Abstract
Objective:
To estimate the incidence of, determine risk factors for, and the consequences of opportunistic infections (OIs) and malignancies among patients with the acquired immune deficiency syndrome (AIDS) in the era of modern combination antiretroviral therapy (cART).
Design&Methods:
Comparison of three enrollment periods (1998–2002, 2003–2005, and 2006–2012), corresponding to changes in predominant cART regimens, among 1889 participants enrolled in a prospective cohort study, the Longitudinal Study of Ocular Complications of AIDS (LSOCA). Incidence of AIDS-related opportunistic infections (OIs) and cancers were estimated. Multivariate logistic and Cox regression models determined the effect of demographic and clinical characteristics on OIs and mortality.
Results:
Between participants enrolled in the 1998–2002 and 2006–2012 enrollment periods, the incidence of OIs decreased from 27/1000 person-years (PY) to 11/1000 PY (P=0.001), and mortality decreased from 41/1000 PY to 18/1000 PY (P< 0.0001) corresponding to improvements in cART regimens.
Conclusions:
Improvements in cART regimens led to a progressive decline in the incidence of OIs and mortality between 1999 and 2013 among patients with AIDS in the era of modern cART.
Keywords: HIV, AIDS, opportunistic infection, AIDS-related cancer, mortality
Summary:
The incidence of AIDS-defining opportunistic infections (OIs) and cancers declined between 1999 and 2013 among patients with AIDS. Low CD4+ T-cell count and high HIV load remain risk factors for OIs and mortality in modern cART era.
INTRODUCTION
With the advent of modern combination antiretroviral therapy (cART) in the mid-1990s, the incidences of human immunodeficiency virus (HIV)-associated opportunistic infections (OIs), cancers, and mortality have decreased substantially [1–15]. Despite the decline in HIV-associated OIs and cancers over the last two decades, their incidences never reached that of people without HIV, and they remain a leading cause of mortality and morbidity [9, 16–25].
Because recommendations for cART are to start it at CD4+ T-cell levels well above those which would diagnose the acquired immunodeficiency syndrome (AIDS) and prior those increasing the risk for OIs, most studies demonstrating the benefits of cART on OIs involve cohorts of patients including patients with earlier stages of HIV infection. Among those at risk for OIs, prophylactic antimicrobial therapy (such as against Pneumocystis jirovicii pneumonia [PJP] and Mycobacterium avium complex [MAC]) also have been effective in decreasing the incidence of OIs [5, 7, 8]. In the modern cART era, OIs still occur, albeit at a substantially reduced rate, and are associated with lower CD4+ T-cell counts and higher amounts of circulating HIV RNA in the blood (HIV load) [20, 26, 27]. Because most of the studies demonstrating the benefit of cART on the incidence of OIs come from cohorts with earlier stages of HIV, there is few data on secular trends in the incidence of OIs among patients with the late-stage of HIV infection, namely AIDS [28–31]. However, AIDS continues to occur, largely due to late diagnosis of HIV infection, but also due to failure in some patients to suppress HIV replication with cART [15, 32–34].
The Longitudinal Study of the Ocular Complications of AIDS (LSOCA) is a 15-year prospective cohort study conducted in the era of modern cART, unique in that, it only enrolls patients with AIDS, and with a wide range of immune function from diverse HIV risk groups. As such, it provides a unique opportunity to evaluate the effect of cART and changes in cART regimens on the incidence of AIDS-related OIs, cancers, and mortality among patients with the late-stage HIV infection, namely AIDS.
PATIENTS and METHODS
The Longitudinal Study of the Ocular Complications of AIDS is a prospective observational study of patients with AIDS conducted in the era of modern cART [35, 36]. Patients age ≥13 years with a diagnosis of AIDS according to the 1993 definition of the Centers for Disease Control and Prevention (CDC) case surveillance were enrolled between dates September 1, 1998 and December 31, 2011 at 19 centers across the United States. Only patients without an ocular OI (namely CMV-Retinitis) are included in this analysis to avoid enrollment bias. After the initial recruitment period, rolling recruitment was used to provide ongoing information on changes in the AIDS epidemic. Demographic information and a detailed medical history, including all OIs and current and previous ART, were obtained at enrollment and confirmed by record review as appropriate. A limited medical and a complete ophthalmic examination were performed [35, 37]. Enrollment laboratory testing included a complete blood count, serum chemistries, CD4+ T-cell counts and HIV loads. The diagnosis of opportunistic infections was made according to the AIDS Clinical Trials Group guidelines [31], information was collected on OIs and AIDS-related cancers; and for the purposes of this analysis, OIs and cancers were those considered AIDS-defining based on CDC revised 1993 AIDS case surveillance definition and the 5 December 2008 CDC OI reporting guidelines (http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5710a2.htm) [38, 39]. Participants were seen every six months in follow-up. The study and the protocol were approved by institutional review boards at all participating centers; enrolled participants provided written informed consent; and the study and procedures adhered to the Declarations of Helsinki.
