Abstract
Background
The triglyceride glucose (TyG) index, a simple surrogate estimate of insulin resistance, has been demonstrated to predict cardiovascular (CV) disease morbidity and mortality in the general population and many patient cohorts. However, to our knowledge, the prognostic usefulness of the TyG index after percutaneous coronary intervention (PCI) in patients with type 2 diabetes mellitus (T2DM) and acute coronary syndrome (ACS) has not been determined. This study aimed to evaluate the association of the TyG index with adverse CV outcomes in patients with T2DM and ACS who underwent PCI.
Methods
The TyG index was calculated using the formula ln[fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2]. The primary endpoint was the composite of all-cause mortality, non-fatal stroke, non-fatal myocardial infarction, or unplanned repeat revascularization. The association between the TyG index and adverse CV outcomes was assessed by Cox proportional hazards regression analysis.
Results
In total, 776 patients with T2DM and ACS who underwent PCI (mean age, 61 ± 10 years; men, 72.2%) were included in the final analysis. Over a median follow-up of 30 months, 188 patients (24.2%) had at least 1 primary endpoint event. The follow-up incidence of the primary endpoint rose with increasing TyG index tertiles. The multivariate Cox proportional hazards regression analysis adjusted for multiple confounders revealed a hazard ratio for the primary endpoint of 2.17 (95% CI 1.45–3.24; P for trend = 0.001) when the highest and lowest TyG index tertiles were compared.
Conclusions
The TyG index was significantly and positively associated with adverse CV outcomes, suggesting that the TyG index may be a valuable predictor of adverse CV outcomes after PCI in patients with T2DM and ACS.
Keywords: Triglyceride glucose index, Adverse cardiovascular outcomes, Type 2 diabetes mellitus, Acute coronary syndrome, Percutaneous coronary intervention
Background
In the last few years, acute coronary syndrome (ACS), the most severe ischaemic heart disease, has been one of the leading causes of death worldwide [1]. Some patients with ACS remain at high risk for recurrent cardiovascular (CV) events despite the use of current guideline-recommended therapies, including prompt coronary revascularization, dual anti-platelet therapy, and intensive lipid-lowering therapy. This risk is particularly high among patients with type 2 diabetes mellitus (T2DM), who account for approximately one-third of ACS cases [2, 3]. Because of the low acceptability of coronary artery bypass grafting (CABG) in Chinese patients with coronary artery disease (CAD), percutaneous coronary intervention (PCI) is currently the most common revascularization strategy, even in patients with diabetes who are more likely to develop multi-vessel CAD. However, PCI was demonstrated to be associated with a higher rate of short- and long-term adverse CV outcomes relative to CABG in diabetic patients with ACS and multi-vessel CAD [4]. Therefore, early identification of diabetic patients with ACS undergoing PCI who have a high residual risk is crucial for better clinical management to reduce future CV events.
Since 1988, insulin resistance (IR) has been established as a crucial mediator of metabolic disorders, T2DM, and atherosclerotic CV disease (CVD) [5–8]. Although the hyperinsulinaemic–euglycaemic clamp is the “gold standard” test for the measurement of IR [9], it is not commonly used in clinical settings due to the complexity of the testing process. IR is significantly associated with the chronic increase in plasma glucose and triglycerides [7]. Accordingly, it was speculated that the combination of plasma glucose and triglycerides could predict IR. Indeed, the product of fasting plasma glucose (FPG) and triglycerides, namely, the fasting triglyceride glucose (TyG) index, has been demonstrated to be significantly correlated with IR measured by the hyperinsulinaemic–euglycaemic clamp test and even perform better than homeostasis model assessment of IR (HOMA-IR) in non-diabetic as well as diabetic patients [10, 11].
Numerous clinical studies have indicated that the TyG index is associated with CVD morbidity and mortality in the general population and many patient cohorts including both non-diabetic and diabetic patients [12–20]; however, no previous study has exclusively investigated the prognostic usefulness of the TyG index for CV events after PCI in patients with T2DM and ACS. Therefore, we examined the relationship between the baseline TyG index and adverse CV outcomes in patients with T2DM and ACS who underwent PCI.
Methods
Study population
The present study is a retrospective analysis derived from a single-centre prospective observational study (ChiCTR1800017417) that aimed to explore the prognostic value of the Logistic Clinical SYNTAX Score and novel risk factors for major adverse CV events in ACS patients undergoing PCI. This prospective observational study was approved by the institutional review board of Beijing Anzhen Hospital, Capital Medical University, and all patients gave their written informed consent before study inclusion. Moreover, this prospective observational study included a prespecified subgroup of patients with diabetes, and all eligible patients were consecutively and prospectively enrolled in a customized database.
A total of 826 patients with T2DM who underwent coronary angiography for ACS and were treated with primary or elective PCI in our CV centre from June 2016 to November 2017 were consecutively and prospectively enrolled in a customized database. The exclusion criteria of this study included patients with prior CABG, cardiogenic shock, left ventricular ejection fraction (LVEF) < 30%, renal dysfunction with creatinine clearance (CrCl) < 15 mL/min or chronic dialysis, extreme body mass index (BMI > 45 kg/m2), suspected familial hypertriglyceridaemia [plasma triglycerides ≥ 500 mg/dL (5.65 mmol/L) or more than one first-degree relative with triglycerides ≥ 500 mg/dL]. Three patients were also excluded because of missing follow-up data despite at least 4 separate attempts to contact them. Ultimately, 776 patients were anonymously and retrospectively included in the final analysis. This study was performed in accordance with the Helsinki Declaration of Human Rights and approved by the institutional review board of Beijing Anzhen Hospital, Capital Medical University. Given the retrospective nature of this study, the requirement for informed consent was waived.
Measurements
Data on demographics, personal medical history, and medication history were collected using a standard questionnaire. The blood pressure on admission was recorded. The concentrations of plasma triglycerides, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and glucose in the first fasting blood samples during the stay in the hospital, which were obtained after 12 h of fasting, were determined at the central laboratory of Beijing Anzhen Hospital. The low-density lipoprotein cholesterol (LDL-C) level was calculated using the Friedewald equation. The TyG index was retrospectively calculated using ln (fasting triglycerides [mg/dL] × fasting glucose [mg/dL]/2). Blood pressure was measured three times on different days, and readings ≥ 140/90 mmHg and antihypertensive medication use were considered criteria for hypertension. The symptoms of diabetes and a casual plasma glucose ≥ 200 mg/dL, FPG ≥ 126 mg/dL, 2-h plasma glucose concentration ≥ 200 mg/dL from a 75-g oral glucose tolerance test, and/or antidiabetic medication use were considered criteria for diabetes. Fasting TC > 200 mg/dL, LDL-C > 130 mg/dL, triglycerides > 150 mg/dL, HDL-C < 40 mg/dL, and/or chronic use of lipid-lowering drugs were considered criteria for dyslipidaemia. CrCl < 60 mL/min was considered the criterion for chronic kidney disease (CKD); CrCl was calculated using the Cockcroft and Gault formula [21]. Patients with previous ischaemic stroke or transient ischaemic attack were defined as having cerebrovascular accident (CVA). Patients with vascular diseases related to the aorta and arteries other than coronaries accompanied by exercise-related intermittent claudication, revascularization surgery, reduced or absent pulsation, angiographic stenosis of more than 50% or combinations of these characteristics were identified as having peripheral artery disease (PAD). Patients with signs/symptoms of congestive heart failure (CHF), current treatment for CHF, or objective evidence of reduced ejection fraction (LVEF < 40%) were considered to have cardiac failure.
