CART antibody uniquely occur in fed rats, consistent with the idea that CART antibody is neutralizing CART released locally in response to a meal.
Several brain regions contain CART neurons that innervate the NTSs (Zheng et al., 2002). However, we provide multiple lines of evidence that NG fibers (from VANs) are a primary source of the endogenous CART in the NTS that controls food intake. First, stimulatory effects of CART antibody on food intake only occurred in fed lean animals when endogenous NG CART expression was elevated, whereas the inhibitory effects of CART were restricted to when NG CART expression was low (i.e., fasted or DIO). Second, removing NG cell bodies and fibers as a source of NTS CART by unilateral nodosectomy prevented hyperphagia following ipsilateral, but not contralateral, injection of CART antibody into the NTS. Most convincingly, KD of CART selectively in NG neurons abolished the eating-stimulatory effects of CART antibody, conclusively demonstrating that the anorexigenic effects of endogenous CART in the NTS results from CART released from vagal afferent fibers. A previous report indicates that intraNTS CART injection causes a rapid and sustained hypothermic response (Skibicka et al., 2009). However, rats with NG CART KD displayed no change in energy expenditure. This may point to different sources of CART-expressing afferent fibers signaling to distinct subpopulations of NTS neurons to regulate energy balance via separate mechanisms or roles for modulators in controlling CART effects in the NTS (Smedh et al., 2019).
The kinetics of CART signaling within the NTS were surprising. There was a noticeable delay in the eating response to CART or CART antibody infusion into the NTS, both requiring 60–90 min to take effect. The reason for the delayed eating effects remains unclear, but the findings are supported by a previous study in which there was no change in sucrose intake up to 30 min after NTS CART injection (Zheng et al., 2002). Importantly, we demonstrate that intraNTS injection of CART activates neurons of the NTS, suggesting that CART acts through a NTS-mediated circuit. Although it is not possible to exclude the possibility that CART is leaking into the 4th ventricle, the dose of CART we injected into the NTS (200 pM in 100 nL) is considerably less than doses of 4th ventricle CART (2–75 μM in 0.1–5 μL) required to produce an anorexic response (Aja et al., 2001a, 2001b, 2006; Smedh et al., 2015; Zheng et al., 2001, 2002). It is plausible that the delayed eating effects in response to NTS administration reflect a physiological mechanism and/or are the consequence of a technical issue. For example, a subthreshold dose, slow dispersion kinetics through the tissue, or a high physiological drive to eat after a fast may have prevented drug efficacy during the first meal. After a fast, rats consume a large first meal followed by a large intermeal interval. By measuring food intake every 30 min, we may lack the time resolution to determine when the second meal occurs. This would be particularly important if extrasynaptic CART plays a modulatory role on postsynaptic gene expression and/or by priming NTS cells to respond to subsequent glutamate release (de Lartigue, 2014). Alternatively, CART may activate a reward circuit that controls satisfaction or motivation to eat and therefore does not affect food intake immediately. This is supported by the recent identification of a polysynaptic reward circuit linking gut-innervating NG neurons to dopaminergic neurons of the substantia nigra (Han et al., 2018). Although it is unknown whether CART-expressing NG neurons are responsible for this effect, it is striking that dopamine release in the dorsal striatum and behaviors associated with the hallmark of reward are mediated by the right NG, but not the left NG (Han et al., 2018), in the same way that CART expression changes are more pronounced in the right NG. CCK recruits this reward circuit, and ablation of the right NG abolished the anorexigenic effects of CCK (Han et al., 2018), suggesting that the right NG may play a critical role in meal termination caused by CCK-mediated satisfaction. While the delayed kinetics of CART raise intriguing questions for future investigations, our findings demonstrate that exogenous CART injection into the NTS increases c-Fos in the NTS and inhibits food intake, whereas blocking endogenous CART in the NTS increases food intake, consistent with an anorexigenic role for CART at the level of the NTS.