Combination ART was defined as any of the following: any three antiretrovirals, one of which was either a protease inhibitor, a non-nucleoside reverse transcriptase inhibitor (NNRTI) or a fusion, integrase, or entry inhibitor; any three nucleoside reverse transcriptase inhibitors, one of which was abacavir or tenofovir (except for the regimens abacavir/tenofovir/lamivudine and didanosine/tenofovir /lamivudine); two full-dose protease inhibitors; a boosted protease inhibitor with either an NNRTI or a fusion inhibitor; or, an integrase inhibitor combined with either a protease inhibitor, NNRTI, entry inhibitor, or fusion inhibitor. If zidovudine and stavudine were present in the same regimen, they were removed from that regimen’s total antiretroviral count due to their known antagonism [28].
In order to assess secular changes in the incidence of OIs and malignancies, participants were grouped into three recruitment periods: 1998–2002; 2003–2005; and 2006–2012. These enrollment periods were selected to coincide with changes in the predominant cART regimen being used (Supplemental Table 1). Because of the initial bolus of recruitment, the initial recruitment period contained slightly over 60% of the participants.
Patient data collected and reported to the Coordinating Center as of 31 December 2012 were included in the analyses. Mortality analyzed throughout the study represents all-cause mortality. Follow-up time was calculated as the time from study entry to first incidence, to death, or to 31 December 2012, for patients under active follow-up or to the date of the last study contact for patients who were lost to follow-up. Mortality and incidence rates were calculated as the number of deaths and number of AIDS defining conditions divided by the number of person years at risk. Relative risks were estimated with Cox proportional hazards. Survival analyses were performed with staggered entries based on time since diagnosis of AIDS. Analyses were performed with SAS/STAT® version 9.3 (Copyright© 2002–2010. SAS Institute, Inc., Cary, NC) and Stata version 12.0 (StataCorp 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP) software packages.
RESULTS
Characteristics of the Study Population
Enrollment characteristics of the three enrollment cohorts of LSOCA are shown as Table 1. Of the 1889 participants, 1180 were enrolled in the first cohort, 329 in the second, and 380 in the third. Consistent with changes in the AIDS epidemic, there was a decrease in the proportion of participants who were white and whose HIV transmission category was male to male sexual contact after the first enrollment period. There also was an increase in the proportion of participants whose AIDS-defining condition was CD4+ T cell lymphopenia, as opposed to an OI, in the third recruitment period vs. the first and second recruitment periods (74% vs. 64 and 62%, P=0.001). Participants in the third recruitment period had higher enrollment CD4+ T cells vs. the first two recruit periods (median 282 vs. 174 and 197 cells/μL, P<0.0001) and lower HIV loads (median log10(copies/mL) 2.0 vs. 3.3 and 2.6, P<0.0001). Although there was an apparent increase in cART use prior to enrollment with each successive recruitment period (76% vs. 83% vs. 94%, P<0.0001), there was no significant difference in the use of cART during follow-up among the three groups, and overall 97% of participants received cART during follow-up.
Table 1.
Patient characteristics at enrollment in the Longitudinal Study of the Ocular Complications of AIDS cohort
| Enrollment Cohort | |||||
|---|---|---|---|---|---|
| 1998–2002 | 2003–2005 | 2006–2012 | Total | P-valuea | |
| Number patients | 1180 | 329 | 380 | 1889 | |
| Median age, years (25th, 75th percentile) | 42 (37,47) | 44 (40,51) | 46 (40, 52) | 43 (38, 49) | < 0.0001 |
| Male (%) | 81 | 77 | 80 | 80 | 0.36 |
| Race (%) | < 0.0001 | ||||
| White | 50 | 34 | 39 | 45 | |
| African American | 33 | 45 | 48 | 38 | |
| Other | 17 | 20 | 13 | 17 | |
| HIV transmission category (%) | 0.02 | ||||
| Male to male sexual contact | 57 | 48 | 51 | 54 | |
| Injection drug useb | 12 | 18 | 16 | 14 | |
| Other | 31 | 34 | 33 | 32 | |
| Any insurance (%) | 82 | 89 | 84 | 83 | 0.08 |
| Median time since AIDS diagnosis, years (25th, 75th percentile) | 4.0 (1.6,6.4) | 5.5 (1.7,8.7) | 4.7 (1.1, 8.2) | 4.3 (1.6, 7.2) | < 0.0001 |
| AIDS diagnosis category (%) | 0.001 | ||||
| CD4+ T-cell lymphopenia | 64 | 62 | 74 | 65 | |
| Opportunistic infection or malignancy | 32 | 34 | 23 | 31 | |
| Enrollment CD4+ T-cells (cells/uL) | |||||
| Median(25th, 75th percentile) | 174 (66,325) | 197 (104,380) | 282 (124,427) | 197 (80,358) | < 0.0001 |
| Percent participants <200 | 55 | 50 | 37 | 51 | <0.0001 |
| Percent participants 200–500 | 34 | 35 | 44 | 36 | |
| Percent participants >500 | 11 | 14 | 19 | 13 | |
| Nadir CD4+ T-cells (cells/uL) | |||||
| Median (25th, 75th percentile) | 43 (13,112) | 44 (14,101) | 50 (16,133) | 44 (14,115) | 0.29 |
| Percent participants <50 | 53 | 53 | 50 | 53 | 0.24 |
| Percent participants ≥50 | 47 | 47 | 40 | 47 | |
| Enrollment HIV load (log10[copies/mL]) | |||||
| Median (25th, 75th percentile) | 3.3 (2.4,4.8) | 2.6 (1.9,4.4) | 2.0 (1.7,2.7) | 2.7 (1.9,4.6) | < 0.0001 |
| Percent participants <2.6 | 27 | 42 | 63 | 37 | < 0.0001 |
| Percent participants 2.6–5 | 32 | 29 | 20 | 29 | |
| Percent participants ≥ 5 | 40 | 29 | 17 | 33 | |
| Maximum prior HIV load (log10copies/ml) | |||||
| Median (25th, 75th percentile) | 5.3 (4.7,5.7) | 5.3 (4.7,5.8) | 5.3 (4.8,5.7) | 5.3 (4.7,5.7) | 0.63 |
| Percent participants <5 | 11 | 11 | 10 | 11 | 0.38 |
| Percent participants ≥5 | 89 | 89 | 90 | 90 | |
| Antiretroviral therapy (%) | |||||
| Any ART prior to enrollmentc | 76 | 83 | 94 | 81 | < 0.0001 |
| Receiving cART at enrollmentc | 82 | 88 | 92 | 85 | < 0.0001 |
| Any cART during follow-upc | 96 | 96 | 97 | 97 | 0.14 |
P-values comparing three cohorts.
