Abstract
Objective:
Discordances between imaging findings of parenchymal neurocysticercosis and seizure expression have been reported, and as such the possibility that neurocysticercosis and seizures may frequently coexist by chance has been raised. In this study, we evaluate the topographic relationship between seizure foci based on semiology and electroencephalography with the location of parenchymal neurocysticercotic lesions.
Methods:
Seizure information, neuroimaging (computed tomography and magnetic resonance imaging [MRI]) and electroencephalographic data from three randomized clinical trials of individuals with parenchymal neurocysticercosis and focal seizures were analyzed. Blinded epileptologists defined a potential seizure onset zone and a symptomatogenic zone for each individual based on semiology. The topographic relationship between semiology, either lesion location or areas of perilesional edema on baseline MRI, and electroencephalographic abnormalities were assessed.
Results:
Fifty-eight patients with one or two parenchymal neurocysticercotic lesions were included in this study. From them, 50 patients (86%; 95% CI, 75%-93%) showed a clinical-topography relationship with the potential seizure onset zone, and 44 (76%) also with the symptomatogenic zone. From the eight patients with no topographic relationship, five had focal seizures 30 days before or after the baseline MRI and showed perilesional edema. All of these five patients showed a clinical-topography relationship between such seizures and an area of perilesional edema, making a total of 55 patients (95%; 95% CI, 85%–99%) with clinical-topography relationship when perilesional edema is considered. Most patients with focal epileptiform discharges (7/8, 88%) had a topographic association between electroencephalographic focality, the potential seizure onset zone and a cysticercotic lesion.
Conclusion:
Seizure semiology and focal epileptiform discharges are topographically related to neurocysticercotic lesions in most patients. These data strongly support seizure origin in the cortex surrounding these lesions.
Keywords: Epilepsy, Cysticercosis, Neurocysticercosis, Taenia solium, Seizures, Peru
1. INTRODUCTION
Neurocysticercosis (NCC), a helminthic infection of the central nervous system caused by the cystic larval stage of the pork tapeworm Taenia solium, is the leading cause of epilepsy in most resource-poor countries (Garcia et al., 2014c; Singh et al., 2013). Neurocysticercosis is a common infection in endemic regions (Moyano et al., 2016), and in field conditions between 10 and 20% of the general population may show NCC lesions in neuroimaging examinations (Montano et al., 2005; Moyano et al., 2016). Only a minority of these individuals, however, would have a history of seizures (Moyano et al., 2016; Prasad et al., 2011).
There are discordances between imaging findings of NCC and seizure expression, both in field studies and in clinical cases (Del Brutto et al., 1992; Duque and Burneo, 2017). In a community-based MRI survey, there was no difference in proportions of patients with seizures regarding number, stage or location of cysticercotic lesion (Prasad et al., 2008). In another study, seizure frequency was not related to the burden of cysticercotic lesions (Kowacs et al., 2006). On the basis of these observations, the possibility that NCC and seizures may frequently coexist by chance has been raised (Kowacs et al., 2006; Prasad et al., 2008; Saito et al., 2016; Sakamoto et al., 1999).
Only a few studies have assessed the topographic relationship between seizure semiology or electroencephalographic (EEG) findings and cysticercotic lesions (Cukiert et al., 1994; Murthy and Reddy, 1998; Singh et al., 2000). Such association was observed in only half of cases. These studies, however, had serious limitations. They were performed with old-fashioned computed tomography (CT) equipment only or were based on anatomical divisions in brain lobes.
Seizure semiology is an extremely valuable tool to assess patients with epilepsy (Tufenkjian and Luders, 2012). In patients with a presumable epileptogenic lesion, semiology plus EEG might accurately determine whether the given lesion is responsible for the observed seizures. More-constrained functional areas in relation to epilepsy are now helping to identify more efficiently the seizure origin (Bonini et al., 2014; Tufenkjian and Luders, 2012). In this study, we took advantage of three historical cohorts of patients with NCC to evaluate the topographic relationship between seizure foci based on semiology and EEG with the location of cysticercotic lesions assessed with magnetic resonance imaging (MRI) and CT.
2. MATERIALS AND METHODS
Individuals with parenchymal NCC and focal seizures (based on semiology) from three clinical trials (Garcia et al., 2014a; Garcia et al., 2014b; Garcia et al., 2016) were selected in order to evaluate whether their seizures and EEG focality were topographically related to NCC lesions. To reduce the likelihood of falsely finding a relationship by chance, we selected patients with only one or two cysticercotic lesions of any stage (viable, degenerating or calcified cysts) located juxtacortically (either in the cortex or in the cortical-subcortical junction).
For topographic purposes, we divided each brain hemisphere in 21 functional areas related to epilepsy (Table 1) (Luders, 2008). For each patient, the areas that could have elicited his/her initial seizure manifestations by local activation or seizure spread were termed potential (suspected) seizure onset zone, and the areas that probably produced the initial manifestations through only local activation were defined as symptomatogenic zone (Rosenow and Luders, 2001). Both zones were defined based on semiology, and hence, from this perspective, a potential seizure onset often included more functional areas than a symptomatogenic zone. For each patient, blinded epileptologists determined both zones and read EEG recordings.
Table 1.
Functional brain areas related to epilepsya (modified from Luders et al. (Luders, 2008))
| Lobe | Functional brain areas |
|---|---|
| Perirolandic regionb | Lateral perirolandic |
| Mesial perirolandic | |
| Frontal lobec | Premotor |
| Negative motor/Broca’s languaged | |
| Dorso-lateral frontal | |
| Frontal pole | |
| Orbitofrontal | |
| Supplementary motor | |
| Pre-supplementary motor | |
| Mesial prefrontal | |
| Anterior cingulate gyrus | |
| Parietal lobee | Postero-superior parietal cortex |
| Postero-inferior parietal cortex | |
| Precuneus | |
| Posterior cingulate gyrus | |
| Temporal lobe | Mesial |
| Neocortical | |
| Occipital lobe | Primary visual cortex |
| Secondary visual cortex | |
| Insular lobe | Anterior insula |
| Posterior insula |
Functional brain areas, for each hemisphere, considered as exclusive categories.
Includes the primary motor and primary somatosensory cortex.
Excludes the primary motor cortex.
Broca’s language area was considered as present only in the dominant hemisphere where overlaps with the negative motor area.