We identify a potential role for defective NG CART signaling in the pathogenesis of obesity. CART expression in the NG was blunted in response to a meal in animals fed an HFHS diet for 8 weeks. This correlated with low levels of endogenous anorexigenic CART in the NTS of DIO animals as demonstrated by the lack of effect of CART antibody in these animals. We demonstrate that knocking down CART protein selectively in the NG increases weight gain. The mechanism driving weight gain seems to be primarily the consequence of hyperphagia from uncompensated increases in meal size and ingestion rate that persist at each meal. A calorie-restricted pair-fed CART KD group was not included, but a key role for hyperphagia in driving weight gain is supported by the following: (1) CART did not reduce energy expenditure (even when controlling for body weight between groups), (2) the anorexigenic effects of endogenous CART in the NTS were lost after CART KD in the NG, and (3) hyperphagia preceded weight gain. We go on to demonstrate a role for reduced CCK sensitivity in DIO rats as a mechanism for blunted CART expression, possibly because of diminished CCKa receptor expression. Whether HFHS-diet-induced disruption in vagal CART signaling is causing the onset of obesity or causes a worsening of the phenotype remains an open question. Future experiments aimed at determining whether disrupted CART expression in the NG develops before or after weight gain in DIO will be important to address this question. More broadly, these data suggest that the vagus nerve influences the long-term regulation of energy homeostasis. Previous studies using vagotomy or subdiaphragmatic vagal deafferentation were used to suggest that the vagus nerve is only implicated in short-term control of food intake, because removing the vagus nerve does not cause weight gain. A major difference between these two surgeries and NG KD of CART is that CART KD does not ablate all vagal signaling from abdominal organs. CART KD retains glutamatergic and other neuropeptide signaling and thus conveys some metabolic information to the brain. Therefore, vagal sensory neurons may play an important yet underappreciated role in energy homeostasis, and more specifically blunted NG CART is involved in weight gain.
The finding that CART remained blunted in the NG even after switching DIO rats from an HFHS diet to chow may provide insight into the lack of clinical success in treating obesity with lifestyle modification. Indeed, dietary interventions can result in modest 5%–10% weight loss in obesity (Look, 2014), with poor long-term body weight maintenance (Look, 2014). Weight gain and obesity were associated with increased ingestion rates (Ohkuma et al., 2015), while slower eating rates were correlated with greater maintenance of weight loss (Karfopoulou et al., 2017), and reducing eating rates in obese individuals can be effective in managing weigh loss (Ford et al., 2009). Because CART KD in the NG increases ingestion rate, future experiments aimed at restoring CART in the NG of DIO animals using either pharmacological methods that mimic a large meal or molecular and genetic tools may be effective treatments alone or in combination with lifestyle modifications. In support of this approach, we demonstrate that the anorexigenic effects of CART in obese rats were retained when we bypassed blunted NG CART expression by injecting CART directly into the NTS. The observation that NTS neurons remain sensitive to CART in DIO provides a proof of concept that future CART-based pharmacotherapies, combined with lifestyle interventions, could be a viable treatment option for obesity.
STAR★METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for reagents should be directed to, and will be fulfilled by the Lead Contact, Guillaume de Lartigue (gdelartigue@ufl.edu). This study did not generate new unique reagents.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
All experiments presented in this study were conducted according to the animal research guidelines from NIH and were approved by the Institutional Animal Care and Use Committee of The J.B. Pierce Laboratory, University of Florida, University of California Davis, and approved by the Canton of Zurich’s Veterinary Office (ETH Zurich).
Cell lines
GH3 cells (CCL 82.1, ATCC; 7month old female Wistar Furth Rat), grown in ATCC-formulated F-12K medium (30–2004) with 2.5% FBS and 1% Pen/Strep at 37C in 5% CO2
Human embryonic kidney 293T cells (HEK293T; CRL3216, ATCC, fetal origin; grown in DMEM (GIBCO) with 10% FBS and 1% Pen/Strep at 37°C in 5% CO2)
Experimental Animals
A total of 281 adult male Wistar rats (Harlan Industries, Indianapolis, IN) weighing 180–230 g (~7 weeks old) were single-housed in a climate-controlled room (22°C ± 2°C) on a 12:12 light cycle (11:00 am ON /11:00 pm OFF). Rats were randomly assigned to receive chow diet (Teklad 2018), or HFHS diet (Research Diets D12451) for 8 weeks. HFHS-fed group were considered obese when the average weight of the group was at least 30% more than average weight of the lean chow-fed control group and each individual HFHS-fed rat weighed more than the heaviest chow-fed rats. For imaging experiments in Figures 1 and 4 and Figures S1 and S2, rats were either fasted 48 h on wire-bottom cage, or fasted 46 h and re-fed respective diet ad libitum for 2 h starting 1 h into the dark phase. Similar feeding paradigms with shorter fasts were used for EIA experiments in Figures 1 and 4, rats (24 h fast), and for behavioral experiments in Figures 2, 5, 6, and 7 (overnight fast). In Figure S2 a separate cohort of rats were either fasted overnight or fed ad libitum and euthanized 3–5 h into the light phase as in Yuan et al. (2016). For knockdown studies 32 adult male Sprague Dawley rats (Charles River, Sulzfeld, Germany) were fed chow diet (Kliba3433, Kaiseraugst, Switzerland).