Injection drug use category includes persons with any injection drug use.
ART = antiretroviral therapy. cART = combination antiretroviral therapy (see Methods).
Incidence of Opportunistic Infections and AIDS-associated Malignancies
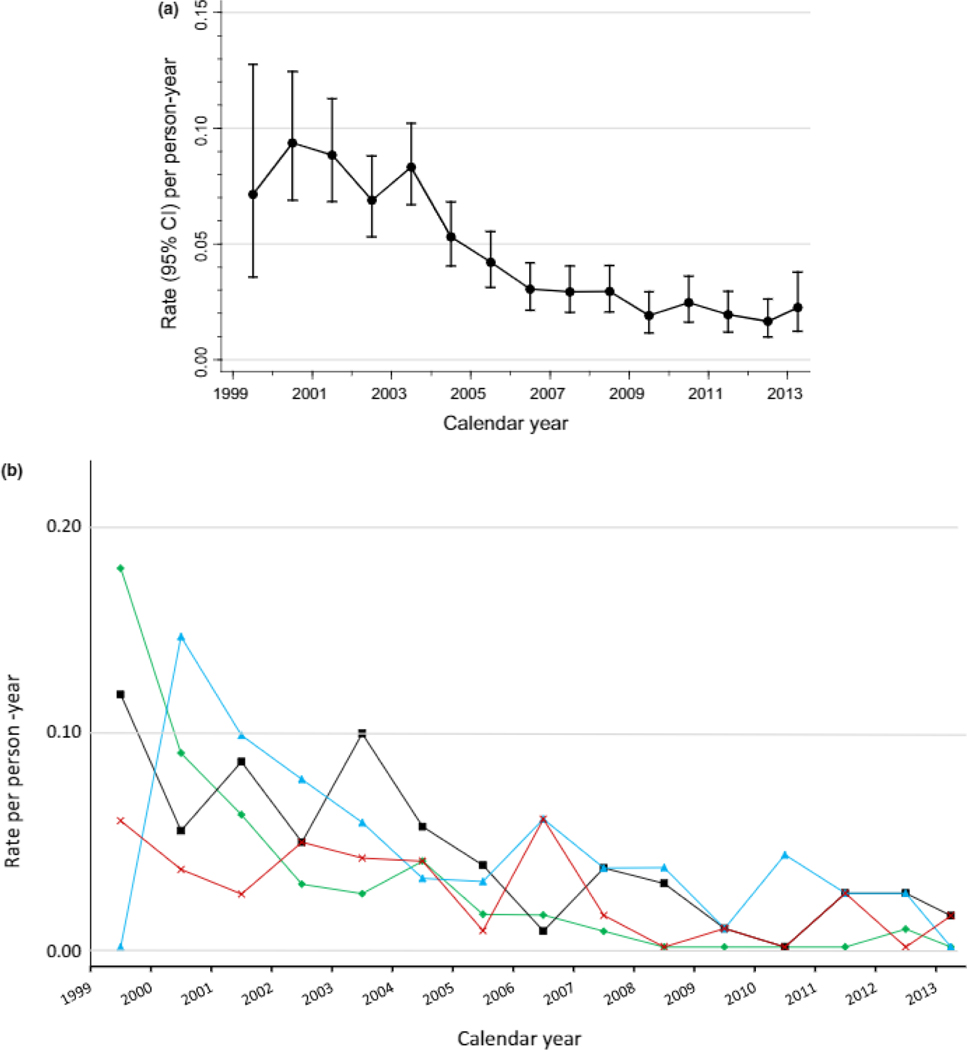
There were 135 incident OI events during 13,689 person-years of observation (20/1000 PY). The incidence of specific OIs by enrollment cohort is shown as Supplemental Table 2 and was lower for participants enrolled in the third enrollment period than in the first two (11/1000 PY vs. 27 and 23/1000 PY, P=0.001). When analyzed by calendar year, the incidence of total OIs decreased from 1999 to 2013 (P < 0.0001) but never reached zero (Figure 1A). The incidences of the four most common OIs (Figure 1B) decreased from 1999 to 2007 (P = 0.001) and then remained constant till 2013 without reaching zero.