Follow-up details
All patients were followed up at 1 month and every 6 months after hospital discharge. The information regarding adverse events was obtained through telephone contact with the patients or their family members by trained personnel blinded to the baseline characteristics and ascertained from a careful review of corresponding medical records. The primary endpoint of the present study was the composite of overall death, non-fatal stroke, non-fatal myocardial infarction (MI), or unplanned repeat revascularization. Stroke was defined as ischaemic cerebral infarction with evidence of neurological dysfunction requiring hospitalization with clinically documented lesions on brain computed tomography or magnetic resonance imaging. MI was defined as an elevated level of cardiac troponin or creatine kinase (MB) greater than the upper limit of the normal range with either ischaemic symptoms or electrocardiographic changes implicating ischaemia. The presence of new pathological Q waves in ≥ 2 contiguous electrocardiogram leads was also diagnosed as MI. Within 1 week after the index PCI, only Q-wave MI was defined as MI. Unplanned repeat revascularization was defined as any non-staged revascularization after the index PCI. Staged revascularization was defined as scheduled revascularization within 90 days after the index PCI without treatment of a coronary artery territory that had been treated during the index PCI or a revascularization status of emergency, urgency, or salvage. The most severe endpoint event was selected for primary endpoint analysis if > 1 event occurred during follow-up (death > stroke > MI > revascularization). If more than one stroke or MI or revascularization occurred, the first stroke or MI or revascularization was selected. The follow-up period of the present study lasted until November 2019.
Statistical analyses
Continuous variables were presented as the mean ± standard deviation if consistent with a normal distribution, otherwise as the median and interquartile range (IQR). Categorical variables were expressed as numbers and percentages. All patients were stratified into three groups (T1 [TyG index < 8.80], T2 [8.80 ≤ TyG index < 9.28], and T3 [TyG index ≥ 9.29]) in accordance with tertiles of the TyG index. The Chi squared test or Fisher’s exact test was used to analyse differences in categorical variables between groups. ANOVA or the Kruskal–Wallis H test was applied to analyse differences in continuous variables between groups. Kaplan–Meier methods were used to derive the event rates at follow-up and to plot time-to-event curves. Differences among Kaplan–Meier estimates of the three groups were evaluated with the log-rank test. We conducted a Cox proportional hazards regression analysis to estimate the hazard ratios (HRs) and their 95% confidence intervals (CIs) of developing the primary endpoint. The TyG index was analysed in two ways: (1) as a categorical variable; and (2) as a continuous variable. Predictors of the incidence of the primary endpoint identified through univariate analysis were also tested in a multivariate analysis. In the multivariate model, the following confounders were chosen because of their clinical importance and statistical significance in the univariate analysis: age (continuous), BMI (continuous), diastolic blood pressure (continuous), HDL-C (continuous), glycosylated haemoglobin (continuous), sex, current smoking, daily drinking, presence of PAD, CKD, cardiac failure, previous MI, past PCI, use of insulin and/or oral antidiabetic agents at discharge, CAD severity, presence of lesions > 20 mm long, use of drug-coated balloon, and complete revascularization. Interaction was tested with a likelihood ratio test, and the proportional hazard assumption was tested by demonstrating no importance of variables multiplied by time as time-dependent variables. Statistical analyses were performed using SPSS version 24.0 software (IBM Corporation, Chicago, IL), and P < 0.05 was considered statistically significant.
Results
The mean age of the 776 patients at baseline was 61 ± 10 years, and 72.2% of patients were men (n = 560). The median follow-up duration was 30 months (IQR, 24–36 months), and during the follow-up period, 188 patients (24.2%) had at least 1 primary endpoint event, which was recorded in 40 (15.6%) patients from the T1 group, 66 (25.4%) from the T2 group, and 82 (31.7%) from the T3 group. In the 188 patients who had at least 1 primary endpoint event, there were 16 deaths (15 deaths from CV causes and 1 death from non-CV causes), 16 cases of non-fatal strokes, 21 cases of non-fatal MI, and 156 cases of unplanned revascularization. Of these, 19 (2.5%) patients suffered two, 2 (0.3%) patients suffered three, and 2 (0.3%) patients suffered four primary endpoint events. The respective incidences of overall death, CV death, non-CV death, non-fatal stroke, non-fatal MI and unplanned repeat revascularization among the TyG index tertiles are shown in Additional file 1: Table S1.
The baseline clinical and laboratory characteristics of the study patients stratified by the primary endpoint are summarized in Table 1. Compared with those without an endpoint event, patients with an endpoint event had higher levels of TyG index. Patients with an endpoint event also showed higher proportions of PAD, cardiac failure, previous MI, and past PCI, elevated concentrations of triglycerides and FPG but lower levels of HDL-C, DBP, and LVEF. Pre-hospital, periprocedural and discharge medications, angiographic findings, and procedural results of the study patients stratified by the primary endpoint are shown in Table 2. Pre-hospital, periprocedural and discharge medications, except for aspirin and insulin, did not differ between patients with and without an endpoint event. Compared with those without an endpoint event, patients with an endpoint event had higher rates of left-main/three-vessel disease, restenotic lesions, and lesions > 20 mm long. As regards procedural results, there were significant differences in the rates of drug-coated balloon use and complete revascularization between patients with and without an endpoint event.
Table 1.
Baseline clinical and laboratory characteristics of the study patients stratified by the primary endpoint
| Variable | No such events n = 588 |
Primary endpoint n = 188 |
P value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 61 ± 10 | 62 ± 10 | 0.498 |
| Male sex, n (%) | 426 (72.4) | 134 (71.3) | 0.755 |
| BMI (kg/m2) | 26.2 ± 3.5 | 25.9 ± 3.1 | 0.298 |
| Medical measurements (on admission) | |||
| SBP (mm Hg) | 132 ± 18 | 131 ± 15 | 0.339 |
| DBP (mm Hg) | 76 ± 11 | 73 ± 10 | 0.001 |
| Risk factors | |||
| Cigarette smoking | |||
| Current smokers, n (%) | 238 (40.5) | 72 (38.3) | 0.596 |
| Former smokers, n (%) | 89 (15.1) | 35 (18.6) | 0.257 |
| Never smokers, n (%) | 261 (44.4) | 81 (43.1) | 0.754 |
| Alcohol intake | |||
| Daily drinkers, n (%) | 56 (9.5) | 18 (9.6) | 0.984 |
| Family history of CHD, n (%) | 172 (29.3) | 66 (35.1) | 0.130 |
| Hypertension, n (%) | 403 (68.5) | 129 (68.6) | 0.984 |
| Dyslipidaemia, n (%) | 483 (82.1) | 163 (86.7) | 0.145 |
| Previous MI, n (%) | 112 (19.0) | 54 (28.7) | 0.005 |
| Past PCI, n (%) | 120 (20.4) | 66 (35.1) | < 0.001 |
| Previous CVA, n (%) | 37 (6.3) | 11 (5.9) | 0.827 |
| PAD, n (%) | 62 (10.5) | 54 (28.7) | < 0.001 |
| CKD, n (%) | 40 (6.8) | 20 (10.6) | 0.087 |
| Cardiac failure, n (%) | 34 (5.8) | 28 (14.9) | < 0.001 |
| LVEF (%) | 65 (60-68) | 63 (58-68) | 0.016 |
| Clinical presentation | |||
| UA, n (%) | 457 (77.7) | 153 (81.4) | 0.287 |
| NSTEMI, n (%) | 82 (13.9) | 18 (9.6) | 0.119 |
| STEMI, n (%) | 49 (8.3) | 17 (9.0) | 0.762 |
| Laboratory measurements (fasting state) | |||
| TC (mg/dL) | 157.6 ± 42.5 | 156.0 ± 37.4 | 0.638 |
| LDL-C (mg/dL) | 93.8 ± 34.9 | 91.6 ± 29.5 | 0.445 |
| HDL-C (mg/dL) | 39.8 ± 9.1 | 37.9 ± 7.9 | 0.008 |
| Triglycerides (mg/dL) | 128.4 (92.1–176.2) | 145.2 (97.4–201.9) | 0.039 |
| FPG (mg/dL) | 123.8 (109.9–147.2) | 138.5 (114.2–169.4) | < 0.001 |
| Glycosylated haemoglobin (%) | 7.2 (6.6–8.1) | 7.3 (6.8–8.2) | 0.140 |
| TyG index | 9.02 ± 0.57 | 9.21 ± 0.53 | < 0.001 |
| TyG index tertiles | < 0.001 | ||
| T1, n (%) | 217 (36.9) | 40 (21.3) | – |
| T2, n (%) | 194 (33.0) | 66 (35.1) | – |
| T3, n (%) | 177 (30.1) | 82 (43.6) | – |
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, CHD coronary heart disease, MI myocardial infarction, PCI percutaneous coronary intervention, CVA cerebrovascular accident, PAD peripheral artery disease, CKD chronic kidney disease, UA unstable angina, NSTEMI non ST-segment elevation myocardial infarction, STEMI ST-segment elevation myocardial infarction, TC total cholesterol, LDL-C low-density lipoprotein-cholesterol, HDL-C high-density lipoprotein-cholesterol, FPG fasting plasma glucose, TyG triglyceride glucose
Table 2.