METHOD DETAILS
Surgical procedures
Nodosectomy
For unilateral NG removal, rats were fasted overnight and anesthetized with isoflurane (2%–4%). the cervical vagus nerve was exposed on one side via a midline cervical incision and blunt dissection. The vagus nerve was cut immediately above and below the NG. After suturing the incision, rats were implanted with a cannula aimed at the right NTS. All rats regained pre-op body weight prior to start of feeding studies.
NTS cannula implantation
Rats were placed in a stereotaxic instrument and implanted with 26-gauge stainless-steel guide cannulas (PlasticsOne) aimed at the right NTS (0.1 mm anterior to occipital suture, −0.5 mm lateral to midline, and 7.8 mm ventral from skull) and fixed with 3 screws into the skull and dental cement (Stoelting). Single intraNTS injections. After 24 h fast on wire floors, rats were anesthetized by isoflurane inhalation (1%–3% in oxygen). The skin over the dorsal neck surface was shaved, sterilized, incised along the midline, and the neck muscles retracted to expose the occipital membrane overlying the dorsal surface of the caudal medulla. With the aid of a surgical microscope, the membrane was opened with a sterile needle to reveal obex on the dorsomedial medullary surface at the caudal tip of the AP. To target tracer delivery into the caudal medial NTS, a glass micropipette (inner tip diameter ~15 μm) filled with either saline or CART (200 pM) was positioned 0.4 mm lateral to obex and then the tip was advanced 0.4 mm below the medullary surface at a 10° angle from the vertical plane. A total of 100 nL was pressure injected using a Picospritzer III injector over the course of 5 min. The glass pipette was removed 1 min after drug delivery. All lean animals received injections of the same solution bilaterally, all obese animals received counterbalanced injections of CART or saline. After injection, the skin incision was sutured, and the animals were monitored in clean cages with no access to food.
NG injection
On surgery day, rats (290–340 g) were anesthetized by isoflurane inhalation (1%–3%) or IP injection of ketamine (88 mg/kg; Ketalar, Cantonal Pharmacy Zurich) and xylazine (5 mg/kg; Rompun 2%, Cantonal Pharmacy Zurich), and NG were exposed. A glass capillary (50 μm tip) was used to administer 1.5 μL viral solution into each NG with a Picospritzer III injector (Parker Hannifin) as previously described (Krieger et al., 2016). Injections were performed bilaterally into NG.
- Viral mediated CART KD
The efficiency of the CART-targeting shRNA construct was initially verified in vitro in GH3 cells and sorting turboGFP-expressing cells (FACS Aria III FCF, Flow Cytometry Facility, University of Zurich). CART–targeting shRNA or control shRNA were packaged into lentiviral particles using HEK293T cells and the pMD2.G and psPAX2 plasmids (gifts from D. Trono, École Polytchnique Fédérale de Lausanne; cat. no. 12259 and 12260; Addgene). The viral constructs were concentrated to 1010 particles/mL using 8% PEG6000 (Millipore), resuspended in PBS, and stored at −80C in 10 ul aliquots,
- Anterograde tracing
10% biotinylated dextran was prepared fresh on the day surgery in 0.1 M PBS and injected bilaterally into NG.
Histological procedures
CART staining. 2 h food intake and stomach content were weighed upon sacrifice. NG were collected, post-fixed in 4% PFA 2 h, and cryoprotected in 0.1 M phosphate buffer containing 25% sucrose at 4°C until cryo-sectioned (10 μm). NG sections from left and right nodose ganglia of one fed and one fasted animal were placed on the same slide (1:5 series) to allow direct comparison. Slides were incubated overnight with CART antibody (1:1000; H003–62; Phoenix Pharmaceuticals) and stained 2 h with donkey anti-rabbit Ig conjugated to AlexaFluor488 (1:500; ThermoFisher Scientific). Specificity of immunostaining was determined by omitting the primary antibody or by pre-incubation of antibody with an excess of CART peptide. Stained sections were mounted on slides and coverslipped with ProLong Gold (Invitrogen) prior to imaging on Zeiss Axio Imager 2 with AxioCam503 using a 20x or 40x objective.
c-Fos staining
Rats were perfused with 4% PFA in PBS 90 minutes following stimulus. The brains were post-fixed in 4% PFA 24 h, and cryoprotected in 0.1 M phosphate buffer containing 25% sucrose at 4°C until cryo-sectioned (30 μm). IHC was conducted according to previously described procedures (Diepenbroek et al., 2017). Briefly, sections were incubated in 50% methanol in PBS 1x for 30 min and washed with 1x PBS. Brain sections were incubated in 10% normal donkey serum for 1 h, followed by an overnight (16 h) incubation with polyclonal rabbit anti c-Fos primary antibody (1:10000; PC05, Calbiochem). Sections were then washed and incubated (2 h) with secondary antibody donkey anti-rabbit Alexa Fluor 488 (1:1000; Jackson ImmunoResearch Laboratories) and coverslipped using prolong Gold.