Excludes the primary somatosensory cortex.
2.1. Study population and data collection
Data was collected from three already published clinical trials assessing antiparasitic treatment (APT) for viable NCC (Garcia et al., 2014a; Garcia et al., 2014b; Garcia et al., 2016). Participants had been recruited from four tertiary-care hospitals in Lima, Peru and followed between April 2006 and October 2013. Common to the original trials, inclusion criteria were age between 16 and 65 years, at least one viable cysticercotic cyst, serological confirmation on enzyme-linked inmmunoelectrotransfer blot assay (EITB, western blot) and at least one seizure in the previous year but a seizure history not longer than 10 years. No patient was receiving APT at the period of enrolment, but it was offered weeks after enrolment as the purpose of the clinical trials. At enrolment, neurologists obtained detailed medical histories from the patients and all available eyewitnesses, and recommended patients to take antiepileptic drugs (AED) regularly during the whole follow-up. Baseline EEG, MRI and CT of the brain were performed. Follow-up lasted at least one year with active seizure surveillance (Garcia et al., 2014a; Garcia et al., 2014b; Garcia et al., 2016). Patients were instructed to recognize events suspected of being a seizure, to register them in a seizure control diary and to immediately report to the study team. The study neurologist interviewed the patient and available eyewitnesses in the day of the event or the following days to determine whether the event was a seizure. Some seizures were witnessed during hospitalization. Parent trials were approved by the main Institutional Review Board (IRB) of the Universidad Peruana Cayetano Heredia and included permission for further use of the data. A separate IRB approval was obtained for this analysis.
2.2. Seizure evaluation and semiology-based zones
Two epileptologists (A.L.E. and W.Z), blinded to neuroimaging and EEG, revised all descriptions of paroxysmal events to confirm those consistent with seizures, and registered all seizure descriptions using standard semiological terminology (Blume et al., 2001; Fisher et al., 2017; Luders et al., 1998). Focal seizures may evolve or not to bilateral tonic-clonic seizures. First, epileptologists independently localized and lateralized potential seizure onset and symptomatogenic zones for seizures occurred before APT onset, and separately, for seizures that occurred after APT. Additionally, both semiology-based zones were localized considering only seizures occurred 30 days before or after the baseline MRI, but prior to APT. Finally, both zones were determined for each patient using all seizures occurred before enrolment and during follow-up as a whole. Localization was performed on the basis of well-known functional areas related to specific seizure manifestations, comprehensively described by Luders et al. (Luders, 2008) (Table 1), and taking into account the number of each seizure type, time of occurrence, the person who reported a given seizure and the neurological assessment for seizures occurred during follow-up. Inter-reader agreement of the two epileptologists for determining whether patients had or had not a clinical-topography association with the potential seizure onset zone, taking into account all seizures before enrolment or during follow-up, was 86% (50 of the 58 patients), and the relaxed kappa was 0.56 (p<0.001). Any discrepancies in the semiology-based zones were resolved by consensus with the intervention of a third epileptologist (J.G.B.). Ictal manifestations with the agreed semiology-based zones of each patient are provided in Supplementary Table B.1.
2.3. MRI evaluation
Based on neuroradiological reports, an epileptologist (A.L.E, blinded to the respective patients’ seizure semiology) reviewed both baseline MRI and CT images, and carefully localized a NCC lesion in one of the functional areas (Table 1). The areas affected by edema around cysticercotic lesions were noted separately. Lesions were further localized in one of the three types of neocortices described by Benarroch et al. (Benarroch, 2008) and many others (Grabowski et al., 2002; Luders, 2008): primary cortex, unimodal association cortex or heteromodal association cortex (Supplementary Fig. B.1). These types of neocortices are based on the degree of neural networks and comparable complexity of receptive stimulus and neural responses. Axial, coronal and sagittal MRI images were obtained from a 1.5-Tesla scanner in T1-weighted, T2-weighted and fluid-attenuated inversion recovery (FLAIR) protocols. Neurocysticercosis cysts whose contents were hyperintense in T2-weighted and hypointense in T1-weighted were considered viable cysts; FLAIR allowed to determine perilesional edema (Del Brutto et al., 2017). CT scan, obtained from a 64-slice scanner with 6-mm slice thickness and no interslice gap, was used to detect calcified NCC cysts. All MRI and some CT were enhanced with contrast to unmask degenerating NCC cysts.
All MRIs were also reviewed to assess the presence of hippocampal sclerosis (defined as the presence of both hippocampal atrophy on T1-weighted and hyperintense signal on T2-weighted or FLAIR MRI), focal cortical dysplasia or other epileptogenic lesions. Although coronal sections were not in alignment with the hippocampus axis, the hippocampus was visible in 3-4 sections.
2.4. EEG
Scalp 16-channel EEGs were performed at baseline during 30 minutes using the 10-20 International system of electrode placement with bipolar and referential recordings. Hyperventilation and photic stimulation were performed in all patients and induced N2 sleep stage in some of them. Additional EEGs were performed after a recent seizure whenever possible. An epileptologist (W.Z), blinded to all clinical information except for the patient’s age, read every EEG twice looking for epileptiform discharges or abnormal slow waves, and localized and lateralized the EEG focalization on the basis of the functional brain areas (Table 1). Epileptiform discharges (e.g., sharp-waves or spikes) were reported separately from slow-waves. Anterior temporal EEG abnormalities were related to the mesial temporal area of the functional brain areas, and posterior temporal EEG abnormalities, to the neocortical temporal area. Inter-reading agreement of the epileptologist for determining an EEG as either normal, with epileptiform discharges, or with only abnormal focal slow activity was 95% (77 out of 81 EEGs), and the relaxed kappa was 0.87 (p<0.001). The four discordant EEGs were read for a third time to reach a conclusion. As EEG tracings were handled by another investigator and presented in groups of ten to be read, the epileptologist was also blinded to whether he was reading a particular exam by first, second or third time.
2.5. Statistical analysis
Results are reported separately for patients with one or two lesions, or for the entire group if subgroup analyses showed similar results. A patient with clinical-topography relationship was defined when a cysticercotic lesion was located in the potential seizure onset zone, determined with all seizures before enrolment and during follow-up. The topographic relationship was also assessed with the symptomatogenic zone. Binomial 95% confidence intervals (CI) were calculated. A nonparametric trend test was used to assess whether the location of NCC lesions influenced the clinical-topography relationship. McNemar’s test was used to assess the potential effect of APT in apparently altering the clinical-topography association. The topographic relationship with brain areas affected by perilesional edema was assessed using semiology-based zones determined with only seizures occurred 30 days before or after the baseline MRI.