Pre-hospital, periprocedural and discharge medications, agiographic findings, and procedural results of the study patients stratified by the primary endpoint
| Variable | No such events n = 588 |
Primary endpoint n = 188 |
P value |
|---|---|---|---|
| Medications before admission | |||
| Aspirin, n (%) | 432 (73.5) | 146 (77.7) | 0.251 |
| P2Y12 inhibitors, n (%) | 231 (39.3) | 79 (42.0) | 0.505 |
| Lipid-lowering drugs, n (%) | 430 (73.1) | 144 (76.6) | 0.346 |
| ACEI/ARBs, n (%) | 180 (30.6) | 74 (39.4) | 0.026 |
| β-blockers, n (%) | 227 (38.6) | 71 (37.8) | 0.837 |
| Insulin, n (%) | 209 (35.5) | 73 (38.8) | 0.415 |
| Oral antidiabetic agents, n (%) | 284 (48.3) | 92 (48.9) | 0.879 |
| Metformin, n (%) | 144 (24.5) | 44 (23.4) | 0.762 |
| Alpha-glucosidase inhibitors, n (%) | 123 (20.9) | 31 (16.5) | 0.185 |
| Sulfonylurea, n (%) | 135 (23.0) | 43 (22.9) | 0.980 |
| Dipeptidyl peptidase 4 inhibitors, n (%) | 8 (1.4) | 4 (2.1) | 0.687 |
| Any antidiabetic treatment, n (%) | 419 (71.3) | 143 (76.1) | 0.199 |
| Periprocedural medications | |||
| Aspirin, n (%) | 588 (100.0) | 182 (96.8) | < 0.001 |
| P2Y12 inhibitors, n (%) | 588 (100.0) | 188 (100.0) | – |
| Unfractionated heparin, n (%) | 482 (82.0) | 156 (83.0) | 0.754 |
| Bivalirudin, n (%) | 77 (13.1) | 23 (12.2) | 0.759 |
| GP IIb/IIIa receptor antagonist, n (%) | 100 (17.0) | 42 (22.3) | 0.100 |
| Medications at discharge | |||
| Aspirin, n (%) | 588 (100.0) | 182 (96.8) | < 0.001 |
| P2Y12 inhibitors, n (%) | 588 (100.0) | 188 (100.0) | – |
| Lipid-lowering drugs, n (%) | 588 (100.0) | 188 (100.0) | – |
| ACEI/ARBs, n (%) | 283 (48.1) | 99 (52.7) | 0.279 |
| β-blockers, n (%) | 432 (73.5) | 130 (69.1) | 0.249 |
| Insulin, n (%) | 188 (32.0) | 76 (40.4) | 0.033 |
| Oral antidiabetic agents, n (%) | 318 (54.1) | 100 (53.2) | 0.831 |
| Metformin, n (%) | 90 (15.3) | 32 (17.0) | 0.574 |
| Alpha-glucosidase inhibitors, n (%) | 214 (36.4) | 64 (34.0) | 0.558 |
| Sulfonylurea, n (%) | 146 (24.8) | 42 (22.3) | 0.488 |
| Dipeptidyl peptidase 4 inhibitors, n (%) | 8 (1.4) | 4 (1.2) | 0.687 |
| Any antidiabetic treatment, n (%) | 408 (69.4) | 150 (79.8) | 0.006 |
| Angiographic findings | |||
| One-vessel disease, n (%) | 68 (11.6) | 6 (3.2) | 0.001 |
| Two-vessel disease, n (%) | 164 (27.9) | 30 (16.0) | 0.001 |
| LM/three-vessel disease, n (%) | 356 (60.5) | 152 (80.9) | < 0.001 |
| Proximal LAD stenosis, n (%) | 291 (49.5) | 103 (54.8) | 0.206 |
| Restenotic lesions, n (%) | 61 (10.4) | 49 (26.1) | < 0.001 |
| Chronic total occlusions, n (%) | 136 (23.1) | 40 (21.3) | 0.597 |
| Trifurcation or bifurcation lesions, n (%) | 450 (76.5) | 148 (78.7) | 0.534 |
| Heavy calcification lesions, n (%) | 193 (32.8) | 65 (34.6) | 0.657 |
| Lesions > 20 mm long, n (%) | 312 (53.1) | 134 (71.3) | < 0.001 |
| Procedural results | |||
| Target vessel territory | |||
| LM, n (%) | 30 (5.1) | 12 (6.4) | 0.499 |
| LAD, n (%) | 291 (49.5) | 91 (48.4) | 0.796 |
| LCX, n (%) | 168 (28.6) | 52 (27.7) | 0.809 |
| RCA, n (%) | 231 (39.3) | 75 (39.9) | 0.882 |
| DES use, n (%) | 492 (83.7) | 154 (81.9) | 0.574 |
| BRS use, n (%) | 27 (4.6) | 5 (2.7) | 0.246 |
| DCB use, n (%) | 32 (5.4) | 22 (11.7) | 0.003 |
| Complete revascularization, n (%) | 376 (63.9) | 80 (42.6) | < 0.001 |
ACEI angiotensin converting enzyme inhibitor, ARB angiotensin II receptor blocker, LM left-main artery, LAD left anterior descending artery, LCX left circumflex artery, RCA right coronary artery, DES drug-eluting stent, BRS bioresorbable scaffold, DCB drug-coated balloon
The baseline clinical and laboratory characteristics of the study patients according to the TyG index tertiles are presented in Table 3. Patients with a high TyG index were more likely to be men. BMI, TC level, LDL-C level, FPG level, triglyceride level and glycosylated haemoglobin level increased, whereas age and HDL-C level decreased in proportion to the TyG index tertiles. The proportion of current smokers and daily drinkers significantly increased with an increase in the TyG index. Pre-hospital, periprocedural and discharge medications, angiographic findings, and procedural results of the study patients according to the TyG index tertiles are listed in Table 4. Medications before admission and periprocedural medications were not different among the different TyG index groups. Medications at discharge, except for oral antidiabetic agents (mainly driven by the difference in α-glucosidase inhibitors), were similar across the groups. Among the patients within the top TyG index tertile, a significantly higher proportion had left-main coronary artery intervention. The rate of drug-eluting stent use was significantly lower in the patients within the top TyG index tertile.
Table 3.