Fig. 1.
Incidence of (a) total and (b) selected most common AIDS-defining opportunistic infections by calendar year. CI, confidence interval
There were 45 incident AIDS-associated malignancies during 13,689 person-years of follow-up (incidence 3.7/1000 PY). The cancer incidence rate decreased by successive enrollment cohorts (Table 2 and Supplemental Table 2). Kaposi sarcoma and lymphomas rates were similar with a slight decline in the last cohort, and both had similarly low rates throughout.
Table 2.
Association of clinical characteristics at enrollment with incidence of AIDS defining opportunistic infections and cancers
| Any OI Incidence (n=135) | Any Cancer Incidence (n=45) | |||
|---|---|---|---|---|
| RRb | Pb | RRb | Pb | |
| Enrollment cohort | ||||
| 1998–2002 | Ref | Ref | ||
| 2003–2005 | 0.83 | 0.48 | 0.84 | 0.69 |
| 2006–2012 | 0.37 | 0.01 | 0.58 | 0.31 |
| AIDS diagnosis category | ||||
| CD4+ T-cell lymphopenia | Ref | Ref | ||
| Opportunistic infection or malignancy | 0.85 | 0.48 | 1.36 | 0.37 |
| Race | ||||
| White | Ref | Ref | ||
| Black | 1.65 | 0.008 | 0.66 | 0.22 |
| Other | 0.68 | 0.22 | 0.84 | 0.69 |
| Sex | ||||
| Male | Ref | Ref | ||
| Female | 1.22 | 0.37 | 0.99 | 0.97 |
| Enrollment median age | ||||
| ≥43 | Ref | Ref | ||
| <43 | 1.35 | 0.09 | 0.98 | 0.94 |
| HIV transmission category Male to male sexual contact | Ref | Ref | ||
| Injection drug usec | 0.86 | 0.62 | 0.57 | 0.29 |
| Other | 1.06 | 0.79 | 0.58 | 0.13 |
| Enrollment CD4+T-Cells (cells/uL) | ||||
| ≥500 | Ref | Ref | ||
| 200–500 | 1.12 | 0.78 | 1.56 | 0.56 |
| <200 | 3.31 | 0.001 | 3.59 | 0.08 |
| Nadir CD4+T-Cells (cells/uL) | ||||
| ≥50 | Ref | Ref | ||
| <50 | 1.71 | 0.003 | 0.89 | 0.72 |
| Enrollment HIV load (log10[copies/ml]) | ||||
| < 2.6 | Ref | Ref | ||
| 2.6–5 | 1.78 | 0.04 | 3.06 | 0.02 |
| ≥ 5 | 4.74 | <.0001 | 4.15 | 0.002 |
| Maximum prior HIV load (log10[copies/ml]) | ||||
| <5 | Ref | Ref | ||
| ≥ 5 | 2.27 | 0.01 | 2.04 | 0.24 |
| Enrollment ARTd | ||||
| No ART | Ref | Ref | ||
| ART (no cART) | 0.70 | 0.32 | 0.57 | 0.34 |
| cART | 0.37 | <.0001 | 0.31 | 0.002 |
Likelihood ratio Chi square P values.
Relative Risk. Cox models with staggered entries based on time since diagnosis of AIDS
Injection drug use category includes persons with any injection drug use.
No ART is no anti-retroviral usage reported at study entry; ART is any anti-retroviral treatment that is not considered cART; cART regimen is at least 2 different ART in combination that qualifies modern cART classifications (see Methods for details).
Risk factors for incident OIs and AIDS-related malignancies are shown in Table 2. Being in the first enrollment cohort, black race, enrollment CD4+ T cells <200 cells/μL, lower nadir CD4+ T cells prior to enrollment, higher HIV load at enrollment, higher maximum HIV load prior to enrollment, and not being on cART therapy were associated with increased risk for OIs (Table 2). Risk factors associated with an increased incidence of AIDS related cancers included enrollment CD4+ T cells <200 cells/μL, higher HIV load at enrollment, and not being on any anti-retroviral therapy (Table 2).
The median (25%,75%) CD4+ T-Cell count was 100 (23,232) at the first follow-up visit reporting an AIDS defining illness (any OI or cancer) and 29% of the OI incidences were observed among patients with CD4+ T-cell count higher than 200 (cells/μL). The median HIV load (log10(copies/mL)) was 4.0 (2.3,5.1) at the first follow-up visit reporting an AIDS defining illness and 14% of the OI incidences were observed among patients with HIV loads < 400 copies/mL.