Anterograde tracer labeling
10% BDA was injected bilaterally into the NG. One week was allowed for the tracer to be transported to label vagal afferent fibers in the NTS. After a 24 h fast on wire floors half the rats were maintained fasted or refed ad libitum chow for 2 h. Food intake was measured, and rats that had consumed more than 10kCal were perfused with 4% PFA in PBS. The brains were collected and post-fixed in 4% PFA for 24 h, followed by storage for up to a week in 0.1 M phosphate buffer containing 25% sucrose at 4°C until cryo-sectioned (30 μm). IHC was conducted according to previously described procedures. For co-labeling of BDA and CART, hindbrain sections from BDA-injected animals were incubated overnight in rabbit anti-CART (1:1000) at 4C, and after washing in 01 M PBS, sections were incubated in Alexa 488-conjugated donkey anti-rabbit (1:500; Invitrogen) and Alexa 594-conjugated streptavidin (1:500) before being coverslipped with Prolong Gold.
Quantification
A semiquantitative method was used to assess the relative intensity of the immunostaining for NG CART (Fang et al., 2002). ImageJ was used to quantify fluorescence intensity of a single full length section of left and right NG from each animal. Only sections with 200 total neurons were considered for analysis. Each NG neuron was outlined and mean gray value was determined using measurement tools in ImageJ from RGB image. The lowest mean intensity was subtracted from all the neurons, and each value was divided by the highest mean absorbance across the whole study (from lean re-fed rats). Therefore each neuron across the whole study resided on the same scale from 0%–100%. Four blinded researchers subjectively scored cells as negative, positive, or weakly positive for CART-LI. Neurons with mean absorbance ≥ 30% were consistently assigned as positive by all individuals, while lower mean absorbances were judged to be negative. Therefore, CART+ was used to refer to neurons with mean absorbance ≥ 30% across all sections. In Figure S1, we used the same protocol as described in Yuan et al. (2016) to express data as relative intensity and intensity quartiles.
Immunofluorescence in the c-fos in the NTS was visualized and imaged using confocal microscopy (Nikon C2) at 10 × and 20 × magnification. Three sections per rat were quantified blinded at the levels of the DVC (bregma −13.6 through − 14.2 mm) using NIS Elements software (AR 3.0, Nikon). The number of Fos+ nuclei or fibers in the visual field were quantified within a region delimited by the drawn contour surrounding the NTS or AP. The detection of Fos+ nuclei from background was based on image analysis of density. Detection of an object required that a group of pixels had a continuous round border with values of OD above threshold, surrounded by a border with optical densities below threshold.
CART and BDA fibers in the NTS were analyzed with Nikon imaging software (NIS-elements) using mean intensity fluorescence tool in “automated Measurements” For each animal, the analyzed sections were binned by calculating the average area across at least two serial sections. The reported values represent the group mean of these binned values. For colocalization between CART and BDA, a Pearson’s correlation coefficient was determined using the “co-localization” tool in Nikon imaging software.
Gene Expression and Protein Analysis
The NTS was mircropunched using anatomical landmarks, and NG from the same animal were pooled before mRNA was extracted using Trizol (Life Technologies). RT quantitative PCR was performed in triplicate using SybR Green on a OneStep Plus instrument (Applied Biosystems), and results were analyzed using the 2ddCt method (Livak and Schmittgen, 2001).
Enzyme Immunoassay
Food intake from each animal was weighed. NG pairs were collected and immediately frozen on dry ice. NG were weighed and homogenized in 2.5 mL solution buffer per gram of tissue (solution buffer: 1% triton-x 100; 7% protease inhibitor cocktail; 92% MPER) for 20–25 s on ice. The homogenate was centrifuged at 10,000 G at 4°C for 15 min. Supernatant was removed and stored at −80°C. Total protein content of the tissue was determined using a Pierce BCA Protein Assay Kit (23225, Thermo Scientific). The CART levels of NG samples were determined using an enzyme immunoassay (EIA) kit (61–102, Phoenix Pharmaceuticals) following the manufacturer’s protocol. The results were read by EIA plate reader (Spectramax 340PC) at 450nm. Concentrations of peptide were calculated from standard curve and normalized to loaded protein.