Baseline clinical and laboratory characteristics of the study patients according to the TyG index tertiles
| Variable | T1 n = 257 |
T2 n = 260 |
T3 n = 259 |
P value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 63 ± 9 | 62 ± 10 | 59 ± 11 | < 0.001 |
| Male sex, n (%) | 173 (67.3) | 201 (77.3) | 186 (71.8) | 0.040 |
| BMI (kg/m2) | 25.5 ± 3.4 | 26.1 ± 3.0 | 26.8 ± 3.8 | < 0.001 |
| Medical measurements (on admission) | ||||
| SBP (mm Hg) | 132 ± 20 | 134 ± 18 | 130 ± 15 | 0.072 |
| DBP (mm Hg) | 74 ± 12 | 76 ± 10 | 76 ± 11 | 0.215 |
| Risk factors | ||||
| Cigarette smoking | ||||
| Current smokers, n (%) | 90 (35.0) | 102 (39.2) | 118 (45.6) | 0.048 |
| Former smokers, n (%) | 41 (16.0) | 51 (19.6) | 32 (12.4) | 0.078 |
| Never smokers, n (%) | 126 (49.0) | 107 (41.2) | 109 (42.1) | 0.144 |
| Alcohol intake | ||||
| Daily drinkers, n (%) | 14 (5.4) | 28 (10.8) | 32 (12.4) | 0.020 |
| Family history of CHD, n (%) | 69 (26.8) | 87 (33.5) | 82 (31.7) | 0.242 |
| Hypertension, n (%) | 183 (71.2) | 175 (67.3) | 174 (67.2) | 0.535 |
| Dyslipidaemia, n (%) | 163 (63.4) | 234 (90.0) | 249 (96.1) | < 0.001 |
| Previous MI, n (%) | 55 (21.4) | 55 (21.2) | 56 (21.6) | 0.992 |
| Past PCI, n (%) | 64 (24.9) | 68 (26.2) | 54 (20.8) | 0.335 |
| Previous CVA, n (%) | 18 (7.0) | 16 (6.2) | 14 (5.4) | 0.753 |
| PAD, n (%) | 32 (12.5) | 38 (14.6) | 46 (17.8) | 0.235 |
| CKD, n (%) | 20 (7.8) | 22 (8.5) | 18 (6.9) | 0.812 |
| Cardiac failure, n (%) | 26 (10.1) | 20 (7.7) | 16 (6.2) | 0.250 |
| LVEF (%) | 63 (60–67) | 65 (60–68) | 65 (60–68) | 0.077 |
| Clinical presentation | ||||
| UA, n (%) | 211 (82.1) | 194 (74.6) | 205 (79.2) | 0.112 |
| NSTEMI, n (%) | 26 (10.1) | 42 (16.2) | 32 (12.4) | 0.117 |
| STEMI, n (%) | 20 (7.8) | 24 (9.2) | 22 (8.5) | 0.840 |
| Laboratory measurements (fasting state) | ||||
| TC (mg/dL) | 141.2 ± 36.6 | 156.1 ± 38.2 | 174.1 ± 42.4 | < 0.001 |
| LDL-C (mg/dL) | 82.3 ± 33.3 | 95.2 ± 32.0 | 102.2 ± 32.7 | < 0.001 |
| HDL-C (mg/dL) | 42.2 ± 10.0 | 38.4 ± 7.7 | 37.4 ± 7.9 | < 0.001 |
| Triglycerides (mg/dL) | 85.0 (70.8–100.1) | 135.5 (113.3–157.6) | 214.3 (169.1–267.4) | < 0.001 |
| FPG (mg/dL) | 116.0 (104.2–126.2) | 124.2 (108.1–146.9) | 147.6 (128.3–175.2) | < 0.001 |
| Glycosylated haemoglobin (%) | 6.9 (6.5–7.8) | 7.3 (6.7–8.1) | 7.6 (6.8–8.4) | < 0.001 |
| TyG index | 8.54 (8.32–8.68) | 9.03 (8.93–9.14) | 9.63 (9.44–9.89) | < 0.001 |
Abbreviations as in Table 1
Table 4.
Pre-hospital, periprocedural and discharge medications, agiographic findings, and procedural results of the study patients according to the TyG index tertiles
| Variable | T1 n = 257 |
T2 n = 260 |
T3 n = 259 |
P value |
|---|---|---|---|---|
| Medications before admission | ||||
| Aspirin, n (%) | 190 (73.9) | 197 (75.8) | 191 (73.7) | 0.843 |
| P2Y12 inhibitors, n (%) | 104 (40.5) | 111 (42.7) | 95 (36.7) | 0.368 |
| Lipid-lowering drugs, n (%) | 196 (76.3) | 193 (74.2) | 185 (71.4) | 0.454 |
| ACEI/ARBs, n (%) | 81 (31.5) | 88 (33.8) | 85 (32.8) | 0.852 |
| β-blockers, n (%) | 108 (42.0) | 104 (40.0) | 86 (33.2) | 0.097 |
| Insulin, n (%) | 88 (34.2) | 108 (41.5) | 86 (33.2) | 0.099 |
| Oral antidiabetic agents, n (%) | 122 (47.5) | 133 (51.2) | 121 (46.7) | 0.557 |
| Metformin, n (%) | 54 (21.0) | 61 (23.5) | 73 (28.2) | 0.154 |
| Alpha-glucosidase inhibitors, n (%) | 50 (19.5) | 58 (22.3) | 46 (17.8) | 0.422 |
| Sulfonylurea, n (%) | 50 (19.5) | 72 (27.7) | 56 (21.6) | 0.069 |
| Dipeptidyl peptidase 4 inhibitors, n (%) | 6 (2.3) | 4 (1.5) | 2 (0.8) | 0.313 |
| Any antidiabetic treatment, n (%) | 182 (70.8) | 197 (75.8) | 183 (70.7) | 0.334 |
| Periprocedural medications | ||||
| Aspirin, n (%) | 255 (99.2) | 260 (100.0) | 255 (98.5) | 0.113 |
| P2Y12 inhibitors, n (%) | 257 (100.0) | 260 (100.0) | 259 (100.0) | – |
| Unfractionated heparin, n (%) | 213 (82.9) | 213 (81.9) | 212 (81.9) | 0.944 |
| Bivalirudin, n (%) | 34 (13.2) | 32 (12.3) | 34 (13.1) | 0.943 |
| GP IIb/IIIa receptor antagonist, n (%) | 40 (15.6) | 50 (19.2) | 52 (20.1) | 0.371 |
| Medications at discharge | ||||
| Aspirin, n (%) | 255 (99.2) | 260 (100.0) | 255 (98.5) | 0.113 |
| P2Y12 inhibitors, n (%) | 257 (100.0) | 260 (100.0) | 259 (100.0) | – |
| Lipid-lowering drugs, n (%) | 257 (100.0) | 260 (100.0) | 259 (100.0) | – |
| ACEI/ARBs, n (%) | 116 (45.1) | 123 (47.3) | 143 (55.2) | 0.055 |
| β-blockers, n (%) | 189 (73.5) | 190 (73.1) | 183 (70.7) | 0.733 |