The topographic congruence between EEG focalization, potential seizure onset zones and cysticercotic lesions was determined separately for focal epileptiform discharges and abnormal slow waves only. Fisher’s exact test was used to compare proportions, and Mann-Whitney test, for comparison of variables with skewed distributions between two groups. All reported p-values are two-sided and those <0.05 are considered statistically significant. STATA v.14.0 (StataCorp, USA) was used.
3. RESULTS
Two-hundred and thirteen patients were enrolled in three clinical trials, seven of them participated in two trials. At enrolment, 65 patients had one or two parenchymal NCC lesions, all of which were located juxtacortically. Among these patients, 58 (89%) had focal seizures at some point and were eligible for this study. Baseline characteristics are summarized in Table 2. All patients received only one AED at the same time, except two patients who received two AED at the same time for less than two months to control seizure recurrence. All patients received APT which were standard albendazole (15 mg/kg/day for 10 or 14 days), increased dose albendazole (22.5 mg/kg/day for 10 days) or combined albendazole (15 mg/kg/day for 10 days) plus praziquantel (50 mg/kg/day for 10 days) depending on the clinical trial (Garcia et al., 2014a; Garcia et al., 2014b; Garcia et al., 2016).
Table 2.
Baseline characteristics
| Characteristic | Total (N=58) |
|---|---|
| Sex male | 35 (60%) |
| Age at enrolment (years), median (IQR; range) | 26 (19–36; 16–58) |
| Length of follow-up (months), mean (SD) | 16.3 (3.9) |
| Antiepileptic drug | |
| Carbamazepine | 31 (53%) |
| Phenytoin | 27 (47%) |
| NCC lesions | |
| Brain lobes with at least one NCC lesiona | |
| Frontal lobe | 22 (38%) |
| Perirolandic region | 17 (29%) |
| Occipital lobe | 14 (24%) |
| Parietal lobe | 10 (17%) |
| Temporal lobe | 6 (10%) |
| Insular lobe | 4 (7%) |
| Type of neocortex with at least one NCC lesiona | |
| Primary cortex | 24 (41%) |
| Unimodal association cortex | 28 (48%) |
| Heteromodal association cortex | 18 (31%) |
| Location of NCC lesions and perilesional FLAIR hyperintensitya | |
| Lateral perirolandic area | 19 (32.8%) |
| Premotor area | 14 (24.1%) |
| Secondary visual area | 12 (20.7%) |
| Primary visual area | 11 (19.0%) |
| Mesial perirolandic area | 9 (15.5%) |
| Negative motor/Broca’s language area | 6 (10.3%) |
| Postero-superior parietal area | 6 (10.3%) |
| Neocortical temporal area | 6 (10.3%) |
| Dorso-lateral frontal area | 5 (8.6%) |
| Supplementary motor area (in mesial frontal lobe) | 5 (8.6%) |
| Posterior insula | 4 (6.9%) |
| Frontal pole | 3 (5.2%) |
| Postero-inferior parietal area | 3 (5.2%) |
| Mesial temporal area | 3 (5.2%) |
| Precuneus | 2 (3.4%) |
| Anterior cingulate gyrus | 2 (3.4%) |
| Posterior cingulate gyrus | 2 (3.4%) |
| Orbitofrontal area | 1 (1.7%) |
| Mesial prefrontal area | 1 (1.7%) |
| Pre-supplementary motor area (in mesial frontal lobe) | 0 |
| Anterior insula | 0 |
| Seizure expression | |
| Age at seizure onset (years), median (IQR; range) | 25 (18–35; 12–57) |
| Length of seizure history at enrolment (months), median (IQR) | 5.9 (1.1–23.8) |
| Occurrence of first seizure ever | |
| >12 months before enrolment | 22 (38%) |
| Between 2 and 12 months before enrolment | 18 (31%) |
| Within 2 months before enrolment | 18 (31%) |
| Occurrence of last focal seizure | |
| Between 2 and 9 months before enrolment | 6 (10%) |
| Within 60 days before enrolment | 26 (45%) |
| During follow-up | 26 (45%) |
Percentages add up to more than 100% because some patients had two lesions in different areas or lobes.
Abbreviations: IQR, interquartile range; NCC, neurocysticercosis; SD, standard deviation.
According to the standard diagnostic criteria (Del Brutto et al., 2017), all patients had a definitive diagnosis based on at least one major neuroimaging criterion (cystic lesions without a discernible scolex, enhancing lesions and/or typical parenchymal brain calcifications), one confirmative neuroimaging criterion (resolution of cysts after antiparasitic treatment: 44 patients had resolution of at least one viable cyst after one course of cysticidal therapy and all the others after one or two further cysticidal treatments) plus a positive EITB assay as an exposure criterion. In addition, 49 patients (84%) also had an absolute criterion, conclusive demonstration of a scolex within a cystic lesion.
3.1. Neuroimaging examination
All patients had both baseline MRI enhanced with contrast and baseline CT, as both neuroimaging were required for inclusion in the parent clinical trials. At enrolment, 45 patients (78%) had viable cysts only, 32 (55%) had one and 13 (22%) had two viable cysts. Seven patients (12%) had one viable and one calcified cyst, and six (10%) had one viable and one degenerating cyst. Location of cysticercotic lesions are presented in Table 2. Perilesional edema was present at least in one NCC lesion in 46 (79.3%) patients. Patients with two lesions more often had, although not statistically significant, at least one lesion in a parietal lobe (7/26 [27%] versus 3/32 [9%], p=0.10) and in the primary cortex (14/26 [54%] versus 10/32 [31%], p=0.11). No patient had hippocampal sclerosis, focal cortical dysplasia or other epileptogenic lesion.