Mortality
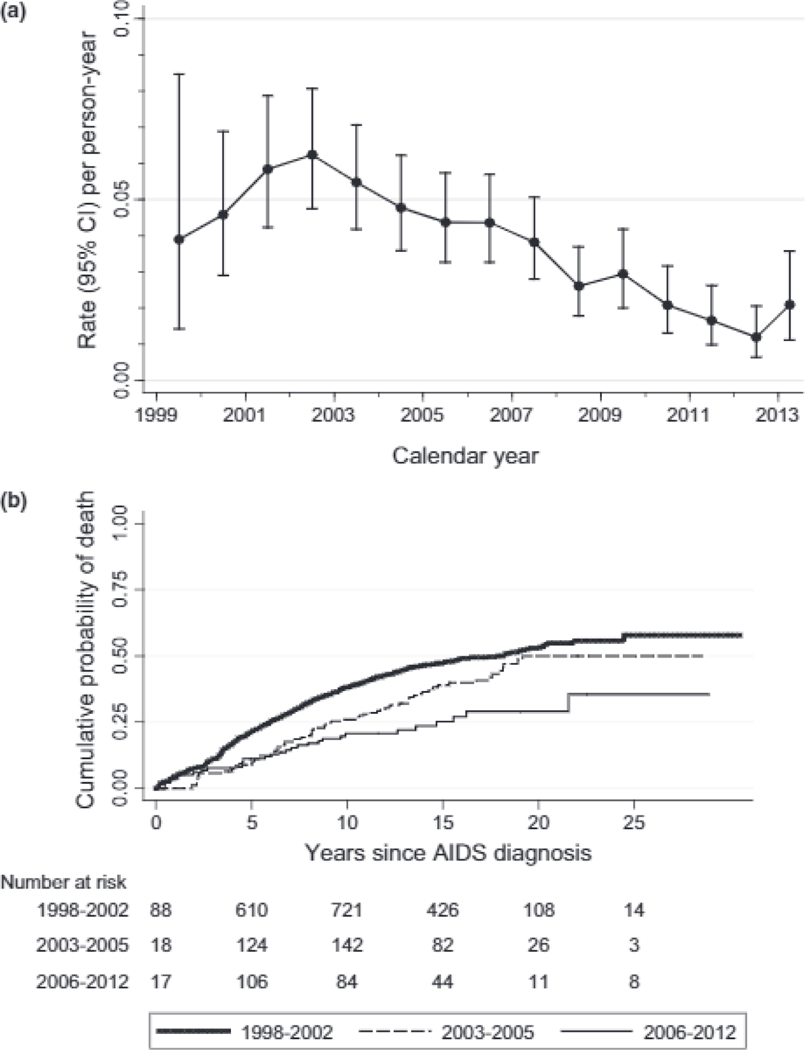
The overall mortality during follow-up was 37/1000 PY. The mortality rate decreased from 2002 to 2013 (Figure 2A; regression P<0.0001). There was a significant decrease in mortality in the third enrollment cohort vs. the first two (18/1000 PY vs. 34/1000 PY and 41/1000 PY, respectively, Figure 2B). The relative risk (RR) for mortality comparing the first enrollment cohorts vs. the third was 0.46 (P=0.0001). Enrollment risk factors for mortality are shown in Table 3. In addition to enrollment cohort, presence of any AIDS-related malignancy at enrollment (RR=1.62, P=0.007) was associated with and increased mortality
Fig. 2.
Incidence of any mortality by calendar year (a) and mortality comparison among enrolment cohorts (b). CI, confidence interval.
Table 3.
Association of cohort, AIDS diagnosis category and AIDS defining illnesses at enrollment with mortality.
| Ratea | No.Deaths/No. at risk | RRb | P | |
|---|---|---|---|---|
| Overall | 37 | 508/1889 | — | — |
| Enrollment cohort | ||||
| 1998–2002 | 41 | 409/1180 | 1.00 | — |
| 2003–2005 | 34 | 72/329 | 0.84 | 0.18 |
| 2006–2012 | 18 | 27/380 | 0.46 | 0.0001 |
| AIDS diagnosis category | ||||
| Opportunistic infection or malignancy | 40 | 188/654 | 1.06 | 0.60 |
| CD4+ T-cell lymphopenia | 36 | 320/1235 | 1.00 | — |
| Opportunistic Infectionsc | ||||
| Any opportunistic infection | 39 | 244/862 | 0.99 | 0.98 |
| No opportunistic infection | 36 | 264/1027 | 1.00 | — |
| Any viral infection | 49 | 2/7 | 2.98 | 0.13 |
| No viral infection | 37 | 506/1882 | 1.00 | — |
| Any parasitic infection | 32 | 19/78 | 1.06 | 0.80 |
| No parasitic infection | 37 | 489/1811 | 1.00 | — |
| Any fungal infection | 39 | 213/746 | 0.99 | 0.96 |
| No fungal infection | 36 | 295/1143 | 1.00 | — |
| Any mycobacterial infection | 44 | 50/158 | 1.02 | 0.92 |
| No mycobacterial infection | 37 | 458/1731 | 1.00 | — |
| Any cancerc | 44 | 43/133 | 1.62 | 0.007 |
| No cancer | 36 | 465/1756 | 1.00 | — |
Incidence per 1000 person years.
Cox models with staggered entries based on time since diagnosis of AIDS. Models adjusted for age, sex, race, HIV transmissin category, nadir CD4+ T-cell count, baseline CD4+ T-cell count, baseline and highest recorded HIV load, and cART.
AIDS defining opportunistic infectins and cancers.