| Insulin, n (%) | 86 (33.5) | 94 (36.2) | 84 (32.4) | 0.653 |
| Oral antidiabetic agents, n (%) | 122 (47.5) | 153 (58.8) | 143 (55.2) | 0.030 |
| Metformin, n (%) | 36 (14.0) | 40 (15.4) | 46 (17.8) | 0.495 |
| Alpha-glucosidase inhibitors, n (%) | 76 (29.6) | 103 (39.6) | 99 (38.2) | 0.036 |
| Sulfonylurea, n (%) | 56 (21.8) | 72 (27.7) | 60 (23.2) | 0.260 |
| Dipeptidyl peptidase 4 inhibitors, n (%) | 4 (1.6) | 6 (2.3) | 2 (0.8) | 0.365 |
| Any antidiabetic treatment, n (%) | 172 (66.9) | 197 (75.8) | 189 (73.0) | 0.073 |
| Angiographic findings | ||||
| One-vessel disease, n (%) | 30 (11.7) | 14 (5.4) | 30 (11.6) | 0.020 |
| Two-vessel disease, n (%) | 70 (27.2) | 71 (27.3) | 53 (20.5) | 0.118 |
| LM/three-vessel disease, n (%) | 157 (61.1) | 175 (67.3) | 176 (68.0) | 0.194 |
| Proximal LAD stenosis, n (%) | 117 (45.5) | 143 (55.0) | 134 (51.7) | 0.091 |
| Restenotic lesions, n (%) | 38 (14.8) | 34 (13.1) | 38 (14.7) | 0.823 |
| Chronic total occlusions, n (%) | 53 (20.6) | 64 (24.6) | 59 (22.8) | 0.555 |
| Trifurcation or bifurcation lesions, n (%) | 199 (77.4) | 205 (78.8) | 194 (74.9) | 0.557 |
| Heavy calcification lesions, n (%) | 90 (35.0) | 96 (36.9) | 72 (27.8) | 0.067 |
| Lesions > 20 mm long, n (%) | 133 (51.8) | 155 (59.6) | 158 (61.0) | 0.072 |
| Procedural results | ||||
| Target vessel territory | ||||
| LM, n (%) | 10 (3.9) | 10 (3.8) | 22 (8.5) | 0.027 |
| LAD, n (%) | 132 (51.4) | 120 (46.2) | 130 (50.2) | 0.461 |
| LCX, n (%) | 83 (32.3) | 60 (23.1) | 77 (29.7) | 0.056 |
| RCA, n (%) | 93 (36.2) | 122 (46.9) | 91 (35.1) | 0.010 |
| DES use, n (%) | 225 (87.5) | 226 (86.9) | 195 (75.3) | < 0.001 |
| BRS use, n (%) | 10 (3.9) | 2 (0.8) | 20 (7.7) | < 0.001 |
| DCB use, n (%) | 16 (6.2) | 16 (6.2) | 22 (8.5) | 0.492 |
| Complete revascularization, n (%) | 165 (64.2) | 148 (56.9) | 143 (55.2) | 0.089 |
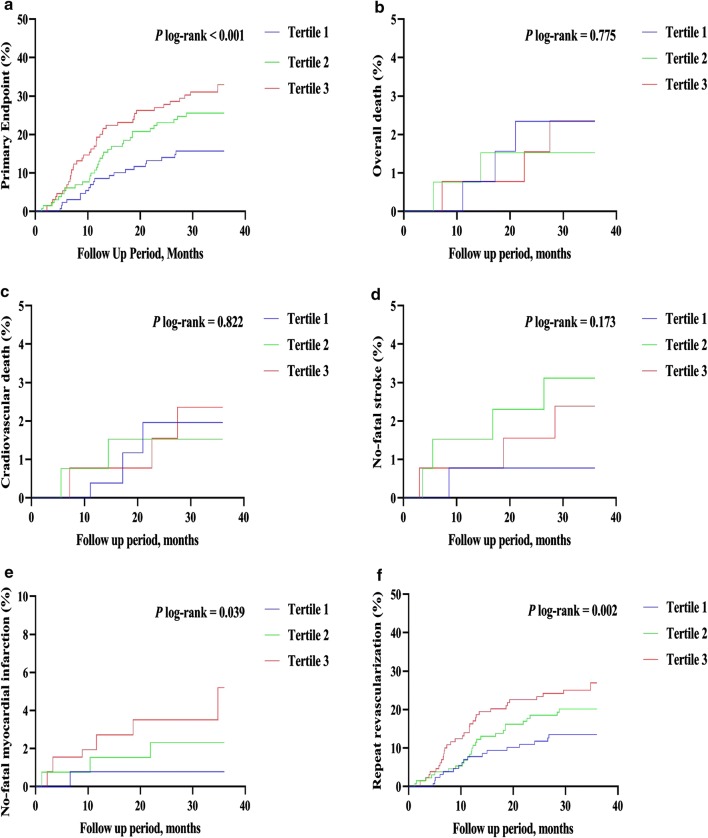
Kaplan–Meier curves of the incidence of the primary endpoint and each component event of the primary endpoint for the TyG index tertiles are presented in Fig. 1. The incidence of the primary endpoint in the T3 group was significantly higher than that in the T1 group (P log-rank < 0.001). This difference was mainly driven by the increase in non-fatal MI (log-rank test, P = 0.039) and unplanned repeat revascularization (log-rank test, P = 0.002) across the TyG index tertiles. However, the incidence of overall death (log-rank test, P = 0.775), CV death (log-rank test, P = 0.822), and non-fatal stroke (log-rank test, P = 0.173) at follow-up were similar among the TyG index tertiles.
Fig. 1.
The TyG index and risk: Kaplan–Meier curves for the incidences of the primary endpoint (a), all-cause death (b), cardiovascular death (c), non-fatal stroke (d), non-fatal myocardial infarction (e), and unplanned repeat revascularization (f) among the 3 study groups based on the TyG index tertiles
The TyG index at baseline was significantly related to the incidence of the primary endpoint. In univariate analysis, the TyG index as a continuous variable was associated with an HR of 1.64 (95% CI 1.15–1.97; P < 0.001). Adjustment for multiple confounders did not attenuate the relationship (HR 1.50, 95% CI 1.16–1.99; P = 0.003) (Additional file 2: Table S2). The incidence of the primary endpoint increased monotonically across the tertiles of the TyG index (P for trend ≤ 0.001) (Fig. 2). Taking T1 as the reference, multivariate analysis revealed that the TyG index for T2 and T3 increased the HRs for the incidence of the primary endpoint (T2: HR 1.60, 95% CI 1.06–2.40; T3: HR 2.15, 95% CI 1.44–3.22) (Table 5).
Fig. 2.
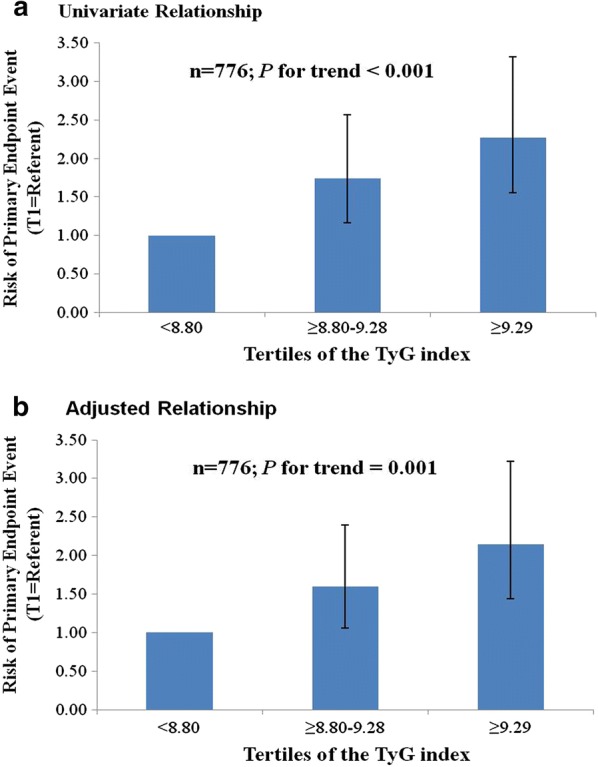
Risk of all-cause death, non-fatal stroke, non-fatal myocardial infarction, or unplanned repeat revascularization according to tertiles of the TyG index. Error bars indicate 95% confidence intervals. The first tertile is the reference. a Univariate relationship. b Relationship adjusted for age, body mass index, diastolic blood pressure, high-density lipoprotein cholesterol, glycosylated haemoglobin, sex, current smoking, daily drinking, presence of peripheral artery disease, chronic kidney disease, cardiac failure, previous myocardial infarction, past percutaneous coronary intervention, use of insulin and/or oral antidiabetic agents at discharge, coronary artery disease severity, presence of lesions > 20 mm long, use of drug-coated balloon, and complete revascularization
Table 5.