3.2. Seizures
A total of 1,092 seizures occurred before enrolment and during follow-up, of which 1,049 (96.1%) were focal seizures. Characteristics of seizures are reported in Table 2. Fifty-two (90%) patients had focal seizures during the follow-up or within 60 days before the enrolment (Table 2). Fifteen patients (26%) had focal seizures which were witnessed during hospitalization. Patients with recent onset of seizures (first seizure ever within two months before enrolment) had a median of 4.5 focal seizures (interquartile range [IQR], 3–9), including all seizures before enrolment and during follow-up, and those with longer seizure history, 8.5 focal seizures (IQR, 3–30.5). Patients with two lesions had a higher number of focal seizures, although this did not reach statistical significance, than those with one lesion (median 9.5 [IQR, 4–39] versus 5 [IQR, 2–17.5], p=0.06), and similar time of seizure history (median 5.5 months [IQR, 1.7–13.3] versus 6.1 [IQR, 1.0-40.3], p=0.88).
3.3. Clinical-topography relationship
For each patient, epileptologists targeted a mean±standard deviation (SD) of 3.2±2.0 brain areas for the potential seizure onset zone, and 2.7±1.7 for the symptomatogenic zone. Considering all seizures before enrolment and during follow-up, 50 patients (86%; 95% CI 75%-93%) showed a clinical-topography relationship with the potential seizure onset zone, and 44 patients (76%) also with the symptomatogenic zone (Table 3).
Table 3.
Topographic relationship among pertinent groups of patients
| Semiology-based zones |
Total sample (N=58) | Recent onset of seizuresa (n = 18) |
Longer seizure history (n = 40) |
P value | One lesion (n = 32) |
Two lesions (n = 26) |
P value |
|---|---|---|---|---|---|---|---|
| Potential seizure onset zone | 50 (86; 75–93) | 17 (94; 74–99) | 33 (83; 68–91) | 0.41 | 25 (78; 61–89) | 25 (96; 81–99) | 0.06 |
| Symptomatogenic zone | 44 (76; 63–85) | 15 (83; 61–94) | 29 (73; 57–84) | 0.51 | 23 (72; 55–84) | 20 (77; 58–89) | 0.77 |
Data presented as n (%; 95% CI)
Patients with their first seizure within two months before enrolment.
Abbreviation: CI, confidence interval.
Proportions of patients with clinical-topography relationship among pertinent subgroups of patients are shown in Table 3. Among the 19 patients with clinical-topography relationship and two lesions in different functional areas, the potential seizure onset zone was topographically related to only one lesion in 16 patients (84%) and to each of both in the other cases.
Six patients had one viable and one calcified cyst in different areas, and all showed a clinical-topography relationship with the potential seizure onset zone. In five patients (83%) semiology was topographically related to only the viable cyst, and in the other patient (17%), to each of both the viable cyst and the calcified cyst. Five patients had one viable and one degenerating cyst in different areas, and four of them had a clinical-topography relationship. Three (75%) of these four patients were related only to the viable cyst, and the other one (25%), only to the degenerating cyst.
Twenty-five patients had focal seizures after APT onset –despite taking AED regularly,– and also had focal seizures before APT. Twenty-two patients (88%) had a clinical-topography association related both to seizures occurred before APT onset and to seizures occurred after that, being analyzed independently (p=0.56, for within-person comparison in favor of similar proportions). Two patients had a clinical-topography association related only to seizures occurred before APT, and one patient, only to seizures occurred after APT onset.
Of the eight patients with no topographic relationship, five had focal seizures 30 days before or after the baseline MRI and showed perilesional edema. All of them showed a clinical-topography relationship between such seizures and an area of perilesional edema (Fig. 1A). Thus, 95% of patients (55/58, 95% CI 85%–99%) had semiology topographically related to a cysticercus when perilesional edema is considered.
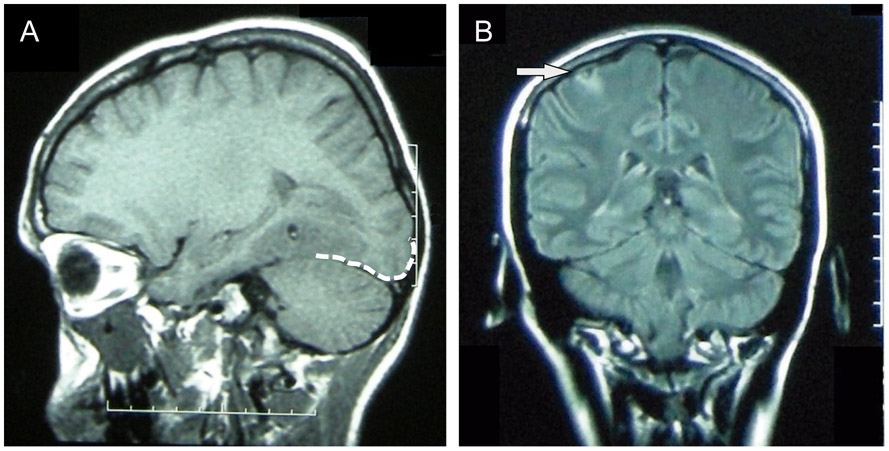
Fig. 1.
MRI images of two representative patients. (A) T1-weighted MRI of a cysticercotic viable cyst localized in a neocortical temporal area showing perilesional edema that reached the occipital lobe (approximation of the occipital lobe cortex demarcated with a white dashed line). The patient had multiple visual seizures from 60 days to seven days before the acquisition of this MRI. (B) Fluid-attenuated inversion recovery (FLAIR) MRI image showing a viable cyst in the right perirolandic area (arrow). This patient experienced a tonic seizure of the left arm and the left-sided face, as she had presented previously, during an EEG that was showing right fronto-central polyspikes at that time.
3.4. Clinical-topography relationship by NCC location
Of the 32 patients with one cysticercotic lesion, one patient had it located in a mesial temporal area, and thus 31 patients were selected to assess whether the location of cysticercotic lesions categorized according to the types of neocortices influences the clinical-topography relationship. Patients with a unique cysticercotic lesion in the primary cortex showed more often a clinical-topography relationship (10/10, 100%) than patients with a unique cysticercotic lesion in the unimodal association cortex (12/14, 86%), and these, in turn, more often than patients with heteromodal association cortex lesions (3/7 [43%]; p=0.005).
3.5. EEG
Forty-four patients (76%) had one EEG, twelve (21%) had two EEGs and two patients (3%) had three EEGs. One EEG was chosen per patient reported to show epileptiform discharges, or in its absence, abnormal slow waves only.