DISCUSSION
The LSOCA cohort is unique in that it enrolled only patients with AIDS, with a wide range of immune function, and was not HIV transmission category restricted [35]. As such it is uniquely positioned to evaluate the impact of cART on AIDS-related OIs, cancers and mortality among patients with late-stage HIV infection, whereas most other cohorts evaluate these outcomes among patients including earlier stages of HIV infection. Our data demonstrate a secular decline in the incidence of OIs and mortality in a cohort of patients with AIDS over the time period 1998–2013 with the largest declines in OIs occurring before 2007. Previous incidence studies of OIs in HIV-infected cohorts (i.e. not restricted to AIDS at enrollment) reported a sharp decline in the 1992–1997 period followed by a more gradual decline in 1998–2002 and low-level stabilized incidences in the 2003–2007 period [7, 18, 20]. Despite the improvements in OI incidence and immune recovery, low-level stabilized incidences of opportunistic illnesses are still evident in this cohort of patients with AIDS in the 2007–2013 calendar period. The most prevalent OIs in LSOCA were the most common OIs seen in the pre-cART era, and they continue to be the most frequently diagnosed in the modern cART era [5, 6, 15, 40, 41]. Because LSOCA enrolled only patients with AIDS, these data uniquely address the effect of cART on late-stage HIV infection. Indeed, the median nadir CD4+ T cell count prior to enrollment of 44 cells/μL indicates that the LSOCA cohort experienced profound levels of immune compromise, and that the benefits of modern cART and the secular trends in the modern cART era are present even among patients with a history of severe immune compromise. Risk factors for opportunistic infections and mortality were those expected: lower CD4+ T cells and higher HIV load.
Patients in the third cohort were more likely to be diagnosed with AIDS based on CD4+T-cell lymphopenia rather than based on an OI or cancer, which might in part explain the lower mortality incidence in the third cohort. However, AIDS diagnosis category did not have a significant effect on mortality, therefore it is unlikely to lead to a survivor bias in the last cohort. Times since AIDS diagnosis to study enrollment was significantly different among the cohorts. To avoid potential survival bias, we used a staggered entry approach anchoring survival analysis to the AIDS diagnosis date for each patient.
Although the LSOCA cohort enrolled only patients with AIDS, it was not HIV transmission category restricted [35]. Previous analyses suggested that the LSOCA cohort is relatively representative of the AIDS epidemic with the exception of a slight under-representation of persons whose HIV transmission category is injection drug use [35]. Therefore, LSOCA is reasonably generalizable to patients with AIDS but not to earlier stages of HIV infection. However, because of late diagnosis of HIV and difficulties controlling HIV replication in some patients despite good follow-up, progression to AIDS does occur [24, 32, 33], so that information on late-stage HIV disease is important, and the LSOCA results uniquely address these patients.
There are limitations to the study. As the outcome of ocular infections (primarily CMV retinitis) was a primary aim of LSOCA, the original cohort oversampled patients with CMV retinitis. However, this analysis focused only on participants without CMV retinitis minimizing the recruitment bias. As such, the findings should be generalizable to AIDS epidemic in the industrialized countries. The slight under-sampling of injection drug users does not appear to limit the generalizability of the study as injection drug use was not a major risk factor for the outcomes of interest, and there were sufficient numbers of injection drug users for subgroup analyses. Nevertheless, caution should be taken in generalizing LSOCA results.
Studies have reported improvements in HIV treatment between 1996–2010, during which cART became less toxic, with increased efficacy and higher adherence rates leading to significant decreases in HIV RNA levels [42, 43]. Our results agree with these reports that focused on the earlier stages of HIV infection, and moreover, show that the efficacy of cART regimens are getting better with time leading to significant improvements in patient immune health and mortality even among patients with AIDS and history of severe immune compromise.
In conclusion, this analysis of LSOCA data demonstrates a substantial decline in per-calendar-year rates for opportunistic infections among patients with AIDS, with the majority of the decline occurring between 2000 and 2009. All-cause mortality per calendar year showed a steady decline between 2002 and 2013 corresponding to a 44% overall reduction in mortality rate and a 64% reduction in mortality relative risk by the 2006–2012 enrollment cohort. These results demonstrate ongoing improvements in the outcomes among patients with late-stage HIV disease.
Supplementary Material
Acknowledgements:
We would like to thank Milana R. Isaacson, Kevin P. May and Alka Ahuja for their help in data collection and analysis. The LSOCA participating centers credit roster is presented as a Supplemental material.
Grant Support: This work was supported by cooperative agreements from the National Eye Institute, the National Institutes of Health, Bethesda, MD to the Icahn School of Medicine at Mount Sinai, New York, NY (U10 EY 08052); The Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD (U10 EY 08057); and the University of Wisconsin, Madison School of Medicine, Madison, WI (U10 EY 08067); Efe Sezgin is supported in part by Johns Hopkins Center for AIDS Research grant (1P30AI094189) from the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, Bethesda, MD, USA; and The Scientific and Technological Research Council of Turkey grant no 116C090.
Footnotes
Potential conflict of interest: Authors have no conflict of interest to declare.