Behavioral Studies
Food Intake Measurement and Meal Pattern Analysis
In a crossover, counterbalanced experimental design, rats were overnight-fasted (24 h) on wire floors or ad libitum-fed and received a 100 nL NTS injection of saline, CART antibody (100 nl, 0–2 ng/ml), or CART (100 nl; 200 pM) 1 h into the dark phase. The final CART antibody concentration (2 ng/ml) was selected based on a preliminary study (Figure 3C), and the highest soluble concentration of CART peptide was used as previously described (Zheng et al., 2002). Each treatment condition was conducted once, each rat receiving a maximum of four injections. Upon completion, rats were euthanized, 100 nL of retrobeads (Lumafluor Inc) were injected and perfused hindbrain tissue was collected for histological verification of cannula placement.
Immediately after intraNTS injection, rats were given access to pre-weighed respective diets, and intake less spillage was recorded manually every 20–30 min for 2 h. For the DIO animal cohort receiving intraNTS CART or saline injections (Figure 7), food intake was continuously monitored by a BioDAQ feeding system. Extra large feeder openings were used for rats with implanted cannulas. Rats were acclimated to BioDAQ cages for 1 week prior to treatments. Notably, rats ate fewer calories in the BioDAQ cages (~5g/day). For CART KD rats, food was available through a niche and placed on scales (XS4001S; Mettler-Toledo) for continuous measurement as described previously (Karimian Azari et al., 2013). Meal patterns were analyzed with custom software (BioDAQ, or LabX meal analyser 1.4, Mettler-Toledo). Data are presented as average of a minimum of 3 days.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses were performed using Prism software (Prism 7.0; GraphPad Software, La Jolla, CA) and R (https://www.r-project.org/). Analyses made use of one- or two-way (repeated-measures) ANOVA and post hoc tests whenever relevant to correct for multiple comparison. All data are presented as means ± SEM. In all cases sample sizes (N) denote number of animals used. To assess potentially spurious results associated with non-normality, all significant effects were confirmed by rerunning the tests using the appropriate non-parametric test. Data are individually plotted (Prism 7, GraphPad), and the corresponding bar plot of the precision measures (mean ± SEM) were overlaid on the figure. The exact value of all N, df, T/F, χ2and p values are reported in the figure legends. An effect was considered statistically significant whenever the corresponding statistic was associated with a p value (Bonferroni or Sidak-corrected when appropriate) equal to or less than 0.05.
DATA AND CODE AVAILABILITY
This study did not generate any unique datasets or code.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CART antibody | Phoenix Pharmaceutical | Cat#H-003-062; RRID: AB_2313614 |
| cFos | Millipore | cat#PC05; RRID: AB_10682209 |
| Streptavidin 594 | ThermoFisher Scientific | Cat# S11227; RRID: AB_2619631 |
| donkey anti-rabbit alexa fluor 488 | ThermoFisher Scientific | AB_2556546, cat#R37118; RRID: AB_2535792 |
| Bacterial and Virus Strains | ||
| Lentivirus | packaged in house | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Cholecystokinin octapeptide | Bachem | H-2080 |
| CART peptide | Phoenix Pharmaceutical | 006-62 |
| retrobeads | Lumafluor Inc | N/A |
| Protease inhibitor cocktail | Sigma | P8340 |
| Triton X-100 | Sigma | X100 |
| Saline | Hospira | 0409-4888-02 |
| Paraformaldehyde | Sigma | P6148 |
| PEG6000 | Millipore | 528877 |
| Sucrose | Sigma | 55-50-1 |
| Prolong Gold | ThermoFisher | P36934 |
| Biotinylated Dextran Amine | ThermoFisher | D1956 |
| Critical Commercial Assays | ||
| Pierce BCA protein assay kit | Thermo Scientific | 23225 |
| CART enzyme immunoassay kit | Phoenix Pharmaceuticals | 61-102 |
| trizol | ThermoFisher | 15596026 |
| sybrGreen | ThermoFisher | 4472903 |
| ProLong Gold | ThermoFisher | P36930 |
| MPER | ThermoFisher | 78501 |
| Pierce BCA Protein Assay kit | ThermoFisher | 23225 |
| Isoflurane | Henry Schein | 11695-6776 |
| Ketamine | Cantonal Pharmacy Zurich | Ketalar |
| Xylazine | Cantonal Pharmacy Zurich | Rompun |
| Experimental Models: Cell Lines | ||
| GH3 cells | ATCC | CCL 82.1 |
| HEK293T cell | ATCC | CRL3216 |
| Experimental Models: Organisms/Strains | ||
| Male Wistar rats | Harlan | RccHan:WIST |
| Male Sprague Dawley rats | Charles River | Strain code 400 |
| Oligonucleotides | ||
| GCGCTGTGTTGCAGATTGAAG | Sigma Aldrich | CART shRNA (SHP001) |
| non-target shRNA | Sigma Aldrich | MFCD07785395; (SHC016) |
| Recombinant DNA | ||
| pLKO.1-puro vectors | Sigma Aldrich | SHC016 |
| pMD2.G | D. Trono, Ecole Polytechnique Federale de Lausanne, available from Addgene | 12259 |
| psPAX2 | D. Trono, Ecole Polytechnique Federale de Lausanne, available from Addgene | 12260 |
| Software and Algorithms | ||
| Prism 7.0 | Graphpad software | https://www.graphpad.com/scientific-software/prism/ |
| R bundled with 64 bit | The R project | https://www.r-project.org/ |
| ImageJ bundled with 64-bit | NIH ImageJ | http://shop.adobe.com; https://imagej.nih.gov |
| NIS Element | Nikon | AR 3.0 |
| Other | ||
| Dental Cement | Stoelting | 51458 |
| Guide Cannula | PlasticsOne | C315G |
| Dummy Cannula | PlasticsOne | C315D |
| Internal Cannula | PlasticsOne | C315I |
Highlights.