Electroencephalograms of nine patients (16%) showed epileptiform discharges. They were performed in a median of 14 days (IQR, 3–47) after a seizure, and all, but one, were limited to one region of the cortex. A epileptologist pointed out, in average, 2.8±1.4 brain areas for focalization. Patients with more than one EEG had higher chances to show epileptiform discharges (6/14 patients [43%] with two or three EEGs versus 3/44 [7%] with one EEG; p=0.004). All patients with epileptiform discharges showed a clinical-topography relationship between the potential seizure onset zone and a cysticercotic lesion. In seven (88%) of the eight patients with focal epileptiform discharges, the sharp-waves or spikes were topographically related to the cysticercotic lesion and the potential seizure onset zone. One patient had an ictal EEG and showed topographic concordance between semiology, focal epileptiform discharges and the cysticercotic lesion (Fig. 1B).
Twelve patients (21%, all of them with clinical-topography relationships) showed interictal focal slow-wave abnormalities only which covered, in average, 4.7±1.9 functional brain areas. Five (42%) showed a topographic relationship between the focal slow waves, the location of a cysticercotic lesion and the potential seizure onset zone. Two (17%) had focal slow waves possibly topographically related to a cysticercotic lesion because of their proximity. The remaining five patients (42%) showed focal slow waves not topographically related to a lesion. All these five patients showed polymorphic (arrhythmic) delta (0–<4 Hz) slow waves in an anterior temporal region from a EEG performed in a median of 20 days (IQR, 15–59) after a seizure.
4. DISCUSSION
This study demonstrates that seizure semiology and focal epileptiform discharges are topographically related to NCC lesions in most patients, as strong evidence that these lesions are the direct cause of seizures or epilepsy in such patients. Our findings support the epileptogenicity of NCC and the seizure origin located in the cortex surrounding a cysticercotic lesion. If this hypothesis is correct, examinations of the cortex surrounding a NCC lesion could provide valuable information on molecular, genetic and electrical mechanisms of seizures and epilepsy in humans.
Around 85% of patients showed a clinical-topography relationship between semiology and a cysticercotic lesion, and this association increased to 95% if perilesional edema is considered. Previous studies had already suggested that clinical-topography discordances could be due to seizure spread (Duque and Burneo, 2017; Murthy and Reddy, 1998; Singh et al., 2000). The definition of a potential seizure onset zone in addition to the symptomatogenic zone in our study did likely account for this effect. We presented the clinical-topography relationships among subgroups of patients and showed no difference in the proportion of patients with clinical-topography relationship among patients with recent onset or longer seizure history. Local inflammation is likely to play a role in seizures associated with NCC, particularly by the time the parasites degenerate due to APT, however the ATP does not seem to affect the clinical-topography relationship in most patients.
Brain cysticercotic cysts may survive viable for years until they are attacked by the host’s immune system and degenerate. Symptoms are more marked during cyst degeneration. In our study, the clinical-topography relationship was more frequently related to only viable cysts, despite an additional calcified cyst or even a degenerating cyst. Potential explanations for this finding may be that cysts can alter the metabolic or electrical properties of the surrounding cortex for long periods and thus establish local seizure foci, or that viable cysts at baseline might have degenerated during follow-up (after treatment) and caused seizures related to these same foci. We also found that patients with heteromodal association cortex lesions have less often a clinical-topography association. Such patients might express their initial seizure manifestations more often after seizure spread, and perhaps, the study epileptologists might have omitted some heteromodal association cortex areas less frequently related to that manifestations. Perilesional edema is a potential risk factor for seizures (Nash et al., 2015), and it was around at least in one NCC lesion at baseline neuroimaging in up to 80% of patients.
A few studies have aimed to examine the clinical-topography relationship in NCC, and showed discordances between imaging findings and electroclinical features (Cukiert et al., 1994; Murthy and Reddy, 1998; Singh et al., 2000). They assessed patients with apparently only calcifications using CT scan only, and the clinical-topography relationship was based on anatomical brain lobes. In such studies, patients with chronic calcified NCC might have developed secondary epileptogenesis in remote brain areas, like hippocampus, producing discordances between electroclinical features and NCC lesions. In fact, there has been proposed that NCC can act as an initial precipitating injury for hippocampal lesions and lead to chronic epilepsy in at least some patients with calcified NCC (Bianchin et al., 2015; Rathore et al., 2013). It might be more relevant in individuals with early age of seizure onset, and our patients with relative older age of seizure onset might miss this possibility (Bianchin et al., 2015). In addition to these differences with those studies, our assessment was based on functional brain areas, which are used in habitual clinical practice for seeking seizure origin in epilepsy units (Bonini et al., 2014; So, 2006; Stephani et al., 2011; Tufenkjian and Luders, 2012). All patients in our study had both CT and MRI as both neuroimaging were required for inclusion in the parent clinical trials. We assessed all consecutive participants of three large trials and as such, our study population is likely to be representative of individuals with both viable NCC and a history of seizures seeking medical care. The relative high proportion of patients with NCC lesions in perirolandic areas, premotor areas and occipital lobes has been identified in other hospital-based studies (Duque and Burneo, 2017; Murthy and Reddy, 1998; Thussu et al., 2008). Of note, the relationship of semiology with the cysticercotic location was not assessed in the parent trials.
We found a topographic relationship between EEG focalization, semiology and cysticercotic lesions in most patients with focal epileptiform discharges but in about half of cases with only focal slow waves, a tendency consistent with another series (Diagana et al., 2005). All EEGs were performed in similar conditions during 30 minutes. Additional EEGs (Monteiro et al., 1995) and EEGs temporally closer to a seizure (Chayasirisobhon et al., 1999; Gupta et al., 2012) have been shown to improve EEG’s sensitivity in NCC, and may have enhanced the electric-topography relationship in our patients with viable NCC. Invasive EEG explorations can accurately locate an abnormality, in contrast to scalp EEG recordings which assess regional abnormalities. As sharp-waves need synchronic discharges of large cortical areas, a precise topographic relationship cannot be defined in our study. We also found that patients with no electric-topography relationship had slow-waves in an anterior temporal region, representing cortical dysfunction. In fact, there has been proposed that NCC can lead to hippocampus damage by repetitive neuroinflammatory responses of the host’s immunity or electric-mediated injury related to seizures, in at least some patients with biological predisposition (Bianchin et al., 2017).