Relationship between the incidence of the primary endpoint and the TyG index expressed as a categorical variable
| Variables | Univariate analysis HR (95% CI) |
P value | Multivariate analysis HR (95% CI) |
P-value |
|---|---|---|---|---|
| TyG index tertiles | ||||
| T1 | Reference | Reference | ||
| T2 | 1.74 (1.17–2.57) | 0.006 | 1.60 (1.06–2.40) | 0.025 |
| T3 | 2.27 (1.56–3.32) | < 0.001 | 2.15 (1.44–3.22) | < 0.001 |
| Age | 1.01 (0.99–1.02) | 0.458 | 0.99 (0.97–1.004) | 0.138 |
| BMI | 0.98 (0.94–1.02) | 0.300 | 0.97 (0.92–1.01) | 0.166 |
| DBP | 0.98 (0.96–0.99) | 0.001 | 0.99 (0.97–1.00) | 0.048 |
| HDL-C | 0.98 (0.96–1.00) | 0.014 | 0.98 (0.96–1.00) | 0.042 |
| Glycosylated haemoglobin | 1.05 (0.94–1.18) | 0.387 | 0.93 (0.82–1.06) | 0.307 |
| Male sex | 0.92 (0.67–1.26) | 0.603 | 0.68 (0.44–1.04) | 0.077 |
| Current smoking | 0.94 (0.70–1.27) | 0.699 | 1.02 (0.71–1.46) | 0.918 |
| Daily drinking | 1.01 (0.62–1.65) | 0.957 | 1.30 (0.77–2.18) | 0.330 |
| Previous MI | 1.52 (1.11–2.09) | 0.009 | 0.88 (0.61–1.27) | 0.500 |
| Past PCI | 1.79 (1.33–2.41) | < 0.001 | 1.72 (1.17–2.53) | 0.006 |
| PAD | 2.82 (2.05–3.87) | < 0.001 | 2.22 (1.52–3.24) | < 0.001 |
| CKD | 1.70 (1.07–2.70) | 0.025 | 1.46 (0.85–2.52) | 0.172 |
| Cardiac failure | 2.27 (1.52–3.40) | < 0.001 | 1.59 (1.00–2.52) | 0.049 |
| Insulin at discharge | 1.39 (1.04–1.87) | 0.025 | 0.95 (0.67–1.36) | 0.797 |
| Metformin at discharge | 1.12 (0.76–1.63) | 0.568 | 1.08 (0.72–1.62) | 0.707 |
| Alpha-glucosidase inhibitors at discharge | 0.89 (0.66–1.21) | 0.457 | 0.76 (0.55–1.06) | 0.104 |
| Sulfonylurea at discharge | 0.89 (0.64–1.26) | 0.524 | 0.95 (0.65–1.38) | 0.784 |
| Dipeptidyl peptidase 4 inhibitors at discharge | 1.34 (0.50–3.60) | 0.566 | 1.24 (0.44–3.48) | 0.685 |
| CAD severity | ||||
| One-vessel disease | Reference | Reference | ||
| Two-vessel disease | 2.08 (0.87–5.01) | 0.101 | 1.30 (0.52–3.24) | 0.579 |
| LM/three-vessel disease | 4.32 (1.91–9.76) | < 0.001 | 2.16 (0.91–5.13) | 0.079 |
| Lesions > 20 mm long | 2.07 (1.51–2.84) | < 0.001 | 1.59 (1.13–2.23) | 0.008 |
| DCB use | 2.07 (1.33–3.23) | 0.001 | 1.23 (0.73–2.08) | 0.430 |
| Complete revascularization | 0.46 (0.35–0.62) | < 0.001 | 0.63 (0.46–0.86) | 0.004 |
Relevant clinical variables like clinical presentation (unstable angina vs. acute MI), age (≤ 70 vs. > 70 years), sex (male vs. female), and BMI (< 28 vs. ≥ 28 kg/m2) were subject to post hoc subgroup analyses for the primary endpoint. When the analysis was stratified by clinical presentation, we found that a higher TyG index was significantly associated with an increased risk of the primary endpoint in patients with unstable angina (adjusted HR 1.52, 95% CI 1.13–2.05; P = 0.006), and the similar result occurred in patients with acute MI (adjusted HR 5.70, 95% CI 1.78–18.30; P = 0.003). When the analysis was stratified by age, we found that a higher TyG index was significantly associated with an increased risk of the primary endpoint in patients aged 70 years and younger (adjusted HR 1.77, 95% CI 1.30–2.42; P < 0.001); however, the similar result did not occur in patients aged over 70 years (adjusted HR 0.44, 95% CI 0.16–1.21; P = 0.110). When the analysis was stratified by sex, we found that a higher TyG index was significantly associated with an increased risk of the primary endpoint in male patients (adjusted HR 1.60, 95% CI 1.14–2.24; P = 0.007); however, the similar result did not occur in female patients (adjusted HR 1.11, 95% CI 0.66–1.88; P = 0.695). When the analysis was stratified by BMI, we found that a higher TyG index was significantly associated with an increased risk of the primary endpoint in patients with BMI < 28 kg/m2 (adjusted HR 1.43, 95% CI 1.06–1.93; P = 0.019), and the similar result occurred in patients with BMI ≥ 28 kg/m2 (adjusted HR 12.07, 95% CI 3.32–43.92; P < 0.001).
Discussion
In the present study, we noticed a significant association between the TyG index and adverse CV outcomes in patients with T2DM and ACS who underwent PCI. Even after adjustment for as many potential confounding risk factors as possible, an independent association of the TyG index with adverse CV outcomes remained. To the best of our knowledge, this is the first study to investigate the prognostic value of the TyG index in diabetic patients with ACS undergoing PCI.
The TyG index was originally studied as a marker of identifying IR, with Pearson’s correlation coefficients of − 0.68 with M rates as measured by the hyperinsulinaemic-euglycaemic clamp test, which is the “gold standard” test in the diagnosis of IR [10], and 0.32 with the HOMA-IR, which is the most widely used method in current clinical practice for the estimation of IR [22]. Compared with several lipid ratios (TC/HDL-C, non-HDL-C/HDL-C, LDL-C/HDL-C, triglyceride/HDL-C, and apolipoprotein B/apolipoprotein A1), the visceral adiposity index, and the lipid accumulation product, the TyG index had a more significant association with HOMA-IR and showed a better discriminatory performance for HOMA-IR [23].
The TyG index, as a surrogate marker of IR, was demonstrated to be a useful predictor of metabolic syndrome and T2DM. Subsequently, a number of clinical studies were conducted to investigate the association of the TyG index with CVD morbidity and mortality in the general population and many patient cohorts, including both non-diabetic and diabetic patients. A population-based, prospective cohort study including 5014 apparently healthy subjects demonstrated that a higher TyG index was associated with an increased risk of incident CVD (CHD, PAD and CVA), independent of diabetic status [12]. Similarly, another population-based but retrospective cohort study including 6078 apparently healthy subjects over 60 years old also demonstrated that an elevated TyG index was significantly associated with an increased risk of developing CVD events, including both fatal and non-fatal CHD and CVA, independent of diabetic status [13]. Alizargar et al. showed that the TyG index was significantly correlated with the total amount of carotid plaque and the intima-media thicknesses of the internal, external, and common carotid arteries in hypertensive and normotensive community-dwelling individuals [14]. Alessandra et al. showed that the TyG index was positively associated with a higher prevalence of symptomatic CAD and could be used as a marker of atherosclerosis in patients with known CVD [15]. Moreover, Jin et al. showed that the TyG index was positively associated with future CV events, including all-cause death, non-fatal MI, stroke and post-discharge revascularization, suggesting that the TyG index may be a useful marker for predicting clinical outcomes in patients with stable CAD [16]. The findings of Mao et al. showed that the TyG index might be an independent predictor of CAD severity as evaluated by the SYNTAX Score and future CV events defined as the composite of cardiac death, non-fatal MMI, target vessel revascularization, CHF, and non-fatal stroke in non-ST-segment elevation ACS [17]. In addition, the findings of Luo et al. showed that a higher TyG index was associated with an increased risk of future CV events, including all-cause death, target vessel revascularization, non-fatal MI, unstable angina requiring hospitalization, CHF, stroke or transient cerebral ischaemia, in ST-segment elevation MI patients undergoing PCI [18]. Furthermore, the association between the TyG index and CV risk has also been specifically explored in patients with diabetes. A cross-sectional study including 888 asymptomatic subjects with T2DM but without a previous history of CHD showed that a higher TyG index was associated with an increased risk of significant coronary artery stenosis [19]. A nested case–control study including 1282 patients with T2DM and stable CAD showed that the TyG index was positively associated with future CV events, which were defined as all-cause death, non-fatal MI, stroke and post-discharge revascularization [20]. Additionally, a cohort study including 25,969 participants without previous diabetes or CVD indicated substantial similarities in the inflammatory profiles associated with diabetes and CVD [24]. Recently, a mediation analysis was performed to quantify the magnitude and relative contributions of several traditional or non-traditional CV risk factors in the pathway from T2DM to increased CV events (MI, stroke and vascular mortality) and demonstrated that the most important pathway contributing to CV events was the presence of IR assessed by the TyG index, followed by elevated triglycerides, the presence of microalbuminuria and reduced kidney function, whereas excess risk was not mediated through elevated systolic blood pressure or high LDL-C [25].