CART is a molecular signal that encodes caloric information for meal termination
Vagal CART synthesis is blunted in obesity partly due to reduced sensitivity to CCK
Knockdown of vagal sensory CART increases food intake by preventing negative feedback
NTS administration of CART inhibits food intake in both lean and obese rats
ACKNOWLEDGMENTS
This work was funded by an American Neurogastroenterology and Motility Society pilot grant (to G.d.L.), ETH Zurich research grant 47 12-2 (to S.J.L. and W.L.), and NIH grants R00 DK094871 (to G.d.L.), R01 DK116004 (to G.d.L.), and R01 DK041004 (to H.E.R.).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2020.01.045.
REFERENCES
- Aja S, Sahandy S, Ladenheim EE, Schwartz GJ, and Moran TH (2001a). Intracerebroventricular CART peptide reduces food intake and alters motor behavior at a hindbrain site. Am. J. Physiol. Regul. Integr. Comp. Physiol 281, R1862–R1867. [DOI] [PubMed] [Google Scholar]
- Aja S, Schwartz GJ, Kuhar MJ, and Moran TH (2001b). Intracerebroventricular CART peptide reduces rat ingestive behavior and alters licking microstructure. Am. J. Physiol. Regul. Integr. Comp. Physiol 280, R1613–R1619. [DOI] [PubMed] [Google Scholar]
- Aja S, Robinson BM, Mills KJ, Ladenheim EE, and Moran TH (2002). Fourth ventricular CART reduces food and water intake and produces a conditioned taste aversion in rats. Behav. Neurosci 116, 918–921. [PubMed] [Google Scholar]
- Aja S, Ewing C, Lin J, Hyun J, and Moran TH (2006). Blockade of central GLP-1 receptors prevents CART-induced hypophagia and brain c-Fos expression. Peptides 27, 157–164. [DOI] [PubMed] [Google Scholar]
- Babic T, Troy AE, Fortna SR, and Browning KN (2012). Glucose-dependent trafficking of 5-HT3 receptors in rat gastrointestinal vagal afferent neurons. Neurogastroenterol. Motil 24, e476–e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Mesgarzadeh S, Ramesh KS, Huey EL, Liu Y, Gray LA, Aitken TJ, Chen Y, Beutler LR, Ahn JS, et al. (2019). Genetic Identification of Vagal Sensory Neurons That Control Feeding. Cell 179, 1129–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhtazad A, Vousooghi N, Garmabi B, and Zarrindast MR (2016). Evaluation of CART peptide level in rat plasma and CSF: Possible role as a biomarker in opioid addiction. Peptides 84, 1–6. [DOI] [PubMed] [Google Scholar]
- Broberger C, Holmberg K, Kuhar MJ, and Hökfelt T (1999). Cocaine- and amphetamine-regulated transcript in the rat vagus nerve: A putative mediator of cholecystokinin-induced satiety. Proc. Natl. Acad. Sci. USA 96, 13506–13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright AN, Griggs J, and Davis DM (2014). The immune synapse clears and excludes molecules above a size threshold. Nat. Commun 5, 5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covasa M, and Ritter RC (1998). Rats maintained on high-fat diets exhibit reduced satiety in response to CCK and bombesin. Peptides 19, 1407–1415. [DOI] [PubMed] [Google Scholar]
- Covasa M, and Ritter RC (2000). Adaptation to high-fat diet reduces inhibition of gastric emptying by CCK and intestinal oleate. Am. J. Physiol. Regul. Integr. Comp. Physiol 278, R166–R170. [DOI] [PubMed] [Google Scholar]
- Covasa M, Grahn J, and Ritter RC (2000). Reduced hindbrain and enteric neuronal response to intestinal oleate in rats maintained on high-fat diet. Auton. Neurosci 84, 8–18. [DOI] [PubMed] [Google Scholar]
- Covasa M, Marcuson JK, and Ritter RC (2001). Diminished satiation in rats exposed to elevated levels of endogenous or exogenous cholecystokinin. Am. J. Physiol. Regul. Integr. Comp. Physiol 280, R331–R337. [DOI] [PubMed] [Google Scholar]
- Daly DM, Park SJ, Valinsky WC, and Beyak MJ (2011). Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J. Physiol 589, 2857–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling RA, Zhao H, Kinch D, Li AJ, Simasko SM, and Ritter S (2014). Mercaptoacetate and fatty acids exert direct and antagonistic effects on nodose neurons via GPR40 fatty acid receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol 307, R35–R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de La Serre CB, de Lartigue G, and Raybould HE (2015). Chronic exposure to low dose bacterial lipopolysaccharide inhibits leptin signaling in vagal afferent neurons. Physiol. Behav 139, 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lartigue G (2014). Putative roles of neuropeptides in vagal afferent signaling. Physiol. Behav 136, 155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lartigue G (2016). Role of the vagus nerve in the development and treatment of diet-induced obesity. J. Physiol 594, 5791–5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lartigue G, Dimaline R, Varro A, and Dockray GJ (2007). Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J. Neurosci 27, 2876–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lartigue G, Dimaline R, Varro A, Raybould H, De la Serre CB, and Dockray GJ (2010). Cocaine- and amphetamine-regulated transcript mediates the actions of cholecystokinin on rat vagal afferent neurons. Gastroenterology 138, 1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lartigue G, Barbier de la Serre C, Espero E, Lee J, and Raybould HE (2012). Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats. PLoS ONE 7, e32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lartigue G, Ronveaux CC, and Raybould HE (2014). Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Mol. Metab 3, 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diepenbroek C, Quinn D, Stephens R, Zollinger B, Anderson S, Pan A, and de Lartigue G (2017). Validation and characterization of a novel method for selective vagal deafferentation of the gut. Am. J. Physiol. Gastrointest. Liver Physiol 313, G342–G352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, and Couceyro P (1995). PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J. Neurosci 15, 2471–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duca FA, Swartz TD, Sakar Y, and Covasa M (2012). Increased oral detection, but decreased intestinal signaling for fats in mice lacking gut microbiota. PLoS ONE 7, e39748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duca FA, Sakar Y, and Covasa M (2013). The modulatory role of high fat feeding on gastrointestinal signals in obesity. J. Nutr. Biochem 24, 1663–1677. [DOI] [PubMed] [Google Scholar]
- Dunn KW, Kamocka MM, and McDonald JH (2011). A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol 300, C723–C742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Djouhri L, Black JA, Dib-Hajj SD, Waxman SG, and Lawson SN (2002). The presence and role of the tetrodotoxin-resistant sodium channel Na(v)1.9 (NaN) in nociceptive primary afferent neurons. J. Neurosci 22, 7425–7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AL, Bergh C, Södersten P, Sabin MA, Hollinghurst S, Hunt LP, and Shield JP (2009). Treatment of childhood obesity by retraining eating behaviour: randomised controlled trial. BMJ 340, b5388. [DOI] [PubMed] [Google Scholar]
- Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, Ferreira TL, Quinn D, Liu ZW, Gao XB, et al. (2018). A Neural Circuit for GutInduced Reward. Cell 175, 887–888. [DOI] [PubMed] [Google Scholar]
- Harding R, and Leek BF (1973). Central projections of gastric afferent vagal inputs. J. Physiol 228, 73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldsinger A, Lu Y, Zhou SY, Wu X, Grabauskas G, Song I, and Owyang C (2012). Cocaine- and amphetamine-regulated transcript is the neurotransmitter regulating the action of cholecystokinin and leptin on short-term satiety in rats. Am. J. Physiol. Gastrointest. Liver Physiol 303, G1042–G1051. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ikramuddin S, Blackstone RP, Brancatisano A, Toouli J, Shah SN, Wolfe BM, Fujioka K, Maher JW, Swain J, Que FG, et al. (2014). Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA 312, 915–922. [DOI] [PubMed] [Google Scholar]
- Karfopoulou E, Brikou D, Mamalaki E, Bersimis F, Anastasiou CA, Hill JO, and Yannakoulia M (2017). Dietary patterns in weight loss maintenance: results from the MedWeight study. Eur. J. Nutr 56, 991–1002. [DOI] [PubMed] [Google Scholar]
- Karimian Azari E, Leitner C, Jaggi T, Langhans W, and Mansouri A (2013). Possible role of intestinal fatty acid oxidation in the eating-inhibitory effect of the PPAR-a agonist Wy-14643 in high-fat diet fed rats. PLoS ONE 8, e74869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish SJ, and Page AJ (2014). Plasticity of gastro-intestinal vagal afferent endings. Physiol. Behav 136, 170–178. [DOI] [PubMed] [Google Scholar]
- Kentish S, Li H, Philp LK, O’Donnell TA, Isaacs NJ, Young RL, Wittert GA, Blackshaw LA, and Page AJ (2012). Diet-induced adaptation of vagal afferent function. J. Physiol 590, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Ling NC, DeSouza EB, and Kuhar MJ (1997). Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J. Neuroendocrinol 9, 823–833. [DOI] [PubMed] [Google Scholar]