Seizure onset zones can only be defined using ictal EEG recordings. In line with this, our method to determine the clinical-topography relationship based on seizure descriptions and interictal EEG may not be completely accurate. In T. solium endemic regions, ictal recordings with video-EEG are limited and expensive, and the low frequency of seizures in NCC patients makes a study with such methods even less viable. Clearly, it is not possible to performed a study with ictal EEG recordings, even though it may be yearned.
Our study is retrospective and, hence, open for information biases which might have affected semiology assessments and thus our results. However, medical records of seizure descriptions were complete on all patients and this allowed setting the limits of the potential onset and symptomatogenic zones with higher inter-rater agreement. The inaccuracy of seizure semiology based on patients or eyewitnesses’ reports is generally recognized, although there is also evidence of reasonable reliability in some studies (So, 2006). A recent study showed that patients had a good recognition of their initial seizure manifestations when they were interviewed promptly (Campora and Kochen, 2016), as the included parent clinical trials carried out. Another study showed strong interobserver agreement on seizure manifestations between neurologists and seizure eyewitnesses (Benbir et al., 2013). We cannot rule out the possibility that visualization of neuroimaging exams by the time of patient’s enrolment may have influenced the study neurologist’s interpretation of seizure manifestations. In the included clinical trials, neurologists obtained seizure manifestations regardless if they observed any neuroimaging. In our study, the possibility of inaccurate reporting of a given seizure may be compensated by the large number of seizures, some of them witnessed during hospitalization.
Our study provides a systematic assessment of the relation between seizure manifestations and lesion topography in NCC in a consecutive series of patients rather than selected cases. Our findings are consistent with many case reports and case series that have demonstrated focal seizures topographically related to cysticercotic lesions (Gupta et al., 2013; Mejia and Nash, 2013; Naddaf et al., 2014). As far as we know, this is the first study to assess the topographic relationship between viable NCC and semiology and epileptiform discharges. Our results support that NCC is responsible for the seizures in most patients, in contrast to what have been raised several times (Kowacs et al., 2006; Prasad et al., 2008; Saito et al., 2016; Sakamoto et al., 1999).
5. CONCLUSION
Seizure semiology and focal epileptiform discharges are topographically related to NCC lesions in most patients. While our findings relate to the clinical-topography relationship and not the epileptogenic zone per se, that needs to be determined by electrophysiological methods, the consistent association supports the epileptogenicity of NCC lesions and the seizure origin in the cortex surrounding these lesions. A better understanding of the processes occurring in such cortex might throw light on the pathophysiology of seizures and epilepsy in human brain.
Supplementary Material
Supplementary Table B.1. Seizure manifestations of each patient with their respective and agreed potential seizure onset and symptomatogenic zones
Supplementary Fig. B.1. Phylogenetic and cytoarchitectonic subdivision of the brain cortex
HIGHLIGHTS.
Fifty-eight patients with one or two parenchymal NCC lesions and focal seizures were selected.
86-95% of patients had seizure semiology topographically related to a NCC lesion.
In patients with focal epileptiform discharges, 88% had semiology and EEG focality topographically related to a NCC lesion.
These data strongly support seizure origin in the cortex surrounding a NCC lesion.
Acknowledgments
STUDY FUNDING
The parent clinical trials were funded by the NINDS-NIH grant NS054805 and the Intramural Research Program of the NIAID-NIH. Partial support from the Fogarty International Center/NIH (training grant D43 TW001140) is also acknowledged. The funders had no role in the design and conduct of the study; in the collection, management, analysis and interpretation of data; in the preparation, writing, review or approval of the report; and in the decision to submit the article for publication.
ABBREVIATIONS
- AED
antiepileptic drug
- APT
antiparasitic treatment
- CI
confidence interval
- CT
computed tomography
- EEG
electroencephalography
- EITB
enzyme-linked inmmunoelectrotransfer blot
- FLAIR
fluid-attenuated inversion recovery
- IQR
interquartile range
- MRI
magnetic resonance imaging
- NCC
neurocysticercosis
- SD
standard deviation.
APPENDIX A. Cysticercosis Working Group in Peru
Other members of the Cysticercosis Working Group in Peru include Robert H. Gilman, MD, DTMH [Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA]; Armando E. Gonzalez, DVM, PhD [School of Veterinary Medicine, Universidad Nacional Mayor de San Marcos, Lima, Peru]; and Victor C. W. Tsang, PhD [Georgia State University, Atlanta, Georgia, USA] (Coordination Board); Silvia Rodriguez, MSc; Manuel Martinez, MD; Manuel Alvarado, MD; Miguel Porras, MD; Victor Vargas, MD; Alfredo Ccjuno, MD (Instituto Nacional de Ciencias Neurológicas, Lima, Peru); Manuela Verastegui, PhD; Mirko Zimic, PhD; Holger Mayta, PhD; Saul Santivanez, MD, PhD; Cristina Guerra, PhD; Yesenia Castillo, MSc; Yagahira Castro, MSc (Universidad Peruana Cayetano Heredia, Lima, Peru); Martin Gavidia, MD; Liliana Rodriguez, MD (Hospital Nacional Edgardo Rebagliati, Essalud, Lima, Peru); Lourdes Rodriguez, MD; Ricardo Illescas, MD; Jose J. Vera, MD (Hospital Nacional Guillermo Almenara, Essalud, Lima, Peru); Enrique Najar, MD; Martin Tipismana, MD; Hugo Umeres, MD (Hospital Nacional Cayetano Heredia, Ministerio de Salud, Lima, Peru); Maria T. Lopez, DVM, PhD; Luis Gomez, DVM; Cesar M. Gavidia, DVM, PhD (School of Veterinary Medicine, Universidad Nacional Mayor de San Marcos, Lima, Peru); Luz M. Moyano, MD, PhD; Ricardo Gamboa, MSc; Claudio Muro; Percy Vichez, MSc (Cysticercosis Elimination Program, Tumbes, Peru); Saul Santivañez, MD, PhD (Johns Hopkings Bloomberg School of Public Health, Baltimore, Maryland, USA); Theodore E. Nash, MD; Siddhartha Mahanty, MD, PhD (NIAID, NIH, Bethesda, MD); John Noh, BS; Sukwan Handali, MD (CDC, Atlanta, GA); Jon Friedland (Imperial College, London, UK).
Footnotes
Other members of the Cysticercosis Working Group in Peru are listed in Appendix A.
DECLARATIONS OF INTEREST
None.