References
- 1.Crum-Cianflone NF, Hullsiek KH, Marconi V, Weintrob A, Ganesan A, Barthel RV, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: authors’ reply. AIDS 2009; 23:1791–1792. [DOI] [PubMed] [Google Scholar]
- 2.Detels R, Tarwater P, Phair JP, Margolick J, Riddler SA, Munoz A, et al. Effectiveness of potent antiretroviral therapies on the incidence of opportunistic infections before and after AIDS diagnosis. AIDS 2001; 15:347–355. [DOI] [PubMed] [Google Scholar]
- 3.Gona P, Van Dyke RB, Williams PL, Dankner WM, Chernoff MC, Nachman SA, et al. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. J Am Med Assoc 2006; 296:292–300. [DOI] [PubMed] [Google Scholar]
- 4.Hessol NA, Kalinowski A, Benning L, Mullen J, Young M, Palella F, et al. Mortality among participants in the Multicenter AIDS Cohort Study and the Women’s Interagency HIV Study. Clin Infect Dis 2007; 44:287–294. [DOI] [PubMed] [Google Scholar]
- 5.Jones JL, Hanson DL, Dworkin MS, Alderton DL, Fleming PL, Kaplan JE, et al. Surveillance for AIDS-defining opportunistic illnesses, 1992–1997. Morb Mortal Wkly Surveill Summ 1999; 48:1–22. [PubMed] [Google Scholar]
- 6.Jones JL, Hanson DL, Dworkin MS, Ward JW, Jaffe HW. Effect of antiretroviral therapy on recent trends in selected cancers among HIV-infected persons. Adult/Adolescent Spectrum of HIV Disease Project Group. J Acquir Immune Defic Syndr 1999; 21 (Suppl 1):S11–17. [PubMed] [Google Scholar]
- 7.Kaplan JE, Hanson D, Dworkin MS, Frederick T, Bertolli J, Lindegren ML, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis 2000; 30 (Suppl 1):S5–14. [DOI] [PubMed] [Google Scholar]
- 8.McNaghten AD, Hanson DL, Jones JL, Dworkin MS, Ward JW. Effects of antiretroviral therapy and opportunistic illness primary chemoprophylaxis on survival after AIDS diagnosis. Adult/Adolescent Spectrum of Disease Group. AIDS 1999; 13:1687–1695. [DOI] [PubMed] [Google Scholar]
- 9.Palella FJ Jr., Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006; 43:27–34. [DOI] [PubMed] [Google Scholar]
- 10.Ledergerber B, Egger M, Erard V, Weber R, Hirschel B, Furrer H, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss HIV Cohort Study. J Am Med Assoc 1999; 282:2220–2226. [DOI] [PubMed] [Google Scholar]
- 11.Long JL, Engels EA, Moore RD, Gebo KA. Incidence and outcomes of malignancy in the HAART era in an urban cohort of HIV-infected individuals. AIDS 2008; 22:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore RD, Chaisson RE. Natural history of HIV infection in the era of combination antiretroviral therapy. AIDS 1999; 13:1933–1942. [DOI] [PubMed] [Google Scholar]
- 13.Murphy EL, Collier AC, Kalish LA, Assmann SF, Para MF, Flanigan TP, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med 2001; 135:17–26. [DOI] [PubMed] [Google Scholar]
- 14.Nesheim SR, Kapogiannis BG, Soe MM, Sullivan KM, Abrams E, Farley J, et al. Trends in opportunistic infections in the pre- and post-highly active antiretroviral therapy eras among HIV-infected children in the Perinatal AIDS Collaborative Transmission Study, 1986–2004. Pediatrics 2007; 120:100–109. [DOI] [PubMed] [Google Scholar]
- 15.Podlekareva D, Mocroft A, Dragsted UB, Ledergerber B, Beniowski M, Lazzarin A, et al. Factors associated with the development of opportunistic infections in HIV-1-infected adults with high CD4+ cell counts: a EuroSIDA study. J Infect Dis 2006; 194:633–641. [DOI] [PubMed] [Google Scholar]
- 16.Bonnet F, Chene G, Thiebaut R, Dupon M, Lawson-Ayayi S, Pellegrin JL, et al. Trends and determinants of severe morbidity in HIV-infected patients: the ANRS CO3 Aquitaine Cohort, 2000–2004. HIV Med 2007; 8:547–554. [DOI] [PubMed] [Google Scholar]
- 17.Bonnet F, Lewden C, May T, Heripret L, Jougla E, Bevilacqua S, et al. Opportunistic infections as causes of death in HIV-infected patients in the HAART era in France. Scand J Infect Dis 2005; 37:482–487. [DOI] [PubMed] [Google Scholar]
- 18.Brooks JT, Kaplan JE, Holmes KK, Benson C, Pau A, Masur H. HIV-Associated Opportunistic Infections-Going, Going, But Not Gone: The Continued Need for Prevention and Treatment Guidelines. Clin Infect Dis 2009; 48:609–611. [DOI] [PubMed] [Google Scholar]
- 19.Buchacz K, Baker RK, Moorman AC, Richardson JT, Wood KC, Holmberg SD, et al. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. AIDS 2008; 22:1345–1354. [DOI] [PubMed] [Google Scholar]
- 20.Buchacz K, Baker RK, Palella FJ Jr., Chmiel JS, Lichtenstein KA, Novak RM, et al. AIDS-defining opportunistic illnesses in US patients, 1994–2007: a cohort study. AIDS 2010; 24:1549–1559. [DOI] [PubMed] [Google Scholar]
- 21.Gebo KA, Fleishman JA, Moore RD. Hospitalizations for metabolic conditions, opportunistic infections, and injection drug use among HIV patients: trends between 1996 and 2000 in 12 states. J Acquir Immune Defic Syndr 2005; 40:609–616. [DOI] [PubMed] [Google Scholar]