- Krieger JP, Arnold M, Pettersen KG, Lossel P, Langhans W, and Lee SJ (2016). Knockdown of GLP-1 Receptors in Vagal Afferents Affects Normal Food Intake and Glycemia. Diabetes 65, 34–43. [DOI] [PubMed] [Google Scholar]
- Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, et al. (1998). Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393, 72–76. [DOI] [PubMed] [Google Scholar]
- Kupari J, Häring M, Agirre E, Castelo-Branco G, and Ernfors P (2019). An Atlas of Vagal Sensory Neurons and Their Molecular Specialization. Cell Rep 27, 2508–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal S, Kirkup AJ, Brunsden AM, Thompson DG, and Grundy D (2001). Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol 281, G907–G915. [DOI] [PubMed] [Google Scholar]
- Lambert PD, Couceyro PR, McGirr KM, Dall Vechia SE, Smith Y, and Kuhar MJ (1998). CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse 29, 293–298. [DOI] [PubMed] [Google Scholar]
- Lee J, Martin E, Paulino G, de Lartigue G, and Raybould HE (2011). Effect of ghrelin receptor antagonist on meal patterns in cholecystokinin type 1 receptor null mice. Physiol. Behav 103, 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, and Williams JA (1985). Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J. Clin. Invest 75, 1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, and Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Look ARG; Look AHEAD Research Group (2014). Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring) 22, 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma T, Hirakawa Y, Nakamura U, Kiyohara Y, Kitazono T, and Ninomiya T (2015). Association between eating rate and obesity: a systematic review and meta-analysis. Int. J. Obes 39, 1589–1596. [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Alhadeff AL, and Grill HJ (2009). Hindbrain cocaine- and amphetamine-regulated transcript induces hypothermia mediated by GLP-1 receptors. J. Neurosci 29, 6973–6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedh U, Scott KA, and Moran TH (2015). Fourth ventricular CART peptide induces c-fos in the area postrema and nucleus of the solitary tract via a CRF-receptor dependent mechanism. Neurosci. Lett 609, 124–128. [DOI] [PubMed] [Google Scholar]
- Smedh U, Scott KA, and Moran TH (2019). Pretreatment with a CRF antagonist amplifies feeding inhibition induced by fourth ventricular cocaineand amphetamine-regulated transcript peptide. BMC Neurosci. 20, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueta C, and De-Miguel FF (2012). Extrasynaptic exocytosis and its mechanisms: a source of molecules mediating volume transmission in the nervous system. Front. Physiol 3, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, et al. (2005). Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308, 1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Baskin DG, and Schwartz MW (2009). Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 150, 1680–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosten G, Harada CM, Haddock C, Gaincotti LA, Kolar GR, Patel R, Guo C, Chen Z, Zhang J, Doyle TM, Dickenson AH, Samson WK, and Salvemini D (2020). GPR160 de-orphanization reveals critical roles in neuropathic pain in rodents. The Journal of Clinical Investivation. 10.1172/JCI133270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Huang Y, Shah S, Wu H, and Gautron L (2016). Levels of Cocaine- and Amphetamine-Regulated Transcript in Vagal Afferents in the Mouse Are Unaltered in Response to Metabolic Challenges. eNeuro 3, ENEURO.0174–16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Patterson C, and Berthoud HR (2001). Fourth ventricular injection of CART peptide inhibits short-term sucrose intake in rats. Brain Res. 896, 153–156. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, and Berthoud HR (2002). CART in the dorsal vagal complex: sources of immunoreactivity and effects on Fos expression and food intake. Brain Res. 957, 298–310. [DOI] [PubMed] [Google Scholar]
- Zhu PC, Thureson-Klein A, and Klein RL (1986). Exocytosis from large dense cored vesicles outside the active synaptic zones of terminals within the trigeminal subnucleus caudalis: a possible mechanism for neuropeptide release. Neuroscience 19, 43–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate any unique datasets or code.