DISCLOSURES
K.R. Duque is partially supported by FIC-NIH training grant D43TW001140. J.G. Burneo has received research funding from the Ontario Brain Institute, Epilepsy Ontario and UCB Canada, and has received honoraria for speaking engagements from UCB Canada and Eisai. J.A. Bustos is partially supported by NIH grants R01 AI116456 and FIC-NIH training grant D43TW001140. H.H. Garcia is supported by NIH grants U19AI129909, U01NS086974, R21NS094976, and FIC-NIH training grant D43TW001140. A.L. Escalaya, W. Zapata, I. Gonzales, H. Saavedra and E.J. Pretell report no disclosures.
ETHICAL STATEMENT
We confirm that we have read the Journal’s polices on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- Benarroch EE, 2008. Mayo Clinic medical neurosciences: organized by neurologic systems and levels, 5th ed. Mayo Clinic Scientific Press; Informa Healthcare, Rochester, MN. [Google Scholar]
- Benbir G, Demiray DY, Delil S, Yeni N, 2013. Interobserver variability of seizure semiology between two neurologist and caregivers. Seizure 22, 548–552. [DOI] [PubMed] [Google Scholar]
- Bianchin MM, Velasco TR, Wichert-Ana L, Araujo D Jr., Alexandre V Jr., Scornavacca F, Escorsi-Rosset SR, dos Santos AC, Carlotti CG Jr., Takayanagui OM, Sakamoto AC, 2015. Neuroimaging observations linking neurocysticercosis and mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsy Res 116, 34–39. [DOI] [PubMed] [Google Scholar]
- Bianchin MM, Velasco TR, Wichert-Ana L, Dos Santos AC, Sakamoto AC, 2017. Understanding the association of neurocysticercosis and mesial temporal lobe epilepsy and its impact on the surgical treatment of patients with drug-resistant epilepsy. Epilepsy Behav 76, 168–177. [DOI] [PubMed] [Google Scholar]
- Blume WT, Luders HO, Mizrahi E, Tassinari C, van Emde Boas W, Engel J Jr., 2001. Glossary of descriptive terminology for ictal semiology: report of the ILAE task force on classification and terminology. Epilepsia 42, 1212–1218. [DOI] [PubMed] [Google Scholar]
- Bonini F, McGonigal A, Trebuchon A, Gavaret M, Bartolomei F, Giusiano B, Chauvel P, 2014. Frontal lobe seizures: from clinical semiology to localization. Epilepsia 55, 264–277. [DOI] [PubMed] [Google Scholar]
- Campora N, Kochen S, 2016. Subjective and objective characteristics of altered consciousness during epileptic seizures. Epilepsy Behav 55, 128–132. [DOI] [PubMed] [Google Scholar]
- Chayasirisobhon S, Menoni R, Chayasirisobhon W, Locke GE, 1999. Correlation of electroencephalography and the active and inactive forms of neurocysticercosis. Clin Electroencephalogr 30, 9–11. [DOI] [PubMed] [Google Scholar]
- Cukiert A, Puglia P, Scapolan HB, Vilela MM, Marino Junior R, 1994. Congruence of the topography of intracranial calcifications and epileptic foci. Arq Neuropsiquiatr 52, 289–294. [DOI] [PubMed] [Google Scholar]
- Del Brutto OH, Nash TE, White AC Jr., Rajshekhar V, Wilkins PP, Singh G, Vasquez CM, Salgado P, Gilman RH, Garcia HH, 2017. Revised diagnostic criteria for neurocysticercosis. J Neurol Sci 372, 202–210. [DOI] [PubMed] [Google Scholar]
- Del Brutto OH, Santibanez R, Noboa CA, Aguirre R, Diaz E, Alarcon TA, 1992. Epilepsy due to neurocysticercosis: analysis of 203 patients. Neurology 42, 389–392. [DOI] [PubMed] [Google Scholar]
- Diagana M, Nsengiyumva G, Tuillas M, Druet-Cabanac M, Bouteille B, Preux PM, Tapie P, 2005. [Electroencephalograms (EEG) in 250 patients with epilepsy in a cysticercosis endemic area in Burundi]. Neurophysiol Clin 35, 1–10. [DOI] [PubMed] [Google Scholar]
- Duque KR, Burneo JG, 2017. Clinical presentation of neurocysticercosis-related epilepsy. Epilepsy Behav 76, 151–157. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, Lagae L, Moshe SL, Peltola J, Roulet Perez E, Scheffer IE, Zuberi SM, 2017. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 58, 522–530. [DOI] [PubMed] [Google Scholar]
- Garcia HH, Gonzales I, Lescano AG, Bustos JA, Pretell EJ, Saavedra H, Nash TE, Cysticercosis Working Group in Peru, 2014a. Enhanced steroid dosing reduces seizures during antiparasitic treatment for cysticercosis and early after. Epilepsia 55, 1452–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia HH, Gonzales I, Lescano AG, Bustos JA, Zimic M, Escalante D, Saavedra H, Gavidia M, Rodriguez L, Najar E, Umeres H, Pretell EJ, Cysticercosis Working Group in Peru, 2014b. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double-blind, randomised controlled trial. Lancet Infect Dis 14, 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia HH, Lescano AG, Gonzales I, Bustos JA, Pretell EJ, Horton J, Saavedra H, Gonzalez AE, Gilman RH, Cysticercosis Working Group in Peru, 2016. Cysticidal Efficacy of Combined Treatment With Praziquantel and Albendazole for Parenchymal Brain Cysticercosis. Clin Infect Dis 62, 1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia HH, Nash TE, Del Brutto OH, 2014c. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol 13, 1202–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski TJ Jr., Anderson SW, Cooper GE, 2002. Disorders of cognitive function. Continuum 8, 7–40. [Google Scholar]
- Gupta RK, Awasthi R, Garg RK, Kumar N, Gupta PK, Singh AK, Sahoo P, Paliwal VK, Prasad KN, Pandey CM, Rathore RK, 2013. T1-weighted dynamic contrast-enhanced MR evaluation of different stages of neurocysticercosis and its relationship with serum MMP-9 expression. AJNR Am J Neuroradiol 34, 997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Awasthi R, Rathore RK, Verma A, Sahoo P, Paliwal VK, Prasad KN, Pandey CM, Narayana PA, 2012. Understanding epileptogenesis in calcified neurocysticercosis with perfusion MRI. Neurology 78, 618–625. [DOI] [PubMed] [Google Scholar]