- 22.Hooshyar D, Hanson DL, Wolfe M, Selik RM, Buskin SE, McNaghten AD. Trends in perimortal conditions and mortality rates among HIV-infected patients. AIDS 2007; 21:2093–2100. [DOI] [PubMed] [Google Scholar]
- 23.Lewden C, May T, Rosenthal E, Burty C, Bonnet F, Costagliola D, et al. Changes in causes of death among adults infected by HIV between 2000 and 2005: The “Mortalite 2000 and 2005” surveys (ANRS EN19 and Mortavic). J Acquir Immune Defic Syndr 2008; 48:590–598. [DOI] [PubMed] [Google Scholar]
- 24.Mocroft A, Sterne JA, Egger M, May M, Grabar S, Furrer H, et al. Variable impact on mortality of AIDS-defining events diagnosed during combination antiretroviral therapy: not all AIDS-defining conditions are created equal. Clin Infect Dis 2009; 48:1138–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smit C, Geskus R, Walker S, Sabin C, Coutinho R, Porter K, et al. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS 2006; 20:741–749. [DOI] [PubMed] [Google Scholar]
- 26.Maurer T, Ponte M, Leslie K. HIV-associated Kaposi’s sarcoma with a high CD4 count and a low viral load. N Engl J Med 2007; 357:1352–1353. [DOI] [PubMed] [Google Scholar]
- 27.Mocroft A, Kirk O, Clumeck N, Gargalianos-Kakolyris P, Trocha H, Chentsova N, et al. The changing pattern of Kaposi sarcoma in patients with HIV, 1994–2003: the EuroSIDA Study. Cancer 2004; 100:2644–2654. [DOI] [PubMed] [Google Scholar]
- 28.Palella FJ, Baker RK, Buchacz K, Chmiel JS, Tedaldi EM, Novak RM, et al. Increased mortality among publicly insured participants in the HIV Outpatient Study despite HAART treatment. AIDS 2011; 25:1865–1876. [DOI] [PubMed] [Google Scholar]
- 29.Puhan MA, Van Natta ML, Palella FJ, Addessi A, Meinert C, Ocular Complications of ARG. Excess mortality in patients with AIDS in the era of highly active antiretroviral therapy: temporal changes and risk factors. Clin Infect Dis 2010; 51:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simmons RD, Ciancio BC, Kall MM, Rice BD, Delpech VC. Ten-year mortality trends among persons diagnosed with HIV infection in England and Wales in the era of antiretroviral therapy: AIDS remains a silent killer. HIV Med 2013; 14:596–604. [DOI] [PubMed] [Google Scholar]
- 31.Jabs DA, Holbrook JT, Van Natta ML, Clark R, Jacobson MA, Kempen JH, et al. Risk factors for mortality in patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology 2005; 112:771–779. [DOI] [PubMed] [Google Scholar]
- 32.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 1999; 5:512–517. [DOI] [PubMed] [Google Scholar]
- 33.Grigoryan A, Hall HI, Durant T, Wei X. Late HIV diagnosis and determinants of progression to AIDS or death after HIV diagnosis among injection drug users, 33 US States, 1996–2004. PLoS One 2009; 4:e4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarcz S, Hsu L, Dilley JW, Loeb L, Nelson K, Boyd S. Late diagnosis of HIV infection: trends, prevalence, and characteristics of persons whose HIV diagnosis occurred within 12 months of developing AIDS. J Acquir Immune Defic Syndr 2006; 43:491–494. [DOI] [PubMed] [Google Scholar]
- 35.Jabs DA, Van Natta ML, Holbrook JT, Kempen JH, Meinert CL, Davis MD. Longitudinal study of the ocular complications of AIDS - 1. Ocular diagnoses at enrollment. Ophthalmology 2007; 114:780–786. [DOI] [PubMed] [Google Scholar]
- 36.Jabs DA, van Natta ML, Kempen JH, Pavan PR, Lim JI, Murphy RL, et al. Characteristics of patients with cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol 2002; 133:48–61. [DOI] [PubMed] [Google Scholar]
- 37.Jabs DA, Van Natta ML, Holbrook JT, Kempen JH, Meinert CL, Davis MD, et al. Longitudinal study of the ocular complications of AIDS: 2. Ocular examination results at enrollment. Ophthalmology 2007; 114:787–793. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Morb Mortal Wkly Rep 2009; 58:1–207. [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morb Mortal Wkly Rep 1992; 41:1–19. [PubMed] [Google Scholar]
- 40.Crum-Cianflone N, Hullsiek KH, Marconi V, Weintrob A, Ganesan A, Barthel RV, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS 2009; 23:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mocroft A, Katlama C, Johnson AM, Pradier C, Antunes F, Mulcahy F, et al. AIDS across Europe, 1994–98: the EuroSIDA study. Lancet 2000; 356:291–296. [DOI] [PubMed] [Google Scholar]
- 42.Moore RD, Bartlett JG. Dramatic decline in the HIV-1 RNA level over calendar time in a large urban HIV practice. Clin Infect Dis 2011; 53:600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sax PE. Antiretroviral therapy: now “it just works”. Clin Infect Dis 2011; 53:605–608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.