Although the mechanism underlying the association of the TyG index with adverse CV outcomes has not been elucidated, it may be linked to IR. In recent years, many studies have indicated the importance of IR not only in atherogenesis but also in advanced plaque progression by promoting apoptosis of macrophages, endothelial cells, and vascular smooth muscle cells [8, 26, 27]. Several metabolic changes caused by IR can induce the development of CVD. For example, IR can induce an imbalance in glucose metabolism that generates chronic hyperglycaemia, which in turn triggers oxidative stress and causes an inflammatory response that leads to vascular endothelial cell damage. IR can also alter lipid metabolism, which then leads to the development of dyslipidaemia and the well-known lipid triad: (1) elevated plasma triglycerides, (2) reduced plasma HDL-C, and (3) the appearance of small dense LDL-C particles. These metabolic changes contribute to atherosclerotic plaque formation [7]. Moreover, IR accompanied by hyperglycaemia and hypertriglyceridaemia has been demonstrated to be correlated with elevated plasminogen activator inhibitor-1 levels, leading to decreased fibrinolytic activity and increased thrombotic events [28, 29]. Furthermore, IR has been shown to be associated with structural and functional arterial wall injuries, such as endothelial dysfunction, impaired vasodilation, increased arterial stiffness, increased intima-media thickness, and increased coronary artery calcification, which are highly predictive of future CV events [30]. Notably, several studies confirmed that in the setting of acute MI, IR was associated with myocardial and microvascular injury. For example, Trifunovic et al. reported that IR was independently associated with poorer myocardial reperfusion assessed by the residual ST-segment elevation and impaired coronary microcirculatory function estimated by coronary flow reserve and potentially associated with larger final infarct size evaluated by the fixed perfusion defect on single-photon emission computed tomography myocardial perfusion imaging in non-diabetic patients with ST-segment elevation MI treated by primary PCI [31]. The poorer myocardial reperfusion, impaired coronary microcirculatory function and larger final infarct size after primary PCI were all related to the subsequent increase in short- and long-term adverse CV events. In fact, the association of IR with adverse CV outcomes has been proven in several clinical studies. A prospective Danish population-based study involving 2493 apparently healthy subjects reported that IR, assessed as HOMA-IR, was associated with incident CV events (CV death, non-fatal ischaemic heart disease, and non-fatal stroke) independent of metabolic syndrome based on both the International Diabetes Foundation and the National Cholesterol Education Program criteria [32]. A similar result was observed in a population of patients who had developed CVD. A multicentre prospective study including 2938 patients with pre-existing CAD revealed that IR, evaluated by HOMA-IR, was a good predictor for CV events (fatal and non-fatal MI and sudden death) [33]. Furthermore, aggressive treatment of diabetes and IR might significantly reduce the risk of CV events. Pioglitazone primarily stimulates peroxisome proliferator-activated receptor (PPAR)-gamma and partially activates PPAR-α, which ameliorates IR and improves glucose and lipid metabolism. Multiple studies have demonstrated that pioglitazone reduces future CV events in association with enhanced insulin sensitivity [8]. In a multicentre, randomized controlled trial including 5238 patients with T2DM who had evidence of macrovascular disease, pioglitazone was demonstrated to reduce the composite of all-cause mortality, non-fatal MI, and stroke [34]. Additionally, in another multicentre, randomized controlled trial involving 3876 patients without diabetes who had a recent history of ischaemic stroke or transient ischaemic attack and who had IR, the patients who received pioglitazone had a lower risk of fatal or non-fatal stroke or MI than those who received placebo [35]. Moreover, a recently published meta-analysis of randomized, controlled trials reported that pioglitazone significantly reduced the composite endpoints of non-fatal MI, non-fatal stroke and CV death in people with IR, pre-diabetes and diabetes mellitus [36].
The TyG index per se was reported to be positively associated with arterial stiffness as assessed by brachial-ankle or carotid-femoral pulse wave velocity, which have been demonstrated to predict the long-term risk of adverse CV events in patients with T2DM and ACS [37–39]. Of note, the TyG index includes triglycerides and glucose in its formula. Fasting triglycerides and glucose were shown to be associated with both long-term and short-term CV risk after ACS, independent of diabetic status [40, 41]. The aforementioned relationships might in part explain the potential association between the TyG index and adverse CV outcomes in diabetic patients with ACS. Moreover, we found that BMI and LDL-C levels were significantly higher in patients in the highest TyG index tertile than in those in other tertiles, whereas HDL-C levels were lower. Therefore, the association of the TyG index with adverse CV outcomes may be partially mediated by these traditional CV risk factors.
Our study also had several important limitations. First, this was a retrospective analysis derived from a single-centre prospective observational study, which could not definitively establish causality. Second, the baseline levels of FPG and triglycerides could be affected by the use of lipid-lowering drugs and antidiabetic agents before admission. However, there were no significant differences among the three groups on the basis of the TyG index tertiles with respect to the use of lipid-lowering drugs and antidiabetic agents before admission. Third, none of the study patients were treated with drugs specifically designed to lower triglycerides, such as fibrates, niacin, and omega-3 fatty acids, before admission and at discharge; therefore, our results may not be applicable to patients treated with such drugs. Fourth, whether the findings from the present study including only Chinese patients can be extrapolated to other ethnic groups will require further studies. Fifth, we did not compare the TyG index with HOMA-IR and the hyperinsulinaemic–euglycaemic clamp test. Finally, we did not record nutritional habits or energy intake. Although we did not adjust for these potential confounding variables, we used adjusted other variables, such as BMI or cholesterol levels which are indirectly related to nutritional habits or energy intake.
Conclusions
The TyG index, which is easily measurable and applicable in clinical practice without the need for complicated techniques or formulas, was significantly associated with a higher risk of CV events in patients with T2DM and ACS who underwent PCI, and this relationship remained significant after adjustment for other confounders. Further prospective, large studies are required to confirm our findings.
Supplementary information
Additional file 1: Table S1. Adverse CV events according to the TyG index tertiles during follow-up.
Additional file 2: Table S2. Relationship between the incidence of the primary endpoint and the TyG index expressed as a continuous variable.
Acknowledgements
We thank Nature Research Editing Service (http://bit.ly/NRES-HS) for their linguistic assistance during the revision of this manuscript.
Abbreviations
- ACS
Acute coronary syndrome
- BMI
Body mass index
- CABG
Coronary artery bypass grafting
- CAD
Coronary artery disease
- CHF
Congestive heart failure
- CI
Confidence interval
- CKD
Chronic kidney disease
- CrCl
Creatinine clearance
- CV
Cardiovascular
- CVA
Cerebrovascular accident
- CVD
Cardiovascular disease
- FPG
Fasting plasma glucose
- HDL-C
High-density lipoprotein cholesterol
- HOMA-IR
homeostasis model assessment of insulin resistance
- HR
Hazard ratio
- IQR
Interquartile range
- IR
Insulin resistance
- LDL-C
Low-density lipoprotein cholesterol
- LVEF
Left ventricular ejection fraction
- MI
Myocardial infarction
- PAD
Peripheral artery disease
- PCI
Percutaneous coronary intervention
- PPAR
Peroxisome proliferator-activated receptor
- T2DM
Type 2 diabetes mellitus
- TC
Total cholesterol
- TyG
Triglyceride glucose
Authors’ contributions
All authors were involved in the conception and design of the study and in the collection, analysis, and interpretation of the data. All authors reviewed the final manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Grant from National Key Research and Development Program of China (2017YFC0908800), Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20150601) and Mission Plan (SML20180601), and Beijing Municipal Health Commission—“Project of Science and Technology Innovation Center” (PXM2019_026272_000006) (PXM2019_026272_000005).
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the institutional review board of Beijing Anzhen Hospital, Capital Medical University. Given the retrospective nature of this study, the requirement for informed consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12933-020-01006-7.