- Kowacs PA, Rogacheski E, Muzzio J, Werneck LC, 2006. The role of the irritative zone and of the number and distribution of calcifications in the severity of epilepsy associated with intracranial calcifications. Arq Neuropsiquiatr 64, 905–911. [DOI] [PubMed] [Google Scholar]
- Luders H, Acharya J, Baumgartner C, Benbadis S, Bleasel A, Burgess R, Dinner DS, Ebner A, Foldvary N, Geller E, Hamer H, Holthausen H, Kotagal P, Morris H, Meencke HJ, Noachtar S, Rosenow F, Sakamoto A, Steinhoff BJ, Tuxhorn I, Wyllie E, 1998. Semiological seizure classification. Epilepsia 39, 1006–1013. [DOI] [PubMed] [Google Scholar]
- Luders HO, 2008. Texbook of epilepsy surgery. Informa Healthcare, London. [Google Scholar]
- Mejia R, Nash TE, 2013. Corticosteroid withdrawal precipitates perilesional edema around calcified Taenia solium cysts. Am J Trop Med Hyg 89, 919–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano SM, Villaran MV, Ylquimiche L, Figueroa JJ, Rodriguez S, Bautista CT, Gonzalez AE, Tsang VC, Gilman RH, Garcia HH, Cysticercosis Working Group in, P., 2005. Neurocysticercosis: association between seizures, serology, and brain CT in rural Peru. Neurology 65, 229–233. [DOI] [PubMed] [Google Scholar]
- Monteiro L, Nunes B, Mendonca D, Lopes J, 1995. Spectrum of epilepsy in neurocysticercosis: a long-term follow-up of 143 patients. Acta Neurol Scand 92, 33–40. [DOI] [PubMed] [Google Scholar]
- Moyano LM, O’Neal SE, Ayvar V, Gonzalvez G, Gamboa R, Vilchez P, Rodriguez S, Reistetter J, Tsang VC, Gilman RH, Gonzalez AE, Garcia HH, Cysticercosis Working Group in, P., 2016. High Prevalence of Asymptomatic Neurocysticercosis in an Endemic Rural Community in Peru. PLoS Negl Trop Dis 10, e0005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy JM, Reddy VS, 1998. Clinical characteristics, seizure spread patterns and prognosis of seizures associated with a single small cerebral calcific CT lesion. Seizure 7, 153–157. [DOI] [PubMed] [Google Scholar]
- Naddaf E, Seeger SK, Stafstrom CE, 2014. Neurocysticercosis in Wisconsin: 3 cases and a review of the literature. WMJ 113, 74–78; quiz 79. [PubMed] [Google Scholar]
- Nash TE, Mahanty S, Loeb JA, Theodore WH, Friedman A, Sander JW, Singh G, Cavalheiro E, Del Brutto OH, Takayanagui OM, Fleury A, Verastegui M, Preux PM, Montano S, Pretell EJ, White AC Jr., Gonzales AE, Gilman RH, Garcia HH, 2015. Neurocysticercosis: A natural human model of epileptogenesis. Epilepsia 56, 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A, Gupta RK, Pradhan S, Tripathi M, Pandey CM, Prasad KN, 2008. What triggers seizures in neurocysticercosis? A MRI-based study in pig farming community from a district of North India. Parasitol Int 57, 166–171. [DOI] [PubMed] [Google Scholar]
- Prasad KN, Verma A, Srivastava S, Gupta RK, Pandey CM, Paliwal VK, 2011. An epidemiological study of asymptomatic neurocysticercosis in a pig farming community in northern India. Trans R Soc Trop Med Hyg 105, 531–536. [DOI] [PubMed] [Google Scholar]
- Rathore C, Thomas B, Kesavadas C, Abraham M, Radhakrishnan K, 2013. Calcified neurocysticercosis lesions and antiepileptic drug-resistant epilepsy: a surgically remediable syndrome? Epilepsia 54, 1815–1822. [DOI] [PubMed] [Google Scholar]
- Rosenow F, Luders H, 2001. Presurgical evaluation of epilepsy. Brain 124, 1683–1700. [DOI] [PubMed] [Google Scholar]
- Saito EK, Nagpal M, Leon A, Mehta B, McMurtray AM, 2016. Topographic congruence of calcified parenchymal neurocysticercosis and other structural brain lesions with epileptiform activity. Trop Parasitol 6, 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto A, Bustamante VC, Garzon E, Takayanagui OM, Santos A, Fernandes RM, Leite JP, Chimelli LM, Assirati JA Jr., 1999. Cysticercosis and epilepsy, in: Kotagal P, Luüders H (Eds.), The epilepsies: etiologies and prevention. Academic Press, San Diego, pp. 275–282. [Google Scholar]
- Singh G, Burneo JG, Sander JW, 2013. From seizures to epilepsy and its substrates: neurocysticercosis. Epilepsia 54, 783–792. [DOI] [PubMed] [Google Scholar]
- Singh G, Sachdev MS, Tirath A, Gupta AK, Avasthi G, 2000. Focal cortical-subcortical calcifications (FCSCs) and epilepsy in the Indian subcontinent. Epilepsia 41, 718–726. [DOI] [PubMed] [Google Scholar]
- So EL, 2006. Value and limitations of seizure semiology in localizing seizure onset. J Clin Neurophysiol 23, 353–357. [DOI] [PubMed] [Google Scholar]
- Stephani C, Fernandez-Baca Vaca G, Maciunas R, Koubeissi M, Luders HO, 2011. Functional neuroanatomy of the insular lobe. Brain Struct Funct 216, 137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thussu A, Chattopadhyay A, Sawhney IM, Khandelwal N, 2008. Albendazole therapy for single small enhancing CT lesions (SSECTL) in the brain in epilepsy. J Neurol Neurosurg Psychiatry 79, 272–275. [DOI] [PubMed] [Google Scholar]
- Tufenkjian K, Luders HO, 2012. Seizure semiology: its value and limitations in localizing the epileptogenic zone. J Clin Neurol 8, 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table B.1. Seizure manifestations of each patient with their respective and agreed potential seizure onset and symptomatogenic zones
Supplementary Fig. B.1. Phylogenetic and cytoarchitectonic subdivision of the brain cortex