References
- 1.Katz P, Leiter LA, Mellbin L, Ryden L. The clinical burden of type 2 diabetes in patients with acute coronary syndromes: prognosis and implications for short- and long-term management. Diab Vasc Dis Res. 2014;11(6):395–409. doi: 10.1177/1479164114546854. [DOI] [PubMed] [Google Scholar]
- 2.Bjarnason TA, Hafthorsson SO, Kristinsdottir LB, Oskarsdottir ES, Johnsen A, Andersen K. The prognostic effect of known and newly detected type 2 diabetes in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2019 doi: 10.1177/2048872619849925. [DOI] [PubMed] [Google Scholar]
- 3.Ray KK, Colhoun HM, Szarek M, Baccara-Dinet M, Bhatt DL, Bittner VA, Budaj AJ, Diaz R, Goodman SG, Hanotin C, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(8):618–628. doi: 10.1016/S2213-8587(19)30158-5. [DOI] [PubMed] [Google Scholar]
- 4.Ramanathan K, Abel JG, Park JE, Fung A, Mathew V, Taylor CM, Mancini GBJ, Gao M, Ding L, Verma S, et al. Surgical versus percutaneous coronary revascularization in patients with diabetes and acute coronary syndromes. J Am Coll Cardiol. 2017;70(24):2995–3006. doi: 10.1016/j.jacc.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 6.Rask-Madsen C, Kahn CR. Tissue-specific insulin signaling, metabolic syndrome, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2012;32(9):2052–2059. doi: 10.1161/ATVBAHA.111.241919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuniga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Pino A, DeFronzo RA. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev. 2019;40(6):1447–1467. doi: 10.1210/er.2018-00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association Consensus Development Conference on insulin resistance. 5–6 November 1997. Diabetes Care. 1998;21(2):310–314. doi: 10.2337/diacare.21.2.310. [DOI] [PubMed] [Google Scholar]
- 10.Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, Jacques-Camarena O, Rodriguez-Moran M. The product of triglycerides and glucose, a simple measure of insulin sensitivity comparison with the euglycemic–hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 11.Vasques AC, Novaes FS, de Oliveira Mda S, Souza JR, Yamanaka A, Pareja JC, Tambascia MA, Saad MJ, Geloneze B. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–e100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Inigo L, Navarro-Gonzalez D, Fernandez-Montero A, Pastrana-Delgado J, Martinez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–197. doi: 10.1111/eci.12583. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Guo B, Chen H, Shi Z, Li Y, Tian Q, Shi S. The role of the triglyceride (triacylglycerol) glucose index in the development of cardiovascular events: a retrospective cohort analysis. Sci Rep. 2019;9(1):7320. doi: 10.1038/s41598-019-43776-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alizargar J, Bai CH. Comparison of carotid ultrasound indices and the triglyceride glucose index in hypertensive and normotensive community-dwelling individuals: a case control study for evaluating atherosclerosis. Medicina. 2018;54(5):71. doi: 10.3390/medicina54050071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Silva A, Caldas APS, Hermsdorff HHM, Bersch-Ferreira AC, Torreglosa CR, Weber B, Bressan J. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol. 2019;18(1):89. doi: 10.1186/s12933-019-0893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin JL, Cao YX, Wu LG, You XD, Guo YL, Wu NQ, Zhu CG, Gao Y, Dong QT, Zhang HW, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10(11):6137–6146. doi: 10.21037/jtd.2018.10.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao Q, Zhou D, Li Y, Wang Y, Xu SC, Zhao XH. The Triglyceride-glucose index predicts coronary artery disease severity and cardiovascular outcomes in patients with non-st-segment elevation acute coronary syndrome. Dis Markers. 2019;2019:6891537. doi: 10.1155/2019/6891537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo E, Wang D, Yan G, Qiao Y, Liu B, Hou J, Tang C. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol. 2019;18(1):150. doi: 10.1186/s12933-019-0957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee EY, Yang HK, Lee J, Kang B, Yang Y, Lee SH, Ko SH, Ahn YB, Cha BY, Yoon KH, et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. 2016;15(1):155. doi: 10.1186/s12944-016-0324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin JL, Sun D, Cao YX, Guo YL, Wu NQ, Zhu CG, Gao Y, Dong QT, Zhang HW, Liu G, et al. Triglyceride glucose and haemoglobin glycation index for predicting outcomes in diabetes patients with new-onset, stable coronary artery disease: a nested case-control study. Ann Med. 2018;50(7):576–586. doi: 10.1080/07853890.2018.1523549. [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 23.Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13:146. doi: 10.1186/s12933-014-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bao X, Borne Y, Johnson L, Muhammad IF, Persson M, Niu K, Engstrom G. Comparing the inflammatory profiles for incidence of diabetes mellitus and cardiovascular diseases: a prospective study exploring the ‘common soil’ hypothesis. Cardiovasc Diabetol. 2018;17(1):87. doi: 10.1186/s12933-018-0733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharif S, Groenwold RHH, van der Graaf Y, Berkelmans GFN, Cramer MJ, Visseren FLJ, Westerink J. Mediation analysis of the relationship between type 2 diabetes and cardiovascular events and all-cause mortality: findings from the SMART cohort. Diabetes Obes Metab. 2019;21(8):1935–1943. doi: 10.1111/dom.13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14(5):575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reardon CA, Lingaraju A, Schoenfelt KQ, Zhou G, Cui C, Jacobs-El H, Babenko I, Hoofnagle A, Czyz D, Shuman H, et al. Obesity and insulin resistance promote atherosclerosis through an IFNgamma-regulated macrophage protein network. Cell Rep. 2018;23(10):3021–3030. doi: 10.1016/j.celrep.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calles-Escandon J, Mirza SA, Sobel BE, Schneider DJ. Induction of hyperinsulinemia combined with hyperglycemia and hypertriglyceridemia increases plasminogen activator inhibitor 1 in blood in normal human subjects. Diabetes. 1998;47(2):290–293. doi: 10.2337/diab.47.2.290. [DOI] [PubMed] [Google Scholar]
- 29.Sobel BE. Insulin resistance and thrombosis: a cardiologist’s view. Am J Cardiol. 1999;84(1a):37j–41j. doi: 10.1016/S0002-9149(99)00357-4. [DOI] [PubMed] [Google Scholar]
- 30.Adeva-Andany MM, Ameneiros-Rodriguez E, Fernandez-Fernandez C, Dominguez-Montero A, Funcasta-Calderon R. Insulin resistance is associated with subclinical vascular disease in humans. World J Diabetes. 2019;10(2):63–77. doi: 10.4239/wjd.v10.i2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trifunovic D, Stankovic S, Sobic-Saranovic D, Marinkovic J, Petrovic M, Orlic D, Beleslin B, Banovic M, Vujisic-Tesic B, Petrovic M, et al. Acute insulin resistance in ST-segment elevation myocardial infarction in non-diabetic patients is associated with incomplete myocardial reperfusion and impaired coronary microcirculatory function. Cardiovasc Diabetol. 2014;13:73. doi: 10.1186/1475-2840-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp-Pedersen C, Madsbad S. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease: a population-based study. J Am Coll Cardiol. 2007;49(21):2112–2119. doi: 10.1016/j.jacc.2007.01.088. [DOI] [PubMed] [Google Scholar]
- 33.Tenenbaum A, Adler Y, Boyko V, Tenenbaum H, Fisman EZ, Tanne D, Lapidot M, Schwammenthal E, Feinberg MS, Matas Z, et al. Insulin resistance is associated with increased risk of major cardiovascular events in patients with preexisting coronary artery disease. Am Heart J. 2007;153(4):559–565. doi: 10.1016/j.ahj.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 35.Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374(14):1321–1331. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao HW, Saver JL, Wu YL, Chen TH, Lee M, Ovbiagele B. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: a systematic review and meta-analysis. BMJ Open. 2017;7(1):e013927. doi: 10.1136/bmjopen-2016-013927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, Kim MJ, Kim MK, Park JS. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17(1):41. doi: 10.1186/s12933-018-0692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao S, Yu S, Chi C, Fan X, Tang J, Ji H, Teliewubai J, Zhang Y, Xu Y. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai Study. Cardiovasc Diabetol. 2019;18(1):95. doi: 10.1186/s12933-019-0898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levisianou D, Foussas S, Skopelitis E, Adamopoulou E, Xenopoulou T, Destounis A, Koukoulis G, Skoularigis I, Melidonis A, Triposkiadis F. Arterial stiffness predicts risk for long-term recurrence in patients with type 2 diabetes admitted for acute coronary event. Diabetes Res Clin Pract. 2013;99(3):315–320. doi: 10.1016/j.diabres.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz GG, Abt M, Bao W, DeMicco D, Kallend D, Miller M, Mundl H, Olsson AG. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 2015;65(21):2267–2275. doi: 10.1016/j.jacc.2015.03.544. [DOI] [PubMed] [Google Scholar]
- 41.Sinnaeve PR, Steg PG, Fox KA, Van de Werf F, Montalescot G, Granger CB, Knobel E, Anderson FA, Dabbous OH, Avezum A. Association of elevated fasting glucose with increased short-term and 6-month mortality in ST-segment elevation and non-ST-segment elevation acute coronary syndromes: the Global Registry of Acute Coronary Events. Arch Intern Med. 2009;169(4):402–409. doi: 10.1001/archinternmed.2008.572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Adverse CV events according to the TyG index tertiles during follow-up.
Additional file 2: Table S2. Relationship between the incidence of the primary endpoint and the TyG index expressed as a continuous variable.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.